Abstract
The inhibition of coagulation factor XI (FXI) presents an attractive approach for anticoagulation as it is not expected to increase the risk of clinically relevant bleeding and is anticipated to be at least as effective as currently available anticoagulants. Fesomersen is a conjugated antisense oligonucleotide that selectively inhibits the expression of FXI. The article describes three clinical studies that investigated the safety, pharmacokinetic (PK), and pharmacodynamic (PD) profiles of fesomersen after subcutaneous (s.c.) injection to healthy participants. The studies included participants from diverse ethnic backgrounds (Caucasian, Japanese, and Chinese). Fesomersen demonstrated good safety and tolerability in all three studies. No major bleeding events were observed. After single‐dose s.c. injection, fesomersen was rapidly absorbed into the systemic circulation, with maximum fesomersen‐equivalent (fesomersen‐eq) concentrations (C max) in plasma observed within a few hours. After reaching C max, plasma fesomersen‐eq concentrations declined in a biphasic fashion. The PD analyses showed that the injection of fesomersen led to dose‐dependent reductions in FXI activity and increases in activated partial thromboplastin time (aPTT). The maximum observed PD effects were reached between Day 15 and 30, and FXI activity and aPTT returned to near‐baseline levels by Day 90 after a single dose. The PK/PD profiles after a single injection were similar among the various ethnic groups. Collectively, the study results suggest that fesomersen has a favorable safety profile and predictable and similar PK and PD profiles across Chinese, Japanese, and Caucasian participants.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Fesomersen, a newly developed antisense oligonucleotide (ASO) targeting factor XI (FXI) messenger ribonucleic acid, shares the same sequence as BAY 2306001 (ISIS 416858) but contains an N‐acetyl galactosamine conjugation to direct the ASO to hepatocytes where FXI is expressed.
WHAT QUESTION DID THIS STUDY ADDRESS?
Safety, pharmacokinetic (PK), and pharmacodynamic (PD) profiles of fesomersen as a single subcutaneous dose of up to 120 mg and the influence of ethnicity (Caucasian, Japanese, and Chinese).
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Injection of fesomersen as a single subcutaneous dose of 40 mg, 80 mg, or 120 mg had a favorable safety profile and was well tolerated in healthy participants of Caucasian, Japanese, and Chinese ethnicities. Similar fesomersen‐equivalent plasma concentration‐time patterns and comparable area under the plasma concentration versus time curves (AUC) were observed in all ethnic groups, while higher maximum observed drug concentration in plasma (C max) was observed in Chinese/Japanese participants compared to Caucasian participants, which were considered to be not clinically relevant. PD profiles were comparable between Chinese/Japanese (Japanese and Chinese) and Caucasian healthy participants: Distinct target engagement and dose response were observed for activated partial thromboplastin time and FXI activity/concentration after fesomersen injection, while no effect on prothrombin time was detected.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
The study result justifies further clinical development of fesomersen as a novel anticoagulant in different regions and ethnicities (Caucasian, Chinese, and Japanese).
INTRODUCTION
Thromboembolic diseases are a major cause of mortality and morbidity worldwide, causing or contributing to acute cardiovascular diseases including acute coronary syndromes, thromboembolic ischemic stroke, and peripheral arterial occlusion. Cardiovascular disease is the primary cause of death in adults around the world. World Health Organization data show that an estimated 17.9 million people died from cardiovascular disease in 2019, which represented 32% of all global deaths. 1
Anticoagulation is an essential component of antithrombotic therapy. However, treatment with traditional anticoagulants, such as heparin and warfarin, is complicated by an increased bleeding risk. 2 , 3 New oral anticoagulants (NOACs), like factor Xa inhibitors (e.g., rivaroxaban and apixaban) and direct thrombin inhibitors (e.g., dabigatran), provide improvements over warfarin/heparin in various approved indications. 4 , 5 However, these therapies still carry a clinically significant risk of bleeding. 3 , 6 Hence, innovative anticoagulants that offer improved efficacy and/or reduced bleeding risk are needed in general and specifically for those patients with a high risk for bleeding events. 2
Factor XI (FXI) is part of the intrinsic pathway of the coagulation cascade, upstream of factor X and factor II, which belong to the common pathway. Targeting FXI/FXIa is not expected to lead to an increased risk for clinically relevant bleeding, but it has the potential to maintain, or even improve, the efficacy benefit provided by current anticoagulants. 3 The expected difference in bleeding risk profile is justified, as inhibition of FXIa affects the intrinsic and propagation pathways, but keeps the extrinsic pathway unaffected, which is activated in case of vessel injury. 3 , 7 Currently, there are no approved FXI inhibitor products available on the market. However, various FXI/FXIa inhibitors are in different stages of clinical development. Such inhibitors include oral small molecule drugs (asundexian, milvexian), monoclonal antibody drugs (osocimab, abelacimab), and antisense oligonucleotides (ASOs). 6
Previous studies with BAY 2306001 (ISIS 416858), an unconjugated 2′‐O‐(2‐methoxyethyl) (2′‐MOE) ASO that has the same base sequence as fesomersen, demonstrated robust, sustained, and dose‐dependent reductions in FXI concentration and activity in healthy volunteers, patients undergoing total knee arthroplasty, and in patients with end‐stage renal disease undergoing dialysis. 2 , 7 , 8 Fesomersen is a conjugated 2′‐MOE ASO drug that targets a region of the FXI messenger ribonucleic acid (mRNA) to selectively and specifically inhibit the expression of FXI. This ASO utilizes ligand‐conjugated antisense (LICA) technology to facilitate its delivery to the hepatocyte. 8 The LICA technology incorporated in fesomersen is a triantennary N‐acetyl galactosamine (GalNAc) moiety, which targets the asialoglycoprotein receptors expressed primarily on the surface of hepatocytes. 8 , 9 The GalNAc conjugate approach results in enhanced ASO delivery to hepatocytes versus non‐parenchymal cells and up to 20‐ to 30‐fold higher potency compared to unconjugated ASOs. 8 , 9 The GalNAc cluster is metabolized to release “free ASOs” following internalization into cells. 8 , 9 The ASO portion is complementary to a region within the 3′ untranslated region of the FXI mRNA and binds to mRNA by Watson–Crick base pairing. This results in the ribonuclease H1‐mediated degradation of FXI mRNA, thus preventing the production of the FXI protein.
In this article, data from three clinical pharmacology studies will be presented to elucidate the safety, pharmacokinetic (PK), and pharmacodynamic (PD) profiles of fesomersen after single‐dose subcutaneous (s.c.) injections to healthy volunteers over three dose levels. In addition, the influence of ethnicity (Caucasian, Japanese, and Chinese) will be assessed.
