Abstract
Breast cancer is the most frequent type of cancer in women, many patients experience recurrences and metastasis. miR-21 (microRNA-21) as biomarker is under investigation for breast cancer. At present, there is very limited information available regarding effect of chemotherapy on miR-21 expression in breast cancer and its correlation with the clinical improvement. Hence, this study was planned to evaluate the effect of chemotherapy on miR-21 in metastatic breast cancer and its relationship with the clinical outcome. Females, aged—18–90 years diagnosed with Invasive Ductal Carcinoma of breast and candidate of neoadjuvant chemotherapy including Adriamycin (60 mg/m2), Cyclophosphamide (600 mg/m2) with or without Taxane (75–175 mg/m2) were included in the study. Before and after 42 days of staring of chemotherapy sample was collected for circulatory miR-21 and RECIST 1.1 criteria was applied to assess the clinical status. Blood samples for routine clinical biomarkers including liver function test and renal function tests was also collected. miR-21 expression before and after chemotherapy was assessed using standard method based on real time PCR. Expression of miR-21, RECIST criteria and other liver and kidney related biomarkers were compared before and after chemotherapy. After neoadjuvant chemotherapy expression of miR-21 was significantly increased by 5.65-fold. There was significant improvement in clinical scores based on RECIST criteria (0.046). No significant correlation was observed between miR-21 expression and difference in RECIST score (r = − 0.122, p = 0.570). Neoadjuvant chemotherapy causes clinical improvement in breast cancer patients however it is not correlated with the miR-21 expression which significantly increased after chemotherapy.
Keywords: Chemotherapy, Breast cancer, microRNA-21, RECIST 1.1, Gene expression
Introduction
Breast cancer is most commonly diagnosed cancer not only in India but also globally. As per the 2020 data, it represents around 12% of all new cases of cancers and is one of the leading causes of cancer deaths in women. [1]. In India, the age adjusted rate of breast cancers in females is 25.8 per 100,000 women and death due to breast cancer is 12.7 per 100,000 women [2]. Looking at the mortality and morbidity associated with the breast cancer, it is important to understand various factors which affects is pathogenesis so that early diagnosis and treatment can be done.
Role of miR (Micro RNA) in pathogenesis of the breast cancer is being explored and early evidences are linking it with the occurrence and invasiveness of the breast cancer [3, 4]. Role of miR in pathology of different types of cancer is complex as some of the miRs acts by inhibiting the tumor suppression mechanisms while others acts by causing the tumor suppression by downregulating genes responsible for cell proliferation [5]. There are various types of miRs found to be involved in the breast cancer, these promotes the tumor pathogenesis by increasing cell proliferation, enhancing invasiveness, promoting angiogenesis and making epigenetic changes [6]. miR-21 is one of the different type of miRNAs which found to be associated with breast cancer [7]. miR-21 was found to be increased in breast cancer and level of this miRNA was found to be high in grade 2 and grade 3 cancers as compared to the grade 1, linking it with the severity of the disease [8]. As per the available studies, miR-21 causes inhibition of tumor suppression gene, Tropomyosin 1 (TM1), inhibits Programmed Cell Death 4 (PDCD4), has anti-apoptotic activity and inhibits Phosphate and Tensin Homolog (PTEN), a tumor suppressor gene [9–12]. In a study done by Yan Li-Xu et al. [13], high expression of miR-21 was associated with advanced tumor stage, lymph node metastasis and poor survival of the patients which indicates role of high miRNA-21 level with the poor prognosis. As this miRNA is involved with tumorigenesis, it may be affected by the therapy, which ideally should decrease the level of miRNA-21 as a surrogate of effect of the therapy, clinical condition and prognosis.
The effect of therapy on miR-21 is explored in few small studies. In a study done by Khalighfard et al. [14], miR-21, miR-155 and miR-10b were compared before and after the radiotherapy, chemotherapy and radiation therapy and all of these miRNAs were found to be downregulated after the therapy. In a similar study the serum miRNA-21 was found to be decreased after the treatment by neoadjuvant chemotherapy and it was also found to be associated with the survival of the patients [15]. Still the issue of effect of therapy particularly drug therapy on miR-21 is not settled, as evidence of no effect of chemotherapy on miRNA-21 level is also available in scientific literature [16]. Looking at the uncertainty of effect of neoadjuvant chemotherapy on miR-21 levels in breast cancer patients due to conflicting results and paucity of data of relationship between miRNA-21 and improvement of clinical scores, it is difficult to consider this miRNA-21 as prognostic and therapeutic biomarkers for breast cancer. To fulfill this uncertainty this study was designed with the aim of evaluating the effect the neoadjuvant chemotherapy on miR-21 levels and to see the relationship between its levels and clinical condition of the patients.
