Abstract
Background:
To evaluate post-operative outcomes and white matter injury (WMI) using brain MRI at term equivalent in neonates with and without severe acute kidney injury (AKI) following surgical necrotizing enterocolitis (NEC).
Methods:
A retrospective cohort study comparing neonates with severe (Stage 2/3) vs. other (no AKI/Stage 1) AKI using KDIGO classification with multivariable models assessing this association in the context of multiple systemic comorbidities.
Results:
Of 103 neonates with surgical NEC, 60 (58%) had severe AKI. Those with severe AKI had lower birth weight (BW; 715 vs. 950g; p = .023), more frequently treated with indomethacin (18.3 vs. 2.4%); p = .014), higher CRP levels at 24 h after NEC onset (14.4 [6.4–19.8] vs. 4.8 [1.6–13.4]; p = .005), higher presence of cholestasis (73.3 vs. 51.2%); p = .023), later age of NEC onset (14 vs. 7d); p = .004), longer length of bowel resected (14.9 vs. 4.3 cm); p = .011), longer post-operative ileus days (14 vs. 9d); p < .001), longer post-operative days at starting enteral feedings (15 vs. 10 d; p < .001), longer days of attainment of full enteral feedings (75 vs. 44.5 d; p = .008) and longer length of stay (140.5 vs. 94 d; p = .028) compared to those without severe AKI. Compared to infants without AKI by serum creatinine, those with AKI had significantly more cases of white matter abnormality (WMA; 90 vs. 36.6%; p < .001) and retinopathy of prematurity (63.9 vs. 35.3%; p = .017). In addition, the presence of AKI Stage 2 and 3 by serum creatinine was independently associated with higher odds of sustaining severe WMI level on an ordinal scale (OR = 6.2; 95% CI = (1.1–35.5); p = .041).
Conclusions:
Neonates with severe AKI following surgical NEC were more likely to experience longer post-operative morbidity and higher WMI by MRI at term.
Keywords: Acute kidney injury, brain injury, postoperative outcomes, preterm infants
Introduction
Necrotizing enterocolitis (NEC) affects 6–10% of preterm infants with a birth weight (BW) less than 1500 g [1,2]. NEC remains a leading cause of death among preterm neonates and leads to increased hospital care and economic burden [3-9]. NEC is one of the most common conditions requiring emergency surgery in a neonatal intensive unit. NEC is associated with a septic shock-like clinical picture and severe systemic inflammatory response that can contribute to multi-organ dysfunction due to intravascular volume depletion, capillary leak syndrome, and hypotension [10]. These hemodynamic changes, surgical stresses, anesthetic agents, and nephrotoxic medications are associated with acute kidney injury (AKI), especially in premature neonates [11]. The impact of NEC on underdeveloped premature kidneys has not been addressed. Animal models of AKI (the cecal ligation and puncture model) and an NEC mouse study have demonstrated that NEC is associated with widespread kidney inflammation [12,13].
AKI affects approximately 30% of neonates admitted to the NICU. They require intravenous fluids and are associated with poor clinical outcomes such as prolonged stay and increased mortality in premature and term neonates [14,15]. Preterm infants with surgical NEC have been shown to have greater pro-inflammatory markers in the serum, severe brain injury, and poor neurodevelopmental outcomes at two years follow-up [16-20]. Many cohort and randomized trials report AKI predicts clinical outcomes in neonatal and pediatric populations with congenital cardiac disease undergoing surgery and cardiopulmonary bypass [21-23]. Following surgical NEC diagnosis, the risk factors for AKI, such as sepsis, hypotension, and nephrotoxic agents, have been previously reported [13,24-30]. Our previous retrospective observational cohort study reported the demographics and clinical outcomes associated with severe AKI in preterm infants with NEC [31] at our institution between 2013 and 2018.
In this report, we have examined the association of severe AKI with other important markers of neonatal morbidity in surgical NEC patients. Compared to prior reports from our group and others, we have carried out a more comprehensive evaluation of a post-operative clinical course and severity of brain abnormality differences between premature surgical NEC neonates with and without severe AKI. This evaluation includes detailed multivariable models assessing whether the presence of AKI in surgical NEC neonates was independently associated with worse post-operative and brain outcomes. Our primary hypotheses were that severe AKI would be independently associated with the prolonged post-operative recovery and severe white matter abnormalities on term equivalent brain MRI, accounting for other known and unknown predictors of adverse outcomes in neonates suffering from surgical NEC.
Methods
Population and study design
The study was conducted at the University of Mississippi Medical Center (UMMC) Neonatal Intensive Care Unit, a Level IV unit with approximately 900–100 admissions yearly and referrals from the entire state. The UMMC Institutional Review Board has approved this retrospective cohort study, with a waiver of informed parental consent to collect clinical data. All infants admitted between January 2013 and 31 December 2018, with an NEC diagnosis (Bell Stage 3) were included in the study [32]. Neonates diagnosed with medical NEC, kidney anomalies, congenital heart disease, spontaneous intestinal perforation, and intestinal atresia were excluded from the analysis.
Demographic and clinical information
We collected demographic data, including BW, gestational age (GA), appropriate for gestational age status (AGA), race, and sex, mode of delivery, outborn status, and Apgar score ≤6 at 5 min. We also gathered maternal information, including chorioamnionitis, antenatal steroids, and pregnancy-induced hypertension (PIH). Additional clinical data included patent ductus arteriosus (PDA), frequency of PDA surgical ligation, duration of mechanical ventilation, inotrope (dopamine) use 24 h after NEC onset, and ibuprofen/indomethacin treatment (before NEC). Our unit does not use Indocin for IVH prophylaxis. Sepsis-related variables included blood culture-proven sepsis at NEC onset and duration/type of antibiotics. We also collected information on the frequency of cholestasis (direct bilirubin >2 mg/dL) at any time after NEC diagnosis.
NEC information
NEC was defined using Bell’s criteria [32], and diagnosis of NEC was made on abdominal X-ray findings, including portal venous gas, pneumatosis, and pneumoperitoneum. The frequency of Bell stage III/surgical NEC was gathered [32]. In addition, we recorded information on the age of NEC diagnosis and the fulminant NEC [33]. Our surgical care and team for managing the NEC remained without much significant change throughout the study duration. We also recorded information on short bowel syndrome (parenteral nutrition >90 d) and surgical morbidity. The surgical morbidity was classified as strictures, fistulas, wound dehiscence, surgical site infections (including abscesses), adhesions, and perforations.
Kidney function data
The Modified Neonatal Staging Criteria described in the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for AKI was used to determine the incidence of kidney injury [11,14,34-36]. We examined all SCr measurements and daily UOP the day before NEC diagnosis, at NEC onset, 24, 48, 72, 96 h, and 7 d following NEC diagnosis. Baseline SCr was defined by the lowest SCr documented in the patient’s clinical record before NEC onset.
Stage 1, AKI was defined as a rise in SCr by 0.3 mg/dL or 1.5–1.9 times above baseline and/or UOP <1 mL/kg/h over the last 24 h. Stage 2, AKI was defined as an increase in SCr 2–2.9 times above baseline and/or UOP <0.5 mL/kg/h. Stage 3, AKI was defined as an increase in SCr 3 times above baseline or SCr >2.5 mg/dL and/or and UOP <0.3 mL/kg/h [36]. The maximum stage AKI was defined by the highest SCr or UOP AKI stage within 4 d after NEC onset. At our center, laboratories measure SCr using the isotope dilution mass spectrometry method. The weighed diapers recorded by bedside nurses in the patient chart were used to collect UOP data. We stratified infants as with severe (Stage 2 and 3 AKI) and without severe (no AKI or Stage 1 AKI) as has been done in previous clinical studies [14,37].
