INTRODUCTION
The prevalence of obesity and metabolic dysfunction continues to rise in the United States. Bariatric or weight loss surgery (WLS) is a highly effective therapeutic option for sustained weight loss in patients with severe obesity or obesity with concomitant metabolic disease.1 However, a growing body of literature suggests an increased risk of alcohol use disorder (AUD) and alcohol-associated liver disease (ALD) in patients who have undergone bariatric procedures. In this review, we discuss the current literature on this phenomenon, theorized mechanisms, and potential risk mitigation strategies for bariatric surgery candidates.
THE FUNDAMENTALS OF BARIATRIC SURGERY
Weight loss in bariatric surgery is primarily achieved through a combination of restricting gastric capacity, disrupting nutrient absorption, and altering neurohormonal regulation. Sleeve gastrectomy (SG) and the Roux-en-Y gastric bypass (RYGB) are the 2 most commonly performed bariatric procedures worldwide.1 SG involves the creation of a small, tubular gastric reservoir, or “sleeve,” by resecting nearly 80% of the greater curvature of the stomach (Figure 1A). In contrast, RYGB requires more complex anatomical disruptions. First, the stomach is divided into a large distal portion and a significantly smaller proximal pouch. The small intestine is then divided ~50–150 cm from the Ligament of Treitz. The distal portion of the divided jejunum is anastomosed to the gastric pouch to create an alimentary or Roux limb through gastrojejunostomy. Meanwhile, the distal stomach remains connected to the duodenum and proximal jejunum which is then anastomosed through a jejunojejunostomy distally to the gastrojejunostomy creating a common channel where biliary and pancreatic enzymes can continue to facilitate digestion (Figure 1B).
FIGURE 1.
Anatomical depictions of various bariatric procedures. (A) Sleeve gastrectomy (B) Roux-en-Y gastric bypass (C) Vertical banded gastroplasty (D) Adjustable gastric banding.
Vertical banded gastroplasty was a historically common but now rarely performed procedure that involved the vertical partitioning of the proximal stomach into a smaller pouch with a restricted outlet bound by mesh or a prosthetic band (Figure 1C).2 Open or laparoscopic adjustable gastric banding (AGB) is another now rarely conducted procedure that involves the placement of a prosthetic band at the entrance of the stomach to create a restricted compartment (Figure 1D).
THEORIZED MECHANISMS OF INCREASED AUD AND ALD RISK AFTER BARIATRIC SURGERY
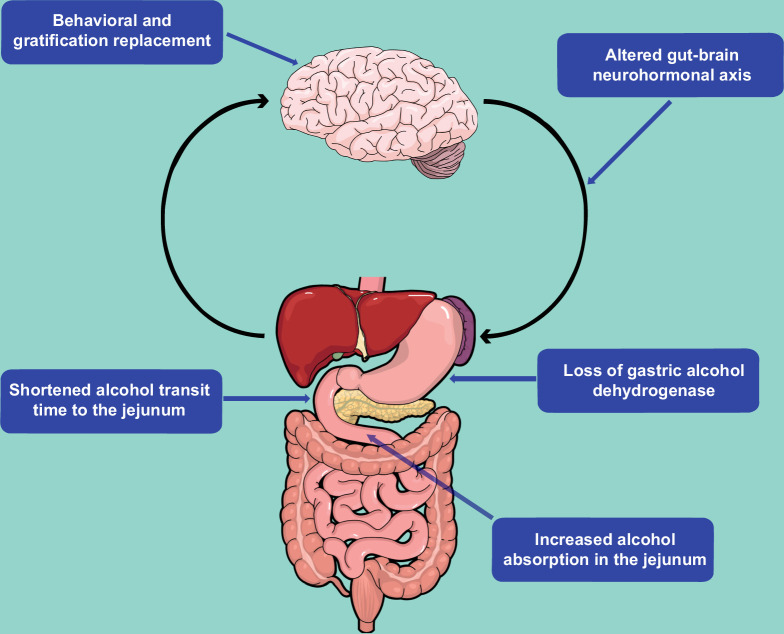
Several anatomic, physiologic, and psychosocial mechanisms have been proposed to explain the observed association between bariatric surgery and subsequent development of AUD and ALD (Figure 2). The “addiction transfer model” posits that one behavior, overeating, is replaced by another, alcohol use, as compensation for the mechanical inability to indulge in large volumes of food after bariatric surgery.3 While the construct of food addiction remains controversial, the proponents of this model suggest that a similar neurophysiological profile of the dopamine-associated reward pathways is triggered in both food-seeking and alcohol-seeking behavior as shown in various animal studies.4 Changes in the gut-brain neurohormonal axis due to anatomical disruption and subsequent downstream dysregulation of digestive hormones such as ghrelin, leptin, glucagon-like peptide-1, and peptide YY have also been proposed as a potential basis for this phenomenon.5
FIGURE 2.
