Abstract
Objective
We aimed to define the clinical features and antimicrobial susceptibility profiles of Burkholderia cepacia complex infections and to determine the predictors for mortality.
Materials and Methods
Our single-center retrospective study included patients with nosocomial B. cepacia complex infection between 2018 and 2022. We evaluated the predictors of 14-day and 28-day mortality by analyzing clinical and microbiological data.
Results
A total of 87 patients were included. Most infections (79.3%) occurred in the intensive care units (ICUs). Among B. cepacia complex isolates, 74.7% were susceptible to trimethoprim-sulfamethoxazole, 70.3% to levofloxacin, 50% to meropenem, and 23.4% to ceftazidime. The rates of 14-day mortality, 28-day mortality, and in-hospital mortality were 41.3% (n=36), 52.8% (n=46), and 64.3% (n=56), respectively. Multivariate analysis revealed neutrophil/lymphocyte ratio (NLR) (odds ratio [OR]=1.05, p=0.024), platelet count (OR=1.00, p=0.011), creatinine (OR=2.14, p=0.006), and aspartate aminotransferase (AST) (OR=1.02, p=0.028) as predictors for 14-day mortality. In addition to NLR (OR=1.07, p=0.014), platelet count (OR=1.00, p=0.039), creatinine (OR=2.05, p=0.008), and AST (OR=1.02, p=0.035), procalcitonin (OR=1.05, p=0.049) was also found as an independent predictor for 28-day mortality. In receiver operating characteristic (ROC) curve analysis for predicting 14-day mortality, area under the ROC curve (AUC) values were 0.684 (p=0.003) in NLR, 0.719 (p<0.001) in platelet count, 0.673 (p=0.003) in procalcitonin, 0.743 (p<0.001) in creatinine, and 0.700 (p<0.001) in AST. In ROC curve analysis for predicting 28-day mortality, AUC values were 0.674 (p=0.002) in NLR, 0.651 (p=0.010) in platelet count, 0.638 (p=0.020) in procalcitonin, 0.730 (p<0.001) in creatinine, and 0.692 (p=0.001) in AST.
Conclusion
Increasing antibiotic resistance and higher mortality rates justify that B. cepacia complex is a significant threat to hospitalized patients, especially in ICUs. Elevated levels of NLR, AST, creatinine, procalcitonin, and decreased platelet may predict poor clinical outcomes and could help clinicians in the management of this notorious bacterial pathogen.
Keywords: antimicrobial susceptibility, antibiotic resistance, Burkholderia cepacia complex, mortality, predictors
Highlights.
Burkholderia cepacia complex is a significant threat to hospitalized patients with invasive devices, including mechanical ventilators, central venous catheters, and urinary catheters in intensive care units.
Elevated levels of neutrophil/lymphocyte ratio, aspartate aminotransferase, creatinine, procalcitonin, and decreased platelet may predict poor clinical outcomes.
The rates of 14-day mortality, 28-day mortality, and in-hospital mortality were 41.3%, 52.8%, and 64.3%, respectively.
Introduction
Burkholderia cepacia complex is aerobic, non-fermentative, multi-drug resistant Gram-negative bacilli containing 24 opportunistic pathogenic species (1). B. cepacia complex members are commonly found in natural environments because they easily adapt to harsh environments due to their genotypic and phenotypic plasticity and ability to mutate rapidly. B. cepacia complex can also grow substantially and survive in water-based environments (2). Unlike most opportunistic pathogens, B. cepacia complex members are not prone to commensal carriage, and these bacteria are usually acquired from hospital settings or the environment (3). Thanks to all these features, they have been easily colonized in medicines and health equipment and reported in several hospital-acquired outbreaks in the last 20 years (4-5). For this reason, the US Food and Drug Administration (FDA) has suggested including B. cepacia complex bacteria in the category of "objectionable microorganisms" (4).
Since B. cepacia complex is cleared by airway mucociliary activity, they rarely cause respiratory tract infections in immunocompetent individuals (6). However, in conditions such as cystic fibrosis and chronic granulomatous disease in which mucociliary activity is impaired, these bacteria colonize the respiratory tract and cause variable clinical pictures ranging from asymptomatic carriage to urinary tract infections, bloodstream infections, and meningitis as well as pneumonia (1, 7, 8).
B. cepacia complex infections have been reported in immunosuppressive and hospitalized patients, especially in the intensive care units (ICUs) (9). The main risk factors in these patients are receiving hemodialysis, having a urinary catheter, central venous catheter, and endotracheal tube (10).
Most B. cepacia complex strains are intrinsically resistant to many antibiotics, such as beta-lactam antibiotics, aminoglycosides, and polymyxins, due to their efflux pump systems and beta-lactamase enzymes. Because of this antimicrobial resistance profile, patients cannot usually be commenced on appropriate empirical antibiotic therapy (11, 12). Therefore, early diagnosis and treatment of the disease gain more importance. However, there are a limited number of studies other than case series describing the clinical course of this infection, which has a high mortality but appears to be rare (13, 14). In this study, we aimed to define the clinical features and antimicrobial susceptibility profiles of B. cepacia complex and determine the predictors for mortality.
Material and Methods
Study Design
This is a cross-sectional, epidemiological study. Patients with isolated B. cepacia complex in their clinical samples between January 2018 and December 2022 were included. Patients under the age of 18 or not hospitalized were excluded. In patients with multiple culture positivity, only the first episode was included in the analysis. Demographic characteristics of patients (age, gender, chronic diseases, and immunosuppressive conditions), laboratory parameters at onset (when culture samples were taken), and clinical results were collected retrospectively through the electronic medical record system.
This study complied with the Declaration of Helsinki, and the Bakırköy Dr. Sadi Konuk Training and Research Hospital Clinical Research Ethics Committee approved the study with decision number 2023-08-25 on April 17, 2023.Written informed consent was waived due to the retrospective nature of this study.
