Abstract
We report a new protocol for the synthesis of substituted benzotriazin-4(3H)-ones which are underrepresented heterocyclic scaffolds with important pharmacological properties. Our method exploits acyclic aryl triazine precursors that undergo a photocyclization reaction upon exposure to violet light (420 nm). Continuous flow reactor technology is exploited to afford excellent yields in only 10 min residence time with no additives or photocatalysts needed. The underlying reaction mechanism appears to be based on an unprecedented variation of the classical Norrish type II reaction with concomitant fragmentation and formation of N–N bonds. Scalability, process robustness, and green credentials of this intriguing transformation are highlighted.
Benzotriazin-4(3H)-ones are important heterocyclic scaffolds with various reported biological activities. These account for applications of this scaffold in active pharmaceutical ingredients such as anesthetics, antidepressants, and agrochemicals.1,2 Furthermore, benzotriazin-4(3H)-ones are versatile building blocks for metal-catalyzed3 as well as photochemical4 denitrogenative transformations toward related heterocycles3a,3d,4a and for different cross-coupling reactions.3e,3f,4b,4c Acid-mediated denitrogenative ortho-hydroxylation5 and heteroannulation reactions yielding benzo[c][1,2]dithiol-3-ones6 represent further useful applications of this valuable albeit underutilized heterocycle.
The most common approach for preparing benzotriazin-4(3H)-ones exploits the diazotization of 2-aminobenzamide or methyl anthranilate; however, the associated use of strong acids and NaNO2 renders this method problematic and limits its scope (Scheme 1a).1,7 Therefore, alternative routes to these attractive scaffolds have been developed in recent years. Modifications include a mild protocol using a polymer-supported nitrite reagent and p-tosic acid.8 Further variations were presented by Liu and collaborators using nitromethane9 or tert-butylnitrite10 as the nitrogen source and avoid the harsh acidic conditions required for diazonium salt formation reactions.
Scheme 1. Strategies toward Benzotriazin-4(3H)-ones.
In addition, Sankararaman and co-workers reported a Pd-catalyzed annulation reaction converting 1,3-diaryltriazenes into benzotriazin-4(3H)-ones in the presence of CO (Scheme 1b).11 Moreover, an oxidative rearrangement of 3-aminoindazoles was reported by Song and co-workers in 2018 (Scheme 1c).12 Lastly, a redox cyclization of benzamides using nitrous oxide after the treatment with sec-butyl lithium was described by Cui and co-workers (Scheme 1d).13 The precedented methodology for accessing the target heterocycle (Scheme 1a–d) clearly shows a lack of mild and green methods that are readily scalable and user-friendly. To contribute to this field, we wished to develop an efficient route to access benzotriazin-4(3H)-ones with and without substituents in the 3-position (N–H vs N–R). To achieve this, we opted to target a photochemical method in combination with continuous flow processing14 which would render a robust and reliable method toward these important heterocyclic scaffolds.
The photochemical flow setup consisted of a Vapourtec E-series reactor and its UV-150 photomodule equipped with a coil reactor (10 mL volume, PFA tubing) and different LEDs as light sources. One peristaltic pump was used as an adjustable back-pressure regulator (BPR). Substrate 1a (Table 1) was used as model substrate during reaction optimization. This was based on knowledge from our previous study15 that showed that the triazine moiety of aryl benzoic acids is susceptible to photochemical N–N single bond fragmentation in the presence of an external secondary amine and subsequent expulsion of an acetamide leaving group. The starting benzamides were constructed via photochemical rearrangement of nitroarenes15 followed by amide coupling as described in the SI.
Table 1. Optimization toward Benzotriazin-4(3H)-one 2a.
| Entrya | Solvent (mM) | Wavelength | Yield (SM%)b |
|---|---|---|---|
| 1 | MeCN (18) | 365 nm | 41 (57) |
| 2 | AcOEt (35) | 365 nm | 30 (54) |
| 3 | MeOH (38) | 365 nm | 97 |
| 4 | MeCN/H2O (2/1) (38) | 365 nm | 96 |
| 5 | DCM (50) | 365 nm | 84 |
| 6 | Acetone (35) | 365 nm | 0 (99) |
| 7 | DCM/MeOH (1/3) (50) | 365 nm | 95 |
| 8 | DCM/MeOH (1/3) (50) | 420 nm | 96c |
| 9d | DCM/MeOH (1/3) (50) | 420 nm | 68 (28) |
| 10 | DCM/MeOH (1/3) (50) | – | 0 (99) |
Reaction conditions: Unless otherwise specified all the reactions were performed at 0.3 mmol scale in the corresponding solvent (homogeneous solution at 22–25 °C) with a residence time of 10 min (flow rate 1 mLmin-1) using the corresponding high-power LEDs with an input power of 50 W and a system pressure of 3 bar.
qNMR yields were calculated using 1,3,5-trimethoxybenzene as the internal standard.
