Abstract
Venous thromboembolism (VTE) is a common vascular disease of venous return disorders, including deep venous thrombosis and pulmonary embolism (PE), with high morbidity and high mortality. However, the relationship between oxidative phosphorylation and NDUFB11 and venous thromboembolism is still unclear. The venous thromboembolism datasets GSE48000 and GSE19151 were downloaded, and the differentially expressed Genes (DEGs) were screened. The protein-protein interaction (PPI) network was constructed. Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) were used for functional enrichment analysis. The comparative toxicogenomics database (CTD) was used to identify the diseases most associated with the core genes. TargetScan was used to screen miRNA regulating central DEGs. Western blotting (WB) experiment and real-time quantitative PCR (RT-qPCR) experiment were performed. A total of 500 DEGs were identified. GO analysis showed that the DEGs were mainly enriched in ATP synthesis coupled electron transport, respiratory electron transport chain, cytoplasm, enzyme binding, nonalcoholic fatty liver disease, oxidative phosphorylation, and Alzheimer disease. Enrichment items were similar to GO and KEGG enrichment items of DEGs. The result of CTD showed that 12 genes (RPS24, FAU, RPLP0, RPS15A, RPS29, RPL9, RPL31, RPL27, NDUFB11, RPL34, COX7B, RPS27L) were associated with chemical and drug-induced liver injury, inflammation, kidney disease, and congenital pure red cell aplasia. WB and RT-qPCR results showed that the expression levels of 12 genes in venous thromboembolism were higher than normal whole blood tissue samples. NDUFB11 is highly expressed in catheter-related venous thromboembolism during continuous blood purification, which may lead to the formation of venous thrombosis through oxidative phosphorylation pathway.
Keywords: bioinformatics, NDUFB11, oxidative phosphorylation, venous thromboembolism
1. Introduction
As an important life support system for critically ill patients, Continuous Renal Replacement Therapy technology has a wide range of application value in the field of treatment of renal injury and non-renal injury, and has become an indispensable support therapy for critically ill patients.[1,2]The establishment of vascular access is a strong guarantee for the smooth progress of continuous blood purification, and the management of vascular access during intermittent treatment is one of the important links. Prolonged catheter indwelling and inappropriate sealing during nursing can easily lead to the formation of venous thrombosis. In the intensive care unit ward, patients often stay in bed for a long time and use of sedatives are common risk factors for venous thrombosis.
Venous thromboembolism (VTE) is caused by venous blood stasis, vascular endothelial cell injury, hypercoagulable state and other factors.[3] VTE, including deep venous thrombosis and pulmonary embolism (PE), is a common disease with multiple factors and high mortality.[4] There are 3 high risk factors for venous thromboembolism. First, the abnormal increase of coagulation factors is easy to induce thrombosis. Secondly, damage to the intima of the blood vessel leads to the release of a large number of procoagulant factors and the formation of thrombosis in the blood vessel. Third, there is a hypercoagulable state, especially in patients with tumors or nephrotic syndrome.[5] Severe VTE in the acute stage can lead to gangrene of the lower limbs and even fatal pulmonary embolism, which directly threatens the life safety of patients. Anticoagulant therapy is the main treatment measure, and surgical thrombectomy can be performed in severe cases.[6] The disease has become a major global health problem and has attracted extensive attention from international academia and society. However, the cause of venous thromboembolism is still unclear. This disease may be related to genetic factors, chromosomal abnormalities, gene fusion and other factors, so it is particularly important to study the molecular mechanism of venous thromboembolism.
Bioinformatics technology is the processing and analysis of various data of the proteome, which is also an important content of proteome research and has become an indispensable part of proteomics research.[7] The development of bioinformatics technology is not only the analysis of genome and proteome data, but also the comprehensive analysis of known or new gene products.[8]
NDUFB11 (NADH: Ubiquinone Oxidoreductase Subunit B11) is a protein-coding gene that encodes a Subunit of the multisubunit NADH: Ubiquinone Oxidoreductase (complex I), this protein has NADH dehydrogenase activity and oxidoreductase activity.[9] Oxidative phosporylation is a biochemical process that occurs in the inner mitochondrial membrane of eukaryotic cells or the cytoplasm of prokaryotes. It is a coupling reaction between the energy released during the oxidation of substances in vivo and the supply of ADP through the respiratory chain and the synthesis of ATP by inorganic phosphate.[10] However, its relationship with catheter-related venous thromboembolism during continuous blood purification is unclear.
