Key Points
-
•
Intensive subcutaneous or IV pegcetacoplan treatment was effective in the management of acute BTH events in patients on pegcetacoplan.
-
•
Intensive subcutaneous or IV treatment with pegcetacoplan was safe and well tolerated.
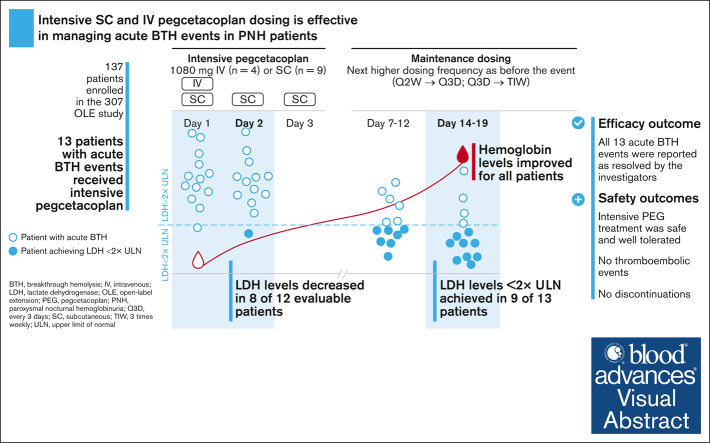
Visual Abstract
Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is characterized by complement-mediated intravascular hemolysis leading to anemia, fatigue, and potentially life-threatening thrombotic complications. Breakthrough hemolysis (BTH) was first described in patients with PNH treated with terminal complement C5 inhibitors when intravascular hemolysis reoccurred despite treatment. Pegcetacoplan, the first proximal complement C3 inhibitor, offers broad hemolysis control in patients with PNH. While experience of managing BTH on C5 inhibitors is documented, very limited guidance exists for proximal complement inhibitors. This interim analysis assessed the effect of intensive treatment with pegcetacoplan following an acute BTH event in a subset of patients enrolled in the ongoing open-label extension study of pegcetacoplan in PNH. Thirteen patients with acute BTH included in the analysis received either a single IV dose of 1080 mg (n = 4) or 1080 mg subcutaneous (SC) dosing on 3 consecutive days (n = 9). A potential, clinically-relevant complement-amplifying condition, such as infection or vaccination, was reported in approximately half of the patients experiencing an acute BTH. Lactate dehydrogenase (LDH) levels decreased between day 1 and day 2 in 8 of 12 evaluable patients and in all 13 patients at day 7 to 12. Nine of 13 patients (69%) achieved LDH <2× the upper limit of normal by day 14 to 19. All adverse events associated with the acute BTH event were considered resolved by the investigators. Overall, intensive treatment with pegcetacoplan was safe and well tolerated. These novel data support effective management of acute BTH events in patients on pegcetacoplan with intensive IV or SC pegcetacoplan dosing. This trial was registered at www.clinicaltrials.gov as #NCT03531255.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, and potentially life-threatening hematological disease characterized by chronic complement-mediated hemolysis, thrombosis, and substantial patient burden.1
Terminal complement C5 inhibitors eculizumab and ravulizumab have improved patient outcomes and survival, with PNH becoming a chronic disease in countries where treatment is available.2,3 C3-mediated extravascular hemolysis (EVH), however, occurs in a significant proportion of patients treated with C5 inhibitors, leaving patients with persistent anemia.4,5
Pegcetacoplan, the first proximal C3 inhibitor developed to address both intravascular hemolysis (IVH) and EVH,6, 7, 8 is approved by the Food and Drug Administration for the treatment of adults with PNH9 and by the European Medicines Agency for the treatment of adults with PNH who remain anemic after at least 3 months of C5 inhibitor therapy.10 In the phase 3 PEGASUS trial (NCT03500549), pegcetacoplan was superior to eculizumab in improving change in hemoglobin levels from baseline to week 16 in adults with PNH and ongoing anemia despite stable eculizumab therapy, with long-term assessment of efficacy and safety of pegcetacoplan through 48 weeks demonstrating sustained improvements in hematological outcomes and quality of life measures.7,11 The ongoing pegcetacoplan open-label extension (OLE) study is evaluating the long-term safety and efficacy of pegcetacoplan in patients with PNH who have completed 1 of the 5 previous pegcetacoplan clinical trials in PNH.12,13
Breakthrough hemolysis (BTH) was first described in patients treated with C5 inhibitors when IVH reoccurred despite inhibitor treatment.2,4 Hemolysis adverse events (AEs) were reported in the PEGASUS trial. Nineteen of 80 patients treated with pegcetacoplan experienced a hemolysis AE; 4 patients in the 16-week randomized controlled period and 15 patients during the 32-week open-label period.11 Most hemolysis events were considered moderate in severity. Hemolysis AEs led to study discontinuation in 5 patients.11 While clinicians had more than 20 years of experience in managing BTH on C5 inhibitors, there is currently very limited guidance on the management of hemolysis on proximal complement inhibitors.
Here, we report an interim analysis of the effect of acute treatment with intensive pegcetacoplan (IV or subcutaneous [SC]) following an acute BTH event in a subset of patients enrolled in the ongoing OLE study as of the March 2022 data cut.
Methods
Study design
The OLE study (study 307; NCT03531255) is an active, multicenter, and nonrandomized trial of adults with PNH (≥18 years) who have completed 1 of the 5 pegcetacoplan trials: phase 1b (PADDOCK [NCT02588833] and PHAROAH [NCT02264639]), phase 2a (PALOMINO [NCT03593200]), and phase 3 (PEGASUS [NCT03500549] and PRINCE [NCT04085601]). The extension study will be 4 years in duration, or until patients are transitioned to commercially available product. Inclusion criteria for intensive pegcetacoplan dosing includes acute BTH as defined by lactate dehydrogenase (LDH) >2× upper limit of normal (ULN) and the presence of at least 1 new or worsening sign or symptom of hemolysis (eg, decreased hemoglobin, hemoglobinuria, fatigue, etc), which in the opinion of the investigator, warrants an acute intervention.
The OLE study was approved by the Institutional Review Board or Independent Ethics Committee at participating trial sites and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Informed consent was obtained from all patients.
Intervention
Patients in the OLE study can receive SC administration of 1080 mg pegcetacoplan twice weekly, every 3 days, or 3 times weekly. As per study protocol, pegcetacoplan dosing frequency may be adjusted from 1080 mg twice weekly to every third day if LDH levels reach >2× ULN. For any patient already receiving 1080 mg every third day, a dose increase to 1080 mg 3 times weekly should be considered if LDH is again >2× ULN.
