Key Points
Question
Is implementation of a regional hospital and nursing home decolonization collaborative (coordinated intervention adopted by participating health care facilities) associated with a reduction in multidrug-resistant organisms (MDROs), infection-related hospitalizations, costs, and deaths?
Findings
In this quality improvement study of 35 health care facilities in Orange County, California, using quasi-experimental design, chlorhexidine bathing and nasal decolonization were associated with significantly lower MDRO prevalence and incident clinical cultures. Infection-related hospitalizations, associated costs, and deaths among nursing home residents also decreased.
Meaning
In this study, a regional decolonization collaborative involving universal decolonization in long-term care facilities and targeted decolonization among hospital patients in contact precautions was associated with lower MDRO carriage, infections, hospitalizations, costs, and deaths.
Abstract
Importance
Infections due to multidrug-resistant organisms (MDROs) are associated with increased morbidity, mortality, length of hospitalization, and health care costs. Regional interventions may be advantageous in mitigating MDROs and associated infections.
Objective
To evaluate whether implementation of a decolonization collaborative is associated with reduced regional MDRO prevalence, incident clinical cultures, infection-related hospitalizations, costs, and deaths.
Design, Setting, and Participants
This quality improvement study was conducted from July 1, 2017, to July 31, 2019, across 35 health care facilities in Orange County, California.
Exposures
Chlorhexidine bathing and nasal iodophor antisepsis for residents in long-term care and hospitalized patients in contact precautions (CP).
Main Outcomes and Measures
Baseline and end of intervention MDRO point prevalence among participating facilities; incident MDRO (nonscreening) clinical cultures among participating and nonparticipating facilities; and infection-related hospitalizations and associated costs and deaths among residents in participating and nonparticipating nursing homes (NHs).
Results
Thirty-five facilities (16 hospitals, 16 NHs, 3 long-term acute care hospitals [LTACHs]) adopted the intervention. Comparing decolonization with baseline periods among participating facilities, the mean (SD) MDRO prevalence decreased from 63.9% (12.2%) to 49.9% (11.3%) among NHs, from 80.0% (7.2%) to 53.3% (13.3%) among LTACHs (odds ratio [OR] for NHs and LTACHs, 0.48; 95% CI, 0.40-0.57), and from 64.1% (8.5%) to 55.4% (13.8%) (OR, 0.75; 95% CI, 0.60-0.93) among hospitalized patients in CP. When comparing decolonization with baseline among NHs, the mean (SD) monthly incident MDRO clinical cultures changed from 2.7 (1.9) to 1.7 (1.1) among participating NHs, from 1.7 (1.4) to 1.5 (1.1) among nonparticipating NHs (group × period interaction reduction, 30.4%; 95% CI, 16.4%-42.1%), from 25.5 (18.6) to 25.0 (15.9) among participating hospitals, from 12.5 (10.1) to 14.3 (10.2) among nonparticipating hospitals (group × period interaction reduction, 12.9%; 95% CI, 3.3%-21.5%), and from 14.8 (8.6) to 8.2 (6.1) among LTACHs (all facilities participating; 22.5% reduction; 95% CI, 4.4%-37.1%). For NHs, the rate of infection-related hospitalizations per 1000 resident-days changed from 2.31 during baseline to 1.94 during intervention among participating NHs, and from 1.90 to 2.03 among nonparticipating NHs (group × period interaction reduction, 26.7%; 95% CI, 19.0%-34.5%). Associated hospitalization costs per 1000 resident-days changed from $64 651 to $55 149 among participating NHs and from $55 151 to $59 327 among nonparticipating NHs (group × period interaction reduction, 26.8%; 95% CI, 26.7%-26.9%). Associated hospitalization deaths per 1000 resident-days changed from 0.29 to 0.25 among participating NHs and from 0.23 to 0.24 among nonparticipating NHs (group × period interaction reduction, 23.7%; 95% CI, 4.5%-43.0%).
Conclusions and Relevance
A regional collaborative involving universal decolonization in long-term care facilities and targeted decolonization among hospital patients in CP was associated with lower MDRO carriage, infections, hospitalizations, costs, and deaths.
This quality improvement study evaluates whether implementation of a decolonization collaborative is associated with reduced regional multidrug-resistant organism prevalence, incident clinical cultures, infection-related hospitalizations, costs, and deaths.
Introduction
Antimicrobial resistance threatens global health.1,2 Compared with antimicrobial-susceptible organisms, infections due to multidrug-resistant organisms (MDROs) are more difficult to treat with increased morbidity, mortality, length of hospitalization, and health care costs.3 Moreover, the emergence of MDROs continues to outpace the development of new antimicrobials, contributing to increasing infections without effective treatments.1 With limited therapeutic options, action is warranted to mitigate MDRO burden and spread, especially in health care settings.
There is a high prevalence of MDROs in long-term care, reaching 40% to 65% in nursing homes (NHs) and 80% in long-term acute care hospitals (LTACHs).4,5,6 These levels exceed the typical hospital prevalence of 10% to 15%, and most cases of MDRO colonization are unknown due to resource constraints that preclude routine screening and limited communication about MDRO status from transferring facilities.6,7 Furthermore, this high prevalence fuels spread as patients colonized with MDROs are shared among NHs, LTACHs, and hospitals.8,9 Thus, coordinated action across regional health care facilities is needed to reduce MDRO burden and interrupt transmission.10
While many MDRO prevention strategies exist, body-surface decolonization using topical antiseptic soap and nasal products has been broadly adopted in high-risk patient populations due to large randomized clinical trials demonstrating reductions in bloodstream infections and MDRO carriage.11,12,13,14,15 Decolonization not only affects MDROs but also provides broad protection against a range of potential pathogens. The Shared Healthcare Intervention to Eliminate Life-threatening Dissemination of MDROs in Orange County (SHIELD-OC) was a 2-part public health endeavor involving simulation modeling16 to identify a high-yield regional strategy for reducing MDROs and infectious sequelae in health care facilities in Orange County, California, the sixth largest US county, and real-world implementation in up to 40 facilities.
Methods
Design
SHIELD-OC was a multicenter quasi-experimental MDRO intervention collaborative led by investigators at the University of California, Irvine (UCI) with support from local, state, and national public health agencies. The design was informed by a previously published model16 of Orange County’s adult nonpsychiatric health care facilities (23 hospitals, 74 NHs, 3 LTACHs) that simulated various interventions and found that decolonization yielded the greatest reductions in MDRO carriage and spread, particularly among interconnected facilities. The goal was to implement a decolonization strategy in a group of 40 facilities with a high degree of patient sharing using network analysis; 47 facilities were invited to obtain 38 participants. This study followed the Standards for Quality Improvement Reporting Excellence (SQUIRE) reporting guideline.
Participating facilities adopted the SHIELD-OC decolonization program as a quality improvement initiative for MDRO prevention. There was a 25-month baseline period (February 1, 2015, to February 28, 2017); a 4-month phase-in period (March 1, 2017, to June 30, 2017); and a 25-month intervention period (July 1, 2017, to July 31, 2019). The phase-in period was excluded from analyses.
The intervention involved universal decolonization in NHs and LTACHs using 2% leave-on chlorhexidine-impregnated cloths for bed bathing and 4% rinse-off chlorhexidine liquid for showering on admission and routinely thereafter. Additionally, all residents (from NHs) or patients (from LTACHs) received twice-daily nasal iodophor (10% povidone-iodine) for 5 days on admission and then Monday through Friday, every other week. Hospitals received refresher training for ongoing universal chlorhexidine bathing in intensive care units (ICUs) and adopted targeted decolonization for all non-ICU patients in contact precautions (CP). Targeted decolonization involved 5 days of chlorhexidine baths and twice daily nasal iodophor. Both participating and nonparticipating facilities maintained their usual bathing frequency. In both groups, residents in NHs generally received a bath or shower 3 times per week, while patients in LTACHs or hospitals were generally offered a daily bath or shower.
Participating facilities were provided coaching calls, in-person training, and a toolkit of protocols, educational materials, checklists, and assessment forms17 (eAppendix 1 in Supplement 1). Adherence was assessed twice monthly using treatment administration records, bathing logs, and discussions with staff, patients, and residents. Project staff reviewed adherence data with nursing leadership, and refresher training was provided as needed. Participating facilities were given a standardized form for adverse events and encouraged to report events.
As a voluntary public health and quality improvement endeavor, SHIELD-OC was deemed exempt from human participant research oversight by the UCI institutional review board. This activity was reviewed by the US Centers for Disease Control and Prevention (CDC) and conducted in accordance with applicable federal law and CDC policy.
Outcomes
Baseline and end of intervention measures were assessed for MDRO carriage (screening) prevalence in participating facilities, incident MDRO clinical (nonscreening) cultures in participating vs nonparticipating facilities, and infection-related hospitalizations and associated costs and deaths among residents in participating vs nonparticipating NHs.
