Abstract
Human cytomegalovirus (HCMV) infection of an astrocytoma cell line (U373) or human fibroblast (HF) cells results in a differential cell distribution of the major envelope glycoprotein gB (UL55). This 906-amino-acid type I glycoprotein contains an extracellular domain with a signal sequence, a transmembrane domain, and a 135-amino-acid cytoplasmic tail with a consensus casein kinase II (CKII) site located at Ser900. Since phosphorylation of proteins in the secretory pathway is an important determinant of intracellular trafficking, the state of gB phosphorylation in U373 and HF cells was examined. Analysis of cells expressing wild-type gB and gB with site-specific mutations indicated that the glycoprotein was equally phosphorylated at a single site, Ser900, in both U373 and HF cells. To assess the effect of charge on gB surface expression in U373 cells, Ser900 was replaced with an aspartate (Asp) or alanine (Ala) residue to mimic the phosphorylated and nonphosphorylated states, respectively. Expression of the Asp but not the Ala gB mutation resulted in an increase in the steady-state expression of gB at the plasma membrane (PM) in U373 cells. In addition, treatment of U373 cells with the phosphatase inhibitor tautomycin resulted in the accumulation of gB at the PM. Interestingly, the addition of a charge at Ser900 trapped gB in a low-level cycling pathway at the PM, preventing trafficking of the protein to the trans-Golgi network or other intracellular compartments. Therefore, these results suggest that a tautomycin-sensitive phosphatase regulates cell-specific PM retrieval of gB to intracellular compartments.
Viral glycoproteins mediate a number of essential functions in the virus life cycle, including entry into the host cell, assembly of viral progeny, and release of infectious virus (24). Glycoprotein maturation occurs through posttranslational modification during sequential transport through the cellular secretory pathway. Viral proteins use cellular trafficking pathways to concentrate at a subcellular location at which capsid envelopment is thought to occur. The mechanisms involved in the localization of glycoproteins to sites of viral assembly are poorly understood.
Human cytomegalovirus (HCMV), a member of the herpesvirus family, demonstrates cell specificity for virus assembly and release (22). The mechanisms for virus assembly and egress are still unclear, although attachment of membrane-bound viral glycoproteins to tegumented capsid is believed to play an important role in this process. The most abundant glycoprotein detected in the HCMV virion envelope is gB (UL55) (6). HCMV gB is synthesized as a 105-kDa polypeptide and processed into a highly glycosylated 130-kDa precursor glycoprotein. After glycosylation, the gB precursor is cleaved by furin to produce a heterodimer protein (gp55 and gp116) (52). gB is a type I glycoprotein containing a signal sequence, an extracellular or luminal domain, a transmembrane (TM) domain, and a 135-amino-acid cytoplasmic tail (5, 20, 38). The cytoplasmic tail contains a consensus casein kinase II (CKII) site, which is phosphorylated both in vitro and in vivo (1, 37, 55).
HCMV infects several different cell types in patients, including monocytes, fibroblasts, endothelial cells, epithelial cells, and stromal cells (8, 9, 11, 13, 18, 19, 23, 28, 32–34, 40, 41, 45, 47–51, 54, 56). However, the vast majority of studies on HCMV replication in vitro have used human fibroblasts (HF). Examination of viral replication in other cell types, such as monocyte-derived macrophages (MDM) and endothelial cells, revealed significant differences in the kinetics of viral replication, viral cytopathic effect, and release of virus from the cell (16, 17, 25). Interestingly, unlike infected HF, in which virus is readily recovered from supernatants, HCMV infection of MDM resulted exclusively in the accumulation of intracellular but not extracellular infectious virus, which was sequestered in numerous intracellular vacuoles whose membranes contained gB. In addition, comparison of HF and MDM by confocal microscopy revealed the presence of gB at the plasma membrane (PM) of HF but not MDM. These observations suggest cell-specific pathways for gB intracellular trafficking.
The surface expression of viral glycoproteins is affected by their steady-state expression, transport to the PM, and rates of internalization from the cell surface. Deletion and point mutational analysis of the C-terminal domain of cell surface receptors and viral glycoproteins has revealed sequence motifs, which are used by adapter molecules to sort the proteins to coated pits, where they become internalized. Internalization signals have been identified for several cellular proteins, including furin, low-density lipoprotein receptor, transferrin receptor, polymeric immunoglobulin (Ig) receptor, and epidermal growth factor receptor. Comparison of the sequences of these proteins indicates that a common structure, rather than sequence, is necessary for internalization. Recent studies on viral glycoproteins have uncovered how viruses have evolved to take advantage of this regulated endocytosis pathway (42, 60).
Recent studies with furin have demonstrated that the state of glycoprotein phosphorylation can affect the steady-state expression of a protein at the PM. Since HCMV gB displayed cell-specific PM expression, we examined the effect of gB phosphorylation on gB trafficking in different cell types. Our results indicate that gB displays a cell-specific steady-state expression of protein at the cell surface, which is regulated by a tautomycin-sensitive phosphatase. In addition, the presence of a charged residue at the phosphorylation site, which mimics the phosphorylation state, results in gB vacuoles that remain near the PM. These data suggest that the cell-specific differences in surface expression of gB are due to altered states of gB phosphorylation, which appears to be mediated by a tautomycin-sensitive phosphatase.
MATERIALS AND METHODS
Isolation and culture of MDM.
MDM cultures were obtained by stimulation of fresh peripheral blood mononuclear cells from the blood of HCMV-seronegative donors with concanavalin A and cultured as previously described (15, 25).
HCMV infection of HF, U373 cells, and MDM.
A recent isolate of HCMV (Po) or the laboratory strain AD169 was used to infect HF, U373 cells, and primary cultures of MDM. The clinical viral isolate Po was isolated from a transplant patient with HCMV disease, passaged through HF, and stored at low passage number at −70°C (25). Frozen samples from this stock were thawed and passaged three additional times through HF before being used to infect MDM. Cell-free supernatants from HCMV-infected HF were used to infect the different cell cultures as previously described (15).
Expression of gB by VV infection in HF and U373 cells.
Vaccinia virus (VV) WR was used in these studies. Recombinant VV (RVV) were constructed by a modification of a previously described method (4, 57). The point mutants gBAla and gBAsp were constructed by PCR with the amino-terminal primer gBwt N-term (5′-TCGTCTGATGCATCCACGGCG-3′) and the carboxy terminal primer gBAla C-term (5′-CTAGCTGAGCGGCCGCTCAGACGTTCTCTTCTTCGTCGGCGTCTTTC-3′) or gBAsp C-term (5′-CTAGCTGAGCGGCCGCTCAGACGTTCTCTTCTTCGTCGTCGTCTTTC-3′). The PCR fragments from the PCR mutagenesis of AD169 gB were digested with NsiI and NotI and cloned into an EcoRI site in Rep4DegBwt, resulting in Rep4DegBAla and Rep4DegBAsp. The genotype of the new clones was confirmed by sequence analysis and digestion with EcoRI. gB was excised from the Rep4De clones with XhoI, and the resulting 2.7-kb fragments were cloned into the VV insertion selection plasmid pZVneo (21) digested with XhoI. The orientation was confirmed by StuI and BglII restriction digest analysis and cycle sequencing. Homologous recombination, selection, and partial purification of recombinant viruses were performed as described by VanSlyke et al. (57). RVV 1-12-11 was chosen for gBwt, 13-24-13 was chosen for gBAsp, and 25-36-33-38 was chosen for gBAla expression in VV. Expression of gBwt and the gB point mutation substitutions gBAla and gBAsp was carried out essentially as described previously (4). In addition, a VV that expresses a dynamin dominant-negative mutant (RVV dynK44A) (7) was constructed as described above.