Theorized mechanisms of increased AUD and ALD risk after bariatric surgery.
Changes in the pharmacokinetic processing of alcohol after bariatric procedures may further augment these behavioral changes by simulating surreptitious binge drinking and exposing patients to higher-than-expected doses of alcohol over a shorter time span. For example, several small physiologic studies have shown that subjects with a history of gastric bypass achieve a higher and faster peak blood alcohol concentration, often in as little as 2–10 minutes of consumption.6,7 Furthermore, it takes longer for the blood alcohol concentration to reach baseline in subjects with a history of bariatric surgery as compared to nonsurgical controls.8 Although direct evidence is lacking, these changes in alcohol metabolism are likely due to a dramatically reduced transit time from ingestion to alcohol absorption in the jejunum. Alcohol degradation may also be partially diminished by the loss of gastric alcohol dehydrogenase. Combined, the increased bioavailability of alcohol, altered neurohormonal reward pathways, and a psychosocial predisposition for harmful behavior are thought to elevate the risk of AUD and ALD after bariatric surgery.
EVIDENCE OF ELEVATED AUD RISK AFTER BARIATRIC SURGERY
An anecdotal rise in AUD risk after bariatric surgery has long been suggested. Although robust evidence is lacking, data from several observational studies over the past decade now support this theorized association (Table 1).
TABLE 1.
Summary of literature on alcohol use and alcohol use disorder after bariatric surgery
| Year | Group | Study type | Intervention (n) | Main outcomes | Alcohol use disorder–related findings |
|---|---|---|---|---|---|
| 2012 | King et al9 | Prospective cohort | RYGB (1360) AGB (490) RYGB + Band (30) SG (50) BPD/DS (15) |
Drinks per day and frequency of alcohol consumption by AUDIT score Evidence of AUD by AUDIT score |
• Drinks per day significantly greater preop and at 2 y postop than at 1-y postop in the RYGB group • Frequency of consumption and AUD significantly greater 2 y postop compared to preop or 1-y postop in the RYGB group • No significant difference in the frequency of consumption or AUD noted in the AGB group |
| 2013 | Conason et al10 | Prospective cohort | RYGB (100) AGB (55) |
Frequency of substance use and self-reported problems by CBQ | • Composite substance use initially decreased from preop to 1-mo postop, but significantly increased from preop to 24 mo postop • Frequency of alcohol consumption significantly lower at 1 and 3 mo postop compared to preop but significantly higher 24 mo postop than preop in the RYGB group • No significant difference of alcohol consumption noted in the AGB group |
| 2013 | Svensson et al11 | Prospective controlled cohort | VBG (1369) Banding (376) GB (265) Matched controls (2037) |
Alcohol consumption and self-reported alcohol problems by SOS dietary questionnaire | • Percentage of individuals reporting at least WHO medium-riska alcohol consumption was highest among the GB group at all time points • Adjusted risk of at least WHO medium-risk alcohol consumptiona and self-reported alcohol problems as compared to controls was highest in the GB and VBG groups but not in the banding group |
| 2013 | Östlund et al12 | Retrospective population-based cohort | GB (4161) VBG or banding (6954) |
Number of hospitalizations for depression, alcohol abuse, other substance abuse, and attempted suicide | • Approximately double the risk of alcohol abuse–related hospitalizations in the GB group |
