Abstract
Previous studies have demonstrated that endogenous tissue-type plasminogen activator (tPA) is upregulated in the brain after an acute ischemic stroke (AIS). While mixed results were observed in genetic models, the pharmacological inhibition of endogenous tPA showed beneficial effects. Treatment with exogenous recombinant tPA exacerbated brain damage in rodent models of stroke. Despite the detrimental effects of tPA on ischemic stroke, recombinant tPA is administered to AIS patients to recanalize the occluded blood vessels because the benefits of its administration outweigh the risks associated with tPA upregulation and increased activity. We hypothesized that tPA knockdown following recanalization would ameliorate sensorimotor deficits and reduce brain injury. Young male and female rats (2–3 months old) were subjected to transient focal cerebral ischemia by occlusion of the right middle cerebral artery. Shortly after reperfusion, rats from appropriate cohorts were administered a nanoparticle formulation containing tPA shRNA or control shRNA plasmids (1 mg/kg) intravenously via the tail vein. Infarct volume during acute and chronic phases, expression of matrix metalloproteinases (MMPs) 1, 3, and 9, enlargement of cerebral ventricle volume, and white matter damage were all reduced by shRNA-mediated gene silencing of tPA following reperfusion. Additionally, recovery of somatosensory and motor functions was improved. In conclusion, our results provide evidence that reducing endogenous tPA following recanalization improves functional outcomes and reduces post-stroke brain damage.
Keywords: tissue-type plasminogen activator, ischemia, recanalization, reperfusion, infarct volume, white matter, sensory function, motor function
1. Introduction
Alteplase, a recombinant tissue-type plasminogen activator (tPA) for patients with acute ischemic stroke (AIS), remains the only pharmacological treatment approved by the Food and Drug Administration for recanalizing the blocked blood vessel in eligible patients. Endovascular mechanical thrombectomy is an additional treatment option employed when alteplase fails to recanalize the occluded blood vessel or when patients are ineligible to receive alteplase (Powers et al., 2018). Recanalization leads to the reperfusion of brain tissue that was previously ischemic. Reperfusion-induced brain injury is commonly referred to as reperfusion injury. Current therapeutic strategies primarily focus only on recanalizing obstructed blood vessels; they do not address the brain damage induced by ischemia and reperfusion. Failure to address the brain damage caused by cerebral ischemia and subsequent reperfusion will lead to severe acute and long-lasting consequences (secondary to reperfusion injury), including additional brain damage and functional impairments.
Numerous studies, including prior research in our lab, indicate that the expression and activity of endogenous tPA increase substantially in the ischemic brain following cerebral ischemia and reperfusion (Wang, Y. F. et al., 1998, Yepes et al., 2000, Lemarchand et al., 2016, Chelluboina, Klopfenstein et al., 2015, Chelluboina, Warhekar et al., 2015, Challa et al., 2022b). The outcomes of experiments using tPA knockout animals yielded mixed results and have been inconclusive (Nagai et al., 1999, Pu et al., 2019, Wang, Y. F. et al., 1998). However, improvements in post-stroke outcomes were observed in rodent stroke models with either genetic deficiency of plasminogen activator inhibitor-1 (PAI-1) (endogenous inhibitor of tPA) or overexpression of neuroserpin (endogenous antagonist of tPA) (Nagai et al., 1999, Cinelli et al., 2001). In addition, beneficial outcomes were observed when tPA was pharmacologically inhibited with neuroserpin following ischemic stroke (Yepes et al., 2000, Lebeurrier et al., 2005). The detrimental role of increased endogenous tPA in the brain after ischemic stroke is apparent from these studies.
Although it had been known that elevated levels of endogenous tPA in the ischemic brain exacerbate brain injury, eligible patients with AIS are administered recombinant tPA within 4.5 hours of symptom onset to facilitate the recanalization of thrombus-obstructed blood vessels (Hacke et al., 2008, Del Zoppo et al., 2009). The exogenously administered recombinant tPA, particularly alteplase, is fibrin-specific and has a plasma half-life of 4–6 minutes. Despite the short plasma half-life of recombinant tPA, the impact of tPA on the coagulation system can last for 24 hours and the residual tPA may still be present within the ischemic brain (Matosevic et al., 2013). Exogenous administration of recombinant tPA to tPA knockout and wild-type animals worsened post-stroke outcomes, indicating that increased levels of tPA in the brain exacerbates brain injury (Wang, Y. F. et al., 1998). While recanalization of the occluded blood vessel is achieved through the administration of recombinant tPA, an additional treatment that decreases endogenous and/or exogenous tPA following recanalization might offer substantial therapeutic benefits for patients with AIS. Furthermore, in cases of recombinant tPA treatment-associated hemorrhage, tPA reversal is needed. Currently, the reversal of tPA effect is achieved indirectly through the administration of cryoprecipitate (Yaghi et al., 2017, Verkerk et al., 2023). At present, no antidote is available for either exogenously administered recombinant tPA or elevated endogenous tPA. While the tPA shRNA used in this study prevents the upregulation of endogenous tPA, it is incapable of neutralizing or acting as an antidote against exogenously administered recombinant tPA or the endogenous tPA itself.
The objective of this study was to investigate the effects of endogenous tPA knockdown (using shRNA-mediated gene silencing) after recanalization on functional and histological outcomes during the acute and chronic phases after transient focal cerebral ischemia and reperfusion. It was hypothesized that endogenous tPA knockdown after recanalization would ameliorate sensorimotor function deficits and reduce brain injury. The results of these studies will serve as proof-of-concept for subsequent assessments according to the Stroke Therapy Academic Industry Roundtable (STAIR) recommendations.
2. Material and methods
2.1. Transient ischemia induction in laboratory animals
A total of 84 Sprague-Dawley rats of both sexes, aged 2–3 months, obtained from Envigo Laboratories, were randomly allocated to experimental groups: the sham group and the ischemia group. The ischemia group consisted of three subgroups: untreated, control shRNA-treated and tPA shRNA-treated. Rats were subjected to transient (1.5 hours or 2 hours) right middle cerebral artery occlusion (MCAO) followed by reperfusion as described recently by our group (Challa et al., 2022b). The modified neurological severity score (mNSS) assessment was performed on rats in the ischemia group at 2–4 hours and day 1 post-ischemia (Nalamolu, Challa et al., 2021, Nalamolu, Chelluboina et al., 2021). Rats that did not exhibit a mNSS between 8 and 12 at 2–4 hours or on day 1 after ischemia were excluded from the study. In addition, animals that died during the study period and animals with postmortem evidence of hemorrhage around the right MCA were excluded from the study. Furthermore, animals euthanized during the study due to excessive loss of body weight and inability to access food and water were excluded. All animal care, procedures, and the conducted experiments were in compliance with the scientific, humane, and ethical principles stated in the Guide for the Care and Use of Laboratory Animals (Publication no. 86–23 revised, National Institutes of Health, U.S. Department of Health and Human Services), in a protocol approved by the Institutional Animal Care and Use Committee of the University of Illinois College of Medicine Peoria (UICOMP), and consistent with the guidelines stated in the “Ischemia Models: Procedural Refinements of In Vivo Experiments”, and the “ARRIVE guidelines 2.0: Updated guidelines for reporting animal research” (Percie du Sert, Nathalie et al., 2017, Percie du Sert, N. et al., 2020).
2.2. ShRNA plasmids preparation and treatment
The tPA shRNA or scrambled sequence shRNA (control shRNA) plasmids were designed, constructed, and synthesized using pSilencer™ 4.1-CMV neo vector (Ambion, Austin, TX) as described earlier (Challa et al., 2022b).The inverted repeat tPA shRNA sequences are shown in table 1. This study utilized one of the positive clones validated through gene sequencing analysis at the University of Illinois at Urbana-Champaign. The shRNA plasmids synthesized from overnight bacterial cultures using QIAGEN plasmid maxi kit (Qiagen, USA) were formulated as nanoparticles using the in vivo-jetPEI reagent (Polyplus transfection, Illkirch, France) and injected intravenously via tail vein at a dose of 1 mg/kg to ischemia-induced rats promptly (within 30 min) following reperfusion (Fig. 1). Figure 1 also depicts the mechanism of action of tPA shRNA. The UICOMP’s Institutional Biosafety Committee approved the synthesis and use of tPA and control shRNA plasmids.
Table 1:
Primers used for the construction of tPA shRNA and real time PCR analysis.
| Gene | Primer Sequence | |
|---|---|---|
| Forward (5’ - 3’) | Reverse (5’ - 3’) | |
| Primers for the construction of tPA shRNA | ||
| tPA shRNA | gatccgtacatagtccataaggaattcaaga gattccttatggactatgtactta |
agcttaagtacatagtccataaggaatctcttga attccttatggactatgtacg |
| Primers for real time PCR analysis | ||
| MMP-1 | cagcagttatttgggctgaaag | tttggtccaacgaggattgt |
| MMP-3 | ggaccagggattaatggagatg | agcattggctgagtgaaaga |
| MMP-9 | cactgtaactgggggcaact | cacttcttgtcagcgtcgaa |
| 18S rRNA | acgtctgccctatcaactttc | ttggatgtggtagccgtttc |
Fig. 1.
