ABSTRACT
Invasive aspergillosis (IA) and mucormycosis are life-threatening diseases, especially among immunocompromised patients. Drug-resistant Aspergillus fumigatus strains have been isolated worldwide, which can pose a serious clinical problem. As IA mainly occurs in patients with compromised immune systems, the ideal therapeutic approach should aim to bolster the immune system. In this study, we focused on Vγ9Vδ2 T cells that exhibit immune effector functions and examined the possibility of harnessing this unconventional T cell subset as a novel therapeutic modality for IA. A potent antifungal effect was observed when A. fumigatus (Af293) hyphae were challenged by Vγ9Vδ2 T cells derived from peripheral blood. In addition, Vγ9Vδ2 T cells exhibited antifungal activity against hyphae of all Aspergillus spp., Cunninghamella bertholletiae, and Rhizopus microsporus but not against their conidia. Furthermore, Vγ9Vδ2 T cells also exhibited antifungal activity against azole-resistant A. fumigatus, indicating that Vγ9Vδ2 T cells could be used for treating drug-resistant A. fumigatus. The antifungal activity of Vγ9Vδ2 T cells depended on cell-to-cell contact with A. fumigatus hyphae, and degranulation characterized by CD107a mobilization seems essential for this activity against A. fumigatus. Vγ9Vδ2 T cells could be developed as a novel modality for treating IA or mucormycosis.
IMPORTANCE
Invasive aspergillosis (IA) and mucormycosis are often resistant to treatment with conventional antifungal agents and have a high mortality rate. Additionally, effective antifungal treatment is hindered by drug toxicity, given that both fungal and human cells are eukaryotic, and antifungal agents are also likely to act on human cells, resulting in adverse effects. Therefore, the development of novel therapeutic agents specifically targeting fungi is challenging. This study demonstrated the antifungal activity of Vγ9Vδ2 T cells against various Aspergillus spp. and several Mucorales in vitro and discussed the mechanism underlying their antifungal activity. We indicate that adoptive immunotherapy using Vγ9Vδ2 T cells may offer a new therapeutic approach to IA.
KEYWORDS: γδ T cell, invasive aspergillosis, filamentous fungi, nitrogen-containing bisphosphonate prodrug
INTRODUCTION
Aspergillus molds, especially Aspergillus fumigatus, are causative agents of invasive aspergillosis (IA), a fatal disease occurring mainly in severely immunocompromised hosts, such as patients with hematologic malignancies and transplant recipients (1–3). Despite the development of novel antifungal agents and improved treatment strategies, the mortality rate of IA remains high at 35% (1). Furthermore, the increase and global spread of azole-resistant A. fumigatus hampers conventional IA treatments and poses a worldwide challenge (4–7). In addition, mucormycosis is an invasive fungal infection caused by over 200 species, including Rhizopus spp. and Cunninghamella spp. (8). These fungal species are naturally resistant to azole and other antifungals, contributing to a high fatality rate of 54% (9).
Recent research advances in the mechanism underlying immune responses to Aspergillus spp. (10–12) indicate that IA resistance to standard therapy may result from host’s failure to induce appropriate immune responses. Indeed, the outcome of invasive mold infections in severely immunocompromised patients depends on host factors, including the resolution of neutropenia (13, 14). This has led to the emergence of immunotherapy as an alternative approach to conventional antifungal drug treatment (15). The antifungal effects of natural killer (NK) cells against A. fumigatus have been demonstrated, and the adoptive transfer of NK cells for treating IA has been explored (16–18).
Besides NK cells, innate or innate-like immune effector cells exist in humans, such as CD4CD8-double negative T cells, including NKT cells and γδ T cells. Human Vγ9Vδ2-bearing γδ T cells (Vγ9Vδ2 T cells) account for 1%–5% of circulating T cells in the peripheral blood, exhibit innate immune-like functions, and can damage infected cells and tumor cells similar to NK cells (19, 20). In a previous study, Vγ9Vδ2 T cells produced a significant level of tumor necrosis factor-α (TNF-α) in response to water-soluble extracts from Aspergillus spp. (21); however, the antigen and mechanism underlying the antigen recognition remain unknown. It is also unknown whether Vγ9Vδ2T cells possess antifungal activity against Aspergillus.
In healthy adults, the majority of peripheral blood γδ T cells express Vγ9Vδ2-bearing T cell receptors (TCRs), which recognize microbial (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP) in the 2-C-methyl-d-erythritol 4-phosphate/1-deoxy-d-xylulose 5-phosphate (MEP/DOXP) pathway and self-isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) in the mevalonate pathway in a butyrophilin (BTN) 2A1/3A1-dependent manner (22, 23).
HMBPP is produced by some pathogenic bacteria, such as Mycobacterium tuberculosis, Mycobacterium bovis, Listeria monocytogenes, Escherichia coli, Salmonella typhimurium, and parasites, such as Plasmodium falciparum and Toxoplasma gondii (24, 25). When Vγ9Vδ2 T cells recognize these pathogen-derived metabolites, they readily proliferate and produce interferon-γ (IFN-γ) and TNF-α (26), mounting a rapid response against the pathogens. The antibacterial activity of Vγ9Vδ2 T cells against M. tuberculosis has been reported (27, 28). A clinical study on allogeneic Vγ9Vδ2 T cell-based immunotherapy in patients with multidrug-resistant tuberculosis demonstrated the regimen to be well tolerated and effective against the pathogen (29), indicating its potential applicability to IA.
We have previously shown that Vγ9Vδ2 T cells from peripheral blood could be expanded ex vivo using PTA, a nitrogen-containing bisphosphonate prodrug and an inhibitor of farnesyl diphosphate synthase (FDPS), and interleukin-2 (IL-2) (30, 31). In addition, a clinical trial of therapeutic administration of Vγ9Vδ2T cells to patients with malignant tumors has revealed that the regimen is well tolerated (32). In this study, we evaluated the antifungal activity of ex vivo expanded/activated human Vγ9Vδ2T cells against Aspergillus spp. and other Mucorales in vitro and explored the mechanism underlying their antifungal activity. Our findings will help develop Vγ9Vδ2 T cells as a novel treatment modality for IA or mucormycosis.
RESULTS
Human Vγ9Vδ2 T cells exhibit direct cytotoxic activity against A. fumigatus filamentous hyphae
To examine whether human Vγ9Vδ2 T cells exhibit antifungal activity against A. fumigatus, we assessed their effect on the viability of A. fumigatus hyphae in a direct co-culture system. We first expanded Vγ9Vδ2 T cells from peripheral blood mononuclear cells (PBMCs) derived from healthy adult volunteers using PTA and IL-2. The initial proportion of CD3+Vδ2+ T cells in PBMCs was 10.7% (Fig S1A). After expansion with PTA/IL-2, the proportion of CD3+Vδ2+ T cells reached 99.5%, with almost all the CD3+Vδ2+ T cells expressing Vγ9. The number of Vγ9Vδ2 T cells increased by 1,008-fold, increasing from 1.31 × 106 cells to 1.32 × 109 cells. During the expansion with PTA/IL-2, Vγ9Vδ2 T cells began to form explicit clusters on day 4, and maximum clustering was attained on day 6. On day 11, the resulting highly homogeneous Vγ9Vδ2 T cells were frozen and stored in a liquid nitrogen tank until used for subsequent assays.
To confirm the effector functions of the PTA/IL-2-expanded Vγ9Vδ2 T cells, we first examined the cell surface markers expressed on Vγ9Vδ2 T cells. As shown in Fig S2, the PTA/IL-2-expanded Vγ9Vδ2 T cells expressed a high level of NKG2D (CD314), an activating C-type lectin-like receptor, and DNAX accessory molecule-1 (DNAM-1, CD226), a type I membrane protein containing 2 Ig-like C2-type domains. However, the expression levels of FasL, a Fas ligand (CD95L), and TRAIL, a 30 kDa transmembrane protein known as TNF-related apoptosis-inducing ligand (CD253, also called TNFSF10 or Apo-2L), were marginal. This indicates that Vγ9Vδ2 T cells exert effector functions through NKG2D and DNAM-1 but not cell death receptor ligands. When Raji, a human malignant Burkitt’s lymphoma cell line, was incubated with the PTA/IL-2-expanded Vγ9Vδ2 T cells, over 30% of Raji cells were killed by Vγ9Vδ2 T cells at an effect-to-target (E/T) ratio of 200, in which Vγ9Vδ2 T cells exhibited the anti-tumor effect in an E/T ratio-dependent manner. Furthermore, the addition of PTA to this system further enhanced the effector functions, with Vγ9Vδ2 T cells killing approximately 80% of Raji cells in 1 h at the E/T ratio of 200, confirming that the PTA/IL-2-expanded Vγ9Vδ2 T cells could exhibit potent effector functions against tumor cells(online supplementary file 1).
