Abstract
CD4-immunoglobulin G2 (IgG2) is a fusion protein comprising human IgG2 in which the Fv portions of both heavy and light chains have been replaced by the V1 and V2 domains of human CD4. Previous studies found that CD4-IgG2 potently neutralizes a broad range of primary human immunodeficiency virus type 1 (HIV-1) isolates in vitro and ex vivo. The current report demonstrates that CD4-IgG2 protects against infection by primary isolates of HIV-1 in vivo, using the hu-PBL-SCID mouse model. Passive administration of 10 mg of CD4-IgG2 per kg of body weight protected all animals against subsequent challenge with 10 mouse infectious doses of the laboratory-adapted T-cell-tropic isolate HIV-1LAI, while 50 mg of CD4-IgG2 per kg protected four of five mice against the primary isolates HIV-1JR-CSF and HIV-1AD6. In contrast, a polyclonal HIV-1 Ig fraction exhibited partial protection against HIV-1LAI at 150 mg/kg but no significant protection against the primary HIV-1 isolates. The results demonstrate that CD4-IgG2 effectively neutralizes primary HIV-1 isolates in vivo and can prevent the initiation of infection by these viruses.
CD4-immunoglobulin G2 (IgG2) is a heterotetramer consisting of two chains of a CD4-human IgG2 heavy-chain fusion protein and two chains of a CD4-human kappa light-chain fusion protein (1). In each case, the V1 and V2 domains of human CD4 replace the Fv portions of the antibody chain. This molecule is being developed for the prophylaxis and therapy of human immunodeficiency virus type 1 (HIV-1) infection. Since CD4 is the high-affinity receptor for HIV-1, CD4-IgG2 has the potential to bind and neutralize all strains of the virus and to minimize the potential for the development of resistant HIV-1 strains. CD4-IgG2 was designed with four gp120 binding sites in order to have a higher avidity for HIV-1 virions or infected cells than monomeric soluble CD4 or dimeric CD4–heavy-chain constructs. In addition, it incorporates the heavy chain of human IgG2 to minimize the possibility of enhancing infection though Fc or complement receptor binding (1).
Several studies have demonstrated that CD4-IgG2 potently neutralizes primary HIV-1 isolates (1, 5, 21). Using a standard in vitro neutralization analysis with 28 primary HIV-1 isolates from different international clades of the virus, including 12 from clade B, it was found that CD4-IgG2 neutralized all strains with median 50% inhibitory concentrations (IC50) of 6.2 μg/ml (B-clade isolates) and 4.8 μg/ml (non-B clade isolates) (21). These in vitro data were extended by the demonstration that CD4-IgG2 effectively neutralized unpassaged primary HIV-1 isolates in viremic plasma samples taken from six HIV-1-infected individuals (an ex vivo assay) (5). In this study, 25 μg of CD4-IgG2 per ml reduced the HIV-1 titers in plasma samples from all donors by between 5- and 625-fold.
The present report focuses on the in vivo efficacy of CD4-IgG2 using the hu-PBL-SCID mouse model, in which severe combined immunodeficiency mice were injected with human peripheral blood lymphocytes and can be infected with HIV-1 (17). Previously, we reported that BAT123, a murine monoclonal antibody directed against the V3 loop of HIV-1LAI, can protect hu-PBL-SCID mice from challenge with this virus strain (20). However, primary isolates of HIV-1 were not sensitive to neutralization by BAT123 in vitro, and the antibody did not offer protection against primary isolates in vivo in hu-PBL-SCID mice (7). More recently, we showed that a potent neutralizing human monoclonal antibody, b12, can protect hu-PBL-SCID mice from challenge with both primary and T-cell-line-adapted strains of HIV-1 (6). We now demonstrate that CD4-IgG2 also neutralizes several strains of HIV-1, including two primary isolates in vivo, by using the hu-PBL-SCID mouse model.
Reconstitution of hu-PBL-SCID mice was performed as described previously (7). Briefly, CB.17 scid/scid mice were injected intraperitoneally (i.p.) with 20 × 106 freshly isolated normal human peripheral blood mononuclear cells (PBMC) suspended in 0.5 ml of phosphate-buffered saline. Two weeks after PBMC transfer, reconstitution was confirmed by analysis of the mouse sera for the presence of human Ig with an enzyme-linked immunosorbent assay (ELISA) kit (SangStat, Menlo Park, Calif.). Only human Ig-positive mice were used for pharmacokinetic and protection studies.
