Abstract
Background
There is an urgent clinical need for developing novel immunoprophylaxis and immunotherapy strategies against Staphylococcus aureus (S. aureus). In our previous work, immunization with a tetra-branched multiple antigenic peptide, named MAP2-3 that mimics lipoteichoic acid, a cell wall component of S. aureus, successfully induced a humoral immune response and protected BALB/c mice against S. aureus systemic infection. In this study, we further investigated whether vaccination with MAP2-3 can elicit immunologic memory.
Methods
BALB/c mice were immunized with MAP2-3 five times. After one month of the last vaccination, mice were challenged with heat-killed S. aureus via intraperitoneal injection. After a 7-day inoculation, the percentage of plasma cells, memory B cells, effector memory T cells, and follicular helper T cells were detected by flow cytometry. The levels of IL-6, IL-21, IL-2, and IFN-γ were measured by real-time PCR and ELISA. Flow cytometry results were compared by using one-way ANOVA or Mann-Whitney test, real-time PCR results were compared by using one-way ANOVA, and ELISA results were compared by using one-way ANOVA or student’s t-test.
Results
The percentage of plasma cells and memory B cells in the spleen and bone marrow from the MAP2-3 immunized mice was significantly higher than that from the control mice. The percentage of effector memory T cells in spleens and lymphoid nodes as well as follicular helper T cells in spleens from the MAP2-3 immunized mice were also higher. Moreover, the levels of IL-6 and IL-21, two critical cytokines for the development of memory B cells, were significantly higher in the isolated splenocytes from immunized mice after lipoteichoic acid stimulation.
Conclusions
Immunization with MAP2-3 can efficiently induce memory B cells and memory T cells.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-09262-8.
Keywords: Staphylococcus aureus (S. aureus), Lipoteichoic acid (LTA), Mimic peptide, Multiple antigenic peptide (MAP)
Background
Staphylococcus aureus (S. aureus) is a common Gram-positive bacterium that can cause a wide spectrum of diseases, ranging from relatively mild skin infections to life-threatening infections, such as bacteremia and septic shock [1]. Since the emergence of antibiotic-resistant strains, such as methicillin-resistant Staphylococcus aureus (MRSA), S. aureus infection has become a growing threat to public health worldwide. As reported, 119,247 S. aureus bloodstream infections with 19,832 associated deaths occurred in the U.S. in 2017 [2]. Meanwhile, the cost and healthcare burden due to S. aureus infection also significantly increased [3]. An effective prophylactic vaccine needs to be developed.
Lipoteichoic acid (LTA) is an essential component of the cell wall that is expressed steadily on the surface of S. aureus. The basic structure of the LTA backbone is formed by conserved, unbranched, repeating units of 1,3-glycerolphosphate that are linked to the cytoplasmic membrane via a glycolipid anchor [4, 5]. Previous works have demonstrated that LTA is essential for the pathogenicity and survival of S. aureus under low-osmolarity conditions [4]. LTA also mediated host cell adhesion [6], biofilm formation [7] as well as penetration of the blood-brain barrier [8]. However, as a thymus-independent antigen (TI antigen), LTA is generally not considered a rational vaccine candidate.
In our previous work, MAP2-3, a tetra-branched multiple antigenic peptide (MAP) mimicking the epitope of LTA, was synthesized. MAP2-3 consists of four copies of the sequence HSGHKEDRQWCQHSGG, whose C-terminus is linked to a non-immunogenic and lysine-based dendritic scaffold [9]. Immunization with MAP2-3 induced S. aureus LTA-specific IgG antibodies, prolonged the survival, and decreased the bacterial burden in the organs of BALB/c mice, thereby protecting mice against S. aureus systemic infection. Moreover, passive immunization with polyclonal anti-MAP2-3 sera reduced bacterial load in the organs of mice with bacteremia and alleviated lung injury and skin lesions in mice models with S. aureus infection. After one month of the last immunization of this peptide, LTA-specific IgG antibody-secreting cells (ASCs) could be detected in splenocytes [9]. As a follow-up study of our previous work, we further investigated whether MAP2-3 immunization can induce the production of memory B cells.
Methods
LTA and MAPs
LTA from S. aureus (Cat No.2515) was purchased from Sigma. MAP2-3 (molecular weight: 7726.3) and the lysine-based, tetra-branched attaching backbone of MAP (named MAPctrl, molecular weight: 402.5) were synthesized with purity above 90% by Hybio Pharmaceutical (Shenzhen, China). MAPs were dissolved in endotoxin-free water (Sigma) at 50 mg/ml and diluted in sterile phosphate-buffered saline (PBS) when used in the assay.
Antibodies
FITC-labeled anti-CD4 (clone: GK1.5), FITC-labeled anti-CD19 (clone: 6D5), PE-labeled anti-B220 (clone: RA3-6B2), PE-labeled anti-CD95 (clone: SA367H8), PE-labeled anti-CD44 (clone: IM7), PE/Cy7-labeled anti-IgD (clone:11-26c.2a), PE/Cy5-labeled anti-CD62L (clone: MEL-14), APC-labeled anti-IgG (clone: Poly4053), APC-labeled anti-CD138 (clone: Syndecan-1), APC-labeled anti-CD80 (clone: 16-10A1), and Pacific Blue-labeled anti-GL7 (clone: GL7) were purchased from BioLegend. CD16/CD32 purified antibody (clone: 93), and FITC-labeled anti-CD38 (clone: 90) were purchased from eBioscience.
Preparation of bacteria
S. aureus (ATCC 25,923) was purchased from Wenzhou Kont Biology and Technology. The bacteria were grown in tryptic soy broth at 37 °C with 250 rpm shaking overnight. Cells were collected, washed, and diluted with sterile PBS to an appropriate concentration.
