Abstract
Objective
Fournier's gangrene (FG) is a rare necrotizing fasciitis affecting genital and perianal areas. This study aimed to provide data on predicting factors, mortality rates, and factors affecting mortality in comparison of survivors and non-survivors in patients with FG.
Materials and Methods
This study included a retrospective analysis of patients diagnosed with FG between 2008 and 2018.
Results
A total of 97 patients with FG were included in this study. Of the patients, 72 (74%) were male. The mean age was 56.03±13.92 years, and the median was 57 (21-90). The mortality rate was 21.6%. The most frequently isolated microorganism from tissue and blood cultures was Escherichia coli (43%-42%). The median Fournier's Gangrene Severity Index (FGSI) and Uludağ FGSI (UFGSI) scores were 4 (0-20) and 5 (1-22), respectively. In the univariate model, mortality risk increases 7.18 times (p=0.001) in patients with two or more comorbidities, 1.31 times as the FGSI score increases (p<0.001), 1.28 times as the UFGSI score increases (p<0.001). When the cut-off value was set as 8 for the FGSI score, the sensitivity was 71.43%, and the specificity was 73.68%. The sensitivity was 73.43%, and the specificity was 75% when the cut-off value was set as 6 for the UFGSI score. In the univariate model, the mortality risk of those with hypotension was 6.07 times higher (p=0.003); as the platelet count increased, mortality risk decreased (odds ratio [OR]=0.99; p=0.02). The mortality risk of those hospitalized in the intensive care unit (ICU) was 16.5 times higher than those followed in the ward (p<0.001). In the multivariate model, this ratio was 6.49.
Conclusion
We concluded that FGSI and UFGSI scores could be used to predict mortality. Management of FG requires a multidisciplinary approach. Empiric treatment should include carbapenems and be de-escalated once getting the culture results. Authors from different centers should report their experiences to help reveal the ideal treatment and evaluate the consequences.
Keywords: Fournier's gangrene, necrotizing fasciitis, severity index, FGSI, UFGSI
Highlights.
Our study consisted of 10 years of data; we considered that resistance rates in our patients increased in recent years.
Of our patients with Fournier's gangrene (FG), 55.7% had a urogenital origin.
All of our patients were evaluated by both Fournier's Gangrene Severity Index (FGSI) and Uludağ FGSI (UFGSI), and scores were statistically significantly higher in non-survivor patients than survivors.
We found that hypotension was statistically significantly higher in non-survivor patients.
Most of our patients had thrombocytopenia, anemia, and hyponatremia, and these parameters were significantly higher in non-survivors.
Introduction
Fournier's gangrene (FG) is necrotizing fasciitis of genital or perianal areas. Typically, FG is an infection of polymicrobial origin and has higher mortality rates if treatment is not initiated earlier (1). The main etiological factors are colorectal (30%-50%) and genitourinary origins (20%-40%), including anorectal infections, genitourinary infections, and trauma or local injuries to the perineal and genital skin (1). In addition, Streptococcus spp., Staphylococcus spp., and Escherichia coli are frequently isolated microorganisms from tissue cultures (2). Risk factors for infection are diabetes mellitus (DM), chronic alcoholism, chronic renal failure, lymphoproliferative diseases, chronic steroid use, and malnutrition (3).
Treatment must be started immediately, including hemodynamic stabilization and infusion of broad-spectrum antibiotics that cover possible agents and surgical debridement. The FG Severity Index (FGSI) developed by Laor et al. has been used for risk assessment (4). In 2010, Yılmazlar et al. modified this scoring system and enlarged the parameters of analysis by adding age and disease severity and published it as the Uludağ FGSI (UFGSI) (5). These scoring systems have been used to predict mortality.
The primary aim of this study was to identify etiological origins and responsible pathogens in patients with FG and help therapeutical approach. The secondary aim was to provide data on predicting factors such as FGSI score, mortality rates, and factors affecting mortality in comparison of survivors and non-survivors in patients with FG.
Materials and Methods
This study included patients diagnosed with FG in the hospital of Ondokuz Mayıs University School of Medicine between 2008 and 2018. Patients who were followed up in general surgery and intensive care units (ICUs) and those consulted by the infectious diseases and clinical microbiology clinic were evaluated retrospectively. Patients were diagnosed with anamnesis, physical examination, and clinical and related laboratory findings besides radiographic examinations.
Our tertiary hospital had 1100 clinical beds and 50 adult intensive care beds. The clinical records were obtained by searching the hospital database. We excluded patients with no definite diagnosis and younger than 18 years. Demographical features, comorbid diseases, laboratory parameters, laboratory risk indicators for necrotizing fasciitis (LRINEC) scores (6), FGSI (Physiological parameters: temperature, heart rate, respiratory rate, serum potassium, sodium, creatinine, hematocrit, white blood count, serum bicarbonate [venous]) and UFGSI (FGSI scores added to dissemination score [urogenital and/or anorectal region, pelvic region, extending beyond the pelvic region] and age score) scores were recorded (Table 1) (4, 5).
Table 1.
