Abstract
Cas9 transgenes can be employed for genome editing in mouse zygotes. However, using transgenic instead of exogenous Cas9 to produce gene-edited animals creates unique issues including ill-defined transgene integration sites, the potential for prolonged Cas9 expression in transgenic embryos, and increased genotyping burden. To overcome these issues, we generated mice harboring an oocyte-specific, Gdf9 promoter driven, Cas9 transgene (Gdf9-Cas9) targeted as a single copy into the Hprt1 locus. The X-linked Hprt1 locus was selected because it is a defined integration site that does not influence transgene expression and breeding of transgenic males generates obligate transgenic females to serve as embryo donors. Using microinjections and electroporation to introduce sgRNAs into zygotes derived from transgenic dams, we demonstrate that Gdf9-Cas9 mediates genome editing as efficiently as exogenous Cas9 at several loci. We show that genome editing efficiency is independent of transgene inheritance, verifying that maternally derived Cas9 facilitates genome editing. We also show that paternal inheritance of Gdf9-Cas9 does not mediate genome editing, confirming that Gdf9-Cas9 is not expressed in embryos. Finally, we demonstrate that off-target mutagenesis is equally rare when using transgenic or exogenous Cas9. Together, these results show that the Gdf9-Cas9 transgene is a viable alternative to exogenous Cas9.
Keywords: Genome editing, SpCas9, germline mutations, CRISPR
Introduction
The ability to create genetically modified mouse models has greatly expanded the study of gene function and pathogenesis of human disease. With the advent of genome editing technologies, these models are generated with greater ease and shorter timelines by directly modifying the genome in mouse zygotes. The CRISPR/Cas9 system has become the preferred technology for mouse germline genome editing with its high precision programmable nuclease and the ease of reagent design (Cong et al., 2013; Doudna & Charpentier, 2014; Liu et al., 2017; Ran et al., 2013; Wang et al., 2013; Yang et al., 2013). The technology surrounding germline mouse genome editing has continued to evolve since initial publications (Andersson-Rolf et al., 2017; Chen et al., 2017; Chu et al., 2016), with newer methods improving editing efficiencies that can facilitate production of more complex alleles, such as electroporation for introduction of reagents into the mouse embryo (Chu et al., 2016; Qin et al., 2015), base and prime editing (Gao et al., 2021; Gaudelli et al., 2017; Kim et al., 2017; Li et al., 2018), Cas endonucleases with alternative PAM sequences (Zetsche et al., 2015; Liu et al., 2019), long single-stranded DNA donors (Miura et al., 2015), two cell microinjection and biotinylated donors (Gu et al., 2018), and AAV donors (Chen et al., 2019).
Concomitant with the rapid development of new approaches for editing in the mouse germline, there has been an influx of mouse models that endogenously express Streptococcus pyogenes Cas9. Genetic supply of Cas9 to zygotes provides a stable, quality source of Cas9 for genome editing and decreases the number of reagents that need to be introduced. However, Cas9 transgenes introduce unique variables during mouse model production that to date have limited their appeal to the scientific community.
Previous studies have generated Cas9-expressing mouse models using randomly targeted transgenes driven by ubiquitous promoters, and subsequently used these models to mediate genome editing in zygotes (Ghassemi et al., 2021; Sakurai et al., 2016; Sakurai et al., 2020). However, multi-copy random integration sites are usually not mapped, can affect the spatiotemporal expression patterns of transgenes, alter expression or function of other genes, and can lead to loss of transgene expression by epigenetic silencing (Bronson et al., 1996; Palmiter & Brinster, 1986; Yoshiki et al., 2022). Moreover, Cas9 expression from random integrated transgenes driven by ubiquitous promoters (Ghassemi et al., 2021; Sakurai et al., 2016; Sakurai et al., 2020) could cause prolonged genome editing within embryos that inherit the transgene, potentially increasing mosaicism and off-target mutagenesis rates in those embryos. Moreover, these unmapped transgenes may not segregate from the targeted locus. Thus, founder animals that inherit these Cas9 transgenes need to be identified so that they are not selected for breeding. The potential for persistent editing in embryos after fertilization has been overcome by using randomly integrated Cas9 transgenes driven by oocyte-specific promoters (Zhang et al., 2016). These transgenes provide a transient, maternally derived source of Cas9 for genome editing in zygotes. However, potential issues with random integration persist.
Separately, other studies have used a safe-harbor locus like the Gt(ROSA)26Sor (Rosa26) for single-copy integration with Cas9 either conditionally or constitutively expressed from a ubiquitous promoter (Alghadban et al., 2020; Cebrian-Serrano et al., 2017; Chu et al., 2016; Platt et al., 2014). The single integration site avoids the previously mentioned issues associated with randomly integrated transgenes. Moreover, if the transgene is expressed in the oocyte, it can provide a maternally derived source of Cas9 without requiring inheritance of the transgene in an embryo for genome editing. However, in embryos that inherit such an allele, continual Cas9 expression from the Rosa26 locus could lead to persistent genome editing. Thus, founders that inherit the activated Rosa26 allele need to be identified and are typically not used for breeding.
The current study aimed to take advantage of a single-copy, targeted Cas9 transgene approach while also limiting Cas9 expression in embryos to a transient, maternally derived source. The mouse strain described herein was generated as a targeted knock-in to the hypoxanthine phosphoribosyltransferase 1 (Hprt1) locus in mouse embryonic stem cells (MESCs). A spontaneous deletion at the mouse Hprt1 locus, Hprt1b-m3, has been well characterized in MESCs (Bronson et al., 1996; Hooper et al., 1987; Yang et al., 2009). Using this allele and existing targeting constructs, efficient single-copy transgene integration can be achieved along with restoration of Hprt1 function for positive selection of successfully target MESCs in Hypoxanthine-Aminopterin-Thymidine (HAT) medium (Korecki et al., 2019; Peeters et al., 2018; Yang et al., 2009). The Hprt1 safe-harbor locus is a neutral integration site as it has minimal influence on the expression of tissue-specific transgenic promoters unlike the Rosa26 locus, which has been reported to override tissue-specific transgene promoters (Tamura et al., 2020; Zambrowicz et al., 1997).
To drive expression of S. pyogenes Cas9 specifically in oocytes, we selected a promoter region from growth differentiation factor 9 (Gdf9) that has been shown to drive Cre transgene expression (Gdf9-Cre) in oocytes (Lan et al., 2004; McGrath et al., 1995; Tora & Vincent, 2021). Previous studies have demonstrated that endogenous Gdf9 mRNA ceases expression by 1.5 days post coitus (McGrath et al., 1995) when maternal transcripts are degraded at the maternal-to-zygotic transition (Tora & Vincent, 2021) and that Gdf9-Cre is expressed in oocytes at all stages of postnatal follicular development (Lan et al., 2004; McPherron & Lee, 1993). Using this promoter, we hypothesized that maternally derived Cas9 mRNA transcript and exogenously provided guide RNAs would permit efficient genome editing in mouse zygotes. Critically, as the source of Cas9 is maternally derived, inheritance would not be necessary for Cas9 expression in embryos.
