Abstract
Background
Joint damage affects the quality of life of persons with hemophilia A. The long-term safety and efficacy of turoctocog alfa pegol (N8-GP) prophylaxis in persons with hemophilia A has been investigated in pivotal phase 3 trials in children, adolescents, and adults (pathfinder program). However, there is a lack of data on joint health in adult persons with hemophilia A treated with N8-GP.
Objectives
To describe the design of the ongoing pathfinderReal study investigating the joint health status in adult persons with hemophilia A after switching to N8-GP.
Methods
pathfinderReal is a multicountry, noninterventional, single-arm study (NCT05621746) of joint health in adult (≥18 years) male persons with hemophilia A who have switched to N8-GP. Patients enrolled in other interventional studies and those who have previously terminated N8-GP treatment will be excluded. Approximately 124 adults with hemophilia A will be enrolled and followed up for a maximum of 24 months. Data from routine clinical assessments of patients’ joint health will be collected. The primary endpoint is change in Hemophilia Joint Health Score (defined as a change in total score of ≤2) from initiation of N8-GP treatment until the end of the study. Secondary endpoints include number of bleeding episodes, number and resolution of target joints, patient-reported outcomes of problem joint score, pain score, and change in physical function levels. An exploratory endpoint is included to measure the number of patients achieving improved Hemophilia Joint Health Score from the initiation of N8-GP until the end of the study.
Conclusion
The pathfinderReal study will provide insights regarding the impact of N8-GP on joint health in persons with hemophilia A in a real-world setting.
Keywords: factor VIII, hemarthrosis, hemophilia A, joint, N8-GP
Essentials
-
•
Joint damage is an important outcome affecting the quality of life of persons with hemophilia.
-
•
Data on joint health in adult persons with hemophilia A treated with N8-GP are lacking.
-
•
We present the design of pathfinderReal, an ongoing study evaluating joint health in adult persons with hemophilia A.
-
•
pathfinderReal aims to investigate outcomes of N8-GP treatment on joint health in persons with hemophilia A in a real-world setting.
1. Introduction
Clinical outcome measures for hemophilia A (HA) include several laboratory and clinical evaluations such as factor VIII (FVIII) levels, FVIII inhibitors, overall bleeding rates, joint bleeding rates, target joints, and quality-of-life (QoL) measures [1,2]. Of all the outcome measures, joint damage largely affects QoL in persons with HA as well as influences the related socioeconomic burden of treatment, including treatment costs and work productivity [[3], [4], [5]]. Recurrent bleeding episodes into the joints may trigger synovitis, leading to progressive osteochondral damage [6] and ultimately resulting in chronic hemophilic arthropathy, which is usually characterized by chronic pain and poor physical function [6,7]. Those joints where at least 3 bleeds occur over a 6-month period are termed target joints [8]. On average, the most frequently involved joints are elbows, knees, and ankles. The functional impact of hemophilic arthropathy can be quantified using the Hemophilia Joint Health Score (HJHS) version 2.1, a validated clinical assessment tool [9]. The HJHS was originally developed to assess joint health in children with hemophilia in order to detect early signs of joint damage and has recently been validated for use in an adult population [10]. HJHS evaluates the status of a joint, including swelling, duration of swelling, muscle atrophy, crepitus of motion, range of motion (extension and flexion loss), joint pain, strength, and gait [[9], [10], [11]].
The current standard of care for HA is long-term prophylaxis, which consists of regular intravenous injections of clotting factor concentrates (either standard half-life or extended half-life products) or regular subcutaneous injections of FVIII mimetics, including episodic treatment in case of breakthrough bleeds [12,13]. In persons with HA, regular prophylaxis with FVIII products is effective in preventing recurrent bleeding events in muscles and joints [13,14]. Turoctocog alfa pegol (N8-GP) is an extended half-life, recombinant, glycoPEGylated FVIII product used for both the prevention and treatment of bleeding episodes in persons with HA [15]. N8-GP offers convenient prophylaxis treatment with a lower frequency of injections (every fourth day) than standard half-life products (on average, every second day or 3 times/wk), with mean trough levels of 3.0 IU/dL and 2.7 IU/dL in adults and adolescents, respectively [16]. This provides a 1.6 times longer half-life in adults compared with standard FVIII products [17]. The long-term safety and efficacy of N8-GP prophylaxis in persons with HA have been investigated in pivotal phase 3 pathfinder clinical trials in children, adolescents, and adults [17,18]. However, there is a scarcity of data regarding joint health in adults treated with N8-GP.