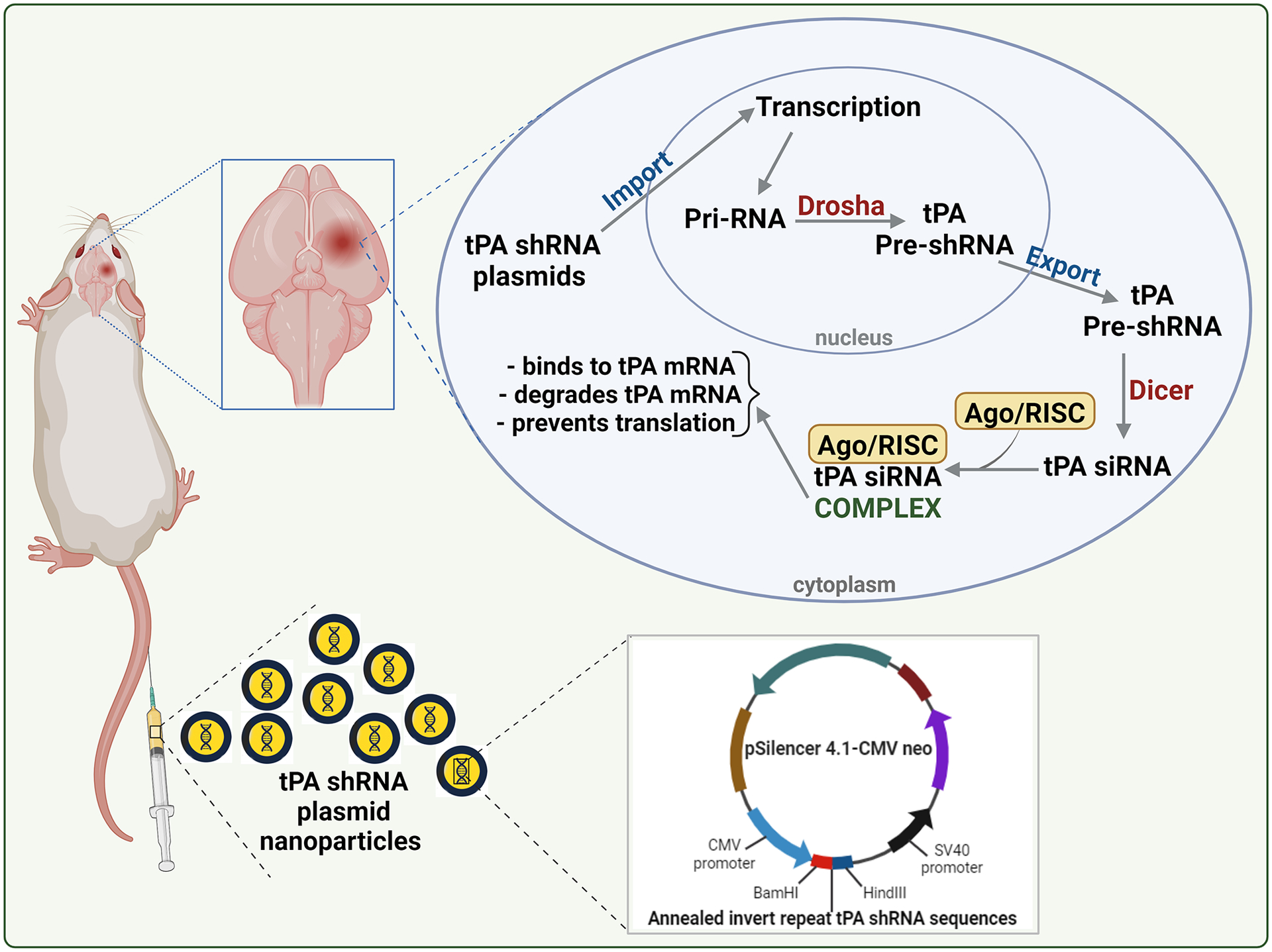
Schematic representation of the tPA shRNA plasmid nanoparticle formulation administration to stroke-induced rats and the mechanism of tPA shRNA-mediated knockdown of tPA. Following the entry of plasmids into the nucleus of a cell, the CMV promoter and Drosha drive the formation of tPA pre-shRNA. Dicer processes tPA pre-shRNA, and the resulting tPA siRNA forms a complex with Ago and RISC. Ago, RISC, and tPA siRNA complex binds and destroys tPA mRNA and thereby prevents tPA protein translation. This figure was created with biorender.com under a paid subscription.
2.3. TTC staining
On post-ischemic day 1, male and female rats from the experimental groups allocated for infarct volume assessment were euthanized. Brains were collected, placed in an adult rat brain matrix (Kent Scientific Corporation, USA), chilled in a freezer at −80 °C until hardened (~10 min), and then cut into 2-mm thick coronal sections. The brain sections were incubated at 37 °C in a freshly prepared 2,3,5-triphenyl tetrazolium chloride (TTC) solution (2% in 1xPBS) for 30–45 min. The non-ischemic (stained red) and total ipsilateral and contralateral areas of each coronal brain sections were traced and measured using the ImageJ analysis software (NIH). The percent infarct volume was calculated using the formula: Infarct volume (%) = [(volume of total contralateral hemisphere – volume of total non-ischemic ipsilateral hemisphere) / volume of total contralateral hemisphere] × 100. This formula takes into account the effect of volume changes in the hemisphere impacted by ischemia and reperfusion.
2.4. Real-time PCR analysis
Rats from appropriate cohorts allocated for real time PCR analysis were euthanized on post-ischemic day 1 and total RNA was extracted from ischemic brain tissues using TRIzol reagent (Invitrogen, Carlsbad, USA). Total RNA (1 μg) from each sample was reverse transcribed to cDNA using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, USA). The real time PCR reaction setup for each cDNA sample was assembled using the iTaq Universal SYBR Green Supermix (Bio-Rad Laboratories, USA). The forward and reverse primers of target genes are listed in table 1. 18S rRNA served as the internal standard. Samples were subjected to the following PCR cycle: [95 °C for 5 min, (95 °C for 30 sec, 60 °C for 30 sec, 72 °C for 30 sec) × 40 cycles, and 72 °C for 5 min] in an iCycler IQ (Multi-Color Real-Time PCR Detection System; Bio-Rad Laboratories, Hercules, California, USA). Data was collected and recorded using the iCycler IQ software (Bio-Rad Laboratories, USA) and expressed as a function of the threshold cycle (Ct). The fold change in target gene expression in the test sample relative to the control sample was calculated using the formula 2^(ΔCt of control)/2^(ΔCt of test).
2.5. Somatosensory and motor function evaluations
Ischemia-induced rats treated with either control shRNA or tPA shRNA were subjected to sticky tape (adhesive tape removal) and rotarod tests as described recently by our group (Nalamolu, Challa et al., 2021, Nalamolu, Chelluboina et al., 2021). Rats were trained to walk on a rotating rotarod for two or three days before ischemia induction. Trained rats that met the set standard (rotarod latency ≥ 60 sec) were included in the rotarod test. The tests were administered prior to ischemia (baseline) and at regular intervals (days 1, 3, 5, 7, and 14) post-ischemia. Trained researchers blinded to treatments conducted the tests and collected the data.
2.6. Animal perfusion, brain collection, and tissue sectioning
Following the final assessment of sensorimotor function on post-ischemic day 14, rats were administered an overdose of sodium pentobarbital (~300 mg/kg, i.p.) and perfused transcardially with ice-cold 1xPBS, followed by 10% buffered formalin solution. Following removal, the brains were postfixed in formalin and then cryoprotected with 30% sucrose in 1xPBS. The brains were embedded in optimal cutting temperature (OCT) compound and stored at −80 °C until sectioned on a cryostat (Leica CM1950). Frozen coronal sections (40-μm thick) were cut along the rostral-caudal axis of the forebrain (bregma: 2.70 mm to −5.8 mm). The sections extended from the frontoparietal cortex (at the level of the forceps minor corpus callosum) to the ventral hippocampus. Separate series of tissue (1 in 15 sections) were collected in 24-well polystyrene plates containing 1xPBS and 0.02% sodium azide. The tissue samples were stored at 4 °C in a refrigerator.
2.7. Cresyl violet staining
One set of tissue (every 15th section, 14 in total) was mounted on glass slides coated with gelatin and chromium alum for each brain. The sections were dried overnight in a 37 °C oven before staining with cresyl violet acetate (0.1% w/v; Electron Microscopy Sciences). Sections were sequentially defatted in xylene, rehydrated in water with graded ethanol (100%, 95%, 70%, and 50%), stained with cresyl violet dye, rinsed in water and 50% ethanol, differentiated in 70% ethanol and 0.1% glacial acetic acid, dehydrated in ethanol (95%, 100%), and lastly cleared in xylene. The slides were then coverslipped with DPX mounting medium and left to dry in a fume hood undisturbed.
2.8. Brain atrophy and infarct volume
Prior to and following cresyl violet staining, each tissue section was scanned at a resolution ≥7500 dpi using a PrimeHisto XE slide scanner (Carolina Biological Supply, USA). In each section, the contralateral brain hemisphere, and the whole brain (contralateral + ipsilateral hemispheres) were manually traced and measured using the ImageJ analysis software (NIH) to obtain an area measurement (mm2). The cross-sectional areas were multiplied by the section thickness (0.04 mm) and the inverse of the section sampling fraction (15) to obtain the volume measurements (mm3). The percentage of atrophy in the lesioned hemisphere was calculated as follows: Percent atrophy = [(volume of the contralateral hemisphere - volume of the ipsilateral hemisphere) / volume of the contralateral hemisphere] × 100. The infarct volume was calculated using the method described by McBride et al (McBride et al., 2015). The ratio of the infarcted area to the entire ipsilateral hemisphere was initially calculated. The fractional amount of infarcted tissue per section was multiplied by the area of the contralateral hemisphere of the brain corresponding to the same section. The sum of the corrected infarct areas across all sections was then multiplied by the distance between sections, as detailed above. Hence, this formula takes into account the effect of volume changes in the hemisphere impacted by ischemia in determining the volume of the infarct.
