Abstract
Introduction
Nephrotoxic medication (NTM) exposure is commonly associated with acute kidney injury (AKI) in the neonatal intensive care unit (NICU). Baby NINJA (Nephrotoxic Injury Negated by Just-in-time Action) is a quality improvement program that assesses for AKI in those exposed to NTM with daily serum creatinine (SCr) levels. However, blood draws for SCr are invasive and have clinical disadvantages. Urinary neutrophil gelatinase associated lipocalin (uNGAL) is a promising indicator of AKI. We tested the hypothesis that uNGAL could reliably screen for NTM-AKI in the Baby NINJA program.
Methods
This two-center prospective study screened 174 NICU subjects, of whom 148 met screening criteria from January 29, 2019, to September 18, 2020. Daily SCr and urine samples were obtained for up to seven days of NTM exposure plus two days after exposure ended or end of AKI. AKI was defined by a SCr rise of 50% from baseline. The highest uNGAL obtained was evaluated to determine its relationship to the diagnosis of AKI. Logistic regression models were used to determine optimal uNGAL cutoffs.
Results
The negative predictive value of a uNGAL value ≥250 ng/mL was 96.8% (95% CI = 93.3%−100%). Urine NGAL ≥400 ng/mL demonstrated the highest ROC-AUC value of 0.72 with a positive likelihood risk for AKI of 2.76 (1.39–4.13).
Discussion/Conclusion
We propose that uNGAL could be used to screen for NTM-AKI and thus replace many blood draws needed in those exposed to NTM. The ideal uNGAL threshold requires further investigation in infants.
Keywords: neonatal intensive care unit, nephrotoxic medication, biomarker, acute kidney injury, neonates, acute renal failure
Introduction
Acute kidney injury (AKI) is common and is associated with morbidity, mortality, and chronic kidney disease in infants admitted to the neonatal intensive care unit (NICU)[1–4]. Nephrotoxic medications (NTM) are modifiable risk factors for AKI [5]. A quality improvement (QI) initiative called Nephrotoxic Injury Negated by Just-in-time Action (NINJA) has shown the ability to significantly reduce AKI in non-ICU and NICU patients[6, 7]. Through daily serum creatinine (SCr) measurements and medication stewardship in the Baby NINJA QI project, we have shown consistent reductions in NTM exposure and AKI rates in the NICU[7].
Multiple challenges exist when evaluating infants to diagnose AKI using SCr. SCr is reflective of kidney function (not injury), with a rise lagging after the onset of kidney damage[8]. While it reflects maternal SCr in the first 1–4 days of life, it can also vary depending on muscle mass and total body water of the infant[9, 10]. Emerging evidence demonstrates urinary biomarkers, specifically urine neutrophil gelatinase-associated lipocalin (uNGAL), may be a reliable marker for tubular injury and kidney injury severity[11–13]. Urinary NGAL is associated with neonatal AKI in instances of hypoxic-ischemic encephalopathy, following cardiopulmonary bypass surgery, and major thoracic and abdominal surgeries [14–16].
The burden of daily blood draws aimed at SCr screening for AKI is invasive, disrupts sleep/wake cycles, is painful, has associated healthcare costs, and is challenging due to blood volume limitations in infants[17, 18]. For these reasons, healthcare providers are reluctant to order daily SCr levels while infants are exposed to NTM. Therefore a non-invasive, cost-effective alternative to screening for AKI is greatly needed. Goldstein et al. demonstrated that uNGAL can reliably detect NTM-AKI in the non-ICU pediatric population with excellent specificity and negative predictive values for ruling out severe AKI[19]. Consequently, urinary NGAL has the potential to increase screening compliance and ensure early and accurate identification of AKI events in at-risk infants.
We performed a 2-center prospective observational study to test the hypothesis that uNGAL could reliably screen for NTM-AKI in the Baby NINJA program. Further, we evaluated any discrepancies found in SCr-diagnosed AKI infants who did not have elevated uNGAL levels and those who had high uNGAL levels without a diagnosis of AKI. We evaluate which uNGAL threshold is optimal for this purpose.
Materials and Methods
This 2-center prospective cohort study was performed at the Children’s of Alabama (COA) 48-bed Level IV NICU and the Cincinnati Children’s Hospital Medical Center (CCHMC) 75-bed Level IV NICU between January 29, 2019, and September 18, 2020 (enrollment for CCHMC began October 17, 2019), pausing from March 13, 2020, to June 1, 2020, due to the COVID pandemic. Infants were screened for NTM exposure, defined as ≥3 NTMs within 24 hours and/or intravenous (IV) aminoglycoside or IV vancomycin for ≥72 hours or ≥3 calendar days) as part of the Baby NINJA QI program (Supplemental Table 1). Any infant regardless of gestational age admitted to the NICU was eligible. For those who met enrollment criteria, a daily SCr was recommended as part of standard of care until two days after end of exposure or end of AKI, whichever occurred last. No medication adjustments were mandated for the QI program with clinical changes made per discretion of the clinical care team[7]. All infants in the QI program during this time were also enrolled in the current study unless they met the following exclusion criteria: a) congenital kidney disease receiving dialysis, b) <72 hours of age at time of first high NTM exposure, c) active urinary tract infection (UTI), d) AKI at time of enrollment, and/or e) those who did not have any SCr or uNGAL values obtained during the study period.
Demographic and clinical characteristics were obtained including primary NTM exposure. AKI was defined, based on the modified KDIGO definition, if the SCr value was at least 0.5mg/dL and increased 50% over the lowest previous SCr or a 0.3 mg/dL increase within 48 hours[20]. The lowest previous SCr prior to, or on, the day of exposure (excluding the first 72 hours of life) served as the baseline SCr value.
