Abstract
Background
Effects of non-pharmaceutical interventions during the pandemic were mainly studied for severe outcomes. Among children, most of the burden of respiratory infections is related to infections which are not medically attended. The perspective on infections in the community setting is necessary to understand the effects of the pandemic on non-pharmaceutical interventions.
Methods
In the unique prospective LoewenKIDS cohort study, we compared the true monthly incidence of self-reported acute respiratory infections (ARI) in about 350 participants (aged 3–4 years old) between October 2019 to March 2020 (pre-pandemic period) and October 2020 to March 2021 (pandemic period). Parents reported children’s symptoms using a diary. Parents were asked to take a nasal swab of their child during all respiratory symptoms. We analysed 718 swabs using Multiplex PCR for 25 common respiratory viruses and bacteria.
Results
During the pre-pandemic period, on average 44.6% (95% CI: 39.5–49.8%) of children acquired at least one ARI per month compared to 19.9% (95% CI: 11.1–28.7%) during the pandemic period (Incidence Rate Ratio = 0.47; 95% CI: 0.41–0.54). The detection of influenza virus decreased absolute by 96%, respiratory syncytial virus by 65%, metapneumovirus by 95%, parainfluenza virus by 100%, human enterovirus by 96% and human bocavirus by 70% when comparing the pre-pandemic to the pandemic period. However, rhinoviruses were nearly unaffected by NPI. Co-detection (detection of more than one virus in a single symptomatic swab) was common in the pre-pandemic period (222 of 390 samples with viral detection; 56.9%) and substantially less common during the pandemic period (46 of 216 samples; 21.3%).
Conclusion
Non-pharmaceutical interventions strongly reduced the incidence of all respiratory infections in preschool children but did not affect rhinovirus.
Keywords: Respiratory tract infections, Birth cohort study, Non-pharmaceutical interventions
Background
The World Health Organisation declared a global pandemic on March 11, 2020, which was caused by the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) and the resulting coronavirus disease of 2019 (COVID-19) [1]. SARS-CoV-2 appeared to affect children less often during the early stages of the COVID-19 pandemic due to mostly asymptomatic or mildly symptomatic cases [2, 3]. However, subsequent studies showed that children are as likely as adults to acquire and transmit SARS-CoV-2 [4–6]. During COVID-19–related lockdown periods, the German government issued, among other non-pharmaceutical interventions (NPI) (i.e. physical distancing, stay-at-home orders, lockdown of non-essential shops, mandatory mask wearing), the closure of schools and day care centres. Given that all respiratory viruses are transmitted via similar mechanisms – aerosols or droplets – NPI targeting SARS-CoV-2 also had the potential to reduce infections by respiratory viruses other than SARS-CoV-2 [7–10]. In Germany, for example, flu season suddenly stopped in week 14 during winter 2019/20 (after the first COVID-19 lockdown was issued in week 12) [11], and an almost undetectable flu season followed during winter 2020/21 (in a period of stepwise increasing restrictions) [12]. In children, studies using data based on hospital records or registers from Australia, Austria, Finland and Japan show that the incidence decreased for different respiratory viruses, e.g. influenza, respiratory syncytial virus and metapneumovirus but not for rhino-/enterovirus and adenovirus during lockdown periods [8, 13–15].
So far, studies have shown reduced frequencies of acute respiratory infections (ARI) and changed viral and bacterial presence during COVID-19 lockdown periods using mostly hospital data or data based on registers from laboratories only. However, most ARI in children are not medically attended [16], and hospital based studies are related to severe infections, thus most of the ARI among preschool children are not included in previous research. In addition, the viral and bacterial profile might be disrupted in hospital-based studies. Furthermore, during the lockdown period, parents were reported to avoid seeing medical doctors for consultations, possibly further changing the viral spectrum detected in medical settings [9, 10, 17].
This study aimed to investigate the impact of COVID-19–related NPI during the winter period 2020/21 compared to the winter period 2019/20 on the occurrence of all ARI, i.e. also those not medically attended, and the respiratory viral and bacterial presence among 3- and 4-year-old children in a population-based cohort in Germany.
Methods
Study sample
We used data and nasal swabs from the birth cohort study LoewenKIDS (Clinicaltrials.Gov Identifier: NCT02654210) which is described in detail elsewhere [18]. Briefly, between 2014 and 2018, we enrolled 782 newborns in five German study centres (336 children in Braunschweig, 174 children in Hannover, 97 children in Bremen, 91 children in Munich and 76 children in Halle). During the first six years of their child’s life, parents are asked to fill in a daily symptom diary and take nasal swabs at each event of respiratory symptoms. The Ethics Committees of the Martin Luther-University Halle-Wittenberg (No. 2016-04), the Medical School Hannover (No. 6794) and the Ludwig Maximilian University Munich (No. 445 − 15), Germany approved the study. Parents received detailed information on the objectives of the cohort study and provided written informed consent.
Symptom diary and ARI episode definition
Parents were asked to record symptoms and to rate their severity on a daily basis in a symptom diary. Symptoms included fever, wheezing, cough with sputum (category A) and runny nose or nasal congestion, sore throat, cough, chills, loss of appetite, increased need to sleep, increased attachment (category B) and were classified as previously described [19]. Participants aged three or four years in the times of interest with at least 80% completeness of daily entries for the symptoms were included in the analysis of the ARI episodes.
Nasal swabs
Parents collected nasal swabs at the beginning of an ARI, which was defined as one or more symptoms of category A or two or more symptoms of category B. Swabs were put in tubes with transport media (eNat or Amies Medium) and transported via regular mail to the laboratory within 48h of collection. Specimens were stored at -80°C until further analysis. We analysed all symptomatic nasal swabs (n = 457) of 206 children aged 3–4 years in the period October 2019 to March 2020 (pre-pandemic period) and all swabs (n = 261) of 162 children aged 3–4 years in the period October 2020 to March 2021 (pandemic period). Participants did not receive the results of the nasal swab testing. There were no restrictions for the selection of nasal swabs regarding the completeness of the symptom diary.
