ABSTRACT
Biological control is a more sustainable and environmentally friendly alternative to chemical fungicides for controlling Fusarium spp. infestations. In this work, Bacillus siamensis Sh420 isolated from wheat rhizosphere showed a high antifungal activity against Fusarium graminearum as a secure substitute for fungicides. Sh420 was identified as B. siamensis using phenotypic evaluation and 16S rDNA gene sequence analysis. An in vitro antagonistic study showed that Sh420’s lipopeptide (LP) extract exhibited strong antifungal properties and effectively combated F. graminearum. Meanwhile, lipopeptides have the ability to decrease ergosterol content, which has an impact on the overall structure and stability of the plasma membrane. The PCR-based screening revealed the presence of antifungal LP biosynthetic genes in this strain’s genomic DNA. In the crude LP extract of Sh420, we were able to discover several LPs such as bacillomycin, iturins, fengycin, and surfactins using ultra-high-performance liquid chromatography–quadrupole time-of-flight mass spectrometry. Microscopic investigations (fluorescent/transmission electron microscopy) revealed deformities and alterations in the morphology of the phytopathogen upon interaction with LPs. Sh420 LPs have been shown in grape tests to be effective against F. graminearum infection and to stimulate antioxidant activity in fruits by avoiding rust and gray lesions. The overall findings of this study highlight the potential of Sh420 lipopeptides as an effective biological control agent against F. graminearum infestations.
IMPORTANCE
This study addresses the potential of lipopeptide (LP) extracts obtained from the strain identified as Bacillus siamensis Sh420. This Sh420 isolate acts as a crucial player in providing a sustainable and environmentally friendly alternative to chemical fungicides for suppressing Fusarium graminearum phytopathogen. Moreover, these LPs can reduce ergosterol content in the phytopathogen influencing the overall structure and stability of its plasma membrane. PCR screening provided confirmation regarding the existence of genes responsible for biosynthesizing antifungal LPs in the genomic DNA of Sh420. Several antibiotic lipopeptide compounds were identified from this bacterial crude extract using ultra-high-performance liquid chromatography–quadrupole time-of-flight mass spectrometry. Microscopic investigations revealed deformities and alterations in the morphology of F. graminearum upon interaction with LPs. Furthermore, studies on fruit demonstrated the efficacy of Sh420 LPs in mitigating F. graminearum infection and stimulating antioxidant activity in fruits, preventing rust and gray lesions.
KEYWORDS: Bacillus siamensis, lipopeptides, antifungal activity, Fusarium graminearum, ergosterol, antioxidant activity
INTRODUCTION
Fusarium head blight (FHB) is a major disease caused by Fusarium graminearum (Fg) that contributes to severe economic losses to wheat and barley crops globally by reducing cellulose, amylose, and protein, reducing yield and productivity (1). Moreover, its secretory mycotoxin deoxynivalenol leads to feed refusal, gastrointestinal dysfunction, vomiting, and reduced immune functions (2). Controlling this toxin-producing pathogen is a significant challenge regarding food safety and food security. Fusarium fungus can infect plants through their roots, seeds, and wounds, especially if the cells that line the root cap are damaged (3).
Although different approaches for controlling FHB are used, removing or burying agricultural wastes infected with Fusarium after harvest is problematic due to limited tillage approaches (4). The utilization of host resistance as a means of reducing FHB is a cost-effective and environmentally friendly method, but so far, only a few extremely resistant wheat cultivars have been identified. Foliar fungicides used during anthesis can aid in scab reduction (5). Because fungicides have detrimental impacts on both human health and the environment, the cost of fungicides is rising, and more significantly, some resistant disease-causing species of fungi may be promoted (6). In the same way, crop rotation is also not an efficient technique to reduce FHB infestation (7). Hence, these strategies have some limitations in one way or another.
Recently, the cost-effective, efficient, and environmentally friendly nature of biological control has made this research a hot topic to inhibit the broad spectrum of fungal pathogens (8). Biocontrol techniques can assist growers in lowering their use of chemicals, hence reducing the development of fungicide resistance in pathogen populations (9). Antagonistic bacteria use diverse mechanisms for the biocontrol of Fusarium, including siderophore-mediated competition for iron, antibiotic production, and induced systemic resistance (10, 11). Many novel fungal biocontrol bacterial species have been discovered recently, which have antagonistic properties against F. graminearum and detoxification activity against its mycotoxin, and researchers are still looking for new strains. Among biocontrol agents, Bacillus species (12), lactic acid bacteria (13), and pseudomonads (14) are the most studied and well-known antibiotic agents due to their natural ability to create endospores and resistance to harsh environmental conditions. The antibiotic activity could be linked to nutrient competition, hydrolytic enzymes, production of antibiotics, and lipopeptides (LPs). These factors are thought to be the main mode of antifungal action by the antagonistic bacteria (15–17). According to gene sequencing, it has been discovered that over 4% of the genes present in Bacillus genome participate in the synthesis of antimicrobial compounds (18). Antimicrobial substances are typically produced through either ribosomal or non-ribosomal synthesis. Ribosomal synthesis gives rise to antimicrobial proteins, bacteriocin, bacteriocin-like inhibitory substances, and subtilin (19), while non-ribosomal peptide synthetases and polyketide synthases are responsible for the production of cyclic lipopeptides belonging to the surfactin, iturin, and fengycin families, as well as polyketides from the difficidin, bacillaene, and microlactin families (20, 21). Bacillus species like Bacillus subtilis, Bacillus pumilus, Bacillus amyloliquefaciens, and Bacillus licheniformis have been reported to produce LPs with high antifungal activity (22–24). LPs are synthesized by a complex of multienzymes called non-ribosomal peptide synthetases (NRPSs). These low-molecular-weight compounds typically consist of short sequence peptides, with generally less than 50 amino acids. Most of the identified LPs are antimicrobial peptides. NRPSs can generate a diverse range of variants based on their amino acid sequence and fatty acid chain length. In Bacillus species, multiple families of LPs and their isoforms can be produced simultaneously.
In this study, we identified Bacillus siamensis Sh420 (GenBank accession number SUB13288549), a gram-positive, rod-shaped, aerobic, and motile bacterium, as a potential inhibitor of F. graminearum. According to recent studies, B. siamensis NKIT9 lipopeptide extract inhibited the growth of Rhizoctonia solani (25). Two lipopeptides like iturin A and bacillomycin F from B. siamensis JFL15 showed good antifungal activity against Colletotrichum nymphaeae, Magnaporthe grisea, and Rhizoctonia solani (26). However, the antifungal potential of LPs produced by B. siamensis against F. graminearum has not yet been systematically studied.
Thus, the main goals of this study were to (i) isolate and identify bacterial strains with promising antifungal potential against F. graminearum, (ii) identify LPs synthesizing genes in the bacterial genome, (iii) identify antifungal LPs in the crude extract of Sh420 on the basis of molecular weight using ultra-high-performance liquid chromatography–quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS), (iv) determine fungicidal activity of LPs to disrupt cell membrane structures and its effect on ergosterol content of Fusarium, and (v) examine alterations in the morphology of Fusarium upon interaction with these LPs using a fluorescent microscope. Additionally, the grape fruit was used to assess the biocontrol capability of LPs, demonstrating how well they could protect the fruit against fungus while also measuring the antioxidant activity and total phenolic content of the lipopeptide-treated fruit. To the best of our knowledge, this is the first report to report the effectiveness of LP antifungal activity against F. graminearum, improvement of grape shelf life after LP treatment, and alterations in antioxidant activity and total phenolics in fruits because of LPs.
MATERIALS AND METHODS
Plant pathogenic fungi
Fusarium graminearum PH1 was obtained from the Institute of Plant Protection, Chinese Academy of Agriculture Science, Beijing, China. This pathogen was preserved in 30% glycerol at −80°C and available for future experimental use.
Isolation, selection, and characterization of bacterial strain
Bacterial strain was isolated from 35 root soil samples taken from the wheat-producing Pinggu and Langfang regions of China. Isolation was carried out with slight modifications in a previous method described by reference (27). A volume of 10 µL of each bacterial dilution solution was spread on Liquid Broth (LB) plates and incubated at 30°C for 1 day. The plates were then sprayed with spores of F. graminearum and incubated at 28°C for 5 days. Bacteria that showed inhibition of fungus were identified as antagonistic against F. graminearum. The serially diluted culture was then streaked onto nutrient agar (NA) plates and incubated at 28°C until single colonies appeared. Single colonies were picked and purified by streaking on fresh NA plates. A collection of about 290 strains was secured, and LPs were extracted from that strain that had high antifungal activity after screening. Pure bacterial isolates were grown in LB broth and kept at −80°C with the addition of 30% (wt/vol) glycerol for further use.