Clinical Data
Infections that developed within the first 48 hours after hospitalization were defined as "community-acquired", and infections that started after 48 hours were defined as "hospital-acquired" (15). Empirical treatment was defined as treatment administered before culture results were obtained. We classified empirical antibiotic therapy as "appropriate" if the isolated microorganism is susceptive and "inappropriate" if resistant. Patients with human immunodeficiency virus (HIV) co-infection, active malignancies, solid organ transplant recipients, and patients receiving immunosuppressive therapy for any reason were defined as immunosuppressive. Chemotherapy, B-cell-depleting agents, tyrosine kinase inhibitors, chronic steroids (≥10 mg/day prednisone or equivalent), tumor necrosis factor α inhibitors, and other cytokine inhibitors were considered immunosuppressive therapies (9). COVID-19 co-infection was defined as co-existence with laboratory-confirmed COVID-19 and B. cepacia complex infection during the hospitalization. Death was evaluated at 14 days and 28 days after culture collection.
Microbiological Data
All the clinical samples were inoculated on 5% sheep blood agar, eosin methylene blue (EMB) agar, chocolate agar and incubated at 37°C for 24-48 hours. Species-level typing and antibiotic susceptibility testing of motile, nonlactose fermenting Gram-negative bacilli with positive oxidase and catalase test were performed using VITEK 2 Compact (bioMérieux, Marcy l'Etoile, France) automated system. In our hospital, the evaluation of antibiotic susceptibility tests has been performed according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) recommendations since 2017 (16). However, the Turkish Working Group on Standardization of Antimicrobial Susceptibility Tests (ADTS) recommends (17) the use of Clinical and Laboratory Standards Institute (CLSI) criteria for the evaluation of B. cepecia antibiotic susceptibility tests until the evaluation criteria are defined in the EUCAST. Therefore, the CLSI criteria were used for B. cepecia complex according to recommendations of the ADTS ( 18). Antimicrobial susceptibility testing was performed using the Kirby Bauer disc diffusion method for trimethoprim-sulfamethoxazole, ceftazidime, meropenem, and the gradient diffusion method for levofloxacin according to the recommendations of the CLSI. The zone diameter breakpoints in the CLSI are as follows:
Trimethoprim-sulfamethoxazole (1.25 /23.75 µg); ≥ 16 mm susceptible, ≤ 10 mm resistant,
Meropenem (10 µg); ≥ 20 mm susceptible, ≤ 15 resistant,
Ceftazidime (30 µg); ≥ 21 mm susceptible, ≤ 17 resistant.
The minimuminhibitory concentration (MIC) breakpoints are as follows in the CLSI: levofloxacin: ≤ 2 mg/L susceptible, ≥ 8 mg/L resistant.
Statistical Analysis
Categorical parameters were expressed as numbers (n) and percentages (%). Continuous variables were expressed as mean ± standard deviation. Categorical variables were compared by Chi-square and Fisher's exact tests. The Mann-Whitney U test was used to compare continuous variables. Univariate and multivariate logistic regression analyses were performed to identify independent predictors for 14-day and 28-day mortality. Receiver operating characteristic (ROC) curve analysis was applied to determine the accuracies of laboratory parameters that were significant in the multivariate analysis. A p-value less than 0.05 was considered statistically significant. The statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) 21.0 (IBM Corp., Armonk, NY, USA).
Results
A total of 118 B. cepacia complex strains were detected during the study. Ten isolates with repeated samples from the same patient and 21 isolates considered colonization were excluded. Finally, 87 patients with B. cepacia complex infections were included in the study. Of these patients, 59.8% (n=52) were male; the mean age was 59.0±18.3 years. The most common comorbidities were coronary artery disease (CAD) (28.7%), hypertension (27.6%), and diabetes mellitus (23.0%). All infections were hospital-acquired; the mean hospital stay before culture positivity was 19.1±10.4 days. Infections with B. cepacia complex were most frequently occurred in the ICUs (79.3%).
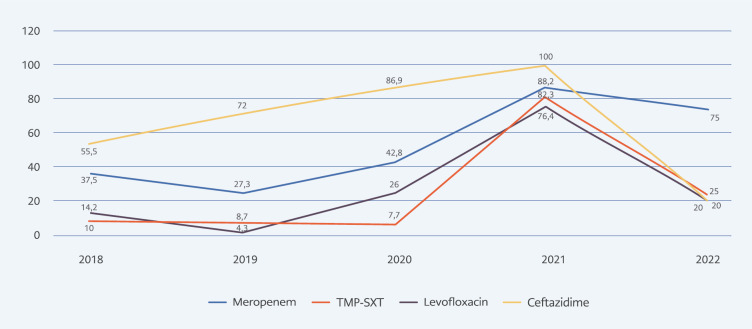
In total, we obtained 57 isolates from endotracheal aspirate cultures, 21 isolates from blood cultures, four isolates from sputum cultures, three isolates from abscess cultures, and two isolates from urine cultures. Of these isolates, 74.7% (n=62/83) were susceptible to trimethoprim-sulfamethoxazole, 70.3% (n=52/74) to levofloxacin, 50% (n=36/72) to meropenem, and 23.4% (n=18/72) to ceftazidime. The resistance rates in B. cepacia complex isolates according to the years are shown in Figure 1. Pre-pandemic (2018-2019 years) and post-pandemic (2020-2022 years) periods were compared in terms of resistance rates. Although meropenem (32.2% vs. 47.7%, p=0.191), trimethoprim-sulfamethoxazole (15.2% vs. 30.0%, p=0.110), levofloxacin (21.5% vs. 26.1%, p=0.651) and ceftazidime (71.0% vs. 80.4%, p=0.336) resistance rates all increased, no statistically significant difference was found (Table 1).
Figure 1.
Distribution of antimicrobial resistance in Burkholderia cepacia complex isolates between 2018-2022.
Table 1.
