Abstract
Objective:
Vital exhaustion (VE) is more strongly associated with cardiovascular disease (CVD) risk for women than men. This study examined whether sex differences in associations of VE with CVD risk markers are accounted for by unique associations of VE with regional adiposity.
Methods:
The study enrolled 143 persons (18–55 years) without diagnosed conditions. VE was assessed by the Maastricht questionnaire. CVD indices were measured using the euglycemic-hyperinsulinemia clamp, resting blood pressure, and blood draws. Regional adiposity was measured using computed tomography and 2-D echocardiography. This cross-sectional study employed a path analysis approach, including relevant covariates.
Results:
Of the cohort, aged 38.7 ± 8.4 years, 65% were men, and 41% were obese. The final model had excellent fit (χ2(36) = 36.5, p = .45; RMSEA = 0.009, CFI = 0.999). For women, but not men, the model indicated paths from VE to: 1) lower insulin sensitivity (B = −0.10, p = .04), and higher total cholesterol to HDL ratio (B = 0.12 p = .09), triglycerides (B = 0.10, p = .08), and C-reactive protein (B = 0.08, p = .09) through visceral adiposity; 2) higher mean arterial pressure (B = 0.14, p = .04), lower insulin sensitivity (B = −0.09, p = .08), and higher C-reactive protein (B = 0.12, p = .07) through subcutaneous adiposity; 3) lower insulin sensitivity (B = −0.07, p = .08) and higher total cholesterol to HDL ratio (B = 0.16, p = .03) through liver adiposity; and 4) higher C-reactive protein (B = 0.08, p = .09) through epicardial adiposity.
Conclusion:
Results extend prior evidence by showing that the association of VE with CVD risk in women is linked with specific regional adiposity elevation. Further study of adiposity-related mechanisms in women who experience early decline in vitality may inform clinical targets for CVD prevention.
Keywords: Cardiovascular disease, Fatigue, Regional adiposity, Sex, Vitality
1. Introduction
Vital exhaustion (VE), characterized by excess fatigue, loss of vigor, increased irritability, and demoralization, has been consistently associated with 1.5 to 2.5-fold increased risk for cardiovascular disease (CVD) diagnosis, recurrent cardiac events, and mortality [1–4]. Although VE for both men and women is linked with conditions commonly associated with preclinical CVD risk, such as obesity, inflammation, and metabolic dysfunction, studies have shown that VE confers greater CVD risk for women than men [5–8]. The underlying biomechanisms mediating greater associations of VE with CVD risk in women are not well understood.
Of the preclinical CVD risk factors, obesity is considered a primary pathway for CVD etiology linked with metabolic and lipidemic dysregulation [9,10]. Studies have shown that VE and other measures of fatigue are highly associated with obesity [5,11,12]. Notably, women with elevated VE have more than two-fold heightened risk of becoming obese [5]. Of relevance, adiposity differs among fat depots and these differences are nonuniformly associated with CVD risk. Studies indicate that, among regional fat depots, visceral and liver adipose tissue are the strongest predictors of CVD morbidity and mortality [13,14]. However, other fat depots, including epicardial and subcutaneous adipose tissue, have also been associated with CVD risk, independent of total adiposity [13,15]. To date, only one prior study has examined adiposity as a mediator of the relationship between VE and CVD risk [16]. This study showed that greater body mass index (BMI) partially mediated the positive association between VE and triglyceride (TG) level [16]. However, no evaluation of sex differences, or other CVD risk indices as outcomes was performed; moreover, regional adiposity differences were not assessed as potential mediators. Notably, patterns of regional adiposity differ in men and women [17]. For instance, men tend to store more fat in the visceral area, whereas women tend to store more fat in the subcutaneous compartment [17]. Thus, sex differences in the linkage of VE with regional adiposity may play a unique role in preclinical CVD pathophysiology.
In this paper, we employed a path analysis approach to examine: 1) whether there are sex differences in the associations of VE with regional adiposity (visceral, subcutaneous, liver, epicardial) and preclinical CVD risk; and 2) whether regional adiposity statistically mediates sex differences in the association of VE with preclinical CVD risk. It is hypothesized that not only will greater VE be associated with CVD risk measures, but these relationships will be stronger in women than men, and mediated primarily by visceral and liver adiposity because these fat depots have been found previously to be most strongly associated with CVD risk [13,14].
2. Methods
2.1. Participants
The study sample (n = 143) was comprised of men and women recruited from South Florida via advertisements and referrals. Inclusion criteria required that participants: a) were aged 18–55 years; b) reported no current or past 10-year history of dependency on substances or alcohol; c) had a negative toxicology screen; d) reported no smoking or other nicotine use in the previous year; e) had normal thyroid stimulating hormone levels (0.4–4.5 mIU/L); and f) for women, were premenopausal, with regular menstrual cycling. Participants were excluded if they: a) had a diagnosis or prescribed medication for a cardiovascular, carbohydrate, cholesterol, metabolic, endocrine, or psychiatric disorder; b) had an electrocardiogram (ECG) arrhythmia indicating impending, recent, or evolving myocardial infarction; or c) were pregnant. The protocol was approved by the institutional review board of the University of Miami and written informed consent was obtained from participants prior to inclusion.
2.2. Procedures
2.2.1. Overview
This study is a secondary analysis of a previously described study examining cardiometabolic function in nondiabetic individuals with and without insulin resistance [18]. Briefly, procedures included an in-person visit for eligibility screening determined via demographic and personal medical and psychiatric history, physical exam, metabolic panel, urine toxicology, pregnancy test, 12-lead ECG, substance use questionnaires and the structured clinical interview for the DSM-IV module E: Substance Use Disorders [19]. During this visit, resting blood pressure, anthropometric measurements, and exercise testing were performed. At the second visit, fasting glucose and insulin, lipid profile, and C-reactive protein (CRP) were obtained from blood samples. A euglycemic-hyperinsulinemia clamp was performed to measure insulin sensitivity (IS), and an echocardiogram was undertaken to assess epicardial adipose tissue. At the third visit, visceral, subcutaneous, and liver adipose tissues were measured using computed tomography. Additionally, participants completed the Maastricht questionnaire (MQ) among other psychosocial surveys.
2.2.2. Vital exhaustion
The MQ [20] is a 21-item survey used to assess fatigue, vigor, irritability, and demoralization (e.g., “Do you ever wake up with a feeling of exhaustion and fatigue,” “Do you often feel tired,” “Do you feel weak all over”). Each item is rated “Yes,” “No,” or “Do not know.” A composite score was calculated from the sum of the items, with values ranging from 0 to 42, wherein higher scores indicated greater VE. The MQ has satisfactory reliability and psychometric validity [21]. Total scores at or above 14 meet the criterion for clinical diagnosis [22].
2.2.3. Regional adiposity
Visceral and subcutaneous adipose tissue volume, and a liver adipose tissue index were obtained using multi-slice computed tomography performed by Siemens Somaton-Sensation-16 scanner (Siemens, Malvern, PA). The areas scanned included an 8-slice scan, where images were obtained from L4–5, 2 slices at 5 cm and 10 cm below the L4–5, and 5 images every 5 cm above L4–5 [23]. Visceral and subcutaneous adipose tissue volume were derived from the sum of the slices, using a triangulation formula multiplied by 0.9391 mg/mL [23]. Liver adiposity was computed by the ratio between spleen and liver attenuation evaluated in Hounsfield units, with higher scores indicating greater adiposity. Epicardial adipose tissue thickness was measured using 2-D M-mode echocardiogram (SONOS 5500, Philips Medical Systems, Andover, MA) as previously validated [24]. Epicardial adiposity was identified as the echofree space between the outer wall of the myocardium and the visceral layer of the pericardium and measured in the parasternal long-axis view on the free wall of the right ventricle. Maximum epicardial adipose tissue thickness was measured at end-systole in three cardiac cycles and expressed as the average.
