Abstract
We investigated the capacity of intramuscular (i.m.) immunization with heterologous-host rotavirus (simian strain RRV) to induce mucosal virus-specific memory B cells in mice. We found that prior i.m. immunization enhanced the magnitude of mucosal virus-specific immunoglobulin A (IgA) production but did not alter the site and timing of induction of virus-specific IgA responses after challenge.
Despite significant efforts, the development of vaccines against mucosal pathogens has been slow. Previous studies have identified several obstacles to the successful development of mucosal vaccines. First, effector B- and T-cell responses at mucosal surfaces are relatively short-lived: mucosal immunoglobulin A (IgA) responses usually wane 4 to 6 months after a primary infection (3, 9, 17, 31), and effector cytotoxic T cells rarely persist at mucosal surfaces for longer than 1 month (4, 14, 22). Second, mucosal pathogens generally have brief incubation periods, often as short as 1 to 3 days. Therefore, a successful mucosal vaccine must either (i) induce a durable effector B- or T-cell response at the mucosal surface or (ii) induce memory B or T cells capable of undergoing rapid expansion and differentiation to mucosal effector cells upon reexposure.
Prior studies have demonstrated that parenteral immunization can induce mucosal immune responses (6, 7, 22) and protection from mucosal infections (5, 7, 11, 13, 20). In addition, parenteral immunization has been shown to enhance mucosal antibody responses following challenge (5, 18, 24, 25, 30). We recently found that intramuscular (i.m.) immunization of mice with heterologous-host rotavirus (simian strain RRV) induced partial protection against challenge with homologous-host rotavirus (murine strain EDIM) (5). In these studies, partial protection was characterized by early resolution of viral shedding. In addition, production of virus-specific IgA by lamina propria (LP) lymphocytes in i.m.-immunized mice was enhanced compared to that in unimmunized mice 6 days after challenge. These findings support the hypothesis that i.m. immunization may induce rotavirus-specific memory B cells that protect against challenge. In this report, we extend our earlier observations and examine the capacity of i.m. immunization with live rotavirus to induce memory B-cell responses in gut-associated lymphoid tissue (GALT).
First, we evaluated the ability of a primary i.m. rotavirus inoculation to induce virus-specific antibody production by peripheral lymph node and GALT lymphocytes. Conventionally reared 6- to 8-week-old female BALB/c mice (Taconic Breeding Laboratories, Germantown, N.Y.) were inoculated i.m. (in the quadriceps femoris muscle) with 2.0 × 106 PFU of simian rotavirus strain RRV (obtained from N. Schmidt, Viral and Rickettsial Disease Laboratory, University of California, Berkeley). Serum collected from these mice prior to inoculation did not contain rotavirus-specific antibodies, as determined by enzyme-linked immunosorbent assay (ELISA). Intestinal and inguinal lymph node (ILN) lymphoid cultures were established 0, 2, 4, 6, 8, 11, 14, and 18 days after i.m. immunization. Using three to four mice per time point, lymphoid cultures of LP fragments, mesenteric lymph node (MLN) fragments, Peyer’s patches (PP), and ILN were established as previously described (2, 6). Supernatant fluids from cultures of 22 to 24 LP fragments, 5 to 6 MLN fragments, 16 to 24 PP, and 5 to 6 ILN per group per time point were tested for the presence of rotavirus-specific and total immunoglobulins (IgA and IgG) by ELISA as described previously (19). Screening dilutions of supernatants from all fragments were tested for the production of total IgA and IgG to ensure tissue viability. The mean quantities of IgA and IgG and the ratio of virus-specific to total IgA or IgG produced by each tissue at each time point were calculated.
Transient production of virus-specific IgA by GALT inductive sites was observed after parenteral immunization. Eleven days after i.m. inoculation, small quantities of virus-specific IgA were produced by lymphocytes in PP and MLN (2.1 and 6.9 ng/ml, respectively). Trace quantities of virus-specific IgA were produced by PP and MLN lymphocytes 14 and 18 days, respectively, after primary i.m. inoculation (data not shown). No virus-specific IgA was produced by LP or ILN lymphocytes after primary i.m. immunization. Six weeks after i.m. immunization, virus-specific IgA production was not detected in intestinal lymphoid cultures (Fig. 1, day 0). However, primary i.m. immunization induced long-lived production of virus-specific IgG by GALT. Virus-specific IgG was first produced by PP and MLN 6 days after primary i.m. immunization (0.6 and 0.2 μg/ml, respectively) and by LP 8 days after primary i.m. immunization (1.0 μg/ml). Production of virus-specific IgG by GALT persisted for at least 6 weeks (Fig. 2, day 0).