Antimicrobial susceptibility profiles of Burkholderia cepacia complex isolates
| Antimicrobial susceptibility | 2018-2019 years |
2020-2022 years |
p | OR |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Meropenem (n=72) | 0.191 | 1.98 | ||
| Susceptible | 19 (67.8) | 23 (52.3) | ||
| Intermediate / Resistant | 9 (32.2) | 21 (47.7) | ||
| TMP-SXT (n=83) | 0.110 | 2.47 | ||
| Susceptible | 28 (84.8) | 35 (70.0) | ||
| Intermediate / Resistant | 5 (15.2) | 15 (30.0) | ||
| Levofloxacin (n=74) | 0.651 | 1.29 | ||
| Susceptible | 22 (78.5) | 34 (73.9) | ||
| Intermediate / Resistant | 6 (21.5) | 12 (26.1) | ||
| Ceftazidime (n=77) | 0.336 | 1.68 | ||
| Susceptible | 9 (29.0) | 9 (19.6) | ||
| Intermediate / Resistant | 22 (71.0) | 37 (80.4) | ||
TMP-SXT: Trimethoprim-sulfamethoxazole, OR: Odds ratio.
51 (58.6%) patients had ventilator-associated pneumonia, 21 (24.1%) patients had bacteremia, 10 (11.5%) patients had nosocomial pneumonia, and 5 (5.7%) patients had other systemic infections. Appropriate empirical antibiotic therapy was initiated in 50.6% (n=44/87) of the patients. 44 (50.6%) patients had concomitant bacterial co-infection. The most common co-pathogens were Acinetobacter sp. (18.4%, n=16/87), Klebsiella sp. (11.5%, n=10/87), Enterococci (9.2%, n=8/87), coagulase-negative Staphylococci (8.0%, n=7/87) and Candida sp. (6.9%, n=6/87), respectively. The rates of 14-day mortality, 28-day mortality, and in-hospital mortality were 41.3% (n=36), 52.8% (n=46), and 64.3% (n=56), respectively.
The 14-day mortality (52.1% vs. 0.0%, p<0.001) and 28-day mortality (65.2% vs. 5.5%, p<0.001) were significantly higher in patients in the ICU than those in the general wards. The frequency of urinary catheterization (UC) and central venous catheterization (CVC) in survivors was lower than in patients who died at 14 days ([UC; 82.4% vs. 100%, p=0.008], [CVC; 68.6% vs. 100%, p<0.001]) and 28 days ([UC; 80.5% vs. 97.8%, p=0.010], [CVC; 61.0% vs. 100%, p<0.001]) (Table 2). Patients who died in 14 days had lower lymphocyte count (1.0±0.7 vs. 1.4±0.7, p=0.006) and platelet count (212±147 vs. 321±120, p<0.001), and higher neutrophil/lymphocyte ratio (NLR) (17.3±13.3 vs. 10.2±11.7, p=0.004), urea (140±140 vs. 59±41, p<0.001), creatinine (2.11±1.86 vs. 0.95±0.90, p<0.001), aspartate aminotransferase (AST) (112±134 vs. 44±27, p=0.002) and procalcitonin (12.4±22.4 vs. 5.1±13.2, p=0.003) than survivors. Similarly, patients who died in 28 days had lower lymphocyte count (1.1±0.7 vs. 1.5±0.7, p=0.003) and platelet count (239±144 vs. 318±128, p=0.015) and higher NLR (16.7±14.5 vs. 9.2±9.2, p=0.005), urea (122±128 vs. 58±45, p<0.001), creatinine (1.87±1.72 vs. 0.93±0.97, p<0.001), AST (99±122 vs. 43±28, p=0.020) and procalcitonin (10.2±20.2 vs. 5.8±14.81, p=0.010) than survivors.
Table 2.
Univariate associations of categorical variables for 14-day and 28-day mortality in patients with Burkholderia cepacia complex
| Parameters | Total n=87n (%) | 14-day outcome |
28-day outcome |
||||
|---|---|---|---|---|---|---|---|
| Mortality n=36 n (%) | Survival n=51 n (%) | p | Mortality n=46 n (%) | Survival n=41 n (%) | p | ||
|
Gender
| |||||||
| Male | 52 (59.8) | 18 (50.0) | 34 (66.7) | 0.071 | 24 (52.2) | 28 (68.3) | 0.128 |
| Female | 35 (40.2) | 18 (50.0) | 17 (33.3) | 22 (47.8) | 13 (31.7) | ||
| Age (mean ± SD) | 59.0±18.3 | 61.9±18.7 | 57.0±12.9 | 0.231 | 61.4±16.9 | 56.3±19.5 | 0.208 |
| Diabetes mellitus | 20 (23.0) | 6 (16.7) | 14 (27.5) | 0.242 | 9 (19.6) | 11 (26.8) | 0.424 |
| Hypertension | 24 (27.6) | 11 (30.6) | 13 (25.5) | 0.605 | 13 (28.3) | 11 (26.8) | 0.882 |