This manuscript outlines the design of the ongoing pathfinderReal study, which is investigating the joint health status in adult persons with HA after switching to N8-GP prophylaxis. The study will assess joint-related clinical outcomes in the patient population, including target joints, number of bleeding episodes, problem joint scores, and pain scores. The study will also evaluate patient-reported outcome (PRO) questionnaires on physical function and activity levels.
2. Methods
2.1. Study design
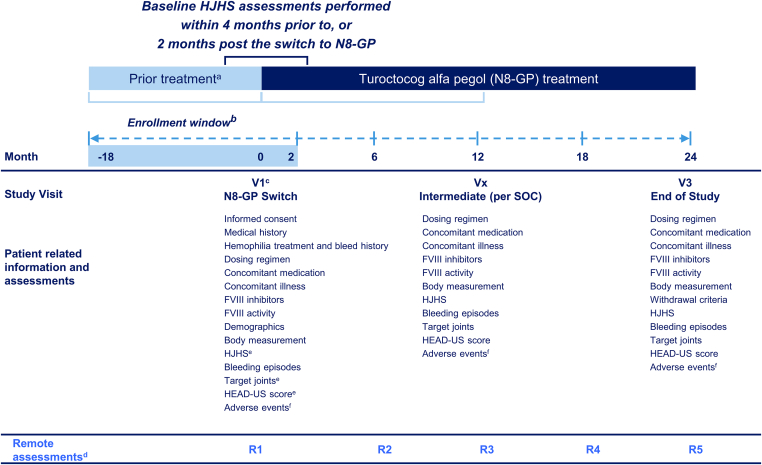
pathfinderReal (NCT05621746) is an international, noninterventional, single-arm study assessing the joint health of adult male persons with HA who have switched to treatment with N8-GP prophylaxis. Patients will be treated with commercially available N8-GP according to the approved local label and clinical practice at the treating physician’s discretion. Patients will be eligible to participate in the study if they have switched to N8-GP in the 18 months preceding enrollment or have decided to switch during the 2 months post enrollment. The decision to initiate treatment with N8-GP is made by the patient and treating physician prior to enrollment in the study. The total study duration for each participant is estimated to be 24 months (Figure). The observation period will be a maximum of 24 months post the switch to N8-GP, during which patient data will be collected; this will include collecting retrospective data for some patients. Independent of routine visits, the study design includes biannual remote direct data capture by participants for PROs (Figure). The study started on November 23, 2022, with the first patient enrollment, and recruitment is ongoing, with each patient observed for a maximum of 24 months.
Figure.
pathfinderReal study design. aPrior treatment: any hemophilia treatment regime other than N8-GP received prior to study enrollment. bThe enrollment window: the date when the patient switched to N8-GP (within 18 months prior to enrollment) or planned to switch (within 2 months post enrollment) to prophylaxis with N8-GP from previous therapy. cVisit 1 may occur prior to enrollment due to criteria that allow patients who have switched to N8-GP in the 18 months prior to enrollment. dRemote PRO measurements: biannual remote direct data capture by participants is planned for the PRO assessments. eAssessments performed within 4 months prior to switch or 2 months after the switch to N8-GP can be used as baseline data. fSites should ask and report on adverse events at each contact with the patient during the course of the study. FVIII, factor VIII; HEAD-US, Hemophilia Early Arthropathy Detection with Ultrasound; HJHS, Hemophilia Joint Health Score; N8-GP, turoctocog alfa pegol; PRO, patient-reported outcome; Rx, remote assessment number; SOC, standard of care; Vx, visit number.
2.2. Study population and eligibility criteria
Overall, patients from 30 to 40 sites across 11 countries (Austria, Canada, Germany, Italy, Denmark, Japan, Saudi Arabia, Spain, Hungary, the United Kingdom, and the United States) are included in the study. The sites were selected based on their adherence to the treatment guidelines on joint health assessments and prescription of N8-GP.
The inclusion criteria were chosen to focus the study on a clinically relevant patient population with HA. The inclusion criteria are as follows: (a) adult males ≥18 years of age at the time of initiating N8-GP who have been diagnosed with severe (FVIII activity, <1%) or moderate (FVIII activity, 1%-5%) HA; (b) those who signed informed consent obtained before study enrollment; (c) those who switched to treatment with N8-GP within 18 months prior to enrollment or planned to switch within 2 months post enrollment, independent of the decision to enroll in the study; and (d) participants with baseline data (HJHS, target joints, and medical history) collected in routine clinical practice within 4 months before or up to 2 months after switching to N8-GP treatment. The key exclusion criteria are as follows: (a) previous participation in this study; (b) history of terminated treatment with N8-GP, either on demand or for prophylaxis; (c) mental incapacity, unwillingness, or language barriers precluding adequate understanding or co-operation; and (d) current participation in interventional clinical trials.