RNA extraction and qPCR analysis
Viral RNA was extracted from 200 μl of nasopharyngeal aspirate specimens using the Quick-DNA/RNATM Viral MagBeadkit (Zymo Research), according to the manufacturer’s instructions. We screened the samples by multiplex PCR with the AllplexTM Respiratory Panel 2–4 and AllplexTM SARS-CoV-2/FluA/FluB/RSV Assay (Seegene Germany GmbH) using the CFX96 Dx System (Bio-Rad). The samples were analysed using the CFX Manager™ Dx Software v3.1 and Seegene Viewer. All assays were carried out as described by the manufacturer’s instructions. The four panels included assays for the following respiratory viruses and bacteria: Influenza A virus (FluA), Influenza B virus (FluB), Respiratory Syncytial Virus (RSV A & B), Adenovirus (AdV), Enterovirus (HEV), Metapneumovirus (MPV), Parainfluenza 1–4 (PIV1-4), Bocavirus (HBoV), Coronaviruses (229E, OC43, NL63), Rhinovirus (HRV), SARS-CoV-2 (N gene, RdRP gene), Bordetella parapertussis (BPP), Bordetella pertussis (BP), Chlamydophila pneumoniae (CP), Haemophilus influenzae (HI), Legionella pneumophila (LP), Mycoplasma pneumoniae (MP) and Streptococcus pneumoniae (SP). Invalid samples with negative internal control values (n = 2) were not included in the analysis.
Statistical analysis
R software (Version 4.0.5) was used for data handling and all statistical analyses. For the classification of symptoms and episodes, we used the R-package lkstaR [20]. Descriptive characteristics were summarised using counts, percentages and means, with a 95% confidence interval (95% CI). For the incidence rate, we divided the number of ARI by the number of person-months in the respective period. We calculated the incidence rate ratio by dividing the incidence rate (number of ARI per person and month) during the pandemic period by the incidence rate of participants during the pre-pandemic period.
Results
Characteristics of the study population
We selected all participants aged 3–4 years in the pre-pandemic and pandemic periods resulting in 323 and 368 participants, respectively. Among those, 257 participants were included in both periods. Table 1 summarises the demographic characteristics of the participants. There is a difference of the age proportion between the pre-pandemic and pandemic period because we did not recruit the same number of children each year. We received 457 nasal swabs during the pre-pandemic period and 261 nasal swabs during the pandemic period.
Table 1.
Socio-demographics of participants enrolled in the study
| Characteristics | Pre-pandemic period N (%) |
Pandemic period N (%) |
|---|---|---|
| Number | 323 | 368 |
| Sex | ||
| Female | 164 (50.8) | 176 (47.8) |
| Male | 157 (48.6) | 188 (51.1) |
| Missing | 2 (0.6) | 4 (1.1) |
| Age (in years) | ||
| 3 | 217 (67.2) | 147 (39.9) |
| 4 | 106 (32.8) | 221 (60.1) |
| Study Region | ||
| Braunschweig | 147 (45.5) | 144 (39.1) |
| Hannover | 73 (22.6) | 77 (20.9) |
| Halle (Saale) | 17 (5.3) | 45 (12.2) |
| Munich | 40 (12.4) | 48 (13.0) |
| Bremen | 45 (13.9) | 51 (13.9) |
| Other | 1 (0.3) | 3 (0.8) |
| Number of persons in household | ||
| 2 | 5 (1.5) | 2 (0.5) |
| 3 | 129 (39.9) | 152 (41.3) |
| 4 | 138 (42.7) | 159 (43.2) |
| 5 | 41 (12.7) | 43 (11.7) |
| ≥ 6 | 6 (1.9) | 7 (1.9) |
| Missing | 4 (1.2) | 5 (1.4) |
| Socio economic status* | ||
| Low | 2 (0.6) | 2 (0.5) |
| Middle | 23 (7.1) | 30 (8.2) |
| High | 293 (90.7) | 330 (89.7) |
| Missing | 5 (1.5) | 6 (1.6) |
| Total of submitted symptomatic nasal swabs | 457 | 261 |
*According to Brandenburg social status index [64]
Incidence and characteristics of ARI during the pre-pandemic and pandemic periods
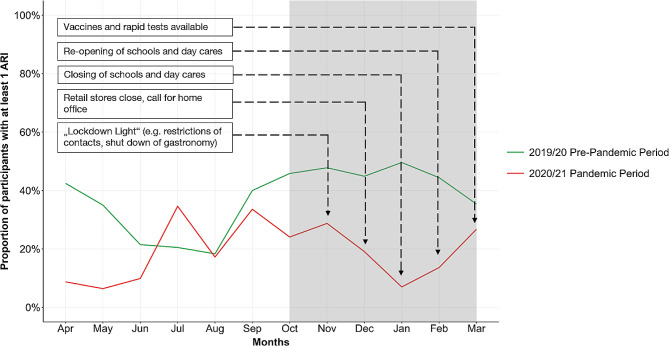
We detected 616 ARI among 222 children during the pre-pandemic winter and 259 ARI among 144 children during the pandemic winter. In the pre-pandemic period, on average 44.6% (95% CI: 39.5–49.8%) of the participants had at least one ARI per month, whereas this number decreased to 19.9% (95% CI: 11.1–28.7%) during the pandemic period. The incidence rate of ARI among children in the pre-pandemic period was 2.8 ARI per person in six months and therefore 0.47 (95% CI: 0.41–0.54) times as high as the rate among children during the pandemic period (1.3 ARI per person in six months). This decrease in the proportion of participants with at least one ARI paralleled the start of NPI in autumn 2020 and the increasing intensity of the interventions (Fig. 1). The lowest incidence, with 6.9% of participants with at least one ARI per month, occurred in January 2021, which paralleled the strictest COVID-19 measures. After the easing of restrictions in February 2021, we observed an increase of ARI. In March 2021, the proportion of ARI reached almost the level observed before the pandemic.
Fig. 1.