Immunocytochemistry.
Uninfected and HCMV-infected cells were grown on chamber slides, fixed at different time points after infection for 20 min at room temperature in buffered picric acid-paraformaldehyde (2% paraformaldehyde, 15% buffered picric acid), and permeabilized with 0.3% Triton X-100 in phosphate-buffered saline. The cells were blocked with 20% normal goat serum in phosphate-buffered saline and incubated for 1 h at 37°C with a 1:100 dilution of one of the following antibodies raised against HCMV gene products: a monoclonal antibody to the N terminus (6) or a polyclonal antibody to the C terminus of gB (the murine monoclonal antibody was a generous gift from William Britt, University of Alabama, Birmingham, Ala.). The polyclonal antibody to the C terminus of gB was generated by immunizing New Zealand White rabbits with glutathione S-transferase (GST)–gB C-terminal tail chimeric protein, where the entire cytoplasmic tail of gB was fused to GST. Injections and boosts were performed as previously described (27). Binding of primary antibody was detected with secondary antibodies conjugated to fluorescein isothiocyanate (FITC; Sigma Chemical Co., St. Louis, Mo.), tetramethylrhodamine isothiocyanate (TRITC; Sigma), or cyanine-5 (Biological Detection Systems, Inc., Pittsburgh, Pa.) raised in the appropriate species and visualized on a Leica confocal laser scanning microscope equipped with a Leitz Fluorovert-FU microscope and argon-krypton laser (CLSM AR/KR-Laser). The Slowfade Antifade kit (Molecular Probes, Inc., Eugene, Oreg.) was used to ensure minimal fluorescence fading.
In vitro phosphorylation of GST-gB constructs.
Fusions of native and mutated gB cytoplasmic tails with GST were produced by PCR amplification of the appropriate full-length gB construct in pZVneo (see above) and cloned into the BamHI site of pGEX 3X (Pharmacia). GST chimeras expressed in bacteria were used for in vitro phosphorylation assays. GST-gB (1 μg) was incubated at 30°C for 20 min in the presence of 0.1 mM [γ-32P]ATP (4,000 cpm/pmol) in a final volume of 30 μl. CKII (10 U; ICOS) was assayed in 50 mM Tris (pH 7.2)–150 mM KCl–10 mM MgCl2. CKI (10 U; ICOS) was assayed in 50 mM Tris (pH 7.5)–150 mM NaCl–60 mM MgCl2.
In vivo phosphorylation of gB.
Confluent HF and U373 cells (5 × 106) cultured in 75-mm2 flasks were infected with RVV at a multiplicity of infection of 5 and incubated at 37°C. At 2 h postinfection (p.i.), the medium was replaced with phosphate-free minimal essential medium (MEM; Gibco) supplemented with 5% dialyzed fetal bovine serum (FBS). At 3 h postinfection, sodium [32P]orthophosphate (3 mCi/5 × 106 cells) was added to the medium and the mixture was incubated for an additional 4 h. After being labeled, the cells were harvested in 1 ml of cold radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitors. The lysates were clarified by centrifugation at 16,000 × g for 10 min at 4°C in an Eppendorf microcentrifuge. The supernatant was transferred to a new tube containing 5 μl of mouse IgG and incubated on ice for 10 min with continuous mixing. Protein A-Sepharose (20 μl) was added, and the mixture was incubated on ice for 10 minutes with continuous mixing. The samples were centrifuged, and the supernatant was transferred to a new tube. The samples were exposed to 20 μl of protein A-Sepharose again to clear the supernatant. The samples were then transferred to a new tube containing 10 μl of gB 7–17 and incubated overnight at 4°C with continuous mixing. This step was followed by addition of 20 μl of protein A-Sepharose; the total mixture was incubated on ice for 2 h with continuous mixing.
Radiolabeling and surface biotinylation of gB.
Radiolabeling and surface biotinylation were used to measure the relative amounts of gB at the PM of U373 cells infected with RVV gBwt, gBAla, or gBAsp. U373 cells infected with RVV gBwt, gBAla, or gBAsp were pulsed-labeled for 12 h with [35S]methionine and [35S]cysteine at 2 days p.i. After removal of the label, the cells were pulsed with NHS-SS-biotin (no. 61105; Pierce, Rockford, Ill.) (stock of 200 mg/ml of dimethyl sulfoxide) at 4°C. After a 1-h labeling period, the cells were rinsed with Hanks balanced salt solution and prepared for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. The cells were harvested in 1 ml of cold RIPA buffer containing protease inhibitors. The lysates were clarified by centrifugation at 16,000 × g for 10 min at 4°C in an Eppendorf microcentrifuge. Biotinylated protein was recovered from sample supernatants by precipitation with 35 μl of a 50% slurry of ImmunoPure immobilized avidin (Pierce), after which the beads were washed. Biotinylated gB was eluted from the avidin beads by boiling in 50 μl of 20 mM Tris-HCl (pH 7.5)–100 mM NaCl–1% SDS buffer for 5 min. The samples were then centrifuged, and the supernatants were transferred to new tubes containing 5 μl of mouse IgG and incubated on ice for 10 min with continuous mixing. Protein A-Sepharose (20 μl) was added, and the mixture was incubated on ice for 10 min with continuous mixing. The samples were centrifuged, and the supernatants were transferred to a new tube. The samples were exposed to 20 μl of protein A-Sepharose again to clear the supernatant. The samples were then transferred to a new tube containing 10 μl of gB 7–17 and incubated overnight at 4°C with continuous mixing. This step was followed by the addition of 20 μl of protein A-Sepharose; the total mixture was incubated on ice for 2 h with continuous mixing. The immunoprecipitated protein was then analyzed by SDS-PAGE.
Internalization experiment.
gB antibody uptake experiments were performed in RVV gBwt- or RVV gBAsp-infected U373 cells. At 6 h postinfection, mouse anti-gB N-terminus antibody was added to the cells for 30 min. The cells were then rinsed and incubated for a 30-min chase period followed by fixation. Nonpermeabilized cells were stained with a cyanine-5–anti-mouse secondary conjugate, rinsed, permeabilized, stained with a TRITC–anti-mouse secondary conjugate, rinsed again, and exposed to rabbit anti-gB C-terminus antibody and then to an FITC–anti-rabbit secondary conjugate.
