Abstract
The occurrence of major depressive disorder is widespread and can be observed in individuals belonging to all societies. It has been suggested that changes in the NO pathway and heightened oxidative stress may play a role in developing this condition. Anethole is a diterpene aromatic compound found in the Umbelliferae, Apiaceae, and Schisandraceae families. It has potential pharmacological effects like antioxidant, anxiolytic, analgesic, anti-inflammatory, antidiabetic, gastroprotective, anticancer, estrogenic, and antimicrobial activities. This study aimed to investigate the potential antidepressant properties of Anethole in a mouse model experiencing maternal separation stress while also examining its impact on oxidative stress and nitrite levels. The research involved the participation of 40 male NMRI mice, separated into five distinct groups to conduct the study. The control group was administered 1 ml/kg of normal saline, while the MS groups were given normal saline and Anethole at 10, 50, and 100 mg/kg doses. The study comprised various behavioural tests, including the open field test (OFT), forced swimming test (FST), and splash test, to assess the effects of Anethole on the mice. In addition to the behavioural tests, measurements were taken to evaluate the total antioxidant capacity (TAC), malondialdehyde (MDA), and nitrite levels in the hippocampus of the mice. According to the findings, maternal separation stress (MS) led to depressive-like conduct in mice, including a rise in immobility duration during the FST and a reduction in the duration of grooming behaviour in the splash test. Additionally, the results indicated that MS correlated with an increase in the levels of MDA and nitrite and a reduction in the TAC in the hippocampus. However, the administration of Anethole resulted in an increase in grooming activity time during the splash test and a decrease in immobility time during the FST. Anethole also exhibited antioxidant characteristics, as demonstrated by its ability to lower MDA and nitrite levels while increasing the TAC in the hippocampus. The results suggest that Anethole may have an antidepressant-like impact on mice separated from their mothers, likely partly due to its antioxidant properties in the hippocampus.
Keywords: Anethole, Oxidative stress, Depression, Maternal separation, Mice, Natural product, Medicinal plant
Subject terms: Drug discovery, Neuroscience, Medical research
Introduction
The prevalence of depression is a significant concern in modern societies, as it can lead to a range of physical health problems1. An estimated 3.8% of the population experience depression, including 5% of adults (4% among men and 6% among women) and 5.7% of adults older than 60 years. Approximately 280 million people in the world have depression2. The depressive disorder will be the second debilitating factor until 20303. Maternal care in infancy is associated with health and mental development during adolescence4. The separation of newborn rodents from their mothers for a specific period, known as maternal separation (MS), is an established animal model used to study the effects of stress on rodents and the development of depression-like behaviours5.
The maternal separation model is frequently used in animal research to examine the effects of stress and depressive behaviours on rodents5. The interaction between a mother and her infant is crucial for developing the brain and behaviour. Early life deficits, such as maternal separation stress in rodents, may result in altered nervous system adaptation, increasing the likelihood of experiencing depressive symptoms later in life6. Studies have shown that maternal separation stress during early life can negatively impact different brain regions in rodents, including the amygdala, prefrontal cortex, and hippocampus. This stress can also lead to neurotransmission alterations and affect the brain's normal functioning7–9. These biological, behavioural, and neurochemical changes have been linked to various neuropsychiatric conditions, including stress and depression in humans and animals during adulthood10,11. The use of antioxidant compounds reduces the symptoms of depression in patients. Additionally, antidepressants are able to reduce some of the markers of oxidative stress and increase some internal antioxidants12.
Oxidative stress is a risk element that raises oxidized lipids and proteins in the central nervous system, ultimately resulting in tissue damage13. Severe depression is associated with decreased antioxidants and oxidative and nitrosative activities14. There is considerable scientific evidence that nitric oxide (NO) may play a role in developing different neurodegenerative and inflammatory disorders15. Antioxidants are essential compounds that help protect cells from the harmful effects of oxidative stress, which can lead to cellular damage and contribute to the development of various diseases. Plant-based antioxidants are particularly beneficial as they contain high levels of these protective compounds. They have been shown to reduce the risk of oxidative damage in the brain and plasma, thereby reducing the likelihood of developing conditions such as inflammation, diabetes, Alzheimer's, and depression16.
Recent studies have focused on identifying new and effective compounds, particularly from plant-based sources, that can serve as complementary or alternative treatments for depression. These efforts are aimed at expanding the range of available treatment options and raising the quality of life with depression17. Anethole (1-methoxy-4-isopropinyl-benzene) is a fragrant terpenoid, colorless, sweet compound and one of the main constituents of essential oils of many plants, including anise, castor oil, and Tarragon18,19. Anethole has many medicinal properties, including anti-inflammatory, antioxidant, anti-neurodegenerative, and analgesic effects20–22.
This study aims to examine the potential antidepressant effects of Anethole in a mouse model of maternal separation stress, taking into account its potential anti-oxidative stress and anti-nitric oxide properties.
Methods
This study is reported in accordance with ARRIVE guidelines.
Maternal separation
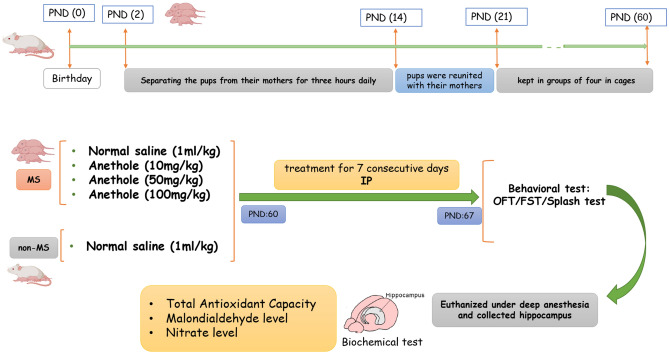
The study involved pregnant NMRI mice obtained from the Pasteur laboratory and kept in conventional laboratory conditions. The maternal separation procedure, which involved separating the pups from their mothers for three hours daily from PND 2 to PND 14, was conducted as per established protocols. After PND 14, the pups were reunited with their mothers until PND 21 and were kept in groups of four in cages until PND 60. The control group, which was not subjected to any manipulation, remained with their mothers in the same cage from PND 0 to PND 21 and were then housed in groups of four in separate cages until PND 6023.
