Abstract
Genes of an influenza A (H5N1) virus from a human in Hong Kong isolated in May 1997 were sequenced and found to be all avian-like (K. Subbarao et al., Science 279:393–395, 1998). Gene sequences of this human isolate were compared to those of a highly pathogenic chicken H5N1 influenza virus isolated from Hong Kong in April 1997. Sequence comparisons of all eight RNA segments from the two viruses show greater than 99% sequence identity between them. However, neither isolate’s gene sequence was closely (>95% sequence identity) related to any other gene sequences found in the GenBank database. Phylogenetic analysis demonstrated that the nucleotide sequences of at least four of the eight RNA segments clustered with Eurasian origin avian influenza viruses. The hemagglutinin gene phylogenetic analysis also included the sequences from an additional three human and two chicken H5N1 virus isolates from Hong Kong, and the isolates separated into two closely related groups. However, no single amino acid change separated the chicken origin and human origin isolates, but they all contained multiple basic amino acids at the hemagglutinin cleavage site, which is associated with a highly pathogenic phenotype in poultry. In experimental intravenous inoculation studies with chickens, all seven viruses were highly pathogenic, killing most birds within 24 h. All infected chickens had virtually identical pathologic lesions, including moderate to severe diffuse edema and interstitial pneumonitis. Viral nucleoprotein was most frequently demonstrated in vascular endothelium, macrophages, heterophils, and cardiac myocytes. Asphyxiation from pulmonary edema and generalized cardiovascular collapse were the most likely pathogenic mechanisms responsible for illness and death. In summary, a small number of changes in hemagglutinin gene sequences defined two closely related subgroups, with both subgroups having human and chicken members, among the seven viruses examined from Hong Kong, and all seven viruses were highly pathogenic in chickens and caused similar lesions in experimental inoculations.
Influenza A virus can infect many species of birds and mammals, but the natural host and reservoir are believed to be free-living aquatic birds belonging to the orders Anseriformes and Charadriiformes (10, 18, 34). Influenza A virus infections are often considered emerging exotic viral diseases in chickens and turkeys because of increased reports of highly virulent influenza outbreaks in Europe, Asia, and North America. Although influenza A viruses are enzootic in wild aquatic birds, the crossover of virus from this reservoir to mammals has been documented only rarely. Crossing this species barrier is thought to require a combination of appropriate virus genetics and environmental factors related to transmission of the virus between species. Host specificity and attenuation of influenza A virus have been attributed to viral hemagglutinin (HA), nucleoprotein (NP), matrix (M), and nonstructural (NS) genes individually or in combinations of viral genes, and host specificity is probably different for each virus because of its unique constellation of genes (31, 33, 35, 43, 44). In experimental studies with humans and nonhuman primates, several different HA subtypes of avian influenza viruses (AIVs) were able to cause infection and in some cases disease (4, 26, 43). Experimental inoculations of humans and nonhuman primates with human-avian influenza reassortant viruses demonstrated that most of these viruses could infect humans and, depending on the source of the avian genes, different genes were linked to attenuation of the reassortant virus (7, 8, 35, 36, 43). In other experimental inoculations, AIVs of many different HA subtypes, including several H5 AIVs, were shown to infect swine, ferrets, hamsters, and cats (20, 26, 49). Natural AIV infections of mammals, including two separate cases of conjunctivitis in humans and epidemic outbreaks in pigs, horses, and seals, have been reported (13, 14, 23, 32, 47).
Experimental or natural infection with AIVs of poultry with subtypes H1 to H4, H6, and H8 to H15, and most AIVs of subtypes H5 and H7, produce subclinical infections with viral replication limited to the respiratory or enteric tract or mild disease with clinical signs and lesions in the respiratory, reproductive, or urinary system (10, 40, 41). A few outbreaks of H5 and H7 AIVs have been highly virulent, producing systemic illness with high mortality and lesions in multiple visceral organs (1, 38). The pathogenesis of highly pathogenic AIVs (HPAIVs) typically involves viral replication and cell death in multiple critical visceral organs, but the predominant cell types for virus replication and lesion production vary among different HPAIVs (5, 17, 22, 25, 38).
In March 1997, an outbreak of HPAIV H5N1 in chickens was reported in Hong Kong (6), and in May 1997, a 3-year-old child from Hong Kong was infected with an H5N1 influenza A virus that likely contributed to the child’s death (37). No other cases were recognized until November and December 1997, when infection by H5N1 influenza viruses resulted in more fatalities (6). The H5 influenza virus subtype had not previously been associated with a naturally acquired human infection, and because the human population is immunologically naive to this HA subtype, it presented a risk of an influenza pandemic. The isolate from the first human case had all avian-like genes, was highly virulent when inoculated into chickens, and was thought to be of recent avian origin (37). The child may have had direct contact with sick chickens before becoming ill (6). The recognition that two viruses from Hong Kong, an avian-like human influenza viruses and an HPAIV chicken isolate, had the same HA and neuraminidase (NA) subtypes indicated the need for a detailed comparison of these two viruses and other related H5N1 isolates.
MATERIALS AND METHODS
Virus purification and biological assays.
A working stock was produced for the human isolates A/Hong Kong/156/97 (HK/156), A/Hong Kong/481/97 (HK/481), A/Hong Kong/482/97 (HK/482), and A/Hong Kong/483/97 (HK/483) and the chicken isolates A/chicken/Hong Kong/220/97 (CK/HK/220), A/chicken/Hong Kong/728/97 (CK/HK/728), and A/chicken/Hong Kong/915/97 (CK/HK/915) of the H5N1 viruses (Table 1) by passage in 10-day-old embryonated chicken eggs. All titrations and analyses were also performed from these stocks. Standard procedures (30) were used for determination of virus titers in chicken embryo fibroblast (CEF) cells, embryonated eggs, and Madin-Darby canine kidney (MDCK) cells as well as hemagglutination titers (HA titers) with chicken erythrocytes. Viral proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as previously described (29).
TABLE 1.
Influenza A (H5N1) viruses examined
| Isolate | Date isolated | Remarks | GenBank accession no. |
|---|---|---|---|
| A/Chicken/HongKong/220/97 | April 1997 | Isolated during HPAIV outbreak | AF046080–AF046087 |
| A/Hong Kong/156/97 | May 1997 | Patient 1a; died | AF046088–AF046095 |
| A/Hong Kong/481/97 | November 1997 | Patient 2; recovered | AF046096 |
| A/Hong Kong/483/97 | November 1997 | Patient 3; died | AF046097 |
| A/Hong Kong/482/97 | December 1997 | Patient 4; died | AF046098 |
| A/Chicken/Hong Kong/728/97 | October 1997 | Isolated from chicken holding farm | AF046099 |
| A/Chicken/Hong Kong/915/97 | December 1997 | Isolated from retail market stall | AF046100 |
Patient numbering as listed in reference 6.
Molecular cloning and sequencing of influenza virus genes.
RNA from all isolates was extracted with RNeasy reagent (Qiagen Corp., Chatsworth, Calif.) from infected allantoic fluid prior to reverse transcriptase-mediated PCR (RT-PCR) amplification. RNA was reverse transcribed by using Superscript II (Life Technologies) reverse transcriptase enzyme with incubation at 45°C for 1 h. PCR was performed at 51°C for 31 cycles. For the NS, M, and NP gene segments, primers to the conserved 12 and 13 bp present on the 5′ and 3′ ends of each viral segment were used. The HA and NA genes were RT-PCR amplified with longer primers but also incorporated the conserved 12 and 13 bp on the 5′ and 3′ ends of the gene. For these five viral genes, full-length cDNA copies were made. The PCR product was electrophoresed in an agarose gel, and the DNA corresponding in size to the gene segment of interest was extracted with an agarose gel DNA extraction kit (Boehringer Mannheim). The DNA was cloned into the pAmp1 (Life Technologies) plasmid vector by using a ligation-independent cloning system. Colonies were screened by PCR with internal primers, positive cultures were grown overnight, and plasmid was extracted by using a High Pure Plasmid Isolation kit (Boehringer Mannheim). Plasmids were sequenced by using a PRISM Ready Reaction DyeDeoxy Terminator Cycle Sequencing kit (Perkin-Elmer) run on a 373A automated sequencer (Perkin-Elmer). The three polymerase genes, PA, PB1, and PB2, were also RT-PCR amplified with the exception of the human PB1 gene, which was cloned similarly to the other viral segments; the genes were amplified in three overlapping parts, and the PCR products were sequenced directly.