| 2017 | King et al13 | Prospective cohort | RYGB (1481) AGB (522) |
Drinks per day and frequency of alcohol consumption by AUDIT score Evidence of AUD by AUDIT score Illicit substance use and treatment |
• Nearly doubled the prevalence of “regular drinking” in both the RYGB and AGB groups from preop to 7 y postop • Prevalence of incident AUD more than doubled in the RYGB group but not the AGB group |
| 2019 | Strømmen et al14 | Retrospective population-based cohort | RYGB (8196) SG (2012) |
Hospitalizations for alcohol-associated diagnoses | • No significant difference seen in the incidence rate of alcohol-associated diagnoses between the RYGB and SG groups |
| 2020 | Maciejewski et al15 | Retrospective cohort study | RYGB (924) SG (1684) RYGB matched control (8038) SG matched controls (1455) |
Alcohol use by AUDIT-C | • In patients without unhealthy alcohol use at baseline, the probability of unhealthy alcohol use increased at both 2 and 8 y postop after both SG and RYGB compared to nonsurgical controls • In patients with unhealthy alcohol use at baseline, the probability of unhealthy alcohol use increased in only the RYGB group |
| 2021 | Mellinger et al16 | Population-based cross-sectional study using the MarketScan insurance claims database | RYGB (102,385) SG (64,687) AGB (27,058) Unclassified (209,058) |
Alcoholic cirrhosis and alcohol misuse | • Significantly elevated the risk of alcohol misuse after RYGB but not AGB |
| 2022 | White et al17 | Prospective cohort | RYGB (155) SG (62) |
Frequency of alcohol use, AUD, risk of alcohol use, and alcohol-associated problems by AUDIT and AUDIT-C | • Similar increase in quantity and frequency of alcohol consumption in both RYGB and SG from preop to postop |
| 2023 | Mahmud et al18 | Retrospective controlled cohort | RYGB (1854) SG (4211) GB (265) Weigh management program (1364) |
Time to AUD-related hospitalization Time to all-cause mortality |
• Significantly elevated adjusted HR of AUD-related hospitalization in the RYGB vs. weight management and SG groups • No risk elevation noted in the SG as compared to the weight management group • Risk noted to be greatest at lower postop BMI • RYGB group had lowest proportion of AUDIT-C score <1 at all time points |
| 2023 | Alvarado-Tapias et al19 | Population-based cross-sectional study using the NIS database | Bariatric surgeryb (537,757) Abdominal surgery (537,757)c |
Prevalence of ALD or psychiatric disorders associated with AUD | • Significantly elevated prevalence of AUD in patients in the bariatric surgery group over time as compared to the abdominal surgery group |
World Health Organization (WHO) medium-risk alcohol consumption=40–60 g pure alcohol consumption per day in men or 20–40 grams in women.
Patients only classified as bariatric surgery or abdominal surgery for analysis. However, 46.88% noted to have undergone RYGB, 33.76% SG, 10.66% AGB, and 8.69% an “other” form of bariatric surgery.
Numbers based on propensity matching.
Abbreviations: AGB, adjustable gastric banding; AUD, alcohol use disorder; AUDIT, Alcohol Use Disorders Identification Test; BMI, body mass index; BPD/DS, biliopancreatic diversion with duodenal switch; CBQ, Compulsive Behaviors Questionnaire; GB, gastric bypass (unspecified); NIS, National Inpatient Sample; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy; SOS, Swedish Obese Subjects study; VBG, vertical banded gastroplasty; WHO, World Health Organization.