Animals
The study was conducted on 40 male NMRI mice weighing 25-30g, randomly distributed into five groups. The control group (Group 1) received intraperitoneal injections of 1 ml/kg of normal saline, while the MS mice in Group 2 were administered 1 ml/kg of normal saline via the same route. The experimental groups (Groups 3, 4, and 5) consisted of MS mice that were treated with different doses of Anethole (Sigma Aldrich, St. Louis, MO, USA), which were 10, 50, and 100 mg/kg, respectively, through i.p. injections. The doses of drug administrations were chosen based on a pilot and previous studies18,24,25.
The mice were treated with Anethole half an hour before the behavioural tests and continued receiving treatment for seven consecutive days. The behavioural tests, including the OFT, FST, and splash, were conducted following the treatment period. Following the completion of the behavioural tests, the mice were humanely euthanized under deep anesthesia, which was induced by administering ketamine (60 mg/kg) and xylazine (10 mg/kg) intraperitoneally. The hippocampi were collected and analyzed for antioxidant capacity, MDA, and nitrite levels (Fig. 1). The tissue was carefully extracted from the mice on a surface cooled with ice and immediately frozen in liquid nitrogen to preserve the biochemical properties of the hippocampus. Subsequently, the frozen tissue samples were transferred to a freezer at -70°C for storage until further analysis26,27. The α error was set at 0.05 and power (1-β) at 0.8, and the required total sample size per group was calculated as 6 in behavioral and molecular evaluations.
Figure 1.
Schematic of experimental design. PND postnatal days, IP intraperitoneal injection, FST forced swimming test, OFT open field test.
All methods were performed according to the relevant guideline and regulations and were approved by the Ethics Committee at Shahrekord University of Medical Sciences. The study was assigned an ethics code of IR.SKUMS.REC.1398.208. All necessary steps were taken to ensure that the welfare and ethical treatment of the mice were maintained throughout the study.
Open field test (OFT)
OFT was used as a paradigm to test locomotion following treatment. In this study, the OFT was done immediately before the FST to consider ambulatory behavior and to confirm that adjustments in motor activity did not affect the immobility time in the FST. The open-field arena was a white plexiglass box with dimensions of 50 cm × 50 cm × 30 cm, containing 16 equal squares. Testing is conducted under dim white-light illumination (about 150 lux). The mice were arranged in the central zone of the open field, which measured 30 × 30 cm, and their movements were recorded for 5 min using a camera. Ethovision software version 8 (Noldus, Netherlands) was utilized to analyze the recorded data, and the number of horizontal and vertical movements made by the mice was accurately measured28.
Splash test
The splash test was employed to evaluate the mice's self-care and motivation abilities. The assessment required applying a 10% sucrose solution on the mice's dorsal coats in their cages. Then, the duration of grooming activities was recorded for 5 min after the spraying sucrose test, which included nose/face cleaning, head washing, and body grooming. The total time spent grooming behavior was considered an index of motivation, self-care behavior, well-being, and mood. Reduced grooming behavior is considered a depression-like behavior in mice29.
Forced swimming test (FST)
The FST was utilized to evaluate depression-like behaviour in mice. The FST is a commonly used method for measuring immobility in rodents. The test involved placing the mice in a cylindrical glass tank with a diameter of 10 cm and a height of 25 cm. The tank was filled with water (19 cm) at a temperature of 24 ± 1 °C. During the 6-min test period, episodes where the mice floated in the water with only necessary movements to keep their heads above water were recorded as immobility. The immobility time was recorded during the final 4 min of the test to measure the depression-like behaviour of the mice30.
Measuring the antioxidant capacity of the hippocampus
The FRAP assay was used to evaluate the antioxidant power of the hippocampus. The assay measured the sample's ability to convert Fe3+-TPTZ to Fe2+. The FRAP reagent comprised TPTZ solution, FeCl3, and acetate buffer. The brain hippocampus was isolated, homogenized, and centrifuged at 10,000 rpm. A floating solution was extracted from the centrifuged sample to assess the antioxidant power. 50 μl of this solution was combined with 1.5 ml of a prepared labour solution at 37 °C. After 10 min, the formation of a complex between Fe3+ and TPTZ produced a blue colour that was measured for absorbance at a wavelength of 593 nm. FeSO4 was used as the standard solution for this method31.
Measuring MDA of the hippocampus
The hippocampus's MDA (malondialdehyde) level was assessed by taking 1 ml of homogenized tissue and incubating it in a glass tube at 37°C for 60 min. 5% trichloroacetic acid and 67% thiobarbituric acid were added to the tube, mixed thoroughly, and centrifuged. The supernatant was transferred to another tube, heated in boiling water, and then the absorbance at 535 nm was measured to determine the MDA level in the hippocampus32.
Measuring nitric oxide of the hippocampus
A colourimetric assay based on the Griess reaction was applied to assess the nitric oxide level in the hippocampus. This involved converting nitrate to nitrite using nitrate reductase enzyme and measuring the nitrite level at 570 nm with the Griess reagent. The Griess reaction produces colour from the diazotization of a sulfonamide by nitrite in an acidic environment, followed by conjugation with an aromatic amine (N-1-naphthyl ethylenediamine (NEDD))33.
