Abstract
Gastrointestinal complications in human immunodeficiency virus (HIV) infection are indicative of impaired intestinal mucosal immune system. We used simian immunodeficiency virus (SIV)-infected rhesus macaques as an animal model for HIV to determine pathogenic effects of SIV on intestinal T lymphocytes. Intestinal CD4+ T-cell depletion and the potential for cytokine responses were examined during SIV infection and compared with results for lymphocytes from lymph nodes and blood. Flow cytometric analysis demonstrated severe depletion of CD4+CD8− single-positive T cells and CD4+CD8+ double-positive T cells in intestinal lamina propria lymphocytes (LPL) and intraepithelial lymphocytes (IEL) during primary SIV infection which persisted through the entire course of SIV infection. In contrast, CD4+ T-cell depletion was gradual in peripheral lymph nodes and blood. Flow cytometric analysis of intracellular gamma interferon (IFN-γ) and interleukin-4 (IL-4) production following short-term mitogenic activation revealed that LPL retained same or higher capacity for IFN-γ production in all stages of SIV infection compared to uninfected controls, whereas peripheral blood mononuclear cells displayed a gradual decline. The CD8+ T cells were the major producers of IFN-γ. There was no detectable change in the frequency of IL-4-producing cells in both LPL and peripheral blood mononuclear cells. Thus, severe depletion of CD4+ LPL and IEL in primary SIV infection accompanied by altered cytokine responses may reflect altered T-cell homeostasis in intestinal mucosa. This could be a mechanism of SIV-associated enteropathy and viral pathogenesis. Dynamic changes in intestinal T lymphocytes were not adequately represented in peripheral lymph nodes or blood.
The gastrointestinal (GI) tract harbors more than 85% of the lymphoid tissue and over 90% of the lymphocytes in the body; peripheral blood, in contrast, represents only 2 to 5% of total lymphocytes. Thus, intestinal tissue may be a significant reservoir of the human immunodeficiency virus type 1 (HIV-1). Since the intestine harbors a large pool of activated lymphocytes, it is also an important site for early viral dissemination as well as for the initial host-virus interactions. GI abnormalities such as nutrient malabsorption, malnutrition, diarrhea, and weight loss are commonly seen in HIV-infected individuals and are indicative of mucosal immune dysregulation (6, 17, 18, 27, 31). The CD4+ T-cell depletion was more pronounced in duodenal mucosa than peripheral blood in individuals with advanced HIV infection. However, our understanding is limited with respect to how the intestinal mucosal immune system is involved in the primary HIV infection and whether immunophenotypic and functional alterations in intestinal immune cells are adequately reflected by circulating immune cells in peripheral blood. The time course and severity of CD4+ T-cell depletion in intestinal mucosa compared to peripheral blood or peripheral lymph nodes are not known. Since pathologic processes occurring in lymphoid organs may determine the outcome of the viral infection, it will be important to evaluate the alterations in intestinal lymphoid cells with respect to T-cell homeostasis and function in HIV infection.
HIV infection is characterized by CD4+ T-cell depletion and depressed proliferative and cytokine responses of peripheral blood mononuclear cells (PBMC) to mitogenic or antigenic stimulations. It is not known whether intestinal T lymphocytes exhibit similar functional defects. The ability of T lymphocytes to produce cytokines in response to pathogenic organisms is crucial in inducing and maintaining effective innate as well as acquired immunity. Since intestinal T lymphocytes are localized at functionally distinct sites, including intestinal epithelium and lamina propria, it will be important to examine isolated cell populations for their cytokine responses. Intraepithelial lymphocytes (IEL) located in the epithelium and lamina propria lymphocytes (LPL) located in the lamina propria are effector cells with the memory phenotype and are important in the virus-specific responses (26, 41, 63). They are immunophenotypically and functionally distinct from peripheral lymphocytes. Studies on intestinal lymphocytes in HIV infection have been limited due to the difficulty in obtaining adequate amounts of tissues in early stages of infection. Suitable animal models of HIV infection are valuable in determining kinetics of CD4+ T-cell depletion and cytokine responses in intestinal mucosal immune cells following viral infection to gain insights into mechanisms of CD4+ T-cell depletion, T-cell homeostasis, and immune dysregulation in HIV infection.
The simian immunodeficiency virus (SIV)-infected rhesus macaques serve as an excellent animal model to study immunopathogenesis of HIV (38, 61). We have previously shown that the GI tract is an early target for SIV infection (28). SIV-infected T lymphocytes and macrophages were detected throughout the intestinal mucosa, and macrophage infection was dominant in the primary acute and terminal stages (28–30). The SIV-associated enteropathy could be documented in primary SIV infection in the absence of detectable opportunistic enteropathogens, indicating an occurrence of immunopathogenic events early in intestine and potentially more severe than those in peripheral blood (28, 61).
We hypothesized that dynamic quantitative and qualitative changes may occur in intestinal T lymphocytes in primary HIV infection which may not be adequately reflected in peripheral lymph nodes and blood. To test this hypothesis, we isolated IEL and LPL from jejunal mucosa from rhesus macaques during primary acute, asymptomatic, and terminal stages of SIV infection and examined immunophenotypic (CD4+ and CD8+ T cell percentages) and functional (gamma interferon [IFN-γ] and interleukin-4 [IL-4] expression and cytotoxic T-cell activity) alterations. Our data demonstrated that there was a severe depletion of intestinal CD4+ T cells in the primary SIV infection which was not adequately mirrored in T lymphocytes from peripheral lymph nodes or blood. However, intestinal T lymphocytes retained a high potential of IFN-γ production through the entire course of SIV infection, in contrast to the PBMC, which showed a decline in that potential. Thus, intestinal CD4+ T-cell depletion accompanied by altered cytokine responses may reflect altered T-cell homeostasis, and this may be a mechanism of viral pathogenesis.
MATERIALS AND METHODS
Animals, viral infection, and tissue collection.
Rhesus macaques (Macaca mulatta) were housed at the California Regional Primate Research Center in accordance with American Association for Accreditation of Laboratory Animal Care standards. The animals were seronegative for SIV, type D retrovirus, and simian T-cell leukemia virus type 1 at onset of the study. Animals were intravenously infected with 102 to 103 50% tissue culture infective doses of SIVmac251. This viral inoculum was previously shown to cause persistent viremia and infection of multiple tissues in vivo, leading to fatal AIDS-like disease (44). The viral infection studies were performed with two groups of animals: group 1 (n = 4) was infected for the short term (1 to 6 weeks), while group 2 (n = 9) was infected for the long term (4 to 18 months).
Four animals in the group 1 were 4 to 5 years of age and had short-term infections encompassing the primary acute phase (1 to 4 weeks postinfection [p.i.]) and early asymptomatic phase (6 weeks p.i.) of infection. Longitudinal immunophenotypic analysis was performed on isolated IEL, LPL, and PBMC. The lymphocytes isolated from jejunal biopsies or peripheral blood samples from the same animals prior to SIVmac251 infection, and from one uninfected healthy animal, provided preinfection control values. Jejunal mucosa, axillary (Ax-LN) and mesenteric (Ms-LN) lymph nodes, and blood samples were collected at necropsy from SIV-infected animals during the primary acute (n = 3) and early asymptomatic (n = 1) stages of infection.
Animals in group 2 were 5 to 15 years of age and had long-term SIV infection encompassing asymptomatic (4 to 9 months p.i.) and simian AIDS (SAIDS) (1 to 1.5 years p.i.) phases. Animals were infected intravenously or vaginally with 102 to 103 50% tissue culture infective doses of a previously characterized SIVmac251 inoculum (courtesy of Chris Miller, California Regional Primate Research Center). Intestinal mucosa and blood samples were collected at necropsy from SIV-infected animals during the long-term asymptomatic (n = 5) and SAIDS (n = 4) phases. Isolated PBMC, IEL, and LPL samples obtained at necropsy from three uninfected animals of comparable age served as negative controls for the animals in group 2.
Detection of SIV-infected cells by in situ hybridization.
Tissue samples were examined for SIV infection by in situ hybridization as previously reported (30), with minor modifications. Cells positive for SIV RNA in intestinal tissues were detected by in situ hybridization of SIV RNA with digoxigenin-UTP-labeled riboprobes. This assay was able to detect cells with active viral replication but was not sensitive enough to detect latently infected cells with low SIV proviral copy number. Formalin-fixed and paraffin-embedded intestinal tissue sections were deparaffinized and treated with proteinase K for 10 min at 37°C, followed by prehybridization for 1 h at 50°C in hybridization solution containing 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 5× Denhardt’s solution, 50% deionized formamide, 10% dextran sulfate, and 1 mg of tRNA per ml. Then the tissue sections were placed in a humidification chamber at 50°C for 16 to 18 h to hybridize with the labeled SIV probe. The unhybridized SIV RNA probe was removed by washing the slides at 50°C in the wash buffer containing 50% formamide and 2× SSC, followed by 1× SSC and 0.5× SSC. The slides were reacted with RNase A for 30 min to hydrolyze the remaining unhybridized SIV RNA probe. Then slides were incubated for 1 h with a monoclonal antibody to digoxigenin conjugated to horseradish peroxidase and counterstained with hematoxylin. The positive controls included SIV-infected PBMC cultures or previously characterized SIV-infected intestinal tissues. The negative controls included intestinal tissues and PBMC from uninfected healthy animals. The slides were microscopically examined for the presence of SIV-positive cells, and each tissue section was scored semiquantitatively from 0 to 5 for viral load as following: 0 (no positive cells), 1 (1 to 10 cells/tissue section), 2 (1 to 5 cells/25× field), 3 (6 to 10 cells/25× field), 4 (11 to 15 cells/25× field), and 5 (>15 cells/25× field).