2.2.4. Subclinical CVD risk indices
Seated oscillometric blood pressure (Dinamap #1846SXP, GE Healthcare Technologies, Waukesha, WI) was measured following a 15-min rest period, wherein the mean was derived from the last 2 of 3 readings. The euglycemic-hyperinsulinemia clamp involved a priming insulin infusion, followed by a constant insulin infusion at 40 μU/m2/min for 150 min using a calibrated IMed Gemini PC-2TX infusion pump (Alaris Medical Systems, San Diego, CA). Glucose infusion was undertaken 4 min after insulin infusion began, and blood glucose was clamped thereafter within 5% of fasting levels. During the steady state phase, the rate of glucose infusion is equal to the rate of total body glucose uptake. A measure of IS was indexed by the whole-body glucose disposal rate (mg/kg•min of fat-free mass), wherein insulin resistance was defined as 4.5 mg/kg•min or lower [25]. Total cholesterol (TC) and TG were measured enzymatically using an autoanalyzer (Cobas Mira Plus; Roche Diagnostics, Branchburg, NJ). High density lipoprotein (HDL) was measured after precipitation with dextran sulfate, low density lipoprotein was calculated by the Friedewald formula, and the total cholesterol to HDL ratio (TC/HDL) was computed. CRP was measured by particle-enhanced immunoturbidimetry (Cobas 6000 analyzer; Roche Diagnostics) [18].
2.2.5. Covariates
Age and education were obtained through self-report. Aerobic capacity was measured to index physical fitness using a maximal graded exercise test (McArdle protocol), performed using an automated electronically-braked cycle ergometer [26]. This measure was defined as the maximal oxygen uptake (VO2 max) taken immediately upon test termination relative to the predicted VO2 max.
2.3. Statistical analysis
Data screening included computing descriptive statistics and intercorrelations among study variables, and evaluating normality. Transformations for variables departing from normality (i.e., VE, TG, CRP) were performed. Complete data for all variables was collected except VO2 max (11% missing). Exploratory procedures, including Little’s MCAR test and significance tests of missingness, were conducted [27]. Multiple imputation was then used to estimate these data, which were confirmed by this testing to be missing at random. Path analysis was conducted using AMOS v24.0 (IBM Corp., Armonk, New York, USA) to assess the direct effect of VE on CVD risk indices (mean arterial pressure [MAP], IS, TC/HDL, TG, CRP), and the indirect effect of VE on these outcomes via the fat depots (visceral, subcutaneous, liver, epicardial). These analyses were done first in the overall sample, and subsequently separated by men and women in a multigroup model. Modification indices were referenced to include any additional covariances specified to improve model fit. Potential confounding factors of age, education, and physical fitness were added as covariates [28–30]. Model trimming was then performed to exclude covariate pathways with a p-value greater than 0.2 [31]. Model fit was assessed by χ2 (p > .05), root-mean-square error of approximation (RMSEA <0.05), and the comparative fit index (CFI ≥0.95) [32,33].
To test for mediation,1 estimates of indirect effects were obtained using bootstrapping procedures. Indices were derived for the proportion of variance explained for each observed variable, and the proportion of variance explained by each indirect path. To test for moderation by sex for each path from VE to regional fat depot and CVD risk index, likelihood ratio tests were performed in which a freely estimated model (i.e., no equality constraints placed across men and women) was compared to a freely estimated model with one path constrained to be equal across men and women [35]. A significant likelihood ratio test indicated that there was a significant decrement in model fit when the specified path was constrained, and therefore suggests that the path is significantly different across groups. Lastly, to assess directionality, reverse modeling analyses were conducted, which included paths from the CVD risk indices to VE mediated by the fat depots. Values are mean (SD) unless otherwise stated.
3. Results
3.1. Descriptive statistics for key variables and participant characteristics
Of the 325 individuals who provided informed consent and were screened, 143 were eligible and completed the study. Of the cohort, 65% were men, and the sample was predominantly Hispanic White (73.4%); other race/ethnicities included Black (14.7%), non-Hispanic White (6.3%), Hispanic Black (1.4%), and others (4.2%). On average, participants were 38.7 (8.4) years old, and reported completing 13.3 (2.6) years of education. Mean BMI was 29.3 (4.7) kg/m2, with 39.2% of the cohort classified as overweight, and 41.3% as obese. For the CVD risk measures, 11% had systolic blood pressure exceeding 140 mmHg and 24% had diastolic blood pressure exceeding 90 mmHg, 55% were considered insulin resistant, 33% had a TC/HDL ratio exceeding 5, 29% had TG levels exceeding 150 mg/dl, and 27% had CRP levels exceeding 3 mg/L.
Table 1 displays the demographic and CVD risk measures for men and women, with group comparisons indicated. The groups were similar in age, education, BMI, and physical fitness. The VE scores ranged from 0 to 34 and overall were relatively low, with no significant difference observed between men and women. However, of the cohort, 8.4% had VE total scores at clinical levels. There were no significant differences between men and women for IS, TG, or CRP; however, men had significantly higher MAP and TC/HDL than women. Measures of fat deposition indicated that visceral adipose tissue volume in men was about 1.5-fold greater than in women. In addition, men had significantly greater liver adiposity than women, whereas women had significantly greater subcutaneous and epicardial adiposity than men. Table 2 presents bivariate correlations of study variables. Notably, VE was associated with greater subcutaneous and liver adiposity in women, but was not associated with any regional fat measures in men. In contrast, VE was not significantly correlated with any CVD risk measure for women, but was associated with lower TC/HDL in men.
Table 1.
Demographic characteristics and CVD risk measures depicted for men and women.
| Men (n = 93) | Women (n = 50) | ||||
|---|---|---|---|---|---|
|
|
|
|
|
||
| Variablesa | M | SD | M | SD | Difference (p) |
|
| |||||
| Age (years) | 39.6 | 8.4 | 37.0 | 8.3 | 0.07 |
| Education (years) | 13.3 | 2.7 | 13.2 | 2.5 | 0.81 |
| BMI (kg/m2) | 29.5 | 4.2 | 28.8 | 5.5 | 0.44 |
| Fitness (% predicted) | − 22.6 | 12.4 | − 25.1 | 14.5 | 0.27 |
| Vital exhaustion | 3.9 | 6.3 | 3.6 | 6.3 | 0.78 |
| VAT volume (L) | 4.57 | 2.01 | 2.75 | 1.24 | <0.001 |
| SAT volume (L) | 7.51 | 3.48 | 9.82 | 4.18 | 0.001 |
| LATb | 0.81 | 0.12 | 0.76 | 0.15 | 0.03 |
| EAT thickness (mm) | 6.33 | 1.32 | 6.83 | 1.49 | 0.04 |
| MAP (mm Hg) | 96.0 | 11.9 | 89.0 | 11.9 | 0.001 |
| IS (mg/kg•min) | 4.8 | 3.3 | 5.3 | 2.9 | 0.42 |
| TC/HDL | 4.8 | 1.4 | 4.1 | 1.4 | 0.004 |
| Triglycerides (mg/dL) | 144.1 | 97.0 | 121.1 | 89.0 | 0.17 |
| CRP (mg/L) | 2.3 | 2.5 | 3.3 | 5.3 | 0.18 |
Abbreviations: BMI body mass index, VAT visceral adipose tissue, SAT subcutaneous adipose tissue, LAT liver adipose tissue, EAT epicardial adipose tissue, MAP mean arterial pressure, IS insulin sensitivity, TC/HDL total cholesterol to high-density lipoprotein ratio, TG triglycerides, CRP C-reactive Protein.
LAT is the ratio between spleen attenuation and liver attenuation evaluated in Hounsfield units, where higher scores indicate greater adiposity.
Table 2.
Bivariate correlations for model variables for men and women.
