Abstract
Objective
The purpose of present study was to comprehensívely explore the efficacy and safety of prothrombín complex concentrate (PCC) to treat massíve bleedíng in patíents undergoing cardiac surgery.
Methods
PubMed®, Embase, and Cochrane Líbrary databases were searched for studíes ínvestigating PCC administratíon duríng cardiac surgery published before September 10, 2022. Mean dífference (MD) wíth 95% confidence interval (CI) was applíed to analyze continuous data, and dichotomous data were analyzed as risk ratio (RR) with 95% CI.
Results
Twelve studies were included in the meta-analysis. Compared with other non-PCC treatment regimens, PCC was not assocíated with elevated mortality (RR=1.18, 95% CI=0.86–1.60, P=0.30, I2=0%), shorter hospital stay (MD=-2.17 days; 95% CI=-5.62–1.28, P=0.22, I2=91%), reduced total thoracic drainage (MD=-67.94 ml, 95% CI=-239.52–103.65, P=0.44, I2=91%), thromboembolíc events (RR=1.10, 95% CI=0.74–1.65, P=0.63, I2=39%), increase ín atríal fibríllatíon events (RR=0.73, 95% CI=0.52–1.05, P=0.24, I2=29%), and myocardial infarction (RR=1.10, 95% CI=0.80–1.51, P=0.57, I2=81%). However, PCC use was associated with reduced intensive care unit length of stay (MD=-0.81 days, 95% CI=-1.48– -0.13, P=0.02, I2=0%), bleeding (MD=-248.67 ml, 95% CI=-465.36– -31.97, P=0.02, I2=84%), and intra-aortic balloon pump/extracorporeal membrane oxygenation (RR=0.65, 95% CI=0.42–0.996, P=0.05, I2=0%) when compared with non-PCC treatment regimens.
Conclusion
The use of PCC in cardiac surgery did not correlate with mortality, length of hospítal stay, thoracic drainage, atríal fibríllatíon, myocardíal ínfarction, and thromboembolíc events. However, PCC sígnificantly improved postoperatíve intensíve care unít length of stay, bleedíng, and intra-aortic balloon pump/ extracorporeal membrane oxygenation outcomes ín patients undergoing cardíac surgery.
Keywords: Cardiac Surgery, Prothrombin Complex Concentrate, Hemorrhage, Mortality, Myocardial Infarction, Meta-Analysís, Systematic Review
INTRODUCTION
Prothrombin complex concentrate (PCC) is a mixture of various coagulation factors and other plasma proteins extracted from the plasma supernatant after precipitation. Although originally used to treat hemophilia, PCC is now more commonly recommended to reverse massive bleeding induced by anticoagulants such as warfarin and the newer direct oral anticoagulants[1,2].
The annual incidence of warfarin-related major bleeding is approximately 1 to 3%, and the case fatality rate is approximately 11%[3]. Patients undergoing cardiac surgery and cardiopulmonary bypass (CPB) frequently experience bleeding and coagulation dysfunction[4,5], necessitating massive blood transfusions, as well as significantly increased mortality. Therefore, active and effective bleeding management critically impacts the prognosis of patients undergoing cardiac surgery. PCC is considered a potentially effective alternative to fresh frozen plasma (FFP) in patients experiencing massive bleeding after cardiac surgery[6,7,8,9]. Drug regimens play a positive role in surgical hemostasis, and common drugs promoting coagulation system functions include PCC, FFP, and recombinant factor VIIa (rFVIIa). Studies have shown that although FFP can be used to treat hemostasis, it could significantly increase vascular volume and lead to decompensated heart failure or transfusion-related lung injury. Therefore, FFP is rarely used for anticoagulant reversal in patients with atrial fibrillation, cardiovascular disease, and ventricular dysfunction[10]. In addition, the use of rFVIIa is reportedly associated with an increased risk of thrombotic events[11]. PCC administration was more effective than FFP in patients who experienced significant bleeding during cardiac surgery, reducing perioperative blood transfusions[12]. In addition, studies have found that a low PCC dosage has been shown to significantly reduce bleeding post-CPB[9]. Furthermore, PCC is superior to FFP to treat bleeding in patients presenting the need for emergency or invasive warfarin reversal[13]. Although PCC exhibits a superior ability to control massive bleeding, the effectiveness and safety of PCC need to be further clarified[14]. Therefore, the purpose of the present study was to comprehensively explore the efficacy and safety of PCC to treat massive bleeding in patients undergoing cardiac surgery by using more recently conducted or published studies.
METHODS
The guidelines from the Preferred Reporting Items for Systematic Review and Meta-Analyses (or PRISMA) statement were employed for this study.
Search Strategy
All studies investigating PCC use during cardiac surgery were obtained by searching the PubMed®, Embase, and Cochrane Library databases for articles published before September 10, 2022. The search terms were as follows: prothrombin complex concentrate, factor IX, factor 9, autoprothrombin II, Christmas factor, plasma thromboplastin component, cardiac surgical procedures, thoracic surgery, heart surgery, and cardiac surgery. Detailed search strategies were shown in Supplementary Method 1. Two reviewers (JPL and YL) independently assessed abstracts and potentially eligible articles identified during the literature selection, and discrepancies were resolved through discussion. The third reviewer (FZ) was consulted in the case of any disagreements.