Prior to HIV-1 challenge experiments, the pharmacokinetics of CD4-IgG2 were examined. CD4-IgG2 was produced in Chinese hamster ovary cells using expression vectors, cell culture, and purification methods described previously (1). The protein was injected i.p. into three hu-PBL-SCID mice at a dose of 10 mg/kg of body weight, and blood samples were obtained from the tail veins at intervals up to 14 days following administration. The levels of CD4-IgG2 in serum were measured by ELISAs. A mean peak serum CD4-IgG2 concentration of 112 μg/ml was obtained 6 h postinjection, and the terminal half-life of CD4-IgG2 was approximately 29 h, which is similar to the result previously obtained in rabbits (1).
Three HIV-1 isolates were used in the challenge studies: HIV-1LAI, a laboratory strain of HIV-1 adapted to grow in transformed T-cell lines (2); HIV-1JR-CSF, a molecularly cloned primary HIV-1 isolate (12), and HIV-1AD6, a primary isolate from an acute seroconvertor (16). CD4-IgG2 neutralizes HIV-1LAI, HIV-1JR-CSF, and HIV-1AD6 with in vitro IC90s of 0.4, 9.9, and 17.7 μg/ml, respectively (1). Virus stocks were prepared from the supernatants of infected PBMC. Briefly, cell-free virus was harvested on days 5 to 7 from acutely infected phytohemagglutinin-stimulated PBMC. Virus stocks were titrated for infectivity in hu-PBL-SCID mice, expressed as the 50% mouse infectives dose (MID50) per milliliter.
Protection experiments were performed with groups of four to eight mice which received either CD4-IgG2 or HuIgG, a polyclonal human IgG fraction purified from an HIV-1-seronegative donor, 1 h prior to inoculation with 10 MID50 of the appropriate HIV-1 strain. Mice were sacrificed 3 weeks after viral challenge, and cells were recovered from peritoneal lavage and spleen as previously described (11, 20). The presence of infectious HIV-1 was determined by coculturing peritoneal lavage cells (2 × 105) and spleen cells (5 × 106) with 2 × 106 phytohemagglutinin-activated peripheral blood lymphocytes from HIV-1-seronegative donors in an end-point dilution culture (10-fold serial dilutions) (9, 10, 20). Cocultures were monitored weekly over 4 weeks for the presence of HIV-1 p24 core antigen in the culture supernatant with a commercial ELISA kit (Abbott). Cultures were considered positive for HIV-1 if a single sample contained >1,000 pg of p24 per ml or if two consecutive samples contained >200 pg of p24 per ml. The positive well containing the fewest spleen cells was taken as the end point, and the viral titers were expressed as tissue culture infectious doses per 106 cells. The statistical significance of differences between experimental groups of mice was calculated by the paired-sample Student t test (Wilcoxon signed-ranks test) or the two-sample (independent groups) Student t test (Wilcoxon rank sum test). The experiments were performed at least twice with similar results.
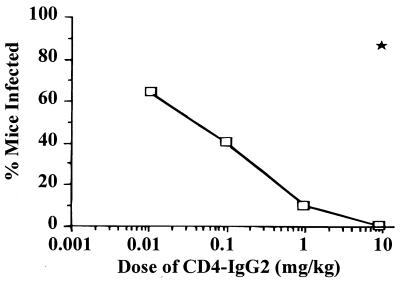
Initially, the ability of CD4-IgG2 to protect hu-PBL-SCID mice against challenge by HIV-1LAI was tested, as this T-cell-line-adapted strain is more sensitive to neutralization in vitro by CD4-IgG2 than are the primary HIV-1 isolates. Doses of CD4-IgG2 ranged from 0.01 to 10 mg/kg, while control mice received 10 mg of HuIgG per kg. The results are shown in Fig. 1. Partial protection was observed at doses from 0.01 to 1 mg of CD4-IgG2 per kg, with complete protection at 10 mg/kg. HuIgG provided no protection when administered at 10 mg/kg.
FIG. 1.
Minimum protective dose of CD4-IgG2 against HIV-1LAI infection. CD4-IgG2 at various doses from 0.01 to 10 mg/kg was injected i.p. into hu-PBL-SCID mice 1 h before HIV-1LAI inoculation (10 MID50). The result for control mice treated with 10 mg of HuIgG per kg is indicated by the solid star. Virus recovery was assessed at sacrifice after 3 weeks. The number of mice in each treatment group is as follows: 0.01 mg/kg, 8; 0.1 mg/kg, 10; 1 mg/kg, 10; and 10 mg/kg, 9.