Data analysis
The obtained data were statistically analyzed using GraphPad Prism 8 software and presented as mean ± SEM. One-way ANOVA followed by Tukey's post hoc test determined significant group differences. A level of statistical significance was set at P < 0.05.
Ethical approval
All methods were performed according to the relevant guideline and regulations.All procedures were carried out under the regulations of the University and the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (ethical code: IR.SKUMS. REC.1398.208) and Guide for the Care and Use of Laboratory Animals (8th edition, National Academies Press).
Results
The effect of anethole on locomotor activity in the OFT
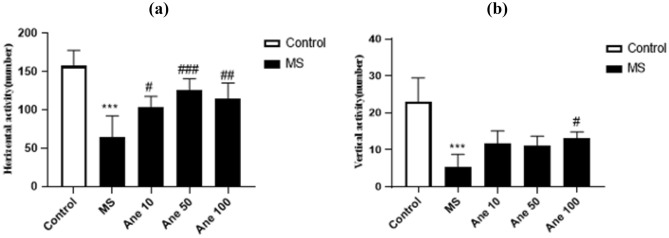
One–way ANOVA analysis showed that there are significant differences in horizontal activity (F (4, 25) = 19.96, P < 0.0001) and vertical activity(F (4, 25) = 17.75, P < 0.0001) among the experimental group. Compared to the control group, the MS group exhibited a substantial decrease in horizontal and vertical movements, with a statistically significant 0.001. However, treatment with Anethole at 10, 50, and 100 mg/kg doses lead to a considerable increase in the number of horizontal movements in the MS group (0.05, 0.001, and 0.01 levels, respectively), and a noteworthy increase in the number of vertical movements at 100 mg/kg dose (P < 0.05) (Fig. 2a, b). The observed results suggest that Anethole supplementation can improve locomotor activity in the MS group. The level of statistical significance was predetermined at P < 0.05.
Figure 2.
The effect of anethole on locomotor activity in the OFT. (a) Horizontal activity and (b) vertical activity. Control: group without MS receiving normal saline; MS: MS group receiving normal saline; Ane 10, Ane 50, and Ane 100: MS group treatment with anethole at doses 10, 50, and 100 mg/kg. ***P < 0.001: comparison of MS group and control group; #P < 0.05, ###P < 0.001 and ##P < 0.01: comparison of MS group with MS group receiving anethole at doses 10, 50, and 100 mg/kg (a). ***P < 0.001: comparison of MS group and control group; #P < 0.05: comparison of MS group with MS group receiving anethole at dose 100 mg/kg (b).
The effect of anethole on grooming activity time in the splash test
One–way ANOVA analysis showed that there are significant differences in the grooming activity time (F (4, 25) = 60.23, P < 0.0001) among the experimental group. The findings revealed a significant reduction in grooming activity time in the MS group compared to the control group at 0.001. However, treatment with Anethole at different doses (50 and 100 mg/kg) significantly increased grooming activity time in the MS group (0.05 and 0.001 levels, respectively). Furthermore, the groups that received Anethole at doses of 10 and 100 mg/kg observed a significant effect at 0.01 level, and between the groups that received Anethole at doses of 50 and 100 mg/kg deliciated substantial differences at 0.05 in grooming activity time (Fig. 3). These results suggest that Anethole supplementation can improve grooming activity in the MS group. The statistical significance level was predetermined at P < 0.05.
Figure 3.
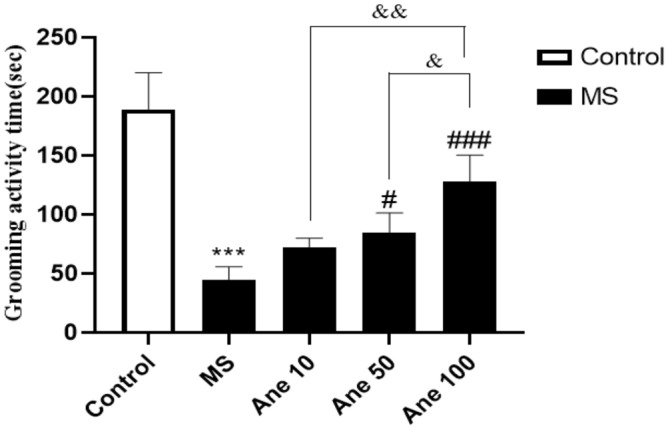
The effect of anethole on grooming time in the splash test. Control: group without MS receiving normal saline; MS: MS group receiving normal saline; Ane 10, Ane 50, Ane 100: MS group treatment with anethole at doses 10, 50, and 100 mg/kg. ***P < 0.001: comparison of MS group and control group; #P < 0.05 and ###P < 0.001: comparison of MS group with MS group receiving anethole at doses 50 and 100 mg/kg; &&P < 0.01 and &P < 0.05: comparison of group receiving anethole at a dose of 10 mg/kg with the group receiving anethole at a dose of 100 mg/kg and comparison of group receiving anethole at a dose of 50 mg/kg with the group receiving anethole at a dose of 100 mg/kg.
The effect of anethole on immobility time in the FST.
One–way ANOVA analysis showed that there are significant differences in the immobility time (F (4, 25) = 63.57, P < 0.0001) among the experimental group. The analysis revealed a significant increase in immobility time in the FST for the MS group compared to the control group at 0.001. However, treatment with Anethole at doses of 10, 50, and 100 mg/kg significantly decreases immobility time in the MS group (0.01, 0.001, and 0.001 levels, respectively). Additionally, a significant difference in immobility time was observed between the group that received Anethole at doses of 10 and 100 mg/kg (P < 0.01), indicating the potential antidepressant effect of Anethole in the MS-induced depression model. Results showed that Anethole supplementation may be an effective treatment for depression. The predetermined significance level for statistical analysis was set at P < 0.05 (Fig. 4).
Figure 4.
