Abstract
Background
Acute otitis media (AOM) is a common childhood disease frequently caused by Streptococcus pneumoniae. Pneumococcal conjugate vaccines (PCV7, PCV10, PCV13) can reduce the risk of AOM but may also shift AOM etiology and serotype distribution. The aim of this study was to review estimates from published literature of the burden of AOM in Europe after widespread use of PCVs over the past 10 years, focusing on incidence, etiology, serotype distribution and antibiotic resistance of Streptococcus pneumoniae, and economic burden.
Methods
This systematic review included published literature from 31 European countries, for children aged ≤5 years, published after 2011. Searches were conducted using PubMed, Embase, Google, and three disease conference websites. Risk of bias was assessed with ISPOR-AMCP-NPC, ECOBIAS or ROBIS, depending on the type of study.
Results
In total, 107 relevant records were identified, which revealed wide variation in study methodology and reporting, thus limiting comparisons across outcomes. No homogenous trends were identified in incidence rates across countries, or in detection of S. pneumoniae as a cause of AOM over time. There were indications of a reduction in hospitalization rates (decreases between 24.5–38.8% points, depending on country, PCV type and time since PCV introduction) and antibiotic resistance (decreases between 14–24%, depending on country), following the widespread use of PCVs over time. The last two trends imply a potential decrease in economic burden, though this was not possible to confirm with the identified cost data. There was also evidence of an increase in serotype distributions towards non-vaccine serotypes in all of the countries where non-PCV serotype data were available, as well as limited data of increased antibiotic resistance within non-vaccine serotypes.
Conclusions
Though some factors point to a reduction in AOM burden in Europe, the burden still remains high, residual burden from uncovered serotypes is present and it is difficult to provide comprehensive, accurate and up-to-date estimates of said burden from the published literature. This could be improved by standardised methodology, reporting and wider use of surveillance systems.
Introduction
Acute otitis media (AOM) is an inflammatory disease of the middle ear associated with middle ear effusion and symptoms like ear pain, fever, irritability, otorrhea, anorexia, and sometimes vomiting or lethargy [1, 2]. Even though AOM can also have a viral etiology, it is more commonly the result of a bacterial infection by Streptococcus pneumoniae (S. pneumoniae), Hemophilus influenzae, or Moraxella catarrhalis, and is often treated with pain medications and/or antibiotics, depending on the patient’s age and severity of symptoms [3, 4].
Pneumococcal conjugate vaccines (PCVs) confer protection against diseases caused by vaccine serotype S. pneumoniae [5]. Since S. pneumoniae is the most common cause of AOM, vaccination of children younger than 5 years with PCVs can reduce the risk of AOM, while further protecting the population and mitigating the spread of antibiotic-resistant serotypes [6–9].
European countries started introducing PCVs in the early 2000s, beginning with PCV7, which protected against 7 pneumococcal serotypes (4, 6B, 9V, 14, 18C, 19F, and 23F) [5, 10]. Previous literature has posited that widespread use of PCVs in higher-income countries has shifted both AOM etiology and pneumococcal serotype distribution among the target populations [11]. The ongoing changes in etiology and emergence of non-vaccine serotypes are a reason for growing concern in the medical community around antibiotic-resistant bacteria as a major cause of treatment failure in pediatric patients with AOM [12, 13]. Furthermore, a growing body of literature has focused on the emergence of residual burden, as serotypes of S. Pneumoniae not covered by available PCVs continue to circulate in the pediatric population [14–18]. As a consequence, higher-valent vaccines have been introduced in immunization programs, such as PCV10 (with additional serotypes 1, 5, and 7F) and PCV13 (with additional serotypes 3, 6A, and 19A) [5]. S1 Fig in the appendix provides an overview of which PCVs were introduced in each of the European countries of interest between 2002 and 2021 [9, 19–28]; by 2011, 70% of the included countries had introduced a PCV into their national immunization program (NIP) for children.
Recently, two higher valency PCVs have been approved by the European Medicines Agency for adult use and are soon expected to have pediatric indication; in addition to the serotypes present in previous PCVs, PCV15 contains 22F and 33F, and PCV20 contains serotypes 8, 10A, 11A, 12F, and 15B [5, 29–31].
Since AOM is one of the most common pediatric infectious diseases, its burden is substantial [4]. A 2012 estimate of the global yearly incidence rate of AOM is 108.5 cases per 1000 person years, with more than 700 million cases estimated to occur every year and 51% of these cases occurring in children [32]. In central Europe, the average incidence rate is 3.6% per year [32]. Incidence rates are highest in children 0–4 years of age, with a peak in the first year of life [32]. In addition, AOM is both a leading cause of antibiotic prescriptions and a major contributor of medical costs in children [4].
However, the incidence and prevalence of AOM, serotype distribution of the AOM-causing bacteria, S. pneumoniae, and the burden of the disease vary between countries and over time. Furthermore, previous reviews of published literature may lack current and comprehensive evidence for a number of reasons: they may no longer be up to date, may not look at all relevant metrics for burden or may be limited in number of countries included [4, 33–35]. Therefore, the objectives of this review were to identify available evidence in published literature related to the burden of AOM over the past 10 years, for 31 European countries, based on the following factors: incidence, etiology, changes in serotype distribution and antibiotic resistance over time for S. pneumoniae, costs and impact on quality of life. The authors then aimed to provide comparable estimates, where possible, that quantified this burden for children <5 years of age around Europe within the context of increased PCV usage.
Methods
This systematic literature review was carried out using methods based on the guidelines by PRISMA, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [36]. The study protocol for this project was registered with PROSPERO, the international prospective register of systematic reviews on 19-Dec-2021. The registration ID is CRD42021292105, and it can be accessed at: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=292105.
Eligibility criteria
To be included in this review, records had to fulfil the inclusion criteria described in Table 1.
Table 1. Inclusion criteria.
| Inclusion Criteria | |
|---|---|
| Population(s) | Children younger than 5 years of age (when specified, children up to 5 years and 11 months were included) in European countries (Austria, Belgium, Bulgaria, Croatia, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, Switzerland, United Kingdom). |
| Intervention/Exposure | Acute otitis media1 (AOM), or at risk of developing AOM |
| Comparisons | Not applicable |
| Outcomes (contain data for any of the following) | • Epidemiological data on AOM’s: ○ Incidence ○ Prevalence ○ Etiology • Serotype data on S. pneumoniae ○ Serotype distribution of S. pneumoniae ○ Antibiotic resistance and susceptibility of S. pneumoniae • Economic data on AOM’s: ○ Resource use ○ Costs • Impact on QoL (humanistic burden) • Vaccine coverage rates (VCR) of PCVs2 |
| Time | Records published since 01 January 20113. |
| Conference abstracts published from January 1st, 2017 to the latest available year, as of November 12th, 2021. | |
| Study design | • Observational studies (for instance cross-sectional, cohort or case-control) • Economic studies (for instance cost of illness) • Systematic reviews |
| Other | For all records, the following should apply: • Available in full text, unless the material is a conference abstract • Available in English |
1. To avoid excluding records that refer to AOM in the full text but do not provide details in the title and abstract, the term “otitis media” was included in the search strategy, but records that did not contain evidence of AOM were excluded.
2. Outcomes on VCR of PCVs were excluded from the present study.
3. **Records published after 01 Jan 2011 might include information collected before 2011. The time period from when the data was collected was extracted as a separate data element in order to differentiate it from the publication date.
Information sources and search strategy
Searches were conducted in PubMed® and Embase®. The search terms (S1 Table) were divided into six blocks (population, exposure, epidemiology and etiology, burden, S. pneumoniae and VCR). Each block included synonyms and MeSH-terms and were searched for in different combinations, also filtering by English language and date of publication (after 1st Jan 2011). As a complement to the database searches, one ad-hoc Google search was performed per country (countries listed in Table 1) using standardized Google searches. In addition, the websites of three relevant conferences were used to search for abstracts: the European Congress of Clinical Microbiology & Infectious Diseases, the European Society for Pediatric Infectious Diseases, and the European Academy of Pediatrics [37–39]. All searches were conducted between 2021, November 11th and 12th.
Selection process
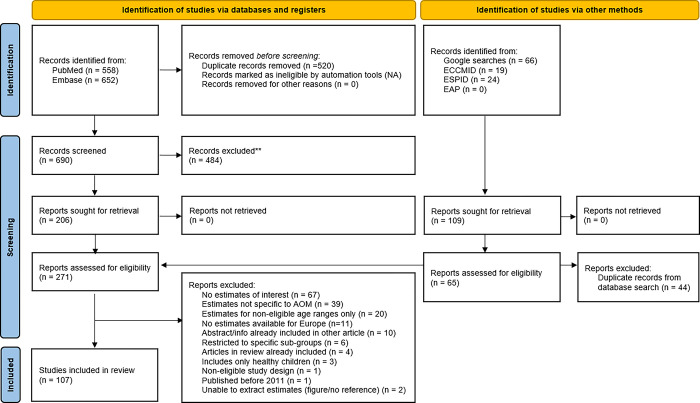
After removal of duplicate records, a two-step process was employed for the selection process. In step one, the titles and abstracts of articles identified in PubMed®, Embase®, Google or the conference websites were assessed and categorized as ‘included,’ ‘unsure,’ or ‘rejected’ independently by two reviewers, based on the eligibility criteria. Discrepancies between reviewers were resolved by consensus; unresolved disputes were referred to a third arbitrator and a consensus reached. In step two, the two reviewers obtained and independently reviewed the full text records in the ‘included’ and ‘unsure’ categories. Reasons for exclusions of records were recorded. Any disagreements were referred to the third arbitrator. A PRISMA flow diagram (Fig 1) was used to visualize the number of records included and excluded at each stage of the review. Records excluded at the full paper stage were tabulated alongside the reason for exclusion in accordance with best practice guidelines [36].
Fig 1. PRISMA flow diagram for the literature search.
Data collection process
All relevant data from the included records was extracted by one reviewer and a quality check was performed by a second reviewer, comparing the extracted content of 10% of the included records to the original work, for correctness. Any major discrepancies resulted in a further review of 5% of the remaining records, and so forth, until free of discrepancies. The type of data extracted is presented in S2 Table. For each of the outcome variables, any stratification by age group, level of complication, etc. was recorded separately if available.
Risk of bias assessment
As different study designs were included in the review, three tools were selected to assess risk of bias for the respective record types. For observational studies and health economic studies that do not involve modelling (for instance, studies assessing economic burden), the ISPOR-AMCP-NPC was chosen due to its applicability for multiple study designs [40]. For health economic studies involving modelling, the ECOBIAS risk of bias tool for model-based economic evaluations was chosen [17]. For systematic reviews, ROBIS was employed since it is a rigorous tool that was specifically designed to assess risk of bias in this type of study [41, 42]. A single reviewer assessed the risk of bias. During this process, the reviewer made a list of the included records that were found to be most difficult to assess. These records were then assessed by a second reviewer and discussed together with the original reviewer to reach a resolution. Unresolved assessments were referred to a senior arbitrator and a consensus reached. There is a potential risk for publication bias in all types of published literature. In order to address this for the context of this study, the reviewers looked for conflicts of interest reported by the authors in each record, as well as their sources of funding to assess whether there could be a potential risk. More advanced methods were not applicable in this review, as the records included are not homogenous in study design. After the risk of bias assessment, each record was categorized as either “high,” “medium” or “low” risk of bias. The results of the assessment are provided in the list of included records, provided in S3 Table.
Synthesis of results
The findings for each of the objectives were grouped into the following objective categories: epidemiology, antibiotic resistance, serotype data, economic burden and quality of life. Data on the outcomes reported in each of the included studies were collected and stratified by country, population and year. As earlier estimates of the burden are available in previously-published literature [4, 33–35], the authors chose to focus on estimates published between 2011 and 2021 (the last 10 years).
Changes in AOM burden were analyzed by comparing estimates across the study period, taking into account the introduction of newer PCVs. Estimates for select metrics (e.g., incidence, direct medical costs, antibiotics reviewed, etc.) were presented together in tables and/or figures, where the comparison of estimates between countries was deemed feasible. For the purpose of comparability, age groups originally reported in years were transformed into months and time intervals reported in months were rounded up to years.
The gaps in the identified data were assessed, and possible relationships in the data were explored and described, where possible. Since no statistical analysis was conducted, absence of data (for instance, from a certain geographical area) was addressed through discussion only. Though records and outcomes related to vaccine coverage rates were included in the scope of the review, the synthesis of these results was not reported in the present study due to risk of bias further described in the Discussion. Nevertheless, the records pertaining to PCV-coverage were still included in the summary statistics as they were indeed part of the original search and extraction process.
