Abstract
The immediate-early gene ie14/vsag of herpesvirus saimiri has homology with murine superantigens. We compared the pathogenesis of infection with either ie14/vsag deletion mutants or wild-type virus C488 in cottontop tamarin monkeys (Saguinus oedipus). Two weeks after infection, all animals developed acute T-cell lymphomas independently of the presence of the viral ie14/vsag gene.
Herpesvirus saimiri is a DNA tumor virus of New World primates and harbors a series of genes with homology to cellular counterparts. Among them is the immediate-early gene ie14/vsag, whose product resembles cellular and retroviral superantigens in mice (1, 12). As previously shown, the gene product IE14/vSag is capable of binding to human major histocompatibility complex class II molecules and of stimulating the proliferation of primary human T cells, similar to superantigens (14). However, selectivity for human Vβ chains has not been observed (11, 13, 14).
Human T cells are transformed to stable growth by herpesvirus saimiri C488 (2). The transformed human cells carry the viral genome as nonintegrated episomes without virion production and express only a few viral genes, which are strongly induced after T-cell stimulation: the transformation-associated genes stpC and tip and ie14/vsag (7, 8, 11). Although classified as a viral immediate-early gene, ie14/vsag was shown not to be required for virus replication (11, 12). Moreover, the deletion of ie14/vsag from virus strain C488 did not affect its capacity to transform simian or human T cells to stable growth in culture (11). To determine if this gene was essential for T-cell lymphoma development in vivo, we compared the pathogenesis in cottontop tamarins infected with wild-type virus carrying an intact ie14/vsag gene with that in cottontop tamarins infected with mutant viruses having this gene deleted.
The ie14/vsag deletion mutants of strain C488, 14-3.11 and 14-4.6, which lack most of the ie14/vsag coding sequence, were generated in independent experiments in order to minimize any bias from spontaneous mutations elsewhere in the herpesvirus genome (3, 5, 11). After approval by the Institutional Animal Care and Use Committee (Biomedical Research Centre, Rijswijk, The Netherlands) wild-type C488 and mutants 14-3.11 and 14-4.6 (107 PFU in 1 ml of cell-free culture supernatant in Dulbecco modified Eagle medium) were individually injected into two naive, purpose-bred Saguinus oedipus monkeys. An intravenous infection at a high dose was done in order to exclude artifacts due to limiting conditions of infection. The animals were mature (400 to 500 g) and in good physical health. Animals R207 and R217 received wild-type virus, B222 and B240 got mutant 14-3.11, and B225 and R222 got mutant 14-4.6. On day 15 or 16 the animals were euthanatized when illness was evident. Autopsy was performed, followed by histopathological examination. Blood samples (1.5 ml each) were taken prior to infection, at weekly intervals, and before euthanasia. Virus isolation experiments were performed on all blood samples obtained after infection. Cells from peripheral blood and from autopsy samples were cultured without interleukin-2 in a mixture of half RPMI 1640 and half CG medium (Vitromex, Selters, Germany) and supplemented with fetal bovine serum (10%), glutamine, and gentamicin (6). Stably growing cells were analyzed by genomic PCR for ie14/vsag and for the neighboring gene orf13/il17 as a positive control (11). Standard flow cytometry analysis was performed with the cell lines and with fresh peripheral blood mononuclear cells (PBMC). For this purpose, the following monoclonal antibodies, which are directed against human epitopes and cross-react with S. oedipus cells, were used: αCD2 (αLeu5b, S5.2; Becton-Dickinson, Heidelberg, Germany), αCD3 (LT3, kindly provided by A. Filatov, Moscow, Russia), αCD4 (αLeu-3a, SK3; Becton-Dickinson or MT301; Dako, Hamburg, Germany), αCD8 (MT1014, kindly provided by E. Rieber, Dresden, Germany), αCD14 (αMY4, 332A-1; Coulter, Krefeld, Germany), αCD20 (αLeu16, L27; Becton-Dickinson), αCD25 (2A3; Becton-Dickinson), αCD28 (αLeu28, L293; Becton-Dickinson), αCD29 (K20; Dako), αCD38 (αLeu17, HB-7; Becton-Dickinson), and αHLA-DR (L243; Becton-Dickinson).
