Abstract
This study aimed to determine the effects of thawing methods such as thawing in a refrigerator, at room temperature, in cold water, and using a microwave oven on the pH, color value, 2-thiobarbituric acid (TBA) value, water-holding capacity, thawing loss, textural attributes, microbial status, and morphology of frozen beef samples. The redness (a*) value of beef samples thawed in cold water for prolonged time and those thawed at room temperature for prolonged time significantly increased compared with that of samples thawed using other methods. The TBA value and thawing loss of beef samples thawed in a refrigerator were significantly lower than those of samples thawed using other methods. Regardless of the thawing method used, beef samples thawed for prolonged time showed significantly high TBA values. With regard to texture, beef samples thawed using a microwave oven showed the maximum hardness compared with those thawed using other methods. The total aerobic bacterial count in fresh beef samples was 1.98 log colony-forming unit (CFU)/g, whereas that in samples thawed at room temperature was 2.49 log CFU/g. Beef samples thawed at room temperature for prolonged time and those thawed using a microwave oven showed irregular structure. These data demonstrated that the thawing condition affects the physicochemical and microbiological qualities of beef, with thawing in a refrigerator resulting in superior beef quality than thawing at room temperature or using a microwave oven.
Keywords: beef quality, color value, microbial status, TBA value, thawing loss
INTRODUCTION
Freezing is a commonly used method to preserve meat for long period of time. Hence, appropriate freezing and thawing procedures are important. The quality of thawed frozen meat is thought to be closely related to the thawing process and condition. This indicates that thawing can affect the sensory attributes of meat (Lagerstedt et al., 2008). Thawing directly changes muscle fibers and cell membranes, which in turn affects the quality deterioration of meat (Leygonie et al., 2012). However, the thawing of meat can lead to microbial growth, reduced nutritional values because of leaching of soluble proteins, and loss of large amounts of meat juice (Min et al., 2016). The quality of meat is classified on the basis of water-holding capacity, pH, and color. Because thawing conditions affect the quality of frozen foods, such as microbial growth, chemical properties, and texture, appropriate thawing of these foods is essential. Moreover, appropriate freezing and thawing can prevent food poisoning. Thawing beef for prolonged time is a common practice at home; however, this process negatively affects the biochemical and physicochemical attributes of meat. Therefore, the loss of food quality depends on the thawing method and thawing temperature (Redmond and Griffith, 2003). Some studies have shown that increased freezing temperature affects drip loss in meat quality (Pietrasik and Janz, 2009; Choi et al., 2018). High-pressure thawing, ohmic thawing, and acoustic thawing are rapid thawing methods used in food technology. Offer and Knight (1988) reported that the thawing method influences pH, protein denaturation, intrafascicular and interfascicular spacing, and sarcomere length. Thawing meat in a refrigerator or in cold water is recommended because an increase of 7.2°C in food temperature is undesirable and the resultant food quality cannot be assured (U.S. Food and Drug Administration, 2022). Storage by freezing can effectively impede physical change; however, the food quality may decrease based on the thawing method. Some studies on meat quality have investigated the effects of various thawing methods (Sage and Ingham, 1998; Medeiros et al., 2000; Yamamoto and Harris, 2001; Park et al., 2012). Different thawing methods, thawing time, and sample volume can affect the physicochemical properties of thawed beef. Moreover, excessive thawing for prolonged time or at high temperature can reduce the quality of beef. However, few studies have conducted quality evaluation of beef thawed for prolonged time, as well as for normal time. Therefore, the present study investigated the effects of different thawing methods, including different thawing times, on the physicochemical properties of beef, such as color, pH, thawing loss, lipid oxidation, and textural and microbiological attributes.
MATERIALS AND METHODS
Reagents
Butylated hydroxyanisole (BHA) and 2-thiobarbituric acid (TBA) were purchased from Sigma-Aldrich. Petrifilm was obtained from 3MTM.