Urine was collected from an indwelling catheter (if already in place as part of clinical care), cotton balls, diaper, or urine collection bag until two days after end of exposure or end of AKI, whichever occurred last. Urine aliquots were stored in cryovials in a −80°C freezer, with COA samples transported to CCHMC hospital lab under appropriate conditions for banking. All frozen urine samples were then batch tested by the Division of Nephrology and Hypertension Biomarker Laboratory using The NGAL Test™, BioPorto Diagnostics assay (Hellrup, Denmark), a turbidometric sandwich assay.
Non-parametric continuous variables are reported as median and interquartile range (IQR) and analyzed using a Wilcoxon rank-sum test. Categorical variables were analyzed using the Wilcoxon rank-sum test and fisher’s exact for chi-square analysis. A p-value of <0.05 was considered statistically significant. SCr and uNGAL compliance were reported as number and percentage of the total possible.
The performance of uNGAL (ng/mL) testing for identification of AKI was performed by calculating a concordance statistic derived from a logistic regression model for cutoffs of 150, 250, 300, 400, 1000, and 2000 ng/mL. In addition, screening measures of sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for each cutoff. Due to the low prevalence of AKI in the study, positive and negative likelihood ratios (LR) were calculated as they provide a more easily interpretable inference of predictive ability. A Fisher’s Exact test was used to evaluate the relationship between uNGAL at the ≥250 ng/mL threshold and AKI. A logistic regression was performed to establish ROC-AUC value to predict optimal uNGAL value to predict SCr-AKI.
Further demographic and clinical characteristics of AKI cases were investigated to ascertain reasons why some subjects that developed AKI had elevations in uNGAL and some did not. Non-transient (true AKI) and transient AKI were delineated, with transient AKI defined as events in which a rapid reversal of elevated SCr occurs within 48 hours of onset as previously reported by Chawla et al.[21]
Data were collected and managed using REDCap electronic data capture tools hosted at CCHMC[22, 23]. SAS 9.4 (Cary, North Carolina) was used for all analyses. The UAB and CCHMC respective IRBs approved the conduct of the study with waiver of parental informed consent.
Results
The study screened 174 infants with 26 that were excluded (11 did not have urine collected, 10 did not have SCr obtained, 2 had UTI at enrollment, 1 had AKI at enrollment, 1 did not have urine after AKI was diagnosed, and 1 had later enrolment). After exclusions, 148 infants were included (Figure 1). The median and IQR gestational age was 32.7 weeks (26.7–37.6) with a median birth weight of 1605 grams (IQR 760–2855) (Table 1). AKI occurred in 6.8% (10/148) infants. There were no differences in demographics between infants with and without AKI except that those with AKI were more likely to receive extracorporeal membrane oxygenation (ECMO, 30.0% vs 5.8%; p-value = 0.03, Table 1). Overall, the most common NTM exposure in both AKI and non-AKI patients was IV vancomycin for 3 or more days (35.8%, 53/148). The types and distribution of NTM exposures in infants with AKI and without AKI are shown in Figure 2 and were not statistically different.
Fig. 1.
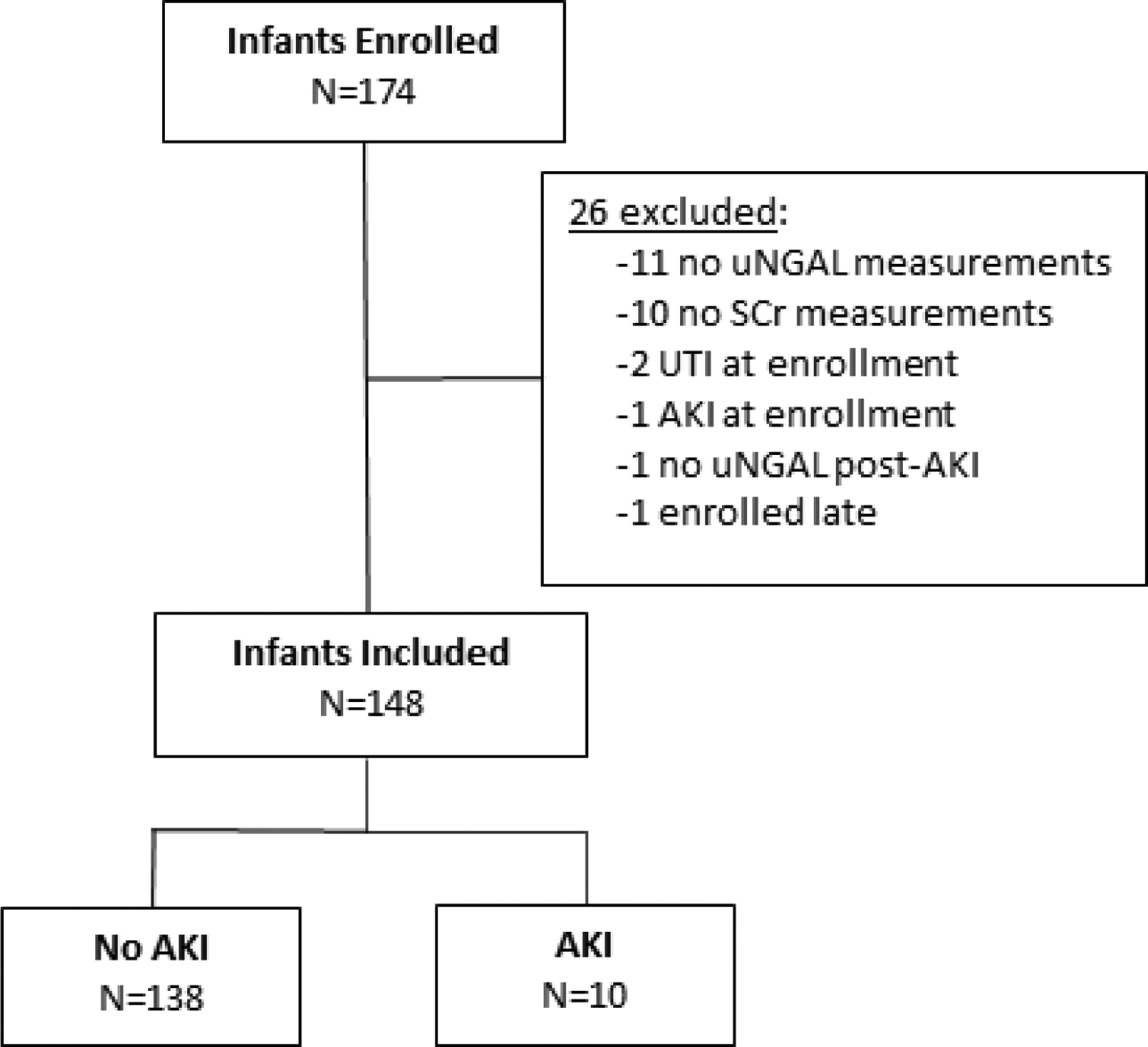
Flowchart for enrollment; uNGAL = urinary neutrophil gelatinase-associated lipocalin, SCr = serum creatinine, UTI = urinary tract infection, AKI = acute kidney injury
Table 1.