88% isolated yield (0.91 mmol scale).
5 min residence time (2 mL/min).
Initial reactions used a high-power LED emitting light at 365 nm in combination with a flow rate of 1 mL min–1. After a short optimization, almost quantitative yields of 2a were obtained. The main challenge at this stage was to find a suitable solvent that fully dissolved the benzamide starting material at an appropriate concentration. Two solvent combinations gave almost quantitative yields MeCN/H2O (entry 4) and MeOH/DCM (entry 7). The use of MeOH alone (entry 3) was discarded, as some precipitation appeared at the end of the reactor. Product isolation after silica gel column chromatography gave a yield of 90% in both cases. Importantly, we were able to also use visible light (420 nm, entry 8) instead of UV-A light with full conversion of the substrate observed in only 10 min. N-Methyl acetamide was found in all cases as the only byproduct of the reaction which was removed by extraction and/or chromatography and can be isolated in near-quantitative yield. An isolated yield of 88% was obtained at 200 mg scale (0.91 mmol) for this reaction (entry 8), following an extractive workup instead of chromatography. Finally, the reaction was attempted in the dark, with no conversion observed (entry 10). Equally, thermal reactions (50 and 100 °C, no irradiation) returned starting material quantitatively, thus demonstrating the necessity of violet light to trigger this process.
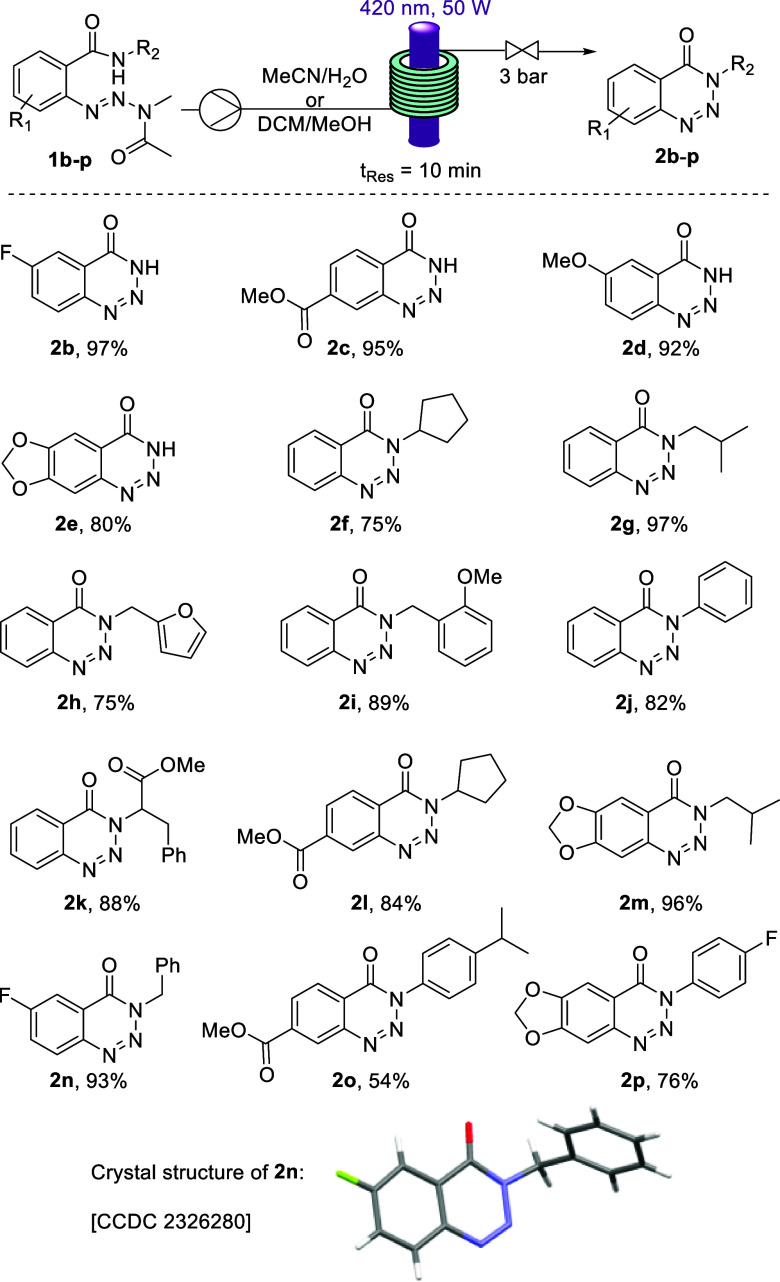
With the optimized conditions in hand, the substrate scope was explored, focusing on both aryl substitution and introduction of different groups at the 3-position. As shown in Scheme 2, various substituents were tolerated on the aryl ring affording the desired products in excellent yields (2b–2e). Secondary amides were also tested as starting materials in combination with different aryl substituents, rendering the corresponding benzotriazin-4(3H)-one products in high yields (75–97%). Notable examples