We hypothesized that NDUFB11 may play a role in continuous blood purification catheter-associated VTE through oxidative phosphorylation pathway. The paper intends to use bioinformatics technology to mine the core genes between venous thromboembolism and normal tissues, and correlation analysis was performed. Public datasets were used to validate the significant roles of oxidative phosphorylation (OXPHOS) and NDUFB11 in venous thromboembolism.
2. Methods
2.1. Venous thromboembolism dataset
Venous thromboembolism dataset GSE48000 and GSE19151 configuration file generated from GPL10558, GPL571 gene expression omnibus database (http://www.ncbi.nlm.nih.gov/geo/) to download. GSE48000 included 107 VTE and 25 normal whole blood tissue samples. GSE19151 included 70 VTE and 63 normal whole blood tissue samples. The differentially expressed genes (DEGs) between VTE and normal samples were identified.
2.2. To batch processing
Merging and debatching of the multiple datasets, GSE48000 and GSE19151 were merged using the R software package. For combination of multiple data sets, use R software package in Silico Merging to merge the data sets to get the merging matrix. The R software package limma removes batch effect. The matrix batch effect after removal was obtained.
2.3. Screening of DEGs
Probe aggregation and background correction of merge matrix of GSE48000 and GSE19151 using R package “limma.” P value were adjusted useing Benjamini-Hochberg method. The fold change (FC) is calculated using false discovery rate. And make a visual representation of the volcano.
2.4. Construction and analysis of protein-protein interaction (PPI) network
Search Tool for the Retrieval of Interacting Genes (STRING) is a search system for known and predicted PPI. STRING database also contains the predicted results using bioinformatics methods. The differential genes were input into STRING to construct PPI network and predict the core genes. PPI network was visualized, core genes are predicted by Cytoscape software. First of all, we import PPI network into the Cytoscape, and then find the module with the best correlation through MCODE. MCC and MNC were used to calculate the best correlated genes. Finally, the list of core genes was obtained after visualization.
2.5. Functional enrichment analysis
Gene Ontology (GO) analysis is a computational method to evaluate gene functions and biological pathways, and it is a key step to endow sequence information with practical biological significance. Kyoto Encyclopedia of Gene and Genome (KEGG) is an online database dedicated to collecting information on genomes, molecular interaction networks, enzyme catalytic pathways, and biochemical products. The genomic information and gene function were linked, and gene function was systematically analyzed. The list of differential genes screened by Wayne map was input into KEGG rest API obtained latest KEGG Pathway gene annotation. Gene set enrichment results were obtained using R package cluster Profiler.
Metascape (http://metascape.org/) can realize cognition of gene or protein function, and can be visually exported. We used Metascape database to analyze functional enrichment of the above differential gene list and derive it.
2.6. Gene Set Enrichment Analysis (GSEA)
GSEA is based on level-specific gene probes that evaluate data from microarrays and is a way to uncover genomic expression data through fundamental knowledge. The samples were divided into venous thromboembolism and normal whole blood tissue. 5 is minimum gene set and 5000 is maximum gene set, 1000 resampling times. The whole genome was analyzed by GO and KEGG.
2.7. Heat map of gene expression
We use R-packet heatmap to map expression of the core genes found in PPI network in GSE48000 and GSE19151, and to visualize difference of core gene expression between venous thromboembolism and normal whole blood tissue samples.
2.8. CTD analysis
CTD is a powerful public database, which predict gene/protein relationships with disease, are used to identify integrated chemical diseases, chemical genes, and gene disease interactions to predict new associations and generate extended networks. We input core gene into CTD, find disease most related to core gene. Excel was used to draw radar map of differential expression of each gene.
2.9. The miRNA
TargetScan (www.targetscan.org) can predict and analyze miRNA and target genes. Screening of miRNAs regulating central DEGs was performed using TargetScan in this study.