To identify dosing regimens that could achieve an immediate increase in pegcetacoplan concentrations, simulations were performed to predict pegcetacoplan exposure following various loading dose strategies with a subsequent increase in maintenance dosing.
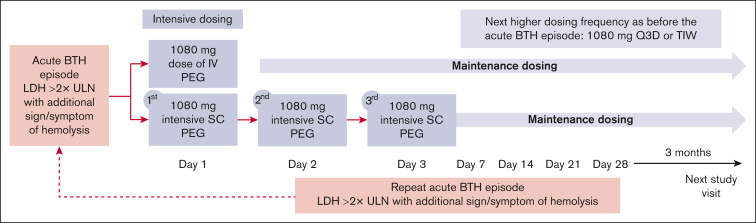
As defined in the study protocol, patients with acute BTH are eligible to receive 1 of 2 intensive pegcetacoplan treatments: a single IV dose of 1080 mg or 1080 mg SC on 3 consecutive days according to investigators’ clinical judgment. The selection of the intensive regimen was based on physician’s choice. The SC preparation of pegcetacoplan comes as a ready-to-use acetate-buffered sorbitol formulation (pegcetacoplan 54 mg/mL, 10 mM acetate buffer, pH 5.0, containing 4.1% w/v sorbitol) in a 20 mL glass vial.10 The IV administration of pegcetacoplan uses the same formulation as used for SC administration. Pegcetacoplan was administered as a 20 mL infusion (either SC or IV during the intensive therapy regimen). IV dosing was administered as an infusion over ∼30 minutes. Of note, a specific IV administration equipment was required for the infusion, which was not available in all countries. The day of first intervention with intensive treatment was defined as day 1 (Figure 1).
Figure 1.
Study design. Patients enrolled in the OLE study who experienced an acute BTH event had the opportunity to receive either intensive subcutaneous or intravenous pegcetacoplan as an acute treatment for BTH. Patients who experienced an acute BTH event but had chosen not to receive intensive pegcetacoplan could continue to participate in the OLE study with the potential to receive a dose adjustment to 1080 mg every third day or 3 times weekly. BTH, breakthrough hemolysis; LDH, lactate dehydrogenase; PEG, pegcetacoplan; Q3D, every 3 days; SC, subcutaneous; TIW, 3 times weekly; ULN, upper limit of normal.
Following intensive SC dosing, maintenance dosing resumes with the third daily SC dose replacing the first dose of the new maintenance dosing regimen. Patients who receive an IV dose continue maintenance SC administration uninterrupted, with the timing of the next dose determined relative to the previous SC dose. Maintenance dosing after either regimen will be at the next higher dosing frequency than that before the episode of acute BTH (ie, subjects previously receiving 1080 mg twice weekly will receive maintenance dosing at 1080 mg every 3 days, and subjects previously receiving 1080 mg every 3 days will receive maintenance dosing at 1080 mg 3 times weekly). Maintenance dosing does not exceed 1080 mg 3 times weekly.
Patients in the OLE study who experienced an acute BTH event but had chosen not to receive intensive pegcetacoplan could continue to participate in the OLE study with the potential to receive a dose adjustment to 1080 mg every third day or 3 times weekly.
Additional management through transfusions to improve anemia-related symptoms or eculizumab dosing to immediately restore adequate complement blockade can also be administered based on investigator judgment. A patient can receive subsequent intensive pegcetacoplan treatment courses for acute BTH, as long as the courses are separated by at least 14 days. Subsequent treatment courses for acute BTH are based on investigator’s decision, provided the subject meets eligibility criteria and can be either intensive IV or SC pegcetacoplan, regardless of which was used for a prior course.
Study population and baseline characteristics
All patients who received intensive pegcetacoplan dosing in the OLE study until the March 2022 data cut were included in this analysis.
High disease activity was assessed using the baseline characteristics on entry into the pegcetacoplan parent studies. Criteria for high disease activity were based on previously used parameters to evaluate the impact of eculizumab on complement-inhibitor–naïve patients14 and expanded to reflect more relevant endpoints in patients treated with complement inhibitors: LDH ≥1.5× ULN; hemoglobin <10 g/dL14; higher-than-label eculizumab dose; detectable CH50 on eculizumab; transfusion dependence (≥4 transfusions within 12 months before parent study entry)7,11; and history of thrombosis. Baseline values collected at entry into the OLE study were LDH, hemoglobin levels, absolute reticulocyte count, and bilirubin levels. Additional information collected included pegcetacoplan dosing regimen at entry into the OLE, dosing regimen before intensive pegcetacoplan dosing, and duration of pegcetacoplan exposure overall by subject. Medical records, records of concomitant medication, and AE reports were reviewed for potential complement-amplifying conditions (CAC) within 30 days before intensive pegcetacoplan treatment.
Outcome measures
The key efficacy outcome was absolute change in LDH from day 1 of intervention through day 21. Values were assessed on day 1 and 2, and after weeks 1, 2, and 3. Other efficacy outcomes included percentage of patients with LDH levels <2× ULN through day 21 following intensive pegcetacoplan treatment and the time to resolution of the AE of acute BTH as reported by the investigator. Levels of hemoglobin before, during, and after the intensive pegcetacoplan treatment were assessed. Outcome measures were evaluated with regard to concomitant red blood cell (RBC) transfusions and eculizumab administration.
Safety assessments included incidence and severity of AEs and serious AEs (SAEs) for up to 21 days after the start of intensive pegcetacoplan treatment. AEs of special interest included infections and thromboembolic events. All discontinuations because of an AE, including hemolysis, were assessed through day 21.
The incidence of recurrent acute BTH events fulfilling the same criteria as for eligibility, and levels of indirect bilirubin and absolute reticulocyte count, were assessed before and after intensive pegcetacoplan dosing as additional outcomes.
Due to the small sample size and the resulting high variability, no formal statistical testing was performed, and descriptive statistics were used to assess the data.
Results
Study population and baseline characteristics
In total, 13 of 137 (9%) patients who entered the OLE study had received intensive pegcetacoplan dosing at the time of the March 2022 data cut: 10 from PEGASUS, 2 from PRINCE, and 1 from PADDOCK. Patients were aged between 20 and 72 years, and 5 (38%) patients were female (Table 1).
Table 1.
