Abstract
Adeno-associated virus (AAV) has emerged as a pivotal delivery tool in clinical gene therapy owing to its minimal pathogenicity and ability to establish long-term gene expression in different tissues. Recombinant AAV (rAAV) has been engineered for enhanced specificity and developed as a tool for treating various diseases. However, as rAAV is being more widely used as a therapy, the increased demand has created challenges for the existing manufacturing methods. Seven rAAV-based gene therapy products have received regulatory approval, but there continue to be concerns about safely using high-dose viral therapies in humans, including immune responses and adverse effects such as genotoxicity, hepatotoxicity, thrombotic microangiopathy, and neurotoxicity. In this review, we explore AAV biology with an emphasis on current vector engineering strategies and manufacturing technologies. We discuss how rAAVs are being employed in ongoing clinical trials for ocular, neurological, metabolic, hematological, neuromuscular, and cardiovascular diseases as well as cancers. We outline immune responses triggered by rAAV, address associated side effects, and discuss strategies to mitigate these reactions. We hope that discussing recent advancements and current challenges in the field will be a helpful guide for researchers and clinicians navigating the ever-evolving landscape of rAAV-based gene therapy.
Subject terms: Drug delivery, Molecular biology
Introduction
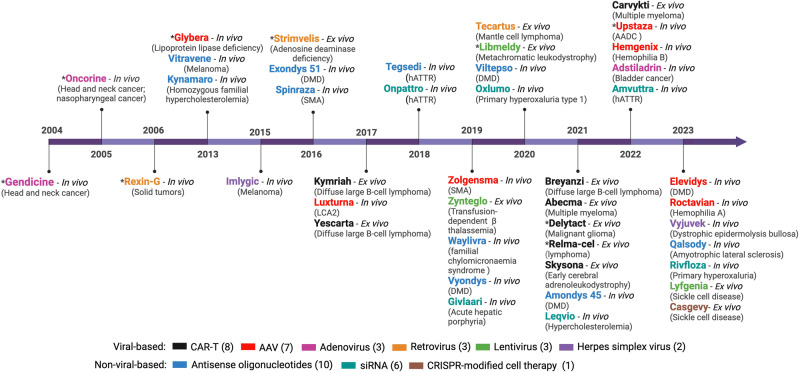
Gene therapy represents a groundbreaking approach for addressing genetic diseases, employing a range of strategies to modify gene expression within target cells using non-viral or viral vehicles.1 One common strategy is gene replacement therapy, where a functional copy of the faulty gene is introduced to living cells. A significant milestone for this strategy was reached in 2017 when the US Food and Drug Administration (FDA) approved the first gene therapy product, Luxturna, a gene replacement therapy for Leber congenital amaurosis type 2 (LCA2). Alternatively, gene silencing therapy aims to suppress or silence a target gene, mainly through RNA interference. For example, Patisiran, a small interfering RNA, is employed in the treatment of hereditary transthyretin amyloidosis.2 Another strategy is genome editing, which can be facilitated by clustered regularly interspaced short palindromic repeats (CRISPR)-based technologies to allow direct modifications of the somatic genome. Modified CRISPR systems that can perform RNA editing are also being developed. Moreover, gene expression may be altered epigenetically via DNA methylation, histone modification, and microRNA regulation. This method offers the advantages of being reversible and versatile, which allows it to be adaptable based on disease progression and responses to treatment. Innovative strategies such as leveraging suppressor tRNAs to enable readthrough of premature stop codons offer avenues to rescue pathologic nonsense mutations and restore gene function under endogenous regulation.3 Irrespective of the chosen strategy, gene therapy can be implemented either ex vivo or in vivo.4 Ex vivo gene therapy involves extracting patient cells, genetically modifying them, and reintroducing them back to the patients. In contrast, in vivo gene therapy directly delivers genetic materials to the target tissues. As of now, a total of 14 ex vivo and 29 in vivo gene therapies have obtained approval mainly through the FDA (Fig. 1). Of these approved gene therapies, 17 are non-viral-based, while 26 are viral-based. Various viral vectors have been studied for in vivo gene therapy, including adenovirus (Ad), retrovirus, lentivirus, and herpes simplex virus (HSV). Adeno-associated virus (AAV) vectors have emerged as the preferred choice in clinical trials and FDA-approved applications (Fig. 1). This is because of their broad tissue tropism, relatively good safety profile, and versatile manufacturing processes. Importantly, AAV is non-pathogenic, rarely integrates into the host genome, and can sustain long-term transgene expression. Moreover, vectors based on some AAV serotypes are inherently capable of efficient cellular entry and transgene expression, which enhances transduction efficacy.5
Fig. 1.
Approved gene therapy products and delivery platforms. Gene therapy products are generally developed for 1) Ex vivo gene therapy where affected patient cells are isolated, genetically modified in cell culture, expanded, enriched, and reinfused into patients to function as a living drug (e.g., CAR-T cells, Lyfgenia, Casgevy) and 2) In vivo gene therapy which is administered directly to patients to achieve therapeutic effects (e.g., Gendicine, Kynamaro, Imlygic, Luxturna, Onpattro). Delivery platforms for gene therapy drugs are primarily categorized into two groups: viral and non-viral-based. Viral-based gene therapy utilizes viruses as gene delivery vectors, including AAV, adenovirus, retrovirus, lentivirus, and herpes simplex virus. Non-viral-based gene therapy includes antisense oligonucleotides, siRNAs, and cell-based CRISPR genome editing. CAR-T chimeric antigen receptor (CAR) T cell therapy, SMA spinal muscular atrophy, AADC aromatic L-amino acid decarboxylase deficiency DMD Duchenne muscular dystrophy, LCA2 Leber congenital amaurosis type 2, hATTR hereditary transthyretin amyloidosis. * indicates non-FDA-approved gene therapy. Figure created with Biorender.com
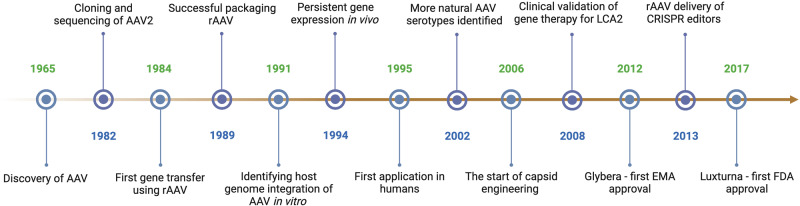
Originally discovered as a contaminant in Ad preparations in the mid-1960s,6,7 wild-type AAV (wtAAV) is a non-enveloped virus containing a single-stranded DNA (ssDNA) genome with inverted terminal repeats (ITRs) at both ends (Fig. 2). Studies of AAV biology led to the successful cloning and sequencing of the wtAAV2 genome.8–11 In the 1990s and 2000s, diverse AAV serotypes with distinct tissue tropisms were identified, thereby introducing avenues for targeted gene delivery approaches.12,13 Studies reported that the wtAAV genome can be integrated into the host chromosome, facilitating stable, long-term transgene expression.14,15 During the 2000s, work was done to engineer AAV capsids to enhance tissue specificity and transduction efficacy as well as to improve the safety of recombinant AAV (rAAV).16 This has advanced to successful rAAV-based clinical trials, particularly for monogenic rare diseases, resulting in regulatory approvals of rAAV gene therapy products.17,18 In parallel, various methods to scale up rAAV manufacturing have been developed, leading to growing interest in rAAV-based gene therapy. More recently, the advent of CRISPR technology has revolutionized the landscape of gene therapy, enabling precise gene editing delivered into cells via rAAV.19
Fig. 2.
Historical milestones in AAV biology research and gene therapy development. Decades of studying AAV biology have led to crucial advances in understanding its structure, biology, vectorology, and gene therapy applications. Historical milestones in AAV research and development are summarized chronologically. These advancements paved the way for successful clinical trials and regulatory approvals for rAAV-based gene therapeutics to treat various human diseases. Figure created with Biorender.com
rAAV-based gene therapy has emerged as a potential cure for once-untreatable genetic diseases owing to its distinctive attributes, including its small size, non-pathogenic nature, versatile tissue tropism, episomally durable transgene expression, replication incompetence, engineerable capsids, and adaptability for diverse payload delivery.16,20 Despite some early successes, numerous concerns and challenges have restrained its widespread applications, particularly for complex disorders. These include limited cargo capacity, immune responses to high systemic doses, potential genotoxicity, challenges in achieving tissue specificity, and manufacturing complexity.16,20 Here, we describe AAV biology and engineering that is the foundation of rAAV gene therapy, discuss key principles of AAV vectorology and the current manufacturing methodologies, and provide overviews on clinical applications and challenges of rAAV gene therapy for treating a wide range of human diseases. Finally, we identify gaps in our current understanding, propose potential solutions to ongoing challenges, and outline future directions in this rapidly advancing field.
AAV biology and vector engineering
AAV as a natural virus
Structure and genome
AAV has an icosahedral capsid composed of 60-mer subunits and a 4.7 kb ssDNA genome flanked by a 145 bp T-shaped ITR at each end. The capsid is assembled by viral proteins (VPs) VP1, VP2, and VP3 at a ratio of 1:1:10.21 VP3 is composed of conserved β-strands which are linked by surface-exposed variable loops. These structures shape the AAV surface morphology and determine AAV serotype-specific functions. The AAV capsid has a channel with a pore-like opening at each five-fold axis, protrusions surrounding the three-fold axis, and a depression at the two-fold axis.
The wtAAV genome contains two main open reading frames encoding four non-structural replication genes (Rep) and three structural capsid genes (Cap) along with an ORF for the assembly-activating protein, which is involved in capsid assembly.22 In addition, the function of membrane-associated accessory protein (MAAP), which is encoded on a different reading frame within the Cap gene, remains unclear.23 The ITRs largely serve as the viral origins of replication and provide the signal for packaging.24
Seroprevalence and non-pathogenic nature
Distinct serotypes of wtAAV are characterized by variations of VPs and ITRs and have been isolated primarily from humans and non-human primates (NHPs).12,13,25 Human seroprevalence studies have revealed that a significant proportion of populations possess neutralizing antibodies (NAbs) against multiple AAV serotypes. A study of 888 human serum samples from healthy volunteers found that NAbs against AAV2 were the most prevalent, followed by AAV1, AAV7, and AAV8.26 Another study showed that the most prevalent NAbs against serotypes other than AAV2 in serum from 552 human samples were AAV1 (74.9%), AAV6 (70.1%), AAV5 (63.9%), AAV8 (60.4%), and AAV9 (57.8%).27
As a Dependoparvovirus within the Parvoviridae family, wtAAV requires essential genes from a helper virus, such as Ad or HSV to facilitate the replication and transcription of its genome, including Ad E1, E2a, E4, and VA RNA genes.28,29 As a result, wtAAV cannot independently replicate and produce new viral particles but can establish a latent infection in host cells by persisting as episomal circular monomers or concatemers. Notably, in in vitro infections, approximately 0.1% of wtAAV2 genomes integrate into a specific region on the long arm of chromosome 19 (19q13-qter), termed the AAVS1 site.30 Although AAV is considered non-pathogenic, recent studies reported that AAV2 was connected with unexplained acute hepatitis in children worldwide.31–33 There is no direct evidence for the mechanism of how AAV2 triggers hepatitis, but it has been suggested that abnormal immune responses to AAV2 might mediate hepatoxicity.34 However, these studies acknowledged that COVID-19 infection may be a contributing factor and reported that most cases of acute hepatitis resolved without prolonged immunosuppression.
rAAV as a delivery vector for gene therapy
Vectorology and tissue tropism
Naturally existing wtAAVs are rapidly evolving, generating a vast genomic diversity classified as viral “clades”. In the past two decades, at least 12 AAV serotypes and over 1000 variants have been identified from Ad stocks, human/NHP tissues, and other mammals or non-mammalian species.5,12,13,25 These serotypes have distinct preferences for various cells or tissues, which is known as tropism (Table 1).35–39 Genomic differences among AAV serotypes are primarily found in the variable regions of the virus capsid sequence, particularly VP3, which play a crucial role in determining the tropism. However, many other processes and interactions with host proteins may affect the tropism, including cell surface receptors, cellular intake, intracellular trafficking, nuclear import, viral uncoating, second-strand DNA synthesis, and genome circularization and concatemerization.
Table 1.
Summary of natural AAV receptors and tissue specificity in humans
| AAV serotype | Origin | Receptor for cellular attachment | Receptors for post-attachment | Tissue tropism in human | |
|---|---|---|---|---|---|
| Primary receptors | Co-receptors | ||||
| AAV1 | NHP | N-linked sialic acid | Unknown | AAVR, GPR108 | Skeletal muscle, CNS, airway, retina, pancreas |
| AAV2 | Human | HSPG | FGFR1, HGFR, LamR, CD9, Tetraspanin | AAVR, GPR108 | Retina, CNS |
| AAV3 | Human | HSPG | FGFR1, HGFR, LamR | AAVR, GPR108 | Liver |
| AAV4 | NHP | O-linked sialic acid | Unknown | GPR108 | Lung |
| AAV5 | Human | N-linked sialic acid | PDGFR | AAVR | Retina, CNS, kidney, pancreas, liver |
| AAV6 | Human | N-linked sialic acid | EGFR | AAVR, GPR108 | Airway, CNS |
| AAV7 | NHP | Unknown | Unknown | Unknown | Liver |
| AAV8 | NHP | Unknown | LamR | AAVR, GPR108 | Liver, CNS, retina |
| AAV9 | Human | Galactose | LamR | AAVR, GPR108 | Heart, CNS |
| rh8 | NHP | Unknown | Unknown | Unknown | CNS |
| rh10 | NHP | Unknown | Unknown | Unknown | CNS, skeletal muscle |
| rh74 | NHP | Unknown | Unknown | Unknown | Skeletal muscle |
CNS central nervous system, FGFR1 fibroblast growth factor receptor 1, HGFR hepatocyte growth factor receptor, PDGFR platelet-derived growth factor receptor, EGFR epidermal growth factor receptor, LamR 37/67 kDa laminin receptor, AAVR adeno-associated virus receptor, GPR108 G protein-coupled receptor 108
The wtAAV2 genome was initially cloned in the 1980s,8,9,11 serving as a template for rAAV. rAAV possesses an identical capsid sequence and structure to that of wtAAV, but lacks any wtAAV protein-coding sequences, instead incorporating therapeutic gene expression cassettes that are within 4.7 kb packaging capacity. The only viral elements in the rAAV genome are the ITRs.16 rAAV is primarily produced by trans-complementing Rep/Cap and Ad helper genes in transiently transfected HEK293 cells. rAAV genomes with AAV2 ITRs can be trans-encapsidated with capsids of different AAV serotypes to alter their transduction properties.
Transduction pathways of rAAVs
Despite an improved understanding of wtAAV biology, the mechanisms by which rAAV interacts with cellular surfaces and delivers transgenes into the nuclei of host cells remain poorly understood. A growing body of research suggests that the virus capsid initially adheres to the cell surface through primary receptors such as glycans, glycoconjugates, or sialic acid, followed by interactions with co-receptor proteins.40 For example, rAAV2 binds to heparan sulphate proteoglycan (HSPG), rAAV1, rAAV4, and rAAV5 primarily interact with sialic acid, and rAAV9 interacts with N-linked galactose.41,42 The distinct binding sites on the capsid are thought to determine its tropism, therefore efforts are being made around these sites to engineer AAV variants with enhanced transduction capabilities for specific cells or tissues.20 Recent genome-wide screening studies identified novel host proteins facilitating rAAV transduction, including the type I transmembrane protein KIAA0319L, which has been designated as the AAV receptor (AAVR).43 Another universal host protein, G protein-coupled receptor 108 (GPR108), plays a role in the transduction of several rAAV serotypes.39 Although knocking out AAVR and GPR108 in vivo reduces transduction, cell-surface binding is largely unaffected,39,44 suggesting that these host proteins primarily contribute to the transduction process post-attachment.
After binding to the cell surface, rAAV is internalized via endocytosis using different mechanisms, which can vary by cell types and rAAV serotypes.40,45,46 rAAV particles inside endosomes are subjected to pH-dependent conformational changes and then trafficked through the trans-Golgi network.47,48 The rAAV particles escape from the endosomes and trans-Golgi network and then enter the nucleus through the nuclear pore complex.49,50 Once the viral particles are inside the nucleus, the ssDNA genome is released and converted into double-stranded DNA (dsDNA) via a process known as second-strand synthesis. Transcription is then initiated from the self-primed ITR at the 3′ end of the genome.51 The genome can be made into a dsDNA structure by mutating an internal ITR motif, which allows for faster replication and enhanced transduction compared to the single-stranded rAAV (ssAAV) genome.52 However, this self-complementary AAV (scAAV) genome design reduces the packaging capacity by half.53,54 The dsDNA genome subsequently undergoes circularization and concatemerization, stabilizing the vector genome for episomal persistence in postmitotic cells.16 Notably, the ITR sequence can also serve as a recombinogenic element to facilitate vector genome recombination.16 The rAAV transduction pathway involves multiple cellular events and may fail or be destroyed by the host at any step, weakening or preventing transduction. Thus, a full understanding of this pathway will help identify additional key host factors affecting the transduction efficacy of rAAV.
rAAV engineering
Both the rAAV capsid sequence and the genomic DNA cargo, including promoters, transgenes, enhancers, and ITRs, are under intense investigation. This section introduces several engineering strategies to modify rAAV for better transduction efficiency and tissue specificity.
Capsid engineering for transgene delivery
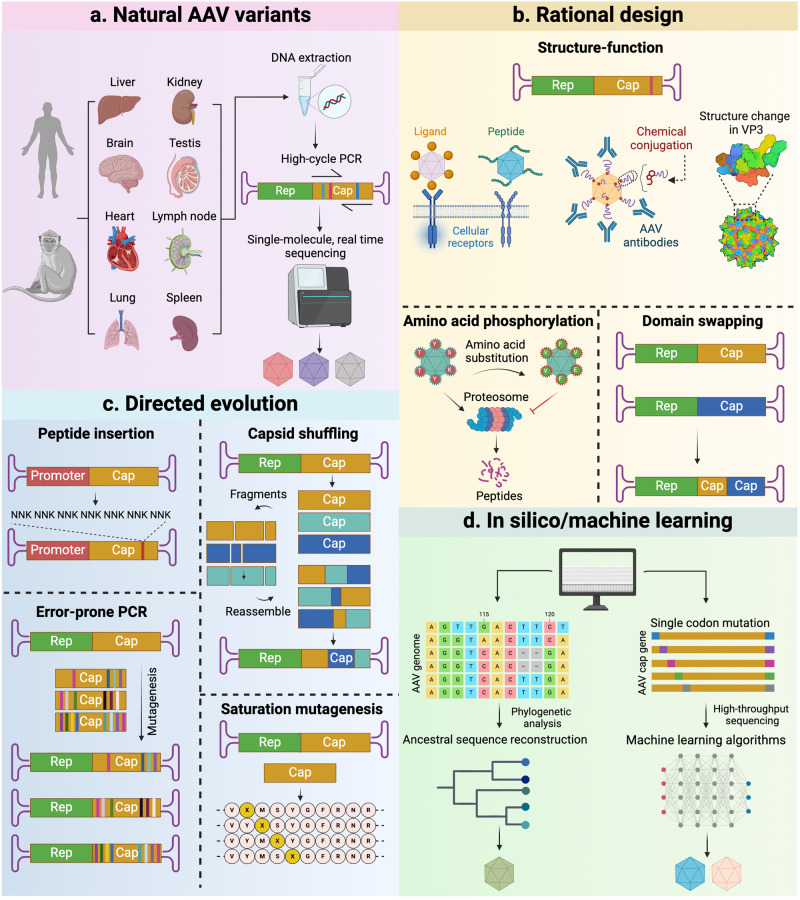
Capsid engineering is a principal strategy for developing rAAV tailored for different clinical applications. It mainly consists of three approaches: isolation of naturally occurring AAV variants, rational design, and directed evolution (Fig. 3). Recently, computer-based approaches using bioinformatic prediction and machine learning (ML) have emerged as novel tools to assist capsid engineering23,55 (Fig. 3).
Fig. 3.
Strategies applied for the development of novel AAV variants. a Natural occurring AAV variants can be isolated from human and NHP tissues using high-cycle PCR and high-throughput sequencing. b Rational design utilizes knowledge of AAV biology to modify the relevant amino acids in AAV capsid to enhance the transduction capability or evade immune surveillance. c Directed evolution is an engineering approach to develop novel AAV variants with designated specificity, including random or defined peptide insertion, capsid shuffling, error-prone PCR, and saturation mutagenesis. d In silico approach utilizes known capsid sequences to reconstruct ancestral AAV sequences. Machine learning is being used for predicting the relationship between specific sequences in the AAV genome with packaging capabilities and tissue tropism by using large datasets of samples transduced with AAVs whose genome had been mutagenized. Figure created with Biorender.com
Naturally occurring AAV variants
Early in AAV development, human tissues were crucial sources for discovering new AAVs. From 1965 to 2004, only a limited number of AAVs were derived primarily from human clinical samples, which were subsequently vectorized and subjected to testing.5 AAV9 was first identified in human liver tissue, yet it has strong brain tropism due to its ability to cross the blood-brain barrier (BBB), making it a common choice for central nervous system (CNS) transduction.13 AAV7 and AAV8 were isolated from the heart and lymph nodes of rhesus monkeys and demonstrated superb tropism in skeletal muscle and liver.12
Studies indicate that 40–80% of people have antibodies against known wtAAV serotypes, highlighting the necessity of discovering more sero-distinctive AAVs.26,56 Screening for AAV serotypes in species other than humans and NHPs has been done to find capsids that can avoid the neutralizing effects of encountering pre-existing antibodies against human AAVs,57–60 but these may have limited transduction in humans. Thus, discovering human-derived AAV variants and screening for candidates with enhanced transduction and specific tissue tropism has been a common approach. AAVv66, an AAV2 variant isolated from a human sample by long-read sequencing, exhibits enhanced production and CNS transduction compared to AAV2.61 These findings indicate that natural variants can provide valuable tools for improved tissue-specific transduction. While only a few groups continue to evaluate natural capsid variants as potential vectors for gene therapy, rAAVs derived from natural AAVs remain the dominant choice in current clinical studies.
Rational design
Rational design approaches attempt to make structural modifications in specific sites on rAAV capsids based on the understanding of rAAV structure and biology. Three key approaches have generally been employed: genetic mutation, insertion of nonviral domains to modify tissue affinity, and chemical modification.
Studies exploring the contributions of specific amino acid residues on rAAV transduction efficacy revealed that phosphorylation of surface tyrosine residues on rAAV2 capsids led to their degradation before nuclear entry.62–65 Modifying these residues to phenylalanine (Y444F/Y500F/Y730F) increased gene delivery in the CNS.66 While rAAV2 is not able to transduce photoreceptors through intravitreal injection, a variant containing substitutions of specific residues (Y272F/Y444F/Y500F/Y730F/T491V) achieved transduction of up to 25% of photoreceptor cells. Moreover, altering surface-exposed residues may enable tissue detargeting, thus improving the safety profile. For example, introducing H527Y and R533S substitutions, which were identified in samples from chimpanzee tissues, into the rAAV9 capsid resulted in reduced transduction in peripheral tissues after intravascular injection into both neonatal mice and a mouse model of a fatal pediatric leukodystrophy.67
Another rational design approach is to introduce functional domains into specific sites of the rAAV capsid. A study inserted a 15-mer binding domain of the human luteinizing hormone receptor (LH-R), resulting in the successful transduction in ovarian cancer cells in an HSPG-independent manner via the LH-R.68 By inserting cell-penetrating peptides into the rAAV9 capsid, a study identified two variants that were able to cross the BBB with improved CNS transduction after systemic delivery.69 The host immune response is a major barrier to rAAV being able to induce effective and long-term transgene expression. Structural studies have demonstrated that the NAbs recognition sites on rAAV capsids might be conserved.70 Engineered rAAV variants were able to evade NAbs in mice, NHPs, and human sera as determined by structural information from cryogenic electron microscopy (cryo-EM) images of rAAV1 capsids complexed with three murine monoclonal antibodies.70 Conjugating the rAAV capsid with biotin-polyethylene glycol (PEG)71 and N-acetylgalactosamine (GalNAc)72 may help evade NAbs, although this is potentially at the cost of altered tissue tropism and reduced transduction efficiency. One study used domain swapping to generate 27 chimeric Cap from rAAV2 and rAAV8 and demonstrated that the liver tropism achieved by rAAV8 was associated with the loop IV domain.73 Additionally, inserting arginine-glycine-aspartic acid (RGD) integrin binding motifs in AAVrh10’s variable region VIII improved cardiac-specific transduction and reduced liver distribution.74
Chemical modification without altering the amino acid composition of the rAAV capsid offers a promising approach in capsid engineering. A minor modification to the surface amino acids can alter the receptor binding affinity to affect transduction and tropism. A study used PEG-N-hydroxysuccinimide with an electrophilic succinimidyl propionic acid functional group to cross-link with lysine residues on rAAV2 capsid to enable NAb evasion.75 Glycated rAAV2 was found to have reduced binding to heparin and monoclonal antibody A20, resulting in remarkably improved transgene expression in muscles.76 One study covalently conjugated antibodies against skeletal muscle-specific protein CACNG1 to a variable region of rAAV9 capsid, leading to engineered capsids that can specifically express transgene in mouse myotubes with reduced liver targeting compared to unconjugated rAAV9.77 Likewise, another study engineered a hybrid capsid of rAAV9 and rAAVrh74 that can specifically bind to the skeletal muscle-enriched receptor integrin alpha V beta (AVB6). One resulting variant, LICA1, had significantly enhanced infectivity in human myotubes and the skeletal muscle of a mouse model of Duchenne Muscular Dystrophy (DMD).78 Alternatively, chemical modification of the capsid can be achieved through genetic code expansion, a versatile platform to incorporate non-canonical amino acids (ncAAs) with desired properties into proteins at a specific site in a gene of interest.79 A recent study using this approach to engineer a novel class of rAAV, termed Nε-AAVs, by inserting single ncAAs via engineered orthogonal prokaryotic tRNA/tRNA synthases.80 These mutant Nε-AAVs were successfully conjugated with functional molecules. In vivo studies demonstrated that the functional molecule conjugated to Nε-AAVs resulted in highly specific uptake in the target cells in xenograft animal models. Another study also showed that the incorporation of a ncAA at D374 in the AAV5 capsid led to enhanced transduction specifically in mouse lungs.81 Despite these advances, rational design approaches are limited by our incomplete understanding of rAAV structure and biology.
Directed evolution
Directed evolution is a form of selective pressure to isolate capsid variants with prevailing properties, such as increased rAAV yield, enhanced transduction, immune response evasion, or specific cell/tissue tropism.16 One directed evolution strategy involves inducing random, unbiased mutations and subjecting capsids to selective pressure for viral fitness. This has been implemented by using error-prone PCR followed by a staggered extension process to generate a library of Cap mutants of VP1 – 3 to identify variants with altered affinities for heparin for immune escaping.82 In addition, a DNA shuffling-based approach has been utilized to generate diverse and extensive libraries of random chimeras by combining capsids from various parent AAV serotypes. This technique has led to the creation of new variants that demonstrate a wide range of cell tropism both in vitro and in vivo.83,84 Saturation mutagenesis of different antigenic footprints were also exploited to engineer an AAV8-derived capsid to evade NAbs and improve liver tropism.85
Surface panning of random peptides on an AAV capsid is another approach for directed evolution. For instance, a Cre recombination-based AAV targeted evolution (CREATE) system allows for attaching short-length peptides to rAAV9 capsid to engineer its properties.86 CREATE was used to generate AAV-PHP.B, an AAV9 variant that can cross the BBB to transduce the CNS in C57BL/6 mice.86 However, follow-up studies demonstrated that the properties of PHP.B capsid did not translate to other mouse strains or NHPs, suggesting a complex interplay between the engineered capsid and host factors which can differ between species.87,88
Subsequently, a novel rAAV evolution platform termed TRACER (tropism redirection of AAV by cell-type-specific expression of RNA) was developed. This platform is based on the recovery of viral RNAs expressed from a bulk capsid library carrying random peptide insertions in a cell-type-specific manner from animal tissue.89 A study in mice with peptide libraries displayed on rAAV9 capsids identified ten leading variants with up to 400-fold higher brain transduction over the parental rAAV9 following systemic administration. The same group recently reported that VCAP-102, a TRACER-engineered rAAV9 variant, displayed 20–90-fold increased transduction in diverse brain regions of NHPs compared to rAAV9.90 Iterative evolution of VCAP-102 led to capsid variants with 6–7-fold further improved BBB penetration. Similarly, TRACER-developed VCAP-100 derivatives showed up to 300-fold liver detargeting and six-fold higher brain transduction in NHPs over its parental AAV5.91
Directed evolution of rAAV capsids leveraging in vivo expression of transgene RNA (DELIVER) is another landmark method, which has led to the identification of highly functional muscle-tropic capsids in mice and NHPs.92 Cross-comparison of muscle-tropic variant capsid sequences in mice and NHPs identified a common RGD motif. Further analysis suggested that RGD-binding integrin heterodimers expressed in the target cells have a strong interaction with the viral variants containing the RGD motif. Researchers recently generated an RGD-embedded 7-mer peptide library inserted in variable region VIII of the capsid and identified variants with more than 20-fold enhancement in skeletal muscle transduction compared to the benchmark capsid.93
Directed evolution is a powerful tool to generate and identify variants with specific properties, but these properties are not always cross-species translatable in primates. For example, AAV-PHP.B showed enhanced CNS transduction in mice but not marmosets compared to AAV9, suggesting the necessity for cross-species evolution in capsid engineering.94 Nevertheless, a study sequentially evolving an rAAV capsid library across multiple species identified rAAV.cc47 as a potent cross-species variant with enhanced transduction capabilities over rAAV9 in the NHP brain and heart.95 7m8 is an AAV2 variant with a 7-mer insertion to strongly transduce the outer retinal layers such as photoreceptor and retinal pigment epithelium (RPE) cells through intravitreal injection mainly in mice.96 A phase II clinical trial (NCT04418427) using ADVM-022 (AAV.7m8-aflibercept) for treating diabetic macular edema (DME) was suspended due to severe adverse effects in several patients 16–36 weeks after receiving high doses (6.0 × 1011 vector genomes (vgs)/eye), including refractory intraocular pressure reductions, underscoring the translational challenges of engineered capsid in the field.