2.9. Lateral Ventricular Enlargement
A volumetric analysis was conducted on the lateral ventricles of the brain to determine the extent of ventricular enlargement after ischemic stroke. Eight serial sections (Sections 3–10) from each brain were examined, extending from the anterior striatum to the dorsal hippocampus (bregma: 1.5 mm to −2.7 mm, with sections 0.6 mm apart). The contralateral and ipsilateral ventricles in each section were outlined and the corresponding areas were determined to the nearest 0.01 μm2. The overall volume of each ventricle was calculated by multiplying the sum of the ventricle areas across all sections by the section thickness (0.04 mm) and the inverse of the section sampling fraction (15). In addition, the total volume of both lateral ventricles was calculated for each brain.
2.10. White Matter Damage
The thickness of the corpus callosum, a structure composed of heavily myelinated nerve fibers connecting the cerebral hemispheres, was measured in unstained tissue sections to determine the extent of white matter damage in the brain after ischemic stroke. In each brain, three serial sections (Sections 5–7) of the corpus callosum were studied at the caudate putamen (0.6 mm apart; approximate bregma 0.3 mm to −0.9 mm). A vertical line was drawn from the dorsal to the ventral margins of the corpus callosum at the midline of each section. The distance of the line superimposed on the corpus callosum was measured to the nearest 0.1 μm using ImageJ. An average of six to ten measurements per section was used for data analysis.
2.11. Statistical analysis
Statistical analysis of the data was performed using GraphPad Prism 8.4.3 for Windows. The data identified as outliers by the Grubbs test were removed from the analysis. The quantitative data from each experiment was tested for normality and equality of variances. Based on the number of groups present in each experiment and the outcome of the normality and variance tests, appropriate statistical tests (Two-way repeated measures ANOVA followed by Sidak’s multiple comparisons test, unpaired t test with or without Welch’s correction, and Pearson r test) were performed to analyze the data. Differences between groups were considered significant at p < 0.05. All data are expressed as mean ± SEM.
3. Results
3.1. Animals excluded from the study
Of 102 rats (82 males and 20 females), 36 (30 males and 6 females), or approximately 35% of the total, were excluded from the study for various reasons. These reasons included post-ischemic neurological scores below 8 or above 12, mortality during the study period, euthanasia due to excessive loss of body weight as a result of their inability to access food and water within the cage, and postmortem signs of hemorrhage surrounding the right MCA.
3.2. ShRNA-mediated gene silencing of tPA following reperfusion decreases infarct volume during the acute phase
The primary sources of tPA in the brain are endothelial cells of cerebral microvessels, neurons, and resident microglia (Cinelli et al., 2001, Siao, Fernandez and Tsirka., 2003). Recently, we reported a significant increase in endogenous tPA expression in the ischemic brains of rats for up to five days after transient focal cerebral ischemia and reperfusion (Challa et al., 2022b). Treatment with tPA shRNA decreases tPA expression by degrading tPA mRNA in cells that produce tPA (Fig. 1). Our laboratory has recently demonstrated that tPA shRNA treatment effectively decreases tPA expression in the ischemic brains of stroke-induced rats (Challa et al., 2022b). In this study, a 1.5-hour MCAO in rats resulted in an infarct of 34.45% ± 6.51% in males and 34.60% ± 4.71% in females, when assessed on post-ischemic day 1 (Fig. 2). tPA shRNA treatment reduced the infarct volume by 78% in males and 66% in females. Statistical analysis (two-way repeated-measures ANOVA) revealed a significant effect of Treatment (p < 0.0001) but no effect of Sex (p = 0.5799) nor Treatment × Sex interaction (p = 0.6055). These results indicated that the mean infarct volume did not differ between males and females within any treatment group. Subsequent post hoc testing (Sidak’s multiple comparisons test) confirmed that the tPA shRNA group showed a statistically significant decrease in infarct volume in both males (p < 0.0001) and females (p = 0.0007), compared to the untreated group.
Fig. 2.
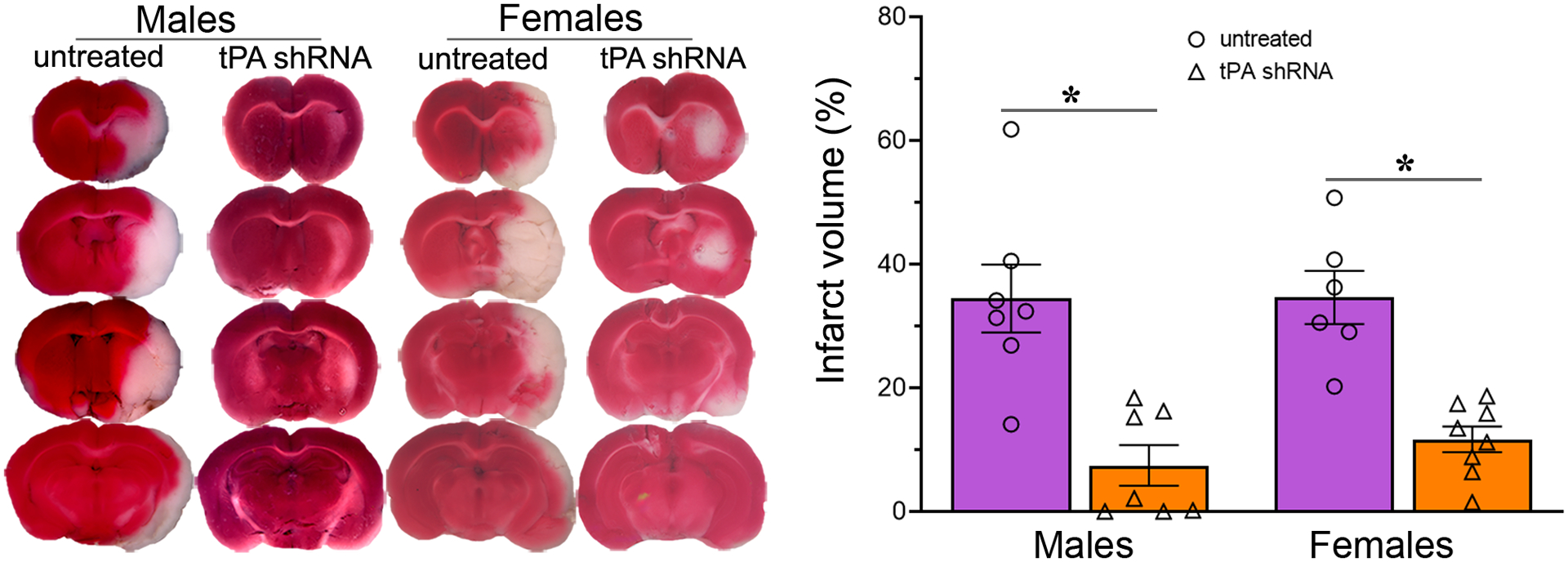
tPA shRNA treatment after reperfusion decreases acute brain damage in both sexes of stroke-induced rats. Representative TTC stained images of untreated and tPA shRNA treated male and female rat brain coronal sections one day after a 1.5-h focal cerebral ischemia. The column scatter plot shows the quantified percent infarct volume in various experimental groups. Columns indicate the mean and error bars indicate the SEM. *p < 0.05 vs untreated group.
3.3. Treatment with tPA shRNA reduces the expression of MMPs in the ischemic brain
On post-ischemic day 1, the mRNA expression of MMP-1, MMP-3, and MMP-9 was decreased by 91%, 73%, and 76%, respectively, in the ischemic brain of tPA shRNA treated rats compared to untreated stroke-induced rats (Fig. 3). Statistical analysis (unpaired t test with or without Welch’s correction, two-tailed) revealed that tPA shRNA treatment significantly decreased the expression of all the studied MMPs: MMP-1 (t = 4.49; p = 0.0107), MMP-3 (t = 5.562; p = 0.0002), and MMP-9 (t = 2.895; p = 0.031).
Fig. 3.