Demographics and high nephrotoxic medication (NTM) exposure characteristics by AKI status in the Neonatal Intensive Care Unit (NICU)
| All patients (N=148) | No AKI (N=138) | AKI (N=10) | P- value** | |
|---|---|---|---|---|
| Demographics | ||||
| Gestational age, wk* | 32.7 (26.7–37.6) | 32.9 (26.7–37.6) | 29.3 (26.9–36.0) | 0.72 |
| Age at exposure, wk* | 5.9 (1.4–13.6) | 6.0 (1.4–13.4) | 4.9 (1.1–17.7) | 0.58 |
| Birth weight, g* | 1605 (760–2855) | 1605 (760–2860) | 1870 (1005–2785) | 0.91 |
| Weight at exposure, g* | 3010 (2165–3925) | 2953 (2150–3850) | 3705 (2480–5480) | 0.15 |
| Gender | 0.75 | |||
| Female | 77 (52.0%) | 71 (51.5%) | 6 (60.0%) | |
| Male | 71 (48.0%) | 67 (48.6%) | 4 (40.0%) | |
| Race | 0.48 | |||
| Caucasian | 86 (58.1%) | 81 (58.7%) | 5 (50.0%) | |
| African American | 54 (36.5%) | 50 (36.2%) | 4 (40.0%) | |
| Asian | 2 (1.4%) | 2 (1.5%) | 0 | |
| Other | 1 (0.7%) | 1 (0.7%) | 0 | |
| Unknown | 5 (3.4%) | 4 (2.9%) | 1 (10.0%) | |
| Ethnicity | 0.77 | |||
| Hispanic | 3 (2.0%) | 3 (2.2%) | 0 | |
| Non-Hispanic | 141 (95.3%) | 131 (94.9%) | 10 (100.0%) | |
| Unknown | 4 (2.7%) | 4 (2.9%) | 0 | |
| Primary Disease at first NTM Exposure | ||||
| Culture positive sepsis | 0.44 | |||
| No | 117 (79.1%) | 110 (79.7%) | 7 (70.0%) | |
| Yes | 31 (21.0%) | 28 (20.3%) | 3 (20.0%) | |
| Culture negative sepsis | 0.68 | |||
| No | 123 (83.1%) | 115 (83.3%) | 8 (80.0%) | |
| Yes | 25 (16.9%) | 23 (16.7%) | 2 (20.0%) | |
| ECMO initiation | 0.03 | |||
| No | 137 (92.6%) | 130 (94.2%) | 7 (70.0%) | |
| Yes | 11 (7.4%) | 8 (5.8%) | 3 (30.0%) | |
| Necrotizing enterocolitis (NEC) | 0.61 | |||
| No | 131 (88.5%) | 121 (87.7%) | 10 (100.0%) | |
| Yes | 17 (11.5%) | 17 (12.3%) | 0 | |
| Pneumonia | 1.00 | |||
| No | 137 (92.6%) | 127 (92.0%) | 10 (100.0%) | |
| Yes | 11 (7.4%) | 11 (8.0%) | 0 | |
| Pulmonary hypertension | ||||
| crisis | 0.78 | |||
| No | 132 (89.2%) | 125 (90.6%) | 7 (70.0%) | |
| Yes | 16 (10.8) | 13 (9.4%) | 3 (30.0%) | |
| Any surgery | 0.71 | |||
| No | 111 (75.0) | 104 (75.4%) | 7 (70.0%) | |
| Yes | 37 (25.0%) | 34 (24.6%) | 3 (30.0%) | |
| Central line placement | 1.00 | |||
| No | 147 (99.3%) | 137 (99.3%) | 10 (10.0%) | |
| Yes | 1 (0.7%) | 7 (0.1%) | 0 | |
| Peritoneal drain | 0.79 | |||
| No | 147 (99.3%) | 137 (99.3%) | 10 (100.0%) | |
| Yes | 1 (0.7%) | 1 (0.7%) | 0 | |
| Intestinal resection | 0.43 | |||
| No | 140 (94.6%) | 130 (94.2%) | 10 (100.0%) | |
| Yes | 8 (5.4%) | 8 (5.8%) | 0 | |
| Other surgery | 0.42 | |||
| No | 118 (79.7%) | 111 (80.4%) | 7 (70.0%) | |
| Yes | 30 (20.3%) | 27 (19.6%) | 3 (30.0%) |
median, IQR, and Wilcoxon rank-sum test for non-normal distributed continuous variables, statistically significant p-value <0.05
Wilcoxon rank-sum test for categorial variables and Fisher’s exact for chi-square analysis, statistically significant p-value <0.05
Fig. 2.