Results
Study selection and general characteristics
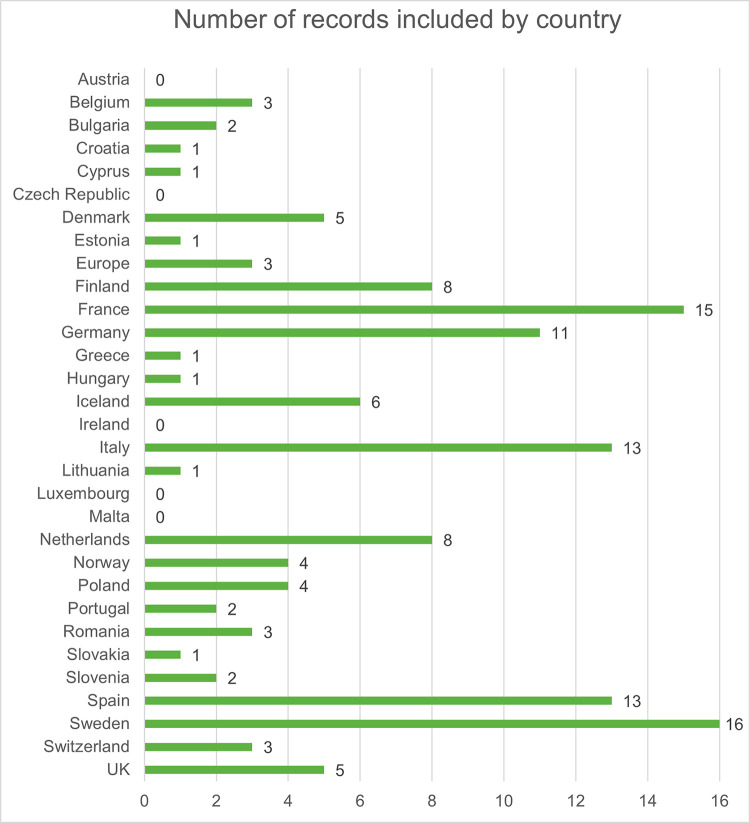
In total, the searches generated 690 unique records from PubMed® and Embase®. In addition, 109 records were sought for retrieval after the ad hoc Google search and the conference abstract website searches. After title and abstract review of all collected records, a total of 271 records were kept for full-text review. Finally, 107 records were included after the full-text review (see the PRISMA flow chart presented in Fig 1 and the PRISMA checklist in S8 Table). The full list of included records, including the risk of bias assessment result, is available in S3 Table. The countries with the highest number of included records were Sweden, Spain, Italy, Germany and France, while no available data were identified for 6 countries (Austria, Czech Republic, Ireland, Latvia, Luxembourg and Malta) (Fig 2).
Fig 2. The number of studies with data for each of the included countries.
Note: some of the included papers reported on more than one outcome.
Many of the included studies had information regarding several of the outcomes of interest. The main proportion of the included studies (69%) had information regarding the epidemiology of AOM, while S. pneumoniae information (serotype distribution and antibiotic resistance) was available in approximately 36%, and economic data in 34%. Even though there was a higher number of papers in the economic outcome category, only 19 of them included QoL-data. Details on which countries had data in each respective area can be found in S4 Table.
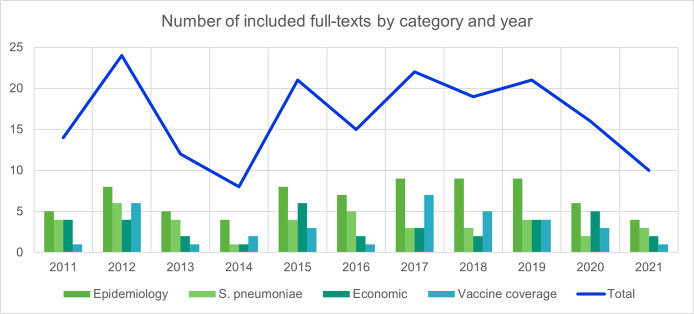
The included studies were rather evenly distributed over the inclusion years (2011–2021), with a peak of epidemiology papers between 2017 and 2019 (Fig 3). For the data synthesis, 78 studies (73%) reported data collected between 2011 to 2021, covering 21 countries.
Fig 3. Number of papers included by year and outcome category.
Note: some of the included papers reported on more than one outcome.
Epidemiology
Incidence of AOM
Of the 61 studies that reported data on incidence rates of AOM, 12 contained information from the period 2011–2021, providing recent estimates for 11 of the 31 included countries (Table 2). One study, Chapman et al 2020, assumed the same incidence rate for several countries, and was therefore excluded from the synthesis of epidemiological data [43]. When needed, incidence rates were transformed to a rate of 100,000 person-years. Incidence rates of AOM ranged from 630 cases (in children aged 3 to <7 years in 2011–2013) to 62,400 cases (in children aged 6 to 12 months in 2008–2014) per 100,000 person-years [44, 45].
Table 2. Incidence rates of AOM cases.
| Location | Population | Data collection period | PCV used in NIP during the period | Incidence rate (per 100,000 person-years) |
|---|---|---|---|---|
| Croatia [46] | 1–59 months | 2012 | None | 23,581 |
| Estonia [47] | 3–71 months | 2011–2012 | None | 13,780 |
| Germany [48] | <24 months | 2012 | PCV10/PCV13 | 3,500 |
| 2017 | PCV10/PCV13 | 2,700 | ||
| 24–59 months | 2012 | PCV10/PCV13 | 4,100 | |
| 2017 | PCV10/PCV13 | 3,400 | ||
| Iceland [44] | <12 months | 2011–2013 | PCV13 | 9,800 |
| 12 –<24 months | 10,580 | |||
| 24 –<36 months | 3,150 | |||
| 36 –<84 months | 630 | |||
| Iceland [45] | <12 months | 2008–2013 | None→PCV7→ PCV10 | 48,300 |
| 2014 | PCV10 | 34,200 | ||
| 12 to <24 months | 2008–2013 | None→PCV7→ PCV10 | 57,200 | |
| 2014 | PCV10 | 51,900 | ||
| 24 to <36 months | 2008–2013 | None→PCV7→ PCV10 | 29,100 | |
| 2014 | PCV10 | 25,500 | ||
| < 36 months | 2005–2015 | None→PCV7→ PCV10 | 41,700 | |
| 2008–2013 | None→PCV7→ PCV10 | 43,600 | ||
| 2014 | PCV10 | 38,000 | ||
| Iceland [49] | 0–84 months | 2012–2014 | PCV10 | 2,350 |
| 2015–2017 | PCV10 | 1,330 | ||
| Italy, Veneto Region [50] | 24–59 months | 2010 | PCV13 | 11,900 |
| 2017 | PCV13 | 12,900 | ||
| Lithuania [47] | 0–71 months | 2011–2012 | None | 18,400 |
| Netherlands, Utrecht [51] | 0–23 months | 2007–2012 | PCV7→ PCV10 | 56,900 |
| Netherlands [52] | 6–12 months | 2008–2014 | PCV7→ PCV10 | 62,400 |
| Poland [47] | 0–71 months | 2011–2012 | PCV7 (risk groups) | 11,570 |
| Romania [47] | 2–71 months | 2011–2012 | None | 14,190 |
| Slovenia [47] | 1–70 months | 2011–2012 | PCV7 (risk groups) | 34,030 |
| Sweden, Skåne Region [53] | 0–35 months | 2011 | PCV10/PCV13 | 30,544 |
| 2012 | PCV10/PCV13 | 37,124 | ||
| 2013 | PCV10/PCV13 | 39,868 | ||
| 2010–2013 | PCV10/PCV13 | 23,697 | ||
| 36–71 months | 2011 | PCV10/PCV13 | 20,109 | |
| 2012 | PCV10/PCV13 | 19,491 | ||
| 2013 | PCV10/PCV13 | 16,454 | ||
| 2010–2013 | PCV10/PCV13 | 17,994 | ||
| ≤ 71 months | 2011 | PCV10/PCV13 | 24,837 | |
| 2012 | PCV10/PCV13 | 23,918 | ||
| 2013 | PCV10/PCV13 | 20,207 | ||
| 2010–2013 | PCV10/PCV13 | 23,640 | ||
| Sweden, Västra Götaland Region [53] | 0–24 months | 2011 | PCV10/PCV13 | 31,800 |
| 2012 | PCV10/PCV13 | 29,432 | ||
| 2013 | PCV10/PCV13 | 25,914 | ||
| 2010–2013 | PCV10/PCV13 | 25,710 | ||
| 25–60 months | 2011 | PCV10/PCV13 | 21,320 | |
| 2012 | PCV10/PCV13 | 19,143 | ||
| 2013 | PCV10/PCV13 | 16,317 | ||
| 2010–2013 | PCV10/PCV13 | 15,270 | ||
| 0–60 months | 2011 | PCV10/PCV13 | 25,671 | |
| 2012 | PCV10/PCV13 | 24,227 | ||
| 2013 | PCV10/PCV13 | 21,411 | ||
| 2010–2013 | PCV10/PCV13 | 25,268 | ||
| Sweden, Västerbotten Region [54] | 0–59 months | 2011 | PCV10/PCV13 | 17,700 |
| 2012 | PCV10/PCV13 | 17,500 | ||
| 2013 | PCV10/PCV13 | 16,200 | ||
| 2014 | PCV10/PCV13 | 16,100 |
As presented in Table 2, incidence estimates in across time were available for 4 countries. (Germany, Iceland, Italy and Sweden). In Germany, the incidence of AOM was estimated to decrease over a 5-year period (2012 to 2017), during which PCV10 and PCV13 were part of the NIP. This decrease was seen in both groups of children younger than 24 months (from 3,500 to 2,700 cases per 100,000 person-years) and between 25–59 months (from 4,100 to 3,400 cases per 100,000 person-years) [48]. A similar decrease was estimated in two separate studies in Iceland after the introduction of PCV10. The methodologies for estimating the incidence differed in the two studies; higher rates were found in the study estimating incidence of AOM from diagnoses in primary care than when assessing middle-ear fluid (MEF) samples of children with ruptured tympanic membranes or middle-ear inflammation [45, 49]. After the introduction of PCVs in three Swedish regions (one introduced PCV10, another PCV13 and the third started with PCV13 and replaced it with PCV10) a decrease in AOM incidence was seen in all three regions, in all age groups with the exception of 0–35 months in Skåne [53]. Contrary to the studies from the other three countries, estimates from the Italian region of Veneto showed a slightly-increasing incidence rate of AOM among children 24–59 months (11,900 to 12,900 cases per 100,000 person-years) when PCV13 was introduced in 2010 compared to 2017 [50, 54]. With limited trend data over time it is difficult to draw conclusions about the impact of PCVs on AOM incidence. The available data implied that the trends in AOM incidence differ between countries (and age groups) after widespread PCV use, with most of the studies estimating a decrease over time.
Hospitalization rates for AOM patients
There were two countries with studies that reported the incidence of hospitalization (i.e., inpatient stay) due to AOM between 2011 and 2021 (Table 3). Three studies (one in Iceland and two in Italy) estimated incidence rates of hospitalization before and after the introduction of PCVs (Table 3). All studies found a decrease of between 24.5% and 38.8% in AOM-related hospitalizations after PCVs were introduced [26, 55, 56], suggesting a reduction in either AOM severity or AOM cases over time.
Table 3. Time trends of changes in AOM hospitalization incidence rates.
| Location | Pop. | Pre-PCV | Post-PCV | Decrease in incidence rate | ||
|---|---|---|---|---|---|---|
| Time interval | Incidence (per 100,000) | Time interval | Incidence rate (per 100,000) | |||
| Iceland [26] | 0–47 months | 2005–2013 (pre PCV10) | 232 | 2011–2018 (post PCV10) | 145 | 38% |
| Italy, Apulia region [55] | <60 months | 2001–2005 (pre PCV7) | 205 | 2006–2011 (post PCV7) | 154.7 | 25% |
| Italy (8 first regions to introduce PCVs) [56] | 91 | 58.9 | 35% | |||
| Italy [56] | 100 | 61.3 | 39% | |||
Etiology
Etiology information was identified in 36 studies. Out of those, 21 contained data from 11 countries on S. pneumoniae detection in AOM patients during the publication period (2011–2021), as shown in Table 4. The remaining 15 studies presented information on detection of S. pneumoniae from before 2011.
