Abstract
Previous studies have shown that human immunodeficiency virus type 1 (HIV-1) exploits dendritic cells (DC) to replicate and spread among CD4+ T cells. To explain the predominance of non-syncytium-inducing (NSI) over syncytium-inducing (SI) strains during the initial viremia of HIV, we investigated the ability of blood monocyte (Mo)-derived DC to transmit HIV-1 to CD4+ cells of the monocytoid lineage. First, we demonstrate that in our system, DC are able to transmit NSI strains, but not SI strains, of HIV-1 to fresh blood Mo and to Mo-derived macrophages (MDM). To establish a productive infection, a 10-fold-lower amount of virus was necessary for DC-mediated transmission of HIV-1 to Mo than in case of cell-free infection. Second, immature CD83− DC (imDC) transmit virus to Mo and MDM with higher efficacy compared to mature CD83+ DC (maDC); this finding is in contrast to data previously obtained with CD4+ T cells. Third, maturation from imDC to maDC efficiently silenced expression of β2-integrins CD11b, CD11c, and CD18 by maDC. Moreover, monoclonal antibody against CD18 inhibited transmission of HIV-1 from imDC to Mo. We propose that the adhesion molecules of the CD11/CD18 family, involved in cell-cell interactions of DC with the microenvironment, may play a major role in imDC-mediated HIV-1 infection of Mo and MDM.
Dendritic cells (DC) play a critical role in the activation of primary immune responses since they are specialized to present antigens to naive T cells in vivo (35). Immature DC (imDC) capture antigen at peripheral sites such as skin, airways, mucosa, or gut and then migrate via afferent lymph to T-cell areas in secondary lymphoid organs. During this homing process, imDC loose their capacity to process antigen but upregulate costimulatory molecules (CD86, CD80, ICAM-1, etc.) on their surfaces and display the mature phenotype. These mature DC (maDC) are able to select antigen-specific T cells from the circulating pool and stimulate them (8, 35).
Since Langerhans cells (i.e., imDC in the epidermis and mucosa) express CD4, the major cellular receptor for human immunodeficiency virus type 1 (HIV-1), their presence in the urogenital mucosa gives rise to the possibility that DC are involved in sexual transmission of HIV infection (10, 24, 33). In rhesus monkeys, it has been observed that simian immunodeficiency virus can be efficiently transferred to lymph nodes within 2 days following mucosal inoculation of the virus (20, 34). Evidence that DC could be directly involved in HIV transmission was deduced from the observation that HIV readily associates with DC and caused DC and T cells to fuse and generate heterokaryons (or syncytia) which are sites of vigorous viral replication in vitro and in vivo (6, 26, 27).
As both macrophages and DC of mucosal epithelia are among the first cells to be associated with virus following sexual contact involving HIV (16, 22, 23, 39), we investigated whether DC are also capable of transmitting HIV to CD4+ cells of monocytoid lineage. For this purpose, we used monocyte (Mo)-derived imDC and maDC populations (3, 28, 40) to study HIV transmission to Mo and Mo-derived macrophages (MDM) in vitro.
MATERIALS AND METHODS
Cell isolation.
Peripheral blood was obtained from the local blood bank as standard buffy coat preparations from normal healthy donors. Peripheral blood mononuclear cells (PBMC) were prepared by centrifugation on a Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density gradient. Mo were separated from PBMC by adherence on gelatin-coated petri dishes as described previously (18). Briefly, 10 ml of PBMC suspension (3 × 106 to 6 × 106 cells/ml) in RPMI 1640–10% fetal calf serum (FCS)–3% human serum was incubated on gelatin-coated (2% gelatin in water) petri dishes for 40 min at 37°C. Nonadherent cells were aspirated, and dishes were washed three times with prewarmed RPMI 1640–10% FCS (RPMI-FCS). Adherent cells (96 to 99% CD14+ Mo) were incubated for 10 min in RPMI-FCS with 5 mM EDTA. Detached Mo were aspirated, washed, and resuspended in RPMI-FCS. To obtain MDM, Mo were cultured for 5 to 7 days in RPMI-FCS supplemented with 5% pooled human AB serum (RPMI-FCS-HS).
Generation of DC from CD14+ Mo.
CD14+ cells isolated by the method described above were cultured at a cell density of 106 cells/ml in six-well plates (Costar, Cambridge, Mass.) in RPMI-FCS at 37°C in a humidified CO2-containing atmosphere. For DC differentiation, 1,000 U of interleukin-4 (IL-4; PromoCell, Heidelberg, Germany) per ml and 800 U of granulocyte-macrophage colony-stimulating factor (GM-CSF; Peprotech, Rocky Hill, N.J.) per ml were added. The cultures were fed with fresh RPMI-FCS and IL-4–GM-CSF every third day, and cells were monitored by light microscopy. On day 6, cells were harvested, washed, and transferred to new six-well plates. Fresh medium with cytokines was added, and cells were cultivated for further 2 days in the presence or absence of stimuli for maturation. Cells cultivated for 8 days in the presence of IL-4 and GM-CSF without stimuli showed the imDC phenotype and did not express CD83. Full differentiation was achieved by adding Mo-conditioned medium (20%) for the last 48 h of the culture period, as described previously (28). Resulting cells expressed the maDC phenotype as revealed by fluorescence-activated cell sorting analysis.
Flow cytometry analysis.
