Abstract
The hallmark features of allergic asthma are type 2 (eosinophilic) inflammation and airways hyperresponsiveness (AHR). Although these features often comanifest in mouse lungs in vivo, we demonstrate in this study that the serine protease Alp1 from the ubiquitous mold and allergen, Aspergillus fumigatus, can induce AHR in mice unable to generate eosinophilic inflammation. Strikingly, Alp1 induced AHR in mice devoid of protease-activated receptor 2/F2 trypsin-like receptor 1 (PAR2/F2RL1), a receptor expressed in lung epithelium that is critical for allergic responses to protease-containing allergens. Instead, using precision-cut lung slices and human airway smooth muscle cells, we demonstrate that Alp1 directly increased contractile force. Taken together, these findings suggest that Alp1 induces bronchoconstriction through mechanisms that are largely independent of allergic inflammation and point to a new target for direct intervention of fungal-associated asthma.
INTRODUCTION
Asthma is a chronic inflammatory disorder of the lower respiratory tract defined by intermittent and reversible airways obstruction (1). Although airway luminal narrowing because of excessive bronchial smooth muscle contraction and mucous accumulation is uniformly present, “asthma” is now considered a catch-all term for a group of heterogeneous disorders and molecular pathways leading to a final common phenotype (2, 3). In most cases, asthmatic lungs feature evidence of type 2 immune responses induced by atopy, including IgE responses to environmental allergens; these findings are typically associated with an influx of eosinophils and Th2 cells into the airways and elevated levels of type 2 cytokines, including IL-4, IL-5, and IL-13 (4). Current treatment approaches target inflammation and bronchial smooth muscle hypercontractility and include inhaled corticosteroids and β2 adrenergic agonists, respectively; newer therapies include neutralizing Abs against specific cytokines or mediators (e.g., anti-IgE, anti-IL-4/5/13) (5). Despite this expanded treatment arsenal, 5–15% of patients have severe, treatment-resistant asthma characterized by persistent symptoms and recurrent exacerbations requiring systemic corticosteroids and/or hospitalization (6, 7).
Allergy to environmental fungi, particularly to allergens from the ubiquitous mold Aspergillus fumigatus, is frequently associated with severe asthma. Clinical syndromes range from severe asthma with fungal sensitization, which is now recognized as a specific disease subset, to allergic bronchopulmonary aspergillosis, a phenotype characterized by high serum IgE and lung tissue damage leading to bronchiectasis (8). The mechanisms underlying A. fumigatus–associated asthma are not fully understood. Protease activity is a common characteristic shared by many environmental allergens that induce asthma, and we and others have studied the role of A. fumigatus–associated proteases in the induction of these phenotypes (9). We found that a specific A. fumigatus–associated serine protease, alkaline protease 1 (Alp1), a known A. fumigatus–derived allergen (Asp f13), was detected in the airways of asthmatics but not healthy controls (10). Immunoreactive Alp1 detected in the epithelium and airway smooth muscle (ASM) bundles in lung biopsies from subjects with asthma increased concomitantly with severe disease and correlated strongly with impairment of lung function and medication (corticosteroid) requirements (11).
In critical mechanistic studies, we demonstrated that application of Alp1 enhanced excitation-contraction signaling in ASM cells (10). In addition, Alp1 promoted airway contraction in response to a bronchoconstrictor, carbachol (CCh), ex vivo in precision-cut lung slices (PCLS) prepared from normal human lung tissue biopsy specimens and also from lungs of naive mice. In related studies, Kheradmand et al. (12) found that A. fumigatus–associated serine protease activity degraded fibrinogen in the airways, leading to activation of TLR4 on airway epithelial cells and macrophages by fibrinogen cleavage products and resulting in increased mucus production (13). Thus, apart from its role as an allergen, these findings suggest that A. fumigatus can elicit innate immune responses and contribute directly to airway remodeling and bronchoconstriction through pathways that are not dependent on generation of IgE.
In this study, we investigated the significance of these in vitro findings and their contributions to the asthmatic phenotype in vivo. Using short-term, repetitive allergen challenges to the lower respiratory tract, we established a model of A. fumigatus–associated asthma in mice that features allergic inflammation, and, most notably, both spontaneous increases in airway resistance as well as airways hyperresponsiveness (AHR) to bronchoconstrictors such as methacholine (MCh). We determined that serine protease activity of A. fumigatus is required to elicit AHR and that the protease Alp1, acting alone, is sufficient to induce this phenotype. Taken together, our findings suggest that fungal proteases may be targets for intervention in severe asthma with fungal sensitization and/or severe allergic asthma.
MATERIALS AND METHODS
Reagents and cells
4-(2-Aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF), Rho-kinase inhibitor Y27632, MCh, and CCh were purchased from Sigma-Aldrich. Well characterized human ASM cells (14) were obtained through the gift of the Hope Organ and Tissue Donor Network. During growth and maintenance, the cells were cultured in serum-containing DMEM/F12 supplemented with 10% FBS (35–010-CV; Corning Life Sciences), 1% penicillin–streptomycin (P0781; Sigma-Aldrich), 1% l-glutamine (25030149; Thermo Fisher Scientific), 1% amphotericin B (15290018; Thermo Fisher Scientific), 0.17% 1 M CaCl2 × 2H2O, and 1.2% 1 M NaOH. Prior to force measurements, the cells were cultured under serum-deprived conditions by replacement of FBS with a 1% insulin–transferrin–selenium supplement (25–800-CR; Corning Life Sciences). All measurements were performed using cells at passage seven.
Alp1 purification
Alp1 protease was purified from A. fumigatus allergen extracts (HollisterStier Allergy) according to a previously described method (10).
Animals
BALB/c, C57BL/6, Δdbl-gata, and protease-activated receptor 2 (PAR2)–deficient (F2rl1−/−) mice were obtained from The Jackson Laboratory. Eight- to twelve-week-old mice were used in experiments. All mice were bred and maintained under pathogen-free conditions at an American Association for the Accreditation of Laboratory Animal Care accredited animal facility at the National Institute of Allergy and Infectious Diseases and housed in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals under an animal study proposal approved by the National Institute of Allergy and Infectious Diseases Animal Care and Use Committee (LAD3E).
Acute allergen challenge model
Mice were challenged intranasally with PBS, A. fumigatus extract (12.5 μg in 40 μl PBS), or heat-inactivated (HI) A. fumigatus extract (95°C for 30 min). In experiments featuring Alp1, mice were challenged intranasally with purified Alp1 (1.25 μg per challenge in 40 μl PBS). Mice were challenged three times per week over a 2-wk period. Lung resistance and inflammation were assessed on day 14 at 48 h after the final allergen challenge as described below.
Protease activity assay
Protease activity was assessed using the Fluoro Protease Assay Kit (G-Biosciences), a method that features FITC-labeled casein as a general protease substrate and carried out according to the manufacturer’s instructions. Samples were diluted in 1× Fluoro Assay Buffer and added to wells of a 96-well fluorometer-compatible titer plate. FITC-conjugated casein assay substrate was added to the wells and incubated at room temperature for 2 h. Fluorescence intensity was determined using a FilterMax F5 multimode microplate reader at an excitation wavelength of 485 nm and an emission wavelength of 530 nm. Buffer without protease was used as a blank for background subtraction.
Airway resistance measurements in live mice
Briefly, mice were anesthetized by i.p. injection of a ketamine and xylazine mixture (100 and 10 mg/kg, respectively). For each mouse, a 0.5-cm incision was made in the trachea, which was then cannulated with an 18-gauge catheter. The cannulated mouse was connected to the flexiVent system (SCIREQ, Montreal, QC, Canada), and vecuronium bromide (0.1 mg/kg) was administered by i.p. injection. After termination of spontaneous respirations, airway responsiveness was measured following inhalation of nebulized saline and increasing concentrations of nebulized MCh (0, 6.25, 12.5, 25, 50, 75 mg/ml). The broadband forced oscillation technique was used to determine Newtonian resistance in the conducting airways (Rn) with the QuickTime 3 perturbation.
