Abstract
Background
To analyze the effects of socioeconomic status (type of insurance and income level) and cancer stage on the survival of patients with liver cancer in Korea.
Methods
A retrospective cohort study was constructed using data from the Healthcare Big Data Platform project in Korea between January 1, 2007, and December 31, 2017. A total of 143,511 patients in Korea diagnosed with liver cancer (International Classification of Diseases, 10th Revision [ICD-10] codes C22, C220, and C221) were followed for an average of 11 years. Of these, 110,443 died. The patient’s insurance type and income level were used as indicators of socioeconomic status. Unadjusted and adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using a Cox proportional hazards regression model to analyze the relationship between the effects of sex, age, and cancer stage at first diagnosis (Surveillance, Epidemiology, and the End Results; SEER), type of insurance, and income level on the survival of patients with liver cancer. The interactive effects of the type of insurance, income level, and cancer stage on liver cancer death were also analyzed.
Results
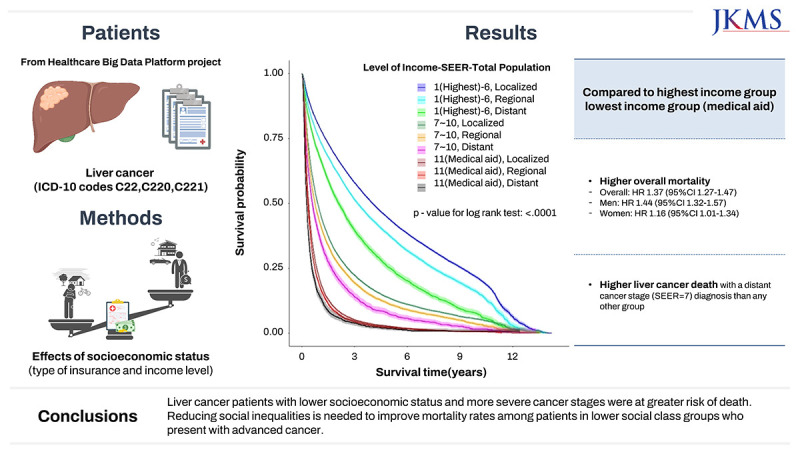
The lowest income group (medical aid) showed a higher risk for mortality (HR (95% CI); 1.37 (1.27–1.47) for all patients, 1.44 (1.32–1.57) for men, and 1.16 (1.01–1.34) for women) compared to the highest income group (1–6) among liver cancer (ICD-10 code C22) patients. The risk of liver cancer death was also higher in the lowest income group with a distant cancer stage (SEER = 7) diagnosis than for any other group.
Conclusion
Liver cancer patients with lower socioeconomic status and more severe cancer stages were at greater risk of death. Reducing social inequalities is needed to improve mortality rates among patients in lower social class groups who present with advanced cancer.
Keywords: Social Inequalities Liver Cancer Death, Social Class, Type of Insured, Medical Aid, Premium, Income, SEER
Graphical Abstract
INTRODUCTION
Liver cancer is a major cause of cancer-related death, and its high mortality makes it one of the four most prevalent cancers (lung, liver, stomach, and colon) contributing to cancer deaths worldwide.1 Social inequalities in liver cancer incidence, survival, and death have been reported globally. Several studies reported the effects of income (or insurance premium) inequalities2,3,4,5,6,7,8,9,10 and type of insurance11,12 on liver cancer or hepatocellular carcinoma (HCC) survival.
Investigations have focused on single risk factors related to social inequalities and the interactions of multiple risk factors. Interactions, including the combined effects of income, heavy alcohol consumption, and hepatitis B virus (HBV) infection on HCC risk,4,13 or the combined effect of socioeconomic status (SES), viral hepatitis, and lifestyles on HCC, have been reported.6
However, the interactive effects between SES and cancer stage on the mortality of liver cancer patients have not been specifically studied. Correlations between cancer severity and social inequalities related to liver cancer death rates have also been little reported. A few studies reported that the risk of death is higher among patients in lower social class groups with advanced liver cancer stages.5,14 Therefore, determining how the interactions of disease severity and SES affect the mortality of liver cancer patients will enable us to offer recommendations on how to reduce cancer-related social inequalities. Thus, more studies exploring the mechanisms related to the underlying social inequalities and the causes for the interdependence of social inequality-based outcomes of patients with liver cancer are needed.
Very few studies on social inequalities and liver cancer outcomes in Korea have been performed, although several studies have reported income-related inequalities in cancer incidence, survival, and overall mortality.2,3,6,8 A previous study reported the combined effect of SES, viral hepatitis, and lifestyles on HCC.6
Therefore, the purpose of this study was to explore the effect of social inequality on liver cancer survival in general and analyze how social inequality affects survival rates according to the cancer stage. In particular, this study analyzed the effects of SES (as indicated by insurer group and insurance premiums) and the cancer stage in the Surveillance, Epidemiology, and the End Results (SEER) database on cancer mortality in Korea using nationwide population data.
METHODS
Study design
We conducted a retrospective cohort study using data collected from the Healthcare Big Data Platform project, which was initiated by the Korea Health Industry Development Institute and the Ministry of Health and Welfare in Korea.15 The dataset was constructed by combining three sources: the Korea National Cancer Incidence (KNCI) database in the Korea Central Cancer Registry (KCCR), the National Cancer Center (NCC),16 the National Health Insurance Sharing Service (NHISS),17 and the Health Insurance Review & Assessment Service (HIRA).18
All cancer patients who registered nationwide with the KNCI database in the KCCR from January 1, 2007, through December 31, 2017, were selected and linked with social insurance registration and health examination data in the NHISS database and medical history data from the HIRA.
Descriptions of the data sources can be found in the “Korea Healthcare Bigdata” online sites.17 The main variables used in this study were age, sex, date of the first diagnosis, cancer stage, and International Classification of Diseases, 10th Revision (ICD-10) codes from the KNCI; sex, age, type of insurance, income level (based on insurance premium), residential address, and general medical examination variables (height, weight, blood pressure, cholesterol level, blood glucose level, past history of disease, alcohol, and smoking), as well as relevant variables related to early medical examinations for cancer (past history of cancer (breast, colorectal, cervical, gastric, and liver)) from the NHISS; and start dates of treatment and ICD codes from the HIRA.
Study population
The retrospective cohort consisting of combined data from the KNCI in the KCCR, the NHISS, and the HIRA included liver cancer patients diagnosed with malignant neoplasms of the liver and intrahepatic bile ducts (ICD-10 code C22) between January 1, 2007, and December 31, 2017, throughout Korea.
Being a liver cancer patient was constructed as a dependent variable. Liver cancer patients were defined as having “malignant neoplasm of liver and intrahepatic bile ducts” according to ICD-10 code C22 and registration in the NCC in Korea. Liver cancer (C22) was the primary tumor site, and the cancers were mainly comprised of HCC (C220) or intrahepatic cholangiocarcinoma (ICC; C221), as defined by the KCCR.
The total number of liver cancer patients (C22) used in this retrospective cohort from January 1, 2007, to December 13, 2017, was 143,511, of which 110,443 died. Of 109,545 HCC (C220) patients, 79,936 died, and of 25,030 ICC (C221) patients, 23,317 died.
Variables
The variables established in this study were age, sex, cancer stage, ICD code, initial diagnosis date from the KNCI, KCCR, and NCC; the type of insurance, and insurance premium as a proxy of income from the NHISS; the start date of treatment (specifically defined as the date of the commencement of medical treatment), the number of days in the hospital, ICD diagnosis code, and HIRA codes for diagnosis, as well as treatment.
The independent variables in this study were constructed as follows. Age variables were binned into five-year intervals and divided into five subgroups with 10-year intervals to reflect the characteristics of a rapid increase in liver cancer incidence in old age: < 40, 40–50, 50–60, 60–70, and > 70 years.
Cancer stage at first diagnosis was informed (described) by the KCCR database, based on the staging system from the SEER database of the United States National Cancer Institute. Cancer stage variables were classified into localized, regionalized, distant, and unknown, as defined by the National Cancer Institute SEER Training Modules in the USA.19 “Localized” refers to a malignancy limited to the organ of origin, with no spread beyond the organ of origin or infiltration past the epithelial basement membrane into the stroma of an organ. “Regionalized” was defined as a tumor that extended beyond the limits of the organ of origin. The “distant” classification indicated a tumor that had spread to body areas distant or remote from the primary tumor, and unknown status was used for cases with insufficient evidence to adequately assign a stage.
The type of insurance and income level were used as proxy indicators of social inequalities. The type of insurance variable was used to divide the participants into self-employed insured and dependents, employee insured and dependents, and medical aid group and dependents.
Insurance premium was used as a variable indicating income. This study used the final premium grade, which is a variable that is common to self-employed insured and dependents and employee-insured and dependents. Insurance premium grades ranged from 1 to 10. The medical aid group was ultimately classified as belonging to the 11th premium variable group, as this group pays almost no premium. The patients were classified into six insurance premium groups (highest [1–2], 3–4, 5–6, 7–8, 9–10, and lowest [11]) and three larger groups (1–6, 7–10, and 11).
Smoking, alcohol, body mass index (BMI), and exercise were selected as health behavior variables for possible liver cancer risk. These variables were recorded in the health examination data conducted by the NHISS at the time closest to January 1, 2007.
Smoking was divided into three categories: 1 (no smoking), 2 (past smoking), and 3 (current smoking). The alcohol variable was classified into five subgroups: 1 (no alcohol), 2 (2–3 times a month), 3 (once or twice a week), 4 (3–4 times a week), and 5 (almost every day). The BMI variable was regrouped into four subgroups, where 1 was a BMI of < 18.4 kg/m2, 2 was a BMI of ≥ 18.4 and < 25 kg/m2, 3 was a BMI of ≥ 25 and < 30 kg/m2), and 4 was a BMI of ≥ 30 kg/m2. The exercise variable was regrouped into five subgroups of 1 (none), 2 (once or twice a week), 3 (3–4 times a week), 4 (5–6 times a week), and 5 (almost every day).
The previous history of disease variable included previous hypertension, cardiovascular disease, cerebrovascular diseases (stroke), diabetes mellitus, hyperlipidemia, anemia, breast cancer, colon cancer, cervix cancer, liver cancer, stomach cancer, and other cancers. This variable was dichotomized into 1 (no) and 2 (yes). A family history of cancer included breast cancer, colon cancer, cervix cancer, liver cancer, stomach cancer, and other cancers and was dichotomized into 1 (no) and 2 (yes).
The Charlson Comorbidity Index (CCI) was used for comorbidities,20 and the methodologies for calculating CCI values using NHISS medical record data were obtained from the HIRA.21 CCI values were calculated using “disease data before being diagnosed with cancer” from NHISS medical record data.21 The CCI variable was regrouped into five subgroups of 1 (none), 2 (one), 3 (two), 4 (three), 5 (four), and 6 (more than five).