Beef samples
Beef samples (sirloin) were purchased from a market in Daegu, Korea, and divided into two beef loaves weighing 200 g each. Next, the samples were placed separately in polyethylene bags and frozen at −18°C for 3 days in a laboratory freezer. Subsequently, the samples were thawed using different thawing methods until the core temperature reached 1°C because thawing times may vary depending on the weight and shape of the frozen mass (Chun et al., 2016; Bai et al., 2023). Thawing methods that are commonly used at home, including thawing in a refrigerator, in cold water, at room temperature, and using a microwave oven, were selected in this study. Thawing at room temperature was performed at 23°C for 3 h and 30 min; meanwhile, thawing in cold water was performed using cold drinking water at 15°C for 2 h. Thawing in a microwave oven was performed using the automatic thawing setting (8 min), and thawing in a refrigerator was performed using a refrigerator at 4°C for 24 h. Additionally, thawing at room temperature, in cold water, and in a refrigerator was performed for prolonged time, which was twice longer than the normal thawing time, to evaluate beef quality after excessive thawing.
Colorimetric parameters
The meat juice was removed from the surface, and color measurements were performed using a Hunter colorimeter (ColorReader CR2, Shenzhen ThreeNH Technology Co., Ltd.) on the basis of certain standards (L*=92.13, a*=0.31, b*=−0.63). The L* (lightness), a* (redness), and b* (yellowness) values of beef samples were measured in triplicate.
pH value
Distilled water (50 mL) was added to 10 g of sample and homogenized (Stomacher 400 Circulator lab blender, Seward Ltd.) for 2 min. The pH of the homogenate was measured using a pH meter (MP220 pH Meter, METTLER TOLEDO) equipped with a thermostat.
Thawing loss
Thawing loss was analyzed on the basis of the method described by Association of Official Analytical Chemists (U.S. Food and Drug Administration, 1999). Beef samples were transferred to centrifugation tubes (MEGA 17R, Hanil) and centrifuged at 100 g for 10 min at 4°C. Next, the weight of samples was measured after removing the released meat juice to measure the amount of meat juice that was released. Thawing loss was calculated as the difference in weight before and after thawing and expressed as the percentage of the weight of the liquid released.
TBA value
The TBA values of thawed samples were measured in accordance with the method of Buege and Aust (1978). A sample weighing 5 g was transferred into a flask containing 50 μL of BHA and 15 mL of distilled water and then homogenized using a homogenizer (T 10 basic ULTRA-TURRAX, IKA) for 30 s. Next, an aliquot (1 mL) of the homogenate was transferred into a test tube and mixed with 2 mL of TBA/trichloroacetic acid solution. This mixture was heated at 90°C for 15 min and centrifugated at 100 g for 10 min using an automatic refrigerated centrifuge. Subsequently, the absorbance of samples was measured using a spectrophotometer (DU 800, Beckman) at 531 nm.
Texture analysis
Texture analysis of beef samples was performed using a textrometer (COMPAC-100, Sun Scientific Co.) with the following settings: Texture Profile Analysis test mode, 12.7-mm diameter probe, 1 mm/s speed, maximum force 2 kg, and 60% sample compression. The meat juice was removed from the surface, and the sample was cut into 2×2×2 cm cubes. The texture parameters were determined from the force-deformation curve.
Microbiological analysis
To determine the total microbial and Enterobacteriaceae counts in the thawed beef samples, 20 g of samples was taken, immediately diluted with 180 mL of 0.85% saline solution in a sterile stomacher bag, and homogenized in a stomacher (Stomacher 400 Circulator lab blender) for 2 min. The diluted sample was homogenized, and 1 mL from each dilution was transferred using a pipette onto sterile culture media in Petrifilm. The Petri plates were incubated at 30°C±1°C for 24 h. Petrifilm aerobic and coliform count plates were used to determine the microbiological and coliform bacterial counts. Plates presenting 30∼300 red colonies were selected and counted, and colony-forming unit (CFU)/mL was determined considering the average of triplicates.
Morphology
The structures of thawed beef samples obtained using different thawing methods were observed under a light microscope. The samples were cut and stored in Bouin’s fixative. Thereafter, they were embedded in paraffin. Tissue sections (10∼15 μm) were stained with Mayer’s hematoxylin and eosin and examined under a light microscope (Axioplan 2, Carl Zeiss). Photographs were taken using an imaging system.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics version 20 (IBM Corp.). The results are expressed as mean±standard deviation, and statistical significance was considered at P<0.05. Differences in continuous variables among different samples were assessed using the t-test and one-way analysis of variance, followed by Duncan’s multiple range test.