Outcome data
Post-operative information, such as post-operative ileus days (defined as infants being NPO after bowel surgery), time to reach full feeds (≥120 mL/kg/d), total parenteral nutrition days, length of stay, and hospital mortality were measured. We defined mortality as death due to any reason prior to hospital discharge.
Neonatal MRI data
Brain MRI without contrasts was routinely obtained at corrected age of 36 weeks, or before discharge, whenever clinically indicated, in all infants with BW less than 1500 g per routine hospital clinical practice. The most common reason for not obtaining MRI was death or transfer to a higher center for bowel transplantation. All term equivalent age (TEA) MRI scans were scored independently by two pediatric neuro-radiologists aware of the infants’ clinical diagnosis, however unware of the initial reported clinical read of Brain MRI findings. We used a scoring system consisting of eight scales for white and gray matter injury developed by Woodward et al. [17]. An objective visual scoring system was used instead of automated software, such as FreeSurfer, which requires acquisition of 3D isovoxel volumetric MRI sequences of the brain. The majority of the brain MRIs performed in this group of patients were 2D non-isovoxel sequences tailored to a faster brain MRI protocol that is used in non-sedated infants. An analysis of any disparities between the initial clinical read and radiologist scoring of white matter loss, gray matter injury, and cerebellar injury was not performed.
We recorded data on cranial ultrasound (CUS) findings from the patient’s clinical record before and after NEC onset. The information was collected on the presence and extent of white-matter echolucency or cystic periventricular leukomalacia, ventriculomegaly, and the highest grade of intraventricular hemorrhage before and after the NEC onset.
Neurodevelopment assessment at two years of age
All infants are routinely offered a comprehensive neurodevelopmental evaluation. Specialists use the Bayley Scales of Infant Development (BSID-III) for all neurodevelopmental assessments. In addition, we recorded psychomotor and cognitive development assessment scores from the clinical record [17].
Statistical methods
We summarized the data as mean and standard deviation (±SD) for parametric continuous variables. Comparisons between infants with severe AKI and without severe AKI were performed using Student’s t-test, Welch’s t-test, and/or ANOVA depending on equality of the variances. For data not normally distributed, median with interquartile range (IQR) [1st quartile; 3rd quartile] estimates were calculated with group differences tested using Kruskal–Wallis test or Mann–Whitney U test. Categorical data were summarized as counts with frequencies as percentages, and group differences were tested using the Chi-squared test or Fisher’s exact test [38]. Association between serum creatinine and urine output criteria for AKI was assessed with the Jonckheere–Terpstra test of ordinal trend and the weighted kappa measure for agreement [39]. Cumulative and multivariable logistic regression was performed to evaluate whether AKI by serum creatinine was an independent risk factor for WMA. For cumulative logistic regression, probabilities modeled are cumulated over the lower ordered values. The odds ratio for continuous variables are expressed per standard deviation of that factor. A p value < .05 was considered significant. The statistical tests were performed in SAS version 9.4 (SAS Institute, Cary, NC).
Results
Cohort characteristics
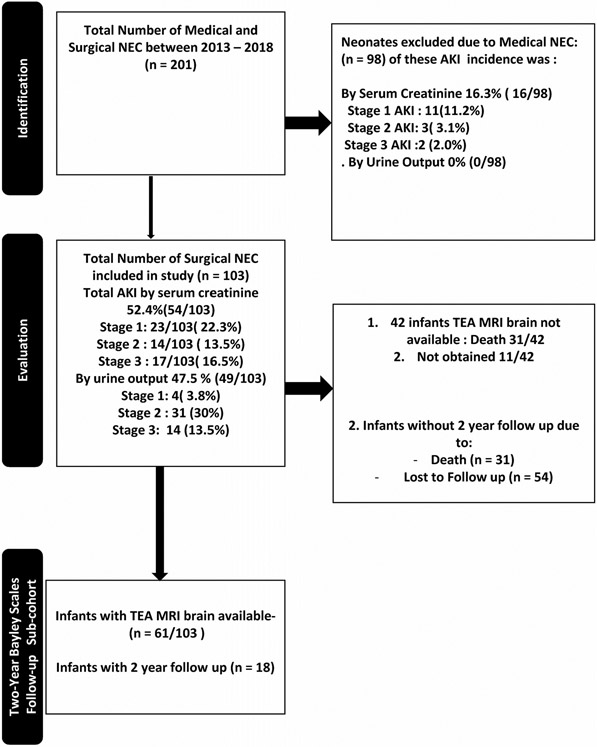
Of the 227 infants evaluated with possible NEC diagnosis, 103 met the inclusion criteria of surgical NEC, while 124 were excluded due to medical NEC or did not meet other inclusion criteria (see Figure 1). The cohort of 103 neonates with surgical NEC had a median (IQR) GA of 26.4 (24.3 – 29.6) weeks, median BW of 780 (650 – 1180) g, was predominantly male (68/103, 66%), African American (77/103, 74.8%), and outborn (63/103, 61.2%). The remaining demographic information is summarized in Table 1.
Figure 1.
Flow diagram showing the patient enrollment.
Table 1.
Demographic and clinical features in neonates with Stage 2 and 3 AKI by both serum creatinine and urine output.
| N | Total N = 103 |
Severe AKI N = 60 |
Non-severe AKI N = 43 |
p Value | |
|---|---|---|---|---|---|
| Prenatal information | |||||
| Pregnancy-Induced Hypertension, n (%) | 103 | 29 (28.2%) | 18 (30%) | 11 (25.6%) | .623 |
| Chronic Hypertension, n (%) | 84 | 15 (17.9%) | 8 (15.4%) | 7 (21.9%) | .451 |
| Chorioamnionitis, n (%) | 102 | 10 (9.8%) | 7 (11.9%) | 3 (7%) | .513 |
| Antenatal Steroids, n (%) | 101 | 73 (72.3%) | 45 (76.3%) | 28 (66.7%) | .288 |
| Infant demographics | |||||
| Gestational age (weeks), median (IQR) | 103 | 26.4 (24.3, 29.6) | 25.4 (24.3, 27.8) | 27 (25, 32) | .069 |
| Birth weight (grams), median (IQR) | 103 | 780 (650, 1180) | 715 (625, 990) | 950 (690, 1600) | .023 |
| Small for gestational age, n (%) | 103 | 35 (34%) | 21 (35.0%) | 14 (32.6%) | .796 |
| Gender (female), n (%) | 103 | 36 (35%) | 19 (31.7%) | 17 (39.5%) | .409 |
| Ethnicity, n (%) | 103 | .029 | |||
| Caucasian | 22 (21.4%) | 9 (15%) | 13 (30.2%) | ||
| African American | 77 (74.8%) | 49 (81.7%) | 28 (65.1%) | ||
| Latino | 2 (2%) | 0 (0%) | 2 (4.7%) | ||
| Other | 2 (2%) | 2 (3.3%) | 0 (0%) | ||
| Mode of delivery, n (%) | 103 | .934 | |||
| C-section | 69 (67%) | 40 (66.7%) | 29 (67.4%) | ||
| Vaginal | 34 (33%) | 20 (33.3%) | 14 (32.6%) | ||
| Apgar Score < 6 at 5 min, n (%) | 101 | 30 (29.7%) | 21 (35.6%) | 9 (21.4%) | .125 |
| Outborn, n (%) | 103 | 63 (61.2%) | 40 (66.7%) | 23 (53.5%) | .176 |
| Infant medical Information prior to NEC | |||||
| Patent ductus arteriosus, n (%) | 103 | 59 (57.3%) | 33 (55.0%) | 26 (60.5%) | .58 |
| Patent ductus arteriosus, indomethacin treated, n (%) | 102 | 12 (11.8%) | 11 (18.3%) | 1 (2.4%) | .014 |
| Patent ductus arteriosus, surgically ligated, n (%) | 103 | 5 (4.9%) | 4 (6.7%) | 1 (2.3%) | .397 |
| Sepsis variables | |||||
| Central line present (days), median (IQR) | 93 | 50 (29, 77) | 57 (36.5, 86.5) | 38 (24, 65) | .067 |
| Positive blood culture sepsis, n (%) | 103 | 32 (31.1%) | 23 (38.3%) | 9 (20.9%) | 0.06 |
| CRP on day of NEC onset, median (IQR) | 89 | 4 (1.2, 8) | 5.05 (1.9, 8.5) | 2.6 (0.9, 6.7) | .153 |
| CRP at 24 h after NEC onset, median (IQR) | 80 | 8.5 (3.1, 19.4) | 14.4 (6.4, 19.8) | 4.8 (1.6, 13.4) | .005 |
| CRP at 48 h after NEC onset, median (IQR) | 66 | 14.9 (3.3, 21.9) | 15.7 (6.7, 24.5) | 4.4 (2.4, 19.8) | .012 |
| CRP at 96 h after NEC onset, median (IQR) | 69 | 7.3 (4.1, 15.6) | 7.6 (4.6, 15.5) | 4.9 (2.6, 16) | .27 |
| CRP at 1 week after NEC onset, median (IQR) | 67 | 5.3 (2.9, 8.3) | 5.2 (2.9, 8.6) | 5.3 (2.9, 7.5) | .958 |
| CRP at 2 weeks after NEC Onset, median (IQR) | 64 | 3.4 (1.8, 5.8) | 4 (2.4, 5.8) | 2.8 (1.4, 5.4) | .103 |
| Cholestasis at NEC Onset, n (%) | 101 | 65 (64.4%) | 44 (73.3%) | 21 (51.2%) | .023 |
NEC: necrotizing enterocolitis; AKI: acute kidney injury; CRP: C-reactive protein
Categorical variables are presented as count (column percentage). Continuous variables are presented as mean (standard deviation) and, if not normally distributed, as median with interquartile range. Continuous measures’ statistical associations with AKI were evaluated with the Kruskal–Wallis test. Differences in categorical measures’ associations were tested using the Chi-square test when expected cell counts were adequate, otherwise Fisher’s exact were used with low expected cell counts.