RESULTS
Steady-state HCMV gB exhibits cell-specific differences in intracellular trafficking.
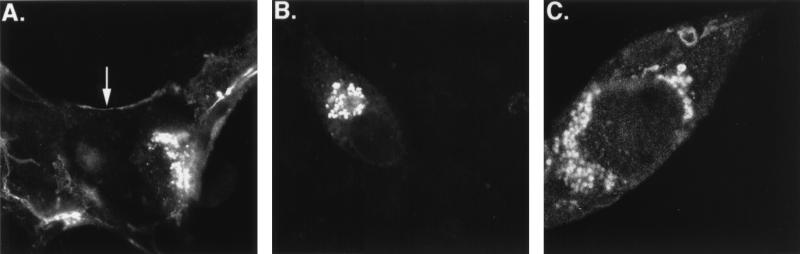
Previous studies of HCMV-permissive cells indicated that production of virus remained exclusively cell associated in U373 cells and MDM, in contrast to HF, which generated significant amounts of supernatant virus (references 14 and 15 and unpublished observations). Since gB is an essential part of the viral envelope, we used confocal microscopy to examine gB accumulation and compartmentalization in these cell types. In HCMV-infected HF, gB was observed in cytoplasmic vacuoles as well as at the PM (Fig. 1A). However, in MDM as well as U373 cells, gB was not detected at the PM and was restricted to intracellular vacuoles (Fig. 1B and C). Similar gB expression patterns were obtained in HF and U373 cells infected with an RVV which expressed wild-type gB (RVVgBwt) (see Fig. 4A and D). The cellular differences in gB localization suggested alteration of the trafficking patterns of this protein in these cells.
FIG. 1.
Confocal images of gB staining in HCMV-infected cells. HF (A), U373 cells (B), and MDM (C) were infected with HCMV as described in Materials and Methods. HF were fixed at 3 days p.i., U373 cells were fixed at 7 days p.i., and MDM were fixed at 14 days p.i. The cells were permeabilized and stained with a monoclonal mouse anti-gB antibody. Magnifications, ×294.
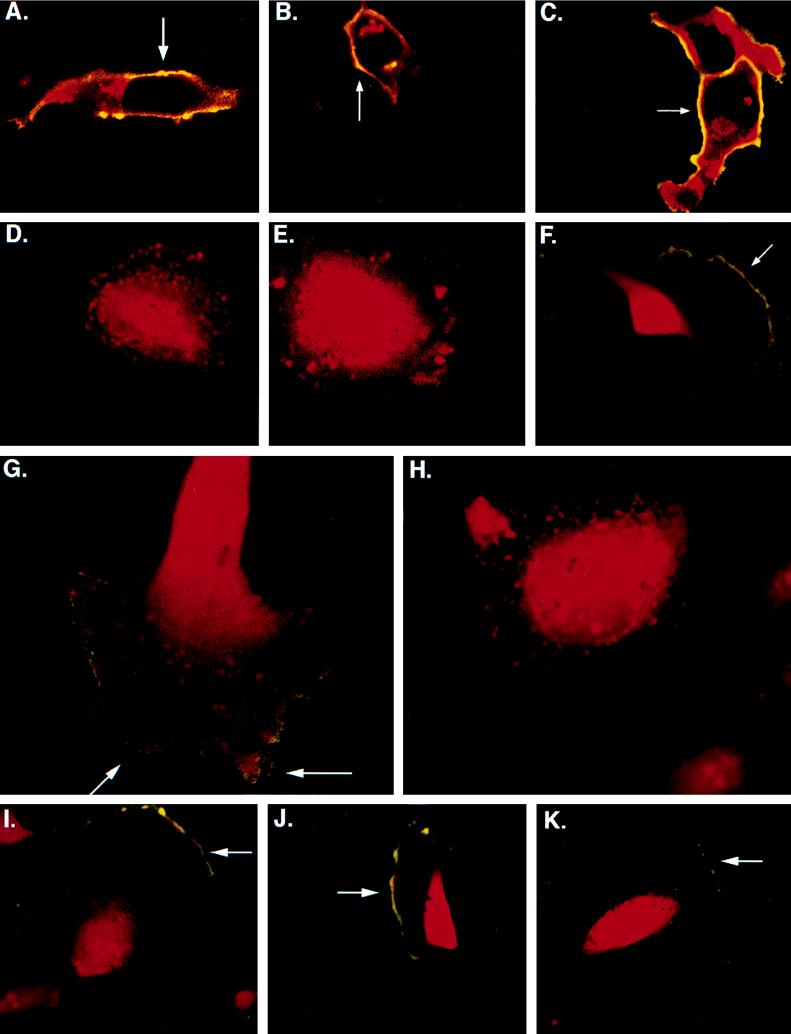
FIG. 4.
Subcellular localization of gB in HF and U373 cells. Confocal images of gB staining of cells infected with RVV gBwt, RVV gBAla, or RVV gBAsp were obtained. (A to C) Infected HF; (D to F) infected U373 cells. The cells were stained before permeabilization or at 4°C (representing surface gB) with mouse anti-gB (green) and after permeabilization (representing total gB) with mouse anti-gB (red). (A and D) RVV gBwt infections; (B and E) RVV gBAla mutant infections; (C and F) RVV gBAsp mutant infections. Surface gB staining is a combination of prepermeabilization at 4°C and postpermeabilization (yellow) (A, B, C, and F). (G and H) Confocal microscopy images demonstrating the presence of gB in VV-infected cells. U373 cells were infected with either RVV gBwt (G) or RVV gBAla (H) and subsequently treated with the phosphatase inhibitor tautomycin. The cells were stained with mouse antibody to gB prepermeabilization or at 4°C (green) (surface gB) and with mouse antibody to gB postpermeabilization (red) (total gB). The accumulation of gB trafficking to the surface was observed only in the RVV gBwt infection (yellow) (G). To demonstrate that transport of gB to the cell surface is not affected by the state of phosphorylation, we coinfected U373 cells with RVV gBwt (I), RVV gBAsp (J), or RVV gBAla (K) and RVV dynK44A. Magnifications, ×303 for panels A to F and I to K and ×473 for panels G and H.
Phosphorylation of HCMV gB occurs only at Ser900.