| Coronary artery disease | 25 (28.7) | 8 (22.2) | 17 (33.3) | 0.262 | 12 (26.1) | 13 (31.7) | 0.565 |
| Chronic kidney disease | 6 (6.9) | 4 (11.1) | 2 (3.9) | 0.195 | 5 (10.9) | 1 (2.4) | 0.124 |
| Asthma/COPD | 10 (11.5) | 5 (13.9) | 5 (9.8) | 0.559 | 6 (13.0) | 4 (9.8) | 0.633 |
| Malignancy | 11 (12.6) | 5 (13.9) | 6 (11.8) | 0.770 | 6 (13.0) | 5 (12.2) | 0.906 |
| COVID-19 co-infection | 31 (35.6) | 14 (38.9) | 17 (33.3) | 0.596 | 18 (39.1) | 13 (31.7) | 0.473 |
| Steroid use | 29 (33.3) | 11 (30.6) | 18 (35.3) | 0.646 | 14 (30.4) | 15 (36.6) | 0.546 |
| Use of immunosuppressive agents (non-steroid) | 15 (17.2) | 6 (16.7) | 9 (17.6) | 0.906 | 7 (15.2) | 8 (19.5) | 0.599 |
| Urinary catheterization | 78 (89.7) | 36 (100) | 42 (82.4) | 0.008 | 45 (97.8) | 33 (80.5) | 0.010 |
| Central venous catheter | 71 (81.6) | 36 (100) | 35 (68.6) | <0.001 | 46 (100) | 26 (61.0) | <0.001 |
|
Type of inpatient unit | |||||||
| General/Surgery ward | 18 (20.7) | 0 (0.0) | 18 (35.3) | <0.001 | 1 (2.2) | 17 (51.5) | <0.001 |
| Intensive care unit | 69 (79.3) | 36 (100) | 33 (64.7) | 45 (97.8) | 24 (58.5) | ||
| Time from hospitalization to the onset of infection (mean ± SD) | 19.1±10.4 | 16.4±6.7 | 20.9±12.1 | 0.071 | 17.1±7.2 | 21.3±12.9 | 0.141 |
| Bacteremia | 21 (24.1) | 8 (22.22) | 13 (25.5) | 0.727 | 13 (28.3) | 8 (19.5) | 0.344 |
| Overall pneumonia | 61 (70.1) | 27 (75.0) | 34 (66.7) | 0.406 | 32 (69.6) | 29 (70.7) | 0.906 |
| Ventilator-associated pneumonia | 51 (58.6) | 25 (69.4) | 26 (51.0) | 0.087 | 30 (65.2) | 21 (51.2) | 0.188 |
| Pneumonia | 10 (11.5) | 2 (5.6) | 8 (15.7) | 0.147 | 2 (4.3) | 8 (19.5) | 0.027 |
| Other infections | 5 (5.7) | 1 (2.85) | 4 (7.8) | 0.320 | 1 (2.2) | 4 (9.8) | 0.132 |
| Appropriate empirical treatment | 44 (50.6) | 16 (44.4) | 28 (54.9) | 0.339 | 23 (50.0) | 21 (51.2) | 0.910 |
| Co-infection | 44 (50.6) | 18 (50.0) | 26 (51.0) | 0.929 | 24 (52.2) | 20 (48.8) | 0.753 |
| Acinetobacter spp. | 16 (18.4) | 7 (19.4) | 9 (17.6) | 0.832 | 8 (17.4) | 8 (19.5) | 0.800 |
| Klebsiella spp. | 10 (11.5) | 5 (13.9) | 5 (9.8) | 0.559 | 5 (10.9) | 5 (12.2) | 0.847 |
| Enterococcus spp. | 8 (9.2) | 2 (5.6) | 6 (11.8) | 0.326 | 3 (6.5) | 5 (12.2) | 0.363 |
|
Antimicrobial susceptibility | |||||||
| Meropenem (n=72) | |||||||
| Susceptible | 36 (50.0) | 12 (44.4) | 24 (53.3) | 0.694 | 15 (44.1) | 21 (55.3) | 0.635 |
| Intermediate | 6 (8.3) | 3 (11.1) | 3 (6.7) | 3 (8.8) | 3 (7.9) | ||
| Resistant | 30 (41.7) | 12 (44.4) | 18 (40.0) | 16 (47.1) | 14 (36.8) | ||
|
TMP-SXT (n=83) | |||||||
| Susceptible | 62 (74.7) | 25 (75.8) | 37 (74.0) | 0.590 | 33 (76.7) | 29 (72.5) | 0.417 |
| Intermediate | 1 (1.2) | 0 (0.0) | 1 (2.0) | 1 (2.3) | 0 (0.0) | ||
| Resistant | 20 (24.1) | 8 (24.2) | 12 (24.0) | 9 (20.9) | 11 (27.5) | ||
|
Levofloxacin (n=74) | |||||||
| Susceptible | 52 (70.3) | 21 (70.0) | 31 (70.5) | 0.921 | 27 (71.1) | 25 (69.4) | 0.990 |
| Intermediate | 4 (5.4) | 2 (6.7) | 2 (4.5) | 2 (5.3) | 2 (5.6) | ||
| Resistant | 18 (24.3) | 7 (23.3) | 11 (25.0) | 9 (23.7) | 9 (25.0) | ||
|
Ceftazidime (n=77) | |||||||
| Susceptible | 18 (23.4) | 5 (16.7) | 13 (27.7) | 0.277 | 8 (21.1) | 10 (25.6) | 0.634 |
| Intermediate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Resistant | 59 (76.6) | 25 (83.3) | 34 (72.3) | 30 (78.9) | 29 (74.4) | ||
COPD: Chronic obstructive pulmonary disease, TMP-SXT: Trimethoprim-sulfamethoxazole, SD: Standard deviation.
Table 3.
Univariate associations of laboratory variables for 14-day and 28-day mortality in patients with Burkholderia cepacia complex.
