Abstract
The study investigated the hepatocurative activity of the bulk alkaloids of Zanthoxylum zanthoxyloides in a tetrachloromethane (CCl4)-induced hepatotoxicity model in rats. The hepatocurative activity of the alkaloids at 200, 400 and 600 mg/kg doses was demonstrated by the assay of both enzymic and non-enzymic parameters. Sections of the liver were also subjected to histological examinations. Mapping techniques and data visualization approaches were adopted in finding relationships between the enzymic and non-enzymic parameters and the treatment groups. The bulk alkaloids caused dose-dependent effects on both the enzymic and non-enzymic parameters. The bulk alkaloids elicited a significant reduction (p < 0.05) in all liver and antioxidant enzymes activities compared with the untreated. The 600 mg/kg dose caused the restoration of the ALP, ALT and AST to 76.16, 10.72 and 11.83 iU/L respectively similar to the standard butylated hydroxytoluene. The 600 mg/kg dose also caused a slight increase in the activities of SOD, catalase and GPx to 11.45. 1.37 and 11.66 iU/L respectively when compared with the untreated rats. In the non-enzymic assays, the 600 mg/kg dose elicited a significant (p < 0.05) upregulation in the total bilirubin (1.18 mg/100 mL), total protein (3.75 g/dL), HDL (1.80 mMol/L) and vitamin C (2.41 mg/dL) and decrease in the CHOL (3.35 g/dL), TAG (1.85 mMol/L), LDL (0.67 mMol/L), BUN (39.55 mg/dL) and MDA (1.13 nMol/mL) when compared with the untreated rats. The restoration of the natural histo-architecture of the CCl4-damaged liver by the alkaloids further evidenced the hepatocurative activity of the bulk alkaloids.
Keyword: Zanthoxylum zanthoxyloides, Alkaloids, Histopathology, Biochemical, Hepatocurative
Introduction
The liver is an essential organ in the body responsible for detoxification, protein synthesis, maintenance and regulation of homeostasis body [1]. It is constantly exposed to toxins, drugs and alcohol which can damage the liver leading to several liver disorders [2, 3]. There may be hepatic failure and death arising from liver injuries. Liver disorders are among the prominent cause of death in the world today, but the available modern drugs used in the management of liver disorders are posed with serious side effects [2]. Many plants are known to have substantial hepatocurative activities and this may be due to plant secondary metabolites (phytochemicals) present in the whole or part of the plant [4, 5].
Carbon tetrachloride (CCl4) is a hepatotoxin responsible for free radical-mediated hepatocellular damage by the activation of the hepatic enzyme. The high activities of liver enzymes as well as total bilirubin concentrations are the markers of hepatic damage. Also, there is an elevation in lipid peroxidation (LPO) as a result of CCl4 intoxication [6, 7]. Antioxidants producing free radical scavenging mechanisms are crucial in protecting the liver against CCl4-induced hepatic damage [8]. Reduced glutathione and several agents such as vitamins C and E, and superoxide dismutase (SOD) decrease CCl4 toxicity in animals and are implicated in detoxifying the toxic effects of the metabolites in animals [2, 9]. Drugs that have been used in the past in the management of liver diseases have many serious side effects. There is an urgent need for active, safe and affordable alternatives.
Zanthoxylum zanthoxyloides Lam. (Family Rutaceae) is extensively used in folk medicine [10]. Decoctions of the roots and stems are commonly used to cure tuberculosis and overall body weakness, as well as digestive disorders, and migraines, and reduce pain during childbirth [11]. The stem barks are employed to treat toothache, sore gums and dental caries [12]. In southern Nigeria, pulped stem bark and root bark are used to stupefy fish when thrown into the water. They are also used to treat cancer. Conjunctivitis is treated with pulped bark when they are applied to the eye [13].
There has been an increased search for natural remedies for liver diseases [14]. Numerous secondary metabolites such as alkaloids, flavonoids, tannins, and saponins have been reported in Z. zanthoxyloides through phytochemical studies [13, 15]. Our previous studies had shown that the alkaloidal constituents are non-toxic and to a great extent responsible for the antioxidant activity [9, 15]. To further understand the mechanism of the antioxidant activity of the alkaloidal constituents, the in-vivo hepatocurative effects on the CCl4-hepato-challenged rats are reported here.
Materials and Methods
Plant Material
Healthy Z. zanthoxyloides leafy vegetables were taken from farms in Orodo, Mbaitolu L.G.A., Imo state, while identification and authentication were done by Prof. Charles N. Mba of the Department of Soil Science, School of Agriculture and Agricultural Technology of Federal University of Technology, Owerri. A voucher specimen (#InterCEDD/901) of Z. zanthoxyloides was thereafter deposited at the herbarium of the International Centre for Ethnomedicine Drug and Development (InterCEDD), Nsukka, Enugu State, Nigeria.
Experimental Animals
Thirty male albino rats (104.53 ± 9.56 g) gotten from the Faculty of Veterinary Medicine, University of Nigeria, Nsukka animal house were used for the hepatocurative assay of the bulk alkaloid. The University of Nigeria Ethics Committee (FBS/2020/TOA/ZZ/002) of 20th March 2020 granted authorization to use animals in this study. This was done following a set of guidelines for the care and use of laboratory animals, "Principles of Laboratory Animal Care" (NIH publication no. 85–23, revised 1985) and/or the declaration of Basel issued on 30th November 2010 as amended on January 2013.