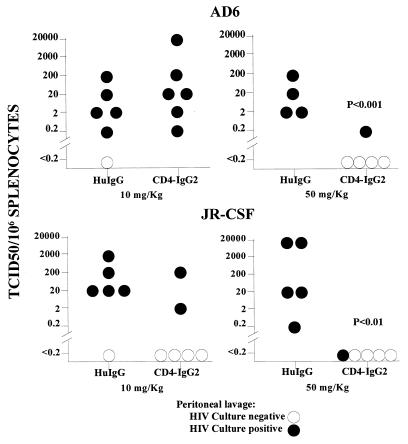
Having demonstrated protection against the laboratory-adapted strain of HIV-1, CD4-IgG2 was tested for its ability to protect hu-PBL-SCID mice challenged with the two primary isolates, HIV-1JR-CSF and HIV-1AD6. Higher concentrations of CD4-IgG2 are required to neutralize these isolates in vitro compared with HIV-1LAI, so doses of 10 and 50 mg of CD4-IgG2 per kg were used for the in vivo protection studies. The results are provided in Fig. 2. At the 10-mg/kg dose level, CD4-IgG2 protected 67% (four of six) of mice challenged with HIV-1JR-CSF but was ineffective in mice challenged with HIV-1AD6. However, at the higher dose (50 mg/kg), CD4-IgG2 protected 80% (four of five) of the mice challenged with either primary HIV-1 isolate. In contrast, all control mice treated with 50 mg of HuIgG per kg became infected and the difference between the treatment and control groups was statistically significant (P ≤ 0.01).
FIG. 2.
Protection by CD4-IgG2 against infection by primary isolates of HIV-1. CD4-IgG2 or HuIgG antibody at doses of 10 or 50 mg/kg was injected i.p. into hu-PBL-SCID mice which were then inoculated with 10 MID50 of HIV-1AD6 or HIV-1JR-CSF. Virus recovery was assessed at sacrifice after 3 weeks. TCID50, 50% tissue culture infective doses.
Based on the pharmacokinetic analysis, the doses of CD4-IgG2 that protected the majority of mice from HIV-1 infection (1 mg/kg for HIV-1LAI and 50 mg/kg for HIV-1JR-CSF or HIV-1AD6) should result in concentrations in serum at 6 h postadministration that are 30- to 60-fold greater than the in vitro IC90s for each isolate. This is in agreement with the BAT123 antibody study, where protection against HIV-1LAI infection of hu-PBL-SCID mice required a concentration in serum 50-fold greater than the in vitro IC90 of BAT123 for this viral strain (7). These results indicate that protection against infection in the hu-PBL-SCID mouse model is a stringent test of in vivo neutralizing capability, presumably because 100% of virus infectivity must be neutralized to prevent infection of the animal.
For comparison with CD4-IgG2, we tested HIVIG, an HIV-1 Ig fraction prepared from human plasma selected for high titers of HIV-1 neutralizing antibodies that was provided by Linda Andrus (New York Blood Center, New York, N.Y.) (13, 19). This HIVIG preparation was administered at 150 mg/kg to hu-PBL-SCID mice challenged with HIV-1LAI, HIV-1JR-CSF, and HIV-1AD6 by the standard procedures described above. At this dose, HIVIG protected 67% (four of six) mice challenged with HIV-1LAI but exhibited no significant protection against the two primary isolates (Table 1). The results obtained with HuIgG and HIVIG against the primary HIV-1 isolates were not significantly different (P > 0.05).
TABLE 1.
Protection by HIVIG against HIV-1 infection of hu-PBL-SCID mice
| HIV-1 isolate | No. (%) of mice infected following treatment witha:
|
|
|---|---|---|
| HuIgG | HIVIG | |
| LAI | 4/5 (80) | 2/6 (33) |
| JR-CSF | 3/4 (75) | 4/5 (80) |
| AD6 | 2/2 (100) | 4/6 (67) |
Mice were treated with 150 mg of HIVIG or HuIgG control per kg 1 h before HIV-1 inoculation (10 MID50) and virus infection was assessed at sacrifice 3 weeks later.
Igs have been used successfully for the therapy and prophylaxis of infections by several viruses including rabies virus, cytomegalovirus, hepatitis B virus, varicella-zoster virus, and respiratory syncytial virus (8). Monoclonal antibodies are also being developed for several of these indications. For antibody-based molecules to be effective against HIV-1 infection, it is important that they effectively neutralize primary isolates of the virus in vivo. Previous studies have demonstrated that antibodies to HIV-1 can protect against infection by T-cell-line-adapted strains of HIV-1 in several animal models of infection (4, 7, 18). However, T-cell-line adapted strains are abnormally sensitive to neutralization in vitro by sera from infected donors or recombinant gp120 vaccinees, soluble CD4 and antibody preparations (3, 14, 15). In addition, many of the antibodies tested in these studies neutralized only a limited range of HIV-1 strains in vitro, based on their specificity for variable domains on the HIV-1 envelope glycoprotein such as the V3 loop (4, 7). A more-recent study, however, has demonstrated that a broadly neutralizing human monoclonal antibody is similar to CD4-IgG2 in its ability to protect hu-PBL-SCID mice from infection by both primary isolates and T-cell-line-adapted strains of HIV-1 (6).