Patient characteristics
| Patient | Parent study | Sex | Medical history of AA/MDS | Prior acute BTH episodes on PEG∗,† | Before entering parent study |
At OLE baseline |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ECU dose |
LDH (U/L) | Hb (g/dL) | CH50 (U/mL) |
RBC transfusions within 12 mo |
Medical history of thrombosis |
LDH (U/L) |
Hb (g/dL) | Reticulocytes (× 109/L) | Bilirubin (μmol/L) | PEG dose frequency before intensive treatment | |||||
| 1 | PEGASUS | Female | no/no | no | 900 mg every 2 wk | 265 | 8.6 | 0 | 2 | yes | 168 | 12.5 | 70 | 7.4 | BIW |
| 2 | PEGASUS | Female | no/no | yes | 900 mg every 2 wk | 177 | 8.1 | 9.43 | 1 | yes | 609 | 11.7 | 120 | 1.9 | Q3D‡ |
| 3 | PEGASUS | Female | no/no | yes | 900 mg every 2 wk | 159 | 8.2 | 2.42 | 1 | yes | 132 | 11.5 | 40 | 6.7 | BIW |
| 4 | PEGASUS | Female | yes/no | yes | 900 mg every 2 wk | 214 | 7.1 | 4.9 | 27 | no | 187 | 10.1 | 60 | 16.1 | BIW |
| 5 | PEGASUS | Female | no/no | yes | 1200 mg every 2 wk | 243 | 10.9 | 2.61 | 9 | no | 162 | 11.9 | 70 | 11.1 | Q3D‡ |
| 6 | PEGASUS | Male | yes/no | no | 900 mg every 2 wk | 213 | 8.7 | 0.13 | 6 | no | 154 | 11.6 | 80 | 8.2 | BIW |
| 7 | PEGASUS | Male | no/no | yes | 1200 mg every 2 wk | 216 | 9.3 | 6.4 | 0 | yes | 181 | 13.5 | 120 | 19.0 | Q3D‡ |
| 8 | PEGASUS | Male | yes/no | no | 900 mg every 2 wk | 188 | 8.5 | 0 | 8 | no | 262 | 11.8 | 80 | 10.4 | BIW |
| 9 | PEGASUS | Male | no/no | no | 900 mg every 2 wk | 503 | 6.7 | 22.35 | 3 | no | 336 | 13.9 | 120 | 5.3 | BIW |
| 10 | PEGASUS | Male | no/no | no | 1200 mg every 2 wk | 225 | 5.5 | 8.21 | 12 | no | 224 | 12.6 | NR | 31.0 | BIW |
| 11 | PADDOCK | Male | yes/no | yes | Treatment naïve | 1866 | 10.4 | 589 | 11 | no | 343 | 15.0 | 80 | 11.1 | TIW‡,§ |
| 12 | PRINCE | Male | no/no | no | Treatment naïve | 3195 | 9.7 | 143 | 16 | yes | 193 | 11.8 | 92 | 31.5 | BIW |
| 13 | PRINCE | Male | yes/no | no | Treatment naïve | 1371 | 8.6 | 72.17 | 15 | no | 283 | 8.0 | 78 | 16.6 | Q3D§ |
AA, aplastic anemia; BIW, twice weekly; BTH, breakthrough hemolysis; ECU, eculizumab; Hb, hemoglobin; LDH, lactate dehydrogenase; MDS, myelodysplastic syndrome; OLE, open-label extension; NR, not reported; PEG, pegcetacoplan; Q3D, every 3 days; RBC, red blood cell; TIW, 3 times weekly.
AE terms reported for acute BTH (hemolysis, BTH, IVH, and acute hemolysis) were used to assess prior acute BTH episodes.
Entire duration of PEG treatment.
Patient had LDH >2× ULN and experienced an AE of BTH that was deemed related to PNH by the investigators.
Patient had LDH >2× ULN and experienced an AE of anemia that was deemed related to PNH by the investigators.
Baseline characteristics on entry into the pegcetacoplan parent studies were used to assess the presence of criteria of high disease activity (Table 1). On entry into the PEGASUS parent study, 3 patients (23%) were receiving a higher-than-label dose of 1200 mg eculizumab and 7 patients (54%) were receiving the label dose of 900 mg every 2 weeks. The remaining 3 patients were treatment-naïve for complement inhibitors when they enrolled in PRINCE or PADDOCK. Eleven patients (85%) had hemoglobin levels <10 g/dL upon enrollment in their parent study (9 of 10 complement-inhibitor–experienced patients; 2 of 3 complement-inhibitor–naïve patients) and 11 patients had detectable CH50 (8 of 10 complement-inhibitor–experienced patients; all 3 complement-inhibitor–naïve patients). One of the patients previously treated with C5 inhibitor had an LDH measurement ≥1.5× ULN before entering the pegcetacoplan parent study. All 3 complement-inhibitor–naïve patients had LDH levels >1000 U/L. Number of RBC transfusions in the 12 months before entering the parent studies ranged from 0 to 27 transfusions (median of 8.0) with ≥4 transfusions administered in 8 patients (62%). Of the 13 patients, 5 (38%) had a medical history of thrombosis before entry into their parent study.
Patient characteristics, including hematological parameters at entry into the OLE, are presented in Table 1. All 13 patients included in this analysis received 1080 mg pegcetacoplan twice weekly on entry into the OLE study. At OLE entry, median LDH levels were 193 U/L (range, 132-609 U/L), and mean hemoglobin levels were 12.0 g/dL (range, 8-15 g/dL).
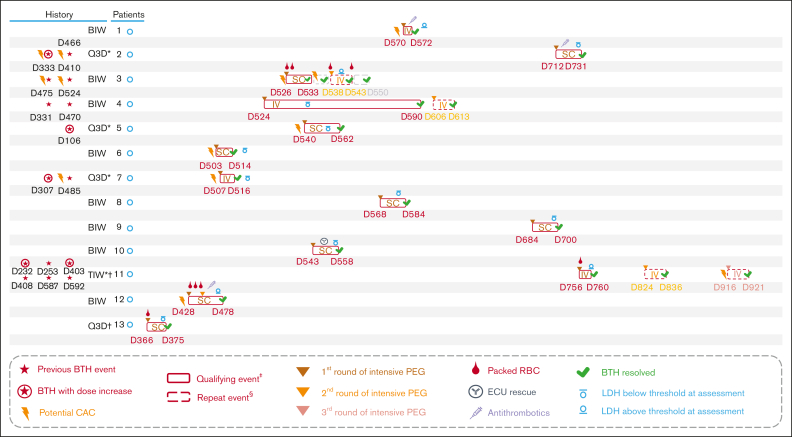
Immediately before beginning the intensive pegcetacoplan dosing regimen, 8 patients (62%) were receiving pegcetacoplan twice weekly, 4 patients (31%) were receiving pegcetacoplan every 3 days, and 1 patient (8%) was receiving pegcetacoplan 3 times weekly (Table 1), all in accordance with dose adjustments to manage increased LDH levels as allowed per study protocol. For 4 of the 5 patients with increased pegcetacoplan doses, AEs of BTH that were deemed related to PNH were reported leading up to the dose increase (Figure 2). The median time on pegcetacoplan treatment in the OLE study until the acute BTH event qualifying for intensive pegcetacoplan dosing was 77 weeks (range, 52-108). The median time from a qualifying acute BTH event until first dose of intensified treatment was 24 hours (range, 0-48).