RYGB, in particular, appears to confer the greatest risk for AUD. The Longitudinal Assessment of Bariatric Surgery-2 study was one of the first and largest studies to examine alcohol consumption and frequency in patients who primarily underwent RYGB or AGB procedures. Patients with a history of RYGB reported an initial decrease or no change in drinks consumed per day, frequency of use, or prevalence of AUD during the first postoperative year followed by a significant increase by the second postoperative year as compared to a preoperative baseline (AUD prevalence: 7.0% preoperatively, 7.9% at 1-year postop, and 10.7% at 2 years postop; p<0.01). No statistically significant changes in behavior or prevalence of AUD were noted in patients who underwent banding (AUD prevalence: 9.3% preoperatively, 5.6% at 1-year postop, and 7.0% at 2 years postop; p=0.24). Those with a history of RYGB were twice as likely to develop AUD than those who underwent AGB (OR, 2.1; 95% CI, 1.4–3.1; p<0.001).9 Subsequent, longer-term studies have shown that this disproportionate increase in volume and frequency of alcohol consumption persists for 5–10 years and beyond after RYGB.10,11,13 The risk of AUD and AUD-related hospitalizations also increases in the years following RYGB when compared to medical controls (HR, 1.7; 95% CI, 1.20–2.41; p=0.003) and SG (HR, 1.98; 95% CI, 1.55–2.53; p<0 .001).12,18
Over the past decade, SG has now replaced RYGB as the predominantly conducted bariatric procedure in the United States. While initial data on AUD after SG were mixed due to inadequate representation in studied populations, newer studies show a similarly increased risk of unhealthy alcohol use among American veterans, American teens, and adults in Norway who have undergone either RYGB or SG.14,15,17 Head-to-head comparisons between SG and AGB or vertical banded gastroplasty are limited due to the relative obsolescence of the latter.
EVIDENCE OF ELEVATED ALD RISK AFTER BARIATRIC SURGERY
While the benefits of WLS in the regression of steatotic liver disease are well established, data on the effect of WLS on ALD are mixed and limited by study size, design, and heterogeneity of studied outcomes.20 Several recent studies, however, suggest that patients who undergo certain weight loss procedures are at greater risk of adverse outcomes related to ALD, specifically alcohol-associated hepatitis (AH) and alcohol-associated cirrhosis (Table 2).
TABLE 2.
Summary of literature on alcohol-associated liver diseases after bariatric surgery
| Year | Group | Study type | Population and intervention (n) | Main outcomes | ALD-related findings |
|---|---|---|---|---|---|
| 2021 | Mellinger et al16 | Population-based cross-sectional study using the MarketScan insurance claims database | RYGB (102,385) SG (64,687) AGB (27,058) Unclassified (209,058) |
Alcohol-associated cirrhosis and alcohol misuse | • Significantly decreased risk of alcohol-associated cirrhosis after SG (HR=0.40) or AGB (HR=0.43) but no association with RYGB (HR=0.98) in the short term • Possibly elevated risk of alcohol-associated cirrhosis in the long term although unable to stratify by procedure type (HR=1.31) |
| 2021 | Fipps et al21 | Multicenter retrospective clinical cohort | Adults who underwent ALD liver transplantation and bariatric surgery: RYGB (16) AGB (2) |
Descriptive characteristics including demographics, anthropometrics, and surgery type | • Patients who underwent ALD liver transplantation and had a history of bariatric surgery were younger, had a higher MELD, female sex, and comorbid mood/anxiety disorders as compared to patients without a history of bariatric surgery |
| 2022 | Yarra et al22 | Population-based cross-sectional using the NIS database | Adults hospitalized for alcohol-associated cirrhosis with prior: RYGB (2542) Controls (7626)b |
Discharge diagnosis of alcoholic hepatitis, acute liver failure, and mortality | • Increased risk of AH (OR, 1.14) and HE (OR, 1.42) among the RYGB group but not ACLF or in-hospital mortality |
| 2023 | Melkebeke et al23 | Single-center prospective cohort | Adults hospitalized for biopsy-proven severe alcoholic hepatitis with prior: RYGB, BPD, or AGB (28) Controls (130) |