Isolation of lymphocytes.
The IEL and LPL were isolated by using modifications of previously described procedures (13, 34, 62, 68). Briefly, samples of jejunum 10 to 20 cm long were collected within the 30 min of necropsy. The tissue was cut into 0.5-cm2 pieces, rinsed in cold phosphate-buffered saline (PBS), and used for sequential isolation of IEL and LPL. The IEL were isolated first by incubating tissue in IEL isolation medium (Hanks’ balanced salt solution [Gibco BRL, Grand Island, N.Y.] containing 0.75 mM EDTA, 100 U of penicillin per ml, 100 U of streptomycin per ml, and 5% fetal calf serum [FCS]), pH 7.2. Subsequently, LPL were isolated by incubating tissue samples in LPL isolation medium (RPMI 1640 medium containing 15 U of collagenase [type II; Sigma], 100 U of penicillin, and 100 U of streptomycin per ml, 5 ml l-glutamine, 5 ml HEPES buffer, 5% FCS). All incubations were performed at 37°C with rapid shaking for 30 min. The cell suspension media were passed through stainless steel screen cups to remove the residual tissue fragments. Cells were washed and resuspended in RPMI 1640 complete (100 U of penicillin per ml, 100 U of streptomycin per ml, 10% FCS) and stored on ice till use. The whole process was repeated at least three times for each cell type.
Isolated cell samples were enriched for lymphocytes by isotonic discontinuous Percoll (Sigma) density gradients (35 and 60% [vol/vol]). Isolated IEL and LPL samples were resuspended in RPMI 1640 complete medium, layered on the top of the gradient, and centrifuged at 1,000 × g for 20 min at 4°C. Lymphocytes formed a band at the interface between the 35 and 60% Percoll layers, which was collected. The number of isolated cells from ∼20-cm-long tissue was in the range of 5 × 106 to 30 × 106 for IEL and 2 × 107 to 8 × 107 for LPL, as determined by counting with a hemocytometer. Viability of lymphocytes was greater than 95%, as determined by trypan blue exclusion staining.
Lymphocytes were also isolated from lymph nodes and peripheral blood samples. The cells were isolated by density centrifugation over Lymphocyte Separation Medium (LSM; Organon-Technica, Durham, N.C.).
Aliquots of freshly isolated cells were stained with fluorescently labeled antibodies for flow cytometric analysis, while the remaining cells were washed in PBS and cryopreserved.
Immunophenotypic analysis of T lymphocytes by flow cytometry.
The CD4+ and CD8+ T-lymphocyte subsets from isolated mononuclear cell samples from peripheral blood, lymph nodes, and intestinal mucosa were examined with mouse anti-human monoclonal antibodies CD4-phycoerythrin (PE) (OKT4; Ortho Diagnostic Systems Inc., Raritan, N.J.), CD8-fluorescein isothiocyanate (FITC) (Becton Dickinson, Mountain View, Calif., and Caltag Laboratories, South San Francisco, Calif.), and biotinylated CD3 (Biosource International, Camarillo, Calif.), followed by streptavidin conjugated to Tricolor (TC; Caltag). These antibodies have been shown to cross-react with macaque lymphocytes. Isotype-matched immunoglobulin G1 (IgG1)-FITC and IgG1-PE antibodies (Becton Dickinson and Caltag) were used as negative controls. Isolated cells were washed in flow buffer (1× PBS with 1% bovine serum albumin and 0.1% sodium azide), and 106 cells were incubated with anti-CD4 or anti-CD8 antibody for 30 min at 4°C. After being washed in 2 ml of flow buffer, the cells were fixed in 0.5 ml of cold 1% paraformaldehyde, pH 7.4. Whole blood samples were used for immunophenotyping PBMC. The EDTA-treated blood samples were stained with anti-CD4 and anti-CD8 antibodies for 15 min at room temperature, followed by a whole-blood lysis Q-prep (Coulter) procedure according to the manufacturer’s instructions. Acquisition of data was performed on a FACScan flow cytometer (Becton Dickinson). Analysis for the group 1 animals was performed with gates set simultaneously on lymphocytes, based on forward scatter (FSC) versus side scatter (SSC) and FL3 (CD3) versus SSC. For group 2 animals, analysis was performed with gates set on lymphocytes according to their forward-versus-side scatter characteristics. A minimum of 5,000 gated events (which amounted to 10,000 to 25,000 total counted events) were collected for each sample in list mode. Data were collected and analyzed by CellQuest software (Becton Dickinson).
Intracellular cytokine measurements by flow cytometry.
Cells were prepared for three-color intracellular cytokine analysis according to previously described (22) procedures, with modifications. Cryopreserved LPL and PBMC were incubated for 12 h at 37°C in complete RPMI 1640 supplemented with 20 μg of ciproflaxin hydrochloride (Miles Inc., Kankakee, Ill.) per ml at a density of 2 × 106 cells/ml. Then cells were stimulated for 4 h with 10 ng of phorbol myristate acetate (PMA; Sigma) per ml, 500 ng of ionomycin (Calbiochem-Novabiochem Corporation, San Diego, Calif.) per ml, and 2 μM monensin (Sigma). Negative controls included cell samples incubated with 2 μM monensin only. Upon stimulation, cells were washed with cytoflow buffer and incubated for 30 min at 4°C with either anti-human CD8-TC (Caltag) or anti-human CD4 (OKT4; Ortho) followed by goat anti-mouse IgG (heavy plus light chain)-TC (Caltag). Cells were washed with 1× PBS and fixed in solution A (Cell Fix & Perm Kit; Caltag). The cells were then washed and resuspended in permeabilization solution B and incubated with mouse anti-human IFN-γ–FITC (Pharmingen, San Diego, Calif.) and mouse anti-human IL-4–PE (Pharmingen). The samples were washed and resuspended in 1× PBS for measuring cell fluorescence. Negative controls included the cell samples stained with isotype-matched mouse antibodies (IgG1-FITC and IgG1-PE; Caltag) resuspended in permeabilizing solution B. Controls for nonspecific binding of antibodies included the cell samples labeled with anti-IFN-γ and anti-IL-4 monoclonal antibodies in 1× PBS. Following this reaction, cells were washed, resuspended in 1× PBS, and analyzed with a FACScan flow cytometer. Cell events were collected by double gating on FSC/SSC and FL3 (CD4 or CD8)/SSC to identify only the cells that satisfy both requirements of being lymphocytes and being positive for desired surface marker. The proportion of cytokine producing T cells for each subset was determined from the FL1-versus-FL2 dot plot. Data collected was analyzed by CellQuest software (Becton Dickinson).
Measurement of SIV-specific CTL activity.
Lymphocytes isolated from the intestinal mucosa were cultured in a limiting-dilution format due to the low number of cells recovered, as previously described (40), in parallel with lymphocytes from blood, spleen, and lymph nodes. Lymphocytes were diluted threefold serially for three dilutions in complete medium with replicates of 28 to 30 wells per dilution in 96-well round bottom plates (Fisher). The cells were stimulated with concanavalin A (10 μg/ml; Sigma) and supplemented with human irradiated PBMC as feeder cells at a concentration of 105 per well and 5% human IL-2 (Schiapparelli Biosystems, Inc. Columbia, Md.). On day 7 of culture, 20 U of recombinant human IL-2 (donated by Cetus Corp., Emeryville, Calif.) per ml was added. Cytotoxicity was measured on day 14. Individual wells were split three ways and assayed for cytolytic function in a 5-h chromium release assay against autologous target cells. Autologous B lymphocytes were transformed by Herpes papio (594Sx1055 producer cell line; provided by M. Sharp, Southwest Foundation for Biomedical Research, San Antonio, Tex.), infected overnight with wild-type vaccinia virus (strain WR) or a recombinant vaccinia virus expressing the p55gag or gp160env of SIVmac239 (provided by L. Giavedoni and T. Yilma, University of California, Davis), and then labeled with 50 μCi of chromium-51 (Na2CrO4; Amersham Holdings, Inc. Arlington Heights, Ill.) per 106 cells. Positive wells were scored from supernatant chromium measured in a liquid scintillation counter (Microbeta 1450; Wallac Biosystems, Gaithersburg, Md.) and had at least 15% specific lysis, based on a bimodal distribution of chromium release.
RESULTS
Early dissemination of SIV infection in intestinal mucosa.
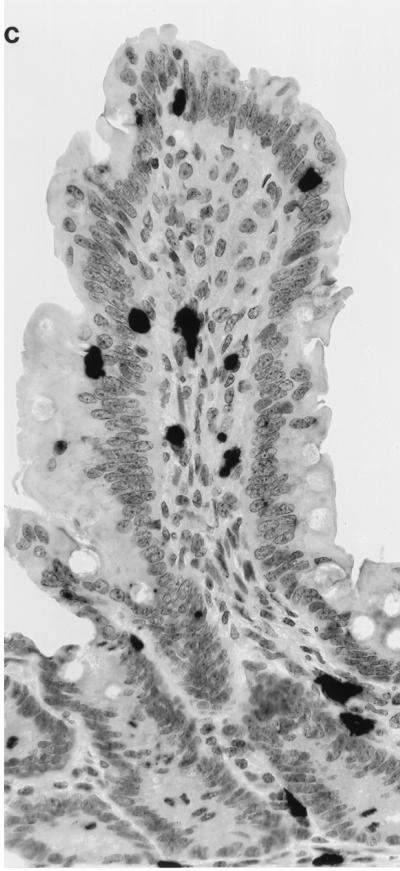
Cells with active SIV replication were localized in intestinal tissue samples by in situ hybridization through the entire SIV infection course (Fig. 1). The SIV-infected cells were detectable as early as 1 week following infection and were found to be present through the entire course of SIV infection (Fig. 1A). The virally infected cells were widely disseminated in the gut-associated lymphoid tissue (GALT; lamina propria, lymphoid follicles, and Peyer’s patches). Many of the IEL and LPL were positive for SIV RNA (Fig. 1C). Comparison of jejunum with Ax-LN and Ms-LN showed comparable levels of infected cells in jejunum and Ax-LN at 2 weeks p.i. during the primary SIV infection (Fig. 1B), whereas Ms-LN had higher levels of infection. While number of infected cells in the Ax-LN decreased at 4 weeks p.i. (level 1), both jejunum and Ms-LN maintained the same level of infection. These results demonstrated that the GALT was an early target of SIV dissemination and remained an active viral reservoir until the terminal stage of viral infection. Although both T cells and macrophages were positive for SIV RNA throughout the SIV infection course, it was noted that macrophage infection was predominant during the primary acute and terminal stages of SIV infection (28).
