ABSTRACT
Hospital-acquired infections (HAIs) represent one of the significant causes of morbidity and mortality worldwide, and controlling pathogens in the hospital environment is of great importance. Currently, the standard disinfection method in the hospital environment is chemical disinfection. However, disinfectants are usually not used strictly according to the label, making them less effective in disinfection. Therefore, there is an emergent need to find a better approach that can be used in hospitals to control pathogenic bacteria in the clinical environment. Bacteriophages (phages) are effective in killing bacteria and have been applied in the treatment of bacterial infections but have not received enough attention regarding the control of contamination in the clinical environment. In this study, we found that various phages remain active in the presence of chemical disinfectants. Moreover, the combined use of specific phages and chemical disinfectants is more effective in removing bacterial biofilms and eliminating bacteria on hard surfaces. Thus, this proof-of-concept study indicates that adding phages directly to chemical disinfectants might be an effective and economical approach to enhance clinical environment disinfection.
IMPORTANCE
In this study, we investigated whether the combination of bacteriophages and chemical disinfectants can enhance the efficacy of reducing bacterial contamination on hard surfaces in the clinical setting. We found that specific phages are active in chemical disinfectants and that the combined use of phages and chemical disinfectants was highly effective in reducing bacterial presence on hard surfaces. As a proof-of-concept, we demonstrated that adding specific phages directly to chemical disinfectants is an effective and cost-efficient strategy for clinical environment disinfection.
KEYWORDS: phage, chemical disinfectant, clinical environmental disinfection
INTRODUCTION
In recent years, hospital-acquired infections (HAIs) have emerged as a significant public health concern worldwide, attracting increased attention (1, 2). HAIs have led to numerous severe and even fatal cases (3). Hospital environmental contamination plays a pivotal role in increasing the risk of HAIs. Therefore, effective disinfection of hospital environmental surfaces is highly crucial in reducing the risk of HAIs. Traditional disinfection methods, such as ultraviolet-C (UV-C) or chemical disinfection, are no longer sufficient to address the challenges posed by drug-resistant bacteria in hospital environments (4). Therefore, it is necessary to explore innovative disinfection methods that can handle the limitations of traditional approaches, while consistently and effectively targeting multidrug-resistant (MDR) bacteria in a hospital environment.
Bacteriophages are viruses that specifically infect bacteria. Initially, they were recognized for their potential as natural bactericidal agents (5, 6). However, research on phages focuses primarily on their application in clinical infection treatment, with limited exploration of their use in environmental disinfection (7–10). While some studies have demonstrated successful applications of phages in food processing plants, livestock farms, and fields, the research on phages in clinical environment disinfection is limited (11–15).
In this study, we conducted further investigations on phage-based strategies in combination with chemical disinfectants to maximize their potential for disinfecting clinical environments. This proof-of-concept study indicates that adding phages to chemical disinfectants might enhance the efficiency of clinical environment disinfection.
RESULTS
The effects of chemical disinfectants on phages
First, we tested whether chemical disinfectants led to inactivation of the phages, and the sensitivity of the eight phages against four different disinfectants was assessed (Tables 1 and 2). The tested concentrations of disinfection are those commonly employed in hospital environments (16–22). Among the eight tested phages, seven are dsDNA phages with the protein capsid, and phiYY is a dsRNA phage belonging to Cystoviridae. Its capsid is encapsulated by an external lipid envelope (23, 24).
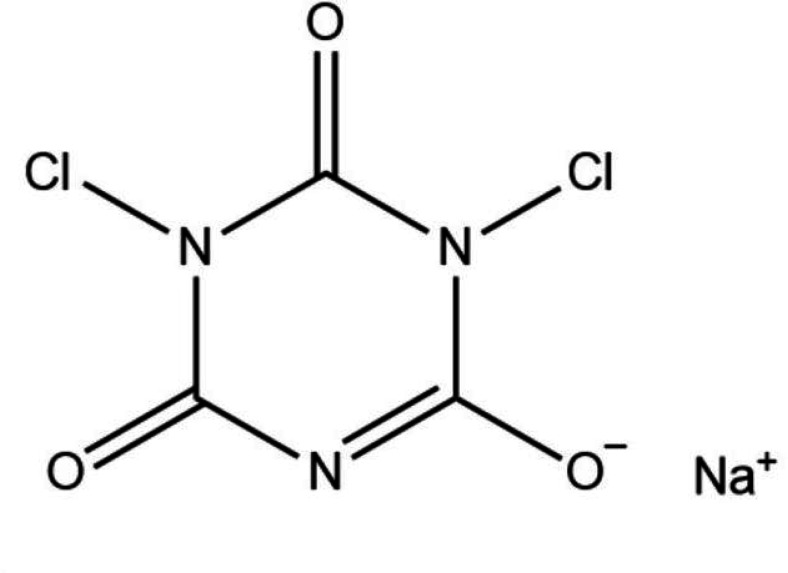
TABLE 1.
Four different types of chemical disinfectants
| Disinfectant | Sodium dichloroisocyanurate |
Benzalkonium chloride | Hydrogen peroxide | Chlorhexidine digluconate |
|---|---|---|---|---|
| Chemical structure |
|
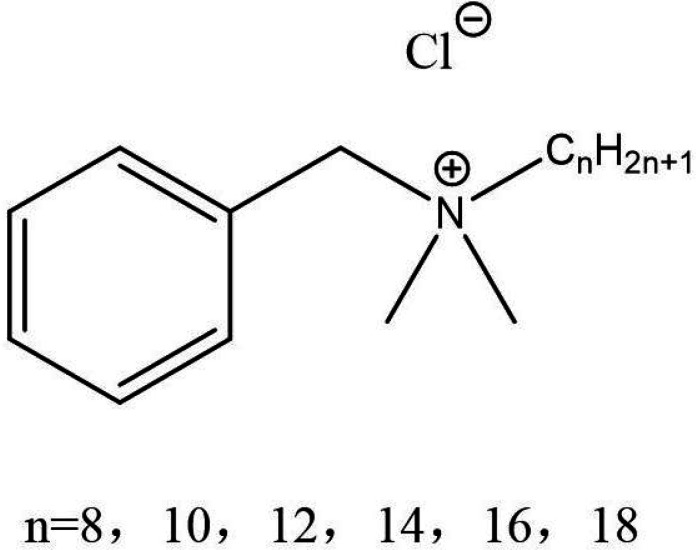
|
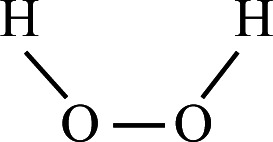
|

|
| Chemical class |
Phenolic compound | Quaternarium ammonum compound (QAC) |
Oxidizing agent | Biguanide |
| Mode of action |
Membrane disruption | Membrane damage | Oxidative damage | Membrane damage |
| Final concentration |
0.05% (500 mg/L) | 0.5% (5000 mg/L) | 3% (30,000 mg/L) | 0.5% (5000 mg/L) |
| Reference | (19) | (20) | (22) | (21) |
TABLE 2.