The bold value signifies the p value is less than 0.05.
NEC data
The median (IQR) NEC onset age was 10 (5 – 23) d. Eighteen neonates (17.5%) had fulminant NEC. The abdominal x-ray showed pneumatosis in 48/103 (46.6%), pneumoperitoneum 55/103 (53.4%), and portal venous gas in 8/103 (7.8%) cases. Forty-one cases were treated with a Penrose drain, and 71/103 (68.9%) required laparotomy in less than 48 h after the NEC onset. The median length of bowel resected was 12 (3.2 – 28.8) cm. Of these, 43/86 (50%) later developed short bowel syndrome. At NEC onset, 88/103 (88%) required assisted ventilation, and 80/103 (77.7%) required inotropic support with dopamine within 24 h of presentation. Among the cases of surgical NEC, 59/103 (57.3%) neonates had PDA, in whom 12/102 (11.8%) had received treatment with cyclooxygenase (COX) inhibitors (indomethacin/ibuprofen) before NEC onset. Blood culture-positive sepsis at the time of NEC onset was seen in 32/103 (31.1%) cases and required antibiotics for a mean time of 9.0 ± 4.8 d. Additional post-operative and clinical characteristics among the patients of NEC are summarized in Tables 1, 3, and 4.
Table 3.
Features in neonates with Stage 2 and 3 AKI by both serum creatinine and urine output.
| Total cohort n = 103 |
Severe AKI n = 60 |
Non-severe AKI n = 43 |
p Value | ||
|---|---|---|---|---|---|
| Clinical presentation, n (%) | 103 | .036 | |||
| Abdominal distension | 92 (89.3%) | 57 (95%) | 35 (81.4%) | – | |
| Bloody stools | 8 (7.8%) | 3 (5%) | 5 (11.6%) | – | |
| Feeding intolerance | 3 (2.9%) | 0 (0%) | 3 (7%) | – | |
| Radiological findings, n (%) | 103 | – | – | – | – |
| Pneumatosis | 48 (46.6%) | 31 (51.7%) | 17 (39.5%) | .224 | |
| Pneumoperitoneum | 55 (53.4%) | 27 (45%) | 28 (65.1%) | .044 | |
| Portal venous gas | 8 (7.8%) | 4 (6.7%) | 4 (9.3%) | .717 | |
| Age of NEC onset (days), median (IQR) | 103 | 10 (5, 23) | 14 (8, 23) | 7 (4, 13) | .004 |
| Fulminant NEC, n (%) | 103 | 19 (18.5%) | 9 (15%) | 10 (23.3%) | .287 |
| Present of penrose drain, n (%) | 98 | 41 (41.8%) | 20 (34.5%) | 21 (52.5%) | .076 |
| Surgery < 48 h, n (%) | 103 | 71 (68.9%) | 43 (71.7%) | 28 (65.1%) | .479 |
| Length of bowel resected (cm), median (IQR) | 101 | 12 (3.2, 28.8) | 14.9 (8, 32) | 4.25 (2, 27.2) | .011 |
| Region of bowel resected, n (%) | 91 | .040 | |||
| Small bowel resected | 58 (63.7%) | 38 (66.7%) | 20 (58.8%) | – | |
| Large bowel resected | 4 (4.4%) | 0 (0%) | 4 (11.8%) | – | |
| Combined large and Small bowel resected | 29 (31.9%) | 19 (33.3%) | 10 (29.4%) | – | |
| Presence of Ileocecal valve, n (%) | 102 | 73 (71.6%) | 39 (65%) | 34 (81%) | .079 |
| Surgical morbidity (infection, adhesions, strictures, dehiscence), n (%) | 103 | 39 (37.9%) | 25 (41.7%) | 14 (32.6%) | .347 |
| Single surgical morbidity (infection, adhesions, strictures, dehiscence), n (%) | 103 | 23 (22.3%) | 15 (25%) | 8 (18.6%) | .442 |
| More than one surgical morbidity (Infection, adhesions, strictures, dehiscence), n (%) | 103 | 13 (12.6%) | 8 (13.3%) | 5 (11.6%) | .797 |
| Adhesions, n (%) | 103 | 16 (15.5%) | 10 (16.7%) | 6 (14%) | .708 |
| Wound dehiscence, n (%) | 103 | 16 (15.5%) | 11 (18.3%) | 5 (11.6%) | .354 |
| Wound infection, n (%) | 103 | 7 (6.8%) | 6 (10%) | 1 (2.3%) | .234 |
| Stricture, n (%) | 103 | 8 (7.8%) | 5 (8.3%) | 3 (7%) | 1 |
| Fistula, n (%) | 103 | 6 (5.8%) | 5 (8.3%) | 1 (2.3%) | .397 |
| Compartment syndrome, n (%) | 103 | 2 (1.9%) | 1 (1.7%) | 1 (2.3%) | 1 |
| Short bowel syndrome, n (%) | 86 | 43 (50%) | 30 (55.6%) | 13 (40.6%) | .181 |
NEC: necrotizing enterocolitis; AKI: acute kidney injury
Categorical variables are presented as count (column percentage). Continuous variables are presented as mean (standard deviation) and, if not normally distributed, as median with interquartile range. Continuous measures’ statistical associations with AKI were evaluated with the Kruskal–Wallis test. Differences in categorical measures’ associations were tested using the Chi-square test when expected cell counts were adequate; otherwise, Fisher’s Exact was used with low expected cell counts.
The bold value signifies the p value is less than 0.05.
Table 4.