This short technical report describes the creation of an Hprt1 targeted, single copy, oocyte-specific Cas9 transgene (Hprt1Gdf9-Cas9). RNA in situ hybridization reveals that expression of the Cas9 transgene is oocyte specific. Genome editing experiments show that the maternally derived Cas9 supplied by oocytes is sufficient to generate interval deletion alleles in mouse zygotes and that genome editing efficiency is similar to that of exogenously supplied Cas9. Genome editing in zygotes does not require inheritance of the Hprt1Gdf9-Cas9 transgene. Moreover, inheritance does not enhance editing efficiency. Critically, paternal inheritance of Cas9 does not enable genome editing in zygotes, verifying that the Cas9 transgene is not expressed from the embryonic genome. Finally, we show that off-target mutagenesis is rare in vivo with either exogenous or maternally derived Cas9 sources.
Results and Discussion
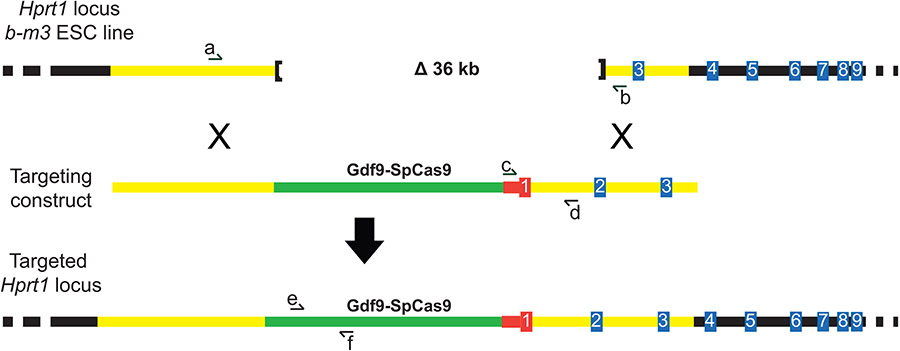
To generate Hprt1Gdf9-Cas9 transgenic mice, a Gdf9 promoter fragment and the open reading frame (ORF) of S. pyogenes Cas9 were cloned into vector pEMS2001 for targeting into the Hprt1 locus of mEMS6131 MESCs (Figure 1A). Following MESC electroporation and HAT medium selection, successful targeting was identified using Hprt1 and Cas9 ORF-specific genotyping screens (Figure 1A and B) (Yang et al., 2009). Of the successfully targeted clones, three were microinjected into C57BL/6NJ albino blastocysts and resulting chimeras were bred for germline transmission of the transgene. Germline transmission (GLT) was initially verified by gender and coat color, and subsequently confirmed by genotyping. PCR was used to confirm GLT in four females born from a single chimeric male (derived from ES cell clone B10) and these females were used to establish a C57BL/6NJ-Hprt1Gdf9-Cas9 colony. Sanger sequencing of the four females was used to confirm the Cas9 ORF and upstream Gdf9 promoter (Figure 1C). Breeding of transgenic dams was used to maintain the colony; breeding of transgenic males was used to generate obligate transgenic females to be used as embryo donors (Figure 2).
Figure 1.

Targeting Gdf9-Cas9 to the Hprt1 locus through a complementation design in MESC. (A) Schematic representing the endogenous deletion locus at Hprt1b-m3 (not drawn to scale). The complementation targeting construct contains Gdf9 promoter sequence, the open reading frame of S. pyogenes Cas9 (SpCas9), promoter and exon 1 sequence from human HPRT1 (shown in red), and intron 1, exon 2, and intron 2 sequence from mouse Hprt1. Homologous recombination introduces the Gdf9-SpCas9 transgene into the genome and restores Hprt1 function. (B) After culturing the electroporated ES cells in Hypoxanthine-Aminopterin-Thymidine (HAT) medium and picking clones, additional PCR-based screening was completed to identify correctly targeted clones. (1) Primers span the 36 kb deletion (Hprt1 b-m3, primers a/b in A) identified clones with admixtures of non-targeted ES cells; only cells still harboring the 36 kb deletion would produce the 380 bp product (pink clone IDs) (2) Positive control PCR to generate a product from the human HPRT1 complementation with successful targeting (HPRT1-CS, primers c/d in A; all clones should have had the 450 bp band) (3) Internal control PCR for Cas9 open reading frame (ORF; 300 bp product, primers e/f in A); clones that were selected for blastocyst injections have green identifiers. (C) Sanger sequencing of a portion of the Gdf9 promoter and the Cas9 ORF confirmed allele integrity following germline transmission from chimeras established from targeted ES cells. Sequencing reads are shown numerically with arrows indicating the orientation of sequencing (1-11).
Figure 2.
Breeding scheme to generate zygotes for genome editing and to achieve germline transmission. Male carriers of the Hprt1Gdf9-Cas9 transgene, delineated in orange, are bred with wild-type females to give rise to obligate Hprt1Gdf9-Cas9/+ daughters. The obligate females thereby do not need to be genotyped prior to superovulation, saving time when optimal age for superovulation is at 4 weeks for C57BL/6NJ mice. The obligate Hprt1Gdf9-Cas9/+ females are mated with wild-type males to generate both male and female zygotes, with and without inheritance of Hprt1Gdf9-Cas9. Zygotes are collected at E0.5, when Cas9 is expressed (delineated in green), and manipulated with genome editing reagents to either (1) culture in vitro for 4 days to generate blastocysts for subsequent genotyping or (2) transfer to pseudopregnant females to produce live-born founder (F0) animals, which are screened for the desired mutation. Male F0s harboring a desired gene edit are preferentially bred over targeted female F0s. The resulting offspring are screened for the on-target mutation. Targeted male progeny from F0 founder sires are preferentially selected as they cannot inherit the Hprt1Gdf9-Cas9 allele from a male F0.
To determine the expression pattern of Cas9 from the Hprt1Gdf9-Cas9 transgene, we performed RNA in situ hybridization (RISH) for endogenous Gdf9 and Cas9. Ovaries from pre-pubescent females from 3 different strains were collected to visualize follicles prior to maturation. Additional tissues were collected, including testes to confirm the absence of Gdf9 and Cas9 expression. In the ovary of Hprt1Gdf9-Cas9 females, Gdf9 and Cas9 expression co-localized to oocytes (Figure 3). Expression of neither Gdf9 nor Cas9 was detected in the testes of Hprt1Gdf9-Cas9 males (Supporting Information [SI] Figure 1). Cas9 expression was also not detected in the heart, liver, or kidney (SI Figures 2-4). In a previous publication that used the Rosa26CAG-Cas9 transgene as maternal source for germline genome editing, expression of Cas9 in the ovary was not characterized (Platt et al., 2014), despite its use for genome editing with maternal transcripts. Curiously, the intensity of Cas9 staining observed in Rosa26CAG-Cas9 oocytes was considerably less than surrounding tissues (Figure 3). As expected, expression of Cas9 from the Rosa26CAG-Cas9 transgene was detected in all tissues assayed (Figure 3 and SI Figures 1-4). Therefore, we conclude that expression of Cas9 from the Hprt1Gdf9-Cas9 transgene is limited to oocytes.
Figure 3.
Expression of Cas9 co-localizes with Gdf9 in oocytes. Hprt1Gdf9-Cas9/+, Rosa26CAG-Cas9 (ubiquitous Cas9 expression, positive control) and wild-type (Gdf9 expression positive control; Cas9 negative control) females were sacrificed for tissue collection between postnatal day (P)21-25. Tissues were fresh frozen for cryosectioning and RNA in situ hybridization. Probes were generated to hybridize to Gdf9 and Cas9.