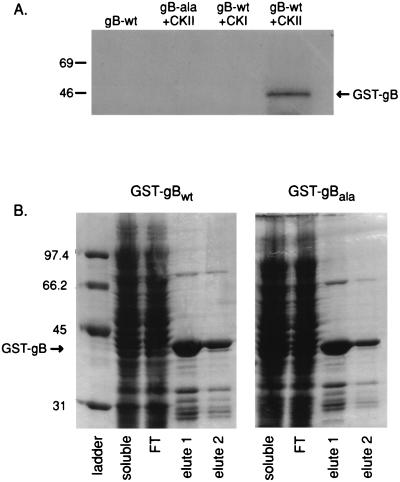
Previous studies have demonstrated that phosphorylation within acidic cluster motifs is an important determinant for protein sorting to intracellular compartments. Recently, the CKII consensus sequence sequence D-Sp900-D-E-E-E-N in the HCMV gB carboxy-terminal tail was shown to be phosphorylated in vitro and in vivo (60). However, other potential phosphorylation sites within gB were not examined in this study. To address this issue, a gB-GST fusion protein was constructed in which the entire 135-amino-acid gB tail was fused to GST (GST-gBwt) to determine the ability of CKII to phosphorylate Ser900 in the context of the entire gB cytoplasmic tail. In addition, the point mutation Ser900 to Ala900 (GST-gBAla) was constructed as a fusion protein and used to determine if CKII phosphorylation of Ser900 was specific for this site in an in vitro phosphorylation experiment. As demonstrated in Fig. 2A, GST-gBwt was an efficient substrate for CKII whereas replacement of Ser900 with Ala abolished phosphorylation. To ensure stable expression of both GST-gBwt and GST-gBAla, expression was analyzed on denaturing SDS-PAGE gels with Coomassie brilliant blue staining. Figure 2B shows that both GST-gB chimeras are stably expressed in similar quantities. In addition, neither phosphorus alone nor CKI plus phosphorus was able to phosphorylate the gB tail; therefore, Ser900 phosphorylation was specific. Thus, the above experiments with point mutations demonstrate that Ser900 is the only amino acid in the gB tail that is phosphorylated by CKII in vitro.
FIG. 2.
In vitro CKII phosphorylation of a GST-gB C-terminal tail chimeric protein. A GST-gBwt or gBAla C-terminal tail chimera was attached to microbeads and treated with either CKII or CKI and [32P]orthophosphate followed by magnetic bead purification and SDS-PAGE. (A) gBwt but not gBAla was phosphorylated by CKII. However, neither of the chimeric proteins was phosphorylated by CKI. (B) Both GST-gBwt and GST-gBAla, were stably expressed as determined by analysis by denaturing SDS-PAGE.
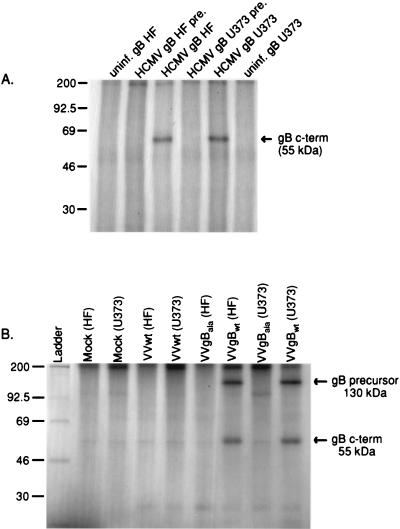
To determine whether gB is phosphorylated in vivo, 32P-labeled HF and U373 cells were infected with HCMV. Immunoprecipitation of gB from HCMV-infected HF and U373 cell lysates indicated that the protein is naturally phosphorylated in vivo in both cell types (Fig. 3A). These results suggest that the cell-specific differences in the presence of gB at the PM of HF and U373 cells is not due to the inability of CKII to phosphorylate the protein in either cell type. To determine whether gB Ser900 is the only residue phosphorylated in vivo, U373 cells and HF were infected by RVV expressing either WT (RVV gBWT) or gB containing Ser900 replaced with an Ala residue (RVV gBAla). While infection of U373 cells and HF with RVV gBWT resulted in phosphorylation of gB in both cell types, mutation of the Ser900 residue abrogated phosphorylation of the glycoprotein (Fig. 3B). Therefore, Ser900 is the only amino acid in gB that is phosphorylated in both U373 cells and HF.
FIG. 3.
gB is phosphorylated in HF and U373 cells in vivo. HCMV-infected (A), RVV gBwt-infected (B), or RVV gBAla-infected (B) HF and U373 cells were labeled with inorganic 32P and then subjected to immunoprecipitation with gB-specific rabbit antisera and a preimmune (pre.) control serum. In vivo-phosphorylated gB was detected in HCMV- and RVV gBwt-infected but not in RVV gBAla-infected HF and U373 cells.
A charge at Ser900 results in cell surface expression of gB in U373 cells.
RVV gBwt, RVV gBAla, and a virus that expresses gB with a point mutation which replaces Ser900 with an aspartate residue (RVV gBAsp) were used to determine if the state of gB phosphorylation affects intracellular routing. The mutants gBAla and gBAsp, with point mutations, were generated to mimic the nonphosphorylated and phosphorylated states of gB Ser900, respectively. Western blot analysis of RVV gBwt-, gBAla-, or gBAsp-infected HF revealed similar levels of gB production (data not shown). In addition, the localization of gB was evaluated by confocal microscopy in HF and U373 cells infected with RVV gBwt, RVV gBAla, or RVV gBAsp. gB was detected at the cell surface of HF infected with RVV gBwt (Fig. 4A) but not at the surface of U373 cells (Fig. 4D). Figures 4A and D show that gB expressed by RVV gBwt retains the differential expression pattern observed with gB expression in both HCMV-infected HF and U373 cells, respectively. Infection of HF or U373 cells with RVV gBAla resulted in a cellular distribution of gB similar to that due to infection with RVV gBwt (compare Fig. 4A and B and compare Fig. 4D and E, respectively). In contrast, when U373 cells were infected with RVV gBAsp, a substantial amount of gB was detected at the cell surface (Fig. 4F). In addition, RVV gBAsp infection of HF resulted in increased expression of gB at the PM compared to that due to RVV gBwt infection (compare Fig. 4A and C).
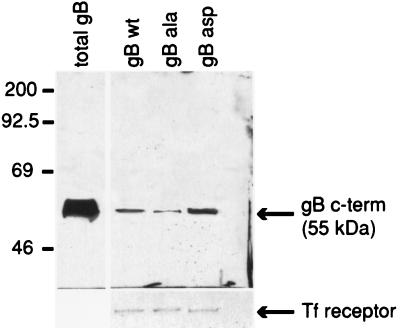
To analyze the overall expression of gB at the cell surface of U373 cell cultures, surface biotinylation of RVV gBwt-, RVV gBAla-, or RVV gBAsp-infected cell monolayers was performed. Specifically, U373 cells infected with RVV gBwt, RVV gBAla, or RVV gBAsp were pulse-labeled for 12 h with [35S]methionine and [35S]cysteine at 2 days p.i. After removal of the label, the cells were pulsed with NHS-SS-biotin at 4°C for 30 min. Surface-biotinylated proteins were immunoprecipitated from culture extracts with immobilized avidin. After the biotin-avidin complexes were disrupted by boiling, immobilized avidin was cleared by centrifugation. gB was then immunoprecipitated from the sample supernatants with a monoclonal antibody and analyzed by SDS-PAGE. Figure 5 demonstrates that substantially more gBAsp than gBAla is detected at the surface of U373 cells.