For the identification of the bacterial strain, genomic DNA was extracted, and PCR reaction of the 16S rDNA gene fragment was done with the universal primers. The obtained sequences were compared with existing sequences in GenBank by using the BLASTN tool and the phylogenetic tree constructed using similar sequences that were taken from NCBI with MEGA 7 based on the neighbor-joining method, and to evaluate the reliability of the tree, 1,000 bootstrap replications were performed accordingly (28). The inhibitory ratio was calculated by using the following formula:
Morphological and physiological characteristics of Sh420 bacterial isolate
A 15-µL culture was streaked onto a LB agar plate and then incubated at 37°C for 1 day. The resulting colony patterns were then examined using a stereomicroscope. For scanning electron microscopic (SEM) examination, bacterial culture broth grown for 1 day is obtained, centrifuged to collect specimens as a pallet, and then washed twice with phosphate buffer. It was then fixed in a 3% glutaraldehyde buffer and stored at 4°C for 1 day. In order to evaluate the colony and cell morphology of Sh420, we followed reference (29).
Culture conditions for maximum lipopeptide production and extraction of antifungal lipopeptides from Sh420
B. siamensis cells were incubated for 72 h at 37°C by shaking at 180 rpm in a specific medium optimal for lipopeptide production (MOLP), as previously used by reference (30), and MOLP media continued to be the preferred medium for subsequent studies.
LPs were isolated by the acid precipitation method with some modifications as described by reference (31). The strain was incubated in 1,000 mL of MOLP medium at 37°C at 180 rpm for 72 h. Then, it was centrifuged at 4°C at 10,000 rpm for 15 min. The supernatant was collected, and the pH was adjusted to 2 by adding 6 mol/L HCl, which appeared as a white precipitation. Now, this supernatant was put at 4°C after 24 h. It was centrifuged, the supernatant obtained was discarded, and the pellet was kept. Ten to fifteen milliliters of methanol solution was added to it, and again, pH was adjusted to 7.0 with 6 mol/L NaOH. The solution was dried with a rotary vacuum evaporator, and the resulting pellet was filtered using 0.22-µm sterile filter to obtain the lipopeptide crude extract.
Antifungal activity of the lipopeptides produced by Sh420
Different concentrations of lipopeptide extract solution, such as 0.5, 2, 4, 5, 7, 8, and 10 mg mL−1 (wt/vol), in distilled water were prepared to test the antifungal activity. A 2-day fungal mycelial plug was placed in the center of 90-mm plates, LPs were inoculated in wells to one side, and distilled water was loaded into the control well on the edges. The plate is then incubated at 28°C. After 7 days, mycelial growth inhibition was determined by the following formula:
Determination of minimal inhibitory concentration and minimal fungicidal concentration of the lipopeptides
Minimal inhibitory concentration (MIC) is the lowest concentration of lipopeptides required to inhibit fungal growth, while minimal fungicidal concentration (MFC) is the smallest concentration of lipopeptides capable of killing the fungi under standardized laboratory conditions. The experiment was carried out in liquid medium according to the protocol described in section “Culture conditions for maximum lipopeptide production and extraction of antifungal lipopeptides from Sh420”. A volume of 500 µL F. graminearum spore suspension of concentration 7 × 106 conidia mL−1 was poured in 1,200-µL Potato Dextrose Broth (PDB) media. Different concentrations of lipopeptides are used against the fungus growth in a 24-well plate to check MIC after 6 days. The plates were incubated at 28°C for 7 days. The results were obtained by observing the presence or absence of fungal growth. The whole content from each well, where there was no growth of F. graminearum, was passed to tubes with PDB medium and incubated at 25°C for 7 days for the determination of MFC. The protocol was designed with some modifications to reference (32).
Determination of ergosterol by UV spectrophotometry
Ergosterol content in the cell membrane of Fg is investigated by a method as reported previously by reference (33). A volume of 100 µL of 107 spore/mL of F. graminearum spore suspension was added to PDB media containing 0, 1, 2, and 4 mg/mL of lipopeptide extract. The mixture was then incubated for 5 days at 28°C. After the incubation period, the cells were collected using centrifugation and suspended in an alcoholic KOH solution (25% wt/vol, 3 mL) for 1 h at 85°C in a water bath. Once the samples were cooled to ambient temperature, sterols were isolated by adding 1 mL of distilled water and 3 mL of n-heptane. The mixture was then vortexed for 3 min to separate the heptane layer, which was subsequently diluted with ethanol before undergoing a spectrophotometric analysis at 240–300 nm wavelengths.
PCR assay for screening of lipopeptide genes in bacterial isolate
Sh420 was screened for the presence of genes encoding various LPs. The PCR parameters were adjusted as done by reference (34). Each reaction was done in triplicate; a negative control was also included in the PCR amplification. The amplified PCR product from each reaction was visualized on 1% agarose gel. Lipopeptide-specific primers and their fragment size used in this study to amplify LP’s biosynthetic genes are enlisted in Table S1.
UPLC-QTOF-MS analysis of antifungal lipopeptides
The lipopeptide crude extract used for inhibition is utilized to identify compounds. One-milligram crude extract was dissolved in 2 mL of 10% methanol and analyzed by UPLC (Acquity UPLC R BEH300, Waters) coupled with high-definition mass spectrometry (Waters synapt G2-si UPLC-QTOF/MS). Mass spectrometry was carried out by positive ionization electrospray. The obtained data were processed by the MassLynx software (Waters).
Microscopic visualization of mycelia treated with lipopeptides using a fluorescent microscope and transmission electron microscopy
A bright field, fluorescent microscope (Himedia) was used to observe Fusarium hyphal and spore structures after treatment with LP extract. Fluorescent stains such as propidium iodide (PI) (stains only dead cells) and calcofluor white (CFW) (Sigma-Aldrich) were used to study the fungal mycelia and spores under fluorescent microscopy (Nikon, Japan). A 30-µmol concentration of PI and CFW was prepared separately from their respective stock solutions. A combination of PI and CFW was prepared in a 1:1 ratio, and 20 mL from both stains was used separately for fungal staining. After putting the combination of stains on mycelial pieces on a slide, treated and control samples were incubated for 6 min in a dark room at 25°C before being examined under a fluorescent microscope. All selected fungal tissues were observed using fluorescent microscopy (Nikon DAPI-FITC-TRITC filter combinations).
For transmission electron microscopy (TEM) investigation, hyphae were fixed for 4 h at room temperature in 2% glutaraldehyde, washed four times with 0.1 M phosphate buffer, and then fixed for 2 h with 1% osmium tetraoxide. The hyphae were coated with gold and palladium using a Nanotech sputter coating device and were then examined using a SEM (HITACHI, S-570, Japan) operating at 15 kV as previously stated by Zhao et al. (35).
Biocontrol assay against F. graminearum in fruits
Conidia were harvested from mature Fusarium. The surface of the grape fruit was sterilized with 5% NaOCl for 5 min and rinsed three times with plenty of sterile water. Wounds of 3 mm were made with a sterile scalpel on the surface of the grapes; then, 12 mL of a 10 mg mL−1 solution of lipopeptides was applied to the injuries and sprayed on the surface of the treated samples. One hour later, when the fruit was dried at room temperature, 0.5 mL of a suspension of F. graminearum 107 conidia mL−1 was inoculated into the wounds. Fruits treated with sterile water act as negative control. Fruits treated with F. graminearum act as positive control. All the treatments were incubated at 25°C, 70% humidity for 5 days. For each treatment, a total of nine fruits were used, and three technical replicates were performed. Results were calculated by the method described in Table 1. The effect was measured and expressed as disease incidence (% of infected fruit).
TABLE 1.