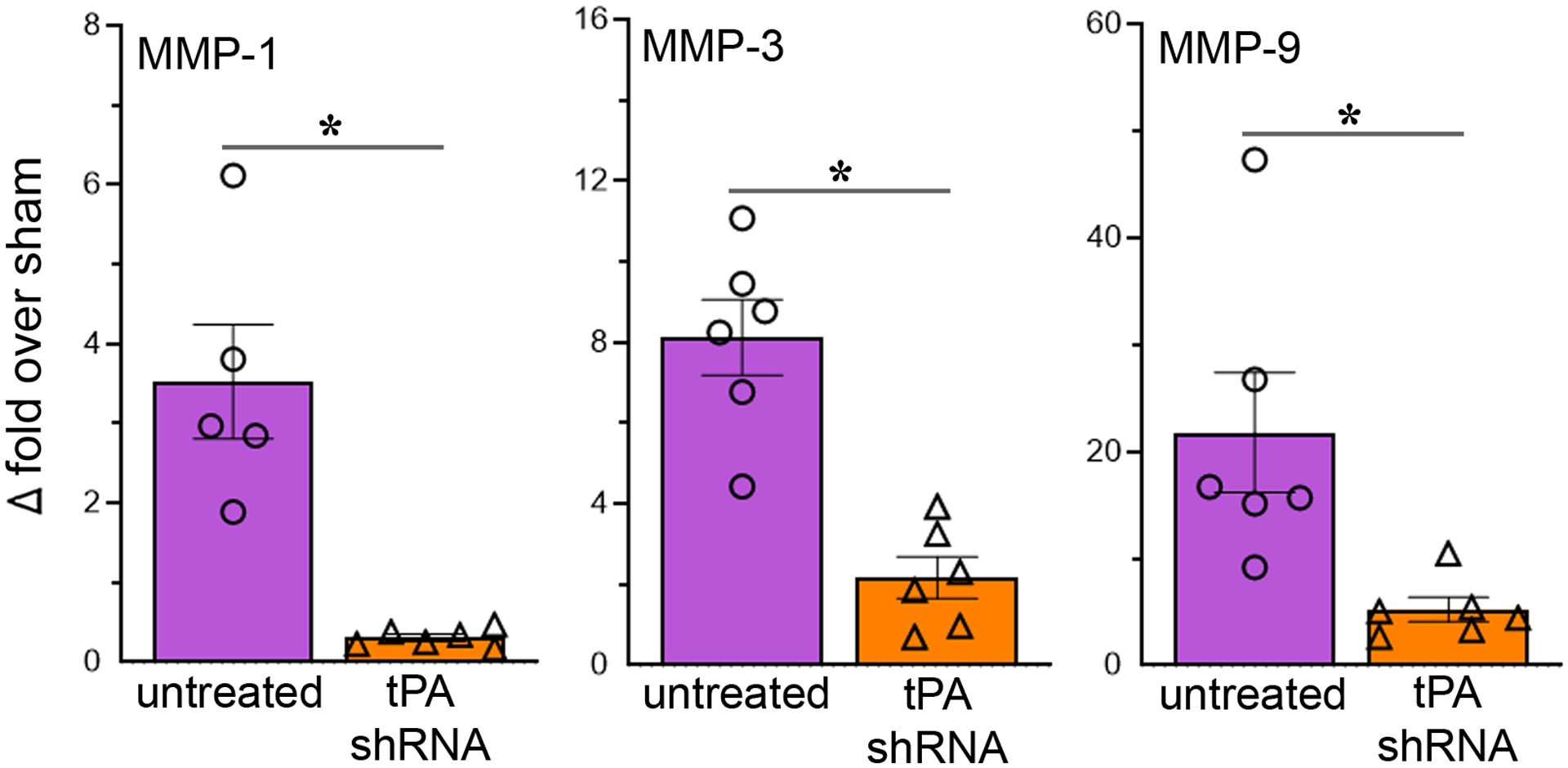
Reducing tPA after reperfusion decreases the expression of MMPs in the ischemic brain. The column scatter plots show the quantified mRNA expression (expressed as fold change over sham) of MMP-1, MMP-3, and MMP-9 in the ischemic brains of rats subjected to 2-h focal cerebral ischemia followed by reperfusion and euthanized on post-ischemic day 1. Columns indicate the mean and error bars indicate the SEM. *p < 0.05 (tPA shRNA vs untreated group).
3.4. Effect of reducing tPA following reperfusion after brief ischemia on survival rate and body weight
The 14-day survival rate was 83% in the tPA shRNA group compared to 71% in the control shRNA group (Fig. 4B). The baseline body weights of the rats in the two groups were not significantly different. As expected, a substantial decline in body weight was observed in all animals from both groups for three to five days after reperfusion (Fig. 4C). Rats in both groups regained or exceeded their initial body weight between post-ischemic days 7 and 14. On day 14, rats in both experimental groups exhibited a significant increase in body weight gain relative to their body weight on day 1. Statistical analysis (two-way repeated-measures ANOVA) revealed that only Time (p < 0.0001) had a significant effect, but not Treatment or the Time × Treatment interaction. The difference in body weights between tPA shRNA and control shRNA groups was not statistically significant (Sidak’s multiple comparisons test) at any of the time points assessed during the study, suggesting that tPA shRNA treatment has no effect on body weight.
Fig. 4.
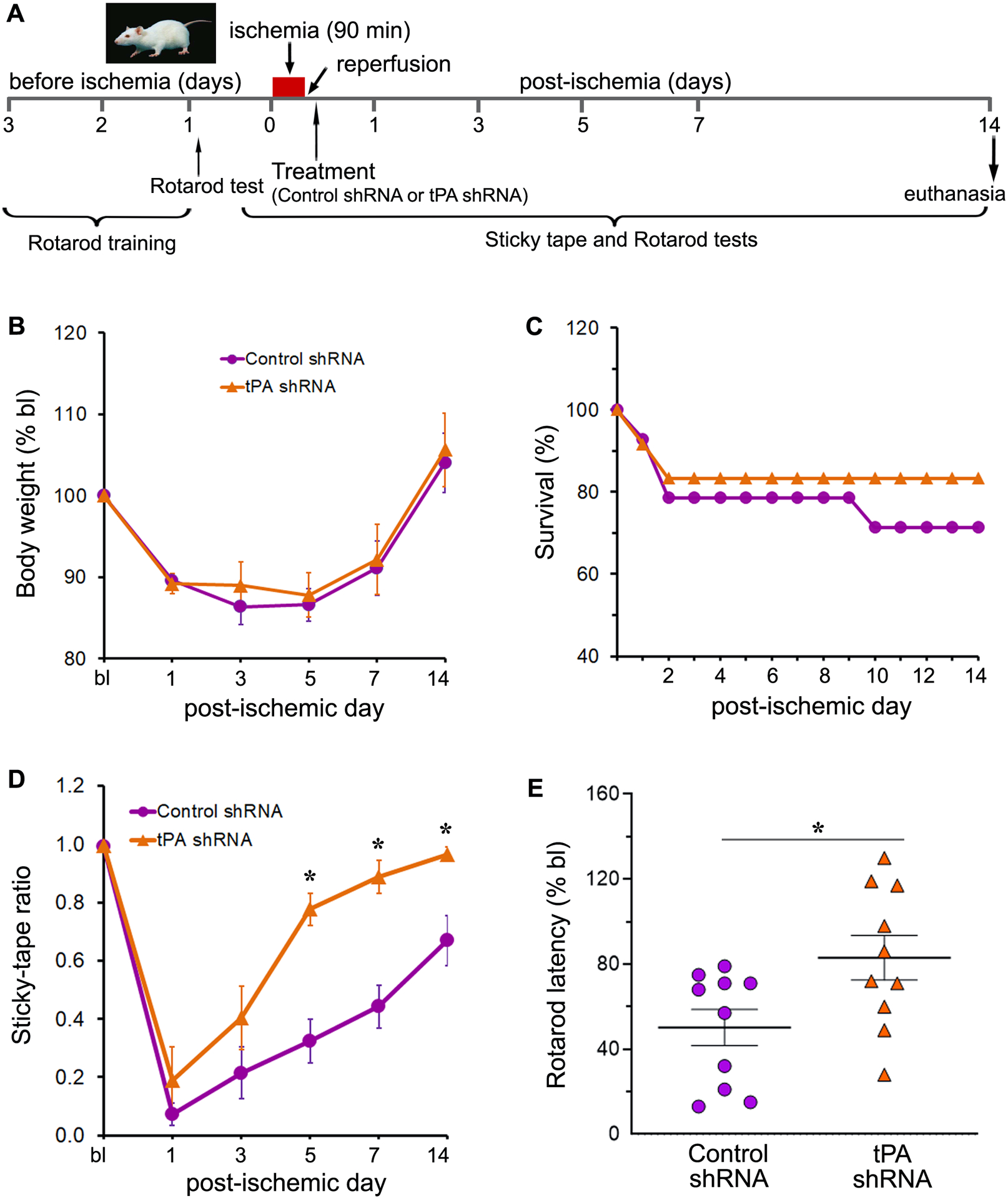
ShRNA-mediated gene silencing of tPA following reperfusion improves sensorimotor function after ischemic stroke. (A) Schematic representation of the experimental design for long-term functional evaluations in rats subjected to transient focal cerebral ischemia and reperfusion followed by treatments as applicable. (B) The line graph shows the changes in body weight, expressed as percentage of baseline (bl), of male rats subjected to 1.5-h focal cerebral ischemia and reperfusion followed by either control shRNA or tPA shRNA treatment. The body weight of rats of both the experimental groups were recorded at baseline and at regular intervals (post-ischemic days 1, 3, 5, 7, and 14). n = 10 (control shRNA group) and 7 (tPA shRNA group). Error bars indicate the SEM. (C) The line graph shows the percent survival of male rats subjected to 1.5-h focal cerebral ischemia and reperfusion followed by either control shRNA or tPA shRNA treatment. n = 14 (control shRNA group) and 12 (tPA shRNA group). (D, E) Rats from both control shRNA and tPA shRNA treated groups were evaluated for the assessment of somatosensory and motor functions at baseline (bl) and at regular intervals after transient ischemia and reperfusion. Post-ischemic sensory and motor function deficits were assessed by the sticky tape test (D) on post-ischemic days 1, 3, 5, 7, and 14, and the accelerating Rotarod performance (E) test on post-ischemic day 14, respectively. Error bars indicate the SEM. n = 10/group. *p < 0.05 vs. control shRNA group.
3.5. Reducing tPA shortly after recanalization facilitates the recovery of somatosensory function
The sticky tape test was performed at regular intervals (baseline and on post-ischemic days 1, 3, 5, 7, and 14) until day 14 to assess somatosensory function in stroke-induced rats (Fig. 4A). The baseline sticky-tape ratio was 0.99 ± 0.00 in the control shRNA group and 1.00 ± 0.00 in the tPA shRNA group. On post-ischemic day 1, the sticky-tape ratio was reduced by 93% in the control shRNA group and by 81% in the tPA shRNA group relative to the baseline ratios for these groups. These results indicate that somatosensory function was impaired following ischemic stroke in both experimental groups, although to a lesser extent in the tPA shRNA group than in the control shRNA group (Fig. 4D). As expected, the sticky-tape ratio gradually increased over time in both groups. By the end of the study (day 14), the sticky-tape ratio exhibited a 816% increase in the control shRNA group and a 408% increase in the tPA shRNA group, relative to the sticky-tape ratios observed on post-ischemic day 1 in these groups. Although the sticky-tape ratio appeared to have improved to a greater extent in the control shRNA group than in the tPA shRNA, the ratio in the tPA shRNA group had attained 97% of its initial (baseline) value, where it had only attained 67% in the control shRNA group. Statistical analysis (two-way repeated-measures ANOVA) showed a significant effect of both Time (p < 0.0001) and Treatment (p = 0.0003). Post hoc testing (Sidak’s multiple comparisons test) revealed that the sticky-tape ratio of the tPA shRNA group was significantly higher than the ratio of the control shRNA group on days 5 (p = 0.0007), 7 (p = 0.0009), and 14 (p = 0.0367). These results suggest that reducing tPA shortly after reperfusion improves the recovery of somatosensory function.