Extraction of Bulk Alkaloids
The crude methanol extract was prepared using the method previously described [3, 16]. A 50 g of the dried methanol extract was dissolved in 10% MeOH before being partitioned in a separatory funnel between dichloromethane and acidic water (0.1 M HCl). The dichloromethane soluble (that is neutral and acidic alkaloid compounds) were collected and evaporated to obtain the alkaloids. Therefore, by treating the aqueous layer with 0.1 M aqueous ammonia and re-extracting it with dichloromethane, the aqueous layer was made basic. As a free base, the alkaloids were collected once more in the dichloromethane layer. When two drops of Dragendorff's reagent were put into the fractions above, an orange-red precipitate formed instantly, indicating the presence of alkaloids [17]. The fractions were then concentrated into a paste and dried under a vacuum. Appropriate concentrations of the bulk alkaloid fractions were made in 3% aqueous tween 80. A volume equivalent to 200, 400 and 600 mg/kg body weight of the bulk alkaloids was measured and used for the hepatocurative activity study.
Biological Activity Study
Hepatocurative Activity of Z. zanthoxyloides
Six groups (A-F) of albino rats (104.53 ± 9.56 g) were grouped with five rats in each cage and granted unrestricted access to water and food. Liver damage was induced with CCl4 in paraffin oil (1:1) on the 1st and 8th days. Rats in group A (normal control) were given 0.5 ml distilled water once daily orally. Group B (untreated) was given 0.5 ml of CCl4 solution intraperitoneal (I.P.) on the 1st and 8th days. Group C (standard control) was treated with 0.5 ml CCl4 on the 1st and 8th day I.P..and 200 mg/kg of butylated hydroxytoluene (BHT) orally, once daily for 14 days. Group D received simultaneously 0.5 ml of CCl4 on the 1st and 8th days of I.P. and 200 mg/kg of bulk alkaloid orally, once daily. Group E received simultaneously 0.5 ml of CCl4 on the 1st and 8th days of I.P. and 400 mg/kg of bulk alkaloid orally, once daily. Group F received simultaneously 0.5 ml of CCl4 on the 1st and 8th days of I.P. and 600 mg/kg of bulk alkaloid orally, once daily. All treatments with doses of the alkaloid and the standard drug lasted for 14 days [18].
Serum Preparation
The rats were fasted overnight after the last daily doses and then sacrificed under chloroform anesthesia. They were made to bleed into centrifuge tubes using an eye puncture. Allowing the blood sample to clot for 15 min and then centrifuged at 3500 rpm for 10 min at 40 ℃ yielded the sera. The biochemical assay was performed within 12 h of the sera preparation [18].
Assay of Biochemical Parameters
The following biochemical parameters were assayed following previously described methods [19–21]: ALP, ALT, AST, total and conjugated (direct) bilirubin, albumin and total protein, cholesterol (CHOL), triacylglycerols (TAG), high-density lipoproteins (HDL) and low-density lipoproteins (LDL), SOD, catalase (CAT), glutathione peroxidase (GPx), vitamins C and E and LPO (malondialdehyde). Others include blood urea nitrogen (BUN) and creatinine.
Tissue Preparation for Histopathological Examinations
Sections of the liver from randomly selected rats in each group were collected for histopathological study. For 48 h, the samples were fixed in 10% phosphate-buffered formalin. Following that, the tissues were cut, dehydrated in 70–100% alcohol, cleared in three grades of xylene, and embedded in molten wax. On solidifying, the blocks were sectioned using a rotary microtome into 5 µm thick sections, floated in a water bath, and incubated at 60 ºC for 30 min. After clearing in three grades of xylene, the 5 m thick sectioned tissues were rehydrated in 90–70% alcohol. Blueing was done with ammonium chloride, and differentiation was done using 1% acid alcohol before counterstaining with eosin. Permanent mounts on degreased glass slides were created using DPX (dibutyl phthalate polystyrene xylene), a mountant [7]. The prepared slides were viewed using × 4, × 10, and × 40 objective lenses on a Motic™ compound light microscope. At × 160 and × 200 magnifications, photomicrographs were obtained at random using a Motic™ 5.0 megapixels microscope camera.
Statistical Analysis
IBM SPSS version 25 windows were used for statistical analysis. All the values were expressed as mean ± standard deviation (SD). The data were evaluated with a one-way ANOVA and a post hoc Turkey test, in the case of normal distribution. Kruskal–Wallis’s test with a post hoc Dunn’s test was used for the non-normal distribution. At p < 0.05, the difference between treatment groups was considered significant.
Results and Discussion
The liver is constantly exposed to toxins, drugs and alcohol which can damage the liver leading to several liver disorders [2, 12]. The study evaluated the curative effects of the Z. zanthoxyloides alkaloids against CCl4-induced toxicity in rats and also employed mapping strategies and visualization methods. It presents a unique viewpoint on the species' medical applications by demonstrating high levels of alkaloids in the leaf composition, which have demonstrated hepatocurative effect via an antioxidant mechanism [9, 15]. Bioactive alkaloids such as piperlonguminine, berberine, fagaronine, skimmianine, arnottianamide, glycozoline, tembetarine, piperlonguminine, zanthosinamide, magnoflorine, berberine, fagaramide, oxychelerythrine, arnottianamide and dioxamin were previously identified in its alkaloid fraction [15]. The abundance of alkaloids suggests a twist in the roles and contribution of alkaloids to the overall radical scavenging activity (RSA) of Z. zanthoxyloides. The alkaloid fraction exhibited higher scavenging activity by intercepting and inhibiting the generation of free radicals, suggesting their role in supportive and complementary treatment of diseases [9]. Treatment with different doses of the alkaloids prevented some CCl4-induced effects showing that they can protect against the effects caused by CCl4 intoxication. The possible mechanism involved in its curative potential could be linked with its ability to decrease reactive oxygen species (ROS) intracellular levels [9]. It reduces the effects of free radicals, in addition to inhibition of lipid peroxidation. Alkaloids are one of the most viable products which might act against oxidative damage [14]. Several alkaloids are known for their use in the management and treatment of liver associated diseases. The alkaloid fractions were found to be rich in alkaloids and therefore exhibited good scavenging activity [15].
