ABSTRACT
The present study aimed to characterize the gut microbiota and serum metabolome changes associated with sleep deprivation (SD) as well as to explore the potential benefits of multi-probiotic supplementation in alleviating SD-related mental health disorders. Rats were subjected to 7 days of SD, followed by 14 days of multi-probiotics or saline administration. Open-field tests were conducted at baseline, end of SD (day 7), and after 14 days of saline or multi-probiotic gavage (day 21). Metagenomic sequencing was conducted on fecal samples, and serum metabolites were measured by untargeted liquid chromatography tandem-mass spectrometry. At day 7, anxiety-like behaviors, including significant decreases in total movement distance (P = 0.0002) and staying time in the central zone (P = 0.021), were observed. In addition, increased levels of lipopolysaccharide (LPS; P = 0.028) and decreased levels of uridine (P = 0.018) and tryptophan (P = 0.01) were detected in rats after 7 days of SD. After SD, the richness of the gut bacterial community increased, and the levels of Akkermansia muciniphila, Muribaculum intestinale, and Bacteroides caecimuris decreased. The changes in the host metabolism and gut microbiota composition were strongly associated with the anxiety-like behaviors caused by SD. In addition, multi-probiotic supplementation for 14 days modestly improved the anxiety-like behaviors in SD rats but significantly reduced the serum level of LPS (P = 0.045). In conclusion, SD induces changes in the gut microbiota and serum metabolites, which may contribute to the development of chronic inflammatory responses and affect the gut-brain axis, causing anxiety-like behaviors. Probiotic supplementation significantly reduces serum LPS, which may alleviate the influence of chronic inflammation.
IMPORTANCE
The disturbance in the gut microbiome and serum metabolome induced by SD may be involved in anxiety-like behaviors. Probiotic supplementation decreases serum levels of LPS, but this reduction may be insufficient for alleviating SD-induced anxiety-like behaviors.
KEYWORDS: sleep deprivation, gut microbiota, metabolomics, anxiety, probiotics
INTRODUCTION
Sleep deprivation (SD) occurs when one does not get enough sleep owing to various factors, such as circadian rhythm disturbance and poor sleeping habits (1). In the United States, 35% of adults have been reported to sleep less than 7 h during a 24-h day (2). SD affects the metabolism of individuals and is linked to obesity, diabetes, cardiovascular diseases, and other health issues related to metabolic disorders (3–6). Identifying metabolic changes helps uncover the potential mechanisms of the adverse effects of SD. Individuals suffering from SD have insulin resistance and altered serum lipoproteins, such as decreased circulating high-density lipoprotein (7, 8). Analysis of the plasma metabolome profile of individuals who suffered from acute SD revealed that 27 metabolites were higher than their sleep status (9). Moreover, SD-induced changes in the gut microbiota also contribute to metabolic diseases (10–12).
SD has been demonstrated to increase the risk of mental health disorders, including depression and anxiety (13, 14), and the potential mechanisms include neuroinflammation caused by the activated innate immune responses in the brain (15–18). Lipopolysaccharide (LPS) is the primary surface membrane component of Gram-negative bacteria, and it participates in host-pathogen interactions with the innate immune system (19). Increased circulating LPS has been identified as a mediator of SD-related systemic inflammation (20, 21). Rats and mice administered LPS show elevated inflammatory cytokine levels that mediate neuroinflammation and anxiety-like behavior (16, 22).
Several lines of evidence have suggested that the gut microbiota bidirectionally communicates with the brain through nerve, metabolism, and immune pathways, namely, the gut-microbiota-brain (GMB) axis (23). A previous study has suggested that anxiety and depression are affected by gut microbiota dysbiosis mediated by the GMB axis (24, 25). Studies have demonstrated that individuals with bipolar disorder exhibit an altered gut microbiome characterized by reduced diversity and decreased abundance of butyrate-producing bacteria, especially Faecalibacterium (26, 27). The circadian rhythm of the host regulates the gut microbiota, especially the feeding/fasting rhythm (28). Gut microbiota dysbiosis and disruption of circadian rhythms have been suggested to play a role in systemic inflammation and psychiatry disorders caused by acute 72-h SD (29).
Preliminary evidence has demonstrated the antidepressant and anxiolytic effects of probiotics (30). Notably, Lactobacillus spp. (such as Lactobacillus acidophilus and Lactobacillus rhamnosus) have been shown to regulate the level of neurotransmitters (such as γ-aminobutyric acid) in the cortex, thereby ameliorating anxiety- and depression-like behaviors in mice (31, 32). Supplementation with Lactobacillus plantarum has been reported to moderate SD-induced immune system stress (33). Bacillus subtilis may exert antidepressant and anti-anxiety functions by increasing serotonin levels through tryptophan production (34). Bifidobacterium supplementation has been shown to attenuate insulin resistance in rhesus macaques induced by long-term SD (35), and Bifidobacterium licheniformis has been shown to prevent depression-like and anxiety-like behaviors in chronic unpredictable mild stress model rats, potentially through increasing the short-chain fatty acid levels in the colon to alter the levels of the neurotransmitters in the brain (36). Enterococcus faecium produce dopamine from dietary sources of L-dopa, thereby alleviating depression- and anxiety-like behaviors (37). Thus, the combination of these probiotics may improve SD-induced psychological symptoms.
This present study aimed to explore the effects of SD on changes in the gut microbiota and serum metabolites as well as to explore their involvement in anxiety-like behavior. The effect of multi-probiotic (including Lactobacillus acidophilus, Bacillus licheniformis, Bacillus subtilis, and Enterococcus faecium) supplementation was also assessed in alleviating SD-induced anxiety-like behavior.
MATERIALS AND METHODS
Sleep deprivation rat model
Thirty 6-week-old male Sprague-Dawley rats were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). All rats were fed water and food ad libitum. The room humidity was 55%–65% with a 12-h light/dark cycle (light phase from 7:00 a.m. to 7:00 p.m.). The Ethics Committee on Animal Experimentation of the Chinese PLA General Hospital approved the study design.
After 1 week of adaption, 30 seven-week-old rats were randomly separated into the saline (SA) control group (SA group) and the multi-probiotic (MP) intervention group (MP group) (0 day). This study conducted SD by disturbing the normal circadian rhythm of rats (1). The sleeping habits of rats are opposite to those of humans, and forcing them to not sleep in the daytime disturbs their sleep-wake cycle, thus causing SD. All rats were forced to be active from 7:00 a.m. to 7:00 p.m. (12 h/day) for 7 days by using a sleep disturbance device (XR-XS 107; Shanghai Xinruan Information Technology Co., Ltd., China), which was a feeding cage equipped with a rod at the bottom and set for operating back and forth periodically (38). The operation interval was set at low frequency (30 s) to disturb the sleep of rats while avoiding increased physical activity/exercise. At the end of the SD period, the rats in the SA group were supplemented with saline, and the rats in the MP group were supplemented with multi-probiotics by gavage for 14 days (i.e., day 21). At 0, 7, and 21 days, open-field tests were conducted to evaluate the behavior performance, and serum and fecal samples were also collected. A flow chart of the experiment is shown in Fig. 1a. Knight et al. reported that cage mate fecal microbiomes become more homogeneous over time as rodents are coprophagic, suggesting that experiments should be replicated across multiple cages to control cage effects (39), and they also reported that single housing may stress mice and should be avoided. Therefore, the present study was conducted with a replicate across multiple cages with more than one rat per cage. After 7 days of SD, rats were transferred to individually ventilated cages (IVCs), and five rats were raised in one IVC. Regarding the coprophagy of rats, rats received SA or MP supplementation separately and were housed in different cages.