Nucleotide and amino acid sequence phylogenetic analysis.
Assembly of sequencing contigs, translation of nucleotide sequence into protein sequence, and initial multiple sequence alignments were performed with the Lasergene (DNASTAR) group of programs. Phylogenetic trees for each gene were generated by using the maximum parsimony method with 100 bootstrap replicates in a heuristic search using the PAUP 3.1 software program (42). Midpoint rooting was used for all genes except the M, NP, and NS genes, where A/equine/Prague/56 was used as the outgroup. The NS, M, and NP analyses used selected gene sequences from GenBank designed to provide a complete tree but enriched with isolates of closely related gene sequences. All full-length or nearly full-length gene segments available in GenBank were included for the remainder of the gene segments.
Animal experiments.
Three- to four-week-old specific-pathogen-free (SPF) White Plymouth Rock (WPR) and adult (37- to 41-week-old) SPF White Leghorn (WL) chickens were used in pathogenicity studies conducted in biosafety level 3 agriculture facilities (2). The chickens were housed in Horsfal-Bauer stainless steel isolation cabinets ventilated under negative pressure with HEPA-filtered air, and care was provided as required by the Institutional Animal Care and Use Committee, based on the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching (8a). Feed and water were provided ad libitum.
For each influenza virus isolate, a modified U.S. Animal Health Association pathogenicity test was performed (45). In the first experiment, 0.2 ml of a 1:10 dilution of a bacterium-free, infectious allantoic fluid was inoculated intravenously (i.v.) into 3-week-old SPF WPR chickens and intranasally (i.n.)/intratracheally (i.t.) into adult SPF WL hens. In addition, 3-week-old WPR chickens received 0.1 ml of a 1:5 dilution of bacterium-free, infectious allantoic fluid i.n. Based on back titration, the doses were 106.9 and 108.1 mean chicken embryo lethal doses (ELD50)/chicken for HK/156 and CK/HK/220, respectively. In the second experiment, the inoculum was standardized to 107.9 ELD50/chicken and given i.v. or i.n. to 4-week-old WPR chickens. In the third experiment, human isolates HK/481, HK/482, HK/483, CK/HK/728, and CK/HK/915 were i.v. inoculated into 4-week-old WPR chickens as in experiment 1, with back titration titers of 107.5, 106.6, 107.2, 104.8, and 105.8, respectively. All chickens in experiment 1 and 3 that died were necropsied. Two 4-week-old WPR, two 4-week-old WPR, and two 41-week-old WL hens were inoculated with sterile allantoic fluid by i.n., i.v., and i.n./i.t. routes, respectively as negative controls. Controls were euthanized on day 3 postinoculation with i.v. sodium pentobarbital (100 mg/kg of body weight) and necropsied.
Histopathology, ultrastructural pathology, and immunohistochemistry.
In experiment 1, tissues were fixed in 10% neutral buffered formalin solution, sectioned, and stained with hematoxylin and eosin. Duplicate sections were stained immunohistochemically to determine influenza virus antigen distribution in individual tissues. A monoclonal antibody against influenza A virus NP, provided by Virginia Hinshaw, University of Wisconsin, was used as the primary antibody as previously described (39).
Lung tissue from one 3-week-old negative control (i.n. route) WPR chicken and three CK/HK/220 AIV-inoculated chickens (i.n. and i.v. routes in 3-week-old WPR chickens and i.n./i.t. in an adult hen) were perfused via the trachea with sodium cacodylate-buffered 1% glutaraldehyde fixative, processed to ultrathin plastic-embedded sections, and stained with lead citrate-uranyl acetate. Sections were viewed on a JEOL transmission electron microscope.
Statistical analysis.
Median death times (MDT) were analyzed by Mann-Whitney rank sum test.
RESULTS
Genetic analysis.