Statistical analysis
We calculated the number of cancer deaths and age-adjusted survival rates (per 100 population) to describe the study population in terms of potential risk factors, as well as in terms of covariates, including sex, age, cancer stage, type of insurance, and income level based on premium grade.
We performed a multivariable proportional hazards regression model using Cox regression model to estimate the adjusted effect of cancer stage and socioeconomic factors on mortality. We tested the proportional hazards assumption in Cox regression using the partial log-likelihood test and confirmed that the hazards were proportional, which meant the relative hazard remained constant over time with different risk factors or covariates (log-likelihood test, P < 0.001). We also tested whether the results were time-dependent, that is, whether the results of this study changed over time, and confirmed that they did not (χ2 test, P > 0.05).
We checked the proportional hazards assumption after fitting a Cox regression model that is the same as identifying time-varying coefficients, and confirmed that the proportional hazards assumption was not violated. Therefore, Cox regression models were fitted without considering time varying covariates.
Survival time was calculated from the first cancer diagnosis date to the date of death. For patients not identified as deceased, survival time was calculated as the time from the date of the first diagnosis to the end of the study period. Kaplan-Meier (KM) survival curves were used to describe the survival pattern of patients with liver cancer in subgroups of each study variable.
Unadjusted and adjusted hazard ratios (HR) and 95% confidence intervals (CIs) were calculated using the Cox proportional hazards regression model to analyze the relationships between the effects of sex, age, cancer stage, type of insurance, and income level (insurance premium grade) on survival. HRs were calculated after adjusting for confounders using several models. Model 1 used univariate HRs, and model 2 used HRs adjusted for sex, age, cancer stage, smoking, alcohol, BMI, exercise, previous histories of each of hypertension or cerebrovascular diseases (stroke), and comorbidities (CCI values).
Since the medical examination variables (health checkups) had a high proportion of missing values (35–40%), the multiple imputation method22 was used employing the SAS statistical package. Procedures included the imputations step (proc MI), the analysis step (proc PHREG), and the combination step (proc MIANALYZE).22 The variables used for the multiple imputation method were smoking, alcohol, BMI, exercise, previous histories of each of hypertension or cerebrovascular diseases (stroke), and comorbidities (CCI values).
We calculated HRs and 95% CIs using imputation methods (i.e., by inserting the variables—smoking, alcohol, BMI, exercise, and a previous history of hypertension or cerebrovascular diseases into the imputation analysis). We compared the results (HRs and 95% CIs) obtained by imputing the missing variables (referred to as model 3) with those obtained in model 2 without imputation of the missing variables.
We created a combination variable using socioeconomic factors and cancer stage to explore the interplay of these risk factors as well as confounding factors and analyzed the effect of the interrelationships on liver cancer death rates. We analyzed how differences in the type of insurance and differences in liver cancer stages interacted and how the differences in income groups and differences in liver cancer stages interacted.
SAS version 9.41 (SAS Institute Inc., Cary, NC, USA) was used for all analyses, and the statistical significance level was set at P < 0.05 for the main effects.
Ethics statement
The present study protocol was reviewed and approved by the Institutional Review Board of Kangwon National University Hospital (approval No. 2019-11-002). Informed consent was waived because of the retrospective nature of the study.
RESULTS
The effect of potential risk factors on the survival of liver cancer patients
The age-adjusted survival rates of liver cancer patients according to potential risk factors (Table 1) showed that the risk of death increased with increasing age (particularly over 70 years old) in male patients, those with distant cancers, lower income, and those receiving medical aid (medical aid subscribers and dependents) (Table 1, Supplementary Fig. 1).
Table 1. The number of cancers, deaths, and age-adjusted survival rates in liver cancer (ICD-10 code C22) patients.
| Variables | Total | Male | Female | ||||
|---|---|---|---|---|---|---|---|
| Total cancer patients (deaths) | Age-adjusted survival rates | Patients (deaths) | Age-adjusted survival rates | Patients (deaths) | Age-adjusted survival rates | ||
| Sex | 143,511 (110,443) | 24.94 (24.75–25.12) | 109,681 (84,332) | 22.11 (21.87–22.36) | 33,830 (26,111) | 27.76 (27.49–28.04) | |
| Age, yr | |||||||
| Age ≤ 40 | 4,218 (2,851) | 31.68 (30.54–32.85) | 3,239 (2,274) | 29.04 (27.83–30.29) | 979 (577) | 43.27 (40.2–46.52) | |
| 40 < Age ≤ 50 | 20,611 (14,433) | 30.39 (29.84–30.96) | 17,737 (12,684) | 28.87 (28.28–29.46) | 2,874 (1,749) | 39.69 (38.01–41.43) | |
| 50 < Age ≤ 60 | 41,808 (28,993) | 30.68 (30.25–31.13) | 35,257 (24,971) | 29.17 (28.7–29.65) | 6,551 (4,022) | 38.03 (36.86–39.24) | |
| 60 < Age ≤ 70 | 38,760 (29,769) | 22.05 (21.67–22.44) | 29,528 (22,911) | 21.42 (20.98–21.86) | 9,232 (6,858) | 23.94 (23.14–24.75) | |
| 70 < Age | 38,114 (34,397) | 10.13 (9.83–10.44) | 23,920 (21,492) | 10.51 (10.12–10.91) | 14,194 (12,905) | 9.48 (8.99–9.98) | |
| History of liver cancer | |||||||
| C220 | 109,545 (79,936) | 26.18 (25.92–26.45) | 87,678 (64,675) | 25.51 (25.22–25.82) | 21,867 (15,261) | 28.34 (27.78–28.91) | |
| C221 | 25,030 (23,317) | 8.28 (8.13–8.43) | 15,717 (14,616) | 8.49 (8.32–8.67) | 9,313 (8,701) | 7.59 (7.3–7.89) | |
| C222 | 174 (43) | 98 (94.47–101.63) | 104 (29) | 69.86 (56.92–84.94) | 70 (14) | 99.45 (95.8–103.2) | |
| C223 | 169 (143) | 21.52 (21.18–21.87) | 104 (92) | 18.03 (17.7–18.36) | 65 (51) | 54.28 (52.55–56.06) | |
| C224 | 157 (121) | 19.94 (19.67–20.2) | 106 (84) | 18 (17.73–18.27) | 51 (37) | 33.43 (32.47–34.42) | |
| C227 | 3,096 (1,966) | 33.48 (33.18–33.78) | 2,356 (1,510) | 34.08 (33.74–34.43) | 740 (456) | 31.5 (30.9–32.1) | |
| C229 | 5,340 (4,917) | 10.1 (9.93–10.26) | 3,616 (3,326) | 9.66 (9.48–9.85) | 1,724 (1,591) | 11.53 (11.17–11.89) | |
| Combined liver cancers | 1,046 (929) | 10.66 (10.48–10.83) | 822 (735) | 10.35 (10.16–10.55) | 224 (194) | 11.58 (11.22–11.95) | |
| Cancer stage (SEER) | |||||||
| 1 Localized | 64,202 (39,566) | 37.36 (37.04–37.67) | 49,077 (30,268) | 37.74 (37.38–38.11) | 15,125 (9,298) | 36.11 (35.48–36.76) | |
| 2 Regional | 34,205 (29,788) | 12.78 (12.59–12.97) | 26,964 (23,463) | 12.79 (12.58–13.01) | 7,241 (6,325) | 12.74 (12.36–13.12) | |
| 7 Distant | 22,244 (21,768) | 2.15 (2.08–2.23) | 16,746 (16,413) | 2.01 (1.92–2.09) | 5,498 (5,355) | 2.62 (2.45–2.8) | |
| 9 Unknown | 22,860 (19,321) | 17.38 (17.16–17.59) | 16,894 (14,188) | 17.38 (17.13–17.63) | 5,966 (5,133) | 17.37 (16.92–17.82) | |
| Type of insurance | |||||||
| Self-employed-subscriber | 38,175 (27,603) | 25.82 (25.56–26.08) | 33,262 (23,809) | 26.67 (26.36–26.98) | 4,913 (3,714) | 23.05 (22.54–23.57) | |
| Self-employed-dependent | 21,877 (18,734) | 11.21 (11.04–11.39) | 13,841 (12,668) | 7.97 (7.80–8.14) | 8,036 (6,066) | 21.73 (21.24–22.24) | |
| Employee-subscriber | 27,959 (18,437) | 29.51 (29.23–29.80) | 26,227 (17,369) | 30.36 (30.04–30.69) | 1,732 (1,068) | 26.75 (26.20–27.31) | |
| Employee-dependent | 45,967 (37,559) | 21.03 (20.79–21.26) | 30,151 (25,124) | 20.32 (20.05–20.59) | 15,816 (12,435) | 23.32 (22.81–23.84) | |
| Medical aid-subscriber | 8,108 (7,228) | 12.67 (12.49–12.86) | 5,627 (5,019) | 11.89 (11.69–12.10) | 2,481 (2,209) | 15.20 (14.79–15.62) | |
| Medical aid-dependent | 1,033 (882) | 15.85 (15.64–16.07) | 310 (263) | 15.48 (15.23–15.72) | 723 (619) | 16.98 (16.54–17.43) | |
| Income level | |||||||
| 1: 1 (highest)–2 | 36,367 (26,574) | 29.38 (29.1–29.66) | 27,389 (19,609) | 30.62 (30.3–30.95) | 8,978 (6,965) | 25.35 (24.81–25.89) | |
| 2: 3–4 | 29,630 (22,484) | 24.04 (23.79–24.3) | 23,044 (17,483) | 24.26 (23.96–24.55) | 6,586 (5,001) | 23.35 (22.84–23.87) | |
| 3: 5–6 | 25,197 (19,475) | 21.38 (21.14–21.62) | 19,955 (15,566) | 21.03 (20.76–21.3) | 5,242 (3,909) | 22.52 (22.01–23.03) | |
| 4: 7–8 | 19,768 (15,482) | 20.25 (20.02–20.49) | 15,533 (12,280) | 19.93 (19.67–20.19) | 4,235 (3,202) | 21.3 (20.81–21.8) | |
| 5: 9–10 | 21,224 (16,897) | 19.73 (19.5–19.96) | 16,169 (13,006) | 19.14 (18.88–19.4) | 5,055 (3,891) | 21.67 (21.18–22.17) | |
| 6: 11 (medical aid) | 9,141 (8,110) | 12.89 (12.7–13.07) | 5,937 (5,282) | 12 (11.8–12.21) | 3,204 (2,828) | 15.77 (15.35–16.2) | |
Values are presented as numbers and age-adjusted survival rates per 100 population.