Inclusion and Exclusion Criteria
Inclusion criteria were as follows: (a) population: adult patients undergoing cardiac surgery; (b) intervention: three- or four-factor PCC; (c) control: non-PCC patients, including FFP, rFVIIa, or no treatment; (d) outcomes: mortality, length of hospital stay, intensive care unit (ICU) stay, blood loss, thoracic drainage, thromboembolic events, and intra-aortic balloon pump (IABP)/ extracorporeal membrane oxygenation (ECMO); the mortality rate was the all-cause mortality rate within 90 days after surgery; if there were multiple time points, the longest time node data within 90 days were selected; except for the length of hospital stay and length of ICU stay, it was 24 hours after surgery; (e) study type: randomized controlled trial (RCT), cohort study, or case-control study.
Exclusion criteria were as follows: repetitive studies, unavailability of data on contacting authors, cardiac surgery without thoracotomy, case reports, letters, and meeting abstracts.
Data Extraction
Based on inclusion and exclusion criteria, two authors independently selected studies for inclusion by reading abstracts and full-text articles. In the event of any disagreement due to inconsistent understanding, a consensus was reached by arbitration and discussion with a third investigator. The following information was extracted from all trials: first author, age, sex, race, sample, body mass index, previous history of heart disease, type of surgery, PCC, and non-PCC.
Quality Assessment
Regarding the quality of the included studies, two reviewers (JPL and YL) independently assessed the quality of RCT according to criteria reported in the Cochrane Handbook[15]. The included studies were assessed based on the following items scored as high, low, and unclear risks: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other sources of bias. The methodological quality of included cohort studies or case-control studies were assessed using the Newcastle-Ottawa Scale (NOS), independently evaluated by two commentators. Studies that achieved six or more stars on the modified NOS were considered high quality. Any disagreement was resolved by discussion and consultation with a third author (FZ) if necessary.
Statistical Analysis
Mean difference (MD) with 95% confidence intervals (CIs) was applied to analyze continuous data, and dichotomous data were analyzed as risk ratios (RRs) with 95% CIs. The I2 statistics were used to assess the heterogeneity of each analysis. I2 was calculated from basic data to represent the size of heterogeneity. A value of 0% represents no heterogeneity, and larger values suggest increased heterogeneity. A fixed-effects model was employed when I2 < 40%, whereas a random-effects model was used when I2 ≥ 40%. All statistical methods were performed according to the Cochrane Handbook[15], and all statistical analyses were performed using RevMan 5.4.1.
RESULTS
Characteristics of Included Trials
Our systematic literature search identified 2,100 potential publications (Figure 1). Based on inclusion and exclusion criteria, we obtained quantitative data for the present meta-analysis by reading all titles, abstracts, and full-text evaluations. Subsequently, 12 studies[6,7,8,16,17,18,19,20,21,22,23,24] assessing 1,799 participants were included (Table 1).
Fig. 1.
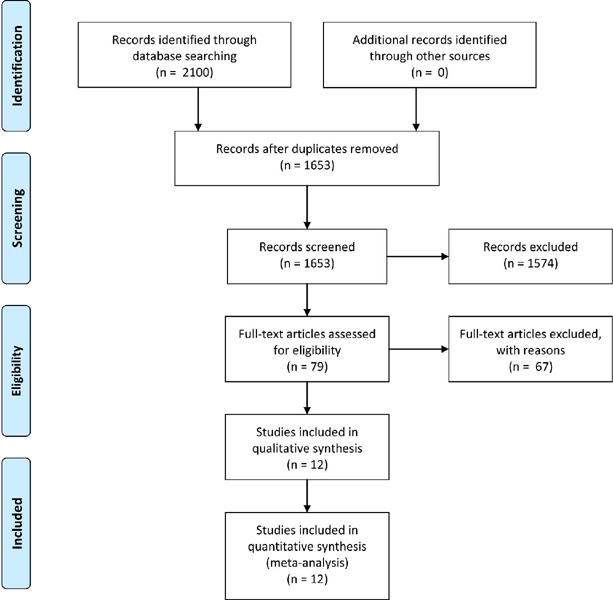
Preferred Reporting Items for Systematic Review and Meta-Analyses (or PRISMA) flow diagram.
Table 1.