| Men | Age | Educ | Fitness | VE | VAT | SAT | LAT | EAT | MAP | IS | TC/HDL | TG | CRP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| age | – | 0.05 | 0.13 | 0.06 | 0.48*** | 0.10 | 0.11 | 0.16 | 0.23* | − 0.30** | 0.25* | 0.13 | 0.18t |
| Educ | – | 0.10 | 0.08 | 0.09 | 0.15 | 0.15 | 0.10 | 0.11 | − 0.06 | 0.01 | − 0.08 | − 0.03 | |
| Fitnessa | – | − 0.15 | 0.11 | 0.19t | 0.18t | − 0.22* | 0.02 | − 0.18t | 0.13 | 0.18t | 0.10 | ||
| VE | – | − 0.11 | − 0.05 | − 0.04 | 0.13 | 0.01 | 0.03 | − 0.22* | − 0.17t | − 0.15 | |||
| VAT | – | 0.58*** | 0.45*** | 0.27** | 0.20t | − 0.56*** | 0.51*** | 0.48*** | 0.44*** | ||||
| SAT | – | 0.32** | 0.15 | 0.16 | − 0.52*** | 0.29** | 0.26* | 0.31** | |||||
| LATb | – | 0.14 | 0.04 | − 0.34** | 0.38*** | 0.32** | 0.11 | ||||||
| EAT | – | 0.10 | − 0.12 | 0.004 | 0.01 | − 0.04 | |||||||
| MAP | – | − 0.25* | 0.30** | 0.25* | 0.18t | ||||||||
| IS | – | − 0.39*** | − 0.41*** | − 0.28** | |||||||||
| TC/HDL | – | 0.59*** | 0.39*** | ||||||||||
| TG | – | 0.23* | |||||||||||
| CRP | – | ||||||||||||
| Women | Age | Educ | Fitness | VE | VAT | SAT | LAT | EAT | MAP | IS | TC/HDL | TG | CRP |
| age | – | − 0.01 | 0.15 | 0.01 | 0.23 | − 0.10 | 0.12 | − 0.01 | 0.16 | 0.04 | 0.23 | 0.20 | − 0.12 |
| Educ | – | 0.04 | − 0.09 | − 0.24 | − 0.44** | − 0.24t | − 0.21 | − 0.15 | 0.07 | − 0.18 | − 0.11 | − 0.27t | |
| Fitnessa | – | − 0.16 | − 0.07 | 0.09 | 0.12 | − 0.16 | 0.02 | − 0.03 | − 0.003 | 0.17 | 0.14 | ||
| VE | – | 0.25t | 0.27t | 0.32* | 0.19 | 0.004 | − 0.12 | 0.09 | 0.12 | 0.14 | |||
| VAT | – | 0.68*** | 0.35* | 0.14 | 0.34* | − 0.58*** | 0.49*** | 0.52*** | 0.49*** | ||||
| SAT | – | 0.49*** | 0.23 | 0.41** | − 0.59*** | 0.41** | 0.47** | 0.61*** | |||||
| LATb | – | 0.35* | 0.19 | − 0.39** | 0.45** | 0.36** | 0.30* | ||||||
| EAT | – | − 0.13 | − 0.18 | 0.20 | 0.25t | 0.36* | |||||||
| MAP | – | − 0.23 | 0.06 | 0.21 | − 0.01 | ||||||||
| IS | – | − 0.47** | − 0.50*** | − 0.48*** | |||||||||
| TC/HDL | – | 0.69*** | 0.39** | ||||||||||
| TG | – | 0.52*** | |||||||||||
| CRP | – | ||||||||||||
abbreviations: Educ education, VE vital exhaustion, see Table 1 for others.
Fitness is defined by maximal oxygen uptake (VO2 max) to a graded exercise test relative to the predicted aerobic capacity.
Higher scores on LAT indicate greater adiposity.
p < 0.10,
p < .05,
p < .01,
p < .001.
3.2. Model results without sex included
The initial model, which did not include sex as a factor, is shown in Fig. 1. The model demonstrated excellent fit (χ2(27) = 22.27, p = .72; RMSEA = 0.000, 90% CI [0.000,0.050], CFI = 1.00). As seen in this figure, although some significant paths from fat depots to CVD risk measures were observed, VE was not significantly associated with fat depots or CVD risk directly or indirectly.
Fig. 1.
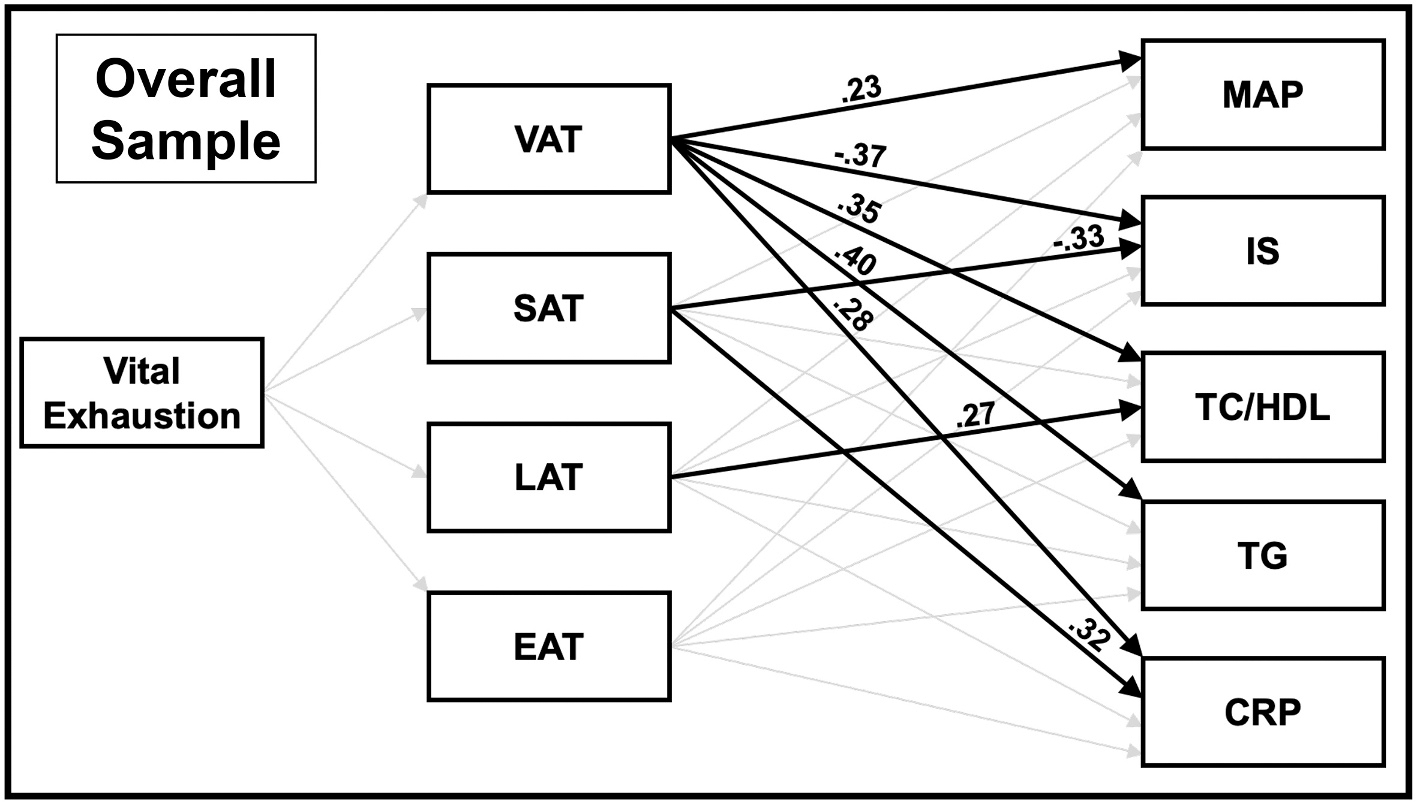
Path analysis model of the overall sample depicting the mediation by regional fat depot of the pathway from vital exhaustion to the CVD risk indices. Direct paths from vital exhaustion to MAP, IS, TC/HDL, TG, and CRP are not displayed in figure (all were nonsignificant, except the path from vital exhaustion to TC/HDL, which approached significance [β = −0.13, p = .06]). Covariates in the model included age, education, and fitness. Paths that were significant are indicated by a solid bold line; paths that approached significance (p < .10) are indicated by a dotted line; and paths that were not significant are indicated by a faded line. Direct paths in this figure are displayed prior to bootstrapping analyses for assessment of mediation. Abbreviations: VAT visceral adipose tissue, SAT subcutaneous adipose tissue, LAT liver adipose tissue, EAT epicardial adipose tissue, MAP mean arterial pressure, IS insulin sensitivity, TC/HDL total cholesterol to high-density lipoprotein ratio, TG triglycerides, CRP C-reactive Protein.
3.3. Model results with sex included
The unconstrained models for men and women are displayed in Fig. 2. Pathway estimates for indirect effects of VE on CVD risk indices via fat depots in men and women are reported in Table 3. The model demonstrated excellent overall fit (χ2(36) = 36.45, p = .45; RMSEA = 0.009, 90% CI [0.000,0.061], CFI = 0.999). Likelihood ratio tests were performed between the freely estimated model (i.e., no paths constrained across groups) and the freely estimated model with one path constrained to be equal across groups to assess for moderation by sex. Significant differences between men and women were observed in the paths from VE to: 1) liver adiposity (χ2diff (1) = 6.47, p = .01), 2) subcutaneous adiposity (χ2diff (1) = 3.91, p = .048), and 3) visceral adiposity (χ2diff (1) = 5.22, p = .02). All other likelihood ratio tests examining group differences in the paths from VE to model outcomes were not significant (ps > 0.05).
Fig. 2.