Bronchoalveolar lavage fluid cytokine and cell analysis
Bronchoalveolar lavage fluid (BALF) was collected by instilling of ice-cold HBSS (1.5 ml per mouse) intratracheally, and collected BALF was centrifuged at 1500 rpm at 4°C for 10 min. Supernatants were stored at 280°C prior to cytokine assays. Total cell numbers were determined by counting on a hemocytometer. Differentials were determined by examining a Diff-Quick–stained cytospin preparation (500 cells per slide at 40× magnification). Cytokines were analyzed in cell-free BALF using a customized Bio-Plex 10-plex Express Kit (Bio-Rad Laboratories) according to instructions provided by the manufacturer. All samples were run in duplicate.
PCLS airway contraction
Mice were sacrificed by isoflurane inhalation and gentle cervical dislocation. Isolation and contraction of mouse PCLS were performed as previously described (15).
Measurement of ASM contraction
ASM cells were serum deprived and cultured to confluence upon deformable polydimethylsiloxane NuSil Gel 8100 (NuSil Technology) substrates prepared in 96-well plates and examined for force changes using contractile force screening as described previously (16, 17). Briefly, based on the displacement of substrate-bound fluorescent beads relative to a cell-free image and with knowledge of substrate stiffness (0.36 kPa) and thickness (0.15 mm), cellular contractile maps were obtained using the approach of monolayer traction cytometry (18). From each map, we calculated the strain energy, a measure of average contractile strength.
Histological analysis
For light microscopic study, lung tissues were fixed in neutral-buffered formalin and embedded in paraffin. Sections were prepared and stained with H&E or periodic acid–Schiff (PAS) stain. Images were acquired at 40× original magnification using a Leica DMI400 light microscope equipped with an Infinity3 charge-coupled device camera (Teledyne Lumenera). Quantification of PAS stain was performed using Fiji (ImageJ). Color deconvolution (haematoxylin periodic acid Schiff PAS) was performed on the image by setting a threshold to define the stained region. Regions of interest were drawn around the airway, excluding the luminal area. Staining of the region of interest was determined using the “limited to threshold” function and dividing by the total airway area.
A. fumigatus–specific IgE
Mouse serum was collected from animals by retro-orbital bleeds. A. fumigatus–specific IgE was measured by ELISA using 96-well plates coated with A. fumigatus extract (5 μg/ml diluted in coating buffer, catalog no. E101; Bethyl Laboratories) overnight at 4°C. Wells were incubated in blocking buffer (PBS/1% BSA [pH 8] plus 0.05% Tween) (catalog no. E101; Bethyl Laboratories) for 1 h prior to addition of sera diluted in blocking buffer. Bound Ab was detected with alkaline phosphatase–labeled anti-mouse IgE (1:1000) (catalog no. A90–115AP; Bethyl laboratories) for 1 h. Relative absorbance was determined using a FilterMax F5 multimode microplate reader (OD450).
RESULTS
Repetitive administration of a fungal extract from A. fumigatus results in a spontaneous increase in airway resistance and hyperresponsiveness to bronchoconstrictors: the role of proteolytic activity
In our previous work, we demonstrated that administration of an extract derived from the fungus A. fumigatus to the airways of BALB/c mice resulted in profound AHR in association with allergic airway inflammation (10). To determine the role of A. fumigatus–derived serine proteases in promoting these respiratory responses, we first assessed protease activity in the commercial allergen extract. Confirming our previous results, we found that the A. fumigatus extract contained readily detectable protease activity. Protease activity was completely abolished by HI (95°C for 30 min) and partially inhibited (~50%) by the serine protease inhibitor AEBSF (30 mM; Fig. 1A). We administered A. fumigatus, HI–A. fumigatus, AEBSF-treated A. fumigatus, or PBS via the intranasal route for a total of six doses over a period of 2 wk and measured airway resistance by plethysmography in mechanically ventilated mice (Fig. 1B). As shown in Fig. 1C, the A. fumigatus–treated mice demonstrated increased airway resistance at baseline prior to the addition of the spasmogen MCh compared with the PBS-challenged controls. Likewise, we also observed significantly more airway resistance in response to MCh in the A. fumigatus–challenged cohort. By contrast, airway resistance in mice challenged with HI–A. fumigatus or AEBSF-treated A. fumigatus was not significantly different from responses induced by PBS alone. From these studies, we conclude that A. fumigatus challenge to the respiratory mucosa induces asthma in mice, which is directly dependent on the serine protease activity of the allergen.
FIGURE 1. Repetitive administration of A. fumigatus induces AHR in mice: the role of proteolytic activity.