In addition, we examined several cell surface makers expressed on Vγ9Vδ2 T cells derived from peripheral blood stimulated with PTA/IL-2 for 0, 3, and 11 days to gain insight into the possible functions of Vγ9Vδ2 T cells in stationary and activated phases. As shown in Fig S4, the expression of CD369 (Dectin-1, CLEC7A), CD282 (TLR-2), CD284 (TLR-4), FasL, and TRAIL was low at all the time points. In contrast, a high level of DNAM-1 expression was observed at all time points. The expression of NKG2D was initially high but decreased on day 3 after stimulation and subsequently resumed to an initial level on day 11. Conversely, CD25 was not expressed in the steady state, but was expressed to a markedly high level on day 3 after stimulation, and the expression gradually decreased thereafter. Programmed death-1 (PD-1, CD279) was slightly expressed on day 0, and the expression level increased on day 3 and returned to the stationary state on day 11.
We subsequently examined the effect of Vγ9Vδ2 T cells on the hyphae of A. fumigatus. When A. fumigatus filamentous hyphae were cultured in the presence of Vγ9Vδ2 T cells, the viability of A. fumigatus was significantly reduced, compared to that in the absence of Vγ9Vδ2 T cells, strongly indicating that Vγ9Vδ2 T cells have potent antifungal activity, which was comparable to that of NK cells (Fig. 1A). Notably, Vγ9Vδ2 T cells exhibit the antifungal activity in a Vγ9Vδ2 T cell number-dependent manner, and antifungal activity of Vγ9Vδ2 T cells after cryopreservation was comparable to that before cryopreservation (Fig. 1A).
Fig 1.

Effect of Vγ9Vδ2 T cells on the viability of filamentous fungi. (A) Dose-dependent antifungal activity of Vγ9Vδ2 T cells against Af293, a wild-type A. fumigatus strain, which was comparable to that of NK cells, and cryopreservation did not significantly alter the antifungal activity of Vγ9Vδ2 T cells. Af293 (A. fumigatus) was challenged by 1 × 106, 3 × 106, or 1 × 107 Vγ9Vδ2 T cells, and the antifungal activity was determined (*P = 0.0012; **P = 0.0003). (B) Species specificity in the antifungal effect of Vγ9Vδ2 T cells on Af293. Af293, Aspergillus terreus, Aspergillus flavus, Aspergillus niger, C. bertholletiae, Rhyzopus oryzae, R. microsporus, Mucor circinelloides, and Rhizomucor pusillus were challenged by Vγ9Vδ2 T cells, and the antifungal effect was observed. Data shown are representative of at least three independent experiments.
When other filamentous fungi species were examined in the same assay, Vγ9Vδ2T cells exhibited similar antifungal activity to all the other Aspergillus species, C. bertholletiae, and R. microsporus (Fig. 1B), but not to Rhizopus oryzae, Rhizomucor pusillus, and Mucor circinelloides.
Vγ9Vδ2 T cells exhibit antifungal activity against azole-resistant and susceptible A. fumigatus strains
We examined the antifungal effect of Vγ9Vδ2 T cells against azole-resistant A. fumigatus strains with different resistance mechanisms (NGS-ER7, MF2108, IFM63240). When azole-resistant A. fumigatus strains (hyphae-form) were co-cultured with Vγ9Vδ2 T cells, the Vγ9Vδ2 T cells exhibited potent antifungal activity against these fungi to a similar level as with the azole-susceptible A. fumigatus strain (Fig. 2). The complex intertwining hyphal layers were observed under an optical microscope after conidial suspensions were incubated for 24 h on a polystyrene plate. We subsequently compared the antifungal effect of Vγ9Vδ2 T cells and voriconazole against A. fumigatus forming the hyphal layers. Vγ9Vδ2 T cells exerted antifungal activity against A. fumigatus-hyphae even in the presence of the hyphal layers. Alternatively, all species of A. fumigatus, including azole-susceptible and several azole-resistant strains, were resistant to voriconazole on the condition of forming the hyphal layer (Fig. 2). Based on these findings, Vγ9Vδ2 T cells could potentially be developed as a novel antifungal modality for the treatment of azole-resistant A. fumigatus and wild-type strains. In addition, Vγ9Vδ2 T cells are effective against A. fumigatus forming hyphal layers resistant to antifungal agents.
Fig 2.
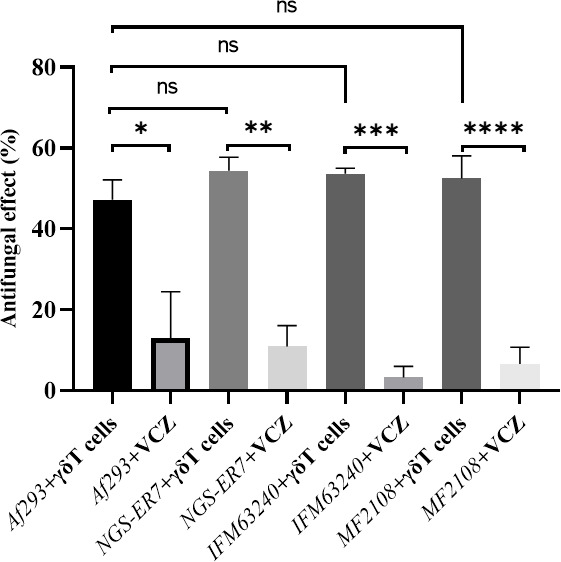
Effect of Vγ9Vδ2 T cells on the viability of azole-resistant A. fumigatus. Some types of azole-resistant A. fumigatus strains were challenged by Vγ9Vδ2 T cells, and the antifungal activity was determined in the presence or absence of voriconazole (*P = 0.0089; **P = 0.0003; ***P < 0.0001; ****P = 0.0003). Data shown are representative of at least three independent experiments.
A. fumigatus-conidia are resistant to the antifungal activity of Vγ9Vδ2 T cells
In previous studies, A. fumigatus-conidia did not exhibit immunogenicity in some human effector cells (17, 29). Therefore, we examined the effect of Vγ9Vδ2 T cells on the viability of A. fumigatus-conidia. When A. fumigatus-conidia were cultured in the presence of Vγ9Vδ2 T cells, the viability of A. fumigatus-conidia remained unaltered judging from the results of the standard colony-forming units (CFUs) assay, indicating that Vγ9Vδ2 T cells failed to recognize the conidial form of A. fumigatus (Fig. 3A), in contrast to A. fumigatus-hyphae. Vγ9Vδ2 T cells likely recognize hyphae, an invasive form of A. fumigatus, but not conidia, an inert form of the fungi.
Fig 3.

Comparison of the effects of the morphological form and metabolic alteration of A. fumigatus on the antifungal activity of Vγ9Vδ2 T cells. (A) Effect of Vγ9Vδ2 T cells on the viability of A. fumigatus-conidia. Af293 (A. fumigatus)-conidia were challenged by Vγ9Vδ2 T cells, and the antifungal activity was determined. (B) Effect of deficiency in galactomannan on the antifungal activity of Vγ9Vδ2 T cells against A. fumigatus. Δuge5, an A. fumigatus-mutant defective in galactomannan (GM), was challenged by Vγ9Vδ2 T cells, and the antifungal activity was determined. Data shown are representative of at least three independent experiments.
Vγ9Vδ2 T cells exert antifungal activity against an A. fumigatus mutant strain which is deficient of galactomannan
To identify the target for the recognition of A. fumigatus by Vγ9Vδ2 T cells, we employed Δuge5, a mutant strain of A. fumigatus, defective in galactomannan (GM) in the cell wall. The antifungal activity against Δuge5 strain was examined using the standard 2,3-bis[2-methoxy-4-nitro-5-sulphenyl]-2H-tetrazolium-5-carboxyanilide sodium salt (XTT) assay. As shown in Fig. 3B, the effect of Vγ9Vδ2 T cells on the viability of the Δuge5 strain was similar to that on the wild-type A. fumigatus strain, indicating GM is not essential for the recognition of A. fumigatus by Vγ9Vδ2 T cells.
A. fumigatus-hyphae suppresses the secretion of both IFN-γ and TNF-α from Vγ9Vδ2 T cells
Th1-type immune responses play a pivotal role in protective immunity against IA (10). It has been reported that Vγ9Vδ2 T cells could produce a significant level of TNF-α in response to water-soluble extracts of A. fumigatus (21). Therefore, we examined the levels of IFN-γ and TNF-α in the culture supernatants of living A. fumigatus-hyphae and Vγ9Vδ2 T cells. Living A. fumigatus-hyphae failed to induce the secretion of IFN-γ and TNF-α from Vγ9Vδ2 T cells (Fig. 4A and C). To examine whether the PTA/IL-2-expanded Vγ9Vδ2 T cells can secrete IFN-γ and TNF-α in response to antigenic stimuli through TCR, we treated Vγ9Vδ2 T cells with HMBPP, which is known to stimulate Vγ9Vδ2 T cells in a Vγ9Vδ2-bearing TCR-dependent manner (26). As shown in Fig. 4B and D, HMBPP-stimulated Vγ9Vδ2 T cells secreted a significant level of IFN-γ and TNF-α, demonstrating that the PTA/IL-2-expanded Vγ9Vδ2 T cells had the ability to secrete IFN-γ and TNF-α in response to TCR stimuli.