FIG. 1.
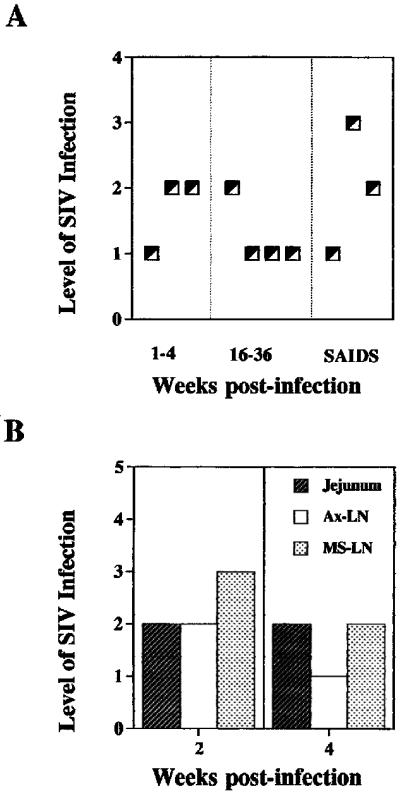
SIV-infected cells were detected in intestinal mucosa through the entire course of SIV infection. SIV-infected cells in jejunal tissues were detected by in situ hybridization, and the level of SIV infection was scored according to the scale described in the Materials and Methods. (A) Levels of SIV infection in jejunal tissue during the primary acute (1 to 4 weeks p.i.), asymptomatic (16 to 36 weeks p.i.), and SAIDS stages. (B) Comparison of the levels of viral infection in jejunum, Ax-LN, and Ms-LN from the same animal during the primary acute phase of SIV infection. (C) SIV-infected cells in intestinal epithelium and lamina propria.
Severe depletion of CD4+CD8− single-positive and CD4+CD8+ double-positive T cells occurred in small intestinal mucosa during primary SIV infection.
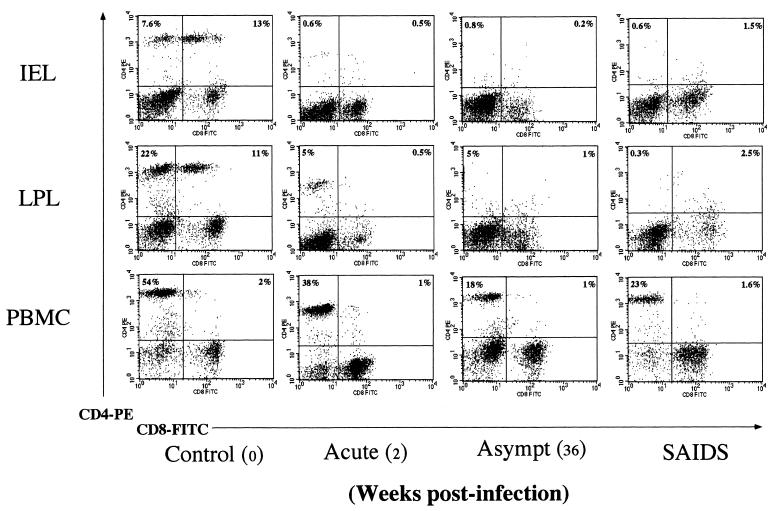
The prevalence of CD4+CD8− and CD4−CD8+ single-positive and CD4+CD8+ double-positive T-lymphocyte subsets was determined in the cell populations of IEL and LPL isolated from jejunal tissues by using two-color flow cytometric analysis (Fig. 2). In the uninfected control animal, IEL had relatively fewer CD4+CD8− single-positive T cells (7.6%) than LPL (22%), as shown in Fig. 2. Both IEL and LPL had remarkably high percentages of CD4+CD8+ double-positive T cells (13 and 11%, respectively). The IEL had a higher percentage of CD8+ T cells (15%) than LPL (22%). Compared to the uninfected animals, a notable change occurred in the CD4+ T-cell percentages from SIV-infected animals.
FIG. 2.
Severe depletion of CD4+CD8− and CD4+CD8+ T cells in jejunum occurs in primary SIV infection. The IEL, LPL, and PBMC were isolated from the animals at different stages of SIV infection (primary acute, asymptomatic [Asympt], and SAIDS) and the CD4+CD8−, CD4+CD8+, and CD4−CD8+ T cells were analyzed by two-color flow cytometry. Freshly isolated cells were stained with PE-conjugated anti-CD4 and FITC-conjugated anti-CD8 and then analyzed on a flow cytometer as described in the text. A lymphocyte gate was set on forward versus side scatter, and percentages of positive cells within this gate are shown. Shown are results for a representative set of animals from different stages of SIV infection, illustrating changes in distribution of CD4+CD8−, CD4+CD8+, and CD4−CD8+ T-cells subsets between different tissue compartments (IEL, LPL, and PBMC) within the same animal.
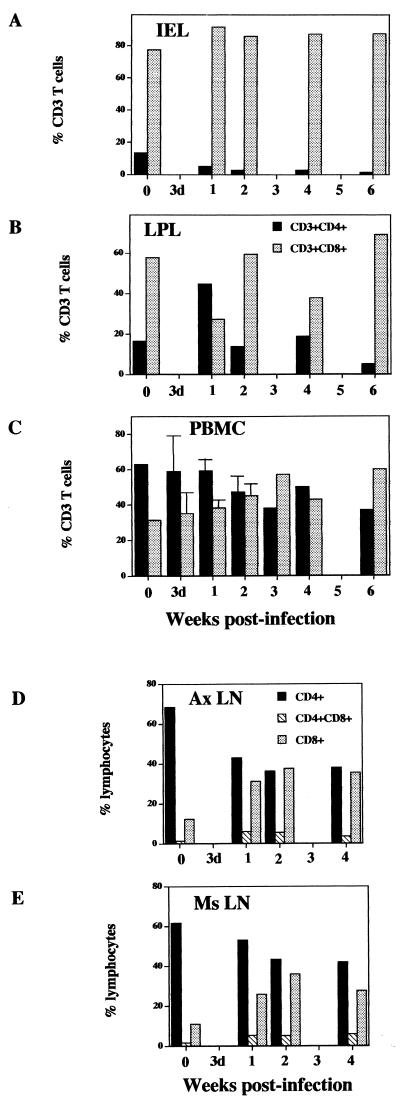
A dramatic depletion of both CD4+CD8− single-positive and CD4+CD8+ double-positive T cells was observed in both IEL and LPL compared to the preinfection values from the same animals as well as uninfected controls (Fig. 2). The CD4+ T-cell loss was more prominent in IEL than LPL. The CD4+ T-cell depletion in IEL was evident as early as 1 week p.i. (5%) compared to the uninfected control (13%) and remained low (<3%) through the primary acute (till 3 weeks p.i.) and early asymptomatic (4 to 6 weeks p.i.) stages (Fig. 3A). A transient increase of CD4+ T cells occurred at 1 week p.i. in the LPL (44%) compared to the uninfected control (16%), which was followed by a rapid depletion appearing at 2 weeks p.i. The CD4+ T-cell depletion was notable in the early asymptomatic stage (5%) at 6 weeks p.i. The prevalence of CD8+ T cells among IEL and LPL showed a relative increase in primary SIV infection that coincided with the CD4+ T-cell depletion (Fig. 3A).
FIG. 3.
The severe CD4+ T-cell depletion in intestinal mucosa during primary SIV infection is not reflected in peripheral blood and lymph node lymphocytes. Lymphocytes were isolated from the five sites indicated from rhesus macaques at 3 days, 1, 2, 3, 4, and 6 weeks post-SIV infection. The percentages of CD4+ and CD8+ T cells were determined by flow cytometry.
No restoration of either CD4+CD8− single-positive or CD4+CD8+ double-positive T cells in intestinal mucosa was detectable in the asymptomatic or terminal stage of SIV infection.
Data on the immunophenotypic alterations in intestinal lymphocytes were obtained from long-term SIV-infected animals (group 2) in the asymptomatic (16 to 36 weeks p.i.) or SAIDS (1 to 1.5 years p.i.) stage. Depletion of CD4+CD8− single-positive and CD4+CD8+ double-positive T-cell subsets of IEL and LPL was observed through the asymptomatic and terminal stages of SIV infection. The percentages of CD4+CD8− single-positive and CD4+CD8+ double-positive IEL were much lower (<1%) in SIV-infected animals than in the uninfected control animals (8 to 17%) in group 2. Similarly, the percentages of CD4+CD8− single-positive and CD4+CD8+ double-positive LPL remained very low (1 to 7%). The prevalence of CD4−CD8+ single-positive T cells in IEL and LPL showed a relative increase in IEL and LPL that coincided with CD4+ T-cell depletion in SIV infection.