The Fournier's Gangrene Severity Index (FGSI) and the Uludağ Fournier's Gangrene Severity Index (UFGSI)*
| Variables | +4 | +3 | +2 | +1 | 0 | +1 | +2 | +3 | +4 |
|---|---|---|---|---|---|---|---|---|---|
|
(A) Physiological parameters
| |||||||||
| Temperature (°C) | >41 | 39-40.9 | - | 38.5-38.9 | 36-38.4 | 34-35.9 | 32-33.9 | 30-31.9 | <29.9 |
| Heart rate | >180 | 140-179 | 110-139 | - | 70-109 | - | 55-69 | 40-54 | <39 |
| Respiratory rate | >50 | 35-49 | - | 25-34 | 12-24 | 10-11 | 6-9 | - | <5 |
| Serum potassium (mmol/L) | >7 | 6-6.9 | - | 5.5-5.9 | 3.5-5.4 | 3-3.4 | 2.5-2.9 | - | <2.5 |
| Serum sodium (mmol/L) | >180 | 160-179 | 155-159 | 150-154 | 130-149 | - | 120-129 | 110-119 | <110 |
| Serum creatinine (mg/100 ml) (×2 for acute renal failure) | >3.5 | 2-3.4 | 1.5-1.9 | - | 0.6-1.4 | - | <0.6 | - | - |
| Hematocrit (%) | >60 | - | 50-59 | 46-49 | 30-45 | - | 20-29 | - | <20 |
| White blood count (×1000/mm3) | >40 | - | 20-39.9 | 15-19.9 | 3-14.9 | - | 1-2.9 | - | <1 |
| Serum bicarbonate, (venous) (mmol/L) | >52 | 41-51 | - | 32-40 | 22-31 | - | 18-21 | 15-17 | <15 |
|
(B) Dissemination score | |||||||||
| Fournier's gangrene confined to the genitourinary and/or anorectal region, add “1” | |||||||||
| Fournier's gangrene confined to the pelvic region, add “2” | |||||||||
| Fournier's gangrene extending beyond the pelvic region, add “6” | |||||||||
|
(C) Age score | |||||||||
| Age ≥60 years, add “1” | |||||||||
| Age <60 years, add “0” | |||||||||
*FGSI=A, UFGSI = A+B+C
A definitive microbial diagnosis in FG is achieved by deep tissue or positive blood cultures rather than superficial tissue cultures because of the misleading results of the superficial wounds (7). The isolates were identified using conventional methods and VITEK® MS (bioMérieux, Marcy l'Etoile, France) automated system. The antibiotic susceptibility was tested with the VITEK ® 2 Compact (bioMérieux, Marcy l'Etoile, France). The susceptibility of the isolates was evaluated according to the criteria of the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST).
The statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) 23.0 (IBM Corp., Armonk, NY, USA). Compliance with normal distribution was evaluated by Kolmogorov-Smirnov and Shapiro-Wilk tests. An independent two-sample t-test was used to compare normally distributed data according to paired groups. Mann-Whitney U test was used to compare the non-normally distributed data within the paired groups. Pearson's chi-squared test, Yates's chi-squared test, and Fisher's exact test were used to compare categorical data; multiple comparisons were analyzed with Bonferroni corrected Z test. Risk factors affecting mortality were analyzed by binary logistic regression. Receiver operating characteristic (ROC) analysis was used to determine the cut-off values of FGSI and UFGSI scores to predict mortality. The results were presented as mean ±standard deviation (SD) and median (minimum-maximum) for quantitative data and as frequency (percent) for categorical data. The statistical significance was set as p< 0.05.
The Ethical Committee of Ondokuz Mayıs University approved the study on October 02, 2019, with approval number 354.
Results
A total of 97 patients with FG were included in this study. Of the patients, 72 (74%) were male, and 25 (26%) were female. The mean age was 56.03±13.92 years, and the median was 57 (21-90). The disease originated from the genitourinary region in 55.7% of patients. Eight (8.3%) patients had a history of trauma. Nosocomial infection after surgery developed in 11 patients (11.5%). Twenty-five patients were hospitalized in the ICU. On admission to the emergency unit, laboratory values of patients were as follows: median white blood cells (WBC) count, 12,300/mm³ (60-99,000/mm3), median C-reactive protein (CRP) 203.5 mg/L (5.7-450 mg/L), mean hemoglobin 10.7±2.2 g/dL, and mean platelet count 271,000 ±148,391 µL. At least one comorbid disease was noted in 56.7% of patients, such as DM, cancer, chronic renal failure, and cirrhosis; 14% of patients had two or more comorbidities associated with mortality (p=0.002). All patients underwent immediate surgical debridement within 24 hours, and the median number of surgical attempts was 1 (1-24). Moreover, 16 patients (17%) underwent colostomy procedures. The median duration of hospital stay was 20 days (1-199), and the mortality rate was 21.6%. The mortality rates in females and males were 28% and 19.4%, respectively.