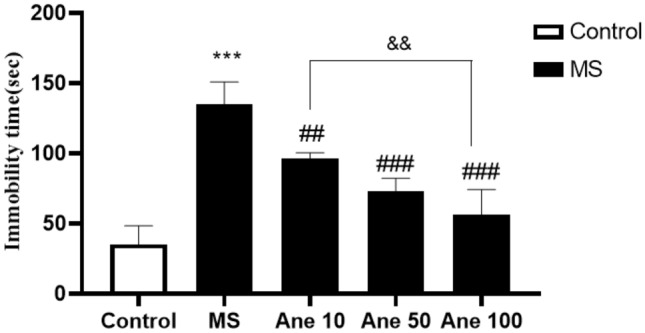
The effect of anethole on immobility time in the FST. Control: group without MS receiving normal saline; MS: MS group receiving normal saline; Ane 10, Ane 50, Ane 100: MS group treatment with anethole at doses 10, 50 and 100 mg/kg. ***P < 0.001: comparison of MS group and control group; ##P < 0.01, ###P < 0.001 and ###P < 0.001: comparison of MS group with MS group receiving anethole at doses 10, 50 and 100 mg/kg; &&P < 0.01: comparison of group receiving anethole at a dose of 10 mg/kg with the group receiving anethole at a dose of 100 mg/kg.
The effect of anethole on antioxidant capacity
One–way ANOVA analysis showed that there are significant differences in the antioxidant capacity (F (4, 25) = 69.92, P < 0.0001) among the experimental group. The study demonstrated a substantial reduction in the antioxidant capacity of the hippocampus in the MS group compared to the control group, with a significant difference at 0.001 level. However, treatment with Anethole at doses of 10, 50, and 100 mg/kg significantly increased hippocampus antioxidant capacity in the MS group at 0.001. Notably, no significant differences in the hippocampus antioxidant capacity were observed among the groups that received Anethole at 0.05 (Fig. 5). These findings suggest that Anethole supplementation may improve hippocampal antioxidant capacity in the MS-induced depression model. A threshold of P < 0.05 was selected as the level of statistical significance.
Figure 5.
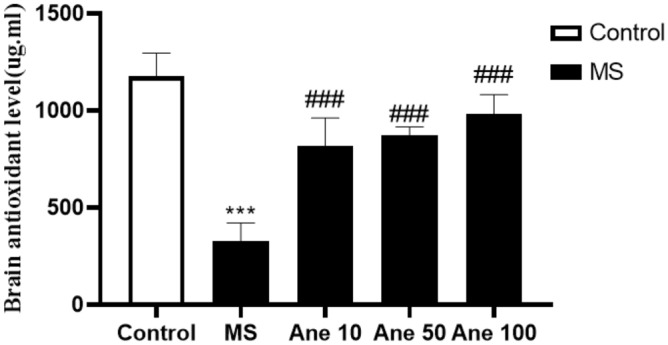
The effect of anethole on antioxidant capacity. Control: group without MS receiving normal saline; MS: MS group receiving normal saline; Ane 10, Ane 50, Ane 100: MS group treatment with anethole at doses 10, 50 and 100 mg/kg. ***P < 0.001: comparison of MS group and control group; ###P < 0.001: comparison of MS group with MS group receiving anethole at doses 10, 50 and 100 mg/kg.
The effect of anethole on MDA level
One–way ANOVA analysis showed that there are significant differences in the MDA level (F (4, 25) = 31.76, P < 0.0001) among the experimental group. The study findings revealed a significant increase in the hippocampus MDA level in the MS group compared to the control group at 0.001 level. However, treatment with Anethole at 10, 50, and 100 mg/kg doses resulted in a significant decrease in the hippocampus MDA level in the MS group (0.01, 0.001, and 0.001 levels, respectively). Additionally, a considerable difference in hippocampus MDA levels was observed between the group that received Anethole at doses of 10 and 100 mg/kg at 0.05 (Fig. 6). The study results imply that Anethole may protect the hippocampus by reducing MDA levels in the MS-induced depression model. A P-value of less than 0.05 was used as the predetermined threshold in the study to assess statistical significance.
Figure 6.
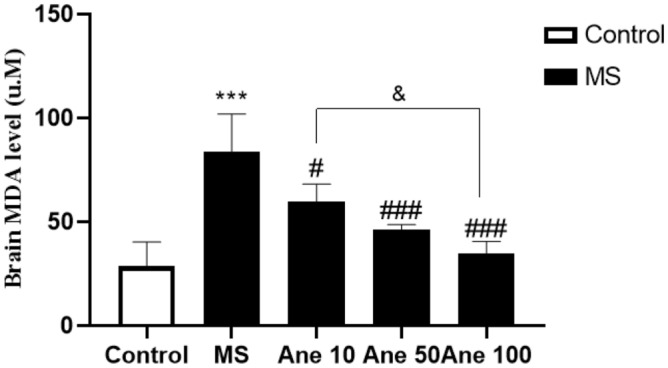
The effect of anethole on MDA level. Control: group without MS receiving normal saline; MS: MS group receiving normal saline; Ane 10, Ane 50, Ane 100: MS group treatment with anethole at doses 10, 50 and 100 mg/kg. ***P < 0.001: comparison of MS group and control group; #P < 0.05,0###P < 0.001 and ###P < 0.001: comparison of MS group with MS group receiving anethole at doses 10, 50 and 100 mg/kg. &P < 0.05: comparison of group receiving anethole at a dose of 10 mg/kg with the group receiving anethole at a dose of 100 mg/kg.