Baseline characteristics, response to steroids, measures of disease severity, and mortality | • Patients with a history of bariatric surgery were nearly a decade younger at presentation for sAH as compared to controls • No difference noted among the groups in terms of disease severity, therapy response, or mortality |
| 2023 | Wang et al24 | Systematic review and meta-analysis | 18 studies of adults with obesity who underwent various bariatric procedures compared to those who did not Bariatric surgery (16,800,287) Controls (10,595,752) |
Adverse liver disease including nonalcohol-associated cirrhosis, alcohol-associated cirrhosis, and liver cancer | • Obese patients who underwent bariatric surgery experienced significantly fewer adverse liver outcomes, including nonalcoholic cirrhosis and liver cancer, than those who did not undergo bariatric surgery (HR=0.07) • However, in a meta-analysis of studies evaluating alcoholic cirrhosis bariatric surgery was associated with an increased risk of developing alcoholic cirrhosis (HR=1.32) |
| 2023 | Anugwom et al25 | Single-center retrospective cohort | Adults hospitalized for clinically diagnosed alcohol-associated hepatitis with prior: RYGB (153) Controls (2481) |
Inpatient mortality | • No difference in inpatient mortality • Increased risk of 30-day readmission, overall mortality, and development of cirrhosis in RYGB group |
| 2023 | Alvarado-Tapias et al19 | Population-based cross-sectional study using the NIS database | Bariatric surgerya (537,757) Abdominal surgery (537,757)b |
Prevalence of ALD or psychiatric disorders associated with AUD | • Increased risk of ALD noted in the bariatric surgery group vs. the control surgery group (OR, 1.29) • Even greater risk of ALD in those with concomitant AUD (OR, 2.47) • Increased risk of cirrhosis in the bariatric surgery group vs. the control surgery group (OR, 1.39) |
Patients only classified as bariatric surgery or abdominal surgery for analysis. However, 46.88% noted to have undergone RYGB, 33.76% SG, 10.66% AGB, and 8.69% an “other” form of bariatric surgery.
Numbers based on propensity matching.
Abbreviations: ACLF, acute liver failure; AGB, adjustable gastric banding; AH, alcohol-associated hepatitis; ALD, alcohol-associated liver disease; AUD, alcohol use disorder; BPD/DS, biliopancreatic diversion with duodenal switch; MELD, Model of End Stage Liver Disease; NIS, National Inpatient Sample; RYGB, Roux-en-Y gastric bypass; sAH, severe alcohol-associated hepatitis; SG, sleeve gastrectomy.
A systematic review of studies exploring ALD and cirrhosis in obese patients noted a significantly lower risk of liver cancer and metabolic dysfunction–associated cirrhosis but an elevated risk of alcohol-associated cirrhosis in patients with a history of bariatric surgery compared to nonsurgical controls (HR, 1.32, 95% CI, 1.35–1.59).24 This increased risk of cirrhosis after bariatric surgery is further supported by 2 large population-based cross-sectional studies utilizing the National Inpatient Sample and MarketScan insurance claims databases.16,19 Outcomes may differ by type of bariatric procedure given lower hazard ratios of alcohol-associated cirrhosis in patients who underwent SG or banding.16 Among patients admitted for alcohol-associated cirrhosis, those with a history of bariatric surgery, specifically RYGB, may have a higher risk of HE and concomitant AH.22
Data on alcohol-associated hepatitis outcomes remain sparse. Two single-center cohort studies suggest that short-term mortality, disease severity, or response to steroids are not impacted by a history of WLS.23,25 However, a history of WLS in those admitted for AH experienced an increased risk of overall mortality (31.4% vs. 24%, p=0.03), 30-day readmission (20.3% vs. 11.7%, p<0.01), and progression to cirrhosis (37.5% vs. 20.9%, p<0.01).25