Selected strains and phages in this study
| Strain | Description | Reference |
|---|---|---|
| Ab9 | Wild-type Acinetobacater baumannii strain | (25) |
| THR60 | Wild-type Klebsiella pneumoniae strain | This study |
| JM110 | Mutant type Escherichia coli strain | (26) |
| PAO1 | Wild-type Pseudomonas aeruginosa strain | (27) |
| PAO1r | P. aeruginosa strain with a rough LPS phenotype | (28) |
| Abp9 | dsDNA A. baumannii bacteriophage | (29) |
| GZ7 | dsDNA K. pneumoniae bacteriophage | This study |
| T5 | dsDNA E. coli bacteriophage | (30) |
| phiYY | dsRNA P. aeruginosa bacteriophage | (23) |
| PaoP5 | dsDNA P. aeruginosa bacteriophage | (24) |
| PaP16-a | dsDNA P. aeruginosa bacteriophage | This study |
| PaP21-1 | dsDNA P. aeruginosa bacteriophage | This study |
| PaP24-X | dsDNA P. aeruginosa bacteriophage | This study |
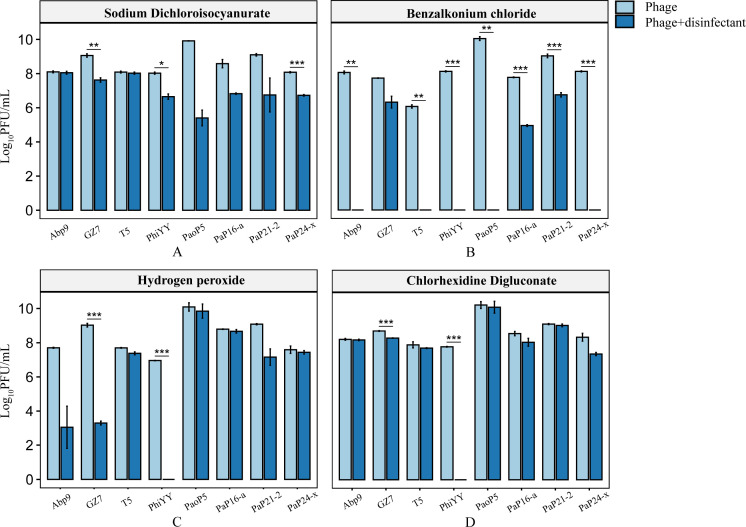
First, the phages were exposed to the disinfectants for 1 hour, and then the remaining viable phages were determined by plaque assays. Among the four chemical disinfectants, benzalkonium chloride (0.5% wt/vol) was very effective in killing the phages, in which most phages (e.g., Abp9, T5, and phiYY) were completely inactivated to undetectable levels (Fig. 1B). However, the other three chemical disinfectants, which are commonly applied in hospital environment disinfection, had a minimal impact on the titers of the phages, except phage phiYY, which was extremely sensitive to all the disinfectants due to the presence of the lipid envelope (23) (Fig. 1).
Fig 1.
The impact of (A) Sodium dichloroisocyanurate (0.05% wt/vol), (B) benzalkonium chloride (0. 5% wt/vol), (C) hydrogen peroxide (3% wt/vol), and (D) chlorhexidine digluconate (0.5% wt/vol) on phages. The data represent the mean of three biological replicates, and the phage titer is expressed in logarithmic form. Chemical disinfectants have different effects on phages. "Phage +disinfectant" stands for the experimental group, and "Phage" stands for the control group. All phages were incubated with chemical disinfectants at 37°C for 1 hour, and then the remaining viable phages were determined by plaque assays. The results showed some phages that could survive in the disinfectants. The experiment includes three biological replicates. The data are represented as means ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001, determined by independent t-test.
Sodium dichloroisocyanurate (0.05% wt/vol) was not adequate for inactivating the phages, which only reduced the titers of some phages (e.g., phiYY and PaoP5) by two to four orders of magnitude (Fig. 1A). Some of the tested phages, such as GZ7, PaP16-a, and PaP21-2, were more resistant to the four types of chemical disinfectants than other phages, which were not inactivated by the four disinfectants (Fig. 1). Thus, we were able to identify specific phages that could survive in the chemical disinfectants.
Phage and chemical disinfectants remove biofilms efficiently
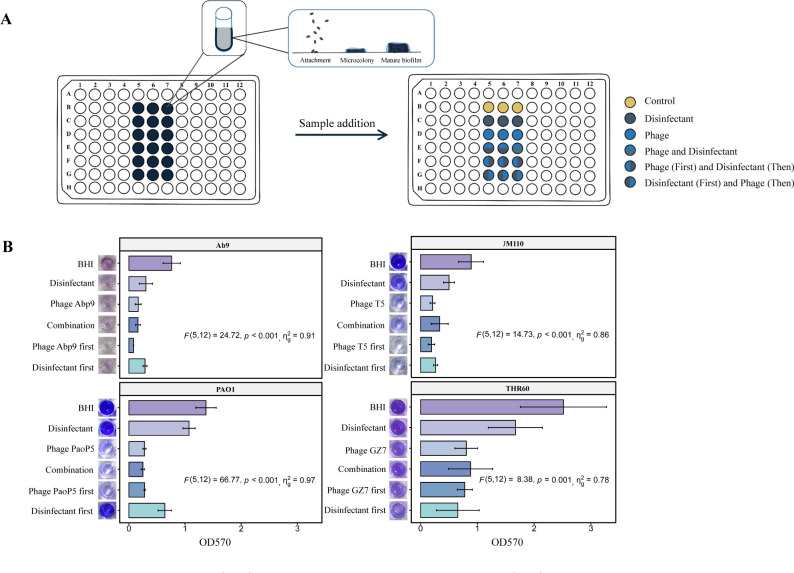
The biofilm is an important source of bacterial contamination on hospital environmental surfaces, and its unique physiological and structural characteristics make it resistant to chemical disinfectants (31). Four types of pathogenic bacteria and their phages were chosen to examine the potential interactions between phage and chemical disinfection for biofilm removal. These strains include A. baumannii Ab9, K. pneumoniae THR60, E. coli JM110, and P. aeruginosa PAO1. Correspondingly, the phages used were Abp9, GZ7, T5, and PaoP5 (Table 2). Then, 24-h biofilms of the four strains were treated with different combinations of phages at a final titer of 1010 PFU/mL and sodium dichloroisocyanurate (0.05% wt/vol) (Fig. 2A).
Fig 2.
(A) Schematic diagram of the biofilm removal experiment. Yellow represents brain heart infusion (BHI). Dark green represents sodium dichloroisocyanurate (0.05% wt/vol). Blue represents phages. Blue above and dark green below represent the simultaneous treatment of bacteria with phage and sodium dichloroisocyanurate, respectively (0.05% wt/vol). Blue on the left and dark green on the right indicate that the phages were applied first, and sodium dichloroisocyanurate (0.05% wt/vol) was used for treatment afterward. Dark green on the left and blue on the right indicate that sodium dichloroisocyanurate (0.05% wt/vol) was used for treatment first, and phages were applied afterward. (B) Results of biofilm clearance experiments. The data represent the mean of three biological replicates, the six graphs on the left of each group show the results of biofilm crystal violet staining, and the bar graph on the right represents the optical density at 570 nm (OD570) values. "Disinfectant" represents sodium dichloroisocyanurate (0.05% wt/vol); "Phage" represents Abp9, T5, PaoP5, or GZ7; "Combination" represents phages and sodium dichloroisocyanurate (0.05% wt/vol); "Phage first" represents the phage used first and then sodium dichloroisocyanurate (0.05% wt/vol); "Disinfectant first" represents sodium dichloroisocyanurate (0.05% wt/vol) used first and then phages. The data were presented as means ± SD. Statistical analysis was conducted using one-way ANOVA. The effect sizes (ηg2) demonstrated the relative magnitude of strain differences observed in each experiment. Inferential statistical comparison resulted in suggestive evidence (P < 0.05).
The biofilm removal rates of K. pneumoniae THR60 and E. coli JM110 were 68% and 76% with phage treatment alone and 65% and 62% with the combination treatment, respectively. The results indicated that the combination treatment was not consistently superior to that with phages. The possible reasons for this discrepancy may include a potential decrease in the lytic activity of the phages in the presence of sodium dichloroisocyanurate or the alteration of bacterial states caused by sodium dichloroisocyanurate, which could impact phage propagation. Therefore, treatments were applied sequentially. The biofilms of P. aeruginosa PAO1 were removed more efficiently when phage treatment was applied first. The removal rate achieved was 80%, but it was less efficient when the disinfectant was used first [F (5, 12)=66.77, P < 0.001] (Table S1). These results suggested that combining phage with sodium dichloroisocyanurate may enhance the efficacy of disinfection.
