Abstract
Engineered probiotics (EPs) can be used to treat/manage chronic and congenital diseases. However, to the best of our knowledge, no systematic review has evaluated the effects of EPs on congenital metabolic disorders in murine models and human subjects. Thus, the present study systematically reviewed interventional studies that assessed the effects of EPs on congenital metabolic disorders. PubMed, Web of Science, and Scopus databases were searched up to February 2023 to retrieve related publications. Seventy-six articles were obtained in the primary step. After screening the titles/abstracts based on the inclusion and exclusion criteria, 11 papers were included. Finally, only seven articles were included after performing full-text evaluation. The included articles evaluated the effects of EPs on managing phenylketonuria (PKU, n=4) and hyperammonemia (n=3). Moreover, these studies examined mice and/or rats (n=6), monkeys (n=1), and humans (n=2). Studies on EPs and hyperammonemia revealed that some wild strains such as Lactobacillus plantarum have an innate ammonia-hyper-consuming potential; thus, there was no need to manipulate them. However, manipulation is needed to obtain a phenylalanine-metabolizing strain. In conclusion, EPs can be used to manage or treat congenital metabolic diseases including PKU.
Keywords: hyperammonemia, phenylketonuria, probiotics
INTRODUCTION
Microbes play an important role in the human body’s metabolic processes. For example, gut microbiota alteration can cause various metabolic diseases (Hur and Lee, 2015). Thus, novel strategies using engineered probiotics (EPs) for the treatment of various diseases, including metabolic disorders and inborn errors of metabolism, need to be developed (Riglar et al., 2017; Barati et al., 2022).
In congenital metabolic disorders including hyperammonemia, secondary ammonia formation occurs when there is a defect in ammonia-detoxifying enzymes [e.g., in urea cycle disorders (UCDs)] or when there is liver damage (e.g., in cirrhosis) (Savy et al., 2018; Zhao et al., 2020). Consequently, this process leads to the accumulation of ammonia (a toxic metabolite), which enters the portosystemic circulation. Circulating ammonia that is not metabolized to urea by the liver can cross into the brain and induce astrocyte edema, resulting in neurological dysfunction, coma, and even death. Therefore, ammonia levels need to remain low and be regulated to prevent these adverse outcomes. Except in cases of liver transplantation, the conventional treatments for hyperammonemia include using antibiotics to decrease ammonia production by urease-positive bacteria and employing nonabsorbable sugars including lactulose, ammonia scavengers, and dietary management to acidify colon content, thereby decreasing ammonia absorption (Walker, 2012; Ribas et al., 2022). While these treatments can help to reduce ammonia levels, they also have undesirable side effects, including nausea, vomiting, and diarrhea, and long-term toxicity and compliance issues. Additionally, no clear evidence shows that these treatments improve survival rates (Auron and Brophy, 2012). Therefore, alternative treatments are needed to safely and effectively address hyperammonemia. Phenylketonuria (PKU) is another inborn metabolic defect, wherein phenylalanine hydroxylase (PAH) mutations result in increased serum phenylalanine (Phe) and decreased serum tyrosine (Tyr) concentrations and metabolites that contain Phe (Smith et al., 2019). High concentrations of Phe can cause irreversible and often severe neurological impairments, including autistic behavior, seizures, tremors, and ataxia (Bilder et al., 2017). The prevailing treatment of PKU involves restricting the dietary intake of Phe to a minimum level required for normal growth. However, nutritional deficiencies, noncompliance, and neuropsychological impairments persist even after such therapies are given (Al Hafid and Christodoulou, 2015).
Currently, researchers are developing EPs capable of enhancing therapeutic efficacy without causing side effects. Probiotics can be genetically modified to consume and break down metabolites in the specific location at which they are formed. Several studies have demonstrated that EPs can be used to treat metabolic diseases (Shen et al., 2015; Isabella et al., 2018). EPs can help reduce hyperammonemia symptoms by aiding the intestine in processing excess ammonia and converting it into nontoxic forms (Kurtz et al., 2019; Jiang et al., 2023). Likewise, EPs can be used to catabolize Phe, providing an alternative treatment option for PKU. Phenylalanine ammonia-lyase (PAL) derived from bacteria has potential therapeutic effects in PKU when administered orally by Escherichia coli Nissle 1917 (EcN) and Lactobacillus reuteri (Durrer et al., 2017; Isabella et al., 2018; Puurunen et al., 2021).
