Abstract
Background
Modern immunosuppressive regimens after kidney transplantation usually use a combination of two or three agents of different classes to prevent rejection and maintain graft function. Most frequently, calcineurin‐inhibitors (CNI) are combined with corticosteroids and a proliferation‐inhibitor, either azathioprine (AZA) or mycophenolic acid (MPA). MPA has largely replaced AZA as a first line agent in primary immunosuppression, as MPA is believed to be of stronger immunosuppressive potency than AZA. However, treatment with MPA is more costly, which calls for a comprehensive assessment of the comparative effects of the two drugs.
Objectives
This review of randomised controlled trials (RCTs) aimed to look at the benefits and harms of MPA versus AZA in primary immunosuppressive regimens after kidney transplantation. Both agents were compared regarding their efficacy for maintaining graft and patient survival, prevention of acute rejection, maintaining graft function, and their safety, including infections, malignancies and other adverse events. Furthermore, we investigated potential effect modifiers, such as transplantation era and the concomitant immunosuppressive regimen in detail.
Search methods
We searched Cochrane Kidney and Transplant's Specialised Register (to 21 September 2015) through contact with the Trials' Search Co‐ordinator using search terms relevant to this review.
Selection criteria
All RCTs about MPA versus AZA in primary immunosuppression after kidney transplantation were included, without restriction on language or publication type.
Data collection and analysis
Two authors independently determined study eligibility, assessed risk of bias and extracted data from each study. Statistical analyses were performed using the random‐effects model and the results were expressed as risk ratio (RR) for dichotomous outcomes and mean difference (MD) for continuous outcomes with 95% confidence intervals (CI).
Main results
We included 23 studies (94 reports) that involved 3301 participants. All studies tested mycophenolate mofetil (MMF), an MPA, and 22 studies reported at least one outcome relevant for this review. Assessment of methodological quality indicated that important information on factors used to judge susceptibility for bias was infrequently and inconsistently reported.
MMF treatment reduced the risk for graft loss including death (RR 0.82, 95% CI 0.67 to 1.0) and for death‐censored graft loss (RR 0.78, 95% CI 0.62 to 0.99, P < 0.05). No statistically significant difference for MMF versus AZA treatment was found for all‐cause mortality (16 studies, 2987 participants: RR 0.95, 95% CI 0.70 to 1.29). The risk for any acute rejection (22 studies, 3301 participants: RR 0.65, 95% CI 0.57 to 0.73, P < 0.01), biopsy‐proven acute rejection (12 studies, 2696 participants: RR 0.59, 95% CI 0.52 to 0.68) and antibody‐treated acute rejection (15 studies, 2914 participants: RR 0.48, 95% CI 0.36 to 0.65, P < 0.01) were reduced in MMF treated patients. Meta‐regression analyses suggested that the magnitude of risk reduction of acute rejection may be dependent on the control rate (relative risk reduction (RRR) 0.34, 95% CI 0.10 to 1.09, P = 0.08), AZA dose (RRR 1.01, 95% CI 1.00 to 1.01, P = 0.10) and the use of cyclosporin A micro‐emulsion (RRR 1.27, 95% CI 0.98 to 1.65, P = 0.07). Pooled analyses failed to show a significant and meaningful difference between MMF and AZA in kidney function measures.
Data on malignancies and infections were sparse, except for cytomegalovirus (CMV) infections. The risk for CMV viraemia/syndrome (13 studies, 2880 participants: RR 1.06, 95% CI 0.85 to 1.32) was not statistically significantly different between MMF and AZA treated patients, whereas the likelihood of tissue‐invasive CMV disease was greater with MMF therapy (7 studies, 1510 participants: RR 1.70, 95% CI 1.10 to 2.61). Adverse event profiles varied: gastrointestinal symptoms were more likely in MMF treated patients and thrombocytopenia and elevated liver enzymes were more common in AZA treatment.
Authors' conclusions
MMF was superior to AZA for improvement of graft survival and prevention of acute rejection after kidney transplantation. These benefits must be weighed against potential harms such as tissue‐invasive CMV disease. However, assessment of the evidence on safety outcomes was limited due to rare events in the observation periods of the studies (e.g. malignancies) and inconsistent reporting and definitions (e.g. infections, adverse events). Thus, balancing benefits and harms of the two drugs remains a major task of the transplant physician to decide which agent the individual patient should be started on.
Keywords: Humans, Kidney Transplantation, Kidney Transplantation/mortality, Azathioprine, Azathioprine/therapeutic use, Cyclosporine, Cyclosporine/therapeutic use, Graft Rejection, Graft Rejection/mortality, Graft Rejection/prevention & control, Immunosuppression Therapy, Immunosuppression Therapy/methods, Immunosuppressive Agents, Immunosuppressive Agents/therapeutic use, Mycophenolic Acid, Mycophenolic Acid/analogs & derivatives, Mycophenolic Acid/therapeutic use, Randomized Controlled Trials as Topic
Plain language summary
Mycophenolic acid versus azathioprine as primary immunosuppression for kidney transplant recipients
After kidney transplantation, patients receive a combination of immunosuppressive medications to prevent rejection of the transplanted kidney. These regimens usually contain a calcineurin‐inhibitor (tacrolimus or cyclosporin A), corticosteroids and an antiproliferative agent (mycophenolic acid (MPA), e.g. mycophenolate mofetil (MMF), or azathioprine (AZA)). MPA is considered to be of stronger immunosuppressive potency than AZA, but the benefits on survival of the graft and its safe use over a long period of time are insufficiently understood.
In this systematic review, we compared the efficacy and safety of MPA versus AZA in randomised controlled trials (RCTs) when given as part of the immunosuppressive regimen immediately after kidney transplantation.
Searches to 21 September 2015 identified 23 studies in which 3301 patients were treated with MPA (all studies used MMF) or AZA. Methodological quality of the studies was limited, e.g. only in two RCTs was the study medication administered in a blinded fashion.
MMF was more effective than AZA for reducing the risk of graft loss (by approximately 20%) and acute rejection (by approximately 30%). No difference in mortality was observed. Moreover, graft function appeared to be similar in both treatments.
When drugs are given to suppress the immune system, this can result in serious side effects such as infections and malignancies. The data on adverse events was limited by relatively short follow up in the studies as some of these side effects occur after several years of treatment. Furthermore, the studies did not focus on these harms and did not use harmonised diagnostic criteria. The incidence of cytomegalovirus infections did not differ between MMF and AZA, but there was a 1.7‐fold increased risk for the more severe, tissue‐invasive cytomegalovirus disease in MMF‐treated patients. Information on malignancies was reported only in five studies; therefore no robust conclusions can be drawn. Gastrointestinal side effects (e.g. nausea, diarrhoea) were more common with MMF‐treatment, whereas bone marrow suppression (e.g. thrombocytopenia) and elevated liver enzymes were observed more frequently in AZA treated patients.
In general, evidence for efficacy outcomes is of high quality and can be seen as considerably robust, but there is less certainty on aspects of safety. Therefore, caregivers should balance potential benefits and harms of MMF and AZA according to individual patient's risks and preferences. Physicians need to individualise the decision between these agents as components of the immunosuppressive regimen.
Summary of findings
Summary of findings for the main comparison. Mycophenolate mofetil (MMF) versus azathioprine (AZA) for primary immunosuppression in kidney transplant recipients.
| MMF compared to AZA for primary immunosuppression in kidney transplant recipients | ||||||
| Patient or population: patients with kidney transplant recipients Settings: primary immunosuppressive regimens (RCTs) Intervention: MMF Comparison: AZA | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| AZA | MMF | |||||
| Death, all cause Follow‐up: 0.5 to 5 years | 49 per 1000 | 47 per 1000 (34 to 63) | RR 0.95 (0.7 to 1.29) | 2987 (16) | ⊕⊕⊕⊝ moderate1 | No evidence for difference due to low precision |
| Graft loss, censored for death Follow‐up: 0.5 to 6 years | 11 per 100 | 9 per 100 (7 to 11) | RR 0.78 (0.61 to 0.98) | 2540 (17) | ⊕⊕⊕⊕ high2 | Statistically significant risk reduction of meaningful magnitude (˜20%) with MMF treatment |
| Malignancy, any Follow‐up: 1 to 6 years | 10 per 100 | 8 per 100 (6 to 11) | RR 0.81 (0.6 to 1.09) | 1735 (5) | ⊕⊝⊝⊝ very low3,4,5 | Statistically not significant favourable point estimate (˜20%) with MMF treatment, but very low quality evidence |
| Acute rejection, steroid resistant/antibody treated As reported in the articles Follow‐up: 0.5 to 3 years | 11 per 100 | 5 per 100 (4 to 7) | RR 0.48 (0.36 to 0.65) | 2914 (15) | ⊕⊕⊕⊕ high | Statistically significant risk reduction of meaningful magnitude (˜50%) with MMF treatment |
| Infection, CMV tissue invasive As reported in the articles Follow‐up: 0.5 to 3 years | 4 per 100 | 7 per 100 (5 to 11) | RR 1.7 (1.1 to 2.61) | 1510 (7) | ⊕⊕⊕⊕ high3,6 | Statistically significantly increased risk of meaningful magnitude (1.7 fold) with MMF treatment |
| Acute rejection, total Any treated acute rejection, including biopsy‐proven Follow‐up: 0.5 to 5 years | 35 per 100 | 23 per 100 (20 to 26) | RR 0.65 (0.57 to 0.73) | 3301 (22) | ⊕⊕⊕⊕ high | Statistically significant risk reduction of meaningful magnitude (˜35%) with MMF treatment |
| Chronic allograft nephropathy Biopsy required in 2 RCTs, one study with optional biopsy Follow‐up: 1 to 6 years | 36 per 100 | 25 per 100 (17 to 36) | RR 0.69 (0.48 to 0.99) | 203 (3) | ⊕⊕⊝⊝ low3,4 | Statistically significant risk reduction of meaningful magnitude (30%) with MMF treatment, but low quality evidence due to sparse data |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
AZA ‐ azathioprine; CMV ‐ cytomegalovirus; MMF ‐ mycophenolate mofetil; RCT ‐ randomised controlled trial
1 Insufficient statistical power to detect a small effect of either treatment on the outcome 2 Large beneficial effect of MMF treatment 3 Considerable risk of reporting bias as data were provided by a limited number of studies only 4 Study populations in which the outcome were reported are a subset not representative for patients enrolled in all trials identified for the review 5 Insufficient statistical power due to sparse data on a potential beneficial effect on the incidence of malignancies with MMF treatment 6 Substantial harm caused by MMF
Background
Description of the condition
While there are several immunosuppressive drugs available, usually a combination of two or three agents of different classes is used to prevent rejection and maintain graft function after kidney transplantation. In most regimens, calcineurin inhibitors (CNI) (cyclosporin A (CsA) or tacrolimus (Tac)), form the cornerstone of treatment and are combined with corticosteroids and a proliferation‐inhibitor with its representatives azathioprine (AZA) and mycophenolic acid (MPA). There are currently two formulations available for MPA, mycophenolate mofetil (MMF) and the more recently approved enteric‐coated mycophenolate sodium (ec‐MPS) (Hardinger 2013; Marcen 2009)
Description of the intervention
MMF was approved by the US Food and Drug Administration (FDA) in 1995 for the prevention of acute rejection in kidney transplant recipients. This was based on the results of three randomised controlled trials (RCT), the pivotal trials, where a total of 1493 patients in North America, Europe and Australia were enrolled. MMF was compared to AZA (MMF TRI Study 1996; MMF US Study 1995) or placebo (European MMF Study Group 1995) in a regimen with concomitant use of CsA (original formulation) and steroids. In all three studies, MMF showed superior ability to prevent acute rejection within the first six months after transplantation.
In the past decade, MPA was tested against AZA in various immunosuppressive regimens in kidney transplantation as a variety of new drugs have been developed, including mammalian target of rapamycin (mTOR) inhibitors (Webster 2006), Tac and a micro‐emulsion formulation of CsA (CsA‐ME) (Webster 2005).
How the intervention might work
Both proliferation inhibitors, MPA and AZA, reduce purine synthesis either through direct inhibition of the cell cycle (AZA) or on the level of nucleotide synthesis (MPA) (Staatz 2007). AZA was one of the first drugs used for immunosuppression in kidney transplantation in the 1960s (Mowbray 1965). Following the results of the pivotal trials, AZA was widely replaced by MPA, particularly MMF, as a component of primary immunosuppressive regimens in most of the developed countries (Halloran 2004; Hardinger 2013), since acute rejection has been shown to be a strong predictor for diminished graft function and reduced graft survival (Pascual 2002).
Why it is important to do this review
Despite MMF being considered to be more effective than AZA in the prevention of acute rejection, its superior effect on long‐term graft function and graft survival has not been shown in RCTs (Srinivas 2005). Instead, similar kidney function and graft survival was found in long‐term follow‐up data of two of the pivotal trials (MMF TRI Study 1996; MMF US Study 1995). The lack of statistical power within the single studies is a crucial aspect that needs to be considered for this phenomenon. Calculations have shown that a sample size of 8 to 10 times the number actually enrolled in the pivotal trials would have been needed to prove a benefit on graft survival (Ekberg 2003). Meta‐analyses are the tool of choice to address the limitation of under‐powered studies.
Observational evidence also highlighted that the use of considerably strong immunosuppressive regimens in recent years has led to acute rejection rates as low as 10% to 15%, sometimes even lower; but that this reduced acute rejection rate has not translated into similar prolongation of long‐term graft survival (Tantravahi 2007). This may be due to side effects directly related to the level of immunosuppression, such as (opportunistic) infections (e.g. Polyoma BK/JC virus or cytomegalovirus (CMV) (Marcen 2009; Staatz 2007) and malignancies (Domhan 2009; Johnston 2010). These complications not only impact patient survival. For example, MMF was reported to be associated with the incidence of the very rare but life threatening progressive multifocal leukoencephalopathy which is caused by JC‐virus activation (FDA 2008). In addition, these complications have been shown to directly impair graft function, e.g. polyomavirus‐associated nephropathy (PVAN). Finally, one of the major causes for death in patients with a functioning graft is cardiovascular disease (CVD) (Israni 2010). Side effects of immunosuppressive agents, particularly CNI and mTOR‐inhibitors, further aggravate classical CVD risk factors, such as hypertension, diabetes and dyslipidaemia (Webster 2005; Webster 2006).
Aside from safety issues due to the general level of immunosuppression, specific adverse events vary for both proliferation‐inhibitors. AZA often provokes leucopenia and may increase the risk of cancer through accumulation of mutagenic metabolites (Domhan 2009). MPA causes more gastrointestinal problems like nausea and diarrhoea and is also contraindicated in pregnancy because of negative effects on foetal development (Sifontis 2006).
The relative efficacy of MMF versus AZA in the prevention of rejection and their impact on long‐term graft survival might also be modulated by the concomitant immunosuppressive therapy and by the overall level of modern transplant therapy. The MYSS Study 2004 compared both drugs in a CsA‐ME based regimen and showed similar acute rejection rates, graft function and survival in both groups up to seven years of follow‐up.
Objectives
This review of RCTs aimed to look at the benefits and harms of MPA versus AZA in primary immunosuppressive regimens after kidney transplantation. Both agents were compared regarding their efficacy for maintaining graft and patient survival, prevention of acute rejection, maintaining graft function, and their safety, including infections, malignancies and other adverse events. Furthermore, we investigated potential effect modifiers, such as transplantation era and the concomitant immunosuppressive regimen in detail.
Methods
Criteria for considering studies for this review
Types of studies
We included all RCTs and quasi‐RCTs (RCTs in which allocation to treatment was performed by somewhat predictable methods) looking at the direct comparison of MPA versus AZA in primary immunosuppressive regimens in kidney transplantation, without restriction on language or publication type.
Types of participants
Inclusion criteria
We included studies investigating children (< 18 years) and adult kidney transplant recipients with any duration of follow‐up in the review, regardless of donor type (living or deceased) or previous transplantation status.
Exclusion criteria
We excluded studies that involved multi‐organ transplantation (e.g. kidney‐pancreas, kidney‐liver) as well as studies in which the intervention was performed in secondary regimens (when the immunosuppressive therapy was changed due to acute rejection, chronic allograft nephropathy (CAN), CNI toxicity or in stable graft function status).
Types of interventions
We included studies in the review in which MPA, namely MMF or ec‐MPS, was tested against AZA in primary immunosuppressive regimens along with any concomitant immunosuppressive therapy (e.g. use of induction antibody treatment, any formulation of CNI (CsA original formulation, CsA‐ME, Tac), various CNI target levels, treatment with or without steroids or mTOR inhibitors). Concomitant immunosuppression regimens needed to be identical in both the intervention and control groups (e.g. studies investigating MMF/CsA versus AZA/Tac were excluded).
Types of outcome measures
Primary outcomes
Graft loss and all‐cause mortality were considered primary outcomes of interest in terms of efficacy and safety, respectively.
Secondary outcomes
Secondary outcomes were acute rejection, CAN, graft function measures (e.g. serum creatinine (SCr), creatinine clearance (CrCl), proteinuria), immunosuppression related (malignancies, infections) and drug specific (e.g. new onset diabetes after transplantation (NODAT), haematological disorders, such as leucopenia, anaemia, elevated liver enzymes) side effects.
Following the suggestions of the GRADE working group (Atkins 2004) we classified outcomes of interest according to clinical importance (critical – high – moderate).
Critical importance
Death and death with a functioning graft
Graft loss, and graft loss censored for death
Malignancies (except non‐melanoma skin cancer).
High importance
Acute rejection, biopsy‐confirmed acute rejection, and steroid resistant/antibody‐treated acute rejection
CAN
Infections of any type, including CMV infection, tissue invasive CMV disease, PVAN
Non‐melanoma skin cancer.
Moderate importance
Kidney function measures: absolute values of measured glomerular filtration rate (GFR), estimated GFR, CrCl, SCr, and proteinuria (in any measurement and metric)
Adverse events, including hypertension, hyperlipidaemia, NODAT, leucopenia, anaemia, nausea, diarrhoea, elevation of liver enzymes or bilirubin.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register (to 21 September 2015) through contact with the Trials' Search Co‐ordinator using search terms relevant to this review (Appendix 1). The Specialised Register contains studies identified from the following sources.
Quarterly searches of the Cochrane Central Register of Controlled Trials CENTRAL
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney and transplant conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies as well as a list of handsearched journals, conference proceedings and current awareness alerts are available in the Specialised Register section of information about the Cochrane Kidney and Transplant.
Searching other resources
We also checked the reference lists of nephrology textbooks, review articles, and identified studies for this review. In particular, we reconciled the studies included in previous systematic reviews addressing MMF versus AZA (Knight 2009; Wang 2004a; Wang 2004b; Wang 2005; Zhang 2004).
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts potentially relevant to the review. All titles and abstracts were screened by at least two authors. Studies not applicable to the review were discarded. Those references that might include relevant data or information on studies were retrieved in full text and the described authors determined if the studies satisfied the inclusion criteria.
Data extraction and management
All articles of eligible studies were retrieved in full text and data relevant for the review were extracted into standardized forms in duplicate independently by two authors.
Data about study design, inclusion and exclusion criteria, items of quality assessment, definitions of primary and secondary study endpoints, etc. were extracted into an Excel file. Information from multiple publications of the same study were reconciled and condensed accordingly
Data on outcomes of interest were extracted in a separate Excel file. Results for dichotomous outcomes were extracted as actual numbers of patients achieving the respective outcome. If only proportions were reported in the studies, we calculated the numbers based on intention‐to‐treat (ITT) population or on‐treatment population as specified in the article.
Studies reported in non‐English language were translatedand data were assessed respectively
Disagreements were resolved via discussion among authors. All data were entered into Review Manager 5 and checked twice.
Assessment of risk of bias in included studies
The following items were assessed using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
Dichotomous outcome results (e.g. death, graft loss, acute rejection) are expressed as risk ratio (RR) with 95% confidence intervals (CI). For treatment effects on continuous scales of measurement (e.g. SCr, CrCl, GFR), the mean difference (MD) was used. The proportion of events per treatment arm at the desired time‐points were extracted from Kaplan Meier curve graphs using planimetric (digitising) software, such as the Engauge Digitizer program (http://digitizer.sourceforge.net/), assuming no censoring. The mean and the standard error (SE)/standard deviation (SD) of continuous outcomes were assessed at the respective time‐points along with the number of patients at risk for the given outcome. If the SD was missing for continuous outcomes, it was imputed based on the median SD of studies in which the relevant outcome was reported.
Dealing with missing data
Any further information required from the original author was requested by written correspondence (e.g. emailing corresponding author) and any relevant information obtained was included in the review. Evaluation of important numerical data such as screened, randomised patients as well as ITT, as‐treated and per‐protocol population was carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) were critically appraised (Higgins 2011).
If information about covariates that were further investigated in meta‐regression analyses (see below) was missing, we imputed the year of transplantation from the year of first publication minus duration of follow‐up minus two years, to account for lag between study completion and publication. If the AZA dose was reported to be body‐weight‐adjusted (mg/kg/d) it was transformed into mg/d using the mean body weight as reported in the study, and by using 70 kg (60 kg in exclusively Asian populations) if information on body weight was missing. Looking at the year of transplantation, it was likely that the original oil‐based formulation of CsA was used in many studies not providing detailed information on which kind of CsA drug was tested, and thus CsA original formulation and studies without this information were grouped and compared to studies reporting the use of CsA‐ME. Studies in which more than one MMF dose was tested, i.e. 3 g versus 2 g (MMF TRI Study 1996; MMF US Study 1995), 2 g versus 1.5 g (Ling 1998) and 2 g versus 1 g (Mendez 1998), were split into two independent studies and each compared to half of the group and events of the patients treated with AZA. In the case of uneven numbers, the nearest integer was used.
Assessment of heterogeneity
Heterogeneity was analysed using a Chi² test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). I² values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity. Considerable clinical heterogeneity was assumed due to a multitude of concomitant immunosuppressive regimens in studies of a long era of kidney transplantation, and over a variety of different study populations and clinical settings.
Assessment of reporting biases
We planned to construct funnel plots to assess for the potential existence of small study bias (Higgins 2011).
Data synthesis
Review Manager 5 was used for all meta‐analyses, using Der Simonian and Laird random‐effects models by default because of clinical heterogeneity rather than fixed‐effects models although we frequently found no evidence for statistical heterogeneity. Summary results, i.e. RR and MD, are presented in forest‐plots according to subgroups of clinically relevant time intervals (≤ 6 months, 6 to 12 months or ≤ 1 year, 1 to 4 years, ≥ 4 years). Moreover, a subgroup longest duration of follow‐up was defined that included data on the longest time interval of each study for the primary study population, i.e. we used six months data provided for the entire study population, rather than 24 months data of only a subgroup of the original study. These study data were further used for meta‐regression analyses (adjusted for duration of follow‐up, see below).
Subgroup analysis and investigation of heterogeneity
Meta‐regression
We performed random effects meta‐regression analyses (Meta‐Analyst for Windows 7, version December 2013, Brown University, Providence, RI, USA; Wallace 2009) to explore possible sources of heterogeneity on the following outcomes: mortality, death‐censored graft loss, malignancy (any), acute rejection (any), CMV viraemia/syndrome, tissue‐invasive CMV disease, SCr, diarrhoea and leucopenia. The logarithmic form of the RR was analysed and back‐transformed regression coefficients are presented as relative risk ratio (RRR) with 95% CI. For continuous outcomes, the MD was modelled and the coefficient with 95% CI is displayed in the table. Furthermore, bubble plots with the size of the bubble reflecting weight of the study in the meta‐regression, visualise the direction of the association between the covariate and the logarithmic RR of MMF versus AZA. Tested covariates included study level factors (year of transplantation, donor type, previous transplantation, dose of the study drugs, antibody induction therapy, maintenance CNI (Tac versus CsA), CsA formulation) and items of study quality and risk of bias (blinding, publication type, industry funding).
The fact that we tested a multitude of factors on a variety of outcomes on a dataset with limited sample size (22 studies) provided a high chance that associations were found or even missed only by chance. Our primary interest was the direction of any effect modification rather than the magnitude of the relative effect. Therefore, and also with our concern about type II error, we have used a threshold of P ≤ 0.10 and presented these results in the respective sections (and highlighted these results accordingly in the table).
Subgroup analyses
To further investigate heterogeneity, subgroup analyses were performed (using Review Manager 5) on the following strata: RCT versus quasi‐RCT, inclusion of children, ITT analysis, publication type and source of funding.
Sensitivity analysis
Sensitivity analyses (performed in Meta‐Analyst) were used to test the robustness of findings. Results from studies were sequentially included or excluded from the analysis with a particular focus on the largest or most dominant studies.
Results
Description of studies
Results of the search
The literature search yielded a total of 2852 citations (Figure 1), including handsearching of the reference lists of included studies and previously published systematic reviews on MMF versus AZA in kidney transplantation. Notably, the reference lists of two Chinese systematic reviews about MMF versus AZA (Wang 2004a; Wang 2004b; Wang 2005; Zhang 2004) were reconciled with the search results of the current review which led to the addition of two eligible studies not identified by the electronic searches.
1.