RESULTS AND DISCUSSION
Colorimetric parameters
The L*, a*, and b* values represent the color of beef samples. The results of instrumental color analysis are shown in Table 1. The L* value of fresh beef sample was 33.43, whereas those of beef samples thawed at room temperature and using a microwave oven decreased significantly to 32.43 and 32.21, respectively. The a* value of fresh beef sample was 27.30, whereas those of beef samples thawed in a refrigerator, in cold water, at room temperature, and using a microwave oven were 28.12, 28.93, 28.83, and 28.03, respectively, showing a significant increase in all thawing methods. The b* value of fresh beef sample was 10.40, whereas those of beef samples thawed in a refrigerator, in cold water, at room temperature, and using a microwave oven were 10.25, 10.38, 11.29, and 11.13, respectively, showing a significant increase in the latter two thawing methods. Furthermore, during prolonged thawing, the L* values of beef samples significantly decreased when they were thawed in a refrigerator, in cold water, and at room temperature (P<0.05). The a* values of beef samples thawed in cold water and at room temperature for prolonged time significantly increased compared with those of samples thawed for normal time (P<0.05). The b* values of beef samples thawed in a refrigerator and at room temperature for prolonged time also significantly increased (P<0.05). The DE values of beef samples thawed in cold water and at room temperature for prolonged time were higher than those of other samples. Freezing and thawing have been reported to affect the a value of meat samples by oxidizing myoglobin (Pan et al., 2021). Suman and Joseph (2013) reported that increasing the temperature tended to increase myoglobin oxidation and discoloration in beef. The increase in the a value by thawing in the present study was consistent with the results of previous studies.
Table 1.
Color values of thawed beef samples
| Thawing condition | L* | a* | b* | ΔE |
|---|---|---|---|---|
| Fresh beef | 33.43±0.37a | 27.30±0.13c | 10.40±0.28c | − |
| Refrigerator | 33.55±0.40a** | 28.12±0.45b | 10.25±0.76c | 0.72±0.13c* |
| Cold water | 33.50±0.11a** | 28.93±0.33b | 10.38±0.12c | 0.82±0.06b |
| Room temperature | 32.43±0.39b** | 28.83±0.36b | 11.29±0.09b | 1.41±0.08a** |
| Microwave oven | 32.21±0.16b | 28.03±0.21b | 11.13±0.38b | 0.84±0.90b |
| Refrigerator II | 32.06±0.33b | 28.09±0.73b | 11.39±0.53ab** | 0.31±0.08c |
| Cold water II | 32.38±0.43b | 29.63±0.28a** | 11.18±0.43b** | 1.59±0.11a** |
| Room temperature II | 31.98±0.31c | 29.44±0.56a** | 11.90±0.08a | 0.85±0.06b |
Values are presented as mean±SD (n=3).
II implies that thawing time was twice the normal thawing time.
Different letters (a-c) indicate a significant difference within the same column (P<0.05).
Means are significantly different between samples thawed for normal and prolonged time by t-test (*P<0.05, **P<0.01).
pH
The changes in the pH of beef samples according to thawing method and thawing time are shown in Table 2. The pH value of fresh beef sample was 6.09, whereas those of beef samples thawed in a refrigerator, in cold water, at room temperature, and using a microwave oven increased significantly to 6.10, 6.11, 6.12, and 6.12, respectively (P<0.05). The pH values of beef samples thawed in a refrigerator, in cold water, and at room temperature for prolonged time were significantly higher than those of samples thawed for normal time (P<0.05). This finding may be because of the increase in pH caused by amino acid leakage during prolonged thawing. Demeyer et al. (1979) reported that the storage of meat increased the pH value because of free amino acids generated by beef containing enzymes, changes in protein buffers, degree of electrolyte dissociation, ammonia production, and amino acid degradation. Farouk et al. (2004) also reported that thawing for prolonged time increases pH by damaging muscle cells and causing drip. In the present study, cell damage in beef samples thawed for prolonged time in a refrigerator, in cold water, and at room temperature may have increased the pH value.