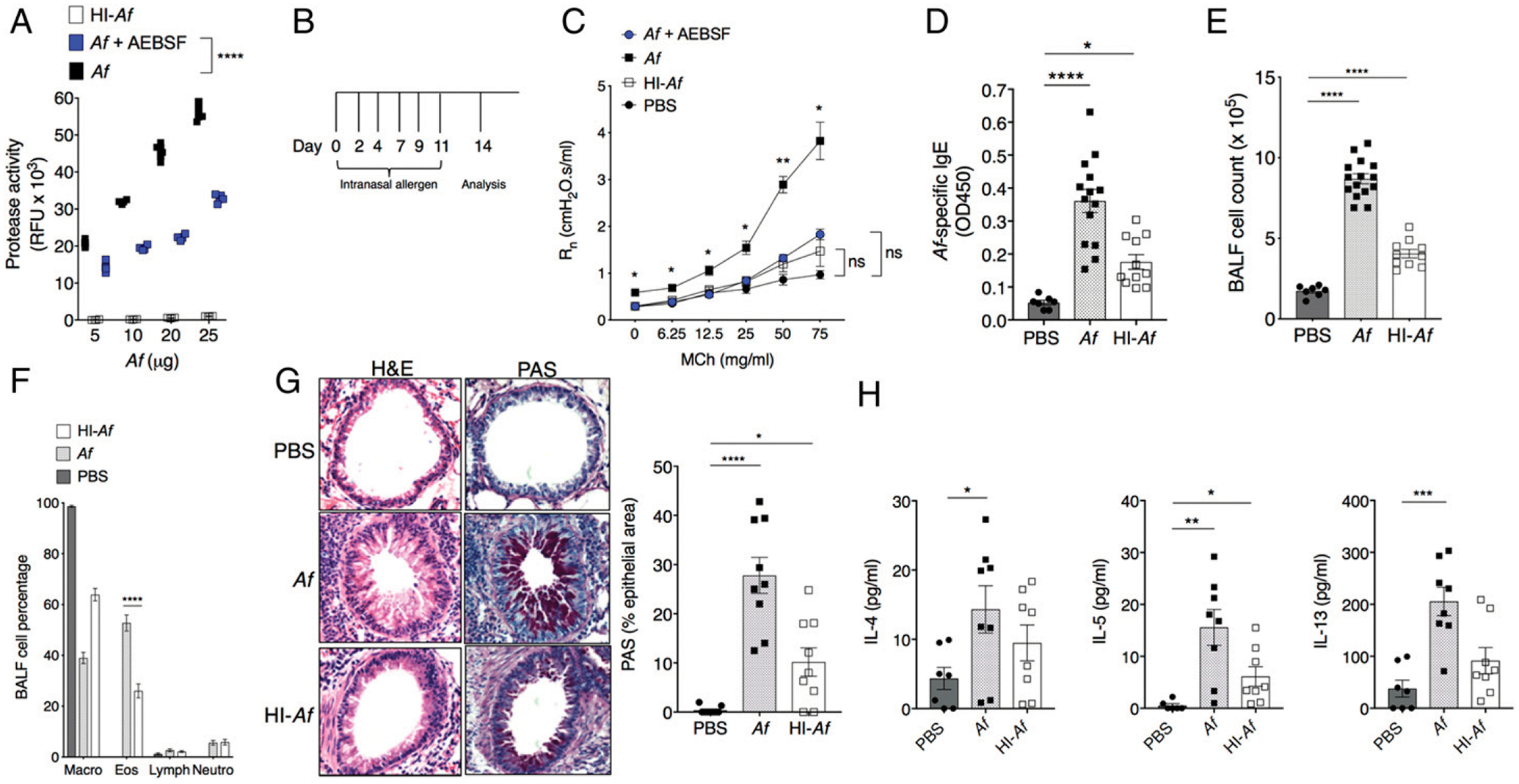
(A) Protease activity of A. fumigatus, HI–A. fumigatus, and AEBSF-treated A. fumigatus (5–25 μg/m1) was determined using a fluoro protease assay as described in Materials and Methods. Values are duplicates from two independent experiments. ****p < 0.0001, t test, corrected for multiple comparisons by Holm-Sidak. (B) Schematic representation of the protocol for intranasal challenge of mice with allergen. Mice were evaluated 48 h after the final challenge. (C) Rn in response to aerosolized MCh in A. fumigatus, HI–A. fumigatus, or AEBSF–A. fumigatus–challenged BALB/c mice. Each symbol represents mean ± SEM from three to eight mice per group analyzed in two independent experiments. *p < 0.04, **p = 0.002, t test corrected for multiple comparisons by Holm-Sidak. (D) Serum levels of A. fumigatus–specific IgE. Values are mean ± SEM of samples assayed in duplicate. *p = 0.01, ****p < 0.0001, Kruskal–Wallis ANOVA with Benjamini, Krieger, and Yekutieli corrections for multiple comparisons. (E and F) Total cell counts (E) and cell composition (F) in BALF. Values are mean ± SEM of 7–15 mice per group. ****p < 0.0001 versus PBS, two-way ANOVA, Tukey multiple comparisons. (G) Lung inflammation and airway mucin evaluated by H& E (left) and PAS (right) staining. Images are representative of nine mice per group. Bar graph on right shows PAS+ area (mean ± SEM) within the airway epithelium. *p = 0.03, ****p < 0.0001, one-way ANOVA, Dunnett multiple comparisons. (H) IL-4, IL-5, and IL-13 levels in BALF; mean ± SEM of seven to eight mice per group. IL-4: *p = 0.03, one-way ANOVA, Dunnett multiple comparisons. IL-5, IL-13: *p = 0.03, **p = 0.001, ***p = 0.0004, Kruskal–Wallis ANOVA, Benjamini, Krieger, and Yekutieli corrections for multiple comparisons. RFU, relative fluorescence units.
Given these results, we proceeded to analyze the impact of these inoculation strategies on airway inflammation. Mice undergoing repetitive challenge with A. fumigatus or HI–A. fumigatus, as described above, developed a systemic allergic response, as indicated by detection of A. fumigatus–specific IgE in serum (Fig. 1D). Likewise, mice treated with A. fumigatus or HI–A. fumigatus developed allergic inflammation within the lower respiratory tract, including eosinophil accumulation in and around the airways (Fig. 1E, 1F) and airway mucous (mucin staining) (Fig. 1G) at levels significantly higher than those detected in response to PBS alone. Notably, HI largely abolishes the increase in BALF Th2 cytokines observed in response to repetitive administration of A. fumigatus, except for IL-5 (Fig. 1H). From this second group of findings, we can conclude that, whereas repetitive administration of A. fumigatus also results in allergic inflammation in the respiratory tract, A. fumigatus–induced AHR is not directly linked to this inflammatory response.
Repetitive administration of the A. fumigatus serine protease Alp1 promotes both AHR and allergic lung inflammation
A. fumigatus can synthesize and secrete many serine proteases, depending on culture conditions in vitro, and may produce a different set of proteases in vivo when colonizing and/or infecting human target tissues (19). We focused on Alp1, as we showed previously that this A. fumigatus–derived protease induced structural and functional changes in ASM in vitro and generated bronchoconstriction in lungs in the PCLS assay ex vivo (10). To determine the role of Alp1 in A. fumigatus–evoked AHR, we challenged mice with native Alp1 purified from A. fumigatus allergen extracts (Fig. 2A). As noted in our previous publication (10), Alp1 had measurable protease activity, and, as indicated in this study, our preparation is~10 times as concentrated as compared with protease activity in the source A. fumigatus extract (Fig. 2B). Interestingly, repetitive challenge with Alp1 did not increase spontaneous airway resistance over that of PBS-treated controls, analogous to what we observed in response to repetitive challenge with A. fumigatus. However, challenge with Alp1 did elicit bronchospasm in response to inhaled MCh that was comparable to that induced by A. fumigatus (Fig. 2C). HI of Alp1 eliminated this response. Furthermore, Alp1 elicited an allergic response in lungs that was on a par with that detected in response to A. fumigatus, including generation of allergen-specific IgE (Fig. 2D), airway and submucosal eosinophilic inflammation, mucous production, and type 2 cytokine response (Fig. 2E–H). In contrast to what we observed with A. fumigatus, HI of Alp1 virtually abolished lung inflammation secondary to mucosal challenge. Thus, repetitive challenge with Alp1 induces both AHR and allergic inflammation in mice, and serine protease activity is required to elicit both responses.
FIGURE 2. Active Alp1 protease induces both AHR and eosinophilic inflammation.