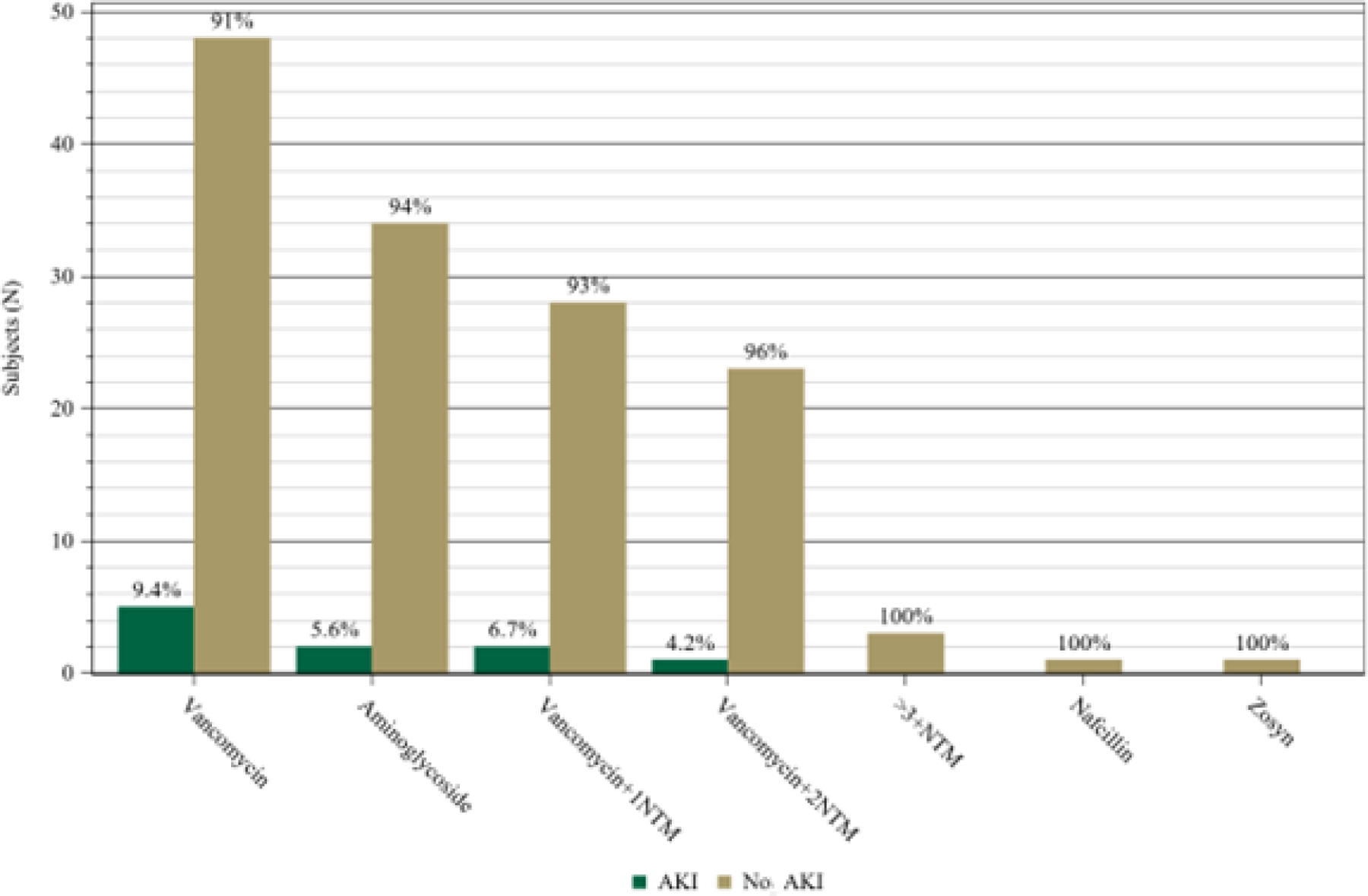
Primary high nephrotoxic medication (NTM) exposure
Daily SCr compliance was reported as a process measure, as lower rates of SCr monitoring could underestimate rates of AKI. Overall SCr compliance was 83.8% in the cohort, 82.8% among those without AKI and 97.1% among infants with AKI. The median number of uNGAL values per subject was five (IQR 2–6) with an overall uNGAL compliance of 67.3% (Supplementary Table 2). The rates of uNGAL compliance were similar in those without AKI (67.7%) to those with AKI (65.8%) (Supplementary Table 2).
The performance of uNGAL to identify AKI at the cutoff values of 150, 250, 300, 400, 1000, and 2000 ng/mL, are outlined in Table 2. All cutoff values had a NPV ≥96%. Infants with uNGAL value ≥250 ng/ml were significantly more likely to have AKI (p-value = 0.03, Supplementary Table 3). The uNGAL value of ≥400 ng/mL had the highest ROC-AUC value of 0.72 (95% CI = 0.57–0.88) with a sensitivity and specificity of 70.0% and 74.6%, respectively (Table 2, Figure 3). The positive likelihood risk (LR) for uNGAL ≥400 ng/mL is 2.76 (95% CI = 1.39–4.13), meaning that those above this uNGAL threshold are 2.76 times as likely to have AKI as those below this threshold.
Table 2.