The effect of anethole on nitrite level
One-way ANOVA analysis showed that there are significant differences in the nitrite level (F (4, 24) = 27.43, P < 0.0001) among the experimental group. The findings indicated a noteworthy increase in the hippocampus nitrite level in the MS group compared to the control group at 0.05 level. However, treatment with Anethole at 50 and 100 mg/kg doses lead to a significant decrease in the hippocampus nitrite level in the MS group (0.01 and 0.001 levels, respectively). Additionally, a significant difference in the hippocampus nitrite level was observed between the group that received Anethole at 10 and 100 mg/kg doses at 0.05 (Fig. 7). Based on these results, it can be inferred that Anethole may have a potential neuroprotective effect against hippocampal damage induced by MS, as evidenced by the decrease in hippocampus nitrite levels. A threshold of P < 0.05 was established as the level of statistical significance.
Figure 7.
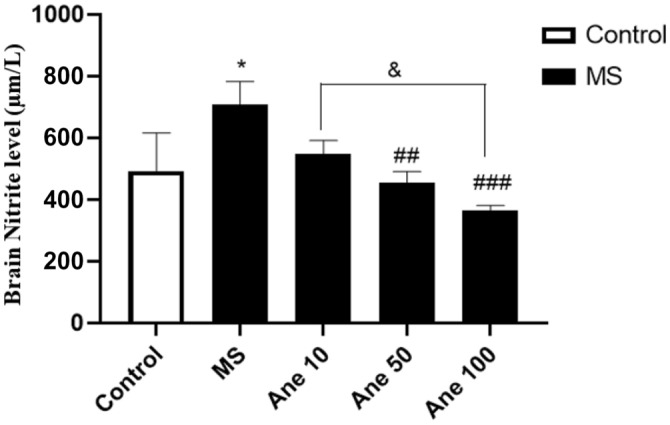
The effect of anethole on nitrite level. Control: group without MS receiving normal saline; MS: MS group receiving normal saline; Ane 10, Ane 50, Ane 100: MS group treatment with anethole at doses 10, 50 and 100 mg/kg. *P < 0.05: comparison of MS group and control group; ##P < 0.01 and ###P < 0.001: comparison of MS group with MS group receiving anethole at doses 50 and 100 mg/kg. &P < 0.05: comparison of group receiving anethole at a dose of 10 mg/kg with the group receiving anethole at a dose of 100 mg/kg.
Discussion
Depression is a prevalent chronic illness worldwide, with increasing incidence rates. It can adversely affect all aspects of an individual's life, as well as their surroundings and loved ones34. There is substantial evidence to suggest that oxidative stress is a key factor in the development of depression and anxiety35. Potential antioxidants that hold a unique or distinctive role in treating depression include natural compounds of plant origin, especially Anethole36. The primary objective of this study was to evaluate the potential antidepressant effects of Anethole, a compound known for its antioxidant and other pharmacological properties, In a murine model of stress induced by maternal separation. The study aimed to examine the underlying mechanisms of Anethole's therapeutic effects, including its impact on oxidative stress and nitric oxide as a potent antioxidant agent.
In the current study, our discoveries revealed that maternal separation led to depression-like behaviours in the animal model. The open-field test results indicated that the MS group exhibited significantly fewer horizontal and vertical movements than the control group. These findings align with prior research by Amini et al. (2017), which similarly demonstrated that maternal separation during the neonatal period resulted in depressive behaviours and reduced movement in the open field test6. Treatment of MS groups with Anethole in OFT in all three doses caused a significant increase in horizontal activities. On the other hand, treatment of MS group with Anethole at a dose of 100 mg/kg caused a considerable rise in the number of vertical movements in comparison to MS group.
The findings from the forced swim test revealed a significant increase in immobility time in the MS group compared to the control group, which is consistent with previous research indicating a link between the MS method and the induction of depressive effects37,38. Furthermore, administering Anethole at all three doses to the MS group significantly reduced immobility time, with the degree of reduction depending on the specific dosage.
A notable difference in immobility time was observed between the groups that received Anethole at 10 and 100 mg/kg doses. Raman et al. (2020) aligned with our study, showing that the trans-anethole combination can reduce immobility time in FST39. In the splash test, a significant reduction in grooming time was observed in the MS group compared to the control group, indicating the adverse effects of maternal separation stress. These results align with a prior study conducted by Lorigooini et al. (2020), which explored the anxiolytic and antidepressant properties of trigonelline in an experimental mouse model designed to induce stress through maternal separation. The previous study also found increased immobility time in the FST, reduced movement in the OFT. It decreased grooming time in the splash test following MS induction, further supporting the negative impact of MS on behaviour40.
In contrast, when the MS group was treated with Anethole at doses of 50 and 100 mg/kg, a significant increase in grooming time was observed, and the degree of increase was dose-dependent. Furthermore, there were significant differences in grooming time between the groups that received Anethole at doses of 10 and 100 mg/kg and between the groups that received Anethole at doses of 50 and 100 mg/kg. The study's behavioural tests also indicated that intraperitoneal injection of Anethole resulted in a significant increase in the number of movements in the OFT, the amount of grooming in the splash test, and a decrease in immobility time in the FST when compared to the MS group. These findings suggest that Anethole may have antidepressant-like effects on behavioural tests, with the most significant impact observed at 100 mg/kg.
In oxidative stress, rising free radicals damage the nervous system in various ways, creating complications such as depression, diabetes, and Parkinson41. The present study results showed that the antioxidant capacity of the brain hippocampus was significantly reduced in the MS group compared to the control group.
Treatment of MS groups in all three doses with Anethole resulted in a substantial elevation of brain antioxidant capacity hippocampus. Some studies have shown that the total serum antioxidant capacity of people with significant depression decreases compared to ordinary people14. In the chronic stress model caused by MS, depression-like behaviours have been observed with increased corticosterone levels, decreased ACTH, and reduced serum antioxidant capacity42,43. Cavalcanti et al. (2012) showed that Anethole had significant antioxidant and antimicrobial properties in improving skin wound conditions44. According to a study conducted by Ryu et al. (2014), Anethole has been found to possess neuroprotective properties in reducing neuronal cell death due to oxygen deprivation. The compound's mechanism of action involves acting as an antioxidant and an anti-stimulant, serving as a mitochondrial protector45. These studies' results align with the present study, which seems that anethole combination with antioxidant properties as free radical scavengers can show antidepressant effects.