In silico- or ML-based design
Computer-assisted rAAV engineering represents a cutting-edge approach that utilizes computational tools to enhance the design and optimization of rAAV. Studies have explored rAAV’s diverse properties from its evolutionary lineage and used reconstruction of the predicted ancestral genome to create novel capsid variants.55,97 Two similar studies computationally established the ancestral capsid library by rational design55 or adopting directed evolution.97 Both studies produced capsid variants with increased thermostability, an indicator of serotype identity, and promising biological properties desired for clinical application. Among these variants, Anc80L65,55 emerged as a promising vector for gene therapy for diseases associated with the inner ear98,99 or the eye.100
ML is extensively used in biomedical research, including medical image analysis, genetics, and drug discovery.101,102 A pioneering study applied ML to understand the rAAV2 capsid.23 They scrutinized the capsid’s fitness landscape, characterizing single-codon substitutions, and employing ML to design precise multi-mutated variants based on their impact on target tissues. Furthermore, deep learning has been used to diversify rAAV2 capsid variants, accurately predicting their viability.103 This approach reveals new possibilities for engineering the rAAV genome beyond the capsid, which has promising innovative applications in future rAAV development.
Engineering the cis-regulatory components of rAAV genome for transgene expression
The rAAV genome consists of ITRs and a transgene expression cassette consisting of the gene of interest and regulatory components. Strategies to engineer the rAAV genome include modifying ITRs or introns as well as inserting tissue-specific promoters for transcriptional regulation and/or using an inducible expression system, codon optimization, and CpG motif reduction of the transgene cDNA, and microRNA (miRNA)-mediated post-transcriptional regulated retargeting.104–107 Each of these components has its own functions, and their individual and collective actions determine transgene expression, cell-type specificity, safety, and durability of rAAV transduction.
Engineering rAAV ITRs
Traditional rAAV carries a single-stranded vector genome. Its transduction efficiency and rate depend on the second-strand synthesis of vector DNA before mRNA transcription occurs, which is the rate-limiting step. To increase transduction efficiency, strategies have been employed to mutate one of the ITRs, generating scAAV with a double-stranded vector genome.53,54 This allows scAAV to bypass the rate-limiting step of dsDNA synthesis, resulting in more rapid and efficient transduction of target cells.20,108–110 However, using scAAV has notable limitations: smaller packaging capacity (2.5 kb in scAAV vs 4.7 kb in ssAAV) and elevated risk of immune responses due to rapid accumulation of transgene products.111,112
The ITRs can be altered to selectively limit encapsidation to either the positive or negative strand of the vector genome.113 This is accomplished by selectively removing the D sequence from one of the two flanking ITRs. ITRs also harbor CpG motifs, which can signal to toll-like receptor (TLR)−9 and induce inflammatory responses. Complete removal of CpG from ITRs did not adversely affect vector genome copy number or transgene expression in treated animals. However, it resulted in a reduced rAAV titer, and whether it diminished inflammatory responses against rAAV remains unclear.114 Overall, ITRs are naturally prone to mutations and can be heterogeneous due to their palindromic nature, high GC content, and secondary structure. Optimizing the GC content in the ITRs could be a potential engineering strategy, ultimately improving rAAV properties.
Optimizing the promoters
Promoters in transgene expression cassettes are selected and optimized for specific needs such as tissue specificity, and expression level. The cytomegalovirus (CMV) or chicken beta-action (CBA) promoters are commonly used because they offer strong and ubiquitous expression, while tissue-specific promoters such as CK8 or MHCK7 provide targeted gene expression in muscle.92 The specificity of transgene expression within targeted tissues is crucial in order to achieve sufficient transduction efficiency with low dosing and minimize off-target effects. For example, a study employed a scAA9 vector carrying an endogenous human survival motor neuron 1 (SMN1) promoter to drive SMN1 expression specifically in neurons.115 This approach demonstrated a remarkable safety profile, notably decreasing liver toxicity, and enhanced therapeutic efficacy in the SMNdelta7 mice with spinal muscular atrophy (SMA). The outcome surpassed the performance of Zolgensma, an FDA-approved treatment utilizing the CMV/CBA promoter, thus underscoring the advantages of tissue-specific promoters. Additional regulatory elements such as upstream enhancers can be engineered to improve expression activity and specificity. Numerous enhancers have been designed in clinical gene therapy vectors with the goal of increasing transgene expression, thereby reducing the viral load.116 Another study reported that a fragment of the mouse methyl-CpG-binding protein-2 promoter enabled robust long-term expression specifically in neurons.117 ML-based multi-omics data allows to design of tissue-specific promoters, resulting in an over 1000-fold dynamic increase in transgene activity between on- and off-target tissues.118
Transgene expression regulation
Transcriptional regulation: The expression of the gene or protein of interest can be up or down-regulated to optimize and fine-tune the desired effect. The tetracycline-inducible system is a widely used strategy to achieve a specific level of expression, although it is unlikely to be translated to humans due to potential immunogenicity issues.119 Alternatively, a system was developed to use the immune suppressive drug rapamycin to activate responsive transcription factors delivered by two separate rAAVs.120 This strategy was subsequently optimized so that only a single rAAV is now required. This has been used to achieve inducible dose-response expression to rapamycin treatment for long-term expression of the desired transgene.121 Importantly, there was only minimal transgene expression without the administration of rapamycin, indicating that this could potentially provide a specific and safe regulatory system.
Post-transcriptional regulation: Another strategy is the use of riboswitches, which are non-coding RNAs that can bind specific metabolites and control gene expression.122 Riboswitches occur naturally, and the principles of ligand-mediated gene regulation are being exploited by engineering aptazymes for mammalian systems with improved regulatory ranges. Using riboswitches over other regulatory systems has the advantages of being compatible with FDA-approved drugs, the small size of the RNA, and little or no immunogenicity. Importantly, riboswitches have very short sequences, which is particularly important when combined with the limited packaging capacity of rAAVs, making it a powerful system for regulating rAAV gene therapy.123,124 Non-coding RNAs are highly versatile tools in transgene regulation, which is further highlighted by incorporating RNA aptamers with cleavable poly A signal (PAS) into the 5′-untranslated region (UTR) of the transgene. In the absence of a small molecule, PAS cleavage leads to mRNA degradation to silence transgene expression. The addition of the small molecule leads to transgene expression through maintaining the integrity of the mRNA, which can be combined with alternative splicing to more precisely control gene expression.125
Some regulatory systems require either exogenous addition or co-expression of certain elements to work correctly. The use of drug-inducible splicing as a mechanism of gene expression regulation has been pursued to mitigate this problem. This approach entails the delivery of a gene containing a premature stop codon to prevent the translation of the protein. The addition of a small molecule splicing inducer facilitates the inclusion of a specifically designed exon into the mature transcript and the exclusion of the stop codon, thereby enabling the translation of the protein of interest.126 The strength of the promoter and dose of the inducer molecule allows for the expression level of the target gene to be precisely activated. However, current technologies rely on intrinsic protein turnover mechanisms to reduce levels of the target protein.
Post-translational regulation: One strategy to modulate the half-life of a protein of interest is the use of a protein degrading system (degron). This allows for a reversible and rapid response to protein removal and recovery. Several of these systems have been established in vitro, but attempts to translate this to in vivo systems have found high levels of toxicity. However, optimizations have generated newer, promising systems for in vivo applications, such as incorporating a SMAsh tag on the protein of interest. A small molecule drug that has already been approved for human use is then introduced to prevent the detachment of the SMAsh tag, which leads to protein degradation.127 Combining inducible expression systems, alternative splicing strategies, and protein degradation approaches has the potential to allow for exquisite control of a target protein’s expression level.
Optimizing the transgene (post-transcriptional level)
One common method in expression cassette engineering involves codon optimization by using computer algorithms to identify rare or suboptimal codons in the transgene to be replaced with preferred ones. Studies have demonstrated significantly higher expression levels in codon-optimized transgenes such as FVIII and the human cystic fibrosis transmembrane conductance regulator (CFTR) in mouse models.128–131 Moreover, a codon-optimized transgene of aspartoacylase (ASPA), the causative gene of Canavan disease, remarkedly restored ASPA protein expression and rescued the lethal disease phenotype in a mouse model.132 While codon optimization can be an effective strategy for improving the expression of the transgene delivered by rAAV, such effects can vary depending on the specific transgenes and the host organism. Codon optimization methods might introduce unexpected immunogenicity and toxicity as well as impair other traits such as tropism. Indeed, a study found that codon-optimization introduced a large quantity of CpG motifs into a rAAV8-based FIX Padua gene therapy for patients with hemophilia B, which could lead to innate immune responses.133 A novel recurrent neural network-based tool was developed to optimize codon usage for specific cell types using data from mouse myocytes, neurons, and hepatocytes. This approach improved protein expression and reduced CpG dinucleotides, holding promise for enhancing tissue-specific gene therapy efficiency.134
Modifying other cis-regulatory elements
Other cis-regulatory elements can be modified in the expression cassette to adjust gene expression and specificity. For example, the woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) is a ~600 bp RNA element that is commonly added downstream of the transgene. The WPRE can enhance transgene expression both in vitro and in vivo by increasing the amount of mRNA transcribed independent of the transgene.135,136 Studies have cautiously reported potential oncogenic activity of the WPRE incorporated in lentivirus vectors delivered to mice.137,138 However, such risk can be reduced by removing the oncogenic sequence from the original WPRE.137
The inclusion of an intron in a transgene expression cassette can also improve gene expression in animals.139 This was supported by another study which showed that an intron increased transgene expression by 40–100-fold in vivo.140 However, the effects of introns on gene expression can be complex and depend on a variety of factors, such as the size and location of introns and the specific target cells. The selection of a proper polyA signal is important for optimizing transgene expression and stability (e.g., beta-globin, SV40, or bovine growth hormone (BGH)). A comparison study found that a modified shorter version of SV40 late polyA (135 bp) showed comparable transgene expression to a BGH polyA (223 bp) in a mouse brain.141 In addition, the inclusion of a Kozak sequence can further enhance the transgene expression.132
Ensuring confined transgene expression to target tissues is vital to avoid toxic overexpression and immunotoxicity. rAAV design incorporates cell-specific promoters as a primary strategy for targeted expression, and fine-tuning strategies are then developed such as adding miRNA binding sites in the 3′-UTR to inhibit expression in cells expressing the complementary miRNA. For example, adding miR-122 binding sites in rAAV9 was reported to enable CNS expression while retargeting liver, heart, and skeletal muscle.104 When rAAV carries a foreign transgene or one encoding a replacement gene in patients who have null mutations in the endogenous copy, the transgene product may be regarded as a non-self-antigen by antigen-presenting cells (APCs), which then process the transgene for major histocompatibility complex (MHC) presentation and immune clearance. One strategy to mitigate transgene immunity is to incorporate APC-specific miRNA binding sites, such as miR-142 and miR-652, into the expression cassette of rAAV to detarget transgene expression from APCs.142,143 The results showed a decrease in transgene-specific immune responses and sustained transgene expression in targeted cells in mice. Such a strategy has been translated in NHPs, in which broadly NAbs against human immunodeficiency virus were durably expressed after delivery by rAAV, thus highlighting this strategy’s potential for clinical translation.144
Expanding rAAV packaging capacity
Another significant challenge in rAAV application is the delivery of transgenes that surpass its 4.7 kb packaging capacity. Examples include therapeutic transgenes like centrosomal protein (CEP290) for Leber’s congenital amaurosis type 10 (~7.5 kb),145 or CRISPR-based tools like cytosine base editor or prime editor 2 (>5 kb).146,147 Various strategies have emerged to overcome this hurdle by dividing a large transgene into two parts, with each encapsulated in an rAAV capsid. Co-introduction of these segmented transgenes into the same cell results in the reconstitution of the full-length transgene and protein. This reconstitution can occur at different stages within the genetic information flow.19 At the DNA level, inter-vector DNA recombination is facilitated by rAAV ITRs, a partial transgene sequence, or an optimized recombinogenic sequence present in both rAAV genomes.148–150 The overlapping sequence can be excised post-transcription through specifically designed splicing signals, resulting in a mature full-length mRNA that is subsequently translated into the desired protein. At the RNA level, trans-splicing between two transcripts from individual vectors, mediated by the splicing donor and acceptor present in each transcript, generates a full-length transcript and protein.151–153 At the protein level, protein reconstitution has been achieved through split inteins, the natural polypeptides capable of excising itself and ligating the nearby protein segments.154–160
rAAV manufacturing
Methodologies
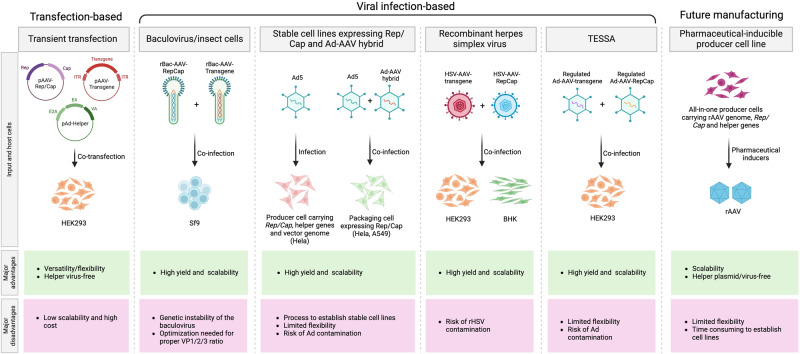
The clinical applications of rAAV highlight the urgent demand for production and purification systems capable of producing substantial quantities and high-quality rAAVs to meet safety, efficacy, stability, and cost requirements (1.0 × 1015–1.0 × 1016 vgs per treatment).161 Because rAAV is unable to self-amplify by reinfection, its production requires the simultaneous expression of helper viral genes and rAAV-specific Rep and Cap genes for replication and packaging of the ITR-flanked vector genomes. Methods for rAAV production have been developed primarily with two strategies: transient transfection and viral infection. For transfection-based rAAV production, plasmid co-transfection into HEK293 cells is the most widely used production system, which was pioneered by Xiao et al. 29 rAAV production through viral infection involves the use of three different viruses, including AAV’s natural helper viruses, Ad and HSV, and baculovirus are used. rAAV production also involves either mammalian (e.g., HeLa, A549, and HEK293) or insect cells (e.g., Spodoptera frugiperda, Sf9) (Fig. 4).5 A newly developed tetracycline-enabled self-silencing Ad (TESSA) represents another infection-based system for large-scale, high-yield rAAV production in HEK293 cells.162 Each of these methods has its unique advantages and drawbacks in terms of flexibility, quality, and scalability, which will be discussed in more detail below. To purify rAAV produced by various methods, gradient sedimentation using either cesium chloride or iodixanol gradient ultracentrifugation is a commonly employed generic procedure for small-scale rAAV preparations.163–165 For large-scale vector production, and particularly for clinic-grade vectors that demand higher quality and quantity, affinity- and/or ion-exchange-based column chromatography is required.166 Finally, the development of stable packaging and producer cell lines is being actively pursued as the preferred next-generation rAAV production platform due to their straightforward scalability for large-scale production at a lower cost.
Fig. 4.
Current approaches to manufacture rAAV. Currently, two main platforms are used for rAAV manufacturing: transfection- and viral infection-based approaches. Plasmid transient transfection of HEK293 cells remains the most widely used method, while stable cell lines, baculovirus (BV) systems, and the HSV type I system offer scalable alternatives for large-scale production. The transfection-free helper virus system TESSA has been developed to produce high-yield rAAVs. Pharmaceutically inducible all-in-one producer cell lines may represent the next generation’s optimal manufacturing platform for rAAV-based drugs. BHK baby hamster kidney. Figure created with Biorender.com
Transient transfection in HEK293 cells
Traditional rAAV production involves transfecting three plasmids carrying vector genome, AAV Rep/Cap genes, and Ad helper genes (VA RNA, E2A, and E4OEF6), typically at equimolar ratios, into HEK293 cells. Another essential Ad helper gene, E1a/b, is inherently expressed in HEK293 cells.167,168 This triple transfection protocol was subsequently optimized to a two-plasmid co-transfection system, involving one plasmid for the rAAV genome and another for Rep/Cap and Ad helper genes.169,170 Current methods can achieve approximately 80% cell transfection rate with peak virus yield at 48–72 h post-transfection.171
There are numerous challenges in viral vector manufacturing that lead to inconsistencies in quality, titers, and purity between batches,172 and the process is very labor-intensive.173 Recent research has focused on modifying the standard manufacturing process at the plasmid level to enhance both yield and purity with reduced cost. One notable recent study showed that low-cis triple transfection (1% or 10% cis plasmid) significantly reduced the transgene plasmid usage compared to the standard triple transfection (100% cis plasmid) and increased the in vivo transduction efficiency.174 Importantly, this method enables the packaging of yield-inhibiting transgene and reduces plasmid backbone contamination in both low-cis rAAV preparations and tissues of mice receiving this rAAV. Others developed a new all-in-one rAAV production system termed AAVone that combines Rep/Cap and Ad helper genes with the rAAV genome into a single plasmid. AAVone yields higher rAAV production compared to the triple transfection method, with lower batch-to-batch variations and reduced levels of replication-competent AAV (rcAAV). It also requires less plasmid DNA and eliminates the need for ratio optimization steps.175
The transient transfection approach presents flexibility by enabling the easy and rapid generation of rAAV with diverse transgenes and capsids. It can be scalable to an extent when using suspension cells, allowing for production to be increased simply by increasing the culture volume. In addition, the transient transfection method is flexible and versatile, and does not require time-consuming processes of establishing and maintaining stable producer cell lines for specific vector products. This facilitates a quicker turnaround time from vector design to production. Notably, this approach suffers from limited scalability and high cost.
Baculovirus production system
The first rAAV gene therapy product to reach the market (uniQure’s Glybera,176 alipogene tiparvovec; now withdrawn from the market) actually utilized the Baculovirus Expression Vector Systems (BEVS), in which baculoviruses encoding Rep and Cap genes and carrying rAAV genome infect insect cells, such as Sf9, to produce rAAV.177 This system was originally optimized for large-scale protein production, but significant advancements have been made to accommodate for rAAV production. The first-generation BEVS involved splitting the Rep gene into two cassettes controlled by different promoters and modifying the Cap gene to include a non-canonical start codon (ACG instead of AUG). These genetically altered AAV viral sequences were incorporated into three separate baculovirus constructs, necessitating simultaneous superinfection of all three in a single cell.177 However, this method was initially successful for rAAV1 production but much less productive for other rAAV serotypes. Second-generation BEVS was more adaptable for additional rAAV serotypes and reduced the number of baculoviruses to two, one carrying Rep/Cap genes (Bac-CapRep) and another carrying the rAAV genome (Bac-Transgene).178 Subsequently, an attenuated Kozak sequence and leaky ribosome scanning were introduced to BEVS to ensure proper AAV gene expression, resulting in rAAV with significantly improved biological potency.179
Recently, BEVS has been further improved so that only one recombinant baculovirus is required for rAAV production. An engineered Sf9 cell line was created by integrating Rep/Cap genes, which then only needs one infection by a baculovirus carrying an ITR-flanked transgene, resulting in higher rAAV yields compared to the conventional three-Bac system.180 Another advancement introduced a highly regulated Rep gene control system to stabilize and allow appropriate Rep expression, leading to the production of potent and high-yield rAAV particles.181
One of the drawbacks of BEVS is the genetic instability of baculovirus. Baculoviruses naturally remove parts of their genomes during replication, which generates mutant viruses, some of which can become defective interfering particles.182,183 During the scaling-up process in cell culture, these mutants may outcompete baculoviruses with intact genomes, potentially reducing rAAV production. However, the genetic instability of baculoviruses can be potentially improved by either deleting specific genes or inserting transgenes into stable loci within the baculovirus genome.184,185 Additionally, BEVS is inherently not capable of assembling capsid proteins into AAV virions with the VP1, VP2, and VP3 at a ratio of 1:1:10, causing a reduction of transduction potency for the resulting rAAVs. A study demonstrated that the incorporation of artificial introns within the rAAV sequence, such as those using the baculoviral polyhedrin promoter, yielded infectious rAAV with high titers across various serotypes, reaching up to 1.0 × 1014 vgs/L.186 Furthermore, a study systematically compared rAAV produced by BEVS (Sf9-rAAV) to that produced by transient transfection (HEK-rAAV) using large-scale suspension cultures and found that Sf9-rAAV may be favored over HEK293-rAAV for its superior yields, full/empty ratio, scalability, and cost-effectiveness.187 However, further studies into the genetics and biology of baculovirus are necessary in order to harness the advantages of the BEVS platform (Fig. 4), such as its high scalability.
Mammalian stable cell lines and Ad-AAV hybrid for rAAV production
Stable cell lines for vector production offer notable advantages, including easy product characterization, scalability, and the capacity to generate higher yields with improved reproducibility and reduced cost.188,189 There are two types of stable cell lines for Ad infection-based rAAV production: packaging and producer cell lines. Packaging cell lines have stably integrated AAV Rep/Cap genes. A major challenge for rAAV production using packaging cell lines is that the cells need to be first infected with AAV’s natural helper virus, wild-type Ad, to initiate endogenous Rep/Cap expression, followed by secondary infection with Ad-AAV hybrid virus from which rAAV genomes will be rescued, replicated, and packaged.190,191 In contrast, producer cell lines have Rep/Cap and Ad helper genes, as well as rAAV genome, stably integrated into the host genomes. In this case, infection by wild-type Ad will activate Rep/Cap gene expression, facilitating the rescue, replication, and packaging of rAAV genomes.192 Stable cell lines derived from A549 and HEK293 cells have been adapted for suspension cultures for scalable rAAV production.193–195
Several HeLa-based cell lines, such as C12, H44, and B50, yielded high titers of infectious and rcAAV-free rAAVs through endogenous expression of Rep/Cap. An A549-derived stable cell line expressing Rep/Cap and a K209 cell line has also been reported to produce high yields of infectious rAAV per cell.193,196
There are several drawbacks to stable cell lines and Ad infection-based rAAV production systems. First, creating stable cell lines is often a complex, time-consuming, serotype-, and vector genome-specific process. Second, ensuring the characterization and stability of these cell lines can be challenging, with potential risks related to the impact of passage history on growth kinetics. Additionally, to ensure the safety of the final gene therapy product, robust downstream purification procedures are essential to eliminate any pathogenic Ad contaminants and oncogenic HeLa DNA.195
Recombinant herpes simplex virus (rHSV) system for rAAV production
rHSV is another natural helper virus capable of providing helper functions for rAAV production.197,198 In order to produce rAAVs using an rHSV system, two distinct rHSVs carrying the AAV Rep/Cap genes and rAAV genome are created for sequential infections of HEK293 or baby hamster kidney cells, followed by downstream processing and purification. The first clinical trial using rAAV1 to treat α1-antitrypsin deficiency was produced using rHSV.199
The notable drawbacks of using the rHSV-based system for rAAV manufacturing include the generation of a sufficient quantity of rHSV particles and the specialized purification steps necessary to effectively eliminate neurotrophic and neurotoxic rHSVs and other associated contaminants from the final rAAV preparations.
Tetracycline-enabled self-silencing adenovirus (TESSA) system
Typical methods for rAAV production involve either helper-free plasmid transfection or the use of a helper virus. However, the helper-free system poses challenges when scaling up and the helper virus approach requires more robust downstream processing and purification to eliminate contaminating helper viruses and highly immunogenic VPs.195,200 A recent innovation introduced a helper Ad designed to inhibit its major late promoter (MLP), thereby restricting its replication and effectively overcoming these limitations.162 This was accomplished by introducing an inducible tetracycline repressor binding site into the MLP. This modified Ad functions normally when doxycycline is present but is restricted to genome amplification and early gene expression (known as the helper functions) without it. Through the utilization of this self-regulating Ad, researchers successfully facilitated the delivery of essential adenoviral helper functions, Rep/Cap genes, and the rAAV genome, resulting in a substantial enhancement in rAAV production with a nearly complete absence of contaminating Ad. Previously, numerous attempts to generate a genetically stable recombinant Ad expressing the AAV Rep gene in HEK293 cells have failed due to the cytotoxic and recombinogenic nature of Rep protein even at a very low level of leaky expression.201 However, the TESSA system tightly regulates the expression of the Rep gene, activating its expression only during rAAV genome rescue, replication, and packaging process, which is a major breakthrough. This system improved the production of various rAAV serotypes in both HEK293 and U87 cells, including rAAV2, rAAV6, rAAV8, and rAAV9.162 Moreover, this system can be scaled up to produce rAAV2 and rAAV6 with a significant improvement on vector yield over the transient transfection approach.202
Next-generation manufacturing platform technology
Selecting which production system to use involves balancing flexibility, scalability, and quality, all of which play a vital role in the development of a safe and effective rAAV product to meet the demand, safety, and efficacy requirements for clinical applications.
To further improve the transient transfection approach, it is crucial to optimize multiple factors during both the production and purification processes, aiming for increased yield and reduced empty rAAVs. Minimizing empty vectors enhances vector potency, which allows for a lower injection dose to be used, thereby reducing immunotoxicity. Ongoing efforts are underway to optimize the upstream processes of rAAV production to improve rAAV yield. For example, adjusting the plasmid ratio by reducing the cis plasmid to 1% or 10% can achieve a yield of rAAV that is comparable to conventional triple transfection. This approach enables the packaging of previously unpackageable transgenes while also reducing backbone plasmid contamination and is proving to be a cost-effective strategy.174
A mechanistic modeling study using triple transfection has identified several key bottlenecks in rAAV production, including inefficient plasmid delivery to the nucleus and poorly coordinated timing of capsid synthesis and viral genome replication leading to the empty capsid.203 Strategies to address these issues include ensuring that viral DNA replication occurs before or in parallel with capsid production, such as early expression of Rep protein or late expression of Cap protein.
Efforts have also been made to identify small-molecule chemical additives that can boost rAAV production.204–206 A library of over 130 small molecules has been assembled to transiently antagonize a broad range of cellular innate antiviral pathways, effectively increasing viral production at scale.205 Imidazole-based small molecules have been shown to improve large-scale rAAV production through multiple signaling pathways.206
Modifying host factors can also affect production. Through a genome-wide screening strategy, studies identified several gain- or loss-of-function targets that can significantly affect rAAV production.207,208 Indeed, a systemic analysis of the cellular transcriptional response to transient plasmid transfection revealed that host cells actively sense rAAV production as an infectious insult and upregulate inflammatory and antiviral responses.209
To reduce empty AAV particles, a study created a hybrid Rep gene from different AAV serotypes with the 3′ end of the AAV2 Rep gene, resulting in a 2–4-fold increase in full capsids for non-AAV2 serotypes, suggesting that enhancing Rep function may improve rAAV production.210
Efforts in optimizing downstream purification processes have also led to improvements in removing empty capsids. The industry has transitioned from ultracentrifugation to column-based chromatography for effective purification of rAAV particles from impurities in large-scale production.211 Anion-exchange chromatography has become a major focus in the field, with modifications to various process parameters, including pH, various elution salts, excipients, surfactants, stabilizers, and osmolytes to enhance purification efficiency.212
Future manufacturing approaches should prioritize maximum flexibility, scalability, and quality while eliminating the need for plasmid transfection, virus infection, and chemical inducibility. Numerous efforts have been directed toward creating a stable and inducible producer cell line with these essential characteristics. One notable study exemplifies this approach by integrating all components necessary for rAAV production into HEK293 cells, including the rAAV genome, Rep/Cap genes, and helper coding sequences sophistically controlled by multiple inducible promoters. The resulting synthetic cell line generates infectious rAAVs upon induction.213 This all-in-one producer cell line approach allows for independent control over replication and packaging activities, ensuring high-quality rAAV production. Subsequent work has further optimized this producer cell line to enhance productivity by reducing the overexpression of the gene of interest, optimizing induction profiles, and mitigating proteasomal degradation of capsid protein by proteosome inhibitors.214 For large-scale vector production, another two-step platform for generating a stable and inducible producer cell line has been developed. Initially, an alpha cell line is created based on a proprietary CAP human cell line or HEK293 cells in suspension with stably integrated Rep and helper genes. This is followed by the integration of the Cap gene and the gene of interest, resulting in a polyclonal producer pool, followed by a single-cell cloning process, clone screening, and selection of the best-performing clone.215 rAAV production in this system is induced by doxycycline. In a proof-of-concept cell line with perfusion bioprocessing for rAAV8-GFP production, the rAAV per cell yield was enhanced 8-fold, with a significantly increased yield of full particles (30–40%) compared to the conventional batch process.