DC differentiation was assessed by indirect immunofluorescence staining followed by flow cytometry analysis. Primary monoclonal antibodies (MAb) used included anti-HLA-DR and anti-CD23 (clones DK22 and MHM6, respectively; Dako, Glostrup, Denmark), immunoglobulin G1 (IgG1) and IgG2a isotype control, anti-CD1a, anti-CD11a, anti-CD11b, and anti-CD18 (clones 1B7.11, S-S.1, OKT-6, TS1/22, OKM-1, and TS1/18, respectively; American Type Culture Collection, Manassas, Va.), anti-CD83 (clone HB15; kindly provided by T. F. Tedder, Durham, N.C.), anti-CD4, anti-CD11c, and anti-CD86 [clones RPA-T4, B-ly6, and 2331(FUN-1); PharMingen, San Diego, Calif.). MAb were detected with fluorescein isothiocyanate-labeled sheep F(ab′)2 polyclonal antibodies to mouse IgG-IgM (An der Grub, Kaumberg, Austria). Flow cytometry analysis was performed with a FACScan (Becton Dickinson, San Jose, Calif.) instrument and Lysis II software.
HIV-1 isolates and infection of cultures.
Primary non-syncytium-inducing (NSI) isolates 92TH024E (TH) and 92RW021A (RW) were from the NIH AIDS Research and Reference Reagent Program (Rockville, Md.). Low-passage-number NSI isolate 676 was kindly provided by P. U. Cameron (Fearflaine, Australia). Syncytium-inducing (SI) strains IIIB and RF, as well as NSI strains Ba-L and SF162, were from MRC (Hertfordshire, England).
Virus stocks were prepared by expansion of inoculum in IL-2 (20 U/ml)-activated PBMC from healthy HIV-1-negative donors. Stocks of different isolates used in one experiment were obtained from PBMC of the same donor. Supernatants were clarified by centrifugation and sterile filtered, and virus was quantified by capture enzyme-linked immunosorbent assay (ELISA) for HIV-1 p24 antigen (p24 Ag). For the first sample, virus was lysed with 1% Nonidet P-40 (NP-40) and the total level of p24 Ag was determined. Virus-free p24 Ag was measured in the second sample in the absence of detergent. Virus stocks containing more than 5% of virus-free p24 Ag were excluded from the following experiments. The infectious dose was determined with PBMC from different donors, and 10 ng of virus-associated p24 Ag per ml corresponded to 20 to 100 50% tissue culture infective doses (TCID50) for all isolates used.
Pulsing of DC with HIV.
Day 8 imDC or maDC were washed and exposed for 2 h at 37°C to different virus concentrations in RPMI-FCS at a cell density of 106 cells/ml. Subsequently, DC were washed with ice-cold RPMI-FCS to remove unbound virus. Aliquots of HIV-loaded cells were lysed with 1% NP-40 in RPMI-FCS, and p24 Ag was determined by capture ELISA.
Infection of Mo and MDM.
Mo and MDM were incubated in RPMI-FCS (106 cells/ml) with different virus concentrations for 2 h at 37°C. Afterwards, the medium was aspirated, and cells were washed three times with RPMI-FCS to remove all free virus and incubated for 14 days in RPMI-FCS-HS at 37°C.
Cocultivation of HIV-loaded DC with Mo and MDM: dose requirement and kinetic experiments.
HIV-loaded DC were added to Mo and MDM (105 cells/well) in RPMI-FCS-HS on 96-well plates at a ratio of 1:10 (104 DC per 105 Mo or MDM); this ratio was optimal for transmission as determined by preliminary experiments. After 14 days, culture supernatants were harvested, and viral p24 Ag was evaluated as described below.
For kinetic experiments, imDC, Mo, and MDM were pulsed with 10 ng of p24 Ag of NSI strain Ba-L per ml for 2 h at 37°C. Afterwards, cells were washed three times to remove unbound virus and cultivated as described above. Viral p24 Ag was measured in supernatants harvested on days 4, 7, 10, 14, and 21.
HIV-1 p24 Ag ELISA; virus input and output.
The p24 Ag ELISA was developed at the Institute of Applied Microbiology, Vienna, Austria, and was performed as described elsewhere (5). Briefly, a mouse MAb against HIV-1 p24 Ag was bound to an ELISA plate (200 ng/well), and lysed samples (diluted 1:1 with 1% NP-40) were added for 1 h at room temperature. Bound HIV-1 p24 Ag was detected with a second biotinylated anti-p24 MAb followed by a streptavidin–β-galactosidase conjugate. Color reaction was developed with resorufin-β-d-galactopyranoside substrate (Sigma, St. Louis, Mo.), and the optical density was measured on an ELISA microplate reader at 550 nm. p24 amounts were calculated from a standard curve by using recombinant HIV-1 p24 Ag. The detection limit of this system is ≤1 pg of p24 Ag per ml, with no detectable cross-reaction with other proteins.
To quantify the efficiency of infection, the virus input was defined as the amount of p24 Ag associated with cells added to each culture per ml (106 Mo or MDM per ml; 105 imDC or maDC per ml). Virus output represents the amount of HIV-1 p24 Ag per milliliter detected in culture supernatant at the end of incubation and is expressed as a multiplicity of virus input.
Immunocytochemical staining of cells in cultures of HIV-pulsed DC with Mo.
For immunostaining, Ba-L-loaded DC and Mo were cultured on eight-well Lab-Tek chamber slides (Nalge Nunc International, Naperville, Ill.). For these experiments, we used maDC which were morphologically and phenotypically stable during cultivation. After the indicated incubation period, medium and nonadherent cells were aspirated from the slides and washed three times with the prewarmed medium, and adherent cells were fixed in methanol-acetone (4:1) at 4°C for 30 min. Immunostaining was performed for 60 min at room temperature with biotinylated anti-HIV-1 p24 Ag MAb (clone 37G12) and anti-HLA-DR MAb (clone DK22). Slides were developed with avidin-β-galactosidase conjugate–5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) substrate for detection of p24 Ag and/or with rabbit anti-mouse Ig-alkaline phosphatase conjugate–Fast Red substrate for the detection of HLA-DR.