Malondialdehyde (MDA) is an essential cellular marker that indicates the level of oxidative stress and antioxidant status, representing the end product of unsaturated fatty acid peroxidation46. The study observed a significant increase in hippocampal MDA levels in the MS group compared to the control group, highlighting the damaging impact of maternal separation stress on the brain. However, administering Anethole at all three doses significantly reduced hippocampal MDA levels, indicating the potent antioxidant properties of the compound. Additionally, the study found a significant difference in the hippocampal MDA levels between the groups that received Anethole at 10 and 100 mg/kg doses, further underlining the dose-dependent effects of the compound. These findings suggest that Anethole supplementation could potentially alleviate oxidative stress in the hippocampus induced by maternal separation stress, thus providing a promising therapeutic strategy for depression and related disorders.
A study examining the anethole activity on pancreatic cancer in the albino mice model of the tumour showed that Anethole caused survival time increase, weight, and tumour volume decrease. Also, this combination decreases MDA and increases glutathione47. Previous research has indicated that Anethole's antioxidant properties are primarily due to its ability to scavenge free radicals, which can lead to elevated intracellular levels of glutathione and glutathione transferase. The compound can also effectively inhibit lipid peroxidation, further highlighting its potent antioxidant effects.48. These results indicate that the Anethole has effectively reduced brain hippocampus MDA.
A decrease in nitric oxide activity in the individual leads to severe depression, characterized by a measurable increase in serum nitrite/nitrate49. The present study results showed that nitric oxide amount in the brain hippocampus significantly increased in MS group compared to the control group. Treatment of MS groups with Anethole at 50 and 100 mg/kg doses reduced considerably nitric oxide amount in the brain hippocampus. There was a significant difference in the nitric oxide level of the brain hippocampus between the groups receiving Anethole at doses of 100 and 10 mg/kg. Wang et al. (2008) showed that inhibition of stimulating nitric oxide prevents chronic depression-like behaviour caused by stress50.
Domiciano et al. (2012) showed that Anethole inhibited the production or release of inflammatory mediators such as nitric oxide, prostaglandins, IL-1, TNF, and IL-1751. Therefore, Anethole's significantly reducing the brain hippocampus nitric oxide improved depressive behaviours. Whereas in the present study, Anethole has shown antidepressant effects, probably observed antidepressant activity of the Anethole in our study may be related to its anti-inflammatory, antioxidant, and oxidative-nitrosative stress-relief effects. Akçan et al. (2018) showed that Anethole with LD50 1820–5000 mg/kg has no chronic toxicity52. The doses used in the present study are much lower than LD50 reported, which seems like other clinical studies performed on anethole36,39,44,51; this compound can be a good option for designing a formulation as a supplement or drug in depression in future studies.
Conclusion
In conclusion, this study delved into the potential antidepressant effects of Anethole, a compound known for its antioxidant properties, particularly in a murine model of stress induced by maternal separation. The behavioral tests, including the open-field test, forced swim test, and splash test, consistently demonstrated that Anethole administration significantly alleviated depression-like behaviors induced by maternal separation, with dose-dependent effects. Moreover, the study unveiled Anethole's significant antioxidant properties, as evidenced by its capacity to elevate the brain hippocampus antioxidant levels and reduce malondialdehyde (MDA), a marker of oxidative stress. The observed reduction in nitric oxide levels in the brain hippocampus further suggests the potential of Anethole in relieving oxidative-nitrosative stress associated with depression. These findings not only underscore the antidepressant potential of Anethole but also highlight its broader therapeutic implications in addressing oxidative stress-related disorders, offering a promising avenue for future research and potential formulations as supplements or drugs in depression treatment.
Acknowledgements
The authors would like to extend their sincere appreciation from Seyyed Mohsen Mortazavi, Amin Soltani, Shirin Asgharian, Sareh Mohammadi, Akram Torki and Vida Naderi for their valuable collaboration in conducting this study.
Abbreviations
- NO
Nitric oxide
- MS
Maternal separation
- PND
Postnatal day
- OFT
Open field test
- FST
Forced swimming test
- Fe3 + -TPTZ
Ferric-tripiridyltriazine
- TBA
Thiobarbituric acid
- NEDD
N-1-naphthyl ethylenediamine
Author contributions
All the authors were involved in the design, Material preparation, data collection and analysis, manuscript writing, and editing. All the authors read and approved the final draft of the manuscript.
Funding
This study was supported by a research grant (3251) from Shahrekord University of Medical Sciences, Shahrekord, Iran.
Data availability
At the Medical Plants Research Center, Shahrekord University of Medical Sciences, data concerning the current study can be obtained. Correspondence and requests for materials should be addressed to Zahra Lorigooini.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Copeland WE, et al. Longitudinal patterns of anxiety from childhood to adulthood: The Great Smoky Mountains Study. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53(1):21–33. doi: 10.1016/j.jaac.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metrics, I.o.H. and Evaluation. Global Health Data Exchange (GHDx). (Institute of Health Metrics and Evaluation, 2021).