Predictive power of maximum urinary neutrophil gelatinase-associated lipocalin (uNGAL) values for acute kidney injury (AKI)
| UNGAL | Test+/− | Sensitivity | Specificity | PPV | NPV | LR+ | LR− | AUC |
|---|---|---|---|---|---|---|---|---|
| ≥ 150 ng/mL | 71/77 | 70.0% (41.6–98.4%) | 53.6% (45.3–61.9%) | 9.9% (2.9–16.8%) | 96.1% (91.8–100%) | 1.51 (0.84–2.18) | 0.56 (0.02–1.10) | 0.62 (0.46–0.77) |
| ≥ 250 ng/mL | 54/94 | 70.0% (41.6–98.4%) | 65.9% (58.0–73.9%) | 13.0% (4.0–21.9%) | 96.8% (93.3–100%) | 2.06 (1.09–3.02) | 0.46 (0.02–0.89) | 0.68 (0.52–0.83) |
| ≥ 300 ng/mL | 49/99 | 70.0% (41.6–98.4%) | 69.6% (61.9–77.2%) | 14.3% (4.5–24.1%) | 97.0% (93.6–100%) | 2.30 (1.20–3.40) | 0.43 (0.02–0.84) | 0.70 (0.54–0.85) |
| ≥ 400 ng/mL | 42/106 | 70.0% (41.6–98.4%) | 74.6% (67.4–81.9%) | 16.7% (5.4–27.9%) | 97.2% (94.0–100%) | 2.76 (1.39–4.13) | 0.40 (0.02–0.78) | 0.72 (0.57–0.88) |
| ≥ 1000 ng/mL | 25/123 | 50.0% (19.0–81.0%) | 85.5% (79.6–91.4%) | 20.0% (4.3–35.7%) | 95.9% (92.5–99.4%) | 3.45 (0.90–6.00) | 0.59 (0.22–0.95) | 0.68 (0.51–0.84) |
| ≥ 2000 ng/mL | 16/132 | 40.0% (9.6–70.4%) | 91.3% (86.6–96.0%) | 25.0% (3.8–46.2%) | 95.5% (91.9–99.0%) | 4.60 (0.31–8.89) | 0.66 (0.32–0.99) | 0.66 (0.49–0.82) |
PPV = positive predictive value; NPV = negative predictive value; LR = likelihood ratio; AUC = area under the curve
Fig. 3.
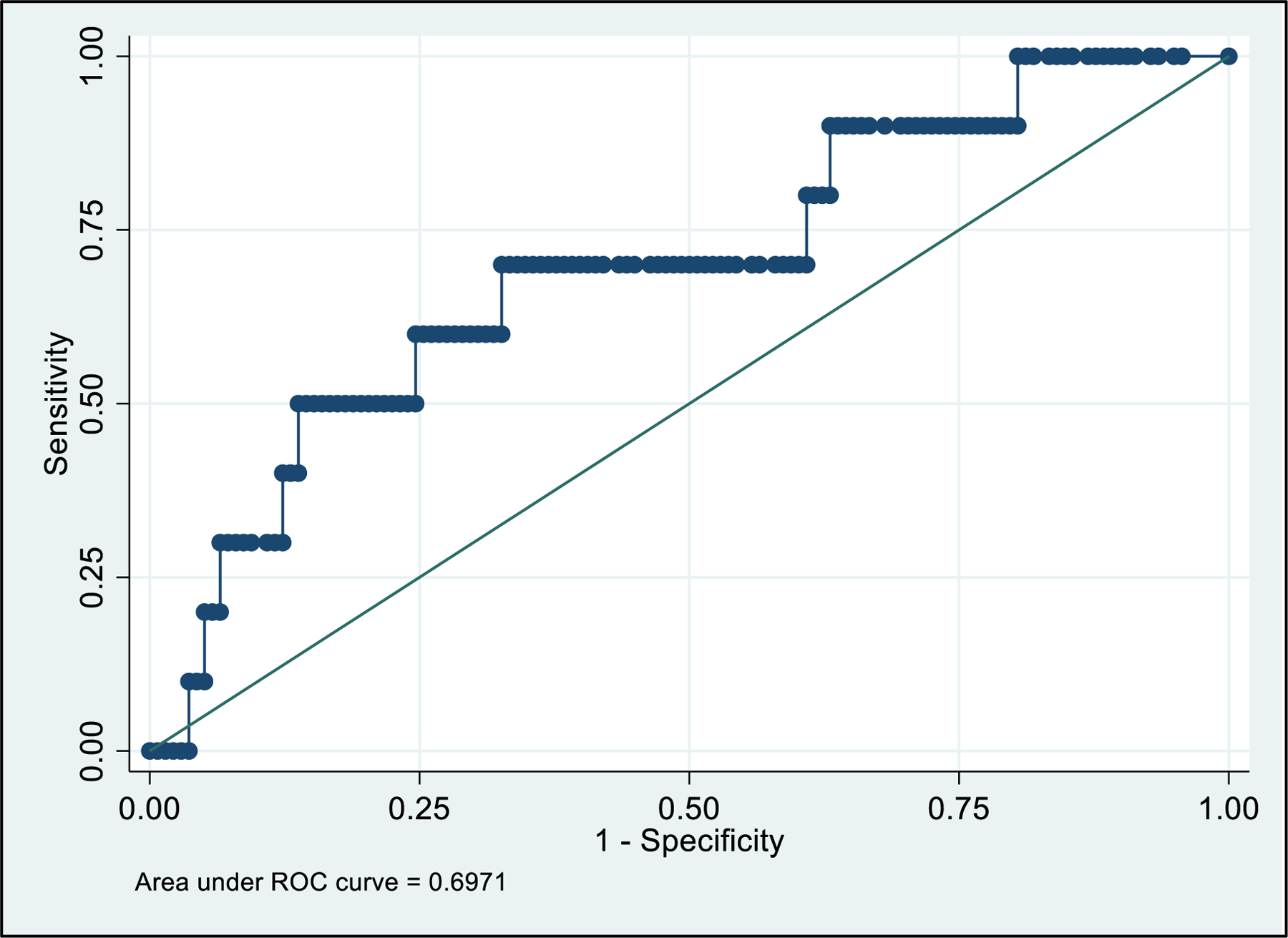
Receiver operator curve (ROC) for maximum urinary neutrophil gelatinase-associated lipocalin (uNGAL) and acute kidney injury (AKI)
Demographic and clinical characteristics of AKI cases were further investigated to evaluate any discrepancies between the diagnosis of AKI and uNGAL values (Table 3). Among the 10 cases of AKI, one had Stage I, three had Stage II, and six had Stage III. Among the 10 cases of AKI, three had transient AKI, six had true AKI (non-transient AKI), and one did not have a SCr value to ascertain if the SCr demonstrated rapid reversal to baseline within 48 hours (transient AKI) and therefore classified as intermediate AKI. Among the three cases of transient AKI, one had Stage I and two had Stage II (Table 3). None of the transient AKI cases exceeded a uNGAL ≥250 ng/mL. The one case of intermediate AKI had Stage III AKI had a maximum uNGAL value of 391 ng/mL.