Inhibition of DC-mediated HIV-1 transmission by MAb. All MAb used were hybridoma supernatants stored sterile without preservatives. IgG2a isotype control, anti-CD11a, anti-CD11b, and anti-CD18 (clones S-S.1, TS1/22, OKM-1, and TS1/18, respectively) were used at 10 μg/ml in RPMI-FCS.
HIV-loaded DC were incubated with MAb for 2 h on ice, washed two times with RPMI-FCS, and added at the ratio 1:10 to Mo. DC-Mo cultures were incubated for 14 days at 37°C, and p24 Ag was measured in culture supernatants by ELISA.
RESULTS
Generation of Mo-derived imDC and maDC.
Purified CD14+ Mo were cultured in RPMI-FCS supplemented with GM-CSF and IL-4 for 8 days. During this incubation period, a homogeneous nonadherent cell population with typical DC morphology and phenotype developed. The small percentage of residual adherent cells in DC cultures, most likely macrophages, was neglected, and only the nonadherent cell population was used. Flow cytometry analysis revealed that in GM-CSF–IL-4 medium-cultivated cells express low levels of the costimulatory molecule CD86 and of the maDC marker CD83, confirming the immature phenotype (Fig. 1A).
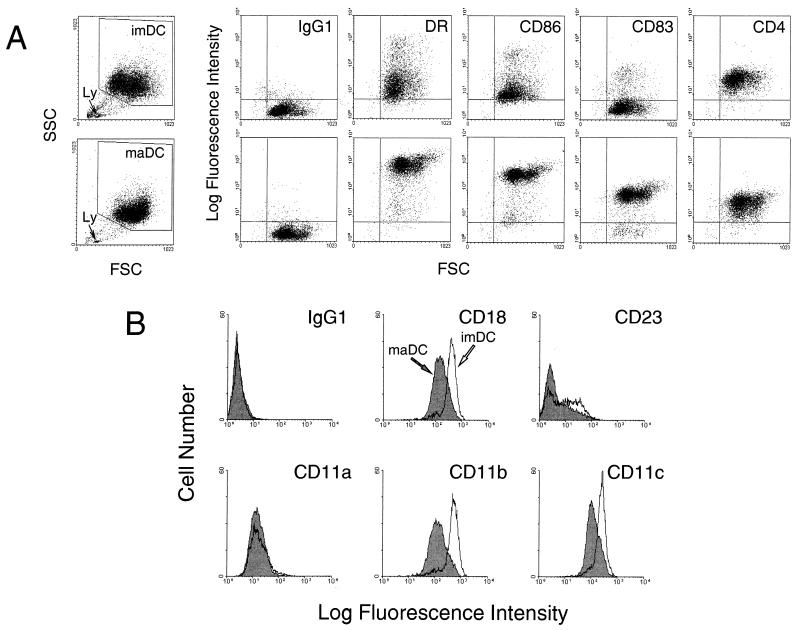
FIG. 1.
(A) Comparative immunofluorescence analysis of surface marker expression by imDC (top) and maDC (bottom). Arrows indicate lymphocytic contamination of imDC and maDC preparations (Ly). SSC, side scatter; FSC, forward scatter. Overlay histograms (B) show expression of adhesion molecules by imDC and maDC.
To convert imDC into maDC, cells kept 6 days in culture were washed and replated in GM-CSF–IL-4 medium supplemented with 20% Mo-conditioned medium for the next 2 days of culture (28). DC generated in the presence of Mo-conditioned medium displayed a fully mature DC phenotype (Fig. 1A), as demonstrated by CD86, CD83, and HLA-DR expression. With regard to the HIV-1 receptor CD4, similar levels of CD4 were expressed on imDC and maDC.
imDC exhibited significantly higher median fluorescence intensities for CD11b (threefold), CD11c (twofold), and CD18 (more than twofold) markers than maDC (Fig. 1B). In contrast, the levels of expression of CD11a on imDC and maDC were similar. Additionally, imDC expressed significantly larger amounts of CD23 than maDC (Fig. 1B); this low-affinity receptor for IgE was reported to bind CD11b and CD11c on Mo (21).
Transmission of HIV-1 to Mo and MDM by imDC and maDC: dose requirement, kinetics, and comparison with cell-free infection.
To investigate whether DC transmit captured virus to Mo and MDM, we explored dose dependence and infection kinetics in vitro. Dose dependence experiments with NSI strain Ba-L showed a higher susceptibility of fully differentiated MDM (Fig. 2B) than of Mo (Fig. 2A) to HIV-1 infection; i.e., less virus was required for the productive infection of MDM. However, when Mo and MDM cultures were infected by cocultivation for 14 days with HIV-loaded imDC, Mo and MDM were susceptible to HIV-1 infection to the same degree. The amount of virus necessary for effective infection of Mo by imDC was 10-fold lower than in the case of cell-free virus (Fig. 2A). In comparison to imDC, maDC transmitted virus to Mo and MDM with lower efficiency; in the case of MDM, maDC-mediated infection was even less effective than that by cell-free virus pulse (Fig. 2B). We only rarely detected low virus production in imDC and maDC cultures alone. Of note, the cocultivation of T cells for 14 days with HIV-loaded maDC resulted in 30-fold-higher p24 Ag output compared to Mo and MDM (not shown).