2.10. Western blotting
Western blotting (WB), also known as immunoblotting, is a method to detect the expression of a certain protein in complex samples according to the specific binding of antigens and antibodies, and can qualitatively and semi-quantitatively analyze proteins. Total protein was extracted and the protein content was determined. After SDS-PAGE electrophoresis and membrane transfer, the protein samples were blocked with 5% skim milk for 1h at room temperature, shaken with Tris Buffered Saline Tween (TBST) at high speed on a shaker, washed for 5 minutes, and repeated 3 times. Primary antibodies were added, incubated overnight at 4 °C, and then shaken and washed 3 times (5 minutes each time) with TBST. Secondary antibody was added and incubated for 1h at room temperature, then TBST was shaken and washed 3 times (5 minutes each time), and the results were analyzed after chemiluminescence solution was developed.
2.11. Real-time quantitative PCR (RT-qPCR)
Rt-qPCR (real-time fluorescent quantitative PCR) is a basic experiment in molecular biology. Through RT-qPCR experiments, cDNA probes can be synthesized, target genes can be obtained, and gene transcription levels can be analyzed. Rt-qPCR mainly includes 3 steps: RNA extraction, reverse transcription into cDNA, and real-time fluorescence quantitative PCR.
2.12. Statistical analysis
Statistical analysis is a method used to process and interpret data to help extract information from data, detect trends, make inferences, and make decisions. The goal of statistical analysis is to understand data through mathematical and computational methods in order to draw conclusions or support decisions. The process of WB processing mainly includes 3 parts: using PS software for typesetting, using Image J for gray value analysis, and prism software was used for mapping analysis.
3. Results
3.1. Functional enrichment analysis
3.1.1. Functional enrichment analysis of DEGs.
In this study, a total of 500 DEGs were identified based on DEGs identified in the debatching merge matrix of GSE197158 and GSE206448 (Fig. 1).
Figure 1.
Functional enrichment analysis of DEGs. Red: Up-regulated; Green: Down-regulated. DEGs = differentially expressed Genes.
We then performed GO and KEGG and Reactome analysis of these differentially expressed genes. According to GO analysis, they were mainly enriched in ATP synthesis coupled electron transport, respiratory electron transport chain, cytoplasm, enzyme binding, nonalcoholic fatty liver disease, oxidative phosphorylation (Fig. 2A–E).
Figure 2.
GO and KEGG, and Reactome analysis. (A) BP, (B) CC, (C) MF, (D) KEGG, (E) REACTOME. KEGG = Kyoto Encyclopedia of Genes and Genomes.
3.2. GSEA
In addition, we performed GSEA enrichment analysis on the whole genome to find possible enrichment terms in non-differentially expressed genes. Enrichment terms were similar to GO and KEGG enrichment terms for differentially expressed genes (Fig. 3A–D).
Figure 3.
Enrichment by the GSEA. (A) BP, (B) CC, (C) MF, (D) KEGG. GSEA = Gene Set Enrichment Analysis, KEGG = Kyoto Encyclopedia of Genes and Genomes.
3.3. Metascape enrichment analysis
The content enriched by Metascape includes GO enriched terms (Fig. 4A) and has an enriched network colored by enriched terms and P value (Figs. 4B,C, and 5).
Figure 4.
Enrichment analysis by Metascape. (A) GO enrichment term, (B) enrichment networks colored by enrichment terms, (C) enrichment networks colored by P value.
Figure 5.
Enrichment analysis by Metascape.
3.4. Construction and analysis of protein-protein interaction (PPI) network
The PPI network of DEGs was constructed from the STRING online database and analyzed by Cytoscape software (Fig. 6A). Two different algorithms (MCC, DMNC) were used to identify central genes (Fig. 6B,C), and Excel was used to take the intersection. 12 core genes (RPS24, FAU, RPLP0, RPS15A, RPS29, RPL9, RPL31, RPL27, NDUFB11, RPL34, COX7B, RPS27L) were obtained.
Figure 6.
Construction and analysis of protein-protein interaction (PPI) network. (A) PPI network of DEGs, (B) MCC identifies central genes, (C) DMNC recognizes central genes. DEGs = differentially expressed Genes.
At the same time, we have made a PPI network with NDFUB2 by using the relevant genes in the process of venous thrombosis, such as protein metabolism, nucleic acid metabolism, TCA pathway, etc, which explains the core significance of NDFUB2 (Fig. 7).
Figure 7.
The PPI network was constructed with NDFUB2. (A) The overview map, (B) the significant hub role of NDUFB11. PPI = protein-protein interaction.