FIG. 5.
The steady-state cell surface expression of gBAsp is greater than that of gBwt and gBAla on the PM of U373 cells. U373 cells were infected with RVV gBwt, RVV gBAla, or RVV gBAsp and then subjected to surface biotinylation to analyze differences in gB PM expression. Specifically, 35S-labeled U373 cells infected with RVV gBwt, RVV gBAla, or RVV gBAsp were pulsed with NHS-SS-biotin and the immunoprecipitated surface gB was analyzed by SDS-PAGE. This figure demonstrates that substantially more gBAsp than gBAla is detected at the surface of U373 cells. These results support the hypothesis that a charge at position 900 in the gB cytoplasmic tail increases surface expression in U373 cells. c-term, C terminus.
These results suggest that phosphorylation of Ser900 plays a key role in the trafficking of gB, since replacing Ser900 with a charged amino acid (Asp) allows surface expression in both U373 cells and HF. One possible explanation for these observations is that the delivery of gB to the PM depends on the phosphorylation state. Alternatively, the state of gB phosphorylation may regulate internalization or recycling at the PM.
Tautomycin treatment of U373 cells results in gBwt cell surface expression.
The altered trafficking of gB in U373 cells infected with gBAsp suggests that the phosphorylation state of Ser900 may play an important role in gB trafficking. However, the experiments described above indicate that gB is equally phosphorylated in both HF and U373 cells. The phosphorylation of gB in these cells may also be influenced by the presence of differential phosphatase activities that regulate the phosphorylation state of gB. To determine whether phosphatases regulate gB cell surface expression, U373 cells infected with RVV gBwt or RVV gBAla were treated with either the phosphatase inhibitor okadaic acid (100 nM; inhibitor of protein phosphatase 2A) or tautomycin (100 nM; inhibitor of protein phosphatases 1 and 2A). While addition of okadaic acid to RVV gBwt- and RVV gBAla-infected U373 cells had no effect on gB localization (data not shown), addition of tautomycin to RVV gBwt-infected U373 cells resulted in gB accumulation at the cell surface (Fig. 4G) whereas an accumulation of gB was not detected in RVV gBAla-infected U373 cells treated with the phosphatase inhibitor (Fig. 4H). These observations suggest that the lack of cell surface expression of gB in U373 cells compared to HF cells is due to a specific phosphatase activity.
Trafficking of gB to the PM is not dependent on the state of gB phosphorylation.
To determine if gB without a charged residue at position 900 trafficks to the PM, U373 cells were coinfected with the RVVs described above, in addition to a VV construct that expresses a dynamin dominant-negative mutant (RVV dynK44A). The dynamin mutation prevents dynamin-mediated transport of surface molecules back to the cytoplasm by blocking clathrin-dependent endocytosis (7). Coinfection of U373 cells with RVV dynK44A and RVV gBwt (Fig. 4I), RVV gBAsp (Fig. 4J), or RVV gBAla (Fig. 4K) resulted in the accumulation of gB at the PM in U373 cells. Surface gB expression was not observed in RVV gBwt-infected U373 cells coinfected with VV expressing wild-type dynamin (data not shown); therefore, expression of gB at the cell PM is not the result of VV infection. These data indicate that both phosphorylated and nonphosphorylated gB can traffic to the cell surface. Furthermore, these observations indicate that the internalization of gB from the PM occurs via a clathrin-dependent pathway. These experiments support the hypothesis that gB trafficks to the PM in a charge-independent manner and is then internalized from the cell surface to an intracellular compartment at a cell-specific rate.
gBAsp remains near the PM upon internalization.
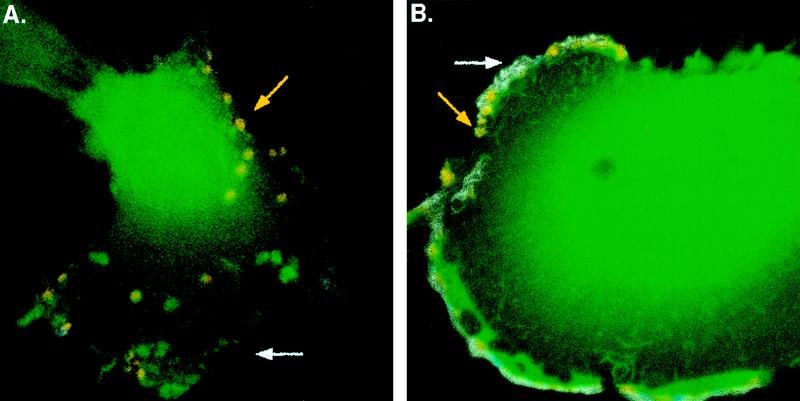
Antibody uptake studies were performed to determine if gB that is internalized at the PM accumulates in cytoplasmic vacuoles. U373 cells were infected with RVV gBwt for 6 h, and the live cells were given a 30-min exposure to a monoclonal antibody specific for the N terminus of gB. After being rinsed, the cells were incubated for 30 min at 37°C, fixed, prepared for immunofluorescence analysis, and examined for antibody internalization by confocal microscopy. Expression of gBwt resulted in the accumulation of the glycoprotein in cytoplasmic vacuoles (Fig. 6A). As a control, antibody to the HCMV tegument protein pp65 was added in parallel experiments and did not stain cells (data not shown). Interestingly, when cells were infected with RVV gBAsp, gB was internalized but remained associated with the PM (Fig. 6B). Thus, in U373 cells, gBwt is rapidly endocytosed upon reaching the cell surface, sorted upon endocytosis, and concentrated on the surface of vacuoles. In contrast, gBAsp internalizes but remains in small vacuoles at the PM.
FIG. 6.
Endocytosis and intracellular targeting of gB in U373 cells. gB antibody uptake experiments were performed in RVV gBwt-infected (A) or RVV gBAsp-infected (B) U373 cells. At 6 h p.i., mouse anti-gB N-terminus antibody was applied to the cells for 30 min. The cells were then rinsed and incubated for a 30-min chase period followed by fixation. Nonpermeabilized cells were stained with a cyanine-5–anti-mouse secondary conjugate (blue; stable surface gB), followed by rinsing, permeabilization, and staining with a TRITC–anti-mouse secondary conjugate (red; internalized gB). The cells were rinsed again and exposed to rabbit anti-gB C-terminus antibody followed by an FITC–anti-rabbit secondary conjugate (green; total gB). Therefore, internalized gB is both green and red (yellow vacuoles), PM gB is blue, red, and green (white PM staining), and gB that was absent from the PM during the 30-min mouse anti-gB N-terminus antibody exposure is green. Magnifications, 473×.