The median LRINEC score was 5 (0-11); in 42% of patients, the score was above 6 and was not associated with mortality. The median FGSI and UFGSI scores were 4 (0-20) and 5 (1-22), respectively. FGSI, UFGSI, number of surgical attempts, two or more associated comorbidities, hemoglobin and platelet counts, serum creatinine and serum sodium levels, hypotension, and hospitalization in ICU were associated with mortality. We found no statistically significant associations between sex, age, etiology, the extent of the disease (genitourinary and/or anorectal region, pelvic region, extending beyond the pelvic region) DM, acute renal failure, WBC, and mortality (p>0.05) (Table 2).
Table 2.
Characteristics of survivors and non-survivors.
| Factors | Non-survivors (n = 21) | Survivors (n = 76) | Total (n=97) | p |
|---|---|---|---|---|
| Female / Male (n, %) | 7 (33.3) / 14 (66.7) | 18 (23.7) / 58 (76.3) | 25 (25.8) / 72 (74.2) | 0.540 |
| Comorbidities (n, %) | 15 (71.4) | 40 (52.6) | 55 (56.7) | 0.197 |
| DM (n=43) | ||||
| Cancer (n=11) | ||||
| CRF (n=10) | ||||
| Other (Cirrhosis, etc.) (n=4) | ||||
| Presence of DM (n, %) | 10 (47.6) | 33 (43.4) | 43 (44.3) | 0.925 |
| Two or more associated comorbidities (n, %) | 8 (38.1) | 6 (7.9) | 14 (14.4) | 0.002 |
| Etiology: Urogenital / Anorectal (n, %) | 8 (44.4) / 10 (55.6) | 41 (58.6) / 29 (41.4) | 49 (55.7) / 39 (44.3) | 0.418 |
| Extent of the disease (n, %) | 0.051 | |||
| Grade 1 | 12 (60) | 61 (83.6) | 73 (78.5) | |
| Grade 2 | 4 (20) | 8 (11) | 12 (12.9) | |
| Grade 3 | 4 (20) | 4 (5.5) | 8 (8.6) | |
| Blood culture (n=39) (n, %) | 3 (25) | 4 (14.8) | 7 (17.9) | 0.654 |
| Acute renal failure (n, %) | 6 (28.6) | 9 (11.8) | 15 (15.5) | 0.086 |
| Hypotension (n, %) | 8 (38.1) | 7 (9.2) | 15 (15.5) | 0.003 |
| Need for ICU (n, %) | 15 (71.4) | 10 (13.2) | 25 (25.8) | <0.001 |
| LRINEC score above 6 (n, %) | 9 (42.9) | 32 (42.1) | 41 (42.3) | 1 |
| UFGSI score above 9 (n, %) | 12 (57.1) | 15 (19.7) | 27 (27.8) | 0.002 |
|
Mean±SD Median (min-max) |
Mean±SD Median (min-max) |
Mean±SD Median (min-max) |
||
| Age (year) | 60.62±12.77 60 (24-81) | 54.76±14.03 55 (21-90) | 56.03±13.92 57 (21-90) | 0.088 |
| Number of surgical attempts | 2.9±2.77 1 (1-10) | 1.89±2.91 1 (1-24) | 2.11±2.9 1 (1-24) | 0.049 |
| WBC (mm3) | 13,352.38±9212.69 12,700 (100-30,000) | 150,17.24±11,870.02 12,150 (60-99,000) | 14,656.8±11,324 12,300 (60-99,000) | 0.56 |
| Hemoglobin (g/dL) | 9.38±1.95 9 (6-12.7) | 11.13±2.13 11.45 (4.9-15.7) | 10.75±2.21 11.1 (4.9-15.7) | 0.001 |
| Platelet (103/µL) | 203±127.83 214 (17-560) | 290.38±148.84 271.5 (10-678) | 271.46±148.39 254 (10-678) | 0.016 |
| Serum creatinine (mg/dL) | 2.42±2.13 1.2 (0.2-7.5) | 1.45±1.67 0.95 (0.3-11.5) | 1.66±1.81 1.08 (0.2-11.5) | 0.01 |
| Serum sodium (mEq/L) | 132.29±7.84 133 (108-149) | 135.43±5.15 136 (123-145) | 134.75±5.94 135 (108-149) | 0.031 |
| Serum potassium (mEq/L) | 4.34±1.23 4 (2.38-8) | 4.27±0.6 4.2 (2.7-6.8) | 4.28±0.77 4.2 (2.38-8) | 0.593 |
| Serum bilirubin (mg/dL) | 0.93±0.79 0.7 (0.01-2.7) | 0.64±0.68 0.4 (0.1-3) | 0.71±0.71 0.4 (0.01-3) | 0.073 |
| Glucose (mg/dL) | 153.24±82.2 138 (43-350) | 203.05±135.08 143.5 (61- 635) | 192.27±126.84 142 (43- 635) | 0.150 |
| CRP (mg/L) | 174.91±102.5 200 (14-330) | 207.4±119.64 207 (5.7-450) | 200.25±115.88 203.5 (5.7-450) | 0.417 |
| LRINEC score | 5.71±2.69 5 (2-11) | 4.74±2.65 4.5 (0-11) | 4.95±2.68 5 (0-11) | 0.175 |
| FGSI score | 7.76±4.86 7 (1-20) | 3.47±3.24 2 (0-13) | 4.4±4.04 4 (0-20) | <0.001 |
| UFSGI score | 10.38±5.48 9 (3-22) | 5.18±3.72 4 (1-14) | 6.31±4.66 5 (1-22) | <0.001 |
CRF: Chronic renal failure, LRINEC: Laboratory risk indicators for necrotizing fasciitis, FGSI: Fournier's Gangrene Severity Index, UFGSI: Uludağ Fournier's Gangrene Severity Index, DM: Diabetes mellitus, ICU: Intensive care unit, WBC: White blood count, CRP: C-reactive protein.