MDRO Point Prevalence (Screening) Surveys
Participating facilities conducted MDRO point prevalence at baseline (between September 2016 and April 2017) and end of intervention (between August 2018 and April 2019). Three hospitals with a delayed intervention launch completed baseline sampling between February 2017 and October 2017. End of intervention sampling occurred 2 years later in the same or adjacent calendar month as baseline.
Nurses from each NH and LTACH sampled 50 randomly selected residents on a single day during baseline with support from project staff. End of intervention sampling was similar except all NH residents were sampled. For hospitalized patients in CP, baseline and end of intervention sampling occurred weekly until 50 unique patients were sampled or until 28 weeks elapsed.
Residents or patients were informed about sampling and allowed to refuse, consistent with MDRO surveillance performed for operational purposes. Written consent was not required. Nurses received standardized training to collect bilateral nares swabs for methicillin-resistant Staphylococcus aureus (MRSA), as well as skin (bilateral axilla and groin) and perirectal swabs, which were processed for MRSA, vancomycin-resistant Enterococci (VRE), extended-spectrum β-lactamase producers (ESBL), and carbapenem-resistant Enterobacterales (CRE). Swabs (BBL CultureSwab; Becton Dickinson) were premoistened and processed within 6 hours.4
Project staff collected resident/patient characteristics from medical records using a standardized form. Wounds and medical devices were identified by direct observation during sampling. NH facility-level characteristics were collected from the US Centers for Medicare & Medicaid Services (CMS) Minimum Data Set, LTCFocus.org, and CMS Nursing Home Compare.18,19,20 LTACH and hospital facility-level characteristics were obtained from publicly available datasets.21
To assess indirect outcomes of regional decolonization, patients transferring into LTACHs, all of whom came from regional hospitals, were sampled on admission during baseline and intervention periods using bilateral nares, axilla and groin, and perirectal swabs.
Incident MDRO Clinical (Nonscreening) Cultures
Countywide reporting of inpatient MDRO-positive clinical cultures (nonscreening) was required of laboratories serving hospitals and NHs by local public health mandate.22 Data included monthly inpatient days and first MRSA, ESBL, or CRE event per person per month, regardless of participation in SHIELD-OC.
Infection-Related Hospitalizations Among NH Residents
Data from the CMS Minimum Data Set18 were linked to state hospitalization data21 to identify infection-related hospitalizations among NH residents using hospital International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes for infection in the first 3 diagnostic positions plus a present-on-admission indicator.23 These publicly available datasets are generally available 18 to 24 months after the close of each calendar year. Datasets were received in the first quarter of 2022 due to pandemic delays. All-cause hospitalizations were also evaluated. Data were analyzed between March 2022 and August 2023.
Costs of each infection-related hospitalization were calculated by multiplying hospitalization charges by a hospital-specific cost-to-charge ratio from annual hospital financial data21 and converted to 2022 US dollars.24 As a conservative measure, the top 3% of hospitalization costs were censored to $100 000. Associated deaths were identified using hospitalization disposition coding.
Statistical Analysis
MDRO Point Prevalence
Prevalence of overall and individual MDROs was assessed during baseline and intervention by facility type. Differences in the odds of MDRO carriage between baseline and intervention were assessed using adjusted generalized linear mixed models accounting for clustering by person (patient or resident) and facility. In these models, LTACHs were combined with NHs as a group of long-term care facilities. Models controlled for individual age, sex, day of stay at time of sampling, history of specific MDROs, and presence of diabetes, invasive medical devices, need for full assistance for all care, incontinence, and wounds, as well as facility characteristics including total licensed beds, occupancy, and proportion of Medicaid-insured patients and residents.
Incident MDRO Clinical (Nonscreening) Cultures
Incident MDRO clinical cultures were evaluated using generalized linear mixed models with negative binomial distributions that compared monthly count data from baseline and intervention periods. For hospitals and NHs, the intervention effect size was based on the group × period interaction term, which assessed whether risk ratios (RR) between baseline and intervention periods differed significantly between participating and nonparticipating facilities. Since all LTACHs participated, generalized linear mixed models for LTACHs assessed period alone. Models accounted for clustering within facility and controlled for facility-level annual admissions, percentage of patients who belong to minoritized racial and ethnic groups, percentage of patients who were Medicaid insured, mean age, and mean Elixhauser comorbidity count.25
Infection-Related Hospitalizations, Associated Costs, and Deaths Among NH Residents
Infection-related hospitalizations and associated deaths were evaluated using Cox proportional hazards regression models with shared frailties, clustering by facility and person (resident). The intervention effect size was based on the group × period interaction term, reflecting the difference in hazard between baseline and intervention periods among participating and nonparticipating NHs. Costs associated with infection-related hospitalizations were assessed using generalized linear mixed models with a Poisson distribution, clustering by facility and person (resident). Models adjusted for individual age, sex, race, Medicaid insurance, diabetes, and cancer. Statistical significance was set at 2-sided P < .05. Analyses were conducted using SAS version 9.4 (SAS Institute) and R version 4.2.1 statistical software (R Foundation for Statistical Computing).
Results
Participating Facilities and Adherence
Among 47 facilities invited to participate, 38 initially enrolled and 35 (16 hospitals, 16 NHs, 3 LTACHs) completed the SHIELD-OC intervention (eFigure 1 in Supplement 1). One hospital and 2 NHs withdrew during phase-in without launching decolonization and were considered nonparticipants. Overall, participating facilities had more licensed beds and annual admissions than nonparticipating facilities (Table). Race and ethnicity categories are included as reported in CMS and hospitalization datasets as demographic characteristics. Participating hospitals had a slightly younger population with fewer median comorbidities vs nonparticipating hospitals but higher proportions of female and White patients. Participating NHs had similar age, sex, and number of comorbidities vs nonparticipating NHs but a greater proportion of White residents and lower proportion of Medicaid-insured residents. Because hospitalization risk is higher for residents receiving postacute care, we note that median facility-level proportion of residents receiving postacute care was similar among participating and nonparticipating NHs, but participating facilities had more residents receiving postacute care at the person level, likely due to their larger size and interconnectivity to regional hospitals. Of note, a separate randomized clinical trial of decolonization in Southern California NHs14 was under way at this time. There was no overlap between SHIELD-OC participants and any NHs in the trial. Furthermore, network analysis assured separation of patient-sharing networks between SHIELD-OC and that trial.
Table. Characteristics of Participating and Nonparticipating Health Care Facilities, SHIELD-OC Regional Decolonization Collaborative 2015-2019a.
