Abstract
Objectives
Patients with SLE have an increased risk of comorbidities and impaired survival. We aimed to assess whether various thresholds of oral CS (OCS) can predict development of infections, comorbidities, malignancies and survival in SLE using data from national health registries in Sweden.
Methods
All incident SLE cases, age >18 years, in Sweden (n = 5309) between 2005 and 2020 and matched population controls (n = 26 545) were included and followed until 2020, a total of 257 942 patient years. Data from national registers were retrieved including information from the National Prescribed Drug Register. Risk factors were analysed using time-dependent Cox regression models.
Results
Compared with no OCS, >0 to <5.0 mg/day, 5.0–7.5 mg/day as well as >7.5 mg/day OCS predicted development of infections (pneumonia, influenza, herpes zoster and urinary tract infection), osteoporosis, osteonecrosis, gastroduodenal ulcers, cataracts, hypertension and mortality (all P < 0.05). OCS >0 to <5.0 mg/day was associated with lower hazard ratios for these comorbidities than higher doses of OCS. Fifteen years after diagnosis, 48% of patients were taking OCS at a median dose of 5.7 mg/day. A small reduction of OCS treatment 5 years after diagnosis in patients diagnosed with SLE 2006–10 compared with 2011–15 was observed, 49% vs 46% respectively (P = 0.039).
Conclusion
Results highlight the potential harm associated with even low OCS dose treatment in SLE and the need to judiciously use OCS at the lowest possible dose to maximize efficacy and minimize harm.
Keywords: SLE, corticosteroids, comorbidities, organ damage, infections, malignancies, survival, mortality, prednisolone, immunosuppressants
Graphical Abstract
Rheumatology key messages.
CS <5.0 mg/day associate with development of infections, comorbidities and impaired survival in SLE.
National SLE inception cohort data show 48% take corticosteroids regularly 15 years after diagnosis.
Compared with 2006–10, OCS use 5 years after diagnosis decreased by 3% in 2011–15.
Introduction
SLE is a heterogeneous, chronic, systemic autoimmune disease, primarily affecting women [1]. It is characterized by production of autoantibodies, immune complex formation and an activated type I IFN system [2]. The disease carries a high clinical burden due to debilitating disease manifestations, irreversible organ damage, and associated comorbidities and complications [3]. As a result, mortality rates in SLE compared with the general population remain elevated and have ranged from 1.5 to 3.0 in studies conducted across the Nordic countries [4, 5]. Excessive organ damage can be the result of both systemic inflammation due to active disease as well as treatment with, for example, CS. Organ systems often affected by damage include the neuropsychiatric, ocular, cardiovascular and musculoskeletal domains [3, 6]. Further, patients with SLE have an increased risk of malignancies including lymphoma, lung cancer and virus-associated cancers [7, 8].
Glucocorticoids remain one of the cornerstones of SLE treatment [9] despite the well-known side effects associated with long-time oral CS (OCS) use [10]. OCS treatment in SLE has been demonstrated to cause osteoporosis, cardiovascular disease, cataracts, diabetes, infections and overall development of irreversible organ damage [10]. The discussion on whether there is an acceptable safe low dose of OCS to be used in SLE has been ongoing for years. In one study, patients treated with an OCS dose of 6 mg or more had increased risk of organ damage accrual [11]. The EULAR recommend the prednisolone dose in SLE not to exceed 7.5 mg/day and to be discontinued when possible [12]. However, it is unknown how this recommendation translates to real world clinical practice. The aim of this study was to describe the use of OCS following diagnosis of SLE, and to investigate the association between OCS dose and comorbidities, and mortality using data from national health registries in Sweden.
Methods
Study design and data sources
This observational cohort study utilized data from the national Swedish health registries: (i) The National Patient Register (NPR) covering all hospital admissions and out-patient specialist visits; (ii) the Prescribed Drug Register (PDR), covering all collected outpatient drug prescriptions from both primary and secondary care using the Anatomical Therapeutic Chemical codes; and (iii) the Cause of Death Register, covering date and cause of death. Individual patient data were linked by the Swedish National Board of Health and Welfare and the data were pseudo-anonymized before analyses.
This study is purely registry-based, and all micro-data are anonymized, so no informed consent was needed. The study was performed in accordance with the Declaration of Helsinki, the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practices, Good Pharmacoepidemiology Practice and the applicable legislation on non-interventional studies and/or observational studies. All data accessed complied with relevant data protection and privacy regulations. The study protocol was approved by the Swedish Ethical Review Authority (registration number 2020-07006).
Study population
Patients aged 18 years or older were included in the study (n = 5309) if they had at least two secondary care visits (i.e. outpatient visits or inpatient stays) with a recorded International Classification of Diseases, 10th Revision (ICD-10) diagnosis code associated with SLE (M32.1, M32.8, M32.9) from 1 July 2005 to 31 December 2019, with the index date being the date of the first observed visit [13]. The baseline period included the index date and the preceding 4-year period to assess selected comorbidities and to confirm that patients had not had prior secondary care visits for SLE.
Patients were followed from the year after index until death, loss to follow-up or end of study, 31 December 2020 (Fig. 1). Individuals without a diagnosis of SLE matched by sex, age and county of residence, were obtained from the NPR at 1:5 ratio (n = 26 488) and used for comparative analyses. Data on SLE medications collected from pharmacies included date of dispensing, defined daily dose based on dosage dispensed, pack size and tablet strengths associated with the drug prescription. Medications administered in the hospital are not recorded in the PDR and were thus not captured.
Figure 1.
Study design
SLE medication definitions
OCS: betamethasone, cortisone, dexamethasone, hydrocortisone, methylprednisolone, prednisolone or prednisone. OCS does not include CS given intravenously at hospitals.
Antimalarial: chloroquine and HCQ.
Immunosuppressant: AZA, mycophenolic acid, CYC, rituximab, sirolimus, belimumab, ciclosporin, tacrolimus or MTX.
Outcomes
Relevant selected SLE and OCS-related comorbidities were defined a priori by ICD-10 diagnoses (Supplementary Table S1, available at Rheumatology online). A history of any of these comorbidities was identified as the presence of an inpatient or outpatient visit for selected comorbidities during baseline. Patients with a selected comorbidity diagnosis prior to index were excluded from the analysis. Additionally, malignancy (Supplementary Table S1, available at Rheumatology online) and mortality were also captured. Non-melanoma skin cancers (ICD-10 code C44) were not included in the definition of malignancy and therefore excluded from the analysis (Supplementary Table S1, available at Rheumatology online).