Animal and ethics statement
BALB/c mice (5–6 weeks old, female) were purchased from the Experimental Animal Center, Southern Medical University, Guangzhou, China. All the animal experiments were approved by the Institutional Animal Care and Use Committee of Southern Medical University (permit number: L2015070) and carried out in strict accordance with national guidelines for animal welfare. The study was conducted in accordance with the ARRIVE guidelines and AVMA guidelines for the euthanasia of animals (2020) for reporting animal experiments.
Immunization with MAPs and inoculation with S. aureus
Vaccination was performed using the method based on our previous study [9]. Briefly, BALB/c mice were randomly divided into three groups: mice immunized with MAP2-3 (herein referred to as “MAP2-3 mice”), mice immunized with MAPctrl (MAPctrl mice), and mice without any peptide immunization used as a blank control. MAPs were injected into mice subcutaneously (100 µg /200 µl/ mouse) five times. The interval between the initial immunization and the second was 3 weeks, while the interval between the following booster immunization was 2 weeks. The first immunization was administered in Freund’s complete adjuvant (Cat No. F5881, Sigma) and the subsequent booster immunizations were administered in Freund’s incomplete adjuvant (Cat No. F5506, Sigma). One month after the last immunization, mice were injected intraperitoneally (i.p) with heat-killed S. aureus (2 × 107 CFU/mouse) to mimic the bacterium infection. After seven days of challenge, mice were anesthetized with 1% pentobarbital sodium (50 mg/kg) by intraperitoneal injection, then euthanized by cervical dislocation. The splenocytes, bone marrow cells, and lymph node cells were isolated followed by flow cytometry analysis and in vitro assays (Fig. 1A).
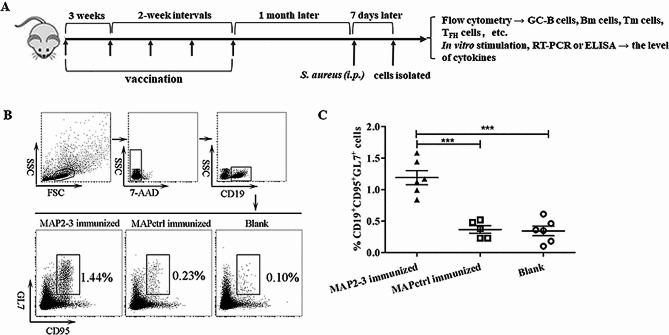
Fig. 1.
Diagram of the experimental design and identification of GC-B cells in the spleen. (A) Mice were immunized with MAP2-3 or MAPctrl 5 times. After one month of the last immunization, all mice were inoculated with heat-killed S. aureus (2 × 107 CFU/mice) via i.p. The splenocytes, lymph node cells, and bone marrow cells were isolated 7 days after the inoculation. The percentage of different immune cells, including GC-B cells, plasma cells, memory B (Bm) cells, memory T (Tm) cells, etc. was identified by flow cytometry, respectively. Isolated splenocytes were stimulated with LTA or heat-killed S. aureus and the level of cytokines was determined by RT-PCR or ELISA. (B) Flow cytometry analysis of GC-B cells. Gating strategy and representative FACS of GC-B cells. (C) The percentage of GC-B cells in spleens. MAP2-3 vs. MAPctrl: p < 0.001; MAP2-3 vs. Blank: p < 0.001. n = 5–6 mice/group. *** p < 0.001
Isolation of splenocytes, bone marrow cells, and lymph node cells
The spleen and lymph nodes were aseptically excised and gently ground followed by filtering through a stainless mesh (size = 70 μm) in 10 ml RPMI 1640 media. Cell suspensions were pelleted and washed one time with 10 ml cold RPMI 1640 media. To lyse erythrocytes, 1×RBC lysis buffer (eBioscience, Cat No. 00-4300-54) was added for 5 min, followed by the addition of 20 ml of cold RPMI 1640 media to stop the lysis. After being washed two times with RPMI 1640, the erythrocyte-free cells were resuspended in the complete RPMI 1640 media.
Bone marrow cells were isolated using the protocol based on Kelly Roney’s work [10]. Briefly, the femur and tibia were isolated from the hind legs of mice. Any additional muscle or connective tissue attached to the bones was removed. An 18 G needle was then inserted into the bone and bone marrow cells were flushed into a tube. Clumps of bone marrow cells were gently dispersed and cell solutions were pipetted through a 70 μm cell strainer. Cells were then washed with PBS and lysed with 1 ml 1× RBC lysis buffer for 1 min. The lysis was stopped by adding 50 ml cold PBS and cells were pelleted after centrifugation at 1500 rpm at 4 °C for 10 min. Cells were washed with RPMI 1640 media and resuspended in an appropriate volume of cold RPMI 1640 media containing 10% calf serum.
Flow cytometry
To reduce the non-specific binding of antibodies to Fc receptors expressed on the cell membrane, cells (2 × 106 /ml) were first incubated with 1 µg/ml of anti-CD16/CD32 at room temperature for 10 min. Cells were then washed and incubated with specific antibodies for 30 min on ice in the dark. Samples were loaded on a BD LSR II flow cytometer and data were analyzed with FlowJo software (version X.0.7, Treestar, Inc). Dead cells were excluded by using 7-AAD in all flow cytometry experiments. Different immune cell types were identified by using specific cell surface markers. GC-B cells and plasma cells were defined as CD19+ CD95+ GL7+ cells and B220− CD138+ cells, respectively. Memory B cells were identified using two gating methods: compared with naïve B cells, memory B cells express membrane IgG (mIgG) or membrane IgG1 (mIgG1) but not membrane IgM (mIgM) [11, 12], thus, CD19+ IgM− IgG+ or CD19+ IgM− IgG1+ were used as surface markers for memory B cells. Besides, memory B cells but not the plasma cells or GC-B cells express B220 [12] and CD38 [13], therefore our second method for identifying memory B cells was to use B220+ CD38+ IgG+ IgD− GL7− as their surface markers. Other immune cell types including follicular helper T cells (TFH, CD4+ CXCR5+ PD-1+) [14], effector memory T cells (TEM, CD4+ CD44+ CD62L−), and central memory T cells (TCM, CD4+ CD44+ CD62L+) were also identified.