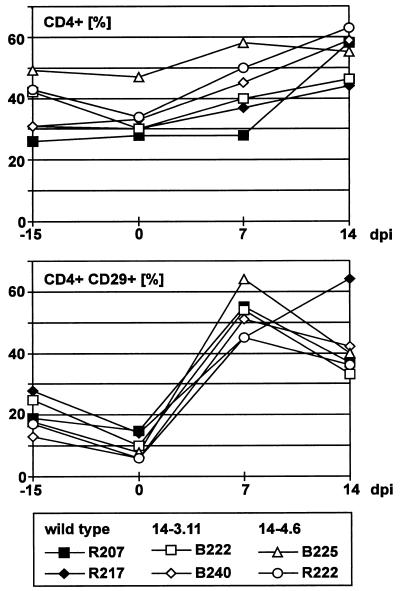
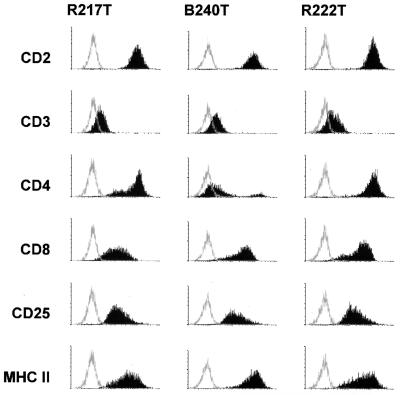
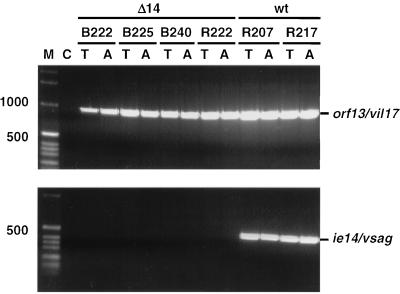
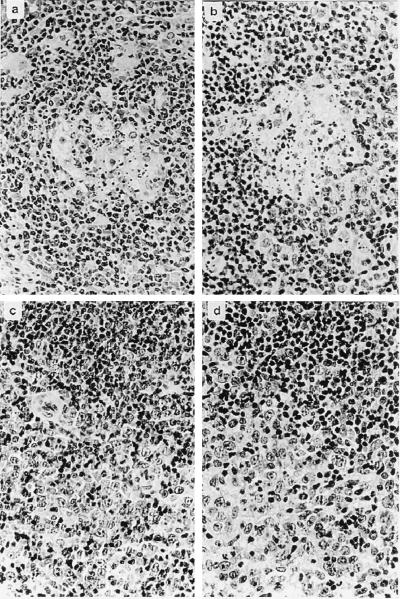
All six animals developed evidence of disease rapidly and almost simultaneously at day 15 or 16 after infection, when they became apathetic and inappetent. In addition, animals R222, B222, B225, and R207 developed severe diarrhea. At necropsy, extranodal solid tumors were not apparent. However, severely enlarged mesenteric lymph nodes were observed in animals B222, B240, R207, R217, and R222. In the same animals, the kidneys had an irregular red-and-white-speckled appearance, suggesting lymphomatous infiltration of renal tissue. The adrenals of animals B222, B240, and R217 were hyperemic and hemorrhagic. Evidence for enteropathy was detected at the necropsies of animals B222, B240, R207, R217, and R222. Fresh PBMC were analyzed by whole-blood flow cytometry. CD4+-cell counts, in particular the relative number of memory-type CD4+ CD29+ cells, increased moderately after virus infection (Fig. 1). Neither double-staining reactions with antibody pairs directed to CD14/CD4, CD20/HLA-DR, CD2/HLA-DR, CD2/CD28, and CD2/CD38 nor the absolute numbers of lymphocytes, T cells (CD2+), and B cells (CD20+) revealed further significant changes after infection. The absolute numbers of granulocytes and monocytes decreased during the course of infection in most animals; however, individual variation was large. Peripheral cells of each blood sample and cells from various organs (thymus, spleen, liver, and kidney and axillar, mesenteric, and inguinal lymph nodes) were cultured in order to expand the lymphoma cells and to isolate the virus. At day 7 after infection, most PBMC samples yielded continuously proliferating T-cell lines, whereas virus isolations remained negative. Two weeks after infection, herpesvirus saimiri was recovered from all animals by cocultivation of PBMC with owl monkey kidney cells (6). Stably growing T-cell cultures were regularly obtained from PBMC (day 14 and at death) and from the thymus, spleen, and lymph nodes at autopsy. These cell lines expressed surface markers which are typically found on activated T cells (Fig. 2). All T cells expressed CD8. The percentage of CD8+ cells coexpressing CD4 varied from 10 to 100%, depending on the cell line but was independent of the virus genotype used for infection. The cell lines obtained from the thymus and axillar lymph nodes of each animal were tested by genomic PCR for the presence of viral genomes and for the maintenance of the respective genotype (wild type or deletion mutant). Whereas cells from wild type-infected animals were positive both for orf13/vil17 and ie14/vsag, those from deletion mutant-infected monkeys contained orf13/vil17 but not ie14/vsag (Fig. 3). Histopathological evaluation confirmed the diagnosis of peripheral pleomorphic T-cell lymphoma with follicular lysis and infiltration of multiple organs, including the kidney and the liver. No consistent difference was detected between animals which had been infected with mutant virus and those infected with wild-type virus C488 (Fig. 4).