The current study demonstrates protection against primary HIV-1 isolates in vivo by CD4-IgG2. Previously, we showed that CD4-IgG2 broadly neutralizes primary HIV-1 isolates in vitro and ex vivo (1, 5, 21). We have now demonstrated that CD4-IgG2 protects hu-PBL-SCID mice against direct challenge with primary HIV-1 isolates as well as a T-cell-line-adapted strain of the virus. The results also suggest that CD4-IgG2 would be more effective than HIVIG in preventing HIV-1 infection in humans, as CD4-IgG2 but not HIVIG protected against primary HIV-1 isolates in vivo. The dose of CD4-IgG2 required to achieve protection in the hu-PBL-SCID mouse model corresponds to a concentration of the molecule in serum considerably in excess of that required to achieve 90% neutralization in vitro. It is not known if the protective dose in the hu-PBL-SCID mouse would translate to an effective human dose on a milligram-per-kilogram basis. One cannot predict the pharmacokinetic or efficacy profile of CD4-IgG2 in humans based solely upon the results in hu-PBL-SCID mice. It is, however, encouraging that protection was demonstrated in a model system where the additional components of bioavailability, distribution, and pharmacologic decay of the reagent are operative. These important parameters that may affect whether HIV-1 infection occurs are not addressed in a simple tissue culture system.
Future studies will analyze the efficacy of CD4-IgG2 in animal models of HIV-1 postexposure prophylaxis and therapy. Clinical trials with the molecule in HIV-1-infected adults and children are also planned.
Acknowledgments
We thank Y. Cao and G. Melcher for providing virus stocks, Linda Andrus for providing HIVIG, J. Binley and E. Garcia for technical assistance, and W. Chen and S. Norton for assistance with graphics.
This work was supported by grants from the National Institutes of Health (AI35522, AI33292, and AI36818) and by Progenics Pharmaceuticals, Inc. M.-C.G. was supported by summer internship awards from the Pediatric AIDS Foundation. R.A.K. is an Elizabeth Glaser Scientist of the Pediatric AIDS Foundation.
REFERENCES
- 1.Allaway G P, Davis-Bruno K L, Beaudry G A, Garcia E B, Wong E L, Ryder A M, Hasel K W, Gauduin M-C, Koup R A, McDougal J S, Maddon P J. Expression and characterization of CD4-IgG2, a novel heterotetramer that neutralizes primary HIV type 1 isolates. AIDS Res Hum Retroviruses. 1995;11:533–539. doi: 10.1089/aid.1995.11.533. [DOI] [PubMed] [Google Scholar]
- 2.Barre-Sinoussi F, Chermann J C, Rey F, Nugeyre M T, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphocyte retrovirus from patient at risk for acquired immunodeficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J. Jitters jeopardize AIDS vaccine trials. Science. 1993;262:980–981. doi: 10.1126/science.8235635. [DOI] [PubMed] [Google Scholar]
- 4.Emini E A, Schleif W A, Nunberg J H, Conley A J, Eda Y, Tokiyoshi S, Putney S D, Matsushita S, Cobb K E, Jett C M, Eichberg J W, Murthy K K. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992;355:728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- 5.Gauduin M C, Allaway G P, Maddon P J, Barbas C F, Burton D R, Koup R A. Effective ex vivo neutralization of human immunodeficiency virus type 1 in plasma by recombinant immunoglobulin molecules. J Virol. 1996;70:2586–2592. doi: 10.1128/jvi.70.4.2586-2592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gauduin M C, Parren P W H I, Weir R, Barbas C F, Burton D R, Koup R A. Passive immunization with a potent neutralizing human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of human immunodeficiency virus type 1. Nat Med. 1997;3:1389–1393. doi: 10.1038/nm1297-1389. [DOI] [PubMed] [Google Scholar]
- 7.Gauduin M C, Safrit J T, Weir R, Fung M S, Koup R A. Pre- and post-exposure protection against human immunodeficiency virus type 1 infection mediated by a monoclonal antibody. J Infect Dis. 1995;171:1203–1209. doi: 10.1093/infdis/171.5.1203. [DOI] [PubMed] [Google Scholar]