Fungal severity calculation scale on grape berries
| Grade | Symptoms |
|---|---|
| 0 | No infection |
| 1 | Very small spot |
| 2 | One infected spot |
| 3 | Two or four infected spots |
| 4 | <50% berry was infected, and sporulation was evident |
| 5 | >50% berry was infected, and sporulation was evident |
Antioxidant activity and estimation of total phenolics of fruit treated with lipopeptides
A weight of 0.5 g of lyophilized fruit sample was mixed with 6 mL of 80% methanol and 0.1% hydrochloric acid, vortexed to obtain a homogeneous mixture, and then stored for 4–5 h at 4°C to assess the fruit’s antioxidant potential and phenolic activity. The supernatant was filtered, and the extract was used for the determination of the phenol content and the Ferric Reducing Antioxidant Power Assay (FRAP). In a test tube, 1 mL of the sample was mixed with 2 mL of phosphate buffer and 2.5 mL of potassium ferricyanide solution, and then, the test tube was wrapped in an aluminum foil. Afterward, the test tube was incubated in a water bath at 50°C for 20 min. After shaking, 2.5 mL of 10% trichloroacetic acid was added and centrifuged at 3,000 rpm for 5–6 min. The upper 2.5 mL of supernatant was taken to a new test tube. One milliliter of distilled water was added to the new tube. Now, 0.5-mL ferric chloride was added to that upper layer, and a thorough mix was given. A bluish compound formation was obtained. Now, 200 µL was taken from the tubes and poured into a 96-well plate. Absorbance optical density (OD) was taken at 700 nm. The higher the absorbance, the higher the antioxidant activity. Ascorbic acid was used as positive control. The percentage of antioxidant activity was calculated using the formula:
For total phenolic estimation, 100 µL of the sample extract and 400 µL of distilled water were added and mixed to dilute the extract, and 150 µL of FC reagent (diluted with distilled water in the ratio 1:1 vol/vol) was added. After vortexing, it was kept at room temperature for 5 min. Then, a bluish color compound was formed by adding 500 µL sodium carbonate and incubating in the dark for 1 h. Then, a microplate reader was used to read the absorbance at 650 nm (Bio-Rad, iMARK, Japan). For 500 µL of methanol, all the reagents were added except fruit extract and considered as blank. Gallic acid was taken as a standard to determine the phenolic content of the samples.
Statistical analyses
CRD design was implemented to conduct the experiment. The data collected were analyzed using ANOVA, for comparing means between groups. Following this, Duncan’s multiple range test was used to make comparisons between pairs of groups.
RESULTS
Antagonistic activity and identification of bacterial isolate
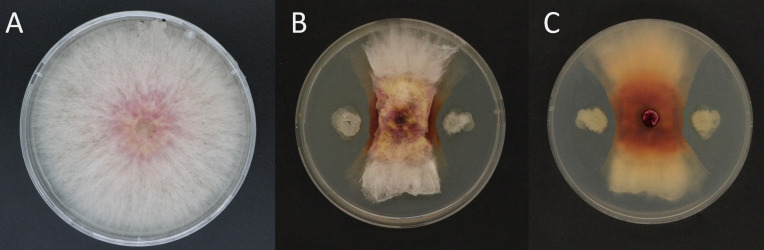
In this research, over 290 bacterial strains were isolated from soil samples of wheat rhizosphere. After screening, we selected Sh420 strain for further analysis, as it exhibited significant antagonistic activity against F. graminearum in a dual culture method and observed that 5 µL of Sh420 with 107 CFU/mL effectively inhibited the mycelia growth indicated by the inhibition zones on Potato Dextrose Agar (PDA) (Fig. 1).
Fig 1.
Antifungal activity of B. siamensis Sh420 against F. graminearum in dual culture plate on PDA media at sixth of incubation at 28°C. (A) CK, a 5-mm agar plug of F. graminearum at the center of PDA plate and (B) front side of plate where Sh420 is inoculated on two sites that are 2.5 cm apart from the F. graminearum colony. (C) Back side of the dual culture plate.
After analyzing the 16S rDNA gene sequence of the isolates using blast software (NCBI database), it was determined that the isolate Sh420 belongs to the Bacillus genus having a correlation of 99.78% with known species (Fig. S1). Hence, it is identified as B. siamensis, which is a gram-positive, rod-shaped bacterium. It can produce a range of enzymes and bioactive compounds. Further study is required to truly understand its possible applications. In a previous study, the LZ88 strain of B. siamensis showed an 81.96% inhibition rate, against brown spot disease in tobacco, caused by Alternaria alternata (36). The partial 16S rDNA gene sequences of strain Sh420 were added to the GenBank database with accession no. SUB13288549.
Morphological and physiological characteristics of Bacillus siamensis Sh420
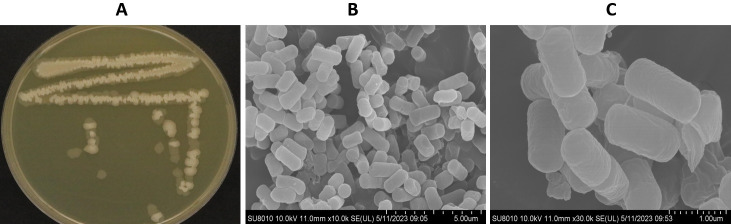
The B. siamensis Sh420 colony had a slightly rough texture and was off-white to creamy in color with well-defined and clear colony edges on LB agar. The colonies were linked together and appeared as a chain when viewed via a stereomicroscope. SEM revealed that Sh420 cells were straight and rod-shaped with round ends, organized in chains, and motile (Fig. 2). The bacterial cell was 1–1.7 µm in length and 2–5 µm in width.
Fig 2.
Morphological features of B. siamensis Sh420. (A) Colony morphology. (B) Bacterial cells in chain form. (C) Single bacterium cells.
Antifungal activity of lipopeptide and screening of lipopeptide genes
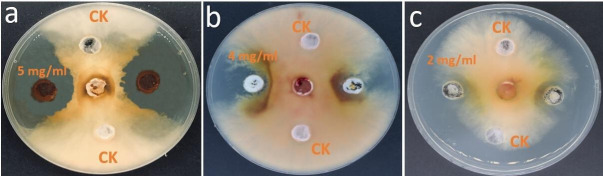
LPs showed antifungal activity against F. graminearum in the well inoculation method. Inhibition rates of 70%, 62%, and 40% of the mycelia diameter were observed for the concentrations of LPs of 5, 4, and 2 mg mL−1, respectively, after 7 days of treatment (Fig. 3). In general, LPs from Sh420 showed antagonistic activity against F. graminearum across a broad spectrum of concentrations in a dose-dependent manner. LPs were screened using certain primers that have been previously researched in Bacillus species-related literature. From these specific genes, we identified many important lipopeptide genes using PCR reaction; those include Loap (antiterminator protein that regulates antibiotic gene clusters), FenB (fengycin), ituA (iturin), itu D (iturin D), BmyA (bacillomycin A), dhbA (bacillibactin), sfp (surfactin), srfA (surfactin), dfnA (difficidin), dfnM (difficidin), bacA (bacilysin), and beaB (bacillaene). These antibiotic genes were detected on gel visualization (Fig. S2).
Fig 3.
Antifungal activity of B. siamensis Sh420 lipopeptides against F. graminearum. Assessment of different concentrations of LPs against fungal growth: reverse side of Petri plates showing inhibition zones and control in the same plate: (A) 5 mg mL−1 LPs, (B) 4 mg mL−1 LPs, and (C) 2 mg mL−1 LPs.
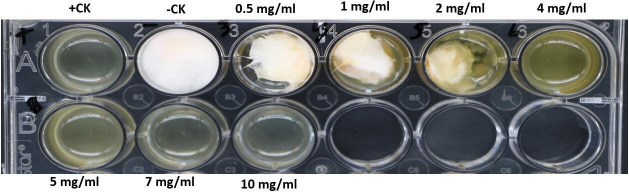
Determination of MIC and MFC of the lipopeptides
Lipopeptides from Sh420 were tested against F. graminearum in 24 multiwell culture plates to check the MIC and MFC. Different concentrations of lipopeptides were tested ranging from 0.5 to 10 mg mL−1. In PDB broth, Fusarium growth was observed to be decreased and stopped with increasing lipopeptide concentrations. At a lipopeptide concentration of 5 mg mL−1, no fungus germinated in PDB after 7 days of incubation. Thus, the MIC of lipopeptide against Fg was determined to be 5 mg mL−1 (Fig. 4).
Fig 4.
MIC and MFC of lipopeptides from Sh420. +CK (positive control) medium inoculated with amphotericin B for fungal growth inhibition, while −CK (negative control) PDB inoculated with spore suspension without any treatment.
Ergosterol content determination
Ergosterol is a critical component of fungal membranes and plays a crucial role in maintaining their structure and function (37). To confirm the impact of the lipopeptide extract, the plasma membrane’s ergosterol content was measured. The effects of lipopeptides on the ergosterol content in the plasma membrane of F. graminearum are shown in (Fig. 5). The cells were treated with different concentrations of lipopeptide extract (0, 1, 2, and 4 mg/mL), and the results demonstrated that the levels of ergosterol in the F. graminearum membranes decreased significantly in a dose-dependent manner, with the maximum inhibition observed at 4 mg/mL. The highest level of absorbance is observed at a wavelength of 282 nm. This finding indicates that lipopeptides have a considerable impact on inhibiting the biosynthesis of ergosterol in F. graminearum, disrupting the production of this essential fungal membrane component. This disruption may lead to impaired growth and function of the fungus.
Fig 5.