CD4+ T-cell depletion was gradual in peripheral lymph nodes and PBMC in contrast to the intestinal mucosa.
The prevalence of CD4+ and CD8+ T-cell subsets in lymphocytes was determined from peripheral blood and Ax-LN and Ms-LN and compared with the prevalence of these subsets in IEL and LPL from SIV-infected and uninfected animals. In uninfected controls, PBMC had higher percentage of CD4+CD8− single-positive T cells than IEL and LPL (Fig. 2). In contrast, PBMC had low (2%) prevalence of CD4+CD8+ double-positive T cells compared to IEL and LPL. The CD4−CD8+ single-positive cells were the prevalent T-cell subset among PBMC, IEL, and LPL.
In SIV-infected animals, a small decline occurred in the percentage of CD4+CD8− T cells in PBMC at 2 weeks p.i. (Fig. 2 and 3). The prevalence of CD4+CD8− T cells in peripheral blood varied among the animals in the asymptomatic stage (6 to 22%) but remained lower than in the uninfected controls (32 to 57%). The CD4+ T-cell depletion was apparent in PBMC during the terminal stage of infection (13 to 37%). The severity of CD4+ T-cell depletion in intestinal mucosa during the primary SIV infection and persistence of CD4+ T-cell loss through asymptomatic stage was not adequately reflected in the peripheral blood compartment. There was no obvious change in the proportion of peripheral CD4+CD8+ double-positive T cells in SIV infection. The percentage of CD4−CD8+ T cells was variable in PBMC but increased considerably (30 to 50%) during the primary SIV infection and remained high through the terminal stage.
In both Ax-LN and Ms-LN, the decline in the percentages of CD4+CD8− single-positive T cells (∼10% drop) was less evident than the decline in IEL and LPL lymphocytes during primary SIV infection (Fig. 3B). There was an increase of CD4−CD8+ single-positive T cells percentages in Ax-LN and Ms-LN at 2 weeks p.i. that correlated with a reduction in percentages of CD4+CD8− single-positive T cells. No major changes were seen in the proportions of CD4+CD8+ double-positive T cells from 1 to 4 weeks p.i. in the lymph nodes.
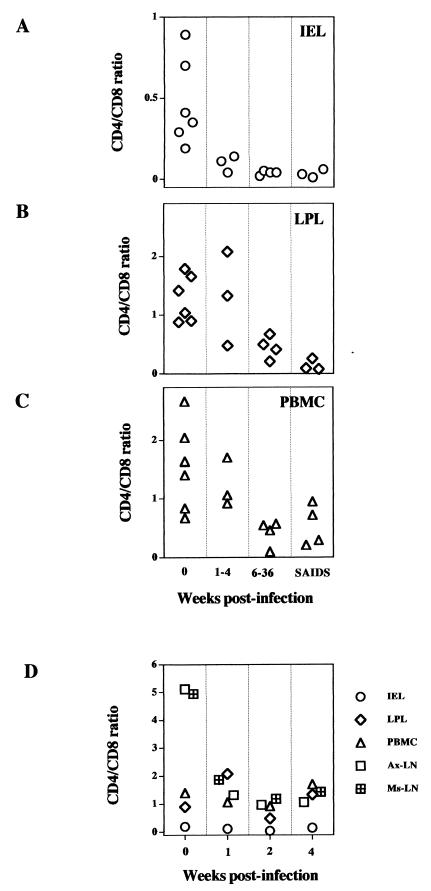
The CD4+ T-cell depletion was reflected in a decline in the CD4/CD8 ratios in IEL, LPL, PBMC, and Ax-LN and Ms-LN lymphocytes (Fig. 4). In IEL, the CD4/CD8 T-cell ratio dropped precipitously from a preinfection value of 0.7 to 0.1 during the primary acute stage and remained less than 0.1 thereafter. In comparison, the decline in the CD4/CD8 T-cell ratio was more gradual in LPL and PBMC during the course of SIV infection. In summary, the severity of the depletion of CD4+CD8− single-positive and CD4+CD8+ double-positive T cells and the qualitative nature of the decline in CD4/CD8 T-cell ratio in the intestinal mucosa were different from those occurring in T cells from peripheral blood and lymph nodes.
FIG. 4.
A severe decline in CD4/CD8 T-cell ratio occurred in intestinal mucosa during primary SIV infection compared to peripheral and lymph node lymphocytes and persisted through the SIV infection course. Lymphocytes were isolated from IEL (A), LPL (B), and PBMC (C) during the primary acute (1 to 4 weeks postinfection), asymptomatic (6 to 36 weeks postinfection), and terminal (SAIDS) stages of SIV infection. Ax-LN and Ms-LN lymphocytes (D) were obtained from macaques only at 1, 2, and 3 weeks postinfection. The percentages of CD4+ and CD8+ T cells were determined by flow cytometry.
Establishment of a flow cytometric assay for the detection of intracellular cytokine production in mitogen-activated simian CD4+ or CD8+ T cells.
We optimized a recently described procedure based on three-color flow cytometry and fluorescence-labeled cytokine antibodies to detect and characterize cytokine responses in specific T-cell subsets in the simian model. The protocol allowed us to detect intracytoplasmic cytokine production in CD4+ or CD8+ T cells of short-term mitogen-activated mononuclear cell populations. The LPL and PBMC from SIV-infected animals and uninfected controls were isolated and analyzed (Fig. 5). Profiles of intracellular cytokine production were determined and presented as percentage of cells positive for the cytokine production for gated CD4+ or CD8+ T cells. Short-term stimulation with PMA by itself did not have any observable effect on the proportion of CD4+ T cells or on the intensity of CD4 expression in PBMC (data not shown). A number of controls were set up to validate the specificity of binding of the labeled cytokine antibodies. Each antibody was tested for staining with fixed but not permeabilized cells. To determine the specificity of anti-human IFN-γ–FITC and anti-human IL-4–PE for monkey IFN-γ and IL-4, the binding was inhibited by unlabeled anti-human IFN-γ and IL-4. Preblocking of cells with unlabeled antibody (100 μg/ml) for IFN-γ and IL-4 resulted in >98% blocking of IFN-γ–FITC and IL-4–PE binding, respectively (data not shown). Preincubation with isotype-matched mouse IgG did not inhibit binding of anticytokine antibodies to the activated and permeabilized cells.
FIG. 5.
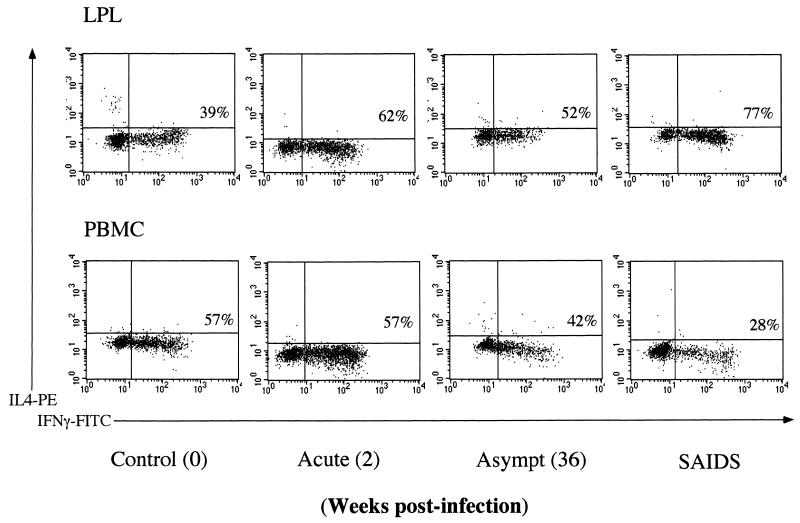
Analysis of intracellular IFN-γ and IL-4 production in CD8+ LPL and PBMC at a single-cell level by three-color flow cytometry. Percentages of CD8+ IFN-γ T cells are shown in the plots. The LPL and PBMC were isolated from rhesus macaques during the primary acute, asymptomatic (Asympt), and terminal stages of SIV infection. Cells were stimulated with PMA-ionomycin, stained with TC-labeled CD8 antibody, fixed, permeabilized, and stained intracellularly with FITC-labeled IFN-γ and PE-labeled IL-4 antibodies. The negative control was stained with an isotype-matched control antibody.
Intestinal T lymphocytes retained the potential of IFN-γ production during all stages of SIV infection.
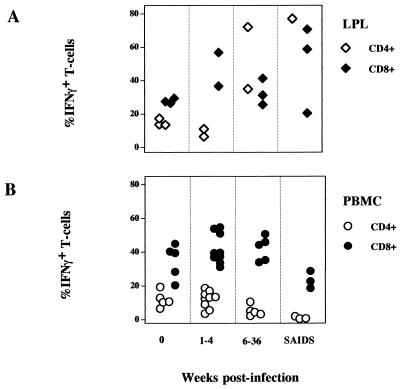
In uninfected animals, the capacity of CD4+ T cells to produce IFN-γ was found to be similar between mitogen-activated PBMC (12%) and LPL (15%). The proportion of CD8+ T cells producing IFN-γ in PBMC (35%) was close to that in LPL (28%) (Fig. 6). The CD8+ T cells appeared to be a more dominant source of IFN-γ production than CD4+ T cells in both LPL and PBMC.
FIG. 6.