- 8.Harris W J. Therapeutic antibodies in infectious disease. In: Harris W J, Adair J R, editors. Antibody therapeutics. Boca Raton, Fla: CRC Press; 1997. pp. 89–118. [Google Scholar]
- 9.Ho D D, Moudgil T, Alam M. Quantitation of human immunodeficiency virus type 1 in the blood of infected persons. N Engl J Med. 1989;321:1621–1625. doi: 10.1056/NEJM198912143212401. [DOI] [PubMed] [Google Scholar]
- 10.Koup R A, Ho D D. Quantitative culture assay for HIV-1 in peripheral blood. In: Aldovini A, Walker B D, editors. Techniques in HIV research. New York, N.Y: Stockton Press; 1994. pp. 107–112. [Google Scholar]
- 11.Koup R A, Hesselton R M, Safrit J T, Somasundran M, Sullivan J L. Quantitative assessment of human immunodeficiency virus type 1 replication in human xenografts of acute-infected Hu-PBL-SCID mice. AIDS Res Hum Retroviruses. 1994;10:279–284. doi: 10.1089/aid.1994.10.279. [DOI] [PubMed] [Google Scholar]
- 12.Koyanagi Y, Miles S, Mitsuyasu R T, Merrill J E, Vinters H V, Chen I S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 13.Lambert J S, Mofenson L M, Fletcher C V, Moye J, Stiehm E R, Meyer III W A, Nemo G J, Mathieson B J, Hirsch G, Sapan C V, Cummins L M, Jiminez E, O’Neill E, Kovacs A, Stek A the Pediatric AIDS Clinical Trials Group Protocol 185 Pharmacokinetic Study Group. Safety and pharmacokinetics of hyperimmune anti-human immunodeficiency virus (HIV) immunoglobulin administered to HIV-infected pregnant women and their newborns. J Infect Dis. 1997;175:283–291. doi: 10.1093/infdis/175.2.283. [DOI] [PubMed] [Google Scholar]
- 14.Mascola J R, Louwagie J, McCutchan F E, Fischer C L, Hegerich P A, Wagner K F, Fowler A K, McNeil J G, Burke D S. Two antigenically distinct subtypes of human immunodeficiency virus type 1: viral genotype predicts neutralization serotype. J Infect Dis. 1994;169:48–54. doi: 10.1093/infdis/169.1.48. [DOI] [PubMed] [Google Scholar]
- 15.Matthews T J. Dilemma of neutralization resistance of HIV-1 field isolates and vaccine development. AIDS Res Hum Retroviruses. 1994;10:631–632. doi: 10.1089/aid.1994.10.631. [DOI] [PubMed] [Google Scholar]
- 16.Moore J P, Cao Y, Ho D D, Koup R A. Development of the anti-gp120 antibody response during seroconversion to human immunodeficiency virus type 1. J Virol. 1994;68:5142–5155. doi: 10.1128/jvi.68.8.5142-5155.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosier D E, Gulizia R J, Baird S M, Wilson D B, Spector D H, Spector S A. Human immunodeficiency virus infection of human-PBL-SCID mice. Science. 1991;251:791–794. doi: 10.1126/science.1990441. [DOI] [PubMed] [Google Scholar]
- 18.Parren P W H I, Ditzel H J, Gulizia R J, Binley J M, Barbas III C F, Burton D R, Mosier D E. Protection against HIV-1 infection in hu-PBL-SCID mice by passive immunization with a neutralizing human monoclonal antibody against the gp120 CD4-binding site. AIDS. 1995;9:F1–F6. doi: 10.1097/00002030-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Prince A M, Horowitz B, Baker L, Shulman R W, Ralph H, Valinsky J, Cundell A, Brotman B, Boehle W, Rey F, Piet M, Reesink H, Lelie N, Tersmette M, Miedema F, Barbosa L, Nemo G, Nastala C L, Allan J S, Lee D R, Eichberg J W. Failure of an HIV immune globulin to protect chimpanzees against experimental challenge with HIV. Proc Natl Acad Sci USA. 1988;85:6944–6948. doi: 10.1073/pnas.85.18.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safrit J T, Fung M S C, Andrews C A, Braun D G, Sun W N C, Chang T W, Koup R A. Hu-PBL-SCID mice can be protected from HIV-1 infection by passive transfer of monoclonal antibody to the principal neutralizing determinant of envelope gp120. AIDS. 1993;7:15–21. doi: 10.1097/00002030-199301000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Trkola A, Pomales A P, Yuan H, Korber B, Maddon P J, Allaway G, Katinger H, Barbas C F, Burton D R, Ho D D, Moore J P. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-immunoglobulin G. J Virol. 1995;69:6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]