RNA isolation and real-time PCR
Splenocytes (4 × 105/well) were added to the 96-well cell culture plates. The cells were stimulated with LTA derived from S. aureus (10 µg/ml) at 37 °C for 24 h. Total RNA was extracted from splenocytes and purified using RNAiso Plus (TaKaRa, Cat No. 9109). cDNA was converted from 1 µg total RNA using the RevertAid First Strand cDNA synthesis kit (Fermentas, Cat No. K1622). Real-time PCR analysis was performed using the Rotor-Gene1000 system (Corbett Research, Bath, UK). Briefly, cDNA was serial diluted and PCR-amplified with SYBR Green PCR mix (Toyobo, Cat No. QPK-201) in triplicates. The program for amplification was set as 1 cycle of 95 °C for 30 s followed by 40 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 60 s. The glyceraldehyde 3-phosphite dehydrogenase (GAPDH) gene was used as an endogenous control to normalize the differences in the amount of total RNA present in the samples. Primer sequences are as follows: IL-21 sense, 5’-ACCCCTGGCTTTCACTGTTT-3’; anti-sense, 5’-CTGAGGCTGGAGCTAGCAGA-3’; IL-6 sense, 5’-TCCAGAAACCGCTATGAAGTT-3’; anti-sense, 5’-TTCATACAATCAGAATTGCCATT-3’ and GAPDH sense, 5’-TGTGTCCGTCGTGGATCTGA-3’; anti-sense, 5’-TTGCTGTTGAAGTCGCAGGA-3’. Data were analyzed using the 2−△△CT method and mRNA levels were calculated as the fold change compared to the control group.
Detection of cytokine in splenocytes incubated with heat-killed S. aureus
Splenocytes (4 × 105/well) were incubated with heat-inactivated S. aureus (2 × 104 CFU/well) at 37 °C for 24 h. The supernatant was then collected and the level of IL-6, IL-2, and IFN-γ was determined by ELISA (BioLegend) according to the manufacturer’s instructions. All measurements were analyzed in triplicates.
Statistical methods
Numeric measurements were expressed as means ± standard error of the mean (SEM). Each experiment under the same condition was repeated two or three times. For analyzing flow cytometry data, if the data conformed to normal distribution and homogeneity of variance, the comparison was performed using one-way ANOVA with the least significant difference (LSD) method. If the data conformed to normal distribution but not homogeneity of variance, the comparison was performed using one-way ANOVA with Dunnett’s T3 test. If the data conformed to neither normal distribution nor homogeneity of variance, the comparison was performed using a Mann-Whitney test. The concentration of cytokines (IL-6, IL-2, and IFN-γ) was compared between groups using either one-way ANOVA or Student’s t-test. Expression of cytokines (IL-6 and IL-21) was compared between groups using one-way ANOVA with Dunnett’s T3 test. The significance of the test will be assessed at alpha = 0.05.
Results
Immunization with MAP2-3 promoted the production of GC-B cells and plasma cells after heat-killed S. aureus inoculation
In response to an antigen challenge, germinal centers (GCs) first form in the secondary lymphoid organ, including the spleen and lymph nodes. Antigen-specific B cells subsequently migrate into the GCs and form GC-B cells [15, 16]. With the stimulation of specific antigens and help from TFH, some GC-B cells can differentiate into plasma cells or memory B cells [17]. Since we previously found that MAP2-3 immunization induced LTA-specific IgG antibodies and LTA-specific antibody-secreting cells (ASCs) [9], we first investigated whether MAP2-3 immunization can induce the production of GC-B cells after heat-killed S. aureus challenge. The percentage of GC-B cells (CD19+ CD95+ GL7+) in MAP2-3 mice was significantly higher than that in control mice (Fig. 1B and C), indicating that MAP2-3 immunization induces the precursors of plasma cells.
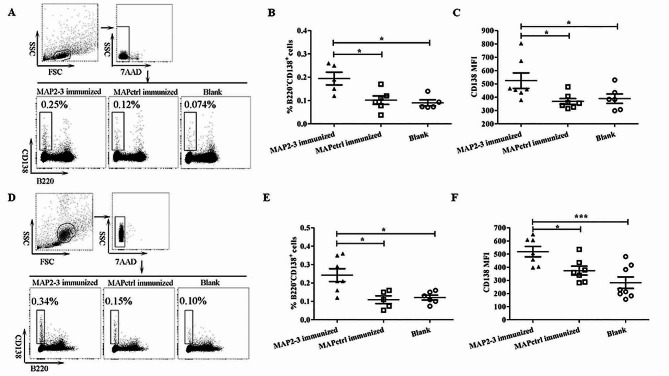
We next examined the percentage of plasma cells (B220- CD138+) in splenocytes after a 7-day S. aureus inoculation. Compared with MAPctrl mice or blank control mice, the percentage of plasma cells in the MAP2-3 mice was significantly higher (Fig. 2A and B). Consistently, the mean fluorescence intensity (MFI) of CD138+ plasma cells in the spleen of MAP2-3 mice was also significantly higher (Fig. 2C). As reported, some plasma cells differentiated from GC-B cells can exit the germinal center and migrate to the bone marrow to secret high-affinity antibodies [15]. Based on this knowledge, we further examined whether the percentage of plasma cells in bone marrow increased after challenge. As shown in Fig. 2D-F, the percentage of B220- CD138+ plasma cells, as well as the MFI of CD138+ plasma cells isolated from bone marrow, was significantly higher in MAP2-3 mice than that from the control mice. All these results indicate that MAP2-3 immunization induced the production of plasma cells in the spleen and bone marrow when the mice were challenged with heat-activated S. aureus.