Table 3.
Demographic and clinical characteristics for AKI cases
| Transient AKI | Indeterminate AKI † | Persistent AKI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ALA-148 | ALA-130 | ALA-123 | ALA-174 | ALA-131 | CIN-023 | ALA-125 | ALA-116 | CIN-053 | ALA-198 | |
| Demographic Characteristics | ||||||||||
| Gestational age, wk/d | 38.2 | 26.6 | 35.5 | 24.5 | 36.0 | 27.2 | 28.4 | 26.5 | 30.0 | 38.1 |
| Birth weight, g | 2950 | 1117 | 2480 | 509 | 2785 | 1260 | 550 | 1005 | 3690 | 2600 |
| Corrected gestational age, wk/d | 50.7 | 31.3 | 36.9 | 68.4 | 36.9 | 32.7 | 78.6 | 44.4 | 31.1 | 41.6 |
| Weight at exposure, g | 5480 | 1340 | 2480 | 8100 | 3250 | 1980 | 8960 | 4540 | 3400 | 4010 |
| Clinical Characteristics | ||||||||||
| Highest AKI severity/stage | 1 | 2 | 2 | 3 | 3 | 3 | 3 | 2 | 3 | 3 |
| Highest uNGAL (ng/mL) | 70.4 | 49.7 | 86 | 391 | 472 | 1010 | 2510 | 2970 | 4510 | 5060 |
| Days in AKI | 1 | 1 | 1 | 1 | 4 | 2 | 2 | 2 | 4 | 9 |
| NTM Exposure | Vancomycin | Vancomycin | Gentamicin | Acyclovir Tobramycin Vancomycin | Gentamicin | Vancomycin | Tobramycin Vancomycin | Vancomycin | Acyclovir Gentamicin Piperacillin/ Tazobactam | Vancomycin |
| Primary disease at first NTM exposure* | PHTN, ECMO | Culture positive sepsis (MSSA), l&D | Surgery (TEF) | Culture positive sepsis (enterovirus), PHTN crisis | Surgery, ECMO(CDH) | Culture positive sepsis | Culture negative sepsis, PHTN crisis | Surgery (SBO with perforation) | NEC/SBP, Surgery (drain placement, bowel resection, central line placement) | Culture negative sepsis, ECMO, Surgery (Exlap) |
| Kidney support therapy | No | No | No | No | Yes | Yes | No | No | Yes | Yes |
Primary disease abbreviation; SBO = small bowel perforation, TEF = tracheoesophageal fistula, PHTN = pulmonary hypertension, l&D = incision and drainage, ECMO = extracorporeal membrane oxygenation, CDH = congenital diaphragmatic hernia. Exlap = exploratory laparotomy, NEC = necrotizing enterocolitis, SBP = small bowel perforation
Missing 1 day of SCr and uNGAL value after patient met modified KDIGO criteria, therefore unable to classify as either transient or persistent AKI
Alternatively, in the infants with true AKI, the median maximum uNGAL value was 2740 ng/mL (IQR 1385–4125). One infant classified as Stage II and five infants classified as Stage III (4/5 of Stage III AKI patients received kidney support therapy). The highest maximum uNGAL was in the patient who had Stage 3 AKI that lasted 9 days.
Discussion/Conclusion
This prospective cohort study evaluated the performance of uNGAL screening to detect NTM-AKI in the Baby NINJA program. We found uNGAL cutoff values ≥250, 300, or 400 ng/mL showed similar sensitivities with excellent NPV values >96%. Due to the low prevalence of AKI in the study population (6.8%, 10/148) LRs were used to determine the usefulness of the test. A statistically significant association of infants with uNGAL ≥250 ng/ml and AKI was noted. Further, a uNGAL value of ≥400 ng/mL showed the strongest predictive power for identifying AKI as it had the highest ROC-AUC 0.72 (95%CI = 0.57–0.88). Furthermore, a uNGAL value of ≥400 ng/mL yielded a positive LR of 2.76 (1.39–4.13; p-value <0.05) meaning that those above this threshold are 2.76 times as likely to have AKI than those below this threshold. Alternatively, a uNGAL value ≤250 ng/mL yielded a negative LR of 0.46 (0.02–0.89; p-value of <0.05). These data suggest uNGAL may effectively screen for AKI in infants exposed to NTM without the need for daily blood draws, reducing barriers to AKI screening adherence among providers and families.
Although not all patients with SCr-based AKI had a positive uNGAL level, all with true AKI had uNGAL levels ≥472ng/mL and were associated with higher levels of AKI (Stage 2 and 3) with longer duration of AKI. In four of the six cases of AKI the peak uNGAL (highest uNGAL recorded) occurred within 48hr of onset of SCr-defined AKI. The three patients who had a quick return of elevated SCr within 48hr without a rise in uNGAL could reflect that those with transient AKI do not reflect true tubular damage, but rather likely had low kidney perfusion that resolved. This is critical as SCr suggests a change in kidney function, while a rise in NGAL suggests tubular injury. Thus, transient AKI suggests a functional change that responds to fluids and not true kidney damage. Alternatively, the six cases of AKI with urine NGAL ≥400 ng/dl all had true AKI with Stage 2 or 3 AKI.
Other studies have evaluated the use of uNGAL when assessing AKI in patients exposed to NTMs. In a two-center, prospective study by Goldstein et al., uNGAL was measured in the non-ICU patients exposed to NTM medications [19]. Similar to our study, the specificity and NPV to predict severe AKI was excellent, supporting that uNGAL thresholds ≤150 ng/mL could serve as to screen for severe AKI in non-ICU pediatric patients. Our study showed similar NPV for all thresholds. Comparable with our study, they also noted low sensitivities and PPVs for uNGAL. However, in our study, the highest AUC threshold was for uNGAL >400 ng/dL.