Basic information regarding included studies.
| Study, year | Age (years) (PCC/Non-PCC) | Female (PCC/Non-PCC) | Sample (PCC/Non-PCC) | BMI (PCC/Non-PCC) | Types of cardiac surgery | PCC group | Non-PCC group |
|---|---|---|---|---|---|---|---|
| Biancari, 2019 | 65.9 (6.7)/65.3 (9.3) | 14-nov. | 101/101 | 27.4 (4.3)/27.1 (3.9) | CABG | PCC: initial dose was 1,000 IU (2,000-3,000 IU) | Fresh frozen plasma with non-PCC |
| Bradford, 2015 | 68/69 | 3-mai. | 41/27 | NA | CABG | PCC: 500 units, every 30 min., maximum dose of 25 units/kg | Non-PCC |
| Cappabianca, 2016 | 69.7 (10.6)/69.2 (11.6) | 91/91 | 225/225 | 24 (4.9)/25 (4.7) | CABG, valve surgery, and proximal aortic procedures | PCC: 500 IU | Non-PCC |
| Fitzgerald, 2018 | 61 (46-70)/60 (50-69) | 45/40 | 117/117 | NA | Valve or isolated CABG | PCC: 15-25 lU/kg in 1000 IU increments | Frozen plasma with non-PCC |
| Green, 2020 | 69 (63-73)/66 (57-74) | 9-set. | 21/21 | 27 (6)/29 (5) | Valve only, major aortic valve only, CABG plus valve, complex/combined procedure | <60 kg: 500 IU; 61-90 kg: 1000 IU; and > 90 kg: 1500 IU | Fresh frozen plasma with non-PCC |
| Harper, 2018 | 60.9 (17.4)/58.5 (19.7) | 17/18 | 53/53 | NA | Mechanical circulatory support, valve transplant, aorta resection plus CABG/valve (s), aorta resection, CABG plus valve (s), congenital/ conduit, CABG, pericardiectomy | Factor IX complex | rFVIIa |
| Harris, 2020 | 65.7 (59-77)/66.9 (59-73) | mai.-19 | 19/60 | 28.7 (24.5-34.3)/29.7 (25.1-33.3) | Isolated CABG, isolated valve | 4-factor PCC: 11.5 (5.3-39.3) units/kg | Non-4-factor PCC |
| Karkouti, 2020 | 66 (50-73)/67 (55-74) | 14/14 | 54/47 | 23.6 (4.5)/23.1 (4.7) | Cardiac surgery | PCC: 1500 IU for patients weighing ≤ 60 kg and 2000 IU for patients weighing > 60 kg | Frozen plasma with non-PCC |
| Ortmann, 2014 | 61 (13)/62 (13) | 19/18 | 45/55 | 28.8(6.1)/27.3(5.0) | Complex cardiac surgery | PCC:15 IU/kg to the nearest 250 IU vial | Fresh frozen plasma with non-PCC |
| Tanaka, 2013 | 55.5 (16.6)/57.8 (12.6) | 15/30 | 50/100 | NA | Valve, aortic, transplant or ventricular assist device implantation | 3-factor PCC: 25 lU/kg, Bebulin or Profilnine upon availability | rFVIIa |
| Zweng, 2018 | 66.9 (12.18)/69.4 (10.5) | 25/34 | 80/80 | NA | CABG, valve, other | The amount of PCC: 500 to 9000 IU | Fresh frozen plasma with non-PCC |
| Alyson, 2021 | 65 (57-73) /69 (58-78) | 21-set. | 61/46 | 25.2 (22.5-29.1)/25 (23-28) | CABG, multivalve procedure, single-valve procedure, CABG and valve, aortic procedure, aortic dissection, ventricular septal defect repair | 4-factor PCC | rFVIIa |
BMI=body mass index; CABG=coronary artery bypass grafting; IU=international units; NA=not available; PCC=prothrombin complex concentrate; rFVIIa=recombinant factor Vlla
Quality Assessment
Among the included studies, there were two RCTs, two cohort studies, and the rest eight studies were case-control studies. Table 2 shows the risk of bias according to criteria reported in the Cochrane Handbook for RCTs, Table 3 shows the NOS scoring system for cohort studies, and Table 4 shows the NOS scoring system for case-control studies. All the included studies were considered high quality.
Table 2.
Quality assessment of randomized controlled trials.
| Author | Year | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessors | Incomplete outcome data | Selective reporting | Other |
|---|---|---|---|---|---|---|---|---|
| Green | 2020 | Low | Low | Low | Low | Low | Low | Unclear |
| Karkouti | 2020 | Low | Low | Low | Low | Low | Low | Unclear |
Table 3.
Quality assessment of cohort studies using Newcastle-Ottawa Scale.
| Author | Year | Selection | Comparability | Outcomes | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur? | Adequacy of follow-up of cohorts | ||||
| Biancari | 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Ortmann | 2014 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 8 |
Table 4.
Quality assessment of case-control studies using Newcastle-Ottawa Scale.
| Author | Year | Selection | Comparability | Outcomes | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Is the case definition adequate? | Representativeness of the cases | Selection of controls | Definition of controls | Comparability of cases and controls on the basis of the design or analysis | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non-response rate | ||||
| Bradford | 2015 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Cappabianca | 2016 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 8 |
| Fitzgerald | 2018 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Harper | 2018 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Harris | 2020 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Tanaka | 2013 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 7 |
| Zweng | 2018 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 8 |
| Alyson | 2021 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
Result of Meta-Analysis
Mortality
Mortality data were available for all 12 included studies[6,7,8,16,17,18,19,20,21,22,23,24], with a total of 1,799 patients. Overall, death occurred in 79 of 867 patients in the PCC group and 72 of 932 patients in the non-PCC group. Accordingly, PCC use was not associated with increased mortality in any patient group (RR=1.18, 95% CI=0.86–1.60, P=0.30, I2=0%) (Figure 2).