Figure 2.
Management of acute BTH events in the OLE study. At the time of March 2022 data cut. ∗Patient had LDH >2× ULN and experienced an AE of BTH that was deemed related to PNH by the investigators. ꝉPatient had LDH >2× ULN and experienced an AE of anemia that was deemed related to PNH by the investigators. ‡Acute BTH event qualifying for administration of first round of intensive pegcetacoplan dosing. §Repeat acute BTH event treated with intensive pegcetacoplan dosing. BIW, twice weekly; BTH, breakthrough hemolysis; CAC, complement-amplifying condition; D, day; ECU, eculizumab; IV, intravenous; LDH, lactate dehydrogenase; PEG, pegcetacoplan; Q3D, every 3 days; RBC, red blood cell; SC, subcutaneous; TIW, 3 times weekly; ULN, upper limit of normal.
Efficacy
Acute BTH events were managed with intensive pegcetacoplan SC dosing in 9 patients (69%) and a single pegcetacoplan IV dose in 4 patients (31%) (Table 2; Figure 2). One patient received a second round of intensive pegcetacoplan dosing to manage the same acute BTH event (Figure 2). Three patients experienced repeat acute BTH events after resolution of the first event and were treated with a second round of intensive pegcetacoplan IV dosing (Table 2; Figure 2). One patient received a third round of intensive treatment for another repeat acute BTH event occurring during the study. Overall, all AEs associated with the acute BTH event treated with intensive pegcetacoplan dosing were considered resolved by the investigators (Figure 2). The median time to resolution of the initial AE of acute BTH (as reported by the investigator) was 15.0 days (interquartile range, 7.0-19.0).
Table 2.
Management of acute BTH events with intensive pegcetacoplan treatment in the OLE study∗
| Patient | Sex | Potential CAC† |
Management of acute BTH event |
Concomitant anticomplement treatment | Concomitant RBC transfusion | Subsequent rounds of intensive treatment‡ | LDH (U/L) prior to event§ |
LDH (U/L) at day 1 | LDH (U/L) at day 2 | LDH (U/L) at day 7-12¦ |
LDH (U/L) at day 14-19‖ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | Nasopharyngitis | IV | No | No | No | 625 | 2615 | 1802 | 469 | 617 |
| 2 | Female | Unexplained | SC | No | No | No | 334 | 1882 | 1607 | 272 | 176 |
| 3 | Female | Respiratory infection | SC | No | Yes | Yes | 224 | 3496¶ | NR | 954 | 2102 |
| 4 | Female | Unexplained | IV | No | No | Yes | 793 | 1425 | 1054 | 357 | 271 |
| 5 | Female | COVID-19 vaccination | SC | No | No | No | 229 | 976 | 1129 | 401 | 157 |
| 6 | Male | Left groin abscess |
SC | No | No | No | 268 | 436 | 448 | 182 | 144 |
| 7 | Male | COVID-19 and meningococcal vaccination |
IV | No | No | No | 201 | 1234 | 869 | 260 | 173 |
| 8 | Male | Unexplained | SC | No | No | No | 398 | 1335¶ | 1261 | 438 | 266 |
| 9 | Male | Unexplained | SC | No | No | No | 662 | 1961 | 1840 | 499 | 326 |
| 10 | Male | Unexplained | SC | Eculizumab 900 mg |
No | No | 193¶ | 1708¶ | 1849¶ (day 3) |
1517¶ | 359¶ |
| 11 | Male | Unexplained | IV | No | Yes | Yes | 456 | 5865 | 3868 | 1173 | 958 |
| 12 | Male | COVID-19 vaccination | SC | No | Yes | Yes | 273 | 6339¶ | 6767¶ | 841 | 452 |
| 13 | Male | Unexplained | SC | No | Yes | No | 402 | 751 | 662 | 397 | 267 |
BTH, breakthrough hemolysis; CAC, complement-amplifying condition; COVID-19, coronavirus disease 2019; NR, not reported; RBC, red blood cell.
In cases of multiple acute BTH events, the data relate to the first acute BTH event. Day 1 of intensive treatment may not necessarily mean day 1 of hemolysis event, therefore, LDH levels before event may not represent baseline values.
Potential CAC within 30 days before intensive pegcetacoplan treatment.
To manage subsequent acute BTH events additional doses of intensive pegcetacoplan IV were administered.
Most recent available measurement before intensive pegcetacoplan treatment.
Dependent on available measurement 7 to 12 days and 14 to 19 days after intensive pegcetacoplan treatment, respectively.
Data from local laboratory; laboratory values from local laboratories were standardized to central laboratory based on normal ranges from respective laboratories.
Concomitant RBC transfusions to improve anemia-related symptoms were administered in 4 of 13 patients (31%) (Table 2; Figure 2). Patients who required RBC transfusions had a mean hemoglobin drop of 3.3 g/dL compared with 2.4 g/dL drop in patients who did not undergo transfusion (mean hemoglobin drop for all patients, 2.7 g/dL). One patient (8%) received a concomitant dose of 900 mg eculizumab to restore adequate complement blockade 5 days after the initiation of intensive pegcetacoplan dosing because of sustained high LDH levels (after 2 weeks: LDH, 359 U/L; hemoglobin, 10.0 g/dL).
Six of the 13 patients (46%) reported a potential clinically-relevant CAC within 30 days before day 1 of intensive treatment (Table 2; Figure 2). Three patients (23%) experienced an AE that could qualify as a CAC (nasopharyngitis, respiratory infection, and left groin abscess) and 3 patients (23%) received a vaccination before the acute BTH event that could also qualify as a CAC (Table 2). Potential CACs were reported in 3 of 4 patients who had an LDH level >10× ULN on day 1 of intensive treatment and 4 of 9 patients who experienced a >2 g/dL drop in hemoglobin. Potential clinically-relevant CACs (fever and acute kidney injury, food poisoning) were additionally reported before half of the repeat acute BTH events that were treated with additional rounds of intensive pegcetacoplan dosing (Figure 2).