Fig 4.

Analyses of the mechanism underlying the antifungal activity of Vγ9Vδ2 T cells against A. fumigatus. (A) Effect of A. fumigatus-hyphae on the secretion of IFN-γ by Vγ9Vδ2 T cells. Vγ9Vδ2 T cells were treated with Af293 (A. fumigatus)-hyphae, and the culture supernatants were examined for IFN-γ (*P = 0.042). (B) Effect of A. fumigatus-hyphae on the secretion of IFN-γ by Vγ9Vδ2 T cells stimulated with HMBPP. Vγ9Vδ2 T cells were treated with HMBPP and Af293 (A. fumigatus)-hyphae, and the culture supernatants were examined for IFN-γ (*P = 0.0037; **P < 0.0001; ***P < 0.0001; ****P < 0.0001). (C) Effect of A. fumigatus-hyphae on the secretion of TNF-α by Vγ9Vδ2 T cells. Vγ9Vδ2 T cells were treated with Af293 (A. fumigatus)-hyphae, and the culture supernatants were examined for TNF-α (*P = 0.0014). (D) Effect of A. fumigatus-hyphae on the secretion of TNF-α by Vγ9Vδ2 T cells stimulated with HMBPP. Vγ9Vδ2 T cells were treated with HMBPP and Af293 (A. fumigatus)-hyphae, and the culture supernatants were examined for TNF-α (*P < 0.0001; **P < 0.0001; ***P < 0.0001; ****P < 0.0001). (E) Effect of direct interaction between A. fumigatus-hyphae and Vγ9Vδ2 T cells on the antifungal activity of Vγ9Vδ2 T cells. Vγ9Vδ2 T cells and Af293 (A. fumigatus)-hyphae were cultured using a cell insert culture system, and the antifungal activity was determined (*P < 0.0001). (F) Effect of supernatants from the culture of Vγ9Vδ2 T cells and A. fumigatus-hyphae on the antifungal activity of Vγ9Vδ2 T cells. Af293 (A. Fumigatus) was challenged by co-culture supernatants of Vγ9Vδ2 T cells and Af293 (A. fumigatus)-hyphae, and the antifungal activity was determined (*P = 0.0002; **P = 0.0003). Data shown are representative of at least three independent experiments.
Subsequently, we examined the effect of A. fumigatus-hyphae on the secretion of these cytokines from HMBPP-stimulated Vγ9Vδ2 T cells. Surprisingly, the addition of A. fumigatus-hyphae markedly decreased both IFN-γ and TNF-α secretion from Vγ9Vδ2 T cells in response to HMBPP (Fig. 4B and D). To examine the effect of A. fumigatus-hyphae on the viability of Vγ9Vδ2 T cells, Vγ9Vδ2 T cells were cocultured in the presence or absence of A. fumigatus-hyphae, and the number of viable Vγ9Vδ2 T cells was counted. The standard trypan blue dye exclusion demonstrated no difference in the number of viable Vγ9Vδ2 T cells between the two groups, indicating that the secretion of both IFN-γ and TNF-α from HMBPP-stimulated Vγ9Vδ2 T cells was significantly suppressed by the presence of A. fumigatus-hyphae.
Antifungal activity of Vγ9Vδ2 T cells against A. fumigatus is contact-dependent
Based on the aforementioned findings, it is evident that Vγ9Vδ2 T cells could exert antifungal effects. Subsequently, we examined the mechanism underlying the immune effector functions of Vγ9Vδ2 T cells against A. fumigatus. To examine the effect of cell-to-cell contact on the antifungal activity of Vγ9Vδ2 T cells, we employed a cell culture insert system comprising a 0.4 µm pore membrane insert. After culturing Vγ9Vδ2 T cells and A. fumigatus-hyphae using the culture insert, the viability of A. fumigatus-hyphae in this system was significantly higher than that in the co-culture system that allowed cell-to-cell contact between Vγ9Vδ2 T cells and A. fumigatus-hyphae (Fig. 4E), indicating that cell-to-cell contact is essential for the antifungal activity of Vγ9Vδ2 T cells. In addition, culture supernatants derived from the co-culture system of Vγ9Vδ2 T cells and A. fumigatus-hyphae demonstrated no antifungal activity against A. fumigatus-hyphae (Fig. 4F), further corroborating the hypothesis that a direct contact between Vγ9Vδ2 T cells and A. fumigatus-hyphae is required for the antifungal activity of Vγ9Vδ2 T cells against A. fumigatus-hyphae.
A. fumigatus-hyphae induced degranulation in Vγ9Vδ2 T cells
The degranulation of intracellular vesicles containing effector molecules is a major mechanism by which immune effector cells exhibit contact-dependent lytic activity against target cells. As CD107a is expressed in intracellular vesicles and translocated onto cell surface membranes during degranulation, the upregulation of CD107a on cell surface membranes can be used as a marker for the degree of degranulation of immune effector cells (33). Therefore, we examined the levels of CD107a expression on Vγ9Vδ2 T cells after incubation with A. fumigatus-hyphae or culture supernatants derived from the co-culture system of Vγ9Vδ2 T cells and A. fumigatus-hyphae. As shown in Fig. 5A and B, the expression of CD107a on Vγ9Vδ2 T cells was significantly upregulated when Vγ9Vδ2 T cells were co-cultured with A. fumigatus-hyphae, compared to those treated with supernatants of the co-culture. Notably, the upregulation of CD107a on Vγ9Vδ2 T cells was dependent on the dose of A. fumigatus-hyphae (Fig. 5A and B), confirming that the cell-to-cell contact between Vγ9Vδ2 T cells and A. fumigatus-hyphae is essential for the antifungal activity of Vγ9Vδ2 T cells.
Fig 5.
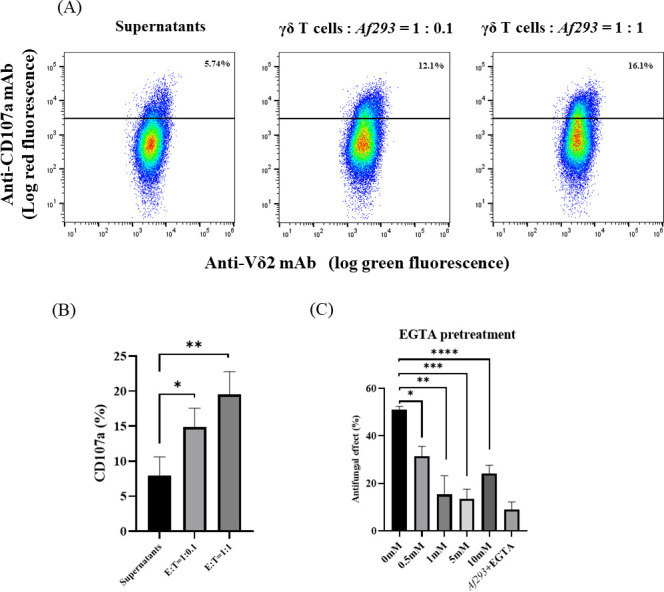
Involvement of degranulation in the antifungal effect of Vγ9Vδ2 T cells against A. fumigatus-hyphae. (A, B) Analyses of degranulation after treatment of Vγ9Vδ2 T cells with A. fumigatus-hyphae. Vγ9Vδ2 T cells were treated with Af293 (A. fumigatus)-hyphae or culture supernatants derived from the co-culture system of Vγ9Vδ2 T cells and A. fumigatus-hyphae, and the degranulation in Vγ9Vδ2 T cells was monitored through CD107a mobility on a flow cytometric analysis (*P = 0.0331; **P = 0.0084). (C) Effect of a Ca2+-chelating inhibitor on the antifungal activity of Vγ9Vδ2 T cells against A. fumigatus. Vγ9Vδ2 T cells were challenged by A. fumigatus-hyphae in the presence or absence of EGTA, a Ca2+-chelating inhibitor, and the antifungal activity of Vγ9Vδ2 T cells against A. fumigatus-hyphae was examined (*P = 0.0014; **P = 0.0015; ***P = 0.0001; ****P = 0.0003). Data shown are representative of at least three independent experiments.