Materials and Methods
The current study was conducted in the Departments of Pharmacology, Biochemistry and Radiation Oncology, AIIMS Jodhpur. The study was a hospital based, single group quasi-experimental study and it commenced after due approval of Institutional Ethics Committee. All consecutive patients who consented for enrollment were included in the study from Feb 2020 to Aug 2021.
Females ≥ 18–≤ 90 years clinically diagnosed as IDC (Invasive Ductal Carcinoma), large operable and locally advanced breast cancer and planned for systemic neoadjuvant chemotherapy or metastatic breast cancer planned for palliative chemotherapy. The applied neoadjuvant chemotherapy regimen was Adriamycin (60 mg/m2), Cyclophosphamide (600 mg/m2) with or without Taxane (75–175 mg/m2). Patients with any uncontrollable comorbidities, severe infections or any past malignancy were excluded.
Before chemotherapy sociodemographic and baseline clinical parameters were recorded. Blood sample was taken for miR-21 analysis. RECIST 1.1 criteria was applied to assess clinical condition of the patients. The RECIST criteria was measured by comparing sum of the longest diameters (SLD) of the target lesions (long axis > or = 10 mm), maximum of 5 for the study in CT scan of post therapy with the baseline and the smallest SLAD during treatment. Lymph nodes could be used as target lesions if short axis diameter (SAD) was > 15 mm. Lymph nodes < 10 mm were considered as normal, between 10 and 14 mm were considered as pathological but not as target lesions. Non target lesions were defined as lesions < 10 mm, non-measurable like ascites, pleural fluid (17). After 3 cycles of chemotherapy (after 42 days of 1st sample) baseline parameters and RECIST 1.1 criteria was used noted again. Blood was also taken for end of the study miR-21 analysis. RECIST 1.1 criteria was assessed by a doctor from the Department of Radiology. The venous blood sample of about 3 ml from each patient was collected in the EDTA vial and was kept at 4 °C which was processed within 24 h for RNA isolation (Figs. 1 and 2).
Fig. 1.

Work plan
Fig. 2.
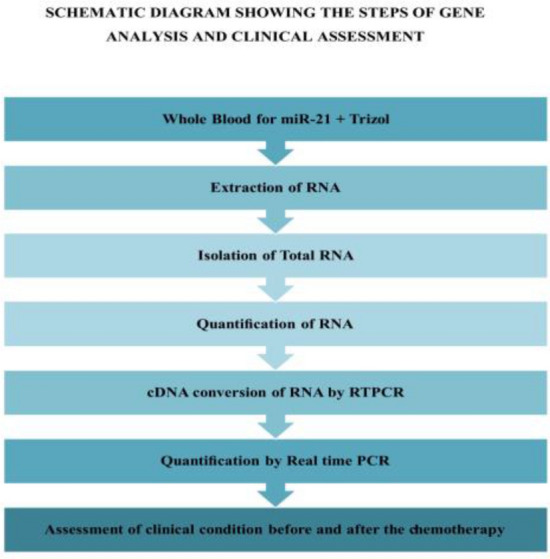
Work flow
Isolation and Quantification of Total RNA
Total RNA was extracted from whole blood using TriZol method. After extraction of RNA from individual samples, the total RNA was quantified and assessed by spectroscopy. The pure RNA will have A260/A280 ratio of ~ 2. RNA was stored at -80ºC until further conversion to cDNA.
cDNA Conversion of RNA by RTPCR
cDNA samples were taken out from minus 80 °C and thawed over ice. Reverse transcription was performed using Qiagen miRCURY LNA RT Kit (339,340)
The converted cDNA was kept at − 80 °C for RTPCR.