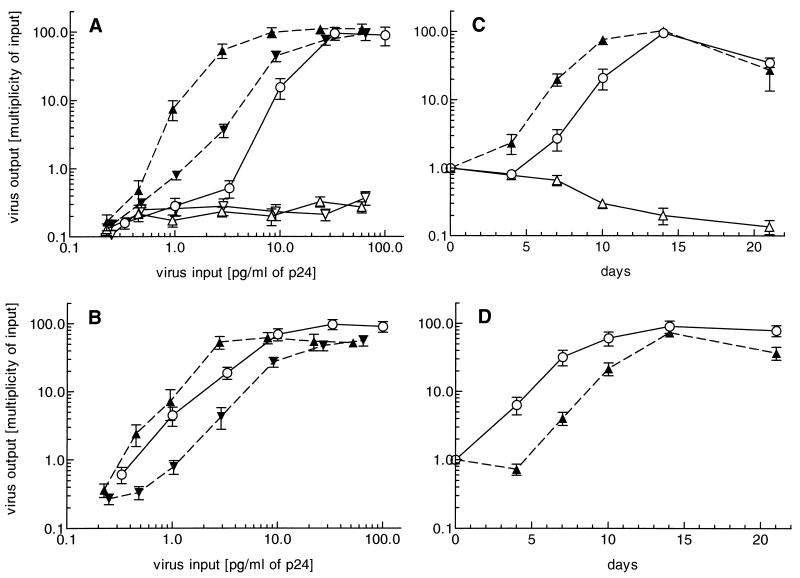
FIG. 2.
Dose requirement of NSI strain Ba-L for productive infection of Mo (A) and MDM (B) cultures. Virus input and output were measured as described in Materials and Methods. (A) Direct infection of Mo (circles), imDC (open triangles), and maDC (open inverted triangles), as well as DC-mediated infection of Mo with Ba-L captured by imDC (closed triangles) or maDC (closed inverted triangles) cultivated at a DC/Mo ratio of 1:10, was monitored after 14 days of incubation. (B) Direct infection of MDM (circles) and imDC-mediated (triangles) and maDC-mediated (inverted triangles) infection of MDM at a DC/MDM ratio of 1:10. The results are expressed as means ± standard errors of the means of data obtained from four independent experiments (donors) of each culture performed in duplicate. (C) Time course of direct infection of Mo (circles) and imDC (open triangles) with strain Ba-L and infection of Mo cultivated with Ba-L-pulsed imDC (closed triangles). (D) Kinetics of direct (circles) and imDC-mediated (triangles) infection of MDM. Mo, MDM, and imDC were pulsed with 10 ng of NSI strain Ba-L per ml, and viral p24 Ag was measured in culture supernatants harvested on days 4, 7, 10, 14, and 21. A typical result obtained with 1 of 12 donors is presented.
For determination of replication kinetics, the optimal virus dose of 10 ng of virus-associated p24 Ag per ml was used to load Mo, MDM, and imDC. Culture supernatants were collected on days 4, 7, 10, 14, and 21 after infection and assayed for extracellular p24 Ag production. The maximum p24 Ag level was observed at day 14 for cell-free virus as well as for DC-mediated infection of Mo and MDM (Fig. 2C and D). imDC-mediated infection of Mo was established more rapidly than infection with cell-free virus: typically HIV replication in Mo infected via imDC was detected 3 days earlier than in case of direct virus pulse (Fig. 2C). In contrast, the very effective infection of MDM by using cell-free Ba-L isolate could not be further enhanced by applying HIV-loaded imDC: HIV infection by means of imDC was slower in MDM cultures (Fig. 2D) but caught up at day 14.
Comparison of different HIV-1 strains with respect to the ability to infect Mo and MDM directly or via imDC-mediated transmission.
A panel of NSI and SI isolates was used to further explore the ability of imDC to mediate HIV-1 infection of Mo and MDM cultures in comparison with cell-free virus pulse. The highest quantity of p24 Ag was detected in cultures infected with the prototype NSI strain Ba-L. NSI isolates SF162, 676, and TH replicated less effectively in Mo. Similarly to the Ba-L isolate, imDC-mediated infection of Mo resulted in up to 10-times-higher p24 Ag levels compared to cell-free virus pulse at the same viral input (Table 1). Interestingly, the primary isolate RW infected MDM only if used as cell-free virus. SI strains RF and IIIB in the amounts used could replicate in neither Mo nor MDM cultures, irrespective of being used as cell-free virus or captured by imDC.
TABLE 1.
Abilities of different HIV isolates to infect Mo and MDM: comparison of infection with cell-free virus and with virus captured by imDCa
| Virus isolate | Phenotype | Virus production
|
|||
|---|---|---|---|---|---|
| Cell-free HIV-1
|
imDC + HIV-1
|
||||
| Mo | MDM | Mo | MDM | ||
| Ba-L | NSI | +++ | +++ | +++ | +++ |
| SF162 | NSI | ++ | +++ | ++ | ++ |
| 676 | NSI | + | ++ | ++ | ++ |
| TH | NSI | + | ++ | ++ | ++ |
| RW | NSI | − | + | − | − |
| RF | SI | − | − | − | − |
| IIIB | SI | − | − | − | − |
Mo, MDM, and imDC were exposed to virus stocks (10 ng of virus-associated p24 Ag/ml) of different HIV-1 isolates for 2 h at 37°C and washed three times to remove free virus. Amount of virus absorbed by cells was determined by p24 Ag ELISA (virus input). After 14 days of incubation, the amount of HIV-1 p24 Ag was determined in culture supernatants (virus output). Virus production was determined as a multiplicity of virus input as follows: −, output lower than virus input; +, output 1- to 5-fold higher than virus input; ++, output 5- to 50-fold higher than virus input; +++, output >50-fold higher than virus input.
Immunocytochemical staining of infected cells in cocultures of HIV-loaded DC and Mo.