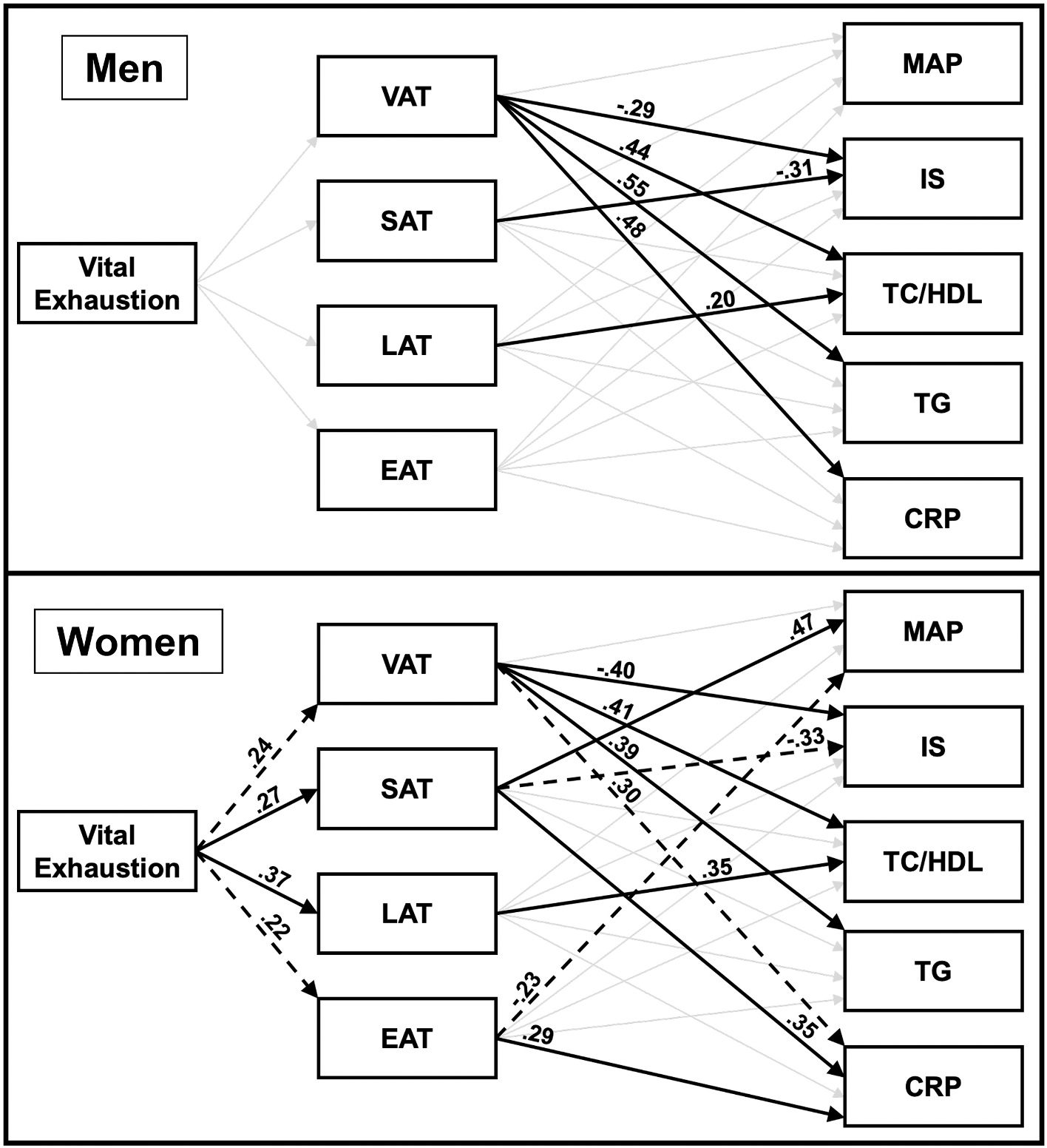
Unconstrained structural models for men and for women showing the mediation by regional fat depot of the pathway from vital exhaustion to the CVD risk indices. Direct paths from vital exhaustion to MAP, IS, TC/HDL, TG, and CRP are not displayed in figure (all were nonsignificant, except the path from vital exhaustion to TC/HDL in men, which approached significance [β = −0.14, p = .099]). Covariates in the models included age, education, and fitness. Paths that were significant are indicated by a solid bold line; paths that approached significance (p < .10) are indicated by a dotted line; and paths that were not significant are indicated by a faded line. Direct paths in this figure are displayed prior to bootstrapping analyses for assessment of mediation. Abbreviations: VAT visceral adipose tissue, SAT subcutaneous adipose tissue, LAT liver adipose tissue, EAT epicardial adipose tissue, MAP mean arterial pressure, IS insulin sensitivity, TC/HDL total cholesterol to high-density lipoprotein ratio, TG triglycerides, CRP C-reactive Protein.
Table 3.
Indirect effects of vital exhaustion on cardiovascular disease risk indices via regional fat depots in men and women.
| Men | Women | |||||
|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
| Path | Coefficient | SE | p | Coefficient | SE | p |
|
| ||||||
| VE to visceral adiposity to mean arterial pressure | − 0.01 | 0.02 | 0.58 | 0.003 | 0.05 | 0.88 |
| VE to visceral adiposity to insulin sensitivity | 0.04 | 0.04 | 0.12 | − 0.10 | 0.07 | 0.04 * |
| VE to visceral adiposity to TC/HDL | − 0.07 | 0.06 | 0.14 | 0.12 | 0.10 | 0.09t |
| VE to visceral adiposity to triglycerides | − 0.07 | 0.06 | 0.13 | 0.10 | 0.08 | 0.08t |
| VE to visceral adiposity to C-reactive protein | − 0.07 | 0.06 | 0.12 | 0.08 | 0.07 | 0.09t |
| VE to subcutaneous adiposity to mean arterial pressure | − 0.002 | 0.02 | 0.75 | 0.14 | 0.09 | 0.04 * |
| VE to subcutaneous adiposity to insulin sensitivity | 0.01 | 0.04 | 0.89 | − 0.09 | 0.07 | 0.08t |
| VE to subcutaneous adiposity to TC/HDL | 0.000 | 0.01 | 0.80 | − 0.004 | 0.09 | 0.98 |
| VE to subcutaneous adiposity to triglycerides | 0.001 | 0.02 | 0.65 | 0.05 | 0.08 | 0.38 |
| VE to subcutaneous adiposity to C-reactive protein | − 0.001 | 0.02 | 0.70 | 0.12 | 0.09 | 0.07t |
| VE to liver adiposity to mean arterial pressure | 0.001 | 0.02 | 0.68 | 0.01 | 0.09 | 0.92 |
| VE to liver adiposity to insulin sensitivity | 0.003 | 0.02 | 0.55 | − 0.07 | 0.07 | 0.08t |
| VE to liver adiposity to TC/HDL | − 0.01 | 0.04 | 0.71 | 0.16 | 0.11 | 0.03 * |
| VE to liver adiposity to triglycerides | − 0.003 | 0.03 | 0.48 | 0.04 | 0.09 | 0.52 |
| VE to liver adiposity to C-reactive protein | 0.004 | 0.02 | 0.60 | − 0.03 | 0.08 | 0.63 |
| VE to epicardial adiposity to mean arterial pressure | 0.002 | 0.01 | 0.50 | − 0.05 | 0.06 | 0.12 |
| VE to epicardial adiposity to insulin sensitivity | 0.002 | 0.01 | 0.51 | − 0.01 | 0.04 | 0.48 |
| VE to epicardial adiposity to TC/HDL | − 0.01 | 0.02 | 0.27 | 0.02 | 0.05 | 0.40 |
| VE to epicardial adiposity to triglycerides | 0.001 | 0.01 | 0.65 | 0.05 | 0.06 | 0.11 |
| VE to epicardial adiposity to C-reactive protein | − 0.01 | 0.02 | 0.32 | 0.08 | 0.06 | 0.09t |
Unstandardized coefficients are reported.
abbreviations: SE standard error, VE vital exhaustion, TC/HDL total cholesterol to high density lipoprotein ratio.
p < 0.10,
p < .05,
p < .01,
p < .001.