METHODS
The safety, tolerability, PK, and PD of s.c. injection of fesomersen were investigated in three phase I, randomized, placebo‐controlled, single‐center, healthy volunteer studies: a first‐in‐human (FIH) study, which enrolled participants in Canada, and two single‐dose escalation studies, one of which enrolled participants from Japan and the other from China.
The overall designs of the three studies are summarized in Table S1. Computer‐generated randomization lists were prepared by sponsors. Appropriate study setup and decision rules were defined in all studies to mitigate potential safety risks during this early phase of clinical development of fesomersen. Four single‐dose cohorts of fesomersen (40 mg, 60 mg, 80 mg, and 120 mg), as well as four multiple‐dose cohorts comprising three cohorts undergoing weekly injection (10 mg, 20 mg, and 30 mg), and one cohort undergoing injection on a 4‐week basis (fesomersen 80 mg) were investigated in the FIH study. This article mainly describes the data from Caucasian participants in the 40 mg, 80 mg, and 120 mg single‐dose cohorts to facilitate interethnic comparison with the single‐dose escalation studies in Chinese and Japanese participants. The blood‐sampling schedules for PK, PD, and anti‐drug antibodies (ADA) for all three studies are listed in Table S2.
The effective protocols were reviewed and approved by independent ethics committees/institutional review boards before the start of each study. Studies met all local legal and regulatory requirements and were conducted in accordance with the currently accepted version of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guideline. 10
Bioanalytical and PK analysis
Fesomersen‐equivalent (eq) concentrations (i.e., total full‐length ASOs [sum of fesomersen, partially conjugated fesomersen, and de‐conjugated fesomersen]) in plasma were determined using a quantitative and validated hybridization electrochemiluminescence assay with a lower limit of quantification of 0.0867 ng/mL. The method validation and analysis of the study samples were performed in compliance with the pertinent guidelines on “Bioanalytical Method Validation”. 11 The details of the bioanalytical analysis are provided in Appendix S1.
Based on the plasma concentration‐time data, PK parameters were calculated using a non‐compartmental analysis approach using Phoenix software (Version 8.1 or higher, Certara™). Fesomersen‐eq in human urine was also determined using a quantitative hybridization enzyme‐linked immunoassay (ELISA) in the FIH and Japan phase I studies.
PD analysis
PD parameters for the FIH and Japanese studies were measured by the Hemostasis Reference Lab (Hamilton, Canada). PD parameters for the Chinese study were measured by Labcorp (Shanghai, China). Both central labs used identical assay systems to exclude methodological bias. Activated partial thromboplastin time (aPTT), prothrombin time (PT), and international normalized ratio (INR) were analyzed on a BCP XP System (Siemens Healthcare GmbH, Erlangen, Germany). Trigger reagents for FXI activity and aPTT were Actin FS and Actin FSL, respectively. PT and INR were triggered with Innovin (all trigger reagents from Siemens Healthcare GmbH, Erlangen, Germany). FXI protein concentration was measured by an ELISA (Affinity Biologicals, Inc., Ancaster, Canada).
ADA
Measurement of ADAs was done batch‐wise as no direct impact on healthy participants' safety was expected. ADA samples were analyzed using a validated ELISA method and the titer of confirmed positive samples was reported. Positive controls were analyzed concurrently with study samples.
Safety and tolerability
Safety and tolerability were assessed by monitoring adverse events (AEs), and by conducting other relevant measurements including physical examinations, monitoring of vital signs, electrocardiogram (ECG) readings, and laboratory examinations of blood and urine samples. The concentration‐QTc interval relationship (C‐QTc) was explored in the FIH and Japanese studies. Paired QTc measurements using heart‐rate correction according to Fridericia's method from triplicate ECGs and plasma concentration values of the study drug were assessed. The details of the C‐QTc analysis are provided in Appendix S1.
Statistical analysis
The healthy participants who received a s.c. single dose of 40 mg, 80 mg, or 120 mg fesomersen, or placebo and were classified as valid for PK, PD, and safety evaluation in the respective single‐dose studies were included in the PK, PD, and safety analysis sets for interethnic evaluation. The baseline characteristics and demographics of the participants were summarized descriptively by ethnic group and dose. The PK parameters of fesomersen‐eq were summarized by geometric mean, coefficient of variation (CV), and minimum and maximum, except for time to reach maximum observed drug concentration in plasma (C max) after single‐dose administration of fesomersen‐eq in plasma (T max), which was summarized utilizing median, minimum, and maximum. An explorative analysis of variance (ANOVA) including the factor “ethnicity” was performed per dose level on the log‐transformed values of area under the plasma concentration versus time curve from zero to infinity after a single dose (AUC) divided by dose (AUC/D) and C max divided by dose (C max/D) assuming log‐normally distributed data. Based on these analyses, point estimates (least‐squares means [LSM]) and exploratory 90% confidence intervals (CIs) for the interethnic ratios were calculated for each dose level by re‐transformation of the logarithmic results.
aPTT, FXI activity, and FXI concentration were considered for interethnic PD analyses. For PD parameters, the maximum (aPTT) and minimum (FXI activity, FXI concentration) ratios to baseline after injection were evaluated. Furthermore, the ratios to baseline at Day 30 (the end of the injection interval in case of multiple injections), Day 60, and Day 90 were evaluated. An explorative analysis of covariance (ANCOVA) including the factor “ethnicity” and log‐transformed baseline as covariates was performed per dose level on the log‐transformed values of these PD parameters. Based on these analyses, point estimates (LSM) and exploratory 90% CIs for the ratios were calculated for each dose level by re‐transformation of the logarithmic results. Placebo data from the different dose groups were pooled by ethnicity for the PD analyses.
No statistical hypotheses were tested. The interethnic evaluation was exploratory and no sample size calculations were performed with respect to interethnic comparisons. No adjustment for multiplicity was performed; CIs were calculated independently. The statistical evaluation was carried out by using SAS software (release 9.4; SAS Institute Inc., Cary, NC, USA).
RESULTS
Baseline characteristics and demographics
A total of 86 participants (Caucasian n = 17, Japanese n = 24, and Chinese n = 45) were randomized and received single injections of fesomersen or placebo. These participants were included in the interethnic sensitivity analyses. Baseline characteristics were generally similar between participants in the Japanese and Chinese studies, whereas Caucasian participants were older and had higher body weights and body mass indices (BMIs) compared to Chinese/Japanese participants (Table 1). Baseline value ranges of FXI activity, FXI concentration, and aPTT were similar between participants of different ethnic backgrounds, while slightly lower mean FXI activity/concentration and slightly higher baseline mean aPTT were observed in Japanese participants. Within each study, baseline characteristics were similar between treatment groups.