Understanding the importance of EPs and their application in the treatment of metabolic congenital diseases can be a turning point in the management of these diseases. To the best of our knowledge, no study has systematically reviewed the role of EPs in the management of congenital metabolic disorders. Thus, in the present study, interventional (human or animal) studies that assessed the effects of EPs on congenital metabolic disorders, including PKU and hyperammonemia, were systematically reviewed.
MATERIALS AND METHODS
Sources of information and search strategy
Three international databases (namely, PubMed/MEDLINE, Scopus, and Web of Science) and important journals (Nature Metabolism, PLOS ONE, Science Translational Medicine), congress, or conference research articles (gray literature) were searched up to February 2023 to gather applicable studies. The articles were restricted to English language, and the references of included studies were hand-searched to identify more potential studies. The final search syntax was carried out based on the key words found in the Emtree and MeSH databases and free-text search terms. The following search method (syntax) was used in PubMed: “((Engineering[tiab] AND probiotic[tiab]) OR “Engineered probiotic”[tiab] OR (Engineered[tiab] AND probiotic[tiab]) OR “genetically modified”[tiab] OR “genetically engineered”[tiab] OR (“genetically modified”[tiab] AND probiotic[tiab])) AND (Phenylketonuria[tiab] OR PKU[tiab] OR Tyrosinemia[tiab] OR Hypertyrosinemia[tiab] OR Homocystinuria[tiab] OR “CBS Deficienc*”[tiab] OR (Deficienc*[tiab] AND CBS[tiab]) OR (Hyperglycinemia[tiab] AND Nonketotic[tiab]) OR (Hyperglycinemia[tiab] AND Non-ketotic[tiab]) OR “non-ketotic hyperglycinemia”[tiab] OR “nonketotic hyperglycinemia”[tiab] OR “maple syrup urine disease”[tiab] OR MSUD[tiab] OR “BCKD Deficiency”[tiab] OR “Keto Acid Decarboxylase Deficiency”[tiab] OR “Branched-Chain alpha-Keto Acid Dehydrogenase Deficiency”[tiab] OR hyperammonemia[tiab]))”.
The present study’s protocol was registered and approved by the Student Research Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (Project No.: 1400/64962).
Eligibility criteria and selection process
Studies that met the following criteria were included: (i) all interventional animal or human studies that (ii) assessed the effects of EP interventions on PKU and hyperammonemia. In vitro studies and books, reviews, editorials, and letters were excluded. EndNote X7 software (Thomson Reuters) was used to export articles, and duplicate papers were removed (one copy of the duplicates was kept). The titles and abstracts of papers were initially screened by two reviewers to select studies for inclusion or potential inclusion. Then, the included articles were confirmed in accordance with the selection criteria. Some articles were excluded because of ineligibility. Two reviewers (MJ and MK) independently performed all steps to conduct the systematic review, including searching, primary and secondary screening by title and abstract, full-text selection, and data extraction. Disparities between the two reviewers were resolved by a third investigator (MB).
Data extraction
The following data were collected from each article: first author, year, study type, subject/disease, host (strain), expressed protein (location), substrate to by-product(s)/promoter inducer, and description of reported results.