Antifungal activity of Vγ9Vδ2T cells against A. fumigatus is mediated by calcium (Ca2+)-dependent degranulation
CD107a-positive intracellular vesicles contain lytic molecules, such as granulysin, perforin, and granzyme B, and the release of these molecules required for their effector functions is Ca2+-dependent (34, 35). Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetra acetic acid A (EGTA) inhibits degranulation owing to its calcium-chelating effect (36, 37). To examine the effect of degranulation in the antifungal activity of Vγ9Vδ2 T cells, we evaluated the antifungal activity of Vγ9Vδ2 T cells after pretreatment with EGTA. As illustrated in Fig. 5C, the antifungal activity of Vγ9Vδ2 T cells against A. fumigatus was significantly suppressed by the pretreatment with EGTA, strongly indicating that degranulation was inexorably linked to the antifungal activity of Vγ9Vδ2 T cells.
DISCUSSION
In this study, we performed in vitro antifungal effects of Vγ9Vδ2 T cells against A. fumigatus and other filamentous fungi that could cause invasive pulmonary infections and explored possible underlying mechanisms. Vγ9Vδ2 T cells exhibited significant antifungal effects against many species of filamentous fungi-hyphae, including azole-susceptible and resistant A. fumigatus, and Mucorales such as C. bertholletiae and R. microsporus. However, Vγ9Vδ2 T cells did not exhibit antifungal effects against the resting conidia of A. fumigatus. In addition, the antifungal effect of Vγ9Vδ2 T cells depended on direct contact between Vγ9Vδ2 T cells and fungi. Direct cell-to-fungus contact upregulated the expression of CD107a, a marker of degranulation, on the surface of Vγ9Vδ2 T cells. Inhibition of degranulation with EGTA pretreatment greatly impaired the antifungal effects of Vγ9Vδ2 T cells. In addition, A. fumigatus-hyphae strongly inhibited the release of IFN-γ and TNF-α from Vγ9Vδ2 T cells.
We previously synthesized PTA, a nitrogen-containing bisphosphonate prodrug, and demonstrated that Vγ9Vδ2 T cells were efficiently expanded in vitro by PTA and IL-2. Since PTA is highly hydrophobic, the prodrug is spontaneously internalized into immune cells, such as dendritic cells and monocytes, where it is hydrolyzed by intracellular esterases to yield 2-(thiazole-2-ylamino) ethylidene-1,1-bisphosphonate (TA), an active form of PTA (38, 39). The resulting TA inhibits intracellular FDPS, which catalyzes the formation of geranyl diphosphate from IPP and DMAPP and that of farnesyl diphosphate from geranyl diphosphate and IPP in the isoprenoid synthetic pathway (31). Inhibition of FDPS by TA results in the intracellular accumulation of IPP and DMAPP, which interact with the B30.2 intracellular domain of BTN3A1 (40). Finally, Vγ9Vδ2 T cells subsequently recognize the PTA-pulsed antigen-presenting cells within the context of BTN2A1/3A1. However, the precise recognition mechanism has not been fully elucidated (22, 41).
Vγ9Vδ2 T cells were successfully expanded by PTA/IL-2 within 11 days, reaching a purity of up to 99.5% without any purification procedure. In this study, we used the highly purified Vγ9Vδ2 T cells and demonstrated their potent cytolytic activity against Raji, a human Burkitt’s lymphoma cell line. As expected, Vγ9Vδ2 T cells also exhibited palpable antifungal activity against A. fumigatus, a causative agent of IA, and other fungi, such as A. terreus, A. flavus, A. niger, C. bertholletiae, and R. microsporus. The antifungal activity of Vγ9Vδ2 T cells against A. fumigatus was comparable to that of NK cells. The advantage of Vγ9Vδ2 T cells over NK cells is that the expansion rate of Vγ9Vδ2 T cells is much greater than that of NK cells. When PBMCs are stimulated with PTA/IL-2, a large number of highly purified Vγ9Vδ2 T cells can be obtained within 11 days without purification, indicating that Vγ9Vδ2 T cell-based adoptive transfer therapy is more practical than that of NK cells.
In addition, Vγ9Vδ2 T cells also exhibited antifungal effects against some azole-resistant A. fumigatus strains with different resistance mechanisms (NGS-ER7, MF2108, IFM63240), strongly indicating that Vγ9Vδ2 T cell-based immunotherapy could serve as an alternative modality for the treatment of IA caused by wild-type and azole-resistant A. fumigatus. Since common mutations in A. fumigatus responsible for drug resistance are generally unrelated to the structure and components of cell walls that are highly antigenic for the host immune system, the antifungal activity of Vγ9Vδ2 T cells is not affected by such azole-resistant mutations. In addition, Vγ9Vδ2 T cells were effective against A. fumigatus forming complex intertwining hyphal layers resistant to antifungal agents. Notably, it was reported that Aspergillus hyphae formed hyphal layers with numerous mycelia intertwined on a polystyrene plate, and the mycelia within the structures were more resistant to antifungal agents including voriconazole than planktonic ones (42, 43).
The existence of azole-resistant A. fumigatus has been reported worldwide (44), posing a clinically significant problem in future (7, 45). In addition, biofilm-forming A. fumigatus has been observed in IA (46) and chronic pulmonary aspergillosis, which are resistant to conventional antifungals (33). Therefore, it is imperative to develop Vγ9Vδ2 T cell-based immunotherapy, which might overcome drug resistance of A. fumigatus or other filamentous fungi.
Whereas various pathogenic bacteria produce HMBPP (47), A. fumigatus does not have the MEP/DOXP pathway and, therefore, does not produce HMBPP, indicating that Vγ9Vδ2 T cells recognize antigenic entities other than HMBPP. Therefore, we employed a mutant A. fumigatus strain to examine the effect of gene mutations on the antifungal activity of Vγ9Vδ2 T cells. In this study, we used an A. fumigatus mutant strain, Δuge5, which is defective in GM, and found that Vγ9Vδ2 T cells exhibited antifungal effects against GM-deficient A. fumigatusas the wild-type A. fumigatus (Af293). The finding indicates that GM might not be the target for the recognition of A. fumigatus by Vγ9Vδ2 T cells. In addition, we examined Vγ9Vδ2 T cells for the expression of several cell surface markers, including PRRs (Dectin-1, TLR2, and TLR4), which have been reported to be associated with Aspergillus infections (48), NK cell-related markers, NKG2D and DNAM-1, cell death receptor ligands (FasL and TRAIL), CD25, an activation marker of Vγ9Vδ2 T cells, and PD-1, a T cell exhaustion marker, on days 0, 3, and 11 after stimulation with PTA/IL-2. Dectin-1 is one of the most well-characterized PRRs recognizing β-d-glucans (48, 49), whereas its expression on Vγ9Vδ2 T cells had not been reported. It was demonstrated that TLR2 and TLR4 were upregulated on Vγ9Vδ2 T cells after stimulation with TLR4 ligands (50), leading to induced antibacterial responses (51). In this study, PRRs were not expressed on Vγ9Vδ2 T cells under a condition we employed. In addition, the expression levels of other cell surface markers remained noticeably unchanged before and after the expanded culture, except for NKG2D, CD25, and PD-1.
Vγ9Vδ2 T cells exhibited antifungal activity against A. fumigatus-hyphae but not conidia. This finding is similar to previous reports indicating that dendritic cells, alveolar macrophages, and NK cells were not activated by resting conidia (17, 52). The hydrophobic layer of A. fumigatus-conidia disappears during germination and transformation into hyphae. Notably, the conidial morphotype of ΔrodA mutant, a strain genetically deficient in the hydrophobic layer, activates dendritic cells and alveolar macrophages, indicating that the hydrophobic layer is involved in the escape of A. fumigatus-conidia from the innate immune cells recognition (52). However, since NK cells are not activated by ΔrodA (7), the mechanism underlying the recognition of A. fumigatus by immune cells might vary among immune cells.
Furthermore, we examined whether the antifungal activity elicited by Vγ9Vδ2 T cells was dependent on soluble factors secreted by Vγ9Vδ2 T cells. Vγ9Vδ2 T cells and NK cells are innate immune effector cells, and NK cells are known to exhibit antifungal activity against A. fumigatus, possibly through the activity of IFN-γ (16). In a previous study, Vγ9Vδ2 T cells secreted a high level of TNF-α in response to water extracts of A. fumigatus (21), and Th1 immunity is considered important for Aspergillus infections (10). Therefore, we examined the level of IFN-γ and TNF-α secreted from Vγ9Vδ2 T cells in response to A. fumigatus-hyphae. Surprisingly, A. fumigatus-hyphae failed to induce the secretion of these cytokines from Vγ9Vδ2 T cells but instead suppressed these cytokine levels when Vγ9Vδ2 T cells were stimulated by HMBPP. In addition, supernatants from the cell culture of A. fumigatus-hyphae with Vγ9Vδ2T cells had little antifungal activity, strongly indicating that the antifungal activity of Vγ9Vδ2 T cells was not mediated by soluble factors, such as IFN-γ or TNF-α.