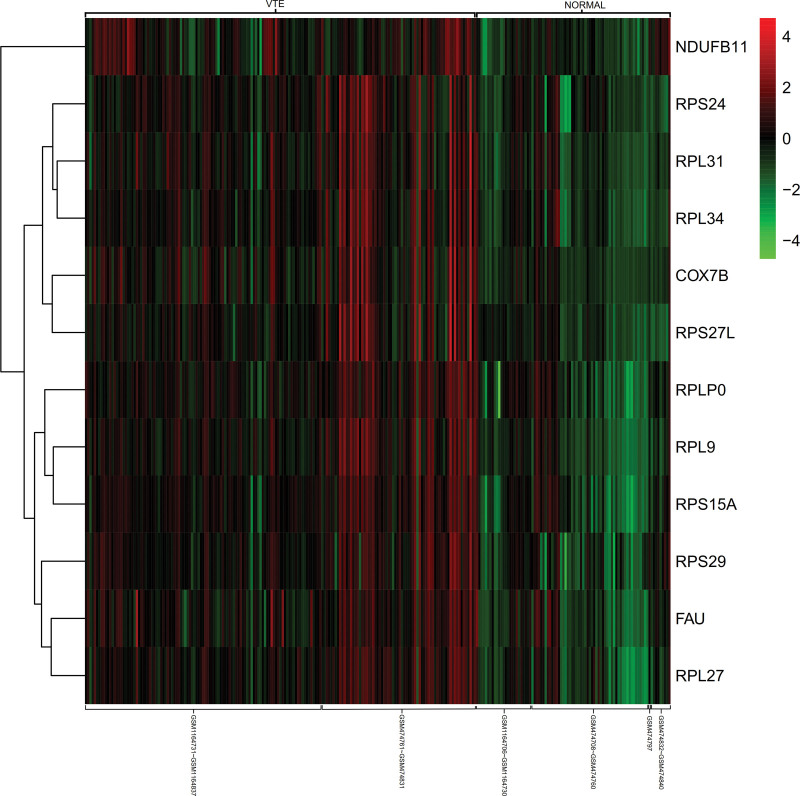
3.5. Heat map of gene expression
Differential expression of core genes between venous thromboembolism and normal whole-blood tissue samples (Fig. 8).
Figure 8.
Heat map of gene expression.
3.6. Analysis of CTD
Core genes was entered into CTD to find diseases related to core genes. 12 genes (RPS24, FAU, RPLP0, RPS15A, RPS29, RPL9, RPL31, RPL27, NDUFB11, RPL34, COX7B, RPS27L) were found to be associated with chemical and drug-induced liver injury, inflammation, kidney disease, and congenital pure red cell aplasia (Fig. 9).
Figure 9.
Analysis of CTD. CTD = comparative toxicogenomics database.
3.7. Western blotting (WB)
The expression levels of 12 genes (RPS24, FAU, RPLP0, RPS15A, RPS29, RPL9, RPL31, RPL27, NDUFB11, RPL34, COX7B, RPS27L) in venous thromboembolism were higher than normal whole blood tissue samples (Fig. 10).
Figure 10.
Analysis of WB.
3.8. RT-qPCR
The relative mRNA expression levels of 12 genes (RPS24, FAU, RPLP0, RPS15A, RPS29, RPL9, RPL31, RPL27, NDUFB11, RPL34, COX7B, RPS27L) in venous thromboembolism were higher than normal whole blood tissue samples (Fig. 11).
Figure 11.
Analysis of RT-qPCR.
3.9. Prediction and functional annotation of miRNA related to hub genes
The hub genes was entered into targetsacan to search for relevant miRNA (Table 1). We found that the related miRNA of RPS24 gene were hsa-miR-19b-3p and hsa-miR-19a-3p. The related miRNA of FAU gene were hsa-miR-6807-3p and hsa-miR-217. The related miRNA of RPS15A gene was hsa-miR-223-3p. The related miRNA of RPS29 gene was hsa-miR-489-3p. The related miRNA of RPL9 gene was hsa-miR-214-5p. The related miRNA of RPL31 gene were hsa-miR-409-3p and hsa-miR-653-5. The related miRNA of RPL34 gene were hsa-miR-23b-3p, hsa-miR-23a-3 and hsa-miR-23c. The related miRNA of COX7B gene were hsa-miR-302c-3p.2 and hsa-miR-520f-3p. The related miRNA of RPS27L gene were hsa-miR-323a-3p and hsa-miR-325-3p.