TABLE 1.
Baseline characteristics and demographics of participants (safety population).
| Placebo | Fesomersen 40 mg | Fesomersen 80 mg | Fesomersen 120 mg | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caucasian | Japanese | Chinese | Caucasian | Japanese | Chinese | Caucasian | Japanese | Chinese | Caucasian | Japanese | Chinese | |
| Dose | n = 5 | n = 6 | n = 9 | n = 4 | n = 6 | n = 12 | n = 4 | n = 6 | n = 12 | n = 4 | n = 6 | n = 12 |
| Age, years | 57.0/8.9 (42–65) | 23.8/4.2 (20–31) | 32.3/5.5 (24–38) | 54.8/15.9 (31–64) | 30.2/6.9 (21–39) | 28.0/6.6 (19–38) | 52.0/15.0 (30–62) | 27.0/9.0 (20–40) | 28.7/5.9 (19–36) | 56.3/3.2 (54–61) | 26.7/5.4 (20–36) | 32.3/3.4 (28–40) |
| Body weight, kg | 78.4/11.2 (66.4–95.9) | 62.8/8.3 (53.0–77.7) | 63.9/6.6 (51.6–74.5) | 79.4/9.8 (65.4–86.9) | 65.5/6.4 (58.0–74.0) | 64.8/6.4 (54.1–74.5) | 73.2/14.0 (54.1–85.8) | 64.5/10.0 (51.3–80.3) | 64.6/7.9 (53.5–75.2) | 76.8/14.1 (59.4–93.5) | 63.0/6.1 (56.5–73.0) | 68.0/8.5 (54.5–83.4) |
| Height, cm | 173.1/4.5 (166.8–179.2) | 172.1/4.0 (166.9–177.0) | 169.2/4.3 (164.3–176.1) | 177.1/8.5 (171.6–189.8) | 170.0/2.8 (165.6–173.0) | 170.6/4.1 (162.7–178.2) | 167.8/14.6 (151.3–184.3) | 171.6/4.8 (163.7–175.6) | 169.2/5.0 (162.5–181.7) | 167.5/1.9 (165.0–169.0) | 169.8/2.7 (167.4–174.5) | 172.3/6.6 (163.4–182.1) |
| BMI, kg/m2 | 26.1/2.6 (23.5–29.9) | 21.2/2.3 (18.5–25.0) | 22.4/2.3 (19.1–26.1) | 25.4/3.3 (21.9–29.5) | 22.7/2.7 (20.3–27.0) | 22.3/1.9 (19.1–24.9) | 25.8/2.4 (23.6–28.1) | 21.9/2.9 (19.0–26.9) | 22.5/2.3 (20.1–26.2) | 27.4/5.0 (20.8–32.7) | 21.9/2.5 (18.6–25.8) | 22.8/1.8 (19.9–25.7) |
| iPDS | n = 5 | n = 5 | n = 9 | n = 4 | n = 6 | n = 11 | n = 4 | n = 6 | n = 12 | n = 4 | n = 6 | n = 12 |
| Baseline FXI activity, U/mL | 0.99/10.5 (0.87–1.12) | 0.78/13.5 (0.64–0.91) | 0.93/13.2 (0.80–1.17) | 1.12/5.5 (1.06–1.18) | 0.78/12.1 (0.65–0.86) | 0.97/7.9 (0.85–1.09) | 1.05/9.4 (0.97–1.17) | 0.77/18.7 (0.65–1.08) | 1.01/9.5 (0.87–1.21) | 0.93/7.5 (0.88–1.03) | 0.76/18.6 (0.57–0.98) | 0.88/15.9 (0.68–1.19) |
| Baseline FXI concentration, U/mL | 1.17/12.4 (0.99–1.31) | 0.95/18.9 (0.71–1.17) | 0.98/9.1 (0.87–1.13) | 1.17/5.6 (1.08–1.22) | 0.93/16.2 (0.73–1.11) | 0.92/13.4 (0.77–1.29) | 1.23/12.2 (1.07–1.44) | 1.02/22.9 (0.78–1.45) | 1.08/12.2 (0.95–1.36) | 1.08/3.9 (1.04–1.13) | 0.93/29.2 (0.63–1.38) | 0.85/22.4 (0.55–1.08) |
| Baseline aPTT, seconds | 26.2/8.5 (24.0–29.8) | 28.5/4.8 (27.0–29.9) | 27.6/5.0 (26.0–29.7) | 25.7/7.5 (23.8–27.4) | 31.1/8.3 (27.8–35.6) | 26.4/6.7 (23.8–30.0) | 24.3/7.4 (22.8–26.9) | 27.2/8.5 (24.3–29.9) | 26.6/5.4 (24.5–29.8) | 24.8/4.2 (23.6–25.8) | 30.1/8.3 (27.4–33.7) | 27.9/7.1 (24.8–31.5) |
Note: The injection site of fesomersen was the abdomen in the Japan and China studies, while multiple injection sites were recorded in the FIH study (arm in most cases, but also thigh or abdomen). Values in the first four rows are mean/SD (min–max) and the last three rows are geometric mean/CV% (min–max).
Abbreviations: aPTT, activated partial thromboplastin time; BMI, body mass index; CV, geometric coefficient of variation; FIH, first in human; FXI, factor XI; iPDS, interethnic evaluation pharmacodynamic analysis set; SD, standard deviation.
PK
A similar plasma concentration‐time pattern was observed in all ethnic groups across the tested dose levels (Figure 1). Following rapid absorption after s.c. injection, C max of fesomersen‐eq in plasma was reached at 1.5–3.5 h (median) after single injections of fesomersen. After reaching C max, mean plasma concentrations of fesomersen‐eq declined in a biphasic fashion, with an initial fast‐disposition phase leading to a drop from the peak of over 90% by 24 h after s.c. injection, followed by a slower elimination phase.
FIGURE 1.