We further examined whether the antifungal activity of Vγ9Vδ2 T cells is dependent on direct contact with A. fumigatus-hyphae. Based on the results of the standard cell culture insert system, which allows for only small soluble molecules to pass through the membrane, the Vγ9Vδ2 T cells exhibited the antifungal activity through direct contact with A. fumigatus-hyphae. Subsequently, we confirmed the degranulation of Vγ9Vδ2 T cells by monitoring the mobility of CD107a upon contact with A. fumigatus-hyphae (33). In addition, the inhibition of degranulation with EGTA significantly decreased the antifungal effect exhibited by Vγ9Vδ2 T cells, indicating the involvement of degranulation in the antifungal activity of Vγ9Vδ2 T cells. CD107a-positive intracellular vesicles contain at least three types of effector molecules: granzymes, granulysin, and perforins (53–55). These mediate cytotoxicity against bacteria and tumor cells through differential mechanisms at the immunological synapse (56, 57). Granulysin has been indicated to exert antifungal activity against Cryptococcus neoformans (58, 59). In addition, the antifungal activity of NK cells against A. fumigatus-hyphae is induced by perforin (17).
This study has some limitations. First, we could not prove the specific type of effector molecules in CD107a-positive intracellular vesicles critical for antifungal activity mediated by Vγ9Vδ2 T cells. Second, although it was indicated that GM itself was not recognized by Vγ9Vδ2 T cells, the antigenic entity of A. fumigatus and Mucorales such as C. bertholletiae and R. microsporus for Vγ9Vδ2 T cells remains unidentified. Third, we prepared hyphal layers resistant to antifungal agents on a polystyrene plate by culturing conidial suspensions as previously reported (42, 43). We referred to these as “biofilms” in the Discussion. In this study, while we confirmed hyphal layers, composed of many mycelia, under an optical microscope, we did not validate the presence of extracellular matrix or perform high-resolution microscopic evaluation.
In conclusion, this study strongly indicates that Vγ9Vδ2 T cell-based adoptive cell therapy could be a novel candidate for the next-generation therapeutics for IA. In addition, immunotherapy using Vγ9Vδ2 T cells may also be effective against other filamentous fungal infections. Given that many individuals with IA are highly immunocompromised, developing immunotherapy that potentially bolsters damaged or exhausted immune systems should be developed for the treatment of IA patients. We are currently planning to establish in vivo experimental systems to examine the effects of Vγ9Vδ2 T cells on IA.
MATERIALS AND METHODS
Derivation of Vγ9Vδ2T cells and NK cells
Human PBMCs were obtained from healthy adult volunteers. Vγ9Vδ2 T cells were derived by stimulating PBMCs with PTA and IL-2 for 11 days. NK cells were ex vivo expanded by treating CD3-depleted PBMCs with IL-2/IL-18 for 10 days. Detailed methods for Vγ9Vδ2 T cells and NK cells expansion are described in Supplementary Methods. All experiments were conducted using Vγ9Vδ2 T cells derived from at least two different donors, demonstrating the generality of the mechanism underlying the antifungal activity of Vγ9Vδ2 T cells.
Preparation of fungal strains
A previously characterized A. fumigatus strain (Af293) was used in this study. All filamentous fungi were grown on potato-dextrose-agar (PDA) slants for 4–7 days at 37℃. Subsequently, spores were harvested by gently scraping the surface of the slants and re-suspended in PBS supplemented with 0.05% Tween 20. Hyphae were removed by passing the suspension through a cell strainer (0.4 mm nylon mesh pore membrane). The conidial suspension was washed twice and re-suspended in RPMI1640 medium. The fungal suspension was used as a target for Vγ9Vδ2 T cells. Suspensions of filamentous fungi species-derived conidia were adjusted to a concentration of 1 × 106 /mL, which were dispensed into wells at a volume of 300 µL per well. Growth of hyphae was achieved by cultivating the conidial suspension at 37℃ for 24 h in polystyrene culture plates. However, A. terreus was incubated for 96 h owing to its low growth speed. In addition to Af293, we used Δuge5, an A. fumigatus mutant, deficient in the uge5 gene, resulting in the absence of Galf necessary for GM synthesis and, by extension, the absence of GM (60). Additionally, we used several azole-resistant A. fumigatus strains, each with a different resistance mechanism: NGS-ER7, MF2108, and IFM63240. NGS-ER7 is an environmental strain isolated in Europe. It shows azole resistance potentially attributed to a tandem repeat in the promoter region of cyp51A (TR46), cyp51A gene mutations (Y121F and T289), and hmg1 gene mutation (E105K, S212P, and Y564H) [minimum inhibitory concentration (MIC): itraconazole = 2, voriconazole >8] (61). In contrast, MF2108 and IFM63240 are clinically isolated strains whose azole resistance is potentially owing to mutations other than cyp51A mutation. MF2108 (MIC: itraconazole >8, voriconazole being >8) has one splice site mutation in the HapE gene (62), whereas IFM63240 (MIC: itraconazole >8, voriconazole = 4) has two mutation (S269F in hmg1 gene and A350T in erg6 gene) (63). Besides A. fumigatus, we included other filamentous fungi causing invasive infections, such as A. terreus (ATCC10690), A. niger (ATCC6275), A. flavus (ATCC9643), C. bertholletiae (MF2710), R. oryzae (MF1166), R. microsporus (IFM46417), M. circinelloides (IFM55051), and R. pusillus (IFM40783). Δuge5 was kindly donated by Dr. Donald C. Sheppard and IFM63240, IFM46417, IFM55051, and IFM40783 by Chiba University. MF2710 and MF1166 were clinical strains collected in Nagasaki University Hospital.
Assessment of antifungal activity
Antifungal activity against filamentous fungal hyphae was assessed through colorimetric assay with XTT (Merck & Co., Darmstadt, Hesse, Germany), in which the absorbance correlated with the number of viable fungal cells. First, Vγ9Vδ2 T cells were added to A. fumigatus or other filamentous fungi hyphae suspension at various effector-to-target ratios and incubated for 24 h. Wells were washed with PBS, and distilled water was added to lyse Vγ9Vδ2 T cells. After the suspension was placed at room temperature for 30 min, an XTT labeling mixture was added to each well at a final concentration of 0.3 mg/mL and incubated at 37°C with 5% CO2. After incubation for 24 h, 100 µL of supernatant samples was transferred to a 96-well flat-bottom plate in duplicate, and the optical density (OD) was measured at 450 nm with a 655-nm reference filter. The antifungal effect was calculated using the following formula: the percent damage = (1−X/Y) × 100, where X is the OD of the samples under the various culture conditions, and Y is that of the negative control (fungus alone). In addition, the antifungal activity exhibited by Vγ9Vδ2 T cells before cryopreservation was evaluated under the same protocol. After the co-culture of filamentous fungi and Vγ9Vδ2 T cells at different durations under the aforementioned conditions, the plate was centrifuged at 5,400 rpm for 2 min, and the supernatants were stored at −80°C.
Cell culture inserts (Nunc) were used to evaluate the effects of the direct contact between Vγ9Vδ2 T cells and fungi and those of soluble factors released from Vγ9Vδ2 T cells on antifungal activity. Given that the cell culture inserts possess pores allowing soluble factors to pass through but preventing Vγ9Vδ2 T cells and fungi from passing, the following culture combinations were employed: Vγ9Vδ2 T cells + fungus//fungus (without contact), fungus//Vγ9Vδ2T cells + fungus (with contact), and fungus//fungus (negative control). The XTT assay evaluated the fungal damage in the right compartment.
To evaluate the effect of HMBPP, which potently induces the release of Th1-type cytokines from Vγ9Vδ2 T cells (28), on the antifungal activity of Vγ9Vδ2 T cells, serially diluted HMBPP solutions were added to the wells when the Vγ9Vδ2 T cells were added to the fungal solution. To examine the effect of exocytosis of cytotoxic lysosomal proteins in fungal damage, Vγ9Vδ2 T cells were preincubated with 0.5–10 mM EGTA (Merck & Co.), a Ca2+ chelating agent, for 30 min at room temperature before the antifungal assay, leading to the inhibition of Ca2+ flux required for degranulation (36, 37).
To evaluate the antifungal activity of Vγ9Vδ2 T cells against Aspergillus conidia, resting conidia were incubated with Vγ9Vδ2 T cells for 6 h. Subsequently, 100 µL of suspensions was spread over PDA agar plates and incubated at 37°C. After 24 h, the number of colonies was counted. Conidial damage was assessed based on the CFUs per mL.