FIG. 1.
Flow cytometry values from fresh blood. The relative CD4+-cell counts increased moderately in infected animals. This increase was most evident for the relative numbers of memory-type CD4+ CD29+ cells. For this analysis, fresh PBMC were costained with monoclonal antibodies directed to CD4 and CD29. dpi, days postinfection.
FIG. 2.
Surface phenotype of tumor cell lines. The surface phenotype is shown for the thymus derived T-cell lines R217T (wild type-infected), B240T (deletion mutant 14-3.11), and R222T (deletion mutant 14-4.6). The histograms show fluorescence intensity in logarithmic scale on the x axis and cell numbers in linear scale on the y axis. MHC, major histocompatibility complex.
FIG. 3.
Presence of viral DNA in tumor cell lines from the thymus and the axillar lymph nodes. Virus DNA was demonstrated in ex vivo T-cell lines from all animals. Whereas the wild-type (wt) control animals showed evidence for the presence of both virus genes analyzed, the deletion was confirmed in the animals which had received mutant viruses without ie14/vsag (Δ14). C, negative control; T, cell line from the thymus; A, cell line derived from the axillar lymph node. The sizes of marker-DNA fragments are given on the left.
FIG. 4.
T-cell lymphoma in S. oedipus. Formalin-fixed tissue sections were stained with hematoxylin and eosin. (a and b) Germinal centers with follicular lysis. (c and d) Infiltration by a characteristic heterogeneous population of neoplastic lymphoid cells from T-cell areas with compression and loss of residual follicular B-cell areas. The photographs shown in panels a and c were obtained from C488 wild type-infected animals, and the photographs shown in panels b and d are from animals infected with ie14/vsag deletion mutant viruses. Original magnification, ×40.
Although experimental T-cell leukemia and lymphoma in various New World primate species have been described for various strains of herpesvirus saimiri (reviewed in reference 9), the pathogenicity of virus strain C488 has not yet been assessed (3, 5). In particular, the course of disease for this virus strain in cottontop tamarins (S. oedipus) has not been described. In this study, we observed that wild-type C488 as well as mutants with ie14/vsag deleted causes a fulminant lethal disease due to uncontrolled T-cell proliferation after a single intravenous infection. The acute onset of this disease argues for a polyclonal transformation event. This conclusion is compatible with the diagnosis of infectious peripheral pleomorphic T-cell lymphoma, as confirmed by surface staining of tumor cell lines. The cells carry markers typical for mature, activated T cells. Although tumor cells can be recovered from the peripheral blood, the cell-type distribution in PBMC seems rather normal. Besides the transformation-associated genes stpC and tip (4, 10), ie14/vsag was one of the leading candidates to contribute to transformation and pathogenesis (1, 12). However, as demonstrated in this study, deletion of this gene did not alter the course of disease, which emphasizes the assumed relevance of stpC/tip for T-cell leukemogenesis by herpesvirus saimiri. We conclude that ie14/vsag is dispensable for lytic virus replication, in vitro transformation, and pathogenicity if nonlimiting infection conditions are applied. It remains to be seen whether ie14/vsag plays a role in perinatal infection or apathogenic persistence in squirrel monkeys (Saimiri sciureus). A similar constellation seems relevant for early transmission of mouse mammary tumor virus. In this context, a Vβ-specific function of the superantigen homolog IE14/vSag in the natural host remains to be elucidated.