| Parameters | Total | 14-day outcome |
28-day outcome |
||||
|---|---|---|---|---|---|---|---|
| Mortality(mean±SD) | Survival(mean±SD) | p | Mortality(mean±SD) | Survival(mean±SD) | p | ||
| Leukocyte count (103/mm3) | 15.1±10.5 | 17.6±14.6 | 13.4±5.8 | 0.228 | 16.9±13.4 | 13.1±5.2 | 0.258 |
| Neutrophil count (103/mm3) | 12.7±9.9 | 15.5±13.5 | 10.8±5.6 | 0.110 | 14.8±12.4 | 10.5±5.1 | 0.098 |
| Lymphocyte count (103/mm3) | 1.3±0.7 | 1.0±0.7 | 1.4±0.7 | 0.006 | 1.1±0.7 | 1.5±0.7 | 0.003 |
| Platelet count (103 μl) | 276±142 | 212±147 | 321±120 | <0.001 | 239±144 | 318±128 | 0.015 |
| Neutrophil/ Lymphocyte ratio | 13.1±12.8 | 17.3±13.3 | 10.2±11.7 | 0.004 | 16.7±14.5 | 9.2±9.2 | 0.005 |
| Platelet/ Lymphocyte ratio | 266±194 | 255±199 | 274±192 | 0.464 | 279±226 | 251±152 | 0.832 |
| Urea (mg/dL) | 92±103 | 140±140 | 59±41 | <0.001 | 122±128 | 58±45 | <0.001 |
| Creatinin (mg/dL) | 1.43±1.48 | 2.11±1.86 | 0.95±0.90 | <0.001 | 1.87±1.72 | 0.93±0.97 | <0.001 |
| ALT (IU/L) | 76±221 | 130±337 | 37±26 | 0.133 | 111±300 | 36±25 | 0.202 |
| AST (IU/L) | 72±94 | 112±134 | 44±27 | 0.002 | 99±122 | 43±28 | 0.020 |
| Albumin (g/L) | 26.4±6.0 | 25.4±4.3 | 27.1±7.0 | 0.229 | 25.3±4.6 | 27.7±7.2 | 0.054 |
| C- reactive protein (mg/dL) | 151±97 | 175±109 | 134±85 | 0.091 | 170±103 | 130±86 | 0.088 |
| Procalcitonin, (ng/mL) | 8.2±18.0 | 12.4±22.4 | 5.1±13.2 | 0.003 | 10.2±20.2 | 5.8±14.81 | 0.010 |
AST: Aspartate aminotransferase, ALT: Alanine aminotransferase.
Multivariate analysis revealed NLR (odds ratio [OR]=1.05, confidence interval [CI]=1.00-1.09, p=0.024), platelet count (OR=1.00, CI=1.00-1.00, p=0.011), creatinine (OR=2.14, CI=1.24-3.67, p=0.006), and AST (OR=1.02, CI=1.00-1.04, p=0.028) as predictors for 14-day mortality. In addition to NLR (OR=1.07, CI=1.01-1.13, p=0.014), platelet count (OR=1.00, CI=1.00-1.00, p=0.039), creatinine (OR=2.05, CI=1.20-3.51, p=0.008), and AST (OR=1.02, CI=1.00-1.04, p=0.035), procalcitonin (OR=1.05, CI=1.00-1.10, p=0.049) was also found as an independent predictor for 28-day mortality (Table 4).
Table 4.
Multivariate analyses of risk factors for mortality due to Burkholderia cepacia complex infections at 14 and 28 days.
| Parameters | P | OR | 95% CI |
|---|---|---|---|
| 14-day mortality | |||
| Neutrophil/Lymphocyte ratio | 0.024 | 1.05 | 1.00-1.09 |
| Platelet count (103 μl) | 0.011 | 1.00 | 1.00-1.00 |
| Creatinine (mg/dL) | 0.006 | 2.14 | 1.24-3.67 |
| Aspartate aminotransferase (IU/L) | 0.028 | 1.021 | 1.00-1.04 |
|
28-day mortality | |||
| Neutrophil/Lymphocyte ratio | 0.014 | 1.07 | 1.01-1.13 |
| Platelet count (103 μl) | 0.039 | 1.00 | 1.00-1.00 |
| Procalcitonin (ng/mL) | 0.049 | 1.05 | 1.00-1.10 |
| Creatinine (mg/dL) | 0.008 | 2.05 | 1.20-3.51 |
| Aspartate aminotransferase (IU/L) | 0.035 | 1.02 | 0.00-1.04 |
OR: Odds ratio, CI: Confidence interval.
In ROC curve analysis for predicting 14-day mortality, area under the ROC curve (AUC) values were 0.684 (p=0.003) in NLR, 0.719 (p<0.001) in platelet count, 0.673 (p=0.003) in procalcitonin, 0.743 ( p<0.001) in creatinine, and 0.700 (p<0.001) in AST. The highest sensitivity and specificity at 14-day mortality were obtained from creatinine, with a sensitivity of 75%, and NLR, with a specificity of 86.3% (Table 5, Figure 2 ).
Figure 2.
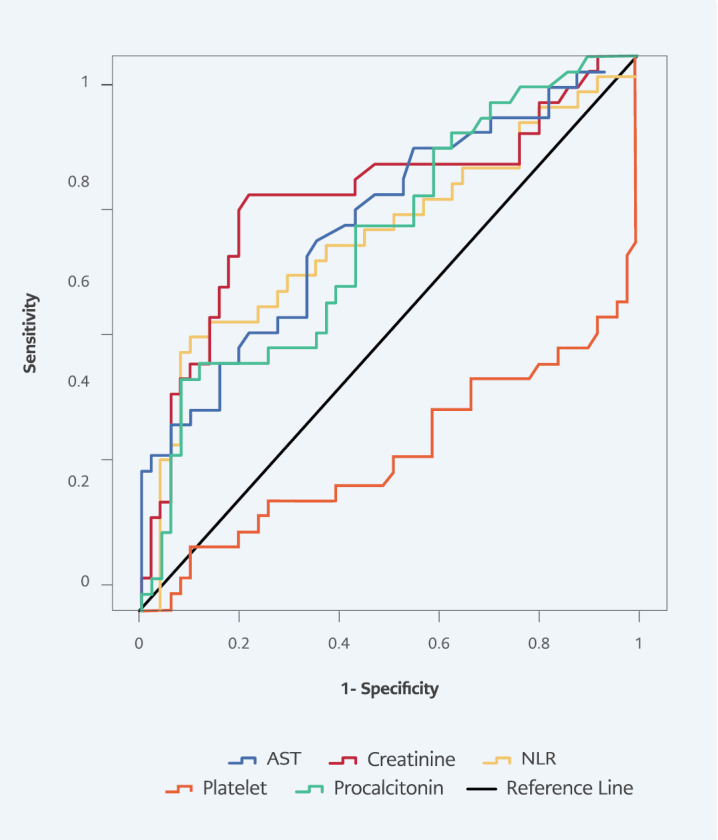
Receiver operating characteristic curves of laboratory parameters for 14-day mortality.
Table 5.