2.3. Study objectives and endpoints
The primary objective of the pathfinderReal study is to evaluate if joint health is maintained in persons with HA after switching to N8-GP. The primary endpoint is to assess the change in HJHS version 2.1 (change in total score of ≤2) from the initiation of N8-GP until the end of the study. The overall score from HJHS evaluates 6 index joints and provides users with a relative indicator of joint health, with a lower HJHS representing better joint health [19]. The scoring range of the HJHS is from 0 (normal, healthy joints) to 124 (maximum severity) [9,11,20,21]. In this study, maintained HJHS is defined as a change in total score of ≤2 points in the 24 months post initiation of N8-GP. This value was determined via a consensus agreement following the review of 11 published studies, which assessed the change in HJHS over time [[22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32]]. The 11 studies, consisting of a total of 537 persons with hemophilia (the majority with severe hemophilia and on prophylaxis), reported an average change in HJHS of +2.
The secondary objective is to assess additional clinically relevant parameters associated with joint health in terms of bleeding patterns, pain, and QoL outcomes. This includes assessing target joints evaluated by the number of cases and by patient-reported problem joints, which are defined as joints having chronic pain and/or limited range of movement due to compromised joint integrity (ie, chronic synovitis and/or hemophilic arthropathy), with or without persistent bleeding [33]. Problem joints are captured as total number (count) and the number per joint (count per location) [33]. In addition, Hemophilia Early Arthropathy Detection with Ultrasound (HEAD-US) scores will be assessed. Secondary endpoints include the following: (a) number of bleeding episodes requiring FVIII treatment from date of switch to N8-GP until the end of the study; (b) number of target joints developed from date of switch to N8-GP until the end of study; (c) number of target joints resolved from date of switch to N8-GP until the end of the study; and (d) change in HEAD-US score calculated using postbaseline measurements of HEAD-US (score unit), up to and including the end of study minus baseline HEAD-US (score unit). In addition, the results from the PRO questionnaires will be evaluated as follows: (a) change in patient-reported problem joint score; (b) change in patient-reported pain scores, as measured by the Brief Pain Inventory Short Form [34,35]; and (c) change in physical function levels and activity, as measured using the 36-Item Short Form Health Survey version 2, from date of switch to N8-GP until the end of the study [34].
An exploratory endpoint is to assess the number of patients with an improved HJHS by the end of the study. An improved HJHS is defined herein as a reduction in total HJHS of >2 points in the 24 months post initiation of N8-GP. There will likely be a lag period before nontransient effects on HJHS are seen after the treatment switch to N8-GP. Therefore, any scores collected within 6 months post switch can be considered unaffected by the new treatment.
2.4. Baseline measurements and study visits
Baseline data are defined as the data collected at the time when a patient switched to N8-GP from their current treatment. This may require retrospective data collection for patients who switched to N8-GP within up to 18 months prior to enrollment. Available data in medical records will be entered in the electronic case report form (eCRF). Data to be collected at baseline are as follows: (a) age (in years); (b) body mass index; (c) number of target joints at the time of switch to N8-GP and details of medical history of joint health, including hemophilic arthropathy of shoulders, elbows, hips, knees, or ankles; and (d) joint replacements in knees or ankles, ankle arthrodesis, and any other hemophilic arthropathy or procedure, as available. Patients will visit the sites according to standard of care frequency. The number of visits and available data may differ between sites, but ideally, patients will visit the clinic at least once per year.
Relevant data regarding patient assessments (Figure) will be entered in the eCRF. For certain assessments (HJHS, HEAD-US, and target joints), data recorded in standard clinical practice 4 months before switch or 2 months after the switch to N8-GP will be considered baseline data. Any visit(s) in the period following the baseline visit until the end of study visit will be categorized as intermediate visits. Additionally, patients will be asked on a biannual basis to enter applicable information themselves into an electronic PRO system. Patients will also be offered the option to complete PROs via paper copies, where appropriate. Patients are allowed to withdraw from the study at any time during the study period. In such circumstances, the physician should collect any outstanding data and document the reason for discontinuation in the eCRF.