Studies on ALD-related liver transplant outcomes after bariatric surgery are also lacking. The sole retrospective cohort study (1416 LT recipients including 18 subjects who had a history of bariatric surgery) published to date showed no impact of WLS on mortality after liver transplantation. However, patients admitted for alcohol-associated liver transplant with a history of bariatric surgery were noted to have higher MELD scores (median MELD of 22 in the bariatric surgery group vs. 18 in the nonbariatric surgery group, p=0.02), younger age at transplantation (median age 50 vs. 57 y, p=0.003), and comorbid mood/anxiety disorders.21
PROPOSED MITIGATION STRATEGIES
Informed by anecdotal evidence of increased substance misuse and psychiatric comorbidity, professional society guidelines have long supported a comprehensive, multidisciplinary approach to preoperative evaluation of patients referred for bariatric procedures. While alcohol screening is typically conducted during this evaluation, only recent guidelines offer additional recommendations on risk mitigation against the development of AUD after bariatric surgery. For example, a 2021 position statement by the American Society for Metabolic and Bariatric Surgery (ASMBS) acknowledges the mounting evidence of increased de novo AUD after bariatric surgery and suggests:
identifying patients at greatest risk of developing postsurgical substance use disorders;
educating patients on these risks, including education on the expected postoperative changes in alcohol metabolism; and
monitoring patients after their procedures.26
Furthermore, while most surgical programs already consider active AUD a contraindication for WLS,27 a 2021 update to the Enhanced Recovery after Surgery Society guidelines adds that a minimum period of 1–2 years of sobriety should be maintained before surgery.28
Despite this growing awareness of AUD risk after WLS, the risk of ALD is underacknowledged and specific risk mitigation strategies are absent from official guidelines. Additional research with larger, prospective studies of contemporary bariatric procedures is needed to better identify risk factors for patients who undergo WLS. One example of a general risk evaluation framework suggested by Mendoza et al29 that providers could consider is:
Patients with obesity and multiple metabolic risk factors should be evaluated for comorbid liver fibrosis by imaging (elastography) or noninvasive testing (eg, fibrosis-4 index for liver fibrosis and/or enhanced liver fibrosis test).
Patients who engage in harmful alcohol consumption should also undergo liver fibrosis testing and be referred to an alcohol cessation program.
In patients with the greatest risk of AUD or ALD, a restrictive bariatric procedure should be offered over bypass surgery.
After bariatric surgery, patients should receive prolonged, multiyear, postop monitoring through alcohol screening tools, biochemical liver tests, and if needed, alcohol metabolite testing.
Patients should also receive long-term psychosocial screening and support during this monitoring period.
CONCLUSIONS
Overall, the preponderance of evidence shows that bariatric surgery is highly beneficial in preventing cardiometabolic and hepatobiliary adverse events in patients with morbid obesity.20,30 However, there is growing support that gastric bypass and SG may increase the risk of AUD. Although data on the risk of ALD after WLS are less established, sufficient evidence exists to merit caution in evaluating candidates, selecting appropriate procedures, prolonging postop monitoring periods, or forgoing WLS altogether in favor of medical management in the highest risk patients (eg, glucagon-like peptide-1 analogs which are now commonly prescribed for therapeutic weight loss and may also reduce alcohol consumption in obese patients).31
Footnotes
Abbreviations: AGB, adjustable gastric banding; AH, alcohol-associated hepatitis; ALD, alcohol-associated liver disease; ASMBS, American Society for Metabolic and Bariatric Surgery; AUD, alcohol use disorder; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy; WLS, weight loss surgery.
Contributor Information
Rahul Grover, Email: rgrover1994@gmail.com.
Brett E. Fortune, Email: bfortune@montefiore.org.
Clara Y. Tow, Email: ctow@montefiore.org.
FUNDING INFORMATION
Financial support and sponsorship: none
CONFLICTS OF INTEREST
Brett E. Fortune consults for WL Gore and Associates. The remaining authors have no conflicts to report.