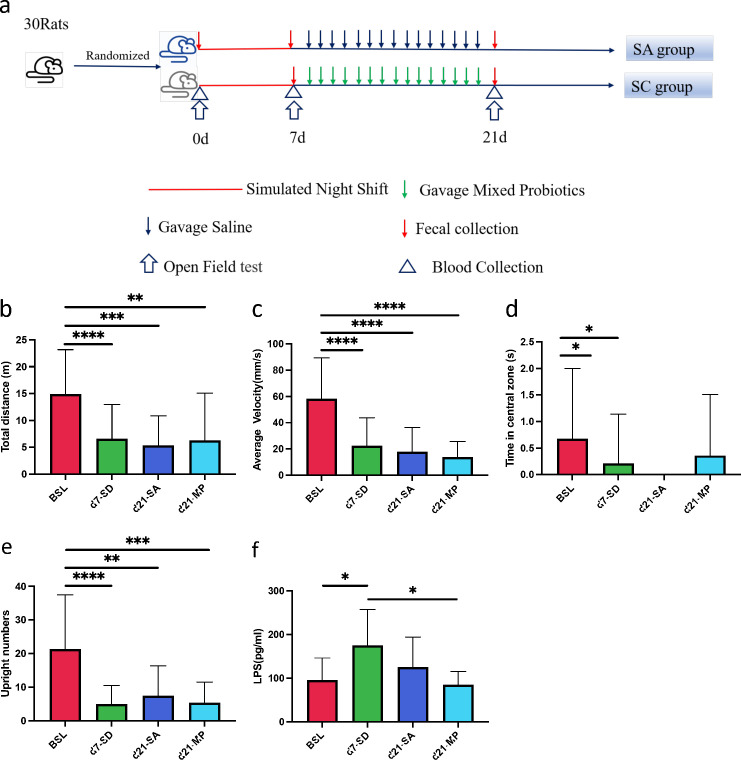
Fig 1.
Rats show anxiety-like behaviors after sleep deprivation (SD) and respond to multi-probiotic supplementation. (a) Study protocols, (b) Total distance, (c) average velocity, (d) time in the central zone, (e) upright numbers, and (f) serum LPS levels of rats. Data are expressed as means ± SD. The significance of the comparison was determined using the Mann-Whitney U test. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. BSL, baseline (N = 30); 7d, SD day 7 (N = 29); SA-21d, saline supplementation for 14 days after SD (N = 15); SC-21d, multi-probiotic supplementation for 14 days after SD (N = 14).
Probiotic supplementation
After 7 days of SD, rats in the SA group were given normal saline (10 mL/kg, 4 mL per rat) by gavage from 2:30 to 3:00 p.m. Rats in the MP group were given mixed probiotics suspended in saline (a total volume of 10 mL/kg), including Bifidobacterium (≥1.0 × 106/CFU), Lactobacillus acidophilus (≥ 1.0 × 106/CFU), Enterococcus (≥1.0 × 106/CFU), Bacillus licheniformis (2.5 × 108/CFU), Bacillus subtilis (5.0 × 107/CFU), and Enterococcus faecium (4.5 × 108/CFU). The Bifidobacterium, Lactobacillus acidophilus, and Enterococcus were from Bifid Triple Viable Capsules (Shanxi Jincheng Haisi Pharmaceutical Co., Ltd.; approval number: National Medicine Standard S19993065). The Bacillus licheniformis was from Zhengchangsheng (Shenyang No. 1 Pharmaceutical Co., Ltd.; approval number: National Medicine Standard S10950019). The Bacillus subtilis and Enterococcus faecium were from live combined Bacillus subtilis and Enterococcus faecium enteric-coated capsules (Beijing Hanmi Pharmaceutical Co., Ltd.; approval number: National Medicine Standard S20030087).
Open-field tests
The open field was a 100 × 100 × 40 cm chamber, and the inner walls of the chamber were painted black. Before the tests, the rats were acclimated in the test room for at least 3 h. Rats were placed in the center of the bottom, and their spontaneous activities were recorded using a 1/3 SONY Super HAD CCD video for 5 min. The total distance moved, average velocities, time in the central zone, and the upright numbers were recorded using the Supermaze system (XR-XZ301, China, Shanghai Xinruan Information Technology Co., Ltd.). After finishing every test, the open field was cleaned with 75% alcohol to eliminate odors. At baseline (0 day), 7 days, and 21 days, the tests were conducted from 1:00 to 5:00 p.m. as previously reported by Andrade et al. (40), who reported that the behavior performance of rats is consistent during this time.
Metagenomic sequencing and data processing
At baseline (0 day), 7 days, and 21 days, approximately 1 g of feces was collected per rat, placed into a 2-mL Eppendorf (EP) microcentrifuge tube, and stored at –80 ℃. The stool was collected by putting rats singly in a sterile can and waiting for defecation from 3:00 to 4:30 p.m. Fecal DNA was extracted using a QIAamp DNA Stool Mini Kit (Qiagen, USA) with the bead-beating step. The quality of extracted DNA was assessed using agarose gel electrophoresis. The genomic library was constructed using 1.5 µg of DNA fragmented using the Covaris E210 (Covaris, USA). According to the manufacturer’s instructions, metagenomic libraries were constructed using an Ovation Ultralow library systems V2 kit (NuGEN, San Carlos, CA). Next-generation sequencing (paired-end, 2 × 150 bp) was performed using the Illumina NovaSeq 6000 System.
Metagenomic sequencing data were processed using bioBakery 3 (41). The removal of low-quality reads and host (rat) sequences was performed using KneadData with default parameters. Taxonomic classification was generated using MetaPhlAn v3.0. The microbial species were annotated using clade-specific marker sequences.
Blood collection and LPS measurement
The plasma samples were collected in ethylenediaminetetraacetic acid (EDTA) tubes from 3:00 to 4:30 p.m. without pre-fasting by cutting the tail and centrifuging at 3,000 rpm for 10 min. The upper serum was preserved in EP tubes and frozen at –80°C within 2 h for further metabolomic tests. Serum LPS was measured using a rat EDT ELISA Kit (The Blue Gene for Life Science Co., Ltd., China) following the manufacturer’s protocol. To avoid contamination of LPS from other sources, the EDTA tubes for whole blood collection were sterilized, and the EP tubes for collecting serum samples were also sterilized using an autoclave. The levels of LPS were measured according to the manufacturer’s protocol, and the labware was free of pyrogens and endotoxins.
Untargeted liquid chromatography tandem-mass spectrometry-based metabolomic profiling
A total of 30 serum samples were included for metabolomic analysis. The metabolites were measured using ultrahigh performance liquid chromatography (1290 Infinity LC, Agilent Technologies) coupled to a quadrupole time-of-flight (AB Sciex TripleTOF 6600). The plasma samples were thawed at 4°C. To remove the serum protein, aliquots (100 µL) were mixed with 400 µL of cold methanol/acetonitrile (1:1, vol/vol) and then centrifuged for 15 min (14,000 × g, 4°C). The supernatant was dried in a vacuum centrifuge and redissolved in 100 µL of acetonitrile/water (1:1, vol/vol) solvent for further analysis. The metabolites were measured in electrospray ionization positive and negative modes using a 2.1 mm × 100 mm ACQUIY UPLC BEH 1.7 µm column (Waters, Ireland). The mobile phase A contained 25 mM ammonium acetate and 25 mM ammonium hydroxide in water, and mobile phase B contained acetonitrile. The following gradient was utilized: 85% B for 1 min, linearly reduced to 65% in 11 min, reduced to 40% in 0.1 min, maintained for 4 min, increased to 85% in 0.1 min, and a 5-min re-equilibration period. To monitor the stability and repeatability of instrument analysis, quality control (QC) samples were prepared by pooling 10 µL of each sample, and the pooled samples were analyzed together with the other samples. The QC samples were inserted regularly and analyzed after every five samples. The selection of samples during the operation was random, and the sample information was unknown to the operator.
Collection of Algorithms of MEtabolite pRofile Annotation was used to annotate isotopes and adducts. The accuracy m/z value compared compound identification of metabolites (<25 ppm) and MS/MS spectra with an in-house database established with available authentic standards. Features were filtered if their relative standard deviations were over 30% in QC samples. An interquartile range filter was applied to maintain the top maximum features. Sample normalization was performed using sums, and Pareto scaling was used to scale the metabolites.