After confirming expression of Cas9 in Hprt1Gdf9-Cas9 oocytes, we performed a functional test to determine if Cas9 expression from the Hprt1Gdf9-Cas9 transgene enables efficient gene editing in mouse zygotes. We selected a gene (Nanos2) previously targeted using exogenous Cas9 mRNA and gRNAs to create an interval deletion null allele (Dawson et al., 2018). Obligate heterozygous Hprt1Gdf9-Cas9 females born from hemizygous Hprt1Gdf9-Cas9/Y sires were superovulated and mated with wild-type males (Figure 2). Fertilized oocytes were microinjected with 2 gRNAs to delete the single exon coding region of Nanos2 and transferred to pseudopregnant females. Live-born mice were genotyped for the deletion allele using primers flanking the target sites by at least 100 bp. Of the 14 mice genotyped, 6 had a deletion allele (43%), which were verified by TA cloning and Sanger sequencing (SI Figure 5 and Table 1). Critically, 2 of the 6 mice harboring a deletion allele did not inherit Hprt1Gdf9-Cas9 (SI Figure 5 and SI Table 1), illustrating that in the absence of Hprt1Gdf9-Cas9 transgene inheritance, maternally derived Cas9 is sufficient for genome editing in zygotes.
Table 1.
The genome editing efficiency of Hprt1Gdf9-Cas9 in mouse zygotes is similar to exogenous Cas9.
| Gene | Route of Administration |
Cas9 Source | Embryos or Mice Screened† |
Genome Editing Detected |
% With Genome Editing |
P value* |
|---|---|---|---|---|---|---|
| Nanos2 | Microinjection | Endogenous | 14 | 6 | 43 | 1 (NS) |
| Exogenous | 29 | 14 | 48 | |||
| Nanos2 | Electroporation | Endogenous | 11 | 7 | 63 | 0.9 (NS) |
| Exogenous | 9 | 2 | 22 | |||
| Cass4 | Microinjection | Endogenous | 11 | 3 | 27 | 1 (NS) |
| Exogenous | 12 | 4 | 33 | |||
| Cass4 | Electroporation | Endogenous | 9 | 2 | 22 | 0.04 |
| Exogenous | 6 | 5 | 83 | |||
| Fam57b | Microinjection | Endogenous | 11 | 4 | 36 | 1 (NS) |
| Exogenous | 26 | 11 | 42 | |||
| Fam57b | Electroporation | Endogenous | 19 | 6 | 32 | 0.15 (NS) |
| Exogenous | 39 | 5 | 13 | |||
| Tyr | Microinjection | Endogenous | 18 | 8 | 44 | 0.0004 |
| Exogenous | 16 | 16 | 100 | |||
| Tyr | Electroporation | Endogenous | 11 | 4 | 36 | 0.14 (NS) |
| Exogenous | 18 | 12 | 67 | |||
| Pooled | Microinjection | Endogenous | 54 | 21 | 39 | 0.08 (NS) |
| Exogenous | 83 | 45 | 54 | |||
| Pooled | Electroporation | Endogenous | 50 | 19 | 38 | 0.70 (NS) |
| Exogenous | 72 | 24 | 33 |
Samples for genotyping, all microinjection attempts were from live-born mice and all electroporation attempts were from cultured blastocysts
Fisher’s Exact test; NS = not significant; Pooled data from all 4 genes.
Previous studies have highlighted the relative ease of introducing CRISPR/Cas9 reagents into mouse zygotes via electroporation when compared to traditional microinjection (Chu et al., 2016; Lusk et al., 2022; O'Hagan et al., 2021). To demonstrate the ability of our Hprt1Gdf9-Cas9 mouse to facilitate genome editing by electroporation, we repeated the exon deletion of Nanos2 as previously tested by microinjection. Fertilized oocytes were electroporated with the same 2 gRNAs targeting Nanos2 and cultured to blastocyst stage for subsequent genotyping. Of the 11 blastocysts genotyped, 7 had a deletion allele (64%), which was verified by Sanger sequencing (Table 1). Again, maternally derived Cas9 was found to be sufficient for genome editing as 5 of the 7 blastocysts harboring a deletion allele did not inherit the Hprt1Gdf9-Cas9 transgene (SI Table 1).
To compare the genome editing efficiency of Cas9 expressed from Hprt1Gdf9-Cas9 to exogenous Cas9, we performed concurrent microinjections and electroporations of zygotes derived from superovulated wild-type C57BL/6NJ females with exogenous Cas9 (mRNA for microinjections; protein for electroporations) and the same Nanos2 gRNAs. Live-born animals derived from microinjections and cultured blastocysts derived from electroporations were genotyped. Critically, no significant difference in genome editing incidence was observed between endogenous and exogenous Cas9 (Table 1).
The ability of Hprt1Gdf9-Cas9 to mediate genome editing in zygotes microinjected or electroporated with gRNAs was tested for an additional three genes (Cass4, Fam57b, and Tyr; Table 1 and SI Table 1) and compared to genome editing efficiency by exogenous Cas9. As observed for Nanos2, no statistical difference in the genome editing of Fam57b was observed between endogenous and exogenous Cas9, either by electroporation or microinjection (Table 1). However, for Cass4 (electroporation) and Tyr (microinjection), the genome editing efficiency of exogenous Cas9 was significantly higher when compared to endogenous Cas9 (p<0.05 and p<0.005, respectively, Table 1). When data from all four genes were pooled and analyzed, no significant difference in genome editing efficiency was observed between exogenous and endogenous Cas9 for microinjections or electroporations (Table 1). Notably, analaysis of data pooled from all four genes also revealed that inheritance of the Hprt1Gdf9-Cas9 transgene did not confer an increase in genome editing efficiency for microinjections or electroporations (Table 2). Associations between Hprt1Gdf9-Cas9 inheritance and genome editing efficiency for individual genes were not analyzed due to small sample sizes (Table S1 in Data S1). Moreover, when data from all four genes were pooled and analyzed, the source of Cas9 had no significant effect on zygote survival to liveborn pups (microinjections) or blastocysts (electroporations) (Table 3). Associations between Cas9 source and survival were not analyzed for individual genes to exclude the influence of session effects on zygote survival (Table S2 in Data S1).
Table 2.
Inheritance of Hprt1Gdf9-Cas9 does not influence genome editing efficiency.
| Route of Administration |
Hprt1Gdf9-Cas9 inheritance |
Embryos or Mice Screened† |
Genome Editing Detected |
% With Genome Editing |
P value* |
|---|---|---|---|---|---|
| Pooled Microinjection | Inherited | 35 | 15 | 43 | 0.16 (NS) |
| Not Inherited | 19 | 6 | 32 | ||
| Pooled Electroporation | Inherited | 18 | 7 | 39 | 1 (NS) |
| Not Inherited | 32 | 12 | 38 |
Samples for genotyping, all microinjection attempts were from live-born mice and all electroporation attempts were from cultured blastocysts
Fisher’s Exact test; NS = not significant
Table 3.