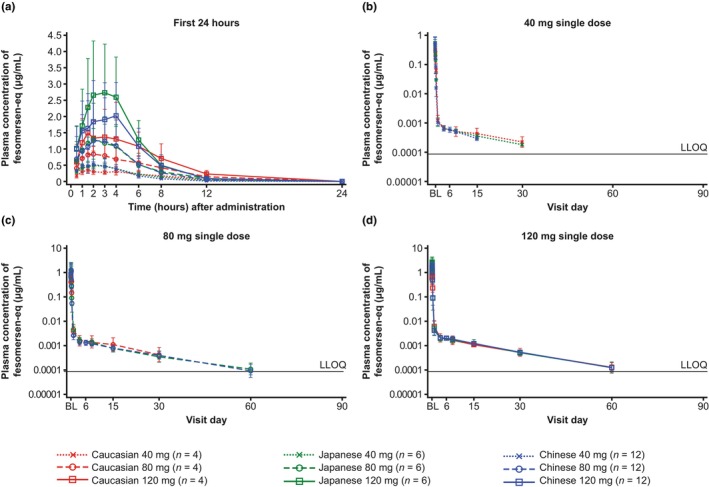
Geometric means with standard deviation range of fesomersen‐eq concentrations (μg/mL) in plasma over time.† (a) Up to 24 h after single‐dose injection (linear scale) and (b–d) over the entire sampling period before reaching the LLOQ for 40 mg, 80 mg, and 120 mg doses (semi‐log scale). Geometric mean concentration was only calculated if two‐thirds or more of individual values ≥LLOQ. †The pharmacokinetics sampling was up to 149 days after injection. BL, baseline; eq, equivalent; LLOQ, lower limit of quantification; fesomersen‐eq concentrations, total full‐length ASOs (sum of fesomersen, partially conjugated fesomersen, and de‐conjugated fesomersen).
Following single injections of fesomersen 40 mg or 80 mg, geometric mean AUCs of fesomersen‐eq were comparable in Chinese, Japanese, and Caucasian participants (Table 2), which was also reflected by the interethnic ratio of AUC/D (Table S3). In the fesomersen 120 mg dose group, geometric mean AUC in Japanese participants was higher than AUC for the Caucasian and Chinese participants, while the overall range overlapped across ethnic groups (Table 2 and Figure S1), which was in accordance with the slightly higher mean estimate for AUC/D in Japanese subjects compared to Caucasian subjects on a descriptive basis while the 90% CI still includes unity (one) (Table S3). AUC from time 0 to 24 h (AUC[0–24]) and AUC from 24 h to infinity (AUC[24–infinity]) were also calculated considering the biphasic disposition pattern. Overall ranges of AUC(0–24) and AUC(24–infinity) overlapped across ethnic groups (Table 2).
TABLE 2.
PK parameters of fesomersen‐eq in plasma as geometric mean/CV (%) (range) (non‐compartmental).
| Single subcutaneous dose | 40 mg | 80 mg | 120 mg | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Geometric mean/CV, % (min–max) | Caucasian (n = 4) | Japanese (n = 6) | Chinese (n = 12) | Caucasian (n = 4 a ) | Japanese (n = 6) | Chinese (n = 12) | Caucasian (n = 4) | Japanese (n = 6) | Chinese (n = 12) |
| AUC (μg × h/mL) | 3.11/32.5 (2.15–4.41) | 3.33/17.7 (2.68–4.42) | 3.18/22.3 (2.21–4.97) | 8.45/53.8 (5.79–15.0) | 8.80/31.1 (6.06–14.1) | 8.79/41.5 (4.95–16.5) | 12.7/40.9 (7.32–16.9) | 17.2/29.2 (11.4–26.2) | 14.0/30.4 (8.37–24.8) |
| AUC/D (h/mL × 10−5) | 7.77/32.5 (5.37–11.0) | 8.33/17.6 (6.70–11.1) | 7.96/22.3 (5.52–12.4) | 10.6/53.8 (7.24–18.7) | 11.0/31.1 (7.57–17.6) | 11.0/41.5 (6.19–20.6) | 10.5/40.9 (6.10–14.1) | 14.3/29.2 (9.51–21.8) | 11.6/30.4 (6.98–20.7) |
| AUC(0–24) (μg × h/mL) | 2.63/33.4 (1.88–3.83) | 2.98/18.3 (2.35–3.97) | 2.90/23.3 (1.94–4.60) | 6.58/50.2 (4.50–13.0) | 7.90/31.3 (5.28–12.7) | 7.91/44.5 (4.51–15.6) | 11.5/42.2 (6.52–15.5) | 15.9/31.2 (10.2–24.9) | 12.8/32.7 (7.26–23.3) |
| AUC(24–infinity) (μg × h/mL) | 0.465/37.4 (0.271–0.581) | 0.355/14.1 (0.303–0.449) | 0.276/20.6 (0.193–0.370) | 0.967/68.3 (0.671–1.97) | 0.885/39.4 (0.498–1.37) | 0.833/29.2 (0.415–1.33) | 1.17/30.2 (0.798–1.58) | 1.19/25.4 (0.736–1.54) | 1.17/14.2 (0.920–1.52) |
| C max (μg/mL) | 0.359/31.5 (0.293–0.564) | 0.509/19.6 (0.385–0.638) | 0.628/45.3 (0.312–1.17) | 0.886/47.1 (0.570–1.61) | 1.40/61.7 (0.561–3.15) | 1.41/84.0 (0.464–4.80) | 1.55/49.9 (0.829–2.58) | 2.83/45.8 (1.45–5.59) | 2.19/46.5 (1.18–4.79) |
| C max/D (/mL × 10−6) | 8.97/31.5 (7.33–14.1) | 12.7/19.6 (9.61–15.9) | 15.7/45.3 (7.81–29.3) | 11.1/47.1 (7.13–20.1) | 17.5/61.7 (7.02–39.4) | 17.6/84.0 (5.80–60.0) | 12.9/49.9 (6.91–21.5) | 23.7/45.7 (12.1–46.6) | 18.3/46.5 (9.83–39.9) |
| t 1/2 (h) | 477.5/21.2 (388–635) | 344.5/9.4 (307–393) | 252.0/22.5 (160–392) | 331.8/22.6 (257–386) | 363.6/28.0 (243–496) | 374.0/26.6 (219–558) | 353.3/13.1 (304–413) | 341.8/19.9 (271–478) | 333.5/14.7 (287–483) |
| T max b (h) | 1.50 (1.00–3.00) | 2.50 (1.50–3.00) | 2.50 (1.00–4.00) | 1.75 (1.50–2.00) | 2.00 (2.00–4.00) | 3.00 (1.50–4.00) | 2.25 (1.50–4.00) | 3.00 (2.00–4.00) | 3.50 (2.00–4.00) |
Abbreviations: AUC, area under the plasma concentration versus time curve from zero to infinity after single dose; AUC(0–24), AUC from zero to 24 h; AUC(24–infinity), AUC from 24 h to infinity; C max, maximum observed plasma concentration; CV, geometric coefficient of variation; D, dose; eq, equivalent; PK, pharmacokinetic; t 1/2, half‐life associated with the terminal slope; T max, time to reach C max.