Diagnostic performance of laboratory parameters in predicting 14-day and 28-day mortality
| Parameters | p | AUC | 95% CI | Cut-off | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
|
14-Day Mortality
| ||||||
| Neutrophil / Lymphocyte ratio | 0.003 | 0.684 | 0.564-0.803 | 14.2 | 52.8 | 86.3 |
| Platelet count | <0.001 | 0.719 | 0.601-0.837 | 280.5 | 63.9 | 58.8 |
| Procalcitonin | 0.003 | 0.673 | 0.558-0.788 | 1.33 | 69.4 | 56.9 |
| Creatinine | <0.001 | 0.743 | 0.629-0.856 | 0.98 | 75.0 | 78.4 |
| Aspartate aminotransferase | 0.001 | 0.700 | 0.586-0.813 | 44.5 | 66.7 | 64.7 |
|
28-Day Mortality | ||||||
| Neutrophil / Lymphocyte ratio | 0.002 | 0.674 | 0.562-0.787 | 15.1 | 43.5 | 92.7 |
| Platelet count | 0.010 | 0.651 | 0.536-0.766 | 245.5 | 47.8 | 61.0 |
| Procalcitonin | 0.020 | 0.638 | 0.522-0.755 | 1.33 | 65.2 | 58.5 |
| Creatinine | <0.001 | 0.730 | 0.622-0.838 | 0.95 | 67.4 | 80.5 |
| Aspartate aminotransferase | 0.001 | 0.692 | 0.581-0.803 | 45.5 | 60.9 | 70.7 |
AUC: Area under ROC curve, CI: Confidence interval.
In ROC curve analysis for predicting 28-day mortality, AUC values were 0.674 ( p=0.002) in NLR, 0.651 (p=0.010) in platelet count, 0.638 (p=0.020) in procalcitonin, 0.730 ( p<0.001) in creatinine, and 0.692 (p=0.001) in AST. The highest sensitivity and specificity at 28-day mortality were obtained from creatinine, with a sensitivity of 67.4%, and NLR, with a specificity of 92.7% (Table 5 , Figure 3).
Figure 3.
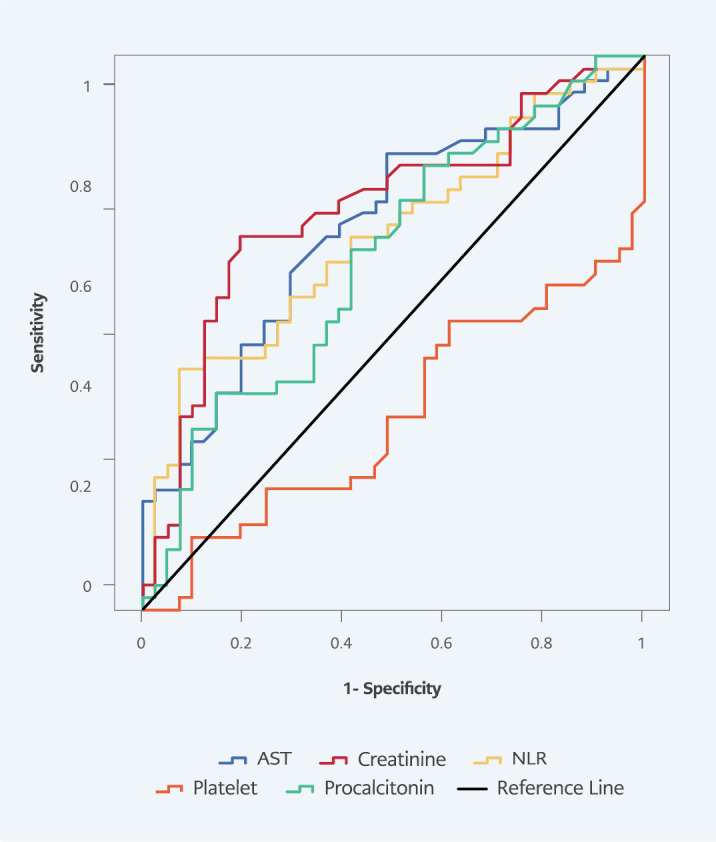
Receiver operating characteristic curves of laboratory parameters for 28-day mortality.
Discussion
In this study, we evaluated the demographic characteristics, laboratory parameters, antimicrobial susceptibility profiles, and clinical outcomes of 87 patients with B. cepacia complex infections in a tertiary care hospital during a five-year period. We demonstrated that antibiotic resistance reached a peak level in 2021, and it decreased in 2022. In addition, NLR, platelet count, AST, and creatinine were independent predictors for 14-day mortality. In addition to these four biomarkers, procalcitonin was a predictor of 28-day mortality.
In our study, the highest antimicrobial resistance rate was against ceftazidime (76.6%), and the lowest resistance rate was against trimethoprim-sulfamethoxazole (25.3%) in B. cepacia complex isolates. In the study of Lee et al., including 216 non-cystic fibrosis patients with B. cepacia complex bacteremia, the rates of resistance to trimethoprim-sulfamethoxazole (7%) and piperacillin-tazobactam (10%) were lower compared to levofloxacin (36%), meropenem (28%), and ceftazidime (25%) (13). In the study of Başbulut et al., including 131 patients with B. cepacia complex, about 5% of B. cepacia complex were pan-resistant; they reported increased pan-resistant isolates by years. When comparing the resistance rates in five-year periods, they demonstrated that meropenem resistance decreased from 64.3% to 22.5% (p<0.001); levofloxacin resistance decreased from 50% to 4.9% in the last period (p<0.001). However, trimethoprim-sulfamethoxazole resistance rate increased from 9.5% to 14.6% in the last period (p>0.05) (19). In the study of El Chakhtoura et al., trimethoprim-sulfamethoxazole, fluoroquinolone, ceftazidime, and meropenem resistance rates were about 6%, 12%, 30%, and 30%, respectively (9). In a Turkish study conducted by Dizbay et al., including 39 patients with B. cepacia infection, they found that piperacillin-tazobactam, cefoperazone-sulbactam, and carbapenems were the most active antibiotics against B. cepacia (20). In another study, they reported that no resistance to ceftazidime, meropenem, and piperacillin-tazobactam was observed (n=27); however, resistance to cefepime (9%), trimethoprim/sulfamethoxazole (15%), and levofloxacin (22%) were observed (21).