Statistical analyses
Numerical variables were described using mean (s.d.). Categorical variables were described using frequency and percentages. The frequency and proportion of individuals receiving OCS during the first year after index to the end of follow-up were analysed.
To examine the effect of selected risk factors on the time to different outcomes, a time-dependent Cox regression analysis was used. Using a time-dependent model allows the values for the risk factors to change over time up until the event. Average OCS dose, use of immunosuppressants and use of antimalarials in the year (12-month period) prior to the year in which the outcome occurred, as well as age and gender, were included as covariates both separately in univariate models and together in a multivariable model. Models were also stratified for county of residence to account for potential differences in health care access between regions, as well as disease severity during the first year after index (Supplementary Data S1 and Table S2, available at Rheumatology online).
Patient follow-up was divided into rolling 12-month periods, starting from the index date (i.e. date of diagnosis) (Fig. 1). For each patient, treatments were analysed from the first year of diagnosis and outcomes were analysed from the patient’s second year after diagnosis (Fig. 1). OCS dose was categorized into steroid-free (0 mg), low dose (>0 to <5.0 mg/day), medium dose (5–7.5 mg/day) and high dose (>7.5 mg/day).
Incomplete years, that is years after index where the patient was not followed up for the complete duration of the year, were excluded. In the analysis for mortality, patients were censored at the time of their last complete year. However, patients who died were followed up during their last year regardless of whether it was complete. P-values <0.05 were considered significant.
Results
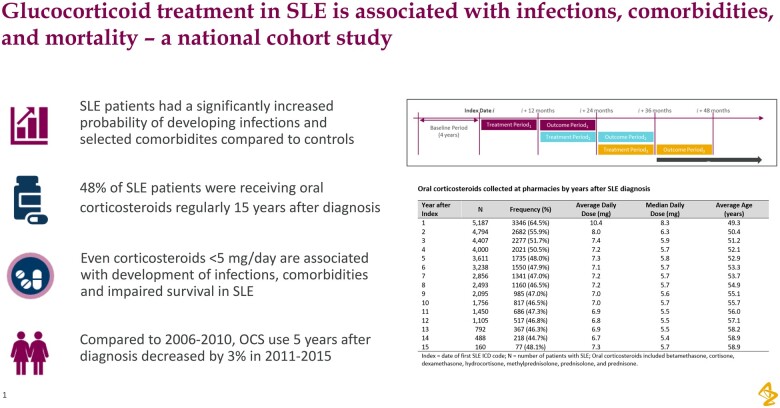
Overall, 5309 incident SLE patients were included in the study and the median follow-up was 7.6 years (Table 1). The breakdown of the SLE population by residence in Sweden is provided in Supplementary Table S2, available at Rheumatology online. In the year following SLE diagnosis, 64.5% of patients were on OCS and >40% were on a dose of at least 10 mg/day. Additionally, 58.9% of patients were receiving antimalarials and 39.5% immunosuppressants (Table 1). During the following 15-year period after SLE diagnosis, the percentage of patients receiving OCS as well as the average daily OCS dose tended to decrease (Table 2). In the first year after diagnosis, OCS at a mean daily dose of 10.4 mg/day and a median dose of 8.3 mg/day were taken (Table 2). Fifteen years later, 48% of patients were taking a mean and median OCS daily dose of 7.3 and 5.7 mg/day, respectively (Table 2). When looking at treatment trends 5 years after diagnosis, 48.7% of patients who were diagnosed with SLE in 2006 were using OCS in 2011, and 43.5% of patients diagnosed with SLE in 2015 were using OCS in 2020 (Supplementary Table S3, available at Rheumatology online). Furthermore, when comparing OCS treatment 5 years after diagnosis in patients diagnosed with SLE in 2006–10 and 2011–15, a small but significant reduction was observed, 49% vs 46% respectively (P = 0.039) (Supplementary Table S3, available at Rheumatology online).
Table 1.
Baseline characteristics and medication use in the first year following SLE diagnosis
| Variable | Statistics | Incident SLE cohort (N = 5309) |
|---|---|---|
| Baseline demographics | ||
| Female | n (%) | 4491 (84.6) |
| Index age | Mean (s.d.) | 49.9 (18.2) |
| OCS use in year after index* | n (%) | 3347 (64.5) |
| OCS, mean dose >7.5 mg/day | n (%) | 1857 (55.5) |
| OCS, mean dose ≥10 mg/day | n (%) | 1389 (41.5) |
| Average OCS dose | Mean (s.d.) | 10.4 (7.3) |
| Median OCS dose | Median (min–max) | 8.3 (0.6–72.5) |
| Antimalarial use in year after index | n (%) | 3125 (58.9) |
| Immunosuppressant use in year after index | n (%) | 2096 (39.5) |
Index: date of first SLE ICD code. OCS include betamethasone, cortisone, dexamethasone, hydrocortisone, methylprednisolone, prednisolone and prednisone; antimalarial medications include chloroquine and HCQ; immunosuppressant medications include AZA, mycophenolic acid, MTX, CYC, rituximab, sirolimus, belimumab, ciclosporin and tacrolimus. OCS: oral CS.
*Subjects with incomplete follow-up during the exposure year were excluded (total n=5187)
Table 2.