ICD-10 = International Classification of Diseases, 10th Revision, SEER = Surveillance, Epidemiology, and the End Results.
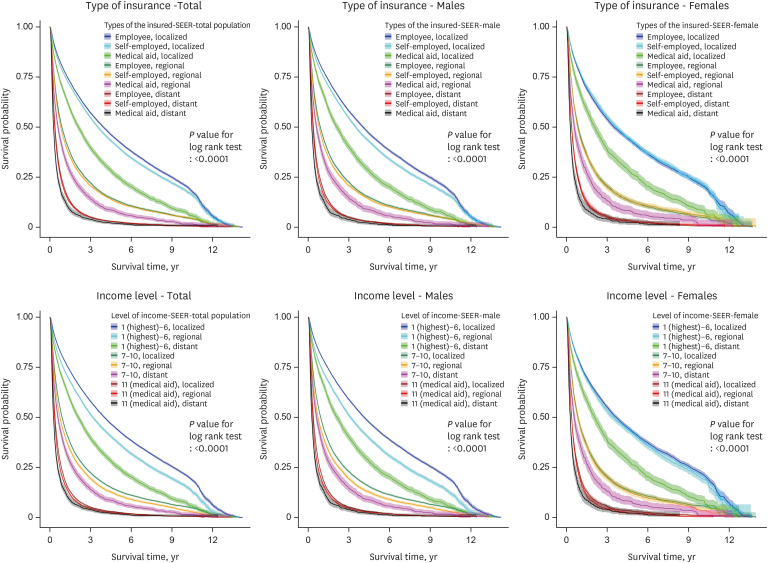
Patients in the lower-level income group and medical aid recipients had lower survival rates than the higher-level income group, and self-employed subscribers, employee subscribers and their dependents, demonstrating social differences in liver cancer survival rates (Table 1).
Younger females had higher survival rates than younger males, indicating a gender difference. Survival differences between the age groups were bigger in females than in males (Table 1).
Inequalities in the risk of death based on insurance type and income level
Table 2 shows the unadjusted and adjusted HRs of death by sex, age, cancer stage, type of insurance, and income level in liver cancer patients (models 1–3). The adjusted covariates were sex, age, cancer stage, smoking, drinking, exercise, BMI, previous history of hypertension or cerebrovascular diseases, and comorbidities (CCI). As a number of covariates had missing values (Supplementary Table 1), we compared two models: model 2, without imputation of the missing variables, and model 3, which included multiple imputations, by Cox regression analysis.
Table 2. Effects of cancer stage and SES on deaths among liver cancer patients (ICD-10 code: C22, C220, C221) in Korea, 2007–2017.
| Variables | Total | Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR1 | HR2 | HR3 | HR1 | HR2 | HR3 | HR1 | HR2 | HR3 | |||
| Liver cancer (C22) | |||||||||||
| Sex | |||||||||||
| Male | 1.00 | 1.00 | 1.00 | ||||||||
| Female | 1.03 (1.02–1.04) | 1.01 (0.98–1.04) | 0.96 (0.94–0.97) | ||||||||
| Age, yr | |||||||||||
| Age ≤ 40 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| 40 < Age ≤ 50 | 1.06 (1.02–1.10) | 1.04 (0.94–1.15) | 1.14 (1.09–1.19) | 1.02 (0.98–1.07) | 1.03 (0.92–1.14) | 1.10 (1.05–1.15) | 1.07 (0.97–1.17) | 1.12 (0.85–1.46) | 1.21 (1.10–1.33) | ||
| 50 < Age ≤ 60 | 1.03 (0.99–1.07) | 1.16 (1.06–1.28) | 1.15 (1.11–1.20) | 0.99 (0.95–1.04) | 1.15 (1.04–1.28) | 1.12 (1.07–1.17) | 1.06 (0.97–1.15) | 1.23 (0.95–1.60) | 1.22 (1.12–1.33) | ||
| 60 < Age ≤ 70 | 1.21 (1.16–1.26) | 1.47 (1.33–1.62) | 1.39 (1.34–1.45) | 1.15 (1.10–1.20) | 1.43 (1.29–1.59) | 1.32 (1.26–1.38) | 1.43 (1.31–1.55) | 1.74 (1.34–2.26) | 1.66 (1.52–1.81) | ||
| 70 < Age | 2.01 (1.94–2.09) | 2.40 (2.18–2.64) | 2.28 (2.20–2.38) | 1.83 (1.75–1.91) | 2.27 (2.04–2.52) | 2.05 (1.96–2.14) | 2.69 (2.47–2.92) | 3.05 (2.35–3.95) | 2.94 (2.79–3.31) | ||
| Cancer stage (SEER) | |||||||||||
| 1 Localized | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| 2 Regional | 2.36 (2.33–2.40) | 2.63 (2.56–2.70) | 2.32 (2.28–2.35) | 2.38 (2.34–2.42) | 2.62 (2.54–2.70) | 2.34 (2.29–2.38) | 2.33 (2.25–2.40) | 2.65 (2.50–2.80) | 2.25 (2.18–2.32) | ||
| 7 Distant | 4.72 (4.64–4.80) | 5.87 (5.71–6.05) | 4.56 (4.48–4.64) | 4.80 (4.71–4.89) | 5.84 (5.65–6.03) | 4.61 (4.52–4.70) | 4.49 (4.33–4.65) | 6.07 (5.71–6.45) | 4.47 (4.32–4.63) | ||
| 9 Unknown | 2.00 (1.96–2.03) | 2.02 (1.95–2.08) | 1.85 (1.82–1.88) | 1.96 (1.92–2.00) | 2.01 (1.94–2.08) | 1.85 (1.82–1.89) | 2.09 (2.02–2.16) | 2.04 (1.90–2.18) | 1.83 (1.77–1.89) | ||
| Type of insurance | |||||||||||
| Self-employed-subscriber | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| Self-employed-dependent | 1.49 (1.46–1.51) | 1.40 (1.35–1.45) | 1.40 (1.38–1.43) | 1.80 (1.77–1.84) | 1.65 (1.58–1.72) | 1.58 (1.55–1.62) | 1.00 (0.96–1.04) | 1.01 (0.94–1.09) | 1.05 (1.01–1.10) | ||
| Employee-subscriber | 0.84 (0.82–0.85) | 1.03 (1.00–1.06) | 0.91 (0.89–0.93) | 0.85 (0.84–0.87) | 1.04 (1.00–1.07) | 0.92 (0.90–0.94) | 0.69 (0.65–0.74) | 1.01 (0.91–1.12) | 0.93 (0.87–1.00) | ||
| Employee-dependent | 1.24 (1.22–1.26) | 1.09 (1.06–1.12) | 1.10 (1.08–1.12) | 1.30 (1.27–1.32) | 1.11 (1.08–1.15) | 1.12 (1.10–1.14) | 1.06 (1.02–1.10) | 0.95 (0.89–1.01) | 0.99 (0.95–1.02) | ||
| Medical aid-subscriber | 1.52 (1.48–1.56) | 1.32 (1.22–1.42) | 1.36 (1.33–1.40) | 1.52 (1.48–1.57) | 1.38 (1.26–1.50) | 1.41 (1.37–1.46) | 1.42 (1.35–1.50) | 1.02 (0.87–1.21) | 1.14 (1.08–1.20) | ||
| Medical aid-dependent | 1.35 (1.26–1.45) | 1.36 (1.10–1.70) | 1.24 (1.16–1.33) | 1.34 (1.19–1.52) | 1.42 (0.97–2.08) | 1.41 (1.25–1.60) | 1.22 (1.12–1.33) | 1.11 (0.84–1.45) | 1.00 (0.91–1.08) | ||
| Income level | |||||||||||
| 1 (highest)–2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| 3–4 | 1.09 (1.07–1.11) | 1.12 (1.09–1.16) | 1.13 (1.11–1.16) | 1.13 (1.11–1.15) | 1.13 (1.10–1.17) | 1.15 (1.13–1.17) | 0.98 (0.95–1.02) | 1.11 (1.04–1.18) | 1.10 (1.06–1.14) | ||
| 5–6 | 1.18 (1.16–1.20) | 1.20 (1.16–1.24) | 1.25 (1.22–1.27) | 1.26 (1.23–1.28) | 1.24 (1.20–1.28) | 1.28 (1.26–1.31) | 0.96 (0.92–0.99) | 1.09 (1.02–1.17) | 1.13 (1.09–1.18) | ||
| 7–8 | 1.23 (1.20–1.25) | 1.18 (1.15–1.23) | 1.30 (1.27–1.32) | 1.31 (1.28–1.34) | 1.20 (1.15–1.25) | 1.33 (1.30–1.36) | 1.01 (0.97–1.05) | 1.16 (1.08–1.24) | 1.21 (1.16–1.26) | ||
| 9–10 | 1.27 (1.25–1.30) | 1.23 (1.19–1.27) | 1.31 (1.28–1.33) | 1.36 (1.33–1.39) | 1.27 (1.23–1.32) | 1.35 (1.32–1.38) | 1.04 (1.00–1.08) | 1.13 (1.06–1.21) | 1.17 (1.12–1.21) | ||
| 11 (medical aid) | 1.53 (1.49–1.57) | 1.37 (1.27–1.47) | 1.43 (1.40–1.47) | 1.58 (1.54–1.63) | 1.44 (1.32–1.57) | 1.54 (1.49–1.58) | 1.36 (1.30–1.42) | 1.16 (1.01–1.34) | 1.21 (1.16–1.27) | ||
| Hepatocellular cancer (C220) | |||||||||||
| Sex | |||||||||||
| Male | 1.00 | 1.00 | 1.00 | ||||||||
| Female | 0.90 (0.88–0.91) | 0.92 (0.89–0.96) | 0.95 (0.93–0.97) | ||||||||
| Age, yr | |||||||||||
| Age ≤ 40 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| 40 < Age ≤ 50 | 1.02 (0.97–1.06) | 1.04 (0.93–1.16) | 1.08 (1.03–1.12) | 0.98 (0.93–1.03) | 1.01 (0.90–1.13) | 1.05 (1.00–1.10) | 1.01 (0.90–1.13) | 1.41 (0.96–2.05) | 1.16 (1.03–1.29) | ||
| 50 < Age ≤ 60 | 0.96 (0.92–1.00) | 1.14 (1.03–1.27) | 1.08 (1.04–1.13) | 0.93 (0.89–0.98) | 1.12 (1.00–1.25) | 1.06 (1.01–1.11) | 0.93 (0.84–1.04) | 1.48 (1.02–2.13) | 1.12 (1.01–1.25) | ||
| 60 < Age ≤ 70 | 1.06 (1.02–1.11) | 1.40 (1.26–1.56) | 1.27 (1.21–1.32) | 1.02 (0.98–1.07) | 1.35 (1.21–1.51) | 1.22 (1.16–1.28) | 1.24 (1.12–1.38) | 2.07 (1.43–2.99) | 1.53 (1.38–1.70) | ||
| 70 < Age | 1.71 (1.63–1.78) | 2.27 (2.04–2.53) | 2.04 (1.95–2.13) | 1.57 (1.50–1.65) | 2.10 (1.87–2.35) | 1.84 (1.75–1.93) | 2.39 (2.16–2.65) | 3.79 (2.62–5.47) | 2.83 (2.55–3.14) | ||
| Cancer stage (SEER) | |||||||||||
| 1 Localized | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| 2 Regional | 2.46 (2.42–2.50) | 2.68 (2.60–2.76) | 2.42 (2.38–2.46) | 2.45 (2.41–2.50) | 2.68 (2.59–2.76) | 2.42 (2.38–2.47) | 2.43 (2.34–2.54) | 2.63 (2.44–2.83) | 2.37 (2.27–2.47) | ||