Grade 1: Genitourinary and/or anorectal region, Grade 2: Pelvic region, Grade 3: Extending beyond the pelvic region.
Risk factors affecting mortality were analyzed using binary logistic regression as univariate and multivariate models. In the univariate model, the mortality risk of those with two or more comorbidities was 7.18 times higher (p=0.001). As FGSI increases, mortality risk increases 1.31 times (p<0.001); likewise, as UFGSI increases, the risk of mortality increases 1.28 times (p<0.001). The mortality risk of patients with hypotension is 6.07 times higher than those without (p=0.003). The mortality risk decreases as the platelet count increases (OR=0.995;p=0.020). However, it was not found significant in the multivariate model (p=0.150). In the univariate model, the mortality risk of those hospitalized in the ICU was 16.5 times higher than those followed in the ward (p<0.001). In the multivariate model, this ratio was 6.47. Other variables were not statistically significant ( p>0.050) (Table 3).
Table 3.
Risk factors affecting mortality with binary logistic regression.
|
Univariate
|
Multivariate
|
|||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Gender (Male) | 1.611 (0.56-4.61) | 0.373 | 0.79 (0.13-4.71) | 0.799 |
| Comorbidities | 2.25 (0.79-6.42) | 0.130 | 1.01 (0.09-10.90) | 0.995 |
| Two or more associated comorbidities | 7.18 (2.14-24.14) | 0.001 | 4.12 (0.55-30.94) | 0.169 |
| Diabetes mellitus | 1.19 (0.45-3.12) | 0.732 | 1.59 (0.09-28.32) | 0.754 |
| Number of surgical attempts | 1.10 (0.95-1.28) | 0.206 | 1.09 (0.89-1.34) | 0.389 |
| Etiology (genitourinary) | 1.77 (0.62-5.02) | 0.285 | --- | --- |
| FGSI | 1.31 (1.13-1.51) | <0.001 | 0.92 (0.54-1.57) | 0.763 |
| UFGSI | 1.28 (1.13-1.45) | <0.001 | 1.27 (0.78-2.08) | 0.34 |
| Hypotension | 6.07 (1.87-19.64) | 0.003 | 0.79 (0.13-4.78) | 0.8 |
| Serum bilirubin (mg/dL) | 1.68 (0.86-3.28) | 0.129 | --- | --- |
| Platelet (103/µL) | 0.99 (0.99-0.99) | 0.020 | 0.99 (0.99-1) | 0.15 |
| Glucose (mg/dL) | 0.99 (0.99-1) | 0.121 | 0.993 (0.987-1) | 0.066 |
| CRP (mg/L) | 0.99 (0.99-1) | 0.410 | --- | --- |
| ESBL | 1.74 (0.33-9.19) | 0.512 | --- | --- |
| Blood culture | 1.92 (0.36-10.32) | 0.449 | --- | --- |
| Need for ICU | 16.5 (5.19-52.48) | <0.001 | 6.486 (1.22-34.38) | 0.028 |
OR: Odds ratio, CI: Confidence interval, FGSI: Fournier's gangrene severity index, UFGSI: Uludağ Fournier's gangrene severity index, ICU: Intensive care unit, WBC: White blood count, CRP: C-reactive protein, ESBL: Extended spectrum beta-lactamases.
The area under the ROC curve (AUC) value of the FGSI parameter was found as 0.78 to be statistically significant in estimating mortality (p<0.001). When the cut-off value was set as 8, the sensitivity was 71.43% and the specificity was 73.68%. In estimating mortality, the AUC value of the UFGSI parameter was found to be 0.79, as statistically significant (p<0.001). When the cut-off value was set as 6, the sensitivity was 73.43%, and the specificity was 75% (Table 4, Figure 1)
Figure 1.
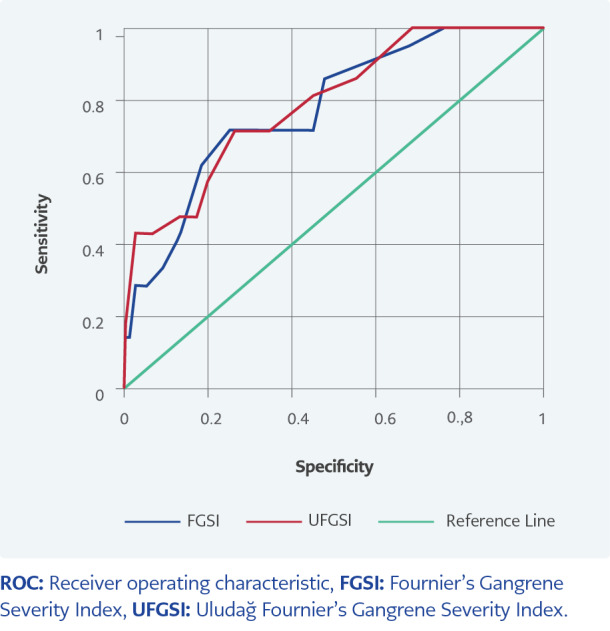
ROC curve graph of FGSI and UFGSI parameters.