Table 4. Detection of S. pneumoniae in children younger than 5 years with AOM in Europe between 2011-2021(% and sample size).
| Location | Population | Period of data collection | PCV used in NIP during the period | Estimate (%, CI)* | Sample size |
|---|---|---|---|---|---|
| Middle ear fluid | |||||
| Finland [59–62] | 0–16 years | 2003–2012 | No PCV → PCV10 | 38.0% | 56 |
| <24 months | No PCV → PCV10 | 43.0% | 14 | ||
| 6–39 months | 2010–2011 | PCV10 | 34.9% | 43 | |
| 5–42 months | PCV10 | 31.1% | 90 | ||
| France [63, 64] | 6–24 months | 2011–2014 | PCV13 | 20.7% | 56 |
| <36 months | 2015–2017 | PCV13 | 17.1% | 175 | |
| Germany [57] | 2–71 months | 2011 | PCV10/PCV13 | 5.8% | 209 |
| 2012 | PCV10/PCV13 | 6.9% | 412 | ||
| 2013 | PCV10/PCV13 | 6.3% | 340 | ||
| 2014 | PCV10/PCV13 | 6.9% | 251 | ||
| 2015 | PCV10/PCV13 | 6.7% | 214 | ||
| Italy, Milan [65] | 6–96 months | 2015–2016 | PCV13 | 27.1% | 48 |
| Poland [66, 67] | 12–35 months | 2010–2014 | PCV7 (R) | 30.0% | 20 |
| 36–71 months | PCV7 (R) | 32.3% | 31 | ||
| <60 months | 2010–2016 | PCV7 (R) | 50.0% | 407 | |
| Romania [68, 69] | <60 months | 2009–2011 | No PCV | 70.3% | 111 |
| <60 months | 2009–2014 | No PCV | 32.9% | 391 | |
| Spain [70] | 3–36 months | 2009–2012 | PCV7 (P) → PCV10/PCV13 (P) | 39.3% | 117 |
| Portugal** [58] | 24–83 months | 2014–2016 | No PCV → PCV13 | 47.0% | 151 |
| Nasopharynx | |||||
| Belgium [71] | 6–30 months | 2016 | PCV13 | 69.2% | 39 |
| 2017 | PCV13 | 64.8%, (55.5%-73.0%) | 122 | ||
| France [72–75] | < 24 months | 2006–2017 | PCV7 →PCV13 | 55.9% | 9,957 |
| 6–24 months | 2011–2012 | PCV13 | 54.5% | 1,790 | |
| 2013–2016 | PCV13 | 54.6% | 3,649 | ||
| 2014 | PCV13 | 56.2% | 7,991 | ||
| 6–35 months | 2011–2018 | PCV13 | 60.8% | 3,964 | |
| Germany [57] | 2–71 months | 2011 | PCV10/PCV13 | 53.1% | 192 |
| 2012 | PCV10/PCV13 | 48.8% | 389 | ||
| 2013 | PCV10/PCV13 | 45.8% | 323 | ||
| 2014 | PCV10/PCV13 | 51.5% | 235 | ||
| 2015 | PCV10/PCV13 | 53.6% | 196 | ||
| Iceland [76] | 12–83 months | 2011 | PCV10 | 65.0% | 420 |
| 2012 | PCV10 | 61.1% | 465 | ||
| 2013 | PCV10 | 70.9% | 471 | ||
| 2014 | PCV10 | 69.6% | 566 | ||
| 2015 | PCV10 | 73.4% | 533 | ||
| 2016 | PCV10 | 65.6% | 541 | ||
| 2017 | PCV10 | 53.8% | 506 | ||
| 2009–2011 | PCV7 (R) →PCV10 | 71.8% | 1,380 | ||
| 2012–2017 | PCV10 | 65.9% | 3,081 | ||
| Portugal [58] | 24–83 months | 2014–2016 | PCV13 | 47.0% | 151 |
| Sweden, Skåne Region [77] | 0–35 months | 2017–2018 | PCV10/PCV13 | 16.1% | 684 |
| 36–83 months | PCV10/PCV13 | 10.5% | 191 | ||
| Nasopharynx and oropharynx | |||||
| Poland [67] | 12–35 months | 2010–2014 | PCV7 (R) | 25.0% | 20 |
| 36–71 months | PCV7 (R) | 35.5% | 31 | ||
Abbreviation: CI, confidence interval. (R) Only introduced to NIP for certain risk groups. (P) Only introduced to NIP in certain regions
Notes
* Of the records included, the authors identified only one instance where the study reported confidence intervals with their Etiology estimate
** Otorrhea samples, not specified as middle ear fluid
The estimates in Table 4 are divided by the source of the sample: either MEF or nasopharynx (NP). Overall, S. pneumoniae was less present in MEF than in NP samples. In MEF samples, the presence of S. pneumoniae ranged from 5.8% in Germany (after the introduction of PCV10/PCV13 to their NIP) and to 70.3% in Romania (which did not include PCVs in their NIP at the time). Estimates from NP samples ranged from 10.5% in Sweden (post-PCV10/PCV13) to 73.4% in Iceland (post-PCV10) [57, 58]. Etiology data sourced from both MEF and NP swabs in the same age groups were available for three countries (France, Germany and Portugal). In all three cases, the estimates varied between sampling methods but were also lower in MEF than in NP samples. For the countries with data across time for the same age group and sampling method, only one country showed a discernable difference in the share of cases caused by S. Pneumoniae; there was about a 50% difference in S. Pneumoniae detection between two studies estimating the etiology of AOM in central Romania (70.3% to 32.9%). However, this difference could in part be explained by differences in the study populations. One study focused on AOM patients with otorrhea or who underwent tympanocentesis, while the other only focused on any presenting AOM cases.
Antibiotic resistance
In total, there were 23 records that included data related to at least one antibiotic (S5 Table). Of these, seven studies had additional information regarding serotype-specific resistance to any of the antibiotics. Most studies were conducted in France, Spain and Belgium.
The included studies had estimates for either resistance, intermediate resistance or non-susceptibility (resistance + intermediate resistance combined) for a total of 19 different antibiotics (S5 Table). The most commonly tested antibiotic was penicillin, which was included in 96% of the records, followed by erythromycin (57%), multiple drugs (multidrug resistance, 39%), tetracycline (17%) and ceftriaxone (17%). Nearly all studies assessing penicillin susceptibility used MEF samples; there was only one study conducted in Belgium (Ekinci et al., 2021) that used NP samples [78].
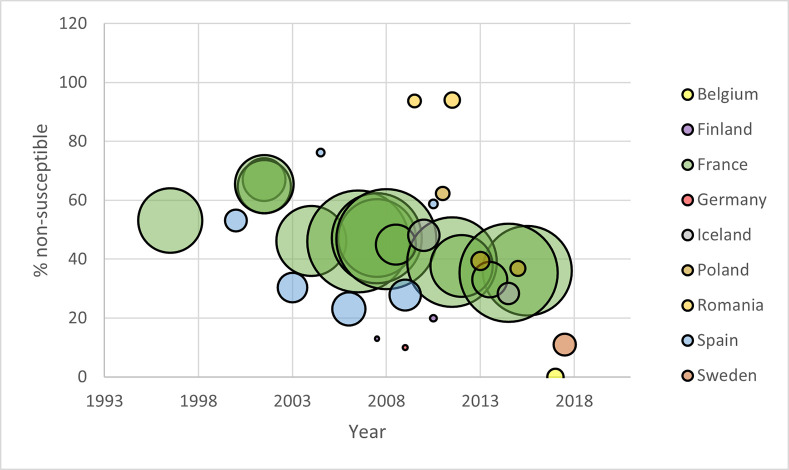
In general, the estimates from the included studies revealed a decrease in the level of penicillin non-susceptibility over time, in countries or periods where PCVs had been introduced (Fig 4). The study reporting on Romania (where no PCV had been introduced into the NIP as of 2021) showed high non-susceptibility (94%) remaining consistent over the time span. Studies from two countries, Finland and Spain, estimated an upward trend between two periods, based on smaller sample sizes (n<40).
Fig 4. Penicillin non-susceptibility1 of S. pneumoniae over time, by country and sample size [11, 12, 60, 66, 68–73, 75–77, 79–86].
Notes: 1. Non-susceptibility measured as resistance + intermediate resistance. 2. The size of the bubble corresponds proportionally with sample size.
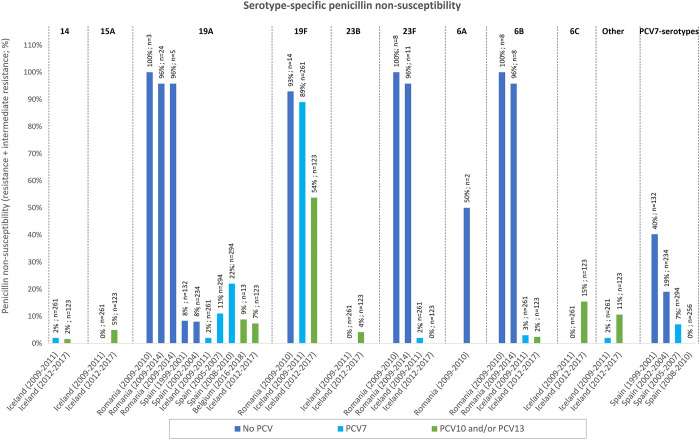
In studies where specific serotypes were assessed, the reported serotypes included 14, 15A, 19A, 19F, 23B, 23F, 6A, 6B, 6C, others (not specified), PCV7-specific serotypes, and serotypes other than 19A. Fig 5 presents the available data for serotype-specific non-susceptibility. Two separate studies estimated that non-susceptibility was especially high in Romania (between 50–100% for the included serotypes), where PCVs were not included in the NIP as of 2021 [68, 69]. The non-susceptibility for many serotypes appeared to be lower in the later PCV-periods compared to the earlier periods, although there were variations between countries and no clear trend was evident. However, when looking at estimates for serotypes not included in PCV13 (or the previous PCVs), one study in Iceland estimated slight increases in non-susceptibility (between 4%-15% over 8 years). This increase in non-susceptibility for non-vaccine serotypes has been documented in other studies [87–90].
Fig 5. Serotype-specific penicillin non-susceptibility during different PCV periods in Iceland, Romania, Spain and Belgium [68, 69, 76, 78, 84, 85].
Notes: 1. No-PCV, early PCV, later PCV.
Serotype distribution
A total of 32 records included data related to serotype distribution. Due to the variability of study populations, sample sizes, serotypes and time span in data collection (25 years, from 1993 to 2018), it was deemed more appropriate to provide a summary of the serotype distributions reported since 2011, by country, for all serotypes covered by PCV20 or lower (Table 5). In total, 17 records contained data collected from 2011 or later, spanning 10 countries. For seven of the records, samples were taken from middle ear fluid (MEF), while 10 sampled serotypes using nasopharyngeal swabbing and one study did not specify the kind(s) of samples taken. As is evident in the table, the gaps in serotype information for countries with available data are considerable, depending on the country. No trends were discernable across countries or studies in the collected data for serotypes covered by existing PCVs.
Table 5. Frequency (%) of PCV-serotypes identified between 2011–2021 by testing source and country.