Table 2.
pH, thawing loss, and TBA values of thawed beef samples
| Thawing condition | pH | Thawing loss (%) | TBA value (MDA mg/kg) |
|---|---|---|---|
| Fresh beef | 6.09±0.00f | − | 0.21±0.02e |
| Refrigerator | 6.10±0.01e | 24.75±0.69e | 0.22±0.00de |
| Cold water | 6.11±0.00de | 26.37±0.07d | 0.23±0.01cd |
| Room temperature | 6.12±0.01cd | 28.33±0.14b | 0.25±0.01b |
| Microwave oven | 6.12±0.01bc | 26.31±0.03d | 0.24±0.01bc |
| Refrigerator II | 6.13±0.01b* | 26.30±0.41d** | 0.23±0.01cd* |
| Cold water II | 6.13±0.00b* | 27.75±0.02c* | 0.25±0.00b** |
| Room temperature II | 6.16±0.01a* | 32.96±0.34a** | 0.29±0.00a** |
Values are presented as mean±SD (n=3).
II implies that thawing time was twice the normal thawing time.
Different letters (a-f) indicate a significant difference within the same column (P<0.05).
Means are significantly different between samples thawed for normal and prolonged time by t-test (*P<0.05, **P<0.01).
TBA, 2-thiobarbituric acid; MDA, malonaldehyde.
Thawing loss
The differences in thawing loss according to thawing conditions are presented in Table 2. The thawing loss values of beef samples thawed in a refrigerator, in cold water, at room temperature, and using a microwave oven were 24.75%, 26.37%, 28.33%, and 26.31%, respectively. No significant difference was observed among the thawing methods at the normal thawing time. The thawing loss value of beef samples thawed for prolonged time at room temperature was 32.96%, which was significantly higher than that of samples thawed under different conditions (P<0.05). Relating to the results of the previous pH experiment, beef samples with high thawing loss also had a high pH. The correlation between pH and thawing loss of thawed beef samples was r2=0.9866. Aaslyng et al. (2003) also reported that pH and thawing loss values showed a positive correlation, which was affected by protein denaturation and an unstable degree of damage during thawing. The thawing loss values of beef samples thawed in a refrigerator, in cold water, and at room temperature for prolonged time were relatively higher than those of samples thawed for normal time. Boles and Swan (2002) reported that a slow thawing rate (overnight at 5°C) and prolonged thawing time increased the amount of thawing loss. The present study also demonstrated that thawing loss at room temperature, which occurred slower than that under other thawing conditions, was higher than that of other thawing methods.
TBA value
The measurement of TBA values is commonly used to determine lipid oxidation in food and is related to flavor (Nissen et al., 2004). The TBA values obtained under various thawing methods are presented in Table 2. The TBA value of fresh beef sample was 0.21 malonaldehyde mg/kg (MDA mg/kg), and a significant difference was observed depending on the thawing method. The TBA values of beef samples thawed in a refrigerator, in cold water, at room temperature, and using a microwave oven for normal time were 0.22, 0.23, 0.25, and 0.24 MDA mg/kg, respectively. Essentially, the TBA value increased under various thawing methods without prolonged thawing time. Thawing at room temperature for prolonged time resulted in a TBA value of 0.29 MDA mg/kg, which was the highest lipid oxidation value compared with that of other thawing methods. The TBA values of beef samples thawed in a refrigerator, in cold water, and at room temperature for prolonged time significantly increased compared with those of samples thawed for normal time (P<0.05). Zhou and Xie (2021) reported an increase in TBA values in mackerel thawed for prolonged time, which is similar to the results of the present study. Estévez et al. (2006) reported that increased lipid oxidation affects the color and other sensory traits of meat. The TBA values of beef samples thawed at room temperature for prolonged time significantly increased from 0.25 to 0.29 MDA mg/kg, which was the highest, followed by samples thawed at room temperature for normal time and samples thawed in cold water for prolonged time. This result indicated that lipid oxidation was increased in meat samples treated physically, such as freezing and thawing. This finding is consistent with the results of Benjakul and Bauer (2001) who reported that physical treatments such as freezing and thawing increased fat acidity in meat. This study demonstrated the effects of thawing on the lipid oxidation of meat while considering factors such as thawing time and temperature. When beef is thawed, free radicals and free metal ions are formed, which could increase lipid oxidation. In the present study, fatty acid oxidation may have increased when the thawing time was prolonged.