Surface decontamination by the phages and sodium dichloroisocyanurate
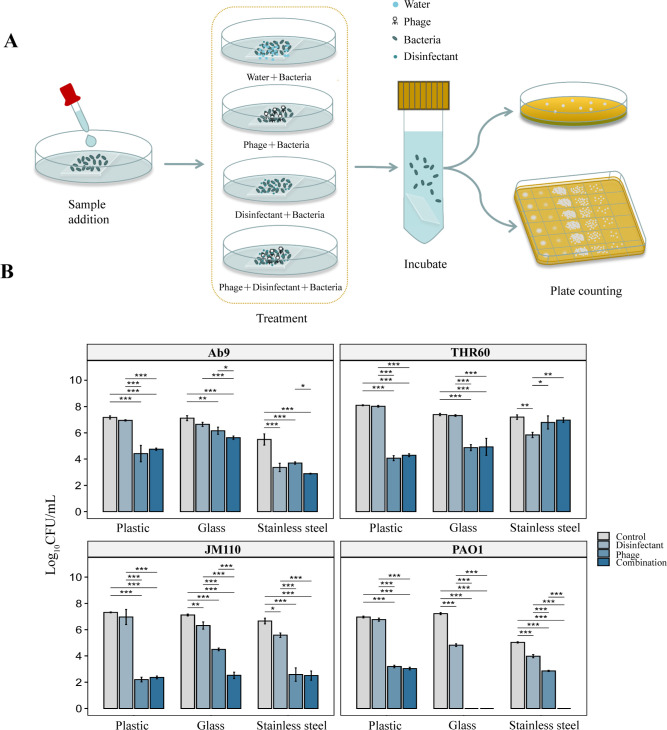
Because the type of the surface material may affect the effectiveness of disinfection (32), as a proof-of-concept experiment, "surface decontamination" studies were performed on three small pieces of distinct material (plastic, glass, and stainless steel) to mimic the hard surfaces in a hospital environment. After contaminating the surface with specific pathogenic bacteria for 1 hour, the materials were treated with phages and sodium dichloroisocyanurate for 2 hours (Fig. 3A). Then, a standard plate count method was used to determine the remaining pathogenic bacteria on the surfaces. For plastics contaminated with A. baumannii Ab9, the colony forming units (CFUs) significantly reduced (three to four orders of magnitude) [F (3, 8)=60.42, P < 0.001] (Table S2)in the phage treatment group and phage–sodium dichloroisocyanurate treatment group when compared with the sodium dichloroisocyanurate treatment alone group. Stainless steel experimentally contaminated with A. baumannii Ab9 showed a statistically significant difference between the phage treatment group and phage–sodium dichloroisocyanurate treatment group [F (3, 8)=53.14, P < 0.001] (Fig. 3B; Table S2).
Fig 3.
(A) Disinfection experiment on hard surfaces of the hospital environment. Sample addition: contaminate hospital environmental surfaces (plastic, glass, and stainless steel) with pathogens. Treatment: treat contaminated hard surfaces with different methods (phage and disinfectant). Incubation: collect bacteria. Count: colony counting. (B) Results of disinfection experiments with phage and sodium dichloroisocyanurate. Treatment with phages alone and the combination of phages with sodium dichloroisocyanurate were effective in reducing the bacterial load on hard surfaces. The data are shown as means ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001, determined by one-way ANOVA.
In addition, we observed notable variations in the disinfection efficacy of phages and sodium dichloroisocyanurate on experimentally contaminated surfaces, depending on the specific pathogens involved (Fig. 3B; Table S2). Specifically, the treatments led to a statistically significant effect in reducing the bacterial load on glass and stainless steel contaminated with P. aeruginosa PAO1 (F (3, 8)=2962.36, P < 0.001) (Fig. 3B; Table S2). When treated with phages alone or in combination with sodium dichloroisocyanurate, the P. aeruginosa PAO1 on glass and stainless steel was significantly reduced by six to seven orders of magnitude. Similarly, on plastic surfaces, the P. aeruginosa PAO1 was decreased by three to four orders of magnitude when treated with phages alone or in combination with sodium dichloroisocyanurate (Fig. 3B; Table S2). This highlights the potential effectiveness of phages in disinfecting environmental surfaces contaminated with P. aeruginosa.
All the studies have demonstrated that the effectiveness of different disinfection methods varies depending on the specific bacteria being targeted and the type of surface material. However, the overall trend observed indicates that both treatment with phages alone and with the combination of phages with sodium dichloroisocyanurate effectively reduced the bacterial load on the surfaces.
DISCUSSION
The significance of HAIs has garnered substantial attention worldwide. In the United States, the Centers for Disease Control and Prevention (CDC) estimates that HAIs contribute to an estimated 99,000 deaths annually (33–35). The emergence of MDR bacteria renders traditional antibiotic therapies less effective in treating HAIs and exacerbates the severity of the issue (36). Extensive research has shown that the hospital environment can serve as a reservoir for pathogens responsible for HAIs (37–44). For example, P. aeruginosa can survive on hospital surfaces for up to 16 months, while K. pneumoniae can survive up to 30 months (45). On the contrary, disinfectants are usually not used strictly according to the label, making them less effective in disinfection (46).
For an extensive period, UV-C and chemical disinfectants have demonstrated promising disinfection efficacy in hospital environments. However, a multitude of concerns have also emerged concurrently. UV-C disinfection is limited in its application due to the presence of patients in the room, and the concentration of chemical disinfectants used must be carefully regulated to ensure both their effectiveness and safety (47–49). Moreover, the constant movement of personnel in hospitals facilitates rapid recolonization of environmental surfaces by bacterial pathogens, further complicating the task of disinfecting the ward environment (50–53). Studies have shown that repeated exposure to sub-inhibitory concentrations of a specific disinfectant may lead bacteria to develop tolerance to the disinfectant (54–59).
The search for alternative disinfectants should prioritize the efficacy, safety, and the ability to target a broad spectrum of drug-resistant pathogens. This will ensure the development of effective disinfection strategies to mitigate the risks associated with HAIs and enhance patient safety. Compared to chemical disinfectants, phages possess several advantages. First, phages represent a vast microbial resource with immense potential for various applications, targeting specific bacteria that may exhibit multiple antibiotic resistance or are prone to forming biofilms. Second, phages are viruses that exclusively infect bacteria and do not affect mammalian cells, making them safe for humans (60, 61). Consequently, using phages for thorough disinfection in hospital environments is considered safe (62, 63). Further research and exploration of phage-based disinfection strategies are needed.
Several studies have investigated the direct application of phages as disinfectants on surfaces in hospital environments, demonstrating their potential in eliminating pathogens effectively(27) (64–66). Additionally, phages have shown effectiveness in removing biofilms formed by various pathogenic bacteria, including P. aeruginosa, A. baumannii, and K. pneumoniae (67, 68). While these studies have provided evidence for the effectiveness of phages in reducing pathogenic microorganisms in hospital environments, there is limited research on the evaluation of combining phages with chemical disinfectants (64, 69). In this article, we focused on studying the effect of disinfectants on phages and discovered that specific phages can tolerate clinical disinfectants. More combination effects were also observed in biofilm removal and bacteria removal from hard surfaces.
Phages lyse bacteria in the presence of chemical disinfectants
Phages will almost certainly encounter chemical disinfectants when they are used for clinical disinfection. Consequently, it is essential to develop phage-based products to assess the potential impact of chemical disinfectants on phages. Many other studies have explored the susceptibility of phages that inactivate bacterial pathogens to chemical disinfectants, with different results. The titers of two E. coli phages were significantly reduced after coincubation with 70% alcohol or chemical disinfectants containing octenidinum dihydrochloride and phenoxyethanol (65). An S. aureus phage was cocultivated with four chemical disinfectants, but only benzalkonium chloride and hydrogen peroxide impacted the phage’s activity.
In contrast, the other chemical disinfectants, trichloromethane and chlorhexidine, did not affect the phage’s activity (66). David et al. found that at least 102–103 PFU/mL of the initial number of phages survived in the presence of widely used traditional disinfectants applied daily in food environments (70). Our study showed that most phages exhibited high resistance to the tested disinfectants, further demonstrating the utility of phages as a disinfectant for bacteria in hospital environments.