RESULTS
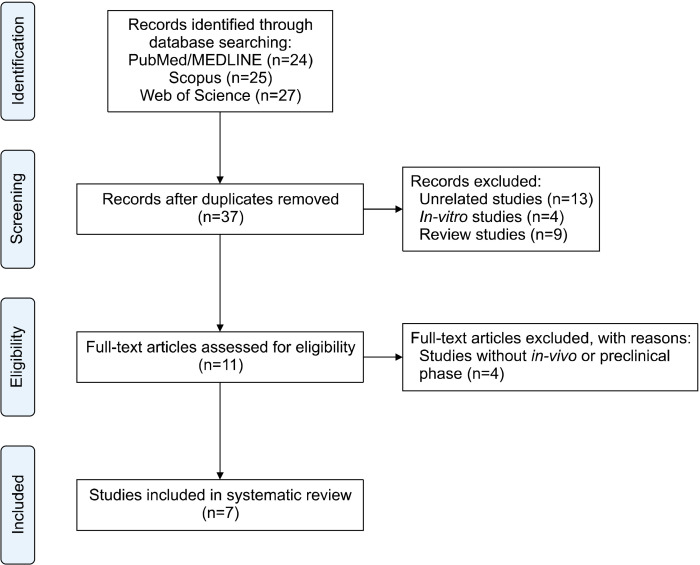
Seventy-six articles were obtained in the primary step. After screening their titles and abstracts based on the inclusion and exclusion criteria, 11 papers were included. Finally, only seven articles were included after performing full-text evaluation. Fig. 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart, comprehensively illustrating the number of identified records, excluded and included articles, and reasons for exclusion. The included articles evaluated the effects of EPs on managing PKU (n=4) and hyperammonemia (n=3). Table 1 shows the details of the data extracted from all included studies. The included studies examined mice and/or rats (n=6), monkeys (n=1), and humans (n=2). The phase 1 (n=1) and phase 1/2a (n=1) results of some EPs were also obtained. The included studies used EcN (n=5), Lactobacillus plantarum (n=1), and L. reuteri (n=1) as hosts and manipulated them to produce intracellular (n=6) or secretory (n=1) recombinant proteins.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart of study selection.
Table 1.
Characteristics of the included studies
| Reference | Study type | Subject/disease | Host | Expressed protein (location) | Substrate to by-product(s)/promoter(s) and its inducer | Result |
|---|---|---|---|---|---|---|
| Jiang et al., 2023 | Experimental mouse model | PahF263S PKU mouse/PKU | EcN | PAL (bacterial surface) | Phe to TCA/PheP and PAL | Compared to control EcN, serum Phe was reduced by 44.4% |
| Puurunen et al., 2021 | Double-blinded RCT (phase 1/2a) | Human/PKU patients and adult healthy volunteers | EcN | PAL (intracellular) and LAAD (bacterial surface) | Phe to TCA and PP/PheP and PAL | The engineered EcN was well tolerated with a maximum dose of 2×1011 CFU Adverse events were mostly GIT problems Dose-responsive increases in TCA and hippuric acid |
| Ochoa-Sanchez et al., 2021 | Experimental rat model | Rat/BDL rat | EcN | Ammonia consuming enzymes argA215 and synthesize butyrate (intracellular) | Ammonia to L-Arg/PfnrS | The EP attenuated hyperammonemia |
| Kurtz et al., 2019 | Experimental mouse model | OTC-deficient mouse and TAC liver-injured mouse/hyperammonemia | EcN | L-Arg biosynthetic enzyme argA215 (intracellular) | Ammonia to L-Arg/PfnrS | Decreased hyperammonemia and improved survival in OTC-deficient mice Reduced hyperammonemia in the TAC liver-injured mice |
| Double-blinded RCT (phase 1) | Human/adult healthy volunteers | EcN | L-Arg biosynthetic enzyme argA215 (intracellular) | Ammonia to L-Arg/PfnrS | Daily doses of up to 1.5×1012 CFU are well tolerated for up to 14 days Increased in urinary nitrate, plasma 15N-nitrate, and urinary 15N-nitrate |
|
| Isabella et al., 2018 | Experimental mouse model | Pahenu2/enu2 PKU mouse/PKU | EcN | PAL (intracellular) and LAAD (bacterial surface) | Phe to TCA and PP/PheP and PAL | Compared to the control group blood Phe was reduced by 38% |
| Experimental monkey model | Cynomolgus monkey/no disease | EcN | PAL (intracellular) and LAAD (bacterial surface) | Phe to TCA and PP/PheP and PAL | Inhibition of Phe increment after an oral Phe challenge | |
| Durrer et al., 2017 | Experimental mouse model | Pahenu2/enu2 PKU mouse/PKU | Lactobacillus reuteri | PAL (intracellular) | Phe to TCA/constitutive Promoter |
Serum Phe significantly reduced compared to the control group |
| Nicaise et al., 2008 | Experimental rat and mouse models | OTC-deficient mouse, TAC liver-injured mouse and CCL4 liver-injured rat/hyperammonemia | Lactobacillus plantarum | Ala dehydrogenase (intracellular) | Ammonia to Ala/NR | In all three models, intervention with the Engineered L. plantarum significantly decreased serum ammonia. Also in acute liver injury model the EP significantly extend the lifespan |
PKU, phenylketonuria; EcN, Escherichia coli Nissle 1917; PAL, phenylalanine ammonia-lyase; TCA, trans-cinnamic acid; Phe, phenylalanine; RCT, randomized controlled trial; LAAD, L-amino acid deaminase; CFU, colony-forming unit; GIT, gastrointestinal tract; BDL, bile-duct-ligated; Arg, arginine; EP, engineered probiotic; OTC, ornithine transcarbamylase; TAC, thioacetamide; PP, phenylpyruvate; CCL4, carbon tetrachloride; Ala, alanine; NR, not reported.