Cytokine assay
IFN-γ and TNF-α levels in the culture supernatants of fungi and Vγ9Vδ2 T cells co-incubated for 24 h were measured using an enzyme-linked immunosorbent assay kit (PeproTech, Cranbury, NJ) according to the manufacturer’s instructions. In brief, capture mAb for human IFN-γ or TNF-α (1 µg/mL in PBS) were placed into the wells of a 96-well flat-bottom plastic plate (Thermo Fisher Scientific, Waltham, Massachusetts, USA). After the plate was incubated at ambient temperature overnight, the wells were washed four times with 300 µL of PBS/0.05% Tween 20 (Nacalai Tesque Inc.) and 300 µL of PBS/1% bovine serum albumin (BSA fraction V, Nacalai Tesque Inc.) was added to the wells. The plate was incubated at room temperature for 2 h, and the wells were washed four times with 300 µL of PBS/0.05% Tween 20. Furthermore, 100 µL of culture supernatants and a serially diluted human IFN-γ or TNF-α standard solution were added to the wells and incubated at room temperature for 2 h. Subsequently, the wells were washed four times with 300 µL of PBS/0.05% Tween 20. Biotin-conjugated detection mAb for human IFN-γ or TNF-α was diluted with PBS/0.05% Tween 20/0.1% BSA for a final concentration of 1 µg/mL, and 100 µL of this solution was added to the wells. After incubation at room temperature for 2 h, the wells were washed four times with 300 µL of PBS/0.05% Tween 20. Furthermore, 100 µL of horseradish peroxidase-conjugated avidin solution was added to the wells and incubated at room temperature for 30 min. The wells were washed four times with 300 µL of PBS/0.05% Tween 20, 100 µL of 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt liquid substrate (Merck & Co.) was added, and the plate was incubated at room temperature for 5 min. Finally, 100 µL of 1% sodium dodecyl sulfate solution in water was added to the wells, and the OD at 405 nm was measured using a NIVO multiplate reader (Revvity, Yokohama, Kanagawa, Japan). As a positive control, HMBPP was added to the mixture of Vγ9Vδ2 T cells and fungi at a final concentration of 100 nM.
Flowcytometric analysis of CD107a on Vγ9Vδ2 T cells
To evaluate the degree of degranulation in Vγ9Vδ2 T cells, the mobilization of CD107a was assessed using a flow cytometer, as this membrane protein translocates to the cell surface during the degranulation process (31). A serially diluted Af293 suspension (25 µL) was placed into a 96-well round-bottom plastic plate (Corning Inc., Corning, NY). After incubating at 37°C for 24 h for hyphae formation, 25 µL of Vγ9Vδ2 T cell suspensions was added to the wells at different effector-to-target ratios, followed by the addition of 5 µL of phycoerythrin-conjugated mouse anti-human CD107a mAb (BioLegend, San Diego, CA).
As controls, supernatants from co-culture of Vγ9Vδ2 T cells with A. fumigatus-hyphae for 24 h were also included. The plate was briefly centrifuged at 500 rpm for 2 min and incubated at 37°C for 2 h. Subsequently, 3 µL of FITC-conjugated mouse anti-human TCR Vδ2 mAb was added to the wells and incubated for an additional 15 min. Finally, Vγ9Vδ2 T cells were harvested and analyzed through a FACSLyric flow cytometer (Becton Dickinson & Co.). The cell population was visualized using a FlowJo software ver. 10 (FlowJo LLC).
Statistical analysis
Statistical significance was determined using the unpaired, two-sided Student t-test, implemented in a GraphPad Prism software ver. 8.4.3. P-value < 0.05 was considered statistically significant. Bars show arithmetic means of the values of the independent experiment ± standard error of the mean.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ms. Ishida for her assistance in the experiments. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
This research was supported by AMED, Grant No. 22fk0108530h0001 to T.T., Grant No. A48 and A90 to Y.T, by JSPS KAKENHI Grant-in-Aid for Scientific Research (C), Grant No. 23K07941 to T.T., by JST START University Ecosystem Promotion Type (Supporting the Creation of Startup Ecosystems in Startup Cities), Japan, Grant No. JPMJST2281 to Y.T., by Grants-in-Aid for Scientific Research from MEXT, Grant No. 16K08844 and 23K06677 to Y.T., and by Research Funding granted by Nagasaki University President Shigeru Kohno to Y.T.
S.K., Conceptualization, Investigation, Validation, Writing—original draft | T.K., Conceptualization, Supervision, Validation, Formal analysis, Data curation, Writing—review and editing, Funding acquisition, project administration | H.N., Formal analysis | D.O., Formal analysis | Y.I., Validation | N.N., Validation | T.H., Formal analysis | K. Takeda, Formal analysis | S.I., Formal analysis | N.I., Validation | M.T., Formal Analysis | N.S., Formal analysis, Validation | A.W., Writing—review and editing | K.I., Writing—review and editing | K.Y., Writing—review and editing | Y.T Tanaka, Supervision, Formal analysis, Data curation, Project administration, Writing—review and editing | H.M., Writing—review and editing, Project administration.
Contributor Information
Takahiro Takazono, Email: takahiro-takazono@nagasaki-u.ac.jp.
Paschalis Vergidis, Mayo Foundation for Medical Education and Research, USA.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.03614-23.
Profiles of Vγ9Vδ2 T cells.
Derivation of Vγ9Vδ2T cells and NK cells.
An accounting of the reviewer comments and feedback.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan SP, Meier-Kriesche HU, Apewokin S, Horn DL. 2012. Clinical epidemiology of 960 patients with invasive Aspergillosis from the PATH alliance registry. J Infect 65:453–464. doi: 10.1016/j.jinf.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 2. Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, Anaissie EJ, Brumble LM, Herwaldt L, Ito J, Kontoyiannis DP, Lyon GM, Marr KA, Morrison VA, Park BJ, Patterson TF, Perl TM, Oster RA, Schuster MG, Walker R, Walsh TJ, Wannemuehler KA, Chiller TM. 2010. Invasive fungal infections among organ transplant recipients: results of the transplant-associated infection surveillance network (TRANSNET). Clin Infect Dis 50:1101–1111. doi: 10.1086/651262 [DOI] [PubMed] [Google Scholar]
- 3. Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, et al. 2010. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the transplant-associated infection surveillance network (TRANSNET) database. Clin Infect Dis 50:1091–1100. [DOI] [PubMed] [Google Scholar]
- 4. Verweij PE, Lucas JA, Arendrup MC, Bowyer P, Brinkmann AJF, Denning DW, Dyer PS, Fisher MC, Geenen PL, Gisi U, Hermann D, Hoogendijk A, Kiers E, Lagrou K, Melchers WJG, Rhodes J, Rietveld AG, Schoustra SE, Stenzel K, Zwaan BJ, Fraaije BA. 2020. The one health problem of azole resistance in Aspergillus fumigatus: current insights and future research agenda. Fungal Biol Rev 34:202–214. doi: 10.1016/j.fbr.2020.10.003 [DOI] [Google Scholar]
- 5. Lestrade PPA, Meis JF, Melchers WJG, Verweij PE. 2019. Triazole resistance in Aspergillus fumigatus: recent insights and challenges for patient management. Clin Microbiol Infect 25:799–806. doi: 10.1016/j.cmi.2018.11.027 [DOI] [PubMed] [Google Scholar]
- 6. Buil JB, Snelders E, Denardi LB, Melchers WJG, Verweij PE. 2019. Trends in azole resistance in Aspergillus fumigatus, vol 176. The Netherlands 1994 -2016. Emerg Infect Dis 25:176–178. doi: 10.3201/eid2501.171925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chowdhary A, Sharma C, Meis JF. 2017. Azole-resistant Aspergillosis: epidemiology, molecular mechanisms, and treatment. J Infect Dis 216:S436–S444. doi: 10.1093/infdis/jix210 [DOI] [PubMed] [Google Scholar]
- 8. Skiada A, Pavleas I, Drogari-Apiranthitou M. 2020. Epidemiology and diagnosis of Mucormycosis: an update. J Fungi (Basel) 6:265. doi: 10.3390/jof6040265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 41:634–653. doi: 10.1086/432579 [DOI] [PubMed] [Google Scholar]
- 10. Pathakumari B, Liang G, Liu W. 2020. Immune defence to invasive fungal infections: a comprehensive review. Biomed Pharmacother 130:110550. doi: 10.1016/j.biopha.2020.110550 [DOI] [PubMed] [Google Scholar]