3.6. Treatment with tPA shRNA following reperfusion improves motor function
The rotarod test was performed at regular intervals (baseline and on post-ischemic days 1, 3, 5, 7, and 14) until day 14 to assess motor function in stroke-induced rats (Fig. 4A). The rotarod latency was reduced on post-ischemic day 1 by 82% in the control shRNA group and by 48% in the tPA shRNA group relative to the baseline latencies for these groups. These results indicate that motor function was impaired in both experimental groups following ischemic stroke, but to a lesser extent in the tPA shRNA group than in the control shRNA group (Fig. 4E). As expected, the rotarod latency gradually increased over time in both groups. By the end of the study (day 14), the rotarod latency exhibited a 185% increase in the control shRNA group and a 59% increase in the tPA shRNA group, relative to the rotarod latencies observed on post-ischemic day 1 in these groups. Although the rotarod latency appeared to have improved to a greater extent in the control shRNA group than in the tPA shRNA group, the latency in the tPA shRNA group had attained 83% of its initial (baseline) value, whereas it had only attained 50% in the control shRNA group. Although the tPA shRNA group exhibited a higher mean latency at all tested time points compared to the control shRNA group, statistical analysis (unpaired t test, two-tailed) revealed a significant effect of treatment only on post-ischemic day 14 (t = 2.434; p = 0.0256). Overall, these results suggest that tPA knockdown shortly following recanalization after transient ischemia improves motor function.
3.7. Reducing tPA expression after reperfusion attenuates infarct volume and white matter damage
The examination of coronal brain sections stained with cresyl violet, which were obtained on post-ischemic day 14, demonstrated a substantial reduction in brain damage in rats treated with tPA shRNA compared to those treated with control shRNA (Fig. 5A). A comparative analysis of sections indicated that the mean infarct volume decreased substantially in all sections examined in the tPA shRNA group when compared to the control shRNA group (Fig. 5B). Statistical analysis (two-way repeated-measures ANOVA) determined that Treatment had a significant effect (p = 0.0283). The results of subsequent post hoc testing (Sidak’s multiple comparisons test) showed that the reduction in infarct volume in rats treated with tPA shRNA was statistically significant only for Section 3 (p = 0.0043), Section 4 (p = 0.0146), and Section 5 (p = 0.0464). Rats treated with tPA shRNA exhibited a decrease in total infarct volume, as anticipated, in comparison to the control shRNA-treated rats (Fig. 5C). Statistical analysis (unpaired t test, two-tailed) indicated that the tPA shRNA group exhibited a significantly smaller total infarct volume than in the control shRNA group (t = 2.426; p = 0.0283). Treatment with tPA shRNA did not significantly reduce the brain atrophy (unpaired t test, two-tailed) that was observed in the control shRNA group (Fig. 5D).
Fig. 5.
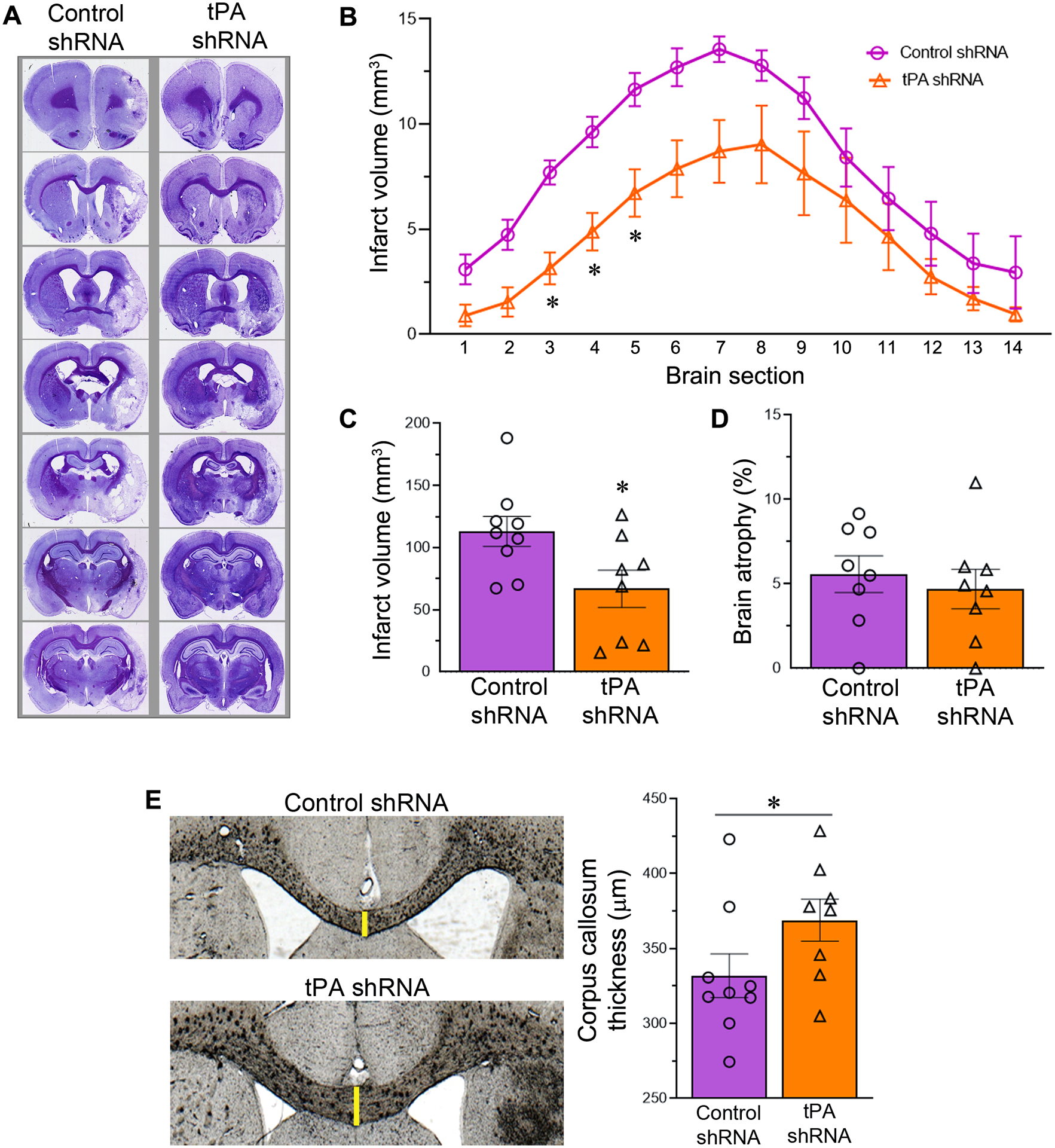
tPA shRNA treatment following transient focal cerebral ischemia and reperfusion attenuates infarct volume and white matter damage. (A) Representative cresyl violet stained coronal brain sections of control and tPA shRNA treated male rats subjected to 1.5-h focal cerebral ischemia followed by reperfusion for 14 days. The resulting stroke infarcts primarily affected the striatum and neocortex. (B) The line graph shows the quantified section by section infarct volume in both the experimental groups. n = 9 (control shRNA group) and 8 (tPA shRNA group). Error bars indicate the SEM. *p < 0.05 vs control shRNA group. (C) The column scatter plot shows the total infarct volume in both the experimental groups. Error bars indicate the SEM. *p < 0.05 vs control shRNA group. (D) The column scatter plot shows the brain atrophy in both the experimental groups. Error bars indicate the SEM. (E) Representative coronal brain sections depict the corpus callosum thickness in control and tPA shRNA treated male rats subjected to 1.5-h focal cerebral ischemia followed by reperfusion for 14 days. The column scatter plot shows the quantified corpus callosum thickness in both control shRNA and tPA shRNA treated rats. Columns indicate the mean and error bars indicate the SEM. *p < 0.05 (tPA shRNA group vs control shRNA group).
To determine the impact of tPA shRNA treatment on post-stroke white matter damage, we measured the corpus callosum thickness in both the control shRNA and tPA shRNA groups (Fig. 5E). The corpus callosum is comprised of millions of myelinated axons and is the largest white matter structure in the brain. As expected, the average thickness of the corpus callosum was greater in the tPA shRNA group than the control shRNA group, indicating less damage. Statistical analysis (unpaired t test with or without Welch’s correction, one-tailed) indicated that the increased corpus callosum thickness in the tPA shRNA group was significant (t = 1.823; p = 0.0441), in comparison to the control shRNA group.
3.8. Lateral ventricle enlargement in the contralateral brain is reduced by tPA shRNA therapy
Cerebral lateral ventricle volume enlargement was observed bilaterally in the control shRNA group (Fig. 6A). Gross examination indicated decreased enlargement of ventricle volume in the tPA shRNA group. Statistical analysis (unpaired t test with or without Welch’s correction, one-tailed) of treatment effect on ventricle volume in both brain hemispheres and whole brain ventricle volume revealed that tPA shRNA treatment, when compared to control shRNA treatment, significantly reduced cerebral ventricle volume only in the contralateral hemisphere (t = 1.824; p = 0.044) (Fig. 6B).