Zygote manipulation produced comparable numbers of animals for genotyping.
| Route of Administration |
Cas9 Source | Zygotes Manipulated |
Embryos or Mice Screened† |
% Assayed | P value* |
|---|---|---|---|---|---|
| Pooled Microinjection | Endogenous | 219 | 54 | 25 | (NS) |
| Exogenous | 428 | 83 | 19 | ||
| Pooled Electroporation | Endogenous | 185 | 50 | 27 | (NS) |
| Exogenous | 280 | 72 | 26 |
Samples for genotyping, all microinjection attempts were from live-born mice and all electroporation attempts were from cultured blastocysts
Fisher’s Exact test; NS = not significant
To validate that Hprt1Gdf9-Cas9 mediates genome editing in mouse zygotes only through a maternally derived (oocyte expressed) source of Cas9, wild-type C57BL/6NJ female mice were superovulated and mated with Hprt1Gdf9-Cas9/Y males to generate female pups obligate for the Hprt1Gdf9-Cas9 transgene (Figure 4A). If Cas9 is expressed from the inherited Hprt1Gdf9-Cas9 transgene after the maternal-to-zygotic transition, genome editing would be observed in female mice. Zygotes were microinjected with the 2 gRNAs targeting Nanos2 and transferred into pseudopregnant females to generate liveborn pups. Males were identified by Sry genotyping and all females inherited the Hprt1Gdf9-Cas9 transgene as expected (Figure 4B). However, none of the females harbored a Nanos2 interval deletion (Figure 4B). Based on these results, we conclude that maternally derived, oocyte expression of Cas9 is necessary for genome editing in zygotes.
Figure 4.
Genome editing is dependent upon maternally supplied Cas9. (A) Male Hprt1Gdf9-Cas9/Y mice were mated to superovulated wild-type C57BL/6N females to generate obligate Hprt1Gdf9-Cas9 female zygotes. Two gRNAs targeting Nanos2 for an interval deletion were electroporated into zygotes and embryos were transferred to pseudopregnant females. (B) Male mice were identified by Sry genotyping and were excluded from analysis. Female mice were genotyped for both Hprt1Gdf9-Cas9 and the Nanos2 interval deletion. PCR genotyping demonstrates that all females were WT for the Nanos2 deletion allele (WT product 629 bp, targeted ~275 bp) and carriers of the Hprt1Gdf9-Cas9 allele. The positive control (+) for the Nanos2 deletion allele was obtained from Cas9 targeting by microinjection. A separate sample was used as a positive control for the Hprt1Gdf9-Cas9 transgene. Wild-type mice were used as negative controls.
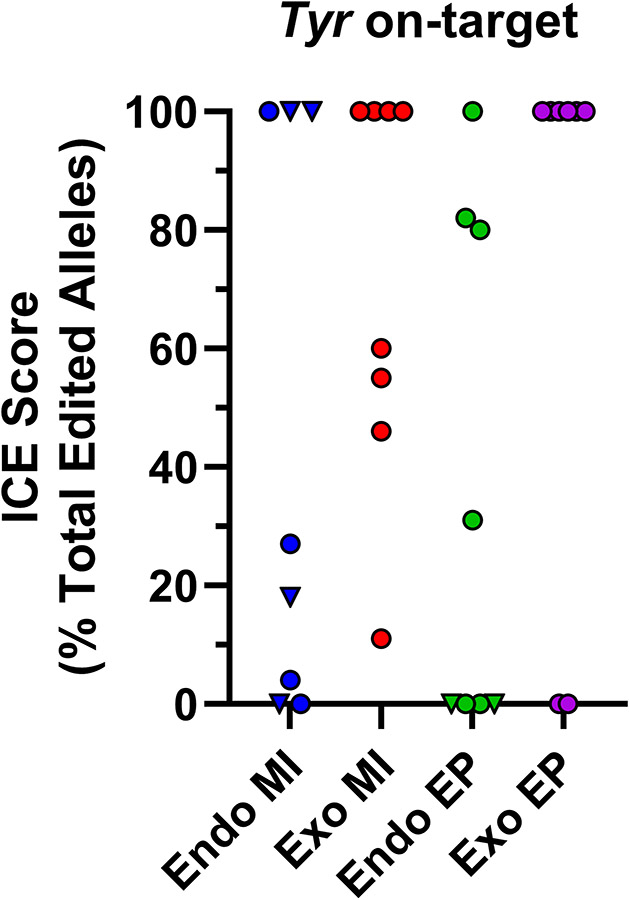
To test for differences in off-target mutagenesis potential when using endogenous versus exogenous Cas9, we used Sanger sequencing and Synthego Inference of CRISPR Editing (ICE) software (Conant et al., 2022) to assess the top 4 predicted off-target sites for the two gRNAs used to target Tyr (Yen et al., 2014). The predicted cut sites for the 2 gRNAs are 8 base pairs apart and can either create individual indel mutations or short interval deletions. Eight embryos or mice per Cas9 source and route of administration, with differing amounts of on-target editing (Figure 5A), and with or without inheritance of Hprt1Gdf9-Cas9 were selected for off-target mutagenesis screening for a total of 32 mice analyzed. From the 8 potential off-target sites screened, mutagenesis was detected for only one site for one gRNA (Figure 5B). Of the 32 animals screened, 3 mice had evidence of mutagenesis at this off-target locus, which translates to 3 events from 256 sites screened (8 loci multiplied by 32 animals) or <1% of all sites screened with an off-target mutagenesis event. With the low rate of off target events observed in the on-target gRNAs for Tyr, additional off-target sites from gRNAs for the other 3 loci were not screened. Previous data has indicated that off-target mutagenesis events are rare in germline genome editing (Peterson et al., 2023). Of the off-target mutagenesis events detected in Tyr, two of the events were identified in the endogenous microinjection samples, both of which had 100% on-target editing and one with Hprt1Gdf9-Cas9 inheritance (Figure 5C). The third off-target event was detected in a mouse with ~50% on target editing generated by exogenous Cas9 microinjection (Figure 5C). Therefore, off-target mutagenesis can occur with both endogenous and exogenous Cas9.
Figure 5.
Off-target genome editing occurs with both endogenous and exogenous Cas9. Zygotes were generated from wild-type and Hprt1Gdf9-Cas9 females and microinjected or electroporated with or without exogenous Cas9, respectively, and two gRNAs targeting Tyr (N=8 animals or blastocysts per Cas9 source/route of administration). Sanger sequencing and ICE analysis of sequencing traces were performed to detect genome editing at the on-target and top 4 predicted off-target sites for each gRNA and to calculate indel allele contribution. (A) Scatter plot of ICE score for each Cas9 source/route of administration for the Tyr on-target site. Endogenous MI and EP samples with inheritance of the Hprt1Gdf9-Cas9 transgene are displayed as triangles, all other samples are circles. (B) Scatter plot of ICE score for each Cas9 source/route of administration for one off-target site. Of the 8 off-target loci screened for the 2 gRNAs employed (4 per guide), mutagenesis was detected at only this off-target site. Of the 2 mice with an off-target event from the Endogenous MI samples, one had inheritance of the Hprt1Gdf9-Cas9 transgene (triangle). (C) Scatter plot depicting off-target ICE score versus on-target score for the only off-target site with identified mutagenesis events to illustrate occurrence of on- and off-target events occurring within the same sample. Endo, endogenous Cas9; Exo, exogenous Cas9; MI, microinjection; EP, electroporation; (T), Hprt1Gdf9-Cas9 transgenic.