Changes in incidence of ARI during the pre-pandemic and pandemic periods. The grey box indicates the winter periods, which were selected for further analysis
During the pre-pandemic winter, an ARI episode lasted on average 12.3 days (95% CI: 11.3–13.3 days) compared to 8.6 days (95% CI: 7.5–9.7 days) during the pandemic winter (Table 2). Symptom patterns were similar for both periods. During the pre-pandemic period, 14.0% (95% CI: 10.8–17.2%) of all participants reported ARI were medically attended and 10.0% (95% CI: 3.5-16.5%) during the pandemic period.
Table 2.
Length of ARI and presence of symptoms during pre-pandemic and pandemic periods
| Pre-pandemic period | Pandemic period | |
|---|---|---|
| Length of ARI in days (Mean, 95% CI) | 12.3 (11.3–13.4) | 8.6 (7.5–9.7) |
| Proportion of days of ARI with …(%, 95% CI) | ||
| runny nose | 71.2 (70.4–71.9) | 70.8 (69.7–71.6) |
| cough | 61.9 (59.7–63.8) | 56.5 (53.2–59.1) |
| increased attachment | 14.3 (13.7–14.9) | 17.3 (16.6–20.2) |
| increased need to sleep | 13.5 (12.8–14.1) | 11.3 (9.5–12.6) |
| loss of appetite | 12.2 (11.4–12.8) | 9.7 (8.2–10.9) |
| fever | 7.5 (6.9–7.9) | 5.4 (4.6–6.1) |
| wheezing | 3.3 (2.3–4.2) | 4.1 (1.4–6.1) |
| sore throat | 3.1 (2.5–3.6) | 4.6 (3.3–5.6) |
| chills | 0.8 (0.5–1.1) | 1.0 (0.4–1.5) |
| Proportion of all ARI that were medically attended (%, 95% CI) | 14.0 (10.8–17.2) | 10.0 (3.5–16.5) |
| Proportion of ARI with hospital admissions | 0.6% | 0.0% |
Distribution of viruses in symptomatic nasal swabs in the pre-pandemic and pandemic periods
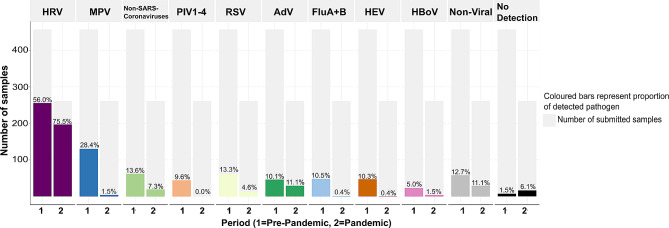
We analysed 455 nasal swabs from 206 children for the pre-pandemic period and 261 nasal swabs from 162 children for the pandemic period using multiplex PCR. We did not detect any bacteria or viruses in 23 samples (3.2%). While the absolute incidence for all tested viruses decreased, the fraction of adenoviruses and bacteria (non-viral) among all swabs remained at the same level, and the fraction of samples containing rhinoviruses even increased (Fig. 2). Other viruses (MPV, PIV1-4, FluA + B, HEV) were not detected during the pandemic period. We did not detect SARS-CoV-2 in any sample.
Fig. 2.
Prevalence (%) of viruses and bacteria detected in both periods in relation to the number of submitted samples. (HRV: Human Rhinovirus; MPV: Metapneumovirus; Non-SARS-Coronaviruses: Human Coronaviruses 229E, OC43, NL63; PIV1-4: Parainfluenza Viruses 1–4; RSV: Respiratory Syncytial Virus; AdV: Adenovirus; FluA + B: Influenza A virus and Influenza B virus; HEV: Human Enterovirus; HBoV: Human Bocavirus; Non-viral includes all bacterial strains: Bordetella parapertussis, Bordetella pertussis, Chlamydophila pneumoniae, Haemophilus influenzae, Legionella pneumophila, Mycoplasma pneumoniae and Streptococcus pneumonia)
Changes in the distribution of viruses and bacteria were accompanied by a substantially decreased number of co-detections. Overall, we detected only viral strains in 57 samples (8.2%), only bacterial strains in 87 samples (12.6%) and viral plus bacterial strains in 549 samples (79.2%, Table 3). During the pre-pandemic period, we observed that 56.9% of all samples had a viral co-detection (more than one virus identified per sample); this fraction decreased to only 21.3% during the pandemic period. While we found up to six viruses in one sample during the pre-pandemic period, we only found up to three viruses in one sample during the pandemic period. The most common combinations for simultaneous presence of viruses were HRV together with MPV (21.9%) or together with AdV (20.8%). For the samples with only bacterial presence, more than 50% of samples had a double detection with HI and SP in both periods. Co-detection of viruses and bacteria were more common in the pre-pandemic period (81.9%) compared to the pandemic period (67.4%). Among them, most samples with viruses showed a co-detection with the bacteria HI and SP, followed by a co-detection with either HI or SP.
Table 3.
Number and characteristics of viral and bacterial co-detection for analysed samples in pre-pandemic and pandemic periods (BPP: Bordetella parapertussis; BP: Bordetella pertussis; CP: Chlamydophila pneumonia; HI: Haemophilus influenza; LP: Legionella pneumophila; MP: Mycoplasma pneumonia; SP: Streptococcus pneumonia)
| Pre-pandemic Period | Pandemic period | |
|---|---|---|
| Samples with ≥ 1 detected virus | ||
| Total number / all valid samples (%) | 390/455 (85.7) | 216/261 (82.8) |
| Average number of detected viruses per sample (95% CI) | 1.8 (1.7–1.9) | 1.2 (1.1–1.3) |
| Number of detected viruses per sample (%) | ||
| 1 | 168 (43.1) | 170 (78.7) |
| 2 | 137 (35.1) | 41 (19.0) |
| 3 | 66 (16.9) | 5 (2.3) |
| 4 | 15 (3.8) | 0 (0.0) |
| 5 | 3 (0.8) | 0 (0.0) |
| 6 | 1 (0.3) | 0 (0.0) |
| Samples with only bacterial presence | ||
| Total number / all valid samples (%) | 58/455 (12.7) | 29/261 (11.1) |
| Number of samples with SP | 21 (36.2) | 8 (27.6) |
| Number of samples with HI | 5 (8.6) | 6 (20.7) |
| Number of samples with SP + HI | 30 (51.7) | 15 (51.7) |
| Number of samples with BPP | 2 (3.4) | 0 (0.0) |
| Samples with only viral presence | ||
| Total number / all valid samples (%) | 17/455 (3.7) | 40/261 (15.3) |
| Samples with viral and bacterial presence | ||
| Total number / all valid samples (%) | 373/455 (81.9) | 176/261 (67.4) |
| Virus + HI | 68 (18.2) | 30 (17.0) |
| Virus + SP | 25 (6.7) | 38 (21.6) |
| Virus + SP + HI | 270 (72.4) | 107 (60.8) |
| Virus + BPP | 2 (0.5) | 0 (0.0) |
| Virus + BPP/MP + HI | 2 (0.5) | 0 (0.0) |
| Virus + BPP/MP + HI + SP | 6 (1.6) | 1 (0.6) |
Discussion
Our results show that the COVID-19 pandemic-related NPI strongly reduced the number of self-reported ARI in healthy 3- and 4-year-old infants in Germany. NPI suppressed the spread of most seasonal viruses except HRV.