Table 1.
A summary of miRNAs that regulate hub genes.
| Gene | MIRNA | |||
|---|---|---|---|---|
| 1 | RPS24 | hsa-miR-19a-3p | hsa-miR-19b-3p | |
| 2 | FAU | hsa-miR-6807-3p | hsa-miR-217 | |
| 3 | RPS15A | hsa-miR-223-3p | ||
| 4 | RPS29 | hsa-miR-489-3p | ||
| 5 | RPL9 | hsa-miR-214-5p | ||
| 6 | RPL31 | hsa-miR-409-3p | hsa-miR-653-5p | |
| 7 | RPL34 | hsa-miR-23b-3p | hsa-miR-23a-3p | hsa-miR-23c |
| 8 | COX7B | hsa-miR-302c-3p.2 | hsa-miR-520f-3p | |
| 9 | RPS27L | hsa-miR-323a-3p | hsa-miR-325-3p | |
| 10 | RPLP0 | none | ||
| 11 | RPL27 | none | ||
| 12 | NDUFB11 | none | ||
4. Discussion
Venous thromboembolism refers to the abnormal coagulation of blood in the venous system, which leads to the blockage of blood vessels and the inability of blood circulation. It is also a common venous reflux disorder in intensive care unit patients.[11] The clinical manifestations of venous thromboembolism include leg pain, tenderness, swelling, venous dilatation, etc. There may be a series of other complications, and even lead to sudden death.[12] Therefore, in-depth exploration of the molecular mechanism of continuous blood purification (CBP) catheter-related venous thromboembolism is extremely important for the research of targeted drugs. The main results of this study were that NDUFB11 was highly expressed in VTE and was mainly enriched in oxidative phosphorylation pathway.
The NDUFB11 gene is located on Xp11.23 and consists of 3 exons, is a non-catalytic component encoding complex I of the mitochondrial respiratory chain and is essential for the assembly of active complex I.[13] The study has shown that exome sequencing in patients with histiocytoid cardiomyopathy reveals a de novo NDUFB11 mutation that plays a role in the pathogenesis of histiocytoid cardiomyopathy.[14] The novel NDUFB11 gene mutation reveals a novel clinical phenotype associated with lactic acidosis and sideroblastic anemia.[15] Patients with NDUFB11 mutations have a more classical mitochondrial phenotype. Congenital sideroblastic anemia caused by NDUFB11 gene mutation. Disorders associated with NDUFB11 include mitochondrial complex I defects, nuclear type 30, and linear skin defects with multiple congenital malformations, 3 associated pathways include respiratory electron transport, chemoosmotically coupled ATP synthesis, and uncoupled protein thermogenesis. NDUFB11 may be particularly important in cardiac tissue.[16] The products of NDUFB11 are involved in the biogenesis of the OXPHOS system, and a variety of subunits, including NDUFB11 mutations, have been identified in patients with cerebral ischemic deficiency, suggesting that these genes play an important role in the assembly and/or stability of cerebral ischemic deficiency.[17] Therefore, it is speculated that NDUFB11 plays a key role in CBP catheter-related venous thromboembolism.
Oxidative phosphorylation refers to the energy released by the oxidation step in the decomposition of organic matter, including sugars, lipids, and amino acids, which drives the synthesis of ATP.[18] In eukaryotic cells, oxidative phosphorylation occurs in mitochondria, and the systems involved in oxidation and phosphorylation are distributed in the inner membrane of mitochondria in the form of a complex, which constitutes the respiratory chain, also known as the electron transport chain.[19] The mitochondrial electron transport chain uses a series of electron transport reactions to generate cellular ATP through oxidative phosphorylation. The result of electron transport is the generation of reactive oxygen species (ROS), which contributes to homeostatic signaling and oxidative stress during pathological processes.[10]
OXPHOS occurs in mitochondria and forms adenosine triphosphate (ATP), which is essential for almost all eukaryotic cells.[20] Impaired mitochondrial oxidative phosphorylation limits self-renewal in T cells exposed to persistent antigens.[21] Oxidative phosphorylation plays a key role in the regulation of cell and tissue metabolism.[18] Oxidative phosphorylation (OXPHOS) can realize biological functions and, more importantly, depend on biomass production.[22] Targeting oxidative phosphorylation can improve the efficacy of combined radiotherapy and immunotherapy.[22] Glucose metabolic flux shifts from mitochondrial oxidative phosphorylation (OXPHOS) to aerobic glycolysis.[23,24] It has also been shown that modulation of oxidative phosphorylation inhibits inflammation.[25] The interaction between oxidative phosphorylation and glycolysis can be used as a potential marker of disease progression and is of relative importance in the growth and maintenance of different cell lines.[26] It has also been reported that wild-type p53 promotes metabolism by inducing inhibition of oxidative phosphorylation.[27] Therefore, oxidative phosphorylation may play an important role in the occurrence and formation of CBP catheter-related venous thromboembolism.