Effect of Alkaloids on Enzymic Parameters
The effects of alkaloids on enzymic parameters (liver function markers and antioxidant enzymes) are shown in Table 1. The alkaloids elicited concentration-dependent effects on the serum concentrations of ALP, ALT and AST. Treatment with 200, 400 and 600 mg/kg of alkaloid caused ALP reduction by 43.9, 32.2 and 21.7% respectively; ALT by 22.1, 21.1 and 20.2% respectively and AST by 29.4, 26.8 and 21.7% compared with the untreated group respectively.
Table 1.
Effect of alkaloids of Z. zanthoxyloides on enzymic parameters of CCl4-intoxicated rats
| Groups | Liver function activities (IU/L) | Antioxidant enzymes (IU/L) | ||||
|---|---|---|---|---|---|---|
| ALP | ALT | AST | SOD | CAT | GPx | |
| A | 75.96 ± 3.4ab | 10.78 ± 0.19ab | 11.68 ± 0.43ab | 11.43 ± 0.04ab | 1.17 ± 0.08a | 11.14 ± 0.75ab |
| B | 92.61 ± 9.50bc | 12.90 ± 0.25bc | 14.36 ± 0.34bc | 10.92 ± 0.04bc | 1.12 ± 0.08b | 9.80 ± 0.15bc |
| C | 76.02 ± 4.36a | 10.74 ± 0.10c | 11.76 ± 0.27c | 11.43 ± 0.01c | 1.27 ± 0.01c | 11.22 ± 0.01c |
| D | 59.24 ± 1.16abc | 10.52 ± 0.01b | 10.92 ± 0.07abc | 11.36 ± 0.02bc | 1.15 ± 0.02c | 10.34 ± 0.01bc |
| E | 68.17 ± 2.29b | 10.62 ± 0.04b | 11.23 ± 0.17b | 11.41 ± 0.02b | 1.23 ± 0.02c | 10.81 ± 0.39b |
| F | 76.16 ± 1.24b | 10.72 ± 0.10b | 11.83 ± 0.40b | 11.45 ± 0.02b | 1.37 ± 0.02bc | 11.66 ± 0.40b |
ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase, SOD superoxide dismutase, CAT catalase, GPx glutathione peroxidase. Values are means ± SD, n = 5. Values for the treatment groups (for each enzyme) with superscripts letters a, b, and c are significant at p < 0.05 with distilled water, CCl4 and BHT respectively
Intoxication with CCl4 increased the activities of liver enzymes in the serum. These enzymes are leaked into circulation following tissue damage. The ability of different doses of the alkaloids to successfully lower the serum activities of ALP, ALT and AST after CCl4 intoxication indicates the hepatocurative potentials of the fractions. The ameliorative effect of 600 mg/kg of the alkaloids produced similar effects to the control. Besides, the ameliorative effect of the extracts of Z. zanthoxyloides on alloxan-induced diabetic rats has reported an increase in the activity of the liver enzymes at higher concentrations of Z. zanthoxyloides [22]. They suggested that the hepatocurative effect of Z. zanthoxyloides may be due to their anti-inflammatory and antioxidant properties. They noted an increase in the activity of the liver enzymes at higher concentrations of Z. zanthoxyloides.
A concentration-dependent increase in the antioxidant enzymes caused by the alkaloids is shown in Table 1. The 200 mg/kg of the alkaloids significantly (p < 0.05) elevated the activity of SOD by 3.85%, while there was no significant difference (p > 0.05) in the activity on treatment with 400 and 600 mg/kg compared with the untreated. A 600 mg/kg of alkaloids significantly (p < 0.05) increased the activity of CAT by 22.3%, with no significant difference in the effect of 200 and 400 mg/kg compared with the untreated. Treatment with 400 and 600 mg/kg of alkaloids did not significantly (p > 0.05) promotes the activity of GPx when compared with the untreated; however, treatment with 200 mg/kg of the alkaloids significantly (p < 0.05) promote GPx activity by 5.51%.
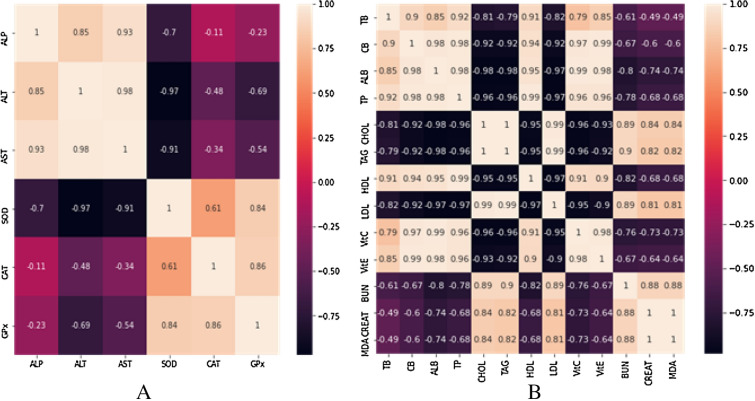
The heat map exhibits distinct patterns of the enzymic parameters (Fig. 1a). Strong positive corelations were existing between the concentrations of ALT and AST, ALP and ALT, and ALP and AST. Similarly, there were also strong positive correlations existing between the concentrations of CAT and GPx, SOD and GPx, CAT and GPx. Meanwhile, a negative strong correlation exists between the concentrations of ALT and SOD, AST and SOD, SOD and ALT, SOD and AST. However, a weak negative correlation exists between the concentrations of ALP and CAT, ALP and GPx, AST and CAT.
Fig. 1.