The observed reduction of ARI incidence during the NPI is in line with other studies focusing on the paediatric population in hospitals or using register data [8, 21, 22]. The reduction is likely to be caused by various NPI, although the impact of individual components is unclear. Respiratory infections, especially in early childhood, are very common and cause not just high socioeconomic burdens [23] but are also associated with the development of chronic diseases later in life [24]. However, fewer infections during NPI likely mean a reduced immunity afterwards. In some parts of the world, a strong atypical resurgence of respiratory infections, like RSV, was observed during summer months for children after easing NPI [25–27]. The high proportion of susceptible individuals and the lack of exposure to certain viruses and bacteria could affect timing and severity of future ARI seasons [28].
We found that the prevalence of viral strains changed before and during NPI with suppression of severe viruses such as FluA + B, PIV1-4, RSV and MPV. The natural season period of some viruses was interrupted through the NPI. For example MPV, which is normally detected in late winter and spring [29], was no longer detectable during the pandemic period [30, 31]. However, some studies reported an off-season outbreak of MPV from May to July 2021 [32, 33]. Similarly, RSV, which mostly occurs between December to March each year [29], could rarely be found during the pandemic period [30, 34–36]. Many hospitals, however, registered a massive off-season outbreak during summer months [27, 30, 34]. Interestingly, influenza virus infection incidence remained low during summer months [37, 38], this could indicate that climatic conditions and seasonality differentially affects the various viruses. In contrast, HRV was less affected by the NPI. HRV infections are highly common in children [39] and can cause light symptoms, which are usually associated with a common cold [40]. However, HRV can also cause severe symptoms leading to lower respiratory tract infections such as bronchitis [41]. In our study, HRV infections usually showed only mild symptoms.
Our findings from a population-based setting are in line with recent reports of registers or hospital data published in various countries which all describe that HRV circulation was unchanged during NPI to control the spread of SARS-CoV-2 [9, 13, 42, 43], but we also detected similar levels of AdV before and during the pandemic. This suggests that non-enveloped viruses were less affected by NPI. A major reason could be the stability of non-enveloped viruses, (e.g., HRV has been proven to be more resistant to detergents for hand washing [44] or disinfectants [45]). HRV and AdV are more effective than other airborne viruses at using the indirect transmission pathway (e.g. hand-to-hand contact followed by self-inoculation) [46–48]. This might explain the high prevalence of HRV in children, as the hygiene behaviour is not yet well established [49]. However, we detected a strong decline in HEV, which also belongs to the non-enveloped viruses. Thus, a comprehensive explanation of our findings is still lacking, and further research is needed to better understand the circulation of enveloped and non-enveloped viruses during NPI. It has been also proposed that surgical masks might not prevent the transmission of HRV [50]. On the other hand, it was discussed that HRV might display some form of colonization and thus be present independently of infection symptoms [51, 52]. If HRV were part of the microbiome, then the fraction of symptomatic swabs without a responsible virus would substantially increase, particularly during the pandemic period when fewer co-detections were present. However, we detected HRV without co-detections in the symptomatic nasal swabs especially during the pandemic period, which points towards HRV being the causative virus in our samples. There is also the possibility that we detected different HRV species. There is evidence that HRV-A and HRV-C are frequently associated with more severe infections, while HRV-B is often associated with asymptomatic infections [53, 54]. However, we did not analyse the species.
The proportion of viral co-detections before the pandemic was surprisingly high compared to most previous studies where co-detection rates up to 40% [55, 56] were reported. However, most of those studies were conducted in hospital settings with severely infected children so that virus patterns might be altered compared to the LoewenKIDS’ community setting. In healthy children, higher co-detections may be normal; however, literature about co-detection in community settings is very limited. An explanation for co-detection could be that some viral shedding is prolonged although the infection already passed. It was also shown that some viruses (e.g., HRV) are able to colonise the nasal microbiome and are therefore non-pathogenic [51, 52]. During the pandemic period, viral co-detection was markedly reduced, which reflects the decline of most viruses.
A recent systematic review and meta-analysis showed that viral co-detections in children do not have an impact on the severity of the disease, where mostly hospital-based studies with clinical outcomes were included [57]. In our analysis, we also did not observe any change in severity of symptoms.
The bacterial presence was not affected by NPI, which is supported by findings for pneumococcal carriage [58, 59]. Detection of viral and bacterial strains in the same sample was rather common, and among them, almost all samples yielded bacterial co-detections with SP and HI. Asymptomatic nasopharyngeal carriage in healthy preschool children has been documented, 64% for HI [60] and at least 50% for SP [61], meaning that children are mostly colonised with those acting commensals after entering daycare [62]. Most of the children in our cohort entered day care in the second year of life [19] potentially explaining the high prevalence of HI and SP in our samples.