Texture
The textural characteristics of thawed beef samples were evaluated using a textrometer, and textural differences based on different thawing methods were examined (Table 3). The maximum resistance of a sample generally characterizes its hardness when the first compression is applied. The hardness value of fresh beef sample was 1,238.63 g, and those of beef samples thawed in a refrigerator, in cold water, at room temperature, and using a microwave oven were 1,324.91, 1,582.46, 1,360.86, and 1,785.13 g, respectively. Beef sample thawed using a microwave oven showed the maximum hardness, gumminess, and chewiness compared with those thawed using other methods. As the beef sample thawed using a microwave oven reached a high temperature, the surface became harder. However, the hardness values of beef samples thawed in a refrigerator, in cold water, and at room temperature for prolonged time were significantly lower than those of samples thawed for normal time (P<0.05). This pattern was also observed in other characteristics such as cohesiveness, springiness, and chewiness, which could be because of water loss by heat and structural changes of beef during the prolonged thawing time. These results indicated that the thawing time and thawing method were important factors that influenced hardness, cohesiveness, springiness, and chewiness of meat. This might be because some of the myofibrillar and connective tissue proteins were decomposed because of self-digestion by proteolytic enzymes present in the muscle, which became flexible (Forrest et al., 1975).
Table 3.
Textural characteristics of thawed beef samples
| Thawing condition | Hardness (g) | Cohesiveness | Springiness (mm) | Gumminess | Chewiness |
|---|---|---|---|---|---|
| Fresh beef | 1,238.63±4.45e | 0.88±0.06a | 5.30±0.18a | 1,094.19±69.81b | 5,797.51±283.33b |
| Refrigerator | 1,324.91±19.50d* | 0.73±0.01bc* | 4.36±0.15bc* | 971.56±12.39c** | 4,237.24±202.49c* |
| Cold water | 1,582.46±9.65b** | 0.72±0.01cd* | 5.27±0.02a* | 1,134.06±5.38b** | 5,972.69±15.95b** |
| Room temperature | 1,360.86±10.53c** | 0.65±0.03e* | 4.19±0.06c* | 889.24±40.27de | 3,724.57±221.69d* |
| Microwave oven | 1,785.13±2.21a | 0.78±0.02b | 4.53±0.06a | 1,386.43±25.57a | 6,285.99±190.15a |
| Refrigerator II | 1,240.70±1.79e | 0.69±0.01cde | 3.57±0.05d | 851.95±7.21de | 3,044.07±23.65f |
| Cold water II | 1,351.94±7.72c | 0.67±0.01de | 3.73±0.05d | 910.33±12.27cd | 3,395.82±86.06e |
| Room temperature II | 1,151.94±7.72f | 0.72±0.06cd | 3.31±0.13e | 829.14±65.79e | 2,736.22±119.41g |
Values are presented as mean±SD (n=3).
II implies that thawing time was twice the normal thawing time.
Different letters (a-g) indicate a significant difference within the same column (P<0.05).
Means are significantly different between samples thawed for normal and prolonged time by t-test (*P<0.05, **P<0.01).
Bacterial counts
The effect of thawing in a refrigerator, in cold water, at room temperature, and using a microwave oven on the microbial properties of beef samples was evaluated. Fresh beef sample was used as a control to validate the efficiency of thawing time on the total bacterial count. The total bacterial count in fresh beef sample was 1.98 log CFU/g, and no coliforms were detected (Table 4). However, the total bacterial counts of beef samples thawed in a refrigerator, in cold water, at room temperature, and using a microwave oven showed significant differences of 2.13, 2.22, 2.49, and 2.43 log CFU/g, respectively. The total bacterial counts of beef samples thawed in cold water and at room temperature for prolonged time were 2.65 and 2.82 log CFU/g, respectively, which were significantly different from those of fresh beef sample. Microbial growth was effectively stopped by freezing but it regain during thawing (Löndahl and Nilsson, 2003). The total bacterial counts were not significantly different between beef samples thawed for normal and prolonged time. Conversely, a previous study on microwave oven thawing reported an increase in microbial growth, which caused deterioration of chemical attributes such as drip loss (Speck and Ray, 1977). Several studies have indicated that it is possible to reduce drip loss and chemical deterioration using the microwave oven thawing method (Virtanen et al., 1997; Kang et al., 2008). However, in the present study, no significant difference was observed in the total bacterial counts of beef samples thawed using a microwave oven compared with fresh beef sample. Moreover, no coliforms were detected in all beef samples.
Table 4.