Heat map plot showing the degree of correlation existing among the concentrations of the enzymic (A) and non-enzymic (B) parameters. ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase, SOD superoxide dismutase, CAT catalase, GPx glutathione peroxidase, TB total bilirubin, CB conjugated bilirubin, ALB albumin, TP total protein, CHOL cholesterol, TAG triacylglycerols, HDL high-density lipoproteins, LDL low-density lipoproteins, Vit C vitamin C, Vit E vitamin E, BUN blood urea nitrogen, MDA malondialdehyde
A representative boxplot is used to visualize differences in the effects of the treatment groups when compared to the control (Fig. 2a). The top and bottom of the boxes are the 75th and 25th percentiles, while the middle line represents the median value. Treatment with 600 mg/kg alkaloid fraction (group F) produced the highest effect on CCl4-intoxicated rats when compared with the standard control, 200 and 400 mg/kg alkaloid fractions (groups C, D and E respectively). Also, those treated with 600 mg/kg alkaloid fraction (group F) produced a similar effect to the standard control (group C).
Fig. 2.
Effects of the treatment groups on enzymic (A) and non-enzymic (B) parameters of the CCl4-intoxicated rats. A: distilled water, B: CCl4, C: CCl4 + BHT (butylated hydroxytoluene), D: CCl4 + 200 mg/kg of alkaloid fraction, E: CCl4 + 400 mg/kg of alkaloid fraction, F: CCl4 + 600 mg/kg of alkaloid fraction. Boxplots for the treatment groups with superscripts letters a, b, and c are significant at p < 0.05 with distilled water, CCl4 and BHT respectively
The ability of the various doses of the alkaloids to reverse the damage caused by CCl4 administration like BHT suggests the antioxidant potential of the alkaloids. This means that they can hinder the build-up of free radicals in animals. The implication is that when the SOD, CAT and GPx internal enzymatic mechanisms fail or become inefficient, as a result of free radicals generation by CCl4, the alkaloids can complement the body’s in vivo scavenging effect of free radicals when consumed [23–25].
Effects of Alkaloids on Non-enzymic Parameters
The effects of alkaloids of Z. zanthooxyloides on non-enzymic parameters are shown in Table 2. The 200 and 600 mg/kg of alkaloids produced a significant elevation of total bilirubin (p < 0.05) by 39.5% and 60.5%. The effects of different doses of the alkaloids on conjugated bilirubin followed similar trends. The 400 and 600 mg/kg of alkaloids significantly (p < 0.05) increased conjugated bilirubin levels from 0.18 to 0.37 mg/100 ml and 0.18 to 0.41 mg/100 ml respectively.
Table 2.
Effects of alkaloids of Z. zanthoxyloides on non-enzymic parameters of CCl4-intoxicated rats
| Groups | Bilirubin (mg/100 mL) | Proteins (g/dL) | Lipid profiles (mMol/L) | Vitamins (mg/dL) | Renal function (mg/dL) | Peroxidation (nMol/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Conjugated | Albumin | Total | CHOL | TAG | HDL | LDL | Vitamin C | Vitamin E | BUN | Creatinine | MDA | |
| A | 1.09 ± 1.14ab | 0.38 ± 0.02ab | 3.73 ± 0.03ab | 3.32 ± 0.24ab | 3.21 ± 0.06ab | 1.79 ± 0.06ab | 1.73 ± 0.07ab | 0.66 ± 0.03ab | 2.64 ± 0.46ab | 1.57 ± 0.09ab | 35.75 ± 0.03a | 0.56 ± 0.12ab | 1.17 ± 0.05ab |
| B | 1.01 ± 0.35bc | 0.18 ± 0.01bc | 2.39 ± 0.18bc | 1.27 ± 0.16bc | 4.65 ± 0.06bc | 2.43 ± 0.01bc | 1.49 ± 0.01bc | 1.10 ± 0.06bc | 0.79 ± 0.10bc | 0.83 ± 0.09bc | 55.58 ± 3.66b | 0.89 ± 0.17bc | 2.11 ± 0.11bc |
| C | 1.17 ± 0.24ac | 0.39 ± 0.02c | 3.75 ± 0.01c | 3.55 ± 0.25c | 3.21 ± 0.06c | 1.84 ± 0.01c | 1.74 ± 0.02c | 0.69 ± 0.02c | 2.48 ± 0.01c | 1.61 ± 0.03c | 36.09 ± 6.29c | 0.58 ± 0.09c | 1.08 ± 0.06c |
| D | 1.12 ± 0.14bc | 0.33 ± 0.01abc | 3.56 ± 0.03abc | 3.27 ± 0.17b | 3.20 ± 0.05b | 1.79 ± 0.01bc | 1.76 ± 0.04b | 0.64 ± 0.01bc | 2.16 ± 0.02bc | 1.34 ± 0.04abc | 24.77 ± 2.14abc | 0.53 ± 0.10b | 1.33 ± 0.02abc |
| E | 1.16 ± 0.12ab | 0.37 ± 0.01b | 3.57 ± 0.02abc | 3.50 ± 0.01b | 3.27 ± 0.01b | 1.84 ± 0.01b | 1.78 ± 0.01b | 0.64 ± 0.01bc | 2.25 ± 0.01bc | 1.45 ± 0.02bc | 35.30 ± 0.97b | 0.54 ± 0.10b | 1.26 ± 0.02bc |
| F | 1.18 ± 0.12ab | 0.41 ± 0.01ab | 3.71 ± 0.02b | 3.75 ± 0.17b | 3.35 ± 0.06bc | 1.85 ± 0.01b | 1.80 ± 0.01b | 0.67 ± 0.07b | 2.41 ± 0.01bc | 1.58 ± 0.04b | 39.55 ± 1.42b | 0.78 ± 0.09d | 1.13 ± 0.01b |
CHOL cholesterol, TAG triacylglycerols, HDL high-density lipoproteins, LDL low-density lipoproteins, BUN blood urea nitrogen, MDA malondialdehyde. Values are means ± SD, n = 5. Values for the treatment groups (for each enzyme) with superscripts letters a, b, and c are significant at p < 0.05 with distilled water, CCl4 and BHT respectively
When there is a blockage in the bile excretion owing to liver damage, levels of serum bilirubin surge [7]. The serum bilirubin concentration is one of the markers used to measure liver-related disease progression. The different doses of the alkaloids caused the restoration following CCl4-induced toxicity, buttressing that the plants might help in the regeneration of hepatocytes, and the healing of hepatic parenchyma.