Table 4.
Receiver operating characteristic (ROC) analysis results.
| Cut-off value | AUC (95% CI) | p | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|---|
| FGSI | ≥8 | 0.78 (0.67-0.87) | <0.001 | 71.43 | 73.68 | 42.86 | 90.32 |
| UFGSI | ≥6 | 0.79 (0.68-0.89) | <0.001 | 73.43 | 75 | 44.12 | 90.48 |
FGSI: Fournier's Gangrene Severity Index, UFGSI: Uludağ Fournier's Gangrene Severity Index, PPV: Positive predictive value, NPV: Negative predictive value, AUC: Area Under the ROC curve.
Deep tissue cultures were obtained during the surgical operation in 75 (77%) patients and blood cultures in 39 (40%). E. coli was the most frequent microorganism isolated from both deep tissue and blood cultures (43%-42%); it was followed by Enterococcus spp. (18%) and Klebsiella pneumoniae (29%), which was also isolated from the blood. Tissue cultures revealed polymicrobial growth in 15 patients (20%). Figure 2 shows blood and tissue culture results. The same bacteria grew in three patients' blood and wound cultures; different growth was observed in one patient's wound and blood cultures. Other patients with growth in blood culture did not have tissue culture. We found no relationship between the growth in blood culture and mortality (p>0.05). Extended-spectrum beta-lactamase (ESBL) and ciprofloxacin resistance rates in E. coliwere 28% and 40%, respectively. The resistance rates in gram-negative bacilli according to years are given in Table 5. In addition, the ampicillin resistance rate was 45% in Enterococcus spp.
Figure 2.
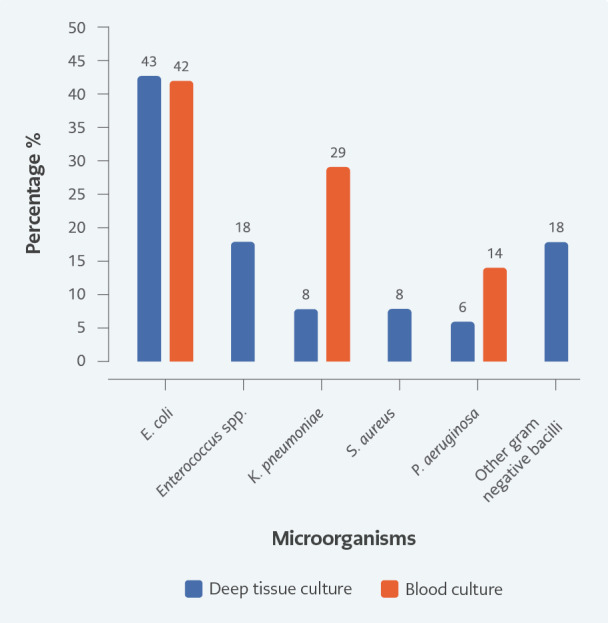
Microorganisms from deep tissue and blood cultures. Percentage
In order of frequency, drug combination preferences were as follows: piperacillin-tazobactam- teicoplanin (13%), imipenem-teicoplanin (49%), ceftriaxone or cefepime-metronidazole (26%) and ciprofloxacin-metronidazole (6%). During treatment, 10% of patients underwent de-escalation according to culture results, ertapenem, ampicillin-sulbactam, trimethoprim-sulfamethoxazole, cefepime-metronidazole, ciprofloxacin-metronidazole were used. Although culture results revealed sensitive growth in 25% (n=12) of patients, no alteration in treatment was made.
Table 5.
ESBL, ciprofloxacin and carbapenem resistance in Gram-negative bacillary by years.
|
Hospitalization date
|
Total | ||
|---|---|---|---|
| 2008-2012 | 2013-2018 | ||
| ESBL, n (%) | |||
| ESBL negative | 4 (80) | 16 (50) | 20 (54.1) |
| ESBL positive | 1 (20) | 16 (50) | 17 (45.9) |
| Ciprofloxacin, n (%) | |||
| Sensitive | 2 (40) | 19 (57.6) | 21 (55.3) |
| Resistance | 3 (60) | 14 (42.4) | 17 (44.7) |
| Carbapenem, n (%) | |||
| Sensitive | 5 (100) | 32 (91.4) | 37 (92.5) |
| Resistance | 0 (0) | 3 (8.6) | 3 (7.5) |
ESBL: Extended spectrum beta-lactamases.
No statistical comparison was made due to the insufficient number of observations.