Postoperative features and outcomes in neonates with Stage 2 and 3 AKI by both serum creatinine and UOP.
| Total N = 103 |
Severe AKI N = 60 |
Non-severe AKI N = 43 |
p Value | ||
|---|---|---|---|---|---|
| Post-operative features and outcomes | |||||
| Post-operative Ileus days (days), median (IQR) | 84 | 13 (9, 17.5) | 14 (12, 20) | 9 (7, 14) | <.001 |
| Post-operative day at starting enteral feedings (days), median (IQR) | 83 | 14 (10, 19) | 15 (13, 23) | 10 (8, 15) | <.001 |
| Day of attainment of full enteral feedings (120 mL/kg), median (IQR) | 69 | 65 (32, 89) | 75 (43, 109) | 44.5 (26, 68.5) | .008 |
| Duration of parenteral nutrition (days), median (IQR) | 102 | 83 (50, 125) | 99 (56.5, 140) | 70 (47, 114) | .078 |
| Breast milk, n (%) | 19 (18.5%) | 4 (6.7%) | 15 (34.9%) | <.001 | |
| Donor milk, n (%) | 103 | 23 (22.3%) | 17 (28.3%) | 6 (14%) | .084 |
| Formula feeds, n (%) | 103 | 52 (50.5%) | 31 (51.7%) | 21 (48.8%) | .777 |
| Breast milk and formula feeds, n (%) | 103 | 17 (16.5%) | 13 (21.7%) | 4 (9.3%) | .096 |
| Post-operative systemic course | |||||
| Assisted ventilation (intubated), n (%) | 100 | 88 (88%) | 55 (94.8%) | 33 (78.6%) | .027 |
| Intubated/invasive ventilation | |||||
| Non-invasive | 7 (7%) | 3 (5.2%) | 4 (9.5%) | – | |
| High flow | 3 (3%) | 0 (0%) | 3 (7.1%) | – | |
| CPAP | 2 (2%) | 0 (0%) | 2 (4.8%) | – | |
| 24 h Presser support, n (%) | 80 (77.7%) | 53 (88.3%) | 27 (62.8%) | .002 | |
| Postnatal use of steroids, n (%) | 60 (58.3%) | 37 (61.7%) | 23 (53.5%) | .407 | |
| Discharge | |||||
| Length of stay (days), median (IQR) | 103 | 121 (72, 177) | 140.5 (88, 180) | 94 (55, 161) | .028 |
| Death, n (%) | 103 | 32 (31.1%) | 19 (31.7%) | 13 (30.2%) | .877 |
NEC: necrotizing enterocolitis; AKI: acute kidney injury
Categorical variables are presented as count (column percentage). Continuous variables are presented as mean (standard deviation) and, if not normally distributed, as median with interquartile range. When the normality assumption was not satisfied, continuous measures’ statistical associations with the Kruskal–Wallis Test. Differences in categorical measures’ associations were tested using the Chi-square test when cell counts were adequate; otherwise, Fisher’s Exact was used with low expected cell counts.
The bold value signifies the p value is less than 0.05.
Acute kidney injury
Of the 103 neonates with surgical NEC, 77/103(74.8%) had AKI by KIDGO criteria. Twenty-six (25.2%) had no AKI, 23/103 (22.3%) had stage 1, 31/103 (30.1%) had Stage 2, and 17/103 (16.5%) had AKI Stage 3 by either highest UOP or SCr staging criteria. The severe AKI (Stage 2 and 3) occurred in 60/103 (58.3%) while 43/103 (41.7%) had Stage 0 and 1 AKI (non-severe). Among those with severe AKI, 15 had AKI by SCr alone, 29 had AKI by UOP only, and 16 had AKI by SCr and UOP (Table 2).
Table 2.
AKI stages by serum creatinine and urine output using KDIGO classification.
| AKI by urine output | ||||||
|---|---|---|---|---|---|---|
| State 0 | Stage 1 | Stage 2 | Stage 3 | Total | ||
| AKI by creatinine | State 0 | 26 53.1% |
3 6.1% |
16 32.7% |
4 8.2% |
49 |
| Stage 1 | 14 60.9% |
0 0 |
8 34.8% |
1 4.4% |
23 | |
| Stage 2 | 5 35.7% |
0 0 |
4 28.6% |
5 35.7% |
14 | |
| Stage 3 | 9 52.9% |
1 5.9% |
3 17.7% |
4 23.5% |
17 | |
| Total | 54 | 4 | 31 | 14 | 103 | |
Frequency and row percent are presented. Shadow area – infants with severe AKI (Stage 2 and 3).
Table 1 also compares baseline demographic characteristics and clinical factors between participants defined with vs. without severe AKI by UOP and/or SCr criteria. Comparing those without severe AKI, infants with severe AKI had significantly lower median (IQR) BW (715 g [625–990] vs. 950 g [690–1600]; p = .023), received Indomethacin more frequently (18 vs. 2.38%; p = .014), more cholestasis (direct bilirubin >2mg/dL) at NEC onset (73.33 vs. 51.22%; p = .023), higher level of CRP at 24 h after NEC onset (14.4 [6.4–19.8] vs. 4.8 [1.6–13.4]; p = .005) and higher level of CRP at 48 h after NEC onset (15.7 [6.65–24.5] vs. 4.4 [2.4–19.8]; p = .012).
Those with severe AKI had older age onset (14 [8-23] vs. 7d [4-13]; p = .004), lower presence of pneumoperitoneum (45 vs. 65%; p = .044) and longer length resected bowel (14.9 cm [8-32] vs. 4.25 cm [2–27.2]; p = .011). Small bowel resection was significantly more frequent (66.7 vs. 58.8%; p = .04) in those with severe AKI. The other NEC features are summarized in Table 3.
Post-operative outcomes
Neonates with severe AKI had a significantly longer period of post-operative ileus (14 d [12-20] vs. 9d [7-14]; p < .001), took a long time to reach full feeds (75 d [43–109] vs. 44.5 d [26–68.5]; p = .008) and had a longer post-operative day at starting enteral feedings (15 d [13-23] vs. 10 d [8-15]; p < .001) than to those without severe AKI. Any mechanical ventilation (94.8 vs. 78.6%; p = .027) and pressor support at 24 h (88.3 vs. 62.8%; p = .002), and length of hospitalization (141 d [88–180] vs. 94 d [55–161]; p = .028) were all significantly higher neonates with severe AKI compared to those without severe AKI. The additional post-operative outcomes are summarized in Table 4.
Brain injury and AKI
In our cohort, 61 infants had brain MRI results available for analysis. Of the 61 infants, 33/61 (54.1%) had some white matter abnormality (WMA) (15/61 (24.6%) had mild WMA, 12/61 (19.7%) had moderate WMA, and 6/61 (9.8%) had severe WMA on term equivalent brain MRI). The infants with severe AKI had significantly more cases of WMA (90 vs. 36.6%; p < .001) and retinopathy of prematurity (63.9 vs. 35.3%; p = .017) than those without severe AKI by serum creatinine. The findings are summarized in Table 5.
Table 5.