Jiang et al. (2023) revealed that EPs with membrane-associated and intracellular PAL decreased serum Phe levels in the PKU mouse model when the enzyme was expressed in EcN. In a similar animal study by Durrer et al. (2017), the oral administration of PAL-producing EP to the PKU mouse model significantly reduced blood Phe levels. Moreover, in their study evaluating the effects of an EP on healthy cynomolgus monkeys and PKU mouse model, Isabella et al. (2018) found that the probiotic exerted beneficial effects in both arms. The only randomized controlled trial (RCT) obtained in our systematic search for PKU is the study of Puurunen et al. (2021), which evaluated the effects of an engineered EcN on healthy subjects and subjects with PKU. In addition, there were three interventional studies regarding EPs and hyperammonemia. Ochoa-Sanchez et al. (2021) and Nicaise et al. (2008) investigated animal models of hyperammonemia, whereas Kurtz et al. (2019) evaluated both animal and human arms.
DISCUSSION
Effects of EPs in managing hyperammonemia
Hyperammonemia is a metabolic disorder characterized by elevated levels of ammonia. It can result from various congenital and acquired conditions. Acquired diseases can be further divided into hyperammonemia because of hepatic and nonhepatic causes. Congenital disorders associated with enzyme defects include UCDs, organic acidosis, congenital lactic acidosis, fatty acid oxidation defects, and dibasic amino acid deficiency (Savy et al., 2018; Zhao et al., 2020). Ammonia is normally produced in the large and small intestines, where it is carried to the liver and converted into urea via the urea cycle. Then, urea, a water-soluble compound, is excreted by the kidneys. If the liver is unable to metabolize ammonia because of enzyme defect or liver cell damage, ammonia levels will accumulate. In addition, ammonia levels can increase if portal vein blood bypasses the liver into the systemic circulation or if certain microbial infections increase ammonia production. Elevated ammonia levels can lead to acute or chronic neurological signs and symptoms depending on the underlying anomaly. Thus, hyperammonemia should be detected and treated promptly to hinder the development of life-threatening complications such as cerebral edema and brain herniation (Walker, 2012; Ribas et al., 2022). Thioacetamide and carbon tetrachloride liver-injured, ornithine transcarbamylase-deficient, and bile-duct ligated animals are popular animal models of hyperammonemia (Nicaise et al., 2008; Kurtz et al., 2019; Ochoa-Sanchez et al., 2021).