| 7 Distant | 5.02 (4.92–5.13) | 6.05 (5.83–6.28) | 4.95 (4.85–5.06) | 4.98 (4.86–5.10) | 5.94 (5.71–6.19) | 4.87 (4.76–4.99) | 5.17 (4.91–5.43) | 6.86 (6.25–7.52) | 5.38 (5.12–5.67) | ||
| 9 Unknown | 1.83 (1.79–1.87) | 1.84 (1.77–1.91) | 1.77 (1.73–1.81) | 1.81 (1.77–1.86) | 1.84 (1.77–1.92) | 1.77 (1.73–1.81) | 1.90 (1.82–1.99) | 1.82 (1.66–1.99) | 1.74 (1.67–1.82) | ||
| Types of insurance | |||||||||||
| Self-employed-subscriber | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| Self-employed-dependent | 1.49 (1.46–1.52) | 1.48 (1.42–1.54) | 1.48 (1.45–1.51) | 1.84 (1.79–1.88) | 1.70 (1.62–1.78) | 1.65 (1.61–1.69) | 1.01 (0.96–1.06) | 1.01 (0.92–1.11) | 1.07 (1.01–1.12) | ||
| Employee-subscriber | 0.83 (0.81–0.85) | 1.01 (0.97–1.04) | 0.89 (0.87–0.91) | 0.84 (0.82–0.86) | 1.01 (0.97–1.05) | 0.90 (0.88–0.92) | 0.66 (0.61–0.73) | 0.96 (0.83–1.11) | 0.90 (0.82–0.98) | ||
| Employee-dependent | 1.16 (1.14–1.18) | 1.06 (1.03–1.10) | 1.09 (1.07–1.11) | 1.23 (1.21–1.26) | 1.09 (1.06–1.14) | 1.12 (1.09–1.14) | 1.02 (0.97–1.07) | 0.87 (0.80–0.95) | 0.96 (0.91–1.00) | ||
| Medical aid-subscriber | 1.48 (1.43–1.52) | 1.35 (1.23–1.48) | 1.45 (1.41–1.50) | 1.49 (1.44–1.55) | 1.37 (1.24–1.51) | 1.47 (1.42–1.53) | 1.46 (1.36–1.56) | 1.14 (0.91–1.43) | 1.21 (1.13–1.30) | ||
| Medical aid-dependent | 1.28 (1.18–1.39) | 1.33 (1.00–1.77) | 1.33 (1.22–1.44) | 1.36 (1.19–1.56) | 1.40 (0.92–2.16) | 1.44 (1.26–1.65) | 1.27 (1.14–1.42) | 1.02 (0.70–1.50) | 1.02 (0.92–1.14) | ||
| Income level | |||||||||||
| 1 (highest)–2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| 3–4 | 1.12 (1.10–1.15) | 1.14 (1.10–1.18) | 1.13 (1.10–1.15) | 1.16 (1.13–1.19) | 1.15 (1.11–1.20) | 1.15 (1.12–1.17) | 0.98 (0.93–1.03) | 1.10 (1.01–1.20) | 1.08 (1.03–1.14) | ||
| 5–6 | 1.25 (1.23–1.28) | 1.24 (1.20–1.29) | 1.26 (1.23–1.29) | 1.33 (1.29–1.36) | 1.27 (1.22–1.32) | 1.29 (1.26–1.32) | 0.98 (0.94–1.04) | 1.14 (1.04–1.25) | 1.15 (1.09–1.21) | ||
| 7–8 | 1.33 (1.30–1.36) | 1.23 (1.18–1.28) | 1.33 (1.30–1.36) | 1.40 (1.37–1.44) | 1.24 (1.18–1.29) | 1.35 (1.32–1.39) | 1.06 (1.00–1.12) | 1.21 (1.10–1.34) | 1.28 (1.21–1.35) | ||
| 9–10 | 1.35 (1.32–1.38) | 1.28 (1.23–1.33) | 1.32 (1.29–1.35) | 1.44 (1.40–1.47) | 1.32 (1.26–1.37) | 1.36 (1.33–1.40) | 1.06 (1.01–1.12) | 1.17 (1.06–1.28) | 1.18 (1.12–1.24) | ||
| 11 (medical aid) | 1.60 (1.55–1.65) | 1.44 (1.32–1.57) | 1.56 (1.51–1.61) | 1.66 (1.60–1.72) | 1.47 (1.33–1.63) | 1.63 (1.58–1.69) | 1.44 (1.36–1.53) | 1.32 (1.08–1.60) | 1.30 (1.23–1.38) | ||
| Cholangiocarcinoma (C221) | |||||||||||
| Sex | |||||||||||
| Male | 1.00 | 1.00 | 1.00 | ||||||||
| Female | 1.03 (1.00–1.06) | 0.99 (0.94–1.05) | 1.01 (0.98–1.04) | ||||||||
| Age, yr | |||||||||||
| Age ≤ 40 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| 40 < Age ≤ 50 | 0.99 (0.87–1.12) | 1.01 (0.77–1.34) | 1.07 (0.94–1.22) | 1.02 (0.86–1.20) | 1.05 (0.74–1.49) | 1.13 (0.96–1.34) | 0.91 (0.74–1.12) | 0.89 (0.56–1.41) | 0.97 (0.79–1.19) | ||
| 50 < Age ≤ 60 | 0.99 (0.88–1.12) | 1.11 (0.85–1.45) | 1.12 (0.99–1.27) | 1.01 (0.87–1.19) | 1.17 (0.83–1.63) | 1.18 (1.00–1.38) | 0.93 (0.77–1.13) | 0.94 (0.61–1.45) | 1.02 (0.84–1.23) | ||
| 60 < Age ≤ 70 | 1.11 (0.98–1.24) | 1.35 (1.04–1.76) | 1.32 (1.17–1.49) | 1.12 (0.96–1.31) | 1.37 (0.98–1.91) | 1.36 (1.17–1.59) | 1.07 (0.89–1.29) | 1.24 (0.81–1.91) | 1.24 (1.03–1.50) | ||
| 70 < Age | 1.65 (1.46–1.85) | 2.04 (1.57–2.65) | 2.03 (1.80–2.29) | 1.60 (1.37–1.86) | 2.02 (1.45–2.83) | 2.02 (1.73–2.37) | 1.71 (1.42–2.06) | 1.91 (1.24–2.95) | 2.03 (1.69–2.45) | ||
| Cancer stage (SEER) | |||||||||||
| 1 Localized | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| 2 Regional | 1.38 (1.33–1.43) | 1.62 (1.53–1.72) | 1.45 (1.40–1.51) | 1.43 (1.37–1.50) | 1.68 (1.56–1.81) | 1.49 (1.42–1.56) | 1.30 (1.22–1.38) | 1.51 (1.36–1.67) | 1.40 (1.32–1.49) | ||
| 7 Distant | 2.53 (2.44–2.62) | 3.30 (3.11–3.51) | 2.76 (2.66–2.86) | 2.70 (2.58–2.83) | 3.46 (3.21–3.72) | 2.89 (2.76–3.03) | 2.26 (2.13–2.4) | 3.08 (2.79–3.41) | 2.57 (2.42–2.73) | ||
| 9 Unknown | 1.60 (1.53–1.67) | 1.64 (1.52–1.76) | 1.53 (1.47–1.60) | 1.64 (1.55–1.73) | 1.73 (1.58–1.89) | 1.58 (1.49–1.66) | 1.53 (1.43–1.64) | 1.49 (1.32–1.68) | 1.47 (1.38–1.58) | ||
| Type of insurance | |||||||||||
| Self-employed-subscriber | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| Self-employed-dependent | 1.26 (1.21–1.32) | 1.24 (1.15–1.33) | 1.23 (1.17–1.28) | 1.56 (1.48–1.65) | 1.47 (1.34–1.61) | 1.45 (1.38–1.54) | 0.91 (0.85–0.98) | 1.02 (0.91–1.15) | 0.96 (0.89–1.04) | ||
| Employee-subscriber | 0.91 (0.87–0.95) | 1.14 (1.07–1.22) | 1.02 (0.97–1.07) | 0.97 (0.92–1.02) | 1.17 (1.08–1.26) | 1.06 (1.00–1.11) | 0.70 (0.62–0.78) | 1.05 (0.89–1.24) | 0.92 (0.81–1.03) | ||
| Employee-dependent | 1.19 (1.15–1.23) | 1.13 (1.07–1.20) | 1.08 (1.04–1.12) | 1.27 (1.22–1.33) | 1.15 (1.07–1.23) | 1.14 (1.09–1.19) | 0.98 (0.92–1.05) | 1.06 (0.95–1.18) | 0.95 (0.89–1.02) | ||
| Medical aid-subscriber | 1.49 (1.40–1.58) | 1.27 (1.07–1.50) | 1.27 (1.20–1.35) | 1.56 (1.44–1.69) | 1.45 (1.18–1.79) | 1.38 (1.27–1.49) | 1.25 (1.14–1.38) | 0.96 (0.73–1.27) | 1.06 (0.96–1.17) | ||
| Medical aid-dependent | 1.40 (1.22–1.60) | 1.29 (0.90–1.85) | 1.20 (1.04–1.37) | 2.13 (1.50–3.02) | 1.72 (0.64–4.62) | 1.91 (1.34–2.70) | 1.11 (0.96–1.30) | 1.09 (0.73–1.61) | 0.95 (0.82–1.11) | ||
| Income level | |||||||||||
| 1 (highest)–2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| 3–4 | 1.05 (1.01–1.09) | 1.09 (1.03–1.15) | 1.07 (1.04–1.12) | 1.06 (1.01–1.11) | 1.09 (1.01–1.17) | 1.08 (1.03–1.13) | 1.04 (0.98–1.11) | 1.09 (0.99–1.21) | 1.07 (1.01–1.14) | ||
| 5–6 | 1.060 (1.02–1.10) | 1.08 (1.02–1.15) | 1.14 (1.10–1.19) | 1.07 (1.02–1.13) | 1.12 (1.04–1.21) | 1.15 (1.09–1.21) | 1.03 (0.96–1.10) | 1.04 (0.93–1.15) | 1.13 (1.05–1.21) | ||
| 7–8 | 1.06 (1.02–1.11) | 1.12 (1.05–1.20) | 1.13 (1.08–1.18) | 1.08 (1.02–1.14) | 1.12 (1.02–1.22) | 1.14 (1.08–1.20) | 1.04 (0.96–1.11) | 1.14 (1.01–1.28) | 1.11 (1.03–1.20) | ||
| 9–10 | 1.10 (1.06–1.15) | 1.14 (1.07–1.22) | 1.14 (1.09–1.19) | 1.13 (1.07–1.19) | 1.17 (1.08–1.27) | 1.17 (1.11–1.23) | 1.05 (0.99–1.13) | 1.10 (0.98–1.22) | 1.10 (1.03–1.18) | ||
| 11 (medical aid) | 1.39 (1.32–1.47) | 1.22 (1.05–1.42) | 1.23 (1.16–1.31) | 1.44 (1.33–1.55) | 1.39 (1.14–1.71) | 1.30 (1.20–1.41) | 1.32 (1.23–1.43) | 1.02 (0.81–1.27) | 1.15 (1.06–1.25) | ||
HR1: univariate HRs; HR2: age, sex, cancer stage, smoking, alcohol consumption, exercise, BMI, hypertension, stroke, commodity (CCI) adjusted HRs; HR3: HR2 with multiple imputation methods.