DISCUSSION
Here we demonstrate that phosphorylation of HCMV gB in HF and U373 cells occurs in vivo only at the CKII site in the cytoplasmic domain and that the phosphorylation state of the gB carboxy-terminal tail is one of the important determinants for intracellular trafficking. We also show that gB plasma membrane expression occurs in a cell-specific manner. Specifically, the steady-state expression of gB at the cell surface in U373 cells is dependent on the phosphorylation state of Ser900 in the gB cytoplasmic tail. Cell-specific differences were shown to be associated with a tautomycin-sensitive phosphatase, not with CKII activity. In addition, our results suggest that gB trafficks to the PM in a dephosphorylation-independent manner. Therefore, the cell-specific difference in the steady-state expression of gB at the cell surface is the result of the state of phosphorylation of Ser900, which affects either the internalization rate of gB from the PM or recycling to the PM. Finally, gB accumulates in cytoplasmic vacuoles upon leaving the PM. These observations suggest that formation of these vacuoles may be required for HCMV capsid envelopment.
The initial finding that gB was not on the PM of HCMV-infected U373 cells is in contrast to previous findings of gB expression in constitutively expressing stable U373 cell lines (55, 59). The difference may be explained either by the overexpression of the glycoprotein in the cell line or by the use of fluorescence-activated cell sorter analysis rather than immunofluorescence to detect gB. In any event, our data are in agreement that gB is present on the U373 PM but the steady-state amount varies greatly depending on the cell type due to the presence of a charged residue at Ser900.
The processes involved in HCMV assembly and egress are controversial and are considered to involve mechanisms similar to those used by other herpesviruses (35). The herpesvirus model suggests that nucleocapsids assembled in the nucleus acquire a temporary envelope by budding through the nuclear membrane, followed by deenvelopment at the outer nuclear membrane (2, 39). Transport across the nuclear membrane is hypothesized to be mediated by gB and gH localization to the nuclear membrane. While the latter hypothesis may be correct, experiments have not been reported which differentiate gB localization at the nuclear membrane from localization at the rough endoplasmic reticulum, which are in close proximity. This issue may be resolved by using double-label experiments with viral envelope antibodies in combination with antibodies to rough endoplasmic reticulum or nuclear membrane markers. The final HCMV envelope is proposed to be acquired in the trans-Golgi network (TGN), since this step is sensitive to brefeldin A treatment (12). This latter step is logical, since several groups of viruses acquire their envelope glycoproteins in the secretory pathway during assembly (44, 53).
The cytoplasmic tails of a number of viral glycoproteins that enter the secretory pathway have been shown to contain selective trafficking signals, which direct proteins to different cellular compartments (10, 29, 31, 42, 46, 60). Surface expression of viral glycoproteins is determined by the cellular steady-state expression of the protein, transport to the PM, and rates of internalization of proteins from the cell surface. Internalization occurs through both clathrin-dependent and -independent pathways. The C-terminal domains of several membrane proteins contain amino acid motifs which constitute internalization signals. These proteins include furin, low-density lipoprotein receptor, transferrin receptor, polymeric Ig receptor, epidermal growth factor receptor, varicella-zoster virus Fc receptor gE, and the simian immunodeficiency virus transmembrane protein gp41 (3, 10, 26, 29, 31, 36, 42, 46, 58, 60). A comparison of the sequences of these proteins indicates that a common structure rather than sequence is necessary for internalization. The varicella-zoster virus gE envelope glycoprotein contains two TGN-targeting sequences in the cytoplasmic domain, an AYRV motif and an acidic amino acid cluster (60). The presence of either of these sequences is sufficient to cause internalization of protein on the PM and targeting to the TGN. The Tyr-dependent motif in the cytoplasmic tail of the simian immunodeficiency virus gp41 transmembrane protein is another example of an internalization signal that regulates glycoprotein expression at the cell surface (42). The signals for internalization of HCMV gB are unknown.
Protein localization to subcellular compartments may also be influenced by secondary modifications that occur in a cell-type-specific manner. For example, the glycoproteins produced by Sindbis virus are modified in the secretory pathway of both vertebrate and insect cells but have cell-specific trafficking patterns which affect the subcellular location of virus assembly (43). Thus, in vertebrate cells, viral assembly and budding occurs at the PM. In contrast, in insect cells, virus buds into intracellular vacuoles, which fuse with the PM and release virus into the extracellular fluid. The Sindbis virus glycoproteins are transiently phosphorylated; inhibitors of phosphorylation prevent the production of infectious virus (30). These observations suggest that the phosphorylation state of Sindbis virus glycoproteins may determine either glycoprotein trafficking or viral assembly. Similarly, HCMV also demonstrates cell specificity for virus release, and the phosphorylation of gB may determine this event.
In summary, understanding the mechanisms involved in gB trafficking may be important in determining the mechanisms of viral envelopment and intracellular sequestration. Future work will determine the importance of gB expression on the PM in these processes.
ACKNOWLEDGMENTS
We thank Sandy Schmid, Shaun Molloy, and Gary Thomas for technical advice and helpful discussion.
This work was supported by Public Health Service grant AI 21640 from the National Institutes of Health (to J.A.N.), the Molecular Hematology Training Program NIH NRSA Training Award (to K.N.F.), and the Knut and Alice Wallenbergs Foundation (to C.S.-N.). C.S.-N. is a scholar of the Wenner-Gren Foundation, Sweden.