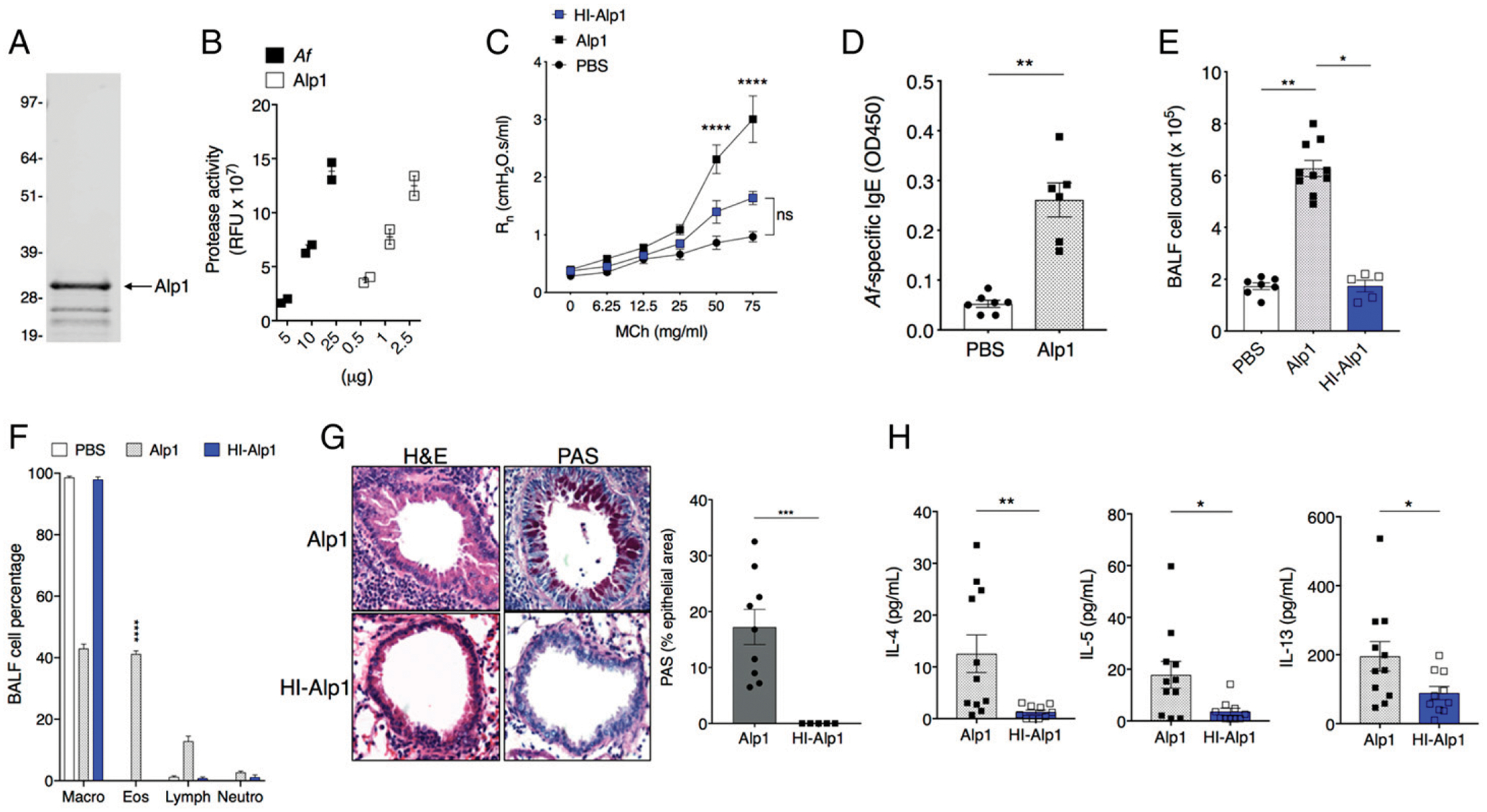
(A) Coomassie-stained SDS-PAGE gel of purified Alp1 (33 kDa). The lower bands most likely represent proteolytic cleavage, as Alp1 is susceptible to proteolysis (12). (B) Protease assay in crude A. fumigatus extracts in comparison with purified Alp1; x-axis denotes total amount of protein in each sample. Values are duplicates from two independent experiments. (C) Rn in response to MCh in PBS, Alp1, or HI-Alp1–challenged BALB/c mice. Values are mean ± SEM of three to seven mice per group. ****p < 0.0001 versus PBS, two-way ANOVA, Tukey multiple comparisons. (D). Serum levels of A. fumigatus–specific IgE determined as in Fig. 1. **p = 0.001, Mann–Whitney U test. (E and F) Total cell counts (E) and cell composition (F) in BALF. (E) *p = 0.01, **p = 0.001, Kruskal–Wallis ANOVA, Dunn multiple comparisons. (F) ****p < 0.0001, one-way ANOVA, Sidak multiple comparisons versus PBS or HI-Alp1. (G) Lung inflammation and airway mucin evaluated by H& E (left) and PAS (right) staining. Images are representative of five to nine mice per group. Original magnification ×10. Bar graph on right shows PAS+ area within the airway epithelium. ***p = 0.001, Mann–Whitney. (H) IL-4, IL-5, and IL-13 levels in BALF. *p < 0.03, **p = 0.002, Mann–Whitney. All symbols in bar graphs represent results from an individual mouse; error bars are mean ± SEM of at least two independent experiments.
Eosinophilic lung inflammation is not required for development of Alp1-evoked AHR in mice
Although eosinophils are frequently found in the airways of patients with asthma, eosinophil-targeted therapies are not universally effective in treating patients with eosinophilic asthma (20). With this in mind, we examined the link between eosinophilic lung inflammation and Alp1-induced AHR using the eosinophil-deficient mouse strain ΔdblGATA on the BALB/c background (21). Repetitive challenge with Alp1 elicited increased airway resistance in response to MCh in ΔdblGATA mice compared with PBS controls (Fig. 3A); this was accompanied by leukocyte infiltration into the airways, primarily neutrophils and lymphocytes (Fig. 3B, 3C). Challenge of eosinophil-deficient ΔdblGATA mice with Alp1 also evoked peribronchial inflammation and mucin in the airways (Fig. 3D) and resulted in increased levels of type 2 cytokines in BALF (Fig. 3E) compared with PBS-challenged controls. The findings in this study are consistent with those presented earlier regarding the contributions of allergic inflammation to AHR in this model. Specifically, these results indicate that eosinophils make no critical contributions to Alp1-mediated AHR.
FIGURE 3. Intranasal inoculation with A. fumigatus or Alp1 induces AHR in the absence of eosinophilic inflammation.
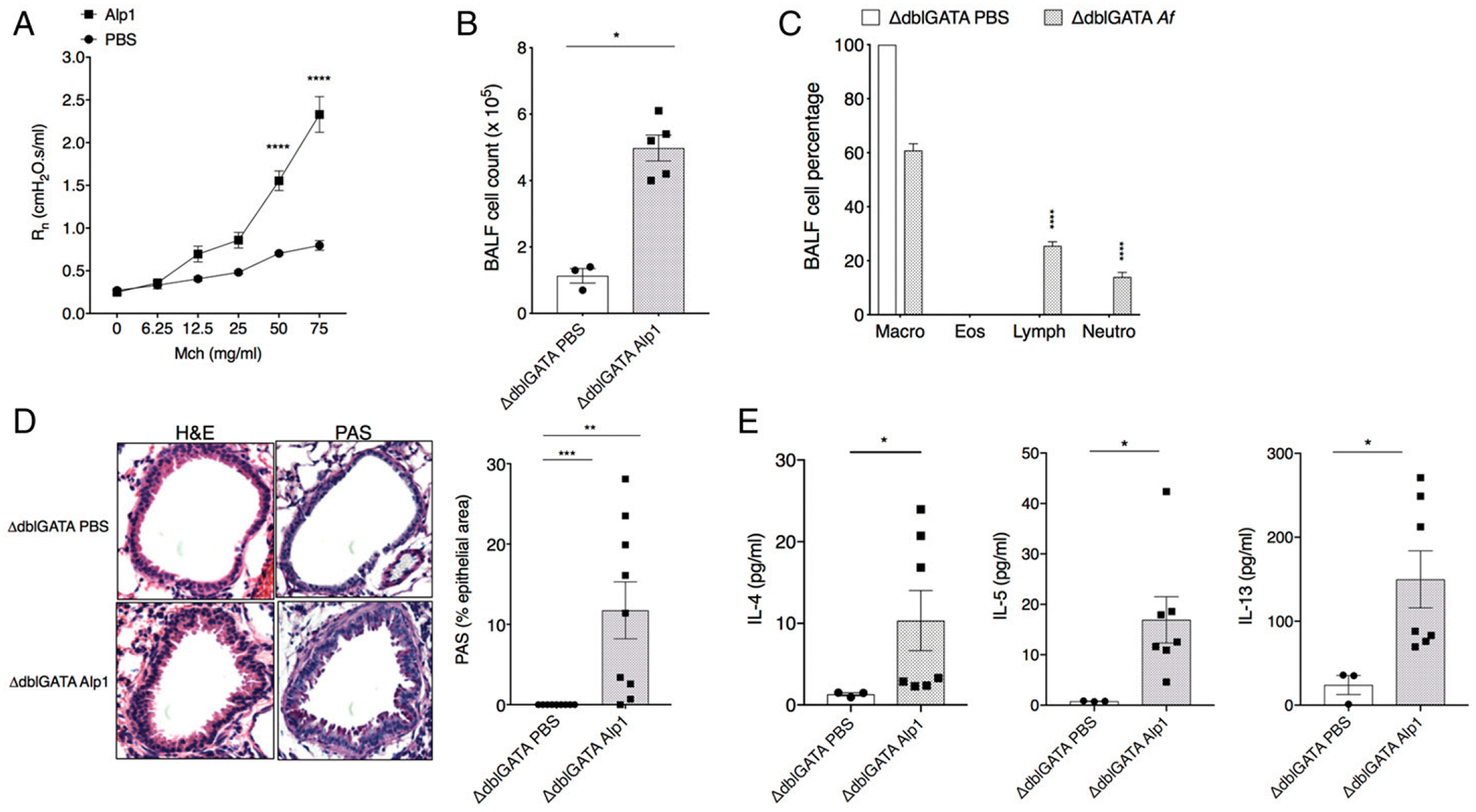
(A) Airway resistance in response to repetitive challenge with A. fumigatus, Alp1, or PBS. Rn in eosinophil-deficient Δdblgata mice in response to aerosolized MCh. Data represent three to six mice per group. ****p < 0.0001, two-way ANOVA, Sidak multiple comparisons. (B and C) Total cell counts (B) and cell composition (C) in BALF. (B) *p = 0.03, Mann-Whitney. (C) ****p < 0.0001, two-way ANOVA, Sidak multiple comparisons. (D) Lung inflammation and airway mucin evaluated by H& E (left) and PAS (right) staining. Images are representative of three to five mice per group. Original magnification ×10. Bar graph on right shows PAS+ area within the airway epithelium. **p = 0.005, ***p = 0.0004, Mann-Whitney. (E) IL-4, IL-5, and IL-13 levels in BALF. *p = 0.01, Mann-Whitney. All symbols in bar graphs represent results from an individual mouse; error bars are mean ± SEM; all results represent at least two independent experiments.