| Characteristic | Nursing homes | Long-term acute care hospitals | Hospitals | ||
|---|---|---|---|---|---|
| Participating | Nonparticipating | Participating | Nonparticipating | ||
| Facility-level characteristics, median (IQR) across facilities | |||||
| Facilities, No. | 16 | 50 | 3b | 16 | 7 |
| Licensed beds | 113 (95-194) | 99 (59-129) | 97.5 (86-109) | 252 (178-329) | 172 (141-188) |
| Annual admissions | 703 (492-860) | 414 (246-631) | 798 (655-873) | 11 854 (4317-18 338) | 4637 (3286-5853) |
| Daily census | 109.9 (87.6-138.7) | 69.3 (48.9-100.0) | 78.2 (66.5-93.9) | 152.3 (71.7-261.4) | 56.9 (41.4-116.8) |
| Length of stay, d | 208.8 (194.2-216.4) | 207.6 (198.1-227.3) | 36.9 (36.1-41.3) | 5.1 (4.7-6.2) | 5.8 (5.3-6.9) |
| Patient characteristics | |||||
| Age, y | 78.3 (72.8-81.2) | 76.6 (70.9-81.3) | 71.7 (71.0-72.4) | 57.9 (54.6-61.2) | 63.8 (51.2-66.5) |
| Sex, % | |||||
| Female | 56.2 (51.5-62.7) | 56.8 (50.9-63.2) | 47.3 (44.4-48.1) | 59.6 (54.2-63.6) | 52.7 (50.5-55.0) |
| Male | 43.8 (37.3-48.5) | 43.2 (36.8-49.1) | 52.7 (51.9-55.6) | 40.4 (36.4-45.8) | 47.3 (45.0-49.5) |
| Postacute, %c | 81.7 (74.4-86.5) | 80.5 (59.8-94.1) | NA | NA | NA |
| Race and ethnicity, %d | |||||
| Asian | 8.8 (6.3-15.0) | 13.0 (7.8-25.8) | 17.2 (15.5-18.8) | 12.2 (6.8-16.5) | 9.6 (4.1-18.7) |
| Black or African American | 2.0 (1.0-4.1) | 2.0 (0.7-3.6) | 2.8 (2.4-3.2) | 2.0 (1.6-3.1) | 4.5 (1.9-4.8) |
| Hispanic | 10.9 (5.3-18.4) | 15.7 (5.6-24.4) | 13.2 (10.6-13.9) | 25.5 (17.5-36.6) | 24.1 (6.3-36.0) |
| White | 78.8 (57.9-86.9) | 59.8 (41.8-74.5) | 72.3 (65.2-73.9) | 75.8 (61.7-81.3) | 69.8 (54.6-90.9) |
| Insurance, %e | |||||
| Medicare | 45.1 (31.6-52.6) | 38.6 (24.6-59.1) | 69.7 (64.5-74.4) | 40.3 (35.9-48.7) | 55.0 (33.6-59.8) |
| Medicaid | 31.3 (21.4-66.7) | 65.0 (30.0-88.2) | 6.4 (3.9-8.2) | 22.3 (12.9-38.7) | 21.5 (8.8-30.6) |
| Other/unknown | 33.3 (20.7-47.7) | 20.6 (5.5-39.9) | 23.8 (21.6-28.2) | 33.2 (23.6-39.8) | 21.8 (15.4-44.7) |
| Comorbidities | |||||
| Diabetes, % | 33.2 (29.3-44.7) | 38.9 (27.9-45.5) | 37.7 (64.5-41.9) | 23.8 (21.5-27.7) | 26.8 (12.2-37.6) |
| Chronic lung disease, % | 20.2 (17.0-23.9) | 21.0 (17.8-24.7) | 37.2 (29.9-42.7) | 17.0 (13.9-18.8) | 18.2 (11.3-26.8) |
| Kidney disease, % | 21.0 (17.9-24.7) | 22.0 (17.1-25.7) | 38.3 (28.1-43.6) | 16.0 (12.4-18.5) | 16.7 (6.3-24.0) |
| Cancer, % | 10.5 (8.3-13.3) | 9.1 (6.0-12.0) | 9.2 (8.6-11.2) | 7.8 (3.1-10.1) | 4.0 (1.7-4.7) |
| Liver disease, % | 2.9 (1.6-4.2) | 2.4 (1.3-3.8) | 7.9 (4.9-10.0) | 6.9 (5.6-7.6) | 6.4 (3.7-8.0) |
| Comorbidity count scoref | 3.5 (3.3-3.7) | 3.5 (3.3-3.7) | 5.13 (4.1-5.9) | 2.9 (2.7-3.2) | 3.1 (2.1-3.9) |
| Baseline MDRO clinical culture rate per 1000 patient-days, mean (IQR) | 0.86 (0.38-1.40) | 0.61 (0.19-0.99) | 7.5 (7.0-8.9) | 6.0 (4.9-7.6) | 6.2 (3.8-8.1) |
| Person-level characteristics | |||||
| Unique persons during the intervention period, No. | 18 585 | 31 040 | 2944g | 419 461 | 70 968 |
| Person-days during the intervention period, No. | 1 330 557 | 3 088 466 | 105 122 | 2 187 149 | 416 244 |
| Length of stay, median (IQR), d | 201.8 (191.3-208.8) | 205.9 (197.0-220.5) | 25.0 (15.0-42.0) | 4.0 (3.0-6.0) | 4.0 (3.0-6.0) |
| Patient characteristics | |||||
| Age, median (IQR), y | 79.0 (69.0-87.0) | 79.0 (67.0-87.0) | 73.0 (63.0-82.0) | 60.0 (37.0-75.0) | 63.0 (47.0-77.0) |
| Sex, No. (%) | |||||
| Female | 10 696 (57.6) | 17 535 (56.5) | 1303 (44.3) | 249 596 (59.5) | 37 666 (53.1) |
| Male | 7889 (42.4) | 13 505 (43.5) | 1641 (55.7) | 169 858 (40.5) | 33 299 (46.9) |
| Postacute, No. (%)c | 14 650 (78.8) | 22 717 (73.2) | NA | NA | NA |
| Race and ethnicity, No. (%)d | |||||
| Asian | 2083 (11.2) | 6289 (20.3) | 510 (17.3) | 66 589 (15.9) | 8380 (11.8) |
| Black or African American | 396 (2.1) | 723 (2.3) | 105 (3.6) | 9533 (2.3) | 3012 (4.2) |
| Hispanic | 2500 (13.5) | 4848 (15.6) | 349 (11.9) | 105 502 (25.2) | 15 282 (21.5) |
| White | 13 808 (74.3) | 19 131 (61.6) | 2052 (69.7) | 289 575 (69.0) | 50 631 (71.3) |
| Insurance, No. (%)e | |||||
| Medicare | 6722 (36.2) | 11 318 (36.5) | 1882 (63.9) | 177 216 (42.2) | 35 734 (50.4) |
| Medicaid | 6117 (32.9) | 13 576 (43.7) | 263 (8.9) | 94 210 (22.5) | 15 795 (22.3) |
| Other/unknown | 8199 (44.1) | 11 593 (37.3) | 188 (6.4) | 20 492 (4.9) | 4523 (6.4) |
| Comorbidities | |||||
| Diabetes, No. (%) | 6246 (33.6) | 10 464 (33.7) | 1177 (40.0) | 100 905 (24.1) | 18 912 (26.6) |
| Chronic lung disease, No. (%) | 3404 (18.3) | 5464 (17.6) | 1065 (36.2) | 68 600 (16.4) | 13 324 (18.8) |
| Kidney disease, No. (%) | 3619 (19.5) | 6052 (19.5) | 1181 (40.1) | 67 723 (16.1) | 12 489 (17.6) |
| Cancer, No. (%) | 1986 (10.7) | 3101 (10.0) | 313 (10.6) | 39 764 (9.5) | 3360 (4.7) |
| Liver disease, No. (%) | 463 (2.5) | 769 (2.5) | 256 (8.7) | 28 560 (6.8) | 4488 (6.3) |
| Comorbidity count score, median (IQR)f | 3.4 (3.3- 3.6) | 3.4 (3.2-3.6) | 5.0 (4.0-7.0) | 3.0 (1.0-5.0) | 3.0 (1.0-5.0) |
Abbreviations: NA, not applicable; SHIELD-OC, Shared Healthcare Intervention to Eliminate Life-threatening Dissemination of MDROs (multidrug-resistant organisms) in Orange County.
All data in this table were collected from the Centers for Medicare & Medicaid Services (CMS) Minimum Data Set (nursing homes) or mandatory hospitalization datasets (long-term acute care hospitals and hospitals) except for MDRO clinical (nonscreening) culture rate per 1000 patient-days. MDRO clinical culture rate was obtained from countywide laboratory reporting. All data are complete without missing values except for person-level long-term acute care characteristics.
All long-term acute care hospitals in the county participated.
Postacute in nursing homes represents the proportion of residents with a length of stay less than 100 days.
Race and ethnicity categories are included as reported in CMS and hospitalization datasets as demographic characteristics. White Hispanic and Black Hispanic race and ethnicity are represented in both categories (eg, White and Hispanic or Black and Hispanic), and thus, the total percentage across categories may exceed 100%.
For nursing homes, total percentage across insurance categories exceeds 100% because Medicare & Medicaid categories include individuals who are dual-eligible for both Medicare & Medicaid.
Elixhauser comorbidity count score is based on the summed count of comorbidities based on diagnostic codes. Higher number indicates greater illness.
Person-level information was not available for 1 of the 3 long-term acute care hospitals that reports their information together with another facility.
Among participating NHs, mean (SD) chlorhexidine adherence was 86.3% (4.2%) and povidone-iodine adherence, 69.5% (14.7%). In LTACHs, mean (SD) chlorhexidine adherence was 94.0% (2.4%) and povidone-iodine adherence, 83.9% (1.3%). Among hospitalized patients in CP, mean (SD) chlorhexidine adherence was 79.3% (9.0%) and povidone-iodine adherence, 69.6% (14.8%). A total of 10 adverse reactions were reported across participating facilities. On examination by an infectious diseases physician, 4 were deemed unrelated to decolonization (3 preexisting candidiasis, 1 preexisting petechiae). One was due to soap in the eye. Of the remaining 5 reports of mild skin irritation, 2 resolved by discontinuing chlorhexidine and 3 resolved without discontinuation. Information about adverse reactions to routine soap in nonparticipating facilities is not available.
Point Prevalence Surveys
Baseline and end of intervention MDRO prevalence by participating facility type are depicted in Figure 1. In NHs, mean (SD) MDRO prevalence decreased from 63.9% (12.2%) to 49.9% (11.3%) (21.9% relative decrease; P < .001); in LTACHs, 80.0% (7.2%) to 53.3% (13.3%) (33.4% relative decrease; P = .01); and among hospitalized patients in CP, 64.1% (8.5%) to 55.4% (13.8%) (13.6% relative decrease; P = .03). Characteristics of the 1690 persons sampled at baseline and 2342 persons sampled at end of intervention are provided in eTable 1 in Supplement 1. Overall, 7% refused sampling.