Discussion
FG is a relatively rare disease with high mortality and morbidity rates with male predominance (8). However, our study group included 72 (74%) male and 25 (26%) female patients. Interestingly, the female ratio was slightly higher in our study group. Sparenborg et al. reported only one female out of 42 patients with FG (9). Eke reported a 1/10 female/male ratio in an analysis including 1726 patients published between 1950 and 1999 and noted the gradual increase in the incidence among women (10). Canbaz et al. reported 33.3% females of the total 18 patients with FG, which is similar to our rates (11). According to findings, women may be vulnerable to FG. In addition, some authors argued that the female sex carries risks for mortality (12, 13). In this study, the mortality rates in females and males were 28% and 19.4%, respectively. The difference was not statistically significant despite relatively higher mortality rates in female patients (p=0.265).
The mean age of patients in our study group was 56.03±13.92 years which was compatible with the reported data (3, 14). We observed that survived patients had a lower mean age, but it was not statistically significant (p=0.08). Older age alone may pose a risk for higher incidence; however, its association with mortality is controversial (8). Previous data supported our results that older age alone is not a risk for mortality (2, 15 , 16).
Comorbid diseases essentially carry the risk for FG and mortality. Some predisposing factors, such as DM, immunodeficiencies, and alcohol intake, contribute to the microenvironment that eases the emergence of infection (17). Goh et al. found DM in 44.5% of 1463 patients with FG and reported that DM was the most accompanying disorder (18). Several studies revealed an association between FG and DM (19, 20). Similarly, 44.3% of our patients had DM. However, as an essential predisposing factor for FG, the aggravating effects of DM on mortality are unclear. We found no statistically significant difference regarding DM presence between survivors and non-survivors patients with FG (p=0.925). As in the study of Wong et al., 14% of our patients had two or more comorbidities which we associated with mortality (p=0.002) (21).
Hypotension was higher in non-survivors, which was statistically significant ( p=0.003). It is a crucial finding of hemodynamic instability, especially as a clinical parameter for sepsis/septic shock in patients with infection (24). Therefore, the initial evaluation of these patients should verify hypotension. In this study, the need for ICU was significantly higher in non-survivors; in the univariate model, the mortality risk of those hospitalized in the ICU was 16.5 times higher than those followed in the ward (p<0.001). In the multivariate model, this ratio was 6.486. Patients with FG may need intensive care during hospitalization and post-surgery. Also, thrombocytopenia is a common laboratory finding in sepsis. A platelet count <100,000/mm3 was found to be an early prognostic marker of 28-day mortality in a study including 1486 patients with septic shock (25). Similarly, thrombocytopenia was significantly higher, particularly in non-survivors (p=0.016). In the univariate model, the mortality risk decreased as the platelet count increased (p=0.020), whereas WBC and CRP did not differ in both survivors and non-survivors (p =0.560, p=0.417).
Despite technological innovations and gained experience, FG still has higher mortality rates. Earlier debridement and broad-spectrum antibiotics are essential in decreasing mortality rates (1, 8, 21). Some authors reported a series revealing approximately 90% mortality rate (22). We found the mortality rate as 21.6%. FGSI and UFGSI scores are often used to predict severity (4, 5). FGSI is the most frequently used scoring system, with 65%-88% sensitivity and 70%-100% specificity (23). All of our patients were evaluated using both scoring systems, and both FGSI and UFGSI scores were statistically significantly higher in non-survivor patients than in survivors ( p<0.001). When the cut-off value of the FGSI score was 8, its sensitivity was 71.43%, and its specificity was 73.68%. When the cut-off value of the UFGSI score was 6, the sensitivity was 73.43%, and the specificity was 75%. We concluded that both scoring systems were beneficial in predicting mortality risk, management of expectations, and developing a therapeutic approach.
Additionally, a LRINEC score of ≥6 should raise the suspicion of necrotizing fasciitis in patients with severe soft tissue infections (6). However, it does not exclude necrotizing fasciitis in patients with low-risk scores (8). In our study, 42% of patients had a LRINEC score above 6, which was not associated with mortality. Since the sensitivity of the LRINEC score is limited, it should not be used alone in clinical decision-making to diagnose FG (8).
Etiologically, FG typically originates from an anorectal region, followed by the genitourinary system (1, 8, 10). Of our patients with FG, 55.7% had a urogenital origin. Although some authors reported higher mortality rates in patients with perianal origin (10, 26), we observed no significant relationship between the etiological origin of FG and mortality (p=0.418). The immediate and repeated surgical debridement to remove necrotizing and infected tissues is of vital importance (3, 27). All 97 patients in this study underwent surgical debridement; the median number of surgical attempts was one (1-21). Moreover, 16 patients (17%) underwent colostomy; the rates of both debridement and colostomy procedures were similar to those in previous studies (27).