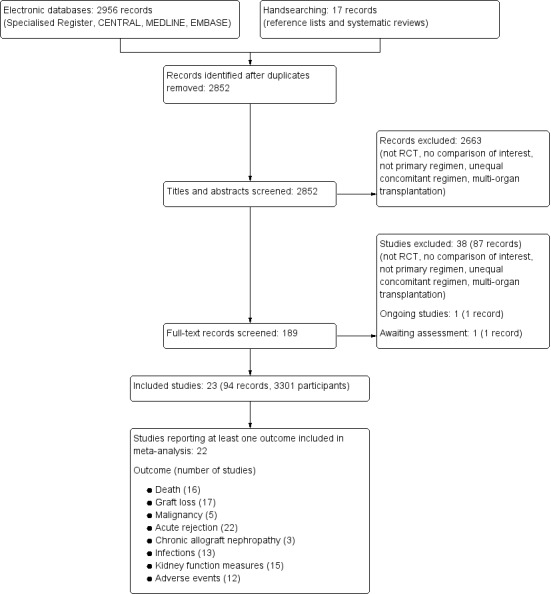
Literature search and identification of studies
In total, 94 reports of 23 studies (Army Hospital 2002; Baltar 2002; Busque 2001; COSTAMP Study 2002; Egfjord 1999; Folkmane 2001; Isbel 1997; Ji 2001; Joh 2005; Johnson 2000; Keven 2003; Ling 1998; Merville 2004; Miladipour 2002; Mendez 1998; MMF TRI Study 1996; MMF US Study 1995; MYSS Study 2004; Sadek 2002; Suhail 2000; Sun 2002b; Tuncer 2002; Weimer 2002) enrolling 3301 patients were included (see Characteristics of included studies). In addition, one ongoing Italian study that aims to investigate MMF versus AZA as sole immunosuppressive treatment after antibody (IL‐2, ATG) induction and CsA‐ME based regimen for one year, was identified (ATHENA Study 2012, see Characteristics of ongoing studies). While 15 studies were reported at least once as full article in a peer reviewed journal (Baltar 2002; COSTAMP Study 2002; Ji 2001; Joh 2005; Johnson 2000; Keven 2003; Merville 2004; Mendez 1998; MMF TRI Study 1996; MMF US Study 1995; MYSS Study 2004; Sadek 2002; Sun 2002b; Tuncer 2002; Weimer 2002), five studies (Busque 2001; Folkmane 2001; Miladipour 2002; Suhail 2000; Tuncer 2002) were published in Transplantation Proceedings only, and three studies (Army Hospital 2002; Egfjord 1999; Isbel 1997) were presented solely as conference abstracts. Nineteen studies published at least one article in English, three studies were published exclusively in Chinese (Ji 2001; Ling 1998; Sun 2002b) and one study was in Spanish language (Baltar 2002). Of the identified 23 studies, one (Isbel 1997) did not provide any information on outcomes relevant for the review.
Prior to publication an additional report was identified (Do 2001a). This appears to be a report of Joh 2005. Details will be assessed in a future update of this review
Included studies
All studies investigated MMF versus AZA, whereas no study used ec‐MPS. Doses of study drugs were reported in 19 studies (Busque 2001; COSTAMP Study 2002; Egfjord 1999; Folkmane 2001; Ji 2001; Joh 2005; Johnson 2000; Keven 2003; Ling 1998; Merville 2004; Miladipour 2002; Mendez 1998; MMF TRI Study 1996; MMF US Study 1995; MYSS Study 2004; Sadek 2002; Suhail 2000; Sun 2002b; Tuncer 2002) and ranged from 1 to 3 g/d for MMF, and 50 to 175 mg/d for AZA. Patients were enrolled in the studies between 1992 and 2002 and 78% (2575 participants) were studied in nine multicentre studies (Busque 2001; COSTAMP Study 2002; Johnson 2000; Merville 2004; Mendez 1998; MMF TRI Study 1996; MMF US Study 1995; MYSS Study 2004; Sadek 2002).
Participants
In 14 studies (Baltar 2002; Busque 2001; Egfjord 1999; Folkmane 2001; Ji 2001; Joh 2005; Johnson 2000; Ling 1998; Merville 2004; Mendez 1998; MMF TRI Study 1996; MMF US Study 1995; MYSS Study 2004; Suhail 2000), only deceased donor transplantation was performed; one study exclusively investigated living donor transplantation (Army Hospital 2002); five studies included both deceased and living (COSTAMP Study 2002; Keven 2003; Sadek 2002; Tuncer 2002; Weimer 2002); and three studies did not report the type of graft donation (Isbel 1997; Miladipour 2002; Sun 2002b). Two studies included children (Johnson 2000; Mendez 1998), eight exclusively enrolled adult recipients (Busque 2001; COSTAMP Study 2002; Keven 2003; Merville 2004; MMF TRI Study 1996; MMF US Study 1995; MYSS Study 2004; Sadek 2002) and no information was provided in the remaining 13 studies (Army Hospital 2002; Baltar 2002; Egfjord 1999; Folkmane 2001; Isbel 1997; Ji 2001; Joh 2005; Ling 1998; Miladipour 2002; Suhail 2000; Sun 2002b; Tuncer 2002; Weimer 2002). The inclusion of patients that previously lost a kidney graft and the values of panel reactive antibodies (PRA) are widely considered measures of baseline immunological risk of the study population; however, this information was limited in the studies.
Patients with previous kidney transplants were included in seven studies (COSTAMP Study 2002; Egfjord 1999; Folkmane 2001; Miladipour 2002; Mendez 1998; MMF TRI Study 1996; Weimer 2002) (ranging from 5.3% to 14.3% of participants), excluded in 10 studies (Army Hospital 2002; Baltar 2002; Busque 2001; Johnson 2000; Merville 2004; MMF US Study 1995; MYSS Study 2004; Sadek 2002; Suhail 2000; Tuncer 2002), and not reported in six studies (Isbel 1997; Ji 2001; Joh 2005; Keven 2003; Ling 1998; Sun 2002b). In only eight studies (Ji 2001; Joh 2005; Johnson 2000; Merville 2004; Mendez 1998; MMF TRI Study 1996; MMF US Study 1995; Weimer 2002), information about PRA was provided, however this information was not described consistently (e.g. as proportion above a certain cut‐off (> 10% or > 20%), or maximum PRA level). Overall, most studies enrolled patients with considerably low to moderate immunological risk.
Concomitant Immunosuppression
A depleting antibody induction therapy (ATG, ALG or OKT3) was used in five studies (Egfjord 1999; Ji 2001; Merville 2004; Mendez 1998; MMF US Study 1995) as initiating immunosuppressive agent in all patients. This therapy was only used in a subset of patients in five studies (e.g. those with higher immunological baseline risk, or patients experiencing delayed graft function) (Busque 2001; Johnson 2000; Keven 2003; Tuncer 2002; Weimer 2002). The remaining 13 studies (Army Hospital 2002; Baltar 2002; COSTAMP Study 2002; Folkmane 2001; Isbel 1997; Joh 2005; Ling 1998; Miladipour 2002; MMF TRI Study 1996; MYSS Study 2004; Sadek 2002; Suhail 2000; Sun 2002b) did not use any antibody induction therapy. All maintenance immunosuppressive regimens were CNI based, while 18 studies used CsA (Army Hospital 2002; Baltar 2002; Egfjord 1999; Folkmane 2001; Isbel 1997; Ji 2001; Joh 2005; Ling 1998; Merville 2004; Miladipour 2002; MMF TRI Study 1996; MMF US Study 1995; MYSS Study 2004; Sadek 2002; Suhail 2000; Sun 2002b; Tuncer 2002; Weimer 2002), four studies used Tac (Busque 2001; COSTAMP Study 2002; Johnson 2000; Mendez 1998) and one study (Keven 2003) reported the use of either CsA or Tac. Of those using CsA, six studies (Egfjord 1999; Merville 2004; MYSS Study 2004; Sadek 2002; Suhail 2000; Weimer 2002) reported treatment with CsA‐ME, two studies (MMF TRI Study 1996; MMF US Study 1995) clearly stated the use of the original CsA solution, one study used both CsA and CsA‐ME (Tuncer 2002), and 10 studies (Army Hospital 2002; Baltar 2002; Folkmane 2001; Isbel 1997; Ji 2001; Joh 2005; Keven 2003; Ling 1998; Miladipour 2002; Sun 2002b) did not clearly specify the CsA formulation.
Target CNI trough levels were reported for all four studies using Tac (C0 levels at month 3: 5 to 15 ng/mL), but in only six studies using CsA (C0levels at month 3: 100 to 500 ng/mL) (Folkmane 2001; Ji 2001; Ling 1998; Merville 2004; MYSS Study 2004; Sadek 2002). Two CsA studies reported the dosage of CsA as being delivered “according to local practice” (MMF TRI Study 1996; MMF US Study 1995) and no information was provided in 11 studies (Army Hospital 2002; Baltar 2002; Egfjord 1999; Isbel 1997; Joh 2005; Keven 2003; Miladipour 2002; Suhail 2000; Sun 2002b; Tuncer 2002; Weimer 2002). Corticosteroids completed the concomitant immunosuppressive regimen in all studies, while in one study (MYSS Study 2004) steroid therapy was withdrawn according to protocol. Notably, IL‐2 receptor antibody induction or mTOR‐inhibitor therapy was not used in the studies identified for the review.
Excluded studies
A total of 87 records (38 studies) were excluded as they did not fulfil the inclusion criteria (see Characteristics of excluded studies). The reasons for exclusion were as follows.
Study design not RCT or quasi‐RCT (nine studies)
Not solely kidney transplantation (two studies); studies enrolling patients undergoing multiorgan transplantation, e.g. simultaneous kidney‐pancreas transplantation were excluded.
Not primary immunosuppressive regimen (18 studies), i.e. the randomisation to MPA versus AZA was not performed at the time of transplantation, but subsequently during the maintenance phase (e.g. due to previous acute rejection, CAN, CNI‐toxicity or in stable graft function status)
Randomised intervention not of interest for the review (eight studies), i.e. not MPA versus AZA
Unequal concomitant regimen (four studies), i.e. different immunosuppressive regimens were administered to patients randomised to treatment and control group (e.g. MMF/CsA versus AZA/Tac.
Risk of bias in included studies
Details of the risk of bias assessment tool (Appendix 2) can be found for each study in Characteristics of included studies and are displayed in Figure 2, Figure 3.
2.
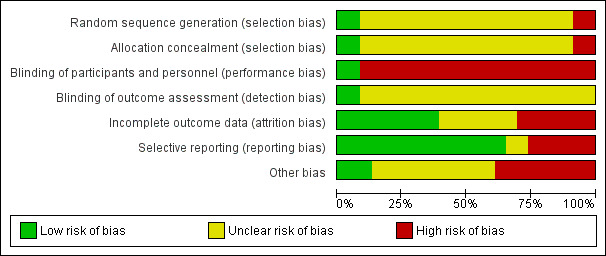
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
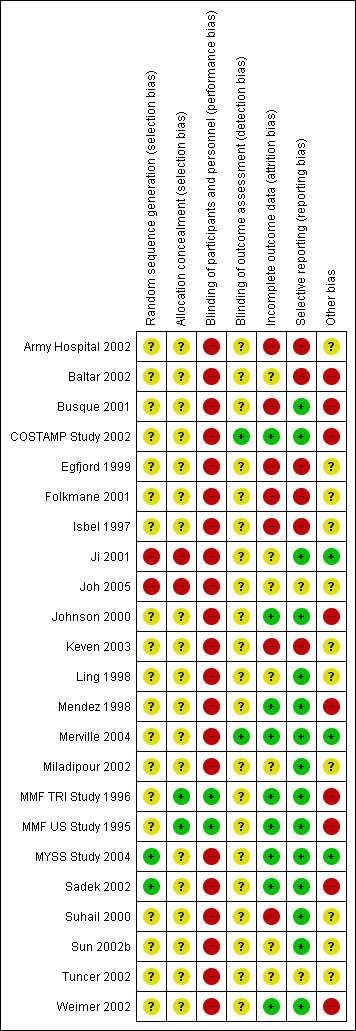
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Details about the methodology of studies were generally limited. Of the included 23 studies, 21 were considered RCTs, while two studies reported allocation methods that classified them as quasi‐RCT (studies in which the method of allocation to the respective treatments was somewhat predictable) (Ji 2001; Joh 2005). In most studies, no detailed information was provided on allocation concealment (19 studies) or the procedure for randomisation (21 studies).
Blinding
Only two studies blinded the intervention of the study drug to patients and study personnel using placebo (MMF TRI Study 1996; MMF US Study 1995).
Incomplete outcome data
Broad descriptions of the course of patients in the study and drop‐out rates were reported in 14 studies (COSTAMP Study 2002; Ji 2001; Joh 2005; Johnson 2000; Keven 2003; Ling 1998; Merville 2004; Mendez 1998; MMF TRI Study 1996; MMF US Study 1995; MYSS Study 2004; Sadek 2002; Suhail 2000; Weimer 2002), while very detailed information about patients (e.g. information about cross‐over treatments, MMF to AZA and vice versa) was available in four studies (Johnson 2000; Mendez 1998; Suhail 2000; Weimer 2002).
Selective reporting
Outcome reporting and outcome details varied substantially among studies (see Figure 1). One study did not report any outcome information relevant for the review (Isbel 1997). Graft‐related outcomes were available for the majority of studies. All 22 studies provided information on acute rejection, 17 reported graft loss (Busque 2001; Egfjord 1999; Folkmane 2001; Ji 2001; Joh 2005; Johnson 2000; Ling 1998; Merville 2004; Miladipour 2002; Mendez 1998; MMF TRI Study 1996; MMF US Study 1995; MYSS Study 2004; Sadek 2002; Suhail 2000; Tuncer 2002; Weimer 2002) and 15 reported a measure of graft function (Army Hospital 2002; Busque 2001; COSTAMP Study 2002; Egfjord 1999; Johnson 2000; Ling 1998; Merville 2004; Miladipour 2002; MMF TRI Study 1996; MMF US Study 1995; MYSS Study 2004; Sadek 2002; Suhail 2000; Sun 2002b; Weimer 2002). Mortality rates were also reported in 16 studies (Busque 2001;COSTAMP Study 2002; Egfjord 1999; Ji 2001; Joh 2005; Johnson 2000; Ling 1998; Mendez 1998; Merville 2004; MMF US Study 1995; MMF TRI Study 1996; MYSS Study 2004; Sadek 2002; Suhail 2000; Tuncer 2002; Weimer 2002). Data on CAN were sparse (three studies, Merville 2004; Tuncer 2002; Weimer 2002). Complications of immunosuppressive therapy were reported much less frequently than efficacy outcomes: any malignancy was reported in five studies (Mendez 1998; MMF TRI Study 1996; MMF US Study 1995; MYSS Study 2004; Sadek 2002) and infections such as Herpes was reported in four studies (COSTAMP Study 2002; Johnson 2000; MMF TRI Study 1996; MMF US Study 1995), and pneumocystis in five studies (Johnson 2000; Mendez 1998; MMF TRI Study 1996; MMF US Study 1995; MYSS Study 2004). Events and details of CMV viraemia/syndrome were reported in 13 studies (COSTAMP Study 2002; Ji 2001; Joh 2005; Johnson 2000; Keven 2003; Merville 2004; Mendez 1998; Miladipour 2002; MMF TRI Study 1996; MMF US Study 1995; MYSS Study 2004; Sadek 2002; Weimer 2002), and CMV tissue‐invasive disease in seven studies (Folkmane 2001; Ji 2001; Johnson 2000; Mendez 1998; MMF TRI Study 1996; MMF US Study 1995; Suhail 2000). Only one study provided information on PVAN (Weimer 2002). Aside from diarrhoea (11 studies) (COSTAMP Study 2002; Ji 2001; Ling 1998; Mendez 1998; Miladipour 2002; MMF TRI Study 1996; MMF US Study 1995; MYSS Study 2004; Sadek 2002; Suhail 2000; Sun 2002b), and leucopenia (12 studies) (Army Hospital 2002; COSTAMP Study 2002; Ji 2001; Ling 1998; Mendez 1998; Miladipour 2002; MMF TRI Study 1996; MMF US Study 1995; MYSS Study 2004; Sadek 2002; Suhail 2000; Sun 2002b), the occurrence of adverse events was inconsistently and rarely reported, and most often not defined in detail.
Other potential sources of bias
Analysis of outcomes by ITT was stated by the authors and supported by details of the presented results in 12 studies (COSTAMP Study 2002; Egfjord 1999; Ji 2001; Johnson 2000; Ling 1998; Mendez 1998; Merville 2004; MMF TRI Study 1996; MMF US Study 1995; MYSS Study 2004; Sadek 2002; Weimer 2002). The type of analysis was unclear in an additional nine studies (Baltar 2002; Busque 2001; Folkmane 2001; Isbel 1997; Joh 2005; Miladipour 2002; Suhail 2000; Sun 2002b; Tuncer 2002) and not performed by ITT in two studies (Army Hospital 2002; Keven 2003). Only two studies (Merville 2004; MYSS Study 2004) clearly stated funding independent from pharmaceutical companies (407 patients, 12%), while nine studies (2252 patients, 68%) reported industry support (Baltar 2002; Busque 2001; COSTAMP Study 2002; Johnson 2000; Mendez 1998; MMF TRI Study 1996; MMF US Study 1995; Sadek 2002; Weimer 2002). For the remaining 11 studies the funding source was unclear (Army Hospital 2002; Egfjord 1999; Folkmane 2001; Isbel 1997; Ji 2001; Joh 2005; Keven 2003; Ling 1998; Miladipour 2002; Suhail 2000; Sun 2002b; Tuncer 2002).
Effects of interventions
See: Table 1
Summary analyses of the comparative efficacy and safety of MMF versus AZA can be found in the section Analyses 1. Outcomes of interest for the review were frequently reported at multiple time points, thus subgroups of clinically meaningful time intervals are displayed. Summary results reported in the text represent longest duration of follow‐up unless stated otherwise.
Primary outcomes
Death
No statistically significant difference for MMF versus AZA treatment was found for all‐cause mortality at any time interval (Analysis 1.1.4 (16 studies, 2987 participants): RR 0.95, 95% CI 0.70 to 1.29; I2 = 0%). Disease‐specific mortality was reported less frequently; therefore no robust conclusions can be drawn. While being clearly not statistically significant, the point estimate for death due to cardio‐, cerebrovascular disease favoured MMF (11 studies: RR 0.66, 95% CI 0.37 to 1.18, P = 0.16), and the point estimate for death due to infectious causes suggested reduced risk in AZA patients (11 studies: RR 1.28, 95% CI 0.57 to 2.91, P = 0.55) (detailed data not shown).
1.1. Analysis.
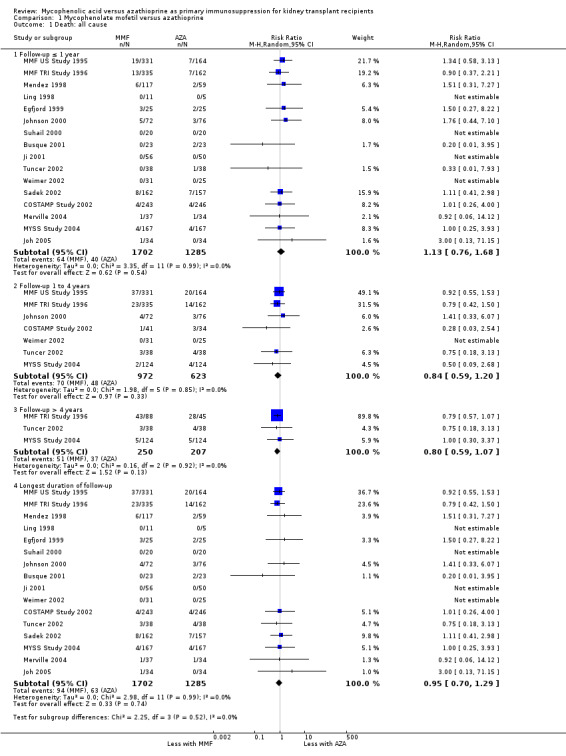
Comparison 1 Mycophenolate mofetil versus azathioprine, Outcome 1 Death: all cause.
Graft loss
Consistently across all time‐intervals, MMF treatment significantly reduced the risk for graft loss including death (Analysis 1.2.4 (15 studies, 2653 participants): RR 0.82, 95% CI 0.67 to 1.00; I2 = 0%) as well as for death‐censored graft loss (Analysis 1.3.4 (17 studies, 2540 participants): RR 0.78, 95% CI 0.62 to 0.99; I2 = 0%). In particular, the risk of graft loss due to rejection was markedly reduced in MMF treated patients (13 studies, RR 0.59, 95% CI 0.41 to 0.86, P < 0.01), while data on graft loss because of any other specific cause was rarely reported (detailed data not shown).
1.2. Analysis.
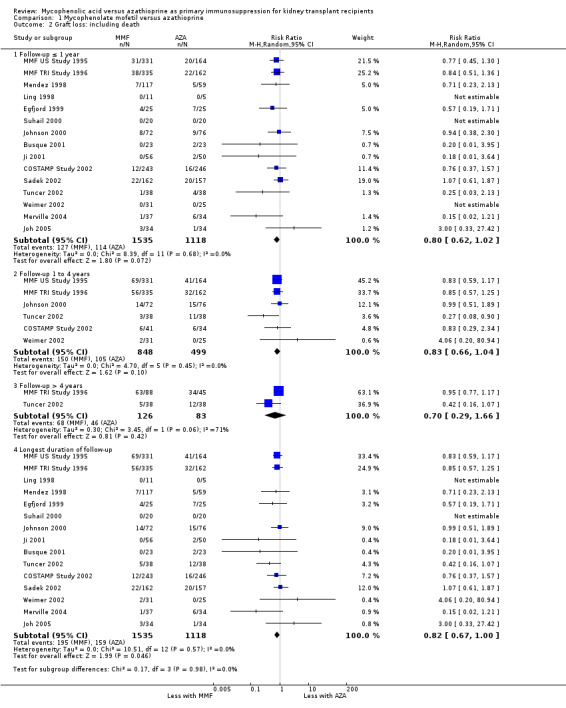
Comparison 1 Mycophenolate mofetil versus azathioprine, Outcome 2 Graft loss: including death.
1.3. Analysis.

Comparison 1 Mycophenolate mofetil versus azathioprine, Outcome 3 Graft loss: censored for death.
Non‐functioning graft
Information regarding primary non‐function of the graft was provided by 11 studies, however, only 18 events were observed by four studies investigating a total of 1601 patients indicating no significant difference between the treatments (Analysis 1.4: RR 0.47, 95% CI 0.19 to 1.18; I2 = 0%).
1.4. Analysis.
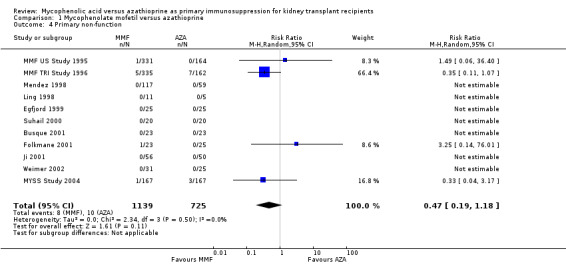
Comparison 1 Mycophenolate mofetil versus azathioprine, Outcome 4 Primary non‐function.
Statistical heterogeneity was not observed for these primary outcomes. Meta‐regression analyses (See Table 2: Meta‐regression analyses) suggested a more pronounced risk for death‐censored graft loss in AZA patients, if higher doses of MMF were used (RRR 0.26, 95%CI 0.06 to 1.24, P = 0.09; Figure 4, panel A). Neither of the remaining study level factors indicated any modification of the treatment effect. In particular, varying baseline risk for death censored graft loss as indicated by the control rate (i.e. the incidence of death censored graft loss in AZA treated patients) was not related to the magnitude of the treatment effect of MMF versus AZA (RRR 0.13, 95% CI 0.01 to 10.70, P = 0.37, Figure 4, panel B).