3.3.1. Model results in men
The model for men explained the following percent of variance in the observed CVD risk outcomes: 23.3% in visceral adiposity, 4.8% in subcutaneous adiposity, 4.5% in liver adiposity, 9.2% in epicardial adiposity, 7.3% in MAP, 38.2% in IS, 33.8% in TC/HDL, 28.1% in TG, and 26.4% in CRP. When fat depots were not included in the model as mediators, there was a significant direct path from VE to lower TC/HDL (β = −0.23, p = .02), which was no longer significant after adding fat depots to the model (β = −0.14, p = .10). The direct paths from VE to the other CVD risk indices were not significant before or after adding fat depots to the model. There were also no significant direct paths from VE to the fat depots, nor indirect paths from VE to the CVD risk indices via the fat depots. Significant direct paths in the model emerged only from regional adiposity to certain CVD indices. Specifically, visceral adiposity was associated with higher TC/HDL (β = 0.44, p < .001), TG (β = 0.55, p < .001), and CRP (β = 0.48, p < .001), and lower IS (β = −0.29, p = .02). In addition, subcutaneous adiposity was related to lower IS (β = −0.31; p = .004), and liver adiposity to higher TC/HDL (β = 0.20, p = .03).
3.3.2. Model results in women
The model for women explained the following percent of variance in the observed CVD risk outcomes: 15.0% in visceral adiposity, 30.6% in subcutaneous adiposity, 22.4% in liver adiposity, 11.2% in epicardial adiposity, 27.1% in MAP, 49.1% in IS, 34.4% in TC/HDL, 39.0% in TG, and 48.4% in CRP. Prior to and after inclusion of fat depots as mediators, the direct paths from VE to the CVD risk indices were not significant. However, there were significant direct and indirect paths from VE to the fat depots and from the fat depots to the CVD indices, which are described as follows.
3.3.3. Direct and indirect paths in women
First, there were paths from VE to visceral adiposity, and from visceral adiposity to insulin sensitivity, total cholesterol to HDL ratio, triglycerides, and C-reactive protein. Specifically, the direct path from VE to greater visceral adiposity approached significance (β = 0.24; p = .07), and there were significant direct paths from visceral adiposity to lower IS (β = −0.40, p = .01), higher TC/HDL (β = 0.41, p = .01), higher TG (β = 0.39, p = .02), and trending to higher CRP (β = 0.30, p = .06). Mediation analyses revealed an indirect path from VE to lower IS through greater visceral adiposity, which accounted for 23.3% of the total effect of VE on IS. Moreover, the indirect paths from VE to higher TC/HDL, TG, and CRP through greater visceral adiposity approached significance (see Table 3).
Second, there were paths from VE to subcutaneous adiposity, and from subcutaneous adiposity to mean arterial pressure, insulin sensitivity, and C-reactive protein. Specifically, there were direct paths from VE to greater subcutaneous adiposity (β = 0.27; p = .02), and from subcutaneous adiposity to higher MAP (β = 0.47, p = .02) and CRP (β = 0.35, p = .04), and a trend to lower IS (β = −0.33, p = .06). Mediation analyses revealed an indirect path from VE to higher MAP through greater subcutaneous adiposity, which accounted for 64.6% of the total effect of VE on MAP. Furthermore, the indirect paths from VE to lower IS and higher CRP via greater subcutaneous adiposity approached significance (see Table 3).
Third, there were paths from VE to liver adiposity, and from liver adiposity to total cholesterol to HDL ratio and insulin sensitivity. Specifically, there was a significant direct path from VE to greater liver adiposity (β = 0.37, p = .003), and from liver adiposity to higher TC/HDL (β = 0.35, p = .02). Mediation analyses showed that this indirect path from VE to TC/HDL through greater liver adiposity was significant and accounted for 32.0% of the total effect of VE on TC/HDL. In addition, the indirect path from VE to lower IS through greater liver adiposity approached significance (see Table 3).
Fourth, there were paths from VE to epicardial adiposity, and from epicardial adiposity to C-reactive protein and mean arterial pressure. Specifically, the path from VE to greater epicardial adiposity approached significance (β = 0.22; p = .099); in addition, there was a significant direct path from epicardial adiposity to higher CRP (β = 0.29, p = .01), and a trend from epicardial adiposity to lower MAP (β = −0.23, p = .08). The indirect path from VE to higher CRP through greater epicardial adiposity approached significance, whereas the path from VE to MAP via epicardial adiposity was not significant (see Table 3).
3.4. Reverse model results
In the reverse model, with CVD risk indices as predictors, fat depots as mediators, and VE as the outcome, the data fit well (χ2(36) = 50.60, p = .054; RMSEA = 0.054, 90%CI [0.000,0.086], CFI = 0.965). However, no significant direct or indirect paths from CVD risk indices and fat depots to VE were observed for men or women.
4. Discussion
The main finding of the present study was that for women, but not men, elevated VE was associated with differences in regional adiposity that, in turn, were associated with unique CVD risk indices. Specifically, in women, evidence suggested indirect paths from elevated VE to: 1) lower IS and higher TC/HDL, TG, and CRP via greater visceral adiposity; 2) higher MAP, lower IS, and higher CRP via greater subcutaneous adiposity; 3) lower IS and higher TC/HDL via greater liver adiposity; and 4) higher CRP via greater epicardial adiposity. These findings were independent of age, education, and physical fitness. As hypothesized, the derived model supports the broad literature indicating that adiposity in visceral and liver fat depots are uniquely implicated in CVD risk [14,36]. Notably, study findings also suggest a pathophysiological role of subcutaneous and epicardial adipose tissue in mediating preclinical CVD risk in women with elevated VE. Overall, study findings are consistent with and extend prior evidence suggesting stronger associations of VE with regional adiposity and markers of CVD risk for women than men [2,3,5,7,11,37].
In the final model, displayed in Fig. 2, evidence for indirect, but not direct, paths from VE to the CVD risk indices were observed. Although many prior studies found associations between VE and CVD risk parameters [7,17,38–40], some others have not [12,41,42]. There were several differences among study methodologies that could explain inconsistencies in the literature including: 1) clinical versus nonclinical samples; 2) eligibility criteria; and 3) variables controlled in analyses. In this context, because VE may already be elevated in clinical cohorts because of disease or medications, the present study used stringent eligibility criteria to exclude persons with diagnosed systemic conditions. Thus, potential confounding by structural and functional alterations of frank disease or side effects of medications was minimized. Moreover, the present findings illustrate the importance of considering sex differences. When sex was not evaluated, as seen in the initial analyses displayed in Fig. 1, the paths from VE to the CVD risk indices were all nonsignificant. It was only when sex differences were examined that modeling revealed significant linkages of VE with CVD risk indices in women, but not men. Thus, it may be concluded that the inclusion of men in our initial model suppressed the significant pathways present among women. This finding may, in part, explain why previous studies that combined men and women found no relationship of VE with CVD risk [12,41].
The present findings derive support from a previous study, which found that VE was only predictive of ischemic stroke in women, but not men [37]. Other findings from a longitudinal study indicated that women with elevated baseline VE, compared to men, were more likely to gain weight and become obese [5]. Another study found associations of VE with proinflammatory and procoagulatory status in healthy women, but not men [7]. Our findings advance this literature by elucidating the unique pathways by which VE may indirectly affect MAP, IS, TC/HDL, TG, and CRP through elevations of adiposity in distinct fat depots. Thus, novel evidence is reported suggesting that body fat deposition may be an underlying biomediator linking VE to worsened preclinical CVD risk in women.
4.1. Potential mechanisms
The final model depicted in Fig. 2 indicates that sex differences were observed in the paths from VE to regional adiposity. One possibility is that sex hormone level or function may explain these female-specific relationships. Specifically, it is known that variations in estrogen, progesterone, and androgen secretion, receptor function, or signaling within the fat depots affect adipocyte expansion and tissue metabolism, which have implications for CVD pathophysiology [43]. In addition, endogenous androgen and estrogen exposure, for example, are thought to affect patterns of adipocyte expansion, with cells either proliferating in number (i.e., hyperplasia) or in size (i.e., hypertrophy) [44]. Of note, adipocyte hypertrophy and not hyperplasia has been associated with greater insulin resistance and proinflammatory status [44]. Thus, estrogen-to-androgen balance is posited to play a role in sex differences in CVD pathophysiology [43].