Enhancing vector genome integrity is another strategy to increase vector potency. A study using a long-read sequencing-based platform and bioinformatic pipeline identified different vector genomic populations from scAAV preparations.216 It concluded that robust DNA secondary structures inherent in vector genome designs can cause a significant truncation, impacting both production and vector efficacy. Another study reported the presence of truncated genomes and a unique genomic species that originated from a nickable ITR incorporating a small portion of payload and a chimeric sequence that joined to the plasmid backbone.217 This heterogeneity was also observed in a vector genome design carrying dual single-guide RNA expression cassettes in tail-to-tail configurations.218 Interestingly, the same group found notable differences in genome heterogeneity between human and insect cell-produced rAAV.219 These findings highlight the urgent need to scrutinize vector genome design and generation of heterogeneity for the efficient production of highly potent rAAV preparations, particularly for clinical vectors.
Gene therapy efficacy and clinical applications
Overview of current clinical trials of rAAV-based gene therapy
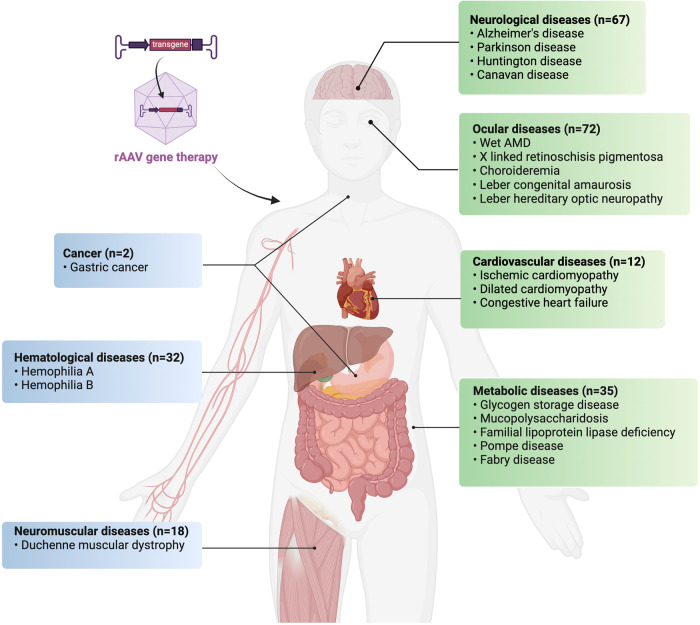
There have been remarkable developments in the clinical use of rAAV over the last few decades, underscoring its tremendous potential in gene therapy for a wide range of genetic and acquired diseases. Indeed, in a relatively short timeframe since the FDA approved Luxturna,220 there have been five additional rAAV gene therapy products introduced to the market (Fig. 1). Although rAAV-based therapies have shown impressive results in some clinical trials, numerous aspects require further investigation, including vector immunogenicity, dose optimization, and long-term safety. While meeting high-yield manufacturing challenges may not be an immediate issue for most monogenic diseases, producing rAAV at a large scale could become a bottleneck as more rAAV gene therapies are being developed for treating chronic prevalent human diseases. Furthermore, the regulatory framework for gene therapies is undergoing rapid changes. In this section, we will explore the latest developments in clinical applications of rAAV in major human diseases (Fig. 5), including ocular, neurological, metabolic, hematological, neuromuscular, cardiovascular, and oncogenic diseases. A table summarizing 238 clinical trials of rAAV-based gene therapies is presented in Supplementary Table 1.
Fig. 5.
Current clinical applications of rAAV in major human diseases. Clinical applications of rAAV across a spectrum of significant human diseases, including ocular, neurological, metabolic, hematological, neuromuscular, cardiovascular diseases, and oncology. Refer to Supplementary Table 1 for a comprehensive list of 238 clinical trials employing rAAV-based gene therapy for the aforementioned diseases. Figure created with Biorender.com
Ocular diseases
Ocular diseases are at the forefront of rAAV gene therapy for several reasons. The eye’s immune-privileged status reduces immune responses to rAAV. Its small volume necessitates low rAAV doses. Many ocular diseases are monogenic, making them suitable for gene therapy. Additionally, the relatively easy accessibility of the eye allows for various rAAV administration routes. Here, we highlight several rAAV-based ocular gene therapies, focusing on the treatment of monogenetic and acquired ocular diseases.
Leber congenital amaurosis type 2 (LCA2)
Voretigene neparvovec-rzyl (Luxturna) is a gene therapy to treat LCA2, a rare form of inherited retinal disease (IRD) caused by mutations in RPE65. This therapy delivers a functional copy of the RPE65 gene, correcting the deficiency in RPE cells, which are crucial for visual acuity. In a landmark phase III clinical trial, 29 LCA patients with confirmed biallelic RPE65 mutations were randomized to receive either Luxturna or no treatments as controls, followed by multi-luminance mobility testing, a measurement of visual function at specific light levels by requiring participants to navigate an obstacle course.221 The results indicate that Luxturna significantly improved functional vision (average of 1.8 light levels) compared to controls (average of 0.2 light levels) within one year. Moreover, full-field sensitivity threshold testing showed greater than 100-fold improvement in the intervention group by day 30, which was maintained over a year. Such beneficial improvements were sustained over 3–4 years in the subsequent follow-up studies.222,223 Due to the favorable results observed in this phase III trial, Luxturna was approved by the FDA in 2017 for the treatment of biallelic RPE65 deficient-associated retinal disease as the first gene therapy for genetic diseases.220
Retinitis pigmentosa (RP)
Given the heterogeneity of causative mutations in RP, various gene therapy strategies have been applied to this common form of IRD. Loss of MERTK, a crucial component in a signaling pathway that controls phagocytosis in the RPE, leads to photoreceptor degeneration and ultimately RP.224 This disease phenotype is autosomal recessive and accounts for ~3% of RP cases. A phase I clinical trial assessed subretinal injection of rAAV2-VMD2-hMERTK in six patients with MERTK-associated RP.224 While three patients showed vision improvement, sustained visual gains were only observed in one patient over 2 years, indicating that long-term investigation is needed.
Retinitis pigmentosa GTPase regulator (RPGR) gene mutation is a common cause of X-linked retinitis pigmentosa (XLRP) and primarily affects males. This is the most severe form of RP and is characterized by progressive vision loss due to dysfunction of photoreceptors and RPE cells.225 Three clinical trials have been conducted to assess the safety and efficacy of rAAV-based gene therapy to treat patients with RPGR mutations.226–228 Early results from one of the trials are encouraging, with some patients showing improvement in visual function and stability of retinal structure after receiving the treatment.226 A post hoc analysis of the gene therapy clinical trial (XIRIUS) and natural history study (XOLARIS) for XLRP revealed that the four participants who received the highest doses of cotoretigene toliparvovec (BIIB112/rAAV8-RPGR) had early improvements in retinal sensitivity and low-luminance visual acuity at month 12.229 In a phase II/III study, however, this gene therapy did not meet the primary endpoint of statistically significant improvement in light sensitivity in treated eyes.
Approximately 15% of RP cases are caused by autosomal dominant gain-of-function rhodopsin (RHO) mutations.230 Gene silencing or knock-out strategies can be used for this type of mutation. A preclinical study was conducted in NHPs using EDIT-103,231 an investigative gene therapy product containing two rAAV5 vectors to remove endogenous RHO through CRISPR/Cas9 and insert a codon-optimized RHO expression cassette that is resistant to targeted Cas9 cleavage to restore functional RHO expression. Early results have shown an encouraging improvement in RHO expression and visual function. However, its efficacy in humans is yet to be assessed.
In addition to gene replacement or editing approaches, other innovative strategies such as optogenetics have been investigated to treat RP independently of the genetic mutation. Optogenetics uses light to manipulate genetically modified cells with light-sensitive proteins. The initial assessment from a phase IIb clinical trial concluded that intravitreal injection of MCO-010, a multi-characteristic opsin encoded in rAAV2 designed to specifically target ON retinal bipolar cells, in patients with RP resulted in vision improvement with no severe ocular or systemic adverse events.232
Age-related macular degeneration (AMD)
AMD is the most common cause of blindness in developed countries. It is a progressive retinal condition featuring geographic atrophy and neovascularization at late stages. The mainstay of the treatment is to target vascular endothelial growth factor (VEGF), a major angiogenic factor involved in the pathogenesis of neovascularization. While anti-VEGF treatments have been proven to be efficient in alleviating disease progress and improving visual function in some patients, they require monthly injections and thus cause a high treatment burden while proving unsuccessful for a large proportion of patients. Gene therapy enables long-lasting, endogenous production of a therapeutic protein, solving many limitations of anti-VEGF agents, such as its short half-lives. As a result, there are an increasing number of clinical trials using rAAV to deliver genes for the treatment of both geographic atrophy and neovascular AMD.
GT005 (Gyroscope) is a rAAV2-based, one-time gene therapy product for geographic atrophy secondary to AMD that is delivered through subretinal injection. GT005 aims to mitigate retinal degeneration caused by an overreactive complement system in the aging retina by increasing the expression of the complement factor I (CFI) protein, a gene addition strategy to reduce inflammation and alleviate the degeneration of retinal cells. EXPLORE and HORIZON are phase II, multicenter, randomized, and controlled (treated vs. untreated patients) trials evaluating the safety and efficacy of GT005 in two different groups of patients with geographic atrophy (EXPLORE: patients with geographic atrophy secondary to AMD and with low CFI expression due to CFI variants; HORIZON: patients only with geographic atrophy secondary to AMD). Interim data indicate that the treatment achieves a sustained increase in vitreous CFI levels and a decrease in downstream proteins of CFI. The effect of the treatment is localized to the eye and no systemic increase in CFI levels has been observed. Nevertheless, the sponsor recently discontinued further development of GT005 because the futility criteria had been met.
sFlt-1 is a chimeric VEGF inhibitory protein consisting of domain 2 of Flt-1 (VEGF receptor-1) coupled to the human immunoglobulin G1 heavy chain Fc fragment.233 Gene therapy for neovascular AMD by using rAAV2 encoding sFlt-1 has been investigated through both intravitreal and subretinal administration.234–237 Intravitreal injection of rAAV2-sFlt-1 in a phase I clinical trial was reported to be safe and well-tolerated, while the expression level of sFlt-1 and the treatment efficacy was variable.238 Similarly, subretinal injection of the same vector was well-tolerated among the elderly.239 However, significant visual improvements were not observed, although this outcome was not the primary endpoint for the study.
RGX-314 (Regenxbio) is being developed as a subretinal treatment for neovascular AMD, consisting of rAAV8 encoding anti-VEGF monoclonal antibody fragments similar to ranibizumab. Initial assessment from a phase I clinical trial (ASCENT) showed that the treatment was safe and well-tolerated. The treatment outcome was dose-dependent, and several patients remained free of supplemental anti-VEGF injections for up to 18 months.
ADVM-022 (Adverum Biotechnologies) is a gene therapy product utilizing an engineered 7m8 vector derived from rAAV2 encoding aflibercept for the treatment of neovascular AMD. In a phase I clinical trial (OPTIC), a single intravitreal injection of ADVM-022 reduced the need for supplemental anti-VEGF injection in over 80% of patients for up to 92 weeks during the follow-up period.240
Neurological diseases
Current FDA-approved rAAV gene therapies for neurological disorders use two fundamentally different routes of delivery for localized versus widespread transgene delivery: stereotactic, anatomically confined rAAV delivery or widespread CNS transduction via intravenous (i.v.) delivery.
Aromatic L-amino acid decarboxylase (AADC) deficiency (AADCD)
AADCD is an autosomal recessive disorder caused by mutations in the dopa decarboxylase gene, leading to AADC enzyme deficiency.241–243 AADC catalyzes the final step in serotonin and dopamine synthesis, with dopamine as a precursor for norepinephrine and epinephrine. This leads to a combined serotonin, dopamine, norepinephrine, and epinephrine deficiency.242
Patients present during the first months of life with muscular hypotonia, dystonia, oculogyric crisis, developmental delay, and autonomic dysfunction.241 A substantial delay between symptom onset (2.7 months) and diagnosis (3.5 years) has been reported.242 In its most severe form, patients miss all developmental milestones, lack head control, never learn to stand or sit, and die before the age of 10 years.241,244
Parkinson’s disease (PD) and AADCD have different etiologies (neuronal loss vs. enzyme deficiency) but result in striatal dopamine deficiency as a shared therapeutic target. This led to the rationale that one therapeutic vector could benefit both diseases. The first attempt to deliver intraluminal (the putamen is part of the striatum) rAAV expressing AADC (rAAV.AADC) was tested in 2008 in PD patients.245–247 Overall, the therapy was well-tolerated, with few procedure-related adverse effects, providing critical data on the safety and feasibility of stereotactic intracranial rAAV.AADC delivery was also associated with clinical improvement.246 Encouraged by the PD trials, this approach was extended to children with AADCD in 2012 in Taiwan.248 Four children, with the oldest being six years of age at the time of treatment, received bilateral intraputaminal infusion of rAAV2.AADC at a total dose of 1.6 × 1011 vgs. All patients showed improved motor scores and subjectively reported improved emotional stability. Interestingly, patients experienced transient post-infusion dyskinesia.248
A follow-up phase I/II trial in 10 patients used a slightly higher dose (1.81 × 1011 vgs), with the oldest patient being 8 years of age at the time of treatment, and no immunosuppression was used. All patients met the primary endpoints of clinical motor performance and cerebrospinal fluid (CSF) biochemistry improvement. Other measures, such as positron emission tomography (PET) imaging, showed significantly improved AADC activity. Again, post-infusion-related transient dyskinesia was noted.249
In 2019, a group in Japan treated six patients in a phase I/II trial with the same approach and a similar vector and dose (2 × 1011 vgs) as the Taiwanese group.250 The patient population, however, was more diverse regarding mutations and age at treatment, with the oldest patient being 19 years old. Similar to the 2017 Taiwanese study, patients experienced transient post-infusion uncoordinated movements of the mouth and extremities. Encouragingly, all patients improved in motor performance and oral food intake.
The anatomical target was selected based on PD pathology defined by dopaminergic neuron loss. However, in AADCD, dopamine-deficient dopaminergic neurons seem to persist. Targeting these dopaminergic neurons in the substantia nigra and ventral tegmental area to emulate physiologic dopamine synthesis was recently evaluated in a safety and efficacy trial.251 Seven patients between 4 to 9 years of age were assigned to two dosing cohorts (8.3 × 1011 and 2.6 × 1012 vgs total dose). All patients experienced improved motor function and mood. In 6/7 patients, oculogyric crises ceased completely. This clinical improvement was consistent with PET imaging results suggesting AADC enzyme activity. As in most trials, transient dyskinesia was observed post-infusion. Unfortunately, a direct comparison of both approaches is difficult at this stage and requires further studies.
Recently, long-term follow-up results from the Taiwanese trials reported significant improvement in motor function in 26 patients, with three patients able to walk.252 Interestingly, treatment age negatively correlated with motor scores, while dose did not.252 The most common treatment-emergent adverse effects (TEAE) were pyrexia and dyskinesia. Dyskinesia resolved in all patients but one. Notably, a positive correlation between dyskinesia severity/duration with treatment age was suggested.252 This correlation could be a critical observation to understand age-risk-benefit and might find equivalents in other CNS diseases. The therapy (Eladocagene Exuparvovec) was eventually licensed and has recently been approved by the European Medicines Agency for patients over 18 months of age (Upstaza).253
Spinal muscular atrophy (SMA)
SMA is a model disease to targets widespread pathology in the brain and spinal cord by i.v. rAAV gene therapy. Over 3000 patients have been treated to date, providing critical insights into efficacy and safety. SMA is classified into variants I–IV.254 Variant I is the most severe form, with symptom onset in the first 6 months of life and death before the age of 2 years.255,256 It is characterized by weakness, motor development regression, and progresses to respiratory failure and death. SMA I is autosomal recessive and caused by loss-of-function mutations in the SMN1 gene.257 SMN2, a gene homolog to SMN1, produces a short version of SMN1. The number of SMN2 copies negatively correlates with the severity of the disease. The incidence varies based on country and ethnicity but is estimated as low as 1 in 7829.258
The first clinical trial results for SMA I were published in 2017.259 15 patients received either 6.7 × 1013 vgs/kg or 2 × 1014 vgs/kg of i.v. rAAV9 expressing SMN1 (rAAV.SMN1) under the control of the CB promoter with a CMV enhancer. The mean age was 6.3 months in the low dose and 3.4 months in the high dose cohort. The main findings were that patients had a reduced need for respiratory ventilator support and improved motor scores. 11/12 patients were able to sit unassisted for several seconds and gained the ability to speak. While the therapy was well-tolerated, elevated liver enzymes were common, but decreased with corticosteroid treatment.259
In two phase III follow-up studies in the US (STR1VE) and the EU (STR1VE-EU), an additional 22 and 33 patients, respectively, were enrolled and received 1.1 × 1014 vgs/kg i.v.260,261 The mean age at dosing was comparable in both studies (3.7 vs. 4.1 months). The US study had co-primary endpoints of unassisted sitting for at least 30 s and ventilator-free survival, while the EU study defined the primary endpoint as unassisted sitting for at least 10 s, and ventilator-free survival as the secondary endpoint. Both studies administered a prednisolone regimen. In STR1VE, 13/22 patients achieved independent sitting for at least 30 s, and in STR1VE-EU, 14/20 patients achieved sitting independently for at least 10 s, with one reaching 30 s. Of note, the EU trial had broader inclusion criteria, with some patients showing signs of more advanced disease, limiting the direct comparison of both studies. In addition, both trials reported elevated liver enzymes and thrombocytopenia after post-infusion.260,261
Long-term follow-up results from the initial START trial were reported in 2021.262 The longest follow-up was 6.2 years, and patients retained motor skills gained during the initial trial. Encouragingly, two patients acquired new motor milestones and could stand with assistance. The safety profile remained very good, with no TEAEs reported.262
The availability of an approved therapy allows the disease to be included in newborn screening, which could identify pre-symptomatic patients. This raises the possibility of prophylactic treatment.
The SPR1NT trial investigated the therapeutic outcome of prophylactic treatment with Onasemnogene abeparvovec (Zolgensma) at 1.1 × 1014 vgs/kg in 14 patients with two SMN2 copies (median age: 21 days) and 15 patients with three SMN2 copies (median age: 32 days).263,264 Prednisolone was given prophylactically and post-treatment. All patients with two SMN2 copies achieved the primary endpoint of independent sitting for over 30 s. 11/14 patients attained this milestone within the normal developmental window, with similar results observed for independent standing. With the understanding that there may be different definitions of walking, 9–10/14 children were able to walk independently, with 4–5 achieving this within the expected developmental window. Importantly, all patients remained free of permanent ventilation and alive at the end of the study. Elevated liver enzymes, cardiac serum markers, thrombocytopenia but no thrombotic microangiopathy (TMA), and transient areflexia/hyporeflexia were reported, which resolved in all but one patient and were classified as unrelated to the treatment.
Patients with three SMN2 copies manifest milder disease, which was reflected in independent standing for at least 3 s and walking for at least five steps in 14/15 children. 14/15 achieved standing and 11/15 achieved walking within the expected developmental window. Similar to the two SMN2 copy groups, all patients with three SMN2 copies remained alive and free from permanent ventilation at the end of the study. Reported TEAEs were comparable to the two SMN2 copies group.
The results in both cohorts emphasize the benefit of early pre-symptomatic treatment, which resulted in degrees of clinical improvement not observed before with Zolgensma. It also highlights the favorable safety profile of the treatment, although there have been reports of more severe hepatotoxicity and even death.265 Zolgensma obtained FDA approval in 2019, and as of March 20, 2023, over 3000 patients have been treated with Zolgensma, according to Novartis. Completed or ongoing studies are now exploring new aspects, including combination therapies or transitioning patients from conventional therapy to Zolgensma.266
Metabolic diseases
Genetic defects causing metabolic disorders can have systemic or organ-specific manifestations, with implications on vector design and route of administration (e.g., lysosomal storage diseases with primary CNS manifestation). Here, Tay Sachs disease (TSD), Sandhoff disease (SD), and Canavan disease (CD) will serve as examples of neurometabolic conditions with currently active clinical trials (NCT04669535, NCT04798235, NCT04833907, and NCT04998396).
TSD and SD are lysosomal storage diseases, categorized as GM2 gangliosidoses resulting from autosomal recessive, loss-of-function mutations in the ubiquitously expressed gene encoding β-N-acetylhexosaminidase (HEX). HEX forms a dimer comprised of subunits α and β (HEXA), β and β (HEXB), or α and α (HEXS). TSD and SD are caused by mutations in the genes encoding the α- and β-subunits, respectively.267 Clinically, both diseases present in infantile, juvenile, and adult forms, which differ in onset and severity.268–270
One challenge is the need for a stochiometric balance of the α- and the β-subunits for optimal functionality of the HEX dimer. With rAAV’s limited packaging capacity, one strategy is simultaneous delivery of the α- and β-subunit with two separate vectors. Alternatively, a bicistronic rAAV has been developed to express the α- and β-subunits together.271,272
In 2022, an expanded access trial in two TSD patients demonstrated the safety of intrathecal (i.t.) infusion of two rAAV.rh8 vectors expressing α and β subunits. The patients were 30 months (1.0 × 1014 vgs; i.t.) and 7 months (4.05 × 1013 vgs; combined bilateral intrathalamic and i.t.) old and received immune suppression with rituximab, sirolimus, and prednisolone. The procedure and vector were well-tolerated. Although the functional outcome was not part of the study’s endpoints, both patients showed signs of disease stabilization. This first human TSD rAAV gene therapy study was a milestone in evaluating intrathalamic delivery and has paved the way for an ongoing clinical trial.273
Currently, two clinical trials are active in Canada (NCT04798235) and the US (NCT04669535). The Canadian phase I/II trial utilizes rAAV9 to deliver the HEXA and HEXB transgene with one vector for infantile TSD or SD patients via a single i.t. dose. In contrast, the trial in the US is designed as a dose escalation study of four different vector doses administered via bilateral intrathalamic route and i.t./cisterna magna combined. The study uses two rAAV.rh8 vectors delivering the HEXA or HEXB transgene. Results from these trials are pending.
CD is an autosomal recessive inherited progressive neurodegenerative disease hallmarked by spongiform degeneration and elevation of N-acetylaspartate (NAA). Loss of function mutations in the enzyme ASPA causes accumulation of NAA in the CNS and urine. Affected children typically never learn to walk, talk, control the movement of their head, or gain any level of independence.274,275 Many patients succumb to the disease within the first years of life. A clinical trial evaluated the safety of intracranial rAAV administration for the first time in the treatment of CD.276 After this clinical trial, studies to optimize vector design could achieve varying degrees of age-dependent prevention and reversal of the disease in mice.132,277–280 It is likely that differences in vector design and route of administration might account for the variable outcomes reported in preclinical studies.
Combining intracerebroventricular (i.c.v.) and i.v. routes of administration were employed to reduce the overall vector dose in an early single-patient trial.281 However, comparative studies are needed to evaluate this strategy.
Currently, there are two clinical trials ongoing, with one actively enrolling at the time of writing (NCT04833907 and NCT04998396). The two trials use different vector designs and routes of administration (intracranial delivery vs. i.v. infusion). This difference in route of administration necessitates differences in dose. Data for both trials have been presented at scientific conferences and look to be promising. With both trials ongoing, direct comparison is not feasible at the time of this writing.
Hematological diseases
Non-oncologic hematologic conditions can be classified into cellular or acellular diseases, meaning that genetic mutations affect cellular function (e.g., sickle cell disease)282 or components of blood (e.g., hemophilia),283 respectively. The distinction is crucial for gene therapy as it determines the target cell/organ.
In cellular hematologic conditions, the target is a cellular blood component, typically bone marrow. Blood cells have a short half-life, so directly targeting them by rAAV loses therapeutic efficacy quickly as the cells turn over. In contrast, bone marrow stem cells constantly divide to produce new cells, which dilutes the rAAV genome. Nevertheless, this may be a desirable effect if the host genome can be edited or if the gene therapy-expressing cells gain a competitive advantage. Acellular components, in contrast, are released by cells into the bloodstream. This provides an opportunity to express the transgene in any cell type that can export proteins into the bloodstream.
Hemophilia A and B are prime examples of hematologic disorders caused by a deficiency in secreted acellular blood components.283 The estimated prevalence for hemophilia A and B are ~12:100,000 and 4:100,000, respectively.284 Hemophilia A and B are caused by factor VIII (FVIII) and factor XI (FXI) deficiency, respectively, and both are recessive X-linked conditions.285 FVIII is synthesized in endothelial cells, whereas FIX is produced in hepatocytes.286–288
Hemophilia A and B patients have a substantially increased risk of bleeding spontaneously or with minor trauma, causing high morbidity and mortality (e.g., intracranial hemorrhage).289,290 In addition, repeated bleeding causes local reactions and disabling cellular degeneration (e.g., hemophilic arthropathy).291 Notably, patients have reduced overall survival compared to the general population.290
Prior to gene therapy, treatment of severe hemophilia A or B required repeated administration of the deficient factor or drug to modulate the coagulation cascade.292–294 Healthcare spending for those drugs has been rising, reaching ~$1.5 billion in the US in 2019.295 Gene therapy emerges as an ideal modality due to its potentially enduring effect and the clinical knowledge (i.e., natural history) in diagnosing and treating hemophilia, a critical aspect in gene therapy development that is often missing in ultra-rare conditions. In addition, FVIII and FIX are secreted, which means ectopic expression in a tissue could produce the factor (e.g., muscle). Hemophilia severity is classified based on plasma FVIII or FIX activity: severe <1%, moderate 1–5%, and normal 5–40%.285 This provides clear target levels to achieve partial (>1%) or complete (>5%) disease correction. Such meaningful thresholds rarely exist or are known in other diseases.
First attempts to develop gene therapy for hemophilia using viral delivery platforms predate the successes of rAAV.296,297 These early non-rAAV studies could not achieve lasting therapeutic factor levels or trigger immune responses against the viral vector.
In 1997, two studies could demonstrate persistent human FIX (hFIX) expression using rAAV (rAAV.hFIX) via intramuscular (i.m.) or intraportal vein (liver-directed) injection.298,299
A possible challenge could arise from FIX activity inhibiting antibodies, which were found after i.m. rAAV.hFIX.300 However, using species-specific transgenes might ameliorate the formation of such antibodies or other immune responses, as demonstrated in hemophilia B dog studies that utilized canine FIX (cFIX).301,302 While intraportal delivery is invasive and dogs required FIX transfusion prior to the procedure, slightly higher cFIX plasma levels were observed versus the i.m. study.301,302 However, this conclusion is unreliable due to differences in study parameters, including vector dose.
In 2000, a phase I trial using i.m. delivery of rAAV.hFIX reported a favorable safety profile,303 which was confirmed three years later after the completion of the study. Notably, no anti-FIX inhibitory or non-inhibitory antibodies were detected, attributed to patient selection (only missense mutations) and limiting the per-site total rAAV dose, which was identified in animal models to correlate with the formation of inhibitory anti-FIX antibodies. However, patients remained below the 1% FIX serum level, which is considered the lowest therapeutic goal.304 A separate phase I/II trial with rAAV2.hFIX was given via hepatic artery delivery and only achieved transient FIX expression, likely due to anti-AAV capsid cellular immune responses causing hepatocyte destruction and transgene loss. This finding spearheaded a major focus on rAAV immunology and its impact on clinical safety and efficacy.305,306 To reach therapeutic efficacy, subsequent efforts to increase FIX expression were undertaken by optimizing several parameters, including the use of newly discovered rAAV capsids, transgene codon optimization, promoter selection, route of administration, and immune suppression. Additional innovation came from the discovery of the Padua variant of FIX in 2009, which has supraphysiologic activity.305 First, a phase I trial combined i.v. delivered hepatotropic rAAV8 and codon-optimized hFIX transgene resulting in 2–12% FIX activity with no immune suppression being used.307 As expected, a humeral as well as T cell-mediated anti-AAV8 capsid immune response was observed. The T cell response correlated with dose generally, but not universally, suggesting patient-specific factors.307 The patients from this trial were included in a follow-up study with four new patients in the high-dose group, which demonstrated overall FIX activity of 1–6% over a median of 3.2 years.308 While the safety profile was favorable, some patients in the high-dose group experienced delayed elevation of liver enzyme levels associated with decreased FIX activity, which resolved with glucocorticoid administration.308
While the change to the rAAV8 capsid and codon-optimization of the transgene showed promising improvements, it did not consistently achieve therapeutic FIX activity. To overcome these challenges, a newly engineered capsid with increased liver tropism and a codon-optimized Padua FIX variant was designed.309 Notably, the dose (5.0 × 1011 vgs/kg) was 4-fold lower than the high dose in the 2011 and 2014 trials (2.0 × 1012 vgs/kg). Despite the reduced dose, the FIX activity reached a mean of 33.7% (range 14–81%).309,310 Prophylactic immunosuppression was not administered and only two patients required prednisolone for transient liver enzyme elevation, highlighting the excellent safety profile. Clinically, the annualized bleeding rate decreased significantly from a mean rate of 11.1 to 0.4.310 Overall, the increased activity of the Padua variant turned out to be highly beneficial and was utilized in subsequent clinical trials.