1. Meta‐regression analyses.
| Death (all cause) | Graft loss (censored for death) |
Malignancy (any) |
Acute rejection (any) | CMV viraemia/syndrome | CMV tissue invasive | Serum creatinine [mg/dl] | Diarrhoea | Leukopenia | |
| Number of studies | 16 | 17 | 5 | 22 | 13 | 7 | 15 | 11 | 12 |
| Study level factors | |||||||||
|
Year of transplantationa per year |
1.01 (0.86 to 1.18) |
0.99 (0.91 to 1.08) |
1.06 (0.92 to 1.22) |
1.03 (0.99 to 1.06) |
0.95 (0.83 to 1.10) |
1.08 (0.14 to 8.30) |
0.04 (‐0.03 to 0.10) |
1.16 (0.91 to 1.49) |
0.84 (0.64 to 1.11) |
|
Donor typeb Both versus deceased only Living only versus deceased only |
1.02 (0.42 to 2.47) (no living donor only studies) |
1.04 (0.57 to 1.90) (no living donor only studies) |
2.04 (0.43 to 9.71) (no living donor only studies) |
1.01 (0.77 to 1.33) 0.46 (0.05 to 4.07) |
1.33 (0.87 to 2.02) (no living donor only studies) |
‐‐ (all studies deceased donor only) |
‐0.08 (‐0.20 to 0.03) ‐0.37 (‐1.46 to 0.73) |
1.35 (0.73 to 2.50) (no living donor only studies) |
0.59 (0.34 to 1.02) 0.72 (0.01 to 35.2) |
|
Previous transplantation Yes versus 1st transplantation only |
0.97 (0.51 to 1.85) |
0.80 (0.48 to 1.34) |
0.76 (0.41 to 1.41) |
0.95 (0.74 to 1.22) |
0.81 (0.53 to 1.24) |
0.73 (0.27 to 1.97) |
‐0.13 (‐0.25 to ‐0.02) |
1.10 (0.77 to 1.57) |
0.71 (0.51 to 0.99) |
| MMF dosec per g/d | 1.20 (0.62 to 2.33) |
0.26 (0.06 to 1.24) |
0.92 (0.47 to 1.79) |
0.90 (0.74 to 1.08) |
1.31 (0.84 to 2.03) |
1.40 (0.63 to 3.10) |
0.08 (‐0.04 to 0.19) |
1.23 (0.88 to 1.72) |
1.60 (1.13 to 2.27) |
|
AZA dosed per mg/d |
0.99 (0.96 to 1.03) |
1.01 (0.98 to 1.03) |
0.98 (0.93 to 1.03) |
1.01 (1.00 to 1.01) |
1.00 (0.99 to 1.01) |
1.01 (0.97 to 1.05) |
0.004 (‐0.001 to 0.009) |
1.00 (0.98 to 1.02) |
1.00 (0.99 to 1.02) |
|
Inductione Some versus no All versus no |
1.06 (0.35 to 3.20) 1.16 (0.59 to 2.28) |
0.87 (0.42 to 1.79) 0.91 (0.54 to 1.54) |
(no studies with induction in some) 1.10 (0.58 to 2.08) |
0.80 (0.41 to 1.58) 0.82 (0.64 to 1.04) |
1.05 (0.60 to 1.85) 0.76 (0.50 to 1.15) |
2.48 (0.10 to 64.07) 1.21 (0.45 to 3.22) |
‐0.22 (‐0.42 to ‐0.01) ‐0.05 (‐0.23 to 0.12) |
(no studies with induction in some) 0.68 (0.49 to 0.96) |
(no studies with induction in some) 1.46 (1.03 to 2.08) |
|
CNI Tac versus CsA |
1.07 (0.40 to 2.82) |
1.03 (0.46 to 2.32) |
0.89 (0.08 to 10.63) |
1.08 (0.81 to 1.44) |
1.16 (0.60 to 2.24) |
1.42 (0.12 to 16.53) |
‐0.19 (‐0.31 to ‐0.06) |
0.54 (0.30 to 0.99) |
0.85 (0.44 to 1.65) |
|
CsA formulation CsA‐ME versus original or unclear |
1.03 (0.26 to 4.10) |
1.12 (0.62 to 2.04) |
1.54 (0.60 to 3.97) |
1.27 (0.98 to 1.65) |
1.24 (0.73 to 2.11) |
0.89 (0.03 to 29.84) |
0.18 (‐0.16 to 0.53) |
0.64 (0.21 to 1.95) |
1.69 (0.79 to 3.63) |
| Study quality/risk of bias factors | |||||||||
|
Blinding Yes versus no or unclear |
0.89 (0.39 to 2.06) |
1.12 (0.69 to 1.83) |
0.72 (0.30 to 1.68) |
0.87 (0.70 to 1.07) |
0.88 (0.11 to 7.13) |
0.42 (0.12 to 1.50) |
‐0.23 (‐0.98 to 0.53) |
7.16 (0.32 to 159.8) |
0.38 (0.02 to 6.15) |
|
Industry funding Yes versus no/unclear |
0.96 (0.42 to 2.19) |
1.60 (0.88 to 2.90) |
0.43 (0.05 to 3.66) |
0.84 (0.66 to 1.07) |
1.53 (0.96 to 2.41) |
1.58 (0.10 to 23.76) |
‐0.14 (‐0.25 to ‐0.02) |
0.39 (0.14 to 1.07) |
0.78 (0.40 to 1.49) |
|
Publication Full manuscript versus abstract or Transplantation Proceedings |
1.09 (0.38 to 3.12) |
1.82 (0.84 to 3.95) |
(all published as full manuscript) |
1.25 (0.80 to 1.93) |
0.14 (0.01 to 2.61) |
0.68 (0.06 to 7.44) |
0.31 (0.06 to 0.57) |
0.49 (0.12 to 2.00) |
0.75 (0.30 to 1.89) |
Meta‐regression was performed on the displayed outcomes, while data classified as “longest duration of follow‐up” were used (see Methods). Displayed are Relative Risk Ratios (RRR), i.e. back‐transformed values of the coefficient of the meta‐regression, or the untransformed coefficient of the meta‐regression for the mean difference (MD) for continuous outcome serum creatinine, along with 95% CI. All regression analyses were adjusted for duration of follow‐up. Statistical significance values of P < 0.20 are highlighted as Italic, values of P < 0.10 are bolded‐Italic , respectively.
Abbreviations: MMF: mycophenolate mofetil, AZA: azathioprine; CNI: calcineurin‐inhibitor; Tac: tacrolimus; CsA: cyclosporin A; CsA‐ME: cyclosporin A microemulsion; CMV: cytomegalovirus
Interpretation Summary effect for the outcome RR < 1 (e.g. acute rejection): RRR < 1 indicate a pronounced risk reduction for higher covariate values, while RRR > 1 indicate attenuated risk reduction. Summary effect for the outcome RR > 1 (e.g. tissue invasive CMV disease): RRR < 1 indicate attenuated risk for higher covariate values, while RRR > 1 indicate increased risk. Summary effect mean difference SCr: positive coefficients indicate greater difference in SCr, i.e. lower SCr values in AZA treated patients for higher covariate values, while negative coefficients indicate reduced difference or even negative difference in SCr, i.e. lower SCr values in MMF treated patients. Examples of various associations displayed in bubble‐plots can be found in Figure 4; Figure 6; Figure 7.
a If missing, year of first publication minus duration of follow‐up minus two years
b Both: living or deceased donor
c Studies in which more than one MMF dose was tested were split into two studies and each compared to half of the group of AZA‐patients/ outcomes
d If reported to be body‐weight‐adjusted (mg/kg/d), transformation into mg/d using the mean body weight as reported in the study, and by using 70 kg (60 kg in exclusively Asian populations) if information on body weight was missing
e Some: antibody induction therapy used in selected patients, e.g. sensitised patients or those experiencing delayed graft function
4.
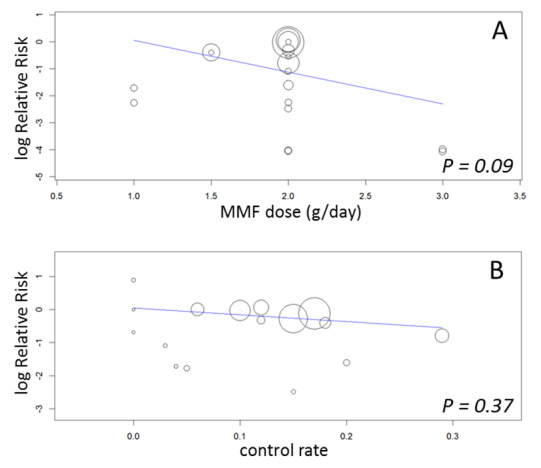
Meta‐regression of logarithmic relative risk of death censored graft loss by MMF dose (panel A) and by control rate (panel B)
Secondary outcomes
Malignancy
The summary effect for any malignancy indicated a reduced risk in MMF‐treated patients (Analysis 1.5.1 (5 studies, 1734 participants): RR 0.81, 95% CI 0.60 to 1.09; I2 = 0%), but this finding was not statistically significant. Similarly, the risk for non‐melanoma skin cancer tended to be reduced by approximately 20% in MMF treated patients, but the association did not reach statistical significance due to the limited number of studies and events (Analysis 1.5.3 (4 studies, 1416 participants): RR 0.78, 95% CI 0.46 to 1.34; I2 = 19%).
1.5. Analysis.
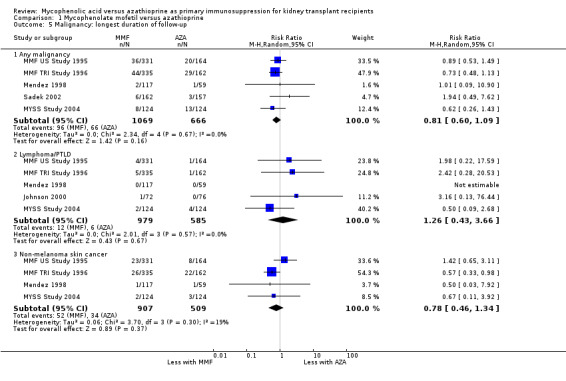
Comparison 1 Mycophenolate mofetil versus azathioprine, Outcome 5 Malignancy: longest duration of follow‐up.
Acute rejection
A consistent risk reduction for any acute rejection (about 35%) was observed with MMF‐treatment across all time intervals (Analysis 1.6.4 (22 studies, 3301 participants): RR 0.65, 95% CI 0.57 to 0.73; I2 = 9%). The effect was approximately 40% for biopsy‐proven acute rejection (Analysis 1.7.4 (12 studies, 2696 participants): RR 0.59, 95% CI 0.52 to 0.68; I2 = 0%) and approximately 50% in steroid‐resistant/antibody‐treated acute rejection (Analysis 1.8.4 (15 studies, 2914 participants): RR 0.48, 95% CI 0.36 to 0.65; I2 = 14%) both with low statistical heterogeneity. In meta‐regression analyses (see Table 2), a higher AZA dose (RRR 1.01, 95% CI 1.00 to 1.01, P = 0.10, Figure 5, panel B) and the use of CsA‐ME rather than the original CsA solution (RRR 1.27, 95% CI 0.98 to 1.65, P = 0.07) tended to attenuate the benefit of MMF versus AZA for acute rejection (i.e. a RR closer to 1, but still favouring MMF treatment). No clear signal was observed for transplantation in the most recent era (RRR 1.03, 95% CI 0.99 to 1.06, P = 0.12, Figure 5, panel A) and a higher MMF dose (RRR 0.90, 95% CI 0.74 to 1.08, P = 0.24, Figure 5, panel C). Moreover, the benefit of MMF over AZA treatment on the reduction of acute rejection was more pronounced with an increased control rate, indicating elevated immunological baseline risk of the study population (RRR 0.34, 95% CI 0.10 to 1.09, P = 0.08, Figure 5, panel D).
1.6. Analysis.

Comparison 1 Mycophenolate mofetil versus azathioprine, Outcome 6 Acute rejection: total.
1.7. Analysis.
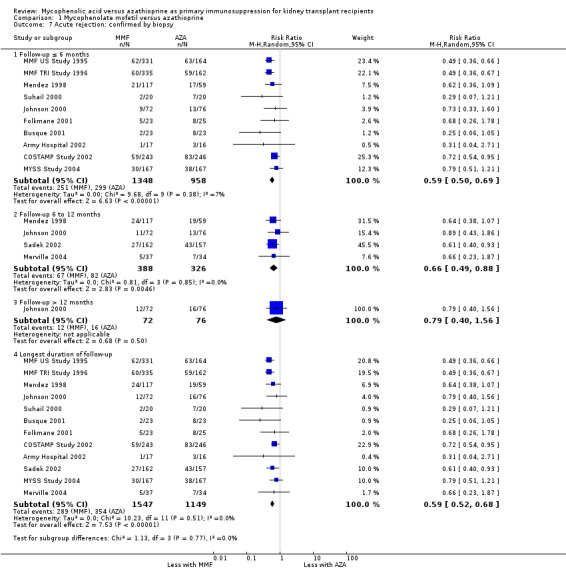
Comparison 1 Mycophenolate mofetil versus azathioprine, Outcome 7 Acute rejection: confirmed by biopsy.
1.8. Analysis.
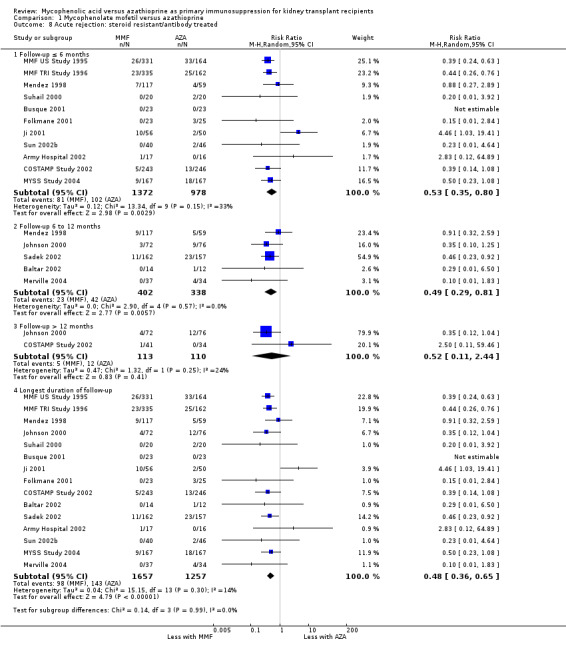
Comparison 1 Mycophenolate mofetil versus azathioprine, Outcome 8 Acute rejection: steroid resistant/antibody treated.
5.
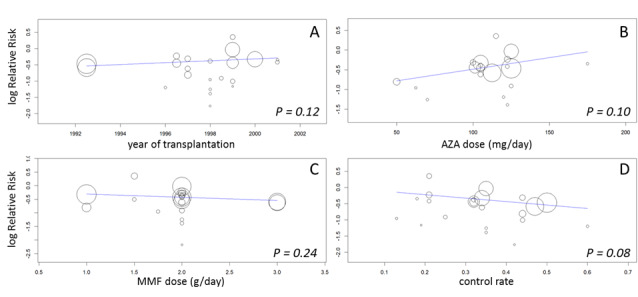
Meta‐regression of logarithmic relative risk of any acute rejection by year of transplantation (panel A), AZA‐dose (panel B), MMF‐dose (panel C) and by control rate (panel D)
Chronic allograft nephropathy
Meta‐analysis showed a significant reduction of the risk for CAN with MMF treatment (Analysis 1.9.4 (3 studies, 203 participants): RR 0.69, 95% CI 0.48 to 0.99; I2 = 0%). Two studies required diagnosis by biopsy (Merville 2004; Weimer 2002) and in one study biopsy was optional (Tuncer 2002).
1.9. Analysis.
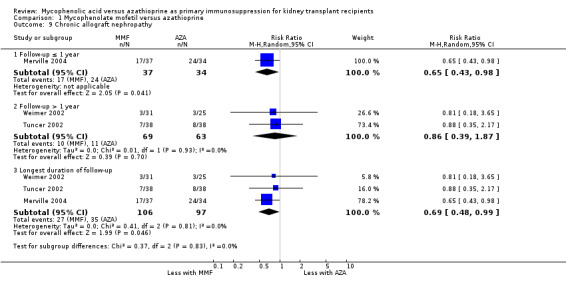
Comparison 1 Mycophenolate mofetil versus azathioprine, Outcome 9 Chronic allograft nephropathy.
Infection
Evidence on infections such as urinary tract infection/cystitis, Herpes zoster, Candida and Aspergillus infections, is limited due to inconsistent and sparse reporting (Analysis 1.10). Only CMV viraemia/syndrome was reported by a substantial number of studies and no clear signal of a benefit for any treatment was found (Analysis 1.11.3 (13 studies, 2880 participants): RR 1.06, 95% CI 0.85 to 1.32; I2 = 24%). However, in seven studies the risk of tissue‐invasive CMV disease was significantly elevated with MMF treatment (Analysis 1.12.3 (7 studies, 1510 participants): (RR 1.70, 95% CI 1.10 to 2.61; I2 = 0%). None of the tested study level factors indicated treatment effect modification in meta‐regression analyses on either CMV viraemia/syndrome or tissue‐invasive CMV disease (See Table 2). Only one study reported no observed events of PVAN (Weimer 2002). Although Pneumocystis carinii/jiroveci pneumonia (PCP) were generally rare diseases in the studies (5 studies, 9 events in 1650 patients), eight of these events occurred in AZA‐treated patients, thus resulting in a statistically significant result favouring MMF treatment (Analysis 1.10: RR 0.19, 95% CI 0.05 to 0.69; I2 = 0%).
1.10. Analysis.
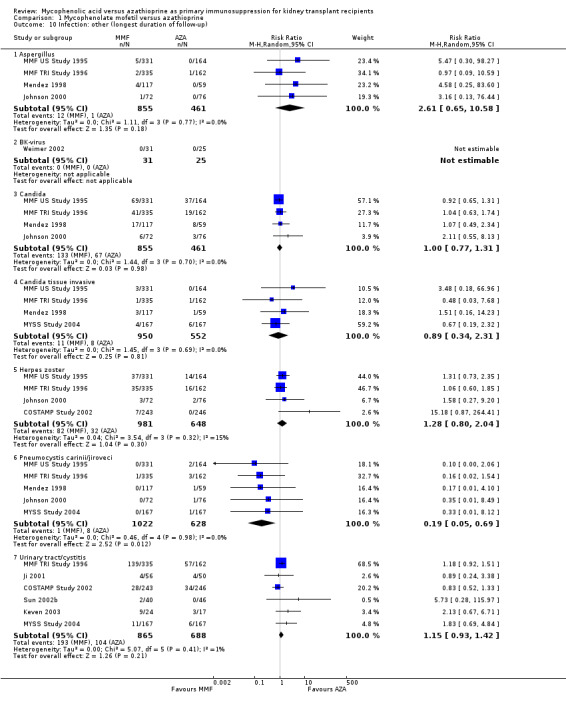
Comparison 1 Mycophenolate mofetil versus azathioprine, Outcome 10 Infection: other (longest duration of follow‐up).
1.11. Analysis.
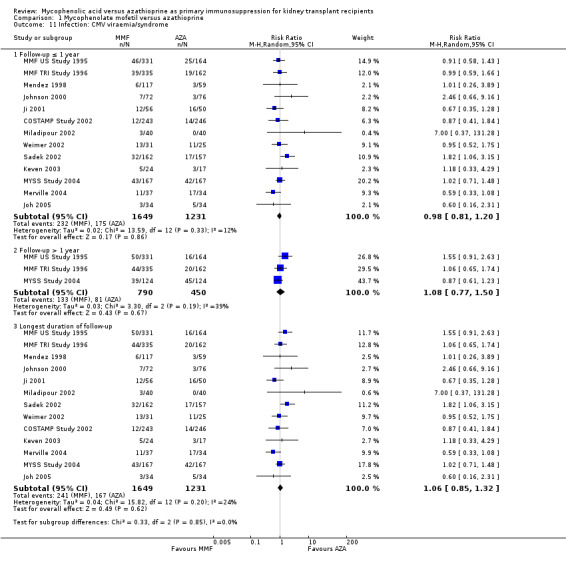
Comparison 1 Mycophenolate mofetil versus azathioprine, Outcome 11 Infection: CMV viraemia/syndrome.
1.12. Analysis.
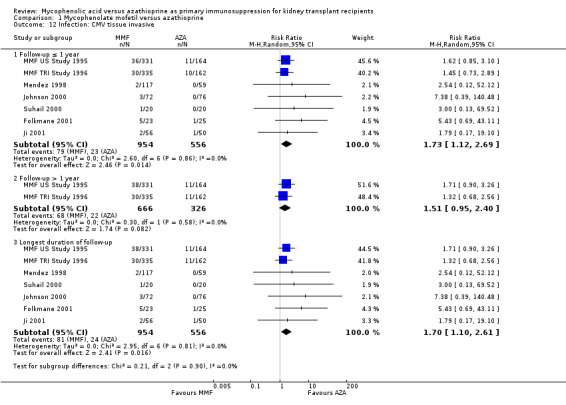
Comparison 1 Mycophenolate mofetil versus azathioprine, Outcome 12 Infection: CMV tissue invasive.
Graft function
While 15 studies reported a measure of graft function, the vast majority did not provide detailed information on either the number of patients in whom these measurements were performed (those with a functioning graft at the various time points) or the standard error/deviation of the reported mean. In general, graft function did not differ substantially at the various time intervals as indicated by point estimates between 0.01 and 0.05 mg/dL. Still, numerically, slightly lower mean values of SCr were observed in AZA treated patients (Analysis 1.13.3 (15 studies, 2233 participants): MD 0.05 mg/dL, 95% CI ‐0.05 to 0.15; I2 = 60%). Substantial heterogeneity was observed. Meta‐regression on study level factors (See Table 2) suggested even greater benefit for AZA treatment if exclusively patients receiving their first graft were studied (MD coefficient ‐0.13, 95% CI ‐0.25 to ‐0.02; P = 0.03). While higher doses of AZA tended to further enhance the benefit for AZA treatment on graft function (MD coefficient 0.004, 95% CI ‐0.001 to 0.009, P = 0.08, Figure 6, panel A), yet no such trend was found for higher doses of MMF although the point estimate of the coefficient suggested possible effect modification (MD coefficient 0.08, 95% CI ‐0.04 to 0.19, P = 0.20, Figure 6, panel B). Further measures of graft function (CrCl or GFR) were less frequently reported but demonstrated similar results (Analysis 1.14). Data on proteinuria were provided by only three studies (Merville 2004; MMF TRI Study 1996; MYSS Study 2004) and no reliable conclusions could be drawn (Analysis 1.15).
1.13. Analysis.
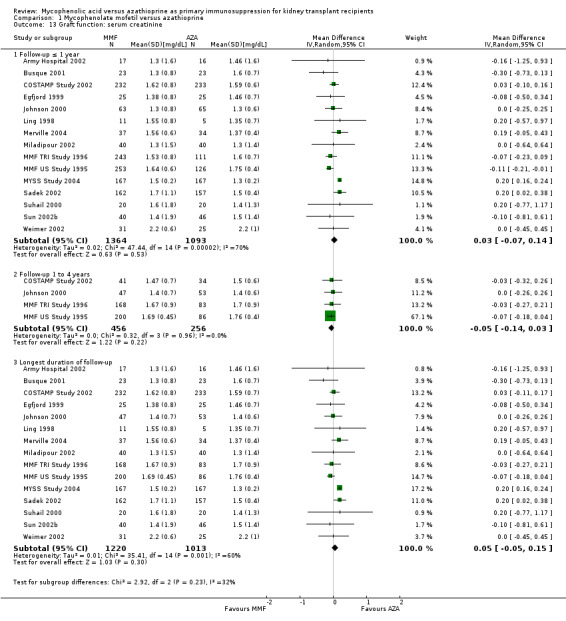
Comparison 1 Mycophenolate mofetil versus azathioprine, Outcome 13 Graft function: serum creatinine.
6.
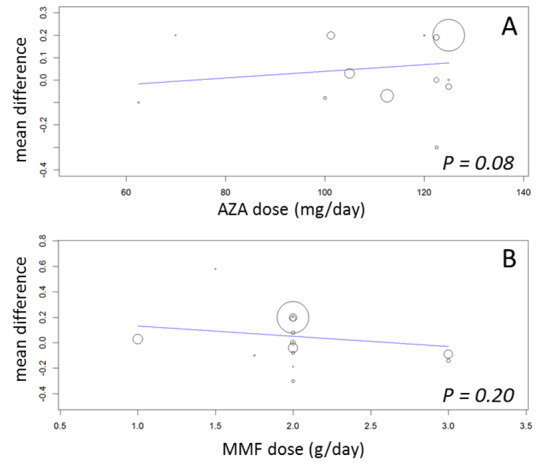
Meta‐regression of mean difference in serum creatinine (mg/dL) by AZA dose (panel A), and by MMF dose (panel B)
1.14. Analysis.
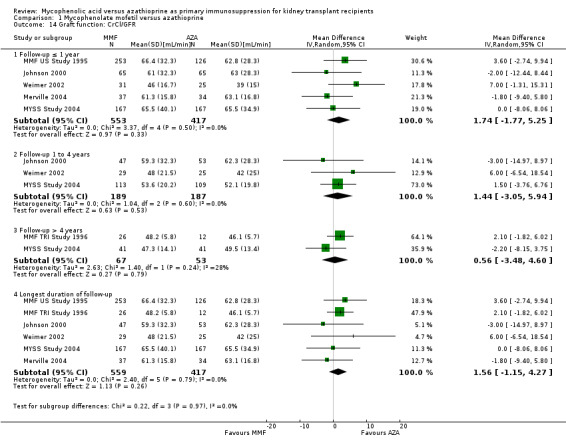
Comparison 1 Mycophenolate mofetil versus azathioprine, Outcome 14 Graft function: CrCl/GFR.
1.15. Analysis.
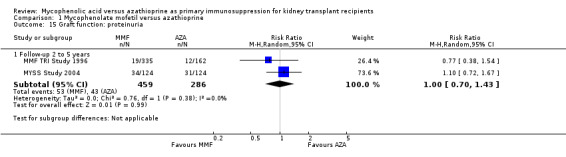
Comparison 1 Mycophenolate mofetil versus azathioprine, Outcome 15 Graft function: proteinuria.
Adverse events
Adverse events and side effects were very inconsistently reported.
Gastrointestinal disorders were more common under MMF therapy with a statistically significant difference for diarrhoea (Analysis 1.17.1 (11 studies, 2638 participants): RR 1.55, 95% CI 1.32 to 1.83; I2 = 0%) and trends for both abdominal pain (Analysis 1.17.2 (3 studies, 1311 participants): RR 1.18, 95% CI 0.97 to 1.44; I2 = 0%) and vomiting (Analysis 1.17.3 (4 studies, 1587 participants): RR 1.27, 95% CI 0.83 to 1.94; I2 = 67%). The only two studies reporting gastrointestinal bleeding suggest a significantly elevated risk in MMF treated patients (Analysis 1.17.4 (575 participants): RR 3.99, 95% CI 1.07 to 14.86; I2 = 0%).
1.17. Analysis.
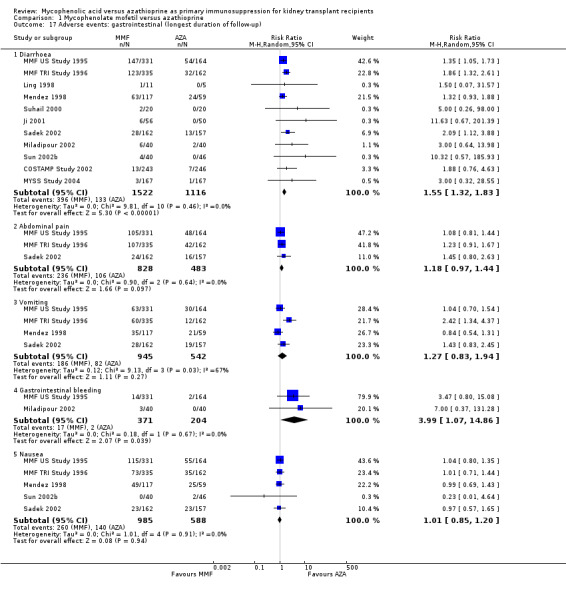
Comparison 1 Mycophenolate mofetil versus azathioprine, Outcome 17 Adverse events: gastrointestinal (longest duration of follow‐up).
Insulin‐treated NODAT was reported in four studies where the maintenance regimen was based on Tac, which itself is a known risk factor for the occurrence of NODAT (Webster 2005). The risk for NODAT was further significantly enhanced by AZA treatment, vice versa reduced by MMF (Analysis 1.18.1 (4 studies, 445 participants): RR 0.57, 95% CI 0.34 to 0.95; I2 = 0%). No clear effect of either treatment on anaemia, leucopenia, or dyslipidaemia was observed. The risk of thrombocytopenia tended to be reduced by MMF treatment (Analysis 1.19.5 (5 studies, 1492 participants): RR 0.73, 95% CI 0.52 to 1.03; I2 = 0%), as well as the risk of elevated liver enzymes (Analysis 1.18.4 (3 studies, 272 participants): RR 0.50, 95% CI 0.21 to 1.23; I2 = 50%).