Of relevance is research demonstrating the differential impact of sex hormones on regional fat deposition. Premenopausal women tend to store fat in subcutaneous regions, whereas men and postmenopausal women tend to store fat in visceral and ectopic regions [44]. Increased visceral adipose tissue has been observed in transgender men who receive intramuscular testosterone therapy and in postmenopausal women who experience a decline in estrogen; in contrast, decreased visceral adiposity has been observed in transgender women who receive estrogen treatment [45–47]. Moreover, studies aimed to correct estrogen deficiency in postmenopausal women found subsequent visceral adipose tissue reductions [48,49]. Thus, a shift in estrogen-to-androgen balance toward androgens remodels body fat distribution resulting in greater visceral and ectopic fat mass, whereas a shift toward estrogen results in greater fat accumulation in peripheral subcutaneous compartments [45–49]. Evidence also indicates that a shift in estrogen-to- androgen ratio toward androgens may decrease adiponectin production, and increase leptin production [50,51]. This adipokine synthesis profile may contribute to metabolic and lipidemic dysregulation and is associated with greater proinflammatory status and oxidative stress and hence elevated CVD risk [44].
It is possible in women that VE is associated with a reduction of the estrogen-to-androgen ratio. This type of hormonal imbalance may negate the typically protective cardiometabolic effects of estrogen in premenopausal women [44]. Indeed, a study of post-menopausal women has shown that treatment with estrogen replacement therapy induced a significant decrease in VE [52]. Therefore, a decrement in estrogen-to-androgen ratio may mechanistically underlie the elevation in VE seen in menopause. In sum, although the literature on the linkage of VE, adiposity, and CVD risk is immature, evidence appears to support the suggestion that, in women, VE may be associated with a shift in estrogen-to-androgen ratio that results in altered adipocyte size, distribution, and function, and consequently CVD pathophysiology.
4.2. Limitations and strengths
First, this study is limited by the cross-sectional design, which restricts establishing definitive causal claims about the directionality of the relationships. Moreover, assessment of mediation hypotheses with cross-sectional data renders path analysis assumptions prone to biased estimates even when correctly specified [53]. However, the reverse model results, wherein there were no significant direct or indirect paths from CVD risk indices to VE via fat deposition, lend support for the directionality of the final model (see Fig. 2). Nevertheless, the study findings cannot eliminate the possibility that there is a reciprocal relationship between VE and CVD risk factors. Future longitudinal research could show how changes in VE and regional adiposity may influence changes in CVD risk indices to better inform directionality.
Second, the present study used computed tomography methods to quantitate regional adiposity, with established and standardized procedures that restricted imaging to the abdominal region. Thus, the findings may not reflect the interrelationships of study outcomes with fat depots present in other areas of the body. Third, the ethnic/racial composition of the cohort, which was predominantly Hispanic/Latino, may not generalize to other populations with different composition. The rapid increase in the Hispanic/Latino persons in the United States in recent decades has piqued interest in better understanding the prevalence of CVD risk in this minority population [54,55]. It is possible that sociocultural factors unique to Hispanic/Latino women relative to men may influence VE, regional adiposity, and CVD risk [55]. Fourth, about 81% of the study sample were overweight or obese, which is higher than the prevalence in the general United States population (approximately 68% [56]). Thus, study generalizability may be limited further.
Fifth, a previous concern in the literature pertains to whether VE, as measured by the MQ survey, has some construct overlap with dysphoria. Thus, depressive symptoms rather than fatigue may be driving the significant associations reported in this study. Prior studies assessing these two constructs suggest that this hypothesis may not have validity. For example, one study, which used factor analysis approach, found that VE items loaded onto a unique factor, relative to items from the depression subscale of the Hospital Anxiety and Depression Scale [57]. Similarly, VE was reported to significantly predict reoccurrence of vascular events when controlling for depression and other relevant cofounders [58]. In addition, VE but not depression has been shown to predict hypertension diagnosis [59]. Thus, the two constructs appear to be independently associated with CVD risk, although additional research may be needed to further establish the extent of divergent validity. Given high rates of undiagnosed depressive disorders in Hispanic/Latino communities and ethnic disparities in mental health treatment [60,61], parsing the independent or joint roles of fatigue and depressive symptoms on CVD risk may have clinical utility for this population. One possibility is to pursue larger-scale analyses that are powered to conduct item-level invariance testing. These analyses would assess whether and how items on the MQ and various depressive and fatigue measures may differentially load on to latent factors for men and women. Elucidating whether a specific subset of questions are predominantly associated with disease onset or progression would have considerable clinical utility toward establishing a more concise, yet more predictive, measure to be used in clinical settings, particularly for women.
Of note, the current study also has major strengths. First, rigorous methodology was used to address possible concerns that extraneous variables could bias the associations between VE and CVD pathophysiology. Specifically, the study enrolled a nonclinical sample, applied strict eligibility criteria, and statistically controlled for potential confounding variables. These procedures reduced the possibility that findings were confounded by structural and functional effects of chronic disease, medical treatment, or substance use. Given the strict eligibility criteria, the majority of participants reported subclinical VE levels, which effectively biased against detecting significant relationships. Yet, theoretically reasonable indirect pathways from VE to CVD indices were observed. Thus, the demonstrated effects in the present nonclinical sample strengthens study findings, suggesting a role for VE in preclinical CVD pathophysiology for women. Second, the women in the present cohort were all premenopausal with regular menstruation. Therefore, it is likely that the power to observe sex differences was strengthened by this study design feature. Third, the path analysis approach, which is an extension of multiple regression, has been shown to provide utility in understanding relational data in multifactorial diseases like CVD [62]. In the present study, the analytic method permitted the evaluation of the mediation hypotheses in a single analysis of a model that included five traditional CVD risk indices as outcome variables, thereby reducing type 1 error [63]. Moreover, this analytic approach enabled testing of direct and indirect relationships among predictor and dependent variables, while controlling for all variables in the model simultaneously [64].
5. Conclusion
In sum, study findings have provided novel evidence in women of distinct pathways wherein VE may confer greater CVD vulnerability through specific fat depots. Given that these associations were observed in a nonclinical sample, results therefore suggest a decline in vitality for women may be an early CVD risk indicator and should be considered in an integrated clinical evaluation approach supporting treatment decision-making processes. To our knowledge, no other study to date has investigated whether specific regional fat depots, rather than total adiposity or body mass, mediate the relationship of VE with CVD risk. Further research is needed to evaluate in women what potential underlying biomechanisms may be operating to facilitate CVD risk through the linkage of VE with regional adiposity.
Acknowledgements
The authors thank the following individuals for their technical contributions to the study: Melanie Ashby, Carmen Baez Garcia, Nancy Gonzalez, Blaire Hall, Jennifer Marks, Meela Parker, and Kanoksri Sarinnapakorn.
Funding
This study was supported by a research grant (HL081817) awarded to B.E.H. and a training grant (HL007426) awarded to N.S. from the National Heart, Lung and Blood Institute of the National Institutes of Health.
Abbreviations:
- BMI
body mass index
- CVD
cardiovascular disease
- CFI
comparative fit index
- CRP
C-reactive protein
- DSM-IV
diagnostic and statistical manual of mental disorders – fourth edition
- Educ
education
- ECG
electrocardiogram
- EAT
epicardial adipose tissue
- HDL
high density lipoprotein
- IS
insulin sensitivity
- LAT
liver adipose tissue
- MQ
Maastricht questionnaire
- VO2 max
maximal oxygen uptake
- MAP
mean arterial pressure
- RMSEA
root-mean-square error of approximation
- SD
standard deviation
- SE
standard error
- SAT
subcutaneous adipose tissue
- TC
total cholesterol
- TC/HDL
total cholesterol to HDL ratio
- TG
triglycerides
- VAT
visceral adipose tissue
- VE
vital exhaustion
Footnotes
Declaration of Competing Interest
The authors have no competing interests to report.
The term, mediation, in this analysis, does not imply causality, but simply describes the possibility that one variable may partially account for the relationship between two other variables. In a path analysis, the total effect of the relationship between an independent variable and an outcome, following the introduction of a possible mediating variable, may be decomposed into a direct and indirect effect [34].