Role of PAR2/F2RL1 in responses to Alp1
Protease-activated receptors, such as PAR2 (F2R-like trypsin receptor 1), are expressed in bronchial epithelial cells and ASM cells (22, 23) and have been implicated in generating responses to protease allergens (24). Given the sequence homology linking Alp1 to other fungal-derived serine proteases (25), we hypothesized that Alp1 may induce AHR by activating PAR2. To test this hypothesis, we administered Alp1 to F2rl1−/− mice (C57/Bl6 background) according to the previously established schedule. Alp1-associated increases in airway resistance and aberrant lung histology in wild-type (WT) and F2rl1−/−mice were indistinguishable from one another, with only slightly diminished numbers of total cells (Fig. 4A–E). This is especially intriguing given that levels of cytokines IL-4, IL-5, and IL-13 detected in BALF were not significantly increased over PBS-challenged C57/Bl6 WT or F2rl1−/− mice in this model (Fig. 4F). Taken together, these studies suggest that Alp1 promotes AHR independent of PAR2 signaling, and possibly through mechanisms independent of type 2 cytokines in the airways.
FIGURE 4. Alp1 promotes AHR independent of PAR2.
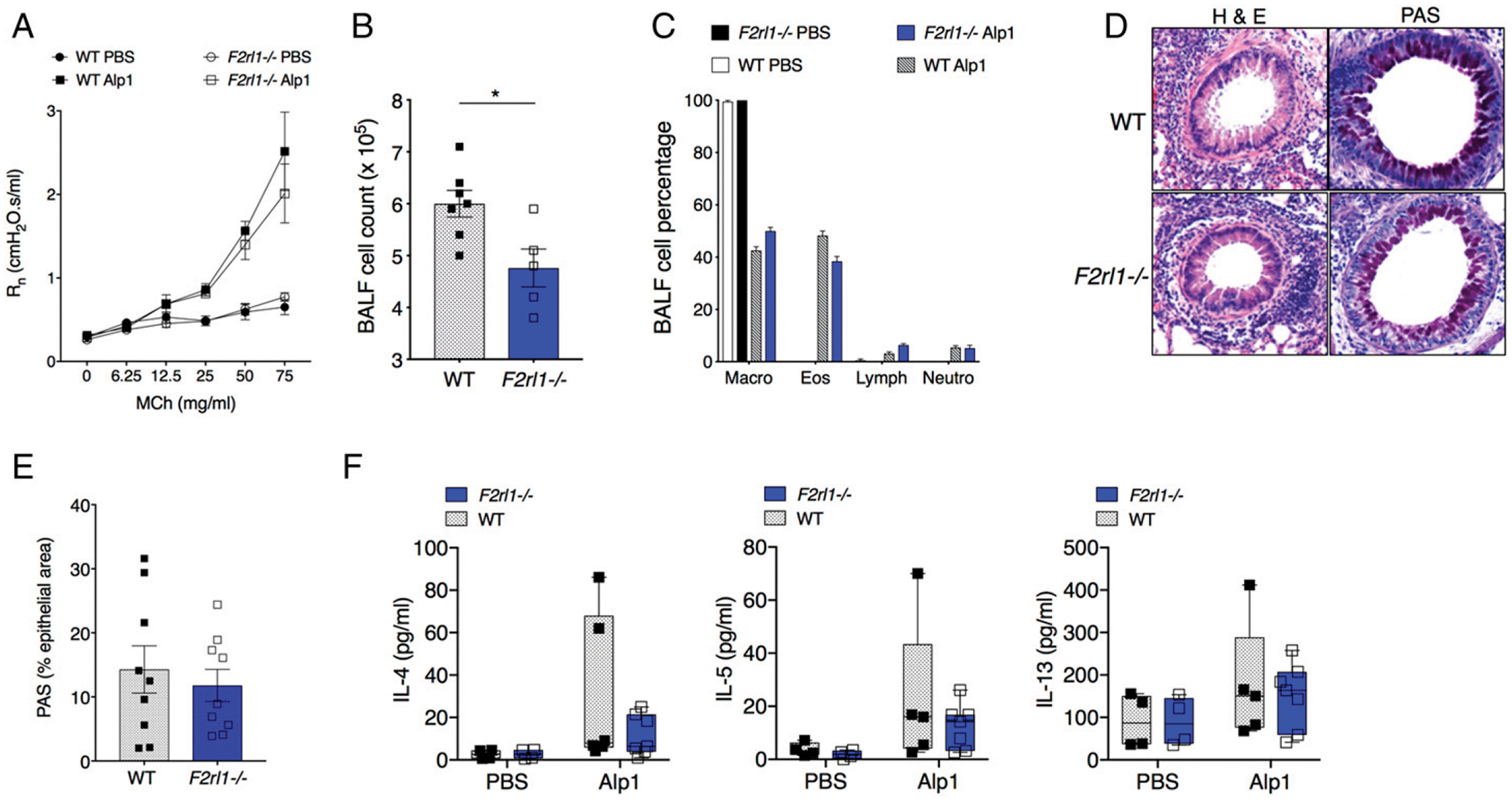
(A) R in Alp1-challenged F2rl1−/− mice that lack PAR2 expression (two to seven mice per group). (B and C) Total cell counts (B) and cell composition (C) in BALF. (B) *p = 0.01, unpaired t test. (D) Lung inflammation and airway mucin evaluated by H& E (left) and PAS (right) staining. Images are representative of three to five mice per group. Original magnification ×10. (E) PAS+ area within the airway epithelium. (F) IL-4, IL-5, and IL-13 levels in BALF. All symbols in bar graphs represent results from an individual mouse; error bars are mean ± SEM.; all results represent at least two independent experiments.