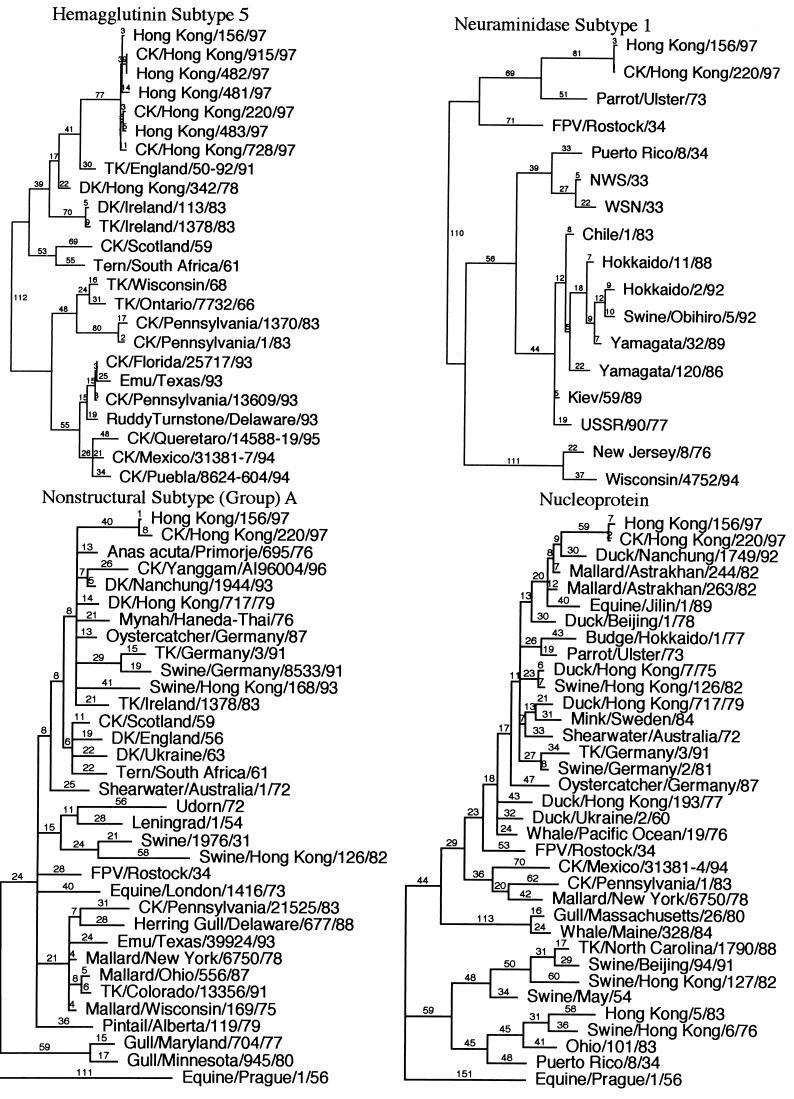
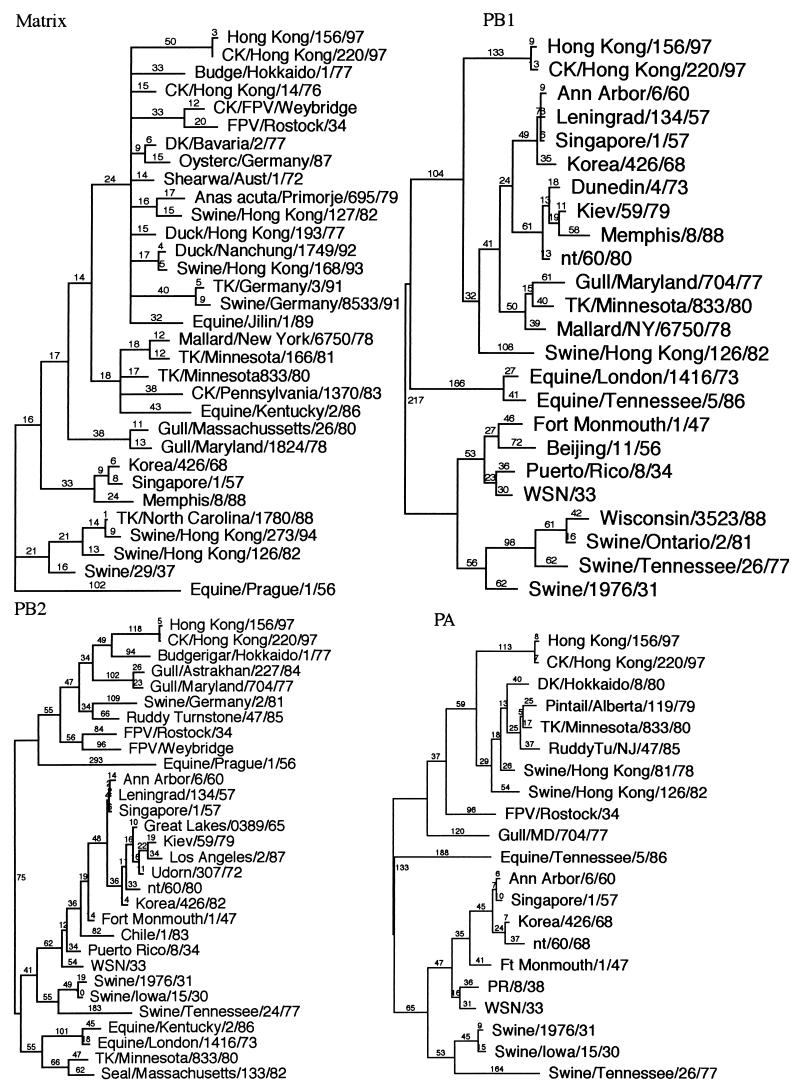
Pairwise sequence comparisons between the eight RNA segments of CK/HK/220 and HK/156 are presented in Table 2. All eight genes had nucleotide differences between them, and all but one, the M gene, had putative amino acid coding differences. Nucleotide sequence homology was greater than 99% for all eight genes in pairwise comparisons. Phylogenetic analysis using parsimony with influenza virus sequences from GenBank showed that all eight genes of these isolates formed a unique branch. However, the HA, M, NS, and NP genes clustered in the Eurasian AIV group (Fig. 1). The N1 and PB2 genes clustered with AIV genes, but because of the limited number of sequences, further distinctions were not possible. The PB1 and PA genes were most closely related by nucleotide sequence similarity to A/swine/Hong Kong/126/82 and A/swine/Hong Kong/81/78, respectively, but these isolates were thought to have been the result of a recent crossover of AIVs to pigs (21). The influenza virus isolates with the closest nucleotide and amino acid sequence similarities are presented in Table 2. Amino acid similarity of the PB1 gene showed that both isolates were most closely related to North American AIVs, but no full-length Eurasian AIV sequences are in the GenBank database. Both HK/156 and CK/HK/220 had an additional amino acid in the 3′ end of the PB1 protein compared to other PB1 sequences found in the GenBank database. This difference was the result of differences in stop codon usage, with these isolates having their PB1 stop codon one codon further downstream than PB1 sequences of the other 23 isolates in the GenBank database.
TABLE 2.
Nucleotide and amino acid comparison of CK/HK/220 and HK/156 with each other and their closest isolates in GenBanka
| Gene | Sequence identity between HK/156 and
CK/HK/220 (no. different/ no. compared)
|
Virus with
the highest % identity with HK/156 (% sequence identity)
|
||
|---|---|---|---|---|
| Nucleotide | Amino acid | Nucleotide | Amino acid | |
| PB2 | 6/2,027 | 3/671 | Budgerigar/Hokkaido/1/77 (89.5) | Ruddy Turnstone/47/85 (96.9) |
| PB1 | 22/2,280 | 4/758 | Swine/Hong Kong/126/82 (89.4) | TK/Minnesota/833/80 (97.8) |
| PA | 15/2,151 | 6/716 | Swine/Hong Kong/81/78 (91.7) | TK/Minnesota/833/80 (96.6) |
| HA | 13/1,740 | 4/568 | TK/England/50-92/91 (92.8) | TK/England/50-92/91 (93.1) |
| NP | 9/1,539 | 3/498 | Mallard/Astrakhan/263/82 (94.2) | DK/New Zealand/76 (98.4) |
| NA | 4/1,370 | 1/450 | Parrot/Ulster/73 (86.4) | Parrot/Ulster/73 (93.3) |
| M | 3/1,002 | DK/Hong Kong/193/77 (94.2) | ||
| M1 | 0/252 | FPV/Dobson/34 (97.6) | ||
| M2 | 0/97 | Swine/Germany/2/81 (95.8) | ||
| NS | 8/865 | Anas acuta/Primorje/695/76 (93.2) | ||
| NS1 | 4/230 | Anas acuta/Primorje/695/76 (90.0) | ||
| NS2 | 3/121 | Swine/Netherlands/25/80 (95.8) | ||
TK, turkey; DK, duck; FPV, fowl plague virus.
FIG. 1.
Phylogenetic analysis using parsimony for all eight influenza virus gene segments based on nucleotide sequence. HK/156 is used as the reference isolate for all eight trees. All trees were constructed by general bootstrap analysis using 100 replicates, using PAUP 3.1 software (42). Branch lengths are provided in each tree. The NP, M, and NS genes are rooted to A/equine/Prague/1/56, and all other trees are midpoint rooted. All isolates in trees are type A influenza virus isolates and are full-length or close to full-length sequences. The NP and M trees used selected isolates to show the main groups but are concentrated with isolates close to the reference isolate. The NS gene tree also uses selected isolates, but only subtype (group) A isolates are presented. Abbreviations used for identifying isolates: CK (chicken), TK (turkey), DK (duck), FPV (fowl plague virus), WSN (mouse neurovirulent derivative of A/WS/33), Aust (Australia).