Fig. 2.
Detection of plasma cells (B220− CD138+ cells) in the spleen and bone marrow. (A) Gating strategy and representative FACS of plasma cells in the spleen. (B) The percentage of plasma cells in the spleen. MAP2-3 vs. MAPctrl: p = 0.022; MAP2-3 vs. Blank: p = 0.016. (C) MFI of CD138 was examined using B220− CD138+ cells in the spleen. MAP2-3 vs. MAPctrl: p = 0.015; MAP2-3 vs. Blank: p = 0.038. n = 5–7 mice/group. (D) Gating strategy and representative FACS of plasma cells in the bone marrow. (E) The percentage of plasma cells in the bone marrow. MAP2-3 vs. MAPctrl: p = 0.026;MAP2-3 vs. Blank: p = 0.034. (F) MFI of CD138 was examined using B220− CD138+ cells in the bone marrow. MAP2-3 vs. MAPctrl: p = 0.021; MAP2-3 vs. Blank: p = 0.0004. n = 5–8 mice/group. p-values: * p < 0.05, *** p < 0.001
MAP2-3 immunization induced the production of memory B cells in the spleen and bone marrow
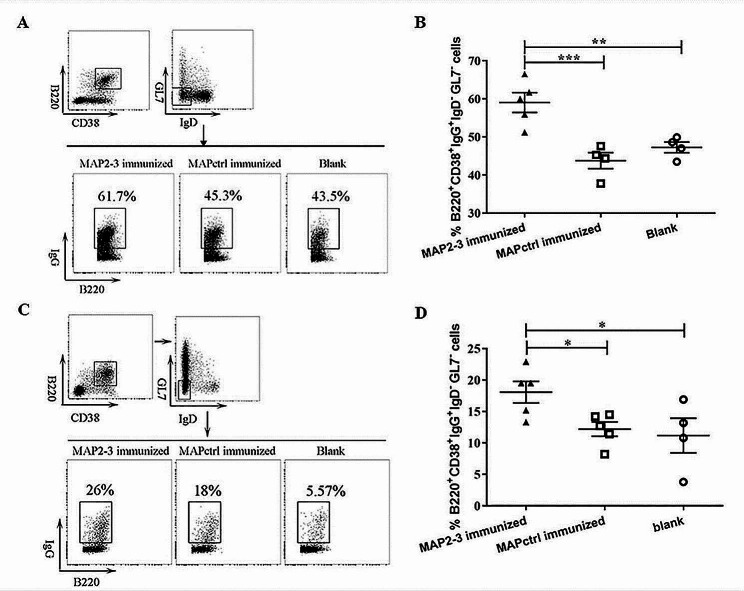
In addition to inducing specific antibodies, immunization with a vaccine candidate can stimulate the production of memory B cells when re-exposure to the specific antigen. As shown in Fig. 3, the percentage of memory B cells (B220+ CD38+ IgG+ IgD− GL7−) was significantly higher in both spleen (Fig. 3A and B) and bone marrow (Fig. 3C and D) after one month of the last immunization followed by S. aureus inoculation. Moreover, the percentage of IgG+ or IgG1+ memory B cells (IgG+/IgG1+ CD19+ B220+ IgM−) in the spleen of MAP2-3 mice was also significantly higher 5 days after the last immunization (supplementary Fig. S1.). Taken together, these results indicated that memory B cells can be induced by MAP2-3 immunization.
Fig. 3.
Detection of memory B cells in the spleen and bone marrow from the immunized mice. (A) Gating strategy and representative FACS of memory B cells in the spleen. (B) The percentage of memory B cells in the spleen. MAP2-3 vs. MAPctrl: p = 0.00057; MAP2-3 vs. Blank: p = 0.003. n = 4–5 mice/group. (C) Gating strategy and representative FACS of memory B cells in the bone marrow. (D) The percentage of memory B cells in the bone marrow. MAP2-3 vs. MAPctrl: p = 0.04; MAP2-3 vs. Blank: p = 0.026. n = 4–5 mice/group. Data are expressed with mean ± SEM. p-values: * p < 0.05, ** p < 0.01, *** p < 0.001
MAP2-3 immunization induced CD80 expression on the surface of memory B cells and induced TFH cells in the spleen
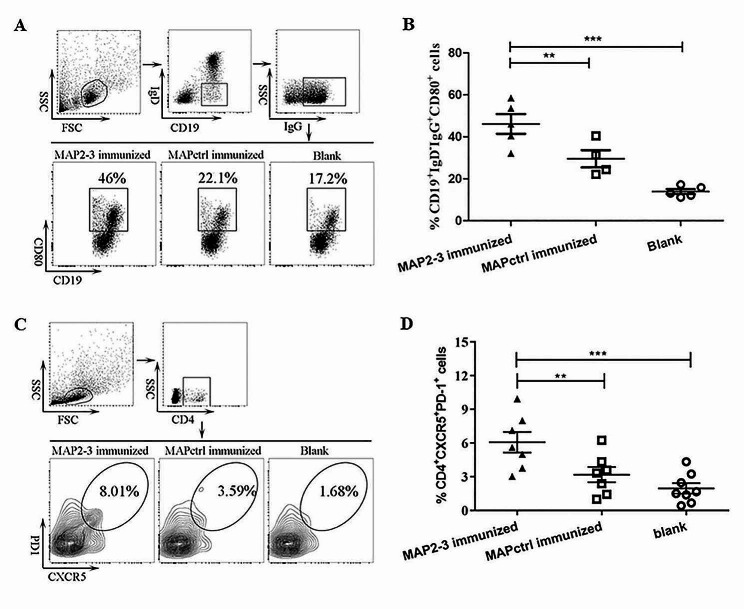
CD80 is one of the critical markers expressed on memory B cells in mice [12], which regulates B-T interactions [18] and promotes follicular T cell generation [19]. Thus, we examined the expression of CD80 on the surface of IgG+ memory B cells and found that the percentage of CD19+ IgD− IgG+ CD80+ memory B cells in splenocytes from MAP2-3 mice was significantly higher than that from the control mice (Fig. 4A and B).
