To the Editor. – Three coronavirus disease 2019 (COVID-19) vaccines targeting the viral spike protein (S)1 have received Food and Drug Administration (FDA) Emergency Use Authorization (EUA)2. We measured levels of anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies in adults after receiving Moderna, Pfizer-BioNTech (Pfizer), or Janssen/J&J (J&J) vaccines.
Methods
Between March and May 2021, we collected blood from 60 adults, who had received at least one of two doses of Moderna (N=48) or Pfizer (N=6) vaccines or one dose of the J&J (N=6) vaccine. Use of the samples was deemed exempt by the National Institutes of Health (NIH) Institutional Review Board.
Plasma was analyzed for antibodies using Roche anti-SARS-CoV-2-S and anti-SARS-CoV-2-nucleocapsid (N) immunoassays on a Cobas-6000 analyzer. Both assays have FDA-EUAs and measure IgG, IgA and IgM against their target proteins. Twelve J&J specimens were additionally analyzed for anti-S using a luciferase immunoprecipitation system (LIPS) assay3.
Results
Participants had a median age of 46.2 years (range 18–64) and 36 of the 60 participants (60%) were women. All Moderna (N=48) and all but one Pfizer vaccine recipients (N=5) were positive for anti-S (Figure, A). This included five Moderna and one Pfizer participants who had received only one dose. All anti-N results were negative, except for four participants with history of SARS-CoV-2 infection (Figure, A).
Figure 1.
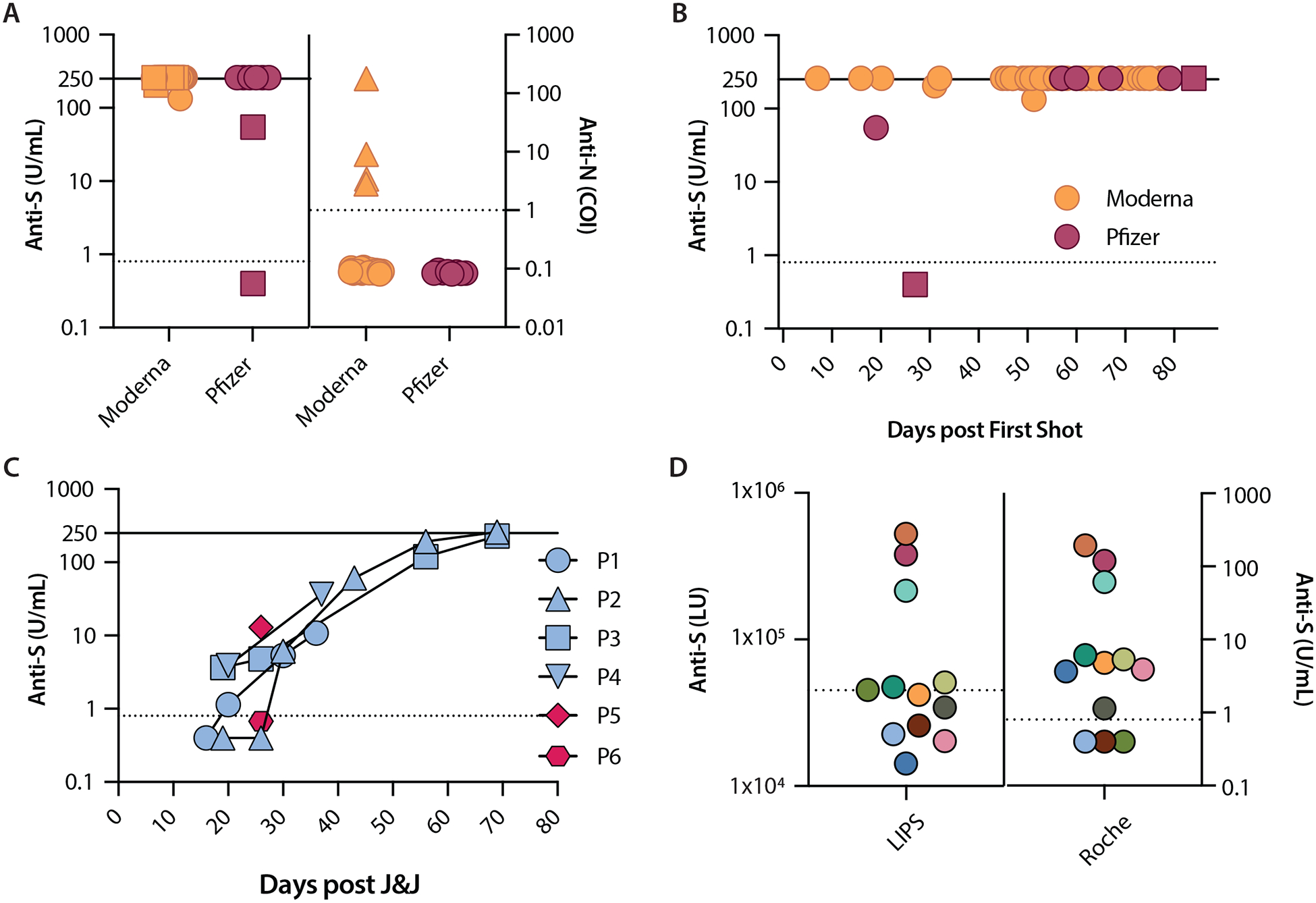
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody levels following Moderna, Pfizer, or J&J vaccination. A. Anti-S and anti-N levels after Moderna and Pfizer vaccines. Squares represent specimens collected after a single shot (made distinct for anti-S only). Triangles represent specimens from participants with a history of SARS-CoV-2 infection (made distinct for anti-N only). B. Anti-S levels over days following first dose of Moderna and Pfizer. Earliest time point is at 7 days (Moderna) and the latest time point is at 84 days (Pfizer). Squares represent two specimens from the same Pfizer participant. C. Anti-S levels in specimens from the six J&J participants were measured once (P5 and P6) and over time (P1 to P4). D. Anti-S levels 12 specimens from J&J participants. Each color represents the same specimen tested with both LIPS and Roche assays. All data are plotted on a logarithmic scale. Anti-S levels are reported in U/mL (Roche) and LU (LIPS); anti-N levels are reported as a cutoff index (COI; signal sample/cutoff). Values ≥250 U/mL are assigned as 260 U/mL for easier visualization. Dashed lines represent cutoff thresholds at 0.8 U/mL (Roche anti-S), 1.0 COI (Roche anti-N), and 45000 LU (LIPS anti-S). Solid lines represent the upper limit of measurement for Roche anti-S assay (250 U/mL). COI: cutoff index; LIPS: luciferase immunoprecipitation system; LU: light units; P: participant.