To investigate sites of viral replication in cocultures of DC with Mo, we used immunocytochemical staining of HIV-1 p24 Ag in cells cultivated on plastic chamber slides. maDC were loaded with Ba-L isolate (10 ng of virus-associated p24 Ag per ml) and added to Mo cultures in the ratio of 1:10. Mock-infected cultures and untreated Mo and maDC were used as controls.
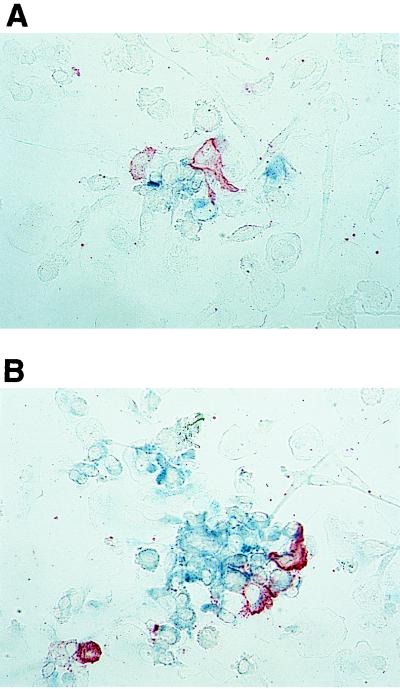
In this system, we were able to detect the first p24 Ag-positive cells after 5 days of coincubation. Viral p24 Ag was almost exclusively observed within Mo attached to or near DC. Of note, DC did not stain for HIV-1 p24 Ag (Fig. 3A). On day 7, p24 Ag-positive cells formed spots of infection. In addition, cells distant from DC were also stained with anti-p24 Ag MAb (Fig. 3B).
FIG. 3.
Immunocytochemical determination of DC-mediated HIV infection of Mo. Mo were incubated in the presence of maDC pretreated with macrophagetropic strain Ba-L (10 ng of virus-associated p24 Ag per ml) for 5 (A) and 7 (B) days. Cells were then fixed and slides were developed with biotinylated anti-p24 Ag MAb–avidin-β-galactosidase conjugate–X-Gal substrate for the detection of p24 Ag (blue color) and with anti-HLA-DR MAb–rabbit anti-mouse Ig-alkaline phosphatase conjugate–Fast Red substrate for the detection of DC (red). (A) Infected cells appeared on day 5 in direct contact with or near DC. DC were not stained with anti-p24 Ag MAb. (B) After 7 days, infected Mo formed spots of infection around DC. The first p24 Ag-positive Mo distant from DC become detectable. Original magnification, ×400.
Uptake of NSI and SI strains by imDC and maDC.
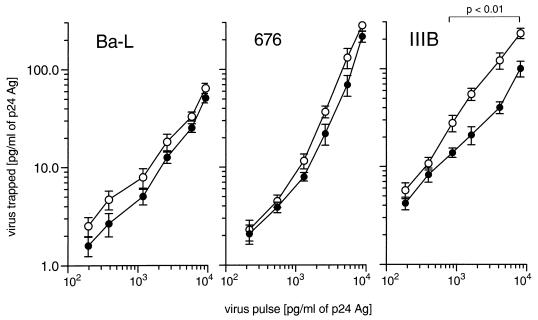
To explain the different efficacies of imDC and maDC for virus transmission to Mo and MDM, we quantified the capture capacities of these DC populations for different HIV-1 strains. imDC and maDC were pulsed with different amounts of the NSI isolates Ba-L or 676 or SI isolate IIIB. Figure 4 shows that imDC regularly trapped more virus than maDC. Except for SI strain IIIB, no significant difference in the trapping capacity was observed for NSI isolates Ba-L and 676. Comparing the efficiency of uptake among the three virus strains, we found that incubation of imDC with 1,000 pg of virus-associated p24 per ml results in the uptake of about 6, 8, and 20 pg of p24 per ml for Ba-L, 676, and IIIB, respectively. Similar results were obtained with all NSI isolates tested (not shown).
FIG. 4.
Pulsing of imDC (open symbols) and maDC (closed symbols) with NSI and SI isolates of HIV-1. DC were exposed to different amounts of the NSI isolates Ba-L and 676 or the SI strain IIIB for 2 h at 37°C.
Inhibition of DC-mediated HIV infection by MAb.
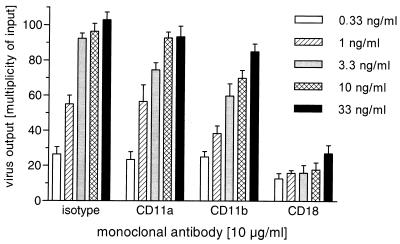
imDC were loaded with the Ba-L isolate, preincubated with MAb, and added to Mo cultures. Figure 5 shows that anti-CD18 MAb caused up to a five-fold decrease of virus replication, within a broad range of the virus amounts used. In addition, we observed an inhibition (∼20%) of p24 Ag production in cultures of imDC pretreated with anti-CD11b MAb. Isotype control and anti-CD11a MAb could not significantly influence the virus transmission from imDC to Mo.
FIG. 5.
Inhibition of imDC-mediated infection of Mo by MAb. imDC were loaded for 2 h at 37°C with different amounts of NSI strain Ba-L (from 0.33 to 33 ng of virus-associated p24 Ag per ml) and subsequently incubated for 2 h at 4°C with different MAb. Finally, cells were washed and added at the ratio of 1:10 to Mo cultures. Virus output was determined by p24 Ag ELISA in the culture supernatants taken on day 14. The results are the means ± standard errors of the means of data from three independent experiments of each culture performed in duplicate.
DISCUSSION
In this study, we used a panel of NSI and SI isolates to demonstrate the ability of DC to mediate transmission of NSI strains of HIV-1 to Mo and MDM in vitro. Moreover, our results suggest that DC may support virus replication in these cells.