SES = socioeconomic status, ICD-10 = International Classification of Diseases, 10th Revision, SEER = Surveillance, Epidemiology, and the End Results, HR = hazard ratio, BMI = body mass index.
The comparison showed that social inequalities in mortality among liver cancer patients were more substantive in covariate-adjusted model 2 (without imputation).
In the relationship between the type of insurance and death from liver cancer (C22), medical aid dependents had a 36% increased risk of death among all liver cancer patients (42% in male patients and 11% in female patients) compared to self-employed subscribers (1.36 [1.10–1.70], 1.42 [0.97–2.08], and 1.11 [0.84–1.45]) in model 2.
In the relationship between income level and death from liver cancer, the liver cancer patients (C22) in the lowest income group (medical aid subscribers and dependents) had about 1.37 times (95% CI, 1.27–1.47) increased risk of death among all patients, 1.44 times (95% CI, 1.32–1.57) increased risk of death among male patients, and 1.16 times (95% CI, 1.01–1.34) increased risk of death among female patients compared to the highest income group.
The liver cancer patients (C22) in the fifth-lowest income group had 1.23 times (95% CI, 1.19–1.27) increased risk of death among all patients, 1.27 times (95% CI, 1.23–1.32) among male patients, and 1.13 times (95% CI, 1.06–1.21) among female patients compared to the highest income group.
The pattern of results derived from model 3 (the imputation model) was similar to that of model 2 (Table 2). The risk of death increased as income decreased and demonstrated a clear reverse-linear relationship.
The risks associated with socioeconomic differences were greater for males than for females. In the comparison of differences between the type of insurance and income levels in males and females, inequalities between the insured groups and the income groups were greater among males than females.
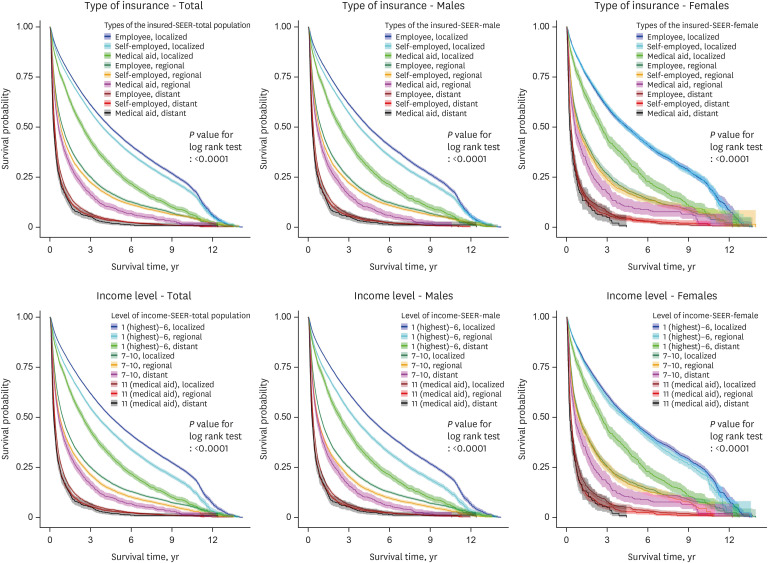
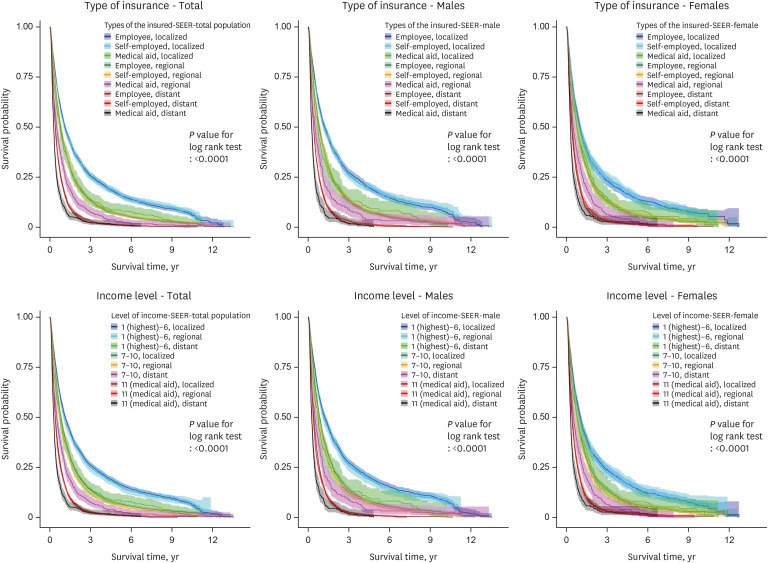
Deaths of HCC patients (code C220), which differed according to social differences, were similar to the results of all liver cancer patients (code C22). However, the strength of the relationship was greater, resulting in greater effects of social inequality. The strength of the relationship in patients with ICC (code C221) was less pronounced than in those with HCC.
The combined effect of type of insurance and cancer stage on liver cancer deaths
The interactive effects between SES (medical aid group or lower-income status) and cancer stage on mortality were statistically significant (P < 0.001). The results showed that the lower the SES, the greater the effect of distant-stage disease on death (C22) (Table 3). Large differences in each insurance group were seen according to different cancer stages. When categorized according to insurance group and cancer stage combinations, the medical aid group with distant-stage cancer had the highest mortality rate (HR, 6.33; 95% CI, 5.35–7.49), followed by the self-employed group with distant cancer (HR, 5.83; 95% CI, 5.57–6.10), and the employee insured group with distant cancer (HR, 5.78; 95% CI, 5.55–6.02). These results were found after adjusting for sex, age, smoking, drinking, exercise, BMI, comorbidities (CCI), and previous histories of hypertension or cerebrovascular diseases (Table 3).
Table 3. The interactive effect of type of insurance, income level, and cancer stage (SEER) on liver cancer (ICD-10 code: C22) mortality in males and females in Korea.