Figure 1. MDRO Point Prevalence (Screening) Among Facilities Participating in the Regional Decolonization Collaborative, Baseline and End of Intervention.
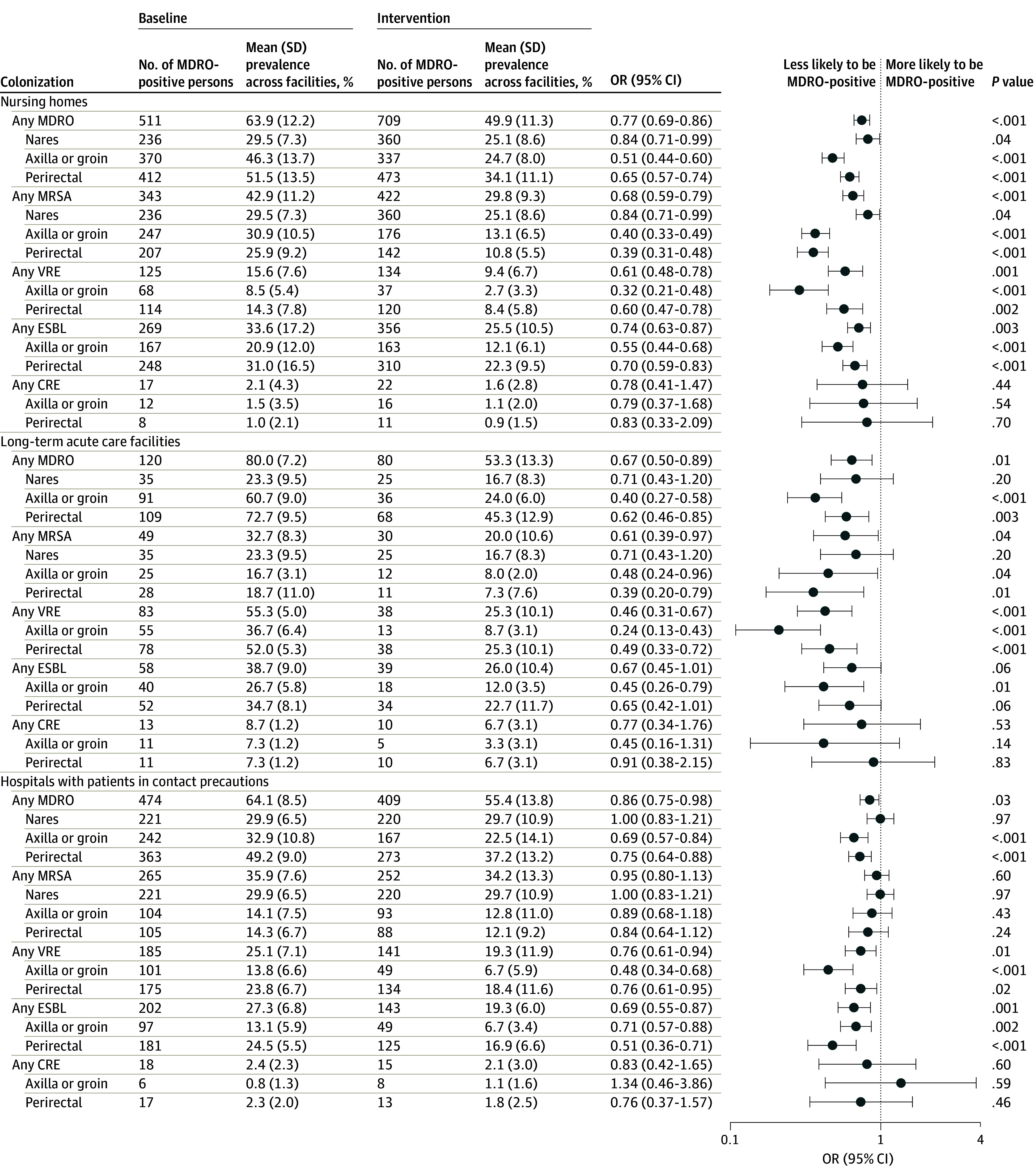
CRE indicates carbapenem resistant Enterobacterales; ESBL, extended spectrum β-lactamase; MDRO, multidrug-resistant organism; MRSA, methicillin-resistant Staphylococcus aureus; and VRE, vancomycin-resistant Enterococci.
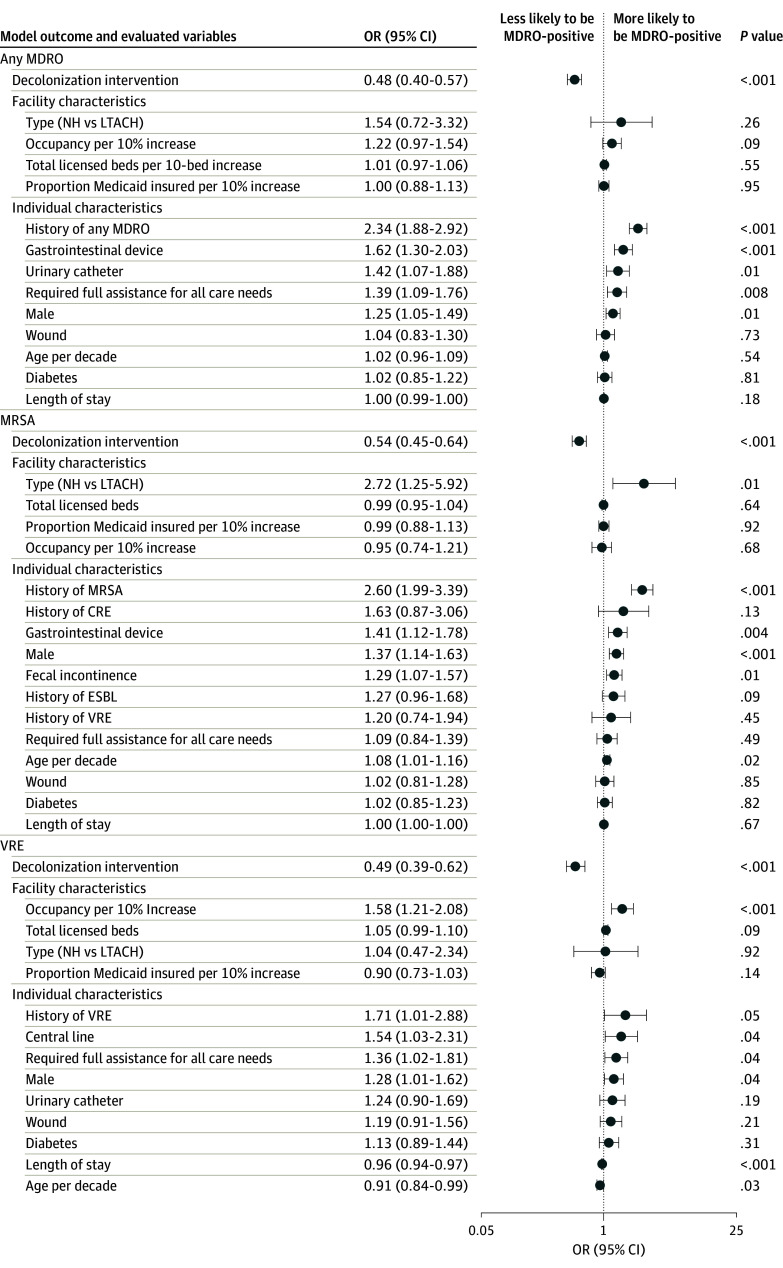
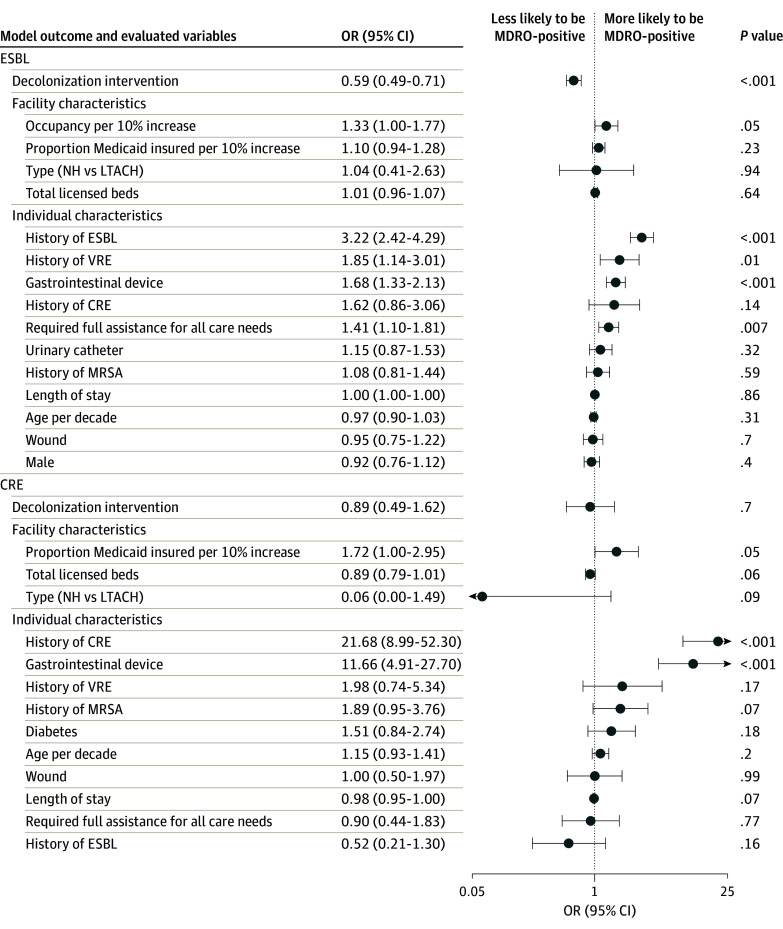
In adjusted analyses, prevalence of MRSA, VRE, ESBL, and any MDRO significantly decreased in NHs and LTACHs (Figure 2 and Figure 3), and prevalence of VRE, ESBL, and any MDRO significantly decreased among hospital patients in CP (eTable 2 in Supplement 1). One hospital was excluded because of the small number of patients in CP. Adjusted ORs for any MDRO were 0.48 (95% CI, 0.40-0.57; P < .001) for NHs and LTACHs and 0.75 (95% CI, 0.60-0.93; P = .01) for hospital patients in CP.