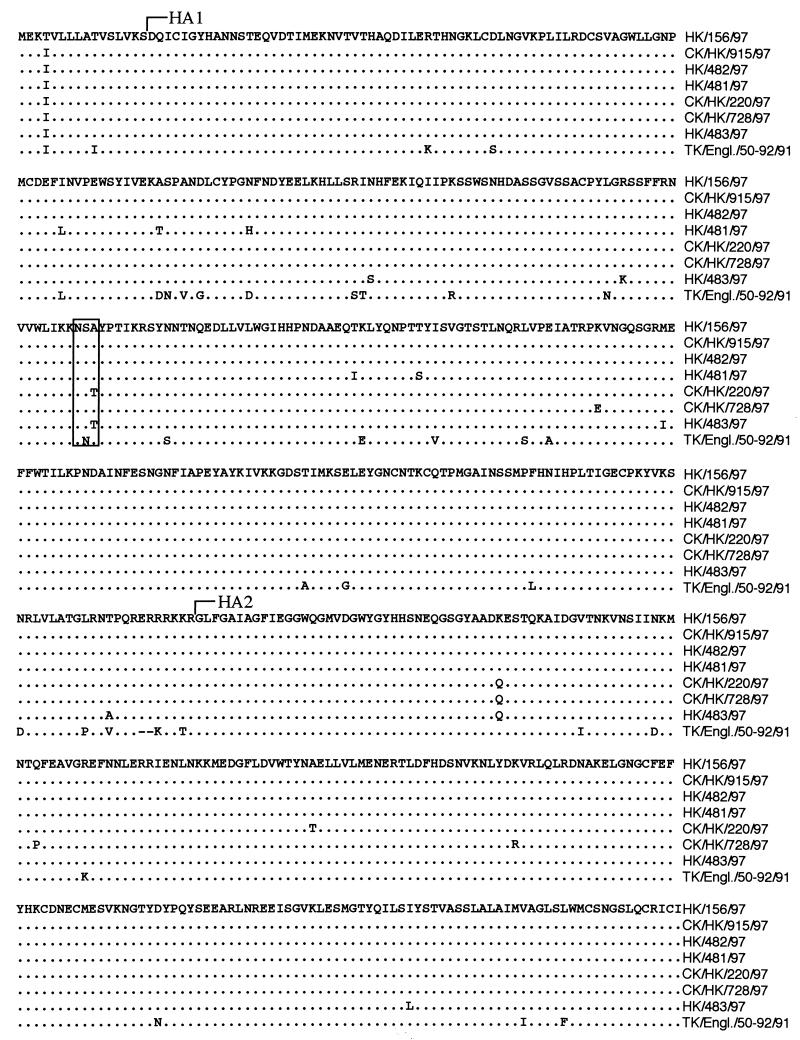
The HA protein differed between HK/156 and CK/HK/220 by four amino acid changes, one in the leader sequence, one in HA1 that caused a putative glycosylation difference, and two in HA2 (Fig. 2). The glycosylation difference was further confirmed by differences in migration of the HA1 proteins when separated on a polyacrylamide gel and stained with Coomassie blue (Fig. 3). The human influenza virus HA1 and HA2 proteins migrated faster than its chicken influenza virus counterparts, but the NP, M1, PA, PB1, and PB2 proteins migrated similarly in the gel. The NA was either unstable, not present in sufficient quantities for staining, as often occurs in influenza virus isolates (28), or obscured by the NP and HA1 bands. Since there was only a single difference in the coding sequence, the difference in the migration of HA1 is most likely due to the difference in glycosylation at position 154. Amino acids in the H5 sequence at positions 154 to 156 correspond to positions 158 to 160 in the H3 solved structure and are located on the outermost loop in the region of the receptor binding pocket. The HA genes of isolates HK/481, HK/482, and CK/HK/915 grouped most closely on the nucleotide and amino acid level with HK/156, with HK/482 and CK/HK/915 having identical amino acid sequences (Fig. 2). HK/483 was most similar to CK/HK/220, including having the potential glycosylation site at position 154. CK/HK/728 was most closely related to CK/HK/220, but it did not have the glycosylation site at position 154. All seven isolates had an insertion of basic amino acids at the HA cleavage site, consistent with their being classified as HPAIV (27, 45).
FIG. 2.
Amino acid sequence alignment of the HA genes from all seven isolates in this study and from A/Turkey/England (TK/Engl.)/50-92/91, the isolate with the closest HA sequence similarity in GenBank. The start sites for the HA1 and HA2 proteins are indicated. Amino acids at positions 154 to 156 are boxed to highlight the glycosylation difference among the different virus isolates.
FIG. 3.
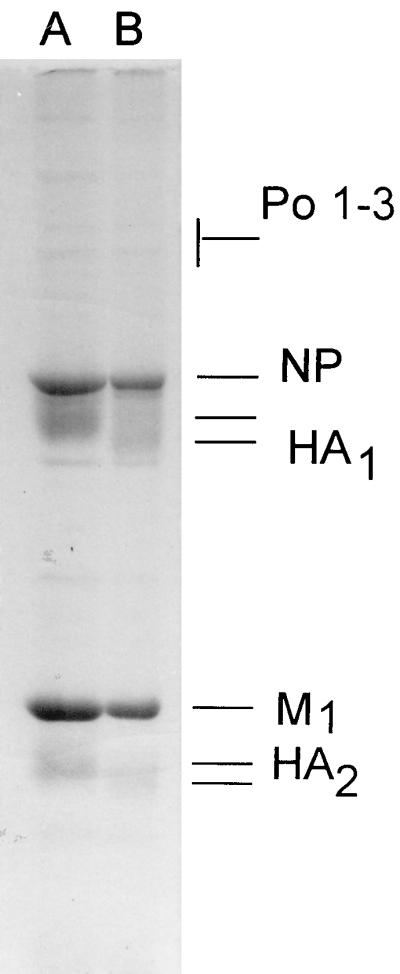
Polyacrylamide gel electrophoresis of purified virus structural proteins. Purified virions were disrupted in sodium dodecyl sulfate and mercaptoethanol and electrophoresed in 10% polyacrylamide gels. Viral polypeptides were identified on the basis of molecular weights. Lane A, CK/HK/220; lane B, HK/156. Po 1–3, polymerase genes PA, PB1, and PB2; NP, nucleoprotein; HA1, hemagglutinin subunit 1; M1, matrix protein 1; HA2, hemagglutinin subunit 2.
Biological characteristics of egg-grown stocks.
Comparisons of biological parameters between the chicken and human H5N1 isolates are presented in Table 3. Stocks of HK/156 had lower HA titers than stocks of CK/HK/220. In addition, the HK/156 plaques in CEF cells were smaller than those of the chicken isolate. The infectivity titers, however, in CEF cells, embryonated chicken eggs, and MDCK cells were roughly comparable for both isolates.
TABLE 3.
Biological characteristics of HK/156 and CK/HK/220 H5N1 isolates
| Isolate | PFU/ml, CEF
|
Plaque size (mm) | HA titer, chicken erythrocytes | Egg titer (ELD50) | 50% Infectious dose, MDCK | |
|---|---|---|---|---|---|---|
| +Trypsin | −Trypsin | |||||
| HK/156 | 4.0 × 106 | 4.7 × 106 | 1–2 | 128 | 108.8 | 105.8 |
| CK/HK/220 | 1.0 × 107 | 8.0 × 106 | 3–4 | 1,024 | 108.8 | 105.8 |
Virulence for chickens.
By criteria developed by the U.S. Animal Health Association, AIVs that kill 75% or more of i.v.-inoculated young chickens within 10 days are classified as highly pathogenic (45). In experiments 1, 2, and 3, the four human and three chicken influenza viruses were 90 to 100% lethal by day 3 postinoculation (Table 4), indicating that the human origin isolates retained their high lethality for chickens. In experiment 1, the MDT were significantly shorter (P < 0.05) for chickens infected with CK/HK/220 than those infected with HK/156 (Table 4), but the inoculum dose was 101.2 ELD50 greater for CK/HK/220 than for HK/156. When the inoculum dose was standardized in experiment 2 to 107.9 ELD50/chicken, the numerical MDT values were still shorter for CK/HK/220 than HK/156 virus, but the differences were not statistically significant (Table 4).
TABLE 4.
Mortality rates and MDTs for chickens inoculated with HK/156 and CK/HK/220 influenza virusesa
| Chickens | Route of inoculation | Expt 1
|
Expt 2
|
||||
|---|---|---|---|---|---|---|---|
| No. of chickens dead/no. inoculated (MDT
[days])
|
Pb | No. of
chickens dead/no. inoculated (MDT [days])
|
P | ||||
| HK/156 | CK/HK/220 | HK/156 | CK/HK/220 | ||||
| 3–4-wk-old WPR | i.n. | 9/10 (5) | 10/10 (2) | <0.0001 | 10/10 (2) | 10/10 (2) | 0.4418 |
| i.v. | 11/11 (2) | 10/10 (1) | 0.0016 | 10/10 (1.5) | 10/10 (1) | 0.1049 | |
| Adult WL | i.n./i.t. | 6/6 (3) | 6/6 (2) | 0.0152 | |||
HK/481, HK/482, HK/483, CK/HK/728, and CK/HK/915 each produced death in eight of eight inoculated chickens in less than 24 h postinoculation (experiment 3).
Determined for MDT by comparing the different viruses by Mann-Whitney sum rank test.
Pathology and immunohistochemistry.