Total aerobic bacterial and coliform counts of thawed beef samples
| Thawing condition | Total aerobic bacteria (log CFU/g) | Coliforms (log CFU/g) |
|---|---|---|
| Fresh beef | 1.98±1.05b | N.D. |
| Refrigerator | 2.13±0.71ab | N.D. |
| Cold water | 2.22±0.76ab | N.D. |
| Room temperature | 2.49±1.32ab | N.D. |
| Microwave oven | 2.43±1.00ab | N.D. |
| Refrigerator II | 2.47±1.06ab | N.D. |
| Cold water II | 2.65±1.07a | N.D. |
| Room temperature II | 2.82±1.72a | N.D. |
Values are presented as mean±SD (n=3).
II implies that thawing time was twice the normal thawing time.
Different letters (a,b) indicate a significant difference within the same column (P<0.05).
CFU, colony-forming unit; N.D., not detected.
Morphology
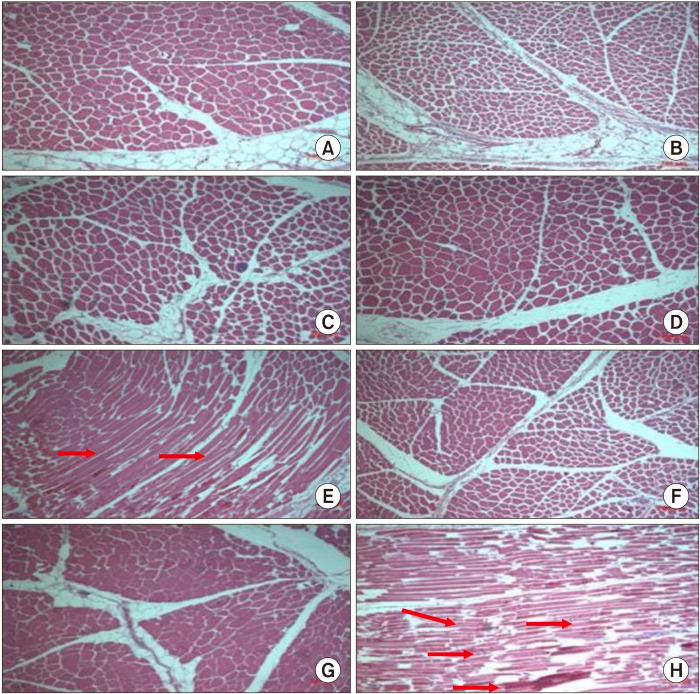
The morphological changes of beef samples thawed using different thawing methods under different thawing times are shown in Fig. 1. Fresh beef sample (Fig. 1A), beef samples thawed in a refrigerator (Fig. 1B), and beef samples thawed in cold water (Fig. 1C) did not show structural damage or tissue changes. However, beef samples thawed using a microwave oven (Fig. 1E) and at room temperature for prolonged time (Fig. 1H) showed uneven tissues or a wide gap between muscle fibers (the arrows in Fig. 1) compared with fresh beef sample. In other thawing conditions, beef samples showed a constant pattern without tissue damage (Park et al., 1999). It seems that this difference in morphology is associated with the loss of thawed beef under different thawing methods and thawing time. The characteristics of muscle tissue affect meat quality, which is associated with moisture content and texture. Therefore, morphology is commonly used to analyze meat quality by microscopy. Structural changes were clearly evident when the beef samples were thawed using a microwave oven (Fig. 1H). These results suggested that the beef samples underwent changes and that muscle tissues were chemically damaged during microwave oven thawing. Thawing using a microwave oven may weaken the protein and lipid structures of the muscle and increase the thawing loss of beef samples.
Fig. 1.
Morphologies of (A) fresh beef sample, (B) beef samples thawed in a refrigerator, (C) beef samples thawed in cold water, (D) beef samples thawed at room temperature, (E) beef samples thawed using a microwave oven, (F) beef samples thawed in a refrigerator for prolonged time, (G) beef samples thawed in cold water for prolonged time, and (H) beef samples thawed at room temperature for prolonged time (×100). The arrows indicate uneven tissues or a wide gap between muscle fibers.
Therefore, the results of this study indicate that thawing frozen beef in a refrigerator is better than thawing it at room temperature. Thawing processes result in a series of physical and chemical changes, which may in turn affect meat quality. Microwave oven thawing can reduce the thawing time; however, beef samples thawed using this method showed a significantly increased bacterial count. In conclusion, the thawing procedure and time affect meat quality characteristics such as thawing loss, color, and lipid oxidation.