The capacity of LPL for intracellular IFN-γ expression was retained through the SIV infection course, while a gradual decline was seen in peripheral blood with disease progression. Frequency of IFN-γ cells was determined among the CD8+ or CD4+ T-cell populations of LPL (A) and PBMC (B) during the course of SIV infection by three-color flow cytometry. Cells were stimulated with PMA-ionomycin, stained for CD8 or CD4, fixed, permeabilized, and stained intracellularly for IFN-γ and IL-4. A negative control was stained with an isotype-matched control antibody.
In animals with primary SIV infection (1 to 4 weeks p.i.), the proportion of CD4+ T cells in PBMC capable of producing IFN-γ was ∼18%, slightly higher than in the uninfected controls. A decline in the capacity of CD4+ T cells to produce IFN-γ was observed during the asymptomatic (∼5%) and SAIDS (<2%) stages of infection. Compared to CD4+ T cells in PBMC, the CD4+ T cells in LPL seemed to retain their capacity to produce IFN-γ following mitogenic stimulation despite the profound CD4+ T-cell depletion associated with SIV infection. The residual lamina propria CD4+ T cells from SIV-infected animals retained very high potential to produce IFN-γ (35 to 77%), while peripheral blood CD4+ T cells from the same animals showed a considerable (<5%) loss in the ability to express IFN-γ.
An increase in the percentages of CD8+ T cells producing IFN-γ was seen in both PBMC (42%) and LPL (47%) during the primary SIV infection (1 and 4 weeks p.i.) compared to the uninfected controls. The CD8+ T cells in both LPL and PBMC had retained the potential to produce IFN-γ in the early asymptomatic phase of infection (42%). A decline in the potential of IFN-γ production in CD8+ PBMC was visible in the terminal disease stage (23%). In contrast, CD8+ T cells from the lamina propria were found to upregulate their capacity to produce IFN-γ with the progression of the disease (50%).
To examine whether there were quantitative differences in the IFN-γ production in LPL, the intensity of IFN-γ production per individual cell (mean fluorescence intensity [MFI]) was determined. The MFI in PBMC from SIV-infected animals remained similar (at ∼60 MFI) for either CD4+ or CD8+ T cells compared to the preinfection control values from the same animals. In contrast, a gradual decline in the intensity of IFN-γ production was observed in both CD4+ and CD8+ LPL in primary SIV infection compared to uninfected controls. The intensity of IFN-γ production of CD4+ LPL was ∼80 MFI in uninfected animals and declined to 54 MFI at 2 weeks p.i. to 31 to 18 MFI at 4 weeks p.i. Similarly, the IFN-γ intensity in CD8+ LPL from uninfected animals was ∼80 MFI, which declined to 56 MFI at 2 weeks p.i., 40 MFI at 4 weeks p.i., and 26 MFI at 6 weeks p.i.
There was no significant change in the percentage of intestinal T cells capable of IL-4 production in SIV-infected animals.
No significant differences were observed in the capacity of CD4+ and CD8+ T cells from both PBMC and LPL to produce IL-4 in uninfected as well as SIV-infected animals (data not shown). Production of IL-4 was between 1 and 4% in both CD4+ and CD8+ subsets of PBMC and LPL samples from SIV-infected (primary acute, asymptomatic, and terminal stages) and uninfected animals. The intensity of IL-4 production per individual cell following mitogenic stimulation of SIV-infected PBMC remained unchanged for either CD4+ or CD8+ T cells compared to the preinfection values obtained from the same animals (∼25 MFI).
Comparison of the potential for IFN-γ production of intestinal T cells with mesenteric and peripheral lymph nodes and blood lymphocytes.
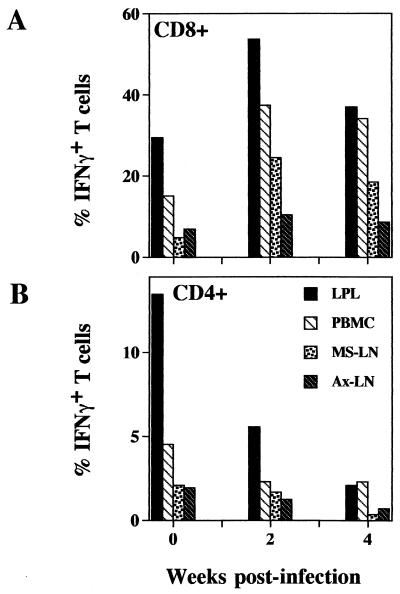
The extents of short-term mitogenic stimulation-induced IFN-γ expression in CD4+ and CD8+ subsets of LPL, PBMC, Ms-LN, and Ax-LN from SIV-infected animals at 2 and 4 weeks p.i. were examined and compared with those from an uninfected animal (Fig. 7). The LPL from the control animal had the highest percentage (30%) of CD8+ T cells expressing IFN-γ compared to CD8+ cells from Ms-LN (5%), Ax-LN (6%), and PBMC (18%). At 2 weeks p.i., the percentages of CD8+ T cells expressing IFN-γ increased in the LPL (54%), PBMC (37%), Ms-LN lymphocytes (25%), and Ax-LN lymphocytes (11%). The difference between LPL and the other three tissue compartments was most pronounced at 2 weeks p.i. The similar pattern of IFN-γ production was seen at 4 weeks post-SIV infection, suggesting that CD8+ T cells in the peripheral and mucosal compartments had retained the ability to produce IFN-γ in the primary acute as well as early asymptomatic stages of infection.
FIG. 7.
Comparison of the potential of intracellular IFN-γ production by CD8+ (A) and CD4+ (B) T cells among LPL, Ms-LN and Ax-LN lymphocytes, and PBMC at 2 and 4 weeks post-SIV infection by flow cytometric analysis. Variable frequencies of INF-γ cells were found among the cells from different tissues.
In the uninfected animal, LPL had the highest percentage of CD4+ T cells expressing IFN-γ compared to PBMC and Ms-LN and Ax-LN lymphocytes. The CD8+ T cells were a richer source of IFN-γ than CD4+ cells (Fig. 6 and 7).
No major differences were observed in the capacity of CD4+ and CD8+ T cells to produce IL-4 among PBMC, LPL, and Ms-LN and Ax-LN lymphocytes.
LPL have CTL activity in primary SIV infection.
LPL, PBMC, and lymphocytes from Ax-LN and Ms-LN were isolated from the animals at 1, 2, or 4 weeks post-SIV infection and examined for the presence of CTL activity specific for SIV gag and env by limiting-dilution analysis. The LPL were positive for CTL activity against SIV env- and/or gag-expressing target cells at all three time points tested. Virus-specific CTL activity was also detected in lymphocytes isolated from Ax-LN and Ms-LN, spleen, and PBMC at 2 and 4 weeks post-SIV infection. However, the level of CTL activity in all these tissues was quite low and did not dilute out in the assay (data not shown). The SIV-specific CTL was more readily detectable in the IEL subpopulations of these animals (data not shown). A similar observation of higher CTL activity in IEL than LPL was noted by other investigators (11).
DISCUSSION
Although information is not available on the viral loads or dissemination in intestinal mucosa during primary HIV infection, several studies have demonstrated the presence of viral proteins or nucleic acids in the intestinal mucosa in later stages of HIV infection (7, 23, 27, 36, 51). In previous and current studies of rhesus macaques intravenously infected with SIV, viral RNA could be readily detected in macrophages and T lymphocytes of the intestinal lamina propria as early as 3 days after infection (28, 61). Though both SIV-infected T cells and macrophages were detected in intestinal mucosa through the entire course of SIV infection, the presence of SIV-infected macrophages was more dominant in the primary acute and terminal stages of SIV infection (28). The severity of the CD4+ T-cell depletion seen in the primary SIV infection may have resulted from the cytopathic effect of direct SIV infection. It has been shown that CD4+ memory T cells in the peripheral blood appeared to be preferentially infected with HIV (59). Since a majority of IEL and LPL were found to be activated memory T cells, they may be readily infected by the virus and contribute to the viral reservoir (56). The CD4+ memory T cells are known routinely to reenter the mucosal surfaces after circulating through the peripheral blood. The homing of infected T cells at intestinal mucosal surfaces may add to viral burden in the tissue due to new infections. Our results demonstrated the presence of virally infected cells in small intestine throughout the entire course of SIV infection, suggesting that the intestinal mucosa served as a potent viral reservoir. It may be important to consider the intestinal tissue while determining the efficacy of antiretrovirus regimen and vaccines.