N = 3 for AUC, AUC/D, AUC(24–infinity), and t 1/2.
T max is presented as median (min–max).
Geometric mean C max of fesomersen‐eq was slightly higher in Japanese and Chinese participants compared to Caucasian participants, but there were overlaps in the overall ranges and large inter‐individual variabilities (Table 2 and Figure S1). The mean estimate of C max/D was higher in Chinese/Japanese participants compared to Caucasian participants for all dose groups on a descriptive basis (Table S3). AUC and C max for fesomersen‐eq in plasma increased slightly more than dose‐proportional or approximately dose‐proportional from 40 mg to 120 mg in the different populations (Table 2 and Figure S1).
T max was similar between ethnic groups, with median T max values across doses of 2.0–3.0 h, 2.5–3.5 h, and 1.5–2.25 h for Japanese, Chinese, and Caucasian participants, respectively (Table 2). Mean elimination half‐life values were also comparable in Chinese, Japanese, and Caucasian participants and ranged from 252 to 477.5 h (10–20 days) across dose ranges investigated (Table 2 and Figure S1).
Urinary excretion of fesomersen‐eq was low (<1.5% of administered dose) for all three dose levels tested in both FIH and Japan phase I studies and will, therefore, not be presented in further detail.
PD
Dose‐dependent decreases of FXI activity from baseline were observed after single‐dose injections of fesomersen 40 mg, 80 mg, or 120 mg for all ethnicities in a similar manner (Figure 2). Geometric mean (CV, %) of the minimum ratios to baseline of FXI activity in Caucasian, Japanese, and Chinese participants with fesomersen 40 mg were 0.596 (12.9), 0.480 (35.2), and 0.570 (27.3), respectively; with fesomersen 80 mg were 0.381 (41.4), 0.369 (21.6), and 0.362 (28.2), respectively; and with fesomersen 120 mg were 0.260 (21.1), 0.360 (71.7), and 0.268 (73.5), respectively.
FIGURE 2.
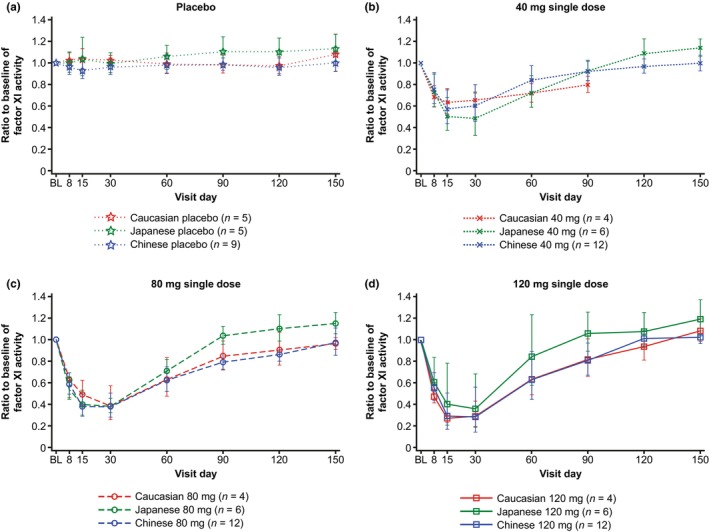
Geometric means with standard deviation range for the ratio to baseline of FXI activity in plasma over time. (a) With placebo and (b–d) with the 40 mg, 80 mg, and 120 mg doses. BL, baseline; FXI, factor XI.
The maximum inhibition of FXI activity was mainly observed between Day 15 and Day 30 in all dose steps across studies. FXI activities returned to near baseline on Day 90 for most participants (Figures 2 and S2). In the exploratory ANCOVA analysis comparing the minimum FXI activity levels (maximum effect, E max) reached after fesomersen injection, the interethnic ratio for Chinese to Caucasian subjects was close to 1 for all dose levels, while the interethnic ratio of Japanese to Caucasian subjects showed more variability, consistent with lower sample size. The same holds true for Day 60 and 90 after injection when FXI activity returned to baseline levels (Table S4). Injection of placebo had no effect on FXI activity (Figure 2).
Dose‐dependent increases in aPTT from baseline were observed after single‐dose injections of fesomersen 40 mg, 80 mg, or 120 mg for all ethnicities in a similar manner (Figure 3). Geometric mean (CV, %) of the maximum ratios to baseline of aPTT in Caucasian, Japanese, and Chinese participants with fesomersen 40 mg were 1.221 (4.8), 1.188 (7.7), and 1.121 (8.7), respectively; with fesomersen 80 mg were 1.414 (3.3), 1.324 (12.1), and 1.241 (8.7), respectively; and with fesomersen 120 mg were 1.397 (5.1), 1.369 (21.0), and 1.308 (13.5), respectively.
FIGURE 3.
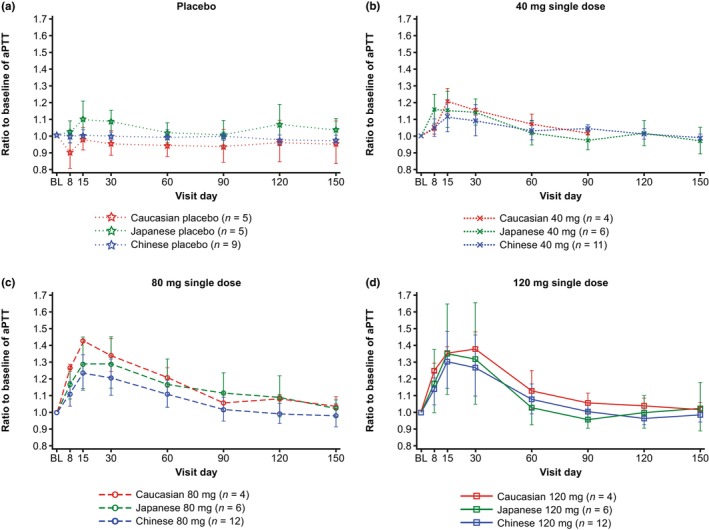
Geometric means with standard deviation range for the ratio to baseline of aPTT in plasma over time. (a) With placebo and (b–d) with 40 mg, 80 mg, and 120 mg doses. aPTT, activated partial thromboplastin time; BL, baseline.