In this study, benzalkonium chloride (0.5% wt/vol) had the most significant effect on the phages. However, results from other studies indicate phages are resistant to benzalkonium chloride. Stachler et al. reported that P. aeruginosa phages resisted benzalkonium chloride (69, 71). Similarly, benzalkonium chloride did not significantly affect the Staphylococcus phage at bacteriostatic concentrations (66). This difference may be due to the benzalkonium chloride concentrations that were used. In this study, we employed the clinical concentration of benzalkonium chloride. Conversely, the previous studies used a relatively low concentration of benzalkonium chloride, which might explain why they did not observe any significant impact on phages. The aforementioned results further illustrate that it is necessary to continue isolating phages that demonstrate resistance to chemical disinfectants if we intend to use them in clinical settings for disinfection. This also emphasizes the importance of identifying and isolating phages for their specific applications in clinical environment disinfection in the future.
The combination of phage and chemical disinfectants can be more effective in removing biofilms
It is widely recognized that bacteria can form biofilms as a survival mechanism in hostile environments, thereby increasing their resistance to antibiotics or disinfectants (71, 72). Therefore, effective removal of biofilms present on surfaces within hospital environments is an essential component of clinical environment disinfection (73). Previous studies have consistently shown that phages were effective in eradicating bacteria within biofilms. Zhang et al. observed approximately 75% reduction in P. aeruginosa biofilms treated with phages for 72 hours. Another study found more than 99% biofilm removal for monophage treatments (69, 74). Comparatively, this study found that phage treatment alone for 2 hours resulted in approximately 74% biofilm removal. However, previous research has indicated that a low phage concentration not only failed to remove biofilms but could also stimulate biofilm growth (75). These contradictory results highlight the importance of phage selection and characterization for future phage-based formulations.
Fewer studies have explored the combinatory effect of phages and chemical disinfectants on biofilms (76, 77). Agún et al. found that combining phages with chlorhexidine cannot increase the removal of biofilms (66). On the contrary, the combination treatment with sodium hypochlorite and phages revealed additive effects, and biofilms were removed entirely (78). Stachler et al. reported that pre-treating P. aeruginosa biofilms with phages followed by treatment with chemical disinfectants proved effective in biofilm removal (69). Consistent with previous studies, our study found that for treating the biofilms formed by A. baumannii Ab9, P. aeruginosa PAO1, and E. coli JM110, the treatment of biofilms with phages followed by treatment with sodium dichloroisocyanurate resulted in more significant removal of biofilms than the treatment with sodium dichloroisocyanurate followed by phage treatment. This phenomenon may be caused by the change in the state of the bacteria after pre-treatment with chemical disinfectants. Applying chemical disinfectants may affect the physiology of the bacteria, which might affect phage replications (79).
The combination of phages and chemical disinfectants can effectively reduce the pathogenic bacteria on the hard surfaces of hospital environments
Previous studies have investigated the impact of chemical disinfectants or phages for decontaminating bacteria-contaminated hard surfaces in hospital environments (80, 81). A Salmonella phage mixture could effectively reduce Salmonella Kentucky on glass and steel surfaces (80). Similarly, Viazis et al. found that the phage mixture BEC8 could reduce E. coli on stainless steel and ceramic surfaces (80). Some phages could decontaminate all types of hard surfaces tested (e.g., glass, plastic, and ceramic) without any significant difference between surface types or bacterial strains (82). Our study found that phages could effectively reduce bacterial load on hard surfaces. Phage PaoP5 effectively reduced the bacterial load on all three hard surfaces tested. This further demonstrated the utility of phages as a treatment for bacteria on hospital surfaces.
To date, fewer studies have examined the effectiveness of phage–disinfectant combinations in decontaminating hard surfaces. Stachler et al. reported that phage treatment before chemical disinfection can enhance the removal of plastic-surface-associated P. aeruginosa (69). In the current study, the combined use of phage and chemical disinfectants significantly reduced pathogenic bacteria on hard surfaces. Considering that phages are safe for humans and even used in therapy, combining phages and disinfectants could be regarded as a preventive control strategy. This approach can help prevent recurrent infections and combat outbreaks caused by specific pathogens in particular environments.
Limitations of the study and future research directions
This is a proof-of-concept study with several limitations. First, this study was conducted under laboratory conditions, which may not fully replicate the complexity and variability of natural hospital environments. The study did not investigate the impact of other factors, such as pathogen load, environmental temperature, or nutrient abundance. However, this study is a proof-of-concept experiment indicating that specific phages can survive in disinfectants and enhance elimination efficacy, which provides valuable data to support further research on related topics in hospital environments (83).
Second, this study focused on a limited number of phages and pathogenic bacteria, and the microbial composition on surfaces in natural clinical environments may be more diverse and complex. Therefore, future studies should consider the microbial diversity on clinical surfaces and investigate whether the presence of non-host bacteria could affect the lytic activity of phages against host bacteria. Moreover, the study focused on a limited number of phages and chemical disinfectants, and there may be other phage–chemical disinfectant combinations worth exploring.
Lastly, considering the strict host specificity of phages, their application for targeted disinfection in clinical settings requires the isolation of specific phages and a thorough evaluation of their interactions with chemical disinfectants before use (84–86). Therefore, future research efforts should focus on developing effective and efficient methods for isolating phages resistant to chemical disinfectants. A phage cocktail comprising multiple phages could offer a more targeted solution, and further investigation of the combination with a phage cocktail is also essential (87). Moreover, synthetic biology technology might be applied to expand the host ranges of phages and enhance tolerance of phage to disinfectants, which deserves further investigation (88, 89).
In summary, this study demonstrates the feasibility and potential of employing phages and chemical disinfectants for surface disinfection in hospital environments.
MATERIALS AND METHODS
Bacterial strains, phages, culture conditions, and disinfectant formulations
This study used four types of pathogenic bacteria and their corresponding phages (Table 2). The bacterial strains were cultured at 37°C in brain heart infusion (BHI) broth, providing suitable conditions for growth. The phages were routinely propagated on their host bacteria at 37°C.
Sodium dichloroisocyanurate (0.05% wt/vol, Chengdu Zhongguang Detergent Co., Ltd., China) (19), benzalkonium chloride (0.5% wt/vol, Shanghai, Jizhi Biochemical Technology Co., Ltd., China) (20), hydrogen peroxide (3% wt/vol, Chongqing, Ball Biotechnology Co., Ltd., China) (22, 90), and chlorhexidine gluconate (0.5% wt/vol, Chongqing, Shengbo biological reagent business department, China) (21, 22) were prepared using sterile water (autoclaved distilled water) and passed through a 0.45-µm filter membrane(Shanghai, Bioengineering Co., Ltd., China. The concentrations of the chemical disinfectants used in the experiments were prepared according to the manufacturers’ references (Table 1).
Preparation of phages
Phages were prepared as previously described (91). First, 10 µL of the phage was added to 3 mL of the logarithmic phase bacteria and then cultured in a shaking incubator at 37°C for 4 hours. Following incubation, the mixture was centrifuged at 21,000 × g for 1 minute, and the supernatant was filtered through a 0.45-µm filter and stored at 4°C.
Phage titers were determined by the double-layer agar (DLA) method (92). Ten microliters of the serial 10-fold diluted phage solution was mixed with host bacteria (100 µL) and BHI soft agar (5 mL), which was then poured onto a BHI agar plate and incubated overnight at 37°C to observe plaque formation.
The impact of chemical disinfectants on phages
To evaluate the sensitivity of the eight phages to different chemical disinfectants, several groups were established: the phage control group (phages plus sterile water), the chemical disinfectant control group (chemical disinfectant plus sterile water), and the experimental group (phages with chemical disinfectant). The samples from the experimental and control groups were incubated at 37°C for 1 hour. After incubation, the surviving phages in the samples were measured by DLA (92). The phage titer obtained in the experimental group was compared with that obtained in the phage control group to determine the survival rate of the phages.