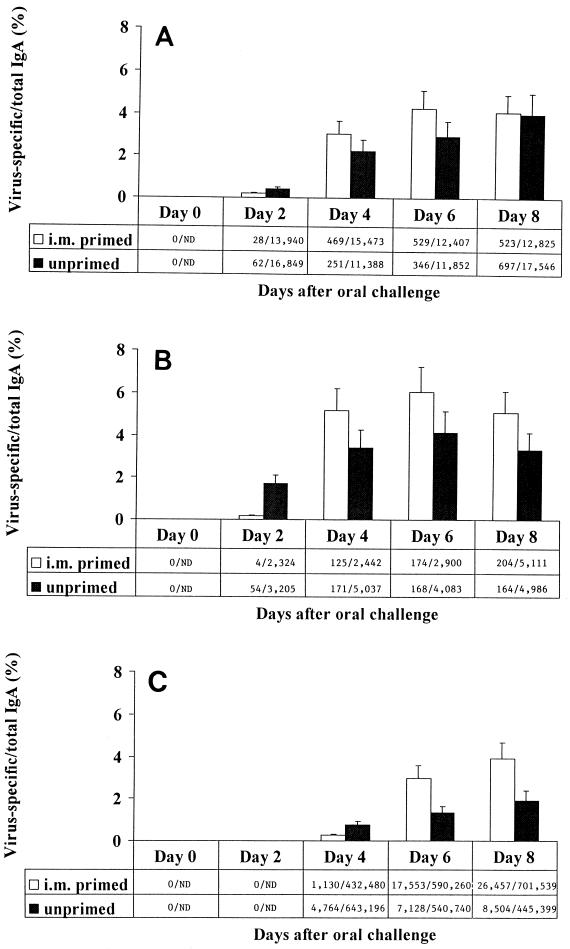
FIG. 1.
Kinetics of virus-specific IgA production by PP (A), MLN (B), and LP (C) from i.m.-immunized and unimmunized animals after oral challenge. Adult BALB/c mice were inoculated i.m. with simian rotavirus strain RRV (i.m. primed). Six weeks after primary i.m. inoculation, naïve (unprimed) and i.m.-primed mice were inoculated orally with EDIM. Lymphoid cultures of systemic and gut-associated tissues were performed 0, 2, 4, 6, and 8 days after oral inoculation. Supernatant fluids were tested for the presence of rotavirus-specific and total IgA by ELISA. Virus-specific antibodies were not detected by ELISA at concentrations of <2 ng/ml. Ratios are rotavirus-specific IgA/total IgA (in nanograms per ml). ND, not done.