The hemophilia B trials uncovered several aspects of rAAV gene therapy in humans that were not anticipated, including a gradual decrease in transgene expression and immune responses. Currently, FDA-approved gene therapy for hemophilia B (i.v. rAAV5 Padua, Hemgenix, Etranacogene dezaparvovec) is on the market, while other rAAV products (verbrinacogene setparvovec (FLT180a) using an rAAVS3 capsid and Fidanacogene elaparvovec, formerly known as SPK-9001 and PF-06838435) are in clinical trials but await FDA approval.
A notable challenge for hemophilia A is the ~7 kb FVIII transgene, exceeding rAAV’s packaging capacity311 and necessitating the use of a truncated form. The therapeutic efficacy of a truncated FVIII was evaluated in nine patients by i.v. rAAV5 delivering a codon-optimized FVIII variant (FVIIISQ; missing the B-domain).312 The results from different dosing groups (6.0 × 1012 vs. 2.0 × 1013 vs. 6.0 × 1013 vgs/kg) demonstrated that the highest dose achieved therapeutic FVIII activity for the 52-week study period. Encouragingly, only the high-dose patients received immune suppression, and no clinically adverse effects were identified.312 However, transgene expression decreased over time.313
A separate trial evaluated a rAAV3-derived engineered capsid expressing FVIIISQ at four doses ranging from 5.0 × 1011 to 2.0 × 1012 vgs/kg in 18 patients.314 While it achieved therapeutic benefit, two of the 18 patients lost transgene expression entirely due to an anti-rAAV capsid immune response despite using glucocorticoids. Of note, attempts to wean several patients off glucocorticoids resulted in a cellular immune response. Although vector properties between the trials by Savita et al. 312 and George et al. 314 might explain the difference in transgene expression and maintenance, it was hypothesized that high expression levels might trigger an unfolded protein response with subsequent FVIII loss.314
A follow-up phase III trial using rAAV5hFVIIISQ enrolled 134 patients receiving a single 6.0 × 1013 vgs/kg i.v. dose.315 This one-time treatment significantly increased FVIII activity and reduced exogenous FVIII use, and the annual bleeding events improved compared to exogenous FVIII prophylaxis. Elevated liver enzymes were found at a median of 8 weeks, which decreased with glucocorticoid treatment in 96.2% of the patients.315 The two-year follow-up data showed a similar trend as in the previous phase I/II trial, with decreased annual bleeding rate and exogenous FVIII use. However, decreasing transgene expression over time was observed as well. Unexpectedly, the FVIII levels in the phase III trial were lower than what was found in the phase I/II trial at one- and two-year follow-up.316 This therapeutic vector recently obtained FDA approval (roctavian, Valoctocogene Roxaparvove, rAAV5hFVIIISQ), and it will be vital to conduct follow-up clinical studies to determine its durability.
Neuromuscular diseases
Neuromuscular diseases comprise conditions affecting neurons and muscle cells,317 with muscular dystrophies primarily affecting muscle cells. DMD is the most common dystrophy,318 and the FDA recently granted approval for an rAAV-based gene therapy.319 DMD is a rare X-linked and progressive disease with a prevalence of ~15.9–19.5/100,000,320 which primarily affects males, although rare cases in females have been reported.321 It is caused by mutations in the dystrophin gene resulting in absent or dysfunctional dystrophin protein. It manifests with muscle weakness, loss of the ability to walk, respiratory impairment, and cardiomyopathy,322–324 with the latter two commonly being the cause of patient mortality.
The dystrophin gene consists of eight promoters and 79 exons to generate a ~11.4 kb cDNA and 427 kDa muscle isoform protein.321 Mutations typically lead to a truncated protein in DMD patients.321 The large dystrophin cDNA size poses a major challenge to rAAV-based gene replacement therapy because it exceeds the packaging capacity, so strategies have focused on identifying the minimally required protein sequences. Two different dystrophin transgenes have been developed: ~6–8 kb mini-dystrophin and ~4 kb microdystrophin.325–328 The mini-dystrophin needs to be divided and delivered by two rAAVs, while the microdystrophin fits into one rAAV.325 Other strategies using rAAV involve exon skipping,329 GALGT2 delivery330 (shown to prevent muscle pathology in mice), and gene editing to correct the dystrophin gene or activate non-muscle dystrophin isoforms using CRISPR.331,332 The focus here is on strategies that have been translated to human application.
In 2006, a trial evaluated a hybrid rAAV2.5 expressing miniature dystrophin by i.m. injection in six patients, aged 5–11 years.333 Patients were divided into two dosing groups: 2 × 1010 and 1.0 × 1011 vgs/kg. While some muscle fibers were found to be mini-dystrophin-positive in 2/6 patients at day 42 post-treatment, the failure to find dystrophin-positive cells at later time points might point towards an immune-mediated process. This is also supported by findings of transgene-specific T cells. Surprisingly, 2/6 patients were positive for T cells against a domain of the mini-dystrophin gene prior to receiving gene therapy, suggesting that the mutated endogenous dystrophin might not be sufficient to induce immune tolerance.333
To achieve broad targeting of affected muscle cells throughout the body, a trial evaluated the safety and therapeutic efficacy of i.v. infusion of 2.0 × 1014 vgs/kg rAAV.rh74.micro-dystrophin in four patients with a mean age of 4.8 years.334 Encouragingly, all patients in this phase I/II trial improved clinically and histopathologically, which correlated with age and disease severity at the time of treatment. In contrast to the earlier trial results from 2010, T-cell activity against the micro-dystrophin protein was not detected. Liver enzyme levels rose after rAAV infusion but remained moderate and normalized with corticosteroid treatment. No signs of TMA were found.334 A randomized, double-blinded, placebo-controlled clinical trial (NCT03769116, delandistrogene moxeparvovec) involving 41 patients was just completed. Notably, this therapy obtained FDA approval in 2023.319
An alternative strategy to gene replacement therapy involves the use of disease-modifying genes. In 2022, a first-in-human trial treated two patients (6.9 and 8.9 years of age) with rAAV.rh47 expressing GALGT2 at 5.0 × 1013 and 2.5 × 1013 vgs/kg per leg via bilateral femoral vein isolated limb perfusion, respectively.335 Isolated limb perfusion has been widely evaluated in animals as an attempt to increase muscle transduction but also reduce the total dose. GALGT2 acts as a β1,4 N-acetylgalactosaminyltransferase in muscle glycosylating α-dystroglycan and has shown positive effects on muscular pathology. No relevant liver toxicity was reported, despite the detection of antibodies against the rAAV capsid and mild T-cell activity against rAAV and the GALGT2 protein. While the patient number was too small to draw significant conclusions, it was noted that the younger patients receiving the higher dose showed greater improvement.335
Other ongoing trials are still active but not recruiting, including a randomized phase I/II trial by Solid Bioscience (NCT03368742) and a non-randomized phase Ib trial by Pfizer (NCT03362502), which both utilize rAAV9 to express truncated dystrophin under the control of muscle-specific promoters. These trials evaluate increasing i.v. doses and include children and adolescents but exclude patients with advanced degrees of cardiac and respiratory impairment.323
Defining eligibility criteria can be particularly challenging and highlights current gaps in understanding and predicting the interaction between rAAV gene therapy and the disease. A recent single-patient DMD trial using i.v. rAAV9 at 1.0 × 1014 vgs/kg expressing dCas9 linked to VP64 to activate expression of cortical dystrophin ended fatally.336 The patient, who was non-ambulatory and had advanced disease at the time of dosing, developed worsening cardiac function on day five and acute respiratory distress syndrome on day six post-dosing, resulting in cardiopulmonary arrest and death on day eight post-treatment. The concluding assessment led to the hypothesis that the patient developed cytokine-mediated capillary leak syndrome, as tests for the humeral or cellular immune response against the rAAV capsid or transgene were negative. There were also no signs of TMA, as has been seen in high-dose DMD trials. A second patient, who was part of the Pfizer trial (NCT03362502), died six days after receiving 2.0 × 1014 vgs/kg of rAAV9. The patient was younger and received double the dose as in the single-patient trial above. Although no autopsy was performed, the current hypothesis centers on an innate immune response in the cardiac muscle causing heart failure (HF).337
Cardiovascular diseases
HF is a syndrome defined by structural or functional alterations of ventricular filling or emptying.338 It is highly prevalent with ~6 million people affected in the US.339 Calcium is crucial in cardiac function and is also a target for conventional drug development. One preclinically successful strategy is the overexpression of ATPase Sarcoplasmic/Endoplasmic Reticulum Ca2+ Transporting 2 (SERCA2a), which pumps calcium into the sarcoplasmic reticulum (SR) during the cardiac relaxation process.340,341
The CUPID trial evaluated overexpressing SERCA2a using rAAV1 (rAAV1.SERCA2a).342,343 It was designed as a phase I/II trial, with the phase I portion being an open-label sequential dose escalation study342 and phase II being randomized, double-blind, and placebo-controlled.343 During the phase I study, a single intracoronary administration for heart-directed rAAV1.SERAC2a delivery was evaluated at 1.4 × 1011, 6.0 × 1011, and 3.0 × 1013 vgs (n = 3 patients per cohort). All treated patients had New York Heart Association (NYHA) Class III HF at the time of treatment. Some patients displayed symptomatic improvement and tolerated the treatment well. One patient died, which was determined to be unrelated to the treatment. Overall, the favorable safety profile encouraged the transition to the phase II portion of the study, which enrolled 25 patients receiving treatment and 14 receiving placebos. Doses were assigned to three cohorts, 6.0 × 1011 (n = 8), 3.0 × 1012 (n = 8), and 1.0 × 1013 vgs (n = 9).343 All patients had NYHA Class III HF at the beginning of the phase II study. Overall, a trend of improvement in the treated patients versus placebo was observed; however, most outcome criteria did not reach statistical significance, except for left ventricular end-diastolic volume. The study confirmed the favorable safety profile observed in phase I.343
The three-year follow-up results of the CUPID phase II could not demonstrate significant improvement in survival. However, a cumulative analysis for non-terminal events reached statistical significance encouraging CUPID 2.344 CUPID 2 assigned a total of 250 patients to the treatment (n = 123) or the placebo group (n = 127).345 Similar to prior trials, critical outcome measures did not show significant differences (e.g., survival). Interestingly, early terminal events were significantly increased in the intervention group.345
Other studies investigated the benefit of rAAV1.SERCA2a on left ventricular end-diastolic volume (AGENT-HF) or safety in patients with concomitant left ventricular assist device (LVAD) (SERCA-LVAD) and the impact of circulating NAbs.346,347 Both trials were terminated after discouraging CUPID 2 trial results emerged.
Although these trials did not result in clinically significant improvement in outcome, they highlight the potential of rAAV gene therapy for cardiac diseases. New, promising cardiac gene therapies using rAAV are being developed, including using rAAV9 to express LAMP2B for the treatment of Danon disease, an X-linked disorder causing severe cardiomyopathy. A phase I (NCT03882437) trial has completed recruitment, and the interim results suggest a favorable safety profile and clinical improvement, with occurrences of TMA and acute kidney injury being resolved by treatment.348,349 A corresponding phase II (NCT06092034) study is actively enrolling males of at least 8 years of age as a single-arm study for administration of a single i.v. infusion. The trial is expected to be completed in 2025.
Cancer
rAAV has shown promise in preclinical studies for cancer, but its translation to use in humans has been limited.350–353 At the time of this writing, only one trial using rAAV for ex vivo gene therapy in gastric cancer is active (NCT02496273). Thus, this section focuses on preclinical achievements and rAAV-based strategies to treat oncologic conditions. For a more detailed understanding, we refer readers to several in-depth reviews.354,355
Anti-tumor strategies using rAAV share similar targets and concepts as conventional anti-tumor regimens. However, gene therapy offers specific advantages, such as modulation or knock-down of gene expression. rAAV transduces dividing and non-dividing cells, enabling broad cell targeting within heterogeneous tumor tissue. This property can be used to deliver cytokine transgenes to tumor cells to recruit immune cells and trigger an anti-tumor immune response.
A proof-of-concept study showed that rAAV-mediated interferon β (IFNβ) expression suppressed colorectal cancer cells in a xenograft mouse model.356 In a non-randomized, non-blinded, retrospective human study, the addition of IFNβ to temozolomide therapy extended survival in glioblastoma (GBM) patients.357 Driving expression of IFNβ by rAAV-transduced (rAAV.IFNβ) tumor cells can overcome IFNβ’s short half-life and boost therapeutic efficacy, as evidenced in an invasive orthotopic xenograft GBM mouse model, where intracranial delivery almost doubled overall survival.358
Taking advantage of rAAV’s adaptable capsid, tumor-directed capsid engineering can increase transduction efficiency and specificity while mitigating possible adverse effects. Indeed, rAAV displaying a Her2 ligand showed increased transduction specificity.359 The expression of HSV thymidine kinase converts ganciclovir into a toxic product which is subsequently incorporated into the tumor DNA to induce cell death. Delivery of this gene reduced tumor burden and extended survival compared to an animal group that received the approved anti-cancer drug Herceptin.360
While engineering capsids to deliver cancer therapeutics offers high specificity, this approach is time-consuming and labor-intensive. An alternative approach is to screen existing rAAV capsid libraries for tropism after delivery by different routes of administration to determine the best possible combination. For example, rAAV7 and nine have been found to achieve high transduction of prostate tissue in mice.361 This enhanced transduction of prostate tissue can be used to knock down miRNAs associated with prostate cancer, which has been found to slow disease progression in a mouse prostate cancer model.362,363
These proof-of-concept studies highlight promising anti-cancer strategies using rAAV. However, critical questions must be addressed before human application is feasible. There are concerns regarding the safety of sustained therapeutic transgene expression, particularly after tumor elimination or relapse. Similar to conventional chemotherapy, recurrent tumors might be resistant to or able to escape the therapy, making ongoing transgene expression ineffective. Given that cytokine therapies often have substantial side effects, unregulated transgene expression could cause additional complications. Improved regulated gene therapy strategies need to be developed together with advances in understanding of tumor biology.
Challenges and limitations in translating rAAV-based gene therapies to clinical practice
rAAV capsid performance is tightly connected to the route of administration, prompting exploration of different delivery routes, including intravascular, direct intra-tissue injection, and delivery into pre-existing body cavities or fluid space.364–371 Each route and capsid selection must be determined in the context of the disease, target area, organ system, and other factors such as patient age (Table 2).
Table 2.
Pros and cons of different routes of administration of rAAV
| Routes of administration | Pros | Cons | Examples |
|---|---|---|---|
| Intravascular | • Widespread transduction | • Transduction of non-target organs | i.v., i.a. |
| • Reach most organs | • Potentially high doses required | ||
| Intra-tissue | • Localized delivery | • Invasive | i.m., i.p. |
| • Decreased spread | |||
| • Possible local reaction | |||
| Intra-cavity | • Spread in preformed space | • Transduction potentially limited to preformed space | s.p., s.r., i.v.t |
| • Potentially uneven distribution | |||
| Intra-fluid space | • Utilization of fluid dynamics to distribute vector | • Long vector travel distance | i.c.v., c.m., i.t. |
i.v. intravenous, i.a. intra-arterial, i.c.v. intracerebroventricular, i.m. intramuscular, i.t. intrathecal delivery, c.m. cisterna magna, i.p. intraparenchymal, s.p. subpial space, s.r. subretinal, i.v.t. intravitreal
A classic example is the BBB protecting the CNS, which presents a barrier that gene therapies need to overcome. Some rAAV capsids efficiently cross the BBB.364–366,372 Targeting the CNS via systemic i.v. delivery is not optimal if the therapeutic target is merely a small brain area, as the required vector dose and possible side effects would be high without achieving superior transduction of the target area. In such cases, direct and localized delivery is critical, as exemplified in AADC.249,251 However, even within a confined delivery area, capsid selection must be scrutinized. rAAV9 displays substantial tissue spread and relatively broad tropism even with localized delivery, while serotypes such as rAAV2 have a more limited transduction capacity.
Another challenge is the translation from preclinical to clinical studies. To mitigate vector costs and risks from high doses as used in i.v. delivery, intra-CSF space administration is widely utilized for widespread CNS transduction.373–375 The challenge of extrapolating findings in mouse, rat, or even larger animal brains to humans is daunting. For example, rAAV9 can achieve global brain transduction in mice, where the distance between the lateral ventricle and cortex is merely a few millimeters. In humans, this distance spans several centimeters. Thus, clinical trials using CSF delivery in humans for widespread CNS transduction have to demonstrate the translatability of this delivery mode.
Immunogenicity of rAAV
In addition to physical barriers, rAAV encounters numerous biological barriers instituted by the immune system, including pre-existing immunity, complement activation, innate pattern and danger receptors, and adaptive B cell and T cell immunity (Fig. 6). Here, we discuss how each immunological barrier negatively impacts vector transduction and what potential solutions are available.
Fig. 6.
Immune responses in rAAV-based gene therapy. a Pre-existing AAV-specific antibodies can interact with rAAV and block its target cell entry. Bacteria-derived endopeptidase IdeS and its homologs can cleave the intact antibody into Fab and Fc fragments, thus reducing its half-life in circulation and eliminating Fc-mediated functions. Alternatively, the capsid can be modified or encapsulated by EVs to prevent antibody recognition. b Complement activation is observed when high doses of rAAV are administered. Though the exact mechanism remains unclear, a leading hypothesis is that rAAV adhering to the cell surface activates the classical pathway by binding to complement C1qs, which cleaves C4 and C2 to form the C3 convertase C4b2b. C3 is then cleaved and forms C5 convertase that cleaves C5 into C5b, which associates with C6–9 to form the membrane attack complex (MAC) that directly causes cell lysis, resulting in liver or kidney injury. Complement activation mediates endothelial damage, thrombotic microangiopathy (TMA), and atypical hemolytic uremic syndrome (aHUS). Cyclic peptide APL-9 is a C1 inhibitor (C1inh) and eculizumab is an inhibitor of C1, C3, and C5, which may suppress the complement activation cascade. c Innate receptors, including TLR2 for virus capsid, TLR9 for unmethylated CpG, and RIG-I/MDA5 for dsRNA, have been shown to promote inflammation. Through a series of signal transduction events, IRF3, IRF7, and NF-κB will become phosphorylated and translocate to the nucleus, where they activate type I interferons (IFN-I), interferon-stimulated genes (ISGs), and proinflammatory cytokines that mediate antiviral immunity and propagate inflammation. Many pathway components can be targeted by pharmacological inhibitors. In addition, the telomere-derived io2 sequence can suppress TLR9-mediated recognition of the transgene. d Transgene and capsid can be processed by the proteasome into peptides, which are presented by MHC-I or MHC-II molecules. CD8 and CD4 T cells that recognize those peptides will become activated. CD8 T cells may directly kill the rAAV-transduced cell, while CD4 T cells can help both CD8 T cells and B cells to enhance their effector function. B cells with specificity to the capsid can release antibodies that eliminate future possibilities of redosing. miRNA-binding sites engineered to the transgene can interact with cellular miRNA, leading to transcript degradation. In antigen-presenting cells, this design may prevent the presentation of transgene-derived peptides to T cells. In addition, anti-CD20 antibody rituximab and mTOR inhibitor rapamycin can be used to reduce adaptive responses. Figure created with Biorender.com
Pre-existing immunity
Pre-existing humoral response against the rAAV capsid, measured as rAAV-specific NAbs, likely comes from the immunological memory of past encounters with wtAAV. Newborns harbor maternal AAV NAbs for the first 6 months.56 After that, the neutralization titer drops but reappears from 7–9 months old onward. The overall seropositive rate ranges from below 10% to over 90%, depending on the serotype and geographical location.376 This is the single most detrimental burden to rAAV gene therapy because even low levels of seropositivity can markedly reduce transgene expression if the vector is given via i.v. administration.377
During active engagement with the foreign antigen, some IgG-expressing B cells will differentiate into long-lived plasma cells that primarily reside in the bone marrow and continue to express antigen-specific IgG long after the resolution of infection. While IgM levels tend to drop sharply after the resolution of the infection, IgG levels can be maintained for a substantially longer time and constitute the main humoral barrier for productive infection.378
NAbs also present the most significant challenge in redosing. Because rAAV genomes are typically maintained as circularized episomes,379 their average number per cell reduces by a factor of two every time the host cell divides and is lost when the host cell dies. Hence, subsequent doses of gene therapy may be required during a patient’s lifetime,380, especially for gene therapies that target organs with active cell turnover, such as the liver. However, redosing is inhibited by NAbs developed in response to the first dose.
rAAV capsid–antibody interactions especially for gene therapies
The antibody footprint of the complementarity-determining region (i.e., where the antibodies touch the antigen) on the AAV capsid surface can be captured through cryo-EM or peptide scanning.21 Some antigenic “hot” areas have been identified, such as the icosahedral 3-fold axes, the 5-fold axes, and the 2/5-fold wall.381 A recent investigation in patients receiving Zolgensma found that ~75% of antibodies are bound to the 2-fold surface.382 While these discoveries help guide capsid engineering toward escape from pre-existing immunity imposed by antibodies,70 it is important to keep in mind that the rAAV is quite small relative to the antibody, such that a few antibodies binding to the virion is sufficient to cover the viral surface and block its cell entry.
Impact on local vs. systemic routes of administration
Intravenous infusion is most susceptible to rAAV-specific circulating antibodies.383 For local injection routes into immune-privileged organs, such as the eye and the CNS, the restriction imposed by rAAV-specific antibodies is less severe and there is a possibility of redosing.384 While pre-existing NAbs are much lower in the vitreous humor than in the serum, the NAb level in the vitreous humor is negatively associated with the integrity of the BBB,385 an important indicator of potential effects of ocular gene therapy. In NHPs receiving ADVM-022 via the intravitreal route, injection in one eye resulted in NAbs in the serum and the injected eye, but not in the uninjected eye.386 Intravitreal injection of the remaining eye 2 months later did not lead to exacerbated inflammation. The transgene expression in the second eye trended lower compared to the first eye but was within the predicted range.
Interestingly, this differential susceptibility to circulating NAbs may be explored as a strategy to limit vector transduction in off-target tissues.387 When performing intrathalamic or i.c.v. injection, the presence of NAbs blocked transgene delivery to the liver but not to the CNS.384,387
Screening for pre-existing immunity
The level of rAAV capsid-specific total antibody (TAb) level can be measured using enzyme-linked immunosorbent assays. To determine patient eligibility for clinical trial participation, many trials employ a cell-based transduction inhibition (TI) assay to measure how dilute the patient’s serum needs to be to allow optimal rAAV transduction.307,388,389 There are instances when the serum is TAb-positive and TI-negative, indicating that not all AAV-specific antibodies inhibit transduction. More intriguingly, some sera are TAb-negative and TI-positive, suggesting the presence of inhibitory serum components that are not antibodies.
Evasion strategies of pre-existing immunity
CD20 is a highly expressed B cell-specific molecule that can be targeted by rituximab, which is given in several gene therapy trials in conjunction with other immune suppressors, such as steroids and rapamycin, to reduce general inflammation as well as B cell- and T cell-mediated immunity.390–392 However, rituximab is not effective at preventing pre-existing humoral immunity because long-lived plasma cells do not express CD20.383
rAAV packaged in extracellular vesicles (EVs) may allow partial escape from NAbs.393–396 The EVs can be coated with immunosuppressive molecules, such as CTLA-4, for further immune suppression.397 Packing rAAVs inside EVs may modify the tissue tropism, which may require additional modifications of the EV similar to modifying the rAAV capsid as discussed above, represents another popular approach for evading pre-existing immunity.
Recently, bacterial endopeptidases that cleave human antibodies have been explored.398 Imilifadase (IdeS), an IgG-degrading enzyme from Streptococcus pyogenes, has been shown to deplete total IgGs and allowed significantly better vector transduction in rAAV-seropositive NHPs.399 Its homolog IdeZ from S. equi ssp. zooepidemicus demonstrated similar effects.400 IdeXork, a proprietary IgG-degrading enzyme, is entering an rAAV-based clinical trial for Late-Onset Pompe disease.401 Across multiple studies, it appears that IdeS-type enzymes can provide an IgG-free window of 5–7 days.399,402,403 One potential caveat is that IdeS seems to function best when the neutralization titer is only moderately high (~1:108 for AAV3B in NHP).402 In one NHP starting with a neutralization titer of 1:696, IdeS reduced the titer to 1:88, which still inhibited transgene expression. Also, an adaptive immune response against IdeS may negate its efficacy. Drawing from transplantation studies, using IdeS the second time may only allow a therapeutic window of 24 h due to the generation of anti-IdeS antibodies.404–406
There are also IgM-cleaving enzymes (IceM) from S. suis and IceMG, which are engineered from IceM and cleaves both IgM and IgG.407,408 In addition to cleaving free-form IgM/G in the serum, these enzymes can cleave IgM from the B cell surface and block complement activation mediated by rAAV9 in vitro.408 Whether these will translate to clinical benefits remains to be studied.
Complement activation
The complement system consists of a series of highly abundant serum proteins and their associated cellular receptors.409,410 Many patients receiving high dose (~1.0 × 1014 vgs/kg) rAAV gene therapy developed TEAEs related to complement activation, including thrombocytopenia, hemolytic anemia, TMA, liver enzyme elevation, acute liver injury, and hemolytic uraemic syndrome (aHUS)-like kidney injury.411,412 Fatality in association with complement activation has been documented.413 It should be noted that as of Jan 13, 2022, nine cases of TMA were recorded out of over 1400 patients (~0.6%) receiving Zolgensma (https://www.fda.gov/media/126109/download).
Complement activation mechanism
Complement activation may happen through one of three pathways: classical (IgM/IgG, C1q, and C4), alternative (C3 spontaneous hydrolysis), and lectin (mannose-binding lectin).409 All three pathways converge to the formation of C3 convertase, C5 convertase, and finally, the membrane-attack complex that kills cells.409
The mechanism of complement activation during rAAV infusion is yet to be elucidated. AAV2 capsid could directly immunoprecipitate C3 and its cleavage products C3b and iC3b,414 but rAAV particles at a dose equivalent of 1 × 1014 vgs/kg in vitro did not activate C3 in pooled human serum without IgG. Ex vivo experiments demonstrated that AAV in whole blood activated complements only when anti-AAV antibodies were present.415 Multiple clinical trials noted the decrease of complement C4 in a subset of rAAV recipients.416,417 In addition, a recent study on patients receiving Zolgensma demonstrated that patients who received steroids alone exhibited higher incidences of TMA and higher AAV9-specific IgM and IgG titers, while patients who received steroids together with rituximab and rapamycin presented lower antibody titers and minimal complement activation.418 The collective evidence seems to suggest that complement activation in rAAV infusion occurs through the classical pathway. However, two observations reveal unresolved conflicts with this hypothesis. First, lower C4 levels were recorded before the development of rAAV-specific IgM and IgG in an AAV-naïve patient. Second, patients are pre-screened to be seronegative for the specific AAV serotype.
After rAAV administration, neither mice nor NHPs seem to share complement activation features observed in humans. In NHPs, complement activation was transiently detected at day 3 after dosing, even before the detection of AAV-specific IgM, and presented as an increase in Bb and sC5b-9 but not C4b.419 In mice, our unpublished results indicate that complement activation is very mild if AAV is given at 2.5 × 1014 vgs/kg dose equivalent. A recent study in mice demonstrated that C3b in serum and C5b-9 staining in the liver became detectable at 3.0 × 1014 vgs/kg, and at a supra-clinical dose of 7.0 × 1014 vgs/kg, mild thrombocytopenia could be detected with the peak of complement activation occurring at day 3.420
Complement suppression strategies
Several complement inhibitors are being tested for use in rAAV gene therapy, including the C5-blocking monoclonal antibody eculizumab.412,413 C3-inhibiting peptide APL-9421 and a naturally occurring serum protein C1 esterase inhibitor (C1-inh).422
Eculizumab is already used for aHUS and has been given to gene therapy recipients following TMA.413 While most patients recover from TMA, one patient eventually dies of acute kidney injury due to new-onset Staphylococcus epidermidis sepsis. Eculizumab lowered serum sC5b-9 and slightly increased platelet count but did not rescue the patient, as complements are essential in defense against bacteria.