Acknowledgments
We are grateful to A. Filatov (Moscow, Russia) and P. Rieber (Dresden, Germany) for kindly providing monoclonal antibodies, to P. van Eerd and P. Frost (Rijswijk, the Netherlands) for veterinary care, and to B. Biesinger (Erlangen, Germany), B. Bröker (Hamburg, Germany), E. Meinl (Erlangen, Germany), and M. Spriggs (Seattle, Wash.) for valuable suggestions.
This study was supported by the Bayerische Forschungsstiftung (Munich, Germany) and the Wilhelm Sander-Stiftung (Neustadt, Germany).
REFERENCES
- 1.Albrecht J, Nicholas J, Biller D, Cameron K, Biesinger B, Newman C, Wittmann S, Craxton M, Coleman H, Fleckenstein B, Honess R. Primary structure of the herpesvirus saimiri genome. J Virol. 1992;66:5047–5058. doi: 10.1128/jvi.66.8.5047-5058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biesinger B, Müller-Fleckenstein I, Simmer B, Lang G, Wittmann S, Platzer E, Desrosiers R, Fleckenstein B. Stable growth transformation of human T-lymphocytes by herpesvirus saimiri. Proc Natl Acad Sci USA. 1992;89:3116–3119. doi: 10.1073/pnas.89.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biesinger B, Trimble J, Desrosiers R, Fleckenstein B. The divergence between two oncogenic herpesvirus saimiri strains in a genomic region related to the transforming phenotype. Virology. 1990;176:505–514. doi: 10.1016/0042-6822(90)90020-r. [DOI] [PubMed] [Google Scholar]
- 4.Biesinger B, Tsygankov A, Fickenscher H, Emmrich F, Fleckenstein B, Bolen J, Bröker B. The product of the herpesvirus saimiri open reading frame 1 (tip) interacts with T cell-specific kinase p56lck in transformed cells. J Biol Chem. 1995;270:4729–4734. doi: 10.1074/jbc.270.9.4729. [DOI] [PubMed] [Google Scholar]
- 5.Desrosiers R, Falk L. Herpesvirus saimiri strain variability. J Virol. 1982;43:352–356. doi: 10.1128/jvi.43.1.352-356.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fickenscher H, Fleckenstein B. Growth-transformation of human T cells. Methods Microbiol. 1998;25:573–602. [Google Scholar]
- 7.Fickenscher H, Biesinger B, Knappe A, Wittmann S, Fleckenstein B. Regulation of the herpesvirus saimiri oncogene stpC, similar to that of T-cell activation genes, in growth-transformed human T lymphocytes. J Virol. 1996;70:6012–6019. doi: 10.1128/jvi.70.9.6012-6019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fickenscher H, Bökel C, Knappe A, Biesinger B, Meinl E, Fleischer B, Fleckenstein B, Bröker B M. Functional phenotype of transformed human αβ and γδ T cells determined by different subgroup C strains of herpesvirus saimiri. J Virol. 1997;71:2252–2263. doi: 10.1128/jvi.71.3.2252-2263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleckenstein B, Desrosiers R. Herpesvirus saimiri and herpesvirus ateles. In: Roizman B, editor. The herpesviruses. Vol. 1. N.Y: Plenum Press; 1982. pp. 253–331. [Google Scholar]
- 10.Jung J U, Desrosiers R C. Association of the viral oncoprotein STP-C488 with cellular ras. Mol Cell Biol. 1995;15:6506–6512. doi: 10.1128/mcb.15.12.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knappe A, Hiller C, Thurau M, Wittmann S, Hofmann H, Fleckenstein B, Fickenscher H. The superantigen-homologous viral immediate-early gene ie14/vsag in herpesvirus saimiri-transformed human T cells. J Virol. 1997;71:9124–9133. doi: 10.1128/jvi.71.12.9124-9133.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicholas J, Smith E, Coles L, Honess R. Gene expression in cells infected with gammaherpesvirus saimiri: properties of transcripts from two immediate-early genes. Virology. 1990;179:189–200. doi: 10.1016/0042-6822(90)90288-3. [DOI] [PubMed] [Google Scholar]
- 13.Spriggs, M. Unpublished results.
- 14.Yao Z, Maraskovsky E, Spriggs M, Cohen J, Armitage R, Alderson M. Herpesvirus saimiri open reading frame 14, a protein encoded by a T lymphotropic herpesvirus, binds to MHC class II molecules and stimulates T cell proliferation. J Immunol. 1996;156:3260–3266. [PubMed] [Google Scholar]