Fig. 6.
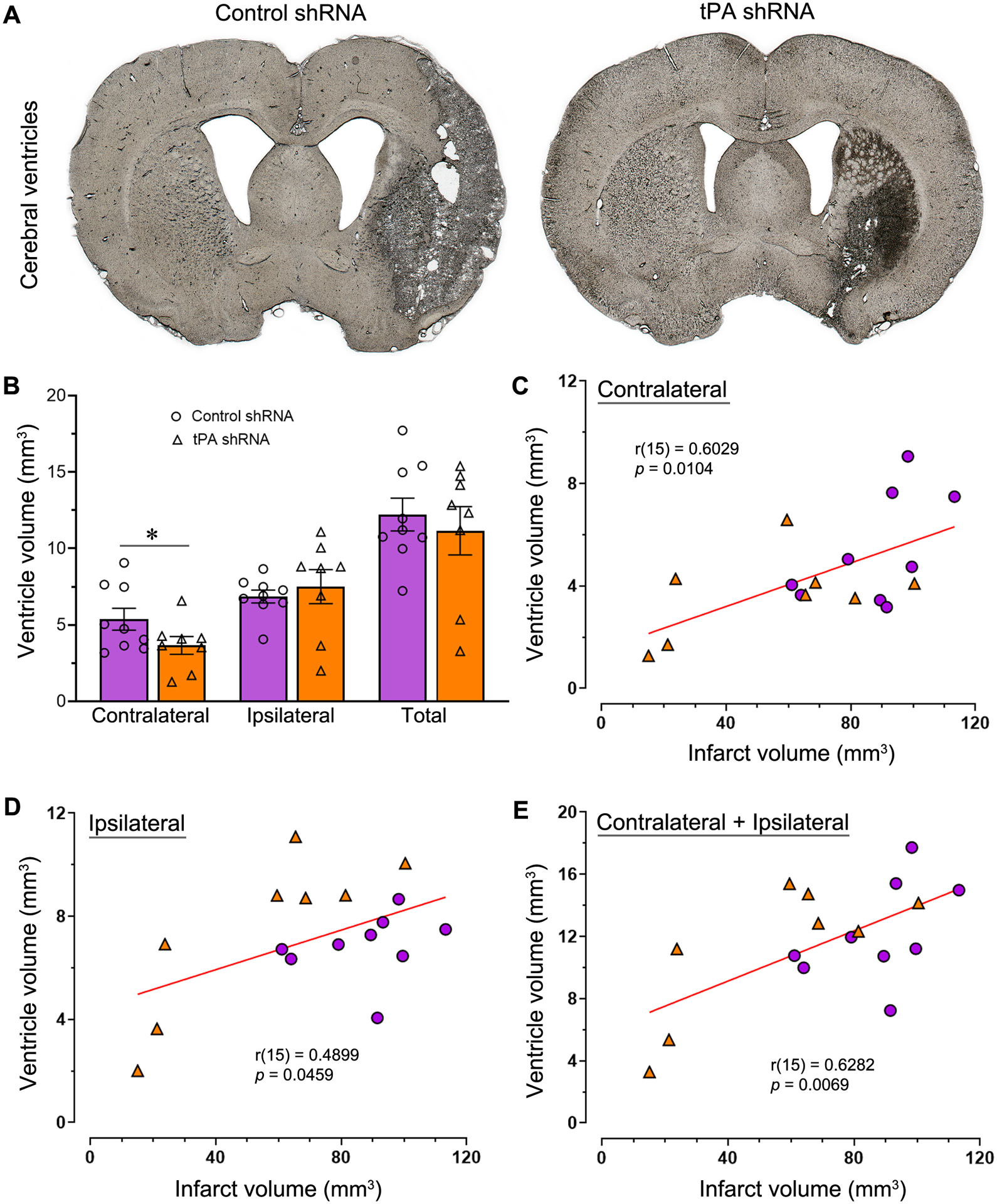
tPA shRNA treatment promptly after reperfusion reduces cerebral ventricle volume enlargement. (A) Representative coronal brain sections from the control shRNA and tPA shRNA groups depicting lateral ventricles. (B) The column scatter plot shows the quantified whole brain ventricle volume as well as the contralateral and ipsilateral ventricle volumes. Columns indicate the mean and error bars indicate the SEM. *p < 0.05 (tPA shRNA group vs control shRNA group). (C, D, E) Scatter plots show the correlation between infarct volume and contralateral brain ventricle volume (C), ipsilateral ventricle volume (D), and whole brain ventricle volume (E). Small circles represent data of the control shRNA group; triangles represent data of the tPA shRNA group.
The relationship between infarct volume and cerebral ventricle volume was examined for all stroke-induced rats, regardless of treatment. As predicted, there was a positive correlation between the volume of the infarct and the volume of the cerebral ventricle (Fig. 6C, D, E). Significant correlations were observed between infarct volume and ventricle volume for the ipsilateral brain (Pearson r(15) = 0.4899; p = 0.0459), the contralateral brain (Pearson r(15) = 0.6029; p = 0.0104), and the whole brain (ipsilateral + contralateral) (Pearson r(15) = 0.6282; p = 0.0069).
4. Discussion
With a few exceptions, the absence or inhibition of endogenous tPA has been associated with more favorable outcomes following ischemic stroke in numerous preclinical studies (Yepes et al., 2000, Nagai et al., 1999, Pu et al., 2019, Cinelli et al., 2001, Lebeurrier et al., 2005, Wang, Y. F. et al., 1998). The adverse effects of tPA on ischemic stroke were confirmed by the poor post-stroke outcomes observed in both wild type and tPA knockout mice after exogenous tPA administration (Wang, Y. F. et al., 1998). Despite the fact that elevated endogenous tPA levels in the brain following ischemic stroke induce detrimental effects, patients with AIS are treated with exogenous recombinant tPA; this is because the recanalization-mediated beneficial effects associated with exogenous tPA treatment outweigh its harmful effects. Although recombinant tPA therapy is necessary to recanalize the occluded arteries in eligible AIS patients, it’s not premature to rule out the possibility that inhibition of tPA following recanalization could be an effective treatment strategy for improving outcomes following ischemic stroke. We believe tPA reduction following confirmed recanalization to be a potentially efficacious approach for improving outcomes in the aftermath of an ischemic stroke. Our recent study demonstrated the effectiveness of administering the non-viral nanoparticle formulation of tPA shRNA plasmids to inhibit tPA expression in the ischemic brains of rats (Challa et al., 2022b). In addition, we observed a reduction in MMP-12 expression, neuroinflammation, and blood-brain barrier (BBB) disruption when tPA was suppressed shortly after recanalization by administering a 1 mg/kg dose of tPA shRNA plasmid nanoparticles (Challa et al., 2022b). In lieu of a prior dose-response study, the systemic dose of shRNA nanoparticles is based on our empirical findings and the manufacturer’s guidelines for nucleic acid quantity, concentration, and injection volume. According to the manufacturer of the transfection reagent in vivo-jetPEI, the recommended maximum amount of plasmid to be given as nanoparticles for intravenous injections in rats is 300 μg. This amount is roughly equivalent to a dose of 1 mg/kg, based on the body weight of the rats used in our experiments during MCAO surgery. In our laboratory, we have established the 1 mg/kg-dose as the standard test dose and have successfully proven the effectiveness of various additional shRNA plasmids at this dose, which have demonstrated therapeutic potential (Chelluboina, Klopfenstein et al., 2015, Chelluboina, Warhekar et al., 2015, Nalamolu, K. R. et al., 2018, Challa et al., 2022a). For this reason, we tested the tPA shRNA plasmids in the previous and current study at a starting dose of 1 mg/kg (Challa et al., 2022b).
In prior studies, we have used both untreated control groups and control groups treated with shRNA. However, we did not observe any statistically significant differences between the two control groups in terms of the expression levels of various molecules, such as claudin-5, MMP-12, MMP-9, myelin basic protein, TNFα, TNFR1, and TNFR2 (Chelluboina, Warhekar et al., 2015, Chelluboina, Klopfenstein et al., 2015, Challa et al., 2022b). Therefore, in this study, we have employed either the untreated or control shRNA groups as the control comparison group for the tPA shRNA group. When we first initiated these investigations four years ago, untreated animals served as the control group in the short-term TTC-staining experiments. In more recent years, we have employed animals treated with control shRNA as the control group, as in our long-term cresyl violet staining experiments. Although both control groups appear to be interchangeable, we believe that the shRNA group is superior and will therefore continue to employ it. Furthermore, by eliminating one of the control groups we have reduced the number of animals used, in accordance with the Guide for the Care and Use of Laboratory Animals (Publication no. 86–23 revised, National Institutes of Health, U.S. Department of Health and Human Services) and our IACUC-recommended three Rs principle (Reduction, Replacement, and Refinement). Our results demonstrate that the prompt administration of tPA shRNA after recanalization results in a reduction in the volume of infarcts, white matter damage, and enlargement of the volume of the cerebral ventricle. Additionally, we found that it facilitates the restoration of sensory and motor function. In contrast to prior investigations that employed tPA gene knockout animals to completely eradicate the target gene, the findings of this study hold translational potential and clinical relevance. This is due to the fact that shRNA treatment for tPA gene knockdown in wild-type or standard animals merely degrades tPA mRNA and inhibits protein translation. Previous research that employed non-specific inhibitors to impede tPA is inconclusive. To our knowledge, this is the first study to conclusively demonstrate the beneficial effects of targeting tPA after recanalization in standard or typical wild-type animals.