include (cyclo)alkyl groups (2f–g), heterocycles (2h), benzylic systems (2i), and aniline derivatives (2j). Moreover, a secondary amide derived from rac-phenylalanine (2k) was also transformed to the heterocyclic target in an 88% yield. Substrates decorated with substituents in both positions (2l–p) gave excellent results except for 2o with a more moderate yield (54%). Interestingly, all the substrates tested afforded the reaction product, with the only deviation from the optimized conditions being the solvent used to provide full solubility of both starting material and product. The structure of product 2n was confirmed by single crystal X-ray crystallography clearly showing the benzotriazin-4(3H)-one scaffold generated.
Scheme 2. Reaction Scope of the Photochemical Transformation.
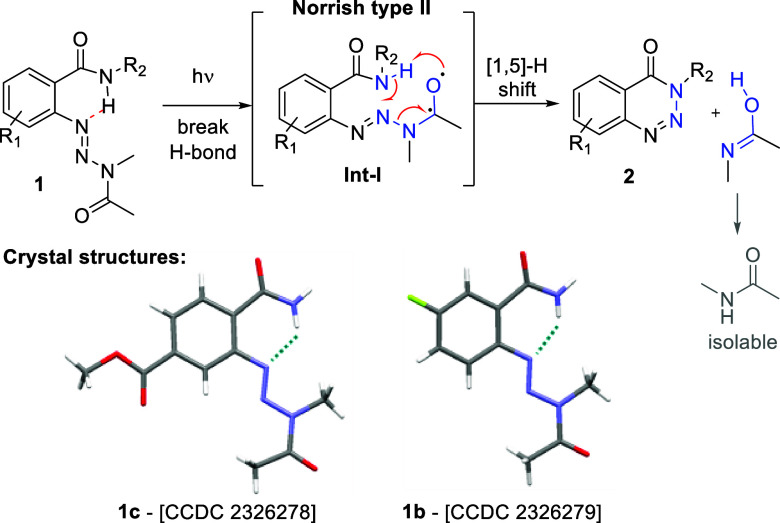
The mechanistic proposal of this intriguing transformation is shown in Scheme 3. X-ray structures secured for selected substrates (i.e., 1b and 1c) indicate a H-bond between the amide N–H and one nitrogen atom of the triazine moiety giving a stable 6-membered ring. Upon exposure to light, it is believed that this H-bond is broken, allowing for a conformational change whereby the carbonyl group can position itself nearby the amide moiety. Photoexcitation of this carbonyl is followed by an ISC from the singlet to the more favorable triplet state. The biradical character of this excited carbonyl then enables the key [1,5]-H shift which is facilitated by the prearranged cyclic transition state in INT-I. This process is reminiscent of the γ-H abstraction in classical Norrish type II reactions and leads to the formation of a new N–N bond as well as the fragmentation of the acetamide moiety, which can be isolated for these reactions. To the best of our knowledge, the presented case is the first of its kind showcasing a Norrish type II process across three contiguous nitrogen atoms.
Scheme 3. Proposed Mechanism for the Photochemical Benzotriazin-4(3H)-one Formation.