Figure 2. Multivariable Regression for Factors Associated With MDRO Carriage in NHs and LTACHs (MDRO, MRSA, and VRE).
Gastrointestinal devices include gastronomy, jejunostomy, nasogastric, and rectal tubes. Models adjust for clustering by facility. CRE indicates carbapenem resistant Enterobacterales; ESBL, extended spectrum β-lactamase; LTACH, long-term acute care hospital; MRSA, methicillin-resistant Staphylococcus aureus; NH, nursing homes; and VRE, vancomycin-resistant Enterococci.
Figure 3. Multivariable Regression for Factors Associated With MDRO Carriage in NHs and LTACHs (ESBL and CRE).
CRE indicates carbapenem resistant Enterobacterales; ESBL, extended spectrum β-lactamase; LTACH, long-term acute care hospital; MRSA, methicillin-resistant Staphylococcus aureus; NH, nursing homes; and VRE, vancomycin-resistant Enterococci.
As a measure of indirect regional outcomes, MDRO prevalence on admission to LTACHs decreased from 58.5% (348 of 595) during baseline to 45.1% (278 of 616) during intervention (OR, 0.58; 95% CI, 0.46-0.73; P < .001), with significant reductions in MRSA, VRE, and CRE (eTable 3 in Supplement 1).
Incident MDRO Clinical (Nonscreening) Cultures
Clinical culture data from the countywide laboratory reporting mandate were available for 34 participating facilities (15 NHs, 3 LTACHs, 16 hospitals) and 50 nonparticipating facilities without specific decolonization activities.
Participating NHs had a mean (SD) of 2.7 (1.9) monthly incident MDRO-positive clinical cultures during baseline and 1.7 (1.1) during decolonization; nonparticipating NHs had 1.7 (1.4) during baseline and 1.5 (1.1) during intervention. In an adjusted model comparing intervention with baseline periods and controlling for annual admissions, there was a 30.4% (95% CI, 16.4%-42.1%) further reduction in incident MDRO-positive clinical cultures in participating NHs (RR, 0.59; 95% CI, 0.51-0.69) compared with nonparticipating NHs (RR, 0.85; 95% CI, 0.76-94) (group × period interaction reduction P < .001) (Figure 4). Among MDRO subsets, ESBL and CRE were significantly reduced.
Figure 4. Incident Cultures in the Intervention vs Baseline Period.
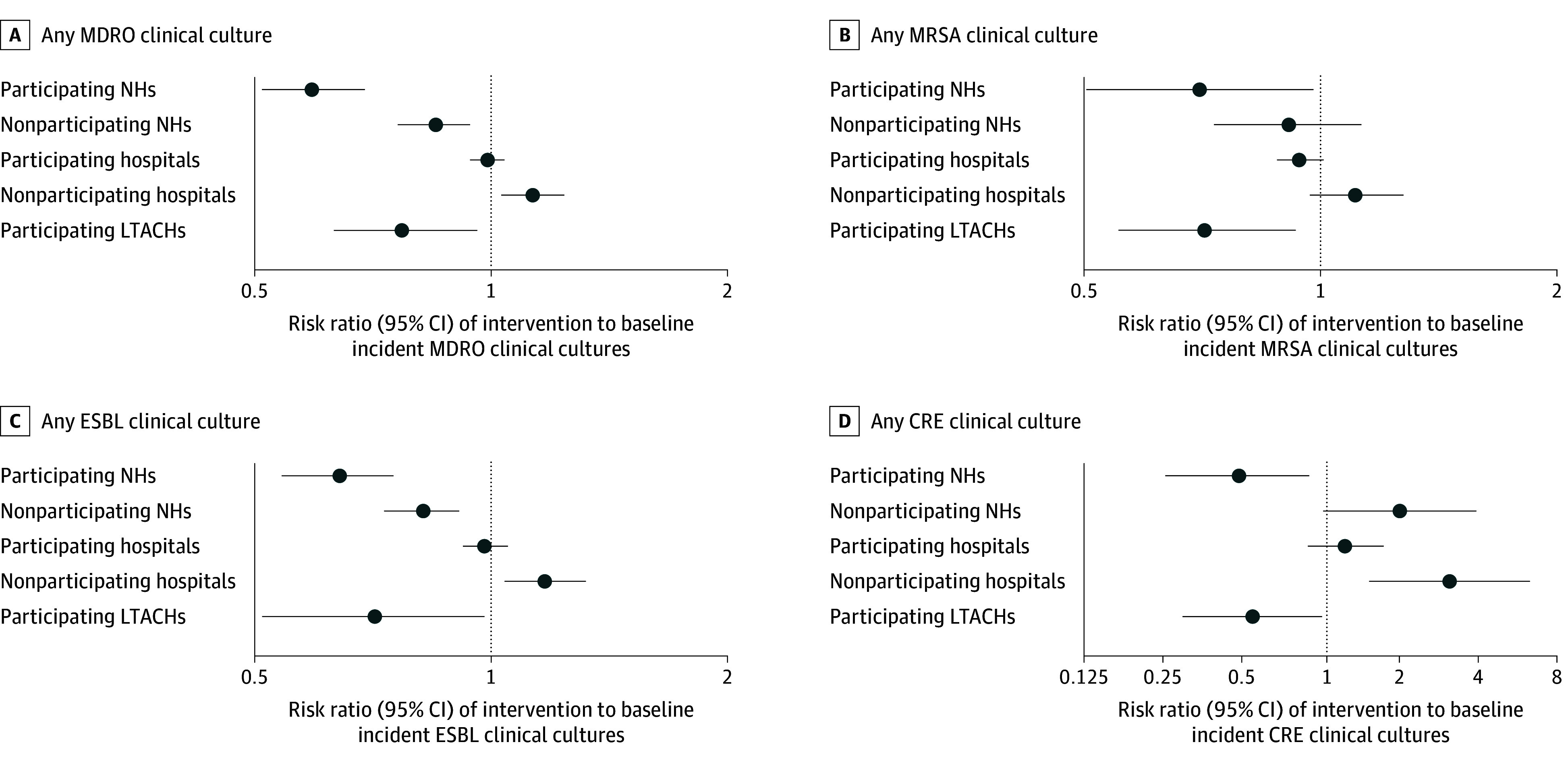
Results are based on generalized linear mixed models that accounted for clustering within facilities and adjusted for facility-level annual admissions, mean age, % White race, % Medicaid-insured, and mean Elixhauser comorbidity count. CRE indicates carbapenem resistant Enterobacterales; ESBL, extended spectrum β-lactamase; LTACH, long-term acute care hospitals; MDRO, multidrug-resistant organism; MRSA, methicillin-resistant Staphylococcus aureus; and NH, nursing homes.
Participating hospitals had a mean (SD) of 25.5 (18.6) monthly incident MDRO-positive clinical cultures during baseline and 25.0 (15.9) during intervention; nonparticipating hospitals had 12.5 (10.1) during baseline and 14.3 (10.2) during intervention. Adjusted models showed a 12.9% (95% CI, 3.3%-21.5%) greater reduction in incident MDRO-positive clinical cultures in participating hospitals (RR, 0.99; 95% CI, 0.94-1.04) compared with nonparticipating hospitals (RR, 1.13; 95% CI, 1.03-1.24) (group × period interaction reduction P = .01). Among specific MDROs, MRSA and ESBL were significantly reduced (Figure 4). As an emerging pathogen, CRE increased in both participating and nonparticipating hospitals but significantly less so among participating hospitals.