A valuable quantitative outcome measure for evaluating the efficacy of treatments in the context of acute ischemic stroke is infarct volume. Previous research had consistently observed a reduction in infarct volume during the acute phase following an ischemic stroke in animals lacking tPA (Wang, Y. F. et al., 1998, Nagai et al., 1999). Moreover, infarcts were larger in tPA knockout animals when exogenous tPA was administered (Wang, Y. F. et al., 1998). In addition, the overexpression or administration of neuroserpin (an antagonist of tPA) reduced the infarct volume and PAI-1 (a major endogenous inhibitor of tPA) knockout animals produced larger lesions (Yepes et al., 2000, Nagai et al., 1999, Cinelli et al., 2001). The collective findings of these studies provide support for the hypothesis that increased endogenous tPA or administered exogenous recombinant tPA contribute to the enlargement of post-ischemic infarct volume. In agreement with the findings of these studies, we also observed a substantial decrease in infarct volume during both the acute phase (day 1 post-ischemia; TTC staining; evaluated in males and females) and the chronic phase (day 14 post-ischemia; cresyl violet staining; evaluated in males only) following cerebral ischemia.
Sex disparities in experimental stroke outcomes are extensively documented (El-Hakim et al., 2021). Recently, we observed similar pathological stroke lesions in both sexes of rats following two hours of focal cerebral ischemia and reperfusion. However, the clinical manifestations of stroke, indicated by neurological scores and sensorimotor impairments, were more severe in males than in females (Nalamolu, Challa et al., 2021, Nalamolu, Chelluboina et al., 2021). In accordance with our prior investigation, we observed comparable infarct volumes in male and female rats that underwent focal cerebral ischemia and reperfusion for a duration of 1.5 hours. Notably, in this investigation, the infarct volume in both sexes was significantly reduced by tPA shRNA administration. This is the first study to demonstrate the impact of tPA knockdown following reperfusion on infarct volume during the chronic phase and in both sexes during the acute phase following ischemic stroke.
The reduction of infarct volume associated with reduced tPA levels could be mechanistically explained by diminished excitotoxicity, BBB disruption, and neuroinflammation (Challa et al., 2022b, Cinelli et al., 2001, Lebeurrier et al., 2005, Abe et al., 2003). At the molecular level, tPA increases the expression and activity of MMPs, particularly MMP-9, which is well known for its role in BBB disruption (Asahi et al., 2001, Sumii and Lo., 2002, Montaner et al., 2003, Horstmann et al., 2003, Wang, X. et al., 2003, Lo, Broderick and Moskowitz., 2004, Kelly, Shuaib and Todd., 2006, Ning et al., 2006, Sandoval and Witt., 2008, Hu et al., 2009, Aoki et al., 2002, Hu et al., 2011). Consistent with these findings, we now demonstrate that a reduction in tPA expression by tPA shRNA treatment decreases the expression of several MMPs, including MMP-1, MMP-3, and MMP-9, whose expression was significantly upregulated on post-ischemic day 1 in stroke-induced rats (Chelluboina, Warhekar et al., 2015, Chelluboina, Klopfenstein et al., 2015). tPA converts inactive plasminogen to plasmin, which in turn activates numerous pro-MMPs to their active forms (Mazzieri et al., 1997, Carmeliet et al., 1997). Furthermore, plasmin induces MMP-12 release by activating the protease-activated receptor-1 and also regulates the activity of MMP-12, which is implicated in post-ischemic BBB disruption, inflammation, and apoptosis (Chelluboina, Klopfenstein et al., 2015, Chelluboina, Warhekar et al., 2015, Molino et al., 1995, Raza et al., 2000, Arruri et al., 2022, Dery et al., 1998). We recently observed that tPA reduction subsequent to recanalization decreased the expression of MMP-12 in the ischemic brain (Challa et al., 2022b). Overall, there is substantial evidence to suggest that infarct volume, BBB disruption, and inflammation can be mitigated by reducing tPA after recanalization.
A longer-term evaluation of multiple outcome parameters is suggested by STAIR for determining the efficacy of new treatments. This study examined the effects of tPA shRNA treatment on various histological parameters and functional outcomes until fourteen days after transient focal cerebral ischemia and reperfusion. Treatment with tPA shRNA improved various histological outcomes (reduced infarct volume, white matter damage, and cerebral lateral ventricle volume enlargement to the contralateral hemisphere) and facilitated somatosensory and motor functions. Demyelination, one of the primary components of white matter damage, is characterized by the loss of the myelin sheath and the death of oligodendrocytes. Axonal demyelination impairs the propagation of impulses and leads to the development of permanent sensorimotor impairments. The involvement of MMP-9 in myelin basic protein (MBP) degradation subsequent to transient focal cerebral ischemia was previously demonstrated in MMP-9 knockout animals (Asahi et al., 2001). Furthermore, elevated MMP-12 has been implicated in brain cell apoptosis, MBP degradation, and myelin structural abnormalities including rarefaction and fragmentation, according to previous research (Chelluboina, Warhekar et al., 2015, Chelluboina, Klopfenstein et al., 2015, Arruri et al., 2022). Reduced expression of MMP-9 and MMP-12 mediated by tPA shRNA treatment might have played a role in the diminished white matter injury and improved sensorimotor function observed in this study. In contrast, severe sensorimotor impairments as well as increased axonal and myelin destruction were reported in tPA gene deficient mice following ischemic stroke (Pu et al., 2019). As stated in the introduction, the outcomes of experiments in tPA knockout animals including the aforementioned study by Pu et al., yielded mixed results and have been inconclusive. The outcomes in tPA knockout animals might be related to the complete absence of tPA in the brain subserving other functions.
A significant correlation between the infarct volume and the volumes of the cerebral lateral ventricles (ipsilateral, contralateral, and total) was observed when the data from both experimental groups (tPA shRNA group and control shRNA group) were analyzed together. As expected, ventricle volume increased in parallel with infarct volume. Treatment with tPA shRNA decreased the volume expansion of the ventricle from the ipsilateral to the contralateral brain. The improved longer-term functional and histological outcomes in this study could be attributed to tPA shRNA treatment-mediated attenuation of brain damage (reduced expression of MMPs 1, 3, 9, and 12, BBB disruption, and neuroinflammation) during the acute phase as demonstrated in this study and our previous study (Challa et al., 2022b).
In summary, endogenous tPA knockdown shortly after recanalization improves long-term functional recovery and attenuates infarct volume, white matter injury, and cerebral ventricle enlargement. Inhibiting tPA following recanalization appears to be a potentially effective treatment that deserves further investigation in accordance with STAIR criteria. The tPA shRNA used in this study decreases the expression of endogenous tPA in the ischemic brain, but it cannot neutralize or serve as an antidote for the exogenously administered recombinant tPA. A potentially effective treatment for AIS could be an antidote for tPA that works on both endogenous tPA and exogenous recombinant tPA. Our future studies will focus on developing and testing the tPA antidote for AIS treatment.
Acknowledgments
We thank Christina Constantinidou for her assistance with the formatting of the manuscript.
Funding
This work was supported by the financial assistance from the OSF HealthCare Illinois Neurological Institute, William E. McElroy Charitable Foundation, and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (Award Number # R01NS102573). The funders had no role in study design, data collection, analysis, data interpretation, the publication decision, or manuscript preparation. The content in this study is the sole responsibility of the authors and does not necessarily reflect the official position of the funders.
Footnotes
Declaration of competing interests
The authors declare no competing interests.
Data availability
All relevant data supporting the key findings of this study are available within the article. Any relevant raw data will be shared upon a reasonable request to the corresponding author.