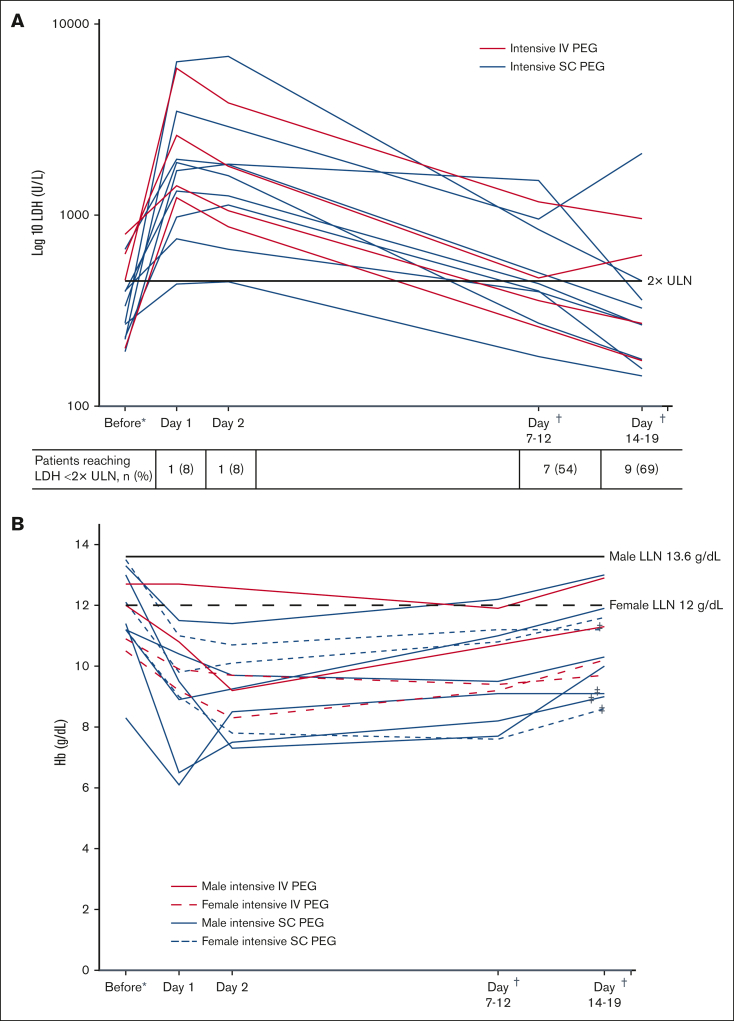
Levels of LDH over time by patient are shown in Table 2 and Figure 3A. LDH levels decreased between day 1 and day 2 in 8 of 12 evaluable patients (4 of 8 patients who underwent intensive SC and 4 of 4 patients who were treated with IV) and in all 13 patients at day 7 to 12. Nine of 13 patients (69%) (SC, 7 of 9; IV, 2 of 4) experienced an LDH of <2× ULN by day 14 to 19. Two patients experienced an increase in LDH levels by day 14 after a prior reduction in levels, 1 of whom had a repeat acute BTH event that was treated with an additional round of intensive pegcetacoplan.
Figure 3.
Levels of LDH and hemoglobin over time during management of the acute BTH event with intensive pegcetacoplan treatment. (A) LDH level on log 10 scale. (B) Hemoglobin level. In cases of multiple acute BTH events, data relate to first acute BTH event reported. All local laboratory values were standardized to a central laboratory based on normal ranges from respective laboratories. ∗Most recent available measurement before intensive pegcetacoplan treatment. †Dependent on available measurement 7 to 12 days and 14 to 19 days after intensive pegcetacoplan treatment, respectively. ‡Patients receiving transfusions during management of acute BTH. Hb, hemoglobin; LDH, lactate dehydrogenase; LLN, lower limit of normal; PEG, pegcetacoplan; ULN, upper limit of normal.
After the initial drop in hemoglobin caused by the acute BTH event, hemoglobin level improved for all patients regardless of administration of RBC transfusions over the course of intervention up to days 14 to 19. One patient experienced only a minimal drop in hemoglobin level after the acute BTH event. Hemoglobin change over time is shown in Figure 3B.
Mean absolute reticulocyte counts and bilirubin were increased above the ULN during the acute BTH episode and reduced from the peak value or returned to normal levels, respectively, with intensive pegcetacoplan treatment, within 14 to 19 days (Table 3). Platelet count remained largely unchanged during the acute BTH episode.
Table 3.
Summary of additional hematological measurements during management of the acute BTH event with intensive pegcetacoplan treatment
| Hematological parameters | Prior to event∗ | At day 1 | At day 2 | At day 7-12† |
At day 14-19† |
|---|---|---|---|---|---|
| Mean (SD) standardized values | |||||
| Absolute reticulocyte count, 109 cells/L (normal reference range, 30-120) |
107.5 (30.8) n = 12 | 140.7 (41.9) n = 9 | 138.0 (35.2) n = 10 | 137.3 (43.8) n = 11 |
136.4 (36.7) n = 11 |
| Indirect bilirubin, μmol/L (normal reference range, 1.7-15.4) |
12.5 (5.0) n = 13 |
30.3 (19.1) n = 11 | 16.7 (8.6) n = 11 | 9.7 (4.7) n = 12 |
11.1 (5.1) n = 11 |
| Direct bilirubin, μmol/L (normal reference range, 0.0-3.4) |
3.3 (1.5) n = 13 |
4.8 (1.9) n = 11 | 4.0 (1.9) n = 11 | 2.8 (1.7) n = 12 |
2.9 (1.5) n = 11 |
| Platelets, 109 cells/L (normal reference range, 140-400) |
143.9 (92.7) n = 13 | 152.2 (97.7) n = 10 | 147.0 (88.3) n = 11 | 143.9 (71.0) n = 12 |
150.6 (83.8) n = 12 |
In cases of repeat acute BTH events, the data relate to first acute BTH event. All local laboratory values were standardized to central laboratory based on normal ranges from respective laboratories. BTH, breakthrough hemolysis; RBC, red blood cell; SD, standard deviation.
Most recent available measurement before intensive pegcetacoplan treatment.
Dependent on available measurement 7-12 days and 14-19 days after intensive pegcetacoplan treatment, respectively.