| Variables | Total | Male | Female | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total cancer patients (deaths) | HRs for combined effect | HRs stratified by cancer stage | Total cancer patients (deaths) | HRs for combined effect | HRs stratified by cancer stage | Total cancer patients (deaths) | HRs for combined effect | HRs stratified by cancer stage | ||||||||
| HR1 | HR2 | HR1 | HR2 | HR1 | HR2 | HR1 | HR2 | HR1 | HR2 | HR1 | HR2 | |||||
| Cancer stage and SES | ||||||||||||||||
| Localized and self-employed | 26,133 (16,056) | 1.00 | 1.00 | 1.00 | 1.00 | 20,334 (12,640) | 1.00 | 1.00 | 1.00 | 1.00 | 5,799 (3,416) | 1.00 | 1.00 | 1.00 | 1.00 | |
| Localized and employee | 33,896 (20,276) | 0.88 (0.87–0.90) | 0.92 (0.89–0.96) | 0.85 (0.83–0.87) | 0.91 (0.88–0.95) | 25,997 (15,470) | 0.86 (0.84–0.88) | 0.92 (0.88–0.96) | 0.83 (0.81–0.85) | 0.91 (0.88–0.95) | 7,899 (4,806) | 0.96 (0.92–1.01) | 0.93 (0.86–1.00) | 0.93 (0.89–0.97) | 0.91 (0.85–0.99) | |
| Localized and medical aid | 4,019 (3,234) | 1.34 (1.29–1.39) | 1.18 (1.05–1.32) | 1.27 (1.23–1.32) | 1.15 (1.02–1.29) | 2,645 (2,158) | 1.39 (1.33–1.46) | 1.24 (1.09–1.42) | 1.35 (1.29–1.42) | 1.20 (1.05–1.37) | 1,374 (1,076) | 1.22 (1.14–1.31) | 1.03 (0.83–1.28) | 1.12 (1.05–1.20) | 0.99 (0.79–1.23) | |
| Regional and self-employed | 14,920 (12,988) | 2.43 (2.37–2.48) | 2.60 (2.50–2.71) | 1.00 | 1.00 | 12,120 (10,571) | 2.41 (2.35–2.48) | 2.57 (2.45–2.69) | 1.00 | 1.00 | 2,800 (2,417) | 2.45 (2.32–2.58) | 2.71 (2.48–2.97) | 1.00 | 1.00 | |
| Regional and employee | 17,340 (15,058) | 2.18 (2.13–2.23) | 2.50 (2.41–2.60) | 0.93 (0.90–0.95) | 0.98 (0.94–1.02) | 13,575 (11,762) | 2.17 (2.11–2.22) | 2.49 (2.39–2.60) | 0.92 (0.90–0.95) | 0.98 (0.94–1.02) | 3,765 (3,296) | 2.23 (2.13–2.34) | 2.52 (2.32–2.73) | 0.94 (0.89–0.99) | 0.97 (0.88–1.06) | |
| Regional and medical aid | 1,846 (1,742) | 2.65 (2.52–2.78) | 3.08 (2.67–3.55) | 1.13 (1.08–1.19) | 1.17 (1.01–1.35) | 1,198 (1,130) | 2.75 (2.59–2.92) | 3.11 (2.64–3.67) | 1.17 (1.10–1.24) | 1.18 (1.00–1.40) | 648 (612) | 2.44 (2.24–2.66) | 2.90 (2.18–3.85) | 1.06 (0.97–1.16) | 1.16 (0.87–1.55) | |
| Distant and self-employed | 9,728 (9,533) | 5.00 (4.88–5.13) | 5.83 (5.57–6.10) | 1.00 | 1.00 | 7,592 (7,446) | 5.00 (4.86–5.15) | 5.73 (5.44–6.04) | 1.00 | 1.00 | 2,136 (2,087) | 5.05 (4.78–5.34) | 6.22 (5.66–6.83) | 1.00 | 1.00 | |
| Distant and employee | 11,233 (11,037) | 4.50 (4.39–4.62) | 5.78 (5.55–6.02) | 0.94 (0.91–0.96) | 0.99 (0.95–1.04) | 8,338 (8,205) | 4.46 (4.34–4.59) | 5.72 (5.47–6.00) | 0.93 (0.90–0.96) | 0.99 (0.94–1.04) | 2,895 (2,832) | 4.69 (4.46–4.94) | 6.02 (5.53–6.56) | 0.97 (0.12–1.03) | 1.01 (0.92–1.11) | |
| Distant and medical aid | 1,217 (1,198) | 5.01 (4.72–5.31) | 6.33 (5.35–7.49) | 1.10 (1.03–1.17) | 1.12 (0.95–1.33) | 772 (762) | 5.24 (4.87–5.65) | 6.48 (5.31–7.91) | 1.13 (1.05–1.22) | 1.13 (0.92–1.38) | 445 (436) | 4.59 (4.15–5.08) | 5.69 (4.16–7.78) | 1.02 (0.92–1.14) | 1.08 (0.78–1.48) | |
| Interaction between income level and cancer stage | ||||||||||||||||
| Localized and 1 (highest)–6 | 41,606 (24,515) | 1.00 | 1.00 | 1.00 | 1.00 | 32,257 (18,908) | 1.00 | 1.00 | 1.00 | 1.00 | 9,349 (5,607) | 1.00 | 1.00 | 1.00 | 1.00 | |
| Localized and 7–10 | 17,634 (11,305) | 1.25 (1.22–1.27) | 1.13 (1.09–1.17) | 1.29 (1.26–1.32) | 1.14 (1.10–1.19) | 13,474 (8,816) | 1.29 (1.25–1.32) | 1.16 (1.11–1.21) | 1.32 (1.29–1.36) | 1.17 (1.12–1.22) | 4,160 (2,489) | 1.12 (1.07–1.18) | 1.04 (0.96–1.13) | 1.18 (1.12–1.23) | 1.11 (1.02–1.20) | |
| Localized and 11 (medical aid) | 4,019 (3,234) | 1.53 (1.48–1.59) | 1.28 (1.14–1.43) | 1.50 (1.45–1.56) | 1.26 (1.12–1.41) | 2,645 (2,158) | 1.63 (1.56–1.70) | 1.36 (1.19–1.56) | 1.63 (1.56–1.70) | 1.32 (1.16–1.51) | 1,374 (1,076) | 1.30 (1.21–1.38) | 1.09 (0.88–1.35) | 1.23 (1.15–1.31) | 1.08 (0.86–1.34) | |
| Regional and 1 (highest)–6 | 21,680 (18,720) | 2.50 (2.45–2.54) | 2.70 (2.61–2.78) | 1.00 | 1.00 | 1,7227 (14,840) | 2.53 (2.47–2.58) | 2.69 (2.60–2.79) | 1.00 | 1.00 | 4,453 (3,880) | 2.38 (2.28–2.48) | 2.69 (2.51–2.88) | 1.00 | 1.00 | |
| Regional and 7–10 | 10,145 (8,941) | 2.94 (2.87–3.01) | 2.96 (2.84–3.08) | 1.15 (1.12–1.18) | 1.09 (1.05–1.14) | 8,121 (7,182) | 3.01 (2.93–3.10) | 2.98 (2.85–3.12) | 1.17 (1.13–1.20) | 1.10 (1.05–1.16) | 2,024 (1,759) | 2.65 (2.52–2.80) | 2.90 (2.65–3.17) | 1.08 (1.02–1.14) | 1.06 (0.97–1.16) | |
| Regional and 11 (medical aid) | 1,846 (1,742) | 3.03 (2.89–3.19) | 3.35 (2.91–3.86) | 1.23 (1.17–1.30) | 1.22 (1.06–1.40) | 1,198 (1,130) | 3.22 (3.03–3.42) | 3.42 (2.90–4.03) | 1.28 (1.20–1.36) | 1.24 (1.05–1.46) | 648 (612) | 2.58 (2.37–2.81) | 3.06 (2.31–4.06) | 1.13 (1.03–1.23) | 1.20 (0.90–1.59) | |
| Distant and 1 (highest)–6 | 13,916 (13,649) | 5.18 (5.07–5.29) | 6.12 (5.91–6.34) | 1.00 | 1.00 | 10,534 (10,354) | 5.27 (5.15–5.41) | 6.13 (5.89–6.38) | 1.00 | 1.00 | 3,382 (3,295) | 4.92 (4.70–5.14) | 6.20 (5.75–6.68) | 1.00 | 1.00 | |
| Distant and 7–10 | 6,760 (6,641) | 5.99 (5.82–6.16) | 6.75 (6.45–7.07) | 1.12 (1.09–1.15) | 1.10 (1.05–1.16) | 5,177 (5,082) | 6.10 (5.91–6.30) | 6.72 (6.37–7.08) | 1.12 (1.08–1.16) | 1.10 (1.04–1.16) | 1,583 (1,559) | 5.63 (5.31–5.96) | 6.95 (6.33–7.63) | 1.10 (1.03–1.17) | 1.10 (1.00–1.21) | |
| Distant and 11 (medical aid) | 1,217 (1,198) | 5.74 (5.41–6.08) | 6.89 (5.83–8.14) | 1.18 (1.11–1.25) | 1.17 (0.99–1.38) | 772 (762) | 6.14 (5.71–6.61) | 7.12 (5.84–8.68) | 1.22 (1.13–1.31) | 1.17 (0.96–1.43) | 445 (436) | 4.86 (4.41–5.37) | 6.04 (4.42–8.24) | 1.07 (0.97–1.19) | 1.11 (0.81–1.53) | |
HR1: age and sex-adjusted HRs; HR2: age, sex, cancer stage, smoking, alcohol, exercise, BMI, hypertension, stroke, and comorbidity (CCI)-adjusted HRs.
SEER = Surveillance, Epidemiology, and the End Results, ICD-10 = International Classification of Diseases, 10th Revision, SES = socioeconomic status, HR = hazard ratio, BMI = body mass index, CCI = Charlson Comorbidity Index.
Male patients had similar risk relationships as those described above, while the order of severity affecting liver cancer death was different in females. Therefore, cancer stage contributed substantially to the death of patients, and the contribution in low social class patients was even higher.
Figs. 1, 2, 3 show the KM graphs of survival probability according to the type of insurance and the cancer stage in C22, C220, and C221 patients. While survival probabilities decreased significantly according to the cancer stage, they also decreased according to the type of insurance. Liver cancer survival rates (probabilities) showed clear gaps depending on insurance type and cancer stage (Figs. 1-3). Interestingly, the employee group showed higher survival probabilities than the self-employed group.
Fig. 1. Survival probability according to the type of insurance, income level, and cancer stage in all, male, and female liver cancer patients (ICD-10 code C22).
ICD-10 = International Classification of Diseases, 10th Revision, SEER = Surveillance, Epidemiology, and the End Results.
Fig. 2. Survival probability according to the type of insurance, income level, and cancer stage in all, male, and female hepatocellular cancer patients (ICD-10 code C220).
ICD-10 = International Classification of Diseases, 10th Revision, SEER = Surveillance, Epidemiology, and the End Results.
Fig. 3. Survival probability according to the type of insurance, income level, and cancer stage in all, male, and female intrahepatic cholangiocarcinoma patients (ICD-10 code C221).
ICD-10 = International Classification of Diseases, 10th Revision, SEER = Surveillance, Epidemiology, and the End Results.
Combined effect of income level and cancer stage on liver cancer deaths
The combined effects of income group and cancer stage showed that the lowest income group (medical aid group) with a distant cancer stage (SEER = 7) had the highest mortality rate (HR, 6.89; 95% CI, 5.83–8.14), followed by the employee-insured group with distant-stage cancer (HR, 6.75; 95% CI, 6.45–7.07) and the self-employed insured group with distant-stage cancer (HR, 6.12; 95% CI, 5.91–6.34) (Table 4). The cancer stage and lower social classes substantially contributed to the death of liver cancer patients (Table 4).
Table 4. Differences in the proportion of cancer stages according to socioeconomic status (type of insurance and income level).