This study holds significant potential clinical significance in the field of clinical medicine, as it can provide crucial insights to enhance the diagnosis, treatment, and care of patients with venous thromboembolism. This research may unveil new treatment methods and strategies aimed at improving patients’ quality of life, extending lifespans, or even achieving a cure for venous thromboembolism. Additionally, it has the potential to aid in the development of earlier and more accurate diagnostic methods, facilitating the identification of underlying health issues and enabling timely interventions, thereby increasing the chances of treatment success. Furthermore, this study could uncover individual variations, paving the way for personalized treatment plans that enhance treatment effectiveness while reducing adverse reactions. By delving deeper into the molecular and cellular mechanisms of the disease, it can provide a more profound understanding of venous thromboembolism, potentially leading to the development of innovative treatment approaches.
Although this paper has carried out rigorous bioinformatics analysis, there are still some shortcomings. Animal experiments with overexpression or knockdown of the gene were not performed in this study to further verify the function. The results showed only computational, in vitro based experiments are also missing. Therefore, in future research, we should conduct in-depth exploration in this aspect.
5. Conclusion
NDUFB11 is highly expressed in catheter-associated venous thromboembolism during continuous blood purification, which may lead to venous thrombosis through oxidative phosphorylation pathway.
Author contributions
Conceptualization: Yanhong Ma.
Data curation: Suzhi Guo.
Formal analysis: Suzhi Guo.
Methodology: Suzhi Guo.
Software: Suzhi Guo.
Writing – original draft: Suzhi Guo.
Writing – review & editing: Yanhong Ma.
Abbreviations:
- ATP
- = adenosine triphosphate,
- CBP
- = continuous blood purification,
- CTD
- = comparative toxicogenomics database,
- DEGs
- = differentially expressed Genes,
- FC
- = fold change,
- GO
- = Gene ontology,
- GSEA
- = Gene Set Enrichment Analysis,
- KEGG
- = Kyoto Encyclopedia of Genes and Genomes,
- OXPHOS
- = oxidative phosphorylation,
- PE
- = pulmonary embolism,
- PPI
- = protein-protein interaction,
- STRING
- = Search Tool for the Retrieval of Interacting Genes,
- VTE
- = Venous thromboembolism.
This study was approved by the Ethics Committee of The Fourth Hospital of Hebei Medical University.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Ma Y, Guo S. High expression of NADH Ubiquinone Oxidoreductase Subunit B11 induces catheter-associated venous thrombosis on continuous blood purification. Medicine 2023;102:48(e36520).