In LTACHs, all of which adopted decolonization, the mean (SD) monthly incident MDRO-positive clinical cultures was 14.8 (8.6) during baseline and 8.2 (6.1) during intervention. Adjusted models showed a 22.5% (95% CI, 4.4%-37.1%) reduction in MDRO-positive clinical cultures (RR, 0.77; 95% CI, 0.63-0.96; P = .02), with significant reductions in MRSA, ESBL, and CRE (Figure 5).
Figure 5. Monthly Infection-Related Hospitalization Rates Among Nursing Homes Residents in Participating (Decolonization) vs Nonparticipating Nursing Homes.
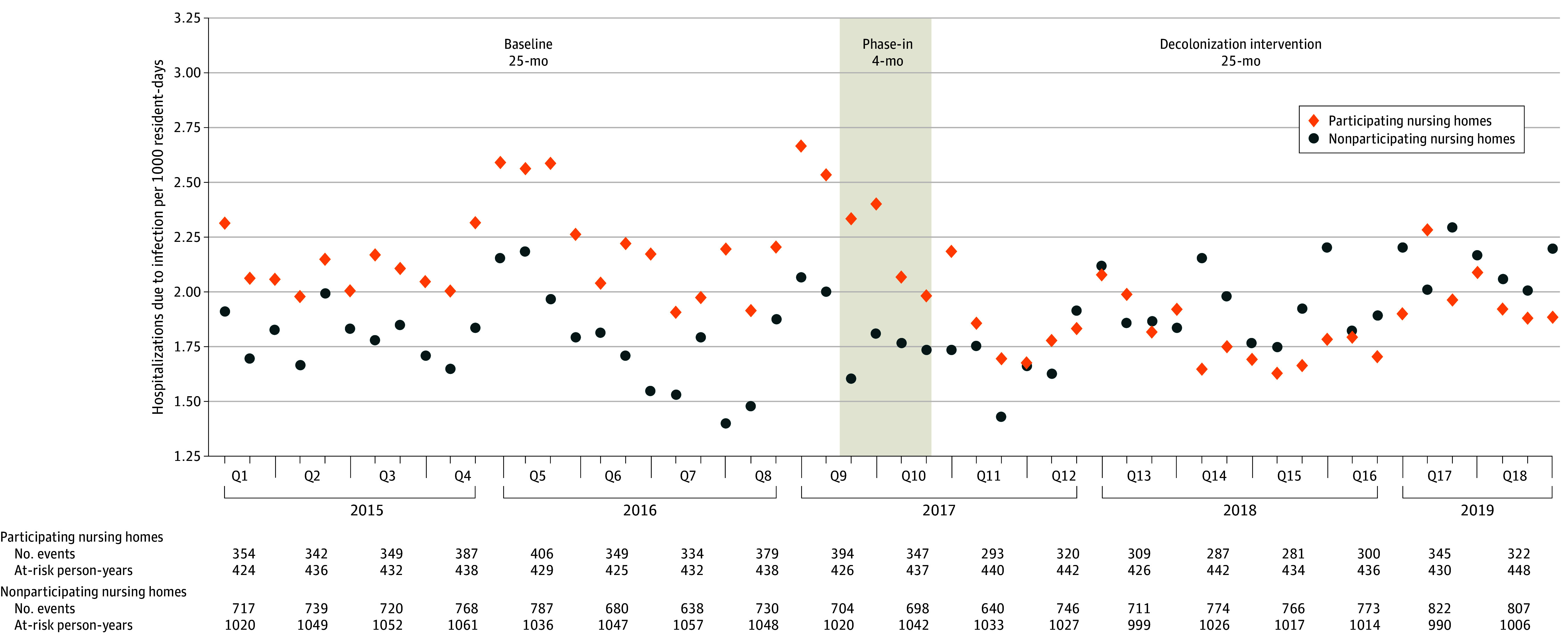
Q indicates quarter.
MDRO-specific adjusted and unadjusted RRs are reported in eTable 4 in Supplement 1. Quarterly rates of MDRO-positive clinical cultures per 1000 resident-days or patient-days are depicted in eFigure 2 in Supplement 1, and rates by year, facility type, and pathogen are depicted in eFigure 3 in Supplement 1. Estimated annual incident MDRO-positive clinical cultures for facilities of varying covariate values, based on our models, are reported in eTable 5 in Supplement 1.
Infection-Related Hospitalizations, Costs, and Deaths
Hospitalization data from NH residents were available for all 16 NHs that adopted decolonization and 50 nonparticipating NHs. In participating NHs, the rate of infection-related hospitalizations per 1000 resident-days was 2.31 (3031 of 1 309 668) during baseline and 1.94 (2580 of 1 330 557) during decolonization; for nonparticipating NHs, 1.90 (6026 of 3 172 387) during baseline and 2.03 (6271 of 3 088 466) during intervention. Based on the group × period interaction in an adjusted model, decolonization was associated with a 26.7% (95% CI, 19.0%-34.5%; P < .001) reduction in infection-related hospitalizations (Figure 5). When evaluating all-cause hospitalizations, decolonization was associated with a 6.7% reduction (95% CI, 1.3%-11.8%; P = .02).
Costs due to infection-related hospitalizations per 1000 resident-days in participating NHs were $64 651 ($84 671 973 of 1 309 668) during baseline and $55 149 ($73 380 460 of 1 330 557) during decolonization; for nonparticipating NHs, $55 151 ($174 961 015 of 3 172 387) during baseline and $59 327 ($183 229 160 of 3 088 466) during intervention. Based on the group × period interaction in an adjusted model, decolonization was associated with a 26.8% (95% CI, 26.7%-26.9%; P < .001) reduction in infection-related hospitalization costs (eTable 6 in Supplement 1). Estimated decolonization costs and associated cost savings for an average 100-occupied bed NH are provided in eAppendix 2 in Supplement 1.
In participating NHs, the rate of deaths from infection-related hospitalizations per 1000 resident-days was 0.29 (379 of 1 309 668) during baseline and 0.25 (326 of 1 330 557) during decolonization; for nonparticipating NHs, 0.23 (731 of 3 172 387) during baseline and 0.24 (744 of 3 088 466) during intervention. Based on the group × period interaction in an adjusted model, decolonization was associated with a 23.7% (95% CI, 4.5%-43.0%; P < .001) reduction in deaths from infection-related hospitalizations. Adjusted and unadjusted results for hospitalization outcomes are provided in eTable 7 in Supplement 1.
Discussion
Mitigating antibiotic resistance remains a global priority. Because MDROs spread across health care facilities as patients are shared among them, multifacility regional collaboration can synergistically interrupt MDRO dissemination beyond what facilities can achieve independently.10,16 While prior regional efforts have generally focused on a single MDRO or facility type,26,27,28,29 the SHIELD-OC strategy prevented multiple MDROs across acute and long-term care settings. This strategy may have been particularly successful because it used patient-sharing patterns to identify target facilities and used simulation modeling to select decolonization as the highest yield intervention.16
The SHIELD-OC regional decolonization intervention was associated with significant reductions in MDRO prevalence and MDRO incident clinical (nonscreening) cultures across all health care facility types. Our findings of a 23% to 30% reduction in MDRO-positive clinical cultures in NHs and LTACHs are consistent with those from randomized clinical trials of universal decolonization in hospital ICUs, non-ICUs, and postdischarge settings.11,12,14,30,31,32 Universal decolonization reduced MRSA-positive clinical cultures by 37% in ICUs,11 reduced MRSA/VRE-positive clinical cultures by 37% in non-ICU inpatients with medical devices,30 and reduced the incidence of MRSA infection by 30% among MRSA carriers after hospital discharge.12 Unlike preceding trials, SHIELD-OC simultaneously demonstrated benefit for MRSA, VRE, ESBL, and CRE—covering both endemic and emerging pathogens.
Notably, the 27% reduction in infection-related hospitalizations among NH residents was similar to the 31% reduction seen in the Protect Trial,14 a randomized clinical trial of universal chlorhexidine and nasal iodophor in NHs. While that trial also demonstrated reductions in NH MDRO prevalence, sampling was limited to nares and skin. SHIELD-OC additionally demonstrated reductions in perirectal carriage and gut-associated pathogens (VRE, ESBL, CRE) despite the intervention’s focus on topical decolonization. This is most likely due to preventing new acquisition of MDROs which then decreases transmission, although the exact mechanism cannot be definitively identified. It is also possible that decreased skin bioburden reduced self-reinoculation of the gastrointestinal tract, allowing spontaneous clearance of MDROs.