Strengths and limitations
This study has several strengths, including the utilisation of data from the prospective, longitudinal birth cohort study LoewenKIDS in a community setting [63]. Parents reported symptoms on a daily basis making it possible to evaluate the true disease burden, because mild diseases are recorded even if a physician was not consulted. Furthermore, parents provided symptomatic nasal swabs whenever the child had symptoms of ARI. Multiplex PCR including 25 major respiratory viruses and bacteria is unusual for regular care; therefore, our study provides a valuable insight into the viral prevalence of ARI during both winter seasons in a healthy paediatric cohort.
This study also has several limitations. First, we cannot exclude that the NPI and the pandemic situation in general had an impact on our participants. It might be possible that parents were more sensitive to perceive symptoms, and we overestimated the number of ARI during the pandemic period. However, participants of our study have been trained for several years to record symptoms routinely. Second, we included nasal swabs which were taken by the parents of the children and not from a health care worker. However, we provided a detailed description on how to take the swab to minimise individual sample techniques and ensure that detection rates were equal. Third, there were ARI episodes in the symptom diary without nasal swabs, and sometimes parents sent nasal swabs without corresponding entries in the symptom diary. However, the numbers were similar in both periods. ARI characteristics of the episodes with nasal swabs did not differ from the episodes without swabs (data not shown), so the missing information should not have an impact on the results. Fourth, the participants of the LoewenKIDS cohort are mostly well educated and affluent [13], (e.g., most of the parents have high academic degrees and could probably work in a home office to avoid contact to other people). This does not reflect the majority of the population in Germany, so our results could be biased by the high socio-economic status of our study population.
Conclusion
The introduction of strict public health measures to control the COVID-19 pandemic in Germany reduced the number of self-reported ARI. Various respiratory viruses and bacteria were differentially affected and might indicate the possibility of various pathways to improve infection control among preschool children. Effects of co-detections should be further studied.
Acknowledgements
We thank all LoewenKIDS families for their efforts and contributions. Moreover, we thank Mareike Kunze, Carla Hartmann and Karin Paduch for excellent technical assistance.
Abbreviations
- 95% CI
95% confidence interval
- AdV
Adenovirus
- ARI
Acute respiratory infections
- BP
Bordetella pertussis
- BPP
Bordetella parapertussis
- COVID
19 Coronavirus disease 2019
- CP
Chlamydophila pneumoniae
- FluA
Influenza A virus
- FluB
Influenza B virus
- HBoV
Human Bocavirus
- HEV
Enterovirus
- HI
Haemophilus influenzae
- HRV
Human Rhinovirus
- LP
Legionella pneumophila
- MP
Mycoplasma pneumoniae
- MPV
Metapneumovirus
- NPI Non
Pharmaceutical interventions
- PIV1-4
Parainfluenza virus 1–4
- RSV
Respiratory Syncytial Virus
- SARS
CoV-2 Severe acute respiratory syndrome coronavirus type 2
- SP
Streptococcus pneumoniae
Author contributions
BK conceptualised and designed the study, carried out the analyses, interpreted the data, drafted the initial manuscript and critically reviewed and revised the manuscript. SD, JH and SL interpreted the data and critically reviewed and revised the manuscript. MW, DO and AB collected data, provided technical assistance and critically reviewed and revised the manuscript. TS, RM and CG conceptualised and designed the study, supervised the data analysis and interpretation and critically reviewed and revised the manuscript. All authors approve the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
This study was funded partly by Helmholtz Centre for Infection Research, Braunschweig, the Martin Luther University Halle-Wittenberg and the German Centre for Infection Research, all located in Germany. T.S. is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy – EXC 2155 – project number 390874280. No other disclosures were reported.
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study followed guidelines stated in the declaration of Helsinki. The study protocol was approved by the Ethics Committees of the Martin Luther University Halle-Wittenberg (No. 2016-04), the Medical School Hannover (No. 6794) and the Ludwig Maximilian University Munich (No. 445 − 15), Germany. Parents received detailed information on the objectives of the cohort study and provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organisation. WHO Director-General’s opening remarks at the media briefing on COVID-19. 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- 2.Ladhani SN, Amin-Chowdhury Z, Davies HG, Aiano F, Hayden I, Lacy J, Sinnathamby M, De Lusignan S, Demirjian A, Whittaker H, Andrews N, Zambon M, Hopkins S, Ramsay M. COVID-19 in children: analysis of the first pandemic peak in England. Arch Dis Child. 2020;105(12):1180–5. doi: 10.1136/archdischild-2020-320042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China. JAMA. 2020;323(13):1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Robert Koch Institut (RKI). Wöchentlicher Lagebericht des RKI zur Coronavirus-Krankheit-2019. 13.01.2022 - aktualisierter Stand für Deutschland. 2022.