Fig. 4.
Detection of CD80+ memory B cells and TFH cells from the immunized mice. (A) Gating strategy and representative FACS of memory B cells with CD80 expressed on the surface. (B) The percentage of memory B cells with CD80 expressed on the surface. MAP2-3 vs. MAPctrl: p = 0.009; MAP2-3 vs. Blank: p < 0.001. n = 4–5 mice/group. (C) Gating strategy and representative FACS of TFH cells in the spleen. (D) The percentage of TFH cells in the spleen. MAP2-3 vs. MAPctrl: p = 0.009895; MAP2-3 vs. Blank: p = 0.000467. n = 7–8 mice/group. p-values: ** p < 0.01, *** p < 0.001
Different from effector helper T cells, such as Th1, Th2, or Th17, which predominantly migrate from the lymphoid tissue to sites of inflammation or infection, TFH cells mainly reside in the spleen and lymph nodes and play an important role in promoting GC-B cells to differentiate into memory B cells and long-lived plasma cells [20]. As shown in Fig. 4C and D, the percentage of CD4+ CXCR5+ PD-1+ TFH cells in spleens from MAP2-3 mice was significantly higher than that from the control mice after the S. aureus challenge.
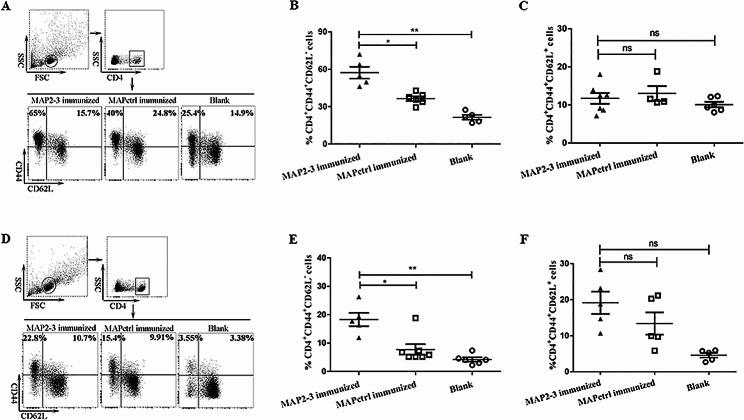
MAP2-3 immunization increased the percentage of effector memory T cells (TEM ) in the spleen or lymph nodes after S. aureus inoculation
The frequency of memory T cells in peripheral immune organs, including the spleen and lymph nodes, was further investigated. The percentage of TEM (CD4+ CD44+ CD62L−) from MAP2-3 mice was significantly higher than the controls in both spleens (Fig. 5A and B) and lymphoid nodes (Fig. 5D and E). However, there is no significant difference in the percentage of TCM (CD4+ CD44+ CD62L+) among the three groups in both the spleen (Fig. 5A and C) and lymphoid nodes (Fig. 5D and F).
Fig. 5.
Detectionof memory T cells in the spleen and lymphoid node from the immunized mice. (A) Gating strategy and representative FACS of memory T cells in the spleen. (B) The percentage of TEM cells in the spleen. MAP2-3vs. MAPctrl: p = 0.022; MAP2-3vs. Blank: p = 0.002. (C) The percentageof TCM cells in the spleen. There is no difference between groups (p> 0.05). n = 4-7 mice/group. (D) Gating strategy and representative FACSof memory T cells in the lymphoid node. (E) The percentage of TEMcells in the lymphoid node. MAP2-3 vs. MAPctrl:p = 0.019; MAP2-3 vs.Blank: p = 0.006. (F) The percentageof TCM cells in the lymphoid node. There is no difference betweengroups (p > 0.05). n = 5-7mice/group. p-values: * p < 0.05, ** p < 0.01, ns p > 0.05.
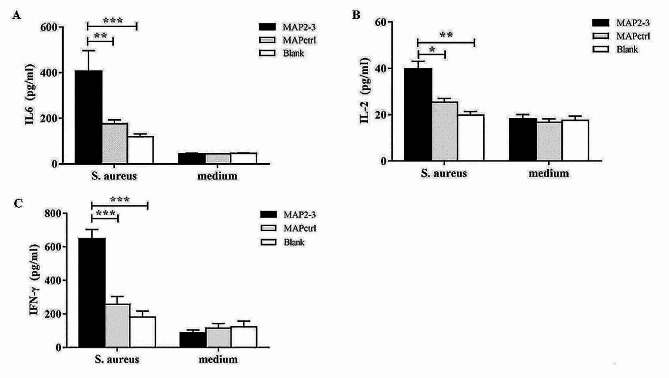
Stimulation with heat-killed S. aureus or LTA increased the level of interleukin-6 (IL-6) in the splenocytes of MAP2-3 mice
Since cytokines are crucial to plasma cells and memory B cells development, the results showed that the concentrations of IL-6, IL-2, and IFN-γ secreted from splenocytes in the MAP2-3 mice were significantly higher than that from the control mice after in vitro heat-killed S. aureus stimulation (Fig. 6A-C). In addition, IL-6 and IL-21 are two important cytokines for TFH cells, plasma cell differentiation, and memory B cells production [20–22], we next measured the levels of IL-6 and IL-21 in the isolated splenocytes stimulated with LTA in vitro. The results showed that the expression of these two cytokines was significantly higher in the MAP2-3 mice as compared to that in the control mice (supplementary Fig. S2).
Fig. 6.