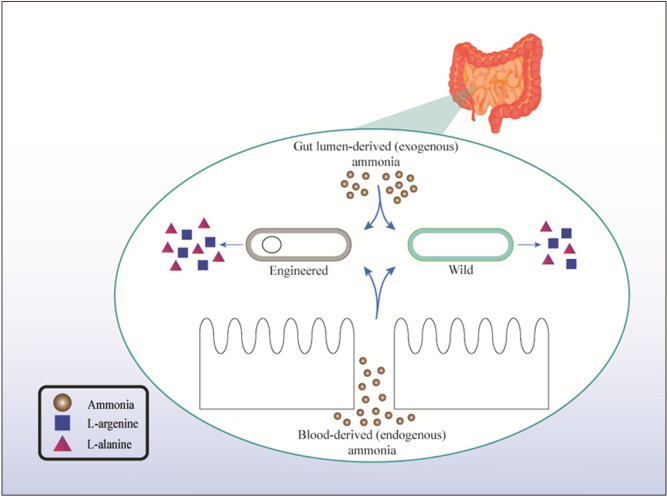
Among the included studies, three examined the effects of EPs on mitigating hyperammonemia. Ochoa-Sanchez et al. (2021) manipulated EcN to deliver two EPs. One EP exhausted and converted ammonia to arginine (Arg), whereas the other was modified in a way to synthesize butyrate (Arg+BUT) by Arg. Both strains were found to reduce hyperammonemia in bile-duct-ligated rats. Some wild probiotic strains can reduce blood ammonia levels. In a pilot study, Yossef et al. (2020) examined the effects of Bacillus subtilis HU58 supplementation on patients with hepatic encephalopathy. They found that supplementation for 4 weeks significantly reduced blood ammonia in patients with baseline ammonia level >60 μg/dL (Yossef et al., 2020). In a similar study, Xia et al. (2018) investigated the beneficial effects of Clostridium butyricum combined with Bifidobacterium infantis in patients with liver cirrhosis. Nicaise et al. (2008) genetically modified L. plantarum to create ammonia hyper-consuming strains. Intervention with the modified strains on acute, chronic, and constitutive hyperammonemia showed that modified and wild strains can reduce blood ammonia levels and improve survival. The researchers also found that innate ammonia transporter is the main mechanism that enables L. plantarum to eliminate ammonia (Nicaise et al., 2008). Kurtz et al. (2019) manipulated EcN to construct an ammonia hyper-consuming strain. Among healthy subjects, the mean baseline serum ammonia level was 28.8±8.8 μmol/L. The researchers examined the beneficial effects of EP for managing hyperammonemia in a phase 1 RCT (Kurtz et al., 2019). As mentioned previously, the gut is one of the main sources of ammonia in the body. Thus, ammonia hyper-consuming strains (either wild or engineered strains) can eliminate gut-derived ammonia (Nicaise et al., 2008; Xia et al., 2018; Kurtz et al., 2019; Yossef et al., 2020; Ochoa-Sanchez et al., 2021). There is a possibility that probiotics can remove blood-derived ammonia; however, no study has confirmed or rejected this hypothesis so far. Fig. 2 shows most of the concepts described in this subsection. It should be noted that the Arg produced by EPs can be metabolized into nitric oxide (NO) and citrulline by NO synthase in the body. NO oxidizes to nitrate and nitrite forms. Then, nitrate and nitrite are excreted in the urine. Thus, the serum levels and urinary excretion of nitrate and nitrite need to be evaluated for the safety assessment of ammonia hyper-consuming EPs (Kurtz et al., 2019).
Fig. 2.
Lumen- and blood-derived ammonia are converted to L-arginine and/or L-alanine by wild and engineered probiotics.
EPs for the management of PKU
PKU is an inborn error of Phe metabolism caused by PAH deficiency. Patients with PKU present with high Phe concentrations in their tissues and hyperphenylalaninemia because of total or partial deficiency of PAH activity and low Tyr concentrations. Phe is an essential amino acid obtained exclusively through diet or proteolysis. The major metabolic pathway of Phe involves its hydroxylation to Tyr by PAH found mainly in the liver but also in the kidneys. Phe is crucial for the synthesis of proteins and Tyr and its derivatives, including dopamine, norepinephrine, and melanin. The main signs and symptoms of PKU are found in the brain, but the pathophysiology of this disease is not well understood. In this context, metabolic alterations including impaired synthesis of proteins and neurotransmitters have been described in animal models and human patients (Schuck et al., 2015). In several studies, EPs were introduced as a therapeutic approach for PKU. Jiang et al. (2023) revealed that membrane-associated and intracellular PAL decreased serum Phe levels in a PKU mouse model when the enzyme was expressed in EcN. PAL catalyzes the conversion of Phe to trans-cinnamic acid (TCA). The by-products are strain-specific and need to be evaluated to determine whether the EP is working well or not. In a phase 1/2a trial, Puurunen et al. (2021) engineered EcN to construct a PKU-specific EP and examined it in healthy subjects and patients with PKU to evaluate its safety. The baseline serum levels of Phe in subjects who received EP and healthy subjects who received placebo were 70.8±7.70 and 64.3±6.04 μmol/L, respectively. On the other hand, in the second arm of the study, the baseline serum levels of Phe were 1,354±339.17 and 1,074±526.63 μmol/L for the EP and placebo groups, respectively. In this study, EcN was engineered to produce PAL and L-amino acid deaminase. TCA and hippuric acid (HPA) were the specific by-products of this EP. Moreover, strain-specific Phe metabolites in plasma (TCA) and urine (HPA) were observed. The results showed that the EP was safe and well tolerated at a maximum acceptable dose of 2×1011 colony-forming units. Adverse events occurred primarily in the gastrointestinal tract and were mild to moderate in severity. The bacteria were eliminated in all participants within 4 days of the last dose (Puurunen et al., 2021). The most important hypothesis regarding the action of PKU-specific EPs is that EPs catabolize Phe content in the gut. Isabella et al. (2018) constructed an EP for PKU condition and examined its biological effects on healthy cynomolgus monkeys. The results showed that the PKU-specific EP inhibits Phe increment after an oral Phe challenge (Isabella et al., 2018). Although the destruction of Phe content in the gut is the main reason for serum Phe reduction after the administration of PKU-specific EP, amino acid absorption mostly occurs in the proximal small intestine (Trommelen et al., 2021), whereas microbial colonization occurs in the distal ileum and colon (Han et al., 2021). As a result, there is a possibility of blood-derived Phe destruction by PKU-specific EPs, which can also be attributed to ammonia hyper-consuming EPs.