2.5. Study size and analysis set
The study sample size is based on a 5% level of significance and a power of 80%, with an assumed SD of 7 and expected mean change after 24 months in HJHS of 0. The primary analysis is planned to include 99 patients to assess the primary endpoint. A proportion of 20% is accounted for patient discontinuation, and therefore, we aim to enroll 124 patients to achieve a sample size of 99 patients completing the study. Descriptions and analysis of effectiveness will be based on the full analysis set, as defined in International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) E9 guidelines [36]. The full analysis set includes all eligible patients based on inclusion and exclusion criteria, and the safety analysis set includes all patients exposed to N8-GP. Patients who initiate immune tolerance induction treatment will be considered withdrawals for statistical analyses, and any data collected during the immune tolerance induction treatment period will be summarized and reported separately.
2.6. Data management and reporting of adverse events
Data management will be assigned to the contract research organization (CRO) and overseen by Novo Nordisk. All information from this study will be captured in an electronic database maintained by the CRO under the supervision of Novo Nordisk and in accordance with country-specific laws. During the study, monitoring will be performed to ensure that the patient has adhered to planned procedures. Monitoring will be performed by a CRO according to the standards set out in Novo Nordisk’s standard operating procedure for a noninterventional study. Relevant data regarding the assessments must be entered in the eCRF, if available.
Collection and reporting of all adverse events to the CRO will be performed in a timely manner from study start to study end dates. Mandatory reporting is required in the event of an overdose, abuse, medication error, or lack of therapeutic effect, with or without an accompanying adverse reaction. The treating physician should report any adverse events (serious and nonserious) to the CRO within 3 calendar days of the event. Bleeding episodes are not considered adverse or serious adverse events unless bleeding is fatal. All serious adverse events and adverse reactions (serious and nonserious) will be followed up until the outcome of the event or reaction is “recovered” or “recovered with sequelae” and queries have been resolved. Cases will be closed with an outcome of “recovering” when the patient has completed the study and is expected by the physician to recover.
2.7. Statistical analysis
The difference in HJHS of patients between study start and end dates will be analyzed by analysis of covariance. For the primary endpoint, the aim is to show that the HJHS does not increase by more than 2 points after 24 months, a cutoff chosen based on existing literature [28,[37], [38], [39]].
Results from statistical analyses will be accompanied by 2-sided 95% CIs and corresponding P values. Categorical data will be summarized by frequency tables, while continuous data will be summarized by mean, SD, median, and minimum and maximum values. Additionally, to investigate the sensitivity of the results of the primary analysis of the primary endpoint with regard to the handling of missing data, a mixed model for repeated measurements with an unstructured covariance structure will be applied. The mixed model for repeated measurements will include number of target joints at baseline and study visits as a fixed factor and baseline age, baseline body mass index, and baseline HJHS as covariates.
2.8. Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting, or dissemination plans of this research.
2.9. Ethics and dissemination
The study is approved by the Institutional Review Board or Independent Ethics Committee from all the participating countries. The study will be conducted in compliance with the Declaration of Helsinki and Good Pharmacoepidemiology Practice [36], Good Pharmacovigilance Practice Module VI guidelines [40], and Ethical Guidelines for Medical and Health Research Involving Human Subjects [41]. All participants will sign written informed consent and are assigned a unique identification number to maintain confidentiality.
3. Discussion
The pathfinderReal study will evaluate the joint health status of adult persons with HA after switching to treatment with N8-GP. To capture real-world data, an extended study enrollment window will allow patients who have switched to N8-GP in the 18 months prior to enrollment or have decided to switch in the 2 months post enrollment to participate. Every patient in the study is planned to visit for routine clinical and biannual patient-reported evaluation over a duration of 24 months. During these visits, the status of joint health will be evaluated based on HJHS and other joint-related clinical outcomes, including target joints, pain, physical function, and bleeding rates.
Encouraging target joint resolution data were collected in the phase 3 pathfinder8 study, which investigated the long-term safety and efficacy of N8-GP in patients of all ages with severe HA. In pathfinder8, a total of 160 patients were enrolled from pathfinder2 and pathfinder5 and were followed up for 104 weeks. A total of 5 patients reported 7 target joints at baseline. By the end of the study, out of these 7 target joints, 3 patients had ≥1 baseline target joint resolved, and of these 3 patients, 2 had all baseline target joints resolved [18]. This suggests that there was a beneficial effect of N8-GP in a small subgroup of patients, indicating the importance of conducting a follow-up study with a larger cohort of patients.