Biofilm removal experiment
The biofilm removal experiment commenced with biofilm preparation. An overnight bacterial culture was diluted in the BHI medium to achieve an optical density at 600 nm (OD600) 0.2. Subsequently, diluted bacterial suspensions were aliquoted and transferred to each well of a 96-well microtitration plate, where the bacterial volume was 200 µL per well. The plate was then incubated at 37°C for 24 hours under static conditions, allowing the formation of a biofilm (93, 94). Following the incubation period, the planktonic phase was carefully removed from the wells, and the biofilm was washed twice with sterile water (200 µL). Finally, the biofilm was left to dry at room temperature. In the biofilm removal experiment, a single phage, sodium dichloroisocyanurate (0.05% wt/vol), or a combination of phage and chemical disinfectant (200 µL) was added to the wells of the 96-well plate. The plate was then incubated at 37°C for 4 hours (69). Following the treatment period, the liquid was carefully removed, and the biofilm was washed twice with sterile water (200 µL). Subsequently, the biofilm was stained with 0.1% crystal violet (200 µL) for 20 minutes. After removing the excess stain, the biofilm was washed twice with sterile water and left to dry at room temperature for 3 hours. To quantify the biomass, 200 µL of 95% ethanol was added to each well for decolorization, and the optical density at 570 nm (OD570) was measured (69, 95).
To investigate the impact of sequential application of phages and chemical disinfectants on biofilm removal, two experiments were conducted. In Experiment 1, phages were applied for 4 hours followed by chemical disinfectants for another 4 hours. In Experiment 2, chemical disinfectants were applied for 4 hours followed by application of phages for another 4 hours. The subsequent assessment of biofilm removal effectiveness was performed as described previously. The control group received the BHI medium only. To ensure consistency in the culture system, the chemical disinfectant was diluted in the BHI liquid medium. The biofilm removal rate was calculated by comparing the biomass in the treatment groups to that in the control group, expressed as a percentage.
Disinfection experiments of hard surfaces by phage and sodium dichloroisocyanurate
To evaluate the efficacy of phages and chemical disinfectants in disinfecting hard surfaces contaminated with pathogens, this study focused on three commonly encountered inanimate hard surface materials in clinical environments: plastic test tube caps (e.g., light switches and toilets), glass slides (e.g., windows), and stainless steel (e.g., door handles). These materials were first disinfected with 75% alcohol and then sterilized by autoclaving in steam for 30 minutes.
The first step involved contaminating the hard surfaces. In summary, the bacterial solution containing 1010 CFU was evenly distributed onto the tested surface (all ca. 25 × 25 mm in size) and spread using a pipette tip. The mixture was dried naturally and incubated at 37°C for 1 hour. The contaminated hard surfaces were subsequently divided into four groups for treatment: the first group served as the control and was treated with the BHI medium alone, the second group was treated with sodium dichloroisocyanurate (0.05% wt/vol) as the disinfectant experimental group, the third group was treated with phages as the phage experimental group, and the fourth group was treated with a combination of phages and sodium dichloroisocyanurate (0.05% wt/vol) as the combination experimental group. All treatment groups were kept at room temperature for 2 hours (96, 97). Following the treatment period, the treated hard surfaces were transferred to a centrifuge tube containing Dey and Engley (D/E) neutralizing broth. The surfaces were incubated in the broth for 10 minutes to ensure complete neutralization of any residual disinfectant and to facilitate the transfer of bacteria from the hard surfaces to the neutralizing broth. Subsequently, the number of bacteria in the neutralizing broth was determined by performing plate colony counting. This allowed for a comparison of the bacterial counts between the control group and the experimental groups (98).
Data analysis
All experiments were conducted with a minimum of three biological replicates to ensure the reliability of the results. The experimental data were subjected to logarithmic transformation and analyzed using SPSS version 18.0 (SPSS Inc., Chicago, Ill., USA). Graphs were created using R version 4.1.3 (Bell Laboratories, Madison, WI, USA) with the ggplot2 package, utilizing the mean and standard deviations of the data sets (99). The laboratory examination indexes between the two groups were analyzed with the independent t-test, and the Bonferroni correction was applied to adjust P-values. Multiple group mean comparisons were performed with one-way analysis of variance (ANOVA) and Tukey test for post hoc comparisons. All tests were bilateral, and P < 0.05 was considered statistically significant.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (NSFC) under award number: 32100116.
We declare no competing financial interest.
Contributor Information
Yu Luo, Email: luoyuhlgl@tmmu.edu.cn.
Shuai Le, Email: leshuai2004@qq.com.
Olaya Rendueles, Centre de Biologie Integrative, Toulouse, France.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.03797-23.
Statistical analysis of Phage and chemical disinfectant remove biofilms efficiently.
Statistical analysis of surface decontamination by the phages and sodium dichloroisocyanurate.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Lemiech-Mirowska E, Kiersnowska ZM, Michałkiewicz M, Depta A, Marczak M. 2021. Nosocomial infections as one of the most important problems of healthcare system. Ann Agric Environ Med 28:361–366. doi: 10.26444/aaem/122629 [DOI] [PubMed] [Google Scholar]
- 2. Raoofi S, Pashazadeh Kan F, Rafiei S, Hosseinipalangi Z, Noorani Mejareh Z, Khani S, Abdollahi B, Seyghalani Talab F, Sanaei M, Zarabi F, et al. 2023. Global prevalence of nosocomial infection: a systematic review and meta-analysis. PLoS One 18:e0274248. doi: 10.1371/journal.pone.0274248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kollef MH, Torres A, Shorr AF, Martin-Loeches I, Micek ST. 2021. Nosocomial infection. Crit Care Med 49:169–187. doi: 10.1097/CCM.0000000000004783 [DOI] [PubMed] [Google Scholar]
- 4. Rock C, Hsu YJ, Curless MS, Carroll KC, Ross Howard T, Carson KA, Cummings S, Anderson M, Milstone AM, Maragakis LL. 2022. Ultraviolet-C light evaluation as adjunct disinfection to remove multidrug-resistant organisms. Clin Infect Dis 75:35–40. doi: 10.1093/cid/ciab896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cisek AA, Dąbrowska I, Gregorczyk KP, Wyżewski Z. 2017. Phage therapy in bacterial infections treatment: one hundred years after the discovery of bacteriophages. Curr Microbiol 74:277–283. doi: 10.1007/s00284-016-1166-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh K, Biswas A, Chakrabarti AK, Dutta S. 2023. Phage therapy as a protective tool against pathogenic bacteria: how far we are? Curr Pharm Biotechnol 24:1277–1290. doi: 10.2174/1389201024666221207114047 [DOI] [PubMed] [Google Scholar]