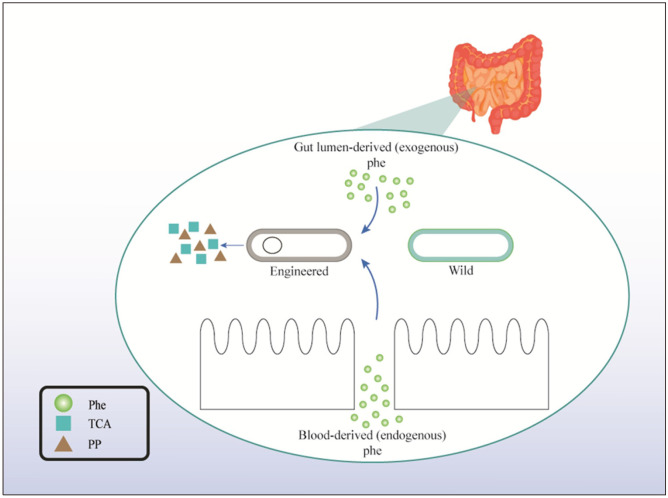
As mentioned previously, the expression of intracellular recombinant PAL in probiotics significantly improves Phe destruction ability. Aside from the intracellular form of PAL, its secretory form also showed the same results in a PKU mouse model. Microbial Phe catabolism is increased by PAL in a secretory fashion by colonic bacteria. The secretion of PAL in the colon ensures that PAL has direct access to free Phe and reduces the metabolic load that enzymes might otherwise impose on microbes. Durrer et al. (2017) manipulated L. reuteri to secrete PAL. The oral administration of L. reuteri-PAL to a PKU mouse model significantly reduced blood Phe. Although the secretion of PAL in the gut lumen makes it susceptible to denaturation and digestion, the preclinical results summarized here revealed that the intracellular and secretory forms of PAL enable the hosts to catabolize Phe (Durrer et al., 2017). Fig. 3 shows the concepts described in this subsection. In the intestine, Phe-consuming EPs convert Phe to TCA and phenylpyruvate. In addition, after the metabolites are absorbed, they are converted into HPA and phenyllactic acid (PLA) in the body. Therefore, aside from serum Phe, the serum and urinary levels of HPA and PLA should be evaluated during the evaluation of the clinical safety of EPs (Puurunen et al., 2021).
Fig. 3.
Lumen- and blood-derived phenylalanine (Phe) are converted to L-arginine and/or L-alanine by wild and engineered probiotics. TCA, trans-cinnamic acid; PP, phenylpyruvate.
In the present systematic review, the application of EPs in the treatment of PKU and hyperammonemia was discussed. PKU-specific EPs have been constructed to produce PAL (intracellular or secretory), and in vivo studies showed that EPs-PAL play an important role in the management of PKU. On the other hand, studies on EPs and hyperammonemia revealed that some wild strains including L. plantarum have an innate ammonia-hyper-consuming potential; thus, there is no need to manipulate them. However, EcN is an important host for the manipulation and construction of ammonia-hyper-consuming strains.