Because C3 interaction with rAAV capsid promoted virus uptake in mouse macrophages,414 complement inhibition may affect rAAV transduction efficiency. This was shown in an ex vivo experiment as APL-9 lowered rAAV uptake by human blood leukocytes in NAb-high serum but not NAb-low serum.421 C1-inh, however, increased liver transduction by ~33% and ~28% in non-immunized and immunized mice, respectively.
Innate immunity
Myriad cell surface, endosomal, and cytosolic receptors detect molecular signatures commonly associated with pathogens to act as an alert system for the cells’ defense mechanisms and initiate an inflammatory response. Activation of innate sensors typically happens within minutes or hours. Because rAAV is virus-based, some of its inherent features are likely to be detected by these innate receptors. The secretion of cytokines triggered by the immune response has been shown to markedly reduce transgene expression and promote adaptive responses.423,424
Innate immune receptors shown to interact with rAAV
Innate receptors that have been shown to interact with rAAV include TLR-2, TLR-9, MDA5, and RIG-I.425 TLR-2 is a cell surface receptor that can recognize a variety of ligands associated with microorganisms, such as lipoproteins, peptidoglycans, and glycolipids.426 It can further expand its ligand recognition repertoire by forming heterodimers with TLR-1 and TLR-6. rAAV2 and rAAV8 both activate NF-κB-mediated cytokine expression in human liver cells in a TLR-2-dependent manner.427 TLR-9 is an endosomal membrane-attached receptor responsible for recognizing unmethylated CpG motifs in microbial DNA. Activation of TLR-9 in rAAV not only results in acute expression of cytokines, such as IL-6 and TNF-α, directly downstream of TLR signaling but also promotes infiltration of cytotoxic CD8+ T cells, leading to the loss of transduced target cells.112,424,428 Depletion of CpG motifs from a rAAV8-based vector to treat hemophilia B led to reduced NAb development.133 MDA5 and RIG-I are cytosolic sensors of dsRNA. The 3′ ITR region of rAAV may have promoter activity for the synthesis of minus-strand RNA, which can anneal to the plus-strand transcripts to form double-strand RNA.423 Both human hepatocytes and primate retina expressed high levels of MDA5 and RIG-I, which mediated IFN-β production following rAAV infection in vitro.423,429
A recent study demonstrated that IL-1 receptor (IL-1R)-MyD88 signaling are critical component involved in rAAV detection, as IL-1R−/− and MyD88−/− mice did not show a transgene-specific T cell response following rAAV8.OVA injection.430 The ligands for IL-1R are IL-1α and IL-1β, which are activated following inflammasome activation,431,432 while MyD88 acts downstream of TLRs and IL-1R.433 Intriguingly, mice deficient in TLR2/3/4/9, NLRP1/3, AIM2, or Caspase-1 exhibited normal or only slightly reduced transgene-specific T cell responses,430 suggesting that the rAAV was detected by a yet to be identified innate immune sensor.
Strategies to mitigate innate receptor signaling
To reduce TLR-9 signaling, CpG motifs in the rAAV cargo can be removed by modifying codons.105 One concern with this approach is that there are differences in the abundance of tRNA molecules, so altering their usage could potentially change the protein folding dynamic and increase the chance of misfolding.434 Therefore, incorporating a methyltransferase during rAAV production may be a good alternative.434 Another possibility is to incorporate TLR-9 inhibitory sequences. A mammalian telomere-derived ODN TTAGGG repeat sequence can form G-tetrads which can bind to TLR-9 in order to inhibit its dimerization and signaling.435 rAAV with this sequence demonstrated reduced immunostimulatory capacity, leading to less T cell infiltration in liver, muscle, and retina across various animal models.436
Pharmacological inhibitors of innate signaling pathways are another option. The blockade of IL-1 (inflammasome cytokine) reduced the frequency of transgene-specific CD8+ T cells,437 while inhibiting IL-6 (TLR cytokine) resulted in reduced antibody responses against the rAAV capsid.438 Inhibiting IRAK4 (TLR kinase) led to lower TLR-9 cytokine expression in vitro, and in vivo testing is underway.439
Adaptive immunity
Adaptive immunity refers to the antigen-specific B cell and T cell responses. B cells can produce antibodies targeting the rAAV capsid, which eliminates the possibility of redosing. Cytotoxic CD8+ T cells can recognize foreign peptides from transgenes and the rAAV capsid, and initiate the killing of transduced cells.306,440,441 CD4+ T cells can help B cell and cytotoxic T cell responses by secreting Th1, Th2, or Th17-type cytokines.441 The formation of adaptive B cell responses follows the same mechanism as pre-existing humoral immunity, so this section will primarily focus on T cell responses.
T cell-mediated adverse effects
Dorsal root ganglia (DRG) are clusters of sensory neurons located along the spinal cord that play a crucial role in transmitting sensory information. NHP, rodent, and piglet models have displayed a high incidence of DRG lesions in a dose-dependent manner.442–444 Symptoms suggesting DRG toxicity, including sensation changes, pain, and DRG enhancement on magnetic resonance imaging (MRI), were observed in one human patient.445 Part of the injury may be transgene-induced, which is thought to be limited in duration and scope, while severe DRG toxicity is associated with the influx of CD8+ T cells and is unaffected by steroids (https://www.fda.gov/media/152000/download). Similarly, myocarditis following systemic rAAV transfer in NHPs was attributed to transgene-specific cytotoxic T cell responses due to the presence of cardiac muscle damage at a later onset, high infiltration of CD8+ T cells in severe myocarditis, and high transgene specificity identified by enzyme-linked immunosorbent spot assays.419
Anti-capsid T-cell responses have also been demonstrated. One hemophilia B patient receiving 2.0 × 1012 vgs/kg AAV2-FIX displayed no anti-transgene T cell response but demonstrated an anti-AAV2 capsid T cell response. This is possibly associated with this patient’s human leukocyte antigen (HLA)-B*0702 allele, which is predicted to have high-affinity binding with a rAAV2 capsid peptide VPQYGYLTL.306 In a different hemophilia B trial using scAAV2/8, none of the six enrolled patients displayed anti-transgene responses.307 However, anti-capsid T-cell responses were clearly present in patients receiving doses at or above 6.0 × 1011 vgs/kg. In two patients, the level of anti-capsid T cell response seems to correlate with a sudden increase in alanine transaminase levels.
Strategies to reduce adaptive immunity
miRNAs are small RNA molecules that can bind to mRNA transcripts and catalyze transcript degradation.446 Cells naturally express certain miRNA molecules to regulate mRNA transcript abundance. For example, miR-142 is highly expressed by APCs.142 By engineering a miR-142 binding site (BS) into the 3′-UTR of the transgene, its translation can be selectively inhibited in APCs, thus reducing antigen presentation to T cells.143 Applying the same principle, miRNA-183-BS, which binds to DRG-enriched miR-183, significantly reduced transgene expression in DRG and lowered DRG toxicity.443
Another potential mitigating strategy is to target the peptide processing and MHC presentation pathway.447 The transgene and capsid sequences are known, and the patient’s HLA types can be determined, so prediction programs are being developed to analyze potential interactions. If required, the transgene and capsid sequences can be redesigned to de-immunize immunodominant peptides when significant interactions are predicted, thereby lowering the likelihood of MHC presentation.
Suppressing innate immune responses can also reduce the intensity of adaptive responses.430 mTOR is a kinase acting downstream of multiple cellular receptors.448 The mTOR inhibitor rapamycin and its derivatives significantly suppressed adaptive B cell and T cell responses, allowing for better transgene expression and redosing in mouse models.449,450 Currently, there is no universal strategy to suppress B-cell and T-cell responses in an antigen-specific manner. Steroids suppress both innate and adaptive immunity and are widely used in rAAV gene therapy.451 While studies have demonstrated that steroids can increase transgene persistence, others report that certain anti-transgene T-cell responses do not respond to steroids.418,452,453
Current immunosuppression drugs
Many trials now use a triple immune suppression regimen that includes steroids, rituximab, and rapamycin.417 Nearly all of the drugs currently used or being investigated for the suppression of immune responses in rAAV clinical trials were originally developed for other diseases, such as autoimmune diseases, transplantations, and tumors (Table 3). The physiology between those diseases and rAAV-based gene therapy can be highly divergent, which calls for further drug development specifically in the context of rAAV.
Table 3.
Immune suppression drugs currently used in gene therapy trials or under investigation in animal models
| Drug | Mechanism of action | Also used in |
|---|---|---|
| Steroids | Binds glucocorticoid receptor, inhibits vasodilation, downregulates inflammatory gene expression | Inflammatory diseases |
| Rituximab | Deplete B cells | B cell lymphoma |
| CD19-specific CAR-T | Deplete B cells | B cell lymphoma |
| Rapamycin/ImmTOR | Inhibits mTOR | |
| Tacrolimus | Inhibits Calcinerin | Organ transplant |
| Anakinra | IL1R antagonist | RA |
| Azathioprine | Inhibits purine synthesis | RA, lupus, Inflammatory bowel diseases, transplant |
| Nipocalimab | Binds FcRn | HDFN |
| Daratumumab | Anti-CD38 | MM |
| Bortezomib | Inhibits 26S proteosome | MM, RA |
| Imlifidase (IdeS) | Cuts IgG | Kidney transplant |
| Soluble CTLA-4 | Binds CD80/86 | |
| Clodronate | Depletes macrophages | Osteoporosis, pain control |
| Hydrogel | Slow release | |
| Extracellular vesicles | Coats AAV | |
| Dasatinib/Acalabrutinib | Kinase inhibitors | Leukemia |
ImmTOR tolerogenic nanoparticles encapsulating rapamycin, mTOR mammalian target of rapamycin, RA rheumatoid arthritis, HDFN hemolytic disease of the fetus and newborn, MM multiple myeloma, CTLA-4 cytotoxic T-lymphocyte–associated antigen 4
Three key areas require attention in advancing our understanding of rAAV immunology. First, there is a notable gap in studies examining the basic immunology of wtAAV, largely related to the fact that until very recently, wtAAV was not known to cause disease.31,33 Second, immunology research tends to focus on primary immune cells, cell lines derived from immune cells, or tumor cells. This overlooks the diverse somatic cells targeted by rAAV gene therapy in vivo (e.g., CNS cells, retinal cells, hepatocytes, muscle cells), each possessing different immunological features. Third, insights gained from animal models may not always reflect the human immune responses observed in clinical trials. Addressing these challenges could involve improved in vitro modeling systems such as mixed cell cultures, 3D organoids, and induced pluripotent stem cells.454 Additionally, the advancement of CRISPR screening technologies may help reveal cellular factors that participate in rAAV-based gene delivery.207 Innovative in vivo systems are also being developed, such as mice-engrafted human bone marrow, liver, and thymus (BLT) systems, which may be helpful for monitoring the activation of human innate sensors and the development of human adaptive immunity.455
Adverse effects/toxicities and management strategies
Safety remains a major concern for therapy development. While rAAV is favored among currently available viral vectors because of its safety profile, it is not without risks. This section focuses on the side effects of rAAV that have clinically drawn attention (Table 4).
Table 4.
Adverse effects and toxicities resulting from rAAV administration
| Toxicity | Pathology | Proposed mechanism | Supportive Treatment | Possible Association |
|---|---|---|---|---|
| Genotoxicity | Tumorigenesis identified in animals but unknown in humans | DNA damage | Unknown | rAAV serotype, promoter/enhancer elements, vector purities |
| Hepatotoxicity | • Liver enzyme elevation | • DNA damage | Corticosteroids | i.v., dose |
| • Drug-induced liver injury (rare) | • ER stress | |||
| Thrombotic microangiopathy | • Endothelial vessel injury | Complement activation | • Corticosteroids | i.v., dose |
| • Microvascular thrombosis | • Complement inhibition | |||
| • Plasmapheresis | ||||
| Dorsal root ganglion | • Immune cell infiltrates | Cellular overload with transgene | Unknown | c.m., i.t., dose |
| • Nerve cell body degeneration | ||||
| Brain MRI findings | • Edema/inflammation | Unknown | Unknown | i.t., i.p., clinical significance unclear |
| • Gliosis | ||||
| • Immune cell infiltrates |
ER endoplasmic reticulum, i.v. intravenous, c.m. cisterna magna, i.t. intrathecal, i.p. intraparenchymal
Genotoxicity (insertional mutagenesis)
wtAAV can integrate into the mammalian genome, facilitated by elements such as Rep protein.456 However, ITRs are the only wtAAV elements present in rAAV, which should reduce the risk for genome integration. However, data in animal models suggest that the chance for viral genome integration is similar between wtAAV and rAAV. The liver appears to be a hotspot for AAV integration, but viral genome integration in other organs such as the heart has been found as well.457 Genomic integration is not detrimental unless it disrupts tumor-suppressing or activates oncogenic genes. The liver is highly transduced, particularly when AAV is delivered by i.v. administration. Thus, hepatocellular carcinoma (HCC) is of particular concern and has been widely described in animal studies.457–465 Interestingly, predisposing factors, such as animal age or underlying liver disease, correlate to the occurrence of HCC in animals. In contrast, no genotoxicity in NHPs or dogs has been described.459 Although wtAAV genome integration has been found in humans, a causative role in tumor development has not been established.466–468 Similarly, the role of therapeutic rAAV in human genome integration and its ability to cause cancer is unclear.469
The growing application of genome editing tools, particularly CRISPR, has led to an observed increase in rAAV genome integration following CRISPR-induced double-strand breaks in animal models, posing another challenge to safety.470,471 Other factors postulated to influence vector genome integration include rAAV serotype, promoter/enhancer elements, and vector impurities. Overall, the possibility and risk of therapeutic genome integration and its role in tumor formation in human patients are not known.
Hepatotoxicity
The administration of high doses of rAAV via i.v. injection is a strategy to achieve widespread transduction of different organs (e.g., muscle and CNS). However, this approach can cause liver toxicity as substantial numbers of viral particles pass through the liver upon entering circulation. Early signs of hepatotoxicity are elevated liver enzymes as monitored by blood testing, which can remain clinically silent or progress to signs of liver injury, failure, and even death.265,472,473 Prophylactic and post-dosing increase of corticosteroids has shown effects to mitigate hepatotoxicity. Liver toxicity after i.v. administration often occurs several weeks to months after treatment, suggesting that an immune response is responsible for the effect.265,306,307,472 This is also supported by earlier reports in hemophilia B, where decreased transgene expression was associated with T cell response against the rAAV capsid and rising liver enzymes.306,474
TMA
TMA is thought to result from endothelial injury with concomitant platelet aggregation, causing the formation of microthrombi, thrombocytopenia, end organ damage through ischemia, and possible death. It can be acquired or genetic475,476 and its association with rAAV gene therapy has only recently become apparent. Although the mechanism is not fully understood, dysregulated complement activation following rAAV infusion has been postulated. In addition to close patient monitoring, current strategies focus on prophylactic immune suppression. Successful treatment of TMA has been reported with supportive care, corticosteroids, eculizumab, and plasmapheresis.412 Most cases of rAAV-associated TMA have been reported for Zolgensma and in DMD trials.412,413,477 It is not known what factors could cause the predisposition to TMA after rAAV administration. Possible patient-specific factors such as the disease phenotype and the course of the underlying disease are starting to emerge.413
Neurotoxicity
DRG toxicity
DRG toxicity causing inflammation and neuronal loss in sensory ganglions with possible extension into dorsal spinal cord columns is a recently noted rAAV-associated side-effect.442,478–481 Current data are primarily from preclinical studies in rodents, monkeys, and piglets where i.v. and i.t. infusion of rAAV was associated with inflammatory infiltrates and neuronal degeneration. DRG toxicity can be preclinically silent, but studies described animal proprioceptive deficits and ataxia.480 Histopathological and clinical changes usually occur but can be delayed, suggesting that toxicity is related to the expression of the transgene rather than the capsid. In support of this, one study reported that no DRG toxicity was observed after the administration of a non-expressing rAAV9 vector.479 Recently, serum neurofilament (NF) levels were proposed as a marker,442 although how serum NF changes are clinically relevant to DRG toxicity is not known. In addition, serum NF changes might be disease-associated and part of the natural history, which would complicate the interpretation of NF levels.
Another challenge is the limited information about DRG toxicity in human patients. In a trial consisting of two patients given i.t. infusion of rAAV9 at a dose of 4.2 × 1014 vgs, one patient developed tingling in his arms and painful shooting pain in the left foot, which correlated with electrophysiologic changes and DRG contrast enhancement on MRI. The second patient received higher levels of immune suppression than the first patient and did not develop clinical signs of DRG toxicity.445 With an increasing number of patients receiving rAAV, the mechanisms, diagnosis, prevention, and treatment of neurotoxicities must be elucidated to improve safety and patient outcomes.
MRI findings
As medical technologies evolve, increased sensitivity facilitates the identification of cellular changes that were previously undetectable. This creates new challenges in how to interpret these findings and determine whether they are of clinical relevance. One example is changes in brain MRI detected after intraparenchymal injections of rAAV.
In preclinical studies, direct intraparenchymal rAAV delivery was associated with lymphocyte aggregation in perivascular spaces (lymphoplasmacytic perivascular cuffing), microglial changes, and neuronal swelling, in the area of the vector administration.482–484 Interestingly, changes were also found in the vehicle control group in one study. However, escalating doses resulted in a proportional increase in regions with T and B cell infiltrates, suggesting that changes are caused by the procedure and the vector.484 Although MRI changes were identified in both the vehicle and vector groups, the features might indicate different etiologies.484 Of note, no clinical changes were observed in this study.
In a human trial for Batten disease, rAAV.rh10 administration expressing CLN2 was associated with post-infusion MRI changes, which continued to be present in most subjects by 12 months post-infusion. Importantly, no apparent clinical signs were attributed to those MRI findings.485 The significance of post-infusion MRI changes is currently unclear, particularly in neurodegenerative disease, where it might be challenging to discern disease-related changes from the procedure and vector-associated effects. However, understanding the underlying molecular and cellular mechanisms will be critical for patient safety and gene therapy success.
Current gaps in knowledge and potential solutions
Persistence
The promise of gene therapy as a single treatment for lifelong therapeutic effects depends on rAAV’s persistence. Though the immunity-mediated loss of rAAV genome or expressed transgene is excluded here (discussed in the section on rAAV immune response), two factors related to rAAV and patient remain regulatory elements of the expression cassette and cellular renewal/survival.
Endogenous genes are tightly regulated in a spatio-temporal manner, meaning that a specific gene is expressed at varying levels in different organs or even different cells within the same organ at a given time point. Widely used generic promoters are unable to accommodate such gene regulatory differences. Loss of transgene expression has been attributed to the deactivation of promoter and other regulatory elements used in the rAAV expression cassette design.486 In addition, promoters without cellular or organ specificity can present a challenge to safety. A possible solution is the use of promoter and regulatory elements derived from the endogenous promoter of the therapeutic transgene.115 However, endogenous regulatory elements typically exceed the packaging limit of rAAV and, therefore, require trimming, possibly excluding essential elements. A promising example is the use of a shortened SMN promoter for the treatment of SMA, which showed improved efficacy and reduced toxicity in mice.487
One challenge of using a partial promoter could be fluctuations in transgene expression during the development of an organism or patient, which could be desired if it reflects the endogenous gene expression but could pose a therapeutic issue if it compromises the therapeutic effect. It is, therefore, critical to better understand the physiologic regulation of the therapeutic gene.
From a host’s perspective, the rAAV genome’s persistence and transgene expression depend on the cells’ half-life time. This can be highlighted in two examples: neurons and the liver. Neurons are considered terminally differentiated, meaning that transduced neurons harbor the rAAV genome throughout the cell’s lifetime. Because the number of neurons does not substantially increase during life, it is unlikely that the rAAV genome would be diluted or even lost due to cellular proliferation. However, a partially treated neurodegenerative disease might be prone to ongoing neuronal loss and would eventually lose the rAAV genome and, thus, transgene expression.
In contrast, the cellular behavior of the liver is dynamic. Hepatocytes can proliferate continuously, with individual hepatocytes living for approximately three years.488 This could profoundly dilute the rAAV genome and even lead to transgene expression loss with the subsequent decline in therapeutic effect. As for most diseases, these aspects are not fully understood, emphasizing the need to better understand the molecular and cellular diseases for successful and long-lasting gene therapy.
While current rAAV therapies in humans have demonstrated encouraging results and life-changing outcomes, it is important to recognize that gene therapy is a relatively young field. The substantial number of patients receiving rAAV gene therapy is a recent accomplishment. It may be premature to conclude on questions of therapeutic effect persistence and the need to manage diminishing therapeutic effects, possibly through strategies such as re-dosing or combination with cell therapy.
Disease-specific needs
Our mechanistic understanding of rAAV transduction has greatly increased over the years. However, whether and how molecular and cellular changes in different pathologies affect rAAV’s performance is poorly understood. For example, disease conditions could change the cellular lineage to affect the population of target cells. An additional complication is that similar dynamics might exist for regulatory elements that control transgene expression.
A goal of gene therapies is to benefit every patient, irrespective of their disease stage and progression. Patient trial eligibility is based on clinical, biochemical, and radiographic assessment. This approach can only approximate the disease stage but has limitations in predicting disease progression or response to treatment. While current data in humans highlight that earlier treatment correlates with better outcomes, the limitations in defining disease stage and correlating it to progression and response to gene therapy potentially exclude many patients who could otherwise have a favorable outcome. For example, two different hypotheses were postulated to explain the fatal outcome in two independent rAAV trials for DMD.336,337 This suggests that the ability to understand disease dynamics, particularly in the context of gene therapy administration, is still limited.
Overcoming these challenges is necessary to continue making advancements in the understanding of disease mechanisms and improvements of rAAV. For example, widespread targeting of muscle tissue for DMD is only likely to be achieved with i.v. delivery. Modifying rAAV capsids to enhance specificity and reduce dose will be crucial for improved safety and efficacy using this delivery method. Although isolated limb perfusion could serve as an alternative, it is unlikely sufficient to reach the entirety of disease pathology. However, combinations of routes of delivery might help to exploit the advantages and simultaneously reduce the disadvantages.
Another example is widespread CNS targeting. Although new routes of administration to confine rAAV to the CNS space (i.e., i.t., i.c.v.) are being tested, the widespread transduction of the CNS seems to benefit from the finely arborized blood vessel supply of the CNS, which is utilized by i.v. rAAV delivery. However, newer capsids might facilitate parenchymal penetration and intraparenchymal spread, thus reducing the disadvantages of i.v. delivery while achieving widespread cellular transduction.
Conclusion
In conclusion, we have discussed the current understanding of AAV biology and manufacturing with a focus on the clinical trials of rAAV-based gene therapy and the challenges facing rAAV when applied to various diseases. The field is currently undergoing significant advancements, marked by progress in both academia and industry. Early successes in treating monogenic diseases have demonstrated the safety and effectiveness of rAAV-based gene therapy. However, challenges persist that must be addressed for a broader range of diseases. One prominent challenge is the effective delivery of rAAV to target tissues, where the choice of administration route can greatly impact the success of therapy. Addressing concerns related to specific cell/tissue targeting and overcoming physical barriers will be essential for expanding the range of targeted diseases. The immunogenicity of rAAV poses another challenge, impacting the safety, durability, and efficacy of gene therapy. Strategies to modulate and mitigate immune responses, including pre-existing immunity in patients, innate immune reactions upon vector administration, viral capsid, and transgene-induced adaptive immune response are essential for patient safety. Understanding the long-term consequences of immune reactions and their potential impact on repeated dosing will be critical. Finally, a better understanding of rAAV integration will inform its potential implications for tumor formation, hepatotoxicity, neurotoxicity, and newly detected adverse effects associated with high doses of rAAV need to be addressed to improve safety and accessibility for patients to this promising area of therapies.
Despite these challenges, several encouraging directions can advance the development of rAAV gene therapy. Research into rAAV-host interactions is vital for informing vector engineering and enhancing transgene expression. Strategies to achieve prolonged transgene expression, including immune evasion or immunosuppressive regimens, can extend the therapeutic benefits and reduce the need for repeated interventions. Tailoring rAAV-based therapies to disease-specific needs, such as customizing tissue, cell- and gene-specific promoters, optimizing transgene design, and engineering capsid variants with specific tissue tropism, represents another exciting direction. These newly engineered rAAVs must exhibit compatibility across species while demonstrating enhanced attributes and safety. Integrating rAAV with CRISPR-based tools, as gene editing technologies advance, offers remarkable potential for precise and targeted genetic modifications. These advancements promise to accelerate more effective treatments across a spectrum of diseases, from monogenic disorders to complex, multifactorial conditions. Overcoming current challenges and embracing these future directions holds the promise of turning gene therapy into a revolutionary power to address numerous human diseases.
Supplementary information
Current clinical trials employing rAAV-based gene therapy for human diseases
Acknowledgements
The authors thank all members of the Gao laboratory and their colleagues in the field for productive collaborations and motivating discussions on AAV and gene therapy. The authors apologize for not being able to be more comprehensive in reviewing all the work of their colleagues due to space limitations. J-H.W. is supported by the Graduate Education Fund of the American Australian Association. G.G. is supported by grants from the University of Massachusetts Chan Medical School (an internal grant) and the NIH (R01NS076991-01, P01HL131471-05, R01AI121135, UG3HL147367-01, R01HL097088, R01HL152723-02, U19AI149646-01, and UH3HL147367-04).
Author contributions
Conceptualization: J.-H.W. and G.G. Methodology: J.-H.W., D.J.G., and G.G. Formal Analysis: J.-H.W., D.J.G., and W.Z. Resources: G.G. Data Curation: J.-H.W., D.J.G., and W.Z. Writing- Original Draft: J.-H.W., D.J.G., and W.Z. Writing- Review & Editing: J.-H.W., D.J.G., W.Z., T.L.G., and G.G. Visualization: J.-H.W. and W.Z. Supervision: G.G. Project Administration: J.-H.W. and G.G. Funding Acquisition: G.G. All authors have read and approved the article.
Competing interests
G.G. is a scientific co-founder of Voyager Therapeutics, Adrenas Therapeutics, and Aspa Therapeutics, and holds equity in these companies. G.G. is an inventor on patents with potential royalties licensed to Voyager Therapeutics, Aspa Therapeutics, and other biopharmaceutical companies. D.J. G. is a cofounder of Aspa Therapeutics and holds equity in the company. D.J.G. is an inventor of patents with potential royalties licensed to Aspa Therapeutics and other biopharmaceutical companies. The remaining authors declare no competing interests. G.G. is one of the Associate Editors of Signal Transduction and Targeted Therapy, but he was not involved in the editorial administration and review of the manuscript.
Footnotes
These authors contributed equally: Jiang-Hui Wang, Dominic J. Gessler, Wei Zhan
Supplementary information
The online version contains supplementary material available at 10.1038/s41392-024-01780-w.