Safety
Intensive treatment with pegcetacoplan was safe and well-tolerated with no different AEs identified compared with those in previous pegcetacoplan studies (Table 4). A total of 22 AEs occurred in 7 patients (54%) in the 21 days after the initiation of intensive pegcetacoplan treatment, with 4 AEs reported during the intensive pegcetacoplan treatment, defined as day 1 for IV and day 1 to 3 for SC (Table 4). The majority of events were mild in severity and not considered related to study drug.
Table 4.
Incidence and severity of AEs in the 21 days following the initiation of intensive pegcetacoplan treatment
| Patients (N = 13) | |
|---|---|
| Patients with ≥1 AE, n (%) | 7 (54) |
| Total AEs, n (%) | 22 |
| Mild | 13 (59) |
| Moderate | 4 (18) |
| Severe | 5 (23) |
| Related to PEG | 10 (45) |
| AEs during intensive dosing, n (%) | 4 (18) |
| Mild | 3 (14) |
| Moderate | 0 |
| Severe | 1 (5) |
| Related to PEG | 2 (9) |
| Serious AEs, n (%) | 3 (14) |
| Related to PEG | 1 (5) |
| AEs leading to treatment discontinuation, n (%) | 0 |
| AEs leading to death, n (%) | 0 |
| AEs by preferred term, n | |
| Infusion site erythema | 7 |
| Hemolysis | 5 |
| Increased LDH | 2 |
| Anemia | 1 |
| Blood creatinine increased | 1 |
| CRP increased | 1 |
| Chest discomfort | 1 |
| Headache | 1 |
| Infusion site induration | 1 |
| Pyrexia | 1 |
| Sepsis | 1 |
| AEs of interest | |
| Infection | 1 |
| Thrombosis | 0 |
AE, adverse event; CRP, C-reactive protein; LDH, lactate dehydrogenase; PEG, pegcetacoplan; SAE, serious adverse event.
There was a total of 3 SAEs from day 1 to day 21 (Table 4). Two events occurred on day 1 before intensive treatment. One was an event of sepsis, deemed possibly related to the study drug, and 1 event of hemolysis that led to intensive pegcetacoplan treatment, unlikely related to the study drug. The third event was an additional episode of hemolysis that led to another round of intensive treatment; this event was triggered by a COVID-19 infection and deemed not related to the study drug. Neither the acute BTH events qualifying the patients for intensive pegcetacoplan treatment nor any of the AEs or SAEs led to treatment discontinuation at the time of data cut. There were no deaths reported.
All AEs reported from day 1 of intensive pegcetacoplan treatment until day 21 are shown in Table 4. Infusion site reactions were the most common AEs (n = 8), accounting for 80% of AEs deemed related to study drug. Three patients experienced 5 acute hemolysis events as reported by the investigator in the 21 days after the initiation of intensive pegcetacoplan treatment. One patient had 3 events (Figure 2), of which 1 was treated with an additional round of pegcetacoplan (as per the protocol in which courses of intensive pegcetacoplan had to be separated by at least 14 days). The other 2 patients had 1 event each, which was also the event leading to intensive pegcetacoplan treatment in both cases. Potential clinically-relevant CACs (fever and acute kidney injury, nasopharyngitis, and vaccination) were reported before the hemolysis events in all 3 patients.
Besides 1 SAE of sepsis that started before the initiation of the intensive treatment, no other AEs of infection, in particular meningitis, were reported within the 21 days after the start of intervention. Despite high LDH levels at the time of the acute BTH, none of the patients experienced a thromboembolic event during the evaluation period. Three patients (23%) received antithrombotic agents (factor Xa inhibitor or low molecular weight heparin) during the acute BTH event (Figure 2).
Discussion
The novel data reported here support effective management of acute BTH events in patients on pegcetacoplan with intensive SC or IV pegcetacoplan dosing. Some patients benefited from additional RBC transfusion to improve anemia-related symptoms.
Mechanistically, the broad control of both IVH and EVH achieved through C3 inhibition leads to increased survival of PNH RBCs, which can approach levels of >90%.7,11 A proposed consequence of this high level of efficacy is a greater proportion of PNH RBCs that are susceptible to lysis with the subsequent potential for severe acute anemia.15,16 Here, we report acute BTH events characterized by a rapid increase in LDH levels coupled with a drop in hemoglobin levels. Intensive pegcetacoplan treatment led to a relatively rapid reduction in LDH levels (<2× ULN in 69% of patients) within 14 to 19 days of treatment initiation. Additionally, hemoglobin levels were stabilized in all patients during the intervention regardless of transfusion status. Overall, all acute BTH events treated with intensive pegcetacoplan dosing were considered resolved by the investigators.
In the setting of C5 inhibitor therapy, hemolysis events can result from incomplete blockade of C5 associated with both residual complement activity and low plasma levels of free eculizumab.17 These pharmacokinetic-driven breakthrough events may require a dose adjustment through an increased dose, a shorter interval between doses, or both to control disease symptoms.18 In addition, clinical conditions triggering complement activation, such as infection, vaccination, or surgery, which occur during complement inhibition can disrupt pharmacodynamics, despite adequate dosing, and increase the risk of breakthrough IVH.15,19,20 While hemolysis events in the context of C5 inhibitor therapy have been identified as largely pharmacokinetic in nature,15 the mechanisms underlying such in patients treated with pegcetacoplan remain an area of research.
In this analysis, approximately half of the patients who received intensive pegcetacoplan dosing experienced acute BTH that can be linked to a potential CAC, such as infection or vaccination, indicating a possible pharmacodynamic influence. Proximal therapeutic strategies for PNH target the alternative complement pathway; however, in vitro data demonstrate that a strong classical pathway activation, for example in relation to a CAC, can lead to C5 activation despite C3 inhibition. C3 bypass activation of C5 occurs as a result of surface-deposited C4b, which can recruit and prime C5 for consecutive proteolytic activation.21,22 Because this analysis is solely based on investigator reporting of AEs or concomitant medication, the number of CACs may be even higher. Based on the small available data set, no conclusions can be drawn on the mechanism underlying acute BTH events under proximal inhibition at this moment and further research is required.
When evaluating disease activity based on our adapted assessment, all 13 patients who received intensive pegcetacoplan dosing in the OLE study fulfilled at least 1 of the criteria for high disease activity at the time of entry into the parent study. The majority of patients in the OLE study (77%) rolled over from the PEGASUS study, a study population with difficult-to-control disease, including 30% of patients on a higher-than-label dose of eculizumab before entry, suggesting a population prone to experiencing hemolysis.7 Intensive pegcetacoplan treatment was effective in the management of acute BTH events, even in patients with difficult-to-control disease, and patients remained on pegcetacoplan throughout the intensive treatment period.