Fig. 2.
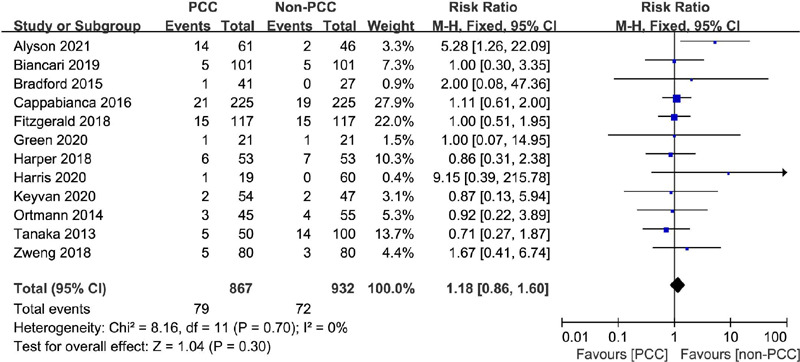
Comparison of mortality between patients treated with prothrombin complex concentrate (PCC) and those not treated with PCC. CI=confidence interval.
Bleeding
Blood loss data were available for six studies involving 927 patients undergoing cardiac surgery[6,8,18,19,20,23]. Patients who received PCC experienced an average blood loss of 353–1159 ml, whereas those in the non-PCC group presented an average blood loss of 480–1644 ml. The total blood loss in the PCC group was significantly decreased (MD=-248.67 ml, 95% CI=-465.36– -31.97, P=0.02, I2=84%) (Figure 3A).
Fig. 3.
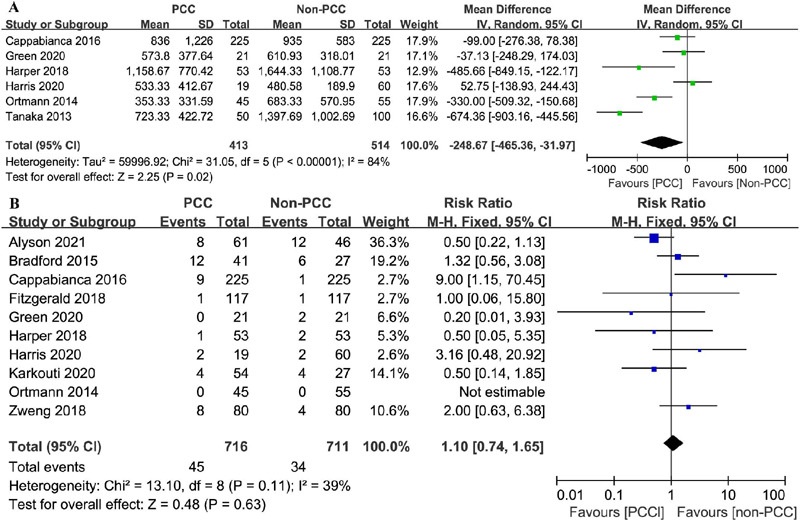
Comparison of bleeding and thromboembolic events between patients treated with prothrombin complex concentrate (PCC) and those not treated with PCC. A) Bleeding; B) thromboembolic events. CI=confidence interval; SD=standard deviation.
Thromboembolic Events
Data on thromboembolic events were recorded in 10 studies[6,7,8,17,18,19,20,21,22,24]. Thromboembolic events occurred in 45 of 716 patients in the PCC group and 34 of 771 patients in the non-PCC group. PCC use was not associated with thromboembolic events (RR=1.10, 95% CI=0.74–1.65, P=0.63, I2=39%) (Figure 3B).
Intra-aortic Balloon Pump/Extracorporeal Membrane Oxygenation
The incidence of IABP/ECMO was recorded in three studies examining 752 patients[6,8,16]. IABP/ECMO was performed in 30 of 371 patients in the PCC group and 48 of 381 patients in the non-PCC group. In all patient groups, the use of PCC slightly reduced IABP/ECMO events (RR=0.65, 95% CI=0.42–0.996, P=0.05, I2=0%) (Figure 4).
Fig. 4.
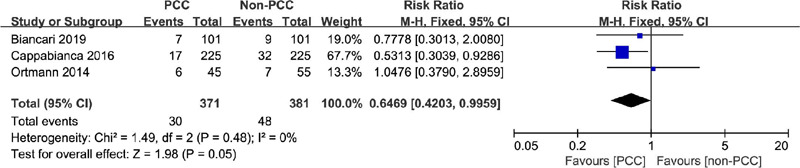
Comparison of intra-aortic balloon pump/extracorporeal membrane oxygenation between patients treated with prothrombin complex concentrate (PCC) and those not treated with PCC. CI=confidence interval.