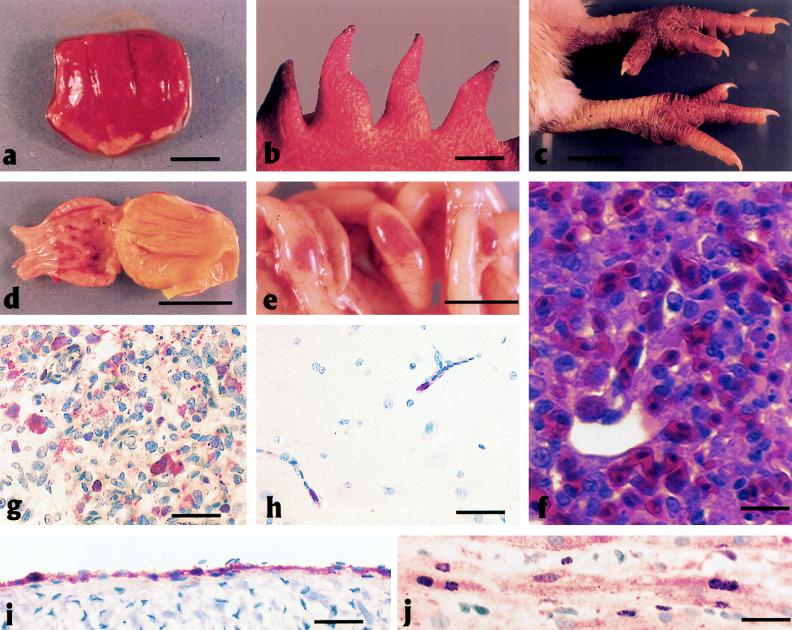
All seven isolates produced gross lesions in chickens consistent with highly pathogenic avian influenza virus or fowl plague virus (10). The most consistent gross lesions included severe pulmonary edema (85%; 78/92) with congestion and hemorrhage (Fig. 4a); necrosis of the comb (Fig. 4b) (67% of hens; 8/12); edema of the brain (68%; 63/92); and petechial to ecchymotic hemorrhages (76%; 70/92) in the skin (Fig. 4c), muscle fascicles, fat pads, serosa, and mucosa of viscera (Fig. 4d) and lymphoid tissues such as the cecal tonsils and Peyer’s patches in the small intestinal mucosa (Fig. 4e). Gross lesions were more common in chickens inoculated i.v. with CK/HK/728 (8.1 lesions/bird), HK/483 (8 lesions/bird), and CK/HK/915 (7.8 lesions/bird) and least common in those inoculated with HK/156 (4.7 lesions/bird). The other viruses had intermediate lesion frequencies of 6/bird, 6.2/bird, and 6.5/bird for HK/482, CK/HK/220, and HK/481, respectively. Chickens inoculated i.v. with AIV had more frequent and more severe lesions than chickens inoculated i.n., irrespective of the virus used.
FIG. 4.
Experimental studies of chickens inoculated with HK/156 or CK/HK/220. (a to e) Photographs of gross lesions; (f to j) photomicrographs of hematoxylin-and-eosin-stained tissue sections or sections stained immunohistochemically to demonstrate AIV NP. (a) Severe congestion, hemorrhage, and edema of the lung from a 3-week-old WPR chicken that died 2 days after i.v. inoculation with HK/156 (bar = 1.5 cm). (b) Ischemic necrosis at the tips of the comb from a 37-week-old WL chicken that died 3 days after i.n./i.t. inoculation with HK/156 (bar = 1 cm). (c) Severe subcutaneous edema and hemorrhage of the feet and shanks from a 3-week-old WPR chicken that died 5 days after i.n. inoculation with HK/156 (bar = 2 cm). (d) Submucosal hemorrhage surrounding ducts of glands in the proventriculus from a 3-week-old WPR chicken that died 2 days after i.v. inoculation with HK/156 (bar = 2 cm). (e) Prominent hemorrhage in lymphoid tissue of Peyer’s patches in the jejunum from a 4-week-old WPR chicken that died 1 day after i.v. inoculation with CK/HK/220 (bar = 1 cm). (f) Severe diffuse pulmonary edema with congestion, hemorrhage, and interstitial pneumonitis in a 4-week-old WPR chicken that died 2 days after i.n. inoculation with CK/HK/220. Scattered necrotic cellular debris is present in blood capillaries (bar = 15 mm). (g) AIV antigen in cytoplasm and nucleus of blood capillary endothelial cells and macrophages and in necrotic debris from the lung of the chicken in panel f (bar = 50 mm). (h) AIV antigen in the cytoplasm and nuclei of blood capillary endothelial cells in the brain of the chicken in panel f (bar = 30 mm). (i) AIV antigen in the cytoplasm and nuclei of endocardial cells from a 4-week-old WPR chicken that died 1 day after i.v. inoculation with CK/HK/220 (bar = 30 mm). (j) AIV antigen in the cytoplasm and nuclei of cardiac myocytes from a 3-week-old WPR chicken that died on day 3 after inoculation with HK/156 (bar = 15 mm).
The principal histologic lesions were hemorrhage, edema, and necrosis in multiple visceral organs and the brain. Moderate to severe diffuse pulmonary edema with congestion, hemorrhage, interstitial pneumonitis and necrosis, and mild-to-moderate degeneration and necrosis of cardiac myocytes were identified most frequently (Fig. 4f). Vascular endothelial cells in vessels throughout the body were hypertrophied. The primary and secondary lymphoid organs had moderate to severe depletion of lymphocytes, and many of the remaining lymphocytes exhibited various stages of cytoplasmic condensation and blebbing with shrunken, round nuclei. These changes are consistent with apoptosis (50).
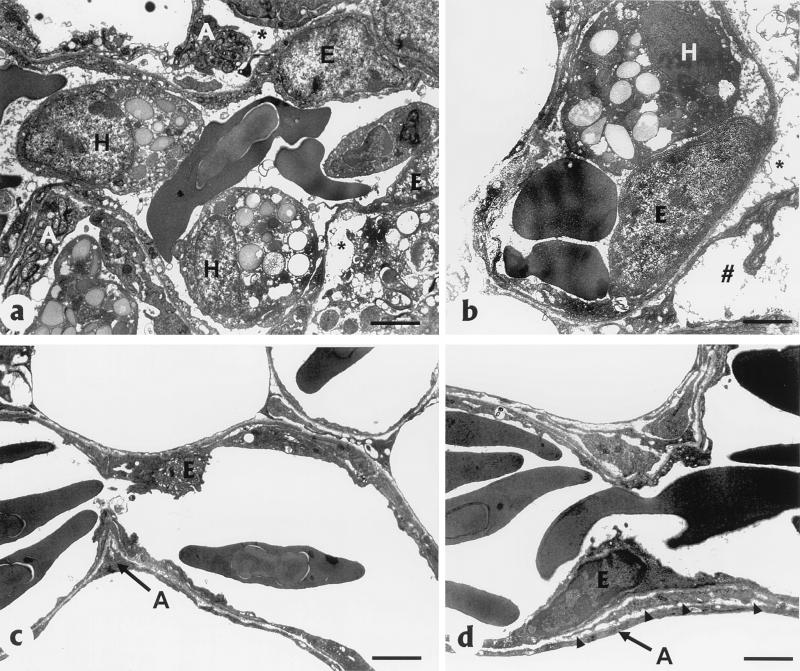
AIV NP was localized in the nuclei and cytoplasm of various cell types in most tissues, but the most consistent and most abundant staining was in endothelial cells lining vascular spaces including capillary beds (Fig. 4g and h), vascular sinuses and glomeruli of the kidney, vascular sinuses of the adrenal, sinusoids of the liver, venules and arterioles, arteries and veins, pulmonary arteries and veins, aorta, and endocardium (Fig. 4i). In addition, AIV antigen was common in cardiac myocytes (Fig. 4j) and macrophages and heterophils within the lungs (Fig. 4g). By contrast, AIV NP was rare in respiratory, intestinal, and kidney epithelium and neurons. AIV NP was absent in apoptotic lymphocytes in primary and secondary lymphoid organs. Ultrastructurally, the air capillary epithelium was frequently separated from the blood capillary endothelium at the basal lamina (Fig. 5a), and the expanded space was typically electron lucent but occasionally contained proteinic material, macrophages, or heterophils. The blood capillary endothelial cells were hypertrophied, and the lumina commonly contained viable, degenerate, or necrotic monocytes and heterophils (Fig. 5b). The air capillary space contained protein fibrils and degenerate heterophils and macrophages. The atrial epithelium was hypertrophied.