Moderna and Pfizer vaccines induced positive anti-S as early as 7 and 19 days, respectively, post first shot. A Pfizer participant with sequential samples was negative at day 27 and positive at day 84 (Figure, B). In J&J vaccine recipients (Figure, C), two participants (P1 and P2), negative at days 16 (P1) and 19 (P2), became positive by days 20 and 30, respectively. P3 and P4 were positive when first tested at days 19 and 20 post vaccine. All four participants showed increases in anti-S levels reaching ≥250 U/mL and 227.6 U/mL in P2 and P3, respectively, on day 69. P5 (positive) and P6 (negative) were only tested once at day 26. The twelve J&J specimens analyzed with a different assay showed even fewer specimens positive for anti-S (N=6) than the Roche assay (N=9) (Figure, D).
Discussion
We found that nearly all vaccinated Moderna participants had anti-S levels that exceeded the upper limit of the Roche assay. By contrast, J&J vaccine recipients had anti-S levels that were negative or low 2 weeks after vaccination and gradually increased over time. Our results suggest that if J&J vaccine recipients were tested 2 weeks after vaccination, many may erroneously be considered vaccine failures. It is possible that the commercial assay is insensitive but there were even fewer positives with the immunoprecipitation assay. Alternatively, the J&J vaccine may induce an effective amnestic response, and/or other antibody or T cell response may correlate with efficacy. Of note, the highly effective varicella vaccine induces antibody responses that are often not detected using commercial assays and thus it is recommended that vaccinated patients not get tested4. Our study is limited by the number of participants, particularly those who had received the Pfizer vaccine, which does not allow for strong conclusions regarding the similarities with or differences from Moderna or J&J in antibody temporal responses. Despite this limitation, the repeated testing of J&J participants indicates that measuring antibody levels within the first weeks of vaccination may give negative results and lead to unwarranted additional doses of vaccine.
Acknowledgements
This work was supported by the intramural research programs of the National Institutes of Health (NIH) Clinical Center, National Institute of Allergy and Infectious Diseases, National Institute of Dental and Craniofacial Research, and the National Heart, Lung, and Blood Institute.
References
- 1.Locht C Vaccines against COVID-19. Anaesth Crit Care Pain Med. 2020;39(6):703–705. doi: 10.1016/j.accpm.2020.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Food and Drug Administration (FDA). COVID-19 Vaccines. Accessed 05/31, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines#eua-vaccines
- 3.Burbelo PD, Riedo FX, Morishima C, et al. Sensitivity in Detection of Antibodies to Nucleocapsid and Spike Proteins of Severe Acute Respiratory Syndrome Coronavirus 2 in Patients With Coronavirus Disease 2019. J Infect Dis. 2020;222(2):206–213. doi: 10.1093/infdis/jiaa273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marin M, Güris D, Chaves SS, Schmid S, Seward JF. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(Rr-4):1–40. [PubMed] [Google Scholar]