Atrial Fibrillation
Data on the occurrence of atrial fibrillation was recorded in two included studies[16,20], with 281 patients in total. Overall, 33 events in 120 patients and 58 events in 161 patients were documented in PCC and non-PCC groups, respectively. PCC use was not associated with an increase in atrial fibrillation events in any patient group (RR=0.73, 95% CI=0.52–1.05, P=0.24, I2=29%) (Figure 5A).
Fig. 5.
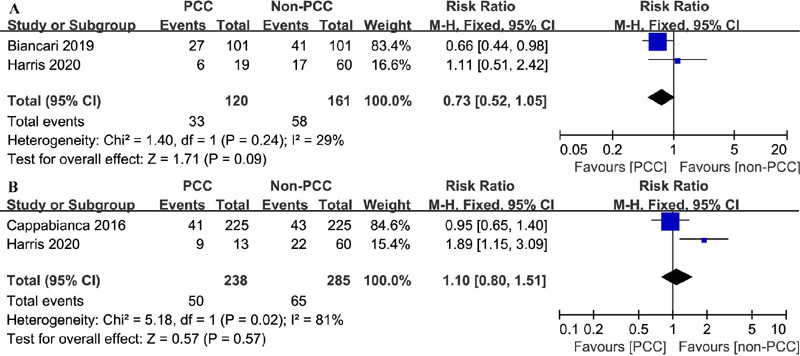
Comparison of atrial fibrillation and myocardial infarction between patients treated with prothrombin complex concentrate (PCC) and those not treated with PCC. A) Atrial fibrillation; B) myocardial infarction. CI=confidence interval.
Myocardial Infarction
Only two studies reported the incidence of myocardial infarction[8,22]. Among 238 patients in the PCC group, 50 presented with myocardial infarction, and among 285 patients in the non-PCC group, 65 exhibited myocardial infarction (RR=1.10, 95% CI=0.80–1.51, P=0.57, I2=81%) (Figure 5B).
Thoracic Drainage
Thoracic drainage was reported in five studies[18,19,20,21,22] evaluating 435 patients who underwent cardiac surgery. The mean thoracic drainage was 485–1165 ml in the PCC group and 396–1648 ml in the non-PCC group, with the total thoracic drainage significantly reduced in the PCC group (MD=-67.94 ml, 95% CI=-239.52–103.65, P=0.44, I2=91%) (Figure 6).
Fig. 6.
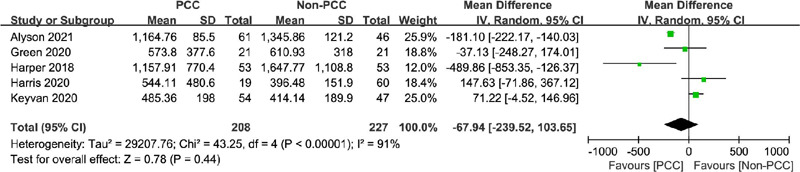
Comparison of thoracic drainage between patients treated with prothrombin complex concentrate (PCC ) and those not treated with PCC. CI=confidence interval; SD=standard deviation.
Hospital Length of Stay
In total, 985 patients were examined in seven studies[6,8,18,19,20,21,22], presenting a mean hospital stay of 7.6–19.8 days and 7–24.4 days in the PCC and non-PCC groups, respectively. PCC use was not associated with a shorter hospital stay (MD=-2.17 days; 95% CI=-5.62–1.28, P=0.22, I2=91%) (Figure 7A).
Fig. 7.
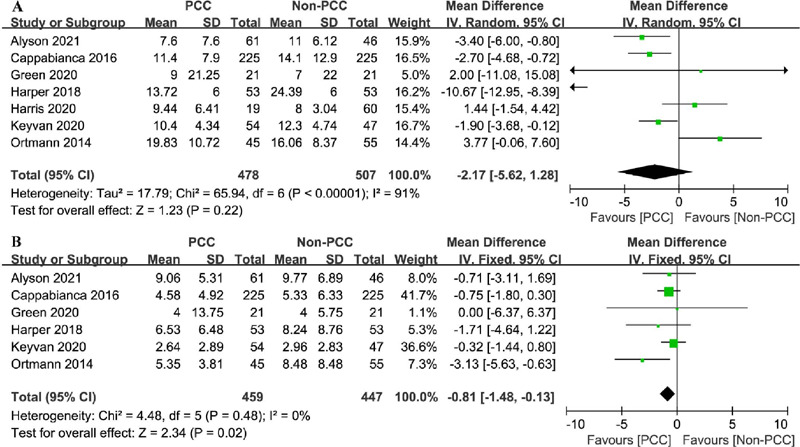
Comparison of hospital length of stay and intensive care unit (ICU) length of stay between patients treated with prothrombin complex concentrate (PCC) and those not treated with PCC. A) Hospital length of stay; B) ICU length of stay. CI=confidence interval; SD=standard deviation.
Intensive Care Unit Length of Stay
A total of 906 patients were evaluated in six studies[6,8,18,19,21,22]. PCC use was associated with a shorter ICU stay (MD=-0.81 days, 95% CI=-1.48– -0.13, P=0.02, I2=0%) (Figure 7B).