The association of uNGAL with AKI has been evaluated in other neonatal populations. Sridharan et al. evaluated neonates admitted to a NICU who had been exposed to one or more NTMs (gentamicin, vancomycin, furosemide, ibuprofen, or acetaminophen)[24]. They found a uNGAL cutoff of 155 ng/mL for identifying potential drug-induced AKI, similar to our study. However, they defined AKI using either changes in SCr and/or urine output whereas we diagnosed AKI based on SCr alone. Further, their list of NTM (5 medications) was more limited than that used in our study (57 medications). Rumpel et al. performed a 4-center prospective study evaluating six urinary biomarkers in those infants who met institutional criteria for therapeutic hypothermia (infants at-risk of decreased renal perfusion) [25]. They found elevations in some biomarkers, including uNGAL, though their incidence of AKI (35%) was lower than previous studies underpowering their analysis. In a large, single center prospective study by Slagle et al., uNGAL had strong prediction performance for AKI[16] at 24 hours post-operatively. They found a cutoff of 144 ng/mL as a potential cutoff for detecting kidney damage and concluded that percent change in uNGAL could also be a useful clinical tool in detecting AKI.
Several plausible explanations may explain why this study demonstrated a higher uNGAL threshold than previous studies. First, premature infants have higher normal values of uNGAL until around 34wga[26]. Second, the majority of the cases in our study were Stage 2 or 3, highlighting more severe cases of AKI than in previous studies. Third, the AKI rates were lower than comparable studies, therefore we had a small sample size to determine an optimal cutoff of uNGAL values.
Our study has several strengths. The study was performed with a large number of subjects in a prospective manner using a well-established and validated QI program. The NICUs in the cohort represented two different geographic areas of the country conferring some applicability to other neonatal populations. In addition, the SCr screening compliance was excellent for monitoring for AKI. However, we acknowledge some limitations. The prevalence of AKI was lower (6.8%) compared to our previous study, which we attribute to the sustainability of a well-established QI program. SCr alone was used to diagnosis AKI, which could have underestimated the incidence of AKI. The study period coincided with the pandemic, which may have led to a reduction in admissions and unplanned surgical interventions, both of which are known risk factors for NTM and AKI. Nevertheless, as the main goal of this study was to evaluate if uNGAL can screen for AKI in infants exposed to NTM, the large non-AKI subjects serve our hypothesis well. Most urine samples were obtained from indwelling catheter or urine collection bag; however, 12.7% (88/688) urine samples were obtained from diaper or cotton balls, the latter of which could affect the validity of biomarker measurements[27]. Urinary NGAL compliance was moderate, potentially underrepresenting elevated uNGAL measurements. Lastly, this study was performed in only Level IV NICUs therefore may not be applicable to other lower acuity units.
In conclusion, we suggest uNGAL has the potential to rule-out AKI in infants exposed to NTM medications in the NICU. With the multiple barriers to daily SCr monitoring in high-risk infants, uNGAL can mitigate the concerns from clinicians and families while further defining AKI events. Similar to Goldstein et al. for non-ICU cohorts, our data suggests that algorithms that utilize uNGAL to screen for AKI in infants at high risk for NTM-AKI can be utilized in the NICU[19]. We suggest that a uNGAL ≤250 ng/mL could be a reliable screening threshold for AKI in the Baby NINJA program. Alternatively, uNGAL ≥400 ng/mL could serve as a cutoff above which a true injury has occurred. However, larger studies should be performed to further define these thresholds and to evaluate if uNGAL cutoffs vary by gestational age and other clinical confounders (i.e., type of NTM exposure, length of exposure, acute kidney disease or presence of CKD). Additionally, studies on cost-analysis and utilization of uNGAL tests in addition to parental preferences are needed to further understand how urine biomarker screening can best be incorporated into traditional screening practices to avoid iatrogenic harm in this at-risk population.
Supplementary Material
Acknowledgement
Special thanks to all of the neonatologists, nephrologists, nurse practitioners, nurses, and the Baby NINJA QI teams who cared for these infants at Children’s of Alabama and Cincinnati Children’s Hospital Medical Center. Thank you to the Division of Nephrology and Hypertension Biomarker Laboratory at CCHMC for their assistance with this project. Lastly, thank you to the Pediatric Center of Excellence in Nephrology and the Center for Clinical and Translational Science and Training for grant support for this project.
Conflict of Interest Statement
All authors declare no real or perceived conflicts of interest that could affect the study design, collection, analysis, and interpretation of data, writing of the report, or the decision to submit for publication. For full disclosure, we provide here an additional list of other author’s commitments and funding sources that are not directly related to this study:
David J Askenazi is a consultant for Baxter, Nuwellis, Medtronic Bioporto, Seastar, and Abbott. His institution receives grant funding for education and research that is not related to this project from NIH, Baxter, Nuwellis, Medtronic, Bioporto, Portero and Seastar. He has patents pending on inventions to improve the kidney care of neonates. He is the founder and Chief Scientific Officer for Zorro-Flow Inc.
Stuart Goldstein receives royalties from Vigilanz Corporation for licensing the NINJA application. He receives extramural grant funding from BioPorto Diagnostics, SeaStar Medical, Baxter Healthcare, Nuwellis and Medtronic. He serves as a consultant for BioPorto Diagnostics, SeaStar Medical, Baxter Healthcare, NuWellis, Medtronic, Renibus, AM Pharma, Otsuka, MediBeacon, and Fresenius. He has stock options from MediBeacon.
Funding Sources
Supported by a Pediatric Center of Excellence in Nephrology NIH P50 DK096418 Pilot and Feasibility Project and the Center for Clinical and Translational Science and Training grant support (UL1TR001425).