References
- Abe Y, Nakamura H, Yoshino O, Oya T, Kimura T, 2003. Decreased neural damage after spinal cord injury in tPA-deficient mice. J. Neurotrauma 20, 43–57. [DOI] [PubMed] [Google Scholar]
- Aoki T, Sumii T, Mori T, Wang X, Lo EH, 2002. Blood-brain barrier disruption and matrix metalloproteinase-9 expression during reperfusion injury: mechanical versus embolic focal ischemia in spontaneously hypertensive rats. Stroke 33, 2711–2717. [DOI] [PubMed] [Google Scholar]
- Arruri V, Chokkalla AK, Jeong S, Chelluboina B, Mehta SL, Veeravalli KK, Vemuganti R, 2022. MMP-12 knockdown prevents secondary brain damage after ischemic stroke in mice. Neurochem. Int 161, 105432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH, 2001. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J. Neurosci 21, 7724–7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Moons L, Lijnen R, Baes M, Lemaitre V, Tipping P, Drew A, Eeckhout Y, Shapiro S, Lupu F, Collen D, 1997. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat. Genet 17, 439–444. [DOI] [PubMed] [Google Scholar]
- Challa SR, Nalamolu KR, Fornal CA, Wang BC, Martin RC, Olson EA, Ujjainwala AL, Pinson DM, Klopfenstein JD, Veeravalli KK, 2022a. Therapeutic efficacy of matrix metalloproteinase-12 suppression on neurological recovery after ischemic stroke: Optimal treatment timing and duration. Front. Neurosci 16, 1012812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challa SR, Nalamolu KR, Fornal CA, Mohandass A, Mussman JP, Schaibley C, Kashyap A, Sama V, Wang BC, Klopfenstein JD, Pinson DM, Kunamneni A, Veeravalli KK, 2022b. The interplay between MMP-12 and t-PA in the brain after ischemic stroke. Neurochem. Int 161, 105436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelluboina B, Klopfenstein JD, Pinson DM, Wang DZ, Vemuganti R, Veeravalli KK, 2015. Matrix Metalloproteinase-12 Induces Blood-Brain Barrier Damage After Focal Cerebral Ischemia. Stroke 46, 3523–3531. [DOI] [PubMed] [Google Scholar]
- Chelluboina B, Warhekar A, Dillard M, Klopfenstein JD, Pinson DM, Wang DZ, Veeravalli KK, 2015. Post-transcriptional inactivation of matrix metalloproteinase-12 after focal cerebral ischemia attenuates brain damage. Sci. Rep 5, 9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinelli P, Madani R, Tsuzuki N, Vallet P, Arras M, Zhao CN, Osterwalder T, Rulicke T, Sonderegger P, 2001. Neuroserpin, a neuroprotective factor in focal ischemic stroke. Mol. Cell. Neurosci 18, 443–457. [DOI] [PubMed] [Google Scholar]
- Del Zoppo GJ, Saveeffrey L, Jauch EC, Adams HPJ, American Heart Association Stroke Council, 2009. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke 40, 2945–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dery O, Corvera CU, Steinhoff M, Bunnett NW, 1998. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am. J. Physiol 274, 1429. [DOI] [PubMed] [Google Scholar]
- El-Hakim Y, Mani KK, Eldouh A, Pandey S, Grimaldo MT, Dabney A, Pilla R, Sohrabji F, 2021. Sex differences in stroke outcome correspond to rapid and severe changes in gut permeability in adult Sprague-Dawley rats. Biol. Sex. Differ 12, 14–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D, ECASS Investigators, 2008. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med 359, 1317–1329. [DOI] [PubMed] [Google Scholar]
- Horstmann S, Kalb P, Koziol J, Gardner H, Wagner S, 2003. Profiles of matrix metalloproteinases, their inhibitors, and laminin in stroke patients: influence of different therapies. Stroke 34, 2165–2170. [DOI] [PubMed] [Google Scholar]
- Hu Q, Chen C, Khatibi NH, Li L, Yang L, Wang K, Han J, Duan W, Zhang JH, Zhou C, 2011. Lentivirus-mediated transfer of MMP-9 shRNA provides neuroprotection following focal ischemic brain injury in rats. Brain Res. 1367, 347–359. [DOI] [PubMed] [Google Scholar]
- Hu Q, Chen C, Yan J, Yang X, Shi X, Zhao J, Lei J, Yang L, Wang K, Chen L, Huang H, Han J, Zhang JH, Zhou C, 2009. Therapeutic application of gene silencing MMP-9 in a middle cerebral artery occlusion-induced focal ischemia rat model. Exp. Neurol 216, 35–46. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Shuaib A, Todd KG, 2006. Matrix metalloproteinase activation and blood-brain barrier breakdown following thrombolysis. Exp. Neurol 200, 38–49. [DOI] [PubMed] [Google Scholar]
- Lebeurrier N, Liot G, Lopez-Atalaya JP, Orset C, Fernandez-Monreal M, Sonderegger P, Ali C, Vivien D, 2005. The brain-specific tissue-type plasminogen activator inhibitor, neuroserpin, protects neurons against excitotoxicity both in vitro and in vivo. Molecular and Cellular Neuroscience 30, 552–558. [DOI] [PubMed] [Google Scholar]
- Lemarchand E, Maubert E, Haelewyn B, Ali C, Rubio M, Vivien D, 2016. Stressed neurons protect themselves by a tissue-type plasminogen activator-mediated EGFR-dependent mechanism. Cell Death Differ. 23, 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH, Broderick JP, Moskowitz MA, 2004. tPA and proteolysis in the neurovascular unit. Stroke 35, 354–356. [DOI] [PubMed] [Google Scholar]
- Matosevic B, Knoflach M, Werner P, Pechlaner R, Zangerle A, Ruecker M, Kirchmayr M, Willeit J, Kiechl S, 2013. Fibrinogen degradation coagulopathy and bleeding complications after stroke thrombolysis. Neurology 80, 1216–1224. [DOI] [PubMed] [Google Scholar]
- Mazzieri R, Masiero L, Zanetta L, Monea S, Onisto M, Garbisa S, Mignatti P, 1997. Control of type IV collagenase activity by components of the urokinase-plasmin system: a regulatory mechanism with cell-bound reactants. EMBO J. 16, 2319–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride DW, Klebe D, Tang J, Zhang JH, 2015. Correcting for Brain Swelling’s Effects on Infarct Volume Calculation After Middle Cerebral Artery Occlusion in Rats. Transl. Stroke Res 6, 323–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molino M, Blanchard N, Belmonte E, Tarver AP, Abrams C, Hoxie JA, Cerletti C, Brass LF, 1995. Proteolysis of the human platelet and endothelial cell thrombin receptor by neutrophil-derived cathepsin G. J. Biol. Chem 270, 11168–11175. [DOI] [PubMed] [Google Scholar]
- Montaner J, Molina CA, Monasterio J, Abilleira S, Arenillas JF, Ribo M, Quintana M, Alvarez-Sabin J, 2003. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation 107, 598–603. [DOI] [PubMed] [Google Scholar]
- Nagai N, De Mol M, Lijnen HR, Carmeliet P, Collen D, 1999. Role of plasminogen system components in focal cerebral ischemic infarction: a gene targeting and gene transfer study in mice. Circulation 99, 2440–2444. [DOI] [PubMed] [Google Scholar]
- Nalamolu KR, Smith NJ, Chelluboina B, Klopfenstein JD, Pinson DM, Wang DZ, Vemuganti R, Veeravalli KK, 2018. Prevention of the Severity of Post-ischemic Inflammation and Brain Damage by Simultaneous Knockdown of Toll-like Receptors 2 and 4. Neuroscience 373, 82–91. [DOI] [PubMed] [Google Scholar]
- Nalamolu KR, Chelluboina B, Fornal CA, Challa SR, Pinson DM, Wang DZ, Klopfenstein JD, Veeravalli KK, 2021. Stem cell treatment improves post stroke neurological outcomes: a comparative study in male and female rats. Stroke Vasc. Neurol 6, 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalamolu KR, Challa SR, Fornal CA, Grudzien NA, Jorgenson LC, Choudry MM, Smith NJ, Palmer CJ, Pinson DM, Klopfenstein JD, Veeravalli KK, 2021. Attenuation of the Induction of TLRs 2 and 4 Mitigates Inflammation and Promotes Neurological Recovery After Focal Cerebral Ischemia. Transl. Stroke Res 12, 923–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning M, Furie KL, Koroshetz WJ, Lee H, Barron M, Lederer M, Wang X, Zhu M, Sorensen AG, Lo EH, Kelly PJ, 2006. Association between tPA therapy and raised early matrix metalloproteinase-9 in acute stroke. Neurology 66, 1550–1555. [DOI] [PubMed] [Google Scholar]
- Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Wurbel H, 2020. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J. Cereb. Blood Flow Metab 40, 1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percie du Sert N, Alfieri A, Allan SM, Carswell HV, Deuchar GA, Farr TD, Flecknell P, Gallagher L, Gibson CL, Haley MJ, Macleod MR, McColl BW, McCabe C, Morancho A, Moon LD, O’Neill MJ, Pérez de Puig I, Planas A, Ragan CI, Rosell A, Roy LA, Ryder KO, Simats A, Sena ES, Sutherland BA, Tricklebank MD, Trueman RC, Whitfield L, Wong R, Macrae IM, 2017. The IMPROVE Guidelines (Ischaemia Models: Procedural Refinements Of in Vivo Experiments). J. Cereb. Blood Flow Metab 37, 3488–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL, American Heart Association Stroke Council, 2018. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 49, e46–e110. [DOI] [PubMed] [Google Scholar]
- Pu H, Shi Y, Zhang L, Lu Z, Ye Q, Leak RK, Xu F, Ma S, Mu H, Wei Z, Xu N, Xia Y, Hu X, Hitchens TK, Bennett MVL, Chen J, 2019. Protease-independent action of tissue plasminogen activator in brain plasticity and neurological recovery after ischemic stroke. Proc. Natl. Acad. Sci. U. S. A 116, 9115–9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza SL, Nehring LC, Shapiro SD, Cornelius LA, 2000. Proteinase-activated receptor-1 regulation of macrophage elastase (MMP-12) secretion by serine proteinases. J. Biol. Chem 275, 41243–41250. [DOI] [PubMed] [Google Scholar]
- Sandoval KE, Witt KA, 2008. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol. Dis 32, 200–219. [DOI] [PubMed] [Google Scholar]
- Siao C, Fernandez SR, Tsirka SE, 2003. Cell type-specific roles for tissue plasminogen activator released by neurons or microglia after excitotoxic injury. J. Neurosci 23, 3234–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumii T, Lo EH, 2002. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke 33, 831–836. [DOI] [PubMed] [Google Scholar]
- Verkerk BS, Lesch C, Cham S, Berger K, 2023. Cryoprecipitate for Alteplase-Related Hemorrhagic Conversion of Acute Ischemic Stroke. J. Pharm. Pract 36, 1253–1259. [DOI] [PubMed] [Google Scholar]
- Wang X, Lee S, Arai K, Lee S, Tsuji K, Rebeck GW, Lo EH, 2003. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat. Med 9, 1313–1317. [DOI] [PubMed] [Google Scholar]
- Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA, 1998. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat. Med 4, 228–231. [DOI] [PubMed] [Google Scholar]
- Yaghi S, Willey JZ, Cucchiara B, Goldstein JN, Gonzales NR, Khatri P, Kim LJ, Mayer SA, Sheth KN, Schwamm LH, American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research, 2017. Treatment and Outcome of Hemorrhagic Transformation After Intravenous Alteplase in Acute Ischemic Stroke: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 48, e343–e361. [DOI] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Wong MK, Coleman TA, Smith E, Cohan SL, Lawrence DA, 2000. Neuroserpin reduces cerebral infarct volume and protects neurons from ischemia-induced apoptosis. Blood 96, 569–576. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data supporting the key findings of this study are available within the article. Any relevant raw data will be shared upon a reasonable request to the corresponding author.