Oral CS collected at pharmacies by years after SLE diagnosis
| Year after index | N | Frequency (%) | Average daily dose (mg) | Median daily dose (mg) | Average age (years) |
|---|---|---|---|---|---|
| 1 | 5187 | 3346 (64.5) | 10.4 | 8.3 | 49.3 |
| 2 | 4794 | 2682 (55.9) | 8.0 | 6.3 | 50.4 |
| 3 | 4407 | 2277 (51.7) | 7.4 | 5.9 | 51.2 |
| 4 | 4000 | 2021 (50.5) | 7.2 | 5.7 | 52.1 |
| 5 | 3611 | 1735 (48.0) | 7.3 | 5.8 | 52.9 |
| 6 | 3238 | 1550 (47.9) | 7.1 | 5.7 | 53.3 |
| 7 | 2856 | 1341 (47.0) | 7.2 | 5.7 | 53.7 |
| 8 | 2493 | 1160 (46.5) | 7.2 | 5.7 | 54.9 |
| 9 | 2095 | 985 (47.0) | 7.0 | 5.6 | 55.1 |
| 10 | 1756 | 817 (46.5) | 7.0 | 5.7 | 55.7 |
| 11 | 1450 | 686 (47.3) | 6.9 | 5.5 | 56.0 |
| 12 | 1105 | 517 (46.8) | 6.8 | 5.5 | 57.1 |
| 13 | 792 | 367 (46.3) | 6.9 | 5.5 | 58.2 |
| 14 | 488 | 218 (44.7) | 6.7 | 5.4 | 58.9 |
| 15 | 160 | 77 (48.1) | 7.3 | 5.7 | 58.9 |
Oral corticosteroids included betamethasone, cortisone, dexamethasone, hydrocortisone, methylprednisolone, prednisolone and prednisone. Index: date of first SLE International Classification of Diseases code; N: number of patients with SLE.
Comorbidities
SLE patients had a significantly increased probability of developing infections compared with controls (Fig. 2A). Among infections, the highest hazard ratios (HRs) were observed for influenza (HR = 7.57, 95% CI 4.34–13.23) and herpes zoster (HR = 7.17, 95% CI 5.74–8.96), but pneumonia and urinary tract infections were also prevalent. The largest difference in cumulative incidence was observed for the diagnosis of pneumonia, affecting 22% of patients compared with 7% of controls (Fig. 2A).
Figure 2.

Cumulative incidence of infections and other comorbidities in SLE patients and controls. Hazard ratios (HRs) were calculated using a Cox regression model. Number at risk at time 0 (number of events): (A) herpes zoster: 5124 (301), influenza: 5183 (56), pneumonia: 4714 (1145) and urinary tract infection: 4871 (1155). (B) Cataracts: 4682 (1750), diabetes: 4980 (460), gastroduodenal ulcers: 4949 (695), hypertension: 4139 (1945), osteonecrosis: 5175 (127), osteoporosis: 5021 (758) and cancers 5078 (424)
We found high, medium and low OCS dose to be associated with an increased hazard of developing infections, with low dose demonstrating lower HRs (Table 3). The highest hazard for high dose of OCS was observed for influenza (HR = 5.73, 95% CI 2.43–13.50). Low OCS dose demonstrated a significant increased risk for all infections studied, all HRs >1.5, with the highest hazard for influenza (HR = 3.20, 95% CI 1.46–6.98). The OCS risks were similar in univariable analyses (Supplementary Table S4, available at Rheumatology online). Treatment with immunosuppressants was associated with herpes zoster and urinary tract infection, while treatment with antimalarials was negatively associated with pneumonia and urinary tract infections (Table 3).
Table 3.
Risk factors associated with development of infections and comorbidities in SLE
| Outcome | Term | Multivariable |
||
|---|---|---|---|---|
| HR | 95% CI | P-value | ||
| Cataracts | Steroid dose: low (ref: none) T-1 | 1.26 | 1.11–1.42 | <0.001 |
| Steroid dose: medium (ref: none) T-1 | 1.57 | 1.35–1.81 | <0.001 | |
| Steroid dose: high (ref: none) T-1 | 2.68 | 2.33–3.10 | <0.001 | |
| Sex: female (ref: male) | 1.22 | 1.07–1.40 | 0.004 | |
| Age | 1.08 | 1.08–1.08 | <0.001 | |
| Immunosuppressants T-1 | 1.18 | 1.05–1.32 | 0.005 | |
| Antimalarial T-1 | 1.22 | 1.11–1.35 | <0.001 | |
| Diabetes | Steroid dose: low (ref: none) T-1 | 1.25 | 0.98–1.60 | 0.067 |
| Steroid dose: medium (ref: none) T-1 | 1.77 | 1.34–2.34 | <0.001 | |
| Steroid dose: high (ref: none) T-1 | 2.67 | 2.03–3.51 | <0.001 | |
| Sex: female (ref: male) | 0.79 | 0.62–1.01 | 0.065 | |
| Age | 1.03 | 1.03–1.04 | <0.001 | |
| Immunosuppressants T-1 | 1.10 | 0.89–1.37 | 0.371 | |
| Antimalarial T-1 | 0.66 | 0.54–0.82 | <0.001 | |
| Gastroduodenal ulcers | Steroid dose: low (ref: none) T-1 | 1.74 | 1.44–2.11 | <0.001 |
| Steroid dose: medium (ref: none) T-1 | 1.90 | 1.50–2.39 | <0.001 | |
| Steroid dose: high (ref: none) T-1 | 2.54 | 2.01–3.22 | <0.001 | |
| Sex: female (ref: male) | 1.00 | 0.81–1.24 | 0.993 | |
| Age | 1.02 | 1.02–1.03 | <0.001 | |
| Immunosuppressants T-1 | 1.02 | 0.86–1.21 | 0.818 | |
| Antimalarial T-1 | 0.89 | 0.76–1.05 | 0.169 | |
| Herpes zoster | Steroid dose: low (ref: none) T-1 | 1.60 | 1.17–2.19 | 0.003 |
| Steroid dose: medium (ref: none) T-1 | 2.02 | 1.41–2.89 | <0.001 | |
| Steroid dose: high (ref: none) T-1 | 3.14 | 2.23–4.42 | <0.001 | |
| Sex: female (ref: male) | 0.89 | 0.65–1.22 | 0.475 | |
| Age | 1.01 | 1.00–1.02 | 0.057 | |
| Immunosuppressants T-1 | 1.61 | 1.25–2.07 | <0.001 | |
| Antimalarial T-1 | 1.07 | 0.85–1.36 | 0.558 | |
| Hypertension | Steroid dose: low (ref: none) T-1 | 1.15 | 1.03–1.29 | 0.011 |
| Steroid dose: medium (ref: none) T-1 | 1.23 | 1.07–1.42 | 0.004 | |
| Steroid dose: high (ref: none) T-1 | 1.60 | 1.38–1.85 | <0.001 | |
| Sex: female (ref: male) | 0.85 | 0.75–0.97 | 0.014 | |
| Age | 1.05 | 1.05–1.05 | <0.001 | |
| Immunosuppressants T-1 | 1.19 | 1.07–1.32 | 0.002 | |
| Antimalarial T-1 | 0.93 | 0.84–1.02 | 0.125 | |
| Influenza | Steroid dose: low (ref: none) T-1 | 3.20 | 1.46–6.98 | 0.004 |