Statistical analysis
Behavior and serum index differences were analyzed using GraphPad Prism 9 (GraphPad Prism Software, Inc., San Diego, CA). As the samples were tied to time series comparisons, the principles reported by Festing et al. were utilized, and further multiple comparisons and Bonferroni correction were also conducted (42). The gut microbiota composition and diversity analysis were performed using MicrobimeAnalyst 5.0 (43). The Mann-Whitney U test was used to compare differences in gut microbiota alpha diversity among the groups. Linear discriminant analysis (LDA) effect size (LEfSe) was used to identify differential bacterial species between groups. One-way analysis of variance (ANOVA) was used to compare the relative abundances of taxa (transformed into a centered-log ratio) among the four groups. Statistical analysis of the metabolites was performed using MetaboAnalyst 5.0. Comparisons between the experimental groups were performed using Student t-test and one-way ANOVA, and P values were adjusted with the false discovery rate (FDR). Heatmap clustering analysis was performed using the Euclidian distance method with Ward’s clustering algorithm. Spearman’s rank correlation was used to analyze the associations among anxiety-like behaviors, gut bacterial taxa, and serum metabolites. Redundancy analysis (RDA) was applied to explore the association between movement indexes and the differential gut bacteria and serum metabolites induced by SD.
RESULTS
Sleep deprivation induces anxiety-like behaviors in rats, which are modestly improved by probiotic supplementation
After SD for 7 days, the open-field test was used to assess the behavior and general activity of rats (Fig. 1a). The total movement distance (P = 0.0002), average velocity (P < 0.0001), staying time in the central zone (P = 0.021), and upright numbers (P < 0.0001) were all significantly decreased after 7 days of SD compared to the baseline level (Fig. 1b through e). These findings suggested that SD induces anxiety-like behavior in rats.
After 14 days of saline (SA group) or multi-probiotic (MP group) gavage, the behaviors of the SD rats did not significantly recover in both groups. However, the mean total movement distance in the MP group was 6,256 ± 8,816 mm, which was slightly higher than the SA group (5,306 ± 5,539 mm; P = 0.73; Fig. 1b). The average velocity was 20.87 mm/s (SD ±29.39 mm/s) and 17.82 ± 18.62 mm/s in the MP and SA groups, respectively (Fig. 1c). The mean staying time in the central zone in the MP group was 0.36 ± 1.15 s and was 0 s for the SA group (Fig. 1d). The mean upright number in the MP group was 5.36 ± 6.17, which was slightly lower than that in the SA group (7.40 ± 8.90; Fig. 1e).
Because LPS has been suggested to induce anxiety- and depression-like behaviors in mice, serum LPS in rats was measured during the SD experiment (Fig. 1f). Serum LPS levels increased significantly from 95.01 ± 51.22 pg/mL at baseline to 174.70 ± 82.19 pg/mL after 7 days of SD (P = 0.028). After 14 days of MP supplementation, serum LPS levels decreased significantly in the MP group at 21 days (84.64 ± 30.25) compared to the levels in the SD group at 7 days (174.70 ± 82.19, P = 0.045). The serum LPS levels in the MP group were lower than those of the SA group (125.0 ± 68.97), although not significantly.
The serum metabolites changed after SD and in response to probiotics supplementation
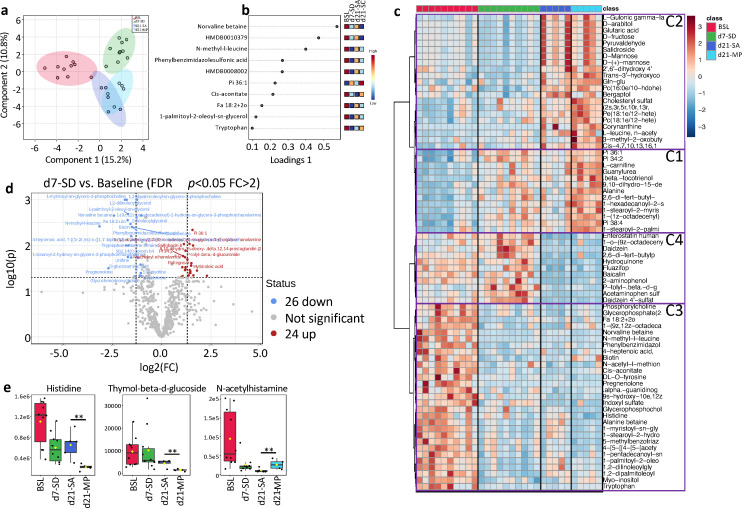
A total of 1,260 metabolites were detected in the rat serum by untargeted liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). Sparse partial least squares discriminant analysis revealed substantial changes in the serum metabolites after 7 days (d7) of SD (Fig. 2a through c). The separation between d7 of SD and baseline was clear (Fig. 2a), and 50 metabolites changed significantly in the serum of the SD group (FDR <0.05 and fold change >2), including 24 increased and 26 decreased metabolites, respectively (Fig. 2d; Table S1). Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis showed that SD significantly increased pyrimidine metabolism (P < 0.05; Fig. S1a).
Fig 2.
Changes in serum metabolites after sleep deprivation and mixed probiotics supplementation. (a) Sparse partial least squares discriminant analysis score plot and (b) loading plot of the serum metabolites. (c) The heatmap shows all serum metabolites in the experimental rats at baseline (BSL), 7 days post-SD (d7), and 14 days of gavage with saline (SA-21d) or mixed probiotics (MP-21d). Four patterns of metabolic changes were classified and indicated as clusters C1–C4. (d) Volcano plot showing the differential metabolites between 7 days post-SD and BSL (FDR <0.05 and fold change >2). Red represents the upregulated differential metabolites at 7 days, and blue represents the downregulated differential metabolites at 7 days. (e) Three metabolites with significantly differential serum levels between the 21d-SA and 21d-MP groups (t-test; P < 0.01 and fold change >2).
Supplementation of either MP or SA for 14 days after SD introduced changes in the serum metabolites (Fig. 2a; Table S2). Different patterns of metabolic changes were observed during the 14 days of recovery. We classified the differentially expressed metabolites into four clusters (Fig. 2c): cluster 1, metabolites that moderately increased during SD and increased further after recovery; cluster 2, metabolites not affected by SD but increased during recovery; cluster 3, metabolites moderately decreased by SD and maintained at low levels during recovery; cluster 4, metabolites not affected by SD but decreased during recovery.
At 14 days after SD cessation, 10 metabolites significantly decreased in the SA group, and 1 metabolite significantly increased in the SA group (FDR <0.05 and fold change >2; Table S3). Compared to baseline, 19 higher and 38 lower serum metabolites were identified in the SA group (FDR <0.05 and fold change >2; Table S4). Rats in the SC group showed no restoration of metabolic homeostasis, with 23 metabolites (11 increased metabolites and 12 decreased metabolites) significantly different from baseline (FDR <0.05 and fold change >2; Table S5). MP did not significantly affect metabolites after SD (FDR >0.05). After 14 days of SA or MP supplementation, histidine and thymol-beta-d-glucoside were lower in the MP group than in the SA group (Fig. 2e), whereas N-acetylhistamine was higher in the MP group than in the SA group (Fig. 2e).