- 7. Fu W, Forster T, Mayer O, Curtin JJ, Lehman SM, Donlan RM. 2010. Bacteriophage cocktail for the prevention of biofilm formation by Pseudomonas aeruginosa on catheters in an in vitro model system . Antimicrob Agents Chemother 54:397–404. doi: 10.1128/AAC.00669-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. J. Santiago A, L. Burgos-Garay M, Kartforosh L, Mazher M, M. Donlan R. 2020. Bacteriophage treatment of carbapenemase-producing Klebsiella pneumoniae in a multispecies biofilm: a potential biocontrol strategy for healthcare facilities. AIMS Microbiol 6:43–63. doi: 10.3934/microbiol.2020003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rajaramon S, Shanmugam K, Dandela R, Solomon AP. 2023. Emerging evidence-based innovative approaches to control catheter-associated urinary tract infection: a review. Front Cell Infect Microbiol 13:1134433. doi: 10.3389/fcimb.2023.1134433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Santiago AJ, Donlan RMJEP. 2020. Bacteriophage infections of biofilms of health care-associated pathogens: Klebsiella pneumoniae EcoSal Plus 9:10. doi: 10.1128/ecosalplus.ESP-0029-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holtappels D, Fortuna K, Lavigne R, Wagemans J. 2021. The future of phage biocontrol in integrated plant protection for sustainable crop production. Curr Opin Biotechnol 68:60–71. doi: 10.1016/j.copbio.2020.08.016 [DOI] [PubMed] [Google Scholar]
- 12. Pinto G, Almeida C, Azeredo J. 2020. Bacteriophages to control Shiga toxin-producing E. coli– safety and regulatory challenges. Crit Rev Biotechnol 40:1081–1097. doi: 10.1080/07388551.2020.1805719 [DOI] [PubMed] [Google Scholar]
- 13. Żbikowska K, Michalczuk M, Dolka B. 2020. The use of bacteriophages in the poultry industry. Animals (Basel) 10:872. doi: 10.3390/ani10050872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li M, Lin H, Jing Y, Wang J. 2020. Broad-host-range Salmonella bacteriophage STP4-a and its potential application evaluation in poultry industry. Poult Sci 99:3643–3654. doi: 10.1016/j.psj.2020.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abd-El Wahab A, Basiouni S, El-Seedi HR, Ahmed MFE, Bielke LR, Hargis B, Tellez-Isaias G, Eisenreich W, Lehnherr H, Kittler S, Shehata AA, Visscher C. 2023. An overview of the use of bacteriophages in the poultry industry: successes, challenges, and possibilities for overcoming breakdowns. Front Microbiol 14:1136638. doi: 10.3389/fmicb.2023.1136638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meyer C, Lucaβen K, Gerson S, Xanthopoulou K, Wille T, Seifert H, Higgins P. 2022. Contribution of RND-type efflux pumps in reduced susceptibility to biocides in Acinetobacter baumannii. Antibiotics (Basel) 11:1635. doi: 10.3390/antibiotics11111635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention . 2008. Guideline for disinfection and sterilization in healthcare facilities, 2008. Atlanta, GA: Centers for Disease Control and Prevention [Google Scholar]
- 18. Meyer C, Lucaβen K, Gerson S, Xanthopoulou K, Wille T, Seifert H, Higgins PG. 2022. Contribution of RND-type efflux pumps in reduced susceptibility to biocides in Acinetobacter baumannii Antibiotics (Basel) 11:1635. doi: 10.3390/antibiotics11111635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joshi LT, Welsch A, Hawkins J, Baillie L. 2017. The effect of hospital biocide sodium dichloroisocyanurate on the viability and properties of Clostridium difficile spores. Lett Appl Microbiol 65:199–205. doi: 10.1111/lam.12768 [DOI] [PubMed] [Google Scholar]
- 20. Kampf G. 2018. Adaptive microbial response to low-level benzalkonium chloride exposure. J Hosp Infect 100:e1–e22. doi: 10.1016/j.jhin.2018.05.019 [DOI] [PubMed] [Google Scholar]
- 21. Block C, Furman M. 2002. Association between intensity of chlorhexidine use and micro-organisms of reduced susceptibility in a hospital environment. J Hosp Infect 51:201–206. doi: 10.1053/jhin.2002.1246 [DOI] [PubMed] [Google Scholar]
- 22. Théraud M, Bédouin Y, Guiguen C, Gangneux JP. 2004. Efficacy of antiseptics and disinfectants on clinical and environmental yeast isolates in planktonic and biofilm conditions. J Med Microbiol 53:1013–1018. doi: 10.1099/jmm.0.05474-0 [DOI] [PubMed] [Google Scholar]
- 23. Yang Y, Lu S, Shen W, Zhao X, Shen M, Tan Y, Li G, Li M, Wang J, Hu F, Le S. 2016. Characterization of the first double-stranded RNA bacteriophage infecting Pseudomonas aeruginosa. Sci Rep 6:38795. doi: 10.1038/srep38795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shen M, Le S, Jin X, Li G, Tan Y, Li M, Zhao X, Shen W, Yang Y, Wang J, Zhu H, Li S, Rao X, Hu F, Lu S. 2016. Characterization and comparative genomic analyses of Pseudomonas aeruginosa phage PaoP5: new members assigned to PAK_P1-like viruses. Sci Rep 6:34067. doi: 10.1038/srep34067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jiang L, Tan J, Hao Y, Wang Q, Yan X, Wang D, Tuo L, Wei Z, Huang G. 2020. Isolation and characterization of a novel myophage Abp9 against pandrug resistant Acinetobacater baumannii. Front Microbiol 11:506068. doi: 10.3389/fmicb.2020.506068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yanisch-Perron C, Vieira J, Messing JJG. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mpl8 and pUC19 vectors. Gene 33:103–119. doi: 10.1016/0378-1119(85)90120-9 [DOI] [PubMed] [Google Scholar]
- 27. Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, Ernst S, Will O, Kaul R, Raymond C, Levy R, Chun-Rong L, Guenthner D, Bovee D, Olson MV, Manoil C. 2003. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci USA 100:14339–14344. doi: 10.1073/pnas.2036282100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shen M, Zhang H, Shen W, Zou Z, Lu S, Li G, He X, Agnello M, Shi W, Hu F, Le S. 2018. Pseudomonas aeruginosa MutL promotes large chromosomal deletions through non-homologous end joining to prevent bacteriophage predation. Nucleic Acids Res 46:4505–4514. doi: 10.1093/nar/gky160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jiang L, Tan J, Hao Y, Wang Q, Yan X, Wang D, Tuo L, Wei Z, Huang G. 2020. Isolation and characterization of a novel myophage Abp9 against pandrug resistant Acinetobacater baumannii. Front Microbiol 11:506068. doi: 10.3389/fmicb.2020.506068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Demerec M, Fano UJG. 1945. Bacteriophage-resistant mutants in Escherichia coli. Genetics 30:119–136. doi: 10.1093/genetics/30.2.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maillard J-Y, Centeleghe I. 2023. How biofilm changes our understanding of cleaning and disinfection. Antimicrob Resist Infect Control 12:95. doi: 10.1186/s13756-023-01290-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen L, Lee W-J, Ma Y, Jang SS, Fong K, Wang S. 2022. The efficacy of different sanitizers against MS2 bacteriophage introduced onto plastic or stainless steel surfaces. Curr Res Food Sci 5:175–181. doi: 10.1016/j.crfs.2022.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Magill SS, O’Leary E, Janelle SJ, Thompson DL, Dumyati G, Nadle J, Wilson LE, Kainer MA, Lynfield R, Greissman S, et al. 2018. Changes in prevalence of health care–associated infections in U.S. hospitals. N Engl J Med 379:1732–1744. doi: 10.1056/NEJMoa1801550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention . 2022. Healthcare-associated infections (HAIs). Atlanta, GA: Centers for Disease Control and Prevention [Google Scholar]
- 35. World Health Organization . 2020. Report on the burden of endemic health care-associated infection worldwide. Geneva, Switzerland: World Health Organization [Google Scholar]
- 36. Hayward C, Ross KE, Brown MH, Whiley H. 2020. Water as a source of antimicrobial resistance and healthcare-associated infections. Pathogens 9:667. doi: 10.3390/pathogens9080667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carling P. 2013. Methods for assessing the adequacy of practice and improving room disinfection. Am J Infect Control 41:S20–5. doi: 10.1016/j.ajic.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 38. Chen LF, Knelson LP, Gergen MF, Better OM, Nicholson BP, Woods CW, Rutala WA, Weber DJ, Sexton DJ, Anderson DJ, CDC Prevention Epicenters Program . 2019. A prospective study of transmission of multidrug-resistant organisms (MDROs) between environmental sites and hospitalized patients—the TransFER study. Infect Control Hosp Epidemiol 40:47–52. doi: 10.1017/ice.2018.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Facciolà A, Pellicanò GF, Visalli G, Paolucci IA, Venanzi Rullo E, Ceccarelli M, D’Aleo F, Di Pietro A, Squeri R, Nunnari G, La Fauci V. 2019. The role of the hospital environment in the healthcare-associated infections: a general review of the literature. Eur Rev Med Pharmacol Sci 23:1266–1278. doi: 10.26355/eurrev_201902_17020 [DOI] [PubMed] [Google Scholar]