| Steroid dose: medium (ref: none) T-1 | 4.60 | 1.99–10.62 | <0.001 | |
| Steroid dose: high (ref: none) T-1 | 5.73 | 2.43–13.50 | <0.001 | |
| Sex: female (ref: male) | 0.76 | 0.38–1.52 | 0.434 | |
| Age | 1.02 | 1.00–1.04 | 0.068 | |
| Immunosuppressants T-1 | 0.98 | 0.55–1.75 | 0.957 | |
| Antimalarial T-1 | 0.82 | 0.46–1.45 | 0.494 | |
| Osteonecrosis | Steroid dose: low (ref: none) T-1 | 1.82 | 1.10–3.01 | 0.020 |
| Steroid dose: medium (ref: none) T-1 | 2.43 | 1.39–4.24 | 0.002 | |
| Steroid dose: high (ref: none) T-1 | 4.13 | 2.44–6.98 | <0.001 | |
| Sex: female (ref: male) | 1.14 | 0.68–1.91 | 0.625 | |
| Age | 1.00 | 0.99–1.02 | 0.443 | |
| Immunosuppressants T-1 | 1.47 | 1.00–2.15 | 0.052 | |
| Antimalarial T-1 | 0.95 | 0.66–1.38 | 0.799 | |
| Osteoporosis | Steroid dose: low (ref: none) T-1 | 2.15 | 1.76–2.62 | <0.001 |
| Steroid dose: medium (ref: none) T-1 | 3.08 | 2.47–3.83 | <0.001 | |
| Steroid dose: high (ref: none) T-1 | 5.11 | 4.11–6.36 | <0.001 | |
| Sex: female (ref: male) | 2.20 | 1.70–2.85 | <0.001 | |
| Age | 1.05 | 1.05–1.06 | <0.001 | |
| Immunosuppressants T-1 | 1.16 | 0.99–1.37 | 0.068 | |
| Antimalarial T-1 | 0.90 | 0.77–1.05 | 0.187 | |
| Pneumonia | Steroid dose: low (ref: none) T-1 | 1.79 | 1.54–2.08 | <0.001 |
| Steroid dose: medium (ref: none) T-1 | 2.12 | 1.77–2.54 | <0.001 | |
| Steroid dose: high (ref: none) T-1 | 3.14 | 2.62–3.76 | <0.001 | |
| Sex: female (ref: male) | 0.95 | 0.80–1.12 | 0.516 | |
| Age | 1.04 | 1.03–1.04 | <0.001 | |
| Immunosuppressants T-1 | 1.08 | 0.95–1.24 | 0.243 | |
| Antimalarial T-1 | 0.82 | 0.72–0.93 | 0.002 | |
| Urinary tract infection | Steroid dose: low (ref: none) T-1 | 1.58 | 1.36–1.84 | <0.001 |
| Steroid dose: medium (ref: none) T-1 | 2.22 | 1.87–2.64 | <0.001 | |
| Steroid dose: high (ref: none) T-1 | 2.73 | 2.28–3.27 | <0.001 | |
| Sex: female (ref: male) | 1.66 | 1.37–2.01 | <0.001 | |
| Age | 1.04 | 1.03–1.04 | <0.001 | |
| Immunosuppressants T-1 | 1.25 | 1.10–1.43 | <0.001 | |
| Antimalarial T-1 | 0.73 | 0.64–0.83 | <0.001 | |
Hazard ratios (HRs) were calculated using a time-dependent Cox regression model. Note: number at risk at time 0 (number of events): cataracts: 4682 (1750), diabetes: 4980 (460), gastroduodenal ulcers: 4949 (695: of which 30 are attributable to K22, 472 are attributable to K29 and 4 are attributable to both K22 and K29), herpes zoster: 5124 (301), hypertension: 4139 (1945), influenza: 5183 (56), osteonecrosis: 5175 (127), osteoporosis: 5021 (758), pneumonia: 4714 (1145), urinary tract infection: 4871 (1155). Immunosuppressants include: AZA, mycophenolic acid, CYC, rituximab, sirolimus, belimumab, ciclosporin, tacrolimus, MTX. Antimalarial medications include chloroquine and HCQ. Steroid dose: low dose >0 to <5.0 mg/day; medium dose 5–7.5 mg/day; high dose >7.5 mg/day. T-1: the value for the variable taken from the previous year while subject is at risk for the outcome at time T.
SLE patients also had a significantly increased probability of developing other comorbidities, including hypertension, osteoporosis, osteonecrosis, cataracts, gastroduodenal ulcers and diabetes, compared with controls (Fig. 2B). The highest HRs were observed for osteonecrosis (HR = 7.24, 95% CI 5.15–10.19) and the largest incidence rate difference was observed for hypertension, affecting 36% of SLE and 19% of controls. When examining the effect of OCS, we found high, medium and low dose to associate with development of all six comorbidities studied, except for low dose OCS and diabetes. Lower HRs were observed for low compared with high OCS (Table 3). The highest hazard for high OCS was observed for osteoporosis (HR = 5.11, 95% CI 4.11–6.36). Low OCS dose was associated with development of all six comorbidities studied, all HRs ≥1.15, and the highest HR for low OCS dose was observed for osteoporosis (HR = 2.15, 95% CI 1.76–2.62). Univariable analysis gave similar results (Supplementary Table S4, available at Rheumatology online). Treatment with immunosuppressants was associated with cataracts and hypertension (Table 3). Treatment with antimalarials was associated with development of cataracts and negatively associated with diabetes (Table 3).
Malignancy
During follow-up of the inception cohort, 424 (8.3%) patients were diagnosed with malignancy compared with 1128 (4.2%) in the control group (HR = 2.12, 95% CI 1.83–2.45) (Fig. 2B). Patients who had been diagnosed with a malignancy prior to SLE diagnosis were excluded. The most common malignancies in SLE patients were ICD-10 code C34, malignant neoplasm of bronchus and lung (n = 95, 1.9%), followed by C83, non-Hodgkin lymphoma (n = 55, 1.1%), C85, diffuse large B cell lymphoma (n = 44, 0.9%), and C43, malignant melanoma of the skin (n = 44, 0.9%) (data not shown). When analysing the effect of OCS on the development of malignancies, OCS was not a predictor of a future malignancy for either high (HR = 0.69, 95% CI 0.48–1.01), medium (HR = 1.12, 95% CI 0.84–1.48) or low (HR = 0.92, 95% CI 0.72–1.17) OCS dose (Table 4). However, treatment with immunosuppressants was associated with increased hazard of malignancies (HR = 1.54, 95% CI 1.24–1.93). These results were similar in the univariable analyses (Supplementary Table S5, available at Rheumatology online).