Treatment of CCl4-intoxicated animals with 600 mg/kg of the alkaloids improved serum total protein and albumin levels by 33.87 and 64.42% respectively. A 200 and 400 mg/kg significantly (p < 0.05) elevated serum albumin from 2.39 to 3.56 g/dl and 2.39 to 3.57 g/dl respectively.
During liver injury, the rate of hepatic synthesis of essential proteins in animals decreases, causing a drop in serum albumin and total protein concentrations [26]. This trend was also reversed following the administration of the different doses of the alkaloids suggesting their potential role in promoting protein synthesis and the function of hepatocytes. The amelioration with 600 mg/kg of the alkaloids demonstrated that serum albumin and total protein concentrations were increased in diabetic rats treated with feed formulated with Z. zanthoxyloides [22].
Treatment with 600 mg/kg of the alkaloid significantly (p < 0.05) reduced the level of cholesterol from 4.65 to 3.35 mmol/L. A 200 mg/kg of alkaloids caused a reduction in serum TAG level from 2.43 to 1.77 mmol/L with no significant difference (p > 0.05), while treatment with 400 and 600 mg/kg of alkaloids significantly (p < 0.05) reduced the level of TAG from 2.43 to 1.84 mmol/L and 2.43 to 1.85 mmol/L respectively.
The study of the consequences of reduced liver function on blood levels of lipoproteins is based on the liver's role as an important organ engaged in lipid metabolism. The amount of cholesterol and fats called triglycerides were measured in the serum of rats, to ascertain the impact of reduced liver function on blood levels of lipoproteins. Intoxication with CCl4 produces fatty liver and hepatic cirrhosis as well as an increase in the total serum cholesterol level [18]. Antioxidants produce beneficial effects on serum lipids [5, 27]. HDL also called good cholesterol can transport excess LDL deposited in walls of blood vessels back to the liver for catabolism. Liver disease resulting from exposure to CCl4 reduces HDL and alters the beneficial effects of HDL [18]. Administration of different doses of the alkaloids to the rats reduced the levels of cholesterol, LDL and TAG but increase the level of HDL in the serum, indicating that they could alleviate dyslipidemia induced by CCl4. Although different doses of the alkaloids reverse these changes, the 600 mg/kg of the alkaloids elicited effects similar to the control which agreed with previously reported effects of Z. zanthoxyloides on serum lipids.
The effects of the 200 and 400 mg/kg alkaloids on serum vitamins showed that serum vitamin C significantly (p < 0.05) increased by 1.37 and 1.46 mg/dl respectively. A 200 and 400 mg/kg of alkaloids significantly (p < 0.05) increased serum vitamin E (0.83 to 1.34 and 0.83 to 1.45 mg/dl) respectively while treatment with 600 mg/kg of alkaloids non-significantly (p > 0.05) increased the serum vitamin E (0.83 to 1.58 mg/dl).
Non-enzymic antioxidants (vitamins C and E) inhibit excessive free radical’s production in animals [28]. They do so by complementing the activities of the enzymic antioxidants. Similarly, the ability of various doses of alkaloids to restore vitamin C and E depletion induced by CCl4 toxicity implies that it can play a critical role in maintaining the animals' natural defense system. The various doses of the fractions were able to preserve the non-enzymic antioxidant system thereby indicating their antioxidant role.
The alkaloids (200 and 400 mg/kg) caused a significant (p < 0.05) reduction in the serum MDA compared with the untreated. The concentration of MDA in the liver is used as a marker for lipid peroxidation [7]. CCl4-induced toxicity significantly increased the level of serum MDA. The simultaneous administration of CCl4 and the alkaloid fractions inhibited the elevation of MDA concentration upon CCl4 administration indicating that the fractions have a preventive effect against hepatic lipid oxidation induced by CCl4, prevention of liver fibrogenesis and pathogenesis of several liver injuries.
When treated with 400 and 600 mg/kg of the alkaloids, the level of BUN was reduced (55.58 to 35.30 mg/dl and 55.58 to 39.55 mg/dl respectively) but there was no significant difference (p > 0.05) when compared with the untreated group. Similarly, the serum creatinine was significantly (p < 0.05) reduced when the CCl4-intoxicated rats were treated with 600 mg/kg of the alkaloids.
The widespread toxic effect of CCl4 makes it difficult to rule out the possibility of additional organ systems being involved in metabolic abnormalities. Some renal function parameters such as urea (BUN) and creatinine were assayed on the sera specimens. Elevated serum creatinine level signifies kidney disease or impaired kidney function [29]. The BUN to creatinine ratio gives a better idea of how well kidneys function, than the creatinine level alone [21]. Elevated serum creatinine and BUN levels signify impaired kidney function or disease. Their elevation serves as an indicator of poor kidney function since the kidney completely filters them from the blood [29]. Their levels in the blood build up due to poor clearance when the kidney becomes impaired for any reason [30]. Various doses of the alkaloids normalized the levels of BUN and creatinine in the serum following CCl4 intoxication like BHT, indicating the curative effects of the studied fractions on the kidney.