REFERENCES
- 1.Bogner E, Anheier B, Offner F, Smuda C, Reschke M, Eickmann M, Radsak K. Nuclear translocation of mutagenized forms of human cytomegalovirus glycoprotein B (gpUL55) J Gen Virol. 1997;78:1647–1651. doi: 10.1099/0022-1317-78-7-1647. [DOI] [PubMed] [Google Scholar]
- 2.Bogner E, Reschke M, Reis B, Reis E, Britt W, Radsak K. Recognition of compartmentalized intracellular analogs of glycoprotein H of human cytomegalovirus. Arch Virol. 1992;126:67–80. doi: 10.1007/BF01309685. [DOI] [PubMed] [Google Scholar]
- 3.Bos K, Wraight C, Stanely K K. TGN38 is maintained in the trans-Golgi network by a tyrosine-containing motif in the cytoplasmic domain. EMBO J. 1993;12:2219–2228. doi: 10.1002/j.1460-2075.1993.tb05870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bresnahan P A, Leduc R, Thomas L, Thorner J, Gibson H I, Brake A J, Barr P J, Thomas G. Human fur gene encodes a yeast KEX2-like endoprotease that cleaves pro-beta-NGF in vivo. J Cell Biol. 1990;111:2851–2859. doi: 10.1083/jcb.111.6.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britt W J. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology. 1984;135:369–378. doi: 10.1016/0042-6822(84)90193-4. [DOI] [PubMed] [Google Scholar]
- 6.Britt W J, Vugler L G. Oligomerization of the human cytomegalovirus major envelope glycoprotein complex gB (gp55-116) J Virol. 1992;66:6747–6754. doi: 10.1128/jvi.66.11.6747-6754.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damke H, Baba T, Warnock D, Schmid S. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dankner W M, McCutchan J A, Richman D D, Hirata K, Spector S A. Localization of human cytomegalovirus in peripheral blood leukocytes by in situ hybridization. J Infect Dis. 1990;161:31–36. doi: 10.1093/infdis/161.1.31. [DOI] [PubMed] [Google Scholar]
- 9.Diosi P, Moldovan E, Tomescu N. Latent cytomegalovirus infection in blood donors. Br Med J. 1969;4:660–662. doi: 10.1136/bmj.4.5684.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doyle C, Roth M G, Sambrook J, Gething M J. Mutations in the cytoplasmic domain of the influenza virus hemagglutinin affect different stages of intracellular transport. J Cell Biol. 1985;100:704–714. doi: 10.1083/jcb.100.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudding L R, Garnett H M. Interaction of strain AD169 and a clinical isolate of cytomegalovirus with peripheral monocytes: the effect of lipopolysaccharide stimulation. J Infect Dis. 1987;155:891–896. doi: 10.1093/infdis/155.5.891. [DOI] [PubMed] [Google Scholar]
- 12.Eggers M, Bogner E, Agricola B, Kern H F, Radsak K. Inhibition of human cytomegalovirus maturation by brefeldin A. J Gen Virol. 1992;73:2679–2692. doi: 10.1099/0022-1317-73-10-2679. [DOI] [PubMed] [Google Scholar]
- 13.Einhorn L, Öst A. Cytomegalovirus infection of human blood cells. J Infect Dis. 1984;149:207–214. doi: 10.1093/infdis/149.2.207. [DOI] [PubMed] [Google Scholar]
- 14.Fish K N, Britt W, Nelson J A. A novel mechanism for persistence of human cytomegalovirus in macrophages. J Virol. 1996;70:1855–1862. doi: 10.1128/jvi.70.3.1855-1862.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fish K N, Depto A S, Moses A V, Britt W, Nelson J A. Growth kinetics of human cytomegalovirus are altered in monocyte-derived macrophages. J Virol. 1995;69:3737–3743. doi: 10.1128/jvi.69.6.3737-3743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fish K N, Soderberg-Naucler C, Mills L, Stenglein S, Nelson J A. Human cytomegalovirus persistently infects aortic endothelial cells. J Virol. 1998;72:5661–5668. doi: 10.1128/jvi.72.7.5661-5668.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fish K N, Stenglein S G, Ibanez C, Nelson J A. Cytomegalovirus persistence in macrophages and endothelial cells. Scand J Infect Dis Supple. 1995;99:34–40. [PubMed] [Google Scholar]
- 18.Foucar E, Mukai K, Foucar K, Sutherland D E, Van Buren C T. Colon ulceration in lethal cytomegalovirus infection. Am J Clin Pathol. 1981;76:788–801. doi: 10.1093/ajcp/76.6.788. [DOI] [PubMed] [Google Scholar]
- 19.Gnann J, Jr, Ahlmen J, Svalander C, Olding L, Oldstone M B, Nelson J A. Inflammatory cells in transplanted kidneys are infected by human cytomegalovirus. Am J Pathol. 1988;132:239–248. [PMC free article] [PubMed] [Google Scholar]
- 20.Gretch D R, Kari B, Rasmussen L, Gehrz R C, Stinski M F. Identification and characterization of three distinctive families of glycoprotein complexes present in the envelopes of human cytomegalovirus. J Virol. 1988;62:875–881. doi: 10.1128/jvi.62.3.875-881.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayflick J S, Wolfgang W J, Forte M A, Thomas G. A unique Kex2-like endoprotease from Drosophila melanogaster is expressed in the central nervous system during early embryogenesis. J Neurosci. 1992;12:705–717. doi: 10.1523/JNEUROSCI.12-03-00705.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho M. Cytomegalovirus: biology and infection. New York, N.Y: Plenum Medical Book Co.; 1991. [Google Scholar]
- 23.Howell C L, Miller M J, Martin W J. Comparison of rates of virus isolation from leukocyte populations separated from blood by conventional and Ficoll-Paque/Macrodex methods. J Clin Microbiol. 1979;10:533–537. doi: 10.1128/jcm.10.4.533-537.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunter E, Swanstrom R. Retrovirus envelope glycoproteins. Curr Top Microbiol Immunol. 1990;157:187–253. doi: 10.1007/978-3-642-75218-6_7. [DOI] [PubMed] [Google Scholar]
- 25.Ibanez C E, Schrier R, Ghazal P, Wiley C, Nelson J A. Human cytomegalovirus productively infects primary differentiated macrophages. J Virol. 1991;65:6581–6588. doi: 10.1128/jvi.65.12.6581-6588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones B G, Thomas L, Molloy S S, Thulin C D, Fry M D, Walsh K A, Thomas G. Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. EMBO J. 1995;14:5869–5883. doi: 10.1002/j.1460-2075.1995.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jupp R, Hoffmann S, Depto A, Stenberg R M, Ghazal P, Nelson J A. Direct interaction of the human cytomegalovirus IE86 protein with the cis repression signal does not preclude TBP from binding to the TATA box. J Virol. 1993;67:5595–5604. doi: 10.1128/jvi.67.9.5595-5604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kondo K, Kaneshima H, Mocarski E S. Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc Nat Acad Sci USA. 1994;91:11879–11883. doi: 10.1073/pnas.91.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazarovits J, Roth M. A single amino acid change in the cytoplasmic domain allows influenza virus hemagglutinin to be endocytosed through coated pits. Cell. 1988;53:743–752. doi: 10.1016/0092-8674(88)90092-x. [DOI] [PubMed] [Google Scholar]
- 30.Liu N, Brown D T. Phosphorylation and dephosphorylation events play critical roles in Sindbis virus maturation. Virology. 1993;196:703–711. doi: 10.1006/viro.1993.1527. [DOI] [PubMed] [Google Scholar]
- 31.Lydy S L, Compans R W. Role of the cytoplasmic domains of viral glycoproteins in antibody-induced cell surface mobility. J Virol. 1993;67:6289–6294. doi: 10.1128/jvi.67.10.6289-6294.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maciejewski J P, Bruening E E, Donahue R E, Mocarski E S, Young N S, St Jeor S C. Infection of hematopoietic progenitor cells by human cytomegalovirus. Blood. 1992;80:170–178. [PubMed] [Google Scholar]