Administration of Alp1 induces bronchoconstriction by promoting ASM contraction
A limitation of plethysmography is that it only measures airway pressures, from which resistance measurements can be derived; it cannot evaluate ASM contraction directly and thereby differentiate between various causes of increased airway resistance. For example, the diameter of allergic airways be may be reduced because of airway remodeling resulting from epithelial and ASM hypertrophy, from increased luminal mucous within the lumen, or bronchial smooth muscle contraction (26). In our earlier work, we showed that Alp1 promoted bronchial contraction even in the absence of prior sensitization and challenge (10). To examine this point more completely, we determined the contribution of bronchoconstriction to the increased airway resistance of allergen-challenged mice by analyzing airway narrowing in mouse PCLS. As shown in Fig. 5A–C, PCLS from A. fumigatus–or Alp1-challenged mice underwent significantly more contraction than those isolated from PBS-treated mice (Emax: PBS = 46.3 ± 1.3%; A. fumigatus = 90.4 ± 0.91%; Alp1 = 77.8 ± 1.2%; p < 0.0001, extra-sum-of-squares F test). Contraction of PCLS from mice challenged with HI–A. fumigatus or HI-Alp1 could not be distinguished from PBS controls (Emax: HI–A. fumigatus = 54 ± 0.95%; HI-Alp1 = 47.3 ± 0.86%). These findings indicate that Alp1 challenge increases airway resistance in mice by promoting bronchoconstriction. In our previous study, we found that Alp1 degraded extracellular matrix components, which led to integrin- and focal adhesion–dependent increases in Ca2+ flux and RhoA activation) in ASM cells in vitro (10). Based on these findings, we hypothesized that Alp1directly increases ASM contractile force. To test this and to extend our findings to humans, we treated human ASM cells with vehicle or Alp1 for 24 h and evaluated them for contractile force differences. Application of Alp1 significantly increased spontaneous ASM contraction, even in the absence of additional stimuli, and this effect was completely blocked by the Rho-associated coiled-coil–containing protein kinase (ROCK) inhibitor Y27632 (Fig. 5D). Thus, we conclude that Alp1 contributes to the development of AHR directly by augmenting bronchial smooth muscle contraction through RhoA-dependent mechanisms.
FIGURE 5. Alp1 induces ASM contraction.
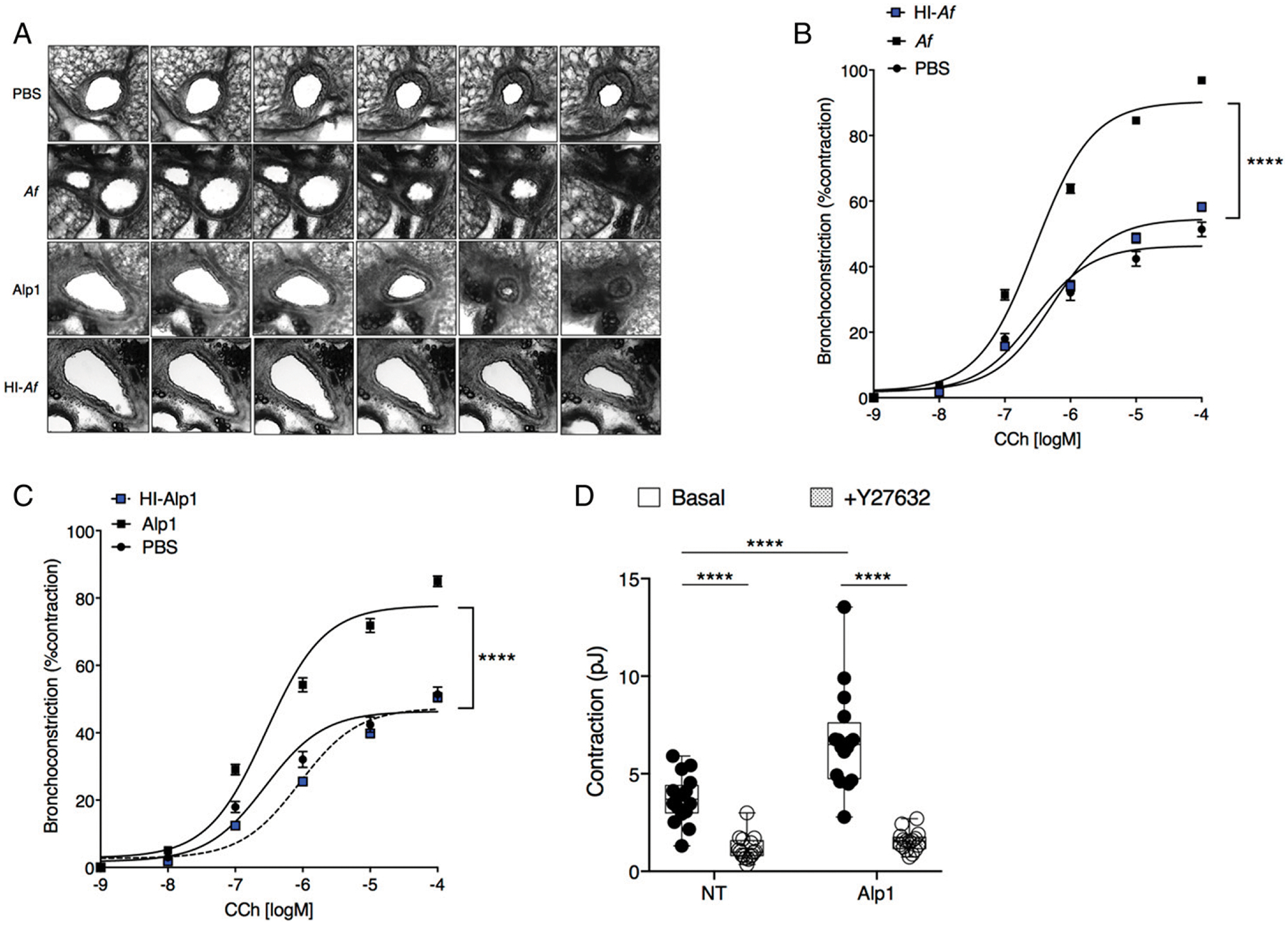
(A–C) Contraction of airways in PCLSs were determined at baseline and in response to CCh stimulation. Original magnification ×10. Results are expressed as the airway luminal area divided by area prior to stimulation. Data are mean ± SEM. of a minimum of 36 slices from four to eight mice per group evaluated in two or more independent experiments, fitted to a curve using the least-squares fit method. ****p < 0.0001, two-way ANOVA. (D) Contraction of human ASM cells were determined after a 24-h treatment with Alp1 (0.005 μg/ml) in the presence or absence of a Rho-kinase inhibitor (Y27632, 10 μM) for an additional 30 min. Results are mean ± SEM of strain energy (pJ) obtained over 16 separate regions of confluent ASM cells across four separate wells. ****p < 0.00001, two-way ANOVA, Tukey multiple comparisons.
DISCUSSION
This study advances our understanding of the role of fungal proteases and their impact on asthma pathogenesis. In this study, we focused on the A. fumigatus–derived serine protease Alp1, as our previous findings suggested that it exacerbates bronchial reactivity by enhancing contraction-related signaling pathways in ASM cells (10). We have extended these studies by demonstrating that Alp1 augments contraction of human ASM cells directly, which is consistent with our previous finding that it increases bronchoconstriction in PCLS from naive mice in the absence of allergic inflammation. Our results suggest that Alp1 enhancement of contraction of cultured ASM cells and airways in PCLS depends on RhoA activation, as both responses were blocked by Y27632, an inhibitor of ROCK. Y27632 is a selective, ATP-competitive inhibitor of both isoforms of ROCK (ROCK1 and ROCK2), with inhibitory constant values that are~700 nM for ROCK2 and the related Rho-activated kinase PRK2. In contrast, inhibitory constant values are 10–24-fold higher for other kinases, including MSK1, RSK2, and PHK (27). Given the potential for off-target effects of pharmacological inhibitors, complementary approaches, such as RhoA knockdown, may be required to confirm the signaling pathways involved. Finally, we show that repetitive challenge with Alp1 was sufficient to induce AHR in mice in a manner that is dependent on its serine protease activity but that did not require eosinophilic lung inflammation or interaction with PAR2.