The distinct patterns of the correlation among the non-enzymic parameters are demonstrated with heat maps (Fig. 1b). There is a strong positive correlation existing between the serum concentrations of CB and TB, CB and ALB, TB and TP, CB and Vit C, ALB and Vit C, ALB and Vit E, CHOL and BUN, TAG and BUN, CHOL and CREAT, LDL and BUN, Vit C and HDL, Vit C and Vit E, LDL and MDA, BUN and CREAT, BUN and MDA. However, a strong negative correlation exists between the serum concentrations of ALB and BUN, TP and BUN, ALB and CREAT, ALB and MDA, Vit C and CHOL, Vit C and TAG, Vit E and CHOL, Vit E and TAG, Vit C and LDL, Vit E and LDL, HDL and LDL, HDL and TAG, HDL and CHOL, Vit C and BUN, Vit C and MDA, LDL and TP, LDL and ALB, LDL and CB.
The differences in the effects of the treatment groups on the non-enzymic parameters are visualized with a representative boxplot (Fig. 2b). Treatment with 600 mg/kg of alkaloid fraction (group F) produced the highest significant (p ˂ 0.05) effect when compared with the standard control (group C), 200 mg/kg alkaloid fraction (group D) and 400 mg/kg alkaloid fraction (group E). There was a gradual increase in enzyme activity as the doses increased. Meanwhile, 600 mg/kg alkaloid fraction (group F) produced a similar effect to the standard control (group C).
Histopathological Effect of the Alkaloids of Z. zanthoxyloides
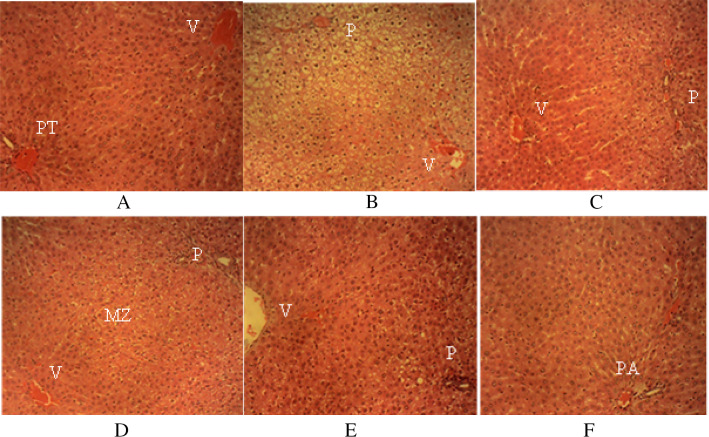
The hepatic histomorphology for rats from each group was observed in the sections of the liver (Fig. 3). The liver sections of the group D rat (plate D) showed a mild cellular swelling of the hepatocytes in the periportal (P) and mid-zonal (MZ) areas of the hepatic lobules with normal hepatocytes around the central veins (V). Mild cellular swelling of the hepatocytes in the P areas of the hepatic lobules in the sections of the liver of group E (plate E) was seen. Sections of the liver of group F rats (plate F) showed the normal hepatic histo-architecture when compared with the untreated (plate A) which showed numerous normal hepatic lobules, with normal hepatocytes organized in radiating interconnecting cords around V. Normal-sized sinusoidal gaps divide the hepatic cords. Normal structures of the portal triads (PT) [hepatic vein, hepatic artery and bile ducts] were also observed. Marked cellular swelling of the hepatocytes in the P and MZ areas of the hepatic lobules was observed in the liver sections of group B rats (plate B). There are normal hepatocytes around V. However, sections of the liver of group C rats (plate C) showed a mild cellular swelling of the hepatocytes in the P areas of the hepatic lobules. The hepatocytes around V are normal.
Fig. 3.
Liver sections of the rats administered with different doses of the bulk alkaloids of Z. zanthoxyloides. Plate A (H & E × 200) for group A; Plates B − F (H & E × 160) for groups B to F; V central veins, PT portal triads, MZ mid-zonal, P periportal, PA portal area
Histopathological examinations further supported the hepatocurative activity of the test fractions. Z. zanthoxyloides alkaloids improved the hepatoprotective damage and restored the histoarchitecture of the kidney and liver [19, 31]. There is tissue architecture injury due to CCl4-intoxication. The histology of the liver section of the rats treated with 600 mg/kg of the alkaloids showed normal hepatic histo-architecture, showing its hepatocurative effects. Examination of the liver tissue of rats treated with 200 and 400 mg/kg of the alkaloids showed a mild cellular swelling of the hepatocytes in the MZ and P areas of the hepatic lobules. There are normal hepatocytes around the central veins.
Conclusion
Liver disease is still a global health challenge and requires an urgent need to find an active and safe drug for treatment. The alkaloids of Z. zanthoxyloides have a significant hepatocurative effect. The alkaloidal content of Z. zanthoxyloides can enhance serum biochemical indicators such as antioxidant markers, liver enzymes, protein profile, kidney function parameters and lipid profile, repair cellular damage and fibrosis in the liver, and control hepatocyte activity since it plays a major role in promoting protein synthesis.
The histopathological examinations of the liver sections show the prevention of hepatocellular damage caused by hepatotoxin (CCl4). The hepatocurative activity of Z. zanthoxyloides demonstrated that the possible mechanism behind this property may be associated with the antioxidant activity of the alkaloids. Due to its potent antioxidant and hepatocurative characteristics, which have considerable effects against free radical damage and may be effective for the prevention of liver tissue damage, it may be a significant raw material for pharmaceutical formulations. The hepatocurative potential of the alkaloid fractions of Z. zanthoxyloides leaves demonstrated in this study suggests its therapeutic benefits in preventing liver injury caused by free-radical generation.