Critically, the design of our Gdf9-Cas9 transgene offers several advantages that make it a potentially more attractive transgenic system for genome editing in mouse zygotes. First, Gdf9-Cas9 is specifically targeted to a defined locus in the mouse genome, overcoming issues associated with randomly integrated Cas9 transgenes, such as the unknown insertion location and potential for silencing after breeding (Sakurai et al., 2016; Sakurai et al., 2020; Zhang et al., 2016). Second, the expression of Gdf9-Cas9 in zygotes is maternally derived, overcoming the potential for persistent Cas9 expression in embryos that inherit ubiquitously expressed randomly integrated or Rosa26 targeted Cas9 transgenes (Alghadban et al., 2020; Cebrian-Serrano et al., 2017; Sakurai et al., 2020). Third, the X-linked status of the Hprt1 locus can significantly reduce the amount of genotyping and breeding needed to track Cas9 transgene inheritance and to accomplish its segregation from an edited locus on any chromosome other than X. Hemizygous Gdf9-Cas9 transgenic sires produce obligate heterozygous daughters. Thus, genotyping is not required to establish cohorts of heterozygous transgenic females to serve as embryo donors for genome editing experiments. Moreover, if male founders harboring a desired genome edit are selected for breeding and their male offspring are subsequently selected for colony expansion, genotyping for Gdf9-Cas9 is not needed to segregate the transgene from a target locus if it is not on the X Chromosome. Gdf9-Cas9 transgenic founders cannot transmit the transgene to their male offspring.
It has been previously reported that the targeted, complemented Hprt1 locus has reduced expression of Hprt1 when compared to the wild-type locus (Yang et al., 2012). HPRT1 deficiency in humans causes Lesch-Nyman Syndrome, with symptoms including impaired kidney function, acute gouty arthritis, and self-mutilating behaviors. Critically, Hprt1 deficiency in mice does not cause these or any other adverse phenotypes, likely due to differences in purine metabolism between mice and humans (Finger et al., 1988; Jinnah et al., 1999). Thus, in male mice harboring Hprt1 targeted transgenes, adverse phenotypes caused by reduced Hprt1 expression are unlikely. In a retrospective analysis of the fertility and fecundity of breeding males within the Hprt1Gdf9-Cas9 colony, no significant changes in the total number of litters or the number of pups per litter were observed when we compared Hprt1Gdf9-Cas9 males in paired matings with wild-type females, and wild-type males mated to Hprt1Gdf9-Cas9 females (SI Figure 6). The hemizygous Hprt1Gdf9-Cas9 males were able to survive and sire litters with the same capacity as their wild-type counterparts.
Collectively, our results demonstrate that the Hprt1 targeted Gdf9-Cas9 transgene is specifically expressed in oocytes, provides a maternally derived source of Cas9 to zygotes, and mediated genome editing in both transgenic and non-transgenic zygotes at similar efficiencies. Averaging genome editing results from 4 loci, Gdf9-Cas9 was shown to be as efficient at mediating genome editing as exogenous sources of Cas9 using both microinjection and electroporation conditions. Moreover, analysis of off-target mutagenesis incidence of two sgRNAs targeting Tyr revealed no difference in off-target potential between Gdf9-Cas9 and exogenous Cas9. Several previous studies have demonstrated that the genome editing efficiency of transgenic sources of Cas9 in mouse zygotes is similar to or slightly better than that of exogenous Cas9 (Alghadban et al., 2020; Cebrian-Serrano et al., 2017). Moreover, in these same studies, the off-target mutagenesis potential of Cas9 transgenes was found to be similar to or slightly lower than that of exogenous Cas9. Thus, the genome editing efficiency of our Gdf9-Cas9 transgene is on par with previously reported transgenic approaches.
Materials and Methods
Plasmid construction.
An adaptor containing AvrII-Acc65i-HindIII-AscI restriction sites was ligated into pGDF-9-iCre (Lan et al., 2004) at Acc65i/HindIII sites. The Gdf9 promoter (−3277/+49) from pGDF-9-iCre (Lan et al., 2004) was released by digestion with Acc65i/HindIII and inserted at Acc65i/HindIII sites of the adaptor-containing vector. An AvrII-AscI fragment containing the Gdf9 promoter (3.3 kb) was then inserted into the Hprt1 complementation targeting vector, pEMS2001 (Yang et al., 2009) at AvrII/AscI sites. The resulting construct was designated as pEMS2001/Gdf9. The SpCas9 open reading frame was released from pX330-U6-Chimeric_BB-CBh-hSpCas9 (Plasmid #42230, Addgene) by digestion with AgeI and EcoRI, filled in 5’-overhang and added 3’-A overhang by dNTP and Taq polymerase, and ligated into pGEM-Teasy (Promega). A NotI-NotI fragment containing Cas9 was then inserted into pEMS2001/Gdf9 at NotI sites to replace 2 kb fragment of iCre-ERT2. The construct was designated as pEMS2001/Gdf9-Cas9, which is 25.8 kb in length. Plasmid DNA was purified with the Qiagen Maxi Kit (Cat#12163, Qiagen), resuspended in Tris-EDTA (TE, pH7.0) buffer, and linearized with I-SceI (Cat#R0694L, New England Biolabs).
ES cell targeting, screening, and chimera production.
Linearized plasmid DNA was electroporated into mEMS6131 MESCs (C57BL/6NTac-Aw-J/Aw-J, Hprt1b-m3/Y) (Korecki et al., 2019). 24–36 hrs post-electroporation, correctly targeted homologous recombinants were selected for using HAT media [FBS ESC media containing 1× HAT (0.1 mM sodium hypoxanthine, 0.4 mM aminopterin, 0.16 mM thymidine), Cat#21060-017, Invitrogen]. Selected clones were lysed for DNA isolation and subsequent allele quality assurance (QA) using PCR-based screening for targeting at the Hprt1b-m3 locus, as previously described (Yang et al., 2009), and integration of Cas9 sequence (see SI Table 3 for primer sequences). Of the clones that passed the PCR based QA, ten were Sanger sequenced to ensure the fidelity of the Cas9 ORF and the Gdf9 promoter. Three of the ten sequenced clones (B07, B08, and B10) were separately microinjected into C57BL/6NTac-Tyrtm1Arte blastocysts to generate chimeric animals. A chimeric male from clone B10 successfully sired offspring from matings with C57BL/6NJ stock females to establish germline transmission of the Hprt1Gdf9-Cas9 allele. GLT was confirmed by gender, coat color inheritance, and subsequent PCR for the Cas9 ORF.
Mouse strains.