Lastly, a series of test reactions were performed to glean some further insight into the reaction (Scheme 4). Substrate 1q, bearing a different acetamide, also afforded benzotriazin-4(3H)-one 2a in almost quantitative yield showing that a bulkier acetamide group is well tolerated. However, when using a triazine substrate lacking the acetamide (i.e., 1r), no reaction was observed, showing that the acetamide moiety with its carbonyl group is crucial. Equally, the N–H group of the amide is crucial, as in the case of tertiary amides the desired Norrish type II process cannot operate. As seen for substrate 1s a new product 3 is observed indicating a more complex outcome as photolysis of the acetamide and subsequent denitrification are accompanied by loss of one ethyl group (see SI for details).
Scheme 4. Further Control Experiments.
An interesting observation was made when testing the feasibility to generate 7-membered ring products using biphenyl substrate 1t. Upon irradiation one major product was isolated which matched structure 4 based on comparison with literature data.16 This indicates that aromatic 6-membered ring products are favored in this case. Clearly, more complex mechanistic pathways are involved in the formation of products 3 and 4 that extend beyond current knowledge in relation to the reactivity of aryl triazines.17 Finally, a competition experiment between the intermolecular triazine formation and the intramolecular reaction described in our previous report was tested; however, in all scenarios tested, the only product observed was benzotriazin-4(3H)-one 2a.
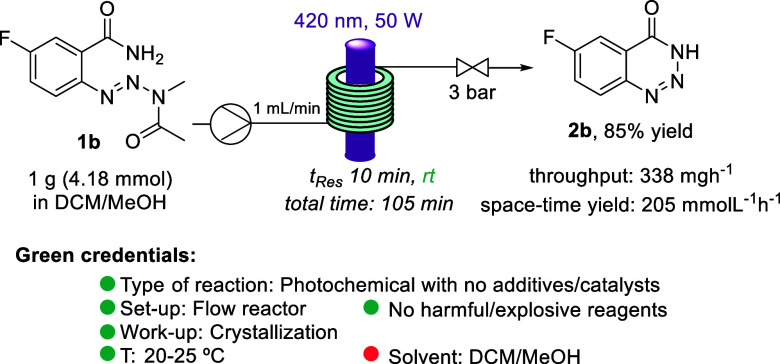
Finally, to demonstrate the scalability and robustness of the flow method, 1 g (4.18 mmol) of substrate 1b was successfully processed (Scheme 5). Although the isolated yield was slightly lower than in the case of the small-scale reaction (85%), productivity and throughput were very high. Moreover, the green credentials of this flow process18 make it a more appealing option compared to reported alternatives. Thus, our protocol shows clear benefits as (1) no additives/catalysts are required; (2) the reaction is fast, uses visible light, and operates at ambient temperature; (3) a miniaturized flow reactor is used that provides for safety and scalability; and (4) the product isolation by simple crystallization avoids chromatography. The only red flag is the use of DCM as cosolvent; however, this can be replaced by a mixture of acetonitrile/water albeit at lower concentration. In contrast to this, previously reported protocols7−11 for generating benzotriazin-4(3H)-ones use potentially explosive diazonium salts, toxic reagents, harsh conditions, and/or large amounts of additives.
Scheme 5. Reaction Scale-up and Green Credentials.
In conclusion, we have developed a new method for the synthesis of benzotriazin-4(3H)-ones, which is based on the photochemical cyclization of amide bearing aryl triazines. The reaction uses violet light (420 nm) and uniquely operates via a nitrogen-centered [1,5]-H shift which is related to more classical Norrish type II reactions19 but unprecedented in this context. Continuous flow processing provides for scalability and process robustness, and the green credentials of this transformation show clear advantages compared to alternative routes toward these underexplored heterocyclic targets. This reaction is characterized by a wide substrate scope affording the desired benzotriazin-4(3H)-one products in high chemical yields with N-methylacetamide being the sole byproduct.
Acknowledgments
J.G.-L. acknowledges the Fundación Ramón Areces for his postdoctoral fellowship (BEVP33P01S12222). The authors thank the School of Chemistry at University College Dublin (UCD) for generous support, as well as Science Foundation Ireland for supporting our research program through grants 12/RC2275_P2, and 20/FFP-P/8712. We are grateful to Dr. Andrew D. Phillips (UCD) for determining the X-ray structures reported in this work.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.4c00248.
The authors declare no competing financial interest.