The bulk alkaloids at doses of 200–600 mg/kg p.o. have a significant hepatocurative effect on the liver of CCl4-induced hepatocellular damage in rats. The CCl4-induced toxicity in the liver and kidney of albino rats can be ameliorated by the alkaloids of Z. zanthoxyloides, supporting its use in the management of liver-associated diseases. There was evidence of significant restoration of the biochemical parameters and liver architecture. Data visualization and mapping approaches adopted were important to promote the identification of target bioactive alkaloids and develop treatment options for liver diseases. Studies to characterize these bioactive alkaloids are ongoing.
Acknowledgements
The authors appreciate Professor Charles N. Mba of the Department of Soil Science, School of Agriculture and Agricultural Technology of Federal University of Technology, Owerri for plant identification.
Authors’ Contributions
We declare that this work was done by the authors named in this article and all liabilities pertaining to claims relating to the content of this article will be borne by the authors. Concept—[TOA]; Design— [CON, NN]; Supervision—[NN, ACE, CUI]; Resources—[TOA]; Materials—[TOA, CON]; Data Collection and/or Processing—[TOA, CON]; Analysis and/or Interpretation—[TOA, CON, CUI, NN]; Literature Search—[TOA, CON, CUI]; Writing—[TOA]; Critical Reviews—[TOA, CON, NN, ACE, CUI]. All authors read and approved the manuscript for publication.
Funding
No funds were received for this research.
Declarations
Conflict of interest
The authors declare no competing interest.
Ethics Approval
The University of Nigeria Ethics Committee (FBS/2020/TOA/ZZ/002) of 20th March, 2020 granted authorization to use animals in this study, following a set of guidelines for the care and use of laboratory animals, "Principles of Laboratory Animal Care" (NIH publication no. 85–23, revised 1985) and/or the declaration of Basel issued on 30th November 2010 as amended on January 2013.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Charles II, Kayode BJ, Kingsley A, Ebenezer D, Sunday O, Raphael E, et al. Histopathological Effect of Varying Dose of Acetylsalicylic Acid (Aspirin) on Liver of Adult Wistar Rats. J Biotechnol Biomed. 2018;1(1):28–33. 10.26502/jbb.2642-9128003
- 2.Faria T, Nascimento C, Vasconcelos S, Stephens P, Saranraj P, Barreto A, et al. Literature Review on the Biological Effects of Taraxacum Officinale Plant In Therapy. Asian J Pharm Res Dev. 2019;7(3):94–9. 10.22270/ajprd.v7i3.502
- 3.Udeh NE, Nnadi CO, Anaga AO, Asuzu IU. Bioactivity-guided fractionation of a methanol leaf extract from Gnetum africanum with potential anti-diabetic activity: (-)-epicatechin as the active principle. J Res Pharm. 2021;25(1):72–9. 10.35333/jrp.2021.293
- 4.Airaodion AI, Ogbuagu EO, Ogbuagu U, Adeniji AR, Agunbiade AP, Airaodion EO. Hepatoprotective effect of Parkia biglobosa on acute ethanol-induced oxidative stress in Wistar Rats. Int Res J Gastroenterol Hepatol. 2019;2(1):1–11. [Google Scholar]
- 5.Nnadi CO, Okorie NH, Nwodo NJ. Evaluation of In Vitro Antiprotozoal and Cytotoxic Activities of Selected Medicinal Plants used in Nigerian Folk Medicine. Trop J Nat Prod Res. 2021;5(4):609–12. 10.26538/tjnpr/v5i4.2
- 6.Brigelius-Flohé R, Flohé L. Regulatory Phenomena in the Glutathione Peroxidase Superfamily. Vol. 33, Antioxidants and Redox Signaling. 2020; 10.1089/ars.2019.7905 [DOI] [PubMed]
- 7.Ani O, Udedi S, Anajekwu B, Ekwealor K. Inhibitory potential and antidiabetic activity of leaf extracts of Vitex doniana. Afr J Biochem Res. 2020;14(3):72–80. doi: 10.5897/AJBR2020.1098. [DOI] [Google Scholar]
- 8.Onoja S, Nnadi C, Udem S, Anaga A. Potential antidiabetic and antioxidant activities of a helliangolide sesquiterpene lactone isolated from Helianthus annuus L. leaves. Acta Pharm. 2020;70(2):215–26. 10.2478/acph-2020-0019. [DOI] [PubMed]
- 9.Ayoka TO, Nwachukwu N, Ene AC, Igwe CU, Ogara AL, Nnadi CO. In-Vitro Antioxidant Activity and Acute Toxicity of the Alkaloidal Constituents of Zanthoxylum zanthoxyloides Leaves. Trop J Nat Prod Res. 2022;6(2):276–80. 10.26538/tjnpr/v6i2.17
- 10.Olushola-Sudoks A, Igbo U, Asiebo G, Damola I, Igwe C. Elemental analysis and phytochemical characterisation of Zanthoxylum zanthoxyloides (Lam.) Zepern and Timler stem bark. J Pharmacogn Phytochem. 2020;9(5):41–6. 10.22271/phyto.2020.v9.i5a.12420.