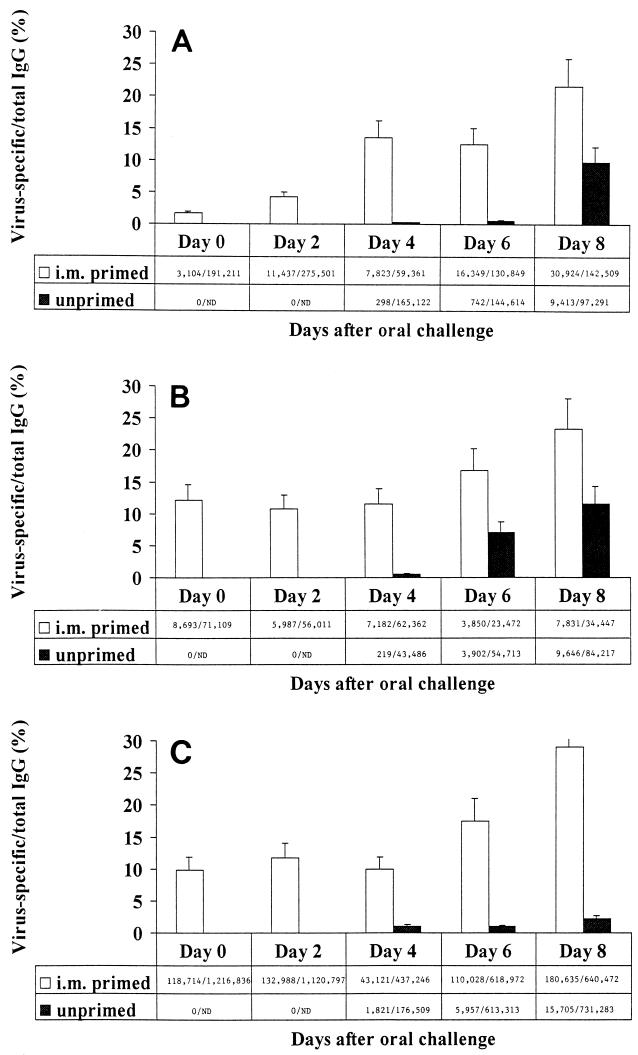
FIG. 2.
Kinetics of virus-specific IgG production by PP (A), MLN (B), and LP (C) from i.m.-immunized and unimmunized animals after oral challenge. Adult BALB/c mice were inoculated i.m. with simian rotavirus strain RRV (i.m. primed). Six weeks after primary i.m. inoculation, naïve (unprimed) and i.m.-primed mice were inoculated orally with EDIM. Lymphoid cultures of systemic and gut-associated tissues were performed 0, 2, 4, 6, and 8 days after oral inoculation. Supernatant fluids were tested for the presence of rotavirus-specific and total IgG by ELISA. Virus-specific IgG was not detected by ELISA at concentrations of <2 ng/ml. Ratios are rotavirus-specific IgG/total IgG (in nanograms per ml). ND, not done.
Next, we examined the ability of i.m. inoculation to induce virus-specific memory B cells committed to IgA secretion in GALT. Six weeks after i.m. inoculation with RRV, naive or previously i.m.-immunized mice were orally inoculated with murine rotavirus strain EDIM (initially obtained from Richard Ward, Children’s Hospital Research Foundation, Cincinnati, Ohio, and propagated as previously described [5]). Mice were orally inoculated (by proximal esophageal intubation) with EDIM at a dose of 60,000 50% shedding doses (by Reed and Muench calculation). Using three to four mice per time point, intestinal and ILN lymphoid cultures were established 0, 2, 4, 6, and 8 days after oral challenge as described above.
We found that i.m. immunization enhanced the magnitude of virus-specific IgA responses by LP lymphocytes after challenge. Six and 8 days after oral challenge, LP lymphocytes from mice previously immunized i.m. produced larger quantities of virus-specific IgA as well as a larger proportion of virus-specific IgA to total IgA than that produced by lymphocytes from unimmunized animals (P < 0.005) (Fig. 1C). However, i.m. immunization did not hasten the onset of virus-specific IgA production by GALT lymphocytes after oral challenge. In both immunized and unimmunized animals, production of virus-specific IgA by PP and MLN first occurred 2 days after challenge while that by LP lymphocytes first occurred 4 days after challenge (Fig. 1). Enhanced production of virus-specific IgA production was also observed in GALT inductive sites. Following challenge, larger quantities of rotavirus-specific IgA were produced by PP (days 4 and 6; P < 0.05) and MLN (days 4, 6, and 8; P < 0.01) lymphocytes from i.m.-immunized than by those from unimmunized mice.
These data suggest that virus-specific memory B cells committed to IgA production were resident in the inductive sites of GALT 6 weeks after i.m. inoculation. Following oral challenge, lymphocytes producing virus-specific IgA were detected initially in PP and MLN. Several days later, lymphocytes producing virus-specific IgA were detected in LP. We hypothesize that memory B cells were induced by parenteral inoculation and homed preferentially to GALT inductive sites (i.e., PP and MLN) compared with effector sites (i.e., LP). Thus, upon oral challenge, the site and time to onset of virus-specific effector B-cell responses were not altered by prior i.m. immunization.