Publication Bias
All funnel plots showed symmetry, and no publication bias was found in any outcome examined.
DISCUSSION
In this meta-analysis, our findings revealed that PCC use was associated with reduced bleeding, IABP/ECMO, and ICU length of stay. In addition, PCC use did not increase mortality, thromboembolic events, atrial fibrillation, myocardial infarction, thoracic drainage, and hospital length of stay.
During aortic balloon counterpulsation, an inflatable balloon contracts and compresses the airbag, which decreases cardiac contractions, increases the cardiac ejection burden, and enhances coronary blood flow during diastole, thereby improving the blood supply to coronary arteries and reducing the cardiac backload[25]. IABP can effectively enhance myocardial blood supply and reduce oxygen consumption. In clinical settings, IABP is used to treat myocardial infarction, cardiogenic shock, and other serious coronary heart diseases or for the prevention and support of cardiac interventional surgery[26]. ECMO is primarily used to provide continuous external respiration and circulation in patients with severe cardiopulmonary failure for maintenance of life[27]. ECMO comprises a membrane lung (artificial lung) and blood pump (artificial heart), which can provide long-term cardiopulmonary support for patients with severe cardiopulmonary failure and afford the critical time required to rescue critically ill patients. No correlation was observed between PCC use and lung reperfusion injury; hence, it was speculated that ECMO in the present study was mainly used for cardiac treatment and could reduce IABP/ ECMO. As blood loss data included in the present study did not specify the specific site of blood loss, it was preliminarily discussed and predicted that the use of PCC might play a role in reducing cardiac blood loss. The analysis showed that PCC use was not associated with a reduction in myocardial infarction and atrial fibrillation, probably due to insufficient data and sample size in both areas. In conclusion, the use of PCC may have a beneficial effect on specific parts of the heart during the treatment of blood loss, and PCC can be used to treat severe perioperative bleeding during cardiac surgery. It is also possible that heterogeneity in several studies influenced the observed results.
PCC can effectively reduce hematoma formation in patients with trauma and quickly reverse the effect of vitamin K antagonist, which has greater advantages than FFP[28]. During initial resuscitation, combined with thromboelastography results, PCC combined with cryoprecipitation or human fibrinogen concentrate could effectively increase the coagulation time. rFVIIa can be considered when the best blood substitute treatment scheme, surgery, and anti-fibrinolysis have been comprehensively exploited, serious acidosis, hypothermia, and hypocalcemia have been corrected, and bleeding could not be effectively controlled (hematocrit > 24%, platelet > 50 × 109/L, and fibrinogen > 1.5–2.0 g/L). Studies have shown that, with the support of the abovementioned standards, the use of rFVIIa can effectively reduce mortality and the amount of blood transfusion required; however, it is necessary to be vigilant against the rFVIIa-induced arterial thrombosis[29]. It should be noted that high-quality studies supporting the use of rFVIIa as a first-line drug are seriously lacking. In addition, excessive PCC use should be avoided to prevent thrombosis. Studies have reported that three-factor complexes significantly increase the risk of thrombosis when compared with four-factor complexes. Therefore, real-time dynamic monitoring of coagulation-related indicators can help reduce the risk of thrombosis.
Limitations
Although this study collected considerable research data and had a large sample size, limitations need to be addressed. First, different PCC types and doses can lead to distinct clinical effects, resulting in under- or over-description of actual effects. Second, not all included studies had strict inclusion or exclusion criteria for research subjects, resulting in population heterogeneity. Third, in some studies, the mean value was derived from the median and quartile, and the original research data were not recorded using the mean and variance, which may impact the accuracy of obtained results.
CONCLUSION
Although the use of PCC in cardiac surgery did not correlate with mortality, length of hospital stay, thoracic drainage, atrial fibrillation, myocardial infarction and thromboembolic events, PCC significantly improved postoperative ICU length of stay, bleeding, and IABP/ECMO outcomes in patients undergoing cardiac surgery.