- 3.Mirowsky J, Ross CE. Social Causes of Psychological Distress. Routledge; 2017. [Google Scholar]
- 4.Feldman R, et al. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent–infant contact. Psychoneuroendocrinology. 2010;35(8):1133–1141. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Sadeghi M, Peeri M, Hosseini M-J. Adolescent voluntary exercise attenuated hippocampal innate immunity responses and depressive-like behaviors following maternal separation stress in male rats. Physiol. Behav. 2016;163:177–183. doi: 10.1016/j.physbeh.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Amini-Khoei H, et al. Oxytocin mitigated the depressive-like behaviors of maternal separation stress through modulating mitochondrial function and neuroinflammation. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2017;76:169–178. doi: 10.1016/j.pnpbp.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Weiss IC, et al. Inheritable effect of unpredictable maternal separation on behavioral responses in mice. Front. Behav. Neurosci. 2011;2011:3. doi: 10.3389/fnbeh.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aksu I, et al. Effect of acute and chronic exercise on oxidant–antioxidant equilibrium in rat hippocampus, prefrontal cortex and striatum. Neurosci. Lett. 2009;452(3):281–285. doi: 10.1016/j.neulet.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 9.Macrì S, Zoratto F, Laviola G. Early-stress regulates resilience, vulnerability and experimental validity in laboratory rodents through mother–offspring hormonal transfer. Neurosci. Biobehav. Rev. 2011;35(7):1534–1543. doi: 10.1016/j.neubiorev.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Roy A. Childhood trauma and neuroticism as an adult: possible implication for the development of the common psychiatric disorders and suicidal behaviour. Psychol. Med. 2002;32(8):1471–1474. doi: 10.1017/S0033291702006566. [DOI] [PubMed] [Google Scholar]
- 11.Diehl LA, et al. Long-lasting effects of maternal separation on an animal model of post-traumatic stress disorder: Effects on memory and hippocampal oxidative stress. Neurochem. Res. 2012;37(4):700–707. doi: 10.1007/s11064-011-0660-6. [DOI] [PubMed] [Google Scholar]
- 12.Maes M, et al. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro) degenerative processes in that illness. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(3):676–692. doi: 10.1016/j.pnpbp.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Al Rouq F, El Eter E. PPAR-γ activator induces neuroprotection in hypercholesterolemic rats subjected to global cerebral ischemia/reperfusion injury: In vivo and in vitro inhibition of oxidative stress. Exp. Gerontol. 2014;51:1–7. doi: 10.1016/j.exger.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Hasler G. Pathophysiology of depression: Do we have any solid evidence of interest to clinicians? World Psychiatry. 2010;9(3):155. doi: 10.1002/j.2051-5545.2010.tb00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirzaei F, Khazaei M. Role of nitric oxide in biological systems: A systematic review. J. Mazandaran Univ. Med. Sci. 2017;27(150):192–222. [Google Scholar]
- 16.Ghaemi SN, et al. Antidepressant treatment in bipolar versus unipolar depression. Am. J. Psychiatry. 2004;161(1):163–165. doi: 10.1176/appi.ajp.161.1.163. [DOI] [PubMed] [Google Scholar]
- 17.Rabiei Z, Rabiei S. A review on antidepressant effect of medicinal plants. Bangladesh J. Pharmacol. 2017;12(1):1–11. doi: 10.3329/bjp.v12i1.29184. [DOI] [Google Scholar]
- 18.Tognolini M, et al. Protective effect of Foeniculum vulgare essential oil and anethole in an experimental model of thrombosis. Pharmacol. Res. 2007;56(3):254–260. doi: 10.1016/j.phrs.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Cabral PHB, et al. Effects of the essential oil of Croton zehntneri and its major components, anethole and estragole, on the rat corpora cavernosa. Life Sci. 2014;112(1–2):74–81. doi: 10.1016/j.lfs.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 20.Shahat AA, et al. Chemical composition, antimicrobial and antioxidant activities of essential oils from organically cultivated fennel cultivars. Molecules. 2011;16(2):1366–1377. doi: 10.3390/molecules16021366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aprotosoaie AC, Costache I-I, Miron A. Anethole and its role in chronic diseases. Drug Discov. Mother Nat. 2016;2016:247–267. doi: 10.1007/978-3-319-41342-6_11. [DOI] [PubMed] [Google Scholar]
- 22.Sheikh BA, et al. Trans-anethole, a terpenoid ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin induced diabetic rats. Biochimie. 2015;112:57–65. doi: 10.1016/j.biochi.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Amini-Khoei H, et al. Experiencing neonatal maternal separation increased pain sensitivity in adult male mice: involvement of oxytocinergic system. Neuropeptides. 2017;61:77–85. doi: 10.1016/j.npep.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Ghasemi-Dehnoo M, et al. Anethole ameliorates acetic acid-induced colitis in mice: Anti-inflammatory and antioxidant effects. Evid.-Based Complem. Altern. Med. 2022;2022:9057. doi: 10.1155/2022/9057451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yadollahi-Farsani Y, et al. Anethole via increase in the gene expression of PI3K/AKT/mTOR mitigates the autistic-like behaviors induced by maternal separation stress in mice. IBRO Neurosci. Rep. 2024;16:1–7. doi: 10.1016/j.ibneur.2023.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghazvini H, et al. Estrogen and progesterone replacement therapy prevent methamphetamine-induced synaptic plasticity impairment in ovariectomized rats. Addict. Health. 2016;8(3):145. [PMC free article] [PubMed] [Google Scholar]