REFERENCES
- 1.Eisenberg D, Shikora SA, Aarts E, Aminian A, Angrisani L, Cohen RV, et al. 2022 American Society of Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) indications for metabolic and bariatric surgery. Obes Surg. 2023;33:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim RB. Bariatric procedures for the management of severe obesity. UpToDate Post, Tod. W2023. [Google Scholar]
- 3.Steffen KJ, Engel SG, Wonderlich JA, Pollert GA, Sondag C. Alcohol and other addictive disorders following bariatric surgery: Prevalence, risk factors and possible etiologies. Eur Eat Disord Rev. 2015;23:442–450. [DOI] [PubMed] [Google Scholar]
- 4.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orellana ER, Covasa M, Hajnal A. Neuro-hormonal mechanisms underlying changes in reward related behaviors following weight loss surgery: Potential pharmacological targets. Biochem Pharmacol. 2019;164:106–114. [DOI] [PubMed] [Google Scholar]
- 6.Klockhoff H, Näslund I, Jones AW. Faster absorption of ethanol and higher peak concentration in women after gastric bypass surgery. Br J Clin Pharmacol. 2002;54:587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steffen KJ, Engel SG, Pollert GA, Li C, Mitchell JE. Blood alcohol concentrations rise rapidly and dramatically after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2013;9:470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagedorn JC, Encarnacion B, Brat GA, Morton JM. Does gastric bypass alter alcohol metabolism? Surg Obes Relat Dis. 2007;3:543–548; discussion 548. [DOI] [PubMed] [Google Scholar]
- 9.King WC, Chen JY, Mitchell JE, Kalarchian MA, Steffen KJ, Engel SG, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012;307:2516–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conason A, Teixeira J, Hsu C-H, Puma L, Knafo D, Geliebter A. Substance use following bariatric weight loss surgery. JAMA Surg. 2013;148:145–150. [DOI] [PubMed] [Google Scholar]
- 11.Svensson P-A, Anveden Å, Romeo S, Peltonen M, Ahlin S, Burza MA, et al. Alcohol consumption and alcohol problems after bariatric surgery in the Swedish obese subjects study. Obesity. 2013;21:2444–2451. [DOI] [PubMed] [Google Scholar]
- 12.Östlund MP, Backman O, Marsk R, Stockeld D, Lagergren J, Rasmussen F, et al. Increased admission for alcohol dependence after gastric bypass surgery compared with restrictive bariatric surgery. JAMA Surg. 2013;148:374–377. [DOI] [PubMed] [Google Scholar]
- 13.King WC, Chen JY, Courcoulas AP, Dakin GF, Engel SG, Flum DR, et al. Alcohol and other substance use after bariatric surgery: Prospective evidence from a U.S. multicenter cohort study. Surg Obes Relat Dis. 2017;13:1392–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strømmen M, Bakken IJ, Klöckner C, Sandvik J, Kulseng B, Holen A. Diagnoses related to abuse of alcohol and addictive substances after gastric bypass and sleeve gastrectomy: A nation-wide registry study from Norway. Surg Obes Relat Dis. 2020;16:464–470. [DOI] [PubMed] [Google Scholar]
- 15.Maciejewski ML, Smith VA, Berkowitz TSZ, Arterburn DE, Mitchell JE, Olsen MK, et al. Association of bariatric surgical procedures with changes in unhealthy alcohol use among US Veterans. JAMA Netw Open. 2020;3:e2028117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mellinger JL, Shedden K, Winder GS, Fernandez AC, Lee BP, Waljee J, et al. Bariatric surgery and the risk of alcohol-related cirrhosis and alcohol misuse. Liver Int. 2021;41:1012–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White GE, Boles RE, Courcoulas AP, Yanovski SZ, Zeller MH, Jenkins TM, et al. A prospective cohort of alcohol use and alcohol-related