Neurodevelopment outcomes in neonates with Stage 1–3 AKI by serum creatinine.
| AKI by serum creatinine | |||||
|---|---|---|---|---|---|
| Variable | Total cohort n = 103 |
AKI present n = 54 |
AKI absent n = 49 |
p Value | |
| MRI corrected GA, median (IQR) | 58 | 40.5 (38.3, 46.1) | 40.4 (38.1, 46.1) | 40.6 (38.3, 48.2) | .821 |
| White matter injury present, n (%) | 61 | 33 (54.1%) | 18 (90.0%) | 15 (36.6%) | <.001 |
| White matter injury abnormality, n (%) | 61 | – | – | – | <.001 |
| No abnormality (5–6) | – | 28 (45.9%) | 2 (10.0%) | 26 (63.4%) | – |
| Mild (7–9) | – | 15 (24.6%) | 6 (30.0%) | 9 (22.0%) | – |
| Moderate (10–12) | – | 12 (19.7%) | 9 (45.0%) | 3 (7.3%) | – |
| Severe (13–15) | – | 6 (9.8%) | 3 (15.0%) | 3 (7.3%) | – |
| Grey matter abnormality Composite score, n (%) | 60 | .432 | |||
| Normal (3–5) | – | 53 (88.3%) | 27 (84.4%) | 26 (92.9%) | – |
| Abnormal (6–9) | – | 7 (11.7%) | 5 (15.6%) | 2 (7.1%) | – |
| Cerebellar injury, n (%) | 57 | 19 (33.3%) | 12 (40%) | 7 (25.9%) | .260 |
| ROP, n (%) | 70 | 35 (50%) | 23 (63.9%) | 12 (35.3%) | .017 |
| Long-term eye complications, n (%) | 27 | .778 | |||
| Myopia | – | 10 (37%) | 7 (41.2%) | 3 (30%) | – |
| Hyperopia | – | 11 (40.7%) | 7 (41.2%) | 4 (40%) | – |
| Strabismus | – | 6 (22.2%) | 3 (17.7%) | 3 (30%) | – |
AKI: acute kidney injury.
Categorical variables are presented as count (column percentage). Continuous variables are presented as mean (standard deviation) and, if not normally distributed, as median with interquartile range. Continuous measures’ statistical associations with AKI were evaluated with the Kruskal–Wallis test. Differences in categorical measures’ associations were tested using the Chi-square test when expected cell counts were adequate; otherwise, Fisher’s Exact was used with low expected cell counts.
The bold value signifies the p value is less than 0.05.
The neonates with severe AKI by serum creatinine had significantly more grade 2 loss of periventricular volume (65 vs. 30%; p < .001), grade 2 ventricular dilatation (65 vs. 25%; p = .001), grade 2 thinning of Corpus Callosum (65 vs. 27.5%; p = .004) compared to neonates without AKI. In addition, seven infants (7/60, 11.7%) had grey matter abnormalities. These infants mainly showed loss of subarachnoid space grade 1 (47/60, 78.3%), grey matter signal abnormality grade 1 (51/60, 85%), and grade one gyral maturation changes (52/60, 86.7%). The data has been summarized in Table 6.
Table 6.
Brain MRI findings in neonates with surgical NEC based on Stage 2 and 3 AKI by serum creatinine.
| Total cohort n = 103 |
Severe AKI N = 31 |
Non-severe AKI N = 72 |
p Value | ||
|---|---|---|---|---|---|
| MRI corrected GA, median (IQR) | 58 | 40.5 (38.3, 46.1) | 39.2 (37.3, 49.1) | 40.6 (39.1, 45.3) | .426 |
| White matter injury present, n (%) | 61 | 33 (54.1%) | 18 (90%) | 15 (36.6%) | <.001 |
| White Matter Injury Abnormality, n (%) | 61 | – | – | – | <.001 |
| No abnormality (5–6) | – | 28 (45.9%) | 2 (10%) | 26 (63.4%) | – |
| Mild (7–9) | – | 15 (24.6%) | 6 (30%) | 9 (22.0%) | – |
| Moderate (10–12) | – | 12 (19.7%) | 9 (45%) | 3 (7.3%) | – |
| Severe (13–15) | – | 6 (9.8%) | 3 (15%) | 3 (7.3%) | – |
| Grade of white matter signal abnormality nature and extent, n (%) | 60 | – | – | – | <.001 |
| Normal | – | 35 (58.3%) | 4 (20%) | 31 (77.5%) | – |
| Mild | – | 12 (20%) | 7 (35%) | 5 (12.5%) | – |
| Moderate | – | 13 (21.7%) | 9 (45%) | 4 (10%) | – |
| Loss in volume of periventricular white matter, n (%) | 60 | – | – | – | <.001 |
| Grade 1 | – | 29 (48.3%) | 3 (15%) | 26 (65%) | – |
| Grade 2 | – | 25 (41.7%) | 13 (65%) | 12 (30%) | – |
| Grade 3 | – | 6 (10%) | 4 (20%) | 2 (5%) | – |
| Extent of cystic abnormality, n (%) | 60 | – | – | – | .039 |
| Grade 1 | – | 45 (75%) | 11 (55%) | 34 (85%) | – |
| Grade 2 | – | 10 (16.7%) | 6 (30%) | 4 (10%) | – |
| Grade 3 | – | 5 (8.3%) | 3 (15%) | 2 (5%) | – |
| Ventricular dilation, n (%) | 60 | .001 | |||
| Grade 1 | – | 29 (48.3%) | 3 (15%) | 26 (65%) | – |
| Grade 2 | – | 23 (38.3%) | 13 (65%) | 10 (25%) | – |
| Grade 3 | – | 8 (13.3%) | 4 (20%) | 4 (10%) | – |
| Thinning of corpus callosum, n (%) | 60 | .004 | |||
| Grade 1 | – | 29 (48.3%) | 4 (20%) | 25 (62.5%) | – |
| Grade 2 | – | 24 (40%) | 13 (65%) | 11 (27.5%) | – |
| Grade 3 | – | 7 (11.7%) | 3 (15%) | 4 (10%) | – |
| Grey matter abnormality composite score, n (%) | 60 | .208 | |||
| Normal (3–5) | – | 53 (88.3%) | 16 (80%) | 37 (92.5%) | – |
| Abnormal (6–9) | – | 7 (11.7%) | 4 (20%) | 3 (7.5%) | – |
| Extent of grey matter signal abnormality, n (%) | 59 | – | – | – | .362 |
| Grade 1 | – | 51 (86.4%) | 16 (80%) | 35 (89.7%) | – |
| Grade 2 | – | 6 (10.2%) | 3 (15%) | 3 (7.7%) | – |
| Grade 3 | – | 2 (3.4%) | 1 (5%) | 1 (2.6%) | – |
| Quality of gyral maturation, n (%) | 60 | – | – | – | .145 |
| Grade 1 | – | 52 (86.7%) | 15 (75%) | 37 (92.5%) | – |
| Grade 2 | – | 6 (10%) | 4 (20%) | 2 (5%) | – |
| Grade 3 | – | 2 (3.3%) | 1 (5%) | 1 (2.5%) | – |
| Size of subarachnoid space, n (%) | 59 | – | – | – | .753 |
| Grade 1 | – | 47 (79.7%) | 15 (75%) | 32 (82.1%) | – |
| Grade 2 | – | 10 (17%) | 4 (20%) | 6 (15.4%) | – |
| Grade 3 | – | 2 (3.4%) | 1 (5%) | 1 (2.6%) | – |
| Cerebellar injury, n (%) | 57 | 19 (33.3%) | 9 (50%) | 10 (25.6%) | .070 |
AKI: acute kidney injury.
Categorical variables are presented as count (column percentage). Continuous variables are presented as mean (standard deviation) and, if not normally distributed, as median with interquartile range. Continuous measures’ statistical associations with AKI are evaluated with the Kruskal–Wallis test. Differences in categorical measures’ associations were tested using the Chi-square test when expected cell counts were adequate; otherwise, Fisher’s exact test was used with low expected cell counts.
The bold value signifies the p value is less than 0.05.
Two-year neurodevelopment follow-up
Out of 103 patients, 18 infants had neurodevelopment follow-up in a high-risk clinic at two years of age. Among those 18 patients, no significant findings in neurodevelopment outcomes between AKI presence and AKI absent patients were found. The results are summarized in Supplemental Table 1.