Glossary
Abbreviations, Acronyms & Symbols
- BMI
Body mass index
- CABG
Coronary artery bypass grafting
- CI
Confidence interval
- CPB
Cardiopulmonary bypass
- ECMO
Extracorporeal membrane oxygenation
- FFP
Fresh frozen plasma
- IABP
Intra-aortic balloon pump
- ICU
Intensive care unit
- IU
International units
- MD
Mean difference
- NA
Not available
- NOS
Newcastle-Ottawa Scale
- PCC
Prothrombin complex concentrate
- RCT
Randomized controlled trial
- rFVIIa
Recombinant factor VIIa
- RR
Risk ratio
- SD
Standard deviation
Supplementary Method 1. Search Strategy
1. PubMed®, 1946 to September 10, 2022
#1 prothrombin complex concentrate
#2 factor IX
#3 factor 9
#4 autoprothrombin II
#5 christmas factor
#6 plasma thromboplastin component
#7 #1 OR #2 OR #3 OR #4 OR #5 OR #6
#8 cardiac surgical procedures
#9 thoracic surgery
#10 heart surgery
#11 cardiac surgery
#12 #8 OR #9 OR #10 OR #11
#13 #7 AND #12
2. Embase, <1974 to September 10, 2022>
#1 ‘prothrombin complex concentrate’/exp
#2 factor IX:ti,ab
#3 factor 9:ti,ab
#4 autoprothrombin II:ti,ab
#5 plasma thromboplastin component:ti,ab
#6 Christmas factor:ti,ab
#7 #1 OR #2 OR #3 OR #4 OR #5 OR #6
#8 cardiac surgical procedures’/exp
#9 thoracic surgery:ti,ab
#10 heart surgery:ti,ab
#11 cardiac surgery:ti,ab
#12 #8 OR #9 OR #10 OR #11
#13 #7 AND #12
3. Cochrane Central Register of Controlled Trials, <Issue 8 of 12, September 2022>
#1 MeSH descriptor: [prothrombin complex concentrate] explode all trees
#2 (factor IX):ti,ab,kw
#3 (factor 9):ti,ab,kw
#4 (autoprothrombin II):ti,ab,kw
#5 (christmas factor):ti,ab,kw
#6 (plasma thromboplastin component):ti,ab,kw
#7 #1 OR #2 OR #3 OR #4 OR #5 OR #6
#8 MeSH descriptor: [cardiac surgical procedures] explode all trees
#9 (thoracic surgery):ti,ab,kw
#10 (heart surgery):ti,ab,kw
#11 (cardiac surgery):ti,ab,kw
#12 #8 OR #9 OR #10 OR #11
#13 #7 AND #12
Footnotes
No financial support.
No conflict of interest.
This study was carríed out at the Department of Blood Transfusion, Zibo Municípal Hospítal, Zíbo, Shandong, People’s Republíc of Chína.
REFERENCES
- 1.Cuker A, Burnett A, Triller D, Crowther M, Ansell J, Van Cott EM, et al. Reversal of direct oral anticoagulants: guidance from the anticoagulation forum. Am J Hematol. 2019;94(6):697–709. doi: 10.1002/ajh.25475. [DOI] [PubMed] [Google Scholar]
- 2.Baskaran J, Lopez RA, Cassagnol M. Prothrombin Complex Concentrate. . StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 3.Eichinger S. Reversing vitamin K antagonists: making the old new again. Hematology Am Soc Hematol Educ Program. 2016;2016(1):605–611. doi: 10.1182/asheducation-2016.1.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodman RC, Harker LA. Bleeding complications associated with cardiopulmonary bypass. Blood. 1990;76(9):1680–1697. [PubMed] [Google Scholar]
- 5.Raphael J, Mazer CD, Subramani S, Schroeder A, Abdalla M, Ferreira R, et al. Society of cardiovascular anesthesiologists clinical practice improvement advisory for management of perioperative bleeding and hemostasis in cardiac surgery patients. Anesth Analg. 2019;129(5):1209–1221. doi: 10.1213/ANE.0000000000004355. Erratum in: Anesth Analg. 2020;130(2):e44. [DOI] [PubMed] [Google Scholar]
- 6.Ortmann E, Besser MW, Sharples LD, Gerrard C, Berman M, Jenkins D P, et al. An exploratory cohort study comparing prothrombin complex concentrate and fresh frozen plasma for the treatment of coagulopathy after complex cardiac surgery. Anesth Analg. 2015;121(1):26–33. doi: 10.1213/ANE.0000000000000689. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald J, Lenihan M, Callum J, McCluskey SA, Srinivas C, van Rensburg A, et al. Use of prothrombin complex concentrate for management of coagulopathy after cardiac surgery: a propensity score matched comparison to plasma. Br J Anaesth. 2018;120(5):928–934. doi: 10.1016/j.bja.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Cappabianca G, Mariscalco G, Biancari F, Maselli D, Papesso F, Cottini M, et al. Safety and efficacy of prothrombin complex concentrate as first-line treatment in bleeding after cardiac surgery. Crit Care. 2016;20:5–5. doi: 10.1186/s13054-015-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnékian V, Camous J, Fattal S, Rézaiguia-Delclaux S, Nottin R, Stéphan F. Use of prothrombin complex concentrate for excessive bleeding after cardiac surgery. Interact Cardiovasc Thorac Surg. 2012;15(3):382–389. doi: 10.1093/icvts/ivs224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yates SG, Sarode R. New strategies for effective treatment of vitamin K antagonist-associated bleeding. J Thromb Haemost. 2015;13(1):S180–S186. doi: 10.1111/jth.12970. [DOI] [PubMed] [Google Scholar]
- 11.Goodnough LT, Levy JH. The judicious use of recombinant factor VIIa. Semin Thromb Hemost. 2016;42(2):125–132. doi: 10.1055/s-0035-1569068. [DOI] [PubMed] [Google Scholar]