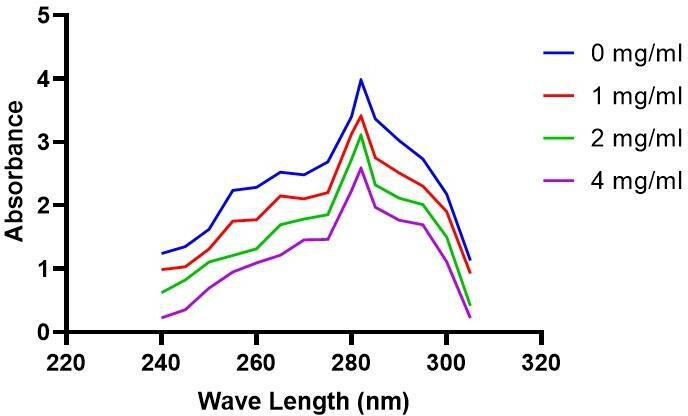
Spectrometric analysis of ergosterol profile of F. graminearum at various concentrations of lipopeptides.
Identification of lipopeptides using UPLC-QTOF/MS analysis
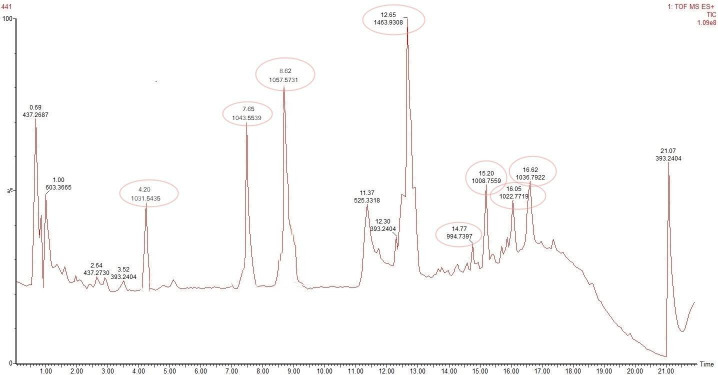
UPLC-QTOF mass spectrometry examination of the purified extract led to the identification of different lipopeptides from various groups. By comparing the mass information obtained from individual peak fractions with the mass data reported for the cyclic lipopeptides from various Bacillus species strains, it is possible to identify the lipopeptide from the crude extract of Sh420 using the total ion chromatogram (TIC) spectrum and corresponding (m/z) values of peaks (Fig. 6). The main lipopeptide compounds of strain Sh420 were identified as bacillomycin D (C14) and two known iturins A2 (C14) and iturin A3-A5 (C-15). Fengycin A (C16) is also found in the extract. Four known surfactins with an acyl chain ranging from C12 to C15 were also detected. Analysis using quadrupole time-of-flight mass spectrometry revealed a total of eight main LP compounds from four different classes of lipopeptides. This investigation detected a [M+H] peak at m/z 1,031.5435 with the molecular formula C48H75N10O15 that represents bacillomycin D. Another [M+H] peak at m/z 1,043.5539 afforded the molecular formula C48H74N12O14, which represents iturin A, and another [M+H] peak at m/z 1,057.5731 corresponding to iturin A3 with the molecular formula C49H76N12O14 is detected. Such similar LP with almost similar mass is reported in previous literature too. At retention time (Rt) of 12.65, a [M+H] peak at m/z 1,463.9308 with the molecular formula C72H110N12O20 (i-Fit D 130.3 and DBE D 23.5) conforms to fengycin A. Furthermore, four other surfactin isomers were identified from the extract. At 14.77 retention time, a peak with protonated molecular ion [M+H] at m/z 994.7397 is identified as surfactin with a C12 fatty acid chain. Other surfactins at Rt 15.20, 16.05, and 16.62 with [M+H] at m/z = 1,008.7559, m/z = 1,036, and m/z = 1,022.7719 with the molecular formula C52H91N7O13 were identified, respectively (Fig. 6), as reported in previous literature compounds with almost similar mass identified during LP’s studies (Table 2) (38, 39).
Fig 6.
TOF-MS ES + TIC of Sh420 LPs with their molecular weight (m/z) and Rt.
TABLE 2.
Lipopeptide production by B. siamensis Sh420 detected by UPLC-QTOF
| Lipopeptide | Chain length | Retention time | Current experimental mass/[M+H]+ | Previously reported mass | Reference |
|---|---|---|---|---|---|
| Bacillomycin D | C14 | 4.20 | 1,031.5435 | 1,031.5431 | (39) |
| Iturin C | C14 | 7.65 | 1,043.5539 | 1,043.528 | (40) |
| Iturin A3 | C15 | 8.62 | 1,057.5731 | 1,057.5830 | (41) |
| Fengycin A | C16 | 12.65 | 1,463.9308 | 1,463.8185 | (41) |
| Surfactin | C12 | 14.77 | 994.7397 | 994.64 | (42) |
| Surfactin | C13 | 15.20 | 1,008.7559 | 1,008.75 | (43) |
| Surfactin | C14 | 16.05 | 1,022.7719 | 1,022.6729 | (39) |
| Surfactin | C15 | 16.62 | 1,036.7922 | 1,036.6954 | (41) |
Effect of Sh420 bacterial strain and its lipopeptides on F. graminearum mycelia and spore structures
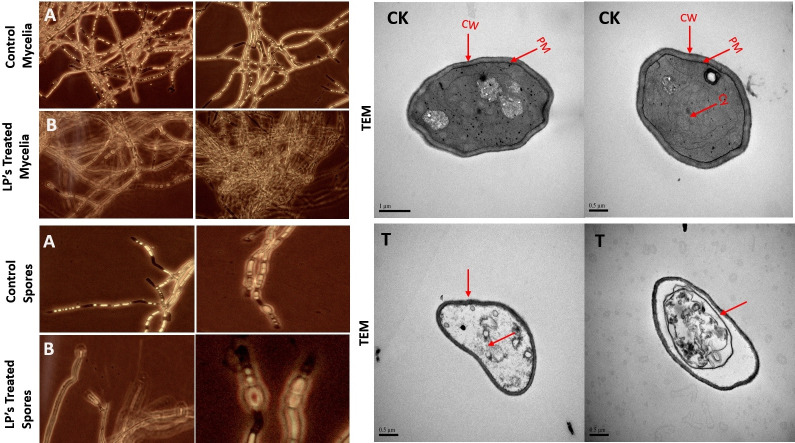
Lipopeptide extract showed significant inhibitory effect and damaging effects against F. graminearum pathogen. Lipopeptide extract effects on Fusarium were analyzed using CFW plus PI staining. The chitin contained in the cell walls of fungi is stained by CFW, which distinguishes between live and dead cells, whereas PI exclusively stains dead cells. Luminous dye was used in fluorescent microscopy (Nikon, Japan), which revealed abnormal hyphae and deformations in the fungus. In samples treated with lipopeptide, considerable numbers of dead Fusarium hyphae and their spores were seen using CFW staining, whereas no or very few damaged or dead cells were evident in control samples. Spores showed swelling and bulging in the treated samples. Furthermore, disintegration and lysis of spore/cells were also observed. However, smooth and normal structures of spores without any deformation were observed in untreated Fusarium (Fig. 7). Burst or swelled portions of the hyphae or spores had blurred images as compared to controls. This may be due to the loss of chitin and glucans from the cell walls. Lipopeptides create pores in fungal hyphae by depolarizing membranes, inhibiting chitin and glucan synthases, and inducing apoptosis. As CFW stained the chitin contained in the cell wall and spores, live fungal mycelia cells and spores were clearly visible, which have intact cell walls; however, burst/ruptured or damaged cells were not clearly visible (blurred) at spore tips and edges. Further analysis using TEM confirmed that Sh420 LPs caused cellular deformities such as loss of integrity, cell wall and plasma membrane damage, cell shrinkage, cytoplasmic displacement, and degeneration of organelles in the fungal hyphae, while control hyphae maintained good cellular shape, dense cytosol, and integrity.
Fig 7.
Effects of lipopeptides on F. graminearum. Control mycelia and spores (A). Treated mycelia and spores (B). Treated fungi showed deformation, swelling, and bulging in hyphae and spores, which are clearly visible in bright field burst or swelled portions of the hyphae, or spores had blurred images as compared to controls. TEM photographs of treated F. graminearum where red arrows showed the abnormal membranes and cell structure as compared to untreated mycelia.