Earlier studies have addressed the role(s) of Aspergillus-derived proteases and their ability to promote AHR in mouse models of allergic inflammation. Among them, Kurup et al. (28) administered high doses (50 μg) of native Alp1 by inhalation over a 4-wk period, followed by assessment of IgE responses, followed by allergen rechallenge. Although Alp1 induced significant increases in total serum IgE, type 2 lung inflammation, and increased airway resistance in response to systemic MCh, the authors did not comment on whether Alp1 used in these studies had detectable protease activity, which may be lacking in some preparations of native or recombinant proteins (29). More recently, two parallel studies evaluated the impact of repeated inhalation of commercially-sourced protease from Aspergillus oryzae (PAO). Millien et al. (13) found that PAO (9 μg, 10−3 endotoxin units/μg protein) elicited airway eosinophil recruitment and AHR, both of which were dependent on TLR4 expression. PAO activated TLR4 to restrict fungal growth and increase epithelial mucous production. In more recent work, the PAO used in these studies was demonstrated to be identical to Alp1 (12). In a separate study, Hiraishi and colleagues (30) demonstrated that three doses of PAO (2–32 μg) administered via the intranasal route over 3 d resulted in eosinophil recruitment to the airways and increased lung levels of type 2 lung cytokines through a mechanism that was independent of TLR2/4; these findings were abolished by HI of the protease, and AHR was not examined.
Thus, although several mechanisms can potentially explain Alp1-induced AHR in mouse models, their relative importance is difficult to assess because of differences in experimental methodology. The source of Aspergillus-derived protease(s), purification strategy, dose, duration of exposure, and strain of mice may influence susceptibility to development of allergic airways inflammation and bronchospasm. The A. fumigatus genome encodes 136 proteases, including serine, cysteine, aspartic, and metal-loproteinases (19). Accordingly, we found that a broadly active serine protease inhibitor, AEBSF, reduced but did not eliminate protease activity in A. fumigatus extracts (Fig. 1). In contrast to the aforementioned studies, we exposed mice to much lower doses of purified Alp1 (1.25 μg) containing substantially less endotoxin activity (<10−4 endotoxin units/μg (10). In addition, it is critical to note that in our studies repetitive inhalation of allergen over a sustained period resulted in a spontaneous increased airway resistance at homeostasis in the absence of MCh challenge and elicited hyperresponsiveness to a bronchoconstrictor, which are both features of allergic asthma in humans. In fact, elicitation of spontaneous bronchospasm by allergen challenge is almost unheard of in mouse models of asthma (31).
We examined the role of eosinophils in Alp1-induced AHR by performing our analyses in eosinophil-deficient ΔdblGATA mice. Although previous studies of OVA sensitization and challenge in this strain yielded conflicting conclusions as to the requirement of eosinophils for allergen-induced AHR in mice (32, 33), ΔdblGATA mice exposed to house dust mite allergens developed AHR equivalent to that observed in WT mice (34). Likewise, ΔdblGATA mice developed AHR in response to Alp1 challenge that was equivalent to WT controls, indicating that airway eosinophils are not required for the development of AHR to Alp1. However, persistence of neutrophils, lymphocytes, and type 2 cytokines in the lungs of these mice may have contributed to bronchial responsiveness. Future studies featuring other mouse strains with impaired immune responses to allergens (e.g., Rag1/2−/−, IgE−/−) may be necessary to fully dissect the relative importance of the allergic inflammatory milieu for Alp1-associated AHR.
We also found that Alp1-induced AHR did not require PAR2 expression, which differs strikingly from previous results obtained from challenge of F2rl1−/− (PAR2-deficient) mice with protease-containing allergens. For example, F2rl1−/− mice had diminished allergic lung inflammation and/or AHR following challenge with the filtrates from the mold Alternaria alternata or from house dust mite allergens (24, 35). In contrast to the findings of Hiraishi et al. (30), who found decreased eosinophilic inflammation in lungs of mice challenged with PAO, we observed essentially equivalent AHR and lung inflammation in WT or of F2rl1−/− mice treated with Alp1, indicating that this allergen acts independently of PAR2. In the case of A. alternata, an alkaline serine protease with limited homology to Alp1 (AASP; ~20 kDa) induced PAR2-dependent eosinophilic lung inflammation. However, AASP-induced relaxation of mouse bronchial rings ex vivo in a prostanoid-dependent fashion. Thus, fungal allergens may contain a mixture of proteases exerting variable effects on airway resistance, some of which may be independent of allergic inflammation.
Pretreatment of A. fumigatus with a broadly active serine protease inhibitor (AEBSF) did not completely abolish protease activity but reduced A. fumigatus–induced AHR to levels similar to that induced by HI A. fumigatus. These results strongly suggest that A. fumigatus–derived serine proteases are major contributors to AHR in this model. Previously, intranasal administration of AEBSF mice was shown to diminish AHR following challenge with cockroach allergen (36), supporting the value of therapeutic application of serine protease inhibitors. Published studies of the A. fumigatus protease “substrate-ome” demonstrated more than 200 proteins that were differentially cleaved by A. fumigatus proteases but not human serum (19), suggesting the feasibility of developing allergen-specific protease inhibitors. Most importantly, our direct measurements of force generation in ASM cells should be easily amenable to high throughput small molecule screening. Development of compounds targeting Alp1’s effects on ASM contractility will further clarify its role(s) in airway hyper-responsiveness in asthma.
Acknowledgments
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health (Projects Z01-AI-000746 and Z01-AI-000943). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Abbreviations used in this article:
- AEBSF
4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride
- AHR
airways hyperresponsiveness
- Alp1
alkaline protease 1
- ASM
airway smooth muscle
- BALF
bronchoalveolar lavage fluid
- CCh
carbachol
- HI
heat-inactivated, heat inactivation
- MCh
methacholine
- PAO
protease from Aspergillus oryzae
- PAR2
protease-activated receptor 2
- PAS
periodic acid–Schiff
- PCLS
precision-cut lung slice
- Rn
Newtonian resistance in the conducting airways
- ROCK
Rho-associated coiled-coil–containing protein kinase
- WT
wild-type
Footnotes
DISCLOSURES
The authors have no financial conflicts of interest.