| Location | Data period | PCVs included during period | Population (n, by order of publication) | PCV7 | PCV10 | PCV13 | PCV15 | PCV20 | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 6B | 9V | 14 | 18C | 19F | 23F | PCV7 serotypes | 1 | 5 | 7F | 3 | 6A | 19A | 1, 3, 5, 6A, 7F, 19A | 3, 6A, 19A | PCV13 serotypes | 22F | 33F | 8 | 10A | 11A | 12F | 15B | ||||
| Middle ear fluid | |||||||||||||||||||||||||||
| France [11] | 2011 | 13 | 0–16 years (n = 152) | 0% | 0% | 0% | 1% | 1% | 10% | 1% | 13% | 38% | 49% | ||||||||||||||
| Germany [57] | 2011 | 10/13 | 2–71 months (n = 12) | 8% | 8% | 33% | 33% | 8% | |||||||||||||||||||
| 2012 | 10/13 | 2–71 months (n = 28) | 4% | 4% | 32% | 21% | 4% | 4% | 18% | ||||||||||||||||||
| 2013 | 10/13 | 2–71 months (n = 21) | 5% | 5% | 38% | 10% | 5% | 5% | |||||||||||||||||||
| 2014 | 10/13 | 2–71 months (n = 17) | 12% | 41% | 6% | 6% | |||||||||||||||||||||
| 2015 | 10/13 | 2–71 months (n = 14) | 43% | 7% | 7% | 7% | |||||||||||||||||||||
| Iceland [76] | 2012–2017 | 10 | 0–23 months (n = 273) | 0% | 1% | 0% | 1% | 0% | 17% | 2% | 2% | 6% | 5% | 0% | 4% | 3% | |||||||||||
| 10 | 24–47 months (n = 273) | 0% | 5% | 0% | 2% | 0% | 11% | 9% | 6% | 10% | 5% | 2% | 1% | 5% | |||||||||||||
| Italy [91] | 2015–2016 | 13 | <24 months (n = 23 Vaccinated) | 0% | 0% | 0% | 9% | 0% | 9% | 0% | 17% | 0% | 0% | 0% | 13% | 0% | 0% | 13% | 0% | ||||||||
| 13 | <24 months (n = 1 Not vaccinated) | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 100% | 0% | 0% | 100% | 0% | ||||||||||
| 13 | 24–59 months (n = 13 Vaccinated) | 8% | 0% | 0% | 0% | 0% | 8% | 0% | 14% | 8% | 0% | 0% | 8% | 0% | 0% | 15% | 0% | ||||||||||
| 13 | 24–59 months (n = 1 Not vaccinated) | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 100% | ||||||||||
| Romania [69] | 2009–2014 | None | <60 months (n = 68) | 12% | 13% | 35% | 16% | 78% | 0% | 3% | 7% | 90% | |||||||||||||||
| Slovakia [92] | 2016–2017 | 10/13 | 0–71 months (n = 295) | 22% | 41% | ||||||||||||||||||||||
| Spain [70] | 2009–2012 | 7→ | 3–36 months (n = 24) | 4% | 4% | 13% | 17% | 4% | 4% | ||||||||||||||||||
| 10/13(P) | |||||||||||||||||||||||||||
| Nasopharynx | |||||||||||||||||||||||||||
| Belgium [71, 78] | 2016 | 13 | 6–30 months (n = 27, n = 79) | 4% | 4% | 0% | 0% | 0% | 0% | 0% | 4% | 14% | 7% | ||||||||||||||
| 2016 | 13 | 4% | 4% | 0% | 0% | 0% | 0% | 0% | 4% | 15% | 7% | ||||||||||||||||
| 2016–2017 | 13 | 0% | 3% | 0% | 0% | 3% | 0% | 3% | 5% | 6% | 10% | ||||||||||||||||
| 2017 | 13 | 0% | 2% | 0% | 0% | 5% | 0% | 4% | 8% | 5% | 6% | 10% | |||||||||||||||
| 2018 | 13 | 0% | 3% | 0% | 0% | 2% | 0% | 6% | 8% | 6% | 7% | 6% | |||||||||||||||
| 2016–2018 | 13 | 0% | |||||||||||||||||||||||||
| France [12, 63, 72, 73, 75, 93] | 2011–2012 | 13 | 6–24 months (n = 976) | 3% | |||||||||||||||||||||||
| 2010–2013 | 13 | 6–24 months (n = NS) | 3% | 8% | 11% | 9% | |||||||||||||||||||||
| 2014 | 13 | 6–24 months (n = 7,991) | 1% | 1% | 7% | ||||||||||||||||||||||
| 2013–2016 | 13 | 6–24 months (n = 1994) | 3% | 10% | |||||||||||||||||||||||
| 2011–2018 | 13 | 6–24 months (n = 2409) | 3% | ||||||||||||||||||||||||
| 2001–2019 | None→ | 6–24 months (n = 10,740) | <3% | <3% | <3% | 5–10% | 5–10% | ||||||||||||||||||||
| 7→13 | |||||||||||||||||||||||||||
| Switzerland [94] | 2011–2015 | 13 | <12 months (n = 108) | 10% | 22% | ||||||||||||||||||||||
| 13 | 24–59 months (n = 108) | 9% | 29% | ||||||||||||||||||||||||
| Not specified | |||||||||||||||||||||||||||
| France [95] | 2015–2018 | 13 | 6–23 months (n = 1,515) | 7% | 2% | 2% | 1% | 5% | 10% | 1% | |||||||||||||||||
Abbreviations (P), only included in NIP for certain regions. NS, not specified
Notes: Distributions reported as 0% represent serotypes that were examined in the studies but were not detected in any sample
Values are rounded with no decimals. Sample sizes vary by study
There were also seven studies, covering five countries, which reported serotype distribution data across time for ‘non-vaccine serotypes,’ referring to serotypes not covered by PCV13 or lower-valency vaccines (e.g., 15A, 6C, 21, etc.). As is shown in Table 6, all seven publications reported evidence of increases in the percentage of AOM cases attributed to non-vaccine serotypes of S. pneumoniae. This evidence suggests an increase in AOM cases involving non-vaccine serotypes across Europe, which would be an indication of increased residual burden after the introduction of PCVs in the included countries.
Table 6. Serotype distributions over time for reported "non-PCV" serotypes.
| Record | Country | Age group | Test location | Serotypes | Time period | PCV in NIP | n | % |
|---|---|---|---|---|---|---|---|---|
| Rybak et al 2018 [73] | France | 6 to 24 months | NP | Non-PCV13 and non-6C serotypes | 2001–2002 | No PCV | 867 | 14.30% |
| 2003–2005 | 7 (R) | 1229 | 32.40% | |||||
| 2006–2010 | 7 | 2488 | 56.10% | |||||
| 2011–2012 | 13 | 976 | 83.70% | |||||
| ≥2013 | 13 | 1994 | 91.70% | |||||
| Kempf et al 2015 [11] | France | 0–16 years | MEF | 15A | 2001 | No PCV | 152 | 0.30% |
| 15A | 2011 | 13 | 341 | 6.60% | ||||
| 23A | 2001 | No PCV | 152 | 0.60% | ||||
| 23A | 2011 | 13 | 341 | 3.90% | ||||
| Allemann et al 2017 [94] | Switzerland | <1 year | NP | Non-PCV13 isolates | 2004–2006 | 7 (R)→ 7 | 314 | 23.60% |
| 2007–2010 | 7 | 188 | 39.40% | |||||
| 2011–2015 | 13 | 109 | 67.90% | |||||
| 2–4 years | NP | Non-PCV13 isolates | 2004–2006 | 7 (R)→ 7 | 251 | 22.70% | ||
| 2007–2010 | 7 | 145 | 35.20% | |||||
| 2011–2015 | 13 | 77 | 62.30% | |||||
| <1 year | MEF | Non-PCV13 isolates | 2004–2006 | 7 (R)→ 7 | 17 | 0.00% | ||
| 2007–2010 | 7 | 20 | 25.00% | |||||
| 2011–2015 | 13 | 16 | 18.80% | |||||
| 2–4 years | MEF | Non-PCV13 isolates | 2004–2006 | 7 (R)→ 7 | 8 | 12.50% | ||
| 2007–2010 | 7 | 16 | 12.50% | |||||
| 2011–2015 | 13 | 8 | 25.00% | |||||
| Ouldali et al 2019 [75] | France | 6–24 months | NP | non-PCV13 serotypes | 2001–2002 | No PCV | 719 | 23.10% |
| 2003–2010 | 7 (R) → 7 → 13 | 2589 | 50.10% | |||||
| 2011–2018 | 13 | 2409 | 89.20% | |||||
| Wouters et al 2019 [71] | Belgium | 6–30 months | NP | 23B | 2016 | 13 | 27 | 11.50% |
| 23B | 2017 | 13 | 79 | 16.50% | ||||
| 23A | 2016 | 13 | 27 | 3.80% | ||||
| 23A | 2017 | 13 | 79 | 3.80% | ||||
| 21 | 2016 | 13 | 27 | 0.00% | ||||
| 21 | 2017 | 13 | 79 | 5.10% | ||||
| Quirk et al 2018 [76] | Iceland | 0-<2 years | MEF | 21 | 2009–2011 | 7 (R) → 10 | 401 | 0.00% |
| 21 | 2012–2017 | 10 | 273 | 3.30% | ||||
| 38 | 2009–2011 | 7 (R) → 10 | 401 | 0.50% | ||||
| 38 | 2012–2017 | 10 | 273 | 0.00% | ||||
| 15A | 2009–2011 | 7 (R) → 10 | 401 | 0.00% | ||||
| 15A | 2012–2017 | 10 | 273 | 1.50% | ||||
| 16F | 2009–2011 | 7 (R) → 10 | 401 | 0.20% | ||||
| 16F | 2012–2017 | 10 | 273 | 0.00% | ||||
| 19C | 2009–2011 | 7 (R) → 10 | 401 | 0.20% | ||||
| 19C | 2012–2017 | 10 | 273 | 0.00% | ||||
| 23A | 2009–2011 | 7 (R) → 10 | 401 | 0.50% | ||||
| 23A | 2012–2017 | 10 | 273 | 6.60% | ||||
| 23B | 2009–2011 | 7 (R) → 10 | 401 | 0.20% | ||||
| 23B | 2012–2017 | 10 | 273 | 3.70% | ||||
| 35B | 2009–2011 | 7 (R) → 10 | 401 | 0.00% | ||||
| 35B | 2012–2017 | 10 | 273 | 2.20% | ||||
| 35F | 2009–2011 | 7 (R) → 10 | 401 | 0.00% | ||||
| 35F | 2012–2017 | 10 | 273 | 0.70% | ||||
| 6C | 2009–2011 | 7 (R) → 10 | 401 | 0.20% | ||||
| 6C | 2012–2017 | 10 | 273 | 12.60% | ||||
| Other | 2009–2011 | 7 (R) → 10 | 401 | 2.70% | ||||
| Other | 2012–2017 | 10 | 273 | 14.70% | ||||
| Alonso et al 2013 [79] | Spain | <5 years | MEF | 6C | 1999–2001 | No PCV | 117 | 0.00% |
| 6C | 2002–2004 | No PCV | 209 | 0.50% | ||||
| 6C | 2005–2007 | No PCV → 7 (P) | 265 | 1.10% | ||||
| 6C | 2008–2010 | 7 (P) → 10/13 (P) | 227 | 4.40% | ||||
| 15A | 1999–2001 | No PCV | 117 | 0.00% | ||||
| 15A | 2002–2004 | No PCV | 209 | 0.00% | ||||
| 15A | 2005–2007 | No PCV → 7 (P) | 265 | 1.10% | ||||
| 15A | 2008–2010 | 7 (P) → 10/13 (P) | 227 | 1.30% | ||||
| 16F | 1999–2001 | No PCV | 117 | 0.90% | ||||
| 16F | 2002–2004 | No PCV | 209 | 1.40% | ||||
| 16F | 2005–2007 | No PCV → 7 (P) | 265 | 2.60% | ||||
| 16F | 2008–2010 | 7 (P) → 10/13 (P) | 227 | 2.60% | ||||
| 21 | 1999–2001 | No PCV | 117 | 0.90% | ||||
| 21 | 2002–2004 | No PCV | 209 | 1.00% | ||||
| 21 | 2005–2007 | No PCV → 7 (P) | 265 | 3.80% | ||||
| 21 | 2008–2010 | 7 (P) → 10/13 (P) | 227 | 1.80% | ||||
| 23A | 1999–2001 | No PCV | 117 | 0.90% | ||||
| 23A | 2002–2004 | No PCV | 209 | 1.00% | ||||
| 23A | 2005–2007 | No PCV → 7 (P) | 265 | 1.50% | ||||
| 23A | 2008–2010 | 7 (P) → 10/13 (P) | 227 | 0.90% | ||||
| 23B | 1999–2001 | No PCV | 117 | 0.00% | ||||
| 23B | 2002–2004 | No PCV | 209 | 1.00% | ||||
| 23B | 2005–2007 | No PCV → 7 (P) | 265 | 1.10% | ||||
| 23B | 2008–2010 | 7 (P) → 10/13 (P) | 227 | 1.30% | ||||
| 31 | 1999–2001 | No PCV | 117 | 0.00% | ||||
| 31 | 2002–2004 | No PCV | 209 | 0.00% | ||||
| 31 | 2005–2007 | No PCV → 7 (P) | 265 | 0.40% | ||||
| 31 | 2008–2010 | 7 (P) → 10/13 (P) | 227 | 2.20% | ||||
| 38 | 1999–2001 | No PCV | 117 | 0.00% | ||||
| 38 | 2002–2004 | No PCV | 209 | 0.00% | ||||
| 38 | 2005–2007 | No PCV → 7 (P) | 265 | 0.40% | ||||
| 38 | 2008–2010 | 7 (P) → 10/13 (P) | 227 | 0.00% | ||||
| Other | 1999–2001 | No PCV | 117 | 0.00% | ||||
| Other | 2002–2004 | No PCV | 209 | 4.30% | ||||
| Other | 2005–2007 | No PCV → 7 (P) | 265 | 3.40% | ||||
| Other | 2008–2010 | 7 (P) → 10/13 (P) | 227 | 2.60% | ||||
| Non-typable | 1999–2001 | No PCV | 117 | 2.60% | ||||
| Non-typable | 2002–2004 | No PCV | 209 | 1.00% | ||||
| Non-typable | 2005–2007 | No PCV → 7 (P) | 265 | 1.50% | ||||
| Non-typable | 2008–2010 | 7 (P) → 10/13 (P) | 227 | 0.00% |
Economic burden
In total, 32 studies were identified which contained data related to the economic burden of AOM. The studies provided data for 21 countries, with some studies providing data for multiple countries. S6 Table provides summary data for those countries, including the types of cost data (direct medical, direct non-medical, indirect costs) and which studies involved economic modelling. Direct medical cost, direct non-medical cost and indirect cost estimates were available in 91%, 55% and 82% of countries, respectively.