Pierce and Gowans also found that parenteral immunization induced memory B cells resident in inductive sites of GALT (23). Antibody-containing cells were first detected in thoracic duct lymph of parenterally immunized rats 2 days after intraduodenal challenge with cholera toxoid. However, antibody-containing cells were not detected in LP of parenterally immunized animals until 4 to 8 days after intestinal challenge. In addition, drainage of thoracic duct lymphocytes after mucosal challenge resulted in a marked reduction of antibody-containing cells in the LP. Similarly, Fuhrman and Cebra found that memory B cells resided in GALT inductive sites after parenteral immunization (10). They demonstrated that comparable quantities of antigen-specific B-cell precursors were induced in PP after intraperitoneal and intraduodenal inoculations. Additionally, 50% of the clonal progeny derived from PP B cells of parenterally immunized animals secreted some IgA. Our studies extend these earlier observations by demonstrating that i.m. immunization induces IgA-committed memory B cells in GALT inductive sites which may undergo differentiation and migration upon mucosal challenge and result in enhanced production of virus-specific IgA by effector cells in the LP of the small intestine.
Recent studies of the expression of homing receptors on lymphocytes and vascular addressins on endothelial cells support the hypothesis that the anatomic location of antigenic stimulation may influence the homing pattern of B cells and, therefore, the location of memory B cells (8, 16, 27, 32). Although 40 to 50% of circulating antigen-specific B cells induced by parenteral inoculation express the mucosal homing receptor α4β7, virtually all express L-selectin (16, 27). The vascular addressin ligand for L-selectin, PNAd, is expressed by high endothelial venules (HEV) in PP and MLN, as well as peripheral lymph nodes (21, 29). PNAd is not expressed by HEV in LP (1, 21). In addition, the level of α4β7 expression by B cells may be lower after i.m. immunization than after oral immunization (12). Therefore, after i.m. immunization, activated B cells, including memory cells committed to IgA production, may preferentially home to tissues which express PNAd, such as PP, MLN, and peripheral lymph nodes. Additional studies examining the homing potential of activated B cells generated by i.m. inoculation are under way.
Finally, we examined the ability of i.m. inoculation to induce virus-specific memory B cells committed to IgG secretion in GALT. Intestinal and ILN lymphoid cultures were established 0, 2, 4, 6, and 8 days after oral challenge of either i.m.-immunized or naïve mice, as described above. We found that virus-specific IgG production by LP lymphocytes after oral challenge of i.m.-immunized mice was enhanced compared with that of unimmunized mice. Enhanced production of virus-specific IgG was first evident in PP of i.m.-immunized animals 2 days after challenge (P < 0.05) (Fig. 2A) and in LP 6 days after challenge (P < 0.0005) (Fig. 2C). Thus, similar to virus-specific IgA responses, enhanced intestinal virus-specific IgG production was first detected in GALT inductive sites and subsequently was found in effector sites. The role of IgG in protection of mucosal surfaces, however, remains unclear. IgG has been shown to migrate across epithelial cells when it is cross-linked to polymeric IgA through a multivalent antigen (15), suggesting that IgG may contribute to mucosal protection through intracellular association with antigen and subsequent activation of complement. IgG may also protect mucosal surfaces through direct neutralization of viral infectivity (26).
Anamnestic B-cell responses have traditionally been thought to be quicker in onset, larger in magnitude, and higher in affinity than primary B-cell responses (28). We found that although i.m. immunization enhanced production of virus-specific IgA by LP lymphocytes upon oral challenge, the period of time from activation and differentiation of memory B cells in PP and MLN to that of effector B cells in LP was not shortened. Because i.m. inoculation cannot hasten the onset of virus-specific IgA production by effector B cells in the LP, it is unlikely to provide complete protection against mucosal infections characterized by short incubation periods (e.g., rotavirus). Additional studies are required to define the immunologic mechanisms by which parenteral immunization induces protection from mucosal pathogens.
Acknowledgments
This work was supported in part by grants 1 K08 AI01367 (S.E.C.) and 1 R01 AI26251 (P.A.O.) from the National Institutes of Health.