Joint damage is a major factor in the socioeconomic burden of hemophilia treatment, influencing treatment costs and health-related QoL (HRQoL) [[3], [4], [5]]. Approximately 90% of people with severe hemophilia suffer from joint disease, which most commonly affects elbows, knees, and ankles [42]. Data from the Cost of Hemophilia across Europe – a Socioeconomic Survey study in persons with severe HA showed that the presence of chronic synovitis had a significant negative impact on HRQoL [43]. A European study on persons with severe HA or hemophilia B with inhibitors reported an increased disease burden due to orthopedic complications, with reduced patient mobility affecting overall QoL [44]. Therefore, the pathfinderReal study aims to supplement the efficacy results of N8-GP by further evaluating joint health and its impact on HRQoL by assessing various PRO measures in persons with HA.
Some of the limitations of the study include the following: (a) retrospective initiators included in the study will be successful initiators, thereby introducing bias. However, this is unavoidable due to the rarity of hemophilia; (b) the noninterventional study design involves recording data in routine practice, as opposed to mandatory clinical assessments at prespecified time points, and may impact the quantity and quality of data collected and their subsequent interpretation; (c) there is likely to be interpatient and intersite variability in the reporting of bleeding episodes requiring FVIII treatment; and (d) missing PRO data for some patients due to the inclusion of patients who have switched to N8-GP within up to 18 months prior to enrollment may limit interpretation.
4. Conclusion
The pathfinderReal study will report the impact of N8-GP prophylaxis on joint health in adults with HA using real-world data. On completion, these data will guide us on the significance of routine clinical evaluation of joint health parameters with the aim of improving clinical practice and well-being of patients treated with N8-GP.
Acknowledgments
The authors would like to thank the patients participating in the study and their families. Medical writing support was provided by Dr Ambika Shivakant Kurbet, PhD, of Novo Nordisk India Pvt Limited, Global Medical Affairs, Bengaluru, India. We also thank Ashfield MedComms GmbH, an Inizio company (Mannheim, Germany), for editorial support, which was funded by Novo Nordisk. The sponsor was involved in the study design, collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. However, the authors are ultimately responsible for opinions, conclusions, and data interpretation.
Funding
This study is funded by Novo Nordisk AG.
Author contributions
All authors contributed substantially to the design, analysis, and interpretation of the work. All authors revised the manuscript critically, provided final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Relationship Disclosure
C.A. has received honoraria for lectures and participation in advisory boards from Bayer, CSL Behring, LFB, Novo Nordisk, Roche, and Sobi. A.N. has received investigator-initiated grant funding from Takeda (Shire), Chugai, and Bayer and has received honoraria from Sanofi, Takeda, Chugai, Bayer, Fujimoto, KMB, Pfizer, JB, Novo Nordisk, Sekisui Medical, and CSL Behring. L.N. has received honoraria for participating in advisory boards from Octapharma, Novo Nordisk, Bayer, Biotest, CSL Behring, Pfizer, and Roche and has received consulting fees from Octapharma, Novo Nordisk, Bayer, Biotest, and CSL Behring. O.B. received reimbursement for attending symposia/congresses and/or received honoraria for speaker bureau and/or participation advisory boards from Bayer, CSL Behring, Novo Nordisk, Takeda, Octapharma, Pfizer, Roche, and Sobi. J.O. received reimbursement for attending symposia/congresses and/or received honoraria and/or funds for research from Bayer, Biogen Idec, BioMarin, Biotest, Chugai, CSL Behring, Freeline, Grifols, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Spark Therapeutics, Swedish Orphan Biovitrum, and Takeda. D.M. reports research grants paid directly to the Institution (McMaster University) from Bayer, Pfizer, Novo Nordisk, Sanofi, Spark, Octapharma, and Roche, and personal fees/honoraria from Sanofi, Sobi, Novo Nordisk, Bayer, Pfizer, and Octapharma for participation in advisory boards, lectures, and preparation of educational material. J.W. is an employee of Novo Nordisk. G.G. has received honoraria for participating in advisory boards for Bayer, Sobi, and Novo Nordisk, has received consulting fees from Hemab, and has received honoraria for lectures from LFB and Bayer. M.E.M. reports no conflicts of interest.
Data availability
We plan to publish the data of the study upon study completion, and data that support the findings of this study will be available on request from the corresponding author.