| Variables | Total | Males | Females | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Level income | Highest (1–6) | Middle (7–10) | Lowest (11) | Highest (1–6) | Middle (7–10) | Lowest (11) | Highest (1–6) | Middle (7–10) | Lowest (11) | ||
| SEER | |||||||||||
| 1 Localized | 41,606 (45.6) | 17,634 (43.0) | 4,019 (44.0) | 32,257 (45.8) | 13,474 (42.5) | 2,645 (44.6) | 9,349 (44.9) | 4,160 (44.8) | 1,374 (42.9) | ||
| 2 Regional | 21,680 (23.8) | 10,145 (24.8) | 1,846 (20.2) | 17,227 (24.5) | 8,121 (25.6) | 1,198 (20.2) | 4,453 (21.4) | 2,024 (21.8) | 648 (20.2) | ||
| 7 Distant | 13,916 (15.3) | 6,760 (16.5) | 1,217 (13.3) | 10,534 (15.0) | 5,177 (16.3) | 772 (13.0) | 3,382 (16.3) | 1,583 (17.0) | 445 (13.9) | ||
| 9 Unknown | 13,992 (15.3) | 6,453 (15.7) | 2,059 (22.5) | 16,509 (23.5) | 16,509 (23.5) | 16,509 (23.5) | 3,622 (17.4) | 1,523 (16.4) | 737 (23.0) | ||
| Total | 91,194 (100.0) | 40,992 (100.0) | 9,141 (100.0) | 70,388 (100.0) | 31,702 (100.0) | 5,937 (100.0) | 20,806 (100.0) | 9,290 (100.0) | 3,204 (100.0) | ||
| Type of insurance | Self-employed subscribers, as well as dependents | Employee subscribers, as well as dependents | Medical aid subscribers, as well as dependents | Self-employed subscribers, as well as dependents | Employee subscribers, as well as dependents | Medical aid subscribers, as well as dependents | Self-employed subscribers, as well as dependents | Employee subscribers, as well as dependents | Medical aid subscribers, as well as dependents | ||
| SEER | |||||||||||
| 1 Localized | 26,133 (43.5) | 33,896 (45.9) | 4,019 (44.0) | 20,334 (43.2) | 25,997 (46.1) | 2,645 (44.6) | 5,799 (44.8) | 7,899 (45.0) | 1,374 (42.9) | ||
| 2 Regional | 14,920 (24.9) | 17,340 (23.5) | 1,846 (20.2) | 12,120 (25.7) | 13,575 (24.1) | 1,198 (20.2) | 2,800 (21.6) | 3,765 (21.5) | 648 (20.2) | ||
| 7 Distant | 9,728 (16.2) | 11,233 (15.2) | 1,217 (13.3) | 7,592 (16.1) | 8,338 (14.8) | 772 (13.0) | 2,136 (16.5) | 2,895 (16.5) | 445 (13.9) | ||
| 9 Unknown | 9,271 (15.4) | 11,457 (15.5) | 2,059 (22.5) | 7,057 (15.0) | 8,468 (15.0) | 1,322 (22.3) | 2,214 (17.1) | 2,989 (17.0) | 737 (23.0) | ||
| Total | 60,052 (100.0) | 73,926 (100.0) | 9,141 (100.0) | 47,103 (100.0) | 56,378 (100.0) | 5,937 (100.0) | 12,949 (100.0) | 17,548 (100.0) | 3,204 (100.0) | ||
Values are presented as numbers (%).
SEER = Surveillance, Epidemiology, and the End Results.
Figs. 1-3 show the KM graphs of survival probability according to income levels and cancer stages in liver cancer (C22), HCC (C220), and ICC (C221) patients. The survival probabilities showed clear linear gaps in different income groups and cancer stages. The survival probabilities decreased according to cancer stage (from localized to regional and distant) and decreased significantly according to income (from the highest income group to the lowest income group) (Figs. 1-3).
Distribution of cancer stage proportions according to the type of insurance and income
Patients in the lowest income group had more severe cancer (regional or distant) than the highest income group. More patients in the lowest income group had unknown cancer stages (SEER = 9) than in the other groups (Supplementary Table 1).
Supplementary Table 1 shows clear linear gaps depending on income groups and cancer stages.
Distribution of covariates according to the type of insurance and income
The distribution of covariates (health behaviors (smoking, alcohol, exercise, and BMI) and history of disease (hypertension or stroke)) from the National Health Insurance Service’s general examination of big data indicated lower smoking rates, drinking rates, and obesity incidence in patients with lower SES (Supplementary Table 1). However, the incidence of missing values for these parameters (covariates) was also highest among these patients (about 36.57% of all liver cancer patients, 35.23% of males, and 40.92% of females). The incidence of missing values differed depending on income level (32.57% in the highest [1–6] income level, 36.32% in the second level of income [7–10], and 77.64% in the lowest level income [11] in all liver cancer patients) (Supplementary Table 1). However, due to the missing values caused by socioeconomic non-participation in health examinations, these factors might not properly reflect the effect on liver cancer.
DISCUSSION
The main result of this study was that social inequality, reflected by different types of medical insurance and income levels, was associated with higher death rates from liver cancer in Korea. The medical aid group, representing the lowest SES, had a higher risk of death than any other group. The risk of liver cancer death was higher in patients in the medical aid group with highly risky distant cancer stages than any other insured group. Lower patient SES was associated with more severe cancer stages and a greater risk of dying from the disease.
Several causes and mechanisms of the risk factors likely influence the effect of social inequalities on mortality among liver cancer patients in Korea. Several previous studies reported on the effect of social inequalities and, in particular, socioeconomic differences on the incidence, survival, or mortality of liver cancer or HCC globally, consistent with the findings of our study.4,5,6,7,8,10,11,12,23,24 However, our study identified social inequalities represented by income level and the type of insurance more deeply and investigated the interactive effects between social inequalities and cancer stage on liver cancer survival, which had not previously been studied in Korea. We found that the risk of death was increased in patients who presented with distant-stage liver cancer at diagnosis, and the effect of cancer stage on death rates was greater in patients with low SES. We showed that SES substantially affected mortality according to cancer severity, even though cancer severity alone greatly affected survival.
The proportion of patients with more severe cancer stages was greater in the lower-income group than in the highest-income group. However, many unknown stage classifications were present in the medical aid group, and if these unknown cancers had been properly diagnosed, the proportion of severe liver cancer might have been even greater in the lower SES groups (Supplementary Table 1).
The link between income inequalities and liver cancer death rates may be due to inequalities in other risk factors and covariates, such as HBV and hepatitis C virus (HCV) infections, alcohol, and smoking.
Several studies reported that HBV and HCV infections and alcohol were risk factors for liver cancer,25,26 and these risk factors were more prevalent in patients in lower social classes.4,6,13 Several other previous studies showed the independent and combined effects of income, heavy alcohol intake, and HBV infection on the risk of HCC4,13 or the combined effect of SES, viral hepatitis, and lifestyles on HCC6 in Korea.
Our study considered the variables of health behaviors, previous history of disease, and comorbidities as covariates and adjusted them in the analysis. However, these covariates did not significantly affect the relationship between socioeconomic factors and liver cancer death rates.
Finally, several earlier studies reported that social inequalities in HBV vaccination, inequalities in healthcare access, and increases in private insurance system participation in Korea could also affect social inequalities and, consequently, liver cancer deaths in Korea. Social differences in HBV vaccination rates in Korea27 and around the world28,29,30,31 were closely related to social inequality in mortality from liver cancer.
Unequal access to healthcare services is a problem in Korea. The World Health Organization reported that inequalities in healthcare access and outcomes due to income inequalities still exist in Korea, and out-of-pocket medical expenses are high in the National Health Insurance System, even though national insurance benefits and coverage were strengthened and expanded recently.32
Increases in the number of private insurance system subscribers in Korea can increase social inequality by limiting medical access for low-income families. For example, inequalities exist with regard to cancer screening in particular, and the main factors contributing to this inequality are private insurance coverage and income level. Heo and Whang33 reported that the overall quality of medical care was low due to limitations in free cancer screening availability for low-income families, whereas high-income families used private screening for high-quality health checkups.
In conclusion, the findings of this study suggest that social inequalities, such as the type of insurance and income level, as well as inequalities in cancer stages at first diagnosis, contribute to poorer liver cancer, HCC, and ICC outcomes. Health behavior variables (smoking, alcohol, exercise, and BMI), previous history of diseases (hypertension and stroke), and comorbidities did not significantly affect social inequalities in deaths from liver cancer, HCC, and ICC in this study.
This study found that liver cancer deaths were greater in males than in females (2007–2017 HCC survival rates: 25.51% in males and 28.34% in females). The HRs for mortality in females versus males were 0.96 (95% CI, 0.94–0.97) in liver cancer and 0.95 (95% CI, 0.89–0.96) in HCC in model 3 (Table 2).
Our findings differ from those of some previous studies. Kwak and Kim34 reported that the survival rate was higher in males than in females. However, our results are consistent with those of Yang et al.,35 who reported “a higher risk of HCC in men compared with women.” They suggested that the role of estrogens and androgens may differ between men and women; that is, “estrogen may protect against hepatocarcinogenesis and promote a more favorable biology once HCC develops,” while “androgens have demonstrated a synergistic oncogenic effect with HBV in men, but not women.”35
In this study, the reason why more males than females died of liver cancer appears to be due to the higher prevalence of HBV infection and alcoholic liver disease among males. In Korea, the rate of hepatitis B surface antigen positivity in men was 5.1% in 1998 and 4.0% in women, whereas in 2019, it was 2.2% in men and 1.8% in women. A total of 1.36 million patients were treated for alcoholic liver disease (including fatty liver, alcoholic hepatitis, alcoholic cirrhosis, and alcoholic liver failure) in 2019, of which 1.16 million were males (84.8%) and 200,000 were females (15.2%), according to statistics from the HIRA service in Korea.26
The current study also showed that socioeconomic inequality in liver cancer deaths was greater in males. The reason is probably that males in Korean society have greater socioeconomic responsibility and status in their families than females, which highlights the social division in the population. Thus, the gap in liver cancer deaths due to socioeconomic differences is larger in males, too.
In Korea, the earned income of men accounted for 67.6% of total earned income in 2022, whereas women accounted for 32.4%.36 The gender wage gap according to Organisation for Economic Co-operation and Development (OECD) was 34.6% in 201737 and was still 31.2% in 2022.38
Therefore, our study findings suggest that the patriarchal family structure in modern society and the economic status of men, which is greater than that of females, increase males’ socioeconomic responsibility and result in higher liver cancer death rates in lower-income groups.
A limitation of this study is that a significant proportion of the covariate values (health behavior (smoking, drinking, exercise, and BMI) and disease history (information on high blood pressure and cerebrovascular diseases) obtained from the National Health Insurance Service’s general health examinations were missing. Approximately 35–40% of the values for these covariates were missing from our data because most of the people with missing values did not participate in general health examinations (screening). This finding is supported by the similar rates of missing values and non-participation in general health screening in Korea, which were 27.9% in 2013 (participation rate, 72.1%) and 24.6% (participation rate, 75.4%) in 2022.39
The missing covariate values showed differences according to SES. The missing values for liver cancer patients were distributed differently according to income level (32.57% in the highest group [income grades 1–6], 36.32% in the middle group [7–10], and 77.64% in the lowest [medical-aid group]), indicating that more values were missing for patients in the low socioeconomic class.
The missing covariates could dilute the research results or cause systemic errors in the research results, as there were more missing values in patients in the lower socioeconomic class (Supplementary Table 1).
Despite the limitations, the study findings make a significant contribution as cancer patients nationwide were comprehensively analyzed over a 10-year period. Adding the liver cancer stage variable, which is one of the most important risk factors for patients, increased the significance of the analysis of the effect of the interrelationship between socioeconomic factors and liver cancer stage on mortality.
The study results suggest that healthcare delivery systems, including diagnosis and treatment, among patients in lower socioeconomic groups must be considered to reduce the mortality rate of patients with liver cancer. It also suggests that reducing inequalities in treatment measures for advanced liver cancer in patients in low socioeconomic groups is urgent, as cancer deaths significantly increase when cancer is diagnosed in advanced stages. Future studies should thoroughly investigate how social inequality in the diagnosis and treatment of liver cancer affects patients’ chances of survival.