Footnotes
FUNDING
None.
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Concept and design: all authors. Analysis and interpretation: all authors. Data collection: MHP. Writing the article: MHP. Critical revision of the article: MK. Final approval of the article: all authors. Statistical analysis: MHP. Overall responsibility: MK.
REFERENCES
- Aaslyng MD, Bejerholm C, Ertbjerg P, Bertram HC, Andersen HJ. Cooking loss and juiciness of pork in relation to raw meat quality and cooking procedure. Food Qual Prefer. 2003;14:277–288. doi: 10.1016/S0950-3293(02)00086-1. [DOI] [Google Scholar]
- Bai X, Li Y, Liang W, Xia X, Bian C. Formation of advanced glycation end products of chicken breast meat induced by freeze-thaw cycles and subsequent cooking. Int J Biol Macromol. 2023;244:125387. doi: 10.1016/j.ijbiomac.2023.125387. https://doi.org/10.1016/j.ijbiomac.2023.125387. [DOI] [PubMed] [Google Scholar]
- Benjakul S, Bauer F. Biochemical and physicochemical changes in catfish (Silurus glanis Linne) muscle as influenced by different freeze-thaw cycles. Food Chem. 2001;72:207–217. doi: 10.1016/S0308-8146(00)00222-3. https://doi.org/10.1016/S0308-8146(00)00222-3. [DOI] [Google Scholar]
- Boles JA, Swan JE. Meat and storage effects on processing characteristics of beef roasts. Meat Sci. 2002;62:121–127. doi: 10.1016/S0309-1740(01)00236-4. [DOI] [PubMed] [Google Scholar]
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/S0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Choi MJ, Abduzukhurov T, Park DH, Kim EJ, Hong GP. Effects of deep freezing temperature for long-term storage on quality characteristics and freshness of lamb meat. Korean J Food Sci Anim Resour. 2018;38:959–969. doi: 10.5851/kosfa.2018.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun HH, Choi EJ, Han AR, Chung YB, Kim JS, Park SH. Changes in quality of Hanwoo bottom round under different freezing and thawing conditions. J Korean Soc Food Sci Nutr. 2016;45:230–238. doi: 10.3746/jkfn.2016.45.2.230. [DOI] [Google Scholar]
- Demeyer DI, Vandekerckhove P, Moermans R. Compounds determining pH in dry sausage. Meat Sci. 1979;3:161–167. doi: 10.1016/0309-1740(79)90033-0. [DOI] [PubMed] [Google Scholar]
- Estévez M, Ventanas S, Cava R. Effect of natural and synthetic antioxidants on protein oxidation and colour and texture changes in refrigerated stored porcine liver pâté. Meat Sci. 2006;74:396–403. doi: 10.1016/j.meatsci.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Farouk MM, Wieliczko KJ, Merts I. Ultra-fast freezing and low storage temperatures are not necessary to maintain the functional properties of manufacturing beef. Meat Sci. 2004;66:171–179. doi: 10.1016/S0309-1740(03)00081-0. [DOI] [PubMed] [Google Scholar]
- Forrest JC, Aberle ED, Hedrick HB, Judge MD, Merkel RA. Principles of meat science. W. H. Freeman; 1975. pp. 145–156. [Google Scholar]
- Kang BS, Kim DH, Lee OS. A study on the changes of pork quality by freezing and thawing methods. Korean J Culin Res. 2008;14:286–292. doi: 10.20878/cshr.2008.14.2.022. [DOI] [Google Scholar]
- Lagerstedt A, Enfält L, Johansson L, Lundström K. Effect of freezing on sensory quality, shear force and water loss in beef M. longissimus dorsi. Meat Sci. 2008;80:457–461. doi: 10.1016/j.meatsci.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Leygonie C, Britz TJ, Hoffman LC. Impact of freezing and thawing on the quality of meat: Review. Meat Sci. 2012;91:93–98. doi: 10.1016/j.meatsci.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Löndahl G, Nilsson T. Freezing: Storage of frozen foods. In: Caballero B, editor. Encyclopedia of Food Sciences and Nutrition. 2nd ed. Academic Press; 2003. pp. 2732–2735. [DOI] [Google Scholar]