Quantification by Real Time PCR (qPCR)
After cDNA conversion, qPCR was carried out by placing the reaction strips in an automated and temperature, controlled cycles consisting of denaturation, annealing and elongation using thermal cycler. Primers used for qPCR were miR-21 QIAGEN miCURY LNA Primer (3,555,396), and for internal control RNU6 (3,450,123) along with QIAGEN miRCURY SYBR Green PCR kits (339,345). For 10 µl reaction 5 µl of 2 × miRCURY SYBR Green master mix, 1 µl of PCR primer mix, 1 µl cDNA template was taken and then volume was made up with RNAse free water. Steps carried were PCR initial heat activation at 95 °C for 2 min, followed by a 2 step cycling process which includes denaturation at 95 °C for 10 s, combined annealing/extension at 56 °C for 60 s. These steps were repeated for 39 times.
The normalization process utilized the 2 method. The delta Cq values of the Pre-chemotherapy patients were calculated by subtracting mean Cq values of the control RNU6B (Qiagen) from the mean Cq values of the cases i.e. target miR-21. Similarly post-chemotherapy delta Cq values were calculated. A subsequent double delta Cq value was calculated by subtracting delta Cq of pre from post chemo. The fold change expression level of miR-21 was calculated using 2(−∆∆ct) (18).
Results
Total 30 subjects were recruited in the study. One patient lost to follow up as she shifted to other center for the treatment. Different parameters of the patients included in the primary and secondary analysis are mentioned in the Table 1. Majority of the patients were from the age group of 41–50. Average age of patients included in the study was 51.3 years (range 30 years to 73 years). Majority of patients were negative for hormonal receptor status. Half of the patients included in the analysis were having right invasive breast cancer and remaining half were having left invasive breast cancer.
Table 1.
Characteristics of the subjects included in the study (n = 30)
| Parameters | Subgroup | Frequency (%) |
|---|---|---|
| Age in years (n = 30) | 30–40 | 4 (13.3) |
| 41–50 | 12 (40) | |
| 51–60 | 7 (17.5) | |
| 61–70 | 6 (20) | |
| 71–80 | 1 (3.3) | |
| Hormonal receptor status (n = 29)* | ER positive | 9 (31) |
| ER negative | 20 (68.9) | |
| PR positive | 4 (13.7) | |
| PR negative | 25 (86.2) | |
| HER2nue positive | 9 (31) | |
| HER2nue negative | 20 (68.9) | |
| Types of breast cancer (n = 30) | Right invasive breast cancer | 15 (50) |
| Left invasive breast cancer | 15 (50) |
*One subject withdrew from the study before the assessment of the other parameters
Evaluation of miRNA-21 Before and After Neoadjuvant Chemotherapy
In five patients the quantifiable amount of RNA was not detected hence only 24 patients were included in the analysis. ∆ Ct (no of cycle threshold) value for pre-chemotherapy and post-chemotherapy was calculated subsequently.
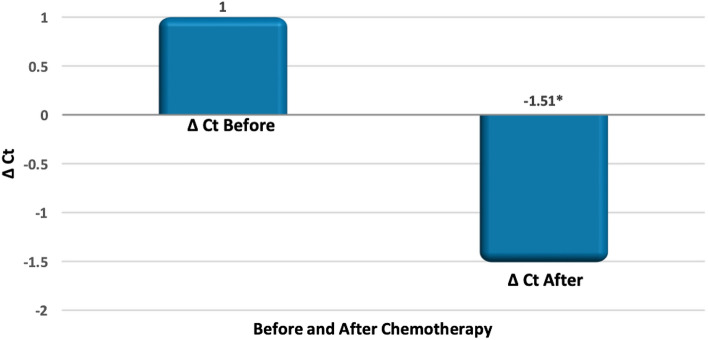
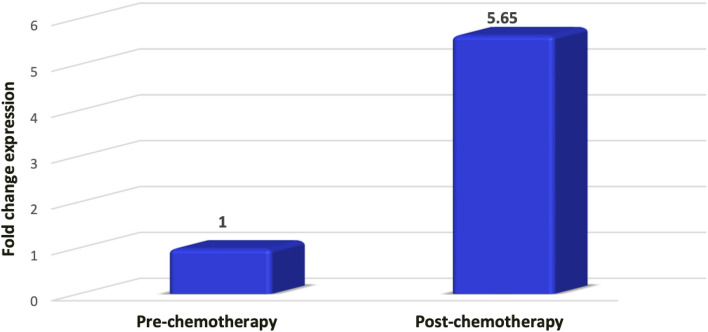
Mean value of delta Ct pre-chemotherapy was 1 (SD = 3.30) and the mean value of delta Ct post-chemotherapy was − 1.51 (SD = 3.28). This difference was statistically significant (p = 0.015) and negative ∆ Ct value post-chemotherapy shows that ∆ Ct decreased after chemotherapy as shown in (Fig. 3). Based on the ∆Ct difference of pre and post chemotherapy, ∆∆Ct value was calculated and subsequently fold change expression post-chemotherapy was calculated using formula 2 (−(∆∆ct)). It was observed that, post chemotherapy the expression of miR-21 was upregulated to 5.65-fold chain as shown in (Fig. 4).