- 5.Dawood FS, Porucznik CA, Veguilla V, Stanford JB, Duque J, Rolfes MA, Dixon A, Thind P, Hacker E, Castro MJE, Jeddy Z, Daugherty M, Altunkaynak K, Hunt D, Kattel U, Meece JK, Stockwell MS. Incidence rates, household infection risk, and clinical characteristics of SARS-CoV-2 infection among children and adults in Utah and New York City, New York. JAMA Pediatr. 2022;176(1):59–67. 10.1001/jamapediatrics.2021.4217 [DOI] [PMC free article] [PubMed]
- 6.Laws RL, Chancey RJ, Rabold EM, Chu VT, Lewis NM, Fajans M. Symptoms and transmission of SARS-CoV-2 among children - Utah and Wisconsin, March-May 2020. Pediatrics. 2021;147(1):1–15. doi: 10.1542/peds.2020-027268. [DOI] [PubMed] [Google Scholar]
- 7.Cowling BJ, Ali ST, Ng TWY, Tsang TK, Li JCM, Fong MW. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health. 2020;5(5):e279–288. doi: 10.1016/S2468-2667(20)30090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan SG, Carlson S, Cheng A, Chilver M, Dwyer DE, Irwin M, Kok J, Macartney K, MacLachlan JH, Minney-Smith CA, Smith DW, Stocks N, Taylor J, Barr IG. Where has all the influenza gone? The impact of COVID-19 on the circulation of influenza and other respiratory viruses, Australia, March to September 2020. Eurosurveillance. 2020;25(47):1–6. 10.2807/1560-7917.ES.2020.25.47.2001847 [DOI] [PMC free article] [PubMed]
- 9.Kuitunen I, Artama M, Mäkelä L, Backman K, Heiskanen-Kosma T, Renko M. Effect of social distancing due to the COVID-19 pandemic on the incidence of viral respiratory tract infections in children in Finland during early 2020. Pediatr Infect Disease J. 2020;39(12):e423–7. doi: 10.1097/INF.0000000000002845. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Y, Li W, Yang B, Qian R, Wu F, He X, Zhu Q, Liu J, Ni Y, Wang J, Mao S. Epidemiological and virological characteristics of respiratory tract infections in children during COVID-19 outbreak. BMC Pediatr. 2021;21(1). [DOI] [PMC free article] [PubMed]
- 11.Buda S, Dürrwald R, Biere B, Buchholz U, Tolksdorf K, Schilling J, Streib V, Preuß U, Prahm K, Haas W. Influenza-Wochenbericht. Kalenderwoche 20/2020. 2020. https://influenza.rki.de/Wochenberichte/2019_2020/2020-20.pdf
- 12.Buda S, Dürrwald R, Biere B, Buchholz U, Tolksdorf K, Schilling J et al. Influenza-Wochenbericht des RKI. Wochenbericht 20/2021. 2021;1–9.
- 13.Diesner-Treiber SC, Voitl P, Voitl JJM, Langer K, Kuzio U, Riepl A, Patel P, Mühl-Riegler A, Mühl B. Respiratory infections in children during a COVID-19 pandemic winter. Front Pead. 2021;9. [DOI] [PMC free article] [PubMed]
- 14.Kuitunen I, Artama M, Haapanen M, Renko M. Rhinovirus spread in children during the COVID-19 pandemic despite social restrictions—A nationwide register study in Finland. J Med Virol. 2021;93(10):6063–7. doi: 10.1002/jmv.27180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takashita E, Kawakami C, Momoki T, Saikusa M, Shimizu K, Ozawa H, Kumazaki M, Usuku S, Tanaka N, Okubo I, Morita H, Nagata S, Watanabe S, Hasegawa H, Kawaoka Y. Increased risk of rhinovirus infection in children during the coronavirus disease-19 pandemic. Influenza Other Respir Viruses. 2021;15(4):488–94. doi: 10.1111/irv.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kusel M, De Klerk N, Holt PG, Landau LI, Sly PD. Occurrence and management of acute respiratory illnesses in early childhood. J Paediatr Child Health. 2007;43(3):139–46. doi: 10.1111/j.1440-1754.2007.01033.x. [DOI] [PubMed] [Google Scholar]
- 17.Donath H, Zielen S, Wittekindt B, Klingebiel T, Graf J, Eckrich M, Walter C, Blumchen K. Effects of the SARS-CoV2-Lockdown on Pediatric Care in the Rhine-Main Area. Klin Padiatr. 2020;233(1):31–6. doi: 10.1055/a-1263-1467. [DOI] [PubMed] [Google Scholar]
- 18.Gottschick, C., Raupach-Rosin, H., Langer, S., Hassan, L., Horn, J., Dorendorf, E., Caputo, M., Bittner, M., Beier, L., Rübsamen, N., Schlinkmann, K. M., Zoch, B., Guzmán, C. A., Hansen, G., Heselich, V., Holzapfel, E., Hübner, J., Pietschmann, T., Pieper, D. H., ? Mikolajczyk, R. Cohort profile: The LoewenKIDS Study - Life-course perspective on infections, the microbiome and the development of the immune system in early childhood. Int J Epidemiol. 2019;48(4):1382–1383. [DOI] [PMC free article] [PubMed]
- 19.Langer S, Horn J, Gottschick C, Klee B, Purschke O, Caputo M et al. Symptom burden and factors associated with acute respiratory infections in the first two years of life—results from the LoewenKIDS cohort. Microorganisms. 2022;(1). [DOI] [PMC free article] [PubMed]
- 20.Purschke O. lkstaR: An R-Package to Analyse the Loewenkids Symptom Diary. Zenodo. 2021. https://zenodo.org/record/4915826#.Ylf5behBw2y
- 21.Buchholz U, Buda S, Prahm K. Abrupter Rückgang Der Raten an Atemwegserkrankungen in Der Deutschen Bevölkerung. Epid Bull. 2020;16:7–9. [Google Scholar]
- 22.Tanislav C, Kostev K. Fewer non-COVID-19 respiratory tract infections and gastrointestinal infections during the COVID-19 pandemic. J Med Virol. 2021;94(1):298–302. doi: 10.1002/jmv.27321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halabi KC, Stockwell MS, Alba L, Vargas C, Reed C, Saiman L. Clinical and socioeconomic burden of rhinoviruses/enteroviruses in the community. Influenza Other Respir Viruses. 2022;16(5):891–6. doi: 10.1111/irv.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Meel ER, Jaddoe VWV, Bønnelykke K, de Jongste JC, Duijts L. The role of respiratory tract infections and the microbiome in the development of asthma: a narrative review. Pediatr Pulmonol. 