We thank Kurt Brown, Jeffrey Brubaker, Fred Clark, and Charlotte Moser for helpful discussions.
REFERENCES
- 1.Berlin C, Bargatze R F, Campbell J J, von Andrian U H, Szabo M C, Hasslen S R, Nelson R D, Berg E L, Erlandsen S L, Butcher E C. Alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 2.Brown K A, Moser C A, Speaker T J, Khoury C A, Offit P A. Enhancement by microencapsulation of rotavirus-specific intestinal immune responses in mice assessed by enzyme-linked immunospot assay and intestinal fragment culture. J Infect Dis. 1995;171:1334–1338. doi: 10.1093/infdis/171.5.1334. [DOI] [PubMed] [Google Scholar]
- 3.Buscho R F, Perkins J C, Knopf H L S, Kapikian A Z, Chanock R M. Further characterization of the local respiratory tract antibody response induced by intranasal instillation of the inactivated rhinovirus 13 vaccine. J Immunol. 1972;108:169–177. [PubMed] [Google Scholar]
- 4.Cebra J J, Cuff C F, Rubin D H. Relationship between alpha/beta T cell receptor/CD8+ precursors for cytotoxic T lymphocytes in the murine Peyer’s patches and the intraepithelial compartment probed by oral infection with reovirus. Immunol Res. 1991;10:321–323. doi: 10.1007/BF02919715. [DOI] [PubMed] [Google Scholar]
- 5.Coffin S E, Moser C A, Cohen S, Clark H F, Offit P A. Immunologic correlates of protection against rotavirus challenge after intramuscular immunization of mice. J Virol. 1997;71:7851–7856. doi: 10.1128/jvi.71.10.7851-7856.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coffin S E, Klinek M, Offit P A. Induction of virus-specific antibody production by lamina propria lymphocytes following intramuscular inoculation with rotavirus. J Infect Dis. 1995;172:874–878. doi: 10.1093/infdis/172.3.874. [DOI] [PubMed] [Google Scholar]
- 7.Conner M E, Crawford S E, Barone C, Estes M K. Rotavirus vaccine administered parenterally induces protective immunity. J Virol. 1993;67:6633–6641. doi: 10.1128/jvi.67.11.6633-6641.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doherty P C. Anatomic environment as a determinant in viral immunity. J Immunol. 1995;155:1023–1027. [PubMed] [Google Scholar]
- 9.Friedman M G, Phillip M, Dagan R. Virus-specific IgA in serum, saliva, and tears of children with measles. Clin Exp Immunol. 1989;75:58–63. [PMC free article] [PubMed] [Google Scholar]
- 10.Fuhrman J A, Cebra J J. Special features of the priming process for a secretory IgA response. J Exp Med. 1981;153:534–544. doi: 10.1084/jem.153.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry J L, Jaikaran E S. A study of polio vaccination in infancy: excretion following challenge with live virus by children given killed or living polio vaccine. J Hyg. 1966;64:105–120. doi: 10.1017/s0022172400040389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmgren J, Svennerholm A-M, Ouchterlony Ö, Andersson Å, Wallerström G, Westerberg-Berndtsson U. Antitoxin immunity in experimental cholera: protection, and serum and local antibody responses in rabbits after enteral and parenteral immunization. Infect Immun. 1975;12:1331–1340. doi: 10.1128/iai.12.6.1331-1340.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howe H A. The quantitation of poliomyelitis virus in the human alimentary tract with reference to coexisting levels of homologous serum neutralizing antibody. Am J Hyg. 1962;75:1–17. doi: 10.1093/oxfordjournals.aje.a120227. [DOI] [PubMed] [Google Scholar]
- 14.Issekutz T B. Kinetics of cytotoxic lymphocytes in efferent lymph from single lymph nodes following immunization with vaccinia virus. Clin Exp Immunol. 1984;56:515–523. [PMC free article] [PubMed] [Google Scholar]
- 15.Kaetzel C S, Robinson J K, Lamm M E. Epithelial transcytosis of monomeric IgA and IgG cross-linked through antigen to polymeric IgA. J Immunol. 1994;152:72–76. [PubMed] [Google Scholar]