References
- 1.Wang D, Gao G. State-of-the-art human gene therapy: part II. Gene therapy strategies and clinical applications. Discov. Med. 2014;18:151–161. [PMC free article] [PubMed] [Google Scholar]
- 2.Adams D, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 2018;379:11–21. doi: 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, et al. AAV-delivered suppressor tRNA overcomes a nonsense mutation in mice. Nature. 2022;604:343–348. doi: 10.1038/s41586-022-04533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Gao G. State-of-the-art human gene therapy: part I. Gene delivery technologies. Discov. Med. 2014;18:67–77. [PMC free article] [PubMed] [Google Scholar]
- 5.Bulcha JT, Wang Y, Ma H, Tai PWL, Gao G. Viral vector platforms within the gene therapy landscape. Sig. Transduct. Target. Ther. 2021;6:53. doi: 10.1038/s41392-021-00487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atchison RW, Casto BC, Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- 7.Hoggan MD, Blacklow NR, Rowe WP. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc. Natl Acad. Sci. 1966;55:1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samulski RJ, Berns KI, Tan M, Muzyczka N. Cloning of adeno-associated virus into pBR322: rescue of intact virus from the recombinant plasmid in human cells. Proc. Natl Acad. Sci. 1982;79:2077–2081. doi: 10.1073/pnas.79.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laughlin CA, Tratschin JD, Coon H, Carter BJ. Cloning of infectious adeno-associated virus genomes in bacterial plasmids. Gene. 1983;23:65–73. doi: 10.1016/0378-1119(83)90217-2. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava A, Lusby EW, Berns KI. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J. Virol. 1983;45:555–564. doi: 10.1128/jvi.45.2.555-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samulski RJ, Chang LS, Shenk T. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J. Virol. 1987;61:3096–3101. doi: 10.1128/jvi.61.10.3096-3101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao GP, et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl Acad. Sci. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao G, et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. J. Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung AK, Hoggan MD, Hauswirth WW, Berns KI. Integration of the adeno-associated virus genome into cellular DNA in latently infected human Detroit 6 cells. J. Virol. 1980;33:739–748. doi: 10.1128/jvi.33.2.739-748.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laughlin CA, Cardellichio CB, Coon HC. Latent infection of KB cells with adeno-associated virus type 2. J. Virol. 1986;60:515–524. doi: 10.1128/jvi.60.2.515-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunbar CE, et al. Gene therapy comes of age. Science. 2018;359:eaan4672. doi: 10.1126/science.aan4672. [DOI] [PubMed] [Google Scholar]
- 18.Keeler AM, Flotte TR. Recombinant adeno-associated virus gene therapy in light of luxturna (and Zolgensma and Glybera): where are we, and how did we get here? Annu. Rev. Virol. 2019;6:601–621. doi: 10.1146/annurev-virology-092818-015530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D, Zhang F, Gao G. CRISPR-based therapeutic genome editing: strategies and in vivo delivery by AAV vectors. Cell. 2020;181:136–150. doi: 10.1016/j.cell.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Samulski RJ. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020;21:255–272. doi: 10.1038/s41576-019-0205-4. [DOI] [PubMed] [Google Scholar]
- 21.Tseng YS, Agbandje-McKenna M. Mapping the AAV capsid host antibody response toward the development of second generation gene delivery vectors. Front. Immunol. 2014;5:9. doi: 10.3389/fimmu.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonntag F, Schmidt K, Kleinschmidt JA. A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proc. Natl Acad. Sci. 2010;107:10220–10225. doi: 10.1073/pnas.1001673107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelsic ED, Ogden PJ, Sinai S, Church GM. Comprehensive AAV capsid fitness landscape reveals a viral gene and enables machine-guided design. Science. 2019;366:1139–1143. doi: 10.1126/science.aaw2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lusby E, Fife KH, Berns KI. Nucleotide sequence of the inverted terminal repetition in adeno-associated virus DNA. J. Virol. 1980;34:402–409. doi: 10.1128/jvi.34.2.402-409.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao G, et al. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc. Natl Acad. Sci. 2003;100:6081–6086. doi: 10.1073/pnas.0937739100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bashirians G, et al. Global seroprevalence of neutralizing antibodies against adeno-associated virus (AAV) serotypes of relevance to gene therapy. Blood. 2022;140:10668–10670. doi: 10.1182/blood-2022-158305. [DOI] [Google Scholar]
- 28.Matsushita T, et al. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998;5:938–945. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
- 29.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 1998;72:2224–2232. doi: 10.1128/JVI.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarty DM, Jr., Young SM, Samulski RJ. Integration of adeno-associated viruS (AAV) and recombinant AAV vectors. Annu. Rev. Genet. 2004;38:819–845. doi: 10.1146/annurev.genet.37.110801.143717. [DOI] [PubMed] [Google Scholar]
- 31.Servellita V, et al. Adeno-associated virus type 2 in US children with acute severe hepatitis. Nature. 2023;617:1–7. doi: 10.1038/s41586-023-05949-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morfopoulou S, et al. Genomic investigations of unexplained acute hepatitis in children. Nature. 2023;617:564–573. doi: 10.1038/s41586-023-06003-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho A, et al. Adeno-associated virus 2 infection in children with non-A–E hepatitis. Nature. 2023;617:555–563. doi: 10.1038/s41586-023-05948-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tacke F. Severe hepatitis outbreak in children linked to AAV2 virus. Nature. 2023;617:471–472. doi: 10.1038/d41586-023-00570-8. [DOI] [PubMed] [Google Scholar]
- 35.Lisowski L, Tay SS, Alexander IE. Adeno-associated virus serotypes for gene therapeutics. Curr. Opin. Pharmacol. 2015;24:59–67. doi: 10.1016/j.coph.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Issa SS, Shaimardanova AA, Solovyeva VV, Rizvanov AA. Various AAV Serotypes and their applications in gene therapy: an overview. Cells. 2023;12:785. doi: 10.3390/cells12050785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verdera HC, Kuranda K, Mingozzi F. AAV vector immunogenicity in humans: a long journey to successful gene transfer. Mol. Ther. 2020;28:723–746. doi: 10.1016/j.ymthe.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pillay S, et al. Adeno-associated Virus (AAV) serotypes have distinctive interactions with domains of the cellular AAV receptor. J. Virol. 2017;91:e00391–17. doi: 10.1128/JVI.00391-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dudek AM, et al. GPR108 is a highly conserved AAV entry factor. Mol. Ther. 2020;28:367–381. doi: 10.1016/j.ymthe.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhungel BP, Bailey CG, Rasko JEJ. Journey to the center of the cell: tracing the path of AAV transduction. Trends Mol. Med. 2021;27:172–184. doi: 10.1016/j.molmed.2020.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Woodard KT, Liang KJ, Bennett WC, Samulski RJ. Heparan sulfate binding promotes accumulation of intravitreally delivered adeno-associated viral vectors at the retina for enhanced transduction but weakly influences tropism. J. Virol. 2016;90:9878–9888. doi: 10.1128/JVI.01568-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen S, Bryant KD, Brown SM, Randell SH, Asokan A. Terminal N-linked galactose is the primary receptor for adeno-associated virus 9. J. Biol. Chem. 2011;286:13532–13540. doi: 10.1074/jbc.M110.210922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pillay S, et al. An essential receptor for adeno-associated virus infection. Nature. 2016;530:108–112. doi: 10.1038/nature16465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dudek AM, et al. An alternate route for adeno-associated virus (AAV) entry independent of AAV receptor. J. Virol. 2018;92:e02213–e02217. doi: 10.1128/JVI.02213-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berry GE, Asokan A. Cellular transduction mechanisms of adeno-associated viral vectors. Curr. Opin. Virol. 2016;21:54–60. doi: 10.1016/j.coviro.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schultz BR, Chamberlain JS. Recombinant adeno-associated virus transduction and integration. Mol. Ther. 2008;16:1189–1199. doi: 10.1038/mt.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sonntag F, Bleker S, Leuchs B, Fischer R, Kleinschmidt JA. Adeno-associated virus type 2 capsids with externalized VP1/VP2 trafficking domains are generated prior to passage through the cytoplasm and are maintained until uncoating occurs in the nucleus. J. Virol. 2006;80:11040–11054. doi: 10.1128/JVI.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao PJ, Samulski RJ. Cytoplasmic trafficking, endosomal escape, and perinuclear accumulation of adeno-associated virus type 2 particles are facilitated by microtubule network. J. Virol. 2012;86:10462–10473. doi: 10.1128/JVI.00935-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicolson SC, Samulski RJ. Recombinant adeno-associated virus utilizes host cell nuclear import machinery to enter the nucleus. J. Virol. 2014;88:4132–4144. doi: 10.1128/JVI.02660-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelich JM, et al. Super-resolution imaging of nuclear import of adeno-associated virus in live cells. Mol. Ther. Methods Clin. Dev. 2015;2:15047. doi: 10.1038/mtm.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fisher KJ, et al. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J. Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCarty DM. Self-complementary AAV vectors; advances and applications. Mol. Ther. 2008;16:1648–1656. doi: 10.1038/mt.2008.171. [DOI] [PubMed] [Google Scholar]
- 53.McCarty DM, et al. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10:2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- 54.Wang Z, et al. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- 55.Zinn E, et al. In silico reconstruction of the viral evolutionary lineage yields a potent gene therapy vector. Cell Rep. 2015;12:1056–1068. doi: 10.1016/j.celrep.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calcedo R, et al. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin. Vaccine Immunol. 2011;18:1586–1588. doi: 10.1128/CVI.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lochrie MA, et al. Adeno-associated virus (AAV) capsid genes isolated from rat and mouse liver genomic DNA define two new AAV species distantly related to AAV-5. Virology. 2006;353:68–82. doi: 10.1016/j.virol.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 58.Arbetman AE, et al. Novel caprine adeno-associated virus (AAV) capsid (AAV-Go.1) is closely related to the primate AAV-5 and has unique tropism and neutralization properties. J. Virol. 2005;79:15238–15245. doi: 10.1128/JVI.79.24.15238-15245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bossis I, Chiorini JA. Cloning of an avian adeno-associated virus (AAAV) and generation of recombinant AAAV particles. J. Virol. 2003;77:6799–6810. doi: 10.1128/JVI.77.12.6799-6810.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mietzsch M, et al. Characterization of the serpentine adeno-associated virus (SAAV) capsid structure: receptor interactions and antigenicity. J. Virol. 2022;96:e00335–22. doi: 10.1128/jvi.00335-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsu HL, et al. Structural characterization of a novel human adeno-associated virus capsid with neurotropic properties. Nat. Commun. 2020;11:3279. doi: 10.1038/s41467-020-17047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhong L, et al. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology. 2008;381:194–202. doi: 10.1016/j.virol.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duan D, Yue Y, Yan Z, Yang J, Engelhardt JF. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J. Clin. Investig. 2000;105:1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhong L, et al. A dual role of EGFR protein tyrosine kinase signaling in ubiquitination of AAV2 capsids and viral second-strand DNA synthesis. Mol. Ther. 2007;15:1323–1330. doi: 10.1038/sj.mt.6300170. [DOI] [PubMed] [Google Scholar]
- 65.Zhong L, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc. Natl Acad. Sci. 2008;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kanaan NM, et al. Rationally engineered AAV capsids improve transduction and volumetric spread in the CNS. Mol. Ther. Nucleic Acids. 2017;8:184–197. doi: 10.1016/j.omtn.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang D, et al. A rationally engineered capsid variant of AAV9 for systemic CNS-directed and peripheral tissue-detargeted gene delivery in neonates. Mol. Ther. Methods Clin. Dev. 2018;9:234–246. doi: 10.1016/j.omtm.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi W, Arnold GS, Bartlett JS. Insertional mutagenesis of the adeno-associated virus type 2 (AAV2) capsid gene and generation of aav2 vectors targeted to alternative cell-surface receptors. Hum. Gene Ther. 2001;12:1697–1711. doi: 10.1089/104303401750476212. [DOI] [PubMed] [Google Scholar]
- 69.Yao Y, et al. Variants of the adeno-associated virus serotype 9 with enhanced penetration of the blood-brain barrier in rodents and primates. Nat. Biomed. Eng. 2022;6:1257–1271. doi: 10.1038/s41551-022-00938-7. [DOI] [PubMed] [Google Scholar]
- 70.Tse LV, et al. Structure-guided evolution of antigenically distinct adeno-associated virus variants for immune evasion. Proc. Natl Acad. Sci. 2017;114:E4812–E4821. doi: 10.1073/pnas.1704766114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yao T, et al. Site-specific PEGylated adeno-associated viruses with increased serum stability and reduced immunogenicity. Molecules. 2017;22:1155. doi: 10.3390/molecules22071155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mevel M, et al. Chemical modification of the adeno-associated virus capsid to improve gene delivery. Chem. Sci. 2019;11:1122–1131. doi: 10.1039/C9SC04189C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shen X, Storm T, Kay MA. Characterization of the relationship of AAV capsid domain swapping to liver transduction efficiency. Mol. Ther. 2007;15:1955–1962. doi: 10.1038/sj.mt.6300293. [DOI] [PubMed] [Google Scholar]
- 74.Andrzejewski S, Karan KR, Stiles KM, Hackett NR, Crysta RG. Engineering of the AAVrh.10 capsid for cardiac gene transfer (ASGCT abstract 1389) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 75.Croyle MA, et al. PEGylated helper-dependent adenoviral vectors: highly efficient vectors with an enhanced safety profile. Gene Ther. 2005;12:579–587. doi: 10.1038/sj.gt.3302441. [DOI] [PubMed] [Google Scholar]
- 76.Horowitz ED, Weinberg MS, Asokan A. Glycated AAV vectors: chemical redirection of viral tissue tropism. Bioconjugate Chem. 2011;22:529–532. doi: 10.1021/bc100477g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Samai Poulami, et al. Antibody conjugated AAV vectors for efficient and specific skeletal muscle directed gene delivery across species (ASGCT abstract 42) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 78.Hong AV, Seul-Petat L, Hierves AA, Poupiot J, Richard I. An integrin-targeting AAV developed by a novel computational rational design methodology presents an improved targeting to the skeletal muscle and reduced tropism to the liver (ASGCT abstract 332) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 79.Chin JW. Expanding and reprogramming the genetic code. Nature. 2017;550:53–60. doi: 10.1038/nature24031. [DOI] [PubMed] [Google Scholar]
- 80.Puzzo F, et al. Aptamer-programmable adeno-associated viral vectors as a novel platform for cell-specific gene transfer. Mol. Ther. Nucleic Acids. 2023;31:383–397. doi: 10.1016/j.omtn.2023.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang H, et al. Non-canonical amino acid incorporation into AAV5 capsid enhances lung transduction in mice. Mol. Ther. Methods Clin. Dev. 2023;31:101129. doi: 10.1016/j.omtm.2023.101129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maheshri N, Koerber JT, Kaspar BK, Schaffer DV. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat. Biotechnol. 2006;24:198–204. doi: 10.1038/nbt1182. [DOI] [PubMed] [Google Scholar]
- 83.Koerber JT, Jang JH, Schaffer DV. DNA shuffling of adeno-associated virus yields functionally diverse viral progeny. Mol. Ther. 2008;16:1703–1709. doi: 10.1038/mt.2008.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li W, et al. Engineering and selection of shuffled AAV genomes: a new strategy for producing targeted biological nanoparticles. Mol. Ther. 2008;16:1252–1260. doi: 10.1038/mt.2008.100. [DOI] [PubMed] [Google Scholar]
- 85.Havlik LP, et al. Coevolution of adeno-associated virus capsid antigenicity and tropism through a structure-guided approach. J. Virol. 2020;94:e00976–20. doi: 10.1128/JVI.00976-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deverman BE, et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 2016;34:204–209. doi: 10.1038/nbt.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hordeaux J, et al. The neurotropic properties of AAV-PHP.B are limited to C57BL/6J mice. Mol. Ther. 2018;26:664–668. doi: 10.1016/j.ymthe.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liguore WA, et al. AAV-PHP.B Administration results in a differential pattern of CNS biodistribution in non-human primates compared with mice. Mol. Ther. 2019;27:2018–2037. doi: 10.1016/j.ymthe.2019.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nonnenmacher M, et al. Rapid evolution of blood-brain-barrier-penetrating AAV capsids by RNA-driven biopanning. Mol. Ther. Methods Clin. Dev. 2021;20:366–378. doi: 10.1016/j.omtm.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moyer Tyler, et al. Directed evolution of an AAV9 library identifies a capsid variant with enhanced brain tropism and liver de-targeting in non-human primates and mice following systemic administration [ASGCT abstract 105] Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 91.Maura D, et al. Stepwise evolution of the AAV5-derived capsid VCAP-100 identifies novel variants with improved CNS transduction and liver detargeting following systemic injection (ASGCT abstract 464) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 92.Tabebordbar M, et al. Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across species. Cell. 2021;184:4919–4938.e22. doi: 10.1016/j.cell.2021.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jin Q, et al. Discovery of novel AAV capsids with enhanced skeletal muscle tropism following directed evolution in cynomolgus macaque (ASGCT abstract 1165) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 94.Matsuzaki Y, et al. Intravenous administration of the adeno-associated virus-PHP.B capsid fails to upregulate transduction efficiency in the marmoset brain. Neurosci. Lett. 2018;665:182–188. doi: 10.1016/j.neulet.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 95.Gonzalez TJ, et al. Cross-species evolution of a highly potent AAV variant for therapeutic gene transfer and genome editing. Nat. Commun. 2022;13:5947. doi: 10.1038/s41467-022-33745-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dalkara D, et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 2013;5:189ra76. doi: 10.1126/scitranslmed.3005708. [DOI] [PubMed] [Google Scholar]
- 97.Santiago-Ortiz J, et al. AAV ancestral reconstruction library enables selection of broadly infectious viral variants. Gene Ther. 2015;22:934–946. doi: 10.1038/gt.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Andres-Mateos E, et al. Choice of vector and surgical approach enables efficient cochlear gene transfer in nonhuman primate. Nat. Commun. 2022;13:1359. doi: 10.1038/s41467-022-28969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Landegger LD, et al. A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat. Biotechnol. 2017;35:280–284. doi: 10.1038/nbt.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carvalho LS, et al. Synthetic adeno-associated viral vector efficiently targets mouse and nonhuman primate retina in vivo. Hum. Gene Ther. 2018;29:771–784. doi: 10.1089/hum.2017.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Libbrecht MW, Noble WS. Machine learning applications in genetics and genomics. Nat. Rev. Genet. 2015;16:321–332. doi: 10.1038/nrg3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Park C, Took CC, Seong JK. Machine learning in biomedical engineering. Biomed. Eng. Lett. 2018;8:1–3. doi: 10.1007/s13534-018-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bryant DH, et al. Deep diversification of an AAV capsid protein by machine learning. Nat. Biotechnol. 2021;39:691–696. doi: 10.1038/s41587-020-00793-4. [DOI] [PubMed] [Google Scholar]
- 104.Xie J, et al. MicroRNA-regulated, systemically delivered rAAV9: a step closer to CNS-restricted transgene expression. Mol. Ther. 2011;19:526–535. doi: 10.1038/mt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Faust SM, et al. CpG-depleted adeno-associated virus vectors evade immune detection. J. Clin. Investig. 2013;123:2994–3001. doi: 10.1172/JCI68205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Young SM, Jr, Samulski RJ. Adeno-associated virus (AAV) site-specific recombination does not require a Rep-dependent origin of replication within the AAV terminal repeat. Proc. Natl Acad. Sci. 2001;98:13525–13530. doi: 10.1073/pnas.241508998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bell P, et al. Effects of self-complementarity, codon optimization, transgene, and dose on liver transduction with AAV8. Hum. Gene Ther. Methods. 2016;27:228–237. doi: 10.1089/hgtb.2016.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fu H, et al. Self-complementary adeno-associated virus serotype 2 vector: global distribution and broad dispersion of AAV-mediated transgene expression in mouse brain. Mol. Ther. 2003;8:911–917. doi: 10.1016/j.ymthe.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 109.Gao GP, et al. High-level transgene expression in nonhuman primate liver with novel adeno-associated virus serotypes containing self-complementary genomes. J. Virol. 2006;80:6192–6194. doi: 10.1128/JVI.00526-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Korolev SE, Solov’ev VV, Tumanian VG. A new method of global search for functional DNA segments using a fractal representation of nucleotide texts. Biofizika. 1992;37:837–847. [PubMed] [Google Scholar]
- 111.Wu T, et al. Self-complementary AAVs induce more potent transgene product-specific immune responses compared to a single-stranded genome. Mol. Ther. 2012;20:572–579. doi: 10.1038/mt.2011.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martino AT, et al. The genome of self-complementary adeno-associated viral vectors increases toll-like receptor 9-dependent innate immune responses in the liver. Blood. 2011;117:6459–6468. doi: 10.1182/blood-2010-10-314518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang XS, Ponnazhagan S, Srivastava A. Rescue and replication of adeno-associated virus type 2 as well as vector DNA sequences from recombinant plasmids containing deletions in the viral inverted terminal repeats: selective encapsidation of viral genomes in progeny virions. J. Virol. 1996;70:1668–1677. doi: 10.1128/jvi.70.3.1668-1677.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pan X, et al. Rational engineering of a functional CpG-free ITR for AAV gene therapy. Gene Ther. 2022;29:333–345. doi: 10.1038/s41434-021-00296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xie Q, et al. Endogenous human SMN1 promoter- driven gene replacement improves the efficacy and safety of AAV9-mediated gene therapy for spinal muscular atrophy (SMA) in mice (ASGCT abstract 263) Mol. Ther. 2022;30:S1–592. [Google Scholar]
- 116.Buck TM, Wijnholds J. Recombinant adeno-associated viral vectors (rAAV)-vector elements in ocular gene therapy clinical trials and transgene expression and bioactivity assays. Int. J. Mol. Sci. 2020;21:4197. doi: 10.3390/ijms21124197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gray SJ, et al. Optimizing promoters for recombinant adeno-associated virus-mediated gene expression in the peripheral and central nervous system using self-complementary vectors. Hum. Gene Ther. 2011;22:1143–1153. doi: 10.1089/hum.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zheng D, et al. Machine-guided design of tissue-specific promoters (Abstract 172) Mol. Ther. 2023;31:S1–794 . [Google Scholar]
- 119.Favre D, et al. Lack of an immune response against the tetracycline-dependent transactivator correlates with long-term doxycycline-regulated transgene expression in nonhuman primates after intramuscular injection of recombinant adeno-associated virus. J. Virol. 2002;76:11605–11611. doi: 10.1128/JVI.76.22.11605-11611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ye X, et al. Regulated delivery of therapeutic proteins after in vivo somatic cell gene transfer. Science. 1999;283:88–91. doi: 10.1126/science.283.5398.88. [DOI] [PubMed] [Google Scholar]
- 121.Rivera VM, et al. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood. 2005;105:1424–1430. doi: 10.1182/blood-2004-06-2501. [DOI] [PubMed] [Google Scholar]
- 122.Breaker RR. Riboswitches and the RNA world. Cold Spring Harb. Perspect. Biol. 2012;4:a003566. doi: 10.1101/cshperspect.a003566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhong G, et al. A reversible RNA on-switch that controls gene expression of AAV-delivered therapeutics in vivo. Nat. Biotechnol. 2020;38:169–175. doi: 10.1038/s41587-019-0357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhong G, Wang H, Bailey CC, Gao G, Farzan M. Rational design of aptazyme riboswitches for efficient control of gene expression in mammalian cells. eLife. 2016;5:e18858. doi: 10.7554/eLife.18858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Luo, L., Jea, J. D.-Y., Wang, Y., Chao, P.-W. & Yen, L. Control of mammalian gene expression by modulation of polyA signal cleavage at 5′ UTR. Nat. Biotechnol. 1–13 10.1038/s41587-023-01989-0. (2024) [DOI] [PubMed]
- 126.Monteys AM, et al. Regulated control of gene therapies by drug-induced splicing. Nature. 2021;596:291–295. doi: 10.1038/s41586-021-03770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Naruse C, et al. A degron system targeting endogenous PD-1 inhibits the growth of tumor cells in mice. NAR Cancer. 2022;4:zcac019. doi: 10.1093/narcan/zcac019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brown HC, et al. Target-cell-directed bioengineering approaches for gene therapy of hemophilia A. Mol. Ther. Methods Clin. Dev. 2018;9:57–69. doi: 10.1016/j.omtm.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ward NJ, et al. Codon optimization of human factor VIII cDNAs leads to high-level expression. Blood. 2011;117:798–807. doi: 10.1182/blood-2010-05-282707. [DOI] [PubMed] [Google Scholar]
- 130.McIntosh J, et al. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood. 2013;121:3335–3344. doi: 10.1182/blood-2012-10-462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Steines B, et al. CFTR gene transfer with AAV improves early cystic fibrosis pig phenotypes. JCI Insight. 2016;1:e88728. doi: 10.1172/jci.insight.88728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gessler DJ, et al. Redirecting N-acetylaspartate metabolism in the central nervous system normalizes myelination and rescues Canavan disease. JCI Insight. 2017;2:e90807. doi: 10.1172/jci.insight.90807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Konkle BA, et al. BAX 335 hemophilia B gene therapy clinical trial results: potential impact of CpG sequences on gene expression. Blood. 2020;137:763–774. doi: 10.1182/blood.2019004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ravi S, Sharma T, Yip M, Gao G, Tai PWL. Improving codon optimization for gene therapy vectors using deep learning (ASGCT abstract 173) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 135.Powell SK, Rivera-Soto R, Gray SJ. Viral expression cassette elements to enhance transgene target specificity and expression in gene therapy. Discov. Med. 2015;19:49–57. [PMC free article] [PubMed] [Google Scholar]
- 136.Patricio MI, Barnard AR, Orlans HO, McClements ME, MacLaren RE. Inclusion of the woodchuck hepatitis virus posttranscriptional regulatory element enhances AAV2-driven transduction of mouse and human retina. Mol. Ther. Nucleic Acids. 2017;6:198–208. doi: 10.1016/j.omtn.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kingsman SM, Mitrophanous K, Olsen JC. Potential oncogene activity of the woodchuck hepatitis post-transcriptional regulatory element (WPRE) Gene Ther. 2005;12:3–4. doi: 10.1038/sj.gt.3302417. [DOI] [PubMed] [Google Scholar]
- 138.Themis M, et al. Oncogenesis following delivery of a nonprimate lentiviral gene therapy vector to fetal and neonatal mice. Mol. Ther. 2005;12:763–771. doi: 10.1016/j.ymthe.2005.07.358. [DOI] [PubMed] [Google Scholar]
- 139.Miao CH, et al. Inclusion of the hepatic locus control region, an intron, and untranslated region increases and stabilizes hepatic factor IX gene expression in vivo but not in vitro. Mol. Ther. 2000;1:522–532. doi: 10.1006/mthe.2000.0075. [DOI] [PubMed] [Google Scholar]
- 140.Lu J, et al. A 5’ noncoding exon containing engineered intron enhances transgene expression from recombinant AAV vectors in vivo. Hum. Gene Ther. 2017;28:125–134. doi: 10.1089/hum.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Choi JH, et al. Optimization of AAV expression cassettes to improve packaging capacity and transgene expression in neurons. Mol. Brain. 2014;7:17. doi: 10.1186/1756-6606-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xiao Y, et al. Circumventing cellular immunity by miR142-mediated regulation sufficiently supports rAAV-delivered OVA expression without activating humoral immunity. JCI Insight. 2019;4:1–12. doi: 10.1172/jci.insight.99052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Muhuri M, et al. Novel combinatorial microRNA-binding sites in AAV Vectors synergistically diminish antigen presentation and transgene immunity for efficient and stable transduction. Front. Immunol. 2021;12:674242. doi: 10.3389/fimmu.2021.674242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Martinez-Navio JM, et al. Adeno-associated virus delivery of anti-HIV monoclonal antibodies can drive long-term virologic suppression. Immunity. 2019;50:567–575.e5. doi: 10.1016/j.immuni.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Maeder ML, et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat. Med. 2019;25:229–233. doi: 10.1038/s41591-018-0327-9. [DOI] [PubMed] [Google Scholar]