B6J.129(Cg)-Gt(ROSA)26Sortm1.1(CAG-cas9*,-EGFP)Fezh/J (Rosa26-Cas9) (RRID:IMSR_JAX:024858), in which a transcriptional stop cassette is removed and S. pyogenes Cas9 expression is ubiquitous, and C57BL/6NJ (RRID:IMSR_JAX:005304) were purchased from The Jackson Laboratory (Bar Harbor, ME); ICR animals [Crl:CD1(ICR)] were purchased from Charles River (Raleigh, NC). Rosa26-Cas9 animals were maintained as homozygotes and Hprt1Gdf9-Cas9 mice were maintained by breeding female heterozygotes to wild-type males. Homozygous Hprt1Gdf9-Cas9 females generated from intercrosses are viable and fertile with no obvious phenotypes. Only heterozygous Hprt1Gdf9-Cas9 females were used as oocyte donors for functional experiments. Animals were housed in AAALAC-accredited animal facility at Baylor College of Medicine. All studies were reviewed and approved by the Institutional Animal Care and Use Committee of BCM and followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The new strain described in this manuscript, C57BL/6NJ-Hprt1tm1(Gdf9-Cas9)Jahe has been registered at Mouse Genome Informatics (MGI:6508529) and has been deposited at the Jackson Laboratory (Stock No. 038997).
RNA In Situ Hybridization.
In situ hybridization (ISH) was performed on 20 μm thick sagittal sections cut from fresh frozen ovary, heart, kidney, liver, and testis mouse tissue. Probes were generated to hybridize to Gdf9 (5’UTR through Ala295) and Cas9 (Val647 through Leu857). A fluorescein (FITC)-labeled probe against Gdf9 was generated using reverse-transcribed mouse cDNA as a template with the following primers: F 5’ – AGAAGACTGGCACGAGGAGA, R 5’ – AGCTTCCTCTTTCACGGGAC. Separately, a digoxigenin (DIG)-labeled mRNA antisense probe against Cas9 was generated from the plasmid DNA as a template using the following primers: F 5’ – TCAGCACCTTGTTGTCGAT, R 5’ – GTGATGAAGCAGCTGAAGC. Both FITC- and DIG- labeled probes were made using RNA labeling kits from Roche.
ISH was performed by the RNA In Situ Hybridization Core at Baylor College of Medicine using an automated robotic platform as previously described (Yaylaoglu et al., 2005) with modifications of the protocol for double ISH. Briefly, both probes were hybridized to the tissue simultaneously. After the described washes and blocking steps the DIG-labeled probes were visualized using tyramide-Cy3 Plus (1/50 dilution, 15-minute incubation, Perkin Elmer). After washes in TNT the remaining HRP-activity was quenched by a 10-minute incubation in 0.2M HCl. The sections were then washed in TNT, blocked in TNB for 15 minutes before a 30-minute room temperature incubation with HRP-labeled sheep anti-FITC antibody (1/500 in TNB, Roche). After washes in TNT the FITC-labeled probe was visualized using tyramide-FITC Plus (1/50 dilution, 15-minute incubation, Perkin Elmer). Following washes in TNT the slides were stained with DAPI, washed again, removed from the machine and mounted in ProLong Diamond (Molecular Probes).
Guide RNA and Cas9 preparation for RNA-guided nuclease (RGN) mixes.
Guide RNAs (gRNAs) for intra-exon interval deletions (Tyr) or full exon deletions (all other genes tested) were selected from a previous publication (Yen et al., 2014) or designed de novo using the Wellcome Trust Sanger Institute Genome Editing website to generate interval deletion alleles (SI Table 4). Single gRNAs were synthesized using DNA templates for in vitro transcription. DNA templates were produced using overlapping oligonucleotides in a high-fidelity PCR reaction (Bassett et al 2013). The PCR products were first purified using the QIAQuick PCR purification kit and used as a template for in vitro transcription of the sgRNA with the MEGAshort script T7 kit (ThermoFisher, AM1354). Following in vitro transcription, RNA was purified using the MEGAclear Transcription Clean-Up Kit (ThermoFisher AM1908). All samples were analyzed by Nanodrop to determine concentration and visualized using the QIAxcel Advanced System using the RNA QC V2.0 kit to check the quality of RNA product before storage at −80°C. Exogenous Cas9 mRNA was purchased from ThermoFisher (A25640) and Cas9 protein was purchased from PNA Bio, Inc (CP01). All sgRNAs were reanalyzed by Nanodrop prior to assembling the microinjection mixtures. Deletion allele microinjection mixes consisted of sgRNA (5 ng/μL each), 50 ng/μL Cas9 mRNA in the exogenous attempts. Deletion allele electroporation mixes consisted of sgRNA (6.6 μM total gRNA concentration), 6 μM Cas9 protein in the exogenous attempts, and 100 pmol ssDNA in the HDR attempts, in a final volume of 10 μL Modified 1x TE.
Introduction of CRISPR/Cas9 reagents to mouse zygotes.
C57BL/6NJ female mice, 24 to 32 days old, were injected with 5 IU/mouse of pregnant mare serum, followed 46.5 hrs later with 5 IU/mouse of human chorionic gonadotropin. The females were then mated to C57BL/6NJ males, and fertilized oocytes were collected at 0.5 dpc. For microinjections the BCM Genetically Engineered Rodent Model (GERM) Core microinjected the RGN mixture into the cytoplasm of 80-100 pronuclear stage zygotes per experiment attempted. For electroporations the GERM Core electroporated the RGN mixture into 40-80 pronuclear stage zygotes per experiment attempted (30V square wave 1 msec pulse, 100 msec interval, 12 pulses total (6x2), BioRad Gene Pulser XCell™ Electroporator). Zygotes were transferred into pseudopregnant ICR females on the afternoon of the microinjections, approximately 25-32 zygotes per recipient female, for live-born pups. Electroporated zygotes were cultured to blastocyst stage and lysed for genotyping.
Standard interval deletion allele mouse genotyping.
Genomic DNA was isolated from tail clips of juvenile mice by proteinase K digestion, followed by ethanol precipitation and resuspension in 1x Tris-EDTA. DNA was amplified by standard PCR using AmpliTaq Gold™ Fast PCR Master Mix (ThermoFisher, 4390939). To detect wild-type and deletion alleles generated by NHEJ, a three-primer scheme was designed. Two primers bind outside the two gRNA sites to PCR amplify deletion products. A third primer was designed to reside within the predicted deleted interval, to PCR amplify a product from the endogenous, wild-type allele. All PCR products were visualized using the QIAxcel Advanced System. Primer sequences for allele PCR can be found in SI Table 5.
Blastocyst culture and genotyping.
Zygotes were cultured in KSOM medium to the blastocyst stage. Blastocysts were washed quickly through 1xPBS and transferred into a single well of a 96-well 0.2 μL thin wall PCR plate containing 10 μL of lysis buffer. Crude DNA extract was generated as described previously (Sakurai et al., 2014), briefly, the lysis mixture was incubated at 56°C for 20 min and then at 95°C for 10 min in a thermocycler with a heated lid, and stored at −20°C until ready for use. Two rounds of standard PCR with nested primers were conducted to ensure sufficient amplification of the region of interest. Non-electroporated blastocysts were screened as wild-type controls. Primer sequences for the PCR reactions are provided in the SI Table 4.
DNA sequencing of CRISPR alleles in mice generated by CRISPR/Cas9.
Genomic DNA was amplified as described above to visualize interval deletion alleles in founder mice. The PCR products were cloned into competent cells using the pGEM Vector System according to the manufacturer’s protocol (Promega, A3600). Clones were screened by PCR for sequences containing the deletion product, and DNA Sanger sequenced by GENEWIZ (South Plainfield, NJ) for direct confirmation of targeted alleles.