Effects of SD and MP supplementation on changing the gut microbiota in rats
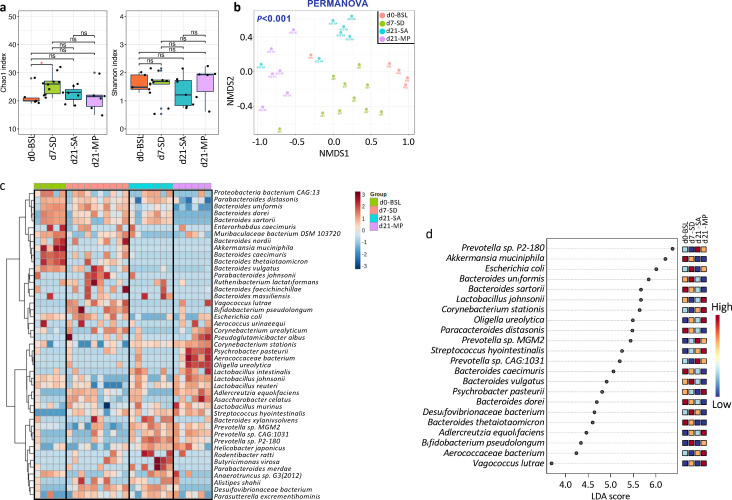
Metagenomic analysis revealed that SD disturbed the gut microbiota homeostasis. The richness of the gut bacterial community (measured by the Chao1 index; P < 0.05) significantly followed SD, while the evenness (measured by the Shannon index) was not influenced (Fig. 3a). The gut bacterial richness dropped during the recovery period in both groups, and the decline was slightly sharper after MP supplementation, despite not significantly. Beta diversity analysis using non-metric multidimensional scaling revealed that the overall gut microbiota composition was influenced by the SD and MP supplementation permutational multivariate analysis of variance (PERMANOVA; P < 0.001; Fig. 3b). The gut microbiota was dominated by Verrucomicrobia (37%) at baseline, which disappeared after 7 days of SD with the enrichment of Proteobacteria (24%) (Fig. S2). Re-growth of Verrucomicrobia was not observed after 14 days of recovery.
Fig 3.
Changes in the gut microbiota after sleep deprivation and mixed probiotic supplementation. (a) At the species level, the alpha diversity of the gut microbiota was measured by the Chao1 index (richness) and Shannon index (diversity). The significance of the comparison was determined using the Mann-Whitney U test (*P < 0.05). (b) Beta diversity was visualized using non-metric multidimensional scaling with Bray-Curtis dissimilarity distances at the species level. Permutational multivariate analysis of variance was performed to assess the statistical significance among the four groups. (c) Heatmap showing the 74 different species identified using one-way ANOVA (FDR <0.005). (d) LEfSe analysis identified bacteria species that significantly differed (FDR <0.05 and LDA >3) among samples collected at baseline (BSL), 7 days post-SD (7d-SD), and 14 days of saline (21d-SA) or mixed probiotics (21d-MP) after SD.
Gut microbiota changes in response to SD and MP supplementation were also detected at the species level (Fig. 3c). LEfSe analysis showed that SD significantly increased the relative abundances of Vagococcus lutrae, Adlercreutzia equolifaciens, Bifidobacterium pseudolongum, Desulfovibrionaceae bacterium, and Streptococcus hyointestinalis; there were lower levels of Akkermansia muciniphila, Muribaculum intestinale, and Bacteroides caecimuris (FDR <0.05 and LDA >3; Fig. 3d). These changes were not reversed after 14 days of recovery (Fig. 3c). After the recovery period, differentially abundant bacterial species were measured between the MP and SA groups. Romboutsia ilealis, Aerococcaceae bacterium, Adlercreutzia equolifaciens, Corynebacterium glutamicum, Psychrobacter pasteurii, Paenalcaligenes hominis, Oligella ureolytica, Corynebacterium stationis, and Lactobacillus johnsonii. Lactobacillus murinus were more enriched in the MP group, while higher abundances of Prevotella sp. P2 180, Parabacteroides distasonis, Prevotella sp. MGM2, Bacteroides sartorii, Parabacteroides merdae, Desulfovibrionaceae bacterium, Bacteroides dorei, and Bacteroides uniformis were detected in SA group (Fig. 3c and d). During the recovery period, there was an increase in the relative abundance of Prevotella sp. MGM2, Prevotella sp. CAG 1031, Prevotella sp. P2 180, and Helicobacter japonicus in the SA and MP groups (Fig. 3c and d) suggesting that changes in these taxa were spontaneous or were caused by the saline solution or the gavage procedure.
Anxiety behaviors are associated with changes in serum metabolism and gut microbiota
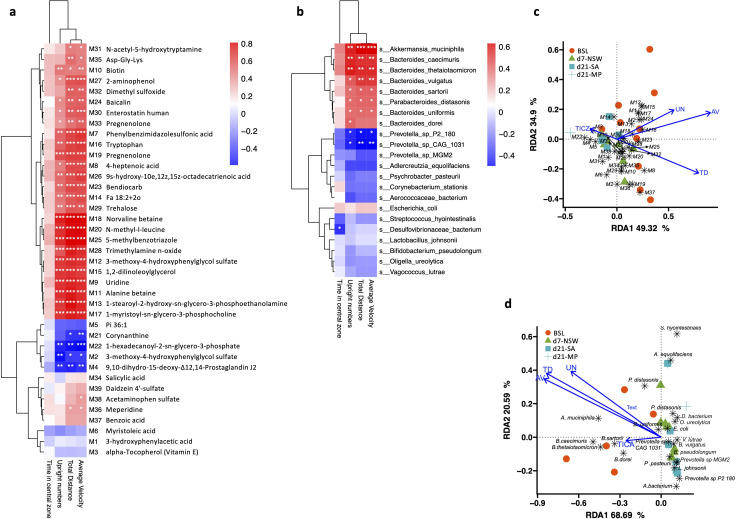
We next explored the associations of behavior performance with changes in the serum metabolites and gut microbiota. We found that changes in the host metabolism and gut microbiota composition induced by SD might be potentially involved in developing anxiety-like behaviors. Spearman’s correlation analysis showed that the average velocity, total distance, and upright numbers were positively correlated with the serum levels of 22 metabolites (top 22 in the heatmap of Fig. 4a; Table S6; P < 0.05). Among these metabolites, phenylbenzimidazolesulfonic acid, uridine, alanine betaine, 1-palmitoyl-2-oleoyl-sn-glycerol, 1-stearoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine, 1,2-dilinoleoylglycerol, tryptophan, 1-myristoyl-sn-glycero-3-phosphocholine, norvaline betaine, N-methyl-l-leucine, 5-methylbenzotriazole, and trimethylamine n-oxide showed positive correlations with these movement indexes (Spearman correlation coefficients R > 0.65). Corynanthine, 1-hexadecanoyl-2-sn-glycero-3-phosphate, 3-methoxy-4-hydroxyphenylglycol sulfate, 9,10-dihydro-15-deoxy-Δ12, and 14-prostaglandin J2 were inversely correlated with the average velocity, total distance, and upright numbers (Fig. 4a).
Fig 4.
The severity of anxiety-like behaviors in rats after sleep deprivation is associated with the changes in serum metabolites and gut microbiota. Heatmaps showing that the (a) serum metabolites and (b) gut bacteria species significantly different from baseline had close relationships with the movement indexes. Significant Spearman correlations are indicated (*P < 0.05, **P < 0.01, and ***P < 0.001). RDA plots show the association of (c) the serum metabolites with four movement indexes and (d) the relationships between the differential taxa from baseline and the movement indexes. TD, total distances; AV, average velocity; UN, upright number; and TICZ, time in the central zone. The arrow length represents the strength of the correlation between the movement indexes and the serum metabolites or microbes.
Correlations were also observed between the differential gut bacteria and the altered movement behaviors (Fig. 4b; Table S7). The average velocity, total distance, and upright numbers positively correlated with Akkermansia muciniphila, Bacteroides caecimuris, Bacteroides thetaiotaomicron, Bacteroides vulgatus, Bacteroides sartorii, Parabacteroides istasonis, and Bacteroides uniformis (Fig. 4b; Table S3; Spearman P < 0.05). Notably, Akkermansia muciniphila showed the strongest correlations with total distance (R = 0.63, P < 0.001), average velocity (R = 0.63, P < 0.001), and upright numbers (R = 0.57, P < 0.01). Two Prevotella spp. (Prevotella sp. P2-180 and Prevotella sp. CAG:1031) were negatively correlated with these movement indexes (Fig. 4b).