ACKNOWLEDGMENTS
We would like to thank the Bigdata Service Team in Korea Central Cancer Registry (KCCR), the National Cancer Center (NCC), the National Health Insurance Sharing Service (NHISS), and the Health Insurance Review & Assessment Service (HIRA) for assistance with data collection.
Footnotes
Funding: This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI19C1320). This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1A1B03035890). This research was funded by Occupational Safety and Health Research Institute (OSHRI) of Korea Occupational Safety and Health Agency (KOSHA), 2018. This research was funded by KOSHA, 2017. This study was supported by 2017 research grant from Kangwon National University (No. 520170451).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Son M.
- Data curation: Son M, Kim HR.
- Formal analysis: Kim HR, Choi M, Heo YJ, Go SH.
- Methodology: Choe SA, Song SY, Lim KH, Ki M, Paek D.
- Software: Son M, Kim HR.
- Validation: Ki M, Heo YJ, Paek D.
- Investigation: Song SY, Lim KH, Paek D.
- Writing - original draft: Son M.
- Writing - review & editing: Choe SA, Ki M, Paek D.
SUPPLEMENTARY MATERIALS
Differences in covariate proportions according to SES (type of insurance)
Survival probability according to the type of insurance and income level in all, male, and female liver cancer patients (ICD-10 code C22).
References
- 1.International Cancer Research Institute, Seoul National University. [Updated 2020]. [Accessed December 31, 2020]. https://cri.snu.ac.kr/en/about/greetings .
- 2.Son M, Kim S, Lee JH, Kim J, Bae S, Oh JH, et al. Reducing Inequalities in Cancer Incidence and Mortality: Developing Epidemiologic Health Inequality Index and Health Policy in Korea. Report of National Cancer Center: 0720340. Sejong, Korea: Ministry of Health and Welfare; 2007. [Google Scholar]
- 3.Kim CW, Lee SY, Moon OR. Inequalities in cancer incidence and mortality across income groups and policy implications in South Korea. Public Health. 2008;122(3):229–236. doi: 10.1016/j.puhe.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Joshi S, Song YM, Kim TH, Cho SI. Socio-economic status and the risk of liver cancer mortality: a prospective study in Korean men. Public Health. 2008;122(11):1144–1151. doi: 10.1016/j.puhe.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Artinyan A, Mailey B, Sanchez-Luege N, Khalili J, Sun CL, Bhatia S, et al. Race, ethnicity, and socioeconomic status influence the survival of patients with hepatocellular carcinoma in the United States. Cancer. 2010;116(5):1367–1377. doi: 10.1002/cncr.24817. [DOI] [PubMed] [Google Scholar]
- 6.Yun EH, Lim MK, Oh JK, Park JH, Shin A, Sung J, et al. Combined effect of socioeconomic status, viral hepatitis, and lifestyles on hepatocelluar carcinoma risk in Korea. Br J Cancer. 2010;103(5):741–746. doi: 10.1038/sj.bjc.6605803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jembere N, Campitelli MA, Sherman M, Feld JJ, Lou W, Peacock S, et al. Influence of socioeconomic status on survival of hepatocellular carcinoma in the Ontario population; a population-based study, 1990-2009. PLoS One. 2012;7(7):e40917. doi: 10.1371/journal.pone.0040917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yim J, Hwang SS, Yoo KY, Kim CY. Contribution of income-related inequality and healthcare utilisation to survival in cancers of the lung, liver, stomach and colon. J Epidemiol Community Health. 2012;66(1):37–40. doi: 10.1136/jech.2009.104554. [DOI] [PubMed] [Google Scholar]
- 9.Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. doi: 10.1155/2017/2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YA, Kang D, Moon H, Sinn D, Kang M, Woo SM, et al. Survival in untreated hepatocellular carcinoma: a national cohort study. PLoS One. 2021;16(2):e0246143. doi: 10.1371/journal.pone.0246143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adler Jaffe S, Myers O, Meisner AL, Wiggins CL, Hill DA, McDougall JA. Relationship between insurance type at diagnosis and hepatocellular carcinoma survival. Cancer Epidemiol Biomarkers Prev. 2020;29(2):300–307. doi: 10.1158/1055-9965.EPI-19-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kronenfeld JP, Ryon EL, Goldberg D, Lee RM, Yopp A, Wang A, et al. Survival inequity in vulnerable populations with early-stage hepatocellular carcinoma: a United States safety-net collaborative analysis. HPB. 2021;23(6):868–876. doi: 10.1016/j.hpb.2020.11.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spadea T, D’Errico A, Demaria M, Faggiano F, Pasian S, Zanetti R, et al. Educational inequalities in cancer incidence in Turin, Italy. Eur J Cancer Prev. 2009;18(3):169–178. doi: 10.1097/CEJ.0b013e3283265bc9. [DOI] [PubMed] [Google Scholar]
- 14.Poulson MR, Blanco BA, Geary AD, Kenzik KM, McAneny DB, Tseng JF, et al. The role of racial segregation in treatment and outcomes among patients with hepatocellular carcinoma. HPB. 2021;23(6):854–860. doi: 10.1016/j.hpb.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Healthcare Big Data Platform project, which was initiated by the Korea Health Industry Development Institute and the Ministry of Health and Welfare in Korea. [Accessed April 28, 2023]. https://hcdl.mohw.go.kr .
- 16.The Korea National Cancer Incidence (KNCI) database from the Korea Central Cancer Registry (KCCR), the National Cancer Center (NCC) [Accessed June 30, 2019]. https://www.ncc.re.kr/main.ncc?uri=english/sub04_Introduction .
- 17.The National Health Insurance Sharing Service (NHISS) [Accessed June 30, 2019]. https://nhiss.nhis.or.kr/bd/ay/bdaya001iv.do .
- 18.The Health Insurance Review & Assessment Service (HIRA) [Accessed June 30, 2019]. https://www.hira.or.kr/main.do .
- 19.National Institutes of Health, U.S. Department of Health and Human Services. USA.gov. National Cancer Institute SEER training modules. [Accessed December 31, 2019]. https://training.seer.cancer.gov/modules_site_spec.html .
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Health Insurance Review and Assessment Service. Building data for analysis of comorbidities. [Updated 2019]. [Accessed December 31, 2019]. https://repository.hira.or.kr/handle/2019.oak/2067 .
- 22.Yun SC. Imputation of missing values. J Prev Med Public Health. 2004;37(3):209–211. [PubMed] [Google Scholar]
- 23.Ji J, Hemminki K. Variation in the risk for liver and gallbladder cancers in socioeconomic and occupational groups in Sweden with etiological implications. Int Arch Occup Environ Health. 2005;78(8):641–649. doi: 10.1007/s00420-005-0015-1. [DOI] [PubMed] [Google Scholar]
- 24.Menvielle G, Kunst AE, Stirbu I, Borrell C, Bopp M, Regidor E, et al. Socioeconomic inequalities in alcohol related cancer mortality among men: to what extent do they differ between Western European populations? Int J Cancer. 2007;121(3):649–655. doi: 10.1002/ijc.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korean Liver Cancer Association (KLCA); National Cancer Center (NCC), Goyang, Korea. 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol. 2019;20(7):1042–1113. doi: 10.3348/kjr.2019.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korean Association for the Study on the Liver. White Paper on Liver Diseases in Korea. Seoul, Korea: YD&P; 2021. [Google Scholar]
- 27.Park B, Choi KS, Lee HY, Jun JK, Park EC. Socioeconomic inequalities in completion of hepatitis B vaccine series among Korean women: results from a nationwide interview survey. Vaccine. 2012;30(40):5844–5848. doi: 10.1016/j.vaccine.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Bhuiyan AR, Kabir N, Mitra AK, Ogungbe O, Payton M. Disparities in hepatitis B vaccine coverage by race/ethnicity: the National Health and Nutrition Examination Survey (NHANES) 2015-2016. Diseases. 2020;8(2):10. doi: 10.3390/diseases8020010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu D, Guo N, Wang J, Nicholas S, Wang Z, Zhang G, et al. Socioeconomic inequality in hepatitis B vaccination of rural adults in China. Hum Vaccin Immunother. 2018;14(2):464–470. doi: 10.1080/21645515.2017.1396401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clouston S, Kidman R, Palermo T. Social inequalities in vaccination uptake among children aged 0-59 months living in Madagascar: an analysis of Demographic and Health Survey data from 2008 to 2009. Vaccine. 2014;32(28):3533–3539. doi: 10.1016/j.vaccine.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 31.Barata RB, Ribeiro MC, de Moraes JC, Flannery B Vaccine Coverage Survey 2007 Group. Socioeconomic inequalities and vaccination coverage: results of an immunisation coverage survey in 27 Brazilian capitals, 2007-2008. J Epidemiol Community Health. 2012;66(10):934–941. doi: 10.1136/jech-2011-200341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. Republic of Korea-WHO country Cooperation Strategy 2019-2023. WPRO/2019/DPM/001. Manila, Philippines: World Health Organization, Westen Pacific Region; 2019. [Google Scholar]
- 33.Heo JH, Whang JN. Income-related inequalities in cancer screening in Korea: using Concentration Index and Decomposition of CI. Pogon Sahoe Yongu. 2014;34(3):59–81. [Google Scholar]
- 34.Kwak M, Kim C. Disparities by age, sex, tumor stage, diagnosis path, and area-level socioeconomic status in survival time for major cancers: results from the Busan Cancer Registry. J Korean Med Sci. 2017;32(12):1974–1983. doi: 10.3346/jkms.2017.32.12.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang D, Hanna DL, Usher J, LoCoco J, Chaudhari P, Lenz HJ, et al. Impact of sex on the survival of patients with hepatocellular carcinoma: a Surveillance, Epidemiology, and End Results analysis. Cancer. 2014;120(23):3707–3716. doi: 10.1002/cncr.28912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Income data of the National Tax Service’s quintile. [Updated 2023]. [Accessed September 11, 2023]. https://www.hidomin.com/news/articleView.html?idxno=523403 .
- 37.OECD gender wage gap 2017. [Updated 2023]. [Accessed September 11, 2023]. https://www.oecd.org/country/korea/thematic-focus/gender-equality-korea-has-come-a-long-way-but-there-is-more-work-to-do-8bb81613/
- 38.OECD gender wage gap 2022. [Updated 2023]. [Accessed September 11, 2023]. https://data.oecd.org/earnwage/gender-wage-gap.htm .
- 39.National Health Insurance Service. Annual health examination rate. [Updated 2023]. [Accessed September 12, 2023]. https://www.nhis.or.kr/announce/wbhaec11407m01.do .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differences in covariate proportions according to SES (type of insurance)
Survival probability according to the type of insurance and income level in all, male, and female liver cancer patients (ICD-10 code C22).