Supplementary Material
References
- Ali Munawar M.; Khalid Z.; Adnan Ahmad H.; Khan M.-u.-A.; Gul S. 1, 2, 3-Benzotriazin-4 (3H)-ones: Synthesis, reactions and applications. Heterocycles 2017, 94, 3–54. 10.3987/REV-16-846. [DOI] [Google Scholar]
- For selected examples, see:; a Caliendo G.; Fiorino F.; Grieco P.; Perissutti E.; Santagada V.; Meli R.; Raso G. M.; Zanesco A.; De Nucci G. Preparation and Local Anaesthetic Activity of Benzotriazinone and Benzoyltriazole Derivatives. Eur. J. Med. Chem. 1999, 34, 1043–1051. 10.1016/S0223-5234(99)00126-9. [DOI] [Google Scholar]; b Wang G.; Chen X.; Deng Y.; Li Z.; Xu X. Synthesis and Nematicidal Activities of 1,2,3-Benzotriazin-4-One Derivatives against Meloidogyne Incognita. J. Agric. Food Chem. 2015, 63, 6883–6889. 10.1021/acs.jafc.5b01762. [DOI] [PubMed] [Google Scholar]; c Zhang F.; Wu D.; Wang G.-L.; Hou S.; Ou-Yang P.; Huang J.; Xu X.-Y. Synthesis and Biological Evaluation of Novel 1,2,3-Benzotriazin-4-One Derivatives as Leukotriene A4 Hydrolase Aminopeptidase Inhibitors. Chin. Chem. Lett. 2017, 28, 1044–1048. 10.1016/j.cclet.2016.12.014. [DOI] [Google Scholar]
- a Miura T.; Yamauchi M.; Murakami M. Synthesis of 1(2H)-Isoquinolones by the Nickel-Catalyzed Denitrogenative Alkyne Insertion of 1,2,3-Benzotriazin-4(3H)-Ones. Org. Lett. 2008, 10, 3085–3088. 10.1021/ol8010826. [DOI] [PubMed] [Google Scholar]; b Yamauchi M.; Morimoto M.; Miura T.; Murakami M. Enantioselective Synthesis of 3,4-Dihydroisoquinolin-1(2H)-Ones by Nickel-Catalyzed Denitrogenative Annulation of 1,2,3-Benzotriazin-4(3H)-Ones with Allenes. J. Am. Chem. Soc. 2010, 132, 54–55. 10.1021/ja909603j. [DOI] [PubMed] [Google Scholar]; c Fang Z.-J.; Zheng S.-C.; Guo Z.; Guo J.-Y.; Tan B.; Liu X.-Y. Asymmetric Synthesis of Axially Chiral Isoquinolones: Nickel-Catalyzed Denitrogenative Transannulation. Angew. Chem., Int. Ed. 2015, 54, 9528–9532. 10.1002/anie.201503207. [DOI] [PubMed] [Google Scholar]; d Thorat V. H.; Upadhyay N. S.; Murakami M.; Cheng C.-H. Nickel-Catalyzed Denitrogenative Annulation of 1,2,3-Benzotriazin-4-(3H)-Ones with Benzynes for Construction of Phenanthridinone Scaffolds. Adv. Synth. Catal. 2018, 360, 284–289. 10.1002/adsc.201701143. [DOI] [Google Scholar]; e Hari Balakrishnan M.; Sathriyan K.; Mannathan S. Nickel-Catalyzed Denitrogenative Cross-Coupling Reaction of 1,2,3-Benzotriazin-4(3H)-Ones with Organoboronic Acids: An Easy Access to Ortho-Arylated and Alkenylated Benzamides. Org. Lett. 2018, 20, 3815–3818. 10.1021/acs.orglett.8b01401. [DOI] [PubMed] [Google Scholar]; f Li S.; Li Y.; Zhu R.; Bai J.; Shen Y.; Pan M.; Li W. Synthesis of Ortho-Methylated Benzamides via Palladium-Catalyzed Denitrogenative Cross-Coupling Reaction of [1,2,3]-Benzotriazin-4(3H)-Ones with DABAL-Me3. Org. Lett. 2023, 25, 5443–5447. 10.1021/acs.orglett.3c01745. [DOI] [PubMed] [Google Scholar]