- 146.Anzalone AV, et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576:149–157. doi: 10.1038/s41586-019-1711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Komor AC, et al. Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C:G-to-T:A base editors with higher efficiency and product purity. Sci. Adv. 2017;3:eaao4774. doi: 10.1126/sciadv.aao4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lai Y, et al. Efficient in vivo gene expression by trans-splicing adeno-associated viral vectors. Nat. Biotechnol. 2005;23:1435–1439. doi: 10.1038/nbt1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nakai H, Storm TA, Kay MA. Increasing the size of rAAV-mediated expression cassettes in vivo by intermolecular joining of two complementary vectors. Nat. Biotechnol. 2000;18:527–532. doi: 10.1038/75390. [DOI] [PubMed] [Google Scholar]
- 150.Sun L, Li J, Xiao X. Overcoming adeno-associated virus vector size limitation through viral DNA heterodimerization. Nat. Med. 2000;6:599–602. doi: 10.1038/75087. [DOI] [PubMed] [Google Scholar]
- 151.Pergolizzi RG, et al. In vivo trans-splicing of 5′ and 3′ segments of Pre-mRNA directed by corresponding DNA sequences delivered by gene transfer. Mol. Ther. 2003;8:999–1008. doi: 10.1016/j.ymthe.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 152.Song Y, et al. Functional cystic fibrosis transmembrane conductance regulator expression in cystic fibrosis airway epithelial cells by AAV6.2-mediated segmental trans-splicing. Hum. Gene Ther. 2009;20:267–281. doi: 10.1089/hum.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Riedmayr LM, et al. mRNA trans-splicing dual AAV vectors for (epi)genome editing and gene therapy. Nat. Commun. 2023;14:6578. doi: 10.1038/s41467-023-42386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li J, Sun W, Wang B, Xiao X, Liu X-Q. Protein trans-splicing as a means for viral vector-mediated in vivo gene therapy. Hum. Gene Ther. 2008;19:958–964. doi: 10.1089/hum.2008.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chew WL, et al. A multifunctional AAV–CRISPR–Cas9 and its host response. Nat. Methods. 2016;13:868–874. doi: 10.1038/nmeth.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Truong D-JJ, et al. Development of an intein-mediated split–Cas9 system for gene therapy. Nucleic Acids Res. 2015;43:6450–6458. doi: 10.1093/nar/gkv601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Villiger L, et al. Treatment of a metabolic liver disease by in vivo genome base editing in adult mice. Nat. Med. 2018;24:1519–1525. doi: 10.1038/s41591-018-0209-1. [DOI] [PubMed] [Google Scholar]
- 158.Levy JM, et al. Cytosine and adenine base editing of the brain, liver, retina, heart and skeletal muscle of mice via adeno-associated viruses. Nat. Biomed. Eng. 2020;4:97–110. doi: 10.1038/s41551-019-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Davis, J. R. et al. Efficient prime editing in mouse brain, liver and heart with dual AAVs. Nat. Biotechnol. 1–12 10.1038/s41587-023-01758-z (2023). [DOI] [PMC free article] [PubMed]
- 160.Tornabene P, et al. Inclusion of a degron reduces levels of undesired inteins after AAV-mediated protein trans-splicing in the retina. Mol. Ther. Methods Clin. Dev. 2021;23:448–459. doi: 10.1016/j.omtm.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Clément N, Grieger JC. Manufacturing of recombinant adeno-associated viral vectors for clinical trials. Mol. Ther. Methods Clin. Dev. 2016;3:16002. doi: 10.1038/mtm.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Su W, et al. Self-attenuating adenovirus enables production of recombinant adeno-associated virus for high manufacturing yield without contamination. Nat. Commun. 2022;13:1182. doi: 10.1038/s41467-022-28738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sena-Esteves M, Gao G. Purification of recombinant adeno-associated viruses (rAAVs) by Iodixanol gradient centrifugation. Cold Spring Harb. Protoc. 2020;2020:095612. doi: 10.1101/pdb.prot095612. [DOI] [PubMed] [Google Scholar]
- 164.Sena-Esteves M, Gao G. Introducing genes into mammalian cells: viral vectors. Cold Spring Harb. Protoc. 2020;2020:095513. doi: 10.1101/pdb.top095513. [DOI] [PubMed] [Google Scholar]
- 165.Su Q, Sena-Esteves M, Gao G. Purification of recombinant adeno-associated viruses (rAAVs) by cesium chloride gradient sedimentation. Cold Spring Harb. Protoc. 2020;2020:095604. doi: 10.1101/pdb.prot095604. [DOI] [PubMed] [Google Scholar]
- 166.Naso MF, Tomkowicz B, Perry WL, Strohl WR. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs. 2017;31:317–334. doi: 10.1007/s40259-017-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Louis N, Evelegh C, Graham FL. Cloning and sequencing of the cellular–viral junctions from the human adenovirus type 5 transformed 293 cell line. Virology. 1997;233:423–429. doi: 10.1006/viro.1997.8597. [DOI] [PubMed] [Google Scholar]
- 168.Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977;36:59–72. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 169.Grimm D, Kern A, Rittner K, Kleinschmidt JA. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum. Gene Ther. 1998;9:2745–2760. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- 170.Tang Q, et al. Two-plasmid packaging system for recombinant adeno-associated virus. BioResearch Open Access. 2020;9:219–228. doi: 10.1089/biores.2020.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wright JF. Manufacturing and characterizing AAV-based vectors for use in clinical studies. Gene Ther. 2008;15:840–848. doi: 10.1038/gt.2008.65. [DOI] [PubMed] [Google Scholar]
- 172.Srivastava A, Mallela KMG, Deorkar N, Brophy G. Manufacturing challenges and rational formulation development for AAV Viral vectors. J. Pharm. Sci. 2021;110:2609–2624. doi: 10.1016/j.xphs.2021.03.024. [DOI] [PubMed] [Google Scholar]
- 173.Hakim CH, et al. Micro-dystrophin AAV vectors made by transient transfection and herpesvirus system are equally potent in treating mdx mouse muscle disease. Mol. Ther. Methods Clin. Dev. 2020;18:664–678. doi: 10.1016/j.omtm.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu H, et al. A modified triple transfection method produces high-quality AAV vectors with much-reduced plasmid demand (ASGCT abstract 48) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 175.Wang Q, et al. AAVone: an all-in-one plasmid system for efficient AAv production (ASGCT abstract 829) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 176.Yla-Herttuala S. Endgame: glybera finally recommended for approval as the first gene therapy drug in the European Union. Mol. Ther. 2012;20:1831–1832. doi: 10.1038/mt.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Urabe M, Ding C, Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum. Gene Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
- 178.Becerra SP, Koczot F, Fabisch P, Rose JA. Synthesis of adeno-associated virus structural proteins requires both alternative mRNA splicing and alternative initiations from a single transcript. J. Virol. 1988;62:2745–2754. doi: 10.1128/jvi.62.8.2745-2754.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kondratov O, et al. Direct head-to-head evaluation of recombinant adeno-associated viral vectors manufactured in human versus insect cells. Mol. Ther. 2017;25:2661–2675. doi: 10.1016/j.ymthe.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Aslanidi G, Lamb K, Zolotukhin S. An inducible system for highly efficient production of recombinant adeno-associated virus (rAAV) vectors in insect Sf9 cells. Proc. Natl Acad. Sci. 2009;106:5059–5064. doi: 10.1073/pnas.0810614106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Moreno F, Lip F, Rojas H, Anggakusuma Development of an insect cell-based adeno-associated virus packaging cell line employing advanced Rep gene expression control system. Mol. Ther. Methods Clin. Dev. 2022;27:391–403. doi: 10.1016/j.omtm.2022.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Airenne KJ, et al. Baculovirus: an insect-derived vector for diverse gene transfer applications. Mol. Ther. 2013;21:739–749. doi: 10.1038/mt.2012.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kohlbrenner E, et al. Successful production of pseudotyped rAAV vectors using a modified baculovirus expression system. Mol. Ther. 2005;12:1217–1225. doi: 10.1016/j.ymthe.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pijlman GP, Vrij J, de, End FJ, van den, Vlak JM, Martens DE. Evaluation of baculovirus expression vectors with enhanced stability in continuous cascaded insect-cell bioreactors. Biotechnol. Bioeng. 2004;87:743–753. doi: 10.1002/bit.20178. [DOI] [PubMed] [Google Scholar]
- 185.Pijlman GP, et al. Relocation of the attTn7 transgene insertion site in bacmid DNA enhances baculovirus genome stability and recombinant protein expression in insect cells. Viruses. 2020;12:1448. doi: 10.3390/v12121448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chen H. Intron splicing-mediated expression of AAV Rep and Cap genes and production of AAV vectors in insect cells. Mol. Ther. 2008;16:924–930. doi: 10.1038/mt.2008.35. [DOI] [PubMed] [Google Scholar]
- 187.Liu S, et al. Systematic comparison of rAAV vectors manufactured using large-scale suspension cultures of Sf9 and HEK293 cells. Mol. Ther. 2024;32:74–83. doi: 10.1016/j.ymthe.2023.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Loo JCvander, Wright JF. Progress and challenges in viral vector manufacturing. Hum. Mol. Genet. 2016;25:R42–R52. doi: 10.1093/hmg/ddv451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yuan Z, Qiao C, Hu P, Li J, Xiao X. A versatile adeno-associated virus vector producer cell line method for scalable vector production of different serotypes. Hum. Gene Ther. 2011;22:613–624. doi: 10.1089/hum.2010.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang H, Xie J, Xie Q, Wilson JM, Gao G. Adenovirus–adeno-associated virus hybrid for large-scale recombinant adeno-associated virus production. Hum. Gene Ther. 2009;20:922–929. doi: 10.1089/hum.2009.125. [DOI] [PubMed] [Google Scholar]
- 191.Gao GP, et al. High-titer adeno-associated viral vectors from a Rep/Cap cell line and hybrid shuttle virus. Hum. Gene Ther. 1998;9:2353–2362. doi: 10.1089/hum.1998.9.16-2353. [DOI] [PubMed] [Google Scholar]
- 192.Clark KR, Voulgaropoulou F, Fraley DM, Johnson PR. Cell lines for the production of recombinant adeno-associated virus. Hum. Gene Ther. 1995;6:1329–1341. doi: 10.1089/hum.1995.6.10-1329. [DOI] [PubMed] [Google Scholar]
- 193.Farson D, et al. Development and characterization of a cell line for large‐scale, serum‐free production of recombinant adeno‐associated viral vectors. J. Gene Med. 2004;6:1369–1381. doi: 10.1002/jgm.622. [DOI] [PubMed] [Google Scholar]
- 194.Jalšić L, et al. Inducible HEK293 AAV packaging cell lines expressing Rep proteins. Mol. Ther. Methods Clin. Dev. 2023;30:259–275. doi: 10.1016/j.omtm.2023.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Martin J, et al. Generation and characterization of adeno-associated virus producer cell lines for research and preclinical vector production. Hum. Gene Ther. Methods. 2013;24:253–269. doi: 10.1089/hgtb.2013.046. [DOI] [PubMed] [Google Scholar]
- 196.Gao GP, et al. Rep/Cap gene amplification and high-yield production of AAV in an A549 cell line expressing Rep/Cap. Mol. Ther. 2002;5:644–649. doi: 10.1006/mthe.2001.0591. [DOI] [PubMed] [Google Scholar]
- 197.Conway JE, et al. High-titer recombinant adeno-associated virus production utilizing a recombinant herpes simplex virus type I vector expressing AAV-2 Rep and Cap. Gene Ther. 1999;6:986–993. doi: 10.1038/sj.gt.3300937. [DOI] [PubMed] [Google Scholar]
- 198.Conway JE, Zolotukhin S, Muzyczka N, Hayward GS, Byrne BJ. Recombinant adeno-associated virus type 2 replication and packaging is entirely supported by a herpes simplex virus type 1 amplicon expressing Rep and Cap. J. Virol. 1997;71:8780–8789. doi: 10.1128/jvi.71.11.8780-8789.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Flotte TR, et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing alpha1-antitrypsin: interim results. Hum. Gene Ther. 2011;22:1239–1247. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Thorne BA, Takeya RK, Peluso RW. Manufacturing recombinant adeno-associated viral vectors from producer cell clones. Hum. Gene Ther. 2009;20:707–714. doi: 10.1089/hum.2009.070. [DOI] [PubMed] [Google Scholar]
- 201.Zhang H-G, et al. Recombinant adenovirus expressing adeno-associated virus cap and rep proteins supports production of high-titer recombinant adeno-associated virus. Gene Ther. 2001;8:704–712. doi: 10.1038/sj.gt.3301454. [DOI] [PubMed] [Google Scholar]
- 202.Ghosh, Bibek. et al. Scaling-up AAV- manufacture gmp production and purification using innovative plasmid-free technology (ASGCT abstract 411). Mol. Ther.31, S1–794 (2023).
- 203.Nguyen TNT, et al. Mechanistic model for production of recombinant adeno-associated virus via triple transfection of HEK293 cells. Mol. Ther. Methods Clin. Dev. 2021;21:642–655. doi: 10.1016/j.omtm.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Leong J. Titer boosting of HEK293-based aav manufacturing process using proprietary small molecule additive and successful scale up to 200L (ASGCT abstract 407) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 205.Sutherland K, et al. Development and scale-up validation of small molecule enhancers for increased viral vector yield (ASGCT abstract 425) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 206.Dietrich N, Lasater S, Rabinowitz J, Israel J. Analysis of mechanisms driving improved adeno-associated vector production by the addition of small molecules (ASGCT abstract 47) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 207.Barnes CR, et al. Genome-wide activation screens to increase adeno-associated virus production. Mol. Ther. Nucleic Acids. 2021;26:94–103. doi: 10.1016/j.omtn.2021.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Maurer AC, et al. Genome-wide CRISPR screen in HEK293 identifies putative cellular restriction factors for AAV manufacturing (ASGCT abstract 965) Mol. Ther. 2020;28:S1–592. [Google Scholar]
- 209.Chung C-H, et al. Production of rAAV by plasmid transfection induces antiviral and inflammatory responses in suspension HEK293 cells. Mol. Ther. Methods Clin. Dev. 2023;28:272–283. doi: 10.1016/j.omtm.2023.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mietzsch M, et al. Improved genome packaging efficiency of adeno-associated virus vectors using rep hybrids. J. Virol. 2021;95:e00773–21. doi: 10.1128/JVI.00773-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Andari JE, Grimm D. Production, processing, and characterization of synthetic AAV gene therapy vectors. Biotechnol. J. 2021;16:e2000025. doi: 10.1002/biot.202000025. [DOI] [PubMed] [Google Scholar]
- 212.Fekete S, et al. Chromatographic strategies for the analytical characterization of adeno-associated virus vector-based gene therapy products. TrAC Trends Anal. Chem. 2023;164:117088. doi: 10.1016/j.trac.2023.117088. [DOI] [Google Scholar]
- 213.Lee Z, Lu M, Irfanullah E, Soukup M, Hu W-S. Construction of an rAAV Producer Cell Line through Synthetic Biology. ACS Synth. Biol. 2022;11:3285–3295. doi: 10.1021/acssynbio.2c00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lu M, et al. Enhancing the production of recombinant adeno‐associated virus in synthetic cell lines through systematic characterization. Biotechnol. Bioeng. 2024;121:341–354. doi: 10.1002/bit.28562. [DOI] [PubMed] [Google Scholar]
- 215.Coronel J, et al. Efficient production of rAAV in a perfusion bioreactor using an ELEVECTA® stable producer cell line. Genet. Eng. Biotechnol. News. 2021;41:S23. doi: 10.1089/gen.41.S2.07. [DOI] [Google Scholar]
- 216.Tai PWL, et al. Adeno-associated virus genome population sequencing achieves full vector genome resolution and reveals human-vector chimeras. Mol. Ther. Methods Clin. Dev. 2018;9:130–141. doi: 10.1016/j.omtm.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Earley LF, et al. Long read sequencing of rAAV vectors illuminates origins of contaminating genomic species (ASGCT abstract 178) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 218.Tran NT, et al. AAV-genome population sequencing of vectors packaging CRISPR components reveals design-influenced heterogeneity. Mol. Ther. Methods Clin. Dev. 2020;18:639–651. doi: 10.1016/j.omtm.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tran NT, et al. Human and insect cell-produced recombinant adeno-associated viruses show differences in genome heterogeneity. Hum. Gene Ther. 2022;33:371–388. doi: 10.1089/hum.2022.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.FDA approves hereditary blindness gene therapy. Nat. Biotechnol. 36, 6 (2018). [DOI] [PubMed]
- 221.Russell S, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bennett J, et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet. 2016;388:661–672. doi: 10.1016/S0140-6736(16)30371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Maguire AM, et al. EfficaCy, safety, and durability of Voretigene Neparvovec-rzyl In Rpe65 mutation-associated inherited retinal dystrophy: results of phase 1 and 3 trials. Ophthalmology. 2019;126:1273–1285. doi: 10.1016/j.ophtha.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 224.Ghazi NG, et al. Treatment of retinitis pigmentosa due to MERTK mutations by ocular subretinal injection of adeno-associated virus gene vector: results of a phase I trial. Hum. Genet. 2016;135:327–343. doi: 10.1007/s00439-016-1637-y. [DOI] [PubMed] [Google Scholar]
- 225.Megaw RD, Soares DC, Wright AF. RPGR: its role in photoreceptor physiology, human disease, and future therapies. Exp. Eye Res. 2015;138:32–41. doi: 10.1016/j.exer.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Cehajic-Kapetanovic J, et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat. Med. 2020;26:354–359. doi: 10.1038/s41591-020-0763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Camara CM-FDL, Cehajic-Kapetanovic J, MacLaren RE. RPGR gene therapy presents challenges in cloning the coding sequence. Expert Opin. Biol. Ther. 2020;20:63–71. doi: 10.1080/14712598.2020.1680635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cideciyan AV, et al. Progression in X-linked retinitis pigmentosa due to ORF15-RPGR mutations: assessment of localized vision changes over 2 years. Investig. Opthalmol. Vis. Sci. 2018;59:4558–4566. doi: 10.1167/iovs.18-24931. [DOI] [PubMed] [Google Scholar]
- 229.Krusenstiern Lvon, et al. Changes in retinal sensitivity associated with Cotoretigene Toliparvovec in X-linked retinitis pigmentosa with RPGR gene variations. JAMA Ophthalmol. 2023;141:275–283. doi: 10.1001/jamaophthalmol.2022.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Massengill MT, Lewin AS. Gene therapy for rhodopsin-associated autosomal dominant retinitis pigmentosa. Int. Ophthalmol. Clin. 2021;61:79–96. doi: 10.1097/IIO.0000000000000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Philippidis A. In through the out door: editas’ CEOs grasp opportunities amidst challenges. GEN Biotechnol. 2022;1:215–217. doi: 10.1089/genbio.2022.29030.aph. [DOI] [Google Scholar]
- 232.Liao D, et al. Optogenetic therapy with MCO-010 for vision restoration in patients with severe sight loss due to retinitis pigmentosa: the phase 2b RESTORE Study (ASGCT abstract 808) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 233.Lukason M, et al. Inhibition of choroidal neovascularization in a nonhuman primate model by intravitreal administration of an AAV2 vector expressing a novel anti-VEGF molecule. Mol. Ther. 2011;19:260–265. doi: 10.1038/mt.2010.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rakoczy EP, et al. Gene therapy with recombinant adeno-associated vectors for neovascular age-related macular degeneration: 1 year follow-up of a phase 1 randomised clinical trial. Lancet. 2015;386:2395–2403. doi: 10.1016/S0140-6736(15)00345-1. [DOI] [PubMed] [Google Scholar]
- 235.Constable IJ, et al. Phase 2a randomized clinical trial: safety and post Hoc analysis of subretinal rAAV.sFLT-1 for wet age-related macular degeneration. EBioMedicine. 2016;14:168–175. doi: 10.1016/j.ebiom.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Constable IJ, et al. Gene therapy in neovascular age-related macular degeneration: three-year follow-up of a phase 1 randomized dose escalation trial. Am. J. Ophthalmol. 2017;177:150–158. doi: 10.1016/j.ajo.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 237.Heier JS, et al. Safety and efficacy of subretinally administered palucorcel for geographic atrophy of age-related macular degeneration: phase 2b study. Ophthalmol. Retin. 2020;4:384–393. doi: 10.1016/j.oret.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 238.Heier JS, et al. Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: a phase 1, open-label trial. Lancet. 2017;390:50–61. doi: 10.1016/S0140-6736(17)30979-0. [DOI] [PubMed] [Google Scholar]
- 239.Rakoczy EP, et al. Three-year follow-up of phase 1 and 2a rAAV.sFLT-1 subretinal gene therapy trials for exudative age-related macular degeneration. Am. J. Ophthalmol. 2019;204:113–123. doi: 10.1016/j.ajo.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 240.Khanani AM, Kiss S, Turpcu A, Hoang C, Osborne A. Phase 1 study of intravitreal gene therapy ADVM-022 for neovascular AMD (OPTIC Trial) Investig. Ophthalmol. Vis. Sci. 2020;61:1154–1154. [Google Scholar]
- 241.Hwu W-L, Chien Y-H, Lee N-C, Li M-H. Natural history of aromatic l-amino acid decarboxylase deficiency in Taiwan. JIMD Rep. 2017;40:1–6. doi: 10.1007/8904_2017_54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wassenberg T, et al. Consensus guideline for the diagnosis and treatment of aromatic l-amino acid decarboxylase (AADC) deficiency. Orphanet J. Rare Dis. 2017;12:12. doi: 10.1186/s13023-016-0522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hyland K, Clayton PT. Aromatic amino acid decarboxylase deficiency in twins. J. Inherit. Metab. Dis. 1990;13:301–304. doi: 10.1007/BF01799380. [DOI] [PubMed] [Google Scholar]
- 244.Bergkvist M, et al. Aromatic L-amino acid decarboxylase deficiency: a systematic review. Futur. Neurol. 2022;17:FNL63. doi: 10.2217/fnl-2022-0012. [DOI] [Google Scholar]
- 245.Eberling JL, et al. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease SYMBOL. Neurology. 2008;70:1980–1983. doi: 10.1212/01.wnl.0000312381.29287.ff. [DOI] [PubMed] [Google Scholar]
- 246.Christine CW, et al. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease SYMBOL. Neurology. 2009;73:1662–1669. doi: 10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Muramatsu S, et al. A Phase I study of aromatic l-amino acid decarboxylase gene therapy for Parkinson’s disease. Mol. Ther. 2010;18:1731–1735. doi: 10.1038/mt.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Hwu W-L, et al. Gene therapy for aromatic l-amino acid decarboxylase deficiency. Sci. Transl. Med. 2012;4:134ra61. doi: 10.1126/scitranslmed.3003640. [DOI] [PubMed] [Google Scholar]
- 249.Chien Y-H, et al. Efficacy and safety of AAV2 gene therapy in children with aromatic l-amino acid decarboxylase deficiency: an open-label, phase 1/2 trial. Lancet Child Adolesc. Heal. 2017;1:265–273. doi: 10.1016/S2352-4642(17)30125-6. [DOI] [PubMed] [Google Scholar]
- 250.Kojima K, et al. Gene therapy improves motor and mental function of aromatic l-amino acid decarboxylase deficiency. Brain. 2019;142:322–333. doi: 10.1093/brain/awy331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Pearson TS, et al. Gene therapy for aromatic L-amino acid decarboxylase deficiency by MR-guided direct delivery of AAV2-AADC to midbrain dopaminergic neurons. Nat. Commun. 2021;12:4251. doi: 10.1038/s41467-021-24524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Tai C-H, et al. Long-term efficacy and safety of eladocagene exuparvovec in patients with AADC deficiency. Mol. Ther. 2022;30:509–518. doi: 10.1016/j.ymthe.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Keam SJ. Eladocagene exuparvovec: first approval. Drugs. 2022;82:1427–1432. doi: 10.1007/s40265-022-01775-3. [DOI] [PubMed] [Google Scholar]
- 254.Oskoui M, et al. The changing natural history of spinal muscular atrophy type 1. Neurology. 2007;69:1931–1936. doi: 10.1212/01.wnl.0000290830.40544.b9. [DOI] [PubMed] [Google Scholar]
- 255.Finkel RS, et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83:810–817. doi: 10.1212/WNL.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Park HB, et al. Survival analysis of spinal muscular atrophy type I. Korean J. Pediatr. 2010;53:965–970. doi: 10.3345/kjp.2010.53.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Mercuri E, Pera MC, Scoto M, Finkel R, Muntoni F. Spinal muscular atrophy—insights and challenges in the treatment era. Nat. Rev. Neurol. 2020;16:706–715. doi: 10.1038/s41582-020-00413-4. [DOI] [PubMed] [Google Scholar]
- 258.Verhaart IEC, et al. Prevalence, incidence and carrier frequency of 5q–linked spinal muscular atrophy—a literature review. Orphanet J. Rare Dis. 2017;12:124. doi: 10.1186/s13023-017-0671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mendell JR, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 260.Day JW, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20:284–293. doi: 10.1016/S1474-4422(21)00001-6. [DOI] [PubMed] [Google Scholar]
- 261.Mercuri E, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy type 1 (STR1VE-EU): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20:832–841. doi: 10.1016/S1474-4422(21)00251-9. [DOI] [PubMed] [Google Scholar]
- 262.Mendell JR, et al. Five-year extension results of the phase 1 START trial of Onasemnogene Abeparvovec in spinal muscular atrophy. JAMA Neurol. 2021;78:834–841. doi: 10.1001/jamaneurol.2021.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Strauss KA, et al. Onasemnogene Abeparvovec for presymptomatic infants with two copies of SMN2 at risk for spinal muscular atrophy type 1: the phase III SPR1NT trial. Nat. Med. 2022;28:1381–1389. doi: 10.1038/s41591-022-01866-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Strauss KA, et al. Onasemnogene Abeparvovec for presymptomatic infants with three copies of SMN2 at risk for spinal muscular atrophy: the phase III SPR1NT trial. Nat. Med. 2022;28:1390–1397. doi: 10.1038/s41591-022-01867-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Feldman AG, et al. Subacute liver failure following gene replacement therapy for spinal muscular atrophy type 1. J. Pediatr. 2020;225:252–258.e1. doi: 10.1016/j.jpeds.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Weiß C, et al. Gene replacement therapy with onasemnogene abeparvovec in children with spinal muscular atrophy aged 24 months or younger and bodyweight up to 15 kg: an observational cohort study. Lancet Child Adolesc. Heal. 2022;6:17–27. doi: 10.1016/S2352-4642(21)00287-X. [DOI] [PubMed] [Google Scholar]
- 267.Leal AF, et al. GM2 gangliosidoses: clinical features, pathophysiological aspects, and current therapies. Int. J. Mol. Sci. 2020;21:6213. doi: 10.3390/ijms21176213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Neudorfer O, et al. Late-onset Tay-Sachs disease: phenotypic characterization and genotypic correlations in 21 affected patients. Genet. Med. 2005;7:119–123. doi: 10.1097/01.GIM.0000154300.84107.75. [DOI] [PubMed] [Google Scholar]
- 269.Maegawa GHB, et al. The natural history of juvenile or subacute GM2 gangliosidosis: 21 new cases and literature review of 134 previously reported. Pediatrics. 2006;118:e1550–e1562. doi: 10.1542/peds.2006-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Bley AE, et al. Natural history of infantile GM2 gangliosidosis. Pediatrics. 2011;128:e1233–e1241. doi: 10.1542/peds.2011-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Woodley E, et al. Efficacy of a bicistronic vector for correction of Sandhoff disease in a mouse model. Mol. Ther. Methods Clin. Dev. 2019;12:47–57. doi: 10.1016/j.omtm.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lahey HG, et al. Pronounced therapeutic benefit of a single bidirectional AAV vector administered systemically in Sandhoff mice. Mol. Ther. 2020;28:2150–2160. doi: 10.1016/j.ymthe.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Flotte TR, et al. AAV gene therapy for Tay-Sachs disease. Nat. Med. 2022;28:251–259. doi: 10.1038/s41591-021-01664-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Bley A, et al. The natural history of Canavan disease: 23 new cases and comparison with patients from literature. Orphanet J. Rare Dis. 2021;16:227. doi: 10.1186/s13023-020-01659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Lotun A, Gessler DJ, Gao G. Canavan disease as a model for gene therapy-mediated myelin repair. Front Cell Neurosci. 2021;15:661928. doi: 10.3389/fncel.2021.661928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Leone P, et al. Long-term follow-up after gene therapy for Canavan disease. Sci. Transl. Med. 2012;4:165ra163. doi: 10.1126/scitranslmed.3003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Lotun, A. et al. Renewal of oligodendrocyte lineage reverses dysmyelination and CNS neurodegeneration through corrected N-acetylaspartate metabolism. Prog. Neurobiol. 102460 10.1016/j.pneurobio.2023.102460 (2023). [DOI] [PMC free article] [PubMed]
- 278.Francis JS, et al. Preclinical biodistribution, tropism, and efficacy of oligotropic AAV/Olig001 in a mouse model of congenital white matter disease. Mol. Ther. Methods Clin. Dev. 2021;20:520–534. doi: 10.1016/j.omtm.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Jonquieres Gvon, et al. Uncoupling N-acetylaspartate from brain pathology: implications for Canavan disease gene therapy. Acta Neuropathol. 2018;135:95–113. doi: 10.1007/s00401-017-1784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Fröhlich D, et al. Dual-function AAV gene therapy reverses late-stage Canavan disease pathology in mice. Front. Mol. Neurosci. 2022;15:1061257. doi: 10.3389/fnmol.2022.1061257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Corti M, et al. Adeno-associated virus-mediated gene therapy in a patient with Canavan disease using dual routes of administration and immune modulation. Mol. Ther. Methods Clin. Dev. 2023;30:303–314. doi: 10.1016/j.omtm.2023.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zarghamian P, Klermund J, Cathomen T. Clinical genome editing to treat sickle cell disease—a brief update. Front. Med. 2023;9:1065377. doi: 10.3389/fmed.2022.1065377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Berntorp E, et al. Haemophilia. Nat. Rev. Dis. Prim. 2021;7:45. doi: 10.1038/s41572-021-00278-x. [DOI] [PubMed] [Google Scholar]
- 284.Soucie JM, Miller CH, Dupervil B, Le B, Buckner TW. Occurrence rates of haemophilia among males in the United States based on surveillance conducted in specialized haemophilia treatment centres. Haemophilia. 2020;26:487–493. doi: 10.1111/hae.13998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Blanchette VS, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J. Thromb. Haemost. 2014;12:1935–1939. doi: 10.1111/jth.12672. [DOI] [PubMed] [Google Scholar]