Fig. 3.
Change in delta Ct post-chemotherapy in comparison to pre-chemotherapy
Fig. 4.
MiRNA-21 expression before and after neoadjuvant chemotherapy
Mean value of the RECIST score before chemotherapy was 78.4 (SD 38.9) which decreased to 62.8 (SD 54.5) after three cycles of chemotherapy with the mean difference of 15.50. This difference was statistically significant (p = 0.046).
Evaluation of Relationship Between Change in miRNA-21 and Change in Clinical Scores
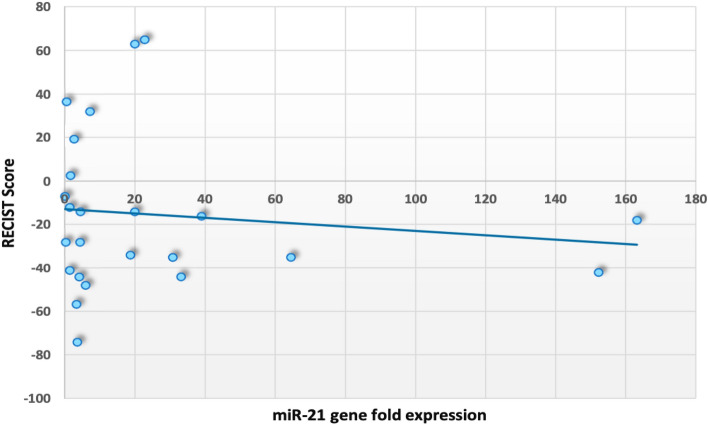
To evaluate the relationship between expression of miR-21 (individual gene fold expression) and difference in pre and post RECIST score, bivariate correlation was done. No significant correlation was observed between miR-21 expression and difference in RECIST score after chemotherapy (r = − 0.122, p = 0.570) (Fig. 5).
Fig. 5.
Scatterplot between individual miR-21 expression and difference in RECIST score
Discussion
The present study was conducted to evaluate the expression of miR-21 in breast cancer patients before and after neoadjuvant chemotherapy and to assess the clinical improvement using RECIST 1.1 scoring and it’s clinical correlation with the miR-21 expression. It was observed that the fold change expression of miR-21 was upregulated by 5.65-fold after chemotherapy which was significant. Similar findings were also observed in some other studies. Al khanbashi et al., evaluated miR-21 expression during 4 cycles of NACT in locally breast cancer patients using RT-qPCR and correlated with clinicopathological features using RECIST 1.1. It was seen that miR-21 levels in fact increased after point C i.e. after 4th cycle of chemotherapy. Whereas, there was also no significant correlation with clinical outcome. miR-21 was seen to be related with tumor progression and recurrences in various studies as discussed, supporting the findings of our study [17]. Sales et al. [16], studied the expression of miR-21 in three groups of patients consisting of NACT treated, chemotherapy naïve and healthy controls respectively. He saw that miR-21 expression was increased in NACT treated and naïve as compared to controls indicating the role of miR-21 as onco miRNA. The Cq values of miR-21 were compared between both NACT treated and naïve and it was seen that NACT treated patients had more Cq values of miR-21. However, when 5 patients with complete response in NACT treated group were compared with healthy controls for miR-21 expression, there was no significant difference. It could be due to possible restoration of miR-21 following NACT and surrounding tissue regeneration [16]. In recent times, as we have seen various miRNAs acting as regulators of gene expressions and response to chemotherapy drugs. Therefore, Mattos et al. studied the role of different miRNAs including miR-21 in Epithelial Mesenchymal Transition and tumor related inflammation pathways. He analysed the NACT response in HER2 positive breast cancer patients. It was seen that among various miRNAs only miR-21 was increased after chemotherapy and was associated with residual disease indicating the mir-21 to be responsible for NACT resistance and a potential biomarker of resistance in HER 2 positive patients. The mechanism of resistance was found to be her2 overexpression, further increases the EMT transition by activating P13K pathway and further increasing the release of interleukin 6 from the cancer cells which further activated NFKB signaling leading to further increase in EMT resulting in cancer progression and NACT resistance [18]. On the contrary, Liu et al. concluded that there was downregulation of miR-21 expression after chemotherapy (21).