- 27.Amiri S, et al. NMDA receptors are involved in the antidepressant-like effects of capsaicin following amphetamine withdrawal in male mice. Neuroscience. 2016;329:122–133. doi: 10.1016/j.neuroscience.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Haj-Mirzaian A, et al. Attenuation of oxidative and nitrosative stress in cortical area associates with antidepressant-like effects of tropisetron in male mice following social isolation stress. Brain Res. Bull. 2016;124:150–163. doi: 10.1016/j.brainresbull.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 29.Amiri S, et al. Streptozotocin induced oxidative stress, innate immune system responses and behavioral abnormalities in male mice. Neuroscience. 2017;340:373–383. doi: 10.1016/j.neuroscience.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Xiao Z-Y, et al. The hippocampus is a critical site mediating antidepressant-like activity of apelin-13 in rats. Neuroscience. 2018;375:1–9. doi: 10.1016/j.neuroscience.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Omidi-Ardali H, et al. Inflammatory responses bridge comorbid cardiac disorder in experimental model of IBD induced by DSS: protective effect of the trigonelline. Inflammopharmacology. 2019;27:1265–1273. doi: 10.1007/s10787-019-00581-w. [DOI] [PubMed] [Google Scholar]
- 32.Fathi F, et al. Neuroprotective effect of pretreatment with Mentha longifolia L. extract on brain ischemia in the rat stroke model. Arch. Biol. Sci. 2015;67(4):1151–1163. doi: 10.2298/ABS150115091F. [DOI] [Google Scholar]
- 33.Rabiei Z, Gholami M, Rafieian-Kopaei M. Antidepressant effects of Mentha pulegium in mice. Bangladesh J. Pharmacol. 2016;11(3):711–715. doi: 10.3329/bjp.v11i3.27318. [DOI] [Google Scholar]
- 34.Andreadou I, et al. Acute doxorubicin cardiotoxicity is successfully treated with the phytochemical oleuropein through suppression of oxidative and nitrosative stress. J. Mol. Cell. Cardiol. 2007;42(3):549–558. doi: 10.1016/j.yjmcc.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Zafir A, Banu N. Modulation of in vivo oxidative status by exogenous corticosterone and restraint stress in rats. Stress. 2009;12(2):167–177. doi: 10.1080/10253890802234168. [DOI] [PubMed] [Google Scholar]
- 36.Freire RS, et al. Synthesis and antioxidant, anti-inflammatory and gastroprotector activities of anethole and related compounds. Bioorgan. Med. Chem. 2005;13(13):4353–4358. doi: 10.1016/j.bmc.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 37.Dang H, et al. Antidepressant effects of ginseng total saponins in the forced swimming test and chronic mild stress models of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2009;33(8):1417–1424. doi: 10.1016/j.pnpbp.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 38.Desbonnet L, et al. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170(4):1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Raman S, Asle-Rousta M, Rahnema M. Protective effect of fennel, and its major component trans-anethole against social isolation induced behavioral deficits in rats. Physiol. Int. 2020;107(1):30–39. doi: 10.1556/2060.2020.00012. [DOI] [PubMed] [Google Scholar]
- 40.Lorigooini Z, et al. Trigonelline through the attenuation of oxidative stress exerts antidepressant-and anxiolytic-like effects in a mouse model of maternal separation stress. Pharmacology. 2020;105(5–6):289–299. doi: 10.1159/000503728. [DOI] [PubMed] [Google Scholar]
- 41.Has ATC. The value of zizyphus mauritiana and myristica fragrans as a new drug discovery in the field of neuroscience. Orient. Neuron Nexus. 2012;3(1):14–18. [Google Scholar]
- 42.Aisa B, et al. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32(3):256–266. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 43.Farooq RK, et al. Is unpredictable chronic mild stress (UCMS) a reliable model to study depression-induced neuroinflammation? Behav. Brain Res. 2012;231(1):130–137. doi: 10.1016/j.bbr.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 44.Cavalcanti JM, et al. The essential oil of Croton zehntneri and trans-anethole improves cutaneous wound healing. J. Ethnopharmacol. 2012;144(2):240–247. doi: 10.1016/j.jep.2012.08.030. [DOI] [PubMed] [Google Scholar]
- 45.Ryu S, et al. Trans-anethole protects cortical neuronal cells against oxygen–glucose deprivation/reoxygenation. Neurol. Sci. 2014;35(10):1541–1547. doi: 10.1007/s10072-014-1791-8. [DOI] [PubMed] [Google Scholar]
- 46.Souza C, et al. Highly palatable diet consumption increases protein oxidation in rat frontal cortex and anxiety-like behavior. Life Sci. 2007;81(3):198–203. doi: 10.1016/j.lfs.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Al-Harbi M, et al. Influence of anethole treatment on the tumour induced by Ehrlich ascites carcinoma cells in paw of Swiss albino mice. Eur. J. Cancer Prevent. 1995;1995:307–318. doi: 10.1097/00008469-199508000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Chainy GB, et al. Anethole blocks both early and late cellular responses transduced by tumor necrosis factor: effect on NF-κB, AP-1, JNK, MAPKK and apoptosis. Oncogene. 2000;19(25):2943–2950. doi: 10.1038/sj.onc.1203614. [DOI] [PubMed] [Google Scholar]
- 49.Singh T, Goel RK. Adjuvant neuronal nitric oxide synthase inhibition for combined treatment of epilepsy and comorbid depression. Pharmacol. Rep. 2017;69(1):143–149. doi: 10.1016/j.pharep.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 50.Wang D, An SC, Zhang X. Prevention of chronic stress-induced depression-like behavior by inducible nitric oxide inhibitor. Neurosci. Lett. 2008;433(1):59–64. doi: 10.1016/j.neulet.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 51.Domiciano TP, et al. Inhibitory effect of anethole in nonimmune acute inflammation. Naunyn-Schmiedeberg's Arch. Pharmacol. 2013;386(4):331–338. doi: 10.1007/s00210-012-0820-5. [DOI] [PubMed] [Google Scholar]
- 52.Akçan R, Lale A, Tümer AR. Trans-anethole: A key compound in Bogma Raki. Acta Med. 2018;49(1):26. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
At the Medical Plants Research Center, Shahrekord University of Medical Sciences, data concerning the current study can be obtained. Correspondence and requests for materials should be addressed to Zahra Lorigooini.