2017;52(10):1363–70. doi: 10.1002/ppul.23795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker RE, Park SW, Yang W, Vecchi GA, Metcalf CJE, Grenfell BT. The impact of COVID-19 nonpharmaceutical interventions on the future dynamics of endemic infections. Proc Natl Acad Sci USA. 2020;117(48):30547–53. doi: 10.1073/pnas.2013182117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh DY, Buda S, Biere B, Reiche J, Schlosser F, Duwe S, Wedde M, Von Kleist M, Mielke M, Wolff T, Dürwald R. Trends in respiratory virus circulation following COVID-19-targeted nonpharmaceutical interventions in Germany, January - September 2020: analysis of national surveillance data. Lancet Reg Health. 2021;6:1–10. doi: 10.1016/j.lanepe.2021.100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maison N, Peck A, Illi S, Meyer-Buehn M, von Mutius E, Hübner J, Von Both U. The rising of old foes: impact of lockdown periods on non-SARS-CoV-2 viral respiratory and gastrointestinal infections. Infection. 2022;50(2):519–24. doi: 10.1007/s15010-022-01756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanz-Muñoz I, Tamames-Gómez S, Castrodeza-Sanz J, Eiros-Bouza JM, de Lejarazu-Leonardo RO. Social distancing, lockdown and thewide use of mask; a magic solution or a double-edged sword for respiratory viruses epidemiology? Vaccines. 2021;9(6):2–5. doi: 10.3390/vaccines9060595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Reeves RM, Wang X, Bassat Q, Brooks WA, Cohen C, Moore DP, Nunes MC, Rath B, Campbell H, Nair H, RESCEU Investigators Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Global Health. 2019;7(8):e1031–45. doi: 10.1016/S2214-109X(19)30264-5. [DOI] [PubMed] [Google Scholar]
- 30.Lumley SF, Richens N, Lees E, Cregan J, Kalimeris E, Oakley S, Morgan M, Segal S, Dawson M, Walker AS, Eyre DW, Crook DW, Beer S, Novak A, Stoesser N, Matthews PC. Changes in paediatric respiratory infections at a UK teaching hospital 2016–2021; impact of the SARS-CoV-2 pandemic. J Infect. 2022;84(1):40–7. doi: 10.1016/j.jinf.2021.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groves HE, Piché-Renaud PP, Peci A, Farrar DS, Buckrell S, Bancej C, Sevenhuysen C, Campigotto A, Gubbay JB, Morris SK. The impact of the COVID-19 pandemic on influenza, respiratory syncytial virus, and other seasonal respiratory virus circulation in Canada: a population-based study. Lancet Reg Health - Americas. 2021;1:100015. doi: 10.1016/j.lana.2021.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein M, Cohen H, Nemet I, Atari N, Kliker L, Fratty IS, Bucris E, Geva M, Mendelson E, Zuckerman NS, Mandelboim M. Human metapneumovirus prevalence during 2019–2021 in Israel is influenced by the COVID-19 pandemic. Int J Infect Dis. 2022;120:205–9. doi: 10.1016/j.ijid.2022.04.037. [DOI] [PubMed] [Google Scholar]
- 33.Kivit C, Groen K, Jongbloed M, Linssen C, van Loo A, van Gorp E, Van Niewkoop S, Van Den Hoogen B, De Kruif M. An off-season outbreak of human metapneumovirus infections after ending of a COVID-19 lockdown. J Infect. 2022;84(5):722–46. doi: 10.1016/j.jinf.2022.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bardsley M, Morbey RA, Hughes HE, Beck CR, Watson CH, Zhao H, Ellis J, Smith GE, Elliot AJ. Epidemiology of respiratory syncytial virus in children younger than 5 years in England during the COVID-19 pandemic, measured by laboratory, clinical, and syndromic surveillance: a retrospective observational study. Lancet Infect Disease. 2023;3099(22):1–11. doi: 10.1016/S1473-3099(22)00525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pellegrinelli L, Galli C, Bubba L, Seiti A, Anselmi G, Primache V, Signorini L, Delbue S, Binda S, Pariana E. Respiratory syncytial virus in pediatric influenza-like illness cases in Lombardy, Northern Italy, during seven consecutive winter seasons (from 2014–2015 to 2020–2021) Influenza Other Respir Viruses. 2021;16(3):481–91. doi: 10.1111/irv.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agha R, Avner JR. Delayed seasonal RSV surge observed during the COVID-19 pandemic. Pediatrics. 2021;148(3):2019–21. doi: 10.1542/peds.2021-052089. [DOI] [PubMed] [Google Scholar]
- 37.Buda S, Dürrwald R, Biere B, Buchholz U, Tolksdorf K, Schilling J et al. Influenza-Monatsbericht Des RKI. 2021;39:1–8.
- 38.Public Health England. Weekly national influenza and COVID-19 surveillance report Executive summary - Week 40 – 6 October 2022. Public Health England. 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1109546/Weekly_Flu_and_COVID-19_report_w40.pdf
- 39.Costa LF, Oliveira Queiróz DA, Lopes Da Silveira H, Bernardino Neto M, De Paula NT, Oliveira TFM, Tolardo AL, Yokosawa J. Human rhinovirus and disease severity in children. Pediatrics. 2014;133(2). [DOI] [PubMed]
- 40.Principi N, Zampiero A, Gambino M, Scala A, Senatore L, Lelii M, Ascolese B, Pelucchi C, Esposito S. Prospective evaluation of rhinovirus infection in healthy young children. J Clin Virol. 2015;66:83–9. doi: 10.1016/j.jcv.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 41.Hayden FG. Rhinovirus and the lower respiratory tract. Rev Med Virol. 2004;14(1):17–31. doi: 10.1002/rmv.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park S, Michelow IC, Choe YJ. Shifting patterns of respiratory virus activity following social distancing measures for Coronavirus Disease 2019 in South Korea. J Infect Disease. 2021;224(11):1900–6. doi: 10.1093/infdis/jiab231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amaddeo A, Cason C, Cozzi G, Ronfani L, Comar M. Social distancing measures for COVID-19 are changing winter season. Arch Dis Child. 2021;106(12):e47. doi: 10.1136/archdischild-2021-322004. [DOI] [PubMed] [Google Scholar]