Footnotes
Handling Editor: Michael Makris
References
- 1.Manco-Johnson M.J., Warren B.B., Buckner T.W., Funk S.M., Wang M. Outcome measures in haemophilia: beyond ABR (annualized bleeding rate) Haemophilia. 2021;27:87–95. doi: 10.1111/hae.14099. [DOI] [PubMed] [Google Scholar]
- 2.Dover S., Blanchette V.S., Srivastava A., Fischer K., Abad A., Feldman B.M. Clinical outcomes in hemophilia: towards development of a core set of standardized outcome measures for research. Res Pract Thromb Haemost. 2020;4:652–658. doi: 10.1002/rth2.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Hara J., Hughes D., Camp C., Burke T., Carroll L., Diego D.G. The cost of severe haemophilia in Europe: the CHESS study. Orphanet J Rare Dis. 2017;12:106. doi: 10.1186/s13023-017-0660-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Hara J., Walsh S., Camp C., Mazza G., Carroll L., Hoxer C., et al. The relationship between target joints and direct resource use in severe haemophilia. Health Econ Rev. 2018;8:1. doi: 10.1186/s13561-018-0185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Hara J., Noone D., Jain M., Pedra G., Landis S., Hawes C., et al. Clinical attributes and treatment characteristics are associated with work productivity and activity impairment in people with severe haemophilia A. Haemophilia. 2021;27:938–946. doi: 10.1111/hae.14302. [DOI] [PubMed] [Google Scholar]
- 6.van Vulpen L.F.D., Holstein K., Martinoli C. Joint disease in haemophilia: pathophysiology, pain and imaging. Haemophilia. 2018;24:44–49. doi: 10.1111/hae.13449. [DOI] [PubMed] [Google Scholar]
- 7.Gualtierotti R., Solimeno L.P., Peyvandi F. Hemophilic arthropathy: current knowledge and future perspectives. J Thromb Haemost. 2021;19:2112–2121. doi: 10.1111/jth.15444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reding M.T., Pabinger I., Lalezari S., Santagostino E., Mancuso M.E. Target joint resolution in patients with haemophilia A receiving long-term prophylaxis with BAY 94-9027. Haemophilia. 2020;26:e201–e204. doi: 10.1111/hae.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman B.M., Funk S.M., Bergstrom B.M., Zourikian N., Hilliard P., van der Net J., et al. Validation of a new pediatric joint scoring system from the International Hemophilia Prophylaxis Study Group: validity of the hemophilia joint health score. Arthritis Care Res (Hoboken) 2011;63:223–230. doi: 10.1002/acr.20353. [DOI] [PubMed] [Google Scholar]
- 10.St-Louis J., Abad A., Funk S., Tilak M., Classey S., Zourikian N., et al. The Hemophilia Joint Health Score version 2.1 validation in adult patients study: a multicenter international study. Res Pract Thromb Haemost. 2022;6 doi: 10.1002/rth2.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hilliard P., Funk S., Zourikian N., Bergstrom B.M., Bradley C.S., McLimont M., et al. Hemophilia joint health score reliability study. Haemophilia. 2006;12:518–525. doi: 10.1111/j.1365-2516.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- 12.Marchesini E., Morfini M., Valentino L. Recent advances in the treatment of hemophilia: a review. Biologics. 2021;15:221–235. doi: 10.2147/BTT.S252580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aledort L., Mannucci P.M., Schramm W., Tarantino M. Factor VIII replacement is still the standard of care in haemophilia A. Blood Transfus. 2019;17:479–486. doi: 10.2450/2019.0211-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuelson Bannow B., Recht M., Negrier C., Hermans C., Berntorp E., Eichler H., et al. Factor VIII: long-established role in haemophilia A and emerging evidence beyond haemostasis. Blood Rev. 2019;35:43–50. doi: 10.1016/j.blre.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Stennicke H.R., Kjalke M., Karpf D.M., Balling K.W., Johansen P.B., Elm T., et al. A novel B-domain O-glycoPEGylated FVIII (N8-GP) demonstrates full efficacy and prolonged effect in hemophilic mice models. Blood. 2013;121:2108–2116. doi: 10.1182/blood-2012-01-407494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowdary P., Carcao M., Holme P.A., Jimenez-Yuste V., Lentz S.R., Moss J., et al. Fixed doses of N8-GP prophylaxis maintain moderate-to-mild factor VIII levels in the majority of patients with severe hemophilia A. Res Pract Thromb Haemost. 2019;3:542–554. doi: 10.1002/rth2.