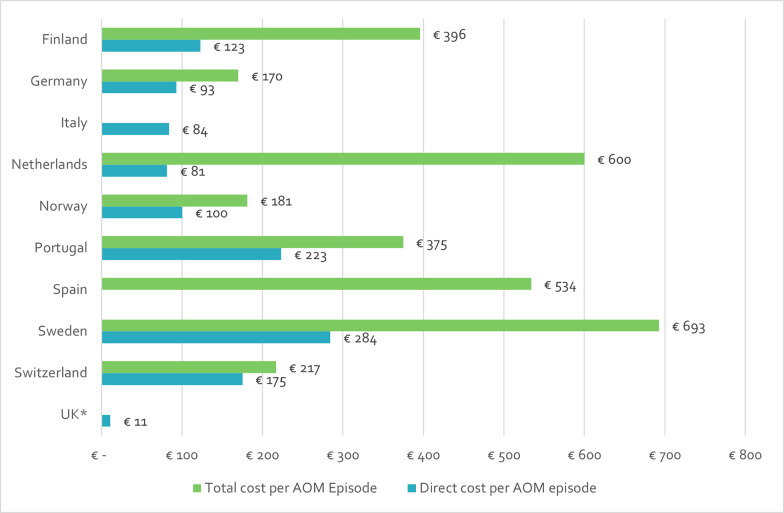
Direct medical costs were the most common economic burden outcome represented in the data. The total direct medical costs estimated by the included records varied by country and record, ranging from €10.98 in the UK to €284.35 in Sweden. Total costs (including direct medical, direct non-medical and indirect costs) were reported for nine countries, across four studies [35, 96–98]. The latest estimates for each country ranged from €170 in the Germany to €693 in Sweden [35, 96, 97]. The most recent estimates of direct medical costs and total costs from each available country are presented together in Fig 6 [35, 96–100]. With the exception of the Netherlands, the variation between countries in the latest estimates for direct and total costs mirrored each other. However, due to variations in the methods and costs included by each study, no conclusions could be drawn from the available evidence.
Fig 6. Estimates for direct medical and total costs per AOM episode (2020 EUR), by country [35, 96–100].
*The UK estimate for average direct medical costs per AOM episode was collected from a conference abstract with limited information about the calculation methods. The explanation for the estimate provided by the authors was that the cost per episode and the contribution of direct/indirect costs varied between countries, potentially reflecting socio-economic differences and variation in AOM management [100]. Therefore, the accuracy of the estimate should be interpreted with caution.
Quality of life (humanistic burden)
Nineteen studies provided QoL data for 19 European countries. Out of those, 10 use the same utility decrement of 0.005 QALY per AOM episode, which was obtained from Oh et al, 1996 and Melegaro and Edmunds, 2004 [101, 102]. Furthermore, one study considered a disutility of 0.09 QALYs for hearing loss due to AOM while two other studies used a disability weight of 0.02 DALYs [46, 103]. The list of studies that report QoL data is presented in S6 Table.
Different disease-specific questionnaires have been used to assess quality of life in AOM. The Otitis Media-6 (OM-6) questionnaire was used in three studies conducted in Denmark. The questionnaire consists of six domains of functional health status (FHS) to measure the child’s physical suffering, hearing loss, speech impairment, emotional distress, activity limitations and the caregiver’s concern, as well as a numerical rating scale (NRS-child) to assess the child’s QoL [104]. Parents answer the questionnaire based on symptoms experienced by the child in the past four weeks and a summary score can be obtained by adjusting the scores to a scale of 0 to 100 [104–106]. Additionally, the Caregiver Impact Questionnaire was used in the study by Heidemann et al 2014 [107]. Two studies used self-developed QoL questionnaires [108, 109].
Discussion
This systematic literature review is, to our knowledge, the first study that has gathered data from published literature on AOM incidence, etiology, antibiotic resistance and serotype distribution for S. pneumoniae, costs of illness and impact on quality of life across Europe. The identified data provided evidence of a shift in residual burden of AOM after the introduction of PCVs for countries where data was available. This was notable on serotype distribution and antibiotic resistance for non-vaccine serotypes. There was also evidence of a decrease in hospitalization rates due to AOM and a general decrease in antibiotic resistance rates in S. pneumoniae, which are contributing factors especially for the economic burden of AOM. However, due to the limited availability of data and the methodological differences of the included literature, the authors advise caution in applying the conclusions across the European region as a whole.
The identified records revealed a wide variation in estimates between countries and over time for all outcomes assessed. Other studies have also acknowledged that the variability of AOM data limited comparison across studies [4, 35]. This data heterogeneity is likely caused by many factors. For instance, the authors encountered 18 different definitions for AOM used in the included studies (S7 Table), which could contribute to the high variability in incidence rates between publications. In addition, the differences in the ages of study populations also hindered a more meaningful comparison of incidence, etiology and serotype distribution estimates between and within the included countries.
Furthermore, differences in clinical practice might affect comparability across studies, such as the choice of sampling and analytical methods in the analysis for pathogen etiology and pneumococcus serotype distribution of AOM. For example, the use of polymerase chain reaction (PCR) versus bacterial culture for the detection of S. pneumoniae can impact the accuracy of estimates [110]. As mentioned in the Etiology section, estimates from countries with data from the same years and target populations, but different sampling methods, varied considerably. This finding might indicate that other factors could explain variation between samples collected from MEF and NP swabs. Based on previous literature, the correlation between bacterial cultures in MEF and the nasopharynx is strong but not absolute, making it difficult to assume the different methods would produce similar results [69, 111–115].
Variations in the aggregation of data also complicated the prospect of comparisons. For example, previous studies often reported the distribution of non-PCV serotypes as a group, without specifying which individual serotypes had been identified. This imposed a challenge, particularly when analyzing older studies, since the group of non-PCV serotypes at that time likely contained some serotypes that were later incorporated into the most recent PCVs [85, 116]. However, since they were not reported individually, this added to the difficulty of identifying trends over time for the grouped serotypes, particularly for the years between PCV7 and PCV10/13 introduction.
A similar complication was also noticed for the economic burden estimates; the types of costs included in the major cost categories varied widely, as did the methods of calculation. This was particularly evident with the reporting of direct medical costs and estimation of cost per AOM episode shown in Fig 6, since the original publication that reported the remarkably low cost per AOM episode in the UK did not specify which costs were considered in their calculations [100]. Moreover, there were variations in non-medical costs (e.g., inclusion of public transportation, estimation of time spent vs. distance travelled, etc.) and indirect costs (e.g., productivity loss vs. caregiver burden, the inputs chosen to estimate those, etc.). The differences in methods, combined with the other factors discussed above, precluded any sort of meaningful comparison between countries or across time in these cost categories.
In addition to the data variability, there were large data gaps in both clinical and economic outcomes for some European countries and certain time-periods; only 11 of the 31 included countries had at least one record reporting on every outcome of interest. For six countries, there were no studies with relevant data identified at all. Furthermore, though the literature searches were limited to the last ten years, almost 30% of the data identified came from before 2011.
Despite these complications, the authors were able to identify a few trends in the data. First, there were indications of a general reduction in hospitalization rate after the introduction of PCVs. This factor should point to a reduction in the economic burden of AOM over time, as a decrease in the occurrence or the average severity and duration of AOM episodes would mean a decrease in the resources and time required to treat those patients. However, time trends in the economic burden estimates that were identified were limited, making confirmation of this hypothesis difficult. Second, a reduction in antibiotic-resistant pneumococci was also identified in the literature, which is line with previous studies [117, 118]. On the other hand, there was evidence of an increase in non-vaccine serotypes over time for countries that had introduced PCVs into their NIP. This serotype replacement phenomenon has already been described for other manifestations of pneumococcal disease. Also, there was limited evidence of an increase in antibiotic resistance within non-vaccine serotypes. Both of these trends suggest that a residual burden remains despite the shift towards higher-valency PCVs.
The natural assumption for addressing residual burden would be to increase the valency of available PCVs. However, recent studies have shown that ‘more is not necessarily better’; a systematic review by Mungall et al in 2022 identified vaccine failures and breakthrough IPD with PCV10 and PCV13 in children up to 5 years and warned of the importance of addressing incomplete protection against certain serotypes [119]. Also, a 2020 study by Løchen et al identified a reduction in the marginal benefit of further increasing the valency of vaccines, as the vanishing of a dominant serotype after PCV introduction mitigates the benefits of targeting and covering additional serotypes [120].
This review had certain limitations when it came to methods and data synthesis. Regarding the former, the exclusion of grey literature, such as surveillance reports or policy documents, from the scope of this review limited the potential for relevant data in some of the outcome categories. However, as this was a review of data available in published literature since 2011, the data gaps are part of the narrative of this review. Additionally, given that AOM was the main focus and also a required search term, many relevant articles concerning PCV-coverage were not captured since these vaccines are also protective against other pneumococcal disease manifestations more well captured in terms of surveillance. Also, records regarding PCV-coverage were still included in the summary statistics as they were indeed part of the original search and extraction process, but there was no synthesis done based on the reported estimates due to the clear risk of bias arising from the factors described above. Finally, additional indicators for a change in AOM burden, such as the rates of physician office visits and antibiotic prescriptions, were not explored in this study, but could have provided further insight.
Conclusions
This study compiled the most recent published evidence on the clinical and economic burden of AOM among European children. Despite the data gaps, it was possible to obtain comparable estimates for reduction in both hospitalization rates and antibiotic resistance of S. pneumoniae, as well as evidence of an increase in residual burden for pneumococcal AOM from non-vaccine serotypes, in the countries with available data. The lack of a homogenous trend in incidence rates across countries and the increased frequency of new serotypes indicate that the burden of pneumococcal AOM is still meaningful, despite the introduction of PCVs. Thus, vaccination programs should be maintained with high coverage rates and re-evaluated when new PCVs become available.
The data variability identified in the present study confirms the need for more standardization when reporting information related to AOM. For instance, future researchers could use the STROBE guidelines for reporting cross-sectional studies when reporting data on AOM [121]. Also, when applicable, the World Health Organization’s recommendations for detecting carriage of S. pneumoniae in NP samples should be followed [122]. Another way to address this issue is with the implementation of surveillance systems to monitor VCR, serotype distribution, etiology, antibiotic resistance, and disease burden. Surveillance systems are instrumental for understanding the burden of pneumococcal diseases, including AOM, which can guide vaccination programs. Even though surveillance systems for invasive pneumococcal diseases exist in all of the European countries included in this study, there is limited information on surveillance of noninvasive forms of pneumococcal disease, like AOM [123]. Thus, it is still difficult to provide comprehensive, accurate and up-to-date estimates of AOM-burden and epidemiology for the European region using published literature. This study confirms that there are still significant data gaps within the published literature, which points to a greater need for surveillance systems monitoring AOM, while also providing some evidence of a reduction in the burden of AOM over time in countries with available data, likely attributed to the introduction of PCVs.
Supporting information
Notes: ®: Vaccine is not administered to the entire population, only to specific risk groups. (P): Vaccine is administered only in certain regions of the country. Sources: [9, 19–28].
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Data Availability
Data underlying the results are in the Supporting Information files.
Funding Statement
This study has been financed by Merck Sharp & Dohme (MSD). MSD was involved in reviewing the study design, data collection and analysis. In addition, MSD has been responsible for the decision to publish as well as reviewing the manuscript preparation. The funder (MSD) provided support in the form of salaries for authors ET and GB. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Venekamp RP, Damoiseaux RA, Schilder AG. Acute Otitis Media in Children. Am Fam Physician. 2017;95(2):109–10. [PubMed] [Google Scholar]
- 2.Gaddey HL, Wright MT, Nelson TN. Otitis Media: Rapid Evidence Review. Am Fam Physician. 2019;100(6):350–6. [PubMed] [Google Scholar]
- 3.Soriano F. Microbial etiologies of acute otitis media. Clin Microbiol Infect. 1997;3 Suppl 3:S23–s5. [PubMed] [Google Scholar]
- 4.Monasta L, Ronfani L, Marchetti F, Montico M, Vecchi Brumatti L, Bavcar A, et al. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One. 2012;7(4):e36226. doi: 10.1371/journal.pone.0036226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wasserman MD, Perdrizet J, Grant L, Hayford K, Singh S, Saharia P, et al. Clinical and Economic Burden of Pneumococcal Disease Due to Serotypes Contained in Current and Investigational Pneumococcal Conjugate Vaccines in Children Under Five Years of Age. Infectious Diseases and Therapy. 2021;10(4):2701–20. doi: 10.1007/s40121-021-00544-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leibovitz E, Broides A, Greenberg D, Newman N. Current management of pediatric acute otitis media. Expert Rev Anti Infect Ther. 2010;8(2):151–61. doi: 10.1586/eri.09.112 [DOI] [PubMed] [Google Scholar]
- 7.Dagan R. Impact of pneumococcal conjugate vaccine on infections caused by antibiotic-resistant Streptococcus pneumoniae. Clin Microbiol Infect. 2009;15 Suppl 3:16–20. doi: 10.1111/j.1469-0691.2009.02726.x [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease control and Prevention (CDC). Pneumococcal Vaccine Recommendations 2021. Available from: https://www.cdc.gov/vaccines/vpd/pneumo/hcp/recommendations.html. [Google Scholar]
- 9.European Center for Disease Control (ECDC). Pneumococcal Disease: Recommended vaccinations 2021. Available from: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=25&SelectedCountryIdByDisease=-1. [Google Scholar]
- 10.Introduction of PCV (Pneumococcal conjugate vaccine) [Internet]. 2022 [cited 09 Sep 2022]. Available from: https://immunizationdata.who.int/pages/vaccine-intro-by-antigen/pneumo_conj.html?ISO_3_CODE=AUT+BEL+BGR+HRV+CYP+CZE+DNK+EST+FIN+FRA+DEU+GRC+HUN+ISL+IRL+LVA+LTU+LUX+MLT+NLD+NOR+POL+PRT+ROU+SVK+SVN+ESP+SWE+CHE+GBR&YEAR=.