Whereas, when patients were analyzed for their clinical response using RECIST 1.1 scoring before and after chemotherapy it was seen that there was significant decrease in the tumor size overall after chemotherapy treatment as compared to baseline proving the efficacy of this chemotherapy regimen. This was supported by the study of Kamal Mohammed et al. [19]. Chunjie Sun et al. evaluated the clinical effects of NACT using RECIST 1.1 and concluded that percentage of tumor cells were decreased proving the efficacy of NACT regimen [20].
However, the correlation between change in expression of miR-21 and clinical scores using RECIST 1.1 was not significant between the groups. Similar results were found in previous studies [16]. In the study, by Al Khanbashi et al. miR-21 levels were assessed in breast cancer patients undergoing NACT and were clinically correlated with RECIST 1.1 scoring. It was found that there was no significant difference in the correlation supporting the findings of our study [17]. It can be attributed due to various reasons including ethnogenetic characteristics of the patients, small sample size and miRNA normalization method which is a sensitive process [16].
As, it is known that breast cancer is the most commonly diagnosed cancer in India and around the world, as well as the leading cause of cancer-related death. The occurrence of metastasis, starting with cell proliferation and migration is the primary cause of cancer mortality. Metastasis is a complicated process and is the chief cause of death in solid tumors but knowledge about mechanism of metastasis is still not sufficient [21]. Therefore, exploring about biomarkers to assess the metastasis, recurrence and survival of tumor would be extremely beneficial for clinicians. According to Asangani et al., miRNAs being regulators of gene expression in breast cancer are being explored for their role as potential candidates for biomarkers (24). As, this miRNA is involved with tumorigenesis, it may be affected by the therapy, which ideally should decrease the expression of miR-21 as a surrogate of effect of the therapy, clinical condition and prognosis.
Non-significant results obtained for the relationship between miR-21 and clinical score may be true non-significance due to less sample size or short duration of the observation as the observations in this study were limited to 3rd cycle of the chemotherapy that is 42 days. In this study, plasma miR-21 level was analyzed but tissue miR-21 was not considered because of the feasibility reason. In the previous studies it was observed that plasma as well as tissue miR-21 behave similarly [13]. There is a paucity of data about the role of miR-21 in breast cancer and whether it can be considered as a marker for successful treatment? This study attempted to answer this question and is one of the early studies, particularly for Indian patients, showing the effect of chemotherapy on miR-21 and is a step in the direction of establishing miR-21 as prognostic biomarker in the breast cancer.
Data Availability
Data is available with the Corresponding Author. Can be submitted on request.
Declarations
Conflict of interest
None Declared.