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Male C., Konigs C., Dey S., Matsushita T., Millner A.H., Zak M., et al. The safety and efficacy of N8-GP (turoctocog alfa pegol) in previously untreated pediatric patients with hemophilia A. Blood Adv. 2023;7:620–629. doi: 10.1182/bloodadvances.2022007529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lentz S.R., Kavakli K., Klamroth R., Misgav M., Nagao A., Tosetto A., et al. Turoctocog alfa pegol (N8-GP) in severe hemophilia A: long-term safety and efficacy in previously treated patients of all ages in the pathfinder8 study. Res Pract Thromb Haemost. 2022;6 doi: 10.1002/rth2.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribeiro T., Abad A., Feldman B.M. Developing a new scoring scheme for the Hemophilia Joint Health Score 2.1. Res Pract Thromb Haemost. 2019;3:405–411. doi: 10.1002/rth2.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuijlaars I.A.R., van der Net J., Feldman B.M., Aspdahl M., Bladen M., de Boer W., et al. Evaluating international Haemophilia Joint Health Score (HJHS) results combined with expert opinion: options for a shorter HJHS. Haemophilia. 2020;26:1072–1080. doi: 10.1111/hae.14180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kempton C.L., Recht M., Neff A., Wang M., Buckner T.W., Soni A., et al. Impact of pain and functional impairment in US adults with haemophilia: patient-reported outcomes and musculoskeletal evaluation in the pain, functional impairment and quality of life (P-FiQ) study. Haemophilia. 2018;24:261–270. doi: 10.1111/hae.13377. [DOI] [PubMed] [Google Scholar]
- 22.Kiialainen A., Niggli M., Kempton C.L., Castaman G., Chang T., Paz-Priel I., et al. Effect of emicizumab prophylaxis on bone and joint health markers in people with haemophilia A without factor VIII inhibitors in the HAVEN 3 study. Haemophilia. 2022;28:1033–1043. doi: 10.1111/hae.14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donoso-Úbeda E., Meroño-Gallut J., López-Pina J.A., Cuesta-Barriuso R. Effect of manual therapy in patients with hemophilia and ankle arthropathy: a randomized clinical trial. Clin Rehabil. 2020;34:111–119. doi: 10.1177/0269215519879212. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y., Chen L., Li K., Shi M., Poon M.C. Severe haemophilia A children on low-dose tertiary prophylaxis showed less joint deterioration and better maintenance of functional independence than children on on-demand treatment: a 6-year follow-up study. Haemophilia. 2020;26:779–785. doi: 10.1111/hae.14016. [DOI] [PubMed] [Google Scholar]
- 25.Zanon E., Tagliaferri A., Pasca S., Ettorre C.P., Notarangelo L.D., Biasioli C., et al. Physical activity improved by adherence to prophylaxis in an Italian population of children, adolescents and adults with severe haemophilia A: the SHAPE Study. Blood Transfus. 2020;18:152–158. doi: 10.2450/2019.0040-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lobet S., Meité N.D., Koninckx M.-I., Van Overstraeten A., Kamagate A.M., Hermans C., et al. Implementation and assessment of a self- and community-based rehabilitation programme in patients with haemophilia from Côte d’Ivoire. Haemophilia. 2019;25:859–866. doi: 10.1111/hae.13833. [DOI] [PubMed] [Google Scholar]
- 27.Chozie N.A., Primacakti F., Gatot D., Setiabudhy R.D., Tulaar A.B.M., Prasetyo M. Comparison of the efficacy and safety of 12-month low-dose factor VIII tertiary prophylaxis vs on-demand treatment in severe haemophilia A children. Haemophilia. 2019;25:633–639. doi: 10.1111/hae.13770. [DOI] [PubMed] [Google Scholar]
- 28.Kuijlaars I.A.R., Timmer M.A., de Kleijn P., Pisters M.F., Fischer K. Monitoring joint health in haemophilia: factors associated with deterioration. Haemophilia. 2017;23:934–940. doi: 10.1111/hae.13327. [DOI] [PubMed] [Google Scholar]
- 29.Oldenburg J., Kulkarni R., Srivastava A., Mahlangu J.N., Blanchette V.S., Tsao E., et al. Improved joint health in subjects with severe haemophilia A treated prophylactically with recombinant factor VIII Fc fusion protein. Haemophilia. 2018;24:77–84. doi: 10.1111/hae.13353. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Y., Xiao J., Yang R., Wu R., Hu Y., Beckmann H., et al. Efficacy of standard prophylaxis versus on-demand treatment with bayer's sucrose-formulated recombinant FVIII (rFVIII-FS) in Chinese children with severe hemophilia A. Pediatr Hematol Oncol. 2017;34:138–148. doi: 10.1080/08880018.2017.1313921. [DOI] [PubMed] [Google Scholar]