- 11.Kempf M, Varon E, Lepoutre A, Gravet A, Baraduc R, Brun M, et al. Decline in antibiotic resistance and changes in the serotype distribution of Streptococcus pneumoniae isolates from children with acute otitis media; a 2001–2011 survey by the French Pneumococcal Network. Clinical Microbiology and Infection. 2015;21(1):35–42. doi: 10.1016/j.cmi.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 12.Angoulvant F, Cohen R, Doit C, Elbez A, Werner A, Béchet S, et al. Trends in antibiotic resistance of Streptococcus pneumoniae and Haemophilus influenzae isolated from nasopharyngeal flora in children with acute otitis media in France before and after 13 valent pneumococcal conjugate vaccine introduction. BMC Infect Dis. 2015;15:236. doi: 10.1186/s12879-015-0978-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zielnik-Jurkiewicz B, Bielicka A. Antibiotic resistance of Streptococcus pneumoniae in children with acute otitis media treatment failure. Int J Pediatr Otorhinolaryngol. 2015;79(12):2129–33. doi: 10.1016/j.ijporl.2015.09.030 [DOI] [PubMed] [Google Scholar]
- 14.Amicizia D, Astengo M, Paganino C, Piazza MF, Sticchi C, Orsi A, et al. Economic burden of pneumococcal disease in children in Liguria, Italy. Hum Vaccin Immunother. 2022;18(5):2082205. doi: 10.1080/21645515.2022.2082205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbieri E, Porcu G, Hu T, Petigara T, Senese F, Prandi GM, et al. A Retrospective Database Analysis to Estimate the Burden of Acute Otitis Media in Children Aged <15 Years in the Veneto Region (Italy). Children (Basel). 2022;9(3). doi: 10.3390/children9030436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu T, Done N, Petigara T, Mohanty S, Song Y, Liu Q, et al. Incidence of acute otitis media in children in the United States before and after the introduction of 7- and 13-valent pneumococcal conjugate vaccines during 1998–2018. BMC Infect Dis. 2022;22(1):294. doi: 10.1186/s12879-022-07275-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izurieta P, Bahety P, Adegbola R, Clarke C, Hoet B. Public health impact of pneumococcal conjugate vaccine infant immunization programs: assessment of invasive pneumococcal disease burden and serotype distribution. Expert Rev Vaccines. 2018;17(6):479–93. doi: 10.1080/14760584.2018.1413354 [DOI] [PubMed] [Google Scholar]
- 18.Talbird SE, Taylor TN, Caporale J, Ismaila AS, Gomez J. Residual economic burden of Streptococcus pneumoniae- and nontypeable Haemophilus influenzae-associated disease following vaccination with PCV-7: a multicountry analysis. Vaccine. 2010;28 Suppl 6:G14–22. doi: 10.1016/j.vaccine.2010.06.080 [DOI] [PubMed] [Google Scholar]
- 19.Ceyhan M, Dagan R, Sayiner A, Chernyshova L, Dinleyici E, Hryniewicz W, et al. Surveillance of pneumococcal diseases in Central and Eastern Europe. Hum Vaccin Immunother. 2016;12(8):2124–34. doi: 10.1080/21645515.2016.1159363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Félix S, Handem S, Nunes S, Paulo AC, Candeias C, Valente C, et al. Impact of private use of the 13-valent pneumococcal conjugate vaccine (PCV13) on pneumococcal carriage among Portuguese children living in urban and rural regions. Vaccine. 2021;39(32):4524–33. doi: 10.1016/j.vaccine.2021.06.035 [DOI] [PubMed] [Google Scholar]
- 21.Koelman DLH, Brouwer MC, van de Beek D. Resurgence of pneumococcal meningitis in Europe and Northern America. Clin Microbiol Infect. 2020;26(2):199–204. doi: 10.1016/j.cmi.2019.04.032 [DOI] [PubMed] [Google Scholar]
- 22.Maraki S, Mavromanolaki VE, Stafylaki D, Hamilos G, Samonis G. The Evolving Epidemiology of Serotype Distribution and Antimicrobial Resistance of Streptococcus pneumoniae Strains Isolated from Adults in Crete, Greece, 2009–2016. Infect Chemother. 2018;50(4):328–39. doi: 10.3947/ic.2018.50.4.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petráš M, Adámková V. Epidemiology of Invasive Pneumococcal Disease in Czech Children under 5 Years of Age after Routine Immunisation. Cent Eur J Public Health. 2016;24(2):133–6. doi: 10.21101/cejph.a4161 [DOI] [PubMed] [Google Scholar]
- 24.Savrasova L, Krumina A, Cupeca H, Zeltina I, Villerusha A, Grope I, et al. Invasive Pneumococcal Disease in Latvia in PCV10 Vaccination Era, 2012–2018. Front Pediatr. 2021;9:532489. doi: 10.3389/fped.2021.532489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Setchanova L, Murdjeva M, Stancheva I, Alexandrova A, Sredkova M, Stoeva T, et al. Serotype changes and antimicrobial nonsusceptibility rates of invasive and non-invasive Streptococcus pneumoniae isolates after implementation of 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in Bulgaria. Braz J Infect Dis. 2017;21(4):433–40. doi: 10.1016/j.bjid.2017.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sigurdsson S, Eythorsson E, Erlendsdóttir H, Hrafnkelsson B, Kristinsson KG, Haraldsson Á. Impact of the 10-valent pneumococcal conjugate vaccine on hospital admissions in children under three years of age in Iceland. Vaccine. 2020;38(12):2707–14. doi: 10.1016/j.vaccine.2020.01.094 [DOI] [PubMed] [Google Scholar]
- 27.Tin Tin Htar M, Christopoulou D, Schmitt HJ. Pneumococcal serotype evolution in Western Europe. BMC Infect Dis. 2015;15:419. doi: 10.1186/s12879-015-1147-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.VIEW-hub. Current Vaccine Intro Status (PCV): IVAC; 2023. Available from: https://view-hub.org/map/?set=current-vaccine-intro-status&group=vaccine-introduction&category=pcv. [Google Scholar]
- 29.European Medicines Agency. Medicines—Search "J07AL02" 2022 [cited 2022 02 May]. Available from: https://www.ema.europa.eu/en/medicines?search_api_views_fulltext=%22J07AL02%22.
- 30.Pfizer Inc. European Medicines Agency Approves Pfizer’s 20-Valent Pneumococcal Conjugate Vaccine Against Invasive Pneumococcal Disease and Pneumonia in Adults 2022 [updated 15 Feb 2022; cited 2022 02 May]. Available from: https://www.pfizer.com/news/press-release/press-release-detail/european-medicines-agency-approves-pfizers-20-valent.
- 31.Merck & Co. Inc. Merck Provides Update on FDA Review of Supplemental Biologics License Application for VAXNEUVANCE™ (Pneumococcal 15-valent Conjugate Vaccine) for Use in Infants and Children—Merck.com 2022 [updated 2022-04-01; cited 2022 02 May]. Available from: https://www.merck.com/news/merck-provides-update-on-fda-review-of-supplemental-biologics-license-application-for-vaxneuvance-pneumococcal-15-valent-conjugate-vaccine-for-use-in-infants-and-children/.
- 32.Schilder AGM, Chonmaitree T, Cripps AW, Rosenfeld RM, Casselbrant ML, Haggard MP, et al. Otitis media. Nature Reviews Disease Primers. 2016;2(1):16063. doi: 10.1038/nrdp.2016.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balsells E, Guillot L, Nair H, Kyaw MH. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: A systematic review and meta-analysis. PLoS One. 2017;12(5):e0177113. doi: 10.1371/journal.pone.0177113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeAntonio R, Yarzabal JP, Cruz JP, Schmidt JE, Kleijnen J. Epidemiology of otitis media in children from developing countries: A systematic review. Int J Pediatr Otorhinolaryngol. 2016;85:65–74. doi: 10.1016/j.ijporl.2016.03.032 [DOI] [PubMed] [Google Scholar]
- 35.Shiri T, Khan K, Keaney K, Mukherjee G, McCarthy ND, Petrou S. Pneumococcal Disease: A Systematic Review of Health Utilities, Resource Use, Costs, and Economic Evaluations of Interventions. Value in Health. 2019;22(11):1329–44. doi: 10.1016/j.jval.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 36.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.European Congress of Clinical Microbiology & Infectious Diseases. Welcome to ECCMID 2021 [2022/02/04]. Available from: https://www.eccmid.org/.
- 38.European Society for Paediatric Infectious Diseases. Come Celebrate 40 at ESPID 2022 2021 [2022/02/24]. Available from: https://espidmeeting.org/.
- 39.European Academy of Paediatrics. European Academy of Paediatrics Home Page 2021 [2022/02/24]. Available from: https://www.eapaediatrics.eu/.