Footnotes
Statement of Ethics
The study performed at each center was approved by the respective Institutional Review Board (IRB) at each participating institution. Written informed consent was excused by both respective ethics boards as this work was part of an existing quality improvement program with the addition of only urine samples for analysis, samples otherwise considered disposable with routine clinical care. The University of Alabama at Birmingham (UAB) IRB approved the study participation including exception from written consent under the study number 300000362. The Cincinnati Children’s Hospital Medical Center (CCCHM) IRB approved study participation including exception from requiring written informed consent under the study number 2019–0971.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author, Christine Stoops, upon reasonable request (christinestoops@uabmc.edu).
References
- 1.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012. Mar;81(5):442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, et al. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. 2012. Apr;59(4):523–30. [DOI] [PubMed] [Google Scholar]
- 3.Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. 2017. Nov;1(3):184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Criss CN, Selewski DT, Sunkara B, Gish JS, Hsieh L, Mcleod JS, et al. Acute kidney injury in necrotizing enterocolitis predicts mortality. Pediatr Nephrol. 2018. Mar;33(3):503–10. [DOI] [PubMed] [Google Scholar]
- 5.Salerno SN, Liao Y, Jackson W, Greenberg RG, McKinzie CJ, McCallister A, et al. Association between Nephrotoxic Drug Combinations and Acute Kidney Injury in the Neonatal Intensive Care Unit. J Pediatr. 2021. 01;228:213–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein SL, Mottes T, Simpson K, Barclay C, Muething S, Haslam DB, et al. A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int. 2016. Jul;90(1):212–21. [DOI] [PubMed] [Google Scholar]
- 7.Stoops C, Stone S, Evans E, Dill L, Henderson T, Griffin R, et al. Baby NINJA (Nephrotoxic Injury Negated by Just-in-Time Action): Reduction of Nephrotoxic Medication-Associated Acute Kidney Injury in the Neonatal Intensive Care Unit. J Pediatr. 2019. 12;215:223–28.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nada A, Bonachea EM, Askenazi DJ. Acute kidney injury in the fetus and neonate. Semin Fetal Neonatal Med. 2017. 04;22(2):90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009. Mar;20(3):672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weintraub AS, Carey A, Connors J, Blanco V, Green RS. Relationship of maternal creatinine to first neonatal creatinine in infants <30 weeks gestation. J Perinatol. 2015. Jun;35(6):401–4. [DOI] [PubMed] [Google Scholar]
- 11.Krawczeski CD, Woo JG, Wang Y, Bennett MR, Ma Q, Devarajan P. Neutrophil gelatinase-associated lipocalin concentrations predict development of acute kidney injury in neonates and children after cardiopulmonary bypass. J Pediatr. 2011. Jun;158(6):1009–15.e1. [DOI] [PubMed] [Google Scholar]
- 12.Stanski N, Menon S, Goldstein SL, Basu RK. Integration of urinary neutrophil gelatinase-associated lipocalin with serum creatinine delineates acute kidney injury phenotypes in critically ill children. J Crit Care. 2019. 10;53:1–7. [DOI] [PubMed] [Google Scholar]
- 13.Hasson D, Menon S, Gist KM. Improving acute kidney injury diagnostic precision using biomarkers. Pract Lab Med. 2022. May;30:e00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005 2005. Apr 2-8;365(9466):1231–8. [DOI] [PubMed] [Google Scholar]
- 15.Essajee F, Were F, Admani B. Urine neutrophil gelatinase-associated lipocalin in asphyxiated neonates: a prospective cohort study. Pediatr Nephrol. 2015. Jul;30(7):1189–96. [DOI] [PubMed] [Google Scholar]
- 16.Slagle CL, Goldstein SL, Gavigan HW, Rowe JA, Krallman KA, Kaplan HC, et al. Association between Elevated Urine Neutrophil Gelatinase-Associated Lipocalin and Postoperative Acute Kidney Injury in Neonates. J Pediatr. 2021. 11;238:193–201.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taksande AM, Vilhekar KY, Jain M, Chitre D. Pain response of neonates to venipuncture. Indian J Pediatr. 2005. Sep;72(9):751–3. [DOI] [PubMed] [Google Scholar]
- 18.Widness JA. Pathophysiology of Anemia During the Neonatal Period, Including Anemia of Prematurity. Neoreviews. 2008. Nov 01;9(11):e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein SL, Krallman KA, Schmerge A, Dill L, Gerhardt B, Chodaparavu P, et al. Urinary neutrophil gelatinase-associated lipocalin rules out nephrotoxic acute kidney injury in children. Pediatr Nephrol. 2021. 07;36(7):1915–21. [DOI] [PubMed] [Google Scholar]
- 20.Kellum JA, Lameire N, Group KAGW. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013. Feb;17(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017. 04;13(4):241–57. [DOI] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009. Apr;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019. 07;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sridharan K, Al Jufairi M, Al Segai O, Al Ansari E, Hashem Ahmed H, Husain Shaban G, et al. Biomarkers in neonates receiving potential nephrotoxic drugs. Eur Rev Med Pharmacol Sci. 2021. Nov;25(22):7078–88. [DOI] [PubMed] [Google Scholar]
- 25.Rumpel J, Spray BJ, Chock VY, Kirkley MJ, Slagle CL, Frymoyer A, et al. Urine Biomarkers for the Assessment of Acute Kidney Injury in Neonates with Hypoxic Ischemic Encephalopathy Receiving Therapeutic Hypothermia. J Pediatr. 2022. 02;241:133–40.e3. [DOI] [PubMed] [Google Scholar]
- 26.Askenazi DJ, Koralkar R, Levitan EB, Goldstein SL, Devarajan P, Khandrika S, et al. Baseline values of candidate urine acute kidney injury biomarkers vary by gestational age in premature infants. Pediatr Res. 2011. Sep;70(3):302–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boohaker L, Halloran B, Wilson L, Berryhill T, Barnes S, Griffin R, et al. Absorbent materials to collect urine can affect proteomics and metabolomic biomarker concentrations. Clin Chem Lab Med. 2019. 05 27;57(6):e134–e37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author, Christine Stoops, upon reasonable request (christinestoops@uabmc.edu).