Considering the different rates of resistance between centers, the importance of local surveillance in determining empirical antibiotic therapy stands out. We observed high resistance rates in B. cepacia isolates in 2021, out of the usual course. In 2022, these resistance rates decreased again. As a result of a detailed retrospective analysis, we determined that resistant cases clustered in the ICUs in a certain period of time. This suggested that an unrecognized silent B. cepacia outbreak had occurred in the ICUs.
An increasing number of studies were published analyzing patients with B. cepacia complex infections and related factors (9, 13, 14, 21). Some studies suggested that underlying diseases may predict worse outcomes (13, 14, 22). However, no robust evidence exists for predicting mortality in patients with B. cepacia complex infections. In addition, limited data exists on the laboratory parameters associated with worse outcomes.
Liao et al. reported that a 4-year long-term outbreak occurred in Taiwan. In their study, among 73 bacteremic patients, 14-day mortality and in-hospital mortality rates were 16.8% and 53.8, respectively (14). Similarly, in our study, 14-day mortality, 28-day mortality, and in-hospital mortality were 41.3%, 52.8%, and 64.3%, respectively. We found that NLR, platelet count, creatinine, and AST were independent predictors for 14-day mortality. Moreover, procalcitonin was also an independent predictor for 28-day mortality in addition to NLR, platelet count, creatinine, and AST. Liao et al. found the presence of malignity and higher sequential organ failure assessment (SOFA) scores as independent risk factors for 14-day mortality. Interestingly, treatment with ceftazidime and the presence of diabetes mellitus were associated with lower mortality rates (14).
In contrast to our study, Lee et al. demonstrated lower mortality rates. They found that the rates of 14-day, 30-day, and in-hospital mortality were 19.4%, 23.1%, and 31.0%, respectively. The independent risk factors of 30-day mortality were found as female gender, liver cirrhosis, septic shock, and catheter-related infection (13). In the study of Ku et al., the overall 28-day mortality rate was 41% (n=11). In their study, univariate analysis revealed that underlying diabetes, inappropriate empirical antibiotherapy, and SOFA score were associated with mortality. In addition, inappropriate empirical antibiotherapy and SOFA scores were identified as independent predictors of mortality (21). In a study conducted by El Chakhtoura, 14-day, 30-day, and 90-day mortality rates were 16%, 25%, and 36%, respectively, inconsistent with our study findings. The researchers revealed that 30-day mortality was associated with age and the Pitt bacteremia score (PBS) (9). In another study, independent risk factors for 14-day mortality were PBS, underlying metastatic cancer, and inappropriate definitive treatment (22). As a result, various factors from different studies have been determined for mortality in patients with B. cepaciacomplex infections. Older adults with comorbid conditions and invasive devices are prone to have poor clinical outcomes; therefore, intensive care implementations will be more efficient for this mortal infection.
Our study had some limitations. First, this study was retrospectively conducted in a single center. Second, the sample size was small to investigate independent factors for mortality. Third, we evaluated all-cause crude mortality. However, to reduce the effect of confounding factors, we studied 14-day mortality. In addition, we included co-infections with various microorganisms in the statistical analysis, but co-infections did not affect mortality. Nevertheless, all deaths in our study cannot be attributed solely to infections with B. cepacia complex. Last, although we used VITEK 2 Compact (bioMérieux, Marcy l'Etoile, France) automated system in addition to the conventional identification methods, molecular analysis could not be performed for further identification and detection of clonality among isolates. Besides limitations, our study had some strengths. First, we harmonized clinical characteristics and microbiological features in this study. Second, various candidate laboratory parameters were included in the multivariate regression model.
In conclusion, increasing antibiotic resistance and higher mortality rates in our study justify that B. cepacia complexis a significant threat to hospitalized patients, especially in ICUs, and patients with B. cepacia complex infections should be evaluated diligently. Elevated levels of NLR, AST, creatinine, procalcitonin, and decreased platelet may predict poor clinical outcomes and could help clinicians in the management of this notorious bacterial pathogen.
Additional Information
Ethical Approval
The Bakırköy Dr. Sadi Konuk Training and Research Hospital Clinical Research Ethics Committee approved the study with decision number 2023-08-25 on April 17, 2023.
Informed Consent
Written informed consent was waived due to the retrospective nature of this study.
Peer-review
Externally peer-reviewed
Author Contributions
Concept – D.B., Z.Ç.; Design – B.K.Y., K.K.Y.; Supervision – D.B., E.C.Ü.; Funding – Y.E.Ö., B.K.Y., O.F.B.; Materials – O.F.B.; Data Collection and/or Processing – B.K.Y., E.C.Ü.; Analysis and/or Interpretation – Y.E.Ö., K.K.Y.; Literature Review – E.C.Ü., Z.Ç.; Writer – Y.E.Ö., O.F.B.; Critical Reviews – D.B., Z.Ç., K.K.Y.
Conflict of Interest
The authors declare no conflict of interest.
Financial Disclosure
The authors declared that this study has received no financial support.