1.18. Analysis.
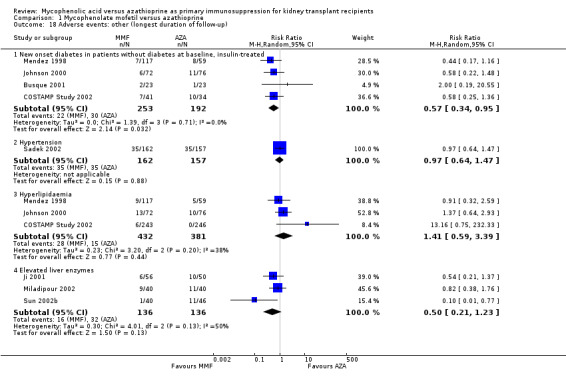
Comparison 1 Mycophenolate mofetil versus azathioprine, Outcome 18 Adverse events: other (longest duration of follow‐up).
1.19. Analysis.
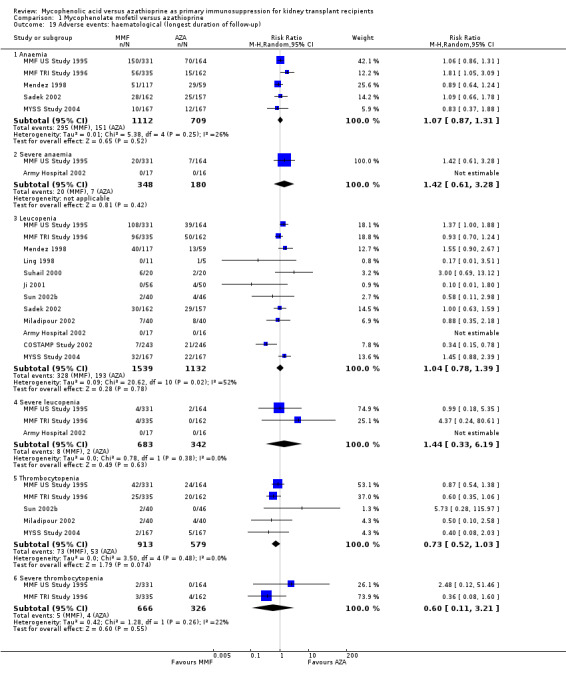
Comparison 1 Mycophenolate mofetil versus azathioprine, Outcome 19 Adverse events: haematological (longest duration of follow‐up).
Investigation of confounding, small study bias and sensitivity analyses
We performed meta‐regression analysis (Table 2) and subgroup‐analyses (Analyses 2 to 6) to investigate potential confounding by various factors (e.g. study quality factors, data‐analysis, publication type) regarding their association with the effect size of MMF versus AZA.
Confounding by study design and data analysis
Blinding of the intervention, which was only performed by the two pivotal trials (MMF TRI Study 1996; MMF US Study 1995), indicated a possible effect modification towards a greater difference in acute rejection favouring MMF treatment (RRR 0.87, 95% CI 0.70 to 1.07, P = 0.19) and a reduced risk for tissue‐invasive CMV disease (RRR 0.42, 95% CI 0.12 to 1.50, P = 0.18), but both results were not statistically significant. The two studies classified as quasi‐RCTs (Ji 2001; Joh 2005) reported a lower risk for CMV viraemia/syndrome as compared to true RCTs (Analysis 2.3). No effects on graft loss, acute rejection, or SCr were found. Stronger effects favouring MMF treatment were reported in studies where ITT analysis was unclear or certainly not performed for graft loss (Analysis 3.1) and acute rejection (Analysis 3.2). In these studies, superior graft function in MMF‐treated patients was reported (Analysis 3.4). No substantial heterogeneity of the results was observed if studies enrolling adults only were compared to studies that also enrolled children (Analysis 4).
2.3. Analysis.
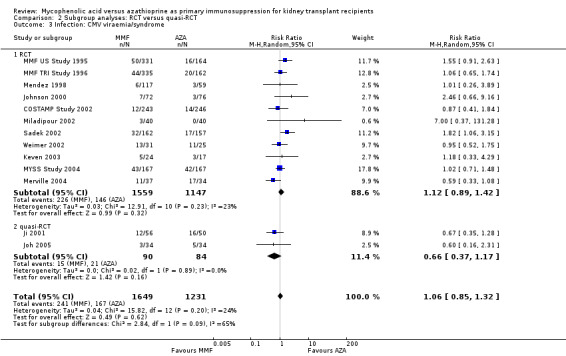
Comparison 2 Subgroup analyses: RCT versus quasi‐RCT, Outcome 3 Infection: CMV viraemia/syndrome.
3.1. Analysis.
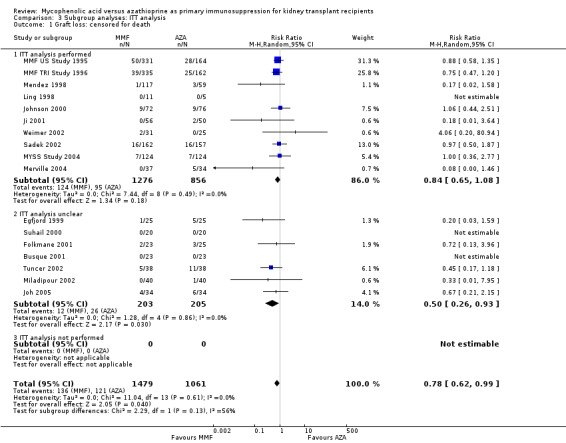
Comparison 3 Subgroup analyses: ITT analysis, Outcome 1 Graft loss: censored for death.
3.2. Analysis.
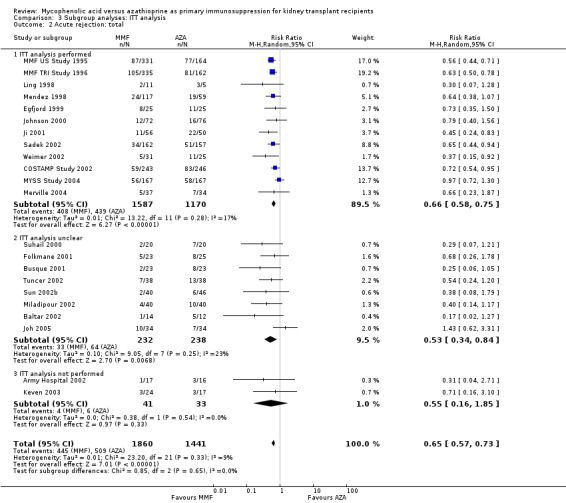
Comparison 3 Subgroup analyses: ITT analysis, Outcome 2 Acute rejection: total.
3.4. Analysis.
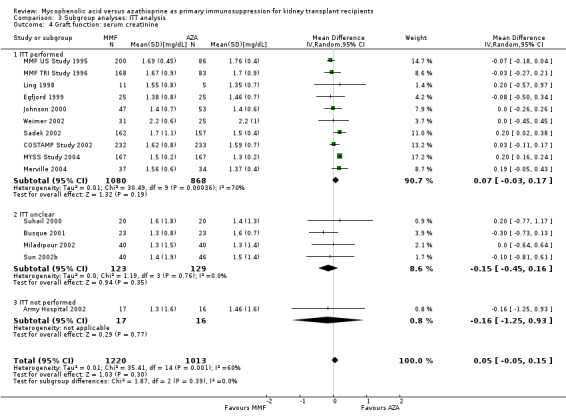
Comparison 3 Subgroup analyses: ITT analysis, Outcome 4 Graft function: serum creatinine.
Confounding by funding source and publication type
Studies clearly reporting industry funding demonstrated a potentially higher risk for CMV viraemia/syndrome in AZA treated patients (Analysis 5.3; meta‐regression RRR 1.53, 95% CI 0.96 to 2.41, P = 0.07). Studies that were explicitly not supported by industry (Merville 2004; MYSS Study 2004) reported a smaller non‐significant benefit in the reduction of acute rejection in MMF treated patients (Analysis 5.2; RRR 0.84, 95% CI 0.66 to 1.07, P = 0.17) and a significant greater mean difference in SCr favouring AZA treatment (Analysis 5.4; meta‐regression MD coefficient ‐0.14, 95% CI ‐0.25 to ‐0.02, P = 0.02). Studies published at least once as a full manuscript in a peer reviewed journal reported a somewhat attenuated risk for death‐censored graft loss (Analysis 6.1; RRR 1.82, 95% CI 0.84 to 3.95, P = 0.13). No other differences were found for the other tested outcomes.
5.3. Analysis.
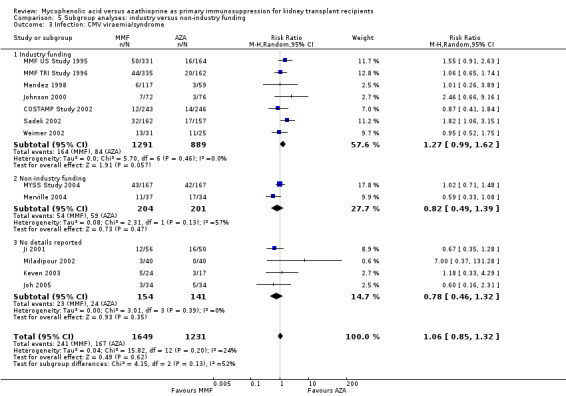
Comparison 5 Subgroup analyses: industry versus non‐industry funding, Outcome 3 Infection: CMV viraemia/syndrome.
5.2. Analysis.
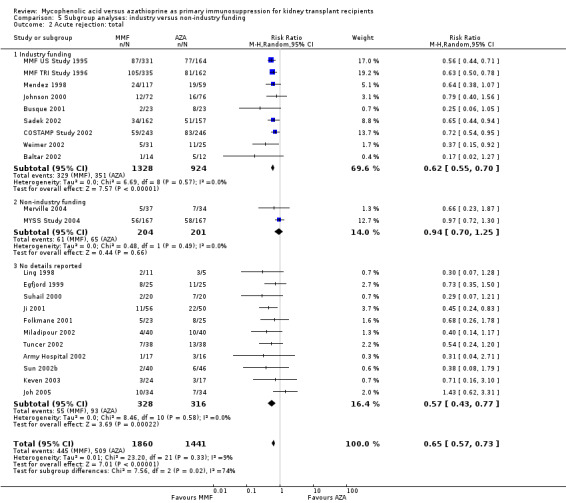
Comparison 5 Subgroup analyses: industry versus non‐industry funding, Outcome 2 Acute rejection: total.
5.4. Analysis.
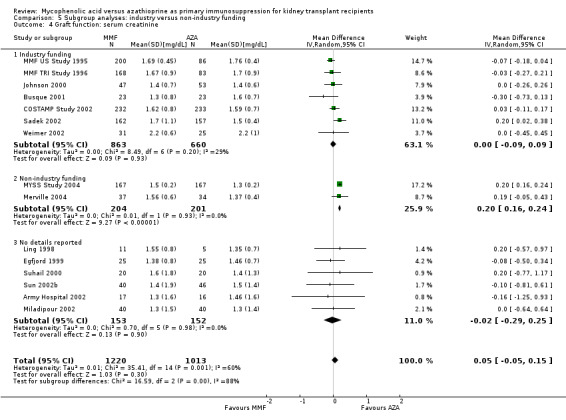
Comparison 5 Subgroup analyses: industry versus non‐industry funding, Outcome 4 Graft function: serum creatinine.
6.1. Analysis.
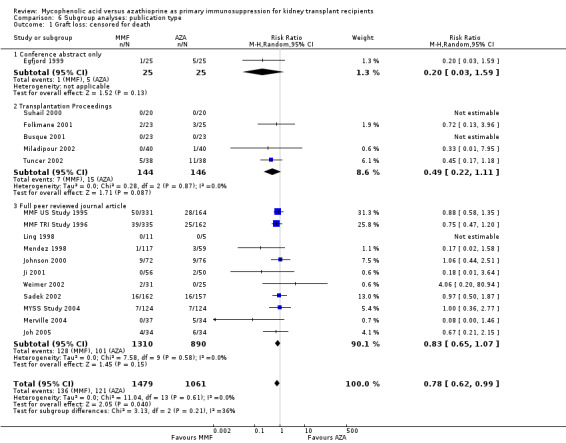
Comparison 6 Subgroup analyses: publication type, Outcome 1 Graft loss: censored for death.
Small study bias and sensitivity analyses
Investigation of funnel plots did not indicate strong signals for asymmetry (Figure 7). However, there are many explanations for why an inverted funnel plot may be asymmetric, including chance, heterogeneity, publication and reporting bias (Sterne 2011). Visual judgment of funnel plots has been shown to be misleading in empirical research (Lau 2006; Terrin 2005).
7.
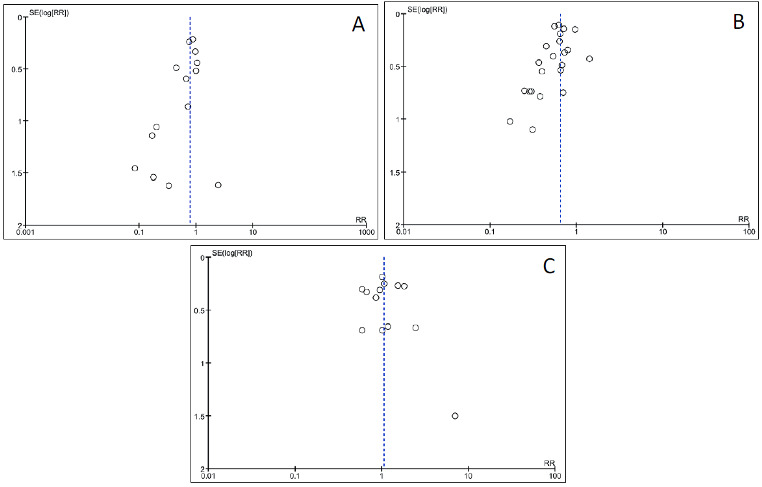
Funnel plots of outcomes. Graft loss: censored for death (panel A, Analysis 1.3); acute rejection: total (panel B, Analysis 1.6); Infection: cytomegalovirus viraemia/syndrome (panel C, Analysis 1.11)
Finally, in sensitivity analyses, the robustness of effect estimates and potential influence of single studies was tested by sequential inclusion and exclusion of each study. In general, the point estimates of all tested outcomes (mortality, death‐censored graft loss, any acute rejection, CMV viraemia/syndrome, tissue‐invasive CMV disease, SCr, diarrhoea and leucopenia) remained fairly stable in each exclusion/inclusion step. Only two studies resulted in an attenuation of significance for death‐censored graft loss (MMF TRI Study 1996: RR 0.79, 95% CI 0.60 to 1.03, P = 0.08; Egfjord 1999: RR 0.79, 95% CI 0.62 to 1.003, P = 0.053, respectively), but did not affect the magnitude of the summary effect. Similarly, by leaving out MMF US Study 1995 in tissue‐invasive CMV disease, the effect estimate did not change markedly, but the association lost significance (RR 1.76, 95% CI 0.89 to 3.48, P = 0.11). No significant changes in the mean difference for SCr were observed which would not make the difference in SCr clinically meaningful (all MD < 0.09 mg/dL).
Discussion
Summary of main results
Summarising the evidence from 23 RCTs identified for this review, MMF was superior over AZA in efficacy outcomes after kidney transplantation. In particular, MMF demonstrated a statistically significant risk reduction of about 20% for any graft loss as well as death‐censored graft loss compared to AZA. A stronger beneficial effect, although not statistically significant, was suggested in studies using higher doses of MMF. The risk of acute rejection was significantly reduced by about 35%, 40% if the rejection was proven by biopsy and 50% for more severe rejection episodes that required antibody treatment. This finding was more pronounced in studies of enhanced overall baseline risk (as indicated by a higher control rate). On the other hand, higher AZA dose and the concomitant use of CsA‐ME rather than CsA suggested an attenuated benefit from MMF over AZA treatment. Although based on sparse data, MMF treatment was related to lower rates of CAN. Graft function did not differ between the two drugs and the observed trend towards slightly lower creatinine levels of 0.05 mg/dL in AZA treated patients may not yield a clinically relevant benefit.
Evidence regarding safety outcomes was more limited since a smaller number of studies reported these. Also definitions of adverse events were rarely provided and likely varied across studies. The non‐significant summary effect for towards lower rates of malignancies in MMF‐treated patients was supported by five studies only. One study reported no events of PVAN and nine events reported in five studies showed the significantly reduced risk for PCP in MMF‐treated patients. Data on CMV viraemia/syndrome were provided by a substantial number of studies (n = 13) showing no difference between the two drugs, however tissue‐invasive CMV disease was significantly less likely in AZA‐treated patients based on seven studies only. The incidence of insulin‐dependent NODAT was reported exclusively in studies investigating Tac‐based regimens and was significantly higher in AZA‐treated patients. Gastrointestinal side effects were more common in MMF‐treated patients, while no significant differences were found for elevated liver enzymes and hematologic disturbances such as leucopenia, anaemia or thrombocytopenia.
Applying current standards to assess methodological quality of the studies (Higgins 2011) indicated that important information on factors used to judge susceptibility for bias were infrequently and inconsistently reported.
Overall completeness and applicability of evidence
Based on an exhaustive search process, we tried to comprehensively collect any published evidence for our research objectives. A large number of published abstracts were screened and many abstracts from conferences in the early and mid‐1990s were retrieved and assessed for eligibility. In total, we included 23 studies enrolling 3301 patients in our review. Study populations varied across continents and patients were treated in a multitude of different health care systems including not only the USA, Canada, various western European countries and Australia, but also China, Singapore, Korea, India, Latvia, Hungary, Turkey, Brazil and Iran. When this information was available, it appeared that studies included patients who were at low to moderate immunological baseline risk as frequently patients received their first kidney graft of a deceased donor. Further details on known markers of immunological risk, such as PRA level, HLA mismatch, or the proportion of patients of African American ancestry were inconsistently and rarely reported.
Quality of the evidence
Items of study quality such as blinding, ITT analysis, allocation concealment can help assess the risk of bias and thus judge the validity of the results. Based on the criteria defined for Cochrane reviews (Higgins 2011), most of the studies of this review lacked sufficient information on methodological items. Many of the studies were sponsored by industry, in particular by the company that held the patent on MMF. Most studies were published at least once as a full manuscript in a peer reviewed journal but a substantial number (eight studies) were conference abstracts or Transplantation Proceedings articles only and thus underwent an abbreviated peer review process. These factors (low methodological quality, industry sponsorship, and publication in non‐peer‐reviewed journals) have the potential to being associated with modification of treatment effects of over‐ or underestimation (Moher 1998; Pittler 2000; Ridker 2006). Overall, although we found evidence suggestive for the effect estimates being associated with study quality/risk of bias factors, we would not claim clinically relevant impact on the summary effects. Studies of lower quality and with unclear ITT analysis tended to overestimate efficacy results as did publications in non‐peer reviewed journals and those sponsored by industry. These studies were also more likely to report attenuated risks for tissue invasive CMV disease and MMF‐specific side effects such as diarrhoea. The most important limitation of our data is the lack of evidence and a considerably large reporting bias in particular for safety outcomes and conditional outcomes, such as graft function which naturally can be measured only in those with a functioning graft. However, numbers of patients at risk or those with a functioning graft were rarely provided.
Potential biases in the review process
We followed high standards to reduce risk of bias in the methodology of this review, such as a comprehensive literature search that was not restricted to publications in English language, article selection and data extraction performed independently by two or more authors and the collection of all potentially relevant outcomes from the included studies. However, the main limitations of the current review are two‐fold: First, while the body of evidence is fairly robust for efficacy outcomes, any conclusion on safety lacks certainty. Only few studies reported data on malignancies, and only CMV‐related diseases/infections were commonly presented. Second, most of the studies did not report outcome data with enough follow‐up to be able to detect development of specific diseases, in particular malignancies, with long induction and latent periods. Finally, most of the studies that investigated MMF versus AZA were performed in the late 1990s and early 2000, a certainly different era of kidney transplantation as we are in nowadays. During the time of these studies, outcomes of interest differed from what we judge important today: CAN or more specifically IF/TA (interstitial fibrosis/tubular atrophy in graft biopsies) and Polyoma BK/JC virus reactivation and PVAN are considered as of higher long‐term importance than acute rejection episodes, which are frequently mild and if diagnosed early can be treated and cured.
Agreements and disagreements with other studies or reviews
Our results are consistent with a systematic review by Knight 2009 who also investigated the comparative efficacy and safety of MMF versus AZA. This review used fixed‐effects models if no statistical heterogeneity was detected while we chose the more conservative random‐effects model by default given the clinical heterogeneity. Moreover, we tried to investigate potential effect modification in detail by a number of a priori defined study level and study quality factors. Another existing systematic review by Wang et al. included studies of secondary regimens (Wang 2004a; Wang 2004b; Wang 2005; Zhang 2004) and is thus not directly comparable.
Observational data can help to understand the findings of the review, in particular how benefits of MMF regarding lower rates of rejection and improved graft survival are balanced against the potential for harm such as infections and malignancies associated with stronger immunosuppressive regimens. Temporal trends in cohort studies have reported diminishing acute rejection rates but higher incidence of Polyoma BK/JC virus infections/reactivations and PVAN during the last decade (Ramos 2009), likely to be caused by the use of stronger immunosuppressive regimens, rather than by a specific agent (Brennan 2005; Snyder 2009). Polyoma BK/JC virus infections/reactivations and PVAN are characterised by impaired graft function and an aggravated risk of graft loss and the rare but life‐threatening progressive multifocal leukoencephalopathy (FDA 2008). The total burden of PVAN especially in the setting of RCTs so far has probably been underestimated since these outcomes were only rarely reported in RCTs in the past.
An about 3‐ to 4‐fold increased risk for cancer in kidney transplant recipients was described when compared to the general population (Domhan 2009). As with infections, the risk is associated with the overall level of immunosuppression rather than a specific drug (Wimmer 2007). We found a non‐significant point estimate suggesting higher risk for PTLD/lymphomas, but also trends towards fewer malignancies in general with MMF treatment, although being of stronger immunosuppressive potency. These findings could be explained by AZA directly causing accumulation of mutagenic metabolites (Domhan 2009), but data are conflicting (Kauffman 2006; Meier‐Kriesche 2003; Morath 2004; Schold 2009).
Authors' conclusions
Implications for practice.
We found that while the risks for graft loss and acute rejection were reduced by MMF treatment, graft function did not differ in a clinically relevant magnitude and evidence regarding safety outcomes was limited. Thus, it is still a major task of the transplant physician to balance benefits and harms of the two drugs and to decide which agent the individual patient should be started on. Patient’s risks and preferences should be considered to individualise this decision.
In this context it should be mentioned, that although none of the included studies tested ec‐MPS against AZA, it is unlikely that ec‐MPS treatment would have considerably changed the evidence derived from MMF‐studies. MPA is the active agent of both, MMF and ec‐MPS, but the latter was developed to limit gastrointestinal disorders since it is absorbed in the gut rather than in the stomach. Studies that have directly compared MMF versus ec‐MPS showed similar efficacy and adverse event profiles including gastrointestinal adverse events (Budde 2004; Salvadori 2004).
Another important aspect is the fact that MPA is contraindicated in pregnancy (Sifontis 2006) and frequently MMF gets replaced by AZA in transplant patients before pregnancy is attempted. The current review did not address the comparison of MPA versus AZA in secondary regimens, including the change of the immunosuppressive regimen in patients with stable graft function. However, Sadek 2002 found that replacement of MMF by AZA three months post‐transplant overall was safe and effective up to 12 months follow‐up in this study.
Finally, a general limitation of how results from meta‐analyses can be applied to the individual patient should briefly be mentioned. Patients enrolled in RCTs (which subsequently get summarised in meta‐analyses) typically differ from each other in their baseline risk for achieving the outcome of interest. Although being equally distributed between treatment and control group, frequently a higher risk group of patients may experience most of the events that drive the main results of the intervention (Kent 2007). The phenomenon of varying treatment effects dependent on baseline risk is likely to be relevant in the setting of kidney transplantation (Wagner 2009). Meta‐analyses and meta‐regression analyses are not helpful to identify the benefits and harms of a particular treatment to the individual patient (Schmid 2004).
Implications for research.
Our review highlights the need for consistent ascertainment and reporting of adverse events in kidney transplant intervention studies (Ioannidis 2004), including infections (e.g. Polyoma BK virus) and malignancies. Further, the deficiencies in the reporting of study quality items point to the need for editors to hold kidney transplant trialists to universal reporting guidelines, such as the CONSORT statement (Moher 2001).
As most of the evidence about MMF versus AZA is based on the late 1990s and early 2000s, it will be interesting how the two drugs compare in the current era. The ongoing ATHENA Study 2012 will provide insights about the two proliferation‐inhibitors in a low‐dose CNI regimen with scheduled CNI withdrawal on CAN and PVAN.
Support for the every‐day decision on which agent (MMF or AZA) a kidney transplant recipient should be started on could be addressed by decision analyses in which the benefits and harms are weighted against each other in various settings. Another approach could be to stratify patients in RCTs at baseline according to immunological risk. The benefits and harms of certain therapies (e.g. MMF versus AZA) could then be investigated across all as well as within subgroups of lower, moderate, and higher risk patients (Wagner 2009). With these endeavours, research in kidney transplantation can make one important step forward to individualise medical therapy and towards choosing the best immunosuppressive regimen for a particular patient.
Acknowledgements
We would like to acknowledge the enormous support and help with this review by all members of the Cochrane Kidney and Transplant Group: Gail Higgins, Ann Jones, Ruth Mitchell, and Narelle Willis. Editorial advice on preparing the protocol was given by Sir Peter Morris, Dr Julio Pascual and Dr Sapna Shah, which is greatly appreciated.
We thank Kerstin Meister, Elisabeth Friedrich (University of Würzburg, Germany) and Audrey Mahoney (Tufts University, Boston, USA), for helping with article retrieval.