FIG. 5.
Lung tissues from a chicken that died 1 day after i.n. inoculation with CK/HK/220 (a and b) and a chicken euthanized 3 days after intranasal sham inoculation (c and d). (a) Hypertrophied blood capillary endothelial cells (E) with luminal heterophils (H) and degeneration of air capillary endothelial cells (A) in the lung of a chicken that died 1 day after i.n. inoculation with CK/HK/220. Note separation (∗) of blood capillary endothelium from air capillary epithelium and proteinic material and inflammatory cells filling the space (bar = 2 nm). (b) Hypertrophied capillary endothelium (E) with luminal heterophil (H) separation of blood capillary endothelium and air capillary epithelium in the lung (∗) and proteinic material in air capillary space ( ) (bar = 2.5 nm). (c) Normal blood capillary endothelium (E) and air capillary epithelium (A) without inflammatory cells (bar = 2 nm). (d) Thin basal lamina (arrowheads) separating blood capillary endothelium (E) and air capillary epithelium (A) (bar = 2.5 nm).
The sham-inoculated controls lacked lesions, and AIV antigen was not demonstrated.
DISCUSSION
The sequence comparison of HK/156 and CK/HK/220 H5N1 influenza viruses showed without doubt that the viruses had a closely related common ancestor, and the chicken virus serves as a reasonable progenitor virus to study the species jump for the human influenza virus. The HA sequence from an additional three human and two chicken isolates provides evidence of two closely related but genetically distinguishable groups of H5N1 influenza virus in Hong Kong. The glycosylation site difference, which is predicted to be near the receptor binding site, did not serve to differentiate between the chicken and human isolates. Comparisons of other H5 AIVs show that two viruses, A/chicken/Scotland/59 and A/turkey/Ontario/773/66, have the glycosylation site, but the other sequenced isolates do not. It appears unlikely that the presence or absence of the glycosylation site at position 154 is important for the virus to cross the species barrier.
The question is, why did this virus so easily cross the species barrier? Further sequencing of all genes from more human and avian influenza virus isolates may help pinpoint required mutational changes. However, based on the sequencing of seven isolates from this outbreak, the HA gene does not appeared to be a strong determinant of host specificity, since no amino acid changes consistently separated the avian and human isolates. Experimental studies with different AIVs have shown that many can infect humans and other mammals with no apparent mutational changes (4, 26, 43). It is possible that this group of H5N1 viruses have a constellation of genes that also allows them to replicate well enough in humans to cause disease in some cases. However, evidence of efficient human-to-human transmission is lacking.
Isolation of influenza A (H5N1) viruses from humans with serious respiratory disease has altered our thinking about the potential for the transmission of AIV to humans specifically and to mammals in general. In several previous outbreaks, direct transmission of AIVs was assumed to be from waterfowl directly to swine, horses, seals, and poultry (10, 13, 14, 32, 47). Since chickens and turkeys are not the normal reservoir for AIVs, infection of these birds also represents a species jump, but presumably the transition from replication in a duck to replication and transmission in chickens is lower than that from ducks to humans. However, when AIV is transmitted to chickens or other poultry from the waterfowl reservoir, the virus begins adaptation to the new host. This idea is supported by studies in long-term poultry outbreaks from a single source (11, 12). The presence of multiple basic amino acids at the HA cleavage site of H5 and H7 AIV isolates is associated with a highly pathogenic phenotype in chickens and turkeys. By contrast, H5 and H7 isolates from waterfowl and other wild birds, with the exception of A/tern/South Africa/61, did not have multiple basic amino acids in the HA cleavage site and were mildly pathogenic in chickens. There are multiple examples of mildly pathogenic viruses acquiring changes at the cleavage site or other places in the HA gene that result in emergence of the highly pathogenic phenotype for chickens (3, 11, 19, 27). The combination of all 10 genes having high sequence similarity, the insertion of additional basic amino acids at the HA cleavage site, and the viruses being highly pathogenic in chickens suggests the H5N1 virus was transmitted from birds to humans, with chickens and not waterfowl being the most likely source. If this virus were to become established in the human population, it has the additional potential to cross back to chickens from humans and cause a severe influenza outbreak in poultry. Transmission of H1 influenza viruses from swine back to turkeys has previously been reported (15, 48).
Both the HK/156 and CK/HK/220 influenza viruses caused severe systemic disease in chickens with lesions, suggesting severe pulmonary hypoxia and generalized cardiovascular collapse as the pathogenic mechanisms involved in virulence and death. These AIVs replicated primarily in vascular endothelial cells, cardiac myocytes, and myeloid inflammatory cells (heterophils, monocytes, and macrophages), but replication was observed less frequently in most parenchymal cells of viscera. Previous studies with H5 and H7 HPAIVs suggested multiple mechanisms involved in disease pathogenesis, primarily dependent on organs involved in major virus replication and lesion development (5, 22, 38). The brain, visceral organs, and blood vessels throughout the body are the predominant sites of HPAIV replication and lesion development (5, 22, 25, 38, 46). However, the generalized involvement and damage to the cardiovascular system are responsible for the major gross lesions typical of HPAIV, i.e., petechial to ecchymotic hemorrhages in the skin and on serosa of visceral organs, widespread subcutaneous edema, and ischemic necrosis of the comb and wattles. With most peracute deaths (<2 days), the predominant lesions are observed in endothelial cells in the cardiovascular system throughout the body (5, 22, 38, 46). In some cases, gross lesions are lacking in poultry that die because the deaths resulted from peracute vascular thrombosis of critical organs, biochemical alterations, or cardiac arrhythmias (24, 38). Prolongation of the disease to 3 to 5 days allows influenza virus to replicate and cause cellular alterations and morphologically identifiable necrosis in multiple parenchymal cells, including neurons, pancreatic acinar epithelium, kidney tubule epithelium, skeletal muscle, and adrenal corticotrophic cells, and hepatocytes (16, 22, 38). Especially in i.v. inoculations, both viral isolates often caused peracute onset and rapid death of birds. Other, more classic lesions become evident in i.n.-inoculated birds that survived for more than 1 day.
With HK/156 and CK/HK/220, the gross, histologic, and ultrastructural alterations in the lung resulted from replication of the virus in blood capillary endothelial cells, with alterations in cell metabolism or cell death leading to protein leakage, edema in the air capillary space, and inflammatory cell infiltration (pneumonitis). Expansion of the blood-air capillary wall and filling of air capillaries with fluid prohibited adequate oxygen-carbon dioxide exchange, resulting in hypoxia. The HPAIVs A/chicken/Queretaro/14588-19/95 (H5N2) (38) and A/turkey/England/50-92/91 (H5N1) (22) produced similar severe pulmonary lesions which can be complicated by bacterial coinfections in the field. From a comparative prospective, severe uncomplicated influenza virus infections of the lungs of humans and pigs have severe diffuse alveolar damage with patchy fibrinous alveolar exudates, hyaline membranes, interstitial edema, and necrosis of bronchiolar mucosa (9, 51).