- 40.Berger ML, Martin BC, Husereau D, Worley K, Allen JD, Yang W, et al. A questionnaire to assess the relevance and credibility of observational studies to inform health care decision making: an ISPOR-AMCP-NPC Good Practice Task Force report. Value Health. 2014;17(2):143–56. doi: 10.1016/j.jval.2013.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whiting P, Savović J, Higgins JPT, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–34. doi: 10.1016/j.jclinepi.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inuganti B, Inuganti A, Vsn M, Hyderboini RK, Chakrawarthy M, Chidirala SR, et al. A Comparison of Amstar and Robis Tools for Methodological Quality Assessment of Systematic Reviews of Alzheimer’s Disease. Value in health. 2018;21:S230. [Google Scholar]
- 43.Chapman R, Sutton K, Dillon-Murphy D, Patel S, Hilton B, Farkouh R, et al. Ten year public health impact of 13-valent pneumococcal conjugate vaccination in infants: A modelling analysis. Vaccine. 2020;38(45):7138–45. doi: 10.1016/j.vaccine.2020.08.068 [DOI] [PubMed] [Google Scholar]
- 44.Sigurdsson S, Kristinsson KG, Erlendsdóttir H, Hrafnkelsson B, Haraldsson Á. Decreased Incidence of Respiratory Infections in Children After Vaccination with Ten-valent Pneumococcal Vaccine. Pediatr Infect Dis J. 2015;34(12):1385–90. doi: 10.1097/INF.0000000000000899 [DOI] [PubMed] [Google Scholar]
- 45.Sigurdsson S, Eythorsson E, Hrafnkelsson B, Erlendsdóttir H, Kristinsson KG, Haraldsson Á. Reduction in All-Cause Acute Otitis Media in Children <3 Years of Age in Primary Care Following Vaccination With 10-Valent Pneumococcal Haemophilus influenzae Protein-D Conjugate Vaccine: A Whole-Population Study. Clin Infect Dis. 2018;67(8):1213–9. [DOI] [PubMed] [Google Scholar]
- 46.Vučina VV, Filipović SK, Kožnjak N, Stamenić V, Clark AD, Mounaud B, et al. Cost-effectiveness of pneumococcal conjugate vaccination in Croatia. Vaccine. 2015;33 Suppl 1:A209–18. doi: 10.1016/j.vaccine.2014.12.043 [DOI] [PubMed] [Google Scholar]
- 47.Usonis V, Jackowska T, Petraitiene S, Sapala A, Neculau A, Stryjewska I, et al. Incidence of acute otitis media in children below 6 years of age seen in medical practices in five East European countries. BMC Pediatr. 2016;16:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu T, Parsons V, Beier D, Galetzka W, Duong M, Qizilbash N, et al. CLINICAL AND ECONOMIC BURDEN OF RECURRENT AOM IN CHILDREN IN GERMANY FROM 2012–2017. ESPID; Virtual Meeting2020. [Google Scholar]
- 49.Sveinsdóttir H, Björnsdóttir JB, Erlendsdóttir H, Hjálmarsdóttir MÁ, Hrafnkelsson B, Haraldsson Á, et al. The effect of the 10-valent pneumococcal nontypeable haemophilus influenzae protein D conjugate vaccine on H. Influenzae in healthy carriers and middle ear infections in Iceland. Journal of Clinical Microbiology. 2019;57(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barbieri E, Cantarutti GP, Hu T, Petigara T, Alimenti C, Prandi GM, et al. A RETROSPECTIVE DATABASE ANALYSIS TO ESTIMATE THE BURDEN OF ACUTE OTITIS MEDIA IN CHILDREN 0–14 YEARS IN THE VENETO REGION, ITALY. ESPID; Virtual Meeting2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fortanier AC, Venekamp RP, De Hoog MLA, Uiterwaal CSPM, Van Der Gugten AC, Van Der Ent CK, et al. Parent-Reported symptoms of acute otitis media during the first year of Life: What is beneath the surface? PLoS ONE. 2015;10(4). doi: 10.1371/journal.pone.0121572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prins-Van Ginkel AC, Bruijning-Verhagen PC, Uiterwaal CSPM, Van Der Ent CK, Smit HA, De Hoog MLA. Acute otitis media during infancy: Parent-reported incidence and modifiable risk factors. Pediatric Infectious Disease Journal. 2017;36(3):245–9. [DOI] [PubMed] [Google Scholar]
- 53.Edmondson-Jones M, Dibbern T, Hultberg M, Anell B, Medin E, Feng Y, et al. Impact of pneumococcal conjugate vaccines on healthcare utilization and direct costs for otitis media in children ≤2 years of age in two Swedish regions. Human Vaccines & Immunotherapeutics. 2021:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johansson Kostenniemi U, Palm J, Silfverdal S-A. Reductions in otitis and other respiratory tract infections following childhood pneumococcal vaccination. Acta Paediatrica. 2018;107(9):1601–9. doi: 10.1111/apa.14345 [DOI] [PubMed] [Google Scholar]
- 55.Martinelli D, Pedalino B, Cappelli MG, Caputi G, Sallustio A, Fortunato F, et al. Towards the 13-valent pneumococcal conjugate universal vaccination: Effectivenessin the transition era between PCV7 and PCV13 in Italy, 2010–2013. Human Vaccines and Immunotherapeutics. 2014;10(1):2709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fortunato F, Martinelli D, Cappelli MG, Cozza V, Prato R. Impact of Pneumococcal Conjugate Universal Routine Vaccination on Pneumococcal Disease in Italian Children. J Immunol Res. 2015;2015:206757. doi: 10.1155/2015/206757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imöhl M, Perniciaro S, Busse A, van der Linden M. Bacterial Spectrum of Spontaneously Ruptured Otitis Media in a 7-Year, Longitudinal, Multicenter, Epidemiological Cross-Sectional Study in Germany. Front Med (Lausanne). 2021;8:675225. doi: 10.3389/fmed.2021.675225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simões A, Alvéolos R, Morales-Aza B, Finn A, Rodrigues F. Multiple bacterial species are more often present in recurrent acute otitis media (raom). ESPID; Ljubljana, Slovenia: 2019. [Google Scholar]
- 59.Laulajainen-Hongisto A, Saat R, Lempinen L, Markkola A, Aarnisalo AA, Jero J. Bacteriology in relation to clinical findings and treatment of acute mastoiditis in children. Int J Pediatr Otorhinolaryngol. 2014;78(12):2072–8. doi: 10.1016/j.ijporl.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 60.Laulajainen-Hongisto A, Saat R, Lempinen L, Aarnisalo AA, Jero J. Children hospitalized due to acute otitis media: how does this condition differ from acute mastoiditis? Int J Pediatr Otorhinolaryngol. 2015;79(9):1429–35. doi: 10.1016/j.ijporl.2015.06.019 [DOI] [PubMed] [Google Scholar]
- 61.Sillanpää S, Kramna L, Oikarinen S, Sipilä M, Rautiainen M, Aittoniemi J, et al. Next-Generation Sequencing Combined with Specific PCR Assays To Determine the Bacterial 16S rRNA Gene Profiles of Middle Ear Fluid Collected from Children with Acute Otitis Media. mSphere. 2017;2(2). doi: 10.1128/mSphere.00006-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sillanpää S, Sipilä M, Hyöty H, Rautiainen M, Laranne J. Antibiotic resistance in pathogens causing acute otitis media in Finnish children. Int J Pediatr Otorhinolaryngol. 2016;85:91–4. doi: 10.1016/j.ijporl.2016.03.037 [DOI] [PubMed] [Google Scholar]
- 63.Cohen R, Biscardi S, Levy C. The multifaceted impact of pneumococcal conjugate vaccine implementation in children in France between 2001 to 2014. Hum Vaccin Immunother. 2016;12(2):277–84. doi: 10.1080/21645515.2015.1116654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levy C, Varon E, Ouldali N, Wollner A, Thollot F, Corrard F, et al. Bacterial causes of otitis media with spontaneous perforation of the tympanic membrane in the era of 13 valent pneumococcal conjugate vaccine. PLoS One. 2019;14(2):e0211712. doi: 10.1371/journal.pone.0211712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marchisio P, Esposito S, Picca M, Baggi E, Terranova L, Orenti A, et al. Prospective evaluation of the aetiology of acute otitis media with spontaneous tympanic membrane perforation. Clinical Microbiology and Infection. 2017;23(7):486.e1–.e6. doi: 10.1016/j.cmi.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 66.Górska-Kot A, Greenberg D, Gastoł K, Zieliński A, Givon-Lavi N. Characterization of acute otitis media otopathogens before the introduction of the pneumococcal conjugated vaccine into the national immunization program in Poland. Int J Pediatr Otorhinolaryngol. 2019;127:109666. doi: 10.1016/j.ijporl.2019.109666 [DOI] [PubMed] [Google Scholar]
- 67.Korona-Glowniak I, Zychowski P, Siwiec R, Mazur E, Niedzielska G, Malm A. Resistant Streptococcus pneumoniae strains in children with acute otitis media–high risk of persistent colonization after treatment. BMC Infectious Diseases. 2018;18(1):478. doi: 10.1186/s12879-018-3398-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Falup-Pecurariu O, Leibovitz E, Mercas A, Bleotu L, Zavarache C, Porat N, et al. Pneumococcal acute otitis media in infants and children in central Romania, 2009–2011: microbiological characteristics and potential coverage by pneumococcal conjugate vaccines. Int J Infect Dis. 2013;17(9):e702–6. doi: 10.1016/j.ijid.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 69.Lixandru RI, Falup-Pecurariu C, Bleotu L, Mercas A, Leibovitz E, Dagan R, et al. Streptococcus pneumoniae Serotypes and Antibiotic Susceptibility Patterns in Middle Ear Fluid Isolates During Acute Otitis Media and Nasopharyngeal Isolates During Community-acquired Alveolar Pneumonia in Central Romania. Pediatr Infect Dis J. 2017;36(2):151–4. doi: 10.1097/INF.0000000000001379 [DOI] [PubMed] [Google Scholar]
- 70.Pumarola F, Salamanca de la Cueva I, Sistiaga-Hernando A, García-Corbeira P, Moraga-Llop FA, Cardelús S, et al. Bacterial etiology of acute otitis media in Spain in the post-pneumococcal conjugate vaccine era. Anales de Pediatria. 2016;85(5):224–31. [DOI] [PubMed] [Google Scholar]
- 71.Wouters I, Desmet S, Van Heirstraeten L, Blaizot S, Verhaegen J, Van Damme P, et al. Follow-up of serotype distribution and antimicrobial susceptibility of Streptococcus pneumoniae in child carriage after a PCV13-to-PCV10 vaccine switch in Belgium. Vaccine. 2019;37(8):1080–6. doi: 10.1016/j.vaccine.2018.12.068 [DOI] [PubMed] [Google Scholar]
- 72.Cohen R, Varon E, Doit C, Schlemmer C, Romain O, Thollot F, et al. A 13-year survey of pneumococcal nasopharyngeal carriage in children with acute otitis media following PCV7 and PCV13 implementation. Vaccine. 2015;33(39):5118–26. doi: 10.1016/j.vaccine.2015.08.010 [DOI] [PubMed] [Google Scholar]
- 73.Rybak A, Levy C, Bonacorsi S, Béchet S, Vié le Sage F, Elbez A, et al. Antibiotic Resistance of Potential Otopathogens Isolated From Nasopharyngeal Flora of Children With Acute Otitis Media Before, During and After Pneumococcal Conjugate Vaccines Implementation. Pediatr Infect Dis J. 2018;37(3):e72–e8. doi: 10.1097/INF.0000000000001862 [DOI] [PubMed] [Google Scholar]
- 74.Ouldali N, Bonacorsi S, Levy C, Bechet S, Vie Le Sage F, Olivier R, et al. CHANGES IN BACTERIAL NASOPHARYNGEAL CARRIAGE IN CHILDREN WITH ACUTE OTITIS MEDIA FOLLOWING PCV13 IMPLEMENTATION: A TIME SERIES ANALYSIS OF A 10 YEARS MULTICENTER PROSPECTIVE SURVEY. ESPID; Malmö, Sweden: 2018. [Google Scholar]
- 75.Ouldali N, Cohen R, Levy C, Gelbert-Baudino N, Seror E, Corrard F, et al. Pneumococcal susceptibility to antibiotics in carriage: a 17 year time series analysis of the adaptive evolution of non-vaccine emerging serotypes to a new selective pressure environment. J Antimicrob Chemother. 2019;74(10):3077–86. doi: 10.1093/jac/dkz281 [DOI] [PubMed] [Google Scholar]
- 76.Quirk SJ, Haraldsson G, Erlendsdóttir H, Hjálmarsdóttir MÁ, van Tonder AJ, Hrafnkelsson B, et al. Effect of Vaccination on Pneumococci Isolated from the Nasopharynx of Healthy Children and the Middle Ear of Children with Otitis Media in Iceland. Journal of Clinical Microbiology. 2018;56(12):e01046–18. doi: 10.1128/JCM.01046-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jinhage M, Hermansson A, Gisselsson-Solén M. Nasopharyngeal cultures in children with AOM–A retrospective study on bacteriological findings and impact on management. International Journal of Pediatric Otorhinolaryngology. 2021;149. [DOI] [PubMed] [Google Scholar]
- 78.Ekinci E, Desmet S, Van Heirstraeten L, Mertens C, Wouters I, Beutels P, et al. Streptococcus pneumoniae Serotypes Carried by Young Children and Their Association With Acute Otitis Media During the Period 2016–2019. Frontiers in Pediatrics. 2021;9(650). doi: 10.3389/fped.2021.664083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alonso M, Marimon JM, Ercibengoa M, Pérez-Yarza EG, Pérez-Trallero E. Dynamics of Streptococcus pneumoniae serotypes causing acute otitis media isolated from children with spontaneous middle-ear drainage over a 12-year period (1999–2010) in a region of northern Spain. PLoS One. 2013;8(1):e54333. doi: 10.1371/journal.pone.0054333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caeymaex L, Varon E, Levy C, Béchet S, Derkx V, Desvignes V, et al. Characteristics and outcomes of acute otitis media in children carrying streptococcus pneumoniae or haemophilus influenzae in their nasopharynx as a single otopathogen after introduction of the heptavalent pneumococcal conjugate vaccine. Pediatric Infectious Disease Journal. 2014;33(5):533–6. doi: 10.1097/INF.0000000000000213 [DOI] [PubMed] [Google Scholar]