- a Wang H.; Yu S. Synthesis of Isoquinolones Using Visible-Light-Promoted Denitrogenative Alkyne Insertion of 1,2,3-Benzotriazinones. Org. Lett. 2015, 17, 4272–4275. 10.1021/acs.orglett.5b01960. [DOI] [PubMed] [Google Scholar]; b Chen F.; Hu S.; Li S.; Tang G.; Zhao Y. Visible-Light-Induced Denitrogenative Phosphorylation of Benzotriazinones: A Metal- and Additive-Free Method for Accessing Ortho-Phosphorylated Benzamide Derivatives. Green Chem. 2021, 23, 296–301. 10.1039/D0GC03613G. [DOI] [Google Scholar]; c Liu B.-X.; Wang F.; Chen Y.; Rao W.-D.; Shen S.-S.; Wang S.-Y. Visible-Light-Promoted Denitrogenative Ortho-Selenylation Reaction of Benzotriazinones: Synthesis of Ortho-Selenylated Benzamides and Ebselen Analogs. Org. Chem. Front. 2022, 9, 2418–2423. 10.1039/D2QO00121G. [DOI] [Google Scholar]
- Madasamy K.; Balakrishnan M. H.; Korivi R.; Mannathan S. Trifluoroacetic Acid-Mediated Denitrogenative Ortho-Hydroxylation of 1,2,3-Benzotriazin-4(3H)-Ones: A Metal-Free Approach. J. Org. Chem. 2022, 87, 8752–8756. 10.1021/acs.joc.2c00354. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Zhang B.; Dong J.; Li J.; Yang S.; Ye L. Assembly of Benzo[c][1,2]Dithiol-3-Ones via Acid-Promoted Denitrogenative Transannulation of Benzotriazinones. Org. Lett. 2022, 24, 9012–9016. 10.1021/acs.orglett.2c03638. [DOI] [PubMed] [Google Scholar]
- a Van Heyningen E. J. 1,2,3-Benzotriazines. J. Am. Chem. Soc. 1955, 77, 6562–6565. 10.1021/ja01629a042. [DOI] [Google Scholar]; b Barker A. J.; Paterson T. McC.; Smalley R. K.; Suschitzky H. 1,2,3-Benzotriazin-4(3H)-Ones and Related Systems. Part 5. Thermolysis of 3-Aryl- and 3-Alkenyl-1,2,3-Benzotriazin-4(3H)-Ones. J. Chem. Soc., Perkin Trans. 1 1979, 2203–2208. 10.1039/p19790002203. [DOI] [Google Scholar]; c Clark A. S.; Deans B.; Stevens M. F. G.; Tisdale M. J.; Wheelhouse R. T.; Denny B. J.; Hartley J. A. Antitumor Imidazotetrazines. 32.1 Synthesis of Novel Imidazotetrazinones and Related Bicyclic Heterocycles to Probe the Mode of Action of the Antitumor Drug Temozolomide. J. Med. Chem. 1995, 38, 1493–1504. 10.1021/jm00009a010. [DOI] [PubMed] [Google Scholar]
- McGrory R.; Faggyas R. J.; Sutherland A. One-Pot Synthesis of N-Substituted Benzannulated Triazoles via Stable Arene Diazonium Salts. Org. Biomol. Chem. 2021, 19, 6127–6140. 10.1039/D1OB00968K. [DOI] [PubMed] [Google Scholar]
- Yan Y.; Niu B.; Xu K.; Yu J.; Zhi H.; Liu Y. Potassium Iodide/Tert-Butyl Hydroperoxide-Mediated Oxidative Annulation for the Selective Synthesis of N-Substituted 1,2,3-Benzotriazine-4(3H)-Ones Using Nitromethane as the Nitrogen Synthon. Adv. Synth. Catal. 2016, 358, 212–217. 10.1002/adsc.201500619. [DOI] [Google Scholar]
- a Yan Y.; Li H.; Niu B.; Zhu C.; Chen T.; Liu Y. Mild and Efficient TBAI-Catalyzed Synthesis of 1,2,3-Benzotriazine-4-(3H)-Ones from Tert-Butyl Nitrite and 2-Aminobenzamides under Acid-Free Conditions. Tetrahedron Lett. 2016, 57, 4170–4173. 10.1016/j.tetlet.2016.07.102. [DOI] [Google Scholar]; b Cai Y.-M.; Zhang X.; An C.; Yang Y.-F.; Liu W.; Gao W.-X.; Huang X.-B.; Zhou Y.-B.; Liu M.-C.; Wu H.-Y. Catalyst-Free Oxidative N-N Coupling for the Synthesis of 1,2,3-Triazole Compounds with tBuONO. Org. Chem. Front. 2019, 6, 1481–1484. 10.1039/C9QO00071B. [DOI] [Google Scholar]