- 11.Ouédraogo L, Nacoulma AP, Compaoré M, Lagnika L, Kiendrebeogo M. Stem bark of Zanthoxylum zanthoxyloïdes a possible substitute of root bark for the conservation of the species in Burkina Faso. Afr J Biotechnol. 2019;18(9):197–205. doi: 10.5897/AJB2019.16743. [DOI] [Google Scholar]
- 12.Dofuor AK, Djameh GI, Ayertey F, Bolah P, Amoa-Bosompem M, Kyeremeh K, et al. Antitrypanosomal effects of zanthoxylum zanthoxyloides (Lam.) Zepern. & Timler extracts on african trypanosomes. Evidence-based Complement Altern Med. 2019;2019:1–14. 10.1155/2019/1730452 [DOI] [PMC free article] [PubMed]
- 13.Tougoma A, Atchrimi S, Denou A, Idah O, Egesie G, Odeh S. Assessment of the antipyretic and anti-inflammatory effects of Zanthoxylum zanthoxyloides stem bark aqueous extract on Wister rats. Issues Biological Sci Pharm Res. 2021;9(3):78–85. 10.15739/ibspr.21.008
- 14.Nnadi CO, Nwodo NJ, Kaiser M, Brun R, Schmidt TJ. Steroid alkaloids from Holarrhena africana with strong activity against trypanosoma brucei rhodesiense. Molecules. 2017;22(7):1–13. doi: 10.3390/molecules22071129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayoka TO, Nwachukwu N, Ene AC, Igwe CU, Nnadi CO. Liquid chromatography-mass spectrometric analyses of potential antioxidant constituents from Zanthoxylum zanthoxyloides leaves : probing into the role of alkaloids. Trop J Nat Prod Res. 2020;4(10):817–23. 10.26538/tjnpr/v4i10.26
- 16.Ayoka TO, Nwachukwu N, Nnadi CO. Identification of potential antioxidant and hepatoprotective constituents of Vitex Doniana By UHPLC/+ESI-QQTOF-MS/MS analysis. Asian J Pharm Clin Res. 2020;13(8):142–8. 10.22159/ajpcr.2020.v13i8.37956
- 17.Gan J, Feng Y, He Z, Li X, Zhang H. Correlations between antioxidant activity and alkaloids and phenols of Maca (Lepidium meyenii) J Food Qual. 2017;2017:1–10. doi: 10.1155/2017/3185945. [DOI] [Google Scholar]
- 18.Acheampong D, Baffour I, Barku W, Addo J, Essuman M, Boye A. Zanthoxylum zanthoxyloides alkaloidal extract improves CCl4-induced hepatocellular carcinoma-like phenotypes in rats. Evidence-Based Complement Altern Med. 2021;1–16. 10.1155/2021/3804379. [DOI] [PMC free article] [PubMed]
- 19.Hadwan MH. New method for assessment of serum catalase activity. Indian J Sci Technol. 2016;9(4):1–6. 10.17485/ijst/2016/v9i4/80499
- 20.Robitaille L, Hoffer LJ. A simple method for plasma total vitamin C analysis suitable for routine clinical laboratory use. Nutr J. 2016;15:40–9. Available from: 10.1186/s12937-016-0158-9 [DOI] [PMC free article] [PubMed]
- 21.Krishnegowda A, Padmarajaiah N, Anantharaman S, Honnur K. Spectrophotometric assay of creatinine in human serum sample. Arab J Chem. 2017;10:2018–2024. doi: 10.1016/j.arabjc.2013.07.030. [DOI] [Google Scholar]
- 22.Aloke C, Nwachukwu N, Ugwuja E, Idenyi J, Nwachi E, Obasi I. Effects of Zanthoxylum zanthoxyloides leaves on blood glucose, lipid profile and some liver enzymes in alloxan induced diabetic rats. Int J Sci Nat. 2012;3(3):497–501. [Google Scholar]
- 23.Parham S, Kharazi AZ, Bakhsheshi-Rad HR, Nur H, Ismail AF, Sharif S, et al. Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants. 2020;9(12):1–36. doi: 10.3390/antiox9121309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gauthier MR, Senhorinho GNA, Scott JA. Microalgae under environmental stress as a source of antioxidants. Algal Res. 2020;52:102104. doi: 10.1016/J.ALGAL.2020.102104. [DOI] [Google Scholar]
- 25.Borquaye LS, Laryea MK, Gasu EN, Boateng MA, Baffour PK, Kyeremateng A, et al. Anti-inflammatory and antioxidant activities of extracts of Reissantia indica, Cissus cornifolia and Grosseria vignei. Cogent Biol. 2020;6(1):1–12. doi: 10.1080/23312025.2020.1785755. [DOI] [Google Scholar]
- 26.Ojochegbe A, Adejoh I, Boniface M, Duniya S, Anna I. Activity of methanol extract of Leptadenia hastata leaves in alcohol-induced activity of methanol extract of Leptadenia hastata leaves in alcohol- induced liver injury. Int J Adv Multidiscip Res. 2019;6(7):11–18. [Google Scholar]
- 27.Ferdous UT, Yusof ZNB. Medicinal prospects of antioxidants from algal sources in cancer therapy. Front Pharmacol. 2021;12:1–22. doi: 10.3389/fphar.2021.593116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moussa Z, Judeh ZMA, Ahmed AS. Nonenzymatic exogenous and endogenous antioxidants. Free Radic Med Biol. 2020;4:1–10. doi: 10.5772/intechopen.87778. [DOI] [Google Scholar]
- 29.Gbate M, Ashamo OM, Kayode AL. Toxicological evaluation of Zanthoxylum zanthoxyloides (Lam) Zepernick & Timler root bark used as biopesticide and medicine. J Med Plants Res. 2021;15(2):108–117. doi: 10.5897/JMPR2020.7081. [DOI] [Google Scholar]
- 30.Olajide J, Sanni M, Omattah G. Effect of methanol leaf extract of Vitex Doniana on cadmium chloride-induced toxicity in kidney and liver tissues of male Wistar Rats. Int J Trend Sci Res Dev. 2018;2(6):1306–1315. [Google Scholar]
- 31.Ayoka TO, Nnadi CO. Lesser-known leafy vegetables of Southeastern Nigeria. (Vitex doniana and Zanthoxylum zanthoxyloides). Not Sci Biol. 2022;14(2):11177–96. 10.15835/nsb14211177