Detectionof cytokines in splenocytes stimulated by heat-killed S. aureus. Afterone month of the last immunization, all mice were inoculated with heat-killed S.aureus for 7 days. Isolated splenocytes were stimulated with heat-killed S.aureus (2×104 CFU/well) for 24 h. The concentrationof IL-6 (A), IL-2 (B), and IFN-γ (C) wasmeasured by ELISA. (A) IL-6, MAP2-3 vs. MAPctrl: p = 0.0065; MAP2-3 vs. Blank: p < 0.001. (B) IL-2, MAP2-3 vs. MAPctrl: p = 0.017; MAP2-3vs. Blank: p = 0.003. (C) IFN-γ, MAP2-3 vs. MAPctrl: p <0.001;MAP2-3 vs. Blank: p < 0.001. n = 6-10 mice/group. p-values:* p < 0.05, ** p < 0.01, *** p < 0.001.
Discussion
In this study, we provide evidence using in vitro experiments that immunization with MAP2-3, a peptide mimicking LTA, could induce immunologic memory after one month of the last immunization followed by heat-killed S. aureus inoculation. First of all, the percentage of plasma cells in the bone marrow and spleen significantly increased. Second, the proportion of memory B cells in the spleen and the bone marrow from the MAP2-3 mice was significantly increased. Third, the percentage of effector memory T cells in the lymph node from the MAP2-3 mice was also significantly increased.
Previous studies have shown that TFH cells play an important role in the differentiation of GC-B cells and the formation of memory B cells [23, 24]. IL-21 secreted from TFH cells is one of the key cytokines for the generation of long-lived plasma cells [25, 26]. In addition, IL-6 plays a role in the formation of GC, promotes antibody production, and maintains TFH cells [27]. Eto D. and colleagues found that the coordination between IL-6 and IL-21 was necessary for TFH cell formation [22]. IFN-γ also effectively activates mononuclear macrophages and promotes the production of IgG antibodies, thereby playing an important anti-infective function in bacteremia and sepsis caused by S. aureus infection [28]. Consistent with the literature, our work shows that the proportion of TFH cells in the spleen of MAP2-3 mice was significantly higher than the controls after the S. aureus challenge. Cytokines, including IL-21, IL-6, IL-2, and IFN-γ were significantly induced in the MAP2-3 mice after in vitro LTA or S. aureus stimulation (Fig. 6 and supplementary Fig. S2). Other cytokines such as IL-4 and TGF-β are also important for B cell proliferation, differentiation, and class switching [14]. However, we failed to detect the induction of these cytokines after LTA or S. aureus stimulation (data not shown). We speculate that the induction of different cytokines is time-dependent and antigen-dose-dependent. Further experiments will be performed to assess the expression of more cytokines (especially secreted by antigen-specific T cells) and their roles in immune responses in mice with MAP2-3 immunization.
We have previously shown that MAP2-3 immunization protected mice from the infection of live S. aureus [9]. In this study, heat-killed S. aureus was used as a surrogate since inactivated bacteria still retain their components and immunogenicity. Previous studies also used inactivated bacteria to determine the immunological response, especially in the flow cytometry assays [29, 30]. Cruciani M’s work further demonstrated that both live S. aureus and inactivated bacteria induce the expression of CD86 and MHC II molecules on dendritic cells and there are no differences in terms of dendritic cell viability and maturation [31]. In future work, we will compare the immune response of MAP2-3 immunized mice to heat-killed S. aureus versus live ones.
Although we demonstrated that memory B cells and related cytokines were induced after peptide immunization, evidence is lacking to prove that these cells are specific for S. aureus. In future work, we will examine the induction of antigen-specific plasma cells, memory B cells, and T cells after stimulation using fluorescein-labeled MAP2-3 peptide, LTA or S. aureus. We will also examine the specificity of peptide-induced immune response by comparing different S. aureus strains and other bacteria.
Peptide-based vaccines are produced almost exclusively using chemical synthetic approaches. This production procedure is simple and fast and there is no biological contamination that may induce allergic responses compared with inactivated vaccine and recombinant protein-based vaccine [32]. However, peptides are poor immunogens on their own and are susceptible to enzymatic degradation compared with protein-derived antigens. Peptide used as a vaccine needs the assistance of an adjuvant or delivery system. Alum, the only widely used adjuvant for humans, is not suitable for peptide antigens due to its weak adjuvant potency [33]. Our previous work also showed that immunization with MAP2-3 emulsified with alum could not effectively induce a humoral immune response (data not shown). In contrast, immunization of MAP2-3 emulsified with Freund’s adjuvant could induce LTA-specific IgG antibodies [9], memory B cells, and memory T cells. However, this adjuvant is not suitable for human vaccination due to its toxicity. Some new adjuvants such as squalene-based emulsions including MF59 and AS03 have been licensed for human application [34, 35]. In addition, alum plus TLR or CpG-ODN and liposomal plus CpG-ODN formulation can elicit a strong humoral immune response [36]. New antigen delivery systems such as polymers [37] and nanoparticles [38] have been recently used for promoting the uptake, transport, and presentation of antigens to APCs. In future experiments, we will examine the effect of novel adjuvants and delivery systems on the immunogenicity of MAP2-3.
Conclusions
Memory B cells in the spleen and bone marrow as well as effector memory T cells in the spleen and lymphoid nodes can be induced after one month of immunization with an LTA-mimicking peptide, MAP2-3.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Li-Chang Liu for handling of experimental animals.