Biocontrol assays of Sh420 lipopeptides against F. graminearum pathogenicity in grapes
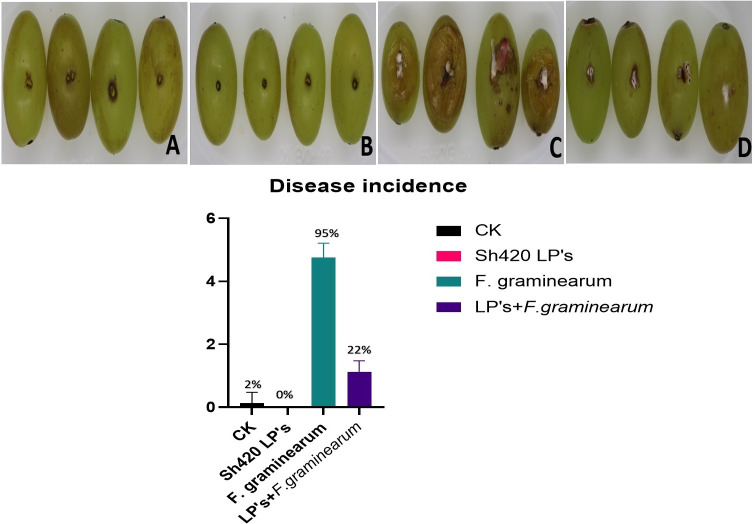
To assess the protective effect of lipopeptides on fruits infected with Fusarium, the MIC/MFC of lipopeptide extract from Sh420 (5 mg mL−1) was used. The lipopeptides were sprayed on and injected too after surface sterilization. The prevalence of disease infections decreased on fruits treated with Sh420 LPs. The disease incidence in grapes treated with Fg was 95%. The disease inhibition in fruits treated with Sh420 LPs was 100%, and no disease symptoms and even gray spots or lesions have appeared on the grapes. On the other hand, the group treated with both LPs and Fusarium showed a disease incidence of 22% (Fig. 8). These findings demonstrated that fruits treated with antifungal LP extract have considerably reduced disease symptoms compared to untreated fruits. Overall, the disease incidence declined significantly in grapes.
Fig 8.
Lipopeptides from Sh420 inhibited disease severity in grapes. Disease severity of gray mold on grapes. (A) Grapes treated with sterile water (negative control). (B) Grapes treated with LPs. (C) Grapes infected with Fusarium. (D) Grapes infected with both F. graminearum and LPs. (E) Graphical representation of disease severity in all four groups.
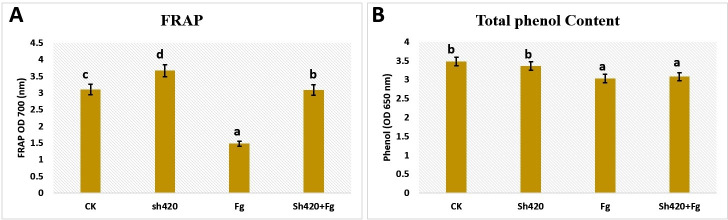
Antioxidant activity on fruit treated with lipopeptides
According to this study, grapes have the highest antioxidant activity. Using MIC of the lipopeptides produced by Sh420 greatly enhanced the antioxidant activity of grape extracts as evaluated by the FRAP experiment (Fig. 9A). The antioxidant activity was highest in LP-treated grapes; however, in the control group, it was relatively low. Antioxidant activity was lowest in grapes exposed to a disease. However, in terms of phenol content, uninfected grapes (CK) showed a significant increase in total phenol content, followed by the LP-treated group. The pathogen-treated group and the grapes treated with both LPs and Fusarium pathogen displayed almost the same trend as a result of exposure to lipopeptides (Fig. 9B).
Fig 9.
Antioxidant activity evaluated with the FRAP assay (A) and total phenols (B) of grapes treated with LPs. Control (CK), Sh420 (lipopeptide-treated), F. graminearum (Fg), and LPs+Fg. Values followed by the same letters did not differ significantly according to Duncan’s multiple range test (P < 0.05). Vertical lines represent the standard errors of the mean.
DISCUSSION
Bacillus LPs are a class of secondary metabolites, which possess a wide range of antimicrobial activities, including inhibitory effects against fungi (44). In this study, we isolated and identified the LPs from B. siamensis and checked its fungicidal potential against F. graminearum that badly affects cereal crops, causing FHB, which leads to significant economic losses worldwide. To avoid these losses and control such pathogens, researchers are looking for various solutions. One of which is the identification of LPs and their potential antibiotic action against various fungal strains. Previous studies have shown that the antifungal action of Bacillus species is caused by the production of LPs such as surfactin, iturin, bacillomycin, and fengycin. These LPs are produced by NRPSs (45). These compounds consist of a peptide chain and a lipid tail, making them amphiphilic and able to interact with cell membranes. Their antifungal activity has been attributed to various mechanisms, including disruption of cell membrane integrity, inhibition of cell wall synthesis, and induction of oxidative stress (46).
Our LP extract showed inhibitory activity against F. graminearum in vitro. The LP extract showed higher inhibitory activity with the increase of their concentration. Likewise, B. subtilis B1 LPs combat the Lasiodiplodia theobromae fungus (47). B. siamensis LPs displayed antifungal activities against multiple fungi like M. grisea, R. solani, and Colletotrichum gloeosporioides (26). Fengycin lipopeptides from B. subtilis BS155 induce membrane damage and organelle dysfunction, disrupt mitochondrial membrane potential, cause oxidative stress, and condense chromatin, ultimately leading to the death of M. grisea hyphal cells (48).
Moreover, ergosterol production is significantly disrupted in F. graminearum due to lipopeptides. LPs work by obstructing the sterol production process, especially when it comes to ergosterol. These fungal sterols consist of two main compounds, ergosterol and 24 (28)-dehydroergosterol. These sterols exhibit their highest level of absorption at a wavelength of 281.5 nm (33). Our results showed maximum absorption at 282 nm. This characteristic absorption pattern provides valuable insights into determining ergosterol levels. This disruption affects both structural integrity and the proper functioning of the membrane. The overall effect of LPs is the prevention of fungal growth since ergosterol also has a hormone-like effect on fungal cells, triggering growth processes. Similarly, according to a study, the Fusarium oxysporum f. sp. Cubense ergosterol level is reduced due to two cyclic LPs from Streptomyces sp. XY006 (49). Likewise, 4-methyl hexanoyl conjugated trimeric battacin lipopeptide displayed antifungal activity by reducing the ergosterol and suppressing the biofilms of Candida albicans (50). Amphotericin B, used against Candida albicans, has a similar action by interacting with ergosterol and causing membrane breakdown, intracellular content leakage, and cell death of fungi (51).
In our investigation, the biosynthetic genes for LPs from the genome of B. siamensis were amplified using specific primers for the detection of various forms of lipopeptides using PCR, which confirmed the presence of gene clusters for various NRP’s lipopeptide while some of the genes are not detected in Sh420. Several current studies have stated that the genomes of Bacillus species like B. amyloliquefaciens (52), B. subtilis (42), and B. siamensis contain biosynthetic genes that express antifungal LPs, including bacillomycin, surfactin, fengycin, bacillibactin, iturin, subtilosin, and bacilysin (53).
We identified the MIC of the LP extract, which was as low as 5 mg mL−1. Thus, the MIC of lipopeptide against Fg was determined to be 5 mg mL−1 (Fig. 4). These observations confirm the strong antifungal activity of the extract. In previous studies, MIC/MFC of LPs from Bacillus methylotrophicus XT1 was 8 mg mL−1 against Botrytis cinerea (39). LPs from B. subtilis SPB1 showed a MIC of 3 mg mL−1 against Fusarium solani (54). MICs depend on the type of bacteria and the quantity of lipopeptides in the extract. Likewise, the MIC concentration of a single peptide from B. amyloliquefaciens against fungal growth was 30 mg L−1 (55). The presence of antifungal LPs in the crude extract of Sh420 was further confirmed by UPLC-QTOF/MS analysis. The LPs were detected based on their mass-to-charge ratio (m/z). The main lipopeptide compounds of strain Sh420 were identified as bacillomycin D, two known iturins, and fengycin A, and four known surfactins with an acyl chain ranging from C12 to C15 were also detected (Table 2). After PCR amplification, many different LPs were shown on the gel, but later, some of these compounds were not found using UPLC-QTOF mass spectrometry analysis. It is possible that their concentrations were too low, in which case their peaks might not have shown. Studies have reported the potential of Bacillus species for producing several antifungal LPs against different fungal infections, which were detected by MS based on their molecular weight (40). LPs with antifungal properties against Sclerotinia sclerotiorum were identified in a study where LC-MS analysis was conducted on the crude mixture of 47 strains of Bacillus (56). The effects of lipopeptides on F. graminearum fungal hyphae and spores were visualized under fluorescent microscopy. In LPs-treated fungi, hyphae and spores had deformation, swelling, and bulging that were clearly evident in bright field, while swollen or ruptured sections of the hyphae or spores exhibited fuzzy or blurred pictures. LPs can cause the formation of pores in fungal hyphae by inducing depolarization of membranes, suppressing the synthesis of chitin and glucans, and triggering apoptosis, which could be attributed to the absence of chitin and glucans from cell walls. In a recent study, LPs were found to inhibit the growth of Verticillium dahliae by inducing cell lysis and hyphal swelling, as well as downregulating genes related to protein catabolism and secondary metabolism signaling pathways and melanin production (57). Mihalache et al. (58) studied the effects of LPs on F. oxysporum, which demonstrated that control hyphae were normal/healthy and exhibited typical conidiophore and microconidia. Conversely, the LP-treated hyphae exhibited shrinkage, perforation, and disintegration with small, curly, and narrow branching (58). Fluorescence microscopy along with SEM showed that Fusarium mycelia and spores showed shivering, formation of pores in the membrane, leakage of protoplasmic substances from cells that leads to death of hyphae, and spores due to LP treatment (59). Lipopeptides produced by B. subtilis YM have the potential to make fungal spores more permeable, preventing their germination (60). Furthermore, lipopeptides from B. subtilis BS-99-H lead to the swelling and distortion of the fungal hyphae in Pestalotiopsis eugeniae (61).