Obtaining deep tissue cultures during debridement is essential to identify responsible microorganisms. We obtained deep tissue in 77% and blood cultures in 40% of our patients during surgery. According to a study, the most frequently encountered microorganisms are the normal bacterial flora of the perineal and genital regions (10). Typically, these are aerobic microorganisms, such as E. coli and K. pneumoniae, and anaerobic microorganisms, such as Bacteroides fragilis and Clostridium spp. (27). In addition, Vibrio spp., Staphylococcus spp., Streptococcus spp., Enterococcus spp., Pseudomonas spp., Proteusspp., and Corynebacterium spp. may be isolated (28). E. coli was the most frequent microorganism isolated from both deep tissue and blood cultures (43%-42%); it was followed by Enterococcus spp. (18%) from tissue and K. pneumoniae (29%) from blood cultures. Cultures from deep tissue in 15 out of 75 patients (20%) resulted in polymicrobial growth. Louro et al. reported Staphylococcus aureus as the most frequently isolated microorganism (46.7%) (29). The extensive distribution of these responsible pathogens is valuable in predicting hospitals' spectrum of microorganisms and resistance. The ESBL and resistance rates to ciprofloxacin in E. coli were 28% and 40%, respectively, whereas ampicillin resistance in Enterococcus spp. was 45%. The rate of ESBL in Gram-negative bacilli was found to be 50%, being very high between 2013 and 2018 compared to previous years. Resistance to antimicrobials is a global healthcare challenge (30); therefore, antibiotic treatment of FG should cover most potential pathogens with broad-spectrum antimicrobial agents (1, 7, 8). During treatment, 10% of patients underwent de-escalation according to culture results. De-escalation following the detection of a responsible pathogen prevents unnecessary drug use and the emergence of resistance (8, 30). Because of the high ESBL rate in our study, empiric treatment should include carbapenems and de-escalation should be decided according to the culture results.
We concluded that the FGSI and UFGSI scores might be useful in predicting mortality, and the management of FG requires a multidisciplinary approach. Empiric treatment should include carbapenems, and de-escalation should be considered once getting the culture results. We recommend that authors from different centers report their experiences to identify the ideal treatment and its consequences.
Additional Information
Ethical Approval
The Ethics Committee of Ondokuz Mayıs University approved the study on October 2, 2019, with the decision number of 354.
Informed Consent
N.A.
Peer-review
Externally peer-reviewed
Author Contributions
Concept – A.A., F.T., T.K.; Design – A.A., F.T., T.K.; Supervision – A.A., F.T., T.K.; Materials – A.K.P.; Data Collection and/or Processing – A.K.P., T.K.; Analysis and/or Interpretation – A.A., F.T.; Literature Review – A.A., F.T., T.K., A.K.P.; Writer – A.A., F.T., T.K.; Critical Reviews – A.A., F.T.; Other – A.K.P., T.K.
Conflict of Interest
The authors declare no conflict of interest.
Financial Disclosure
The authors declared that this study has received no financial support.
Scientific Presentation
The study was partially presented as a poster at Bakırköy Surgery Days on February 28 - March 2, 2019.
References
- Mallikarjuna MN, Vijayakumar A, Patil VS, Shivswamy BS. Fournier's gangrene: current practices. ISRN Surg. 2012;2012 doi: 10.5402/2012/942437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uluğ M, Gedik E, Girgin S, Çelen MK, Ayaz C. The evaluation of microbiology and Fournier's gangrene severity index in 27 patients. Int J Infect Dis. 2009;13(6):424–430. doi: 10.1016/j.ijid.2009.01.021. [DOI] [PubMed] [Google Scholar]
- Jeong HJ, Park SC, Seo IY, Rim JS. Prognostic factors in Fournier gangrene. Int J Urol. 2005;12(12):1041–1044. doi: 10.1111/j.1442-2042.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- Laor E, Palmer LS, Tolia BM, Reid RE, Winter HI. Outcome prediction in patients with Fournier's gangrene. J Urol. 1995;154(1):89–92. [PubMed] [Google Scholar]
- Yilmazlar T, Ozturk E, Ozguc H, Ercan I, Vuruskan H, Oktay B. Fournier's gangrene: an analysis of 80 patients and a novel scoring system. Tech Coloproctol. 2010;14(3):217–223. doi: 10.1007/s10151-010-0592-1. [DOI] [PubMed] [Google Scholar]
- Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535–1541. doi: 10.1097/01.ccm.0000129486.35458.7d. [DOI] [PubMed] [Google Scholar]
- Stevens DL, Bisno AL, Chambers HF, et al. Executive summary: practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):147–159. doi: 10.1093/cid/ciu444. [DOI] [PubMed] [Google Scholar]
- Montrief T, Long B, Koyfman A, Auerbach J. Fournier gangrene: a review for emergency clinicians. J Emerg Med. 2019;57(4):488–500. doi: 10.1016/j.jemermed.2019.06.023. [DOI] [PubMed] [Google Scholar]