In contrast to other studies, we used Mo-derived DC that can be derived directly from fresh isolated blood Mo under the influence of GM-CSF and IL-4 (25, 29). These cells expressed typical DC markers on their surfaces and exhibited the immature phenotype. In addition, when imDC were cultured with an appropriate combination of GM-CSF–IL-4 and Mo-conditioned medium (3, 28), they expressed high levels of HLA class II, CD86, and CD83—the specific markers of maDC.
When imDC and maDC were loaded with NSI or SI strains of HIV-1 and further cultivated in culture medium (i.e., without cytokine supplementation), we could only rarely observe p24 Ag production. In contrast, it has been reported that different populations of DC are susceptible to direct HIV-1 infection (2, 7, 36). Experiments carried out in our laboratory have shown that DC produced large amounts of virus after 7 days of cultivation only in the presence of IL-4 and GM-CSF (15a). Therefore, we conclude that productive infection of DC strongly depends on the culture conditions (4).
When Mo and MDM were infected with cell-free virus stock of NSI strain Ba-L, vigorous virus infection occurred. Dose requirement and kinetics studies showed that MDM were more susceptible to HIV-1 infection than fresh Mo (14, 32). In contrast to the results obtained with cell-free virus, transmission experiments with HIV-pulsed imDC demonstrated that for productive infection of Mo a 10-fold-lower viral input was necessary. Comparing imDC and maDC for transmission capacity, we found that the latter were less effective, although levels of uptake of NSI strains by imDC and maDC were not significantly different. These data raised the possibility that imDC were able not only to transmit the HIV infection to Mo and MDM but also to upregulate virus replication in Mo.
Kinetic studies have shown that HIV-1 infection of Mo and MDM was established within 7 to 10 days in cultures pulsed with NSI strain Ba-L. Peak viral production was detected after 14 days of culture. Cocultivation with HIV-1-exposed imDC led to infection of Mo earlier than in the case of a cell-free virus pulse. Conversely, imDC could not accelerate HIV infection in MDM cultures. These data correspond with the suggestion that imDC, in addition to delivering virus, exert a stimulatory effect on Mo.
To investigate the possibility that DC can be stimulated to produce virus upon cocultivation with Mo, we used immunocytochemical methods for staining infected cells in cultures of DC with Mo. The first infected cells were detected after 5 days, whereas spots of infection around the DC appeared after 7 days of cultivation. Furthermore, double staining revealed that Mo but not DC in these cultures were stained with anti-p24 Ag MAb. However, a level of viral replication below the detection limit of immunocytochemical staining cannot be excluded.
Using a panel of seven HIV-1 isolates, we investigated differences in transmission of NSI and SI strains via imDC to Mo and MDM. Although prototypic NSI strain Ba-L more readily infected Mo and MDM, NSI isolates SF162, 676, and TH also replicated well in Mo and MDM cultures. Of note, at the same viral input amount, up to 10-times-higher p24 Ag production was determined with imDC-mediated infection of Mo than in the case of a cell-free virus pulse. The primary NSI isolate RW infected MDM only. SI strains RF and IIIB at the inoculum quantity used could establish productive infection neither in Mo nor in MDM, irrespective of being used as cell-free virus or captured in imDC. However, an inoculum dose of 10 ng of virus-associated p24 Ag per ml corresponded to 50 and 100 TCID50 in the case of isolates RF and IIIB, as determined by using PBMC for the infection assay.
Requirements of NSI and SI isolates are different: chemokine receptor 5 (CCR5) functions as an HIV-1 coreceptor for NSI isolates (1, 9), whereas SI strains use the CXCR4 chemokine receptor as their main coreceptor (15, 31). DC were found to express both CCR5 and CXCR4 and thus should be equally permissive to NSI and SI isolates (2, 19, 38). Although Mo and MDM express both CCR5 and CXCR4, only CCR5 was functional in a fusion assay (2, 31, 38). Therefore, our results conform with the theoretical expectation.
Surprisingly, maDC were less effective in transmitting virus to Mo and MDM than imDC, although amounts of virus trapped by these DC populations after pulsing with HIV-1 were not significantly different. These data are in agreement with the fact that CD4 is expressed similarly on imDC and maDC. Since HIV-1 was shown to be taken up by macropinocytosis (3), viral particles may be internalized more rapidly by imDC than by maDC, which lost endocytic activity during the maturation (30).
Flow cytometry analysis revealed significantly reduced expression of CD11b, CD11c, and CD18 adhesion molecules on the surface of maDC in comparison with imDC. Since β2-integrins CD11b/CD18 and CD11c/CD18 were reported to serve as adhesion molecules in cell-cell interaction of DC with other cells in the microenvironment (11), we propose that imDC, expressing high levels of β2-integrins, bind to monocytic cells via intercellular adhesion molecule 1 (ICAM-1 or CD54, principal ligand of β2-integrins) with higher avidity than maDC, hence favoring HIV-1 transmission to target cells. In addition, about 50% of imDC express CD23; this low-affinity receptor for IgE was reported to bind and activate human Mo via CD11b/CD18 and CD11c/CD18 (21).
Therefore, we carried out inhibition experiments using MAb against β2-integrins to affect HIV-1 transmission of imDC to Mo. We showed that anti-CD18 MAb significantly inhibited infection of monocytes via imDC, suggesting the important role of β2-integrins in this transmission process. In addition, weak (∼20%) inhibition of HIV-1 transmission was observed with anti-CD11b MAb. These data correlate with the blocking capacity of the MAb used in our experiments, since OKM1 is directed against the epitope located in the C-terminal region and is known to only partially inhibit the binding of ligands to CD11b (12). Nevertheless, other molecules in addition to β2-integrins could be involved in the transmission of virus from DC to monocytic cells by strengthening attachment and/or activating the target cells.