Table 4.
Risk factors associated with development of malignancy and mortality in SLE
| Outcome | Term | Multivariable |
||
|---|---|---|---|---|
| HR | 95% CI | P-value | ||
| Malignancy | Steroid dose: low (ref: none) T-1 | 0.92 | 0.72–1.17 | 0.491 |
| Steroid dose: medium (ref: none) T-1 | 1.12 | 0.84–1.48 | 0.449 | |
| Steroid dose: high (ref: none) T-1 | 0.69 | 0.48–1.01 | 0.055 | |
| Sex: female (ref: male) | 0.93 | 0.71–1.22 | 0.594 | |
| Age | 1.04 | 1.03–1.05 | <0.001 | |
| Immunosuppressants T-1 | 1.54 | 1.24–1.93 | <0.001 | |
| Antimalarial T-1 | 0.98 | 0.79–1.21 | 0.843 | |
| Mortality | Steroid dose: low (ref: none) T-1 | 1.59 | 1.42–1.78 | <0.001 |
| Steroid dose: medium (ref: none) T-1 | 2.19 | 1.92–2.49 | <0.001 | |
| Steroid dose: high (ref: none) T-1 | 3.75 | 3.31–4.26 | <0.001 | |
| Sex: female (ref: male) | 0.83 | 0.75–0.93 | 0.001 | |
| Age | 1.09 | 1.09–1.10 | <0.001 | |
| Immunosuppressants T-1 | 0.87 | 0.78–0.97 | 0.013 | |
| Antimalarial T-1 | 0.61 | 0.55–0.68 | <0.001 | |
Hazard ratios (HRs) were calculated using a time-dependent Cox regression model. Immunosuppressants include: AZA, mycophenolic acid, MTX, CYC, rituximab, sirolimus, belimumab, ciclosporin and tacrolimus. Antimalarial medications include chloroquine and HCQ. Steroid dose: low dose >0 to <5.0 mg/day; medium dose 5–7.5 mg/day; high dose >7.5 mg/day. T-1 the value for the variable taken from the previous year while subject is at risk for the outcome at time T.
Mortality
In total, 873 patients died during study follow-up and the most common causes of death in SLE were circulatory diseases (269; 30.8%), neoplasms (222; 25.4%) and musculoskeletal disease (99; 11.3%) including SLE (data not shown). Lupus was listed as the cause of death for 78 (8.9%) patients. Patients taking OCS in a given year were at an increased hazard for mortality in the following year compared with patients not on OCS (Table 4). High OCS demonstrated a higher HR of 3.75 (95% CI 3.31–4.26), medium OCS an HR of 2.19 (95% CI 1.92–2.49) and low OCS an HR of 1.59 (95% CI 1.42–1.78). These results were similar in univariable analyses (Supplementary Table S5, available at Rheumatology online). Use of both immunosuppressants and/or antimalarials were negatively associated with mortality (Table 4).
Discussion
In this nationwide inception cohort study including >5300 patients with SLE, we found that a majority of patients were taking OCS on a daily basis, with more than half of patients collecting an average dose above 7.5 mg/day during the first year following diagnosis. As can be expected after initial treatment of active disease, the percentage of patients on OCS decreased during the following years. However, 48% were still taking OCS both 5 and 15 years after diagnosis, retrieving an average dose of 7.3 mg/day and a median dose of 5.7 mg/day from pharmacies. The mean dose was generally higher than the median dose, possibly reflecting a minority of patients receiving high doses of OCS. In a prospective study of steroid treatment in the SLICC inception cohort, 57% were on an average OCS dose of 5.5 mg/day, 5 years after diagnosis [14]. The higher percentage on OCS in this study might reflect inclusion of multi-ethnic younger patients with more severe SLE compared with our Caucasian-dominated cohort. Also, ascertainment of steroid dose differed—in the SLICC cohort the dose was recorded by the physician and the present study data included retrieved doses at pharmacies. Regarding treatment trends over time, previous studies have shown no OCS reduction [14].
Use of antimalarial treatment in SLE has been recommended by EULAR and has been shown to prevent flares [12]. We found that 58.9% of incident SLE patients received an antimalarial medication in the year after diagnosis. This is consistent with other studies conducted in Sweden [3, 15]. In the Cox regression analysis, treatment with an antimalarial was associated with development of cataracts, potentially due to an increased frequency of eye check-ups. However, further follow-up of this observation is of interest.
We compared incidence of infections between SLE and the general population and found increased risk for pneumonia, urinary infection, herpes zoster and influenza. The observed HRs of 3.7 and 7.2 for pneumonia and herpes zoster infection is in line with previous studies demonstrating a relative risk of >2.5 for these infections in SLE [16]. Numerous risk factors for infections in SLE have been described including medical treatment [17], older age and aberrations of the immune system associated with SLE [18] including active disease [19]. Similar to what has been described by others [20, 21], we found an association between high OCS dose and development of infections. This is maybe not surprising given the effects of OCS on the immune system such as impeding access of neutrophils and monocytes to sites of inflammation [22]. However, in an in vitro experiment only medium to high OCS dose impaired granulocyte phagocytosis [23]. Therefore, we found it noteworthy that even low OCS dose was associated with an increased hazard of infections, a finding recently observed also by Abe et al. who showed a low OCS dose of 5.1–7.5 mg/day to increase risk of infections in SLE [24]. Furthermore, we found immunosuppressant treatment to increase the hazard for urinary tract and herpes zoster infections, which has been well described previously [17, 25]. Unfortunately, we had no data on septicaemia or vaccination. Furthermore, the number of infections in SLE could be overestimated due to close follow-up. In summary, we observed an increased cumulative incidence of infections in SLE associated with high, medium and low OCS dose [3, 21] highlighting the need to use OCS carefully.