A hallmark of HIV infection is the marked depletion of CD4+ T cells in PBMC and peripheral lymph nodes which contributes to overt immunodeficiency during the advanced stages of disease. The decline in CD4+ T cells in PBMC is progressive and is most pronounced during the later stages of infection. Our data demonstrated that a rapid depletion of CD4+ T cells occurred in both LPL and IEL of the intestinal mucosa early during the primary SIV infection. Recently, CD4+ T-cell depletion in LPL of macaques infected with pathogenic molecular clone SIVmac239 was reported (64). We did not detect any obvious restoration of CD4+ T-cell population during the asymptomatic stage of SIV infection or thereafter. Although no information is available on intestinal CD4+ T-cell depletion in primary HIV infection, a more pronounced CD4+ T-cell depletion in intestinal biopsies was observed compared to PBMC in patients with advanced HIV infection (8, 39, 57, 58). The mechanisms of early CD4+ T-cell depletion in intestinal mucosa and its implications in the intestinal microenvironment are not fully known. A number of mechanisms may account for the CD4+ T-cell depletion in HIV or SIV infection. These include direct and indirect cytopathic effects of viral infection on infected and uninfected CD4+ T cells and/or altered lymphocyte regeneration and/or trafficking. We have found numerous SIV infected cells in jejunal tissues in primary SIV infection. A direct cytopathic effect of viral infection on CD4+ T cells through direct killing of the infected cells by high rates of viral replication is likely to be one of the key mechanisms of CD4+ T-cell depletion in early SIV infection (10, 19, 32, 67). Alternatively, the induction of apoptosis may play a large role in this process. We have detected many apoptotic cells in intestinal mucosa during primary SIV infection which could be induced by the virally expressed genes (data not shown). The HIV-1 encoded vpr and tat genes have been shown to induce apoptosis in T cells (46, 60). Removal of infected cells by the cytotoxic T cells or antibodies could contribute to the CD4+ T-cell depletion (48). It has been established that memory CD4+ T cells are preferentially affected by HIV infection (59). The majority of the T cells residing in intestinal mucosa, both IEL and LPL, express memory markers and thus may be the favored target of the virus. A bystander effect due to immune activation in intestinal tissue may be another mechanism of uninfected CD4+ T-cell depletion. The GALT is known to harbor many activated T cells due to antigenic stimulation in this environment that may facilitate apoptosis of intestinal immune cells. Other mechanisms of bystander cell elimination using the Fas receptor-ligand pathway include CD4 cross-linking induced apoptosis (53) and expression of FasL ligand by infected monocytic cells in the lymph nodes (3). The severity of CD4+ T-cell depletion in SIV-infected jejunal tissues suggest that killing of bystander cells may be an important mechanism in the intestine. A different explanation is offered by a model of cytokine-induced lymphocyte extravasation that proposes that CD4+ T-cell depletion from the peripheral blood may be a result of altered lymphocyte migration and sequestration in the lymph nodes, which correlated with increased plasma IFN-γ and tumor necrosis factor alpha levels (55). Exposure of resting CD4+ T lymphocytes to HIV transiently upregulated expression of CD62L (L-selectin), the receptor for homing to lymph nodes, in SCID mice (66).
A high proportion (10 to 15%) of both IEL and LPL from the uninfected animals were CD4+CD8+ double-positive T cells. In contrast, PBMC harbored 5 or <5% of CD4+CD8+ double-positive cells. The function of these cells is still not completely understood. However, they have been shown to have helper T-cell function and the capability to produce either Th1 or Th2 cytokines (24). The origin of these cells has been elucidated in the mouse model as extrathymic since it was shown that CD4+ single-positive IEL became CD4+CD8+ double positive by acquiring CD8 expression under the influence of the intestinal epithelium (49). Our results have demonstrated depletion of CD4+CD8+ double-positive T cells in IEL and LPL by 2 weeks post-SIV infection and coincided with the depletion of CD4+CD8− single-positive cells.
In contrast to IEL and LPL, the decline in CD4+ T cells was more gradual in PBMC and Ms-LN and Ax-LN lymphocytes and became more apparent in advanced stages of SIV infection. This was coincident with a distinct increase in the proportion of CD8+ T cells. In HIV infection, an increased number of CD8+ T cells in peripheral blood has been attributed to the existence of T-cell homeostatic mechanisms to maintain a constant level of circulating T cells and to compensate for the loss of CD4+ T cells (2, 25, 45). Alterations in intestinal T-cell homeostasis in primary HIV infection are not known. Implications of jejunal CD4+ T-cell depletion in the primary SIV infection on the local immune functions are not completely defined. It will be important to examine whether innate responses assume a greater role in local host defenses against infections and may compensate partially for the loss of CD4+ T cells.
We found that the percentage of CD4−CD8− double-negative lymphocytes in LPL seemed to increase in intestinal lymphocyte samples throughout the course of SIV disease (70 to 80%) compared to the uninfected controls (45%). Since this population was within the lymphocyte gate, it may consist of null cells, natural killer cells, antibody-dependent cellular cytotoxic effectors, B cells, and others. The immunophenotypic and functional characteristics of the CD4−CD8− double-negative cells (non-B cells) in the intestine are not fully known and will be of interest to examine in SIV-infected tissues.
Both HIV and SIV infections are characterized by the development of T-cell anergy, which is evidenced by decreased proliferative responses of PBMC to mitogenic or antigenic stimulations. During the primary SIV infection, the high potential of CD4+ and CD8+ T cells to produce IFN-γ in the peripheral blood was observed, which could be indicative of healthy host immune system and T lymphocytes. A steady decline in the potential of CD4+ T cells was seen thereafter, which could be attributed to CD4+ T-cell depletion and anergy. It was interesting that CD8+ PBMC retained potential to produce IFN-γ during the asymptomatic stage and showed a decline only in the terminal stage. Recent studies have reported relatively intact responses of PBMC from HIV-infected asymptomatic individuals to mitogenic stimulations by this procedure (5). Contradictory results have been reported regarding a shift from a Th1 to a Th2 type response in PBMC of HIV-infected patients during disease progression (9, 33, 50). IL-4 production by PBMC cultures in vitro was found to be either normal, decreased, or increased (4, 15, 16, 20, 21, 35, 43, 47, 69). Our results showed no increase in the potential of CD4+ and CD8+ T cells to express IL-4 during the course of SIV infection.
The CD8+ lamina propria lymphocytes had retained high potential of IFN-γ production at all stages of SIV infection (Fig. 6). This could be attributed to the active and persistent SIV infection in the lamina propria. The majority of CD8+ LPL were CD69+ (an early cell activation marker), indicating an activated status of these cells (data not shown). Most CD8+ T cells in the intestinal mucosa have the memory phenotype (our unpublished results and reference 56). Therefore, increased IFN-γ production from CD8+ LPL could reflect an activated cellular immune response to SIV infection in the intestinal mucosa. In advanced HIV infection, many intestinal CD8+ T cells were activated HLA-DR+ and cytotoxic CD11a+ and had the potential to function in local immune responses (58). Such activated cytotoxic CD8+ T cells could recognize HIV antigens (12, 37, 52, 65) and may aid in control of infection.
The proportion of CD4+ T cells in LPL declined considerably in SIV infection. However, the few hundred detectable residual CD4+-positive T cells in samples during asymptomatic and SAIDS phases showed a very high capacity for activation and IFN-γ expression (Fig. 6). An upregulation in capacity to produce IFN-γ in CD4+ and CD8+ T cells in the lamina propria of the intestinal tissue may indicate an activated cellular immune response to SIV infection.
Retaining the potential of IFN-γ production by intestinal T cells may be crucial in suppressing viral infection. However, this may reflect dysregulation in cytokine balance leading to pathological symptoms observed in the GI tract. IFN-γ was shown to directly alter the epithelial barrier function by increasing the tight junction permeability (1, 42), and coculturing of human LPL producing IFN-γ with HT29 colonic epithelial cells resulted in killing of HT29 cells (14). An increased IFN-γ expression may also alter the local cytokine network and lead to further recruitment of inflammatory cells into the site of infection, which may contribute to viral pathogenesis. Thus, the increased IFN-γ production may alter the integrity of the intestinal epithelium following infection and may be one of the major contributing factors in SIV-associated enteropathy.
The LPL were positive for the CTL activity specific against SIV gag and env as early as 1 week post-SIV infection. This activity was present through the primary acute and asymptomatic stages of infection. The high potential of IFN-γ expression following mitogenic stimulation coincided with the virus-specific activity in LPL. These results pointed to the potentially very crucial function of LPL in suppressing viral infection in intestinal mucosa. The CTL activity was detectable more readily in IEL than in LPL (our unpublished results and reference 11).
Comparison of IFN-γ expression in jejunal LPL with T lymphocytes from other lymph nodes and blood within the same animal at 2 weeks p.i. (Fig. 7) showed that peripheral lymphoid compartments (PBMC and Ax-LN) did not accurately represent the extent of T-cell activity present in mucosal lymphoid tissues. Our results demonstrated that an increased percentage of intestinal T cells were able to become activated and express IFN-γ in SIV infection and harbored SIV-specific CTL activity. On the contrary, this ability was reduced in PBMC, emphasizing the importance of studying intestinal lymphoid tissue as well as PBMC in order to get an in-depth understanding of HIV immunopathogenesis.
In summary, the small intestinal mucosa is an early site for dynamic changes in T-cell populations and perturbations in CD4+ and CD8+ T cells that may lead to altered T-cell homeostasis in the intestinal microenvironment. It will be important to examine the role of these events in the disease outcome since it could be an important mechanism of viral pathogenesis. Most of the studies on HIV pathogenesis and on antiretrovirus therapy and vaccines rely heavily on the analysis of PBMC. Our studies have indicated that all the immunophenotypic and functional changes in intestinal T cells are not adequately mirrored by PBMC. Therefore, it will be important to take this point into consideration while studying the restoration of immune functions and CD4 replenishment in antiretrovirus therapy and vaccine development.
ACKNOWLEDGMENTS
We are grateful to Chris Miller for providing some of the tissue samples and to Linda Antipa, Ross Tarara, and Don Canfield at the California Regional Primate Center for their invaluable help in this project. We thank Jeanette Rheinhardt for invaluable support in the in situ hybridization analysis, Carol Oxford for technical support with the flow cytometer, and Jennifer Collins for cytotoxicity assays.
This work was supported by grants from the National Institutes of Health (R01-DK43183 and NCRR-00169) and the Universitywide AIDS Research Program, University of California.