Analysis of off-target and on-target genome editing.
The top six potential off-target sites for each gRNA used for the Tyr intra-exon deletion allele were identified using the Wellcome Trust Sanger Institute Genome Editing website (http://www.sanger.ac.uk/htgt/wge/). Flanking PCR primers were designed to amplify 500-600 bp amplicons around each off-target locus and the on-target locus. PCR amplicons were Sanger sequenced and trace files were aligned to annotated genomic sequence files for the on-target and off-target loci and analyzed by the ICE tool from Synthego (Conant et al., 2022) to give a percentage ICE score of off-target indels produced per sequencing trace when compared to wild-type amplicons. See SI Table 6 for off-target loci and primer sequences for sequenced amplicons.
Statistics.
Fisher’s exact tests were used to assess statistical differences between the number of animals or blastocysts with genome editing (interval deletion) as detected by PCR genotyping.
Supplementary Material
Acknowledgements:
We thank the Genetically Engineered Rodent Models and In Situ Hybridization Cores at Baylor College of Medicine for assistance with mouse production and characterization.
Funding:
Resources accessed through the cores were supported by National Institutes of Health grants P30CA12512 and S10 OD016167 to the Dan L. Duncan Comprehensive Cancer Center and In Situ Hybridization Core, respectively. This research was supported by National Institutes of Health grants UM1 HG006348 and U54 OD030165 to JDH, and The University of British Columbia, Faculty of Medicine (PM007960) to EMS.
Footnotes
Conflict of interest: The authors have no financial or non-financial competing interests to declare.
Ethics statement: All animal work presented here was reviewed and approved by the Baylor College of Medicine Institutional Animal Care and Use Committee.
Data availability:
The data supporting this study are contained within the manuscript itself. The Hprt1Gdf9-Cas9 transgenic mice has been deposited at The Jackson Laboratory (JAX Stock No. 038997).
References
- Alghadban S, Bouchareb A, Hinch R, Hernandez-Pliego P, Biggs D, Preece C, & Davies B (2020). Electroporation and genetic supply of Cas9 increase the generation efficiency of CRISPR/Cas9 knock-in alleles in C57BL/6J mouse zygotes. Sci Rep, 10(1), 17912. 10.1038/s41598-020-74960-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson-Rolf A, Mustata RC, Merenda A, Kim J, Perera S, Grego T, Andrews K, Tremble K, Silva JC, Fink J, Skarnes WC, & Koo BK (2017). One-step generation of conditional and reversible gene knockouts. Nat Methods, 14(3), 287–289. 10.1038/nmeth.4156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson SK, Plaehn EG, Kluckman KD, Hagaman JR, Maeda N, & Smithies O (1996). Single-copy transgenic mice with chosen-site integration. Proc.Natl.Acad.Sci.U.S.A, 93(17), 9067–9072. http://www.ncbi.nlm.nih.gov/pubmed/8799155 (Not in File) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebrian-Serrano A, Zha S, Hanssen L, Biggs D, Preece C, & Davies B (2017). Maternal Supply of Cas9 to Zygotes Facilitates the Efficient Generation of Site-Specific Mutant Mouse Models. PLoS One, 12(1), e0169887. 10.1371/journal.pone.0169887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Du Y, He X, Huang X, & Shi YS (2017). A Convenient Cas9-based Conditional Knockout Strategy for Simultaneously Targeting Multiple Genes in Mouse. Sci Rep, 7(1), 517. 10.1038/s41598-017-00654-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu VT, Weber T, Graf R, Sommermann T, Petsch K, Sack U, Volchkov P, Rajewsky K, & Kuhn R (2016). Efficient generation of Rosa26 knock-in mice using CRISPR/Cas9 in C57BL/6 zygotes. BMC Biotechnol, 16, 4. 10.1186/s12896-016-0234-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant D, Hsiau T, Rossi N, Oki J, Maures T, Waite K, Yang J, Joshi S, Kelso R, Holden K, Enzmann BL, & Stoner R (2022). Inference of CRISPR Edits from Sanger Trace Data. CRISPR J, 5(1), 123–130. 10.1089/crispr.2021.0113 [DOI] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, & Zhang F (2013). Multiplex genome engineering using CRISPR/Cas systems. Science, 339(6121), 819–823. 10.1126/science.1231143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson EP, Lanza DG, Webster NJ, Benton SM, Suetake I, & Heaney JD (2018). Delayed male germ cell sex-specification permits transition into embryonal carcinoma cells with features of primed pluripotency. Development, 145(6). 10.1242/dev.156612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudna JA, & Charpentier E (2014). Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science, 346(6213), 1258096. 10.1126/science.1258096 [DOI] [PubMed] [Google Scholar]
- Finger S, Heavens RP, Sirinathsinghji DJ, Kuehn MR, & Dunnett SB (1988). Behavioral and neurochemical evaluation of a transgenic mouse model of Lesch-Nyhan syndrome. J Neurol Sci, 86(2-3), 203–213. 10.1016/0022-510x(88)90099-8 [DOI] [PubMed] [Google Scholar]
- Gao P, Lyu Q, Ghanam AR, Lazzarotto CR, Newby GA, Zhang W, Choi M, Slivano OJ, Holden K, Walker JA 2nd, Kadina AP, Munroe RJ, Abratte CM, Schimenti JC, Liu DR, Tsai SQ, Long X, & Miano JM (2021). Prime editing in mice reveals the essentiality of a single base in driving tissue-specific gene expression. Genome Biol, 22(1), 83. 10.1186/s13059-021-02304-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, & Liu DR (2017). Programmable base editing of A*T to G*C in genomic DNA without DNA cleavage. Nature, 551(7681), 464–471. 10.1038/nature24644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassemi B, Jamalkhah M, Shokri G, Kehtari M, Soleimani M, Shamsara M, & Kiani J (2021). Improved efficiency of genome editing by constitutive expression of Cas9 endonuclease in genetically-modified mice. 3 Biotech, 11(2), 56. 10.1007/s13205-020-02580-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper M, Hardy K, Handyside A, Hunter S, & Monk M (1987). HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature, 326(6110), 292–295. http://www.ncbi.nlm.nih.gov/pubmed/3821905 (Not in File) [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Jones MD, Wojcik BE, Rothstein JD, Hess EJ, Friedmann T, & Breese GR (1999). Influence of age and strain on striatal dopamine loss in a genetic mouse model of Lesch-Nyhan disease. J Neurochem, 72(1), 225–229. 10.1046/j.1471-4159.1999.0720225.x [DOI] [PubMed] [Google Scholar]
- Kim K, Ryu SM, Kim ST, Baek G, Kim D, Lim K, Chung E, Kim S, & Kim JS (2017). Highly efficient RNA-guided base editing in mouse embryos. Nat Biotechnol, 35(5), 435–437. 10.1038/nbt.3816 [DOI] [PubMed] [Google Scholar]