- 33.Maciejewski J P, Bruening E E, Donahue R E, Sellers S E, Carter C, Young N S, St. Jeor S. Infection of mononucleated phagocytes with human cytomegalovirus. Virology. 1993;195:327–336. doi: 10.1006/viro.1993.1383. [DOI] [PubMed] [Google Scholar]
- 34.Minton E J, Tysoe C, Sinclair J H, Sissons J G. Human cytomegalovirus infection of the monocyte/macrophage lineage in bone marrow. J Virol. 1994;68:4017–4021. doi: 10.1128/jvi.68.6.4017-4021.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mocarski E S J. Cytomegaloviruses and their replication. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. [Google Scholar]
- 36.Molloy S S, Thomas L, VanSlyke J K, Stenberg P E, Thomas G. Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J. 1994;13:18–33. doi: 10.1002/j.1460-2075.1994.tb06231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norais N, Hall J A, Gross L, Tang D, Kaur S, Chamberlain S H, Burke R I, Marcus F. Evidence for a phosphorylation site in cytomegalovirus glycoprotein gB. J Virol. 1996;70:5716–5719. doi: 10.1128/jvi.70.8.5716-5719.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pereira L, Hoffman M, Gallo D, Cremer N. Monoclonal antibodies to human cytomegalovirus: three surface membrane proteins with unique immunological and electrophoretic properties specify cross-reactive determinants. Infect Immun. 1982;36:924–932. doi: 10.1128/iai.36.3.924-932.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radsak K, Brucher K, Britt W, Shiou H, Schneider D, Kollert A. Nuclear compartmentation of gB of human cytomegalovirus. Virology. 1990;177:515–522. doi: 10.1016/0042-6822(90)90516-t. [DOI] [PubMed] [Google Scholar]
- 40.Rice G P, Schrier R D, Oldstone M B. Cytomegalovirus infects human lymphocytes and monocytes: virus expression is restricted to immediate-early gene products. Proc Natl Acad Sci USA. 1984;81:6134–6138. doi: 10.1073/pnas.81.19.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rinaldo C, Jr, Black P H, Hirsch M S. Interaction of cytomegalovirus with leukocytes from patients with mononucleosis due to cytomegalovirus. J Infect Dis. 1977;136:667–678. doi: 10.1093/infdis/136.5.667. [DOI] [PubMed] [Google Scholar]
- 42.Sauter M M, Pelchen-Matthews A, Bron R, Marsh M, LaBranche C C, Vance P J, Romano J, Haggarty B S, Hart T K, Lee W M, Hoxie J A. An internalization signal in the simian immunodeficiency virus transmembrane protein cytoplasmic domain modulates expression of envelope glycoproteins on the cell surface. J Cell Biol. 1996;132:795–811. doi: 10.1083/jcb.132.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schlesinger S, Schlesinger M. Togaviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 825–841. [Google Scholar]
- 44.Schmelz M, Sodeik B, Ericsson M, Wolffe E J, Shida H, Hiller G, Griffiths G. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans-Golgi network. J Virol. 1994;68:130–170. doi: 10.1128/jvi.68.1.130-147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schrier R D, Nelson J A, Oldstone M B. Detection of human cytomegalovirus in peripheral blood lymphocytes in a natural infection. Science. 1985;230:1048–1051. doi: 10.1126/science.2997930. [DOI] [PubMed] [Google Scholar]
- 46.Shafer W, Stroh A, Berghofer S, Seiler J, Vey M, Kern M F, Klenk H D, Garten W. Two independent targeting signals in the cytoplasmic domain determine trans Golgi network localization and endosomal trafficking of the proprotein convertase furin. EMBO J. 1995;14:2424–2435. doi: 10.1002/j.1460-2075.1995.tb07240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sinzger C, Grefte A, Plachter B, Gouw A S, The T H, Jahn G. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J Gen Virol. 1995;76:741–750. doi: 10.1099/0022-1317-76-4-741. [DOI] [PubMed] [Google Scholar]
- 48.Sinzger C, Muntefering H, Loning T, Stoss H, Plachter B, Jahn G. Cell types infected in human cytomegalovirus placentitis identified by immunohistochemical double staining. Virchows Arch A. 1993;423:249–256. doi: 10.1007/BF01606887. [DOI] [PubMed] [Google Scholar]
- 49.Sinzger C, Plachter B, Grefte A, The T H, Jahn G. Tissue macrophages are infected by human cytomegalovirus in vivo. J Infect Dis. 1996;173:240–245. doi: 10.1093/infdis/173.1.240. [DOI] [PubMed] [Google Scholar]
- 50.Sinzger C, Plachter B, Stenglein S, Jahn G. Immunohistochemical detection of viral antigens in smooth muscle, stromal, and epithelial cells from acute human cytomegalovirus gastritis. J Infect Dis. 1993;167:1427–1432. doi: 10.1093/infdis/167.6.1427. [DOI] [PubMed] [Google Scholar]
- 51.Söderberg C, Larsson S, Bergstedt-Lindqvist S, Möller E. Definition of a subset of human peripheral blood mononuclear cells that are permissive to human cytomegalovirus infection. J Virol. 1993;67:3166–3175. doi: 10.1128/jvi.67.6.3166-3175.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spaete R R, Thayer R M, Probert W S, Masiarz F R, Chamberlain S H, Rasmussen L, Merigan T C, Pachl C. Human cytomegalovirus straine Towne glycoprotein B is processed by proteolytic cleavage. Virology. 1988;167:207–225. doi: 10.1016/0042-6822(88)90071-2. [DOI] [PubMed] [Google Scholar]
- 53.Sturman L S, Holmes K V. The molecular biology of coronaviruses. Adv Virus Res. 1983;28:35–112. doi: 10.1016/S0065-3527(08)60721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor-Wiedeman J, Sissons J G, Borysiewicz L K, Sinclair J H. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991;72:2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- 55.Tugizov S, Wang Y, Qadri I, Navarro D, Maidji E, Pereira L. Mutated forms of human cytomegalovirus glycoprotein B are impaired in inducing syncytium formation. Virology. 1995;209:580–591. doi: 10.1006/viro.1995.1290. [DOI] [PubMed] [Google Scholar]
- 56.Turtinen L W, Saltzman R, Jordan M C, Haase A T. Interactions of human cytomegalovirus with leukocytes in vivo: analysis by in situ hybridization. Microb Pathog. 1987;3:287–297. doi: 10.1016/0882-4010(87)90062-3. [DOI] [PubMed] [Google Scholar]
- 57.VanSlyke J K, Thomas L, Thomas G. In: Methods in neuroscience. Smith I A, editor. Orlando, Fla: Academic Press, Inc.; 1995. pp. 45–64. [Google Scholar]
- 58.Voorhees P, Diegnan E, van Donselaar E, Humphrey J, Marks M S, Peters P J, Bonifacino J S. An acid sequence within the cytoplasmic tail domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO J. 1995;14:4961–4975. doi: 10.1002/j.1460-2075.1995.tb00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng Z, Maidji E, Tugizov S, Pereira L. Mutations in the carboxyl-terminal hydrophobic sequence of human cytomegalovirus glycoprotein B alter transport and protein chaperone binding. J Virol. 1996;70:8029–8040. doi: 10.1128/jvi.70.11.8029-8040.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu Z, Hao Y, Gershon M D, Ambron R T, Gershon A A. Targeting of glycoprotein I (gE) of varicella-zoster virus to the trans-Golgi network by an AYRV sequence and an acidic amino acid-rich patch in the cytosolic domain of the molecule. J Virol. 1996;70:6563–6575. doi: 10.1128/jvi.70.10.6563-6575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]