- 40. Russotto V, Cortegiani A, Raineri SM, Giarratano A. 2015. Bacterial contamination of inanimate surfaces and equipment in the intensive care unit. J Intensive Care 3:54. doi: 10.1186/s40560-015-0120-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sexton T, Clarke P, O’Neill E, Dillane T, Humphreys H. 2006. Environmental reservoirs of methicillin-resistant Staphylococcus aureus in isolation rooms: correlation with patient isolates and implications for hospital hygiene. J Hosp Infect 62:187–194. doi: 10.1016/j.jhin.2005.07.017 [DOI] [PubMed] [Google Scholar]
- 42. Dancer SJ. 2014. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev 27:665–690. doi: 10.1128/CMR.00020-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boyce JM, Havill NL, Guercia KA, Moore BA. 2022. Microbial burden on environmental surfaces in patient rooms before daily cleaning—analysis of multiple confounding variables. Infect Control Hosp Epidemiol 43:1142–1146. doi: 10.1017/ice.2021.349 [DOI] [PubMed] [Google Scholar]
- 44. Singrün C, Hsam SLK, Hartl L, Zeller FJ, Mohler V. 2003. Powdery mildew resistance gene Pm22 in cultivar Virest is a member of the complex Pm1 locus in common wheat (Triticum aestivum L. em Thell.). Theor Appl Genet 106:1420–1424. doi: 10.1007/s00122-002-1187-7 [DOI] [PubMed] [Google Scholar]
- 45. Otter JA, Yezli S, Salkeld JAG, French GL. 2013. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am J Infect Control 41:S6–11. doi: 10.1016/j.ajic.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 46. Gao W, Liu Z, Feng Z, XJCJoN L. 2010. Chemical disinfectant application: problems and countermeasures. Plant Pathol J 20:69–70. [Google Scholar]
- 47. Sholtes K, Simons R, Beck S, Adeli B, Sun Z. 2020. UV 101: overview of ultraviolet disinfection. International Ultraviolet Association [Google Scholar]
- 48. Kreitenberg A, Martinello RA. 2021. Perspectives and recommendations regarding standards for ultraviolet-C whole-room disinfection in healthcare. J Res Natl Inst Stand Technol 126:126015. doi: 10.6028/jres.126.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Resendiz M, Blanchard D, West GF. 2023. A systematic review of the germicidal effectiveness of ultraviolet disinfection across high-touch surfaces in the immediate patient environment. J Infect Prev 24:166–177. doi: 10.1177/17571774231159388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ledwoch K, Dancer SJ, Otter JA, Kerr K, Roposte D, Rushton L, Weiser R, Mahenthiralingam E, Muir DD, Maillard JY. 2018. Beware biofilm! Dry biofilms containing bacterial pathogens on multiple healthcare surfaces; a multi-centre study. J Hosp Infect 100:e47–e56. doi: 10.1016/j.jhin.2018.06.028 [DOI] [PubMed] [Google Scholar]
- 51. Costa DM, Johani K, Melo DS, Lopes LKO, Lopes Lima LKO, Tipple AFV, Hu H, Vickery K. 2019. Biofilm contamination of high‐touched surfaces in intensive care units: epidemiology and potential impacts. Lett Appl Microbiol 68:269–276. doi: 10.1111/lam.13127 [DOI] [PubMed] [Google Scholar]
- 52. Han JH, Sullivan N, Leas BF, Pegues DA, Kaczmarek JL, Umscheid CA. 2015. Cleaning hospital room surfaces to prevent health care–associated infections. Ann Intern Med 163:598–607. doi: 10.7326/M15-1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Doll M, Stevens M, Bearman G. 2018. Environmental cleaning and disinfection of patient areas. Int J Infect Dis 67:52–57. doi: 10.1016/j.ijid.2017.10.014 [DOI] [PubMed] [Google Scholar]
- 54. Martínez-Suárez JV, Ortiz S, López-Alonso V. 2016. Potential impact of the resistance to quaternary ammonium disinfectants on the persistence of Listeria monocytogenes in food processing environments. Front Microbiol 7:638. doi: 10.3389/fmicb.2016.00638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang K, Chen M-L, Zhu D. 2023. Exposure to benzalkonium chloride disinfectants promotes antibiotic resistance in sewage sludge microbiomes. Sci Total Environ 867:161527. doi: 10.1016/j.scitotenv.2023.161527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim M, Weigand MR, Oh S, Hatt JK, Krishnan R, Tezel U, Pavlostathis SG, Konstantinidis KT. 2018. Widely used benzalkonium chloride disinfectants can promote antibiotic resistance. Appl Environ Microbiol 84:e01201-18. doi: 10.1128/AEM.01201-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cadena M, Kelman T, Marco ML, Pitesky M. 2019. Understanding antimicrobial resistance (AMR) profiles of Salmonella biofilm and planktonic bacteria challenged with disinfectants commonly used during poultry processing. Foods 8:275. doi: 10.3390/foods8070275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kampf G. 2018. Biocidal agents used for disinfection can enhance antibiotic resistance in Gram-negative species. Antibiotics (Basel) 7:110. doi: 10.3390/antibiotics7040110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nasr AM, Mostafa MS, Arnaout HH, Elshimy AAA. 2018. The effect of exposure to sub-inhibitory concentrations of hypochlorite and quaternary ammonium compounds on antimicrobial susceptibility of Pseudomonas aeruginosa. Am J Infect Control 46:e57–e63. doi: 10.1016/j.ajic.2018.04.201 [DOI] [PubMed] [Google Scholar]
- 60. Jensen KC, Hair BB, Wienclaw TM, Murdock MH, Hatch JB, Trent AT, White TD, Haskell KJ, Berges BK. 2015. Isolation and host range of bacteriophage with lytic activity against methicillin-resistant Staphylococcus aureus and potential use as a fomite decontaminant. PLoS One 10:e0131714. doi: 10.1371/journal.pone.0131714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mathieu J, Yu P, Zuo P, Da Silva MLB, Alvarez PJJ. 2019. Going viral: emerging opportunities for phage-based bacterial control in water treatment and reuse. Acc Chem Res 52:849–857. doi: 10.1021/acs.accounts.8b00576 [DOI] [PubMed] [Google Scholar]
- 62. Liu D, Van Belleghem JD, de Vries CR, Burgener E, Chen Q, Manasherob R, Aronson JR, Amanatullah DF, Tamma PD, Suh GA. 2021. The safety and toxicity of phage therapy: a review of animal and clinical studies. Viruses 13:1268. doi: 10.3390/v13071268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Speck P, Smithyman A, Millard A. 2016. Safety and efficacy of phage therapy via the intravenous route. FEMS Microbiol Lett 363:fnv242. doi: 10.1093/femsle/fnv242 [DOI] [PubMed] [Google Scholar]
- 64. Ledda M, Aviran S. 2018. PATTERNA: transcriptome-wide search for functional RNA elements via structural data signatures. Genome Biol 19:28. doi: 10.1186/s13059-018-1399-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cieślik M, Harhala M, Orwat F, Dąbrowska K, Górski A, Jończyk-Matysiak E. 2022. Two newly isolated enterobacter-specific bacteriophages: biological properties and stability studies. Viruses 14:1518. doi: 10.3390/v14071518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Agún S, Fernández L, González-Menéndez E, Martínez B, Rodríguez A, García P. 2018. Study of the interactions between bacteriophage phiIPLA-RODI and four chemical disinfectants for the elimination of Staphylococcus aureus contamination. Viruses 10:103. doi: 10.3390/v10030103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Song J, Ruan H, Chen L, Jin Y, Zheng J, Wu R, Sun D. 2021. Potential of bacteriophages as disinfectants to control of Staphylococcus aureus biofilms. BMC Microbiol 21:57. doi: 10.1186/s12866-021-02117-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liu S, Lu H, Zhang S, Shi Y, Chen Q. 2022. Phages against pathogenic bacterial biofilms and biofilm-based infections: a review. Pharmaceutics 14:427. doi: 10.3390/pharmaceutics14020427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stachler E, Kull A, Julian TR, McBain AJ. 2021. Bacteriophage treatment before chemical disinfection can enhance removal of plastic-surface-associated Pseudomonas aeruginosa. Appl Environ Microbiol 87:e0098021. doi: 10.1128/AEM.00980-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tomat D, Balagué C, Aquili V, Verdini R, Quiberoni A. 2018. Resistance of phages lytic to pathogenic Escherichia coli to sanitisers used by the food industry and in home settings. Int J of Food Sci Tech 53:533–540. doi: 10.1111/ijfs.13626 [DOI] [Google Scholar]