- 44.Turner RB, Fuls JL, Rodgers ND. Effectiveness of hand sanitizers with and without organic acids for removal of rhinovirus from hands. Antimicrob Agents Chemother. 2010;54(3):1363–4. doi: 10.1128/AAC.01498-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Savolainen-Kopra C, Korpela T, Simonen-Tikka ML, Amiryousefi A, Ziegler T, Roivainen M, Hovi T. Single treatment with ethanol hand rub is ineffective against human rhinovirus-hand washing with soap and water removes the virus efficiently. J Med Virol. 2012;84(3):543–7. doi: 10.1002/jmv.23222. [DOI] [PubMed] [Google Scholar]
- 46.Winther B, McCue K, Ashe K, Rubino J, Hendley JO. Rhinovirus contamination of surfaces in homes of adults with natural colds: transfer of virus to fingertips during normal daily activities. J Med Virol. 2011;83(5):906–9. doi: 10.1002/jmv.22027. [DOI] [PubMed] [Google Scholar]
- 47.Gwaltney JM. Hand-to-hand transmission of Rhinovirus Colds. Ann Intern Med. 1978;88(4):463. doi: 10.7326/0003-4819-88-4-463. [DOI] [PubMed] [Google Scholar]
- 48.Leung NHL. Transmissibility and transmission of respiratory viruses. Nat Rev Microbiol. 2021;19:528–45. doi: 10.1038/s41579-021-00535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aiello AE, Coulborn RM, Perez V, Larson EL. Effect of hand hygiene on infectious disease risk in the community setting: a meta-analysis. Am J Public Health. 2008;98(8):1372–81. doi: 10.2105/AJPH.2007.124610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leung NHL, Chu DKW, Shiu EYC, Chan KH, McDevitt JJ, Hau BJP, Yen H, Li Y, Ip DKM, Peiris JSM, Seto WH, Leung GM, Milton DK, Cowling BJ. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26(5):676–80. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Zalm MM, van Ewijk BE, Wilbrink B, Uiterwaal CSPM, Wolfs TFW, van der Ent CK. Respiratory pathogens in children with and without respiratory symptoms. J Pediatr. 2009;154(3):396–e4001. doi: 10.1016/j.jpeds.2008.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Benten I, Koopman L, Niesters B, Hop W, Van Middelkoop B, De Waal L, Van Drunen CM, Osterhaus ADME, Neijens HJ, Fokkens WJ. Predominance of rhinovirus in the nose of symptomatic and asymptomatic infants. Pediatr Allergy Immunol. 2003;14(5):363–70. doi: 10.1034/j.1399-3038.2003.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wildenbeest JG, van der Schee MP, Hashimoto S, Benschop KS, Minnaar RP, Sprikkelman AB, Wolthers KC. Prevalence of rhinoviruses in young children of an unselected birth cohort from the Netherlands. Clin Microbiol Infect. 2016;22(8):e736739–736715. doi: 10.1016/j.cmi.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee WM, Lemanske RF, Jr, Evans MD, Vang F, Pappas T, Gangnon R, Gern JE. Human rhinovirus species and season of infection determine illness severity. Am J Respir Crit Care Med. 2012;186(9):886–91. doi: 10.1164/rccm.201202-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goka EA, Vallely PJ, Mutton KJ, Klapper PE. Single and multiple respiratory virus infections and severity of respiratory disease: a systematic review. Paediatr Respir Rev. 2014;15:363–70. doi: 10.1016/j.prrv.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang G, Hu Y, Wang H, Zhang L, Bao Y, Zhou X. High incidence of multiple viral infections identified in Upper respiratory tract infected children under three years of age in Shanghai, China. PLoS ONE. 2012;7(9). [DOI] [PMC free article] [PubMed]
- 57.Scotta MC, Chakr VCBG, de Moura A, Becker RG, de Souza APD, Jones MH, Pinto LA, Sarria EE, Pitrez PM, Stein RT, Mattiello R. Respiratory viral coinfection and disease severity in children: a systematic review and meta-analysis. J Clin Virol. 2016;80:45–56. doi: 10.1016/j.jcv.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Danino D, Ben-Shimol S, van der Beek BA, Givon-Lavi N, Avni YS, Greenberg D, Dagan R. Decline in Pneumococcal Disease in Young Children during the Coronavirus Disease 2019 (COVID-19) pandemic in Israel Associated with suppression of Seasonal Respiratory viruses, despite persistent pneumococcal carriage: a prospective cohort study. Clin Infect Dis. 2022;75:e1154–64. doi: 10.1093/cid/ciab1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rybak A, Levy C, Angoulvant F, Auvrignon A, Gembara P, Danis K, Ouldali N. Association of nonpharmaceutical interventions during the COVID-19 pandemic with invasive pneumococcal disease, pneumococcal carriage, and respiratory viral infections among children in France. JAMA Netw Open. 2022:E2218959. [DOI] [PMC free article] [PubMed]
- 60.Farjo RS, Foxman B, Patel MJ, Zhang L, Pettigrew MM, McCoy SI, Marrs CF, Gilsdorf JR. Diversity and sharing of Haemophilus influenzae strains colonizing healthy children attending day-care centers. Pediatriatric Infect Disease J. 2004;23(1):41–6. doi: 10.1097/01.inf.0000106981.89572.d1. [DOI] [PubMed] [Google Scholar]
- 61.Bogaert D, Van Belkum A, Sluijter M, Luijendijk A, De Groot R, Rümke HC, Verbrugh HA, Hermans PWM. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet. 2004;363(9424):1871–182. doi: 10.1016/S0140-6736(04)16357-5. [DOI] [PubMed] [Google Scholar]
- 62.Hashida K, Shiomori T, Hohchi N, Kitamura T, Udaka T, Suzuki H. Survey of nasopharyngeal carriage of Haemophilus Influenzae and Streptococcus Pneumoniae in infants at Day Care centers. Nippon Jibiinkoka Gakkai Kaiho. 2006;109(12):821–9. doi: 10.3950/jibiinkoka.109.821. [DOI] [PubMed] [Google Scholar]
- 63.Langer S, Klee B, Gottschick C, Mikolajczyk R. Birth cohort studies using symptom diaries for assessing respiratory diseases–a scoping review. PLoS ONE. 2022;17. [DOI] [PMC free article] [PubMed]
- 64.Böhm A, Ellsäßer G, Lüdecke K. Der Brandenburger sozialindex: Ein Werkzeug für die gesundheits- und sozialberichterstattung auf landes- und kommunaler ebene Bei Der analyse Von einschülerdaten. Gesundheitswesen. 2007;69(10):555–9. doi: 10.1055/s-2007-992772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.