The SHIELD-OC collaborative found not only direct benefits to participating facilities but also indirect benefits. MDROs noted on admission to LTACHs were sharply reduced within the 2-year intervention, suggesting reduced MDRO prevalence among patients transferring from regional hospitals. In Israel, a national intervention to control CRE in long-term care decreased CRE incidence by 50% and nearly eliminated CRE prevalence 8 years into implementation.26 Mathematical models of regional CRE spread suggested that coordinated action across interconnected health care facilities continued to accrue decreases in CRE acquisitions up to a 55% reduction over 15 years.10 These findings suggest that the benefits of regional decolonization may accumulate with sustained adoption.
Compared with hospitals, NHs and LTACHs achieved greater adherence with the decolonization protocol and experienced greater reductions in MDRO prevalence and incident clinical cultures. This greater benefit could be due, in part, to greater adherence from universal vs targeted decolonization and longer lengths of stays of NH residents and LTACH patients, which provide more time for decolonization to accrue effects and reduce importation of new pathogens due to less frequent turnover. These differences, compounded by the more medically complex population in long-term care, may explain the 27% reduction in infection-related hospitalizations from NHs and the associated reductions in hospitalization costs and related deaths.
Decolonization only works if products are correctly applied.33,34 Initial training was needed to ensure proper application, and ongoing training was needed due to high staff turnover and gaps in bathing practices.35,36 Nevertheless, the SHIELD-OC intervention was implemented by usual facility staff with existing leadership support, suggesting that reported gains should be achievable if similar adherence is attained.
The success of SHIELD-OC in reducing MDRO carriage, infections, hospitalizations, and associated costs and deaths led to a regional NH incentive program supported by CalOptima, the sole Medicaid provider in Orange County, and offered to countywide NHs. The incentive program covered the cost of chlorhexidine and iodophor and provided additional nurses who trained and supported 28 enrolled NHs from July 2019 to June 2022 before the program was terminated due to COVID-19 pandemic–associated budgetary constraints. When the incentive program ended, 21 of 28 NHs opted to continue the decolonization intervention.
Limitations
First, this study’s limitations include the quasi-experimental nonrandomized design component. Participating facilities were selected based on their high degree of shared patients, and thus, were more interconnected and tended to be larger than nonparticipating facilities. The greater proportion of residents receiving postacute care and higher rate of baseline hospitalization in participating vs nonparticipating NHs reflects this. Differences among participant groups underscore the importance of adjusting for facility and population characteristics in our analyses. That a similar reduction in MDRO prevalence and infection-related hospitalizations was observed in NHs in a randomized clinical trial where NH groups were balanced provides confirmation.14
Second, while analyses accounted for differences in facility size, and patient and resident characteristics, data on activities such as hand hygiene, contact/barrier precautions, or antibiotic stewardship were lacking and may have confounded observed results. Requirements for antimicrobial stewardship programs were emerging in California during this time,37 while recommendations for enhanced barrier precautions in NHs largely postdated the intervention.38,39 Due to limited resources, NHs generally struggle to handle multiple quality improvement initiatives simultaneously. During the study, decolonization was the only campaign among participating facilities. Nevertheless, secular trends and unmeasured contextual factors highlight the value of the comparison group in our analyses.
Third, this intervention benefitted from contributed chlorhexidine-impregnated cloths for bed bathing, which may have affected use of this product vs liquid soap typically used in NHs. Furthermore, it is not possible to know whether increased attention to bathing contributed to observed benefits over and above the switch to an antiseptic bathing product. However, decolonization trials performed in ICUs, where daily bathing is standardized and performed by nurses, reported a benefit from the chlorhexidine itself.11,31,32
Fourth, while recruitment of interconnected facilities was a strength of SHIELD-OC, other regions may not be able to recruit facilities in this manner, although a helpful tool exists.40 Fifth, chlorhexidine resistance testing was not performed. Although prior large-scale trials with resulting widespread adoption have not identified differential emergence of chlorhexidine resistance.11,12,31,41 Sixth, while SHIELD-OC was geographically limited, Orange County is a large metropolitan county with a socioeconomically and demographically diverse population of 3.2 million, suggesting potential generalizability across a range of populations.
Conclusion
In this study, a regional decolonization collaborative involving universal decolonization in long-term care facilities and targeted decolonization among hospital patients in contact precautions was associated with lower MDRO carriage, infections, hospitalizations, costs, and deaths.
eTable 1. Characteristics of Sampled Patients and Residents in Participating SHIELD-OC Facilities, Baseline and End-Intervention Periods
eTable 2. Multivariable Regression for Factors Associated with MDRO Carriage in Hospital Patients on Contact Precautions
eTable 3. Indirect Effects of Regional Decolonization: Reduction in MDRO Prevalence Upon Admission to Long-Term Acute Care Hospitals
eTable 4. Baseline Versus Decolonization Intervention Incident Clinical (Non-Screening) Cultures by Multidrug-Resistant Organism Type Comparing Participating and Nonparticipating Facilities
eTable 5. Estimated Annual Incident Multidrug-Resistant Organism (MDRO)-Positive Clinical (Non-Screening) Cultures for Facilities Varying by Size and Mean Number of Patient Comorbidities
eTable 6. Costs and Length-of-Stay for Infection-Related Hospitalizations Arising From Nursing Homes
eTable 7. Group Comparisons for Adjusted and Unadjusted Nursing Home Infection-Related Hospitalization Outcomes
eFigure 1. SHIELD Orange County Intervention Flow Diagram of Healthcare Facilities, Participants and Comparators
eFigure 2. Quarterly Multidrug-Resistant Organism (MDRO)-Positive Clinical (Non-Screening) Cultures per 1,000 Patient Days among Participating (Decolonization) Versus Non-Participating Healthcare Facilities
eFigure 3. Multidrug-Resistant Organism (MDRO)-Positive Clinical (Non-Screening) Cultures per 1,000 Patient Days by Year, Pathogen, and Participant Group
eAppendix 1. SHIELD-OC Project Related Support
eAppendix 2. Value and Evidence Base for Universal Decolonization in Nursing Homes
Data Sharing Statement
References
- 1.Centers for Disease Control and Prevention . Antibiotic Resistance Threats in the United States. Department of Health and Human Services; 2019. [Google Scholar]
- 2.World Health Organization . Ten threats to global health in 2019. Published 2019. Accessed October 31, 2023. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
- 3.Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42(suppl 2):S82-S89. doi: 10.1086/499406 [DOI] [PubMed] [Google Scholar]
- 4.McKinnell JA, Singh RD, Miller LG, et al. The SHIELD Orange County Project: Multidrug-resistant Organism Prevalence in 21 Nursing Homes and Long-term Acute Care Facilities in Southern California. Clin Infect Dis. 2019;69(9):1566-1573. doi: 10.1093/cid/ciz119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mody L, Foxman B, Bradley S, et al. Longitudinal assessment of multidrug-resistant organisms in newly admitted nursing facility patients: implications for an evolving population. Clin Infect Dis. 2018;67(6):837-844. doi: 10.1093/cid/ciy194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKinnell JA, Miller LG, Singh RD, et al. High prevalence of multidrug-resistant organism colonization in 28 nursing homes: an “iceberg effect”. J Am Med Dir Assoc. 2020;21(12):1937-1943.e2. doi: 10.1016/j.jamda.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimasaki T, Segreti J, Tomich A, Kim J, Hayden MK, Lin MY; CDC Prevention Epicenters Program . Active screening and interfacility communication of carbapenem-resistant Enterobacteriaceae (CRE) in a tertiary-care hospital. Infect Control Hosp Epidemiol. 2018;39(9):1058-1062. doi: 10.1017/ice.2018.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin MY, Lyles-Banks RD, Lolans K, et al. ; Centers for Disease Control and Prevention Epicenters Program . The importance of long-term acute care hospitals in the regional epidemiology of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis. 2013;57(9):1246-1252. doi: 10.1093/cid/cit500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee BY, Bartsch SM, Wong KF, et al. The importance of nursing homes in the spread of methicillin-resistant Staphylococcus aureus (MRSA) among hospitals. Med Care. 2013;51(3):205-215. doi: 10.1097/MLR.0b013e3182836dc2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slayton RB, Toth D, Lee BY, et al. Vital signs: estimated effects of a coordinated approach for action to reduce antibiotic-resistant infections in health care facilities—United States. MMWR Morb Mortal Wkly Rep. 2015;64(30):826-831. doi: 10.15585/mmwr.mm6430a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang SS, Septimus E, Kleinman K, et al. ; CDC Prevention Epicenters Program; AHRQ DECIDE Network and Healthcare-Associated Infections Program . Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368(24):2255-2265. doi: 10.1056/NEJMoa1207290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang SS, Singh R, McKinnell JA, et al. ; Project CLEAR Trial . Decolonization to reduce postdischarge infection risk among MRSA carriers. N Engl J Med. 2019;380(7):638-650. doi: 10.1056/NEJMoa1716771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayden MK, Lin MY, Lolans K, et al. ; Centers for Disease Control and Prevention Epicenters Program . Prevention of colonization and infection by Klebsiella pneumoniae carbapenemase-producing enterobacteriaceae in long-term acute-care hospitals. Clin Infect Dis. 2015;60(8):1153-1161. doi: 10.1093/cid/ciu1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller LG, McKinnell JA, Singh RD, et al. Decolonization in nursing homes to prevent infection and hospitalization. N Engl J Med. 2023;389(19):1766-1777. doi: 10.1056/NEJMoa2215254 [DOI] [PubMed] [Google Scholar]
- 15.Huang SS, Septimus EJ, Kleinman K, et al. Nasal iodophor antiseptic vs nasal mupirocin antibiotic in the setting of chlorhexidine bathing to prevent infections in adult ICUs: a randomized clinical trial. JAMA. 2023;330(14):1337-1347. doi: 10.1001/jama.2023.17219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartsch SM, Wong KF, Mueller LE, et al. Modeling interventions to reduce the spread of multidrug-resistant organisms between health care facilities in a region. JAMA Netw Open. 2021;4(8):e2119212. doi: 10.1001/jamanetworkopen.2021.19212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.UCI Health . SHIELD: Eliminating multidrug-resistant organisms. Accessed February 28, 2024. https://www.ucihealth.org/shield
- 18.Centers for Medicare & Medicaid Services . Minimum Data Set (MDS) 3.0 for Nursing Homes and Swing Bed Providers. Accessed March 1, 2023. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/NHQIMDS30
- 19.Brown University Center for Gerontology and Healthcare Research . LTCFocus. Accessed August 1, 2021. https://ltcfocus.org/
- 20.Centers for Medicare & Medicaid Services Nursing Home Compare . Updated August 1, 2021. Accessed October 1, 2023. https://www.medicare.gov/nursinghomecompare
- 21.California Office of Statewide Health Planning and Development . California Department of Health Care Access and Information. Accessed August 1, 2021. https://hcai.ca.gov/
- 22.Orange County Healthcare Agency . County Health Order for Reportable Diseases. Updated July 5, 2016. Accessed June 1, 2021. https://www.ochealthinfo.com/sites/hca/files/import/data/files/57152.pdf
- 23.Agency for Healthcare Research and Quality . HCUP Clinical Classifications Software (CCS) for ICD-9-CM. Healthcare Cost and Utilization Project (HCUP). Accessed June 1, 2023. http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp
- 24.US Bureau of Labor Statistics . Consumer price index, medical care component (US city average, not seasonally adjusted) Series ID: CUSR0000SAM. Accessed September 28, 2023. http://www.bls.gov/data
- 25.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 26.Ben-David D, Masarwa S, Fallach N, et al. ; Israel LTCF CRE Working Group . Success of a national intervention in controlling carbapenem-resistant Enterobacteriaceae in Israel’s long-term care facilities. Clin Infect Dis. 2019;68(6):964-971. doi: 10.1093/cid/ciy572 [DOI] [PubMed] [Google Scholar]
- 27.Schwaber MJ, Lev B, Israeli A, et al. ; Israel Carbapenem-Resistant Enterobacteriaceae Working Group . Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin Infect Dis. 2011;52(7):848-855. doi: 10.1093/cid/cir025 [DOI] [PubMed] [Google Scholar]
- 28.Ostrowsky BE, Trick WE, Sohn AH, et al. Control of vancomycin-resistant enterococcus in health care facilities in a region. N Engl J Med. 2001;344(19):1427-1433. doi: 10.1056/NEJM200105103441903 [DOI] [PubMed] [Google Scholar]
- 29.Lin M, Froilan MC, Makhija J, et al. Regional impact of a CRE Intervention Targeting High Risk Postacute Care Facilities (Chicago PROTECT). Infect Control Hosp Epidemiol. 2020;41:S48-S49. doi: 10.1017/ice.2020.531 [DOI] [Google Scholar]
- 30.Huang SS, Septimus E, Kleinman K, et al. ; ABATE Infection trial team . Chlorhexidine versus routine bathing to prevent multidrug-resistant organisms and all-cause bloodstream infections in general medical and surgical units (ABATE Infection trial): a cluster-randomised trial. Lancet. 2019;393(10177):1205-1215. doi: 10.1016/S0140-6736(18)32593-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368(6):533-542. doi: 10.1056/NEJMoa1113849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milstone AM, Elward A, Song X, et al. ; Pediatric SCRUB Trial Study Group . Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet. 2013;381(9872):1099-1106. doi: 10.1016/S0140-6736(12)61687-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Supple L, Kumaraswami M, Kundrapu S, et al. Chlorhexidine only works if applied correctly: use of a simple colorimetric assay to provide monitoring and feedback on effectiveness of chlorhexidine application. Infect Control Hosp Epidemiol. 2015;36(9):1095-1097. doi: 10.1017/ice.2015.124 [DOI] [PubMed] [Google Scholar]
- 34.Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Daily skin cleansing with chlorhexidine did not reduce the rate of central-line associated bloodstream infection in a surgical intensive care unit. Intensive Care Med. 2010;36(5):854-858. doi: 10.1007/s00134-010-1783-y [DOI] [PubMed] [Google Scholar]
- 35.Gandhi A, Yu H, Grabowski DC. High nursing staff turnover in nursing homes offers important quality information: study examines high turnover of nursing staff at US nursing homes. Health Aff. 2021;40(3):384-391. doi: 10.1377/hlthaff.2020.00957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen KP, Singh RD, Saavedra R, et al. Not as simple as it seems: extensive facility and training gaps in nursing home bathing. Infect Control Hosp Epidemiol. 2023;44(9):1490-1493. doi: 10.1017/ice.2023.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.California Department of Public Health . California Antimicrobial Stewardship Program Initiative. Updated January 17, 2023. Accessed January 3, 2024. https://www.cdph.ca.gov/Programs/CHCQ/HAI/Pages/CA_AntimicrobialStewardshipProgramInitiative.aspx
- 38.California Department of Public Health . Enhanced Standard Precautions for Skilled Nursing Facilities, 2019. Updated June 10, 2019. Accessed January 3, 2024. https://www.cdph.ca.gov/Programs/CHCQ/LCP/Pages/AFL-19-22.aspx
- 39.Centers for Disease Control and Prevention . Consideration for use of enhanced barrier precautions in skilled nursing facilities. Published 2019. Updated July 28, 2021. Accessed January 3, 2024. https://www.cdc.gov/hicpac/workgroup/EnhancedBarrierPrecautions.html
- 40.Octaria R, Cincotta S, Healy J, et al. An interactive patient transfer network and model visualization tool for multidrug-resistant organism prevention strategies. Antimicrob Steward Healthc Epidemiol. 2023;3(S2):s120-s122. doi: 10.1017/ash.2023.403 [DOI] [Google Scholar]
- 41.Hayden MK, Lolans K, Haffenreffer K, et al. Chlorhexidine and mupirocin susceptibility of methicillin-resistant Staphylococcus aureus isolates in the REDUCE-MRSA trial. J Clin Microbiol. 2016;54(11):2735-2742. doi: 10.1128/JCM.01444-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of Sampled Patients and Residents in Participating SHIELD-OC Facilities, Baseline and End-Intervention Periods
eTable 2. Multivariable Regression for Factors Associated with MDRO Carriage in Hospital Patients on Contact Precautions
eTable 3. Indirect Effects of Regional Decolonization: Reduction in MDRO Prevalence Upon Admission to Long-Term Acute Care Hospitals
eTable 4. Baseline Versus Decolonization Intervention Incident Clinical (Non-Screening) Cultures by Multidrug-Resistant Organism Type Comparing Participating and Nonparticipating Facilities
eTable 5. Estimated Annual Incident Multidrug-Resistant Organism (MDRO)-Positive Clinical (Non-Screening) Cultures for Facilities Varying by Size and Mean Number of Patient Comorbidities
eTable 6. Costs and Length-of-Stay for Infection-Related Hospitalizations Arising From Nursing Homes
eTable 7. Group Comparisons for Adjusted and Unadjusted Nursing Home Infection-Related Hospitalization Outcomes
eFigure 1. SHIELD Orange County Intervention Flow Diagram of Healthcare Facilities, Participants and Comparators
eFigure 2. Quarterly Multidrug-Resistant Organism (MDRO)-Positive Clinical (Non-Screening) Cultures per 1,000 Patient Days among Participating (Decolonization) Versus Non-Participating Healthcare Facilities
eFigure 3. Multidrug-Resistant Organism (MDRO)-Positive Clinical (Non-Screening) Cultures per 1,000 Patient Days by Year, Pathogen, and Participant Group
eAppendix 1. SHIELD-OC Project Related Support
eAppendix 2. Value and Evidence Base for Universal Decolonization in Nursing Homes
Data Sharing Statement