REFERENCES
- 1.Adams R B, Planchon S M, Roche J K. IFN-gamma modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J Immunol. 1993;150:2356–2363. [PubMed] [Google Scholar]
- 2.Adleman L M, Wofsy D. T-cell homeostasis: implications in HIV infection. J Acquired Immune Defic Syndr. 1993;6:144–152. [PubMed] [Google Scholar]
- 3.Badley A D, McElhinny J A, Leibson P J, Lynch D H, Alderson M R, Paya C V. Upregulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes. J Virol. 1996;70:199–206. doi: 10.1128/jvi.70.1.199-206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barcellini W, Rizzardi G P, Borghi M O, Fain C, Lazzarin A, Meroni P L. Th1 and Th2 cytokine production by peripheral blood mononuclear cells from HIV-infected patients. AIDS. 1994;8:757. doi: 10.1097/00002030-199406000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Caruso A, Licenziati S, Canaris A D, Cantalamessa A, Corulli M, Benzoni B, Peroni L, Balsari A, Turano A. Characterization of T cell subsets involved in the production of IFN-gamma in asymptomatic HIV-infected patients. AIDS Res Hum Retroviruses. 1996;12:135–141. doi: 10.1089/aid.1996.12.135. [DOI] [PubMed] [Google Scholar]
- 6.Chlebowski R, Grosvenor M, Bernhard N, Morales L, Bulcavage L. Nutritional status, gastrointestinal dysfunction, and survival in patients with AIDS. Am J Gastroenterol. 1989;84:1288–1293. [PubMed] [Google Scholar]
- 7.Clayton F, Reka S, Cronin W J, Torlakovic E, Sigal S H, Kotler D P. Rectal mucosal pathology varies with human immunodeficiency virus antigen content and disease stage. Gastroenterology. 1992;103:919–933. doi: 10.1016/0016-5085(92)90026-u. [DOI] [PubMed] [Google Scholar]
- 8.Clayton F, Snow G, Reka S, Kotler D P. Selective depletion of rectal lamina propria rather than lymphoid aggregate CD4 lymphocytes in HIV infection. Clin Exp Immunol. 1997;107:288–292. doi: 10.1111/j.1365-2249.1997.236-ce1111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clerici M, Shearer G M. A TH1→TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 10.Coffin J M. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 11.Couedel-Courteille A, Le Grand R, Tulliez M, Guillet J G, Venet A. Direct ex vivo simian immunodeficiency virus (SIV)-specific cytotoxic activity detected from small intestine intraepithelial lymphocytes of SIV-infected macaques at an advanced stage of infection. J Virol. 1997;71:1052–1057. doi: 10.1128/jvi.71.2.1052-1057.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Culmann B, Gomard E, Kieny M P, Guy B, Dreyfus F, Saimot A G, Sereni D, Levy J P. An antigenic peptide of the HIV-1 NEF protein recognized by cytotoxic T lymphocytes of seropositive individuals in association with different HLA-B molecules. Eur J Immunol. 1989;19:2383–6. doi: 10.1002/eji.1830191231. [DOI] [PubMed] [Google Scholar]
- 13.Davies M D J, Parrott D V M. Preparation and purification of lymphocytes from the epithelium and lamina propria of murine small intestine. Gut. 1981;22:481–488. doi: 10.1136/gut.22.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deem R L, Shanahan F, Targan S R. Triggered human mucosal T cells release tumour necrosis factor-alpha and interferon-gamma which kill human colonic epithelial cells. Clin Exp Immunol. 1991;83:79–84. doi: 10.1111/j.1365-2249.1991.tb05592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz-Mitoma F, Kumar A, Karimi S, Kryworuchko M, Daftarian M P, Creery W D, Filion L G, Cameron W. Expression of IL-10, IL-4 and interferon-gamma in unstimulated and mitogen-stimulated peripheral blood lymphocytes from HIV-seropositive patients. Clin Exp Immunol. 1995;102:31–39. doi: 10.1111/j.1365-2249.1995.tb06632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckwalanga M, Marussig M, Tavares M D, Bouanga J C, Hulier E, Pavlovitch J H, Minoprio P, Portnoi D, Renia L, Mazier D. Murine AIDS protects mice against experimental cerebral malaria: down-regulation by interleukin 10 of a T-helper type 1 CD4+ cell-mediated pathology. Proc Natl Acad Sci USA. 1994;91:8097–8101. doi: 10.1073/pnas.91.17.8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehrenpreis E D, Ganger D R, Kochvar G T, Patterson B K, Craig R M. D-xylose malabsorption: characteristic finding in patients with the AIDS wasting syndrome and chronic diarrhea. J Acquired Immune Defic Syndr. 1992;5:1047–1050. [PubMed] [Google Scholar]
- 18.Ehrenpreis E D, Patterson B K, Brainer J A, Yokoo H, Rademaker A W, Glogowski W, Noskin G A, Craig R M. Histopathologic findings of duodenal biopsy specimens in HIV-infected patients with and without diarrhea and malabsorption. Am J Clin Pathol. 1992;97:21–28. doi: 10.1093/ajcp/97.1.21. [DOI] [PubMed] [Google Scholar]
- 19.Embretson J, Zupancic M, Ribas J L, Burke A, Racz P, Tenner-Ratz K, Haase A T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;362:359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 20.Emilie D, Fior R, Crevon M C, Maillot M C, Boue F, Galanaud P. Cytokines from lymphoid organs of HIV-infected patients: production and role in the immune disequilibrium of the disease. Res Immunol. 1994;145:595–600. doi: 10.1016/s0923-2494(05)80039-2. [DOI] [PubMed] [Google Scholar]
- 21.Estaquier J, Idziorek T, Zou W, Emilie D, Farber C M, Bourez J M, Ameisen J C. T helper type 1/T helper type 2 cytokines and T cell death: preventive effect of interleukin 12 on activation-induced and CD95 (FAS/APO-1)-mediated apoptosis of CD4+ T cells from human immunodeficiency virus-infected persons. J Exp Med. 1995;182:1759–1767. doi: 10.1084/jem.182.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrick D A, Schrenzel M D, Mulvania T, Hsieh B, Ferlin W G, Lepper H. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 23.Fox C H, Kotler D, Tierney A, Wilson C S, Fauci A S. Detection of HIV-1 RNA in the lamina propria of patients with AIDS and gastrointestinal disease. J Infect Dis. 1989;159:467–471. doi: 10.1093/infdis/159.3.467. [DOI] [PubMed] [Google Scholar]
- 24.Fujihashi K, Yamamoto M, McGhee J R, Beagley K W, Kiyono H. Function of alpha beta TCR+ intestinal intraepithelial lymphocytes: Th1- and Th2-type cytokine production by CD4+CD8− and CD4+CD8+ T cells for helper activity. Int Immunol. 1993;5:1473–1481. doi: 10.1093/intimm/5.11.1473. [DOI] [PubMed] [Google Scholar]
- 25.Galai N, Margolick J B, Astemborski J, Vlahov D. Existence and failure of T-cell homeostasis prior to AIDS onset in HIV-infected injection drug users. Clin Immunol Immunopathol. 1996;79:134–141. doi: 10.1006/clin.1996.0060. [DOI] [PubMed] [Google Scholar]
- 26.Halstensen T S, Brandtzaeg P. Phenotypic characterization of human intraepithelial lymphocytes. In: Kiyono H, McGhee J R, editors. Mucosal immunology: intraepithelial lymphocytes. New York, N.Y: Raven Press Ltd.; 1993. p. 147. [Google Scholar]
- 27.Heise C, Dandekar S, Kumar P, Duplantier R, Donovan R M, Halsted C H. Human immunodeficiency virus infection of enterocytes and mononuclear cells in human jejunal mucosa. Gastroenterology. 1991;100:1521–1527. doi: 10.1016/0016-5085(91)90648-5. [DOI] [PubMed] [Google Scholar]
- 28.Heise C, Miller C J, Lackner A, Dandekar S. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J Infect Dis. 1994;169:1116–1120. doi: 10.1093/infdis/169.5.1116. [DOI] [PubMed] [Google Scholar]
- 29.Heise C, Vogel P, Miller C J, Halsted C H, Dandekar S. Simian immunodeficiency virus infection of the gastrointestinal tract of rhesus macaques. Functional, pathological, and morphological changes. Am J Pathol. 1993;142:1759–1771. [PMC free article] [PubMed] [Google Scholar]
- 30.Heise C, Vogel P, Miller C J, Lackner A, Dandekar S. Distribution of SIV infection in the gastrointestinal tract of rhesus macaques at early and terminal stages of AIDS. J Med Primatol. 1993;22:187–193. [PubMed] [Google Scholar]
- 31.Hellerstein M K, Kahn J, Mudie H, Viteri F. Current approach to the treatment of human immunodeficiency virus-associated weight loss: pathophysiologic considerations and emerging management strategies. Semin Oncol. 1990;17:17–33. [PubMed] [Google Scholar]
- 32.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 33.Hyjek E, Lischner H W, Hyslop T, Bartkowiak J, Kubin M, Trinchieri G, Kozbor D. Cytokine patterns during progression to AIDS in children with perinatal HIV infection. J Immunol. 1995;155:4060–4071. [PubMed] [Google Scholar]
- 34.James S P, Graeff A S, Zeitz M. Predominance of helper-inducer T cells in mesenteric lymph nodes and intestinal lamina propria of normal nonhuman primates. Cell Immunol. 1987;107:372–383. doi: 10.1016/0008-8749(87)90245-0. [DOI] [PubMed] [Google Scholar]