problems before and after metabolic and bariatric surgery in adolescents. Ann Surg. 2022;278:e519–e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahmud N, Panchal S, Abu-Gazala S, Serper M, Lewis JD, Kaplan DE. Association between bariatric surgery and alcohol use-related hospitalization and all-cause mortality in a Veterans Affairs cohort. JAMA Surg. 2023;158:162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarado-Tapias E, Marti-Aguado D, Kennedy K, Fernández-Carrillo C, Ventura-Cots M, Morales-Arraez D, et al. Bariatric surgery is associated with alcohol-related liver disease and psychiatric disorders associated with AUD. Obes Surg. 2023;33:1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patton H, Heimbach J, McCullough A. AGA clinical practice update on bariatric surgery in cirrhosis: Expert review. Clin Gastroenterol Hepatol. 2021;19:436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fipps DC, Goetze RE, Clark MM, Mara K, Watt KD, Jowsey-Gregoire SG, et al. Liver transplantation after bariatric surgery: A clinical cohort study. Obes Surg. 2021;31:3700–3706. [DOI] [PubMed] [Google Scholar]
- 22.Yarra P, Dunn W, Younossi Z, Kuo YF, Singal AK. Association of previous gastric bypass surgery and patient outcomes in alcohol-associated cirrhosis hospitalizations. Dig Dis Sci. 2023;68:1026–1034. [DOI] [PubMed] [Google Scholar]
- 23.Van Melkebeke L, Broekhoven AGC, Ostyn T, Korf H, Coenraad MJ, Vangoitsenhoven R, et al. Patients with a history of bariatric surgery are 8 years younger at presentation with severe alcoholic hepatitis. Obes Surg. 2023;33:284–292. [DOI] [PubMed] [Google Scholar]
- 24.Wang G, Huang Y, Yang H, Lin H, Zhou S, Qian J. Impacts of bariatric surgery on adverse liver outcomes: A systematic review and meta-analysis. Surg Obes Relat Dis. 2022;19:717–26. [DOI] [PubMed] [Google Scholar]
- 25.Anugwom C, Thomson M, Freese RL, Lake JR, Lim N. Lower survival and higher rates of cirrhosis in patients with ROUX-EN-Y gastric bypass hospitalised with alcohol-associated hepatitis. BMJ Open Gastroenterol. 2023;10:e001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carter J, Chang J, Birriel TJ, Moustarah F, Sogg S, Goodpaster K, et al. ASMBS position statement on preoperative patient optimization before metabolic and bariatric surgery. Surg Obes Relat Dis. 2021;17:1956–1976. [DOI] [PubMed] [Google Scholar]
- 27.Parikh M, Johnson JM, Ballem N. ASMBS position statement on alcohol use before and after bariatric surgery. Surg Obes Relat Dis. 2016;12:225–230. [DOI] [PubMed] [Google Scholar]
- 28.Stenberg E, Dos Reis Falcão LF, O'Kane M, Liem R, Pournaras DJ, Salminen P, et al. Guidelines for perioperative care in bariatric surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations: A 2021 update. World J Surg. 2022;46:729–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendoza YP, Becchetti C, Watt KD, Berzigotti A. Risks and rewards of bariatric surgery in advanced chronic liver diseases. Semin Liver Dis. 2021;41:448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doumouras AG, Wong JA, Paterson JM, Lee Y, Sivapathasundaram B, Tarride JE, et al. Bariatric surgery and cardiovascular outcomes in patients with obesity and cardiovascular disease: A population-based retrospective cohort study. Circulation. 2021;143:1468–1480. [DOI] [PubMed] [Google Scholar]
- 31.Chuong V, Farokhnia M, Khom S, Pince CL, Elvig SK, Vlkolinsky R, et al. The glucagon-like peptide-1 (GLP-1) analogue semaglutide reduces alcohol drinking and modulates central GABA neurotransmission. JCI Insight. 2023;8:e170671. [DOI] [PMC free article] [PubMed] [Google Scholar]