The maximum increase of aPTT was mainly observed between Day 15 and 30 in all dose steps across studies. aPTT returned to near baseline on Day 90 for most participants in all dose arms (Figures 3 and S3). In the exploratory ANCOVA analysis of aPTT regarding E max and return to baseline (i.e., aPTT at Day 60/90) after fesomersen injection, all point estimates of interethnic ratios for Chinese/Japanese to Caucasian participants were close to 1, implying overall similar changes in aPTT for both Chinese/Japanese and Caucasian participants after injection of fesomersen (Table S4). Injection of placebo had no effect on aPTT (Figure 3).
In all three studies, FXI concentration changed in a similar manner with the FXI activity, and aPTT increased with decreasing FXI activity in a consistent manner as illustrated by the scatterplot (Figure 4).
FIGURE 4.
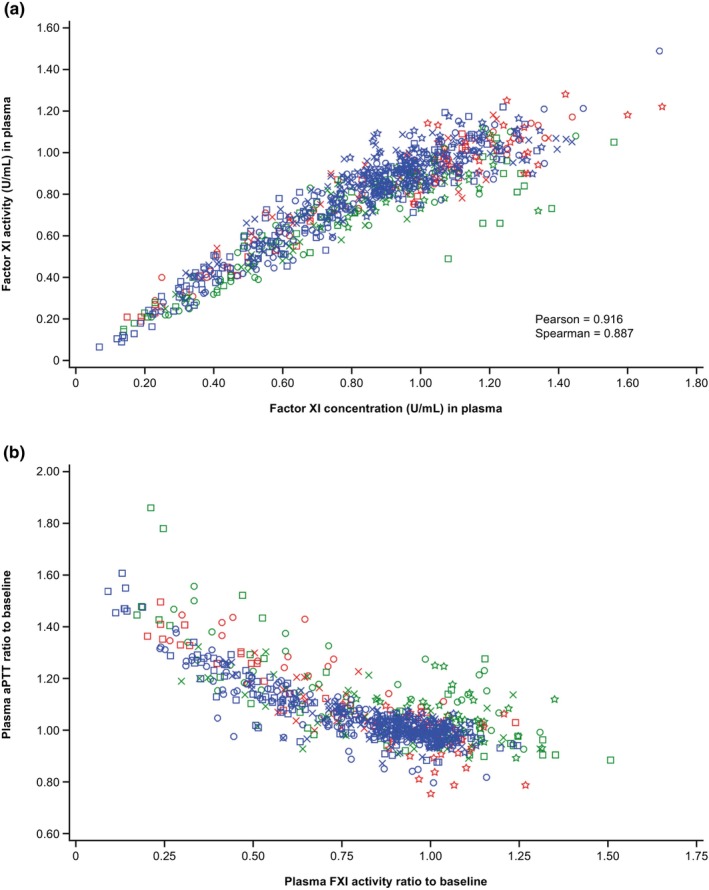
Correlation of FXI activity and FXI concentration (a); and correlation of aPTT and FXI activity (b). All available time points are included in the plots. aPTT, activated partial thromboplastin time; FXI, factor XI.
As expected, the injection of fesomersen or placebo had no relevant effect on PT or INR.
ADA
ADA formation, while occasionally found positive prior to injection in all studies, remained largely unaltered after the injection of either fesomersen or placebo (Table 3). Furthermore, the occurrence of positive ADA formation was comparable across different dose cohorts in all studies, negating the presence of ethnic differences after a single fesomersen injection. Moreover, the rare incidences of positive ADA formation observed within the study did not discernibly influence either PK or PD.
TABLE 3.
Frequency of participants with confirmed anti‐drug antibody formation.
| Time, day | Positive anti‐drug antibody formation, n/N | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | 40 mg | 80 mg | 120 mg | |||||||||
| Caucasian | Japanese | Chinese | Caucasian | Japanese | Chinese | Caucasian | Japanese | Chinese | Caucasian | Japanese | Chinese | |
| N = 5 | N = 6 | N = 9 | N = 4 | N = 6 | N = 12 | N = 4 | N = 6 | N = 12 | N = 4 | N = 6 | N = 12 | |
| 1, pre‐dose | 0/5 | 1/6 | 2/9 | 0/4 | 0/6 | 3/12 | 2/4 | 0/6 | 2/12 | 0/4 | 1/6 | 1/12 |
| 2 | N/A | 1/6 | 2/9 | N/A | 0/6 | 2/12 | N/A | 0/6 | 0/12 | N/A | 0/6 | 0/12 |
| 8 | N/A | 1/6 | 2/9 | N/A | 1/6 | 2/12 | N/A | 0/6 | 3/12 | N/A | 1/6 | 1/12 |
| 15 | N/A | 1/6 | 2/9 | N/A | 2/6 | 3/12 | N/A | 0/6 | 2/12 | N/A | 2/6 | 1/12 |
| 30 | N/A | 1/6 | 2/9 | N/A | 2/6 | 3/12 | N/A | 0/6 | 2/12 | N/A | 2/6 | 2/12 |
| 60 | 0/5 | 1/6 | 2/9 | 0/4 | 2/6 | 2/12 | 3/4 | 0/6 | 2/12 | 2/4 | 1/6 | 2/12 |
| 90 | N/A | 1/6 | 2/9 | N/A | 2/6 | 2/11 | N/A | 0/6 | 2/12 | N/A | 1/6 | 3/12 |
| 120 | 0/4 | 1/6 | 2/9 | N/A | 2/6 | 4/11 | 2/4 | 0/6 | 2/12 | 2/4 | 1/6 | 3/12 |
| 150 | 0/4 | 1/6 | 2/9 | N/A | 2/6 | 2/11 | 1/4 | 0/6 | 2/12 | 1/4 | 2/6 | 4/11 |
Abbreviation: N/A, not applicable.
Safety and tolerability
No deaths, no participant discontinuations due to AEs, and no treatment‐related serious AEs (SAEs) were reported. One SAE (appendicitis) was reported in the placebo cohort in the Japanese study, which was not considered related to study intervention by the investigator, nor related to procedures required by the study protocol. All treatment‐related treatment‐emergent AEs were of mild intensity. The details of treatment‐emergent AEs are provided in Tables S5–S7.
No local cutaneous reactions at the injection site or flu‐like reactions were reported in any study. With regards to bleeding‐related AEs, one Caucasian participant in the FIH 120 mg cohort reported an AE of mild epistaxis 2 days after fesomersen injection that lasted 5 min and resolved spontaneously. The event did not appear to be accompanied by a reduction in platelet levels or to low FXI activity levels. Another Japanese participant in the 80 mg cohort had gingival bleeding 23 days after fesomersen injection. He recovered from this within 11 days without any treatment.