The unanswered question from the current investigation is, will this H5N1 virus cause a pandemic? As efforts continue to control the avian outbreak of HPAIV in Hong Kong and hopefully reduce or eliminate the chicken-to-human spread of the virus, it is still unclear if human-to-human transmission is occurring at low levels. Repeated chances at replication in humans may allow this virus to become better adapted to humans and allow efficient human-to-human transmission. Increased transmission of the viral H5 and N1 genes may also occur by the reassortment of these genes with naturally circulating human influenza A viruses that could form a new virus that can readily spread from human to human. In either scenario, a virus capable of causing a pandemic could occur. This outbreak provides a clear indication that some AIVs have the potential to directly infect humans without a swine intermediate as a “mixing vessel,” and we must increase our vigilance in detecting these new subtypes of human influenza viruses.
ACKNOWLEDGMENTS
We thank Patsy Decker, Suzanne DeBlois, and John Latimer for technical support; Walstine Steffens and Mary Ard of the Electron Microscopy Center, Department of Veterinary Pathology, University of Georgia, for assistance with ultrastructural analysis; Les Sims and Kitman Dyrting, Agriculture and Fisheries Department, Hong Kong, for supply of avian isolates; and Kennedy Shortridge of the University of Hong Kong and Robert Webster of St. Jude Children’s Research Hospital, Memphis, Tenn., for assistance in receiving some of the viral isolates used.
This work was supported by USDA/ARS Cris project 6612-32000-016.
REFERENCES
- 1.Alexander D J. Avian influenza. Recent developments. Vet Bull. 1982;52:341–359. [Google Scholar]
- 2.Barbeito M S, Abraham G, Best M, Cairns P, Langevin P, Sterritt W G, Barr D, Meulepas W, Sanchez-Vizcaino J M, Saraza M, Requena E, Collado M, Mani P, Breeze R, Brunner H, Mebus C A, Morgan R L, Rusk S, Siegfried L M, Thompson L H. Recommended biocontainment features for research and diagnostic facilities where animal pathogens are used. Rev Sci Tech Off Int Epizoot. 1995;14:873–887. doi: 10.20506/rst.14.3.880. [DOI] [PubMed] [Google Scholar]
- 3.Bashiruddin J B, Gould A R, Westbury H A. Molecular pathotyping of two avian influenza viruses isolated during the Victoria 1976 outbreak. Aust Vet J. 1992;69:140–142. doi: 10.1111/j.1751-0813.1992.tb07485.x. [DOI] [PubMed] [Google Scholar]
- 4.Beare A S, Webster R G. Replication of avian influenza viruses in humans. Arch Virol. 1991;119:37–42. doi: 10.1007/BF01314321. [DOI] [PubMed] [Google Scholar]
- 5.Brown C C, Olander H J, Senne D A. A pathogenesis study of highly pathogenic avian influenza virus H5N2 in chickens, using immunohistochemistry. J Comp Pathol. 1992;107:341–348. doi: 10.1016/0021-9975(92)90009-j. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Isolation of avian influenza A (H5N1) from humans—Hong Kong, May–December, 1997. Morbid Mortal Weekly Rep. 1997;46:1204–1207. [PubMed] [Google Scholar]
- 7.Clements M L, Snyder M H, Buckler-White A J, Tierney E L, London W T, Murphy B R. Evaluation of avian-human reassortant influenza A/Washington/897/80 × A/Pintail/119/79 virus in monkeys and adult volunteers. J Clin Microbiol. 1986;24:47–51. doi: 10.1128/jcm.24.1.47-51.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clements M L, Subbarao E K, Fries L F, Karron R A, London W T, Murphy B R. Use of single-gene reassortant viruses to study the role of avian influenza A virus genes in attenuation of wild-type human influenza A virus for squirrel monkeys and adult human volunteers. J Clin Microbiol. 1992;30:655–662. doi: 10.1128/jcm.30.3.655-662.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Craig J V, Dean W F, Eckroade R J, Harris G C, Janni K A, Johnson H S, Siegel P B. Guide for the care and use of animals in agricultural research and teaching. Champaign, Ill: NASULGC; 1988. Guidelines for poultry; pp. 39–46. [Google Scholar]
- 9.Dea S, Bilodeau R, Sauvageau R, Montpetit C, Martineau G P. Antigenic variant of swine influenza virus causing proliferative and necrotizing pneumonia in pigs. J Vet Diagn Invest. 1992;4:380–392. doi: 10.1177/104063879200400403. [DOI] [PubMed] [Google Scholar]
- 10.Easterday B C, Hinshaw V S, Halvorson D A. Influenza. In: Calnek B W, Barnes H J, Beard C W, McDougald L R, Saif Y M, editors. Diseases of poultry. Ames: Iowa State University Press; 1997. pp. 583–605. [Google Scholar]
- 11.Garcia M, Crawford J M, Latimer J W, Rivera-Cruz M V Z E, Perdue M L. Heterogeneity in the hemagglutinin gene and emergence of the highly pathogenic phenotype among recent H5N2 avian influenza viruses from Mexico. J Gen Virol. 1996;77:1493–1504. doi: 10.1099/0022-1317-77-7-1493. [DOI] [PubMed] [Google Scholar]
- 12.Garcia M, Suarez D L, Crawford J M, Latimer J W, Slemons R D, Swayne D E, Perdue M L. Evolution of H5 subtype avian influenza A viruses in North America. Virus Res. 1997;51:115–124. doi: 10.1016/s0168-1702(97)00087-7. [DOI] [PubMed] [Google Scholar]
- 13.Guan Y, Shortridge K F, Krauss S, Li P H, Kawaoka Y, Webster R G. Emergence of avian H1N1 influenza viruses in pigs in China. J Virol. 1996;70:8041–8046. doi: 10.1128/jvi.70.11.8041-8046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y, Wang M, Kawaoka Y, Gorman O, Ito T, Saito T, Webster R G. Characterization of a new avian-like influenza A virus from horses in China. Virology. 1992;188:245–255. doi: 10.1016/0042-6822(92)90754-d. [DOI] [PubMed] [Google Scholar]
- 15.Hinshaw V S, Webster R G, Bean W J, Downie J, Senne D A. Swine influenza-like viruses in turkeys: potential source of virus for humans? Science. 1983;220:206–208. doi: 10.1126/science.6298942. [DOI] [PubMed] [Google Scholar]
- 16.Hooper P T, Russell G W, Selleck P W, Stanislawek W L. Observations on the relationship in chickens between the virulence of some avian influenza viruses and their pathogenicity for various organs. Avian Dis. 1995;39:458–464. [PubMed] [Google Scholar]
- 17.Hooper P T, Russell G W, Selleck P W, Stanislawek W L. Observations on the relationship in chickens between the virulence of some avian influenza viruses and their pathogenicity for various organs. Avian Dis. 1995;39:458–464. [PubMed] [Google Scholar]
- 18.Kawaoka Y, Chambers T M, Sladen W L, Webster R G. Is the gene pool of influenza viruses in shorebirds and gulls different from that in wild ducks? Virology. 1988;163:247–250. doi: 10.1016/0042-6822(88)90260-7. [DOI] [PubMed] [Google Scholar]
- 19.Kawaoka Y, Naeve C W, Webster R G. Is virulence of H5N2 influenza viruses in chickens associated with loss of carbohydrate from the hemagglutinin? Virology. 1984;139:303–316. doi: 10.1016/0042-6822(84)90376-3. [DOI] [PubMed] [Google Scholar]
- 20.Kida H, Ito T, Yasuda J, Shimizu Y, Itakura C, Shortridge K F, Kawaoka Y, Webster R G. Potential for transmission of avian influenza viruses to pigs. J Gen Virol. 1994;75:2183–2188. doi: 10.1099/0022-1317-75-9-2183. [DOI] [PubMed] [Google Scholar]