Data availability
The data that support these findings are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
References
- [1].Appels A, Mulder P, Excess fatigue as a precursor of myocardial infarction, Eur. Heart J. 9 (7) (1988) 758–764, 10.1093/eurheartj/9.7.758. [DOI] [PubMed] [Google Scholar]
- [2].Cohen R, Bavishi C, Haider S, Thankachen J, Rozanski A, Meta-analysis of relation of vital exhaustion to cardiovascular disease events, Am. J. Cardiol. 119 (8) (2017) 1211–1216, 10.1016/j.amjcard.2017.01.009. [DOI] [PubMed] [Google Scholar]
- [3].Frestad D, Prescott E, Vital exhaustion and coronary heart disease risk: a systematic review and meta-analysis, Psychosom. Med. 79 (3) (2017) 260–272, 10.1097/PSY.0000000000000423. [DOI] [PubMed] [Google Scholar]
- [4].Williams JE, Mosley TH, Kop WJ, Couper DJ, Welch VL, Rosamond WD, Vital exhaustion as a risk factor for adverse cardiac events (from the Atherosclerosis Risk in Communities [ARIC] study), Am. J. Cardiol. 105 (12) (2010) 1661–1665, 10.1016/j.amjcard.2010.01.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Iversen LB, Strandberg-Larsen K, Prescott E, Schnohr P, Rod NH, Psychosocial risk factors, weight changes and risk of obesity: the Copenhagen City Heart Study, Eur. J. Epidemiol. 27 (2) (2012) 119–130, 10.1007/s10654-012-9659-9. [DOI] [PubMed] [Google Scholar]
- [6].Suarez EC, Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity, Brain Behav. Immun. 22 (6) (2008) 960–968, 10.1016/j.bbi.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Toker S, Shirom A, Shapira I, Berliner S, Melamed S, The association between burnout, depression, anxiety, and inflammation biomarkers: C-reactive protein and fibrinogen in men and women, J. Occup. Health Psychol. 10 (4) (2005) 344–362, 10.1037/1076-8998.10.4.344. [DOI] [PubMed] [Google Scholar]
- [8].Valentine RJ, McAuley E, Vieira VJ, Baynard T, Hu L, Evans EM, et al. , Sex differences in the relationship between obesity, C-reactive protein, physical activity, depression, sleep quality and fatigue in older adults, Brain Behav. Immun. 23 (5) (2009) 643–648, 10.1016/j.bbi.2008.12.003. [DOI] [PubMed] [Google Scholar]
- [9].Piche ME, Poirier P, Lemieux I, Despres JP, Overview of epidemiology and contribution of obesity and body fat distribution to cardiovascular disease: an update, Prog. Cardiovasc. Dis. 61 (2) (2018) 103–113, 10.1016/j.pcad.2018.06.004. [DOI] [PubMed] [Google Scholar]
- [10].Klaus JR, Hurwitz BE, Llabre MM, Skyler JS, Goldberg RB, Marks JB, et al. , Central obesity and insulin resistance in the cardiometabolic syndrome: pathways to preclinical cardiovascular structure and function, J. Cardiometabolic Syndr. 4 (2) (2009) 63–71, 10.1111/j.1559-4572.2008.00038.x. [DOI] [PubMed] [Google Scholar]
- [11].Bryant MJ, Stevens J, Truesdale KP, Mosley T, Chambless L, Obesity and vital exhaustion: analysis of the atherosclerosis risk in the communities study, Obesity (Silver Spring) 16 (7) (2008) 1545–1551, 10.1038/oby.2008.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lim W, Hong S, Nelesen R, Dimsdale JE, The association of obesity, cytokine levels, and depressive symptoms with diverse measures of fatigue in healthy subjects, Arch. Intern. Med. 165 (8) (2005) 910–915, 10.1001/archinte.165.8.910. [DOI] [PubMed] [Google Scholar]
- [13].Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS, Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors, Circulation 132 (17) (2015) 1639–1647, 10.1161/CIRCULATIONAHA.114.015000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kotronen A, Westerbacka J, Bergholm R, Pietilainen KH, Yki-Jarvinen H, Liver fat in the metabolic syndrome, J. Clin. Endocrinol. Metab. 92 (9) (2007) 3490–3497, 10.1210/jc.2007-0482. [DOI] [PubMed] [Google Scholar]
- [15].Iacobellis G, Willens HJ, Echocardiographic epicardial fat: a review of research and clinical applications, J. Am. Soc. Echocardiogr. 22 (2009) 1311–1319, 10.1016/j.echo.2009.10.013. [DOI] [PubMed] [Google Scholar]
- [16].Igna CV, Julkunen J, Vanhanen H, Vital exhaustion, depressive symptoms and serum triglyceride levels in high-risk middle-aged men, Psychiatry 187 (2011) 363–369, 10.1016/j.psychres.2010.10.016. [DOI] [PubMed] [Google Scholar]
- [17].Mansour MF, Chan CWJ, Laforest S, Veilleux A, Tchernof A, Sex differences in body fat distribution, in: Symonds ME (Ed.), Adipose Tissue Biology, 2nd edn, Springer International Publishing, Cham, Switzerland, 2017, pp. 257–300. [Google Scholar]
- [18].Hurwitz BE, Schneiderman N, Marks JB, Mendez AJ, Gonzalez A, Llabre MM, et al. , Adaptation of beta-cell and endothelial function to carbohydrate loading: influence of insulin resistance, Diabetes 64 (7) (2015) 2550–2559, 10.2337/db15-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].First MB, Spitzer RL, Gibbon M, Williams JBW, Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP), Biometrics Research, New York State Psychiatric Institute, New York, 2002. [Google Scholar]
- [20].Appels A, Höppener P, Mulder P, A questionnaire to assess premonitory symptoms of myocardial infarction, Int. J. Cardiol. 17 (1) (1987) 15–24, 10.1016/0167-5273(87)90029-5. [DOI] [PubMed] [Google Scholar]
- [21].Meesters C, Appels A, An interview to measure vital exhaustion. II. Reliability and validity of the interview and correlations of vital exhaustion with personality characteristics, Psychol. Health 11 (4) (1996), 10.1080/08870449608401990, 573–58. [DOI] [Google Scholar]
- [22].Appels A, Bär F, van der Pol G, Erdman R, Assman M, Trijsburg W, et al. , Effects of treating exhaustion in angioplasty patients on new coronary events: results of the randomized Exhaustion Intervention Trial (EXIT), Psychosom. Med. 67 (2) (2005) 217–223, 10.1097/01.psy.0000151485.38411.36. [DOI] [PubMed] [Google Scholar]
- [23].Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, de la Bretonne J, et al. , Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity, Metab. Clin. Exp. 50 (4) (2001) 425–435, 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- [24].Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mauro U, et al. , Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction, Obes. Res. 11 (2003) 304–310, 10.1038/oby.2003.45. [DOI] [PubMed] [Google Scholar]
- [25].Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G, Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR), J. Clin. Invest. 100 (5) (1997) 1166–1173, 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Beltz NM, Gibson AL, Janot JM, Kravitz L, Mermier CM, Dalleck LC, Graded exercise testing protocols for the determination of VO2max: historical perspectives, progress, and future considerations. Bosch A, editor, J. Sports Med. (2016) 3968393, 10.1155/2016/3968393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. , Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls, BMJ 338 (2009), 10.1136/bmj.b2393b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang Y, Beydoun MA, The obesity epidemic in the United States–gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis, Epidemiol. Rev. 29 (2007) 6–28, 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- [29].Junghaenel DU, Christodoulou C, Lai JS, Stone AA, Demographic correlates of fatigue in the US general population: results from the patient-reported outcomes measurement information system (PROMIS) initiative, J. Psychosom. Res. 71 (3) (2011) 117–123, 10.1016/j.jpsychores.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McAuley PA, Kokkinos PF, Oliveira RB, Emerson BT, Myers JN, Obesity paradox and cardiorespiratory fitness in 12,417 male veterans aged 40 to 70 years, Mayo Clin. Proc. 85 (2) (2010) 115–121, 10.4065/mcp.2009.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bentler PM, Mooijaart A, Choice of structural model via parsimony: a rationale based on precision, Psychol. Bull. 106 (1989) 315–317, 10.1037/0033-2909.106.2.315. [DOI] [PubMed] [Google Scholar]
- [32].Hooper D, Coughlan J, Mullen M, Structural equation modelling: guidelines for determining model fit, Electron. J. Bus. Res. Methods 6 (1) (2008) 53–60, 10.21427/D7CF7R. [DOI] [Google Scholar]