In this study, we also evaluated the protective effects of LPs from Sh420 against mold infestation and lesions in fruits (grapes). Since no disease symptoms or lesions were seen on fruits treated with Sh420 lipopeptides, the disease was completely inhibited in fruits treated with LPs, and no lesions or even gray spots were seen on the grapes. Disease incidence in grapes treated only with fungal spores was 95%. Likewise, the grapes treated with both LPs and Fusarium showed disease incidence of 22% (Fig. 6). According to earlier studies, grapevines are protected from B. cinerea infection and given localized protection by lipopeptides generated from B. subtilis (62). Similarly, LPs by B. subtilis Y17B possess significant biocontrol abilities to combat A. alternata fruit rot in cherry fruit (42). LPs produced are recognized by plant cells through specific receptors and activate different signaling pathways, including the MAPK cascade, leading to the activation of defense-related genes. This recognition and activation of plant defense responses are important in plant–microbe interactions, as they contribute to the plant’s ability to defend against pathogens (63).
This study has found that Sh420 LPs have the potential to activate the antioxidant properties in fruit and protect them from getting rusty/brownish. The greatest increase in antioxidant activity was seen in fruits treated with LPs. By exposing the fruit to LPs produced by Sh420, the total phenolic content was significantly increased in control and LP-treated grapes, followed by the combined treatment of LPs and pathogen. The findings suggest that the antibiotic effects of LPs and the accumulation of antioxidant compounds may be linked to the fruit’s ability to resist pathogens. In short, LPs have a significant antibiotic and biocontrol potential against molds; hence, they can be used for plants for the competitive inhibition of phytopathogens.
Conclusion
The study focused on examining the effectiveness of Sh420 lipopeptides in preventing the growth of F. graminearum, a fungus responsible for causing FHB in grains. It was found that the lipopeptides produced by the strain were able to control the growth of F. graminearum by inhibiting ergosterol synthesis and stimulating antioxidant activity in fruits. The results showed that Sh420 had a significant inhibitory potential to control fungal pathogens. It also has the potential to protect the fruits against molds and lesions and act as an oxidant, making it a promising alternative to chemical fungicides for reducing damage caused by Fusarium.
ACKNOWLEDGMENTS
Conceptualization was performed by S.H. and T.B.; formal analysis was performed by S.H., G.W., and M.A.; data curation was performed by S.H., I.J., S.S., X.Z., and B.B.; writing (original draft) was performed by S.H.; writing (review and editing) was performed by F.X., T.B., and M.A.; resources/validation/investigation was performed by I.J. and G.W.; supervision was performed by F.X.
Contributor Information
Fuguo Xing, Email: xingfuguo@caas.cn.
Renato Kovacs, University of Debrecen, Debrecen, Hungary.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.04008-23.
Gene primers, phylogenetic analysis, and gel documentation.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Hameed A, Poznanski P, Noman M, Ahmed T, Iqbal A, Nadolska-Orczyk A, Orczyk W. 2022. Barley resistance to Fusarium graminearum infections: from transcriptomics to field with food safety concerns. J Agric Food Chem 70:14571–14587. doi: 10.1021/acs.jafc.2c05488 [DOI] [PubMed] [Google Scholar]
- 2. Jia B, Lin H, Yu S, Liu N, Yu D, Wu A. 2023. Mycotoxin deoxynivalenol-induced intestinal flora disorders, dysfunction and organ damage in broilers and pigs. J Hazard Mater 451:131172. doi: 10.1016/j.jhazmat.2023.131172 [DOI] [PubMed] [Google Scholar]
- 3. Reyna M, Pia Macor E, Carolina Vilchez A, Laura Villasuso A. 2023. Response in barley roots during interaction with Bacillus subtilis and Fusarium graminearum. Biological Control 179:105128. doi: 10.1016/j.biocontrol.2022.105128 [DOI] [Google Scholar]
- 4. Leslie JF, Moretti A, Mesterházy Á, Ameye M, Audenaert K, Singh PK, Richard-Forget F, Chulze SN, Ponte EMD, Chala A, Battilani P, Logrieco AF. 2021. Key global actions for mycotoxin management in wheat and other small grains. Toxins (Basel) 13:725. doi: 10.3390/toxins13100725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paul PA, Bradley CA, Madden LV, Dalla Lana F, Bergstrom GC, Dill-Macky R, Wise KA, Esker PD, McMullen M, Grybauskas A, Kirk WW, Milus E, Ruden K. 2018. Effects of pre-and postanthesis applications of demethylation inhibitor fungicides on Fusarium head blight and deoxynivalenol in spring and winter wheat. Plant Disease 102:2500–2510. doi: 10.1094/PDIS-03-18-0466-RE [DOI] [PubMed] [Google Scholar]
- 6. Cowger C, Arellano C, Marshall D, Fitzgerald J. 2019. Managing Fusarium head blight in winter barley with cultivar resistance and fungicide. Plant Dis 103:1858–1864. doi: 10.1094/PDIS-09-18-1582-RE [DOI] [PubMed] [Google Scholar]
- 7. Czaban J, Wróblewska B, Sułek A, Mikos M, Boguszewska E, Podolska G, Nieróbca A. 2015. Colonisation of winter wheat grain by Fusarium spp. and mycotoxin content as dependent on a wheat variety, crop rotation, a crop management system and weather conditions. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 32:874–910. doi: 10.1080/19440049.2015.1019939 [DOI] [PubMed] [Google Scholar]
- 8. Mohamad OAA, Li L, Ma J-B, Hatab S, Xu L, Guo J-W, Rasulov BA, Liu Y-H, Hedlund BP, Li W-J. 2018. Evaluation of the antimicrobial activity of endophytic bacterial populations from Chinese traditional medicinal plant Licorice and characterization of the bioactive secondary metabolites produced by Bacillus atrophaeus against Verticillium dahliae. Front Microbiol 9:924. doi: 10.3389/fmicb.2018.00924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rojas EC, Jensen B, Jørgensen HJL, Latz MAC, Esteban P, Ding Y, Collinge DB. 2020. Selection of fungal endophytes with biocontrol potential against Fusarium head blight in wheat. Biological Control 144:104222. doi: 10.1016/j.biocontrol.2020.104222 [DOI] [Google Scholar]
- 10. Wang H, Liu R, You MP, Barbetti MJ, Chen Y. 2021. Pathogen biocontrol using plant growth-promoting bacteria (PGPR): role of bacterial diversity. Microorganisms 9:1988. doi: 10.3390/microorganisms9091988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim M-J, Radhakrishnan R, Kang S-M, You Y-H, Jeong E-J, Kim J-G, Lee I-J. 2017. Plant growth promoting effect of Bacillus amyloliquefaciens H-2-5 on crop plants and influence on physiological changes in soybean under soil salinity. Physiol Mol Biol Plants 23:571–580. doi: 10.1007/s12298-017-0449-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khan M, Salman M, Ahmad Jan S, Khan Shinwari Z. 2021. Biological control of fungal phytopathogens: a comprehensive review based on Bacillus species. MOJBM 6:90–92. doi: 10.15406/mojbm.2021.06.00137 [DOI] [Google Scholar]
- 13. Manjarres Melo JJ, Álvarez A, Ramirez C, Bolivar G. 2021. Antagonistic activity of lactic acid bacteria against phytopathogenic fungi isolated from cherry tomato (solanum lycopersicum var. cerasiforme). Curr Microbiol 78:1399–1408. doi: 10.1007/s00284-021-02416-w [DOI] [PubMed] [Google Scholar]