The help of Drs Mei Chung, Cindy Huang (Tufts University, Boston, USA) and Dr Kai Hu (University of Würzburg, Germany) for translating Chinese articles and Dr Jose Calvo (Tufts University, Boston, USA) for translating Spanish articles, respectively, is greatly appreciated.
Dr Thomas Trikalinos (Brown University, Providence, USA) and Gowri Raman (Tufts University, Boston, USA) helped with abstract screening and study selection, data management was supported by Ilonka Pecik (University of Würzburg, Germany) and statistical analyses with Meta‐Analyst were made possible with the help of Drs Trikalinos and Byron Wallace (Brown University, Providence, USA), which is all thankfully acknowledged.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Mycophenolate mofetil versus azathioprine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death: all cause | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Follow‐up ≤ 1 year | 16 | 2987 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.76, 1.68] |
| 1.2 Follow‐up 1 to 4 years | 7 | 1595 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.20] |
| 1.3 Follow‐up > 4 years | 3 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.59, 1.07] |
| 1.4 Longest duration of follow‐up | 16 | 2987 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.70, 1.29] |
| 2 Graft loss: including death | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Follow‐up ≤ 1 year | 15 | 2653 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.62, 1.02] |
| 2.2 Follow‐up 1 to 4 years | 6 | 1347 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.66, 1.04] |
| 2.3 Follow‐up > 4 years | 2 | 209 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.29, 1.66] |
| 2.4 Longest duration of follow‐up | 15 | 2653 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.67, 1.00] |
| 3 Graft loss: censored for death | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Follow‐up ≤ 1 year | 15 | 2384 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.49, 0.94] |
| 3.2 Follow‐up 1 to 4 years | 6 | 1512 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.13] |
| 3.3 Follow‐up > 4 years | 4 | 525 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.60, 1.25] |
| 3.4 Longest duration of follow‐up | 17 | 2540 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.62, 0.99] |
| 4 Primary non‐function | 11 | 1864 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.19, 1.18] |
| 5 Malignancy: longest duration of follow‐up | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Any malignancy | 5 | 1735 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.60, 1.09] |
| 5.2 Lymphoma/PTLD | 5 | 1564 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.43, 3.66] |
| 5.3 Non‐melanoma skin cancer | 4 | 1416 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.46, 1.34] |
| 6 Acute rejection: total | 22 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Follow‐up ≤ 6 months | 19 | 3128 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.55, 0.75] |
| 6.2 Follow‐up 6 to 12 months | 10 | 2086 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.58, 0.74] |
| 6.3 Follow‐up > 12 months | 5 | 603 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.61, 0.99] |
| 6.4 Longest duration of follow‐up | 22 | 3301 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.57, 0.73] |
| 7 Acute rejection: confirmed by biopsy | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Follow‐up ≤ 6 months | 10 | 2306 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.50, 0.69] |
| 7.2 Follow‐up 6 to 12 months | 4 | 714 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.49, 0.88] |
| 7.3 Follow‐up > 12 months | 1 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.40, 1.56] |
| 7.4 Longest duration of follow‐up | 12 | 2696 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.52, 0.68] |
| 8 Acute rejection: steroid resistant/antibody treated | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Follow‐up ≤ 6 months | 11 | 2350 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.35, 0.80] |
| 8.2 Follow‐up 6 to 12 months | 5 | 740 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.29, 0.81] |
| 8.3 Follow‐up > 12 months | 2 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.11, 2.44] |
| 8.4 Longest duration of follow‐up | 15 | 2914 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.36, 0.65] |
| 9 Chronic allograft nephropathy | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Follow‐up ≤ 1 year | 1 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.43, 0.98] |
| 9.2 Follow‐up > 1 year | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.39, 1.87] |
| 9.3 Longest duration of follow‐up | 3 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.48, 0.99] |
| 10 Infection: other (longest duration of follow‐up) | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Aspergillus | 4 | 1316 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [0.65, 10.58] |
| 10.2 BK‐virus | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Candida | 4 | 1316 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.77, 1.31] |
| 10.4 Candida tissue invasive | 4 | 1502 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.34, 2.31] |
| 10.5 Herpes zoster | 4 | 1629 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.80, 2.04] |
| 10.6 Pneumocystis carinii/jiroveci | 5 | 1650 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.05, 0.69] |
| 10.7 Urinary tract/cystitis | 6 | 1553 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.93, 1.42] |
| 11 Infection: CMV viraemia/syndrome | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Follow‐up ≤ 1 year | 13 | 2880 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.81, 1.20] |
| 11.2 Follow‐up > 1 year | 3 | 1240 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.77, 1.50] |
| 11.3 Longest duration of follow‐up | 13 | 2880 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.85, 1.32] |
| 12 Infection: CMV tissue invasive | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 Follow‐up ≤ 1 year | 7 | 1510 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.12, 2.69] |
| 12.2 Follow‐up > 1 year | 2 | 992 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.95, 2.40] |
| 12.3 Longest duration of follow‐up | 7 | 1510 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [1.10, 2.61] |
| 13 Graft function: serum creatinine | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 Follow‐up ≤ 1 year | 15 | 2457 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.07, 0.14] |
| 13.2 Follow‐up 1 to 4 years | 4 | 712 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.14, 0.03] |
| 13.3 Longest duration of follow‐up | 15 | 2233 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.05, 0.15] |
| 14 Graft function: CrCl/GFR | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 Follow‐up ≤ 1 year | 5 | 970 | Mean Difference (IV, Random, 95% CI) | 1.74 [‐1.77, 5.25] |
| 14.2 Follow‐up 1 to 4 years | 3 | 376 | Mean Difference (IV, Random, 95% CI) | 1.44 [‐3.05, 5.94] |
| 14.3 Follow‐up > 4 years | 2 | 120 | Mean Difference (IV, Random, 95% CI) | 0.56 [‐3.48, 4.60] |
| 14.4 Longest duration of follow‐up | 6 | 976 | Mean Difference (IV, Random, 95% CI) | 1.56 [‐1.15, 4.27] |
| 15 Graft function: proteinuria | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 Follow‐up 2 to 5 years | 2 | 745 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.70, 1.43] |
| 16 Graft function: proteinuria | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 16.1 Follow‐up ≤ 1 year | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Adverse events: gastrointestinal (longest duration of follow‐up) | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 Diarrhoea | 11 | 2638 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.32, 1.83] |
| 17.2 Abdominal pain | 3 | 1311 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.97, 1.44] |
| 17.3 Vomiting | 4 | 1487 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.83, 1.94] |
| 17.4 Gastrointestinal bleeding | 2 | 575 | Risk Ratio (M‐H, Random, 95% CI) | 3.99 [1.07, 14.86] |
| 17.5 Nausea | 5 | 1573 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.85, 1.20] |
| 18 Adverse events: other (longest duration of follow‐up) | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 New onset diabetes in patients without diabetes at baseline, insulin‐treated | 4 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.34, 0.95] |
| 18.2 Hypertension | 1 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.64, 1.47] |
| 18.3 Hyperlipidaemia | 3 | 813 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.59, 3.39] |
| 18.4 Elevated liver enzymes | 3 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.21, 1.23] |
| 19 Adverse events: haematological (longest duration of follow‐up) | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.1 Anaemia | 5 | 1821 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.87, 1.31] |
| 19.2 Severe anaemia | 2 | 528 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.61, 3.28] |
| 19.3 Leucopenia | 12 | 2671 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.78, 1.39] |
| 19.4 Severe leucopenia | 3 | 1025 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.33, 6.19] |
| 19.5 Thrombocytopenia | 5 | 1492 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.52, 1.03] |
| 19.6 Severe thrombocytopenia | 2 | 992 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.11, 3.21] |
| 20 Total cholesterol | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 Follow‐up ≤ 1 year | 2 | 219 | Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐15.65, 10.51] |
1.16. Analysis.
Comparison 1 Mycophenolate mofetil versus azathioprine, Outcome 16 Graft function: proteinuria.
1.20. Analysis.
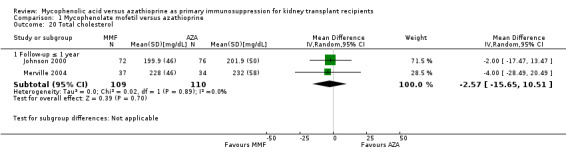
Comparison 1 Mycophenolate mofetil versus azathioprine, Outcome 20 Total cholesterol.
Comparison 2. Subgroup analyses: RCT versus quasi‐RCT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Graft loss: censored for death | 17 | 2540 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.62, 0.99] |
| 1.1 RCT | 15 | 2366 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.62, 1.01] |
| 1.2 quasi‐RCT | 2 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.19, 1.67] |
| 2 Acute rejection (total) | 22 | 3301 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.57, 0.73] |
| 2.1 RCT | 20 | 3127 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.58, 0.72] |
| 2.2 quasi‐RCT | 2 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.25, 2.40] |
| 3 Infection: CMV viraemia/syndrome | 13 | 2880 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.85, 1.32] |
| 3.1 RCT | 11 | 2706 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.89, 1.42] |
| 3.2 quasi‐RCT | 2 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.37, 1.17] |
| 4 Graft function, serum creatinine | 15 | 2233 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.05, 0.15] |
| 4.1 RCT | 15 | 2233 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.05, 0.15] |
| 4.2 quasi‐RCT | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
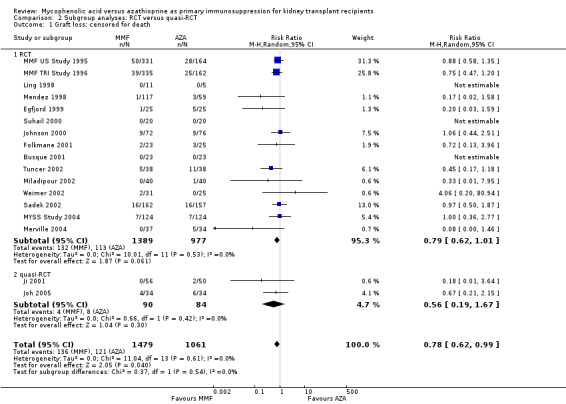
Comparison 2 Subgroup analyses: RCT versus quasi‐RCT, Outcome 1 Graft loss: censored for death.
2.2. Analysis.
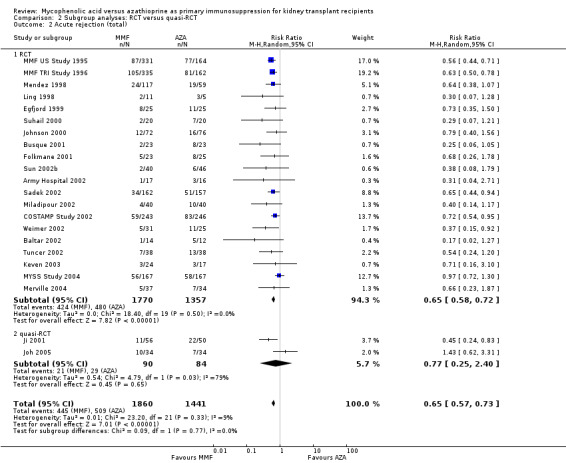
Comparison 2 Subgroup analyses: RCT versus quasi‐RCT, Outcome 2 Acute rejection (total).
2.4. Analysis.
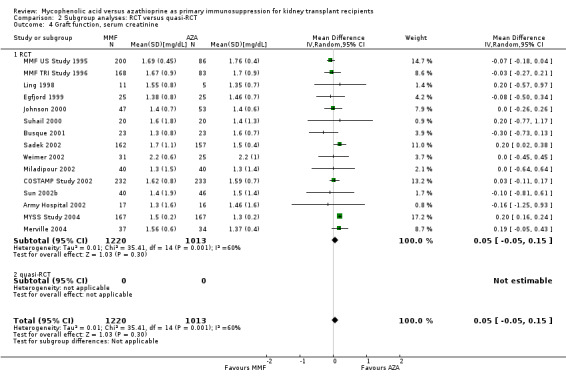
Comparison 2 Subgroup analyses: RCT versus quasi‐RCT, Outcome 4 Graft function, serum creatinine.
Comparison 3. Subgroup analyses: ITT analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Graft loss: censored for death | 17 | 2540 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.62, 0.99] |
| 1.1 ITT analysis performed | 10 | 2132 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.65, 1.08] |
| 1.2 ITT analysis unclear | 7 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.26, 0.93] |
| 1.3 ITT analysis not performed | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Acute rejection: total | 22 | 3301 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.57, 0.73] |
| 2.1 ITT analysis performed | 12 | 2757 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.58, 0.75] |
| 2.2 ITT analysis unclear | 8 | 470 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.34, 0.84] |
| 2.3 ITT analysis not performed | 2 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.16, 1.85] |
| 3 Infection: CMV viraemia/syndrome | 13 | 2880 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.85, 1.32] |
| 3.1 ITT analysis performed | 10 | 2691 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.83, 1.34] |
| 3.2 ITT analysis unclear | 2 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.13, 16.10] |
| 3.3 ITT analysis not performed | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.33, 4.29] |
| 4 Graft function: serum creatinine | 15 | 2233 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.05, 0.15] |
| 4.1 ITT performed | 10 | 1948 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.03, 0.17] |
| 4.2 ITT unclear | 4 | 252 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.45, 0.16] |
| 4.3 ITT not performed | 1 | 33 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐1.25, 0.93] |
3.3. Analysis.

Comparison 3 Subgroup analyses: ITT analysis, Outcome 3 Infection: CMV viraemia/syndrome.
Comparison 4. Subgroup analyses: adults only versus children included.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Graft loss: censored for death | 17 | 2540 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.62, 0.99] |
| 1.1 Adults only | 6 | 1676 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.64, 1.10] |
| 1.2 Children included | 2 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.10, 3.15] |
| 1.3 No details reported | 9 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.28, 0.95] |
| 2 Acute rejection: total | 22 | 3301 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.57, 0.73] |
| 2.1 Adults only | 8 | 2292 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.57, 0.79] |
| 2.2 Children included | 2 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.46, 1.04] |
| 2.3 No details reported | 12 | 685 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.40, 0.71] |
| 3 Infection: CMV viraemia/syndrome | 13 | 2880 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.85, 1.32] |
| 3.1 Adults only | 7 | 2246 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.84, 1.45] |
| 3.2 Children included | 2 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.62, 4.09] |
| 3.3 No details reported | 4 | 310 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.54, 1.24] |
| 4 Graft function: serum creatinine | 15 | 2233 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.05, 0.15] |
| 4.1 Adults only | 7 | 1772 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.06, 0.19] |
| 4.2 Children included | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.26, 0.26] |
| 4.3 No details reported | 7 | 361 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.24, 0.22] |
4.1. Analysis.
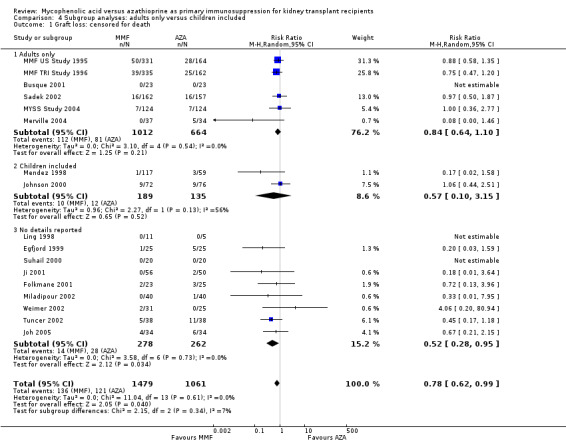
Comparison 4 Subgroup analyses: adults only versus children included, Outcome 1 Graft loss: censored for death.
4.2. Analysis.
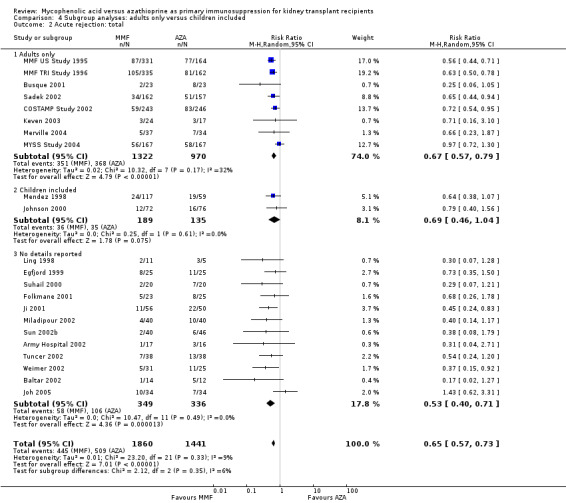
Comparison 4 Subgroup analyses: adults only versus children included, Outcome 2 Acute rejection: total.
4.3. Analysis.
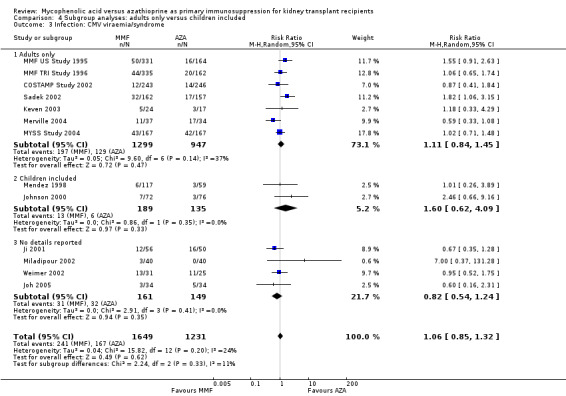
Comparison 4 Subgroup analyses: adults only versus children included, Outcome 3 Infection: CMV viraemia/syndrome.
4.4. Analysis.
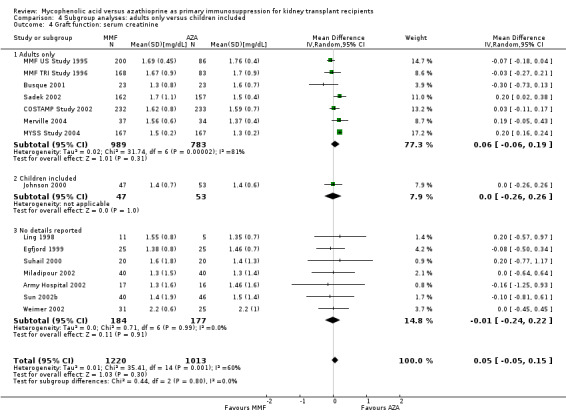
Comparison 4 Subgroup analyses: adults only versus children included, Outcome 4 Graft function: serum creatinine.
Comparison 5. Subgroup analyses: industry versus non‐industry funding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Graft loss: censored for death | 17 | 2540 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.62, 0.99] |
| 1.1 Industry funding | 7 | 1737 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.66, 1.12] |
| 1.2 Non‐industry funding | 2 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.03, 4.74] |
| 1.3 No details reported | 8 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.88] |
| 2 Acute rejection: total | 22 | 3301 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.57, 0.73] |
| 2.1 Industry funding | 9 | 2252 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.55, 0.70] |
| 2.2 Non‐industry funding | 2 | 405 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.70, 1.25] |
| 2.3 No details reported | 11 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.43, 0.77] |
| 3 Infection: CMV viraemia/syndrome | 13 | 2880 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.85, 1.32] |
| 3.1 Industry funding | 7 | 2180 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.99, 1.62] |
| 3.2 Non‐industry funding | 2 | 405 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.49, 1.39] |
| 3.3 No details reported | 4 | 295 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.46, 1.32] |
| 4 Graft function: serum creatinine | 15 | 2233 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.05, 0.15] |
| 4.1 Industry funding | 7 | 1523 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.09, 0.09] |
| 4.2 Non‐industry funding | 2 | 405 | Mean Difference (IV, Random, 95% CI) | 0.20 [0.16, 0.24] |
| 4.3 No details reported | 6 | 305 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.29, 0.25] |
5.1. Analysis.
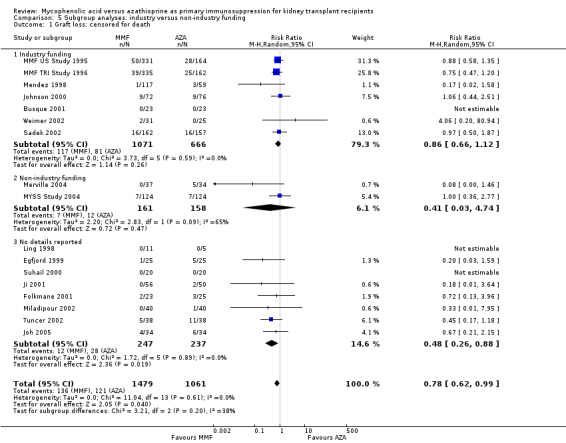
Comparison 5 Subgroup analyses: industry versus non‐industry funding, Outcome 1 Graft loss: censored for death.
Comparison 6. Subgroup analyses: publication type.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Graft loss: censored for death | 17 | 2540 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.62, 0.99] |
| 1.1 Conference abstract only | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.03, 1.59] |
| 1.2 Transplantation Proceedings | 5 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.22, 1.11] |
| 1.3 Full peer reviewed journal article | 11 | 2200 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.65, 1.07] |
| 2 Acute rejection: total | 22 | 3301 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.57, 0.73] |
| 2.1 Conference abstract only | 2 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.34, 1.33] |
| 2.2 Transplantation Proceedings | 5 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.29, 0.74] |
| 2.3 Full peer reviewed journal article | 15 | 2928 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.57, 0.76] |
| 3 Infection: CMV viraemia/syndrome | 13 | 2880 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.85, 1.32] |
| 3.1 Conference abstract only | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Transplantation Proceedings | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.37, 131.28] |
| 3.3 Full peer reviewed journal article | 12 | 2800 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.84, 1.30] |
| 4 Graft function: serum creatinine | 15 | 2233 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.05, 0.15] |
| 4.1 Conference abstract only | 2 | 83 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.48, 0.30] |
| 4.2 Transplantation Proceedings | 3 | 166 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.49, 0.18] |
| 4.3 Full peer reviewed journal article | 10 | 1984 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.03, 0.18] |
6.2. Analysis.
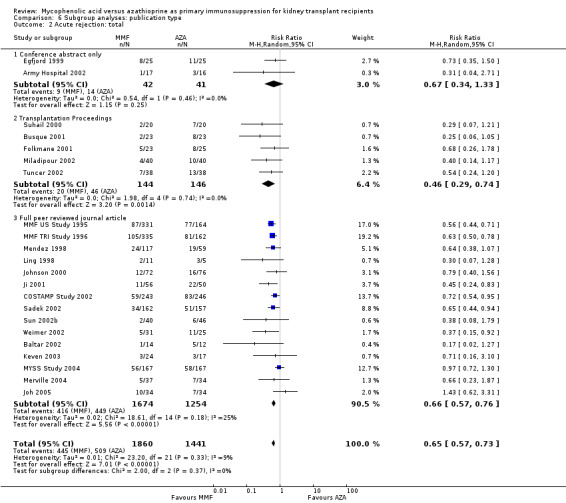
Comparison 6 Subgroup analyses: publication type, Outcome 2 Acute rejection: total.
6.3. Analysis.
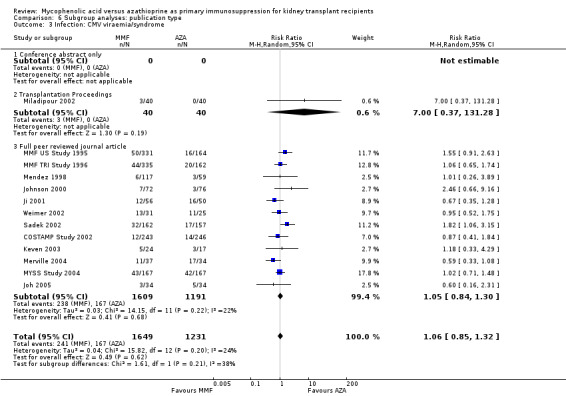
Comparison 6 Subgroup analyses: publication type, Outcome 3 Infection: CMV viraemia/syndrome.
6.4. Analysis.
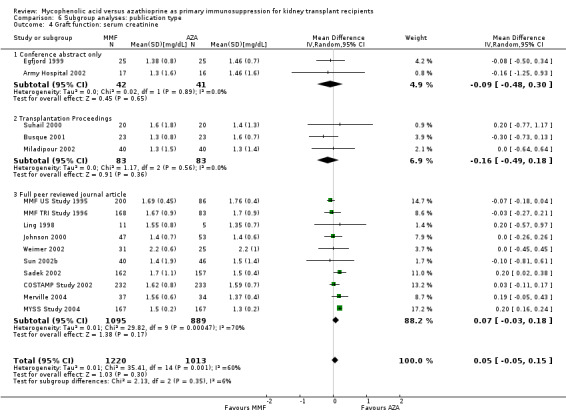
Comparison 6 Subgroup analyses: publication type, Outcome 4 Graft function: serum creatinine.