The present systematic review attempted to point out the importance and specific role of EPs in the management of congenital metabolic disorders. The authors tried to summarize and discuss the included studies related to the application of EPs in the management of congenital metabolic disorders. The most important limitation of the present systematic review is that only a limited number of studies were included because of the subject’s novelty. Three databases (namely, Scopus, PubMed, and Web of Science) were systematically searched in the present study. Other databases including Embase and Cochrane need to be searched to find more possible/available studies. Although the results obtained from the enrolled studies revealed that EPs have great potential to control PKU and hyperammonemia, there are a limited number of animal and human studies in this field. Thus, more animal studies and consequent RCTs are needed to verify the effects of EPs on congenital metabolic disorders.
ACKNOWLEDGEMENTS
We also appreciate the “Student Research Committee” and the “Research & Technology Chancellor” in Shahid Beheshti University of Medical Sciences for their financial support of this study.
Footnotes
FUNDING
This study is related to the project number (No. 1400/64962) from Student Research Committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Concept and design: MB, MJ, SHD. Analysis and interpretation: MB, EM, MK. Data collection: MJ, AK, MB, SKM. Writing the article: MB, MJ, MA, EM, AHT, MK, AAG, AF. Critical revision of the article: SHD. Final approval of the article: all authors. Obtained funding: MB. Overall responsibility: MB, SHD.
References
- Al Hafid N, Christodoulou J. Phenylketonuria: a review of current and future treatments. Transl Pediatr. 2015;4:304–317. doi: 10.3978/j.issn.2224-4336.2015.10.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auron A, Brophy PD. Hyperammonemia in review: pathophysiology, diagnosis, and treatment. Pediatr Nephrol. 2012;27:207–222. doi: 10.1007/s00467-011-1838-5. [DOI] [PubMed] [Google Scholar]
- Barati M, Jabbari M, Abdi Ghavidel A, Nikmehr P, Arzhang P, Aynehchi A, et al. The engineered probiotics for the treatment of chronic diseases: A systematic review. J Food Biochem. 2022;46:e14343. doi: 10.1111/jfbc.14343. https://doi.org/10.1111/jfbc.14343. [DOI] [PubMed] [Google Scholar]
- Bilder DA, Kobori JA, Cohen-Pfeffer JL, Johnson EM, Jurecki ER, Grant ML. Neuropsychiatric comorbidities in adults with phenylketonuria: A retrospective cohort study. Mol Genet Metab. 2017;121:1–8. doi: 10.1016/j.ymgme.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Durrer KE, Allen MS, Hunt von Herbing I. Genetically engineered probiotic for the treatment of phenylketonuria (PKU); assessment of a novel treatment in vitro and in the PAHenu2 mouse model of PKU. PLoS One. 2017;12:e0176286. doi: 10.1371/journal.pone.0176286. https://doi.org/10.1371/journal.pone.0176286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Lu Y, Xie J, Fei Y, Zheng G, Wang Z, et al. Probiotic gastrointestinal transit and colonization after oral administration: a long journey. Front Cell Infect Microbiol. 2021;11:609722. doi: 10.3389/fcimb.2021.609722. https://doi.org/10.3389/fcimb.2021.609722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur KY, Lee MS. Gut microbiota and metabolic disorders. Diabetes Metab J. 2015;39:198–203. doi: 10.4093/dmj.2015.39.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isabella VM, Ha BN, Castillo MJ, Lubkowicz DJ, Rowe SE, Millet YA, et al. Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. Nat Biotechnol. 2018;36:857–864. doi: 10.1038/nbt.4222. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Sun B, Qian F, Dong F, Xu C, Zhong W, et al. Expression of phenylalanine ammonia lyase as an intracellularly free and extracellularly cell surface-immobilized enzyme on a gut microbe as a live biotherapeutic for phenylketonuria. Sci China Life Sci. 2023;66:127–136. doi: 10.1007/s11427-021-2137-3. https://doi.org/10.1007/s11427-021-2137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz CB, Millet YA, Puurunen MK, Perreault M, Charbonneau MR, Isabella VM, et al. An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. Sci Transl Med. 2019;11:eaau7975. doi: 10.1126/scitranslmed.aau7975. https://doi.org/10.1126/scitranslmed.aau7975. [DOI] [PubMed] [Google Scholar]