Injection of fesomersen had no clinically relevant effect on vital signs or safety‐monitoring ECG readings. Based on the concentration‐QTc analyses using a linear mixed‐effects approach, the estimated slope of placebo‐adjusted, baseline‐corrected QTc interval using Fridericia's formula (ΔQTcF) with fesomersen‐eq plasma concentration was flat (0.00018 ms/[μg/L]) and statistically not significant (p = 0.9004) in the FIH study pooled analysis, and slightly negative (−0.00095 ms/[μg/L]) with statistical significance (p = 0.0406) in the Japanese study, indicating that there was no positive relationship between fesomersen‐eq plasma concentrations and QTc prolongation (Figure S4). The upper limit of the two‐sided 90% CI of the estimated placebo‐adjusted ΔQTcF changes did not cross the +10 ms threshold at the highest measured plasma concentration (2580 μg/L in Caucasian participants and 5588 μg/L in Japanese participants, both in the 120 mg cohort).
DISCUSSION
Three phase I clinical studies assessed the safety, PD, and PK profiles of fesomersen in healthy participants across different regions. Overall, the injection of fesomersen as a single s.c. dose up to 120 mg had a favorable safety profile and was well tolerated in all three studies. There were no reported local cutaneous reactions at the injection site or flu‐like reactions, which are often observed with other ASO drugs, 12 , 13 , 14 and no severe bleeding potentially related to inhibition of FXI production. The QTc analyses of healthy participants suggested no effect of fesomersen on the cardiac re‐ and de‐polarization durations for single doses up to 120 mg and multiple doses up to 80 mg. These findings are consistent with prior experience with ASOs as a chemical class, which have neither been associated with QT prolongation nor proarrhythmic risk at clinically relevant dose levels. 9 , 15
A slight imbalance in baseline characteristics between the three studies was observed, which is reflective of differences in study designs (such as age, gender, and BMI), and reflective of demographic differences between regions/countries (North America, China, and Japan). The imbalance should be interpreted with caution due to the small sample size of these studies.
Consistent plasma concentration‐time patterns were observed in all ethnic groups across all dose levels. Fesomersen‐eq appeared to be rapidly absorbed into the systemic circulation after a single s.c. injection, with C max observed within a few hours after injection. After reaching C max, mean plasma concentrations of fesomersen‐eq declined in a biphasic fashion over time with an initial fast‐disposition phase mainly reflecting rapid and extensive distribution to tissues, followed by a slower elimination phase, which may reflect slow elimination of the drug from tissues. AUC after single‐dose fesomersen s.c. injection was overall comparable among all ethnic groups, while higher C max was observed in Chinese/Japanese participants compared to Caucasian participants, which was in line with the lower body weight of Chinese/Japanese participants. However, the differences in mean C max were not considered to be clinically relevant based on PD results (FXI activity and aPTT). Previously it has been reported that ethnicity was not a significant covariate affecting the PK of another ASO drug, inotersen, but a small impact on PK by body weight and lean body mass was reported. 16
A dose‐dependent decrease in FXI activity from baseline was observed across all ethnicities. Consequently, dose‐dependent increases in aPTT from baseline were also observed across all ethnicities. No effect on PT was observed. These observations support a specific inhibitory effect of fesomersen on the intrinsic pathway. Maximum PD effects in terms of both FXI activity inhibition and aPTT prolongation were mainly observed between Day 15 and Day 30 in all dose steps across the studies. Both aPTT and FXI activity returned to near baseline on Day 90 for most participants. Consistent with the mechanism of action, in which fesomersen targets the mRNA rather than pre‐existing coagulation protein in the blood, a close correlation between FXI activity and FXI concentration was observed.
Despite the fast absorption of fesomersen into the systemic circulation, maximal PD effect was observed after approximately 2 weeks, and it subsequently took about 3 months for the PD effect to dissipate in most participants after a single dose, which is in line with the mechanism of action of fesomersen. Fesomersen acts by binding to the mRNA of FXI, which leads to its degradation. As a result, the expression of FXI protein decreases, disrupting the balance of blood FXI protein levels gradually. Consequently, the concentration of blood FXI protein decreases, leading to a reduction in FXI activity and an elongated aPTT. Once fesomersen is slowly eliminated from the liver, FXI mRNA gradually recovers, resulting in the recovery of FXI protein. The delay in the onset of PD effects is largely attributable to the long half‐life of existing FXI protein in the blood. The extended PD effect is primarily caused by the slow elimination of fesomersen from tissue (terminal half‐life: 10–20 days) and FXI protein reproduction rate.
The PD profile after single‐dose fesomersen injection was largely comparable between Chinese/Japanese and Caucasian participants, although two Japanese participants in the 120 mg group had a lower PD response. Baseline characteristics including the demographics of these two participants were in line with the overall Japanese cohort. Their systemic exposure was within the range of most participants, and no special pattern of ADA was identified for them. In theory, genetic polymorphisms in the binding region of the ASO could lead to suboptimal binding and hence result in insufficient inhibition or PD response. We tested this hypothesis using genomic sequencing of the respective region in all Japanese participants. On the basis of genomic Sanger sequencing in both directions of 300 base pairs surrounding the binding region, all obtained sequences were identical (data not shown). Therefore, the difference in PD response in the two Japanese participants could not be explained by existing polymorphism. Alternatively, lower intrahepatocellular drug concentrations could have contributed to the observed results, possibly due to reduced uptake of drugs by liver cells. However, this hypothesis could not be tested as intrahepatocellular drug concentrations measured in healthy participants are not ethical.
Pre‐existing ADAs were occasionally detected and remained mostly unchanged after injection. As most people have been exposed to the GalNAc moiety, this might have triggered an immune response in some participants, and therefore in our studies, pre‐existing ADAs could have been observed. ASOs are smaller than typical antibody drugs and have fewer potential epitopes. Therefore, they are less likely to cause immunogenicity. 17 Additionally, no significant influence of ADA formations on the PKs or PDs was observed in the studies (data not shown). Therefore, it appears that those ASO antibodies were not neutralizing antibodies; if they were, the PD effect was expected to be diminished.
Several FXI inhibitors, including monoclonal antibodies, small molecule inhibitors, and ASOs are currently in various stages of clinical development for multiple indications. Although all these drugs target thrombotic disease, fesomersen may be more suitable for the management of chronic diseases owing to its delayed, but predictable onset combined with a long duration of action. Patient compliance can be improved by reducing the frequency of injection.