Abbreviations
- 7-AAD
7-aminoactinomycin D
- ASCs
antibody-secreting cells
- ATCC
American type culture collection
- APC
Allophycocyanin
- APCs
antigen presenting cells
- Bm
memory B cells
- CD
cluster of differentiation
- cDNA
complementary DNA
- CFU
colony-forming units
- CXCR5
C-X-C chemokine receptor type 5
- ELISA
enzyme linked immunosorbent assay
- FACS
fluorescence activated cell sorting
- Fc
fragment crystallizable
- FCM
flow cytometry
- FITC
fluorescein isothiocyanate
- GAPDH
Glyceraldehyde 3-phosphite dehydrogenase
- GC
germinal center
- GC-B cells
germinal center B cells
- h
hour
- IL-6
interleukin 6
- IL-21
interleukin 21
- IL-2
interleukin 2
- IFN-γ
interferon gamma
- i.p
intraperitoneally
- mIgD
membrane immunoglobulin D
- mIgG/mIgG1
membrane immunoglobulin G or G1
- mIgM
membrane immunoglobulin M
- LTA
lipoteichoic acid
- LSD
the least significant difference
- MAP
multiple antigenic peptide
- MFI
the mean fluorescence intensity
- mg
milligram
- min
minute
- ml
milliliter
- mRNA
messenger RNA
- MRSA
methicillin-resistance Staphylococcus aureus
- OD
optical density
- PBS
phosphate-buffered saline
- PD-1
programmed cell death-1
- PE
Phycoerythrin
- PE/Cy5
Phycoerythrin/Cyanine 5
- PE/Cy7
Phycoerythrin/Cyanine 7
- RBC
red blood cell
- rpm
revolutions per minute
- s
second
- RT-PCR
real-time polymerase chain reaction
- S aureus
Staphylococcus aureus
- SEM
standard error of the mean
- TI-antigen
thymus-independent antigen
- TCM
central memory T cells
- TEM
effector memory T cells
- TFH
follicular helper T cells
- Th
helper T cells
- µl
microliter
- µg
microgram
- µm
micrometer
Author contributions
BYL designed the experiments and wrote the paper. XYY, XRH, and ZXH performed the experiments and analyzed the data. PZ provided the key reagents. All authors read and approved the final manuscript.
Funding
This work was supported by the Guangdong Basic and Applied Basic Research Foundation (2021A1515011229) to BYL and a Grant from the National Natural Science Foundation of China (31270980) to BYL. The funders had no role in study design, data collection and analysis, and the decision to publish or preparation of the manuscript.
Data availability
All data generated or analyzed during this study are included in this article and its supplementary information files.
Declarations
Ethics approval and consent to participate
All the animal experiments were approved by the Institutional Animal Care and Use Committee of Southern Medical University (permit number: L2015070) and carried out in strict accordance with ARRIVE guidelines.
Consent for publication
Not applicable.
Conflict of interest
The peptide sequence in MAP2-3, HSGHKEDRQWCQHSGG has been acquired National Invention Patent of China (Patent No. ZL 2016 1 0832921.3 licensed to Southern Medical University). BYL, XRH, PZ, and ZXH are the inventors of this patent. XYY declares that she has no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xia-Yu Yi and Xiao-Rui Hou contributed equally to this work.
References
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339(8):520–32. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Kourtis AP, Hatfield K, Baggs J, Mu Y, See I, Epson E, et al. Vital signs: Epidemiology and recent trends in Methicillin-resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections - United States. MMWR Morb Mortal Wkly Rep. 2019;68(9):214–9. doi: 10.15585/mmwr.mm6809e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee AS, de Lencastre H, Garau J, Kluytmans J, Malhotra-Kumar S, Peschel A, et al. Methicillin-resistant Staphylococcus aureus. NAT REV DIS PRIMERS. 2018;4:18033. doi: 10.1038/nrdp.2018.33. [DOI] [PubMed] [Google Scholar]
- 4.Percy MG, Grundling A. Lipoteichoic acid synthesis and function in gram-positive bacteria. ANNU REV MICROBIOL. 2014;68:81–100. doi: 10.1146/annurev-micro-091213-112949. [DOI] [PubMed] [Google Scholar]
- 5.Schneewind O, Missiakas D. Lipoteichoic acids, phosphate-containing polymers in the envelope of gram-positive bacteria. J BACTERIOL. 2014;196(6):1133–42. doi: 10.1128/JB.01155-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abachin E, Poyart C, Pellegrini E, Milohanic E, Fiedler F, Berche P, et al. Formation of D-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. MOL MICROBIOL. 2002;43(1):1–14. doi: 10.1046/j.1365-2958.2002.02723.x. [DOI] [PubMed] [Google Scholar]
- 7.Fabretti F, Theilacker C, Baldassarri L, Kaczynski Z, Kropec A, Holst O, et al. Alanine esters of enterococcal lipoteichoic acid play a role in biofilm formation and resistance to antimicrobial peptides. INFECT IMMUN. 2006;74(7):4164–71. doi: 10.1128/IAI.00111-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheen TR, Ebrahimi CM, Hiemstra IH, Barlow SB, Peschel A, Doran KS. Penetration of the blood-brain barrier by Staphylococcus aureus: contribution of membrane-anchored lipoteichoic acid. J Mol Med (Berl) 2010;88(6):633–9. doi: 10.1007/s00109-010-0630-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi XY, Huang ZX, Hou XR, Zhu P, Wang XY, Luo HB, et al. Immunization with a peptide mimicking lipoteichoic acid protects mice against Staphylococcus aureus infection. VACCINE. 2019;37(31):4325–35. doi: 10.1016/j.vaccine.2019.06.024. [DOI] [PubMed] [Google Scholar]