- Medeiros LC, Sanik MM, Miller EH, McCombs K, Miller C. Performance and microbiological growth in ground meat associated with the use of thawing trays. J Food Qual. 2000;23:409–419. doi: 10.1111/j.1745-4557.2000.tb00567.x. [DOI] [Google Scholar]
- Min SG, Hong GP, Chun JY, Park SH. Pressure ohmic thawing: a feasible approach for the rapid thawing of frozen meat and its effects on quality attributes. Food Bioprocess Technol. 2016;9:564–575. doi: 10.1007/s11947-015-1652-3. [DOI] [Google Scholar]
- Nissen LR, Byrne DV, Bertelsen G, Skibsted LH. The antioxidative activity of plant extracts in cooked pork patties as evaluated by descriptive sensory profiling and chemical analysis. Meat Sci. 2004;68:485–495. doi: 10.1016/j.meatsci.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Offer G, Knight P. The structural basis of the water-holding in meat. In: Lawrie R, editor. Development in Meat Science. Elsevier Applied Science; 1988. pp. 63–243. [Google Scholar]
- Pan N, Dong C, Du X, Kong B, Sun J, Xia X. Effect of freeze-thaw cycles on the quality of quick-frozen pork patty with different fat content by consumer assessment and instrument-based detection. Meat Sci. 2021;172:108313. doi: 10.1016/j.meatsci.2020.108313. https://doi.org/10.1016/j.meatsci.2020.108313. [DOI] [PubMed] [Google Scholar]
- Park BH, Kim YO, Kee HJ, Cho YJ, Choi HK. The effect of fig conserve additive on the physicochemical characteristics of beef obtained from various breeds. J Korean Soc Food Sci Nutr. 1999;28:511–519. [Google Scholar]
- Park MH, Kwon JE, Kim SR, Won JH, Ji JY, Hwang IK, et al. Physicochemical and microbiological properties of pork by various thawing methods. J East Asian Soc Diet Life. 2012;22:298–304. [Google Scholar]
- Pietrasik Z, Janz JA. Influence of freezing and thawing on the hydration characteristics, quality, and consumer acceptance of whole muscle beef injected with solutions of salt and phosphate. Meat Sci. 2009;81:523–532. doi: 10.1016/j.meatsci.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Redmond EC, Griffith CJ. Consumer food handling in the home: a review of food safety studies. J Food Prot. 2003;66:130–161. doi: 10.4315/0362-028X-66.1.130. [DOI] [PubMed] [Google Scholar]
- Sage JR, Ingham SC. Survival of Escherichia coli O157:H7 after freezing and thawing in ground beef patties. J Food Prot. 1998;61:1181–1183. doi: 10.4315/0362-028X-61.9.1181. [DOI] [PubMed] [Google Scholar]
- Speck ML, Ray B. Effects of freezing and storage on microorganisms in frozen foods: a review. J Food Prot. 1977;40:333–336. doi: 10.4315/0362-028X-40.5.333. [DOI] [PubMed] [Google Scholar]
- Suman SP, Joseph P. Myoglobin chemistry and meat color. Annu Rev Food Sci Technol. 2013;4:79–99. doi: 10.1146/annurev-food-030212-182623. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration, author. Food code 1999. 1999. [cited 2022 Jun 26]. Available from: https://www.fda.gov/food/fda-food-code/food-code-1999 .
- U.S. Food and Drug Administration, author. Food code 2022. 2022. [cited 2023 Jan 6]. Available from: https://www.fda.gov/food/fda-food-code/food-code-2022 .
- Virtanen AJ, Goedeken DL, Tong CH. Microwave assisted thawing of model frozen foods using feed-back temperature control and surface cooling. J Food Sci. 1997;62:150–154. doi: 10.1111/j.1365-2621.1997.tb04388.x. [DOI] [Google Scholar]
- Yamamoto SA, Harris LJ. The effects of freezing and thawing on the survival of Escherichia coli O157:H7 in apple juice. Int J Food Microbiol. 2001;67:89–96. doi: 10.1016/S0168-1605(01)00438-X. [DOI] [PubMed] [Google Scholar]
- Zhou PC, Xie J. Effect of different thawing methods on the quality of mackerel (Pneumatophorus japonicus) Food Sci Biotechnol. 2021;30:1213–1223. doi: 10.1007/s10068-021-00966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]