In summary, fesomersen was well tolerated in healthy participants in Caucasian, Japanese, and Chinese studies as a single s.c. dose up to 120 mg. All ethnic groups displayed similar concentration‐time patterns and comparable AUC values. The PD profiles of Chinese, Japanese, and Caucasian healthy participants were comparable, as evidenced by distinct target engagement and dose response observed for aPTT and FXI activity after fesomersen injection.
AUTHOR CONTRIBUTIONS
All authors wrote the manuscript and approved the final version. T.L., K.H., H.C., Y.M., S.S., and R.Y. designed the studies. All authors analyzed the data.
FUNDING INFORMATION
IONIS and Bayer AG were the sponsors of the clinical trials.
CONFLICT OF INTEREST STATEMENT
T.L., K.H., E.K., H.C., Y.M., F.F., S.W., S.S., and A.S. are employees of and may hold stock in Bayer AG. K.T. was a former employee of Bayer AG at the time of the studies. R.Y. is an employee of Ionis Pharmaceuticals, Inc., and held stock in the company at the time of the studies.
Supporting information
Appendix S1.
ACKNOWLEDGEMENTS
The authors thank all volunteers in these trials, investigators of respective studies, and Bayer and IONIS employees who contributed to the project design, study conduct, and analysis. Interethnic analysis programming was provided by Christine Kalteis and Otto Schramek, both of Bayer AG. C‐QTc analysis was provided by Valentin Demmel and Alexander Staller, both of Nabios GmbH/Banook Group. Medical writing and editorial support were provided by Sara Shaw, PhD; Moamen Hammad, PhD; and Melissa Ward, BA from Scion (a division of Prime, London, UK) and funded by Bayer.
Liu T, Hashizume K, Krieg E, et al. Pharmacokinetics, pharmacodynamics, and safety of fesomersen, a novel antisense inhibitor of factor XI, in healthy Chinese, Japanese, and Caucasian volunteers. Clin Transl Sci. 2024;17:e13784. doi: 10.1111/cts.13784
REFERENCES
- 1. World Health Organization (WHO) . Fact sheet on cardiovascular diseases (CVDs). https://www.who.int/news‐room/fact‐sheets/detail/cardiovascular‐diseases‐(cvds). Accessed May 24, 2023.
- 2. Walsh M, Bethune C, Smyth A, et al. Phase 2 study of the factor XI antisense inhibitor IONIS‐FXI(Rx) in patients with ESRD. Kidney Int Rep. 2022;7:200‐209. doi: 10.1016/j.ekir.2021.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weitz JI, Fredenburgh JC. Factors XI and XII as targets for new anticoagulants. Front Med (Lausanne). 2017;4:19. doi: 10.3389/fmed.2017.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bauer KA. Pros and cons of new oral anticoagulants. Hematology Am Soc Hematol Educ Program. 2013;2013:464‐470. doi: 10.1182/asheducation-2013.1.464 [DOI] [PubMed] [Google Scholar]
- 5. Lee CJ, Badhwar G, Ansell JE. Oral IIa inhibitors. Hematol Oncol Clin North Am. 2010;24:739‐753, ix. doi: 10.1016/j.hoc.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 6. Fredenburgh JC, Weitz JI. News at XI: moving beyond factor Xa inhibitors. J Thromb Haemost. 2023;21:1692‐1702. doi: 10.1016/j.jtha.2023.04.021 [DOI] [PubMed] [Google Scholar]
- 7. Büller HR, Bethune C, Bhanot S, et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372:232‐240. doi: 10.1056/NEJMoa1405760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Willmann S, Marostica E, Snelder N, et al. PK/PD modeling of FXI antisense oligonucleotides to bridge the dose‐FXI activity relation from healthy volunteers to end‐stage renal disease patients. CPT Pharmacometrics Syst Pharmacol. 2021;10:890‐901. doi: 10.1002/psp4.12663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Y, Yu RZ, Henry S, Geary RS. Pharmacokinetics and clinical pharmacology considerations of GalNAc(3)‐conjugated antisense oligonucleotides. Expert Opin Drug Metab Toxicol. 2019;15:475‐485. doi: 10.1080/17425255.2019.1621838 [DOI] [PubMed] [Google Scholar]
- 10. Dixon JR Jr. The International Conference on Harmonization GoodClinical Practice guideline. Qual Assur. 1998;6:65‐74. doi: 10.1080/105294199277860 [DOI] [PubMed] [Google Scholar]
- 11. Kadian N, Raju KS, Rashid M, et al. Comparative assessment of bioanalytical method validation guidelines for pharmaceutical industry. J Pharm Biomed Anal. 2016;126:83‐97. doi: 10.1016/j.jpba.2016.03.052 [DOI] [PubMed] [Google Scholar]
- 12. Chambergo‐Michilot D, Alur A, Kulkarni S, Agarwala A. Mipomersen in familial hypercholesterolemia: an update on health‐related quality of life and patient‐reported outcomes. Vasc Health Risk Manag. 2022;18:73‐80. doi: 10.2147/vhrm.S191965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Benson MD, Waddington‐Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:22‐31. doi: 10.1056/NEJMoa1716793 [DOI] [PubMed] [Google Scholar]
- 14. Esan O, Wierzbicki AS. Volanesorsen in the treatment of familial chylomicronemia syndrome or hypertriglyceridaemia: design, development and place in therapy. Drug Des Devel Ther. 2020;14:2623‐2636. doi: 10.2147/dddt.S224771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qu Y, Henderson KA, Harper TA Jr, Vargas HM. Scientific review of the proarrhythmic risks of oligonucleotide therapeutics: are dedicated ICH S7B/E14 studies needed for low‐risk modalities? Clin Pharmacol Ther. 2024. doi: 10.1002/cpt.3204 [DOI] [PubMed] [Google Scholar]
- 16. Yu RZ, Collins JW, Hall S, et al. Population pharmacokinetic‐pharmacodynamic modeling of inotersen, an antisense oligonucleotide for treatment of patients with hereditary transthyretin amyloidosis. Nucleic Acid Ther. 2020;30:153‐163. doi: 10.1089/nat.2019.0822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stebbins CC, Petrillo M, Stevenson LF. Immunogenicity for antisense oligonucleotides: a risk‐based assessment. Bioanalysis. 2019;11:1913‐1916. doi: 10.4155/bio-2019-0133 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.