- 10.Roney K. Bone marrow-derived dendritic cells. Methods Mol Biol. 2019;1960:57–62. doi: 10.1007/978-1-4939-9167-9_4. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Li G, Wan Z, Liu C, Zeng Y, Liu W. How b cells remember? A sophisticated cytoplasmic tail of mIgG is pivotal for the enhanced transmembrane signaling of IgG-switched memory B cells. Prog Biophys Mol Biol. 2015;118(3):89–94. doi: 10.1016/j.pbiomolbio.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Cancro MP, Tomayko MM. Memory B cells and plasma cells: the differentiative continuum of humoral immunity. IMMUNOL REV. 2021;303(1):72–82. doi: 10.1111/imr.13016. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi Y, Ohta H, Takemori T. Fas is required for clonal selection in germinal centers and the subsequent establishment of the memory B cell repertoire. Immunity. 2001;14(2):181–92. doi: 10.1016/S1074-7613(01)00100-5. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein JS, Herman EI, Lainez B, Licona-Limon P, Esplugues E, Flavell R, et al. TFH cells progressively differentiate to regulate the germinal center response. NAT IMMUNOL. 2016;17(10):1197–205. doi: 10.1038/ni.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suan D, Sundling C, Brink R. Plasma cell and memory B cell differentiation from the germinal center. CURR OPIN IMMUNOL. 2017;45:97–102. doi: 10.1016/j.coi.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Good-Jacobson KL, Shlomchik MJ. Plasticity and heterogeneity in the generation of memory B cells and long-lived plasma cells: the influence of germinal center interactions and dynamics. J IMMUNOL. 2010;185(6):3117–25. doi: 10.4049/jimmunol.1001155. [DOI] [PubMed] [Google Scholar]
- 17.Kurosaki T, Kometani K, Ise W, Memory B. cells. NAT REV IMMUNOL 2015;15(3):149– 59. [DOI] [PubMed]
- 18.Tomayko MM, Steinel NC, Anderson SM, Shlomchik MJ. Cutting edge: Hierarchy of maturity of murine memory B cell subsets. J IMMUNOL. 2010;185(12):7146–50. doi: 10.4049/jimmunol.1002163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Good-Jacobson KL, Song E, Anderson S, Sharpe AH, Shlomchik MJ. CD80 expression on B cells regulates murine T follicular helper development, germinal center B cell survival, and plasma cell generation. J IMMUNOL. 2012;188(9):4217–25. doi: 10.4049/jimmunol.1102885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinuesa CG, Linterman MA, Yu D, MacLennan IC, Follicular Helper T, Cells ANNU REV IMMUNOL. 2016;34:335–68. doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- 21.Eivazi S, Bagheri S, Hashemzadeh MS, Ghalavand M, Qamsari ES, Dorostkar R, et al. Development of T follicular helper cells and their role in disease and immune system. BIOMED PHARMACOTHER. 2016;84:1668–78. doi: 10.1016/j.biopha.2016.10.083. [DOI] [PubMed] [Google Scholar]
- 22.Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (tfh) differentiation. PLoS ONE. 2011;6(3):e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crotty S. Follicular helper CD4 T cells (TFH) ANNU REV IMMUNOL. 2011;29:621–63. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 24.Crotty ST. Follicular Helper Cell Biology: a decade of Discovery and diseases. Immunity. 2019;50(5):1132–48. doi: 10.1016/j.immuni.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zotos D, Coquet JM, Zhang Y, Light A, D’Costa K, Kallies A, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J EXP MED. 2010;207(2):365–78. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J EXP MED. 2010;207(2):353–63. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopf M, Herren S, Wiles MV, Pepys MB, Kosco-Vilbois MH. Interleukin 6 influences germinal center development and antibody production via a contribution of C3 complement component. J EXP MED. 1998;188(10):1895–906. doi: 10.1084/jem.188.10.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao YX, Nilsson IM, Tarkowski A. The dual role of interferon-gamma in experimental Staphylococcus aureus septicaemia versus arthritis. Immunology. 1998;93(1):80–5. doi: 10.1046/j.1365-2567.1998.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolata JB, Kuhbandner I, Link C, Normann N, Vu CH, Steil L, et al. The fall of a dogma? Unexpected high T-cell memory response to Staphylococcus aureus in humans. J Infect Dis. 2015;212(5):830–8. doi: 10.1093/infdis/jiv128. [DOI] [PubMed] [Google Scholar]
- 30.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, et al. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature. 2012;484(7395):514–8. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 31.Cruciani M, Sandini S, Etna MP, Giacomini E, Camilli R, Severa M, et al. Differential responses of human dendritic cells to live or inactivated Staphylococcus aureus: impact on cytokine production and T helper expansion. Front Immunol. 2019;10:2622. doi: 10.3389/fimmu.2019.02622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skwarczynski M, Toth I. Peptide-based synthetic vaccines. CHEM SCI. 2016;7(2):842–54. doi: 10.1039/C5SC03892H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perrie Y, Kirby D, Bramwell VW, Mohammed AR. Recent developments in particulate-based vaccines. Recent Pat Drug Deliv Formul. 2007;1(2):117–29. doi: 10.2174/187221107780831897. [DOI] [PubMed] [Google Scholar]
- 34.Firdaus FZ, Skwarczynski M, Toth I. Developments in Vaccine adjuvants. Methods Mol Biol. 2022;2412:145–78. doi: 10.1007/978-1-0716-1892-9_8. [DOI] [PubMed] [Google Scholar]
- 35.Shi S, Zhu H, Xia X, Liang Z, Ma X, Sun B. Vaccine adjuvants: understanding the structure and mechanism of adjuvanticity. VACCINE. 2019;37(24):3167–78. doi: 10.1016/j.vaccine.2019.04.055. [DOI] [PubMed] [Google Scholar]
- 36.Reidel IG, Camussone C, Guillermo A, Suarez Archilla, Calvinho LF, Veaute C. Liposomal and CpG-ODN formulation elicits strong humoral immune responses to recomibinant Staphylococcus aureus antigens in heifer calves. Vet Immunol Immunopathol. 2019;212:1–8. doi: 10.1016/j.vetimm.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Buyuktimkin B, Wang Q, Kiptoo P, Stewart JM, Berkland C, Siahaan TJ. Vaccine-like controlled-release delivery of an immunomodulating peptide to treat experimental autoimmune encephalomyelitis. Mol Pharm. 2012;9(4):979–85. doi: 10.1021/mp200614q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao G, Azuar A, Toth I, Skwarczynski M. A potent vaccine delivery system. Bio Protoc. 2021;11(7):e3973. doi: 10.21769/BioProtoc.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary information files.