- 31.Verma S.P., Dutta T.K., Mahadevan S., Nalini P., Basu D., Biswal N., et al. A randomized study of very low-dose factor VIII prophylaxis in severe haemophilia - a success story from a resource limited country. Haemophilia. 2016;22:342–348. doi: 10.1111/hae.12838. [DOI] [PubMed] [Google Scholar]
- 32.Groen W., van der Net J., Lacatusu A.M., Serban M., Helders P.J., Fischer K. Functional limitations in Romanian children with haemophilia: further testing of psychometric properties of the Paediatric Haemophilia Activities List. Haemophilia. 2013;19:e116–e125. doi: 10.1111/hae.12090. [DOI] [PubMed] [Google Scholar]
- 33.Burke T., Rodriguez-Santana I., Chowdary P., Curtis R., Khair K., Laffan M., et al. Humanistic burden of problem joints for children and adults with haemophilia. Haemophilia. 2023;29:608–618. doi: 10.1111/hae.14731. [DOI] [PubMed] [Google Scholar]
- 34.Buckner T.W., Wang M., Cooper D.L., Iyer N.N., Kempton C.L. Known-group validity of patient-reported outcome instruments and hemophilia joint health score v2.1 in US adults with hemophilia: results from the Pain, Functional Impairment, and Quality of life (P-FiQ) study. Patient Prefer Adherence. 2017;11:1745–1753. doi: 10.2147/PPA.S141392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barry V., Lynch M.E., Tran D.Q., Antun A., Cohen H.G., DeBalsi A., et al. Distress in patients with bleeding disorders: a single institutional cross-sectional study. Haemophilia. 2015;21:e456–e464. doi: 10.1111/hae.12748. [DOI] [PubMed] [Google Scholar]
- 36.Baber N. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use (ICH) Br J Clin Pharmacol. 1994;37:401–404. doi: 10.1111/j.1365-2125.1994.tb05705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nolan B., Mahlangu J., Pabinger I., Young G., Konkle B.A., Barnes C., et al. Recombinant factor VIII Fc fusion protein for the treatment of severe haemophilia A: final results from the ASPIRE extension study. Haemophilia. 2020;26:494–502. doi: 10.1111/hae.13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klamroth R., Windyga J., Radulescu V., Collins P.W., Stasyshyn O., Ibrahim H.M., et al. Rurioctocog alfa pegol PK-guided prophylaxis in hemophilia A: results from the phase 3 PROPEL study. Blood. 2021;137:1818–1827. doi: 10.1182/blood.2020005673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Callaghan M.U., Negrier C., Paz-Priel I., Chang T., Chebon S., Lehle M., et al. Long-term outcomes with emicizumab prophylaxis for hemophilia A with or without FVIII inhibitors from the HAVEN 1-4 studies. Blood. 2021;137:2231–2242. doi: 10.1182/blood.2020009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.European Medicines Agency Guideline on good pharmacovigilance practices (GVP). Module VI – management and reporting of adverse reactions to medicinal products (Rev 1) https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-module-vi-management-and-reporting-adverse-reactions-medicinal-products-rev-1-superseded_en.pdf 2014 [accessed November 01, 2024].
- 41.Eba J., Nakamura K. Overview of the ethical guidelines for medical and biological research involving human subjects in Japan. Jpn J Clin Oncol. 2022;52:539–544. doi: 10.1093/jjco/hyac034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saulyte Trakymiene S., Ingerslev J., Rageliene L. Utility of the Haemophilia Joint Health Score in study of episodically treated boys with severe haemophilia A and B in Lithuania. Haemophilia. 2010;16:479–486. doi: 10.1111/j.1365-2516.2009.02178.x. [DOI] [PubMed] [Google Scholar]
- 43.O'Hara J., Walsh S., Camp C., Mazza G., Carroll L., Hoxer C., et al. The impact of severe haemophilia and the presence of target joints on health-related quality-of-life. Health Qual Life Outcomes. 2018;16:84. doi: 10.1186/s12955-018-0908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morfini M., Haya S., Tagariello G., Pollmann H., Quintana M., Siegmund B., et al. European study on orthopaedic status of haemophilia patients with inhibitors. Haemophilia. 2007;13:606–612. doi: 10.1111/j.1365-2516.2007.01518.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We plan to publish the data of the study upon study completion, and data that support the findings of this study will be available on request from the corresponding author.