- 11. Park SJ, Mehrad B. 2009. Innate immunity to Aspergillus species. Clin Microbiol Rev 22:535–551. doi: 10.1128/CMR.00014-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hohl TM. 2017. Immune responses to invasive Aspergillosis: new understanding and therapeutic opportunities. Curr Opin Infect Dis 30:364–371. doi: 10.1097/QCO.0000000000000381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kontoyiannis DP, Selleslag D, Mullane K, Cornely OA, Hope W, Lortholary O, Croos-Dabrera R, Lademacher C, Engelhardt M, Patterson TF. 2018. Impact of unresolved neutropenia in patients with neutropenia and invasive Aspergillosis: a post hoc analysis of the SECURE trial. J Antimicrob Chemother 73:757–763. doi: 10.1093/jac/dkx423 [DOI] [PubMed] [Google Scholar]
- 14. Koehler P, Salmanton‐García J, Gräfe SK, Koehler FC, Mellinghoff SC, Seidel D, Steinbach A, Cornely OA. 2019. Baseline predictors influencing the prognosis of invasive Aspergillosis in adults. Mycoses 62:651–658. doi: 10.1111/myc.12926 [DOI] [PubMed] [Google Scholar]
- 15. Einsele H, Löffler J, Kapp M, Rasche L, Mielke S, Grigoleit UG. 2015. Immunotherapy for viral and fungal infections. Bone Marrow Transplant 50 Suppl 2:S51–S54. doi: 10.1038/bmt.2015.96 [DOI] [PubMed] [Google Scholar]
- 16. Bouzani M, Ok M, McCormick A, Ebel F, Kurzai O, Morton CO, Einsele H, Loeffler J. 2011. Human NK cells display important antifungal activity against Aspergillus fumigatus, which is directly mediated by IFN--γ release. J Immunol 187:1369–1376. doi: 10.4049/jimmunol.1003593 [DOI] [PubMed] [Google Scholar]
- 17. Schmidt S, Tramsen L, Hanisch M, Latgé JP, Huenecke S, Koehl U, Lehrnbecher T. 2011. Human natural killer cells exhibit direct activity against Aspergillus fumigatus hyphae, but not against resting Conidia. J Infect Dis 203:430–435. doi: 10.1093/infdis/jiq062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Soe WM, Lim JHJ, Williams DL, Goh JG, Tan Z, Sam QH, Chotirmall SH, Ali NABM, Lee SC, Seet JE, Ravikumar S, Chai LYA. 2020. Using expanded natural killer cells as therapy for invasive Aspergillosis. J Fungi (Basel) 6:231. doi: 10.3390/jof6040231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vantourout P, Hayday A. 2013. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol 13:88–100. doi: 10.1038/nri3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chien Y, Meyer C, Bonneville M. 2014. γδ T cells: first line of defense and beyond. Annu Rev Immunol 32:121–155. doi: 10.1146/annurev-immunol-032713-120216 [DOI] [PubMed] [Google Scholar]
- 21. Hebart H, Bollinger C, Fisch P, Sarfati J, Meisner C, Baur M, Loeffler J, Monod M, Latgé J-P, Einsele H. 2002. Analysis of T-cell responses to Aspergillus fumigatus antigens in healthy individuals and patients with hematologic malignancies. Blood 100:4521–4528. doi: 10.1182/blood-2002-01-0265 [DOI] [PubMed] [Google Scholar]
- 22. Harly C, Guillaume Y, Nedellec S, Peigné CM, Mönkkönen H, Mönkkönen J, Li J, Kuball J, Adams EJ, Netzer S, Déchanet-Merville J, Léger A, Herrmann T, Breathnach R, Olive D, Bonneville M, Scotet E. 2012. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood 120:2269–2279. doi: 10.1182/blood-2012-05-430470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Altincicek B, Kollas A, Eberl M, Wiesner J, Sanderbrand S, Hintz M, Beck E, Jomaa H. 2001. LytB, a novel gene of the 2-C-methyl-D-erythritol 4-phosphate pathway of isoprenoid biosynthesis in Escherichia coli. FEBS Lett 499:37–40. doi: 10.1016/s0014-5793(01)02516-9 [DOI] [PubMed] [Google Scholar]
- 24. Eberl M, Hintz M, Reichenberg A, Kollas A-K, Wiesner J, Jomaa H. 2003. Microbial isoprenoid biosynthesis and human γδ T cell activation. FEBS Lett 544:4–10. doi: 10.1016/s0014-5793(03)00483-6 [DOI] [PubMed] [Google Scholar]
- 25. Belmant C, Espinosa E, Poupot R, Peyrat MA, Guiraud M, Poquet Y, Bonneville M, Fournié JJ. 1999. 3-formyl-1-butyl pyrophosphate a novel Mycobacterial metabolite-activating human gamma delta T cells. J Biol Chem 274:32079–32084. doi: 10.1074/jbc.274.45.32079 [DOI] [PubMed] [Google Scholar]
- 26. Dunne MR, Mangan BA, Madrigal-Estebas L, Doherty DG. 2010. Preferential Th1 cytokine profile of phosphoantigen-stimulated human Vγ9Vδ2 T cells. Mediators Inflamm 2010:704941. doi: 10.1155/2010/704941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen CY, Yao S, Huang D, Wei H, Sicard H, Zeng G, Jomaa H, Larsen MH, Jacobs Jr WR, Wang R, Letvin N, Shen Y, Qiu L, Shen L, Chen ZW. 2013. Phosphoantigen/IL2 expansion and differentiation of Vγ2Vδ2 T cells increase resistance to tuberculosis in nonhuman primates. PLoS Pathog 9:e1003501. doi: 10.1371/journal.ppat.1003501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qiu L, Huang D, Chen CY, Wang R, Shen L, Shen Y, Hunt R, Estep J, Haynes BF, Jacobs WR, Letvin N, Du G, Chen ZW. 2008. Severe tuberculosis induces unbalanced up-regulation of gene networks and overexpression of IL-22, MIP-1Alpha, CCL27, IP-10, CCR4, CCR5, CXCR3, PD1, PDl2, IL-3, IFN-beta, TIM1, and TLR2 but low antigen-specific cellular responses. J Infect Dis 198:1514–1519. doi: 10.1086/592448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liang J, Fu L, Li M, Chen Y, Wang Y, Lin Y, Zhang H, Xu Y, Qin L, Liu J, Wang W, Hao J, Liu S, Zhang P, Lin L, Alnaggar M, Zhou J, Zhou L, Guo H, Wang Z, Liu L, Deng G, Zhang G, Wu Y, Yin Z. 2021. Allogeneic Vγ9Vδ2 T-cell therapy promotes pulmonary lesion repair: an open-label, single-arm pilot study in patients with multidrug-resistant tuberculosis. Front Immunol 12:756495. doi: 10.3389/fimmu.2021.756495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tanaka Y, Murata-Hirai K, Iwasaki M, Matsumoto K, Hayashi K, Kumagai A, Nada MH, Wang H, Kobayashi H, Kamitakahara H, Okamura H, Sugie T, Minato N, Toi M, Morita CT. 2018. Expansion of human γδ T cells for adoptive Immunotherapy using a bisphosphonate prodrug. Cancer Sci 109:587–599. doi: 10.1111/cas.13491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okuno D, Sugiura Y, Sakamoto N, Tagod MSO, Iwasaki M, Noda S, Tamura A, Senju H, Umeyama Y, Yamaguchi H, Suematsu M, Morita CT, Tanaka Y, Mukae H. 2020. Comparison of a novel bisphosphonate prodrug and zoledronic acid in the induction of cytotoxicity in human Vγ2Vδ2 T cells. Front Immunol 11:1405. doi: 10.3389/fimmu.2020.01405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kakimi K, Matsushita H, Masuzawa K, Karasaki T, Kobayashi Y, Nagaoka K, Hosoi A, Ikemura S, Kitano K, Kawada I, Manabe T, Takehara T, Ebisudani T, Nagayama K, Nakamura Y, Suzuki R, Yasuda H, Sato M, Soejima K, Nakajima J. 2020. Adoptive transfer of zoledronate-expanded autologous Vγ9Vδ2 T-cells in patients with treatment-refractory non-small-cell lung cancer: a multicenter open-label, single-arm, phase 2 study. J Immunother Cancer 8:e001185. doi: 10.1136/jitc-2020-001185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods 281:65–78. doi: 10.1016/s0022-1759(03)00265-5 [DOI] [PubMed] [Google Scholar]
- 34. Yagi H, Conroy PJ, Leung EWW, Law RHP, Trapani JA, Voskoboinik I, Whisstock JC, Norton RS. 2015. Structural basis for Ca2+-mediated interaction of the perforin C2 domain with lipid membranes. J Biol Chem 290:25213–25226. doi: 10.1074/jbc.M115.668384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sparrow EL, Fowler DW, Fenn J, Caron J, Copier J, Dalgleish AG, Bodman-Smith MD. 2020. The cytotoxic molecule granulysin is capable of inducing either chemotaxis or fugetaxis in dendritic cells depending on maturation: a role for Vδ2+ γδ T cells in the modulation of immune response to tumour?. Immunology 161:245–258. doi: 10.1111/imm.13248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lyubchenko TA, Wurth GA, Zweifach A. 2001. Role of calcium influx in cytotoxic T lymphocyte lytic granule exocytosis during target cell killing. Immunity 15:847–859. doi: 10.1016/s1074-7613(01)00233-3 [DOI] [PubMed] [Google Scholar]