AKI independent effect assessment
While holding all other variables constant, the presence of AKI Stage 2 and 3 by serum creatinine was independently associated with higher odds of sustaining a more severe ordinal grade of white matter injury (WMI; OR = 6.2; 95% CI = (1.1–35.5); p = .041). In contrast to the grade of WMI, AKI Stage 2 and 3 by serum creatinine was numerically but not independently significantly associated with the binary presence of any WMA (OR = 12.9; 95% CI = (0.7–241); p = .09) in this multivariable analysis. Similarly, AKI Stage 2 and 3 by serum creatinine was numerically but not independently significantly associated with the presence of binary grade 3 or 4 WMA vs. Grade 1 or 2 WMA (OR = 9.8; 95% CI = (0.8–116); p = .072. Detailed results are summarized in Table 7 and Appendix Q. For the subset of 28 with US data, AKI Stage 3 or 4 by serum creatinine remained a significant predictor for higher grades of WMBI (OR = 14.9 [95% CI: 2.3, 98.7], p = .005 adjusted for ordinal severity of IVH on head US.
Table 7.
Factors associated with white matter brain injury on multivariable logistic regression analysis.
| Variable | Factors associated with any white matter brain injury (present vs. absent) |
Factor associations with Gade 3 or 4 white matter brain injury (present vs. absent) |
Factor associations with white matter brain injury (ordinal levels) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p | Odds ratio | 95% CI | p | Odds ratio | 95% CI | p | ||||
| AKI by serum creatinine (Stage 2/3 vs. Stage 1 or normal) | 12.9 | 0.7 | 241 | 0.087 | 9.8 | 0.8 | 116 | 0.072 | 6.2 | 1.1 | 35.5 | .041 |
| Indomethacin use (vs. not use) | 0.5 | 0.1 | 12.9 | 0.706 | 2.1 | 0.1 | 46.2 | 0.633 | 0.6 | 0.1 | 4.9 | .666 |
| Birth weight (g) | 6.7 | 1.0 | 44.5 | 0.092 | 3.6 | 0.5 | 44.5 | 0.161 | 3.6 | 1.0 | 12.6 | .072 |
| Length of bowel resected (cm) | 0.5 | 0.06 | 4.8 | 0.562 | 0.6 | 0.1 | 4.8 | 0.588 | 0.4 | 0.1 | 1.8 | .251 |
| Post-operative days at starting enteral feedings (days) | 2.8 | 0.5 | 15.7 | 0.240 | 4.8 | 0.9 | 25.3 | 0.069 | 2.6 | 0.8 | 8.3 | .097 |
| Day of attainment of full enteral feedings (120 mL/kg) | 1.6 | 0.5 | 5.2 | 0.386 | 1.7 | 0.5 | 5.9 | 0.378 | 1.8 | 0.9 | 3.8 | .112 |
| 24 h Pressor support, n (%) | 35.8 | 0.7 | >999.9 | 0.073 | 5.0 | 0.1 | 210 | 0.396 | 15.9 | 1.1 | 231 | .043 |
| CRP at 24 h after NEC onset | 0.8 | 0.2 | 3.2 | 0.766 | 0.5 | 0.1 | 1.7 | 0.268 | 0.9 | 0.4 | 2.2 | .835 |
| Age of NEC onset (days) | 1.2 | 0.4 | 3.8 | 0.766 | 3.1 | 0.9 | 10.4 | 0.068 | 1.3 | 0.6 | 2.8 | .544 |
| Length of stay (days) | 4.9 | 0.7 | 35.6 | 0.100 | 3.5 | 0.5 | 25.7 | 0.213 | 3.3 | 1.0 | 9.6 | .042 |
Odds ratio for continuous variables are expressed per standard deviation of that factor.
The bold value signifies the p value is less than 0.05.
Discussion
Our study evaluated the association between AKI in patients with surgical NEC and the early post-operative outcomes, WMI outcomes, and neurodevelopmental outcomes at two years of age. We have previously shown that neonates with surgical NEC who had severe AKI had a significantly longer length of hospitalization. Those with severe AKI were outborn, received more antenatal steroids, and had blood culture-positive sepsis at NEC onset [31]. This report provides a broader understanding of the outcomes in those with severe AKI. Although this study did not show a statistically significant difference in the mortality rates between those with severe AKI vs. non-severe/no AKI (31.6 vs. 30.2%; p = .877), we document significant morbidity in the neonates with severe AKI. Most notably, within this cohort, severe AKI was significantly associated with treatment with indomethacin, higher CRP levels at 24 h after NEC onset, higher presence of cholestasis, higher age of NEC onset, a longer length of bowel resected, longer post-operative ileus days, more days before attainment of full enteral feedings, and longer of hospitalizations.
Our data also shows that severe AKI is significantly and independently associated with a more severe level of WMA (OR = 6.2; 95% CI = (1.1–35.5); p = .041). Consistent with this finding in direction and magnitude, severe AKI was numerically associated with any WMA (OR = 12.9; 95% CI = (0.7–241); p = .09) and severe Grade 3 or 4 WMA (OR = 9.8; 95% CI = (0.8–116); p = .072). While the numerical results from all three multivariable analyses are similar in direction and magnitude, the “loss of information” from collapsing ordinal information into binary categories and inherently limited single-center sample size/power (as evident from the wide odds ratio confidence intervals) for a relatively rare condition likely accounts for the lack of statistical significance in the binary vs. ordinal analyses.
Severe AKI (Stage 2 and 3) was observed in 58% of our cohort, which was higher than the incidence of KIDGO-defined AKI Stage 2 and 3 from the Assessment of Worldwide AKI Epidemiology in Neonates study (16%), one of the largest neonatal AKI study [14]. Previously published studies of neonates with NEC have estimated any AKI incidence of 39–54% primarily by serum creatinine criteria [28-30]. By KIDGO criteria, 75% had any AKI. While AKI by SCr criteria is similar in our surgical NEC cohort to these prior reported medical and surgical NEC cohorts, the addition of the KIDGO urine output criteria may increase the sensitivity to detect AKI.
In our cohort, infants with severe AKI had significant post-operative morbidities. These infants took four weeks longer to reach full enteral feeding and wean from parenteral nutrition. In addition, the infants with severe AKI had median post-operative ileus five days longer than other groups, which may be explained due to bowel wall edema due to decreased kidney function in the severe AKI group. However, we do not know much AKI is associated with long-term morbidities, such as time to reach full feeds or duration of parenteral nutrition. These long-term morbidities may be more related to intestinal factors, such as length of bowel loss after laparotomy. Unfortunately, there is no study reporting these morbidities in preterm infants undergoing abdominal surgery.
Several reports show that severe AKI is associated with prolonged hospitalization in other sick pediatric populations, even when adjusting for the disease severity [40,41]. In this cohort, we noted that severe AKI had 46 days longer NICU hospital stay (141 d [88–180] vs. 94 d [55–161]; p = .028).
In our study, infants with severe AKI by serum creatinine had higher rates of white matter abnormalities on term equivalent brain MRI (90 vs. 36.6%; p < .001). In the small sub-cohort available for longer-term assessment, significantly lower Bayley neurodevelopmental scores were observed at two years of corrected age. We noticed that periventricular volume loss and Grade 2-periventricular dilation were predominant lesions on brain MRI. In our cohort, AKI is significantly and independently associated with a more severe ordinal level of WMA [42]. These findings suggest AKI may be more than just a confounder or epiphenomenon merely associated with other clinical and demographic causal factors determining outcome in preterm infants with surgical NEC. Although there are several possible explanations for our findings, some of the possible reasons for our observed associations between AKI and brain injury result from both hypovolemic insults as well as systemic inflammation. Hypovolemia would result in AKI and ischemic brain injury from compromised perfusion. The AKI is likely not to be an isolated phenomenon but instead represents remote multi-organ dysfunction involving the lungs, heart, liver, brain, and intestine through inflammatory pathways involving increased oxidative stress, neutrophil migration, and cytokine expression shown in animal studies [43]. Several studies have shown that preterm infants with surgical NEC have severe WMI on the brain MRI, higher serum pro-inflammatory markers, and poor neurodevelopmental outcome at two years of corrected age [16-20]. Animal studies have reported surgical NEC leads to systemic inflammation and causes neuronal injury via microglial activation, inflammatory pathway activation, and brain barrier disruption [44-47]. Mechanistically, we hypothesize that severe AKI in neonates with surgical NEC may exacerbate the brain injury by acting as a catalyst or modifier of neuro-inflammation. Further studies are needed to understand the role of severe kidney injury with brain injury in neonates with NEC.