These relationships between movement, serum metabolites, and gut microbiota were also accessed using redundancy analysis. The total distance, average velocity, and upright numbers were strongly associated with the serum metabolites or gut microbiota, and the relation between time in the central zone and serum metabolites or gut microbiota was weak compared to the other associations (Fig. 4c and d).
DISCUSSION
The present study demonstrated that SD induces anxiety-like behavior in rats, agreeing with human and other animal studies (44–48). The induced anxiety-like behaviors were sustained after 14 days of recovery, suggesting that the effects of SD on psychology may last for a long time even if the SD ended. Similarly, it has been reported that the degradation of performance patterns caused by SD persists for days even after restoring normal sleep (49). Another study has demonstrated that the mood of adolescents was impaired by a week of partial SD and was not fully recovered after two nights of normal sleep (50). Thus, some physical and phycological changes induced by SD may be irreversible and need further intervention to improve the recovery.
In the present study, LPS was increased significantly in the serum of rats after SD, which may be involved in the development of chronic inflammatory responses and contribute to the occurrence of anxiety-like behaviors. Previous studies have reported that peripheral low-grade inflammation is increasingly observed in patients with psychiatric disorders (51–54). For example, elevated concentration of C-reactive protein (CRP; >3 mg/L) has been detected in 21%–34% of patients with depression, along with increased concentrations of IL-6 and other inflammatory cytokines in blood and in cerebrospinal fluid (51–54). In the present study, SD increased the serum level of LPS, a biologically active substance in the outer membrane of Gram-negative bacteria, which are known to activate innate immunity and promote inflammatory responses (55). Artificially increasing peripheral LPS levels induces neuroinflammation (56), which causes anxiety-like behavior via the immune-kynurenine pathway or modulating neuronal plasticity (15, 16, 57). In the present study, the relative abundances of some Gram-negative bacteria (Akkermansia muciniphila and Bacteroides caecimuris) were decreased after SD, while those of some Gram-positive bacteria (Streptococcus hyointestinalis, Adlercreutzia equolifaciens, Bifidobacterium pseudolongum, Aerococcaceae bacterium, and Vagococcus lutrae) increased after SD. Because the circadian rhythm disruption related to SD causes gut barrier impairment (58–60), the translocation of Gram-negative bacteria from the gut to the circulation may explain their decreased levels in the intestine. An increase in Gram-negative bacteria and LPS production induced by SD may be involved in the development of anxiety-like behaviors. In the present study, there was a decreased relative abundance of Akkermansia muciniphila in SD rats. Notably, Akkermansia muciniphila has been suggested to play critical roles in multiple neuropsychiatric disorders through the GMB axis, including depression, anxiety, Alzheimer’s disease, cognitive impairment, substance use disorders, amyotrophic lateral sclerosis, multiple sclerosis, autism spectrum disorders, epilepsy, Parkinson’s disease, and stroke (61). Given the immunomodulatory and anti-inflammatory effects of Akkermansia muciniphila (62–64), we proposed that SD induces an increase in LPS-producing bacteria and a reduction in beneficial microbes, resulting in peripheral low-grade inflammation and intestinal barrier injury, thereby participating in the development of anxiety- and depression-like behaviors.
Growing evidence has indicated a link between host metabolism and circadian rhythms, suggesting that alterations in the metabolism may also be involved in SD-induced anxiety-like behaviors (65). In the present study, four clusters of metabolites that changed in different trajectories during SD and recovery stages were identified, and some of the varied metabolites may influence host psychological status and contribute to the development of anxiety-like behaviors. The present data revealed that upregulated pyrimidine metabolism in SD rats may be linked to anxiety-like behaviors, which agreed with recent studies, reporting that depression is related to purine and pyrimidine metabolism disorders (66). Among the decreased metabolites after SD, uridine has been identified as a neuroprotective factor that improves memory and suppresses depression (67, 68). As the availability of serotonin (5-hydroxytryptophan) depends on tryptophan, dietary intake of tryptophan is essential for maintaining mental health and well-being (69). In addition, tryptophan deficiency has been shown to cause depression, and tryptophan supplementation may alleviate anxiety and improve mood (70). Thus, a reduced level of tryptophan in SD rats may also contribute to anxiety-like behaviors. Moreover, the present data revealed that a decreased serum level of trimethylamine-N-oxide (TMAO) correlated with anxiety-like behaviors in SD rats. Some studies have reported that animals or humans with depression or anxiety disorders have higher TMAO levels than healthy controls, which may cause neuroinflammation (71, 72). However, decreasing TMAO levels has no significant influence on anxiety, depression-like behaviors, and memory formation in adult mice (73). Thus, further investigation is required to determine the role of TMAO in the development of psychiatric, cognitive, and behavioral disorders.
It has been reported that 14 days of probiotic supplementation moderates the impact of anticipatory stress on the immune system in the time prior to the night shift (74). However, improvement in depression or anxiety behaviors has not been reported. In the present study, 14 days of MP supplementation did not thoroughly recover the anxiety-like behaviors caused by SD. Clinical trials have suggested that probiotic administration lasting longer than a month yields small but significant effects for patients with depression, but the impact of probiotics does not differ from placebo (30). Thus, the potential therapeutic function of probiotics for depression and anxiety should be further evaluated by randomized clinical trials with psychiatric samples.
The present study had several limitations. Firstly, there was no control group without gavage; therefore, it was impossible to investigate the impact of saline on metabolism or gut microbiota. However, Zhang et al. reported that the gut microbiota of rats during adulthood is fairly stable without other interventions (75). Based on this evidence, we assumed that the gut microbiota of rats with normal sleeping cycles on the same day (7-day SD) was similar to baseline and that the before-and-after study was a feasible research design. Secondly, some physiological variables of rats, such as sleep duration, markers of circadian timing, and timing of food intake, were not measured in the present study. Thirdly, the serum and fecal samples were collected from different animals, complicating the detection of potential bacterial taxa involved in specific metabolic changes. Finally, the time required to recover from anxiety associated with SD without any intervention was not measured.
In conclusion, the present study provided evidence that SD induces anxiety disorders and that increased serum LPS may play an important role. The dysbiosis of gut microbiota and changes in serum metabolism show significant correlations with SD-induced anxiety behaviors, and the mechanism may involve a chronic inflammation response of the gut that affects the gut-brain axis. Moreover, probiotic supplementation has limited ability to improve SD-induced anxiety-like behaviors; however, it significantly reduces the production of LPS, which may reduce the influence of chronic inflammation.
Contributor Information
Xuefeng Gao, Email: xfgao@smu.edu.cn.
Yunsheng Yang, Email: sunnyddc@plagh.org.
Zikai Wang, Email: wangzikai301@126.com.
Zhenjiang Zech Xu, State Key Laboratory of Food Science and Resources, Nanchang, China.
DATA AVAILABILITY
The raw data of metagenomic sequencing have been uploaded to the Sequence Read Archive database (https://trace.ncbi.nlm.nih.gov/Traces/home/) and are available for download via accession number PRJNA1049576.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.01437-23.
Changes in metabolic functional pathways caused by sleep deprivation.
Changes in the relative abundances of gut bacteria phyla after SD, and 14 days of saline or mixed probiotics gavage.
Legends for Fig. S1 and S2 and Tables S1 to S7.
Different metabolites between baseline day and 7-day sleep deprivation.
Different metabolites among baseline day, 7-day sleep deprivation, and 14-day saline or mixed probiotic supplementation.
Different metabolites between 7-day sleep deprivation (d7-SD) and 14-day saline supplementation (d21-SA).
Different metabolites between baseline day and 14-day saline supplementation (d21-SA).
Different metabolites between baseline day and 14-day mixed probiotic supplementation (d21-MP).