- 37. Caroline J, Rafae BP, Guilherme C, Sabrina A, Zhitao L, Sumit SS, Ângela C, Dhelio BP, Ricardo TG, Jeffrey DD, Judy L. 2021. γδ T cells suppress Plasmodium falciparum blood-stage infection by direct killing and phagocytosis. Nat Immunol 22:347–357. doi: 10.1038/s41590-020-00847-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Matsumoto K, Hayashi K, Murata-Hirai K, Iwasaki M, Okamura H, Minato N, Morita CT, Tanaka Y. 2016. Targeting cancer cells with a bisphosphonate prodrug. ChemMedChem 11:2656–2663. doi: 10.1002/cmdc.201600465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tanaka Y, Iwasaki M, Murata-Hirai K, Matsumoto K, Hayashi K, Okamura H, Sugie T, Minato N, Morita CT, Toi M. 2017. Anti-tumor activity and immunotherapeutic potential of a bisphosphonate prodrug. Sci Rep 7:5987. doi: 10.1038/s41598-017-05553-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sandstrom A, Peigné CM, Léger A, Crooks JE, Konczak F, Gesnel MC, Breathnach R, Bonneville M, Scotet E, Adams EJ. 2014. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T cells. Immunity 40:490–500. doi: 10.1016/j.immuni.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rigau M, Ostrouska S, Fulford TS, Johnson DN, Woods K, Ruan Z, McWilliam HEG, Hudson C, Tutuka C, Wheatley AK, et al. 2020. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science 367:6478. doi: 10.1126/science.aay5516 [DOI] [PubMed] [Google Scholar]
- 42. Mowat E, Butcher J, Lang S, Williams C, Ramage G. 2007. Development of a simple model for studying the effects of antifungal agents on multicellular communities of Aspergillus fumigatus. J Med Microbiol 56:1205–1212. doi: 10.1099/jmm.0.47247-0 [DOI] [PubMed] [Google Scholar]
- 43. Mowat E, Lang S, Williams C, McCulloch E, Jones B, Ramage G. 2008. Phase-dependent antifungal activity against Aspergillus fumigatus developing multicellular filamentous biofilms. J Antimicrob Chemother 62:1281–1284. doi: 10.1093/jac/dkn402 [DOI] [PubMed] [Google Scholar]
- 44. Buil JB, Hare RK, Zwaan BJ, Arendrup MC, Melchers WJG, Verweij PE. 2019. The fading boundaries between patient and environmental routes of triazole resistance selection in Aspergillus fumigatus. PLoS Pathog 15:e1007858. doi: 10.1371/journal.ppat.1007858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lestrade PP, Bentvelsen RG, Schauwvlieghe A, Schalekamp S, van der Velden WJFM, Kuiper EJ, van Paassen J, van der Hoven B, van der Lee HA, Melchers WJG, de Haan AF, van der Hoeven HL, Rijnders BJA, van der Beek MT, Verweij PE. 2019. Voriconazole resistance and mortality in invasive Aspergillosis: a multicenter retrospective cohort study. Clin Infect Dis 68:1463–1471. doi: 10.1093/cid/ciy859 [DOI] [PubMed] [Google Scholar]
- 46. Loussert C, Schmitt C, Prevost MC, Balloy V, Fadel E, Philippe B, Kauffmann-Lacroix C, Latgé JP, Beauvais A. 2010. In vivo biofilm composition of Aspergillus fumigatus. Cell Microbiol 12:405–410. doi: 10.1111/j.1462-5822.2009.01409.x [DOI] [PubMed] [Google Scholar]
- 47. Davey MS, Morgan MP, Liuzzi AR, Tyler CJ, Khan MWA, Szakmany T, Hall JE, Moser B, Eberl M. 2014. Microbe-specific unconventional T cells induce human neutrophil differentiation into antigen cross-presenting cells. J Immunol 193:3704–3716. doi: 10.4049/jimmunol.1401018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gresnigt MS, Netea MG, van de Veerdonk FL. 2012. Pattern recognition receptors and their role in invasive Aspergillosis. Ann N Y Acad Sci 1273:60–67. doi: 10.1111/j.1749-6632.2012.06759.x [DOI] [PubMed] [Google Scholar]
- 49. Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, Kolls JK, Brown GD. 2005. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog 1:e42. doi: 10.1371/journal.ppat.0010042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cui Y, Kang L, Cui L, He W. 2009. Human gamma delta T cell recognition of lipid A is predominately presented by CD1b or CD1c on dendritic cells. Biol Direct 4:47. doi: 10.1186/1745-6150-4-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Devilder MC, Allain S, Dousset C, Bonneville M, Scotet E. 2009. Early triggering of exclusive IFN-gamma responses of human Vg9Vd2 t cells by TLR-activated myeloid and plasmacytoid dendritic cells. J Immunol 183:3625–3633. doi: 10.4049/jimmunol.0901571 [DOI] [PubMed] [Google Scholar]
- 52. Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, Elluru SR, Clavaud C, Paris S, Brakhage AA, Kaveri SV, Romani L, Latgé J-P. 2009. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460:1117–1121. doi: 10.1038/nature08264 [DOI] [PubMed] [Google Scholar]
- 53. Hanson DA, Kaspar AA, Poulain FR, Krensky AM. 1999. Biosynthesis of granulysin, a novel cytolytic molecule. Mol Immunol 36:413–422. doi: 10.1016/s0161-5890(99)00063-2 [DOI] [PubMed] [Google Scholar]
- 54. Peña SV, Krensky AM. 1997. Granulysin, a new human cytolytic granule-associated protein with possible involvement in cell-mediated cytotoxicity. Semin Immunol 9:117–125. doi: 10.1006/smim.1997.0061 [DOI] [PubMed] [Google Scholar]
- 55. Peña SV, Hanson DA, Carr BA, Goralski TJ, Krensky AM. 1997. Processing, subcellular localization, and function of 519 (granulysin), a human late T cell activation molecule with homology to small, lytic, granule proteins. J Immunol 158:2680–2688. [PubMed] [Google Scholar]
- 56. Lieberman J. 2010. Anatomy of a murder: how cytotoxic T cells and NK cells are activated, develop, and eliminate their targets. Immunol Rev 235:5–9. doi: 10.1111/j.0105-2896.2010.00914.x [DOI] [PubMed] [Google Scholar]
- 57. Spencer CT, Abate G, Sakala IG, Xia M, Truscott SM, Eickhoff CS, Linn R, Blazevic A, Metkar SS, Peng G, Froelich CJ, Hoft DF. 2013. Granzyme a produced by γ(9)δ(2) T cells induces human macrophages to inhibit growth of an intracellular pathogen. PLoS Pathog 9:e1003119. doi: 10.1371/journal.ppat.1003119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, Ganz T, Thoma-Uszynski S, Melián A, Bogdan C, Porcelli SA, Bloom BR, Krensky AM, Modlin RL. 1998. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 282:121–125. doi: 10.1126/science.282.5386.121 [DOI] [PubMed] [Google Scholar]
- 59. Ma LL, Wang CL, Neely GG, Epelman S, Krensky AM, Mody CH. 2004. NK cells use perforin rather than granulysin for anticryptococcal activity. J Immunol 173:3357–3365. doi: 10.4049/jimmunol.173.5.3357 [DOI] [PubMed] [Google Scholar]
- 60. Lee MJ, Gravelat FN, Cerone RP, Baptista SD, Campoli PV, Choe SI, Kravtsov I, Vinogradov E, Creuzenet C, Liu H, Berghuis AM, Latgé JP, Filler SG, Fontaine T, Sheppard DC. 2014. Overlapping and distinct roles of Aspergillus fumigatus UDP-glucose 4-epimerases in galactose metabolism and the synthesis of galactose-containing cell wall polysaccharides. J Biol Chem 289:1243–1256. doi: 10.1074/jbc.M113.522516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nakano Y, Tashiro M, Urano R, Kikuchi M, Ito N, Moriya E, Shirahige T, Mishima M, Takazono T, Miyazaki T, Izumikawa K. 2020. Characteristics of azole-resistant Aspergillus fumigatus attached to agricultural products imported to Japan. J Infect Chemother 26:1021–1025. doi: 10.1016/j.jiac.2020.05.008 [DOI] [PubMed] [Google Scholar]
- 62. Ito Y, Takazono T, Koga S, Nakano Y, Ashizawa N, Hirayama T, Tashiro M, Saijo T, Yamamoto K, Imamura Y, Miyazaki T, Yanagihara K, Izumikawa K, Mukae H. 2021. Clinical and experimental phenotype of azole-resistant Aspergillus fumigatus with a HapE splice site mutation: a case report. BMC Infect Dis 21:573. doi: 10.1186/s12879-021-06279-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hagiwara D, Arai T, Takahashi H, Kusuya Y, Watanabe A, Kamei K. 2018. Non-Cyp51A azole-resistant Aspergillus fumigatus isolates with mutation in HMG-CoA reductase. Emerg Infect Dis 24:1889–1897. doi: 10.3201/eid2410.180730 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Profiles of Vγ9Vδ2 T cells.
Derivation of Vγ9Vδ2T cells and NK cells.
An accounting of the reviewer comments and feedback.