Overall, intensive treatment with pegcetacoplan was safe and well-tolerated, and most AEs were mild or moderate in severity. Patients have increased risk of thrombosis during hemolytic events23 and 3 of the 13 patients received antithrombotic agents during the acute BTH event, however no thromboembolic events were reported. The administration of antithrombotic agents was not defined in the study protocol.
Although hemoglobin levels stabilized in all patients during the intervention regardless of transfusion status, absolute changes in hemoglobin levels because of intensive pegcetacoplan treatment should be interpreted with caution in patients who received RBC transfusions during acute BTH management. In addition, the presented levels for hemoglobin and LDH before intensive pegcetacoplan treatment were those most recently available and may not necessarily reflect steady-state values. A further limitation is the timing of LDH measurements after intensive treatment, which might have affected the accuracy of the time to resolution of acute BTH. Overall, the small sample size of the study limits conclusions that can be drawn, in particular when assessing any potential differences between intensive SC and IV dosing.
Further investigation is required to complement the clinical data presented, in particular data on pharmacokinetics and pharmacodynamics, to better understand the optimization of pegcetacoplan treatment in individual patients and to allow identification of biomarkers indicative of BTH risk to prevent their occurrence. While the intensive treatment regimen might prevent pharmacokinetic BTH in patients with insufficient dosing and mitigate BTH in patients who were well dosed at the time of CAC, self-limitation of the BTH event owing to resolution of the underlying CAC cannot be ruled out without complement activity data collected at the time of BTH and during treatment with intensive pegcetacoplan.
Although progress has been made in the management of hemolysis during pegcetacoplan treatment, risk-mitigation strategies continue to be necessary. Strategies should focus on patient and clinician education, and patients switching from C5 inhibitors to pegcetacoplan should be closely observed for signs of hemolysis.24,25 If a hemolysis event occurs, immediate RBC transfusion, pegcetacoplan dose adjustment, or short-term administration of eculizumab to control the acute episode should be considered. Patients can remain on pegcetacoplan, with current prescribing information allowing for a pegcetacoplan dose increase from twice weekly to up to every 3 days in cases of LDH >2× ULN.9,10 Patients should be alerted to possible CACs and in the event of pharmacodynamic influence, the identified CAC should be treated in parallel with increased pegcetacoplan dosing per approved prescribing information.24,25
The management of acute BTH events in patients with PNH on complement inhibitors is an evolving field. With continued and expanded use of proximal inhibitors, management will become more optimal and patients at higher risk of acute BTH events easier to identify.
Conflict-of-interest disclosure: M.G. reports consultancy with BioCryst, Regeneron, and Swedish Orphan Biovitrum AB; received speaker’s fees from Alexion, AstraZeneca, Novartis, and Swedish Orphan Biovitrum AB; and served as scientific advisory board member for Alexion, AstraZeneca, BioCryst, and Novartis. R.K. reports consultancy with Swedish Orphan Biovitrum AB and Otsuka; received speaker’s fees from Alexion, Swedish Orphan Biovitrum AB, Jazz, Astellas, and Biologix; served as scientific advisory board member for Alexion, Swedish Orphan Biovitrum AB, Jazz, Abbvie, and Amgen; and received research funding from Novartis and Swedish Orphan Biovitrum AB. J.P. reports consultancy, honoraria, and membership on an entity's board of directors or advisory committees with Apellis Pharmaceuticals, Blueprint Medicines, Bristol Myers Squibb, F. Hoffmann-La Roche Ltd, Grunenthal, and MSD Merck Sharp & Dohme; reports consultancy and membership on an entity's board of directors or advisory committees with Amgen; participated in speakers bureau with Chugai and Pfizer; and reports membership on an entity's board of directors or advisory committees and speakers bureau with Alexion, Boehringer Ingelheim, and Novartis. C.d.C. reports research funding from Alexion Pharmaceuticals and Apellis Pharmaceuticals; consultancy with Apellis Pharmaceuticals; and received honoraria from Novartis, Alexion Pharmaceuticals, BioCryst, and Apellis Pharmaceuticals. J.S. reports research support from Alexion, Apellis Pharmaceuticals, Novartis, Ra Pharma, Sanofi-Genzyme; reports consultancy with BioCryst; received speaker’s fees from Alexion, Novartis, and Swedish Orphan Biovitrum AB; and served as scientific advisory board member for Link Pharmaceuticals, Novartis, Sanofi-Genzyme, Swedish Orphan Biovitrum AB. R.H. is presently employed by Swedish Orphan Biovitrum AB and hold company shares. L.T. is a consultant to Swedish Orphan Biovitrum AB. M.Y. is currently employed by Apellis Pharmaceuticals, Inc and hold company shares. R.P.d.L. reports consultancy, honoraria, and research funding with Alexion, Novartis, and Pfizer; received research funding from Amgen; and reports consultancy and honoraria with Apellis Pharmaceuticals and Swedish Orphan Biovitrum AB.
Acknowledgments
The authors thank Jessica Savage for significant review of the manuscript.
The study was funded by Apellis Pharmaceuticals, Inc. The analysis was funded by Swedish Orphan Biovitrum AB. Medical writing support was provided by Miriam Souto and Elizabeth Oliver of nspm ltd (Meggen, Switzerland) and funded by Swedish Orphan Biovitrum AB and Apellis Pharmaceuticals, Inc.
Authorship
Contribution: The sponsor (Apellis Pharmaceuticals) and the authors designed the study; trial investigators collected the data; all authors contributed to the analysis or interpretation of the data as well as revision of the manuscript; the authors vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol; Swedish Orphan Biovitrum AB and Apellis Pharmaceuticals reviewed the manuscript during the development process; and the authors maintained full editorial control of the manuscript, all of whom provided their final approval of the manuscript submitted for publication; and all authors analyzed the data and had full access to all clinical trial data.
Footnotes
Presented in abstract form at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 10-13 December 2022.
Data access will be granted in response to qualified research requests. Individual participant data will not be shared. All requests are evaluated by a cross-functional panel of experts within Sobi, and a decision on sharing will be based on the scientific merit and feasibility of the research proposal, maintenance of personal integrity, and commitment to publication of the results. To request access to study data, data sharing request form (available on www.sobi.com) should be sent to medical.info@sobi.com.