Of note, our transmission experiment results are in contrast to studies done in systems exploring DC and T lymphocytes, which found that only maDC are able to transmit HIV infection to unstimulated CD4+ T cells (37). The mucosa of both symptomatic and asymptomatic HIV-infected individuals contain T cells and DC, two cell types that together support HIV-1 replication in vitro (16, 27). Infected cells in the mucosa are multinucleated syncytia which express viral p24 Ag and DC markers (16, 17). Besides DC, macrophages are also among the first cells to be infected following mucosal contact with HIV (22, 23, 39). In our system, imDC transmitted HIV-1 to Mo and MDM more effectively compared to maDC, suggesting that imDC could interact with monocytic cells whereas maDC favor interaction with T cells.
In conclusion, the findings presented here demonstrate that imDC, pulsed with NSI isolates of HIV-1, transmit the virus to fresh blood Mo and MDM with higher efficiency, as observed by direct infection of these cells with cell-free HIV virions. Moreover, we observed the inhibition of HIV transmission by MAb against β2-integrins. We are now investigating the molecular events leading to transmission of HIV and induction of virus replication in the presence of DC. These studies should yield additional insights into the role of DC and monocytes/macrophages in HIV pathogenesis.
ACKNOWLEDGMENTS
We thank Nikolaus Romani and Martin Thurnher for helpful discussion.
This work was supported by grant 6278 from Jubiläumsfond der Oestereichischen Nationalbank, Vienna, the Ludwig-Boltzmann Institute for AIDS Research, the BMWV Austria, and the State of Tyrol.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α MIP-β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Ayehunie S, Garcia-Zepeda E A, Hoxie J A, Horuk R, Kupper T S, Luster A D, Ruprecht R M. Human immunodeficiency virus-1 entry into purified blood dendritic cells through CC and CXC chemokine coreceptors. Blood. 1997;90:1379–1386. [PubMed] [Google Scholar]
- 3.Bender A, Sapp M, Schuler G, Steinman R M, Bhardwaj N. Improved method for generation of dendritic cells from nonoproliferating progenitors in human blood. J Immunol Methods. 1996;196:121–135. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 4.Blauvelt A, Asada H, Wayne Saville M, Klaus-Kovtun V, Altman D J, Yarchoan R, Katz S I. Productive infection of dendritic cells by HIV-1 and their ability to capture virus are mediated through separate pathways. J Clin Invest. 1997;100:2043–2053. doi: 10.1172/JCI119737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, Gruber G, Tauer C, Steindl F, Jungbauer A, Katinger H. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retroviruses. 1994;10:359–369. doi: 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- 6.Cameron P U, Freudenthal P S, Barker J M, Gezelter S, Inaba K, Steinman R M. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ cells. Science. 1992;257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 7.Canque B, Rosenzwejg M, Camus S, Yagello M, Bonet M-L, Guigon M, Gluckman J C. The effect of in vitro human immunodeficiency virus infection on dendritic-cell differentiation and function. Blood. 1996;88:4215–4228. [PubMed] [Google Scholar]
- 8.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 9.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di-Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 10.De Panfilis G, Manara G C, Ferrari C, Torresani C. Simultaneous colloidal gold immunoelectronmicroscopy labeling of CD1a, HLA-DR, and CD4 surface antigens of human epidermal Langerhans cells. J Invest Dermatol. 1988;91:547–552. doi: 10.1111/1523-1747.ep12476912. [DOI] [PubMed] [Google Scholar]
- 11.De Panfilis G, Soligo D, Manara G C, Ferrari C, Torresani C. Adhesion molecules on the plasma membrane of epidermal cells. I. Human resting Langerhans cells express two members of the adherence-promoting CD11/CD18 family, namely, H-Mac-1 (CD11b/CD18) and gp 150, 95 (CD11c/CD18) J Invest Dermatol. 1989;93:60–69. doi: 10.1111/1523-1747.ep12277352. [DOI] [PubMed] [Google Scholar]
- 12.Diamond M S, Garcia-Aguilar J, Bickford J K, Corbi A L, Springer T A. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol. 1993;120:1031–1043. doi: 10.1083/jcb.120.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 14.Fais S, Borghi P, Gherardi G, Logozzi M, Belardelli F, Gessani S. Human immunodeficiency virus type 1 induces cellular polarization, intercellular adhesion molecule-1 redistribution, and multinucleated giant cell generation in human primary monocytes but not in monocyte-derived macrophages. Lab Invest. 1996;75:783–790. [PubMed] [Google Scholar]
- 15.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 15a.Frank, I. Unpublished data.