- Nicaise C, Prozzi D, Viaene E, Moreno C, Gustot T, Quertinmont E, et al. Control of acute, chronic, and constitutive hyperammonemia by wild-type and genetically engineered Lactobacillus plantarum in rodents. Hepatology. 2008;48:1184–1192. doi: 10.1002/hep.22445. https://doi.org/10.1002/hep.22445. [DOI] [PubMed] [Google Scholar]
- Ochoa-Sanchez R, Oliveira MM, Tremblay M, Petrazzo G, Pant A, Bosoi CR, et al. Genetically engineered E. coli Nissle attenuates hyperammonemia and prevents memory impairment in bile-duct ligated rats. Liver Int. 2021;41:1020–1032. doi: 10.1111/liv.14815. [DOI] [PubMed] [Google Scholar]
- Puurunen MK, Vockley J, Searle SL, Sacharow SJ, Phillips JA, 3rd, Denney WS, et al. Safety and pharmacodynamics of an engineered E. coli Nissle for the treatment of phenylketonuria: a first-in-human phase 1/2a study. Nat Metab. 2021;3:1125–1132. doi: 10.1038/s42255-021-00430-7. [DOI] [PubMed] [Google Scholar]
- Ribas GS, Lopes FF, Deon M, Vargas CR. Hyperammonemia in inherited metabolic diseases. Cell Mol Neurobiol. 2022;42:2593–2610. doi: 10.1007/s10571-021-01156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riglar DT, Giessen TW, Baym M, Kerns SJ, Niederhuber MJ, Bronson RT, et al. Engineered bacteria can function in the mammalian gut long-term as live diagnostics of inflammation. Nat Biotechnol. 2017;35:653–658. doi: 10.1038/nbt.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savy N, Brossier D, Brunel-Guitton C, Ducharme-Crevier L, Du Pont-Thibodeau G, Jouvet P. Acute pediatric hyperammonemia: current diagnosis and management strategies. Hepat Med. 2018;10:105–115. doi: 10.2147/HMER.S140711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck PF, Malgarin F, Cararo JH, Cardoso F, Streck EL, Ferreira GC. Phenylketonuria pathophysiology: on the role of metabolic alterations. Aging Dis. 2015;6:390–399. doi: 10.14336/AD.2015.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen TC, Albenberg L, Bittinger K, Chehoud C, Chen YY, Judge CA, et al. Engineering the gut microbiota to treat hyperammonemia. J Clin Invest. 2015;125:2841–2850. doi: 10.1172/JCI79214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith N, Longo N, Levert K, Hyland K, Blau N. Phase I clinical evaluation of CNSA-001 (sepiapterin), a novel pharmacological treatment for phenylketonuria and tetrahydrobiopterin deficiencies, in healthy volunteers. Mol Genet Metab. 2019;126:406–412. doi: 10.1016/j.ymgme.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Trommelen J, Tomé D, van Loon LJC. Gut amino acid absorption in humans: Concepts and relevance for postprandial metabolism. Clin Nutr Open Sci. 2021;36:43–55. doi: 10.1016/j.nutos.2020.12.006. [DOI] [Google Scholar]
- Walker V. Severe hyperammonaemia in adults not explained by liver disease. Ann Clin Biochem. 2012;49:214–228. doi: 10.1258/acb.2011.011206. [DOI] [PubMed] [Google Scholar]
- Xia X, Chen J, Xia J, Wang B, Liu H, Yang L, et al. Role of probiotics in the treatment of minimal hepatic encephalopathy in patients with HBV-induced liver cirrhosis. J Int Med Res. 2018;46:3596–3604. doi: 10.1177/0300060518776064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yossef S, Clark F, Bubeck SS, Abernethy J, Bayne T, Krishnan K, et al. An oral formulation of the probiotic, Bacillus subtilis HU58, was safe and well tolerated in a pilot study of patients with hepatic encephalopathy. Evid Based Complement Alternat Med. 2020;2020:1463108. doi: 10.1155/2020/1463108. https://doi.org/10.1155/2020/1463108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Walline JH, Gao Y, Lu X, Yu S, Ge Z, et al. Prognostic role of ammonia in critical care patients without known hepatic disease. Front Med. 2020;7:589825. doi: 10.3389/fmed.2020.589825. https://doi.org/10.3389/fmed.2020.589825. [DOI] [PMC free article] [PubMed] [Google Scholar]