- Korecki AJ, Hickmott JW, Lam SL, Dreolini L, Mathelier A, Baker O, Kuehne C, Bonaguro RJ, Smith J, Tan CV, Zhou M, Goldowitz D, Deussing JM, Stewart AF, Wasserman WW, Holt RA, & Simpson EM (2019). Twenty-Seven Tamoxifen-Inducible iCre-Driver Mouse Strains for Eye and Brain, Including Seventeen Carrying a New Inducible-First Constitutive-Ready Allele. Genetics, 211(4), 1155–1177. 10.1534/genetics.119.301984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan ZJ, Xu X, & Cooney AJ (2004). Differential oocyte-specific expression of Cre recombinase activity in GDF-9-iCre, Zp3cre, and Msx2Cre transgenic mice. Biol Reprod, 71(5), 1469–1474. 10.1095/biolreprod.104.031757 [DOI] [PubMed] [Google Scholar]
- Li X, Wang Y, Liu Y, Yang B, Wang X, Wei J, Lu Z, Zhang Y, Wu J, Huang X, Yang L, & Chen J (2018). Base editing with a Cpf1-cytidine deaminase fusion. Nat Biotechnol, 36(4), 324–327. 10.1038/nbt.4102 [DOI] [PubMed] [Google Scholar]
- Liu ET, Bolcun-Filas E, Grass DS, Lutz C, Murray S, Shultz L, & Rosenthal N (2017). Of mice and CRISPR: The post-CRISPR future of the mouse as a model system for the human condition. EMBO Rep, 18(2), 187–193. 10.15252/embr.201643717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusk SJ, McKinney A, Hunt PJ, Fahey PG, Patel J, Chang A, Sun JJ, Martinez VK, Zhu PJ, Egbert JR, Allen G, Jiang X, Arenkiel BR, Tolias AS, Costa-Mattioli M, & Ray RS (2022). A CRISPR toolbox for generating intersectional genetic mouse models for functional, molecular, and anatomical circuit mapping. BMC Biol, 20(1), 28. 10.1186/s12915-022-01227-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath SA, Esquela AF, & Lee SJ (1995). Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol, 9(1), 131–136. 10.1210/mend.9.1.7760846 [DOI] [PubMed] [Google Scholar]
- McPherron AC, & Lee SJ (1993). GDF-3 and GDF-9: two new members of the transforming growth factor-beta superfamily containing a novel pattern of cysteines. J Biol Chem, 268(5), 3444–3449. https://www.ncbi.nlm.nih.gov/pubmed/8429021 [PubMed] [Google Scholar]
- O'Hagan D, Kruger RE, Gu B, & Ralston A (2021). Efficient generation of endogenous protein reporters for mouse development. Development, 148(13). 10.1242/dev.197418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, & Brinster RL (1986). Germ-line transformation of mice. Annu Rev Genet, 20, 465–499. 10.1146/annurev.ge.20.120186.002341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters SB, Korecki AJ, Simpson EM, & Brown CJ (2018). Human cis-acting elements regulating escape from X-chromosome inactivation function in mouse. Hum Mol Genet, 27(7), 1252–1262. 10.1093/hmg/ddy039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson KA, Khalouei S, Hanafi N, Wood JA, Lanza DG, Lintott LG, Willis BJ, Seavitt JR, Braun RE, Dickinson ME, White JK, Lloyd KCK, Heaney JD, Murray SA, Ramani A, & Nutter LMJ (2023). Whole genome analysis for 163 gRNAs in Cas9-edited mice reveals minimal off-target activity. Commun Biol, 6(1), 626. 10.1038/s42003-023-04974-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt RJ, Chen S, Zhou Y, Yim MJ, Swiech L, Kempton HR, Dahlman JE, Parnas O, Eisenhaure TM, Jovanovic M, Graham DB, Jhunjhunwala S, Heidenreich M, Xavier RJ, Langer R, Anderson DG, Hacohen N, Regev A, Feng G, … Zhang F (2014). CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell, 159(2), 440–455. 10.1016/j.cell.2014.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, & Zhang F (2013). Genome engineering using the CRISPR-Cas9 system. Nat Protoc, 8(11), 2281–2308. 10.1038/nprot.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Kamiyoshi A, Kawate H, Mori C, Watanabe S, Tanaka M, Uetake R, Sato M, & Shindo T (2016). A non-inheritable maternal Cas9-based multiple-gene editing system in mice. Sci Rep, 6, 20011. 10.1038/srep20011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Kamiyoshi A, Kawate H, Watanabe S, Sato M, & Shindo T (2020). Production of genetically engineered mice with higher efficiency, lower mosaicism, and multiplexing capability using maternally expressed Cas9. Sci Rep, 10(1), 1091. 10.1038/s41598-020-57996-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Watanabe S, Kamiyoshi A, Sato M, & Shindo T (2014). A single blastocyst assay optimized for detecting CRISPR/Cas9 system-induced indel mutations in mice. BMC Biotechnol, 14, 69. 10.1186/1472-6750-14-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura S, Yasuoka Y, Miura H, Takahashi G, Sato M, & Ohtsuka M (2020). Thy1 promoter activity in the Rosa26 locus in mice: lessons from Dre-rox conditional expression system. Exp Anim, 69(3), 287–294. 10.1538/expanim.20-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tora L, & Vincent SD (2021). What defines the maternal transcriptome? Biochem Soc Trans, 49(5), 2051–2062. 10.1042/BST20201125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, & Jaenisch R (2013). One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell, 153(4), 910–918. 10.1016/j.cell.2013.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GS, Banks KG, Bonaguro RJ, Wilson G, Dreolini L, de Leeuw CN, Liu L, Swanson DJ, Goldowitz D, Holt RA, & Simpson EM (2009). Next generation tools for high-throughput promoter and expression analysis employing single-copy knock-ins at the Hprt1 locus. Genomics, 93(3), 196–204. 10.1016/j.ygeno.2008.09.014 [DOI] [PubMed] [Google Scholar]
- Yang H, Wang H, Shivalila CS, Cheng AW, Shi L, & Jaenisch R (2013). One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell, 154(6), 1370–1379. 10.1016/j.cell.2013.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaylaoglu MB, Titmus A, Visel A, Alvarez-Bolado G, Thaller C, & Eichele G (2005). Comprehensive expression atlas of fibroblast growth factors and their receptors generated by a novel robotic in situ hybridization platform. Dev Dyn, 234(2), 371–386. 10.1002/dvdy.20441 [DOI] [PubMed] [Google Scholar]
- Yen ST, Zhang M, Deng JM, Usman SJ, Smith CN, Parker-Thornburg J, Swinton PG, Martin JF, & Behringer RR (2014). Somatic mosaicism and allele complexity induced by CRISPR/Cas9 RNA injections in mouse zygotes. Dev Biol, 393(1), 3–9. 10.1016/j.ydbio.2014.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiki A, Ballard G, & Perez AV (2022). Genetic quality: a complex issue for experimental study reproducibility. Transgenic Res, 31(4-5), 413–430. 10.1007/s11248-022-00314-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG, & Soriano P (1997). Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci U S A, 94(8), 3789–3794. https://www.ncbi.nlm.nih.gov/pubmed/9108056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhou J, Han J, Hu B, Hou N, Shi Y, Huang X, & Lou X (2016). Generation of an Oocyte-Specific Cas9 Transgenic Mouse for Genome Editing. PLoS One, 11(4), e0154364. 10.1371/journal.pone.0154364 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this study are contained within the manuscript itself. The Hprt1Gdf9-Cas9 transgenic mice has been deposited at The Jackson Laboratory (JAX Stock No. 038997).