- 35.Klein S A, Dobmeyer J M, Dobmeyer T S, Pape M, Ottmann O G, Helm E B, Hoelzer D, Rossol R. Demonstration of the Th1 to Th2 cytokine shift during the course of HIV-1 infection using cytoplasmic cytokine detection on single cell level by flow cytometry. AIDS. 1997;11:1111–1118. doi: 10.1097/00002030-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Kotler D P, Reka S, Borcich A, Cronin W J. Detection, localization, and quantitation of HIV-associated antigens in intestinal biopsies from patients with HIV. Am J Pathol. 1991;139:823–830. [PMC free article] [PubMed] [Google Scholar]
- 37.Kundu S K, Merigan T C. Equivalent recognition of HIV proteins, Env, Gag and Pol, by CD4+ and CD8+ cytotoxic T-lymphocytes. AIDS. 1992;6:643–649. [PubMed] [Google Scholar]
- 38.Letvin N L. Animal models for AIDS. Immunol Today. 1990;11:322–326. doi: 10.1016/0167-5699(90)90127-u. [DOI] [PubMed] [Google Scholar]
- 39.Lim S G, Condez A, Lee C A, Johnson M A, Elia C, Poulter L W. Loss of mucosal CD4 lymphocytes is an early feature of HIV infection. Clin Exp Immunol. 1993;92:448–454. doi: 10.1111/j.1365-2249.1993.tb03419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lohman B L, Miller C J, McChesney M B. Antiviral cytotoxic T lymphocytes in vaginal mucosa of simian immunodeficiency virus-infected rhesus macaques. J Immunol. 1995;155:5855–5860. [PMC free article] [PubMed] [Google Scholar]
- 41.Lundqvist C, Baranov V, Hammarstrom S, Athlin L, Hammarstrom M L. Intra-epithelial lymphocytes. Evidence for regional specialization and extrathymic T cell maturation in the human gut epithelium. Int Immunol. 1995;7:1473–1487. doi: 10.1093/intimm/7.9.1473. [DOI] [PubMed] [Google Scholar]
- 42.Madara J L, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989;83:724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maggi E, Mazzetti M, Ravina A, Annunziato F, de Carli M, Piccinni M P, Manetti R, Carbonari M, Pesce A M, del Prete G, et al. Ability of HIV to promote a TH1 to TH0 shift and to replicate preferentially in TH2 and TH0 cells. Science. 1994;265:244–248. doi: 10.1126/science.8023142. [DOI] [PubMed] [Google Scholar]
- 44.Mandell C P, Jain N C, Miller C J, Dandekar S. Bone marrow monocyte/macrophages are an early cellular target of pathogenic and nonpathogenic isolates of simian immunodeficiency virus (SIVmac) in rhesus macaques. Lab Invest. 1995;72:323–333. [PubMed] [Google Scholar]
- 45.Margolick J B, Donnenberg A D, Munoz A, Park L P, Bauer K D, Giorgi J V, Ferbas J, Saah A J. Changes in T and non-T lymphocyte subsets following seroconversion to HIV-1: stable CD3+ and declining CD3− populations suggest regulatory responses linked to loss of CD4 lymphocytes. The Multicenter AIDS Cohort Study. J Acquired Immune Defic Syndr. 1993;6:153–161. [PubMed] [Google Scholar]
- 46.McCloskey T W, Ott M, Tribble E, Khan S A, Teichberg S, Paul M O, Pahwa S, Verdin E, Chirmule N. Dual role of HIV Tat in regulation of apoptosis in T cells. J Immunol. 1997;158:1014–1019. [PubMed] [Google Scholar]
- 47.Meyaard L, Hovenkamp E, Keet I P M, Hooibrink B, de Jong I H, Otto S A, Miedema F. Single-cell analysis of IL-4 and IFN-γ production by T cells from HIV-infected individuals. J Immunol. 1996;157:2712–2718. [PubMed] [Google Scholar]
- 48.Minowada J, Nishizaki C, Toki M, Kinzl P, Otani T, Matsuo Y. In vitro study using leukemia cell line panel to demonstrate rapid thymic T cell death due to contact with HIV-1 carrier cell clones. Leukemia. 1997;11:714–722. doi: 10.1038/sj.leu.2400615. [DOI] [PubMed] [Google Scholar]
- 49.Mosley R L, Klein J R. Repopulation kinetics of intestinal intraepithelial lymphocytes in murine bone marrow radiation chimeras. Transplantation. 1992;53:868–874. doi: 10.1097/00007890-199204000-00030. [DOI] [PubMed] [Google Scholar]
- 50.Navikas V, Link J, Wahren B, Persson C, Link H. Increased levels of interferon-gamma (IFN-gamma), IL-4 and transforming growth factor-beta (TGF-beta) mRNA expressing blood mononuclear cells in human HIV infection. Clin Exp Immunol. 1994;96:59–63. doi: 10.1111/j.1365-2249.1994.tb06230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nelson J A, Wiley C A, Reynolds K C, Reese C E, Margaretten W, Levy J A. Human immunodeficiency virus detected in bowel epithelium from patients with gastrointestinal symptoms. Lancet. 1988;1:259–262. doi: 10.1016/s0140-6736(88)90348-0. [DOI] [PubMed] [Google Scholar]
- 52.Nixon D F, McMichael A J. Cytotoxic T-cell recognition of HIV proteins and peptides. AIDS. 1991;5:1049–1059. [PubMed] [Google Scholar]
- 53.Oyaizu N, Adachi Y, Hashimoto F, McCloskey T W, Hosaka N, Kayaganaki N, Yagita H, Pahwa S. Monocytes express Fas ligand upon CD4 cross-linking and induce CD4+ T cells apoptosis: a possible mechanism of bystander cell death in HIV infection. J Immunol. 1997;158:2456–2463. [PubMed] [Google Scholar]
- 54.Rosenberg Y J, Cafaro A, Brennan T, Greenhouse J G, McKinnon K, Bellah S, Yalley-Ogunro J, Gartner S, Lewis M G. Characteristics of the CD8+ lymphocytosis during primary simian immunodeficiency virus infections. AIDS. 1997;11:959–968. doi: 10.1097/00002030-199708000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Rosenberg Y J, Cafaro A, Brennan T, Greenhouse J G, Villinger F, Ansari A A, Brown C, McKinnon K, Bellah S, Yalley-Ogunro J, Elkins W R, Gartner S, Lewis M G. Virus-induced cytokines regulate circulating lymphocyte levels during primary SIV infections. Int Immunol. 1997;9:703–712. doi: 10.1093/intimm/9.5.703. [DOI] [PubMed] [Google Scholar]
- 56.Schieferdecker H L, Ullrich R, Hirseland H, Zeitz M. T cell differentiation antigens on lymphocytes in the human intestinal lamina propria. J Immunol. 1992;149:2816–2822. [PubMed] [Google Scholar]
- 57.Schneider T, Jahn H U, Schmidt W, Riecken E O, Zeitz M, Ullrich R. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Berlin Diarrhea/Wasting Syndrome Study Group. Gut. 1995;37:524–529. doi: 10.1136/gut.37.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schneider T, Ullrich R, Bergs C, Schmidt W, Riecken E O, Zeitz M. Abnormalities in subset distribution, activation, and differentiation of T cells isolated from large intestine biopsies in HIV infection. The Berlin Diarrhoea/Wasting Syndrome Study Group. Clin Exp Immunol. 1994;95:430–435. doi: 10.1111/j.1365-2249.1994.tb07014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schnittman S M, Lane H C, Greenhouse J, Justement J S, Baseler M, Fauci A S. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc Natl Acad Sci USA. 1990;87:6058–6062. doi: 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stewart S A, Poon B, Jowett J B, Chen I S. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stone J D, Heise C C, Miller C J, Halsted C H, Dandekar S. Development of malabsorption and nutritional complications in simian immunodeficiency virus-infected rhesus macaques. AIDS. 1994;8:1245–1256. doi: 10.1097/00002030-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Taguchi T, McGhee J R, Coffman R L, Beagley K W, Eldridge J H, Takatsu K, Kiyono H. Analysis of Th1 and Th2 cells in murine gut-associated tissues. J Immunol. 1990;145:68–77. [PubMed] [Google Scholar]
- 63.Trejdosiewicz L K, Smart C J, Oakes D J, Howdle P D, Malizia G, Campana D, Boylston A W. Expression of T-cell receptors TcR1 (gamma/delta) and TcR2 (alpha/beta) in the human intestinal mucosa. Immunology. 1989;68:7–12. [PMC free article] [PubMed] [Google Scholar]
- 64.Veazey R S, DeMaria M A, Chalifoux L V, Shvetz D E, Pauley D R, Knight H L, Rosenzweig M, Johnson R P, Desrosiers R C, Lackner A A. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 65.Walker B D, Flexner C, Paradis T J, Fuller T C, Hirsch M S, Schooley R T, Moss B. HIV-1 reverse transcriptase is a target for cytotoxic T lymphocytes in infected individuals. Science. 1988;240:64–66. doi: 10.1126/science.2451288. [DOI] [PubMed] [Google Scholar]
- 66.Wang L, Robb C W, Cloyd M W. HIV induces homing of resting T lymphocytes to lymph nodes. Virology. 1997;228:141–152. doi: 10.1006/viro.1996.8397. [DOI] [PubMed] [Google Scholar]
- 67.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, Saag M S, Shaw G M. Viral dynamics in human immunodeficiency virus 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 68.Zeitz M, Greene W C, Peffer N J, James S P. Lymphocytes isolated from the intestinal lamina propria of normal nonhuman primates have increased expression of genes associated with T cell activation. Gastroenterology. 1988;94:647–655. doi: 10.1016/0016-5085(88)90235-1. [DOI] [PubMed] [Google Scholar]
- 69.Zhang M, Gong J, Iyer D V, Jones B E, Modlin R L, Barnes P F. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J Clin Invest. 1994;94:2435–2442. doi: 10.1172/JCI117611. [DOI] [PMC free article] [PubMed] [Google Scholar]