Characteristics of studies
Characteristics of included studies [ordered by year of study]
MMF US Study 1995.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment |
| Allocation concealment (selection bias) | Low risk | The study was double‐blind |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was double‐blind. Investigators and patients were blinded until all patients had been in the study for 1 year |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis reported; four patients never received study drug and were excluded from some of the analysis; all patients followed‐up or accounted for |
| Selective reporting (reporting bias) | Low risk | The published report included all expected outcomes regarding efficacy and safety |
| Other bias | High risk | This study was sponsored by a grant from Syntex (U.S.A.), Inc., Palo Alto, CA. Syntex (funder) was unblinded to provide the results of the efficacy analysis for all patients as of 6 months after transplant and the results of the analyses of safety data collected for patients as of the data cut‐off date of March 4, 1994 |
MMF TRI Study 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment |
| Allocation concealment (selection bias) | Low risk | The study was double‐blind |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinding of investigators and patients continued throughout the 3 years of this study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis reported, six patients never received study drug and were excluded from some of the analysis; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | The published report included all expected outcomes regarding efficacy and safety |
| Other bias | High risk | This study was supported by Roche Pharmaceuticals |
Isbel 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported, presumably open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis unclear; number randomised or number of patients at the end of the study not reported, data only available from conference abstract |
| Selective reporting (reporting bias) | High risk | No relevant data for this review ‐ data only available from conference abstract |
| Other bias | Unclear risk | Funding source not reported |
Mendez 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated, but no information on allocation method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis reported; eleven patients never received study drug or kidney graft and were excluded from the analyses; all patients followed up or accounted for. 11 patients in the AZA group discontinued AZA and crossed over to the MMF group |
| Selective reporting (reporting bias) | Low risk | The published report included all expected outcomes regarding efficacy and safety |
| Other bias | High risk | This study was supported by a grant from Fujisawa Healthcare, Inc, Deerfield, IL. |
Ling 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated, but no information on allocation method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information on blinding available (no blinding assumed) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis unclear; all patients followed‐up |
| Selective reporting (reporting bias) | Low risk | The published report included most expected outcomes regarding efficacy and safety |
| Other bias | Unclear risk | Funding source not reported |
Egfjord 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated, but no information on allocation method used is available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information on blinding available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis unclear; number randomised or number of patients at the end of the study was not reported. (data only available from conference abstract) |
| Selective reporting (reporting bias) | High risk | The published report included few outcomes regarding efficacy and safety. (data only available from conference abstract) |
| Other bias | Unclear risk | Funding source not reported |
Johnson 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated, but no information on method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis reported; all patients followed up or accounted for; twelve patients discontinued study drug |
| Selective reporting (reporting bias) | Low risk | The published report included all expected outcomes regarding efficacy and safety. Relevant outcomes (efficacy and safety) for this review have been reported |
| Other bias | High risk | This study was supported by a grant from Fujisawa Healthcare, Inc, Deerfield, IL |
Suhail 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated, but no information on method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis unclear; number randomised or number of patients at the end of the study was not reported |
| Selective reporting (reporting bias) | Low risk | The published report included most expected outcomes regarding efficacy and safety |
| Other bias | Unclear risk | Funding source not reported |
Folkmane 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated, but no information on allocation method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information on blinding available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis unclear; number randomised or number of patients at the end of the study was not reported |
| Selective reporting (reporting bias) | High risk | The published report included few outcomes regarding efficacy and safety |
| Other bias | Unclear risk | Funding source not reported |
Busque 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated, but no information on allocation method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis unclear; number randomised or number of patients at the end of the study was not reported |
| Selective reporting (reporting bias) | Low risk | The published report included most expected outcomes regarding efficacy and safety |
| Other bias | High risk | The study was supported by Fujisawa Canada Inc. |
Ji 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐RCT, patients allocated in alternating sequence |
| Allocation concealment (selection bias) | High risk | Patients were consecutively enrolled and were allocated to the treatment arms in alternating sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis unclear; all patients followed‐up |
| Selective reporting (reporting bias) | Low risk | The published report included most expected outcomes regarding efficacy and safety |
| Other bias | Low risk | The study was funded by a university grant |
Weimer 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated, but no information on allocation method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis reported; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | The published report included most expected outcomes regarding efficacy and safety |
| Other bias | High risk | The study was supported in part by the Fujisawa, Roche, Novartis, Biotest and Fresenius companies |
Sun 2002b.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated, but no information on allocation method used is available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information on blinding available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis unclear; all patients followed‐up. |
| Selective reporting (reporting bias) | Low risk | The published report included most expected outcomes regarding efficacy and safety. |
| Other bias | Unclear risk | Funding source not reported |
Tuncer 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated, but no information on allocation method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "non‐blinded study" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis unclear; all patients were followed up |
| Selective reporting (reporting bias) | Unclear risk | The published report included few outcomes regarding efficacy and safety |
| Other bias | Unclear risk | Funding source not reported |
Army Hospital 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated, but no information on allocation method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information on blinding available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis unclear; number randomised and number of patients at the end of the study not reported. (data only available from conference abstract) |
| Selective reporting (reporting bias) | High risk | The published report included few outcomes regarding efficacy and safety. (data only available from conference abstract) |
| Other bias | Unclear risk | Funding source not reported |
Sadek 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “...randomization was [...] performed with the Almedica Drug Labeling System (Almedica Service Corp., Allendale, New Jersey). The numbers assigned to each center were sequential within each stratum.” |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated, but no information on allocation method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis reported, all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | The published report included all expected outcomes regarding efficacy and safety |
| Other bias | High risk | This study was supported by a grant from Novartis Pharma AG, Basel, Switzerland |
Miladipour 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated, but no information on allocation method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information on blinding available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis unclear; all patients followed up |
| Selective reporting (reporting bias) | Low risk | The published report included most outcomes regarding efficacy and safety |
| Other bias | Unclear risk | Funding source not reported |
COSTAMP Study 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated, but no information on allocation method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “The histological evaluation of the biopsy was performed by the local histopathologist who was blinded towards the patient’s treatment.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis reported, all patients followed up or accounted for. The majority of patients (452 patients; 92 %) completed the study |
| Selective reporting (reporting bias) | Low risk | The published report included most expected outcomes regarding efficacy and safety |
| Other bias | High risk | This study was supported by Fujisawa GmbH, Munich, Germany |
Baltar 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated, but no information on allocation method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis unclear; all patients followed up |
| Selective reporting (reporting bias) | High risk | The published report included very few outcomes regarding efficacy |
| Other bias | High risk | The study was supported by Roche |
Keven 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated, but no information on allocation method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information on blinding available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT analysis: eight patients not analysed due to reported reasons (e.g. diarrhoea, proteinuria, etc.); however, all patients followed up |
| Selective reporting (reporting bias) | High risk | The published report included very few outcomes regarding efficacy and safety |
| Other bias | Unclear risk | Funding source not reported |
Merville 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated, but no information on allocation method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “All biopsies (from donors and recipients) were blindly and centrally examined by the same pathologist (CD).” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis reported; all patients followed up or accounted for; 3 patients, 2 in the MMF group and one in the AZA group, were excluded after randomisation for discontinuation of the randomised drug and a total of 71 individuals was finally available for analysis |
| Selective reporting (reporting bias) | Low risk | The published report included all expected outcomes regarding efficacy and safety |
| Other bias | Low risk | The study was financially supported partly by the Association Promotion Transplantation Renale and the Centre Hospitalier Universitaire de Bordeaux (non‐industry) |
MYSS Study 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation was centralised at the Laboratory of Biostatistics of the Clinical Research Centre for Rare Diseases Aldo e Cele Daccò of the Mario Negri Institute for Pharmacological Research, under the responsibility of an independent investigator who was not involved in design or performance of the study.” |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated, but no information on allocation method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis reported, two patients did not receive study drug or kidney graft and were excluded from the analyses; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | The published report included all expected outcomes regarding efficacy and safety |
| Other bias | Low risk | No conflicts of interest declared. The study was supported by non‐industry funding |
Joh 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Concomitant immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐RCT: "When two cases of kidney transplantation took place from the same cadaveric donor in our hospital, transplantation was performed on a first come first serve base. MMF was administered to the early‐transplanted patient and AZA to the following patient and vice versa in the next case" |
| Allocation concealment (selection bias) | High risk | Patients were consecutively enrolled and were allocated to the treatment arms in alternating sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis unclear; all patients were followed up |
| Selective reporting (reporting bias) | Unclear risk | The published report included few outcomes regarding efficacy and safety |
| Other bias | Unclear risk | Funding source not reported |
ALG ‐ antilymphocyte globulin; ATG ‐ antithymocyte globulin; AZA ‐ azathioprine; CAN ‐ chronic allograft nephropathy; CrCl ‐ creatinine clearance; CsA ‐ cyclosporin A, CsA‐ME ‐ cyclosporin A microemulsion; GI ‐ gastrointestinal; Hb ‐ haemoglobin; HBV ‐ hepatitis B virus; HIV ‐ human immunodeficiency virus; HLA ‐ human leucocyte antigen; HTLV ‐ human T‐lymphotropic virus; ITT ‐ intention‐to‐treat; MMF ‐ mycophenolate mofetil; NODAT ‐ new onset diabetes after transplantation; PRA ‐ panel reactive antibody; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; SD ‐ standard deviation; SE ‐ standard error; Tac ‐ tacrolimus; WCC ‐ white cell count
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Araujo 1999 | Not primary regimen |
| Asci 2002 | Not primary regimen |
| Baek 2004 | Not RCT |
| Bataille 2010 | Unequal concomitant regimen |
| Benfield 1999 | Randomised to OKT3 and CsA (not AZA and MMF) |
| Boletis 1999b | Not RCT |
| Brennan 2005 | Not comparison of interest |
| Cransberg 2007 | Not primary regimen, no comparison of interest |
| El‐Agroudy 2009 | Not primary regimen |
| Ettenger 1995 | Not comparison of interest |
| Griffin 2003 | Not primary regimen |
| Ha 2004 | Not primary regimen |
| Hernandez 2007 | No comparison of interest |
| Jain 2001 | Not primary regimen |
| Jirasiritham 2000 | Not primary regimen |
| Kasiske 1997 | Not RCT |
| Khosroshahi 2006a | not RCT |
| Kim 1999 | not RCT |
| Langman 1996 | Not primary regimen |
| Lezaic 2005 | Not primary regimen |
| Lison 2004 | Not comparison of interest |
| Makhdoomi 2005 | Not RCT |
| Mandelbaum 1998 | not RCT |
| Merion 2000 | Multi‐organ transplantation |
| Metcalfe 2002 | Not primary regimen |
| MMF 1998 | Not primary regimen |
| MO2ART Study 2003 | Not comparison of interest |
| Nowacka‐Cieciur 2000 | Not primary regimen, no comparison of interest |
| Oliveira 1999 | No RCT |
| Schurter 1997 | Not primary regimen |
| Smak Gregoor 2000 | Not primary regimen |
| Touchard 2005 | Not primary regimen |
| Tsinalis 2000 | Unequal concomitant regimen |
| Vacher‐Coponat 2006 | Unequal concomitant regimen |
| van der Mast 2000 | Not primary regimen |
| Vanrenterghem 1998 | Not comparison of interest |
| Woeste 2002 | Multi‐organ transplantation, unequal concomitant regimen |
| Wuthrich 2000 | Not primary regimen |
AZA ‐ azathioprine; CsA ‐ cyclosporin A; MMF ‐ mycophenolate mofetil; RCT ‐ randomised controlled trial
Legend: no RCT: study design not RCT or quasi‐RCT; multi‐organ transplantation: not solely kidney transplantation, exclusion of studies enrolling patients undergoing multi‐organ transplantation, e.g. simultaneous kidney‐pancreas transplantation; not primary regimen: the randomisation to MMF versus AZA was not performed at the time of transplantation, but subsequently during the maintenance phase (e.g. due to previous acute rejection, chronic allograft nephropathy, calcineurin‐inhibitor‐toxicity or in stable graft function status); no comparison of interest: randomised intervention not of interest for the review, i.e. not MMF versus AZA; unequal concomitant regimen, i.e. patients randomised to intervention and control were treated with different immunosuppressive regimens (e.g. MMF/cyclosporin A versus AZA/tacrolimus)
Characteristics of ongoing studies [ordered by study ID]
ATHENA Study 2012.
| Trial name or title | A randomized, prospective, multicenter trial to compare the effect on chronic allograft nephropathy prevention of mycophenolate mofetil versus azathioprine as the sole immunosuppressive therapy for kidney transplant recipients (ATHENA) |
| Methods | RCT, multicentre (6) Year of transplantation: 2007 to 2012 Study duration: 4 years |
| Participants | Estimated enrolment: 224 Country: Italy Deceased donor: 100% Previous transplantation: 0% Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment group
Control group
Concomitant regimen
|
| Outcomes | Primary endpoint
Secondary endpoints
|
| Starting date | May 2007 |
| Contact information | Norberto Perico, MD; Mario Negri Institute for Pharmacological Research; Milano, Italy |
| Notes | The study was registered at clinicaltrials.gov (#NCT00494741) on June 29, 2007 This study is ongoing, but not recruiting participants (last update February 24, 2014) Estimated completion date: September 2016 |
AZA ‐ azathioprine; CAN ‐ chronic allograft nephropathy; CsA‐ME ‐ cyclosporin A emulsion; HIV ‐ human immunodeficiency virus; IL‐2 ‐ interleukin 2; IV ‐ intravenous; MMF ‐ mycophenolate mofetil; PRA ‐ panel reactive antibody; RATG ‐ rabbit antithymocyte globulin; WCC ‐ white cell count
Differences between protocol and review
During the preparation of the review a few aspects were implemented in the current version of the review that was not pre‐specified in the protocol.
Within the presentation of results according to primary and secondary outcomes, we highlighted the clinical importance of outcomes as suggested by the GRADE working group (Atkins 2004). We adopted the classification from the KDIGO workgroup of experts in the fields of kidney transplantation and methodology (KDIGO 2009).
We tested all covariates pre‐specified in the protocol regarding confounding of the meta‐analysis results and to investigate heterogeneity using meta‐regression and subgroup‐analyses. Herein, the effects of blinded administration of the study drug and studies excluding previous kidney transplantation were tested in meta‐regression, rather than in subgroup‐analyses.
Contributions of authors
Draft the protocol: MW, EB, KU, AW
Study selection: MW, EB, AE
Data extraction from studies and entering into RevMan: MW, AE, IP
Study translation: JC and EB (Spanish); MC, CH and KH (Chinese)
Statistical analyses: MW, CS
Interpretation of results: MW, EB, KU, AW
Draft the final review: MW, AE, EB, KU, AW
Disagreement resolution: KU, EB, AW
Update the review: MW, AW
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Kidney Foundation, USA.
(Fellowship training program of the National Kidney Foundation Center for Clinical Practice Guideline Development and Implementation at Tufts Medical Center)
-
Agency for Healthcare Research and Quality, USA.
(Grant # R01‐HS018574)
Declarations of interest
In the past 5 years, MW received travel grants from Genzyme
In the past 5 years, CS received grants from Blue Cross Blue Shield, TEVA Pharma and Glaxo Smith Kline
AE, AW, EB and KU do not have any known conflicts of interest
New
References
References to studies included in this review
Army Hospital 2002 {published data only}
- Army Hospital (R&R). Mycophenolate versus AZA‐in‐de‐nova renal transplantation ‐ a short term study [abstract]. Indian Journal of Nephrology 2002;12(4):226. [CENTRAL: CN‐00460299] [Google Scholar]
Baltar 2002 {published data only}
- Baltar J, Ortega F, Rebollo P, Gomez E, Laures A, Alvarez‐Grande J. Changes in health‐related quality of life in the first year of kidney transplantation [Cambios en la calidad de vida relacionada con la salud durante el primer ano del trasplante renal]. Nefrologia 2002;22(3):262‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Baltar J, Ortega F, Rebollo P, Rodriguez M, Alvarez‐Grande J. Health related quality of life (HRQOL) in two inmunosuppressor therapy regimens [abstract]. Nephrology Dialysis Transplantation 1999;14(9):A274. [CENTRAL: CN‐00483117] [Google Scholar]
Busque 2001 {published data only}
- Busque S, Shoker A, Landsberg D, McAlister V, Halloran P, Shapiro J. Canadian multicentre kidney trial of prograf/AZA vs. neoral/MMF [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome, Italy. 2000. [CENTRAL: CN‐00433620]
- Busque S, Shoker A, Landsberg D, McAlister V, Halloran P, Shapiro J. Canadian multicentre trial of prograf/AZA vs. prograf/MMF vs. Neoral/MMF in renal transplantation [abstract]. Transplantation 2000;69(8 Suppl):S114. [Google Scholar]
- Busque S, Shoker A, Landsberg D, McAlister V, Halloran P, Shapiro J, et al. Canadian multicentre trial of tacrolimus/azathioprine/steroids versus tacrolimus/mycophenolate mofetil/steroids versus neoral/mycophenolate mofetil/steroids in renal transplantation. Transplantation Proceedings 2001;33(1‐2):1266‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
COSTAMP Study 2002 {published data only}
- Mucha K, Foroncewicz B, Paczek L, Pazik J, Lewandowska D, Krawczyk A, et al. 36‐month follow‐up of 75 renal allograft recipients treated with steroids, tacrolimus, and azathioprine or mycophenolate mofetil. Transplantation Proceedings 2003;35(6):2176‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wlodarczyk Z, Glyda M, Paczek L, Ostrowski M, Marcinkowski W, Klinger M, et al. Long‐term results of steroid withdrawal following renal transplantation in tacrolimus‐based immunosuppression regimens ‐ results of multicenter study [abstract no: 25]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego, CA. 2004. [CENTRAL: CN‐00550597]
- Wlodarczyk Z, Walaszewski J, Perner F, Vitko S, Ostrowski M. Tacrolimus/MMF/steroids compared to tacrolimus/AZA/steroids in renal transplantation: differences in efficacy and feasibility of steroid withdrawal [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002. [CENTRAL: CN‐00416944]
- Wlodarczyk Z, Walaszewski J, Perner F, Vitko S, Ostrowski M, Bachleda P, et al. Freedom from rejection and stable kidney function are excellent criteria for steroid withdrawal in tacrolimus‐treated kidney transplant recipients. Annals of Transplantation 2002;7(3):28‐31. [MEDLINE: ] [PubMed] [Google Scholar]
- Wlodarczyk Z, Walaszewski J, Perner F, Vitko S, Ostrowski M, Bachleda P, et al. Steroid withdrawal at 3 months after kidney transplantation: a comparison of two tacrolimus‐based regimens. Transplant International 2005;18(2):157‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wlodarczyk Z, Walazewski J, Perner F, the COSTAMP Study Group. Freedom from rejection and stable renal function are excellent criteria for steroid withdrawal in tacrolimus therapy [abstract]. American Journal of Transplantation 2003;3(Suppl 5):556. [CENTRAL: CN‐00448396] [PubMed] [Google Scholar]
Egfjord 1999 {published data only}
- Egfjord M, Ladefoged J, Olgaard K. Mycophenolate mofetil (MMF) vs azathioprin (AZA) in combination with prednisone, cyclosporin, and ATGAM for prevention of acute renal graft rejection [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):727A. [CENTRAL: CN‐00550650] [Google Scholar]
- Ladefoged J, Egfjord M, Olgaard K. Open randomized study of mofetil vs azathioprin combined with prednisone and cyclosporin for prevention of acute renal graft rejection [abstract]. Nephrology Dialysis Transplantation 1999;14(9):A275. [CN‐00483826] [Google Scholar]
Folkmane 2001 {published data only}
- Folkmane I, Bicans J, Amerika D, Chapenko S, Murovska M, Rosentals R. Low rate of acute rejection and cytomegalovirus infection in kidney transplant recipients with basiliximab. Transplantation Proceedings 2001;33(7‐8):3209‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Folkmane I, Bicans J, Chapenko S, Murovska M, Rosentals R. Results of renal transplantation with different immunosuppressive regimens. Transplantation Proceedings 2002;34(2):558‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Folkmane I, Chapenko S, Amerika D, Bicans J, Murovska M, Rosentals R. beta‐herpes virus activation after kidney transplantation with mycophenolate mofetil‐based maintenance immunosuppression. Transplantation Proceedings 2001;33(3):2384‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Folkmane I, Chapenko S, Murovska M, Rosental R. Low rate of acute rejection and cytomegalovirus infection in renal transplant recipients with basiliximab [abstract no: 1037]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00400939] [DOI] [PubMed]
Isbel 1997 {published data only}
- Isbel NM, Smith KG, Leydon JA, Walker RG, Becker GJ. Mycophenolate mofetil suppresses the humoral response to influenza vaccination in renal transplant recipients [abstract no: P1014]. Nephrology 1997;3(Suppl 1):S327. [CENTRAL: CN‐00460991] [Google Scholar]
Ji 2001 {published data only}
- Ji YL, Yang YQ, Yang GB, Yu XQ, Jiang ZP, Shen QR, et al. The clinical investigation of mycophenolate mofetil for the prevention of acute rejection. Academic Journal of Sun Yat‐Sen University of Medical Sciences 2001;22(3):215‐7. [Google Scholar]
Joh 2005 {published data only}
- Joh JW, Lee HH, Lee DS, Lee KW, Lee SK, Kim SJ. The influence of mycophenolate mofetil and azathioprine on the same cadaveric donor renal transplantation. Journal of Korean Medical Science 2005;20(1):79‐81. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lee KW, Lee SK, Park JH, Woo DH, Oh HY, et al. The influence of mycophenolate (MMF) and azathioprine (AZA) in the same cadaveric renal transplantation [abstract no: FC5‐01]. Nephrology 2003;8(Suppl 1):A18. [Google Scholar]
Johnson 2000 {published data only}
- Ahsan N, Johnson C, Gonwa T, Halloran P, Stegall M, Hardy M, et al. Randomized trial of tacrolimus plus mycophenolate mofetil or azathioprine versus cyclosporine oral solution (modified) plus mycophenolate mofetil after cadaveric kidney transplantation: results at 2 years. Transplantation 2001;72(2):245‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ahsan N, Milton S, Hershey P, Johnson C, Gonwa T, Halloran P, et al. Diabetes mellitus with neoral (cyclosporine) vs. prograf (tacrolimus) based regimens after kidney transplant [abstract no: 356]. Transplantation 1998;65(12):S91. [CENTRAL: CN‐00653714] [Google Scholar]
- Gonwa T, Johnson C, Ahsan N, Alfrey EJ, Halloran P, Stegall M, et al. Randomized trial of tacrolimus + mycophenolate mofetil or azathioprine versus cyclosporine + mycophenolate mofetil after cadaveric kidney transplantation: results at three years. Transplantation 2003;75(12):2048‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gonwa T, Johnson C, Ahsan N, Halloran P, Stegall M, Hardy M, et al. Comparative trial of prograf (tacrolimus) in combination with azathioprine or mycophenolate mofetil vs neoral (cyclosporine) with mycophenolate mofetil after kidney transplantation [abstract no: 930]. Transplantation 1999;67(7):S239. [CN‐00764289] [DOI] [PubMed] [Google Scholar]
- Gonwa TA, FK506/MMF Study Trial Group. Two year follow‐up of a randomized trial of FK506 + MMF vs FK506 + AZA vs CyA + MMF [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00433630]
- Gonwa TA, Johnson C, Ahsan N, Halloran P, Stegall M, Hardy M, et al. Two year follow up of a randomised multicenter kidney transplant study comparing tacrolimus (PG) + azathioprine (AZA) vs cyclosporin (Neoral) + mycophenolate mofetil (MMF) vs tacrolimus + MMF [abstract]. Transplantation 2000;69(8 Suppl):S113. [CENTRAL: CN‐00509214] [Google Scholar]
- Johnson C, Ahsan N, Gonwa T, Halloran P, Stegall M, Hardy M, et al. Randomized comparative trial of prograf (tacrolimus) in combination with azathioprine or mycophenolate mofetil vs. neoral (cyclosporine) with mycophenolate mofetil after kidney transplantation [abstract no: 662]. Transplantation 1998;65(12):S168. [CENTRAL: CN‐00583255] [DOI] [PubMed] [Google Scholar]
- Johnson C, Ahsan N, Gonwa T, Halloran P, Stegall M, Hardy M, et al. Randomized trial of tacrolimus (Prograf) in combination with azathioprine or mycophenolate mofetil versus cyclosporine (Neoral) with mycophenolate mofetil after cadaveric kidney transplantation. Transplantation 2000;69(5):834‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Johnson C, Gonwa T, Light J, Hardy M, Ahsan N, et al. Randomized trial of Prograf+MMF or azathioprine versus neoral+MMF after cadaveric kidney transplantation: results at three years.[abstract no: F‐FC012]. Journal of the American Society of Nephrology 2002;13:3A. [CENTRAL: CN‐00433632] [Google Scholar]
Keven 2003 {published data only}
- Keven K, Sahin M, Kutlay S, Sengul S, Erturk S, Erbay B. Immunoglobulin deficiency in kidney allograft recipients: comparative effects of mycophenolate mofetil and azathioprine [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):646A. [DOI] [PubMed] [Google Scholar]
- Keven K, Sahin M, Kutlay S, Sengul S, Erturk S, Ersoz S, et al. Immunoglobulin deficiency in kidney allograft recipients: comparative effects of mycophenolate mofetil and azathioprine. Transplant Infectious Disease 2003;5(4):181‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ling 1998 {published data only}
- Ling JY, Zhu Y, Shun FK. Combined use of MMF with low dosage of cyclosporine A in renal transplantation. Chinese Journal of Organ Transplantation 1998;19(3):175‐6. [Google Scholar]
Mendez 1998 {published data only}
- Mendez R. FK 506 and mycophenolate mofetil in renal transplant recipients: six‐month results of a multicenter, randomized dose ranging trial. FK 506 MMF Dose‐Ranging Kidney Transplant Study Group. Transplantation Proceedings 1998;30(4):1287‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Miller J. Tacrolimus and mycophenolate mofetil in renal transplant recipients: one year results of a multicenter, randomized dose ranging trial. FK506/MMF Dose‐Ranging Kidney Transplant Study Group. Transplantation Proceedings 1999;31(1‐2):276‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Miller J, FK506/MMF Dose‐Ranging Kidney Transplant Study Group. Tacrolimus and mycophenolate mofetil in renal transplant recipients: six and twelve month results of a multicenter, randomized dose ranging study [abstract no: 752]. Transplantation 1998;65(12):S190. [CENTRAL: CN‐00671869] [Google Scholar]
- Miller J, Mendez R, Pirsch JD, Jensik SC. Safety and efficacy of tacrolimus in combination with mycophenolate mofetil (MMF) in cadaveric renal transplant recipients. FK506/MMF Dose‐Ranging Kidney Transplant Study Group. Transplantation 2000;69(5):875‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Merville 2004 {published data only}
- Berge F, Durang D, Merville P, Morel D, Mourad G, Potaux L. Beneficial effect of mycophenolate mofetil on the incidence of chronic allograft nephropathy: a randomized protocol biopsy‐based study [abstract no: 1614]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00602028]
- Merville P, Berge F, Deminiere C, Morel D, Chong G, Durand D, et al. Interest of mycophenolate mofetil in the prevention of chronic allograft nephropathy: a randomized biopsy‐based study [abstract no: 1076]. American Journal of Transplantation 2002;2(Suppl 3):409. [CENTRAL: CN‐00416277] [Google Scholar]
- Merville P, Berge F, Deminiere C, Morel D, Chong G, Durand D, et al. Lower incidence of chronic allograft nephropathy at 1 year post‐transplantation in patients treated with mycophenolate mofetil. American Journal of Transplantation 2004;4(11):1769‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Miladipour 2002 {published data only}
- Miladipour AH, Ghods AJ, Nejadgashti H. Effect of mycophenolate mofetil on the prevention of acute renal allograft rejection. Transplantation Proceedings 2002;34(6):2089‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MMF TRI Study 1996 {published data only}
- A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. Transplantation 1996;61(7):1029‐37. [MEDLINE: ] [PubMed] [Google Scholar]
- Clayton P, McDonald S, Chapman J, Chadban S. Mycophenolate vs azathioprine for kidney transplantation ‐ 15 year follow‐up of a randomised trial [abstract no: 111]. Immunology & Cell Biology 2011;89(7):A1. [EMBASE: 70655945] [Google Scholar]
- Clayton P, McDonald S, Chapman J, Chadban S. Mycophenolate vs azathioprine for kidney transplantation: 15 year follow‐up of a randomized trial [abstract no: 172]. Nephrology 2011;16(Suppl 1):69. [EMBASE: 70532520] [Google Scholar]
- Clayton PA, McDonald SP, Chapman JR, Chadban SJ. Mycophenolate versus azathioprine for kidney transplantation: a 15‐year follow‐up of a randomized trial. Transplantation 2012;94(2):152‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Halloran P, Mathew T, Tomlanovich S, Groth C, Hooftman L, Barker C. Mycophenolate mofetil in renal allograft recipients: a pooled efficacy analysis of three randomized, double‐blind, clinical studies in prevention of rejection. The International Mycophenolate Mofetil Renal Transplant Study Groups. [erratum appears in Transplantation 1997 Feb 27;63(4):618]. Transplantation 1997;63(1):39‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hayry P, International Mycophenolate Mofetil Renal Transplant Study Group. Mycophenolate mofetil (MMF) for acute rejection of cadavers renal transplants: results of a randomized, double‐blind, multicenter study [abstract]. 14th Annual Meeting. American Society of Transplant Physicians (ASTP); 1995 May 14‐17; Chicago, IL. 1995.