- 16.Kantele A, Kantele J M, Savilahti E, Westerholm M, Arvilommi H, Lazarovits A, Butcher E C, Makela P H. Homing potential of circulating lymphocytes in humans depends on the site of activation. J Immunol. 1997;158:574–579. [PubMed] [Google Scholar]
- 17.Kaul T N, Welliver R C, Wong D T, Udwadia R A, Riddlesberger K, Ogra P L. Secretory antibody response to respiratory syncytial virus infection. Am J Dis Child. 1981;135:1013–1016. doi: 10.1001/archpedi.1981.02130350017007. [DOI] [PubMed] [Google Scholar]
- 18.Keren D F, McDonald R A, Carey J L. Combined parenteral and oral immunization results in an enhanced mucosal immunoglobulin A response to Shigella flexneri. Infect Immun. 1988;56:910–915. doi: 10.1128/iai.56.4.910-915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khoury C A, Brown K A, Kim J E, Offit P A. Rotavirus-specific intestinal immune response in mice assessed by enzyme-linked immunospot assay and intestinal fragment culture. Clin Diagn Lab Immunol. 1994;1:722–728. doi: 10.1128/cdli.1.6.722-728.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNeal M M, Sheridan J F, Ward R L. Active protection against rotavirus infection of mice following intraperitoneal immunization. Virology. 1992;191:150–157. doi: 10.1016/0042-6822(92)90176-p. [DOI] [PubMed] [Google Scholar]
- 21.Michie S A, Streeter P R, Bolt P A, Butcher E C, Picker L J. The human peripheral lymph node vascular addressin. Am J Pathol. 1993;143:1688–1698. [PMC free article] [PubMed] [Google Scholar]
- 22.Offit P A, Cunningham S L, Dudzik K I. Memory and distribution of virus-specific cytotoxic T lymphocytes (CTLs) and CTL precursors after rotavirus infection. J Virol. 1991;65:1318–1324. doi: 10.1128/jvi.65.3.1318-1324.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierce N F, Gowans J L. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975;142:1550–1563. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierce N F, Sack R B, Sircar B K. Immunity to experimental cholera: enhanced duration of protection after sequential parenteral-oral administration of toxoid to dogs. J Infect Dis. 1977;135:888–896. doi: 10.1093/infdis/135.6.888. [DOI] [PubMed] [Google Scholar]
- 25.Pierce N F, Cray W C, Jr, Sircar B K. Induction of a mucosal antitoxin response and its role in immunity to experimental canine cholera. Infect Immun. 1978;21:185–193. doi: 10.1128/iai.21.1.185-193.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prince G A, Hemming V G, Horswood R L, Baron P A, Murphy B R, Chanock R M. Mechanism of antibody-mediated viral clearance in immunotherapy of respiratory syncytial virus infection of cotton rats. J Virol. 1990;64:3091–3092. doi: 10.1128/jvi.64.6.3091-3092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quiding-Jabrink M, Nordstrom I, Granstrom G, Kilander A, Jertborn M, Butcher E C, Lazarovits A I, Holmgren J, Czerkinsky C. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunization. J Clin Invest. 1997;99:1281–1286. doi: 10.1172/JCI119286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sprent J. T and B memory cells. Cell. 1994;76:315–322. doi: 10.1016/0092-8674(94)90338-7. [DOI] [PubMed] [Google Scholar]
- 29.Streeter P R, Rouse B T, Butcher E C. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988;107:1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svennerholm A-M, Lange S, Holmgren J. Correlation between intestinal synthesis of specific immunoglobulin A and protection against experimental cholera in mice. Infect Immun. 1978;21:1–6. doi: 10.1128/iai.21.1.1-6.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Velazquez F R, Matson D O, Calva J J, Guerrero L, Morrow A L, Carter-Campbell S, Glass R I, Esters M K, Pickering L K, Ruiz-Palacios G M. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med. 1996;335:1022–1028. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 32.Williams M B, Butcher E C. Homing of naive and memory T lymphocyte subsets to Peyer’s patches, lymph nodes, and spleen. J Immunol. 1997;159:1746–1752. [PubMed] [Google Scholar]