Spearman's correlation analysis between movement behaviors and serum metabolites.
Spearman's correlation analysis between movement behaviors and gut microbes.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Bandyopadhyay A, Sigua NL. 2019. What is sleep deprivation? Am J Respir Crit Care Med 199:11–p12. doi: 10.1164/rccm.1996P11 [DOI] [PubMed] [Google Scholar]
- 2. Gonzalez A, Tyminski Q. 2020. Sleep deprivation in an American homeless population. Sleep Health 6:489–494. doi: 10.1016/j.sleh.2020.01.002 [DOI] [PubMed] [Google Scholar]
- 3. Bayon V, Leger D, Gomez-Merino D, Vecchierini MF, Chennaoui M. 2014. Sleep debt and obesity. Ann Med 46:264–272. doi: 10.3109/07853890.2014.931103 [DOI] [PubMed] [Google Scholar]
- 4. Larcher S, Benhamou PY, Pépin JL, Borel AL. 2015. Sleep habits and diabetes. Diabetes Metab 41:263–271. doi: 10.1016/j.diabet.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 5. Tobaldini E, Costantino G, Solbiati M, Cogliati C, Kara T, Nobili L, Montano N. 2017. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci Biobehav Rev 74:321–329. doi: 10.1016/j.neubiorev.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 6. Borel AL. 2019. Sleep apnea and sleep habits: relationships with metabolic syndrome. Nutrients 11:2628. doi: 10.3390/nu11112628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van den Berg R, Mook-Kanamori DO, Donga E, van Dijk M, van Dijk JG, Lammers G-J, van Kralingen KW, Prehn C, Adamski J, Romijn JA, van Dijk KW, Corssmit EPM, Rensen PCN, Biermasz NR. 2016. A single night of sleep curtailment increases plasma acylcarnitines: novel insights in the relationship between sleep and insulin resistance. Arch Biochem Biophys 589:145–151. doi: 10.1016/j.abb.2015.09.017 [DOI] [PubMed] [Google Scholar]
- 8. Aho V, Ollila HM, Kronholm E, Bondia-Pons I, Soininen P, Kangas AJ, Hilvo M, Seppälä I, Kettunen J, Oikonen M, et al. 2016. Prolonged sleep restriction induces changes in pathways involved in cholesterol metabolism and inflammatory responses. Sci Rep 6:24828. doi: 10.1038/srep24828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davies SK, Ang JE, Revell VL, Holmes B, Mann A, Robertson FP, Cui N, Middleton B, Ackermann K, Kayser M, Thumser AE, Raynaud FI, Skene DJ. 2014. Effect of sleep deprivation on the human metabolome. Proc Natl Acad Sci U S A 111:10761–10766. doi: 10.1073/pnas.1402663111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gutierrez Lopez DE, Lashinger LM, Weinstock GM, Bray MS. 2021. Circadian rhythms and the gut microbiome synchronize the host’s metabolic response to diet. Cell Metab 33:873–887. doi: 10.1016/j.cmet.2021.03.015 [DOI] [PubMed] [Google Scholar]
- 11. Benedict C, Vogel H, Jonas W, Woting A, Blaut M, Schürmann A, Cedernaes J. 2016. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol Metab 5:1175–1186. doi: 10.1016/j.molmet.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Poroyko VA, Carreras A, Khalyfa A, Khalyfa AA, Leone V, Peris E, Almendros I, Gileles-Hillel A, Qiao Z, Hubert N, Farré R, Chang EB, Gozal D. 2016. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci Rep 6:35405. doi: 10.1038/srep35405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ben Simon E, Vallat R, Barnes CM, Walker MP. 2020. Sleep loss and the socio-emotional brain. Trends Cogn Sci 24:435–450. doi: 10.1016/j.tics.2020.02.003 [DOI] [PubMed] [Google Scholar]
- 14. Pires GN, Bezerra AG, Tufik S, Andersen ML. 2016. Effects of acute sleep deprivation on state anxiety levels: a systematic review and meta-analysis. Sleep Med 24:109–118. doi: 10.1016/j.sleep.2016.07.019 [DOI] [PubMed] [Google Scholar]
- 15. Kim YK, Jeon SW. 2018. Neuroinflammation and the immune-kynurenine pathway in anxiety disorders. Curr Neuropharmacol 16:574–582. doi: 10.2174/1570159X15666170913110426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng Z-H, Tu J-L, Li X-H, Hua Q, Liu W-Z, Liu Y, Pan B-X, Hu P, Zhang W-H. 2021. Neuroinflammation induces anxiety- and depressive-like behavior by modulating neuronal plasticity in the basolateral amygdala. Brain Behav Immun 91:505–518. doi: 10.1016/j.bbi.2020.11.007 [DOI] [PubMed] [Google Scholar]
- 17. Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. 2010. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol 185:5796–5805. doi: 10.4049/jimmunol.1001026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manchanda S, Singh H, Kaur T, Kaur G. 2018. Low-grade neuroinflammation due to chronic sleep deprivation results in anxiety and learning and memory impairments. Mol Cell Biochem 449:63–72. doi: 10.1007/s11010-018-3343-7 [DOI] [PubMed] [Google Scholar]
- 19. Kogut MH, Lee A, Santin E. 2020. Microbiome and pathogen interaction with the immune system. Poult Sci 99:1906–1913. doi: 10.1016/j.psj.2019.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Atwater AQ, Immergluck LC, Davidson AJ, Castanon-Cervantes O. 2021. Shift work predicts increases in lipopolysaccharide-binding protein, interleukin-10, and leukocyte counts in a cross-sectional study of healthy volunteers carrying low-grade systemic inflammation. Int J Environ Res Public Health 18:13158. doi: 10.3390/ijerph182413158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Su H, Zhang C, Zou X, Lu F, Zeng Y, Guan H, Ren Y, Yuan F, Xu L, Zhang M, Dong H. 2020. Jiao-tai-wan inhibits inflammation of the gut-brain-axis and attenuates cognitive impairment in Insomnic rats. J Ethnopharmacol 250:112478. doi: 10.1016/j.jep.2019.112478 [DOI] [PubMed] [Google Scholar]
- 22. Li M, Li C, Yu H, Cai X, Shen X, Sun X, Wang J, Zhang Y, Wang C. 2017. Lentivirus-mediated interleukin-1β (IL-1β) knock-down in the hippocampus alleviates lipopolysaccharide (LPS)-induced memory deficits and Anxiety- and depression-like behaviors in mice. J Neuroinflammation 14:190. doi: 10.1186/s12974-017-0964-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morais LH, Schreiber HL, Mazmanian SK. 2021. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol 19:241–255. doi: 10.1038/s41579-020-00460-0 [DOI] [PubMed] [Google Scholar]
- 24. Foster JA, McVey Neufeld KA. 2013. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 36:305–312. doi: 10.1016/j.tins.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 25. Feng L, Zhou N, Li Z, Fu D, Guo Y, Gao X, Liu X. 2022. Co-occurrence of gut microbiota dysbiosis and bile acid metabolism alteration is associated with psychological disorders in Crohn’s disease. FASEB J 36:e22100. doi: 10.1096/fj.202101088RRR [DOI] [PubMed] [Google Scholar]