References
- 1.Peffault de Latour R, Hosokawa K, Risitano AM. Hemolytic paroxysmal nocturnal hemoglobinuria: 20 years of medical progress. Semin Hematol. 2022;59(1):38–46. doi: 10.1053/j.seminhematol.2022.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Brodsky RA, Young NS, Antonioli E, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111(4):1840–1847. doi: 10.1182/blood-2007-06-094136. [DOI] [PubMed] [Google Scholar]
- 3.Kulasekararaj AG, Hill A, Langemeijer S, et al. One-year outcomes from a phase 3 randomized trial of ravulizumab in adults with paroxysmal nocturnal hemoglobinuria who received prior eculizumab. Eur J Haematol. 2021;106(3):389–397. doi: 10.1111/ejh.13564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Risitano AM, Notaro R, Marando L, et al. Complement fraction 3 binding on erythrocytes as additional mechanism of disease in paroxysmal nocturnal hemoglobinuria patients treated by eculizumab. Blood. 2009;113(17):4094–4100. doi: 10.1182/blood-2008-11-189944. [DOI] [PubMed] [Google Scholar]
- 5.Hill A, Rother RP, Arnold L, et al. Eculizumab prevents intravascular hemolysis in patients with paroxysmal nocturnal hemoglobinuria and unmasks low-level extravascular hemolysis occurring through C3 opsonization. Haematologica. 2010;95(4):567–573. doi: 10.3324/haematol.2009.007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Castro C, Grossi F, Weitz IC, et al. C3 inhibition with pegcetacoplan in subjects with paroxysmal nocturnal hemoglobinuria treated with eculizumab. Am J Hematol. 2020;95(11):1334–1343. doi: 10.1002/ajh.25960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillmen P, Szer J, Weitz I, et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2021;384(11):1028–1037. doi: 10.1056/NEJMoa2029073. [DOI] [PubMed] [Google Scholar]
- 8.Wong RSM, Navarro-Cabrera JR, Comia NS, et al. Pegcetacoplan controls hemolysis in complement inhibitor-naive patients with paroxysmal nocturnal hemoglobinuria. Blood Adv. 2023;7(11):2468–2478. doi: 10.1182/bloodadvances.2022009129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.EMPAVELI (pegcetacoplan) US prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/215014s002lbl.pdf
- 10.Aspaveli (pegcetacoplan) EMA summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/aspaveli-epar-product-information_en.pdf
- 11.Peffault de Latour R, Szer J, Weitz IC, et al. Pegcetacoplan versus eculizumab in patients with paroxysmal nocturnal haemoglobinuria (PEGASUS): 48-week follow-up of a randomised, open-label, phase 3, active-comparator, controlled trial. Lancet Haematol. 2022;9(9):e648–e659. doi: 10.1016/S2352-3026(22)00210-1. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman K, Machaidze Z, Yeh M, Weitz IC. Evaluation of the long-term safety and efficacy of pegcetacoplan treatment for paroxysmal nocturnal hemoglobinuria patients: an extension study. Blood. 2021;138(1):2175. [Google Scholar]
- 13.Patriquin CJ, Bogdanovic A, Griffin M, et al. Long-term safety and efficacy of pegcetacoplan treatment in adults with paroxysmal nocturnal hemoglobinuria. Blood. 2022;140(1):2921–2923. [Google Scholar]
- 14.Schrezenmeier H, Röth A, Araten DJ, et al. Baseline clinical characteristics and disease burden in patients with paroxysmal nocturnal hemoglobinuria (PNH): updated analysis from the International PNH Registry. Ann Hematol. 2020;99(7):1505–1514. doi: 10.1007/s00277-020-04052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Risitano AM, Peffault de Latour R, Marano L, Frieri C. Discovering C3 targeting therapies for paroxysmal nocturnal hemoglobinuria: achievements and pitfalls. Semin Immunol. 2022;59 doi: 10.1016/j.smim.2022.101618. [DOI] [PubMed] [Google Scholar]
- 16.Notaro R, Luzzatto L. Breakthrough hemolysis in PNH with proximal or terminal complement inhibition. N Engl J Med. 2022;387(2):160–166. doi: 10.1056/NEJMra2201664. [DOI] [PubMed] [Google Scholar]
- 17.Peffault de Latour R, Fremeaux-Bacchi V, Porcher R, et al. Assessing complement blockade in patients with paroxysmal nocturnal hemoglobinuria receiving eculizumab. Blood. 2015;125(5):775–783. doi: 10.1182/blood-2014-03-560540. [DOI] [PubMed] [Google Scholar]
- 18.Kelly R, Arnold L, Richards S, et al. Modification of the eculizumab dose to successfully manage intravascular breakthrough hemolysis in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;112(11):3441. [Google Scholar]
- 19.Brodsky RA. Eculizumab: another breakthrough. Blood. 2017;129(8):922–923. doi: 10.1182/blood-2017-01-760496. [DOI] [PubMed] [Google Scholar]
- 20.Harder MJ, Kuhn N, Schrezenmeier H, et al. Incomplete inhibition by eculizumab: mechanistic evidence for residual C5 activity during strong complement activation. Blood. 2017;129(8):970–980. doi: 10.1182/blood-2016-08-732800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mannes M, Dopler A, Zolk O, et al. Complement inhibition at the level of C3 or C5: mechanistic reasons for ongoing terminal pathway activity. Blood. 2021;137(4):443–455. doi: 10.1182/blood.2020005959. [DOI] [PubMed] [Google Scholar]
- 22.Risitano AM, Frieri C, Urciuoli E, Marano L. The complement alternative pathway in paroxysmal nocturnal hemoglobinuria: from a pathogenic mechanism to a therapeutic target. Immunol Rev. 2023;313(1):262–278. doi: 10.1111/imr.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill A, Kelly RJ, Hillmen P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood. 2013;121(25):4985–4996. doi: 10.1182/blood-2012-09-311381. [DOI] [PubMed] [Google Scholar]
- 24.Griffin M, Muus P, Munir T, et al. Experience of compassionate-use pegcetacoplan for paroxysmal nocturnal hemoglobinuria. Blood. 2023;141(1):116–120. doi: 10.1182/blood.2022017266. [DOI] [PubMed] [Google Scholar]
- 25.Hillmen P, Risitano AM, Peffault de Latour R. Pegcetacoplan versus eculizumab in PNH. Reply. N Engl J Med. 2021;385(18):1725–1726. doi: 10.1056/NEJMc2106424. [DOI] [PubMed] [Google Scholar]