- 14. Sun X, Xu Y, Chen L, Jin X, Ni H. 2021. The salt-tolerant phenazine-1-carboxamide-producing bacterium Pseudomonas aeruginosa NF011 isolated from wheat rhizosphere soil in dry farmland with antagonism against Fusarium graminearum. Microbiol Res 245:126673. doi: 10.1016/j.micres.2020.126673 [DOI] [PubMed] [Google Scholar]
- 15. Kumar K, Pal G, Verma A, Verma SK. 2021. Role of rhizospheric bacteria in disease suppression during seedling formation in millet, p 263–274. In Plant, soil and microbes in tropical ecosystems. Springer. [Google Scholar]
- 16. Sarwar A, Brader G, Corretto E, Aleti G, Ullah MA, Sessitsch A, Hafeez FY. 2018. Qualitative analysis of biosurfactants from Bacillus species exhibiting antifungal activity. PLoS One 13:e0198107. doi: 10.1371/journal.pone.0198107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Medeot DB, Fernandez M, Morales GM, Jofré E. 2020. Fengycins from Bacillus amyloliquefaciens MEP218 exhibit antibacterial activity by producing alterations on the cell surface of the pathogens xanthomonas axonopodis pv. vesicatoria and Pseudomonas aeruginosa PA01. Front Microbiol 10:3107. doi: 10.3389/fmicb.2019.03107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caulier S, Nannan C, Gillis A, Licciardi F, Bragard C, Mahillon J. 2019. Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front Microbiol 10:302. doi: 10.3389/fmicb.2019.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tajbakhsh M, Karimi A, Fallah F, Akhavan MM. 2017. Overview of ribosomal and non-ribosomal antimicrobial peptides produced by gram positive bacteria. Cell Mol Biol 63:20–32. doi: 10.14715/cmb/2017.63.10.4 [DOI] [PubMed] [Google Scholar]
- 20. Mora I, Cabrefiga J, Montesinos E. 2015. Cyclic lipopeptide biosynthetic genes and products, and inhibitory activity of plant-associated Bacillus against phytopathogenic bacteria. PLoS One 10:e0127738. doi: 10.1371/journal.pone.0127738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baltz RH. 2017. Gifted Microbes for genome mining and natural product discovery. J Ind Microbiol Biotechnol 44:573–588. doi: 10.1007/s10295-016-1815-x [DOI] [PubMed] [Google Scholar]
- 22. Desmyttere H, Deweer C, Muchembled J, Sahmer K, Jacquin J, Coutte F, Jacques P. 2019. Antifungal activities of Bacillus subtilis lipopeptides to two venturia Inaequalis strains possessing different tebuconazole sensitivity. Front Microbiol 10:2327. doi: 10.3389/fmicb.2019.02327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yan F, Li C, Ye X, Lian Y, Wu Y, Wang X. 2020. Antifungal activity of lipopeptides from Bacillus amyloliquefaciens MG3 against colletotrichum gloeosporioides in loquat fruits. Biological Control 146:104281. doi: 10.1016/j.biocontrol.2020.104281 [DOI] [Google Scholar]
- 24. Chen Y, Liu SA, Mou H, Ma Y, Li M, Hu X. 2017. Characterization of lipopeptide biosurfactants produced by Bacillus licheniformis MB01 from marine sediments. Front Microbiol 8:871. doi: 10.3389/fmicb.2017.00871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sharma A, Kaushik N, Sharma A, Bajaj A, Rasane M, Shouche YS, Marzouk T, Djébali N. 2021. Screening of tomato seed bacterial endophytes for antifungal activity reveals lipopeptide producing Bacillus siamensis strain NKIT9 as a potential bio-control agent. Front Microbiol 12:609482. doi: 10.3389/fmicb.2021.609482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu B-H, Lu Y-Q, Ye Z-W, Zheng Q-W, Wei T, Lin J-F, Guo L-Q. 2018. Genomics-guided discovery and structure identification of cyclic lipopeptides from the Bacillus siamensis JFL15. PLoS One 13:e0202893. doi: 10.1371/journal.pone.0202893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu S, Wang Y, Hu J, Chen X, Qiu Y, Shi J, Wang G, Xu J. 2021. Isolation and characterization of Bacillus amyloliquefaciens MQ01, a bifunctional biocontrol bacterium with antagonistic activity against Fusarium graminearum and biodegradation capacity of zearalenone. Food Control 130:108259. doi: 10.1016/j.foodcont.2021.108259 [DOI] [Google Scholar]
- 28. Imade FN, Humza M, Dada OA, Ullah S, Jahan I, Eseigbe D, Geng H, Zheng Y, Xing F, Liu Y. 2023. Isolation and characterization of novel soil bacterium, Klebsiella pneumoniae strain GS7-1 for the degradation of zearalenone in major cereals. Food Control 143:109287. doi: 10.1016/j.foodcont.2022.109287 [DOI] [Google Scholar]
- 29. Jahan I, Tai B, Ma J, Hussain S, Du H, Guo L, Wang G, Adegoke TV, Ma L, Xing F. 2023. Identification of a novel Bacillus velezensis IS-6 nudix hydrolase Nh-9 involved in ochratoxin A detoxification by transcriptomic profiling and functional verification. J Agric Food Chem 71:10155–10168. doi: 10.1021/acs.jafc.3c01910 [DOI] [PubMed] [Google Scholar]
- 30. Ahimou F, Jacques P, Deleu M. 2000. Surfactin and Iturin A effects on Bacillus subtilis surface hydrophobicity. Enzyme Microb Technol 27:749–754. doi: 10.1016/s0141-0229(00)00295-7 [DOI] [PubMed] [Google Scholar]
- 31. Ma Y, Kong Q, Qin C, Chen Y, Chen Y, Lv R, Zhou G. 2016. Identification of lipopeptides in Bacillus megaterium by two-step ultrafiltration and LC–ESI–MS/MS. AMB Express 6:79. doi: 10.1186/s13568-016-0252-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frikha-Gargouri O, Ben Abdallah D, Ghorbel I, Charfeddine I, Jlaiel L, Triki MA, Tounsi S. 2017. Lipopeptides from a novel Bacillus methylotrophicus 39b strain suppress agrobacterium crown gall tumours on tomato plants. Pest Manag Sci 73:568–574. doi: 10.1002/ps.4331 [DOI] [PubMed] [Google Scholar]
- 33. Kocsis B, Kustos I, Kilár F, Nyul A, Jakus PB, Kerekes S, Villarreal V, Prókai L, Lóránd T. 2009. Antifungal unsaturated cyclic mannich ketones and amino alcohols: study of mechanism of action. Eur J Med Chem 44:1823–1829. doi: 10.1016/j.ejmech.2008.10.038 [DOI] [PubMed] [Google Scholar]
- 34. Aydi Ben Abdallah R, Stedel C, Garagounis C, Nefzi A, Jabnoun-Khiareddine H, Papadopoulou KK, Daami-Remadi M. 2017. Involvement of lipopeptide antibiotics and chitinase genes and induction of host defense in suppression of Fusarium wilt by endophytic Bacillus spp. in tomato. Crop Protection 99:45–58. doi: 10.1016/j.cropro.2017.05.008 [DOI] [Google Scholar]
- 35. Zhao Y, Selvaraj JN, Xing F, Zhou L, Wang Y, Song H, Tan X, Sun L, Sangare L, Folly YME, Liu Y. 2014. Antagonistic action of Bacillus subtilis strain SG6 on Fusarium graminearum. PLoS ONE 9:e92486. doi: 10.1371/journal.pone.0092486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xie Z, Li M, Wang D, Wang F, Shen H, Sun G, Feng C, Wang X, Chen D, Sun X. 2021. Biocontrol efficacy of Bacillus siamensis LZ88 against brown spot disease of tobacco caused by Alternaria alternata. Biological Control 154:104508. doi: 10.1016/j.biocontrol.2020.104508 [DOI] [Google Scholar]
- 37. Ermakova E, Zuev Y. 2017. Effect of ergosterol on the fungal membrane properties. all-atom and coarse-grained molecular dynamics study. Chem Phys Lipids 209:45–53. doi: 10.1016/j.chemphyslip.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 38. DeFilippi S, Groulx E, Megalla M, Mohamed R, Avis TJ. 2018. Fungal competitors affect production of antimicrobial lipopeptides in Bacillus subtilis strain B9–5. J Chem Ecol 44:374–383. doi: 10.1007/s10886-018-0938-0 [DOI] [PubMed] [Google Scholar]