- 26. Lu Q, Lai J, Lu H, Ng C, Huang T, Zhang H, Ding K, Wang Z, Jiang J, Hu J, Lu J, Lu S, Mou T, Wang D, Du Y, Xi C, Lyu H, Chen J, Xu Y, Liu Z, Hu S. 2019. Gut microbiota in bipolar depression and its relationship to brain function: an advanced exploration. Front Psychiatry 10:784. doi: 10.3389/fpsyt.2019.00784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lucidi L, Pettorruso M, Vellante F, Di Carlo F, Ceci F, Santovito MC, Di Muzio I, Fornaro M, Ventriglio A, Tomasetti C, Valchera A, Gentile A, Kim Y-K, Martinotti G, Fraticelli S, Di Giannantonio M, De Berardis D. 2021. Gut microbiota and bipolar disorder: an overview on a novel biomarker for diagnosis and treatment. Int J Mol Sci 22:7. doi: 10.3390/ijms22073723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Voigt RM, Forsyth CB, Green SJ, Engen PA, Keshavarzian A. 2016. Circadian rhythm and the gut microbiome. Int Rev Neurobiol 131:193–205. doi: 10.1016/bs.irn.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 29. Yang D-F, Huang W-C, Wu CW, Huang C-Y, Yang Y-C, Tung Y-T. 2023. Acute sleep deprivation exacerbates systemic inflammation and psychiatry disorders through gut microbiota dysbiosis and disruption of circadian rhythms. Microbiol Res 268:127292. doi: 10.1016/j.micres.2022.127292 [DOI] [PubMed] [Google Scholar]
- 30. Liu RT, Walsh RFL, Sheehan AE. 2019. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev 102:13–23. doi: 10.1016/j.neubiorev.2019.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M, Ouwehand A, Leyer G, Carcano D, Colombel J-F, Ardid D, Desreumaux P. 2007. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med 13:35–37. doi: 10.1038/nm1521 [DOI] [PubMed] [Google Scholar]
- 32. Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. 2011. Ingestion of lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 108:16050–16055. doi: 10.1073/pnas.1102999108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Choi SK, Saier MH. 2005. Regulation of sigL expression by the catabolite control protein CcpA involves a roadblock mechanism in Bacillus subtilis: potential connection between carbon and nitrogen metabolism. J Bacteriol 187:6856–6861. doi: 10.1128/JB.187.19.6856-6861.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dhaliwal J, Singh DP, Singh S, Pinnaka AK, Boparai RK, Bishnoi M, Kondepudi KK, Chopra K. 2018. Lactobacillus plantarum MTCC 9510 supplementation protects from chronic unpredictable and sleep deprivation-induced behaviour, biochemical and selected gut microbial aberrations in mice. J Appl Microbiol 125:257–269. doi: 10.1111/jam.13765 [DOI] [PubMed] [Google Scholar]
- 35. Zhao Y, Shu Y, Zhao N, Zhou Z, Jia X, Jian C, Jin S. 2022. Insulin resistance induced by long-term sleep deprivation in rhesus macaques can be attenuated by Bifidobacterium. Am J Physiol Endocrinol Metab 322:E165–E172. doi: 10.1152/ajpendo.00329.2021 [DOI] [PubMed] [Google Scholar]
- 36. Feng S, Meng C, Liu Y, Yi Y, Liang A, Zhang Y, Hao Z. 2023. Bacillus licheniformis prevents and reduces anxiety-like and depression-like behaviours. Appl Microbiol Biotechnol 107:4355–4368. doi: 10.1007/s00253-023-12580-7 [DOI] [PubMed] [Google Scholar]
- 37. Villageliú D, Lyte M. 2018. Dopamine production in Enterococcus faecium: a microbial endocrinology-based mechanism for the selection of probiotics based on neurochemical-producing potential. PLoS One 13:e0207038. doi: 10.1371/journal.pone.0207038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ramesh V, Kaushal N, Gozal D. 2009. Sleep fragmentation differentially modifies EEG delta power during slow wave sleep in socially isolated and paired mice 2:64–75. http://www.sleepscience.org.br/details/139. [Google Scholar]
- 39. Knight R, Vrbanac A, Taylor BC, Aksenov A, Callewaert C, Debelius J, Gonzalez A, Kosciolek T, McCall L-I, McDonald D, Melnik AV, Morton JT, Navas J, Quinn RA, Sanders JG, Swafford AD, Thompson LR, Tripathi A, Xu ZZ, Zaneveld JR, Zhu Q, Caporaso JG, Dorrestein PC. 2018. Best practices for analysing microbiomes. Nat Rev Microbiol 16:410–422. doi: 10.1038/s41579-018-0029-9 [DOI] [PubMed] [Google Scholar]
- 40. Andrade MMM, Tomé MF, Santiago ES, Lúcia-Santos A, de Andrade TGCS. 2003. Longitudinal study of daily variation of rats' behavior in the elevated plus-maze. Physiol Behav 78:125–133. doi: 10.1016/s0031-9384(02)00941-1 [DOI] [PubMed] [Google Scholar]
- 41. Beghini F, McIver LJ, Blanco-Míguez A, Dubois L, Asnicar F, Maharjan S, Mailyan A, Manghi P, Scholz M, Thomas AM, Valles-Colomer M, Weingart G, Zhang Y, Zolfo M, Huttenhower C, Franzosa EA, Segata N. 2021. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. Elife 10:e65088. doi: 10.7554/eLife.65088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Festing MFW, Altman DG. 2002. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J 43:244–258. doi: 10.1093/ilar.43.4.244 [DOI] [PubMed] [Google Scholar]
- 43. Chong J, Liu P, Zhou G, Xia J. 2020. Using microbiomeanalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat Protoc 15:799–821. doi: 10.1038/s41596-019-0264-1 [DOI] [PubMed] [Google Scholar]
- 44. Perotta B, Arantes-Costa FM, Enns SC, Figueiro-Filho EA, Paro H, Santos IS, Lorenzi-Filho G, Martins MA, Tempski PZ. 2021. Sleepiness, sleep deprivation, quality of life, mental symptoms and perception of academic environment in medical students. BMC Med Educ 21:111. doi: 10.1186/s12909-021-02544-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goldstein-Piekarski AN, Greer SM, Saletin JM, Harvey AG, Williams LM, Walker MP. 2018. Sleep deprivation, and the anxious brain. J Cogn Neurosci 30:565–578. doi: 10.1162/jocn_a_01225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ben Simon E, Rossi A, Harvey AG, Walker MP. 2020. Overanxious and underslept. Nat Hum Behav 4:100–110. doi: 10.1038/s41562-019-0754-8 [DOI] [PubMed] [Google Scholar]
- 47. Wang X, Wang Z, Cao J, Dong Y, Chen Y. 2021. Melatonin ameliorates anxiety-like behaviors induced by sleep deprivation in mice: role of oxidative stress, neuroinflammation, autophagy and apoptosis. Brain Res Bull 174:161–172. doi: 10.1016/j.brainresbull.2021.06.010 [DOI] [PubMed] [Google Scholar]
- 48. Zhang DD, Li HJ, Zhang HR, Ye XC. 2022. Poria cocos water-soluble polysaccharide modulates anxiety-like behavior induced by sleep deprivation by regulating the gut dysbiosis, metabolic disorders and TNF-α/NF-κB signaling pathway. Food Funct 13:6648–6664. doi: 10.1039/d2fo00811d [DOI] [PubMed] [Google Scholar]
- 49. Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, Russo MB, Balkin TJ. 2003. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res 12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x [DOI] [PubMed] [Google Scholar]
- 50. Lo JC, Ong JL, Leong RLF, Gooley JJ, Chee MWL. 2016. Cognitive performance, sleepiness, and mood in partially sleep deprived adolescents: the need for sleep study. Sleep 39:687–698. doi: 10.5665/sleep.5552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Osimo EF, Baxter LJ, Lewis G, Jones PB, Khandaker GM. 2019. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med 49:1958–1970. doi: 10.1017/S0033291719001454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N, Raison CL, Miller BJ, Lanctôt KL, Carvalho AF. 2017. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand 135:373–387. doi: 10.1111/acps.12698 [DOI] [PubMed] [Google Scholar]
- 53. Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M. 2015. Cumulative meta-analysis of Interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun 49:206–215. doi: 10.1016/j.bbi.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. 2010. A meta-analysis of cytokines in major depression. Biol Psychiatry 67:446–457. doi: 10.1016/j.biopsych.2009.09.033 [DOI] [PubMed] [Google Scholar]