- [33].Hu LT, Bentler PM, Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives, Struct. Equ. Model. Multidiscip. J. 6 (1) (1999) 1–55, 10.1080/10705519909540118. [DOI] [Google Scholar]
- [34].Albert JM, Cho JI, Liu Y, Nelson S, Generalized causal mediation and path analysis: extensions and practical considerations, Stat. Methods Med. Res. 28 (6) (2019) 1793–1807, 10.1177/0962280218776483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tarka P, An overview of structural equation modeling: its beginnings, historical development, usefulness and controversies in the social sciences, Qual. Quant. 52 (1) (2018) 313–354, 10.1007/s11135-017-0469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wajchenberg BL, Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome, Endocr. Rev. 21 (6) (2000) 697–738, 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- [37].Kornerup H, Marott JL, Schnohr P, Boysen G, Barefoot J, Prescott E, Vital exhaustion increases the risk of ischemic stroke in women but not in men: results from the Copenhagen City Heart Study, J. Psychosom. Res. 68 (2) (2010) 131–137, 10.1016/j.jpsychores.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Janszky I, Lekander M, Blom M, Georgiades A, Ahnve S, Self-rated health and vital exhaustion, but not depression, is related to inflammation in women with coronary heart disease, Brain Behav. Immun. 19 (6) (2005) 555–563, 10.1016/j.bbi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- [39].Koertge JC, Ahnve S, Schenck-Gustafsson K, Orth-Gomer K, Wamala SP, Vital exhaustion in relation to lifestyle and lipid profile in healthy women, Int. J. Behav. Med. 10 (1) (2003) 44–55, 10.1207/S15327558IJBM1001_04. [DOI] [PubMed] [Google Scholar]
- [40].Meyer T, Stanske B, Kochen MM, Cordes A, Yüksel I, Wachter R, et al. , Elevated serum levels of interleukin-10 and tumor necrosis factor are both associated with vital exhaustion in patients with cardiovascular risk factors, Psychosomatics 51 (3) (2010) 248–256, 10.1016/S0033-3182(10)70692-7. [DOI] [PubMed] [Google Scholar]
- [41].Hoekstra T, Barbosa-Leiker C, Twisk JW, Vital exhaustion and markers of low-grade inflammation in healthy adults: the Amsterdam Growth and Health Longitudinal Study, Stress. Health 29 (5) (2013) 392–400, 10.1002/smi.2485. [DOI] [PubMed] [Google Scholar]
- [42].Shirom A, Toker S, Melamed S, Berliner S, Shapira I, Burnout and vigor as predictors of the incidence of hyperlipidemia among healthy employees, Appl. Psychol. Health Well-Being 5 (2013) 79–98, 10.1111/j.1758-0854.2012.01071.x. [DOI] [PubMed] [Google Scholar]
- [43].Le Magueresse-Battistoni B, Adipose tissue and endocrine-disrupting chemicals: does sex matter? Int. J. Environ. Res. Public Health 17 (24) (2020) 10.3390/ijerph17249403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bracht JR, Vieira-Potter VJ, Santos R De Souza, Öz OK, Palmer BF, Clegg DJ, The role of estrogens in the adipose tissue milieu, Ann. N. Y. Acad. Sci. 1461 (1) (2020) 127–143, 10.1111/nyas.14281. [DOI] [PubMed] [Google Scholar]
- [45].Elbers JM, Asscheman H, Seidell JC, Megens JA, Gooren LJ, Long-term testosterone administration increases visceral fat in female to male transsexuals, J. Clin. Endocrinol. Metab. 82 (7) (1997) 2044–2047, 10.1210/jcem.82.7.4078. [DOI] [PubMed] [Google Scholar]
- [46].Elbers JM, Asscheman H, Seidell JC, Gooren LJ, Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals, Am. J. Phys. 276 (2) (1999) E317–E325, 10.1152/ajpendo.1999.276.2.E317. [DOI] [PubMed] [Google Scholar]
- [47].Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR, Increased visceral fat and decreased energy expenditure during the menopausal transition, Int. J. Obes. (2005) 32 (6) (2008) 949–958, 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Papadakis GE, Hans D, Gonzalez Rodriguez E, Vollenweider P, Waeber G, Marques-Vidal P, et al. , Menopausal hormone therapy is associated with reduced total and visceral adiposity: the OsteoLaus Cohort, J. Clin. Endocrinol. Metab. 103 (5) (2018) 1948–1957, 10.1210/jc.2017-02449. [DOI] [PubMed] [Google Scholar]
- [49].Gambacciani M, Ciaponi M, Cappagli B, Piaggesi L, De Simone L, Orlandi R, et al. , Body weight, body fat distribution, and hormonal replacement therapy in early postmenopausal women, J. Clin. Endocrinol. Metab. 82 (2) (1997) 414–417, 10.1210/jcem.82.2.3735. [DOI] [PubMed] [Google Scholar]
- [50].Hong SC, Yoo SW, Cho GJ, Kim T, Hur JY, Park YK, et al. , Correlation between estrogens and serum adipocytokines in premenopausal and postmenopausal women, Menopause (New York, NY) 14 (5) (2007) 835–840, 10.1097/GME.0b013e31802cddca. [DOI] [PubMed] [Google Scholar]
- [51].Page ST, Herbst KL, Amory JK, Coviello AD, Anawalt BD, Matsumoto AM, et al. , Testosterone administration suppresses adiponectin levels in men, J. Androl. 26 (1) (2005) 85–92, 10.1002/j.1939-4640.2005.tb02876.x. [DOI] [PubMed] [Google Scholar]
- [52].de Moraes SA, Szklo M, Knopman D, Park E, Prospective assessment of estrogen replacement therapy and cognitive functioning: atherosclerosis risk in communities study, Am. J. Epidemiol. 154 (8) (2001) 733–739, 10.1093/aje/154.8.733. [DOI] [PubMed] [Google Scholar]
- [53].Maxwell SE, Cole DA, Bias in cross-sectional analyses of longitudinal mediation, Psychol. Methods 12 (1) (2007) 23–44, 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- [54].Daviglus ML, Talavera GA, Avilés-Santa ML, Allison M, Cai J, Criqui MH, et al. , Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States, Jama 308 (17) (2012) 1775–1784, 10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lopez-Jimenez F, Lavie CJ, Hispanics and cardiovascular health and the “Hispanic Paradox”: what is known and what needs to be discovered? Prog. Cardiovasc. Dis 57 (3) (2014) 227–229, 10.1016/j.pcad.2014.09.007. [DOI] [PubMed] [Google Scholar]
- [56].Williams EP, Mesidor M, Winters K, Dubbert PM, Wyatt SB, Overweight and obesity: prevalence, consequences, and causes of a growing public health problem, Curr. Obes. Rep. 4 (3) (2015) 363–370, 10.1007/s13679-015-0169-4. [DOI] [PubMed] [Google Scholar]
- [57].Kudielka BM, von Känel R, Gander ML, Fischer JE, The interrelationship of psychosocial risk factors for coronary artery disease in a working population: do we measure distinct or overlapping psychological concepts? Behav. Med. (Washington, DC) 30 (1) (2004) 35–43, 10.3200/BMED.30.1.35-44. [DOI] [PubMed] [Google Scholar]
- [58].Balog P, Thege B Konkoly, The role of vital exhaustion in predicting the recurrence of vascular events: a longitudinal study, Int. J. Clin. Health Psychol. 19 (1) (2019) 75–79, 10.1016/j.ijchp.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Balog P, Falger PRJ, Szabó G, Rafael B, Székely A, Thege B Konkolÿ, Are vital exhaustion and depression independent risk factors for cardiovascular disease morbidity? Health Psychol. 36 (8) (2017) 740–748, 10.1037/hea0000495. [DOI] [PubMed] [Google Scholar]
- [60].Lewis-Fernández R, Das AK, Alfonso C, Weissman MM, Olfson M, Depression in US Hispanics: diagnostic and management considerations in family practice, J. Am. Board Fam. Med. 18 (4) (2005) 282–296, 10.3122/jabfm.18.4.282. [DOI] [PubMed] [Google Scholar]
- [61].Simpson SM, Krishnan LL, Kunik ME, Ruiz P, Racial disparities in diagnosis and treatment of depression: a literature review, Psychiatry Q 78 (1) (2007) 3–14, 10.1007/s11126-006-9022-y. [DOI] [PubMed] [Google Scholar]
- [62].Kopp M, Falger P, Appels A, Szedmak S, Depressive symptomatology and vital exhaustion are differentially related to behavioral risk factors for coronary artery disease, Psychosom. Med. 60 (6) (1998) 752–758, 10.1097/00006842-199811000-00018. [DOI] [PubMed] [Google Scholar]
- [63].Peres-Neto PR, How many statistical tests are too many? The problem of conducting multiple ecological inferences revisited, Mar. Ecol. Prog. Ser. 176 (1999) 303–306, 10.3354/meps176303. [DOI] [Google Scholar]
- [64].Anderson JC, Gerbing DW, Structural equation modeling in practice: a review and recommended two-step approach, Psychol. Bull. 103 (3) (1988) 411–423, 10.1037/0033-2909.103.3.411. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support these findings are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.