In the present study we found SLE to be associated with increased development of comorbidities including osteoporosis, osteonecrosis, hypertension, diabetes, gastroduodenal ulcers and cataracts. Osteoporosis and secondary fractures are well-known risks in patients with SLE and are multifactorial, reflecting not only use of CS but persisting inflammation, physical inactivity and vitamin D insufficiency [26]. For osteonecrosis, the high HR for patients on CS, especially high doses, reflect the strong association, also highlighted in previous studies [27, 28]. However, we also show steroid doses of <5 mg/day to associate with osteonecrosis development. This could be due to higher steroid doses given previously during disease or to a steroid burst, >40 mg/day given during a few weeks followed by a dose of 2.5 mg/day during the year prior to the event. But it could also be related to the low-dose regime, in line with a recent longitudinal study in SLE demonstrating steroid doses <10 mg/day to be associated with osteonecrosis [29]. Next, we found an increased incidence of cataracts, affecting 27% after 15 years, which is in line with data from Carli et al. who found 29% of patients taking OCS at 5.5 mg/day to develop cataracts [30]. Hypertension is a well described risk factor for cardiovascular disease, and like others [31] we found an increased incidence of hypertension after SLE diagnosis [31]. Regarding diabetes, we observed a 2% increased cumulative incidence in SLE. Given the known association between OCS and diabetes development, a higher incidence might have been expected, but our results are in line with previous observations [3, 32].
There has long been a debate about whether there is a safe glucocorticoid dose to be used in SLE [10]. Here we demonstrate that, compared with no OCS treatment, not only high but also low OCS dose (<5.0 mg/day) is associated with development of comorbidities. Several previous studies have demonstrated OCS treatment to be associated with organ damage development in SLE [29, 33, 34], but the effect of a low dose has been disputed. In contrast to our results, some studies have shown low OCS dose not to associate with organ damage development [11, 35]. The reason for this could be lack of power in these smaller studies. Patients with high disease activity are typically treated with high OCS dose and thus the observed associations between high OCS and development of comorbidities could reflect a more active and severe SLE disease [11]. However, recent studies have demonstrated the association between OCS and SLICC/ACR Damage Index development to remain significant also when adjusting for disease activity [34, 36], suggesting OCS itself to cause organ damage. EULAR guidelines for SLE recommend the OCS dose to be ≤7.5 mg/day and, when possible, withdrawn [12]. Our results, demonstrating an increased risk of comorbidities also for patients on an OCS dose of <5 mg, suggest these recommendations could be too conservative.
Analogous to what has been described by others we found patients with SLE to have an increased risk of developing malignancies [37]. After excluding non-melanoma skin cancers including basal cell carcinoma, we found haematological and lung cancer to be the most common malignancies which is in line with earlier reports [3, 7]. In our regression analysis, we found high, medium and low OCS dose not to be associated with cancer development. These results are in agreement with previous studies evaluating glucocorticoids and the risk of malignancies. Askling et al. found no association between GCA/PMR, typically treated with high OCS, and development of lymphoma [38], and a large SLE study showed no connection between cumulative OCS dose and malignancies [39]. In our study, we found no association between OCS and malignancies in SLE.
There are several known risk factors for development of malignancies in SLE and we found immunosuppressants to be associated with increased incidence of cancer. In total, 48% of patients were taking at least one immunosuppressant after SLE diagnosis and until the end of follow-up, where AZA, MTX and mycophenolic acid were the most common drugs prescribed. Thus, our results are in line with the increased risk of cervical neoplasia [8] and haematological malignancies [40]. Unfortunately, we were not able to correct for other known cancer risk factors such as smoking [41, 42]. Taken together, our data highlight the importance of vigilance regarding malignancies in patients with SLE, especially in patients taking immunosuppressants.
Despite better treatment and medical care, patients with SLE still have impaired survival compared with the general population [43]. We confirm these findings, and like others, observed cardiovascular disease and malignancies to be the most common causes of death [44]. Next, we observed an association between high, medium and low OCS dose and impaired survival, with the highest risk in patients taking >7.5 mg/day. Bultink et al. recently showed cumulative glucocorticoid dose to associate with impaired survival in SLE but, unlike the present study, found no dose-dependent effect [45]. The connection between OCS and mortality could be due to development of infections and comorbidities described above, known to be associated with impaired survival in SLE [37, 44, 46]. Furthermore, high doses of OCS are often included in the treatment of neoplasms. It can also be a proxy for high disease activity, known to be associated with increased mortality in SLE [47]. The negative association between immunosuppressants and mortality could be due to treatment inhibition in patients with malignancies and in weak patients of old age.
One important strength of our study is the use of full-population, high-quality health registries, including all patients in Sweden with an SLE diagnosis in secondary care during the study period. Use of nationwide mandatory registry data, including data of drugs retrieved at pharmacies, ensured that selection bias and loss to follow-up was minimized and strengthened the generalizability of our findings. However, there are some limitations. As this is an inception cohort study starting in 2005, the majority of patients were observed for 5–10 years while fewer patients were followed for >10 years, thus limiting the data available for long term follow-up. OCS use could be overestimated since it was not known whether the amount dispensed was actually taken by patients, or whether patients took different doses within the period of time. Also, this study did not account for periods of high doses of glucocorticoids that a patient may have been exposed to previously in their treatment history, which could affect the association with infections and comorbidities such as osteonecrosis. We report categories of average yearly OCS dose based on thresholds of >0 to <5 mg, 5–7.5 mg and >7.5 mg, and the results should not be interpreted by these fixed boundaries but rather that higher steroid exposure is a greater predictor of adverse outcomes than lower exposure. OCS remain a mainstay of SLE care, and a future study could explore cumulative glucocorticoid exposure. Furthermore, data regarding cardiovascular disease, septicaemia and immunosuppressive treatment given i.v. at hospitals such as CYC and CS were not available. In addition, we did not have access to information regarding ethnicity and disease activity.
Conclusion
In this nationwide SLE inception cohort including >5300 patients, we observed a high proportion of patients with SLE to take OCS not only at disease onset, but also over time. Furthermore, we found not only high (>7.5 mg) but also medium (5–7.5 mg) and low (<5 mg) OCS dose to significantly predict development of infections, comorbidities and survival in SLE. HRs were lower for low OCS dose than high OCS dose. These results highlight the potential harm also associated with low OCS dose treatment in SLE and the need to judiciously use OCS at the lowest possible dose to maximize efficacy and minimize harm. Consequently, there is a need for new treatments to prevent organ damage and premature death in SLE.