- International Mycophenolate Mofetil Study Group. A long‐term randomized multicenter study of mycophenolate mofetil (MMF) in cadaveric renal transplantation: results at 3 years [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago, IL. 1997:175. [CENTRAL: CN‐00509246]
- Keown PA, Sullivan SD, Best JH, Garrison LP, Krueger H, Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. Economic evaluation of mycophenolate mofetil (MMF) for prevention of acute graft rejection after cadaveric renal transplantation in Canada [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago, IL. 1997:239. [CENTRAL: CN‐00509270]
- Mathew T, Halloran P, Groth C, Tomlanovich S, Hooftman L. Adverse event profile of mycophenolate mofetil (MMF) in cadaveric renal transplant patients ‐ a 1‐year follow‐up [abstract]. Nephrology 1997;3(Suppl 1):S71. [CENTRAL: CN‐00653750] [Google Scholar]
- Mathew T, International Mycophenolate Mofetil Renal Transplantation Study Group. A randomized, double‐blind, multicenter study of mycophenolate mofetil (MMF) for acute rejection of cadaveric renal transplants [abstract]. ISN XIII International Congress of Nephrology; 1995 Jul 2‐6; Madrid, Spain. 1995:335. [CENTRAL: CN‐00509342]
- Mathew TH. A blinded, long‐term, randomized multicenter study of mycophenolate mofetil in cadaveric renal transplantation: results at three years. Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. [erratum appears in Transplantation 1998 Sep 27;66(6):817]. Transplantation 1998;65(11):1450‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MMF US Study 1995 {published data only}
- Mycophenolate mofetil for the prevention of acute rejection of primary cadaveric kidney transplants: status of the MYC 1866 study at 1 year. The U.S. Mycophenolate Mofetil Study Group. Transplantation Proceedings 1997;29(1‐2):348‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mycophenolate mofetil in cadaveric renal transplantation. US Renal Transplant Mycophenolate Mofetil Study Group. American Journal of Kidney Diseases 1999;34(2):296‐303. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ferguson RM, US Renal Transplant Mycophenolate Mofetil Study Group. Efficacy of mycophenolate mofetil for the prevention of acute rejection in first cadaveric renal transplantation. [abstract]. ISN XIII International Congress of Nephrology; 1995 Jul 2‐6; Madrid, Spain. 1995:335.
- Gonwa TA, US Renal Transplant Mycophenolate Mofetil Study Group. Safety of mycophenolate mofetil (MMF) vs azathioprine (AZA) in primary cadaveric renal transplant (Cad TX) [abstract]. 14th Annual Meeting American Society of Transplant Physicians (ASTP); 1995 May 10‐14;Chicago (IL). 1995.
- Halloran P, Mathew T, Tomlanovich S, Groth C, Hooftman L, Barker C. Mycophenolate mofetil in renal allograft recipients: a pooled efficacy analysis of three randomized, double‐blind, clinical studies in prevention of rejection. The International Mycophenolate Mofetil Renal Transplant Study Groups. [erratum appears in Transplantation 1997 Feb 27;63(4):618]. Transplantation 1997;63(1):39‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kimball JA, Pescovitz MD, Book BK, Norman DJ. Reduced human IgG anti‐ATGAM antibody formation in renal transplant recipients receiving mycophenolate mofetil. Transplantation 1995;60(12):1379‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mathew T, Halloran P, Groth C, Tomlanovich S, Hooftman L. Adverse event profile of mycophenolate mofetil (MMF) in cadaveric renal transplant patients ‐ a 1‐year follow‐up [abstract]. Nephrology 1997;3(Suppl 1):S71. [Google Scholar]
- Neylan JF. Immunosuppressive therapy in high‐risk transplant patients: dose‐dependent efficacy of mycophenolate mofetil in African‐American renal allograft recipients. U.S. Renal Transplant Mycophenolate Mofetil Study Group. Transplantation 1997;64(9):1277‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Neylan JF, Deierhoi MH. Dose‐dependent prevention of acute renal transplant rejection in African‐Americans receiving mycophenolate mofetil (MMF) [abstract]. 14th Annual Meeting. American Society of Transplant Physicians (ASTP); 1995 May 14‐17; Chicago, IL. 1995.
- Neylan JF, Deierhoi MH, US Renal Transplant Mycophenolate Mofetil Study Group. Dose‐dependant prevention of acute renal transplant rejection in African‐Americans receiving mycophenolate mofetil (MMF) [abstract]. ISN XIII International Congress of Nephrology; 1995 Jul 2‐6; Madrid, Spain. 1995:390. [CENTRAL: CN‐00509384]
- Sollinger HW. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. U.S. Renal Transplant Mycophenolate Mofetil Study Group. Transplantation 1995;60(3):225‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Garrison LP Jr, Best JH. The cost effectiveness of mycophenolate mofetil in the first year after primary cadaveric transplant. U.S. Renal Transplant Mycophenolate Mofetil Study Group. Journal of the American Society of Nephrology 1997;8(10):1592‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tomlanovich S, Cho S, Hodge E, Miller J, Neylan J, Hoofman L, et al. Mycophenolate mofetil in cadaveric renal transplantation: 3‐year data [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago, IL. 1997:175. [CENTRAL: CN‐00509515]
- Weinstein SS, US Renal Transplant Mycophenolate Mofetil Study Group. Safety of mycophenolate mofetil (MMF) vs. azathioprine (AZA) in primary cadaveric renal transplant (CAD TX) [abstract]. ISN XIII International Congress of Nephrology; 1995 Jul 2‐6; Madrid, Spain. 1995:335. [CENTRAL: CN‐00509560]
MYSS Study 2004 {published data only}
- Gotti E, Perico N, Gaspari F, Cattaneo D, Lesti MD, Ruggenenti P, et al. Blood cyclosporine level soon after kidney transplantation is a major determinant of rejection: insights from the Mycophenolate Steroid‐Sparing Trial. Transplantation Proceedings 2005;37(5):2037‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Perico N, Ruggenenti P, Gotti E, Gaspari F, Cattaneo D, Valente U, et al. In renal transplantation blood cyclosporine levels soon after surgery act as a major determinant of rejection: insights from the MY.S.S. trial. Kidney International 2004;65(3):1084‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Perico N, Ruggenenti P, Gotti E, Gaspari F, Cattaneo D, Valente U, et al. In renal transplantation low blood cyclosporine levels soon after surgery is a determinant of rejection: insights from the MY.S.S. trial [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):11A. [CENTRAL: CN‐00601980] [Google Scholar]
- Remuzzi G, Cravedi P, Costantini M, Lesti M, Ganeva M, Gherardi G, et al. Mycophenolate mofetil versus azathioprine for prevention of chronic allograft dysfunction in renal transplantation: the MYSS follow‐up randomized, controlled clinical trial. Journal of the American Society of Nephrology 2007;18(6):1973‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Remuzzi G, Lesti M, Gotti E, Ganeva M, Dimitrov BD, Ene‐Iordache B, et al. Mycophenolate mofetil versus azathioprine for prevention of acute rejection in renal transplantation (MYSS): a randomised trial. Lancet 2004;364(9433):503‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sadek 2002 {published data only}
- Sadek S, Medina J, Arias M, Sennesael J, Squifflet JP, Vogt B, et al. Short‐term combination of mycophenolate mofetil with cyclosporine as a therapeutic option for renal transplant recipients: a prospective, multicenter, randomized study. Transplantation 2002;74(4):511‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sadek S, Vogt B, Beauregard‐Zllinger L, Prestele H, Neoral Phase IV Study Group. Short‐term combination of mycophenolate mofetil with cyclosporine as a safe therapeutic option for renal transplant recipients [abstract]. Transplantation 2000;69(8 Suppl):S160. [CENTRAL: CN‐00447538] [DOI] [PubMed] [Google Scholar]
- Sadek SA, Vogt B, Beauregard‐Zollinger L, Neoral Phase IV Study Group. Short‐term mycophenolate mofetil combined with cyclosporine compared to standard maintenance regimens of cyclosporine with mycophenolate mofetil or azathioprine in kidney transplantation: interim analysis [abstract no: 927]. Transplantation 1999;67(7):S238. [CENTRAL: CN‐00766427] [Google Scholar]
- Vogt B, Sadek S, Phase IV Neonatal Study Group. Short‐term combination of mycophenolate mofetil with cyclosporine as a safe therapeutive option in renal transplantation [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00448227]
Suhail 2000 {published data only}
- Suhail SM, Vathsala A, Lou HX, Woo KT. Safety and efficacy of mycophenolate mofetil for prophylaxis in Asian renal transplant recipients. Transplantation Proceedings 2000;32(7):1757‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vathsala A, Lou H, Suhail SM, Woo K. Does 6 months prophylaxis with mycophenolate mofetil reduce acute rejection in renal transplantation? [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):711A. [CENTRAL: CN‐00583822] [Google Scholar]
Sun 2002b {published data only}
- Sun CC, Hao JW, Sun J, Yang DA. A comparison between therapeutic effects of mycophenolate mofetil and azathioprine in the management of patients after renal transplantation. Yiyao Dao Bao [Herald of Medicine] 2002;21(9):544‐6. [CENTRAL: CN‐00429261] [Google Scholar]
Tuncer 2002 {published data only}
- Tuncer M, Gurkan A, Erdogan O, Demirbas A, Suleymanlar G, Ersoy FF, et al. Mycophenolate mofetil in renal transplantation: five years experience. Transplantation Proceedings 2002;34(6):2087‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weimer 2002 {published data only}
- Weimer R, Deisz S, Dietrich H, Renner F, Bodeker RH, Daniel V, et al. Impact of maintenance immunosuppressive regimens‐‐balance between graft protective suppression of immune functions and a near physiological immune response. Transplant International 2011;24(6):596‐609. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weimer R, Deisz S, Dietrich H, Yildiz S, Staak A, Renner F, et al. Impact of maintenance immunosuppressive regimens on immunological parameters of graft outcome [abstract no: P101]. Transplant International 2007;20(Suppl 2):120. [Google Scholar]
- Weimer R, Deisz S, Renner F, Dietrich H, Daniel V, Kamali‐Ernst S, et al. Different impact of maintenance immunosuppressive regimens on the immune response of renal transplant recipients [abstract no: 112]. Transplantation 2008;86(2 Suppl):40. [CENTRAL: CN‐00671788] [Google Scholar]
- Weimer R, Deisz S, Renner F, Dietrich H, Suesal C, Kamali‐Ernst S, et al. Impact of steroid withdrawal on the immune response of renal transplant recipients [abstract no: O‐249]. Transplant International 2009;22(Suppl 2):66. [Google Scholar]
- Weimer R, Ettrich M, Renner F, Dietrich H, Susal C, Deisz S, et al. ATG induction in renal transplant recipients: Long‐term hazard of severe infection is associated with long‐term functional T cell impairment but not the ATG‐induced CD4 cell decline. Human Immunology 2014;75(6):561‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weimer R, Staak A, Streller S, Dietrich H, Daniel V, Feustel A, et al. Effects of three immunosuppressive regimens on immunological risk parameters: results of a prospective randomized study in renal transplant recipients [abstract no: 055]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):18. [CENTRAL: CN‐00509558] [DOI] [PubMed] [Google Scholar]
- Weimer R, Staak A, Susal C, Streller S, Yildiz S, Pelzl S, et al. ATG induction therapy: long‐term effects on Th1 but not on Th2 responses. Transplant International 2005;18(2):226‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weimer R, Streller S, Staak A, Heilke M, Li D, Dietrich H, et al. Effects of three immunosuppressive regimens on CD4 helper function, B cell monocyte and cytokine responses in renal transplant recipients: 4‐month follow‐up of a prospective randomized study. Transplantation Proceedings 2002;34(6):2377‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weimer R, Suesal C, Staak A, Yildiz S, Pelzl S, Renner F, et al. sCD30 and neopterin as risk factors of chronic renal transplant rejection ‐ impact of different immunosuppressive regimens [abstract no: 637]. American Journal of Transplantation 2005;5(Suppl 11):318. [CENTRAL: CN‐00644289] [Google Scholar]
- Weimer R, Suesal C, Staak A, Yildiz S, Pelzl S, Renner F, et al. sCD30 and neopterin as risk factors of chronic renal transplant rejection ‐ impact of different immunosuppressive regimens [abstract no: SP424]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v160. [Google Scholar]
- Weimer R, Suesal C, Yildiz S, Pelzl S, Renner F, Dietrich H, et al. SCD30 and neopterin as risk factors of chronic renal transplant rejection ‐ impact of CsA, TACR and MMF [abstract]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego, CA. 2004. [CENTRAL: CN‐00644336]
- Weimer R, Susal C, Yildiz S, Pelzl S, Renner F, Dietrich H, et al. SCD30 and neopterin as risk factors of chronic renal transplant rejection ‐ impact of different immunosuppressive regimens [abstract]. Transplantation 2004;78(2 Suppl):111. [CENTRAL: CN‐00509559] [Google Scholar]
- Weimer R, Susal C, Yildiz S, Staak A, Pelzl S, Renner F, et al. Post‐transplant sCD30 and neopterin as predictors of chronic allograft nephropathy: impact of different immunosuppressive regimens. American Journal of Transplantation 2006;6(8):1865‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weimer R, Susal C, Yildiz S, Streller S, Pelzl S, Staak A, et al. sCD30 and neopterin as risk factors of chronic renal transplant rejection: impact of cyclosporine A, tacrolimus, and mycophenolate mofetil. Transplantation Proceedings 2005;37(4):1776‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Araujo 1999 {published data only}
- Araujo MR, Oliveira AC, Abensur H, Marcondes M, Romao JE, Zatz R, et al. Mycopheolate mofetil (MMF) in the treatment of chronic renal allograft rejection: a three‐year follow‐up [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):719A. [CENTRAL: CN‐00550469] [Google Scholar]
Asci 2002 {published data only}
- Asci G, Toz H, Ok E, Sezis M, Basci A. No benefit from mycophenolate mofetil in renal transplant recipients with chronic allograft nephropathy [abstract no: O56]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):18. [MEDLINE: ] [Google Scholar]
Baek 2004 {published data only}
- Baek H, Huh W, Kim JA, Kim Y, Kim DJ, Oh H, et al. Efficacy of mycophenolate mofetil and azathiporine therapy in paired cadaveric renal transplantation: five‐year experience [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:402. [CENTRAL: CN‐00509077]
Bataille 2010 {published data only}
- Bataille S, Moal V, Gaudart J, Indreies M, Purgus R, Dussol B, et al. Cytomegalovirus risk factors in renal transplantation with modern immunosuppression. Transplant Infectious Disease 2010;12(6):480‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Benfield 1999 {published data only}
- Benfield MR, Herrin J, Feld L, Rose S, Stablein D, Tejani A. Safety of kidney biopsy in pediatric transplantation: a report of the Controlled Clinical Trials in Pediatric Transplantation Trial of Induction Therapy Study Group. Transplantation 1999;67(4):544‐7. [MEDLINE: ] [Google Scholar]
- Benfield MR, Symons JM, Bynon S, Eckhoff D, Herrin J, Harmon W, et al. Mycophenolate mofetil in pediatric renal transplantation. Pediatric Transplantation 1999;3(1):33‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Benfield MR, Tejani A, Harmon WE, McDonald R, Stablein DM, McIntosh M, et al. A randomized multicenter trial of OKT3 mAbs induction compared with intravenous cyclosporine in pediatric renal transplantation. Pediatric Transplantation 2005;9(3):282‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tejani A. A randomized prospective multicenter trial of T‐cell antibody induction therapy in pediatric renal transplantation [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000.
- Tejani A, Harmon W, Benfield M, Elshihabi I, McDonald R, Stablein D, et al. A randomized prospective multicenter trial of T‐cell antibody induction therapy in pediatric renal transplantation [abstract]. Transplantation 2000;69(8 Suppl):S111. [CENTRAL: CN‐00402826] [Google Scholar]
- Tejani A, Harmon W, Benfield M, Rose S, Stablein D, Strom T, et al. A randomized prospective multicenter trial of T‐cell antibody induction therapy in pediatric renal transplantation [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):709A. [Google Scholar]
Boletis 1999b {published data only}
- Boletis JN, Stamatiadis D, Markis F, Konstandinidou I, Theodosis I, Mansour M, et al. Mycophenolate mofetil in renal transplantation [abstract]. Nephrology Dialysis Transplantation 1999;14:2975. [CENTRAL: CN‐00278914] [Google Scholar]
Brennan 2005 {published data only}
- Agha IA, Hardinger KL, Bohl D, Ansari A, Dyk P, Koch M, et al. Preemptive withdrawal of AZA or MMF prevents progression of BK viremia to BK nephropathy: a prospective randomized controlled trial of BK virus infection after renal transplantation [abstract]. American Journal of Transplantation 2004;4(Suppl 8):200. [CENTRAL: CN‐00509045] [Google Scholar]
- Bohl DL, Storch GA, Ryschkewitsch C, Gaudreault‐Keener M, Schnitzler MA, Major EO, et al. Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. American Journal of Transplantation 2005;5(9):2213‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brennan DC, Agha I, Bohl DL, Schnitzler MA, Hardinger KL, Lockwood M, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction.[erratum appears in Am J Transplant. 2005 Apr;5(4 Pt 1):839]. American Journal of Transplantation 2005;5(3):582‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brennan DC, Bohl DL, Storch GA, Major EO, Agha IA, Schnitzler MA. High donor antibody level and HLA C7 predict sustained BK‐polyoma viremia: results of a randomized prospective trial [abstract no: P817]. Transplantation 2004;78(2 Suppl):488. [CENTRAL: CN‐00644222] [Google Scholar]
- Hardinger KL, Bohl DL, Schnitzler MA, Lockwood M, Storch GA, Brennan DC. A randomized, prospective, pharmacoeconomic trial of tacrolimus versus cyclosporine in combination with thymoglobulin in renal transplant recipients. Transplantation 2005;80(1):41‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cransberg 2007 {published data only}
- Cransberg K, Cornelissen M, Lilien M, Hoeck K, Davin JC, Nauta J. Maintenance immunosuppression with mycophenolate mofetil and corticosteroids in pediatric kidney transplantation: temporary benefit but not without risk. Transplantation 2007;83(8):1041‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
El‐Agroudy 2009 {published data only}
- El‐Agroudy AE, El‐Dahshan KF, Wafa EW, Sheashaa HA, Gad ZA, Ismail AM, et al. Safe conversion of mycophenolate mofetil to azathioprine in kidney transplant recipients with sirolimus‐based immunosuppression. Nephrology 2009;14(2):255‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- El‐Dahshan K, El‐Agroudy A, Wafa E, Gad Z. Safe conversion of mycophenolate mofetil to azathioprine in kidney transplant recipients with sirolimus‐based immunosuppression [abstract no: Su711]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. [DOI] [PubMed]
Ettenger 1995 {published data only}
- Ettenger R, Mentser BW, Potter D, Cohen A. Mycophenolate mofetil (MMF) in pediatric (PED) renal transplantation (TX): a report of the PED MMF study group [abstract]. Journal of the American Society of Nephrology 1995;6(3):1082. [CENTRAL: CN‐00483891] [Google Scholar]
Griffin 2003 {published data only}
- Griffin MD, Slezak JM, Kozel TK, Bergstralh EJ, Schwab TR, Gloor JM, et al. Renal function loss in chronic allograft nephropathy: results of a two‐year study of mycophenolate mofetil substitution for azathioprine [abstract]. American Journal of Transplantation 2003;3(Suppl 5):223. [CENTRAL: CN‐00445554] [Google Scholar]
Ha 2004 {published data only}
- Ha J, Yun IJ, Lee JH, Choi SJ, Kang JM, Kim SJ. Azathioprine combination therapy can stably reduce cyclosporine dose as mycophenolate mofetil combination therapy for the recipients showing stable renal function after renal transplantation [abstract no: P‐19]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00583458]
Hernandez 2007 {published data only}
- Hernandez D, Miquel R, Porrini E, Fernandez A, Gonzalez‐Posada JM, Hortal L, et al. Randomized controlled study comparing reduced calcineurin inhibitors exposure versus standard cyclosporine‐based immunosuppression. Transplantation 2007;84(6):706‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jain 2001 {published data only}
- Jain S, Metcalfe M, White SA, Furness PN, Nicholson ML. Chronic allograft nephropathy: a prospective randomised trial of cyclosporin reduction with or without mycophenolate mofetil. Transplantation Proceedings 2001;33(3):2165‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jain S, Metcalfe M, White SA, Furness PN, Nicholson ML. Randomized trial comparing mycophenolate mofetil and azathioprine to allow cyclosporin reduction in chronic allograft nephropathy [abstract no: PO513W]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00445890]
- Nicholson ML, Jain S, Metcalfe M, White SA, Furness PN. Chronic allograft nephropathy: a prospective randomised trial of cyclosporin reduction with or without mycophenolate mofetil [abstract no: 437]. Transplantation 2000;69(8 Suppl):S227. [CENTRAL: CN‐00446949] [DOI] [PubMed] [Google Scholar]
Jirasiritham 2000 {published data only}
- Jirasiritham S, Sumethkul V, Mavichak V, Chalermsanyakorn P. The treatment of chronic rejection with mycophenolate mofetil versus azathioprine in kidney transplantation. Transplantation Proceedings 2000;32(7):2040‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kasiske 1997 {published data only}
- Kasiske BL, Johnson HJ, Heim‐Duthoy KL, Rao VK, Dahl DC, Jacobs DM, et al. Does mycophenolate mofetil improve already good results from cyclosporine induction early after renal transplantation? [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:236. [CENTRAL: CN‐00509263]
Khosroshahi 2006a {published data only}
- Khosroshahi HT, Asghari A, Estakhr R, Baiaz B, Ardalan MR, Shoja MM. Effects of azathioprine and mycophenolate mofetil‐immunosuppressive regimens on the erythropoietic system of renal transplant recipients. Transplantation Proceedings 2006;38(7):2077‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Khosroshahi HT, Estakhri R, Shoja MM. Effects of azathioprine and mycophenolate mofetil based immunosuppressive regimen on the erthropoietic system of the renal transplant recipients [abstract no: SP735]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv263. [CENTRAL: CN‐00626063] [DOI] [PubMed] [Google Scholar]
Kim 1999 {published data only}
- Kim JK, Shin YH, Park YG, Hur D, Kim MS, Lee SR. Clinical observation of mycophenolate mofetil for the prevention of acute rejection in renal transplantation [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):734A. [CENTRAL: CN‐00550544] [Google Scholar]
Langman 1996 {published data only}
- Langman LJ, LeGatt DF, Halloran PF, Yatscoff RW. Pharmacodynamic assessment of mycophenolic acid‐induced immunosuppression in renal transplant recipients. Transplantation 1996;62(5):666‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lezaic 2005 {published data only}
- Lezaic V, Marinkovic J, Ristic S, Dokic Z, Radivojevic D, Blagojevic R, et al. Conversion of azathioprine to mycophenolate mofetil and chronic graft failure progression [abstract]. Transplantation 2004;78(2 Suppl):268. [CENTRAL: CN‐00509315] [DOI] [PubMed] [Google Scholar]
- Lezaic VD, Marinkovic J, Ristic S, Dokic ZM, Basta Jovanovic G, Radivojevic DM, et al. Conversion of azathioprine to mycophenolate mofetil and chronic graft failure progression. Transplantation Proceedings 2005;37(2):734‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lison 2004 {published data only}
- Eilts V, Zantvoort F, Wullstein HG, Hillebrand GF, Gobmann J, Kachel HG, et al. Mycophenolate mofetil ‐ a solution for cyclosporine induced hypertension in kidney transplanted patients? [abstract no: T‐PO50008]. Nephrology 2005;10(Suppl 1):A209. [CENTRAL: CN‐00583349] [Google Scholar]
- Lison AE, Eilts V, Zantvoort F, Wullstein H. Mycophenolate mofetil‐ a solution for cyclosporine induced hypertension in kidney transplanted patients? [abstract]. Transplantation 2004;78(2 Suppl):659. [CENTRAL: CN‐00527138] [Google Scholar]
Makhdoomi 2005 {published data only}
- Makhdoomi K, Ahmadpoor P, Ghafari A, Yekta Z. Comparison of 1 year allograft function with mycofenolate mofetil versus azathioprine in renal transplant patients [abstract no: T‐PO50033]. Nephrology 2005;10(Suppl):A216. [Google Scholar]
Mandelbaum 1998 {published data only}
- Mandelbaum AP, Wiesel M, Ksoll‐Ruddek D, Zeier MG. Long‐term results of mycophenolate‐mofetil (MMF) as compared to azathioprine (AZA) in renal transplantation [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):686A. [CENTRAL: CN‐00446574] [Google Scholar]
Merion 2000 {published data only}
- Merion RM, Henry ML, Melzer JS, Sollinger HW, Sutherland DE, Taylor RJ. Randomized, prospective trial of mycophenolate mofetil versus azathioprine for prevention of acute renal allograft rejection after simultaneous kidney‐pancreas transplantation. Transplantation 2000;70(1):105‐11. [MEDLINE: ] [PubMed] [Google Scholar]
Metcalfe 2002 {published data only}
- Brook NR, Metcalf MS, Jain S, Bicknell GR, Nicholson ML, Harper SJ. A randomised trial of mycophenolate mofetil versus azathioprine as calcineurin inhibitor sparing agents in the treatment of chronic allograft nephropathy [abstract no: P‐97]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550372] [DOI] [PubMed]