References
- Tavares M, Kozak M, Balola A, Sá-Correia I. Burkholderia cepacia complex bacteria: a feared contamination risk in water-based pharmaceutical products. Clin Microbiol Rev. 2020;33(3):e00139-19. doi: 10.1128/CMR.00139-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn Y, Kim JM, Ahn H, Lee YJ, LiPuma JJ, Hussong D, et al. Evaluation of liquid and solid culture media for the recovery and enrichment of Burkholderia cenocepacia from distilled water. J Ind Microbiol Biotechnol. 2014;41(7):1109–1118. doi: 10.1007/s10295-014-1442-3. [DOI] [PubMed] [Google Scholar]
- Mahenthiralingam E, Urban TA, Goldberg JB. The multifarious, multireplicon Burkholderia cepacia complex. Nat Rev Microbiol. 2005;3(2):144–156. doi: 10.1038/nrmicro1085. [DOI] [PubMed] [Google Scholar]
- Sutton S, Jimenez L. A review of reported recalls involving microbiological control 2004-2011 with emphasis on FDA considerations of “objectionable organisms”. Am Pharm Rev. 2012;15:42–57. [Google Scholar]
- Tüfekci S, Şafak B, Nalbantoğlu B, Samancı N, Kiraz N. Burkholderia cepacia complex bacteremia outbreaks among non-cystic fibrosis patients in the pediatric unit of a university hospital. Turk J Pediatr. 2021;63(2):218–222. doi: 10.24953/turkjped.2021.02.005. [DOI] [PubMed] [Google Scholar]
- Parke JL, Gurian-Sherman D. Diversity of the Burkholderia cepacia complex and implications for risk assessment of biological control strains. Annu Rev Phytopathol. 2001;39:225–258. doi: 10.1146/annurev.phyto.39.1.225. [DOI] [PubMed] [Google Scholar]
- Greenberg DE, Goldberg JB, Stock F, Murray PR, Holland SM, Lipuma JJ. Recurrent Burkholderia infection in patients with chronic granulomatous disease: 11-year experience at a large referral center. Clin Infect Dis. 2009;48(11):1577–1579. doi: 10.1086/598937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho CP, Dos Santos SC, Madeira A, Mira NP, Moreira AS, Sá-Correia I. Long-term colonization of the cystic fibrosis lung by Burkholderia cepacia complex bacteria: epidemiology, clonal variation, and genome-wide expression alterations. Front Cell Infect Microbiol. 2011;1:12. doi: 10.3389/fcimb.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Chakhtoura NG, Saade E, Wilson BM, Perez F, Papp-Wallace KM, Bonomo RA. A 17-year nationwide study of Burkholderia cepacia complex bloodstream infections among patients in the United States Veterans Health Administration. Clin Infect Dis. 2017;65(8):1253–1259. doi: 10.1093/cid/cix559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa SA, Ramos CG, Leitão JH. Burkholderia cepacia complex: emerging multihost pathogens equipped with a wide range of virulence factors and determinants. Int J Microbiol. 2011 doi: 10.1155/2011/607575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp-Wallace KM, Becka SA, Taracila MA, Zeiser ET, Gatta JA, LiPuma JJ, et al. Exploring the role of the Ω-loop in the evolution of ceftazidime resistance in the penA β-lactamase from Burkholderia multivorans, an important cystic fibrosis pathogen. Antimicrob Agents Chemother. 2017;61(2):e01941-16. doi: 10.1128/AAC.01941-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podnecky NL, Rhodes KA, Schweizer HP. Efflux pump-mediated drug resistance in Burkholderia . Front Microbiol. 2015;6:305. doi: 10.3389/fmicb.2015.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YM, Park KH, Moon C, Kim DY, Lee MS, Kim T, et al. Management and outcomes of Burkholderia cepacia complex bacteremia in patients without cystic fibrosis: a retrospective observational study. Eur J Clin Microbiol Infect Dis. 2020;39(11):2057–2064. doi: 10.1007/s10096-020-03960-2. [DOI] [PubMed] [Google Scholar]
- Liao CH, Chang HT, Lai CC, Huang YT, Hsu MS, Liu CY, et al. Clinical characteristics and outcomes of patients with Burkholderia cepacia bacteremia in an intensive care unit. Diagn Microbiol Infect Dis. 2011;70(2):260–266. doi: 10.1016/j.diagmicrobio.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Monegro AF, Muppidi V, Regunath H. Hospital-acquired infections. StatPearls. [February 12, 2023]. [August 29, 2023]. https://www.ncbi.nlm.nih.gov/books/NBK441857/ [PubMed]
- Breakpoint tables for interpretation of MICs and zone diameters. European Committee on Antimicrobial Susceptibility Testing (EUCAST) Jun 29, 2023. [August 29, 2023]. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_13.0_Breakpoint_Tables.pdf
- TMC-ADTS Restricted Notification Table 2022. İstanbul: Turkish Microbiology Society. [August 29, 2023]. https://www.tmc-online.org/userfiles/file/TMC-ADTS%20Kısıtlı%20Bildirim%20Tabloları%202022%20(pdf%20dosyası-).pdf Turkish.
- Performance Standards for Antimicrobial Susceptibility Testing. 27th ed. CLSI supplement M100. Wayne, AP: Clinical and Laboratory Standards Institute. [August 29, 2023]. https://clsi.org/media/1469/m100s27_sample.pdf
- Başbulut E, Bilgin M, İşler H. [Evaluation of antibiotic resistance of Burkholderia species in the last 10 years in a tertiary care hospital in Turkey] Flora. 2022;27(1):103–112. doi: 10.1016/10.5578/flora.20229909. Turkish . [DOI] [Google Scholar]
- Dizbay M, Tunccan OG, Sezer BE, Aktas F, Arman D. Nosocomial Burkholderia cepacia infections in a Turkish university hospital: a five-year surveillance. J Infect Dev Ctries. 2009;3(4):273–277. doi: 10.3855/jidc.124. [DOI] [PubMed] [Google Scholar]
- Ku NS, Han SH, Kim CO, Baek JH, Jeong SJ, Jin SJ, et al. Risk factors for mortality in patients with Burkholderia cepacia complex bacteraemia. Scand J Infect Dis. 2011;43(10):792–797. doi: 10.3109/00365548.2011.589076. [DOI] [PubMed] [Google Scholar]
- Chang TH, Chuang YC, Wang JT, Sheng WH. Clinical characteristics and outcomes of non-cystic fibrosis patients with Burkholderia cepacia complex bacteremia at a medical center in Taiwan. J Microbiol Immunol Infect. 2022;55(6 Pt 2):1301–1309. doi: 10.1016/j.jmii.2021.09.009. [DOI] [PubMed] [Google Scholar]