We observed severe brain injury in neonates with AKI by serum creatinine compared to AKI by urine output. Rapid recovery of urine output within 24 h after NEC onset may explain this observation, which needs further study. In contrast, higher serum creatinine indicates severe kidney injury, which takes longer to recover [31]. In our cohort, as in other published studies [36,48,49], there was no significant association or agreement between serum creatinine and urine output criteria for diagnosing AKI. The criteria for diagnosing AKI in neonates deserve further investigation in large multi-center studies.
The strengths of this study include a detailed description of the largest reported (to our knowledge) consecutive cohort of neonates with surgical NEC. This description includes contemporary measures of neonatal AKI, brain MRI findings, detailed post-operative course measures, laboratories, and 2-year Bayley neurodevelopmental outcomes in a small but important sub-cohort. We successfully captured the urine output data, which was not included in previously published reports. Additionally, severe AKI determined by serum creatinine was a more helpful predictor than AKI by urine output. Our data may help guide bedside management and risk stratification of patients with surgical NEC and counseling families for short-term and long-term outcomes.
Limitations include a single-center experience, perhaps reducing the study’s generalizability. In this Mississippi-based cohort, 75% of the neonates with surgical NEC were African American. While this is likely partly due to the demographics of Mississippi, this may also be related to adverse social determinants of health and/or genetic risk for kidney disease. We did not have complete data of pre-NEC head ultrasound. However, in the subset of surgical NEC neonates with complete US data, US status does not appear to be a significant confounder explaining the observed AKI and WMBI severity significant association. The diaper weights to reflect UOP also leads to measurement limitations. This may have affected the agreement between serum creatinine and urine output AKI definitions. We did not have anesthesia exposure data to evaluate its effect on the brain outcomes [50]. The retrospective study design lacks uniformity and completeness in each neonate’s available data, including incomplete follow-up after discharge. Finally, NEC is a relatively rare condition. While ours is a relatively high volume neonatal intensive care unit center, sample size also limits our power to detect associations between clinical factors, NEC, AKI, and outcomes. Further, multiple comparisons yield a higher probability of type I errors. While our multivariable analyses suggest otherwise, the significant and numerical associations observed between severe AKI had brain injury on the brain MRI may still plausibly arise from confounding with other known, unmeasured, and/or unknown factors.
Conclusion
In conclusion, neonates with severe AKI had significant post-operative morbidities and white matter abnormalities on the brain MRI compared to those without severe AKI. While severe AKI may plausibly be just a marker of severe illness remains, data and multivariable analyses from our surgical NEC experience credence to the alternative hypothesis that severe AKI contributes causally to greater morbidity and prolonged postoperative clinical course and slower recovery in neonates with surgical NEC.
Future direction
In the future, both retrospective and prospective studies (particularly multi-center to enhance sample size) with additional clinical data (e.g. end-organ (kidney and brain) perfusion by near-infrared spectroscopy [51]) and functional/tubular urinary and brain biomarkers, which may help providers predict AKI and brain injury at an early stage. Studies that evaluate kidney protective strategies to prevent AKI and neuroprotective strategies to prevent brain injury and its consequences are needed to improve the post-operative recovery clinical and neurodevelopmental outcomes in neonates with surgical NEC. There is a need to develop a scoring system including AKI to identify the high-risk neonates with advanced NEC and develop improved therapeutic options. These high-risk infants with severe AKI should be followed post-discharge with a pediatric nephrologist and developmental specialist to identify long-term sequela, including chronic kidney disease.
Supplementary Material
Funding
William B. Hillegass, MD, Ph.D. is partially supported by the IDeA grant U54GM115428. Dr. Parvesh Garg is partially supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number U54GM115428. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health and The Mississippi Center for Clinical and Translational Research for supporting NEC research and Mississippi IDeA Network of Biomedical Research Excellence.
Footnotes
Disclosure statement
The authors disclose no conflicts. All authors declare no real or perceived conflicts of interest that could affect the study design, collection, analysis, and interpretation of data, writing of the report, or the decision to submit for publication. PMG designed the study; PMG, AB, JP, DA collected and analyzed the data; MZ and WBH participated in the data analysis. PMG, DA, MZ, WBH, JFP, and KS wrote the article. All the authors approved the manuscript. For full disclosure, we provide here an additional list of other author’s commitments and funding sources that are not directly related to this study: David J Askenazi is a consultant for Baxter, Nuwellis, Medtronic Bioporto, the AKI Foundation, and SeaStar. In addition, he receives grant funding for education and studies not related to this project from Baxter, Nuwellis, and Medtronic. He has patents pending in the area of urine collection and kidney support therapy.
Supplemental data for this article is available online at https://doi.org/10.1080/14767058.2022.2121917
Informed consent
Patient consent was not required as per IRB.
References
- [1].Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364(3):255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sankaran K, Puckett B, Lee DS, et al. Variations in incidence of necrotizing enterocolitis in Canadian neonatal intensive care units. J Pediatr Gastroenterol Nutr. 2004;39:366–372. [DOI] [PubMed] [Google Scholar]
- [3].Sjoberg Bexelius T, Ahle M, Elfvin A, et al. Intestinal failure after necrotising enterocolitis: incidence and risk factors in a Swedish population-based longitudinal study. BMJ Paediatr Open. 2018;2(1):e000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Allin BSR, Long AM, Gupta A, et al. One-year outcomes following surgery for necrotising enterocolitis: a UK-wide cohort study. Arch Dis Child Fetal Neonatal Ed. 2018;103(5):F461–f466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Knell J, Han SM, Jaksic T, et al. Current status of necrotizing enterocolitis. Curr Probl Surg. 2019;56(1):11–38. [DOI] [PubMed] [Google Scholar]
- [6].Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314(10):1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Santulli TV, Schullinger JN, Heird WC, et al. Acute necrotizing enterocolitis in infancy: a review of 64 cases. Pediatrics. 1975;55(3):376–387. [PubMed] [Google Scholar]
- [8].Mowitz ME, Dukhovny D, Zupancic JAF. The cost of necrotizing enterocolitis in premature infants. Semin Fetal Neonatal Med. 2018;23(6):416–419. [DOI] [PubMed] [Google Scholar]
- [9].Ganapathy V, Hay JW, Kim JH, et al. Long term healthcare costs of infants who survived neonatal necrotizing enterocolitis: a retrospective longitudinal study among infants enrolled in Texas Medicaid. BMC Pediatr. 2013;13(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schwarz CE, Dempsey EM. Management of neonatal hypotension and shock. Semin Fetal Neonatal Med. 2020;25(5):101121. [DOI] [PubMed] [Google Scholar]
- [11].Jetton JG, Askenazi DJ. Acute kidney injury in the neonate. Clin Perinatol. 2014;41(3):487–502. [DOI] [PubMed] [Google Scholar]