Supplementary Material
Contributor Information
Martina Frodlund, Department of Biomedical and Clinical Sciences, Division of Inflammation and Infection/Rheumatology, Linköping University, Linköping, Sweden.
Andreas Jönsen, Department of Clinical Sciences Lund, Rheumatology, Lund University, Lund, Sweden.
Lauren Remkus, AstraZeneca A/S, Copenhagen, Denmark.
Gunilla Telg, AstraZeneca Nordics, Sodertalje, Sweden.
Fabian Söderdahl, Statisticon AB, Uppsala, Sweden.
Dag Leonard, Rheumatology, Department of Medical Sciences, Uppsala University, Uppsala, Sweden.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
The dataset supporting the conclusions of this article are available upon request.
Author contributions
All authors significantly contributed to the work reported either in the study conception, design, execution; data acquisition, analysis and interpretation; or in all of these areas. All authors also participated in drafting, revising or critically reviewing the article; read and approved the final version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work. All authors consent to publish these data.
Funding
This work was supported by AstraZeneca.
Disclosure statement: L.R. and G.T. are fulltime employees of AstraZeneca. F.S. is employed at Statisticon of which AstraZeneca is a client. M.F. has received consultancy fees from GSK and AstraZeneca. A.J. has received consultancy fees from GSK and AstraZeneca. D.L. has received consultancy fees from GSK, AstraZeneca and Otsuka.
References
- 1. Bengtsson AA, Ronnblom L.. Systemic lupus erythematosus: still a challenge for physicians. J Intern Med 2017;281:52–64. [DOI] [PubMed] [Google Scholar]
- 2. Ronnblom L, Leonard D.. Interferon pathway in SLE: one key to unlocking the mystery of the disease. Lupus Sci Med 2019;6:e000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frodlund M, Reid S, Wettero J. et al. The majority of Swedish systemic lupus erythematosus patients are still affected by irreversible organ impairment: factors related to damage accrual in two regional cohorts. Lupus 2019;28:1261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ingvarsson RF, Landgren AJ, Bengtsson AA, Jonsen A.. Good survival rates in systemic lupus erythematosus in southern Sweden, while the mortality rate remains increased compared with the population. Lupus 2019;28:1488–94. [DOI] [PubMed] [Google Scholar]
- 5. Lerang K, Gilboe IM, Steinar Thelle D, Gran JT.. Mortality and years of potential life loss in systemic lupus erythematosus: a population-based cohort study. Lupus 2014;23:1546–52. [DOI] [PubMed] [Google Scholar]
- 6. Goncalves MJ, Sousa S, Ines LS. et al. Characterization of damage in Portuguese lupus patients: analysis of a national lupus registry. Lupus 2015;24:256–62. [DOI] [PubMed] [Google Scholar]
- 7. Ladouceur A, Clarke AE, Ramsey-Goldman R, Bernatsky S.. Malignancies in systemic lupus erythematosus: an update. Curr Opin Rheumatol 2019;31:678–81. [DOI] [PubMed] [Google Scholar]
- 8. Wadstrom H, Arkema EV, Sjowall C, Askling J, Simard JF.. Cervical neoplasia in systemic lupus erythematosus: a nationwide study. Rheumatology (Oxford) 2017;56:613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sprague RG, Power MH.. Observations on the physiologic effects of cortisone and ACTH in man. Arch Intern Med (Chic) 1950;85:199–258. [DOI] [PubMed] [Google Scholar]
- 10. Stojan G, Petri M.. The risk benefit ratio of glucocorticoids in SLE: have things changed over the past 40 years? Curr Treatm Opt Rheumatol 2017;3:164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thamer M, Hernan MA, Zhang Y, Cotter D, Petri M.. Prednisone, lupus activity, and permanent organ damage. J Rheumatol 2009;36:560–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fanouriakis A, Kostopoulou M, Alunno A. et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Anna Rheum Dis 2019;78:736–45. [DOI] [PubMed] [Google Scholar]
- 13. Arkema EV, Jonsen A, Ronnblom L. et al. Case definitions in Swedish register data to identify systemic lupus erythematosus. BMJ Open 2016;6:e007769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Little J, Parker B, Lunt M. et al. Glucocorticoid use and factors associated with variability in this use in the Systemic Lupus International Collaborating Clinics Inception Cohort. Rheumatology (Oxford) 2018;57:677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bengtsson C, Ohman ML, Nived O, Dahlqvist SR.. Cardiovascular event in systemic lupus erythematosus in northern Sweden: incidence and predictors in a 7-year follow-up study. Lupus 2012;21:452–9. [DOI] [PubMed] [Google Scholar]
- 16. Pego-Reigosa JM, Nicholson L, Pooley N. et al. The risk of infections in adult patients with systemic lupus erythematosus: systematic review and meta-analysis. Rheumatology (Oxford) 2021;60:60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singh JA, Hossain A, Kotb A, Wells G.. Risk of serious infections with immunosuppressive drugs and glucocorticoids for lupus nephritis: a systematic review and network meta-analysis. BMC Med 2016;14:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Torres-Ruiz J, Mejia-Dominguez NR, Zentella-Dehesa A. et al. The Systemic Lupus Erythematosus Infection Predictive Index (LIPI): a clinical-immunological tool to predict infections in lupus patients. Front Immunol 2018;9:3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nived O, Sturfelt G, Wollheim F.. Systemic lupus erythematosus and infection: a controlled and prospective study including an epidemiological group. Q J Med 1985;55:271–87. [PubMed] [Google Scholar]
- 20. Petri M, Genovese M.. Incidence of and risk factors for hospitalizations in systemic lupus erythematosus: a prospective study of the Hopkins Lupus Cohort. J Rheumatol 1992;19:1559–65. [PubMed] [Google Scholar]
- 21. Rua-Figueroa I, Lopez-Longo J, Galindo-Izquierdo M. et al. Incidence, associated factors and clinical impact of severe infections in a large, multicentric cohort of patients with systemic lupus erythematosus. Semin Arthritis Rheum 2017;47:38–45. [DOI] [PubMed] [Google Scholar]