Ethical approval
The study was approved by the Institutional Ethics Committee of All India Institute of Medical Science, Jodhpur (AIIMS/IEC/2020/2059).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Malvia S, Bagadi SA, Dubey US, Saxena S. Epidemiology of breast cancer in Indian women: breast cancer epidemiology. Asia-Pac J Clin Oncol. 2017;13:289–295. doi: 10.1111/ajco.12661. [DOI] [PubMed] [Google Scholar]
- 3.Farazi TA, Horlings HM, ten Hoeve JJ, Mihailovic A, Halfwerk H, Morozov P, et al. MicroRNA sequence and expression analysis in breast tumors by deep sequencing. Cancer Res. 2011;71:4443–4453. doi: 10.1158/0008-5472.CAN-11-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang J, Ahmad A, Sarkar FH. The role of MicroRNAs in breast cancer migration invasion and metastasis. IJMS. 2012;13:13414–13437. doi: 10.3390/ijms131013414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corsini LR, Bronte G, Terrasi M, Amodeo V, Fanale D, Fiorentino E, et al. The role of microRNAs in cancer: diagnostic and prognostic biomarkers and targets of therapies. Expert Opin Ther Targets. 2012 doi: 10.1517/14728222.2011.650632. [DOI] [PubMed] [Google Scholar]
- 6.Shah NR. MicroRNAs in pathogenesis of breast cancer: Implications in diagnosis and treatment. WJCO. 2014;5:48. doi: 10.5306/wjco.v5.i2.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qi L, Bart J, Tan LP, Platteel I, van der Sluis T, Huitema S, et al. Expression of miR-21 and its targets (PTEN, PDCD4, TM1) in flat epithelial atypia of the breast in relation to ductal carcinoma in situ and invasive carcinoma. BMC Cancer. 2009;9:163. doi: 10.1186/1471-2407-9-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iorio MV, Ferracin M, Liu C-G, Veronese A, Spizzo R, Sabbioni S, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 9.Zhu S, Si M-L, Wu H, Mo Y-Y. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) J Biol Chem. 2007;282:14328–14336. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 10.Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the MicroRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283:1026–1033. doi: 10.1074/jbc.M707224200. [DOI] [PubMed] [Google Scholar]
- 11.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 Is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 12.Meng F, Henson R, WehbeJanek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan L-X, Huang X-F, Shao Q, Huang M-Y, Deng L, Wu Q-L, et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14:2348–2360. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khalighfard S, Alizadeh AM, Irani S, Omranipour R. Plasma miR-21, miR-155, miR-10b, and Let-7a as the potential biomarkers for the monitoring of breast cancer patients. Sci Rep. 2018;8:17981. doi: 10.1038/s41598-018-36321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yadav P, Mirza M, Nandi K, Jain SK, Kaza RCM, Khurana N, et al. Serum microRNA-21 expression as a prognostic and therapeutic biomarker for breast cancer patients. Tumour Biol. 2016;37:15275–15282. doi: 10.1007/s13277-016-5361-y. [DOI] [PubMed] [Google Scholar]
- 16.Sales ACV, Gomes da Silva IIF, Leite MCB, Coutinho LL, Reis RBAC, Castoldi A, et al. Mirna21 expression in the breast cancer Tumor tissue is independent of neoadjuvant chemotherapy. Breast Cancer. 2020;12:141–151. doi: 10.2147/BCTT.S269519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Khanbashi M, Caramuta S, Alajmi AM, Al-Haddabi I, Al-Riyami M, Lui W-O, et al. Tissue and serum miRNA profile in locally advanced breast cancer (LABC) in response to neo-adjuvant chemotherapy (NAC) treatment. PLoS ONE. 2016;11:e0152032. doi: 10.1371/journal.pone.0152032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeMattos-Arruda L, Bottai G, Nuciforo PG, DiTommaso L, Giovannetti E, Peg V, et al. MicroRNA-21 links epithelial-to-mesenchymal transition and inflammatory signals to confer resistance to neoadjuvant trastuzumab and chemotherapy in HER2-positive breast cancer patients. Oncotarget. 2015;6:37269–37280. doi: 10.18632/oncotarget.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamal RM, Saad SM, Moustafa AFI, Gomaa MM, Mokhtar O, Gouda I, et al. Predicting response to neo-adjuvant chemotherapy and assessment of residual disease in breast cancer using contrast-enhanced spectral mammography: a combined qualitative and quantitative approach. Egypt J Radiol Nucl Med. 2020;51:161. doi: 10.1186/s43055-020-00275-2. [DOI] [Google Scholar]
- 20.Sun C, Shi L, Gu Y, Hu Y, Wang J, Liu Y, et al. Clinical effects of neoadjuvant chemotherapy in treating breast cancer. Cancer Biother Radiopharm. 2021;36:174–179. doi: 10.1089/cbr.2019.3545. [DOI] [PubMed] [Google Scholar]
- 21.Riggio AI, Varley KE, Welm AL. The lingering mysteries of metastatic recurrence in breast cancer. Br J Cancer. 2021;124:13–26. doi: 10.1038/s41416-020-01161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available with the Corresponding Author. Can be submitted on request.