- 12.Roman M, Biancari F, Ahmed AB, Agarwal S, Hadjinikolaou L, Al-Sarraf A, et al. Prothrombin complex concentrate in cardiac surgery: a systematic review and meta-analysis. Ann Thorac Surg. 2019;107(4):1275–1283. doi: 10.1016/j.athoracsur.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein JN, Refaai MA, Milling TJ Jr, Lewis B, Goldberg-Alberts R, Hug BA, et al. Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomised trial. Lancet. 2015;385(9982):2077–2087. doi: 10.1016/S0140-6736(14)61685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erdoes G, Koster A, Ortmann E, Meesters MI, Bolliger D, Baryshnikova E, et al. A European consensus statement on the use of four-factor prothrombin complex concentrate for cardiac and non-cardiac surgical patients. Anaesthesia. 2021;76(3):381–392. doi: 10.1111/anae.15181. [DOI] [PubMed] [Google Scholar]
- 15.Higgins J P, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928–d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biancari F, Ruggieri VG, Perrotti A, Gherli R, Demal T, Franzese I, et al. Comparative analysis of prothrombin complex concentrate and fresh frozen plasma in coronary surgery. Heart Lung Circ. 2019;28(12):1881–1887. doi: 10.1016/j.hlc.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 17.Bradford CD, Stahovich MJ, Dembitsky W P, Adamson RM, Engelbert JJ, Perreiter AS. Safety of prothombin complex concentrate to control excess bleeding during continuous flow LVAD insertion. ASAIO J. 2015;61(5):509–513. doi: 10.1097/MAT.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 18.Green L, Roberts N, Cooper J, Agarwal S, Brunskill SJ, Chang I, et al. Prothrombin complex concentrate vs. fresh frozen plasma in adult patients undergoing heart surgery - a pilot randomised controlled trial (PROPHESY trial) Anaesthesia. 2021;76(7):892–901. doi: 10.1111/anae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harper PC, Smith MM, Brinkman NJ, Passe MA, Schroeder DR, Said SM, et al. Outcomes following three-factor inactive prothrombin complex concentrate versus recombinant activated factor VII administration during cardiac surgery. J Cardiothorac Vasc Anesth. 2018;32(1):151–157. doi: 10.1053/j.jvca.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Harris JE, Varnado S, Herrera E, Salazar E, Colavecchia AC. Evaluation of postoperative clinical outcomes in jehovah's witness patients who receive prothrombin complex concentrate during cardiac surgery. J Card Surg. 2020;35(4):801–809. doi: 10.1111/jocs.14463. [DOI] [PubMed] [Google Scholar]
- 21.Karkouti K, Bartoszko J, Grewal D, Bingley C, Armali C, Carroll J, et al. Comparison of 4-factor prothrombin complex concentrate with frozen plasma for management of hemorrhage during and after cardiac surgery: a randomized pilot trial. JAMA Netw Open. 2021;4(4):e213936. doi: 10.1001/jamanetworkopen.2021.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz A, Ahuja T, Arnouk S, Lewis TC, Marsh K, Papadopoulos J, et al. A comparison of prothrombin complex concentrate and recombinant activated factor VII for the management of bleeding with cardiac surgery. J Intensive Care Med. 2022;37(2):231–239. doi: 10.1177/0885066620984443. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka KA, Mazzeffi MA, Grube M, Ogawa S, Chen EP. Three-factor prothrombin complex concentrate and hemostasis after high-risk cardiovascular surgery. Transfusion. 2013;53(4):920–921. doi: 10.1111/trf.12110. [DOI] [PubMed] [Google Scholar]
- 24.Zweng I, Galvin S, Robbins R, Bellomo R, Hart GK, Seevanayagam S, et al. Initial experience of the use of 3-factor prothrombin complex concentrate and thromboembolic complications after cardiac surgery. Heart Lung Circ. 2019;28(11):1706–1713. doi: 10.1016/j.hlc.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Senst B, Kumar A, Diaz RR. Cardiac Surgery. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 26.Akyurekli Y, Taichman GC, Keon WJ. Effectiveness of intra-aortic balloon counterpulsation on systolic unloading. Can J Surg. 1980;23(2):122–126. [PubMed] [Google Scholar]
- 27.Bernhardt AM, Schrage B, Schroeder I, Trummer G, Westermann D, Reichenspurner H. Extracorporeal membrane oxygenation. Dtsch Arztebl Int. 2022;119(13):235–244. doi: 10.3238/arztebl.m2022.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chai-Adisaksopha C, Hillis C, Siegal DM, Movilla R, Heddle N, Iorio A, et al. Prothrombin complex concentrates versus fresh frozen plasma for warfarin reversal. A systematic review and meta-analysis. Thromb Haemost. 2016;116(5):879–890. doi: 10.1160/TH16-04-0266. [DOI] [PubMed] [Google Scholar]
- 29.Payen JF, Berthet M, Genty C, Declety P, Garrigue-Huet D, Morel N, et al. Reduced mortality by meeting guideline criteria before using recombinant activated factor VII in severe trauma patients with massive bleeding. Br J Anaesth. 2016;117(4):470–476. doi: 10.1093/bja/aew276. [DOI] [PubMed] [Google Scholar]