- 22. Fauci AS, Dale DC, Balow JE.. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med 1976;84:304–15. [DOI] [PubMed] [Google Scholar]
- 23. Herzer P, Lemmel EM.. Inhibition of granulocyte function by prednisolone and non-steroid anti-inflammatory drugs. Quantitative evaluation with NBT test and its correlation with phagocytosis. Immunobiology 1980;157:78–88. [DOI] [PubMed] [Google Scholar]
- 24. Abe K, Ishikawa Y, Kita Y. et al. Association of low-dose glucocorticoid use and infection occurrence in systemic lupus erythematosus patients: a prospective cohort study. Arthritis Res Ther 2022;24:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morand EF, Furie R, Tanaka Y. et al. ; TULIP-2 Trial Investigators. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med 2020;382:211–21. [DOI] [PubMed] [Google Scholar]
- 26. Salman-Monte TC, Torrente-Segarra V, Vega-Vidal AL. et al. Bone mineral density and vitamin D status in systemic lupus erythematosus (SLE): a systematic review. Autoimmun Rev 2017;16:1155–9. [DOI] [PubMed] [Google Scholar]
- 27. Gladman DD, Dhillon N, Su J, Urowitz MB.. Osteonecrosis in SLE: prevalence, patterns, outcomes and predictors. Lupus 2018;27:76–81. [DOI] [PubMed] [Google Scholar]
- 28. Kaneko K, Chen H, Kaufman M. et al. Glucocorticoid-induced osteonecrosis in systemic lupus erythematosus patients. Clin Transl Med 2021;11:e526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen HL, Shen LJ, Hsu PN. et al. Cumulative burden of glucocorticoid-related adverse events in patients with systemic lupus erythematosus: findings from a 12-year longitudinal study. J Rheumatol 2018;45:83–9. [DOI] [PubMed] [Google Scholar]
- 30. Carli L, Tani C, Querci F. et al. Analysis of the prevalence of cataracts and glaucoma in systemic lupus erythematosus and evaluation of the rheumatologists' practice for the monitoring of glucocorticoid eye toxicity. Clin Rheumatol 2013;32:1071–3. [DOI] [PubMed] [Google Scholar]
- 31. Munguia-Realpozo P, Mendoza-Pinto C, Sierra Benito C. et al. Systemic lupus erythematosus and hypertension. Autoimmun Rev 2019;18:102371. [DOI] [PubMed] [Google Scholar]
- 32. Castro LL, Lanna CCD, Ribeiro ALP, Telles RW.. Recognition and control of hypertension, diabetes, and dyslipidemia in patients with systemic lupus erythematosus. Clin Rheumatol 2018;37:2693–8. [DOI] [PubMed] [Google Scholar]
- 33. Bruce IN, O’Keeffe AG, Farewell V. et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis 2015;74:1706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Al Sawah S, Zhang X, Zhu B. et al. Effect of corticosteroid use by dose on the risk of developing organ damage over time in systemic lupus erythematosus-the Hopkins Lupus Cohort. Lupus Sci Med 2015;2:e000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ruiz-Arruza I, Ugarte A, Cabezas-Rodriguez I. et al. Glucocorticoids and irreversible damage in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2014;53:1470–6. [DOI] [PubMed] [Google Scholar]
- 36. Apostolopoulos D, Kandane-Rathnayake R, Louthrenoo W. et al. Factors associated with damage accrual in patients with systemic lupus erythematosus with no clinical or serological disease activity: a multicentre cohort study. Lancet Rheumatol 2020;2:e24–30. [DOI] [PubMed] [Google Scholar]
- 37. Zhang M, Wang Y, Wang Y, Bai Y, Gu D.. Association between systemic lupus erythematosus and cancer morbidity and mortality: findings from cohort studies. Front Oncol 2022;12:860794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Askling J, Klareskog L, Hjalgrim H. et al. Do steroids increase lymphoma risk? A case-control study of lymphoma risk in polymyalgia rheumatica/giant cell arteritis. Ann Rheum Dis 2005;64:1765–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hsu CY, Lin MS, Su YJ. et al. Cumulative immunosuppressant exposure is associated with diversified cancer risk among 14 832 patients with systemic lupus erythematosus: a nested case-control study. Rheumatology (Oxford) 2017;56:620–8. [DOI] [PubMed] [Google Scholar]
- 40. Bernatsky S, Joseph L, Boivin JF. et al. The relationship between cancer and medication exposures in systemic lupus erythaematosus: a case-cohort study. Ann Rheum Dis 2008;67:74–9. [DOI] [PubMed] [Google Scholar]
- 41. Bernatsky S, Ramsey-Goldman R, Petri M. et al. Smoking is the most significant modifiable lung cancer risk factor in systemic lupus erythematosus. J Rheumatol 2018;45:393–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li XB, Cao NW, Chu XJ. et al. Antimalarials may reduce cancer risk in patients with systemic lupus erythematosus: a systematic review and meta-analysis of prospective studies. Ann Med 2021;53:1687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tektonidou MG, Lewandowski LB, Hu J, Dasgupta A, Ward MM.. Survival in adults and children with systemic lupus erythematosus: a systematic review and Bayesian meta-analysis of studies from 1950 to 2016. Ann Rheum Dis 2017;76:2009–16. [DOI] [PubMed] [Google Scholar]
- 44. Bernatsky S, Boivin JF, Joseph L. et al. Mortality in systemic lupus erythematosus. Arthritis Rheum 2006;54:2550–7. [DOI] [PubMed] [Google Scholar]
- 45. Bultink IEM, de Vries F, van Vollenhoven RF, Lalmohamed A.. Mortality, causes of death and influence of medication use in patients with systemic lupus erythematosus vs matched controls. Rheumatology (Oxford) 2021;60:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nived O, Jonsen A, Bengtsson AA, Bengtsson C, Sturfelt G.. High predictive value of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for survival in systemic lupus erythematosus. J Rheumatol 2002;29:1398–400. [PubMed] [Google Scholar]
- 47. Alarcón GS, McGwin G Jr, Bastian HM. et al. ; LUMINA Study Group. Systemic lupus erythematosus in three ethnic groups. VII [correction of VIII]. Predictors of early mortality in the LUMINA cohort. Arthritis Rheum 2001;45:191–202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting the conclusions of this article are available upon request.