- 55. Rathinam VAK, Zhao Y, Shao F. 2019. Innate immunity to intracellular LPS. Nat Immunol 20:527–533. doi: 10.1038/s41590-019-0368-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lopes PC. 2016. LPS and neuroinflammation: a matter of timing. Inflammopharmacology 24:291–293. doi: 10.1007/s10787-016-0283-2 [DOI] [PubMed] [Google Scholar]
- 57. Liu H, Huang X, Li Y, Xi K, Han Y, Mao H, Ren K, Wang W, Wu Z. 2022. TNF signaling pathway-mediated microglial activation in the PFC underlies acute paradoxical sleep deprivation-induced anxiety-like behaviors in mice. Brain Behav Immun 100:254–266. doi: 10.1016/j.bbi.2021.12.006 [DOI] [PubMed] [Google Scholar]
- 58. Bishehsari F, Engen PA, Adnan D, Sarrafi S, Wilber S, Shaikh M, Green SJ, Naqib A, Giron LB, Abdel-Mohsen M, Keshavarzian A. 2021. Abnormal food timing and predisposition to weight gain: role of barrier dysfunction and microbiota. Transl Res 231:113–123. doi: 10.1016/j.trsl.2020.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Martel J, Chang SH, Ko YF, Hwang TL, Young JD, Ojcius DM. 2022. Gut barrier disruption and chronic disease. Trends Endocrinol Metab 33:247–265. doi: 10.1016/j.tem.2022.01.002 [DOI] [PubMed] [Google Scholar]
- 60. Pagel R, Bär F, Schröder T, Sünderhauf A, Künstner A, Ibrahim SM, Autenrieth SE, Kalies K, König P, Tsang AH, Bettenworth D, Divanovic S, Lehnert H, Fellermann K, Oster H, Derer S, Sina C. 2017. Circadian rhythm disruption impairs tissue homeostasis and exacerbates chronic inflammation in the intestine. FASEB J 31:4707–4719. doi: 10.1096/fj.201700141RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xu R, Zhang Y, Chen S, Zeng Y, Fu X, Chen T, Luo S, Zhang X. 2023. The role of the probiotic Akkermansia muciniphila in brain functions: insights underpinning therapeutic potential. Crit Rev Microbiol 49:151–176. doi: 10.1080/1040841X.2022.2044286 [DOI] [PubMed] [Google Scholar]
- 62. Huck O, Mulhall H, Rubin G, Kizelnik Z, Iyer R, Perpich JD, Haque N, Cani PD, de Vos WM, Amar S. 2020. Akkermansia muciniphila reduces Porphyromonas gingivalis-induced inflammation and periodontal bone destruction. J Clin Periodontol 47:202–212. doi: 10.1111/jcpe.13214 [DOI] [PubMed] [Google Scholar]
- 63. Bian X, Wu W, Yang L, Lv L, Wang Q, Li Y, Ye J, Fang D, Wu J, Jiang X, Shi D, Li L. 2019. Administration of Akkermansia muciniphila ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front Microbiol 10:2259. doi: 10.3389/fmicb.2019.02259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Raftar SKA, Ashrafian F, Abdollahiyan S, Yadegar A, Moradi HR, Masoumi M, Vaziri F, Moshiri A, Siadat SD, Zali MR. 2022. The anti-inflammatory effects of Akkermansia muciniphila and its derivates in HFD/CCL4-induced murine model of liver injury. Sci Rep 12:2453. doi: 10.1038/s41598-022-06414-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wei H, Gu J, Jiang X, Deng N, Wu J, Zou L, Zhu Y, Tan B. 2021. Anxiety disturbs the blood plasma metabolome in acute coronary syndrome patients. Sci Rep 11:12897. doi: 10.1038/s41598-021-92421-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lu Z, Li S, Aa N, Zhang Y, Zhang R, Xu C, Zhang S, Kong X, Wang G, Aa J, Zhang Y. 2022. Quantitative analysis of 20 purine and pyrimidine metabolites by HILIC-MS/MS in the serum and hippocampus of depressed mice. J Pharm Biomed Anal 219:114886. doi: 10.1016/j.jpba.2022.114886 [DOI] [PubMed] [Google Scholar]
- 67. Dobolyi A, Juhász G, Kovács Z, Kardos J. 2011. Uridine function in the central nervous system. Curr Top Med Chem 11:1058–1067. doi: 10.2174/156802611795347618 [DOI] [PubMed] [Google Scholar]
- 68. Carlezon WA, Mague SD, Parow AM, Stoll AL, Cohen BM, Renshaw PF. 2005. Antidepressant-like effects of uridine and omega-3 fatty acids are potentiated by combined treatment in rats. Biol Psychiatry 57:343–350. doi: 10.1016/j.biopsych.2004.11.038 [DOI] [PubMed] [Google Scholar]
- 69. Lindseth G, Helland B, Caspers J. 2015. The effects of dietary tryptophan on affective disorders. Arch Psychiatr Nurs 29:102–107. doi: 10.1016/j.apnu.2014.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Roth W, Zadeh K, Vekariya R, Ge Y, Mohamadzadeh M. 2021. Tryptophan metabolism and gut-brain homeostasis. Int J Mol Sci 22:2973. doi: 10.3390/ijms22062973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pu J, Liu Y, Gui S, Tian L, Yu Y, Wang D, Zhong X, Chen W, Chen X, Chen Y, Chen X, Gong X, Liu L, Li W, Wang H, Xie P. 2022. Effects of pharmacological treatment on metabolomic alterations in animal models of depression. Transl Psychiatry 12:175. doi: 10.1038/s41398-022-01947-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Le J, Peng R, Li Y. 2022. Trimethylamine-N-oxide and precursors as novel potential biomarkers for anxiety disorder. Lab Med 53:177–182. doi: 10.1093/labmed/lmab063 [DOI] [PubMed] [Google Scholar]
- 73. Mao J, Zhao P, Wang Q, Chen A, Li X, Li X, Liu T, Tao Z, Wang X, Du Y, Gong M, Song L, Gao Y, Shi H. 2021. Repeated 3,3-dimethyl-1-butanol exposure alters social dominance in adult mice. Neurosci Lett 758:136006. doi: 10.1016/j.neulet.2021.136006 [DOI] [PubMed] [Google Scholar]
- 74. West NP, Hughes L, Ramsey R, Zhang P, Martoni CJ, Leyer GJ, Cripps AW, Cox AJ. 2020. Probiotics, anticipation stress, and the acute immune response to night shift. Front Immunol 11:599547. doi: 10.3389/fimmu.2020.599547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang X, Yang Y, Su J, Zheng X, Wang C, Chen S, Liu J, Lv Y, Fan S, Zhao A, Chen T, Jia W, Wang X. 2021. Age-related compositional changes and correlations of gut microbiome, serum metabolome, and immune factor in rats. Geroscience 43:709–725. doi: 10.1007/s11357-020-00188-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Changes in metabolic functional pathways caused by sleep deprivation.
Changes in the relative abundances of gut bacteria phyla after SD, and 14 days of saline or mixed probiotics gavage.
Legends for Fig. S1 and S2 and Tables S1 to S7.
Different metabolites between baseline day and 7-day sleep deprivation.
Different metabolites among baseline day, 7-day sleep deprivation, and 14-day saline or mixed probiotic supplementation.
Different metabolites between 7-day sleep deprivation (d7-SD) and 14-day saline supplementation (d21-SA).
Different metabolites between baseline day and 14-day saline supplementation (d21-SA).
Different metabolites between baseline day and 14-day mixed probiotic supplementation (d21-MP).
Spearman's correlation analysis between movement behaviors and serum metabolites.
Spearman's correlation analysis between movement behaviors and gut microbes.
Data Availability Statement
The raw data of metagenomic sequencing have been uploaded to the Sequence Read Archive database (https://trace.ncbi.nlm.nih.gov/Traces/home/) and are available for download via accession number PRJNA1049576.