References
- [1].Tandukar S, Palevsky PM. Continuous renal replacement therapy: who, when, why, and how. Chest. 2019;155:626–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Karkar A. Continuous renal replacement therapy: Principles, modalities, and prescription. Saudi J Kidney Dis Transpl. 2019;30:1201–9. [DOI] [PubMed] [Google Scholar]
- [3].Khan F, Tritschler T, Kahn SR, et al. Venous thromboembolism. Lancet. 2021;398:64–77. [DOI] [PubMed] [Google Scholar]
- [4].Phillippe HM. Overview of venous thromboembolism. Am J Manag Care. 2017;23(20 Suppl):S376–82. [PubMed] [Google Scholar]
- [5].Bartholomew JR. Update on the management of venous thromboembolism. Cleve Clin J Med. 2017;84(12 Suppl 3):39–46. [DOI] [PubMed] [Google Scholar]
- [6].Witmer C, Raffini L. Treatment of venous thromboembolism in pediatric patients. Blood. 2020;135:335–43. [DOI] [PubMed] [Google Scholar]
- [7].Azad RK, Shulaev V. Metabolomics technology and bioinformatics for precision medicine. Brief Bioinform. 2019;20:1957–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Huang X, Liu S, Wu L, et al. High throughput single cell RNA sequencing, bioinformatics analysis and applications. Adv Exp Med Biol. 2018;1068:33–43. [DOI] [PubMed] [Google Scholar]
- [9].Reinson K, Kovacs-Nagy R, Õiglane-Shlik E, et al. Diverse phenotype in patients with complex I deficiency due to mutations in NDUFB11. Eur J Med Genet. 2019;62:103572. [DOI] [PubMed] [Google Scholar]
- [10].Nolfi-Donegan D, Braganza A, Shiva S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020;37:101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cohen D. Current issues in venous thromboembolism. Postgrad Med. 2021;133:1–2. [DOI] [PubMed] [Google Scholar]
- [12].Werth S. [Venous thromboembolism - update 2019]. Ther Umsch. 2018;75:496–501. [DOI] [PubMed] [Google Scholar]
- [13].Furuyama K, Kaneko K. Iron metabolism in erythroid cells and patients with congenital sideroblastic anemia. Int J Hematol. 2018;107:44–54. [DOI] [PubMed] [Google Scholar]
- [14].Shehata BM, Cundiff CA, Lee K, et al. Exome sequencing of patients with histiocytoid cardiomyopathy reveals a de novo NDUFB11 mutation that plays a role in the pathogenesis of histiocytoid cardiomyopathy. Am J Med Genet A. 2015;167:2114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Torraco A, Bianchi M, Verrigni D, et al. A novel mutation in NDUFB11 unveils a new clinical phenotype associated with lactic acidosis and sideroblastic anemia. Clin Genet. 2017;91:441–7. [DOI] [PubMed] [Google Scholar]
- [16].Petruzzella V, Tessa A, Torraco A, et al. The NDUFB11 gene is not a modifier in Leber hereditary optic neuropathy. Biochem Biophys Res Commun. 2007;355:181–7. [DOI] [PubMed] [Google Scholar]
- [17].van Rahden VA, Fernandez-Vizarra E, Alawi M, et al. Mutations in NDUFB11, encoding a complex I component of the mitochondrial respiratory chain, cause microphthalmia with linear skin defects syndrome. Am J Hum Genet. 2015;96:640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wilson DF. Oxidative phosphorylation: regulation and role in cellular and tissue metabolism. J Physiol. 2017;595:7023–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wu Z, Ho WS, Lu R. Targeting Mitochondrial Oxidative Phosphorylation in Glioblastoma Therapy. Neuromolecular Med. 2022;24:18–22. [DOI] [PubMed] [Google Scholar]
- [20].Braun HP. The Oxidative Phosphorylation system of the mitochondria in plants. Mitochondrion. 2020;53:66–75. [DOI] [PubMed] [Google Scholar]
- [21].Vardhana SA, Hwee MA, Berisa M, et al. Impaired mitochondrial oxidative phosphorylation limits the self-renewal of T cells exposed to persistent antigen. Nat Immunol. 2020;21:1022–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Boreel DF, Span PN, Heskamp S, et al. Targeting Oxidative Phosphorylation to Increase the Efficacy of Radio- and Immune-Combination Therapy. Clin Cancer Res. 2021;27:2970–8. [DOI] [PubMed] [Google Scholar]
- [23].Li T, Han J, Jia L, et al. PKM2 coordinates glycolysis with mitochondrial fusion and oxidative phosphorylation. Protein Cell. 2019;10:583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Evans KW, Yuca E, Scott SS, et al. Oxidative phosphorylation is a metabolic vulnerability in chemotherapy-resistant triple-negative breast cancer. Cancer Res. 2021;81:5572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bai X, Liao Y, Sun F, et al. Diurnal regulation of oxidative phosphorylation restricts hepatocyte proliferation and inflammation. Cell Reports. 2021;36:109659. [DOI] [PubMed] [Google Scholar]
- [26].Petrella G, Ciufolini G, Vago R, et al. The interplay between oxidative phosphorylation and glycolysis as a potential marker of bladder cancer progression. Int J Mol Sci . 2020;21:8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kim J, Yu L, Chen W, et al. Wild-Type p53 promotes cancer metabolic switch by inducing PUMA-dependent suppression of oxidative phosphorylation. Cancer Cell. 2019;35:191–203.e8. [DOI] [PubMed] [Google Scholar]