- 16.Frankel S S, Wenig B M, Burke A P, Mannan P, Thompson L D R, Abbondanzo S L, Nelson A M, Pope M, Steinman R M. Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid. Science. 1996;272:115–117. doi: 10.1126/science.272.5258.115. [DOI] [PubMed] [Google Scholar]
- 17.Frankel S S, Tenner-Racz K, Racz P, Wenig B M, Hansen C H, Heffner D, Nelson A M, Pope M, Steinman R M. Active replication of HIV-1 at the lymphoepithelial surface of the tonsil. Am J Pathol. 1997;151:89–96. [PMC free article] [PubMed] [Google Scholar]
- 18.Freundlich B, Avdalovic N. Use of gelatin/plasma coated flasks for isolating human peripheral blood monocytes. J Immunol Methods. 1983;62:31–37. doi: 10.1016/0022-1759(83)90107-2. [DOI] [PubMed] [Google Scholar]
- 19.Granelli-Piperno A, Moser B, Pope M, Chen D, Wei Y, Isdell F, O’Doherty U, Paxton W, Koup R, Mojsov S, Bhardwaj N, Clark-Lewis I, Baggiolini M, Steinman R M. Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J Exp Med. 1996;184:2433–2438. doi: 10.1084/jem.184.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joag S V, Adany I, Li Z, Foresman L, Pinson D M, Wang C, Stephens E B, Raghavan R, Narayan O. Animal model of mucosally transmitted human immunodeficiency virus type 1 disease: intravaginal and oral deposition of simian/human immunodeficiency virus in macaques results in systemic infection, elimination of CD4+ T cells, and AIDS. J Virol. 1997;71:4016–4023. doi: 10.1128/jvi.71.5.4016-4023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lecoanet-Henchoz S, Gauchat J F, Aubry J P, Graber P, Life P, Paul-Eugene N, Ferrua B, Corbi A L, Dugas B, Plater-Zyberk C, Bonnefoy J-Y. CD23 regulates monocyte activation through a novel interaction with the adhesion molecules CD11b-CD18 and CD11c-CD18. Immunity. 1995;3:119–125. doi: 10.1016/1074-7613(95)90164-7. [DOI] [PubMed] [Google Scholar]
- 22.Miller C J, Vogel P, Alexander N J, Dandekar S, Hendrickx A G, Marx P A. Pathology and localization of simian immunodeficiency virus in the reproductive tract of chronically infected male rhesus macaques. Lab Invest. 1994;70:255–262. [PubMed] [Google Scholar]
- 23.Miller C J, Vogel P, Alexander N J, Sutjipto S, Hendrickx A G, Marx P A. Localization of SIV in the genital tract of chronically infected female rhesus macaques. Am J Pathol. 1992;141:655–660. [PMC free article] [PubMed] [Google Scholar]
- 24.Niedecken H, Lutz G, Bauer R, Kreysel H W. Langerhans cell as primary target and vehicle for transmission of HIV. Lancet. 1987;ii:519–520. doi: 10.1016/s0140-6736(87)91843-5. [DOI] [PubMed] [Google Scholar]
- 25.Peters J H, Giesler R, Thiele B, Steinbach F. Dendritic cells: from ontogenetic orphans to myelomonocytic descendants. Immunol Today. 1996;17:273–278. doi: 10.1016/0167-5699(96)80544-5. [DOI] [PubMed] [Google Scholar]
- 26.Pope M, Betjes M G H, Romani N, Hirmand H, Cameron P U, Hoffman L, Gezelter S, Schuler G, Steinman R M. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 27.Pope M, Gezelter S, Gallo N, Hoffman L, Steinman R M. Low levels of HIV-1 infection in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ T cells. J Exp Med. 1995;182:2045–2056. doi: 10.1084/jem.182.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romani N, Reider D, Heuer M, Ebner S, Kämpgen E, Eibl B, Niederwieser D, Schuler G. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Methods. 1996;196:137–151. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 29.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells maintained by granulocyte/macrophage colony stimulating-factor plus interleukin-4 and downregulated by tumor necrosis factor-α. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simmons G, Wilkinson D, Reeves J D, Dittmar M T, Beddows S, Weber J, Carnegie G, Desselberger U, Gray P W, Weiss R A, Clapham P R. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonza S, Maerz A, Uren S, Violo A, Hunter S, Boyle W, Crowe S. Susceptibility of human monocytes to HIV type 1 infection in vitro is not dependent on their level of CD4 expression. AIDS Res Hum Retroviruses. 1995;11:769–776. doi: 10.1089/aid.1995.11.769. [DOI] [PubMed] [Google Scholar]
- 33.Soto-Ramirez L E, Renjifo B, McLane M F, Marlink R, O’Hara C, Sutthent R, Wasi C, Vithayasai P, Vithayasai V, Apichartpiyakul C, Auewarakul P, Cruz V P, Chui D-S, Osathanondh R, Mayer K, Lee T-H, Essex M. HIV-1 Langerhans’ cell tropism associated with heterosexual transmission of HIV. Science. 1996;271:1291–1293. doi: 10.1126/science.271.5253.1291. [DOI] [PubMed] [Google Scholar]
- 34.Spira A I, Marx P A, Patterson B K, Mahoney J, Koup R A, Wolinsky S M, Ho D D. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinman R M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 36.Tsunetsugu-Yokota Y, Akagawa K, Kimoto H, Suzuki K, Iwasaki M, Yasuda S, Hausser G, Hultgren C, Meyerhans A, Takemori T. Monocyte-derived cultured dendritic cells are susceptible to human immunodeficiency virus infection and transmit virus to resting T cells in the process of nominal antigen presentation. J Virol. 1995;69:4544–4547. doi: 10.1128/jvi.69.7.4544-4547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weissman D, Li Y, Ananworanich J, Zhou L-J, Adelsberger J, Tedder T F, Baseler M, Fauci A S. Three populations of cells with dendritic morphology exist in peripheral blood, only one of which is infectable with human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1995;92:826–830. doi: 10.1073/pnas.92.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaitseva M, Blauvelt A, Lee S, Lapham C K, Klaus-Kovtun V, Mostowski H, Manischewitz J, Golding H. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat Med. 1997;3:1369–1375. doi: 10.1038/nm1297-1369. [DOI] [PubMed] [Google Scholar]
- 39.Zambruno G, Giannetti A, Bertazzoni U, Girolomoni G. Langerhans cells and HIV infection. Immunol Today. 1995;16:520–524. doi: 10.1016/0167-5699(95)80044-1. [DOI] [PubMed] [Google Scholar]
- 40.Zhou L-J, Tedder T F. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–2592. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]