- [12].Bao YW, Yuan Y, Chen JH, et al. Kidney disease models: tools to identify mechanisms and potential therapeutic targets. Zool Res. 2018;39(2):72–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Garg PM, Tatum R, Ravisankar S, et al. Necrotizing enterocolitis in a mouse model leads to widespread renal inflammation, acute kidney injury, and disruption of renal tight junction proteins. Pediatr Res. 2015;78(5):527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jetton JG, Boohaker LJ, Sethi SK, et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. 2017;1(3):184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Charlton JR, Boohaker L, Askenazi D, et al. Incidence and risk factors of early onset neonatal AKI. Clin J Am Soc Nephrol. 2019;14(2):184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hintz SR, Barnes PD, Bulas D, et al. Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics. 2015;135(1):e32–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Woodward LJ, Anderson PJ, Austin NC, et al. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355(7):685–694. [DOI] [PubMed] [Google Scholar]
- [18].Shin SH, Kim EK, Yoo H, et al. Surgical necrotizing enterocolitis versus spontaneous intestinal perforation in white matter injury on brain magnetic resonance imaging. Neonatology. 2016;110(2):148–154. [DOI] [PubMed] [Google Scholar]
- [19].Merhar SL, Ramos Y, Meinzen-Derr J, et al. Brain magnetic resonance imaging in infants with surgical necrotizing enterocolitis or spontaneous intestinal perforation versus medical necrotizing enterocolitis. J Pediatr. 2014;164(2):410–412.e411. [DOI] [PubMed] [Google Scholar]
- [20].Maheshwari A, Schelonka RL, Dimmitt RA, et al. Cytokines associated with necrotizing enterocolitis in extremely-low-birth-weight infants. Pediatr Res. 2014; 76(1):100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ueno K, Shiokawa N, Takahashi Y, et al. Kidney disease: improving global outcomes in neonates with acute kidney injury after cardiac surgery. Clin Exp Nephrol. 2020;24(2):167–173. [DOI] [PubMed] [Google Scholar]
- [22].Schroeder LW, Buckley JR, Stroud RE, et al. Plasma neutrophil Gelatinase-Associated lipocalin is associated with acute kidney injury and clinical outcomes in neonates undergoing cardiopulmonary bypass. Pediatr Crit Care Med. 2019;20:957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].MacDonald C, Norris C, Alton GY, et al. Acute kidney injury after heart transplant in young children: risk factors and outcomes. Pediatr Nephrol. 2016;31(4):671–678. [DOI] [PubMed] [Google Scholar]
- [24].Wu Y, Hua X, Yang G, et al. Incidence, risk factors, and outcomes of acute kidney injury in neonates after surgical procedures. Pediatr Nephrol. 2020;35(7):1341–1346. [DOI] [PubMed] [Google Scholar]
- [25].Mallory PP, Selewski DT, Askenazi DJ, et al. Acute kidney injury, fluid overload, and outcomes in children supported with extracorporeal membrane oxygenation for a respiratory indication. ASAIO J. 2020;66(3):319–326. [DOI] [PubMed] [Google Scholar]
- [26].Starr MC, Boohaker L, Eldredge LC, et al. Acute kidney injury and bronchopulmonary dysplasia in premature neonates born less than 32 weeks’ gestation. Am J Perinatol. 2020;37(03):341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Stoops C, Boohaker L, Sims B, et al. The association of intraventricular hemorrhage and acute kidney injury in premature infants from the assessment of the worldwide acute kidney injury epidemiology in neonates (AWAKEN) study. Neonatology. 2019;116(4):321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Criss CN, Selewski DT, Sunkara B, et al. Acute kidney injury in necrotizing enterocolitis predicts mortality. Pediatr Nephrol. 2018;33(3):503–510. [DOI] [PubMed] [Google Scholar]
- [29].Bakhoum CY, Basalely A, Koppel RI, et al. Acute kidney injury in preterm infants with necrotizing enterocolitis. J Matern Fetal Neonatal Med. 2019;32(19):3185–3190. [DOI] [PubMed] [Google Scholar]
- [30].Sanchez C, Garcia MA, Valdes BD. Acute kidney injury in newborns with necrotizing enterocolitis: risk factors and mortality. Bol Med Hosp Infant Mex. 2019;76:210–214. [DOI] [PubMed] [Google Scholar]
- [31].Garg PM, Britt AB, Ansari MAY, et al. Severe acute kidney injury in neonates with necrotizing enterocolitis: risk factors and outcomes. Pediatr Res. 2021;90(3):642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lambert DK, Christensen RD, Baer VL, et al. Fulminant necrotizing enterocolitis in a multihospital healthcare system. J Perinatol. 2012;32(3):194–198. [DOI] [PubMed] [Google Scholar]
- [34].Selewski DT, Charlton JR, Jetton JG, et al. Neonatal acute kidney injury. Pediatrics. 2015;136(2):e463–e473. [DOI] [PubMed] [Google Scholar]
- [35].Jetton JG, Guillet R, Askenazi DJ, et al. Assessment of worldwide acute kidney injury epidemiology in neonates: design of a retrospective cohort study. Front Pediatr. 2016;4:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zappitelli M, Ambalavanan N, Askenazi DJ, et al. Developing a neonatal acute kidney injury research definition: a report from the NIDDK neonatal AKI workshop. Pediatr Res. 2017;82(4):569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Basu RK, Kaddourah A, Terrell T, et al. Assessment of worldwide acute kidney injury, renal angina and epidemiology in critically ill children (AWARE): a prospective study to improve diagnostic precision. J Clin Trials. 2015;5:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mehta C, Patel N. A network algorithm for performing fisher’s exact test in r × c contingency tables. J Am Statist Assoc. 1983;78(382):427–434. [Google Scholar]
- [39].Jonckheere AR. A distribution-free k-sample test against ordered alternatives. Biometrika. 1954;41(1–2):133–145. [Google Scholar]
- [40].Alkandari O, Eddington KA, Hyder A, et al. Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit Care. 2011;15(3):R146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kirkley MJ, Boohaker L, Griffin R, et al. Acute kidney injury in neonatal encephalopathy: an evaluation of the AWAKEN database. Pediatr Nephrol. 2019;34(1):169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Garg PM, Paschal JL, Zhang M, et al. Brain injury in preterm infants with surgical necrotizing enterocolitis: clinical and bowel pathological correlates. Pediatr Res. 2022;91(5):1182–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yap SC, Lee HT. Acute kidney injury and extrarenal organ dysfunction: new concepts and experimental evidence. Anesthesiology. 2012;116(5):1139–1148. [DOI] [PubMed] [Google Scholar]
- [44].Adén U, Favrais G, Plaisant F, et al. Systemic inflammation sensitizes the neonatal brain to excitotoxicity through a pro-/anti-inflammatory imbalance: key role of TNFalpha pathway and protection by etanercept. Brain Behav Immun. 2010;24(5):747–758. [DOI] [PubMed] [Google Scholar]
- [45].Brunse A, Abbaspour A, Sangild PT. Brain barrier disruption and Region-Specific neuronal degeneration during necrotizing enterocolitis in preterm pigs. Dev Neurosci. 2018;40(3):198–208. [DOI] [PubMed] [Google Scholar]
- [46].Niño DF, Zhou Q, Yamaguchi Y, et al. Cognitive impairments induced by necrotizing enterocolitis can be prevented by inhibiting microglial activation in mouse brain. Sci Transl Med. 2018;10:eaan0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Biouss G, Antounians L, Li B, et al. Experimental necrotizing enterocolitis induces neuroinflammation in the neonatal brain. J Neuroinflamm. 2019;16(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Libório AB, Branco KM, Torres de Melo Bezerra C. Acute kidney injury in neonates: from urine output to new biomarkers. Biomed Res Int. 2014;2014:601568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Stojanović V, Barišić N, Radovanović T, et al. Acute kidney injury in premature newborns-definition, etiology, and outcome. Pediatr Nephrol. 2017;32(10):1963–1970. [DOI] [PubMed] [Google Scholar]
- [50].Orser BA, Suresh S, Evers AS. SmartTots update regarding anesthetic neurotoxicity in the developing brain. Anesth Analg. 2018;126(4):1393–1396. [DOI] [PubMed] [Google Scholar]
- [51].Harer MW, Adegboro CO, Richard LJ, et al. Non-invasive continuous renal tissue oxygenation monitoring to identify preterm neonates at risk for acute kidney injury. Pediatr Nephrol. 2021;36(6):1617–1625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.