- Brook NR, Metcalfe MS, Waller JR, Jain S, Hosgood SA, Nicholson ML. A prospective randomised trial of mycophenolate mofetil and azathioprine after calcineurin reduction in renal allografts with established chronic allograft nephropathy [abstract]. American Journal of Transplantation 2004;4(Suppl 8):485. [CENTRAL: CN‐00509106] [Google Scholar]
- Metcalfe MS, Jain S, Waller JR, Saunders RN, Bicknell GR, Nicholson ML. A randomized trial of mycophenolate mofetil versus azathioprine as calcineurin inhibitor sparing agents in the treatment of chronic allograft nephropathy. Transplantation Proceedings 2002;34(5):1812‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MMF 1998 {published data only}
- Mycophenolate mofetil for the treatment of a first acute renal allograft rejection: The Mycophenolate Mofetil Acute Renal Rejection Study Group.[erratum appears in Transplantation 1998 Apr 15;65(7):followi]. Transplantation 1998;65(2):235‐41. [MEDLINE: ] [PubMed] [Google Scholar]
- Mycophenolate Mofetil Acute Renal Rejection Study Group. Mycophenolate mofetil for the treatment of a first acute renal allograft rejection: three‐year follow‐up. The Mycophenolate Mofetil Acute Renal Rejection Study Group. Transplantation 2001 Apr 27;71(8):1091‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pescovitz MD. Mycophenolate mofetil for the treatment of renal transplant rejection: 3 years of follow‐up [abstract no: 929]. Transplantation 1999;67(7):S239. [CENTRAL: CN‐00765711] [Google Scholar]
- Pirsch J, Best JH, 1912 Renal Transplant Mycophenolate Mofetil Study Group. An economic evaluation of mycophenolate mofetil (MMF) versus azathioprine (AZA) as adjunctive treatment for acute renal allograft rejection [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (IL). 1997:239. [CENTRAL: CN‐00509418]
- Pirsch JD, Pescovitz MD, Ferguson R, Deierhoi M, Hooftman L, Navarro M. Mycophenolate mofetil for the treatment of first acute renal allograft rejection [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (IL). 1997:261. [CENTRAL: CN‐00509419]
MO2ART Study 2003 {published data only}
- Balshaw R, Marra C, Nashan B, Hagenmeyer EG, Kalo Z, Keown P. Two‐hour post‐dose cyclosporine levels in renal transplantation: a cost‐effective strategy for reducing graft rejection [abstract no: 3316]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415222]
- Buchler M, Chadban S, Cole E, Midtvedt K, Thervet E, Prestele H, et al. Evolution of the absorption profile of cyclosporine A in renal transplant recipients: a longitudinal study of the de novo and maintenance phases. Nephrology Dialysis Transplantation 2006;21(1):197‐202. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Buchler M, Chadban S, Jost L, Rodriguez A, Beauregard L, Balshaw R. Excellent renal tolerability of neoral C‐2h monitoring in de novo kidney transplantation: initial results of the MO2ART trial [abstract no: 1038]. American Journal of Transplantation 2002;2(Suppl 3):399. [CENTRAL: CN‐00550435] [Google Scholar]
- Chadban S, Pilmore H, Goodman D, Hutchison B, MO2ART SG. Minimal rejection and excellent graft function by neoral C2 monitoring in renal transplantation: interim results of MO2ART [abstract]. Transplantation Society of Australia & New Zealand (TSANZ). 21st Annual Scientific Meeting; 2003 Apr 9‐11; Canberra, Australia. 2003:65. [CENTRAL: CN‐00444732]
- Chadban S, Pilmore H, Goodman D, Jose M, Hutchison B, Cole E. Optimal C2 targets after month 3 for renal transplant recipients receiving cyclosporin‐microemulsion based triple therapy ‐ the MO2ART trial [abstract no: 93]. Transplantation Society of Australia & New Zealand (TSANZ). 22nd Annual Scientific Meeting; 2004 Mar 31‐Apr 2; Canberra, Australia. 2004:83. [CENTRAL: CN‐00583658]
- Chadban S, Pilmore H, Goodman D, Jose M, Hutchison B, MO2ART ISG. Cyclosporin based immunosuppression with C2‐monitoring for recipients with delayed graft function ‐ MO2ART trial [abstract]. Transplantation Society of Australia & New Zealand (TSANZ). 22nd Annual Scientific Meeting; 2004 Mar 31‐Apr 2; Canberra, Australia. 2004:33. [CENTRAL: CN‐00527100]
- Curtis JJ, Thervet E, Vincenti F, Rodriguez A, Soergel M, Barbeito R. Outcomes of 117 cases of delayed graft function from two clinical trials involving early C2 monitored neoral and antibody therapy [abstract]. Transplantation 2004;78(2 Suppl):5. [CENTRAL: CN‐00509145] [Google Scholar]
- Pfeffer P, Stefoni S, Agost Carreno C, Thervet E, Fornairon S, Keown P. Monitoring of 2‐hour neoral absorption in renal transplantation (MO2ART): interim analysis shows low incidence of acute rejection in the early post‐graft period. [abstract no: 1037]. American Journal of Transplantation 2002;2(Suppl 3):399. [CENTRAL: CN‐00416456] [Google Scholar]
- Pilmore H, Chadban S, G, Goodman, Jose, Hutchison, et al. Improved efficacy and tolerability of neoral by C2‐monitoring in de novo renal transplant recipients [abstract]. Transplantation Society of Australia & New Zealand (TSANZ). 22nd Annual Scientific Meeting; 2004 Mar 31‐Apr 2; Canberra, Australia. 2004:96. [CENTRAL: CN‐00509417]
- Servais A, Meas‐Yedid V, Buchler M, Morelon E, Olivo‐Marin JC, Thervet E. Quantification of interstitial fibrosis by image analysis on routine renal biopsy 1 year after transplantation in patients managed by C2 monitoring of cyclosporine microemulsion. Transplantation Proceedings 2007;39(8):2560‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stefoni S, Midtved K, Cole E, Thervet E, Cockfield S, Buchler M, et al. Efficacy and safety outcomes among de novo renal transplant recipients managed by C2 monitoring of cyclosporine a microemulsion: results of a 12‐month, randomized, multicenter study. Transplantation 2005;79(5):577‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stefoni S, Midtvedt K, Cole E, Thervet E, Cockfield S, Buchler M, et al. Excellent efficacy and tolerability of cs‐monitoring for cyclosporine microemulsion (CSA‐ME, neoral): results of MO2ART, a 12‐month randomized international study [abstract]. American Journal of Transplantation 2004;4(Suppl 8):238‐9. [CENTRAL: CN‐00509492] [Google Scholar]
- Thervet E, Pfeffer P, Scolari MP, Toselli L, Pallardo LM, Chadban S, et al. Clinical outcomes during the first three months posttransplant in renal allograft recipients managed by C2 monitoring of cyclosporine microemulsion. Transplantation 2003;76(6):903‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Toselli L, Peffer P, Stefoni S, Therver E, Fornairon S, Keown P. Minimal rejection and excellent graft function by neoral c‐2h monitoring in renal transplantation: interim results of MO2ART, a randomized prospective international study [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416774]
Nowacka‐Cieciur 2000 {published data only}
- Nowacka‐Cieciura E, Kaminska B, Cieciura T, Gradowska L, Pazik J, Lao M, et al. Randomised open clinical trial of conversion from mycophenolate mofetil to azathioprine in cadaveric renal transplantation. Transplant International 2000;13(Suppl 1):S68‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oliveira 1999 {published data only}
- Oliveira JG, Ramos JP, Xavier P, Sampaio S, Magalhaees M, Mandes A, et al. Lymphocyte subsets in peripheral blood and inside the allograft in renal tx treated with AZA versus MMF: CD3DR andICAM‐1 down regulation with MMF [abstract]. Nephrology Dialysis Transplantation 1999;14(9):A276. [CENTRAL: CN‐00583426] [Google Scholar]
Schurter 1997 {published data only}
- Schurter G, Glicklich D, Greenstein SM, Schreiber T, Mallis M, Clemetson S, et al. Mycophenolate mofetil (MMF) therapy for chronic rejection in renal transplant recipients [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (IL). 1997:133. [CENTRAL: CN‐00509469]
Smak Gregoor 2000 {published data only}
- Smak Gregoor PJ, Gelder T, Besouw NM, mast P, kuiper P, Ijzermans JN, et al. Long‐term follow‐up of a prospective, randomised study on minimising immunosuppressive medication from one year after kidney transplantation [abstract no: 0016]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416672]
- Smak Gregoor PJ, Gelder T, Besouw NM, Mast BJ, IJzermans JN, Weimar W. Randomized study on the conversion of treatment with cyclosporine to azathioprine or mycophenolate mofetil followed by dose reduction. Transplantation 2000;70(1):143‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Smak Gregoor PJ, Gelder T, Besouw NM, Mast BJ, Kuiper P, IJzermans JN, et al. Five‐year follow‐up of a prospective, randomised study on minimising immunosuppressive medication from one year after kidney transplantation [abstract no: 1276]. American Journal of Transplantation 2003;3(Suppl 5):479. [CENTRAL: CN‐00447778] [Google Scholar]
- Gelder T, Kuiper P, Besouw NM, Mast B, Smak Gregoor PJ, Ijzermans JN, et al. Randomised trial comparing conversion of maintenance treatment with ciclosporine and prednisone to azathioprine or mycophenolate mofetil with prednisone one year after kidney transplantation [abstract no: 248]. Transplantation 1998;65(12):S64. [CENTRAL: CN‐00583809] [Google Scholar]
Touchard 2005 {published data only}
- Touchard G, Bridoux F, Etienne I, Toupance O, Lavaud S, Hurault de Ligny B, et al. Efficacy and safety of maintenance neoral monotherapy compared to bitherapy neoral+MMF or neoral+AZA in renal transplantation [abstract no: 1198]. American Journal of Transplantation 2005;5(Suppl 11):462. [CENTRAL: CN‐00793401] [Google Scholar]
Tsinalis 2000 {published data only}
- Tsinalis D, Binet I, Dickenmann M, Steiger J, Brunner F, Thiel G. Cost of medical care after renal transplantation comparing cyclosporine‐mycophenolate to tacrolimus‐azathioprine ‐ a randomised controlled study [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000.
Vacher‐Coponat 2006 {published data only}
- Al‐Massarani G, Vacher‐Coponat H, Paul P, Widemann A, Arnaud L, Loundou A, et al. Impact of immunosuppressive treatment on endothelial biomarkers after kidney transplantation. American Journal of Transplantation 2008;8(11):2360‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Legris T, Picard C, Moal V, Burtey S, Loundou A, Purgus R, et al. Humoral immunity after kidney transplantation: impact of two randomized immunosuppressive protocols. Annals of Transplantation 2013;18:622‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vacher‐Coponat H, Brunet C, Moal V, Loundou A, Bonnet E, Lyonnet L, et al. Tacrolimus/mycophenolate mofetil improved natural killer lymphocyte reconstitution one year after kidney transplant by reference to cyclosporine/azathioprine. Transplantation 2006;82(4):558‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vacher‐Coponat H, Indreies M, Moal V, Purgus R, Moussi JF, Dussol B, et al. Cost effectiveness comparison for two immunosuppressive regimens in kidney transplantation [abstract no: 1634]. Transplantation 2008;86(2 Suppl):541. [CENTRAL: CN‐00679019] [Google Scholar]
- Vacher‐Coponat H, Moal V, Indreies M, Purgus R, Loundou A, Burtey S, et al. A randomized trial with steroids and antithymocyte globulins comparing cyclosporine/azathioprine versus tacrolimus/mycophenolate mofetil (CATM2) in renal transplantation. Transplantation 2012;93(4):437‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van der Mast 2000 {published data only}
- Mast BJ, Besouw NM, Kuiper P, Vaessen LM, Ijzermans JN, Gelder T, et al. A longitudinal study of TGF‐beta1 protein levels in renal allograft recipients converted from CsA to MMF or AZA. Clinical Transplantation 2000;14(1):66‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vanrenterghem 1998 {published data only}
- Forsythe J. Tacrolimus and mycophenolate mofetil in cadaveric renal transplant recipients. The European Multicentre Tacrolimus/MMF Study Group. Transplantation Proceedings 1999;31(7A):69S‐71S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morales JM, Andres A, Morales E, Herrero JC, Cubas A, Praga M, et al. Tacrolimus, mycophenolate mofetil and corticosteroids as primary immunosuppression after renal transplantation at the Hospital 12 de Octubre, Madrid. Transplantation Proceedings 1999;31(7A):75S‐77S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Squifflet JP, Backman L, Claesson K, Dietl KH, Ekberg H, Forsythe JL, et al. Dose optimization of mycophenolate mofetil when administered with a low dose of tacrolimus in cadaveric renal transplant recipients. Transplantation 2001;72(1):63‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Squifflet JP, Hooff JP, Vanrenterghem Y. The Benelux experience with the combination of tacrolimus and mycophenolate mofetil. Transplantation Proceedings 1999;31(7A):72S‐74S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vanrenterghem Y, Squifflet JP, Forsythe J, Heeman U, Backman L, Taube D, et al. Co‐administration of tacrolimus and mycophenolate mofetil in cadaveric renal transplant recipients. Transplantation Proceedings 1998;30(4):1290‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hooff JP, Squifflet JP, Vanrenterghem Y. Benelux experience with a combination of tacrolimus and mycophenolate mofetil: 4‐year results. Transplantation Proceedings 2002;34(5):1591‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Woeste 2002 {published data only}
- Woeste G, Wullstein C, Dette K, Pridohl O, Lubke P, Bechstein WO. Tacrolimus/mycophenolate mofetil vs cyclosporine A/azathioprine after simultaneous pancreas and kidney transplantation: five‐year results of a randomized study. Transplantation Proceedings 2002;34(5):1920‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Woeste G, Wullstein C, Pridohl O, Dette K, Bechstein WO. Five year results of a randomized study comparing FK506/MMF and ciclosporin A/azathioprine after simultaneous pancreas and kidney transplantation [abstract]. 5th International Conference on New Trends in Clinical and Experimental Immunosuppression; 2002 Feb 7‐10; Geneva, Switzerland. 2002. [CENTRAL: CN‐00817724]
Wuthrich 2000 {published data only}
- Binswanger U, Ambuehl P, Cicvara Muzar S, Knoflach A. Randomized conversion from Mycophenolate to Azathioprine: follow‐up after 2 years. [abstract no: 1592]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00602119]
- Inderbitzin M, Muzar SC, Ambuhl PM, Knoflach A, Binswanger U. Randomized conversion from mycophenolate mofetil to azathioprine: follow‐up after 2 years [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):896A. [CENTRAL: CN‐00602120] [Google Scholar]
- Wuthrich RP, Cicvara S, Ambuhl PM, Binswanger U. Randomized trial of conversion from mycophenolate mofetil to azathioprine 6 months after renal allograft transplantation. Nephrology Dialysis Transplantation 2000;15(8):1228‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Do 2001a {published data only}
- Do JH, Huh W, Kim JA, Lee HR, Choi SC, Han HJ. The influence of mycophenolate (MMF) and azathioprine (AZA) in the same cadaveric renal transplantation. Korean Journal of Nephrology 2001;20(6):949‐54. [CENTRAL: CN‐01044485] [Google Scholar]
References to ongoing studies
ATHENA Study 2012 {published data only}
- Perico N. A randomized, prospective, multicenter trial to compare the effect on chronic allograft nephropathy prevention of mycophenolate mofetil versus azathioprine as the sole immunosuppressive therapy for kidney transplant recipients. www.clinicaltrials.gov/ct2/show/NCT00494741 (accessed 30 November 2015).
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Budde 2004
- Budde K, Curtis J, Knoll G, Chan L, Neumayer HH, Seifu Y, et al. Enteric‐coated mycophenolate sodium can be safely administered in maintenance renal transplant patients: results of a 1‐year study. American Journal of Transplantation 2004;4(2):237‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Domhan 2009
- Domhan S, Zeier M, Abdollahi A. Immunosuppressive therapy and post‐transplant malignancy. Nephrology Dialysis Transplantation 2009;24(4):1097‐103. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ekberg 2003
- Ekberg H. Graft survival benefit to be expected of new immunosuppressive regimens. Transplantation Reviews 2003;17(4):187‐93. [EMBASE: 2003503607] [Google Scholar]
European MMF Study Group 1995
- Placebo‐controlled study of mycophenolate mofetil combined with cyclosporin and corticosteroids for prevention of acute rejection. European Mycophenolate Mofetil Cooperative Study Group. Lancet 1995;345(8961):1321‐5. [MEDLINE: ] [PubMed] [Google Scholar]
FDA 2008
- U.S Food, Drug Administration. CellCept (mycophenolate mofetil capsules) FDA Medwatch. 2008. http://www.fda.gov/safety/medwatch/safetyinformation/safety‐relateddruglabelingchanges/ucm119284.htm (accessed 4 November 2015).
Halloran 2004
- Halloran PF. Immunosuppressive drugs for kidney transplantation. New England Journal of Medicine 2004;351(26):2715‐29. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hardinger 2013
- Hardinger KL, Brennan DC. Novel immunosuppressive agents in kidney transplantation. World Journal of Transplantation 2013;3(4):68‐77. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ioannidis 2004
- Ioannidis JP, Evans SJ, Gotzsche PC, O'Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Israni 2010
- Israni AK, Snyder JJ, Skeans MA, Peng Y, Maclean JR, Weinhandl ED, et al. Predicting coronary heart disease after kidney transplantation: Patient Outcomes in Renal Transplantation (PORT) Study. American Journal of Transplantation 2010;10(2):338‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Johnston 2010
- Johnston O, Jaswal D, Gill JS, Doucette S, Fergusson DA, Knoll GA. Treatment of polyomavirus infection in kidney transplant recipients: a systematic review. Transplantation 2010;89(9):1057‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kauffman 2006
- Kauffman HM, Cherikh WS, McBride MA, Cheng Y, Hanto DW. Post‐transplant de novo malignancies in renal transplant recipients: the past and present. Transplant International 2006;19(8):607‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDIGO 2009
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. American Journal of Transplantation 2009;9 Suppl 3:S1‐155. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kent 2007
- Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA 2007;298(10):1209‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Knight 2009
- Knight SR, Russell NK, Barcena L, Morris PJ. Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: a systematic review. Transplantation 2009;87(6):785‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lau 2006
- Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ 2006;333(7568):597‐600. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marcen 2009
- Marcen R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs 2009;69(16):2227‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meier‐Kriesche 2003
- Meier‐Kriesche HU, Steffen BJ, Hochberg AM, Gordon RD, Liebman MN, Morris JA, et al. Mycophenolate mofetil versus azathioprine therapy is associated with a significant protection against long‐term renal allograft function deterioration. Transplantation 2003;75(8):1341‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;357(9263):1191‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Morath 2004
- Morath C, Mueller M, Goldschmidt H, Schwenger V, Opelz G, Zeier M. Malignancy in renal transplantation. Journal of the American Society of Nephrology 2004;15(6):1582‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mowbray 1965
- Mowbray JF, Cohen SL, Doak PB, Kenyon JR, Owen K, Percival A, et al. Human cadaveric renal transplantation. Report of twenty cases. British Medical Journal 1965;2(5475):1387‐94. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pascual 2002
- Pascual M, Theruvath T, Kawai T, Tolkoff‐Rubin N, Cosimi AB. Strategies to improve long‐term outcomes after renal transplantation. New England Journal of Medicine 2002;346(8):580‐90. [DOI] [PubMed] [Google Scholar]
Pittler 2000
- Pittler MH, Abbot NC, Harkness EF, Ernst E. Location bias in controlled clinical trials of complementary/alternative therapies. Journal of Clinical Epidemiology 2000;53(5):485‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ramos 2009
- Ramos E, Drachenberg CB, Wali R, Hirsch HH. The decade of polyomavirus BK‐associated nephropathy: state of affairs. Transplantation 2009;87(5):621‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ridker 2006
- Ridker PM, Torres J. Reported outcomes in major cardiovascular clinical trials funded by for‐profit and not‐for‐profit organizations: 2000‐2005. [Erratum appears in JAMA. 2006 Jun 21;295(23):2726]. JAMA 2006;295(19):2270‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Salvadori 2004
- Salvadori M, Holzer H, Mattos A, Sollinger H, Arns W, Oppenheimer F, et al. Enteric‐coated mycophenolate sodium is therapeutically equivalent to mycophenolate mofetil in de novo renal transplant patients. American Journal of Transplantation 2004;4(2):231‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schmid 2004
- Schmid CH, Stark PC, Berlin JA, Landais P, Lau J. Meta‐regression detected associations between heterogeneous treatment effects and study‐level, but not patient‐level, factors. Journal of Clinical Epidemiology 2004;57(7):683‐97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schold 2009
- Schold JD, Kaplan B. AZA/tacrolimus is associated with similar outcomes as MMF/tacrolimus among renal transplant recipients. American Journal of Transplantation 2009;9(9):2067‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sifontis 2006
- Sifontis NM, Coscia LA, Constantinescu S, Lavelanet AF, Moritz MJ, Armenti VT. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation 2006;82(12):1698‐702. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Snyder 2009
- Snyder JJ, Israni AK, Peng Y, Zhang L, Simon TA, Kasiske BL. Rates of first infection following kidney transplant in the United States. Kidney International 2009;75(3):317‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Srinivas 2005
- Srinivas TR, Kaplan B, Schold JD, Meier‐Kriesche HU. The impact of mycophenolate mofetil on long‐term outcomes in kidney transplantation. Transplantation 2005;80(2 Suppl):S211‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Staatz 2007
- Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clinical Pharmacokinetics 2007;46(1):13‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tantravahi 2007
- Tantravahi J, Womer KL, Kaplan B. Why hasn't eliminating acute rejection improved graft survival?. Annual Review of Medicine 2007;58:369‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Terrin 2005
- Terrin N, Schmid CH, Lau J. In an empirical evaluation of the funnel plot, researchers could not visually identify publication bias. Journal of Clinical Epidemiology 2005;58(9):894‐901. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wagner 2009
- Wagner M, Balk EM, Kent DM, Kasiske BL, Ekberg H. Subgroup analyses in randomized controlled trials: the need for risk stratification in kidney transplantation. American Journal of Transplantation 2009;9(10):2217‐22. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallace 2009
- Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta‐Analyst: software for meta‐analysis of binary, continuous and diagnostic data. BMC Medical Research Methodology 2009;9:80. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2004a
- Wang K, Zhang H, Li Y, Wei Q, Li H, Yang Y, et al. Efficacy of mycophenolate mofetil versus azathioprine after renal transplantation: a systematic review. Transplantation Proceedings 2004;36:2071‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 2004b
- Wang K, Zhang H, Li Y, Wei Q, Li H, Yang Y, et al. Safety of mycophenolate mofetil versus azathioprine in renal transplantation: a systematic review. Transplantation Proceedings 2004;36(7):2068‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 2005
- Wang KJ, Zhang HT, Li YP, Lu YP, Wei Q, Li H, et al. Safety of mycophenolate mofetil versus azathioprin in renal transplantation: a systematic review. Chinese Journal of Evidence‐Based Medicine 2005;5(5):365‐74. [EMBASE: 2005250232] [Google Scholar]
Webster 2005
- Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta‐analysis and meta‐regression of randomised trial data. BMJ 2005;331(7520):810. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Webster 2006
- Webster AC, Lee VW, Chapman JR, Craig JC. Target of rapamycin inhibitors (TOR‐I; sirolimus and everolimus) for primary immunosuppression in kidney transplant recipients. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD004290.pub2] [DOI] [PubMed] [Google Scholar]
Wimmer 2007
- Wimmer CD, Rentsch M, Crispin A, Illner WD, Arbogast H, Graeb C, et al. The janus face of immunosuppression ‐ de novo malignancy after renal transplantation: the experience of the Transplantation Center Munich. Kidney International 2007;71(12):1271‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhang 2004
- Zhang H, Wang K, Li Y, Gao L, Liu J, Cai Y. Systematic review of randomized controlled trials about comparison mycophenolate mofetil and azathioprine after renal transplantation. Chinese Journal of Evidence‐Based Medicine 2004;4(2):79‐91. [Google Scholar]
References to other published versions of this review
Wagner 2009a
- Wagner M, Balk EM, Webster AC, Raman G, Trikalinos TA, Schmid CH, et al. Mycophenolic acid versus azathioprine as primary immunosuppression for kidney transplant recipients. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007746] [DOI] [PMC free article] [PubMed] [Google Scholar]