- 81.Cohen R, Bingen E, Levy C, Thollot F, Boucherat M, Derkx V, et al. Nasopharyngeal flora in children with acute otitis media before and after implementation of 7 valent pneumococcal conjugate vaccine in France. BMC Infect Dis. 2012;12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Couloigner V, Levy C, François M, Bidet P, Hausdorff WP, Pascal T, et al. Pathogens implicated in acute otitis media failures after 7-valent pneumococcal conjugate vaccine implementation in France: distribution, serotypes, and resistance levels. Pediatr Infect Dis J. 2012;31(2):154–8. doi: 10.1097/INF.0b013e3182357c8d [DOI] [PubMed] [Google Scholar]
- 83.Grevers G, Wiedemann S, Bohn JC, Blasius RW, Harder T, Kroeniger W, et al. Identification and characterization of the bacterial etiology of clinically problematic acute otitis media after tympanocentesis or spontaneous otorrhea in German children. BMC Infect Dis. 2012;12:312. doi: 10.1186/1471-2334-12-312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pérez-Trallero E, Marimón JM, Alonso M, Ercibengoa M, García-Arenzana JM. Decline and rise of the antimicrobial susceptibility of Streptococcus pneumoniae isolated from middle ear fluid in children: influence of changes in circulating serotypes. Antimicrob Agents Chemother. 2012;56(7):3989–91. doi: 10.1128/AAC.00501-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pumarola F, Marès J, Losada I, Minguella I, Moraga F, Tarragó D, et al. Microbiology of bacteria causing recurrent acute otitis media (AOM) and AOM treatment failure in young children in Spain: shifting pathogens in the post-pneumococcal conjugate vaccination era. Int J Pediatr Otorhinolaryngol. 2013;77(8):1231–6. doi: 10.1016/j.ijporl.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 86.Sillanpää S, Oikarinen S, Sipilä M, Kramna L, Rautiainen M, Huhtala H, et al. Moraxella catarrhalis Might Be More Common than Expected in Acute Otitis Media in Young Finnish Children. J Clin Microbiol. 2016;54(9):2373–9. doi: 10.1128/JCM.01146-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang SS, Platt R, Rifas-Shiman SL, Pelton SI, Goldmann D, Finkelstein JA. Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001 and 2004. Pediatrics. 2005;116(3):e408–13. doi: 10.1542/peds.2004-2338 [DOI] [PubMed] [Google Scholar]
- 88.Kaur R, Pham M, Yu KOA, Pichichero ME. Rising Pneumococcal Antibiotic Resistance in the Post-13-Valent Pneumococcal Conjugate Vaccine Era in Pediatric Isolates From a Primary Care Setting. Clin Infect Dis. 2021;72(5):797–805. doi: 10.1093/cid/ciaa157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kawaguchiya M, Urushibara N, Aung MS, Ito M, Takahashi A, Habadera S, et al. High prevalence of antimicrobial resistance in non-vaccine serotypes of non-invasive/colonization isolates of Streptococcus pneumoniae: A cross-sectional study eight years after the licensure of conjugate vaccine in Japan. J Infect Public Health. 2020;13(8):1094–100. doi: 10.1016/j.jiph.2020.04.012 [DOI] [PubMed] [Google Scholar]
- 90.Kim SH, Chung DR, Song JH, Baek JY, Thamlikitkul V, Wang H, et al. Changes in serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates from adult patients in Asia: Emergence of drug-resistant non-vaccine serotypes. Vaccine. 2020;38(38):6065–73. doi: 10.1016/j.vaccine.2019.09.065 [DOI] [PubMed] [Google Scholar]
- 91.Marchisio P, Esposito S, Picca M, Baggi E, Terranova L, Orenti A, et al. Serotypes not Included in 13-Valent Pneumococcal Vaccine as Causes of Acute Otitis Media with Spontaneous Tympanic Membrane Perforation in a Geographic Area with High Vaccination Coverage. Pediatr Infect Dis J. 2017;36(5):521–3. doi: 10.1097/INF.0000000000001485 [DOI] [PubMed] [Google Scholar]
- 92.Macaj LP M., Madarova L., Kralinsky K. Streptoccocus pneumoniae as a cause of acute otitis media in slovak children in pneumococcal vaccination era. ESPID; Ljubljana, Slovenia: 2019. [Google Scholar]
- 93.Cohen R, Levy C, Ouldali N, Béchet S, Gaudelus J, Hau I, et al. 18 years of surveillance of nasopharyngeal pneumococcal carriage before, during, and after PCV7 then PCV13 implementation in children with acute otitis media. ECCMID2020. [Google Scholar]
- 94.Allemann A, Frey PM, Brugger SD, Hilty M. Pneumococcal carriage and serotype variation before and after introduction of pneumococcal conjugate vaccines in patients with acute otitis media in Switzerland. Vaccine. 2017;35(15):1946–53. doi: 10.1016/j.vaccine.2017.02.010 [DOI] [PubMed] [Google Scholar]
- 95.Cohen R, Ben-Shimol S, Levy C, Ouldali N, Danino D, Beek BAVD, et al. POTENTIAL SEROTYPE COVERAGE OF THIRD-GENERATION PCVS IN ISRAEL AND FRANCE IN CHILDREN 6–23 MONTHS OLD. ESPID; Virtual Meeting: 2020. [Google Scholar]
- 96.Boonacker CWB, Broos PH, Sanders EAM, Schilder AGM, Rovers MM. Cost effectiveness of pneumococcal conjugate vaccination against acute otitis media in children: a review. Pharmacoeconomics. 2011;29(3):199–211. doi: 10.2165/11584930-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 97.van Uum RT, Venekamp RP, Pasmans CTB, de Wit GA, Sjoukes A, van der Pol AC, et al. Cost of childhood acute otitis media in primary care in the Netherlands: economic analysis alongside a cluster randomised controlled trial. BMC health services research. 2021;21(1):193. doi: 10.1186/s12913-021-06157-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Speets AM, Wolleswinkel JH, Forsgren A, Sobocki PA. Use of medical resources and indirect costs of otitis media in Sweden. Scand J Public Health. 2011;39(2):137–46. doi: 10.1177/1403494810393553 [DOI] [PubMed] [Google Scholar]
- 99.Ansaldi F, Pugh S, Amicizia D, Di Virgilio R, Trucchi C, Orsi A, et al. Estimating the Clinical and Economic Impact of Switching from the 13-Valent Pneumococcal Conjugate Vaccine (PCV13) to the 10-Valent Pneumococcal Conjugate Vaccine (PCV10) in Italy. Pathogens. 2020;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liese JG, Giaquinto C, Silfverdal SA, Carmona A, Larcombe J, Garcia-Sicilia J, et al. The clinical and economic burden of acute otitis media: A large prospective observational cohort study in Europe. Value in Health. 2011;14(7):A508–A9. [Google Scholar]
- 101.Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine. 2004;22(31):4203–14. doi: 10.1016/j.vaccine.2004.05.003 [DOI] [PubMed] [Google Scholar]
- 102.Oh PI, Maerov P, Pritchard D, Knowles SR, Einarson TR, Shear NH. A cost-utility analysis of second-line antibiotics in the treatment of acute otitis media in children. Clinical therapeutics. 1996;18(1):160–82. doi: 10.1016/s0149-2918(96)80188-3 [DOI] [PubMed] [Google Scholar]
- 103.Robberstad B, Frostad CR, Akselsen PE, Kværner KJ, Berstad AK. Economic evaluation of second generation pneumococcal conjugate vaccines in Norway. Vaccine. 2011;29(47):8564–74. doi: 10.1016/j.vaccine.2011.09.025 [DOI] [PubMed] [Google Scholar]
- 104.Heidemann CH, Godballe C, Kjeldsen AD, Johansen EC, Faber CE, Lauridsen HH. The Otitis Media-6 questionnaire: psychometric properties with emphasis on factor structure and interpretability. Health Qual Life Outcomes. 2013;11:201. doi: 10.1186/1477-7525-11-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Indius JH, Alqaderi SK, Kjeldsen AD, Heidemann CH. Middle ear disease in Danish toddlers attending nursery day-care—Applicability of OM-6, disease specific quality of life and predictors for middle ear symptoms. Int J Pediatr Otorhinolaryngol. 2018;110:130–4. doi: 10.1016/j.ijporl.2018.04.031 [DOI] [PubMed] [Google Scholar]
- 106.Heidemann CH, Lauridsen HH, Kjeldsen AD, Faber CE, Johansen ECJ, Godballe C. Quality-of-Life Differences among Diagnostic Subgroups of Children Receiving Ventilating Tubes for Otitis Media. Otolaryngology—Head and Neck Surgery (United States). 2015;153(4):636–43. doi: 10.1177/0194599815569491 [DOI] [PubMed] [Google Scholar]
- 107.Heidemann CH, Lauridsen HH, Kjeldsen AD, Faber CE, Johansen EC, Godballe C. Caregiver Quality of Life and Daily Functioning in Relation to Ventilating Tube Treatment. Otolaryngol Head Neck Surg. 2014;151(2):341–7. doi: 10.1177/0194599814529911 [DOI] [PubMed] [Google Scholar]
- 108.van Brink J, Gisselsson-Solen M. Quality of life in Swedish children receiving grommets–An analysis of pre- and postoperative results based on a national quality register. International Journal of Pediatric Otorhinolaryngology. 2019;120:44–50. doi: 10.1016/j.ijporl.2019.02.009 [DOI] [PubMed] [Google Scholar]
- 109.Holl K, Rosenlund M, Giaquinto C, Silfverdal SA, Carmona A, Larcombe J, et al. The Impact of Childhood Acute Otitis Media on Parental Quality of Life in a Prospective Observational Cohort Study. Clin Drug Investig. 2015;35(10):613–24. doi: 10.1007/s40261-015-0319-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aly BH, Hamad MS, Mohey M, Amen S. Polymerase Chain Reaction (PCR) Versus Bacterial Culture in Detection of Organisms in Otitis Media with Effusion (OME) in Children. Indian J Otolaryngol Head Neck Surg. 2012;64(1):51–5. doi: 10.1007/s12070-011-0161-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Emaneini M, Gharibpour F, Khoramrooz SS, Mirsalehian A, Jabalameli F, Darban-Sarokhalil D, et al. Genetic similarity between adenoid tissue and middle ear fluid isolates of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis from Iranian children with otitis media with effusion. Int J Pediatr Otorhinolaryngol. 2013;77(11):1841–5. doi: 10.1016/j.ijporl.2013.08.024 [DOI] [PubMed] [Google Scholar]
- 112.Hotomi M, Yamanaka N, Billal DS, Sakai A, Yamauchi K, Suzumoto M, et al. Genotyping of Streptococcus pneumoniae and Haemophilus influenzae isolated from paired middle ear fluid and nasopharynx by pulsed-field gel electrophoresis. ORL J Otorhinolaryngol Relat Spec. 2004;66(5):233–40. doi: 10.1159/000081119 [DOI] [PubMed] [Google Scholar]
- 113.Kaur R, Czup K, Casey JR, Pichichero ME. Correlation of nasopharyngeal cultures prior to and at onset of acute otitis media with middle ear fluid cultures. BMC Infect Dis. 2014;14:640. doi: 10.1186/s12879-014-0640-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu Q, Gill S, Xu L, Gonzalez E, Pichichero ME. Comparative Analysis of Microbiome in Nasopharynx and Middle Ear in Young Children With Acute Otitis Media. Front Genet. 2019;10:1176. doi: 10.3389/fgene.2019.01176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yatsyshina S, Mayanskiy N, Shipulina O, Kulichenko T, Alyabieva N, Katosova L, et al. Detection of respiratory pathogens in pediatric acute otitis media by PCR and comparison of findings in the middle ear and nasopharynx. Diagn Microbiol Infect Dis. 2016;85(1):125–30. doi: 10.1016/j.diagmicrobio.2016.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Knerer G, Ismaila A, Pearce D. Health and economic impact of PHiD-CV in Canada and the UK: a Markov modelling exercise. J Med Econ. 2012;15(1):61–76. doi: 10.3111/13696998.2011.622323 [DOI] [PubMed] [Google Scholar]
- 117.Ben-Shimol S, Givon-Lavi N, Greenberg D, Stein M, Megged O, Bar-Yochai A, et al. Impact of pneumococcal conjugate vaccines introduction on antibiotic resistance of Streptococcus pneumoniae meningitis in children aged 5 years or younger, Israel, 2004 to 2016. Euro Surveill. 2018;23(47). doi: 10.2807/1560-7917.ES.2018.23.47.1800081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Andrejko K, Ratnasiri B, Hausdorff WP, Laxminarayan R, Lewnard JA. Antimicrobial resistance in paediatric Streptococcus pneumoniae isolates amid global implementation of pneumococcal conjugate vaccines: a systematic review and meta-regression analysis. The Lancet Microbe. 2021;2(9):e450–e60. doi: 10.1016/S2666-5247(21)00064-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mungall BA, Hoet B, Nieto Guevara J, Soumahoro L. A systematic review of invasive pneumococcal disease vaccine failures and breakthrough with higher-valency pneumococcal conjugate vaccines in children. Expert Rev Vaccines. 2022;21(2):201–14. doi: 10.1080/14760584.2022.2012455 [DOI] [PubMed] [Google Scholar]
- 120.Løchen A, Croucher NJ, Anderson RM. Divergent serotype replacement trends and increasing diversity in pneumococcal disease in high income settings reduce the benefit of expanding vaccine valency. Sci Rep. 2020;10(1):18977. doi: 10.1038/s41598-020-75691-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Strengthening the reporting of observational studies in epidemiology (STROBE). STROBE Checklists 2022. Available from: https://www.strobe-statement.org/checklists/.
- 122.Satzke C, Turner P, Virolainen-Julkunen A, Adrian PV, Antonio M, Hare KM, et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine. 2013;32(1):165–79. doi: 10.1016/j.vaccine.2013.08.062 [DOI] [PubMed] [Google Scholar]
- 123.European Centre for Disease Prevention and Control. Surveillance Atlas of Infectious Diseases for Invasive Bacterial Diseases (2018 data): Invasive H. influenzae disease, invasive meningococcal disease and invasive pneumococcal disease. Stockholm: ECDC; 2020. [Google Scholar]