- Sparenborg JD, Brems JA, Wood AM, Hwang JJ, Venkatesan K. Fournier's gangrene: a modern analysis of predictors of outcomes. Transl Androl Urol. 2019;8(4):374–378. doi: 10.21037/tau.2019.03.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke N. Fournier's gangrene: a review of 1726 cases. Br J Surg. 2000;87(6):718–728. doi: 10.1046/j.1365-2168.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- Canbaz H, Cağlikülekçi M, Altun U, et al. [Fournier's gangrene: analysis of risk factors affecting the prognosis and cost of therapy in 18 cases] Ulus Travma Acil Cerrahi Derg. 2010;16(1):71–76. [PubMed] [Google Scholar]
- Czymek R, Frank P, Limmer S, Schmidt A, Jungbluth T, Roblick U, et al. Fournier's gangrene: is the female gender a risk factor? Langenbecks Arch Surg. 2010;395(2):173–180. doi: 10.1007/s00423-008-0461-9. [DOI] [PubMed] [Google Scholar]
- Taviloglu K, Cabioglu N, Cagatay A, Yanar H, Ertekin C, Baspinar I, et al. Idiopathic necrotizing fasciitis: risk factors and strategies for management. Am Surg. 2005;71(4):315–320. [PubMed] [Google Scholar]
- Villanueva-Sáenz E, Martínez Hernández-Magro P, Valdés Ovalle M, Montes Vega J, Alvarez-Tostado F JF. Experience in management of Fournier's gangrene. Tech Coloproctol. 2002;6(1):5–10. doi: 10.1007/s101510200001. discussion 11-3. [DOI] [PubMed] [Google Scholar]
- Tenório CEL, Lima SVC, Albuquerque AV, Cavalcanti MP, Teles F. Risk factors for mortality in Fournier's gangrene in a general hospital: use of simplified founier gangrene severe index score (SFGSI). Int Braz J Urol. 2018;44(1):95–101. doi: 10.1590/S1677-5538.IBJU.2017.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiakos EP, Bagias G, Patapis P, Sotiropoulos D, Kanavidis P, Machairas A. Current concepts in the management of necrotizing fasciitis. Front Surg. 2014;1:36. doi: 10.3389/fsurg.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkis P, Farran F, Khoury R, et al. Fournier's gangrene: a review of the recent literature. Prog Urol. 2009;19:75–84. doi: 10.1016/j.purol.2008.09.050. French. [DOI] [PubMed] [Google Scholar]
- Goh T, Goh LG, Ang CH, Wong CH. Early diagnosis of necrotizing fasciitis. Br J Surg. 2014;101(1):e119-25. doi: 10.1002/bjs.9371. [DOI] [PubMed] [Google Scholar]
- Dworkin MS, Westercamp MD, Park L, McIntyre A. The epidemiology of necrotizing fasciitis including factors associated with death and amputation. Epidemiol Infect. 2009;137(11):1609–1614. doi: 10.1017/S0950268809002532. [DOI] [PubMed] [Google Scholar]
- Hsiao CT, Weng HH, Yuan YD, Chen CT, Chen IC. Predictors of mortality in patients with necrotizing fasciitis. Am J Emerg Med. 2008;26(2):170–175. doi: 10.1016/j.ajem.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am. 2003;85(8):1454–1460. [PubMed] [Google Scholar]
- Rello J, Valenzuela-Sánchez F, Ruiz-Rodriguez M, Moyano S. Sepsis: a review of advances in management. Adv Ther. 2017;34(11):2393–2411. doi: 10.1007/s12325-017-0622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery-Antier N, Binquet C, Vinault S, Meziani F, Boisramé-Helms J, Quenot JP EPIdemiology of Septic Shock Group. Is thrombocytopenia an early prognostic marker in septic shock? Crit Care Med. 2016;44(4):764–772. doi: 10.1097/CCM.0000000000001520. [DOI] [PubMed] [Google Scholar]
- Stone HH, Martin JD Jr. Synergistic necrotizing cellulitis. Ann Surg. 1972;175(5):702–711. doi: 10.1097/00000658-197205000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roghmann F, von Bodman C, Löppenberg B, Hinkel A, Palisaar J, Noldus J. Is there a need for the Fournier's gangrene severity index? Comparison of scoring systems for outcome prediction in patients with Fournier's gangrene. BJU Int. 2012;110(9):1359–1365. doi: 10.1111/j.1464-410X.2012.11082.x. [DOI] [PubMed] [Google Scholar]
- Koukouras D, Kallidonis P, Panagopoulos C, et al. Fournier's gangrene, a urologic and surgical emergency: presentation of a multi-institutional experience with 45 cases. Urol Int. 2011;86(2):167–172. doi: 10.1159/000321691. [DOI] [PubMed] [Google Scholar]
- Yanar H, Taviloglu K, Ertekin C, et al. Fournier's gangrene: risk factors and strategies for management. World J Surg. 2006;30(9):1750–1754. doi: 10.1007/s00268-005-0777-3. [DOI] [PubMed] [Google Scholar]
- Voelzke BB, Hagedorn JC. Presentation and diagnosis of Fournier gangrene. Urology. 2018;114:8–13. doi: 10.1016/j.urology.2017.10.031. [DOI] [PubMed] [Google Scholar]
- Louro JM, Albano M, Baltazar J, et al. Fournier's gangrene: 10-year experience of a plastic surgery and burns department at a tertiary hospital. Acta Med Port. 2019;32(5):368–374. doi: 10.20344/amp.11003. [DOI] [PubMed] [Google Scholar]
- Christaki E, Marcou M, Tofarides A. Antimicrobial resistance in bacteria: mechanisms, evolution, and persistence. J Mol Evol. 2020;88(1):26–40. doi: 10.1007/s00239-019-09914-3. [DOI] [PubMed] [Google Scholar]