- 21.Kida H, Shortridge K F, Webster R G. Origin of the hemagglutinin gene of H3N2 influenza viruses from pigs in China. Virology. 1988;162:160–166. doi: 10.1016/0042-6822(88)90405-9. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi Y, Horimoto T, Kawaoka Y, Alexander D J, Itakura C. Pathological studies of chickens experimentally infected with two highly pathogenic avian influenza viruses. Avian Pathol. 1996;25:285–304. doi: 10.1080/03079459608419142. [DOI] [PubMed] [Google Scholar]
- 23.Kurtz J, Manvell R J, Banks J. Avian influenza virus isolated from a woman with conjunctivitis. Lancet. 1996;348:901–902. doi: 10.1016/S0140-6736(05)64783-6. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 24.McKenzie B E, Easterday B C, Will J A. Light and electron microscopic changes in the myocardium of influenza-infected turkeys. Am J Pathol. 1972;69:239–254. [PMC free article] [PubMed] [Google Scholar]
- 25.Mo I P, Brugh M, Fletcher O J, Rowland G N, Swayne D E. Comparative pathology of chickens experimentally inoculated with avian influenza viruses of low and high pathogenicity. Avian Dis. 1997;41:125–136. [PubMed] [Google Scholar]
- 26.Murphy B R, Hinshaw V S, Sly D L, London W T, Hosier N T, Wood F T, Webster R G, Chanock R M. Virulence of avian influenza A viruses for squirrel monkeys. Infect Immun. 1982;37:1119–1126. doi: 10.1128/iai.37.3.1119-1126.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perdue M L, Garcia M, Senne D, Fraire M. Virulence-associated sequence duplication at the hemagglutination cleavage site of avian influenza viruses. Virus Res. 1997;49:173–186. doi: 10.1016/s0168-1702(97)01468-8. [DOI] [PubMed] [Google Scholar]
- 28.Perdue M L, Latimer J, Greene C, Holt P. Consistent occurrence of hemagglutinin variants among avian influenza virus isolates of the H7 subtype. Virus Res. 1994;34:15–29. doi: 10.1016/0168-1702(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 29.Perdue M L, Latimer J W, Crawford J M. A novel carbohydrate addition site on the hemagglutinin protein of a highly pathogenic H7 subtype avian influenza virus. Virology. 1995;213:276–281. doi: 10.1006/viro.1995.1571. [DOI] [PubMed] [Google Scholar]
- 30.Purchase H G, Arp L H, Domermuth C H, Pearson J E. A laboratory manual for the isolation and identification of avian pathogens. Dubuque, Iowa: Kendall/Hunt; 1989. [Google Scholar]
- 31.Rogers G N, Paulson J C. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983;127:361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- 32.Scholtissek C, Burger H, Bachmann P A, Hannoun C. Genetic relatedness of hemagglutinins of the H1 subtype of influenza A viruses isolated from swine and birds. Virology. 1983;129:521–523. doi: 10.1016/0042-6822(83)90194-0. [DOI] [PubMed] [Google Scholar]
- 33.Scholtissek C, Burger H, Kistner O, Shortridge K F. The nucleoprotein as a possible major factor in determining host specificity of influenza H3N2 viruses. Virology. 1985;147:287–294. doi: 10.1016/0042-6822(85)90131-x. [DOI] [PubMed] [Google Scholar]
- 34.Slemons R D, Johnson D C, Osborn J S, Hayes F. Type-A influenza viruses isolated from wild free-flying ducks in California. Avian Dis. 1974;18:119–124. [PubMed] [Google Scholar]
- 35.Snyder M H, Buckler-White A J, London W T, Tierney E L, Murphy B R. The avian influenza virus nucleoprotein gene and a specific constellation of avian and human virus polymerase genes each specify attenuation of avian-human influenza A/Pintail/79 reassortant viruses for monkeys. J Virol. 1987;61:2857–2863. doi: 10.1128/jvi.61.9.2857-2863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snyder M H, Clements M L, Herrington D, London W T, Tierney E L, Murphy B R. Comparison by studies in squirrel monkeys, chimpanzees, and adult humans of avian-human influenza A virus reassortants derived from different avian influenza virus donors. J Clin Microbiol. 1986;24:467–469. doi: 10.1128/jcm.24.3.467-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subbarao K, Klimov A, Katz J, Regenery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T, Shaw M, Xu X, Fukuda K, Cox N. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 38.Swayne D E. Pathobiology of H5N2 Mexican avian influenza virus infections of chickens. Vet Pathol. 1997;34:557–567. doi: 10.1177/030098589703400603. [DOI] [PubMed] [Google Scholar]
- 39.Swayne D E, Beck J R, Perdue M L, Brugh M, Slemons R D. Assessment of the ability of ratite-origin influenza viruses to infect and produce disease in rheas and chickens. Avian Dis. 1996;40:438–447. [PubMed] [Google Scholar]
- 40.Swayne D E, Slemons R D. Comparative pathology of a chicken-origin and two duck-origin influenza virus isolates in chickens: the effect of route of inoculation. Vet Pathol. 1994;31:237–245. doi: 10.1177/030098589403100211. [DOI] [PubMed] [Google Scholar]
- 41.Swayne D E, Slemons R D. Comparative pathology of intravenously inoculated wild duck- and turkey-origin type A influenza virus in chickens. Avian Dis. 1995;39:74–84. [PubMed] [Google Scholar]
- 42.Swofford D. PAUP: phylogenetic analysis using parsimony. Version 3. Champaign: Illinois Natural History Survey; 1989. [Google Scholar]
- 43.Tian S F, Buckler White A J, London W T, Reck L J, Chanock R M, Murphy B R. Nucleoprotein and membrane protein genes are associated with restriction of replication of influenza A/Mallard/NY/78 virus and its reassortants in squirrel monkey respiratory tract. J Virol. 1985;53:771–775. doi: 10.1128/jvi.53.3.771-775.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Treanor J J, Snyder M H, London W T, Murphy B R. The B allele of the NS gene of avian influenza viruses, but not the A allele, attenuates a human influenza A virus for squirrel monkeys. Virology. 1989;171:1–9. doi: 10.1016/0042-6822(89)90504-7. [DOI] [PubMed] [Google Scholar]
- 45.U.S. Animal Health Association. Proceedings of the 98th Annual Meeting of the U.S. Grand Rapids, Mich: Animal Health Association. U.S. Animal Health Association; 1994. Report of the Committee on Transmissible Diseases of Poultry and Other Avian Species. Criteria for determining that an AI virus isolation causing an outbreak must be considered for eradication; p. 522. [Google Scholar]
- 46.Van Campen H, Easterday B C, Hinshaw V S. Virulent avian influenza A viruses: their effect on avian lymphocytes and macrophages in vivo and in vitro. J Gen Virol. 1989;70:2887–2895. doi: 10.1099/0022-1317-70-11-2887. [DOI] [PubMed] [Google Scholar]
- 47.Webster R G, Hinshaw V S, Bean W J, van Wyke K L, Geraci J R, St Aubin D J, Petursson G. Characterization of an influenza A virus from seals. Virology. 1981;113:712–724. doi: 10.1016/0042-6822(81)90200-2. [DOI] [PubMed] [Google Scholar]
- 48.Wood G W, Banks J, Brown I, Strong I, Alexander D J. The nucleotide sequence of the HA1 of the haemagglutinin of an H1 avian influenza isolate from turkeys in Germany provides additional evidence suggesting recent transmission from pigs. Avian Pathol. 1997;26:347–355. doi: 10.1080/03079459708419217. [DOI] [PubMed] [Google Scholar]
- 49.Wood J M, Webster R G, Nettles V F. Host range of A/Chicken/Pennsylvania/83 (H5N2) influenza virus. Avian Dis. 1985;29:198–207. [PubMed] [Google Scholar]
- 50.Wyllie A H, Kerr J F R, Currie A R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 51.Yeldandi A V, Colby T V. Pathologic features of lung biopsy specimens from influenza pneumonia cases. Hum Pathol. 1994;25:47–53. doi: 10.1016/0046-8177(94)90170-8. [DOI] [PubMed] [Google Scholar]