- 39. Toral L, Rodríguez M, Béjar V, Sampedro I. 2018. Antifungal activity of lipopeptides from Bacillus XT1 CECT 8661 against botrytis cinerea. Front Microbiol 9:1315. doi: 10.3389/fmicb.2018.01315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shahid I, Han J, Hanooq S, Malik KA, Borchers CH, Mehnaz S. 2021. Profiling of metabolites of Bacillus spp. and their application in sustainable plant growth promotion and biocontrol. Front Sustain Food Syst 5:605195. doi: 10.3389/fsufs.2021.605195 [DOI] [Google Scholar]
- 41. de Souza CG, Martins F, Zocolo GJ, Figueiredo JEF, Canuto KM, de Brito ES. 2018. Simultaneous quantification of lipopeptide Isoforms by UPLC-MS in the fermentation broth from Bacillus subtilis CNPMS22. Anal Bioanal Chem 410:6827–6836. doi: 10.1007/s00216-018-1281-6 [DOI] [PubMed] [Google Scholar]
- 42. Ahmad T, Xing F, Nie C, Cao C, Xiao Y, Yu X, Moosa A, Liu Y. 2023. Biocontrol potential of lipopeptides produced by the novel Bacillus subtilis strain Y17B against postharvest alternaria fruit rot of cherry. Front Microbiol 14:1150217. doi: 10.3389/fmicb.2023.1150217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Malfanova N, Franzil L, Lugtenberg B, Chebotar V, Ongena M. 2012. Cyclic lipopeptide profile of the plant-beneficial endophytic bacterium Bacillus subtilis HC8. Arch Microbiol 194:893–899. doi: 10.1007/s00203-012-0823-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao H, Shao D, Jiang C, Shi J, Li Q, Huang Q, Rajoka MSR, Yang H, Jin M. 2017. Biological activity of lipopeptides from Bacillus. Appl Microbiol Biotechnol 101:5951–5960. doi: 10.1007/s00253-017-8396-0 [DOI] [PubMed] [Google Scholar]
- 45. Cochrane SA, Vederas JC. 2016. Lipopeptides from Bacillus and paenibacillus spp.: a gold mine of antibiotic candidates. Med Res Rev 36:4–31. doi: 10.1002/med.21321 [DOI] [PubMed] [Google Scholar]
- 46. Wang Y, Zhang C, Liang J, Wu L, Gao W, Jiang J. 2020. Iturin A extracted from Bacillus subtilis WL-2 affects phytophthora infestans via cell structure disruption, oxidative stress, and energy supply dysfunction. Front Microbiol 11:536083. doi: 10.3389/fmicb.2020.536083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sajitha KL, Dev SA, Maria Florence EJ. 2016. Identification and characterization of lipopeptides from Bacillus subtilis B1 against sapstain fungus of rubberwood through MALDI-TOF-MS and RT-PCR. Curr Microbiol 73:46–53. doi: 10.1007/s00284-016-1025-9 [DOI] [PubMed] [Google Scholar]
- 48. Zhang L, Sun C. 2018. Fengycins, cyclic lipopeptides from marine Bacillus subtilis strains, kill the plant-pathogenic fungus magnaporthe grisea by inducing reactive oxygen species production and chromatin condensation. Appl Environ Microbiol 84:e00445-18. doi: 10.1128/AEM.00445-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang X, Du Z, Chen C, Guo S, Mao Q, Wu W, Wu R, Han W, Xie P, Zeng Y, Shan W, Wang Z, Yu X. 2023. Antifungal effects and biocontrol potential of lipopeptide-producing streptomyces against banana Fusarium wilt fungus Fusarium oxysporum f. sp. cubense. Front Microbiol 14. doi: 10.3389/fmicb.2023.1177393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. De Zoysa GH, Glossop HD, Sarojini V. 2018. Unexplored antifungal activity of linear battacin lipopeptides against planktonic and mature biofilms of C. albicans. Eur J Med Chem 146:344–353. doi: 10.1016/j.ejmech.2018.01.023 [DOI] [PubMed] [Google Scholar]
- 51. Gray KC, Palacios DS, Dailey I, Endo MM, Uno BE, Wilcock BC, Burke MD. 2012. Amphotericin primarily kills yeast by simply binding ergosterol. Proc Natl Acad Sci U S A 109:2234–2239. doi: 10.1073/pnas.1117280109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alvarez F, Castro M, Príncipe A, Borioli G, Fischer S, Mori G, Jofré E. 2012. The plant‐associated Bacillus amyloliquefaciens strains MEP218 and ARP23 capable of producing the cyclic lipopeptides iturin or surfactin and fengycin are effective in biocontrol of sclerotinia stem rot disease. J Appl Microbiol 112:159–174. doi: 10.1111/j.1365-2672.2011.05182.x [DOI] [PubMed] [Google Scholar]
- 53. Wang S-Y, Herrera-Balandrano DD, Wang Y-X, Shi X-C, Chen X, Jin Y, Liu F-Q, Laborda P. 2022. Biocontrol ability of the Bacillus amyloliquefaciens group, B. amyloliquefaciens, B. velezensis, B. nakamurai, and B. siamensis, for the management of fungal postharvest diseases: a review. J Agric Food Chem 70:6591–6616. [DOI] [PubMed] [Google Scholar]
- 54. Mnif I, Hammami I, Triki MA, Azabou MC, Ellouze-Chaabouni S, Ghribi D. 2015. Antifungal efficiency of a lipopeptide biosurfactant derived from Bacillus subtilis SPB1 versus the phytopathogenic fungus, Fusarium solani. Environ Sci Pollut Res Int 22:18137–18147. doi: 10.1007/s11356-015-5005-6 [DOI] [PubMed] [Google Scholar]
- 55. Zhang Q-X, Zhang Y, Shan H-H, Tong Y-H, Chen X-J, Liu F-Q. 2017. Isolation and identification of antifungal peptides from Bacillus amyloliquefaciens W10. Environ Sci Pollut Res Int 24:25000–25009. doi: 10.1007/s11356-017-0179-8 [DOI] [PubMed] [Google Scholar]
- 56. Farzand A, Moosa A, Zubair M, Rashid Khan A, Hanif A, Tahir HAS, Gao X. 2019. Marker assisted detection and LC-MS analysis of antimicrobial compounds in different Bacillus strains and their antifungal effect on Sclerotinia sclerotiorum. Biological Control 133:91–102. doi: 10.1016/j.biocontrol.2019.03.014 [DOI] [Google Scholar]
- 57. Ali H, Afzal MN, Muhammad D. 2009. Effect of sowing dates and plant spacing on growth and dry matter partitioning in cotton (gossypium hirsutum L.). Pak J Bot 41:2145–2155. [Google Scholar]
- 58. Mihalache G, Balaes T, Gostin I, Stefan M, Coutte F, Krier F. 2018. Lipopeptides produced by Bacillus subtilis as new biocontrol products against fusariosis in ornamental plants. Environ Sci Pollut Res Int 25:29784–29793. doi: 10.1007/s11356-017-9162-7 [DOI] [PubMed] [Google Scholar]
- 59. Kumar K, Verma A, Pal G, White JF, Verma SK. 2021. Seed endophytic bacteria of pearl millet (pennisetum glaucum L.) promote seedling development and defend against a fungal phytopathogen. Front Microbiol 12:774293. doi: 10.3389/fmicb.2021.774293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chitarra GS, Breeuwer P, Nout MJR, van Aelst AC, Rombouts FM, Abee T. 2003. An antifungal compound produced by Bacillus subtilis YM 10–20 inhibits germination of penicillium roqueforti conidiospores. J Appl Microbiol 94:159–166. doi: 10.1046/j.1365-2672.2003.01819.x [DOI] [PubMed] [Google Scholar]
- 61. Lin H, Chen T, Liu S. 2010. Bioactivity of antifungal substance Iturin A produced by Bacillus subtilis strain BS-99-H against Pestalotiopsis eugeniae, a causal pathogen of wax apple fruit rot. Plant Pathology Bulletin 19:225–233. [Google Scholar]
- 62. Farace G, Fernandez O, Jacquens L, Coutte F, Krier F, Jacques P, Clément C, Barka EA, Jacquard C, Dorey S. 2015. Cyclic lipopeptides from Bacillus subtilis activate distinct patterns of defence responses in grapevine. Mol Plant Pathol 16:177–187. doi: 10.1111/mpp.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Waewthongrak W, Leelasuphakul W, McCollum G. 2014. Cyclic lipopeptides from Bacillus subtilis ABS–S14 elicit defense-related gene expression in citrus fruit. PLoS One 9:e109386. doi: 10.1371/journal.pone.0109386 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene primers, phylogenetic analysis, and gel documentation.