- 71. Smith AW. 2005. Biofilms and antibiotic therapy: is there a role for combating bacterial resistance by the use of novel drug delivery systems? Adv Drug Deliv Rev 57:1539–1550. doi: 10.1016/j.addr.2005.04.007 [DOI] [PubMed] [Google Scholar]
- 72. Ciofu O, Tolker-Nielsen T. 2019. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents—how P. aeruginosa can escape antibiotics. Front Microbiol 10:913. doi: 10.3389/fmicb.2019.00913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chen Y, Liao K, Huang Y, Guo P, Huang H, Wu Z, Liu M. 2020. Determining the susceptibility of carbapenem resistant Klebsiella pneumoniae and Escherichia coli strains against common disinfectants at a tertiary hospital in China. BMC Infect Dis 20:88. doi: 10.1186/s12879-020-4813-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang Y, Hu Z. 2013. Combined treatment of Pseudomonas aeruginosa biofilms with bacteriophages and chlorine. Biotechnol Bioeng 110:286–295. doi: 10.1002/bit.24630 [DOI] [PubMed] [Google Scholar]
- 75. Zhang B, Yu P, Wang Z, Alvarez PJJ. 2020. Hormetic promotion of biofilm growth by polyvalent bacteriophages at low concentrations. Environ Sci Technol 54:12358–12365. doi: 10.1021/acs.est.0c03558 [DOI] [PubMed] [Google Scholar]
- 76. Abedon ST. 2015. Ecology of anti-biofilm agents I: antibiotics versus bacteriophages. Pharmaceuticals (Basel) 8:525–558. doi: 10.3390/ph8030525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. García-Cruz JC, Huelgas-Méndez D, Jiménez-Zúñiga JS, Rebollar-Juárez X, Hernández-Garnica M, Fernández-Presas AM, Husain FM, Alenazy R, Alqasmi M, Albalawi T, Alam P, García-Contreras R. 2023. Myriad applications of bacteriophages beyond phage therapy. PeerJ 11:e15272. doi: 10.7717/peerj.15272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chandra M, Thakur S, Chougule SS, Narang D, Kaur G, Sharma N.. 2015. Combined effect of disinfectant and phage on the survivality of S. Typhimurium and its biofilm phenotype. Internet J Food Saf 17:25–31. [Google Scholar]
- 79. Ferriol-González C, Domingo-Calap P. 2020. Phages for biofilm removal. Antibiotics (Basel) 9:268. doi: 10.3390/antibiotics9050268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Viazis S, Labuza TP, Diez‐Gonzalez F. 2014. Bacteriophage mixture inactivation kinetics against Escherichia coli O157:H7 on hard surfaces. J Food Saf 35:66–74. doi: 10.1111/jfs.12160 [DOI] [Google Scholar]
- 81. Woolston J, Parks AR, Abuladze T, Anderson B, Li M, Carter C, Hanna LF, Heyse S, Charbonneau D, Sulakvelidze A. 2014. Bacteriophages lytic for Salmonella rapidly reduce Salmonella contamination on glass and stainless steel surfaces. Bacteriophage 3:e25697. doi: 10.4161/bact.25697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. D’Accolti M, Soffritti I, Piffanelli M, Bisi M, Mazzacane S, Caselli E. 2018. Efficient removal of hospital pathogens from hard surfaces by a combined use of bacteriophages and probiotics: potential as sanitizing agents. Infect Drug Resist 11:1015–1026. doi: 10.2147/IDR.S170071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Glonti T, Pirnay J-P. 2022. In vitro techniques and measurements of phage characteristics that are important for phage therapy success. Viruses 14:1490. doi: 10.3390/v14071490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Plumet L, Ahmad-Mansour N, Dunyach-Remy C, Kissa K, Sotto A, Lavigne J-P, Costechareyre D, Molle V. 2022. Bacteriophage therapy for Staphylococcus aureus infections: a review of animal models, treatments, and clinical trials. Front Cell Infect Microbiol 12:907314. doi: 10.3389/fcimb.2022.907314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kortright KE, Chan BK, Koff JL, Turner PE. 2019. Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 25:219–232. doi: 10.1016/j.chom.2019.01.014 [DOI] [PubMed] [Google Scholar]
- 86. Hatfull GF, Dedrick RM, Schooley RT. 2022. Phage therapy for antibiotic-resistant bacterial infections. Annu Rev Med 73:197–211. doi: 10.1146/annurev-med-080219-122208 [DOI] [PubMed] [Google Scholar]
- 87. Kaur G, Agarwal R, Sharma RK. 2021. Bacteriophage therapy for critical and high-priority antibiotic-resistant bacteria and phage cocktail-antibiotic formulation perspective. Food Environ Virol 13:433–446. doi: 10.1007/s12560-021-09483-z [DOI] [PubMed] [Google Scholar]
- 88. Sun Q, Shen L, Zhang B-L, Yu J, Wei F, Sun Y, Chen W, Wang S. 2023. Advance on engineering of bacteriophages by synthetic biology. Infect Drug Resist 16:1941–1953. doi: 10.2147/IDR.S402962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Strathdee SA, Hatfull GF, Mutalik VK, Schooley RT. 2023. Phage therapy: from biological mechanisms to future directions. Cell 186:17–31. doi: 10.1016/j.cell.2022.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ríos-Castillo AG, González-Rivas F, Rodríguez-Jerez JJ. 2017. Bactericidal efficacy of hydrogen peroxide‐based disinfectants against Gram‐positive and Gram‐negative bacteria on stainless steel surfaces. J Food Sci 82:2351–2356. doi: 10.1111/1750-3841.13790 [DOI] [PubMed] [Google Scholar]
- 91. Arai T, Kuroda S. 1962. Fine structure of spiny spores of Streptomyces. J Bacteriol 83:924. doi: 10.1128/jb.83.4.924-924.1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kropinski AM, Mazzocco A, Waddell TE, Lingohr E, Johnson RP. 2009. Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol Biol 501:69–76. doi: 10.1007/978-1-60327-164-6_7 [DOI] [PubMed] [Google Scholar]
- 93. Stepanović S, Vuković D, Hola V, Di Bonaventura G, Djukić S, Cirković I, Ruzicka F. 2007. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 115:891–899. doi: 10.1111/j.1600-0463.2007.apm_630.x [DOI] [PubMed] [Google Scholar]
- 94. Wilson C, Lukowicz R, Merchant S, Valquier-Flynn H, Caballero J, Sandoval J, Okuom M, Huber C, Brooks TD, Wilson E, Clement B, Wentworth CD, Holmes AE. 2017. Quantitative and qualitative assessment methods for biofilm growth: a mini-review. Res Rev J Eng Technol 6 [PMC free article] [PubMed] [Google Scholar]
- 95. Stiefel P, Rosenberg U, Schneider J, Mauerhofer S, Maniura-Weber K, Ren Q. 2016. Is biofilm removal properly assessed? Comparison of different quantification methods in a 96-well plate system. Appl Microbiol Biotechnol 100:4135–4145. doi: 10.1007/s00253-016-7396-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Abuladze T, Li M, Menetrez MY, Dean T, Senecal A, Sulakvelidze A. 2008. Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef by Escherichia coli O157: H7. Appl Environ Microbiol 74:6230–6238. doi: 10.1128/AEM.01465-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. McLean S, Dunn L, Palombo E. 2010. Bacteriophage biocontrol has the potential to reduce enterococci on hospital fabrics, plastic and glass. World J Microbiol Biotechnol 27:1713–1717. [Google Scholar]
- 98. Otter JA, Vickery K, Walker JT, deLancey Pulcini E, Stoodley P, Goldenberg SD, Salkeld JAG, Chewins J, Yezli S, Edgeworth JD. 2015. Surface-attached cells, biofilms and biocide susceptibility: implications for hospital cleaning and disinfection. J Hosp Infect 89:16–27. doi: 10.1016/j.jhin.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 99. Wickham H, Wickham H. 2016. Data analysis. Springer, Cham. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical analysis of Phage and chemical disinfectant remove biofilms efficiently.
Statistical analysis of surface decontamination by the phages and sodium dichloroisocyanurate.