- Chandrasekhar A.; Sankararaman S. Selective Synthesis of 3-Arylbenzo-1,2,3-Triazin-4(3H)-Ones and 1-Aryl-(1H)-Benzo-1,2,3-Triazoles from 1,3-Diaryltriazenes through Pd(0) Catalyzed Annulation Reactions. J. Org. Chem. 2017, 82, 11487–11493. 10.1021/acs.joc.7b02023. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Wang Y.; Lou Y.; Song Q. Oxidative Rearrangement of 3-Aminoindazoles for the Construction of 1,2,3-Benzotriazine-4(3H)-Ones at Ambient Temperature. Org. Lett. 2018, 20, 6494–6497. 10.1021/acs.orglett.8b02813. [DOI] [PubMed] [Google Scholar]
- Lai Z.; Wang C.; Li J.; Cui S. Redox Cyclization of Amides and Sulfonamides with Nitrous Oxide for Direct Synthesis of Heterocycles. Org. Lett. 2020, 22, 2017–2021. 10.1021/acs.orglett.0c00397. [DOI] [PubMed] [Google Scholar]
- For recent flow photochemistry reviews, see:; a Plutschack M. B.; Pieber B.; Gilmore K.; Seeberger P. H. The Hitchhiker’s Guide to Flow Chemistry. Chem. Rev. 2017, 117, 11796–11893. 10.1021/acs.chemrev.7b00183. [DOI] [PubMed] [Google Scholar]; b Donnelly K.; Baumann M. Scalability of Photochemical Reactions in Continuous Flow Mode. J. Flow Chem. 2021, 11, 223–241. 10.1007/s41981-021-00168-z. [DOI] [Google Scholar]; c Buglioni L.; Raymenants F.; Slattery A.; Zondag S. D. A.; Noël T. Technological Innovations in Photochemistry for Organic Synthesis: Flow Chemistry, High-Throughput Experimentation, Scale-up, and Photoelectrochemistry. Chem. Rev. 2022, 122, 2752–2906. 10.1021/acs.chemrev.1c00332. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Xiouras C.; Kuijpers K.; Fanfair D.; Dorbec M.; Gielen B. Enabling Technologies for Process Intensification in Pharmaceutical Research and Manufacturing. Curr. Opin. Chem. Eng. 2023, 41, 100920. 10.1016/j.coche.2023.100920. [DOI] [Google Scholar]
- García-Lacuna J.; Baumann M. Modular Photochemical Flow Synthesis of Structurally Diverse Benzyne and Triazine Precursors. Adv. Synth. Catal. 2023, 365, 2628–2635. 10.1002/adsc.202300414. [DOI] [Google Scholar]
- Bjørsvik H.-R.; González R. R.; Liguori L. Investigations of a Novel Process to the Framework of Benzo[c]cinnoline. J. Org. Chem. 2004, 69, 7720–7727. 10.1021/jo049102o. [DOI] [PubMed] [Google Scholar]
- Liu T.; Wu H.; Zhang Q.; Wang C. Recent Advances in the Chemistry of Aryltriazene. Org. Biomol. Chem. 2023, 21, 2059–2068. 10.1039/D2OB02267B. [DOI] [PubMed] [Google Scholar]
- a McElroy C. R.; Constantinou A.; Jones L. C.; Summerton L.; Clark J. H. Towards a Holistic Approach to Metrics for the 21st Century Pharmaceutical Industry. Green Chem. 2015, 17, 3111–3121. 10.1039/C5GC00340G. [DOI] [Google Scholar]; b Dallinger D.; Kappe C. O. Why Flow Means Green - Evaluating the Merits of Continuous Processing in the Context of Sustainability. Curr. Op. Green Sust. Chem. 2017, 7, 6–12. 10.1016/j.cogsc.2017.06.003. [DOI] [Google Scholar]; c Baumann M.; Moody T. S.; Smyth M.; Wharry S. Evaluating the Green Credentials of Flow Chemistry towards Industrial Applications. Synthesis 2021, 53, 3963–3976. 10.1055/a-1541-1761. [DOI] [Google Scholar]
- Dantas J. A.; Correia J. T. M.; Paixão M. W.; Corrêa A. G. Photochemistry of Carbonyl Compounds: Application in Metal-Free Reactions. ChemPhotoChem. 2019, 3, 506–520. 10.1002/cptc.201900044. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.