REFERENCES
- 1.Mims JW 2015. Asthma: definitions and pathophysiology. Int. Forum Allergy Rhinol 5(Suppl. 1): S2–S6. [DOI] [PubMed] [Google Scholar]
- 2.Gelfand EW, and Schedel M. 2018. Molecular endotypes contribute to the heterogeneity of asthma. Immunol. Allergy Clin. North Am 38: 655–665. [DOI] [PubMed] [Google Scholar]
- 3.Chung KF, and Adcock IM. 2019. Precision medicine for the discovery of treatable mechanisms in severe asthma. Allergy. DOI: 10.1111/all.13771. [DOI] [PubMed] [Google Scholar]
- 4.Lambrecht BN, Hammad H, and Fahy JV. 2019. The cytokines of asthma. Immunity 50: 975–991. [DOI] [PubMed] [Google Scholar]
- 5.Wechsler ME 2018. Current and emerging biologic therapies for asthma and COPD. Respir. Care 63: 699–707. [DOI] [PubMed] [Google Scholar]
- 6.Gibson PG, and McDonald VM. 2017. Management of severe asthma: targeting the airways, comorbidities and risk factors. Intern. Med. J 47: 623–631. [DOI] [PubMed] [Google Scholar]
- 7.Teague WG, Phillips BR, Fahy JV, Wenzel SE, Fitzpatrick AM, Moore WC, Hastie AT, Bleecker ER, Meyers DA, Peters SP, et al. 2018. Baseline features of the severe asthma research program (SARP III) cohort: differences with age. J. Allergy Clin. Immunol. Pract 6: 545–554.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muldoon EG, Strek ME, and Patterson KC. 2017. Allergic and noninvasive infectious pulmonary aspergillosis syndromes. Clin. Chest Med 38: 521–534. [DOI] [PubMed] [Google Scholar]
- 9.Kubo M 2017. Innate and adaptive type 2 immunity in lung allergic inflammation. Immunol. Rev 278: 162–172. [DOI] [PubMed] [Google Scholar]
- 10.Balenga NA, Klichinsky M, Xie Z, Chan EC, Zhao M, Jude J, Laviolette M, Panettieri RA Jr., and Druey KM. 2015. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat. Commun 6: 6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basu T, Seyedmousavi S, Sugui JA, Balenga N, Zhao M, Kwon Chung KJ, Biardel S, Laviolette M, and Druey KM. 2018. Aspergillus fumigatus alkaline protease 1 (Alp1/Asp f13) in the airways correlates with asthma severity. J. Allergy Clin. Immunol 141: 423–425.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landers CT, Tung HY, Knight JM, Madison MC, Wu Y, Zeng Z, Porter PC, Rodriguez A, Flick MJ, Kheradmand F, and Corry DB. 2019. Selective cleavage of fibrinogen by diverse proteinases initiates innate allergic and antifungal immunity through CD11b. J Biol Chem. 294: 8834–8847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Millien VO, Lu W, Shaw J, Yuan X, Mak G, Roberts L, Song LZ, Knight JM, Creighton CJ, Luong A, et al. 2013. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science 341: 792–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comer BS, Camoretti-Mercado B, Kogut PC, Halayko AJ, Solway J, and Gerthoffer WT. 2014. MicroRNA-146a and microRNA-146b expression and anti-inflammatory function in human airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol 307: L727–L734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madigan LA, Wong GS, Gordon EM, Chen WS, Balenga N, Koziol-White CJ, Panettieri RA Jr., Levine SJ, and Druey KM. 2018. RGS4 overexpression in lung attenuates airway hyper-responsiveness in mice. Am. J. Respir. Cell Mol. Biol 58: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park CY, Zhou EH, Tambe D, Chen B, Lavoie T, Dowell M, Simeonov A, Maloney DJ, Marinkovic A, Tschumperlin DJ, et al. 2015. High-throughput screening for modulators of cellular contractile force. Integr. Biol 7: 1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshie H, Koushki N, Kaviani R, Tabatabaei M, Rajendran K, Dang Q, Husain A, Yao S, Li C, Sullivan JK, et al. 2018. Traction force screening enabled by compliant PDMS elastomers. Biophys. J 114: 2194–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trepat X, Wasserman MR, Angelini TE, Millet E, Weitz DA, Butler JP, and Fredberg JJ. 2009. Physical forces during collective cell migration. Nat. Phys 5: 426–430. [Google Scholar]
- 19.Watson DS, Feng X, Askew DS, Jambunathan K, Kodukula K, and Galande AK. 2011. Substrate specifity profiling of the Aspergillus fumigatus proteolytic secretome reveals consensus motifs with pre-dominance of Ile/Leu and Phe/Tyr. PLoS One 6: e21001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morjaria JB, Emma R, Fuochi V, Polosa R, and Caruso M. 2019. An evaluation of mepolizumab for the treatment of severe asthma. Expert Opin. Biol. Ther 19: 491–500. [DOI] [PubMed] [Google Scholar]
- 21.Fulkerson PC, Fischetti CA, McBride ML, Hassman LM, Hogan SP, and Rothenberg ME. 2006. A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc. Natl. Acad. Sci. USA 103: 16418–16423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidlin F, Amadesi S, Vidil R, Trevisani M, Martinet N, Caughey G, Tognetto M, Cavallesco G, Mapp C, Geppetti P, and Bunnett NW. 2001. Expression and function of proteinase-activated receptor 2 in human bronchial smooth muscle. Am. J. Respir. Crit. Care Med 164: 1276–1281. [DOI] [PubMed] [Google Scholar]
- 23.Homma T, Kato A, Bhushan B, Norton JE, Suh LA, Carter RG, Gupta DS, and Schleimer RP. 2016. Role of Aspergillus fumigatus in triggering protease-activated receptor-2 in airway epithelial cells and skewing the cells toward a T-helper 2 bias. Am. J. Respir. Cell Mol. Biol 54: 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yee MC, Nichols HL, Polley D, Saifeddine M, Pal K, Lee K, Wilson EH, Daines MO, Hollenberg MD, Boitano S, and DeFea KA. 2018. Protease-activated receptor-2 signaling through b-arrestin-2 mediates Alternaria alkaline serine protease-induced airway inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol 315: L1042–L1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen HD, Tam MF, Tang RB, and Chou H. 2007. Aspergillus and Penicillium allergens: focus on proteases. Curr. Allergy Asthma Rep 7: 351–356. [DOI] [PubMed] [Google Scholar]
- 26.Wagers S, Lundblad LK, Ekman M, Irvin CG, and Bates JH. 2004. The allergic mouse model of asthma: normal smooth muscle in an abnormal lung? J. Appl. Physiol 96: 2019–2027. [DOI] [PubMed] [Google Scholar]
- 27.Davies SP, Reddy H, Caivano M, and Cohen P. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J 351: 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurup VP, Xia JQ, Shen HD, Rickaby DA, Henderson JD Jr., Fink JN, Chou H, Kelly KJ, and Dawson CA. 2002. Alkaline serine proteinase from Aspergillus fumigatus has synergistic effects on Asp-f-2-induced immune response in mice. Int. Arch. Allergy Immunol 129: 129–137. [DOI] [PubMed] [Google Scholar]
- 29.Moser M, Menz G, Blaser K, and Crameri R. 1994. Recombinant expression and antigenic properties of a 32-kilodalton extracellular alkaline protease, representing a possible virulence factor from Aspergillus fumigatus. Infect. Immun 62: 936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiraishi Y, Yamaguchi S, Yoshizaki T, Nambu A, Shimura E, Takamori A, Narushima S, Nakanishi W, Asada Y, Numata T, et al. 2018. IL-33, IL-25 and TSLP contribute to development of fungal-associated protease-induced innate-type airway inflammation. Sci. Rep 8: 18052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenberg HF, and Druey KM. 2018. Modeling asthma: pitfalls, promises, and the road ahead. J. Leukoc. Biol 104: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh ER, Sahu N, Kearley J, Benjamin E, Kang BH, Humbles A, and August A. 2008. Strain-specific requirement for eosinophils in the recruitment of T cells to the lung during the development of allergic asthma. J. Exp. Med 205: 1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohtomo T, Kaminuma O, Yamada J, Kitamura N, Abe A, Kobayashi N, Suko M, and Mori A. 2010. Eosinophils are required for the induction of bronchial hyperresponsiveness in a Th transfer model of BALB/c background. Int. Arch. Allergy Immunol 152(Suppl. 1): 79–82. [DOI] [PubMed] [Google Scholar]
- 34.Fattouh R, Al-Garawi A, Fattouh M, Arias K, Walker TD, Goncharova S, Coyle AJ, Humbles AA, and Jordana M. 2011. Eosinophils are dispensable for allergic remodeling and immunity in a model of house dust mite-induced airway disease. Am. J. Respir. Crit. Care Med 183: 179–188. [DOI] [PubMed] [Google Scholar]
- 35.de Boer JD, Van’t Veer C, Stroo I, van der Meer AJ, de Vos AF, van der Zee JS, Roelofs JJ, and van der Poll T. 2014. Protease-activated receptor-2 deficient mice have reduced house dust mite-evoked allergic lung inflammation. Innate Immun. 20: 618–625. [DOI] [PubMed] [Google Scholar]
- 36.Saw S, and Arora N. 2015. Protease inhibitor reduces airway response and underlying inflammation in cockroach allergen-induced murine model. Inflammation 38: 672–682. [DOI] [PubMed] [Google Scholar]