- 286.Shahani T, et al. Human liver sinusoidal endothelial cells but not hepatocytes contain factor VIII. J. Thromb. Haemost. 2014;12:36–42. doi: 10.1111/jth.12412. [DOI] [PubMed] [Google Scholar]
- 287.Fahs SA, Hille MT, Shi Q, Weiler H, Montgomery RR. A conditional knockout mouse model reveals endothelial cells as the principal and possibly exclusive source of plasma factor VIII. Blood. 2014;123:3706–3713. doi: 10.1182/blood-2014-02-555151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Tatsumi K, et al. Hepatocyte is a sole cell type responsible for the production of coagulation factor IX in vivo. Cell Med. 2012;3:25–31. doi: 10.3727/215517912X639496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Zanon E, Pasca S. Intracranial haemorrhage in children and adults with haemophilia A and B: a literature review of the last 20 years. Blood Transfus. 2018;17:378–384. doi: 10.2450/2019.0253-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Hassan S, et al. Mortality, life expectancy, and causes of death of persons with hemophilia in the Netherlands 2001–2018. J. Thromb. Haemost. 2021;19:645–653. doi: 10.1111/jth.15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Glorioso TJ, et al. Natural history of hemophilic joint disease using sensitive imaging with magnetic resonance imaging (MRI) Blood. 2013;122:209. doi: 10.1182/blood.V122.21.209.209. [DOI] [Google Scholar]
- 292.Lusher JM, Arkin S, Abildgaard CF, Schwartz RS. Recombinant factor VIII for the treatment of previously untreated patients with hemophilia A- safety, efficacy, and development of inhibitors. N. Engl. J. Med. 1993;328:453–459. doi: 10.1056/NEJM199302183280701. [DOI] [PubMed] [Google Scholar]
- 293.Powell JS, et al. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N. Engl. J. Med. 2013;369:2313–2323. doi: 10.1056/NEJMoa1305074. [DOI] [PubMed] [Google Scholar]
- 294.Srivastava A, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1–e47. doi: 10.1111/j.1365-2516.2012.02909.x. [DOI] [PubMed] [Google Scholar]
- 295.Hernandez I, Rowe D, Gellad WF, Good CB. Trends in the use of conventional and new pharmaceuticals for hemophilia treatments among medicaid enrollees, 2005-2020. JAMA Netw. Open. 2021;4:e2112044. doi: 10.1001/jamanetworkopen.2021.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Kay MA, et al. In vivo hepatic gene therapy: complete albeit transient correction of factor IX deficiency in hemophilia B dogs. Proc. Natl Acad. Sci. 1994;91:2353–2357. doi: 10.1073/pnas.91.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Kay MA, et al. In vivo gene therapy of hemophilia B: sustained partial correction in factor IX-deficient dogs. Science. 1993;262:117–119. doi: 10.1126/science.8211118. [DOI] [PubMed] [Google Scholar]
- 298.Herzog RW, et al. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc. Natl Acad. Sci. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Snyder RO, et al. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat. Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- 300.Monahan P, et al. Direct intramuscular injection with recombinant AAV vectors results in sustained expression in a dog model of hemophilia. Gene Ther. 1998;5:40–49. doi: 10.1038/sj.gt.3300548. [DOI] [PubMed] [Google Scholar]
- 301.Herzog RW, et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat. Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- 302.Snyder RO, et al. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat. Med. 1999;5:64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- 303.Kay MA, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat. Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 304.Manno CS, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 305.Simioni P, et al. X-linked thrombophilia with a mutant factor IX (Factor IX Padua) N. Engl. J. Med. 2009;361:1671–1675. doi: 10.1056/NEJMoa0904377. [DOI] [PubMed] [Google Scholar]
- 306.Manno CS, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 307.Nathwani AC, et al. Adenovirus-associated virus vector–mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Nathwani AC, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.George LA, et al. Spk-9001: adeno-associated virus mediated gene transfer for hemophilia B achieves sustained mean factor IX activity levels of >30% without immunosuppression. Blood. 2016;128:3. doi: 10.1182/blood.V128.22.3.3. [DOI] [Google Scholar]
- 310.George LA, et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N. Engl. J. Med. 2017;377:2215–2227. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.High KA, Anguela XM. Adeno-associated viral vectors for the treatment of hemophilia. Hum. Mol. Genet. 2016;25:R36–R41. doi: 10.1093/hmg/ddv475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Savita R, et al. AAV5–factor VIII gene transfer in severe hemophilia A. N. Engl. J. Med. 2017;377:2519–2530. doi: 10.1056/NEJMoa1708483. [DOI] [PubMed] [Google Scholar]
- 313.Pasi KJ, et al. Multiyear follow-up of AAV5-hFVIII-SQ gene therapy for hemophilia A. N. Engl. J. Med. 2020;382:29–40. doi: 10.1056/NEJMoa1908490. [DOI] [PubMed] [Google Scholar]
- 314.George LA, et al. Multiyear factor VIII expression after AAV gene transfer for hemophilia A. N. Engl. J. Med. 2021;385:1961–1973. doi: 10.1056/NEJMoa2104205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Ozelo MC, et al. Valoctocogene Roxaparvovec gene therapy for hemophilia A. N. Engl. J. Med. 2022;386:1013–1025. doi: 10.1056/NEJMoa2113708. [DOI] [PubMed] [Google Scholar]
- 316.Mahlangu J, et al. Two-year outcomes of Valoctocogene Roxaparvovec therapy for hemophilia A. N. Engl. J. Med. 2023;388:694–705. doi: 10.1056/NEJMoa2211075. [DOI] [PubMed] [Google Scholar]
- 317.Morrison B. Neuromuscular diseases. Semin. Neurol. 2016;36:409–418. doi: 10.1055/s-0036-1586263. [DOI] [PubMed] [Google Scholar]
- 318.Mercuri E, Bönnemann CG, Muntoni F. Muscular dystrophies. Lancet. 2019;394:2025–2038. doi: 10.1016/S0140-6736(19)32910-1. [DOI] [PubMed] [Google Scholar]
- 319.Mullard A. FDA approves first gene therapy for Duchenne muscular dystrophy, despite internal objections. Nat. Rev. Drug Discov. 2023;22:610. doi: 10.1038/d41573-023-00103-y. [DOI] [PubMed] [Google Scholar]
- 320.Ryder S, et al. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J. Rare Dis. 2017;12:79. doi: 10.1186/s13023-017-0631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Duan D, Goemans N, Takeda S, Mercuri E, Aartsma-Rus A. Duchenne muscular dystrophy. Nat. Rev. Dis. Prim. 2021;7:13. doi: 10.1038/s41572-021-00248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 3: primary care, emergency management, psychosocial care, and transitions of care across the lifespan. Lancet Neurol. 2018;17:445–455. doi: 10.1016/S1474-4422(18)30026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018;17:347–361. doi: 10.1016/S1474-4422(18)30025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018;17:251–267. doi: 10.1016/S1474-4422(18)30024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Duan D. Micro-dystrophin gene therapy goes systemic in Duchenne muscular dystrophy patients. Hum. Gene Ther. 2018;29:733–736. doi: 10.1089/hum.2018.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Davies KE, Guiraud S. Micro-dystrophin genes bring hope of an effective therapy for Duchenne muscular dystrophy. Mol. Ther. 2019;27:486–488. doi: 10.1016/j.ymthe.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Zhang Y, Duan D. Novel mini–dystrophin gene dual adeno-associated virus vectors restore neuronal nitric oxide synthase expression at the sarcolemma. Hum. Gene Ther. 2012;23:98–103. doi: 10.1089/hum.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.England SB, et al. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature. 1990;343:180–182. doi: 10.1038/343180a0. [DOI] [PubMed] [Google Scholar]
- 329.Wein N, et al. Systemic delivery of an AAV9 exon-skipping vector significantly improves or prevents features of Duchenne muscular dystrophy in the Dup2 mouse. Mol. Ther. Methods Clin. Dev. 2022;26:279–293. doi: 10.1016/j.omtm.2022.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Martin PT, et al. Overexpression of Galgt2 in skeletal muscle prevents injury resulting from eccentric contractions in both mdx and wild-type mice. Am. J. Physiol. Cell Physiol. 2009;296:C476–C488. doi: 10.1152/ajpcell.00456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Qi LS, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152:1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Mali P, et al. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat. Biotechnol. 2013;31:833–838. doi: 10.1038/nbt.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Mendell JR, et al. Dystrophin immunity in Duchenne’s muscular dystrophy. N. Engl. J. Med. 2010;363:1429–1437. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Mendell JR, et al. Assessment of systemic delivery of rAAVrh74.MHCK7.micro-dystrophin in children with Duchenne muscular dystrophy. JAMA Neurol. 2020;77:1122–1131. doi: 10.1001/jamaneurol.2020.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Flanigan KM, et al. A first-in-human phase I/IIa gene transfer clinical trial for Duchenne muscular dystrophy using rAAVrh74.MCK.GALGT2. Mol. Ther. Methods Clin. Dev. 2022;27:47–60. doi: 10.1016/j.omtm.2022.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Lek A, et al. Death after high-dose rAAV9 gene therapy in a patient with Duchenne’s muscular dystrophy. N. Engl. J. Med. 2023;389:1203–1210. doi: 10.1056/NEJMoa2307798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Lek A, Atas E, Hesterlee SE, Byrne BJ, Bönnemann CG. Meeting report: 2022 muscular dystrophy association summit on “safety and challenges in gene transfer therapy”. J. Neuromuscul. Dis. 2023;10:327–336. doi: 10.3233/JND-221639. [DOI] [PubMed] [Google Scholar]
- 338.Heidenreich PA, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 339.Roger VL. Epidemiology of heart failure. Circ. Res. 2021;128:1421–1434. doi: 10.1161/CIRCRESAHA.121.318172. [DOI] [PubMed] [Google Scholar]
- 340.Bers DM. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 341.Sakata S, et al. Restoration of mechanical and energetic function in failing aortic-banded rat hearts by gene transfer of calcium cycling proteins. J. Mol. Cell. Cardiol. 2007;42:852–861. doi: 10.1016/j.yjmcc.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Jaski BE, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID trial), a first-in-human phase 1/2 clinical trial. J. Card. Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Jessup M, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID) Circulation. 2011;124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Zsebo K, et al. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure. Circ. Res. 2014;114:101–108. doi: 10.1161/CIRCRESAHA.113.302421. [DOI] [PubMed] [Google Scholar]
- 345.Greenberg B, et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet. 2016;387:1178–1186. doi: 10.1016/S0140-6736(16)00082-9. [DOI] [PubMed] [Google Scholar]
- 346.Hulot J, et al. Effect of intracoronary administration of AAV1/SERCA2a on ventricular remodelling in patients with advanced systolic heart failure: results from the AGENT‐HF randomized phase 2 trial. Eur. J. Heart Fail. 2017;19:1534–1541. doi: 10.1002/ejhf.826. [DOI] [PubMed] [Google Scholar]
- 347.Lyon AR, et al. Investigation of the safety and feasibility of AAV1/SERCA2a gene transfer in patients with chronic heart failure supported with a left ventricular assist device – the SERCA-LVAD TRIAL. Gene Ther. 2020;27:579–590. doi: 10.1038/s41434-020-0171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Greenberg B, et al. Abstract 10727: results from first-in-human clinical trial of RP-A501 (AAV9:LAMP2B) gene therapy treatment for danon disease. Circulation. 2021;144:A10727–A10727. doi: 10.1161/circ.144.suppl_1.10727. [DOI] [Google Scholar]
- 349.Rossano J, et al. Abstract 11117: phase 1 danon disease results: the first single dose intravenous (IV) gene therapy (RP-A501) with recombinant adeno-associated virus (AAV9:LAMP2B) for a monogenic cardiomyopathy. Circulation. 2022;146:A11117–A11117. doi: 10.1161/circ.146.suppl_1.11117. [DOI] [Google Scholar]
- 350.Ng SSM, et al. A novel glioblastoma cancer gene therapy using AAV-mediated long-term expression of human TERT C-terminal polypeptide. Cancer Gene Ther. 2007;14:561–572. doi: 10.1038/sj.cgt.7701038. [DOI] [PubMed] [Google Scholar]
- 351.Watanabe M, et al. Adeno-associated virus 2-mediated intratumoral prostate cancer gene therapy: long-term maspin expression efficiently suppresses tumor growth. Hum. Gene Ther. 2005;16:699–710. doi: 10.1089/hum.2005.16.699. [DOI] [PubMed] [Google Scholar]
- 352.Ma H-I, et al. Intratumoral gene therapy of malignant brain tumor in a rat model with angiostatin delivered by adeno-associated viral (AAV) vector. Gene Ther. 2002;9:2–11. doi: 10.1038/sj.gt.3301616. [DOI] [PubMed] [Google Scholar]
- 353.Kanazawa T, et al. Suicide gene therapy using AAV-HSVtk/ganciclovir in combination with irradiation results in regression of human head and neck cancer xenografts in nude mice. Gene Ther. 2003;10:51–58. doi: 10.1038/sj.gt.3301837. [DOI] [PubMed] [Google Scholar]
- 354.Hacker UT, Bentler M, Kaniowska D, Morgan M, Büning H. Towards clinical implementation of adeno-associated virus (AAV) vectors for cancer gene therapy: current status and future perspectives. Cancers. 2020;12:1889. doi: 10.3390/cancers12071889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Santiago-Ortiz JL, Schaffer DV. Adeno-associated virus (AAV) vectors in cancer gene therapy. J. Control. Release. 2016;240:287–301. doi: 10.1016/j.jconrel.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.He LF, et al. Suppression of cancer growth in mice by adeno-associated virus vector-mediated IFN-beta expression driven by hTERT promoter. Cancer Lett. 2008;286:196–205. doi: 10.1016/j.canlet.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 357.Motomura K, et al. Benefits of interferon‐β and temozolomide combination therapy for newly diagnosed primary glioblastoma with the unmethylated MGMT promoter. Cancer. 2011;117:1721–1730. doi: 10.1002/cncr.25637. [DOI] [PubMed] [Google Scholar]
- 358.GuhaSarkar D, Neiswender J, Su Q, Gao G, Sena‐Esteves M. Intracranial AAV‐IFN‐β gene therapy eliminates invasive xenograft glioblastoma and improves survival in orthotopic syngeneic murine model. Mol. Oncol. 2017;11:180–193. doi: 10.1002/1878-0261.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Münch RC, et al. Displaying high-affinity ligands on adeno-associated viral vectors enables tumor cell-specific and safe gene transfer. Mol. Ther. 2013;21:109–118. doi: 10.1038/mt.2012.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Münch RC, et al. Off-target-free gene delivery by affinity-purified receptor-targeted viral vectors. Nat. Commun. 2015;6:6246. doi: 10.1038/ncomms7246. [DOI] [PubMed] [Google Scholar]
- 361.Ai J, Wang D, Wei Q, Li H, Gao G. Adeno-associated virus serotype vectors efficiently transduce normal prostate tissue and prostate cancer cells. Eur. Urol. 2016;69:179–181. doi: 10.1016/j.eururo.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 362.Yi, X. et al. Adeno-associated virus-mediated intraprostatic suppression of MIR375 inhibits tumor progression in the TRAMP mouse model of prostate cancer. Genes Dis. 101182 10.1016/j.gendis.2023.101182 (2023). [DOI] [PMC free article] [PubMed]
- 363.Ai J, et al. rAAV-based and intraprostatically delivered miR-34a therapeutics for efficient inhibition of prostate cancer progression. Gene Ther. 2022;29:418–424. doi: 10.1038/s41434-021-00275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Yang B, et al. Global CNS transduction of adult mice by intravenously delivered rAAVrh.8 and rAAVrh.10 and nonhuman primates by rAAVrh.10. Mol. Ther. 2014;22:1299–1309. doi: 10.1038/mt.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Lu Y, et al. Efficient transduction of corneal stroma by adeno-associated viral serotype vectors for implications in gene therapy of corneal diseases. Hum. Gene Ther. 2016;27:598–608. doi: 10.1089/hum.2015.167. [DOI] [PubMed] [Google Scholar]
- 366.Ai J, et al. Adeno-associated virus serotype rh.10 displays strong muscle tropism following intraperitoneal delivery. Sci. Rep.-uk. 2017;7:40336. doi: 10.1038/srep40336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Taghian T, et al. A safe and reliable technique for CNS delivery of AAV vectors in the cisterna magna. Mol. Ther. 2020;28:411–421. doi: 10.1016/j.ymthe.2019.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Passini MA, et al. Intraventricular brain injection of adeno-associated virus type 1 (AAV1) in neonatal mice results in complementary patterns of neuronal transduction to AAV2 and total long-term correction of storage lesions in the brains of β-glucuronidase-deficient mice. J. Virol. 2003;77:7034–7040. doi: 10.1128/JVI.77.12.7034-7040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Bravo-Hernandez M, et al. Spinal subpial delivery of AAV9 enables widespread gene silencing and blocks motoneuron degeneration in ALS. Nat. Med. 2020;26:118–130. doi: 10.1038/s41591-019-0674-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Dane AP, Wowro SJ, Cunningham SC, Alexander IE. Comparison of gene transfer to the murine liver following intraperitoneal and intraportal delivery of hepatotropic AAV pseudo-serotypes. Gene Ther. 2013;20:460–464. doi: 10.1038/gt.2012.67. [DOI] [PubMed] [Google Scholar]
- 371.Foley CP, et al. Intra-arterial delivery of AAV vectors to the mouse brain after mannitol mediated blood brain barrier disruption. J. Control Release. 2014;196:71–78. doi: 10.1016/j.jconrel.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Foust KD, et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Lukashchuk V, Lewis KE, Coldicott I, Grierson AJ, Azzouz M. AAV9-mediated central nervous system–targeted gene delivery via cisterna magna route in mice. Mol. Ther. Methods Clin. Dev. 2016;3:15055. doi: 10.1038/mtm.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Bey K, et al. Intra-CSF AAV9 and AAVrh10 administration in nonhuman primates: promising routes and vectors for which neurological diseases? Mol. Ther. Methods Clin. Dev. 2020;17:771–784. doi: 10.1016/j.omtm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Hinderer C, et al. Widespread gene transfer in the central nervous system of cynomolgus macaques following delivery of AAV9 into the cisterna magna. Mol. Ther. Methods Clin. Dev. 2014;1:14051. doi: 10.1038/mtm.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Klamroth R, et al. Global seroprevalence of pre-existing immunity against AAV5 and other AAV serotypes in people with hemophilia A. Hum. Gene Ther. 2022;33:432–441. doi: 10.1089/hum.2021.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Gross DA, Tedesco N, Leborgne C, Ronzitti G. Overcoming the challenges imposed by humoral immunity to AAV vectors to achieve safe and efficient gene transfer in seropositive patients. Front. Immunol. 2022;13:857276. doi: 10.3389/fimmu.2022.857276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Isho B, et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci. Immunol. 2020;5:eabe5511. doi: 10.1126/sciimmunol.abe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Penaud-Budloo M, et al. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J. Virol. 2008;82:7875–7885. doi: 10.1128/JVI.00649-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.McElroy A, Sena-Esteves M, Arjomandnejad M, Keeler AM, Gray-Edwards HL. Redosing adeno-associated virus gene therapy to the central nervous system. Hum. Gene Ther. 2022;33:889–892. doi: 10.1089/hum.2022.170. [DOI] [PubMed] [Google Scholar]
- 381.Emmanuel SN, et al. Structurally mapping antigenic epitopes of adeno-associated virus 9: development of antibody escape variants. J. Virol. 2022;96:e0125121. doi: 10.1128/JVI.01251-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Hsi J, et al. Structural and kinetic characterization of anti-AAV9 monoclonal antibodies derived from patients post-zolgensma treatment (ASGCT abstract 14) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 383.Ertl, H. C. J. Circumventing B cell responses to allow for redosing of adeno-associated virus vectors. Hum. Gene Ther. 10.1089/hum.2023.162 (2023). [DOI] [PubMed]
- 384.Wang D, et al. Adeno-associated virus neutralizing antibodies in large animals and their impact on brain intraparenchymal gene transfer. Mol. Ther. Methods Clin. Dev. 2018;11:65–72. doi: 10.1016/j.omtm.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Lee S, et al. Relationship between neutralizing antibodies against adeno-associated virus in the vitreous and serum: effects on retinal gene therapy. Transl. Vis. Sci. Technol. 2019;8:14. doi: 10.1167/tvst.8.2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Poulsen K, et al. Bilateral Ixo-vec NHP tolerability and efficacy following a staggered dosing interval between eyes - gene therapy nAMD (ASGCT abstract 76) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 387.Horiuchi M, et al. Intravenous immunoglobulin prevents peripheral liver transduction of intrathecally delivered AAV vectors. Mol. Ther. - Methods Clin. Dev. 2022;27:272–280. doi: 10.1016/j.omtm.2022.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Falese L, et al. Strategy to detect pre-existing immunity to AAV gene therapy. Gene Ther. 2017;24:768–778. doi: 10.1038/gt.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Rangarajan S, et al. AAV5-factor VIII gene transfer in severe hemophilia A. N. Engl. J. Med. 2017;377:2519–2530. doi: 10.1056/NEJMoa1708483. [DOI] [PubMed] [Google Scholar]
- 390.Mingozzi F, et al. Pharmacological modulation of humoral immunity in a nonhuman primate model of AAV gene transfer for hemophilia B. Mol. Ther. 2012;20:1410–1416. doi: 10.1038/mt.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Mingozzi F, et al. Prevalence and pharmacological modulation of humoral immunity to AAV vectors in gene transfer to synovial tissue. Gene Ther. 2013;20:417–424. doi: 10.1038/gt.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Widdowson P, et al. A Bi-Cistronic AAV Vector aimed at addressing the complex pathology associated with geographic atrophy (ASGCT abstract 709) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 393.Du W, et al. Shuttle peptides-mediated local rnp delivery of editing complex in the sensory organs of the inner ear and retina in vivo in adult mice (ASGCT abstract 29) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 394.Salvia SL, et al. EV-AAVs as a novel gene delivery vector to the heart: new -evaluations for EV-AAVs and free AAV separation (ASGCT abstract 202) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 395.Cheng M, et al. Neutralizing antibody evasion and transduction with purified extracellular vesicle-enveloped adeno-associated virus vectors. Hum. Gene Ther. 2021;32:1457–1470. doi: 10.1089/hum.2021.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Kauffman JJ, Saad NY. Development of therapeutic extracellular vesicle enveloped-AAV vectors for muscle gene therapy (ASGCT abstract 1227) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 397.Rosario A, et al. EVADER reduces immunogenic response to AAV8-FIX capsid (ASGCT abstract 1432) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 398.Pawel-Rammingen U, von, Johansson BP, Bjorck L. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 2002;21:1607–1615. doi: 10.1093/emboj/21.7.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Leborgne C, et al. IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat. Med. 2020;26:1096–1101. doi: 10.1038/s41591-020-0911-7. [DOI] [PubMed] [Google Scholar]
- 400.Elmore ZC, et al. Gene transfer from neutralizing antibodies with an IgG-degrading enzyme. JCI Insight. 2020;5:e139881. doi: 10.1172/jci.insight.139881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Selecta Biosciences and Astellas Announce Exclusive Licensing and Development Agreement for Xork IgG Protease. (2023).
- 402.Ros-Ganan I, et al. Optimising the IgG-degrading enzyme treatment regimen for enhanced adeno-associated virus transduction in the presence of neutralising antibodies. Clin. Transl. Immunol. 2022;11:e1375. doi: 10.1002/cti2.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Uzcátegui N, et al. Neutralizing antibody depletion with imlifidase—a potential way to enable AAV based gene therapy in seropositive patients (ASGCT abstract 726) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 404.Winstedt L, et al. Complete removal of extracellular IgG antibodies in a randomized dose-escalation phase I study with the bacterial enzyme IdeS-A novel therapeutic opportunity. PLoS One. 2015;10:e0132011. doi: 10.1371/journal.pone.0132011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Lorant T, et al. Safety, immunogenicity, pharmacokinetics, and efficacy of degradation of anti-HLA antibodies by IdeS (imlifidase) in chronic kidney disease patients. Am. J. Transplant. 2018;18:2752–2762. doi: 10.1111/ajt.14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Huang E, Maldonado AQ, Kjellman C, Jordan SC. Imlifidase for the treatment of anti-HLA antibody-mediated processes in kidney transplantation. Am. J. Transplant. 2022;22:691–697. doi: 10.1111/ajt.16828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Seele J, et al. Identification of a novel host-specific IgM protease in Streptococcus suis. J. Bacteriol. 2013;195:930–940. doi: 10.1128/JB.01875-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Smith T, et al. Newly engineered IgM and IgG cleaving enzymes for AAV gene therapy (ASGCT abstract 210) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 409.Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Complement system part I—molecular mechanisms of activation and regulation. Front. Immunol. 2015;6:262. doi: 10.3389/fimmu.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Merle NS, Noe R, Halbwachs-Mecarelli L, Fremeaux-Bacchi V, Roumenina LT. Complement system part II: role in immunity. Front. Immunol. 2015;6:257. doi: 10.3389/fimmu.2015.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Mullard A. Gene therapy community grapples with toxicity issues, as pipeline matures. Nat. Rev. Drug Discov. 2021;20:804–805. doi: 10.1038/d41573-021-00164-x. [DOI] [PubMed] [Google Scholar]
- 412.Chand DH, et al. Thrombotic microangiopathy following Onasemnogene Abeparvovec for spinal muscular atrophy: a case series. J. Pediatr. 2021;231:265–268. doi: 10.1016/j.jpeds.2020.11.054. [DOI] [PubMed] [Google Scholar]
- 413.Guillou J, et al. Fatal thrombotic microangiopathy case following adeno-associated viral SMN gene therapy. Blood Adv. 2022;6:4266–4270. doi: 10.1182/bloodadvances.2021006419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Zaiss AK, et al. Complement is an essential component of the immune response to adeno-associated virus vectors. J. Virol. 2008;82:2727–2740. doi: 10.1128/JVI.01990-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Xicluna R, et al. Immunosafety of AAV gene therapy vectors: induction of cytokine release and complement activation (ASGCT abstract 707) Mol. Ther. 2022;30:S1–592. [Google Scholar]
- 416.Emami MR, et al. Innate and adaptive AAV-mediated immune responses in a mouse model of Duchenne muscular dystrophy. Mol. Ther. Methods Clin. Dev. 2023;30:90–102. doi: 10.1016/j.omtm.2023.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Servais, L., Horton, R., Saade, D., Bonnemann, C. & Muntoni, F. Proceedings of 261st ENMC Workshop: Management of Safety Issues Arising Following AAV Gene Therapy. 17th–19th June 2022, Hoofdorp, The Netherlands. Neuromuscul. Disord. 10.1016/j.nmd.2023.09.008 (2023). [DOI] [PubMed]
- 418.Salabarria SM, et al. Thrombotic microangiopathy following systemic AAV administration is dependent on anti-capsid antibodies. J. Clin. Investig. 2023;134:e173510. doi: 10.1172/JCI173510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Hordeaux J, et al. Immune transgene-dependent myocarditis in macaques after systemic administration of adeno-associated virus expressing human acid alpha-glucosidase. Front. Immunol. 2023;14:1094279. doi: 10.3389/fimmu.2023.1094279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Buchlis G, et al. High-dose AAV toxicity in mice: serotype-dependent hepatocellular damage and complement deposition and activation (ASGCT abstract 137) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 421.Smith CJ, et al. Pre-existing humoral immunity and complement pathway contribute to immunogenicity of adeno-associated virus (AAV) vector in human blood. Front. Immunol. 2022;13:999021. doi: 10.3389/fimmu.2022.999021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Ross N, et al. Effects of complement component 1 (C1) inhibition on AAV-based gene transfer efficacy and immunogenicity in mice (ASGCT abstract 1202) Mol. Ther. 2022;30:S1–592. [Google Scholar]
- 423.Shao W, et al. Double-stranded RNA innate immune response activation from long-term adeno-associated virus vector transduction. JCI Insight. 2018;21:e120474. doi: 10.1172/jci.insight.120474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Zhu J, Huang X, Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J. Clin. Investig. 2009;119:2388–2398. doi: 10.1172/JCI37607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Muhuri M, et al. Overcoming innate immune barriers that impede AAV gene therapy vectors. J. Clin. Investig. 2021;131:e143780. doi: 10.1172/JCI143780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Oliveira-Nascimento L, Massari P, Wetzler LM. The role of TLR2 in infection and immunity. Front. Immunol. 2012;3:79. doi: 10.3389/fimmu.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Hosel M, et al. Toll-like receptor 2-mediated innate immune response in human nonparenchymal liver cells toward adeno-associated viral vectors. Hepatology. 2012;55:287–297. doi: 10.1002/hep.24625. [DOI] [PubMed] [Google Scholar]
- 428.Ashley SN, Somanathan S, Giles AR, Wilson JM. TLR9 signaling mediates adaptive immunity following systemic AAV gene therapy. Cell. Immunol. 2019;346:103997. doi: 10.1016/j.cellimm.2019.103997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Reichel FF, et al. AAV8 can induce innate and adaptive immune response in the primate eye. Mol. Ther. 2017;25:2648–2660. doi: 10.1016/j.ymthe.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Kumar, S. R. P. et al. TLR9-independent CD8+ T cell responses in hepatic AAV gene transfer through IL-1R1-MyD88 signaling. Mol. Ther. 10.1016/j.ymthe.2023.11.029 (2023). [DOI] [PMC free article] [PubMed]
- 431.Dinarello CA. Overview of the IL‐1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018;281:8–27. doi: 10.1111/imr.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Rathinam VA, Fitzgerald KA. Inflammasome complexes: emerging mechanisms and effector functions. Cell. 2016;165:792–800. doi: 10.1016/j.cell.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Janssens S, Beyaert R. A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem. Sci. 2002;27:474–482. doi: 10.1016/S0968-0004(02)02145-X. [DOI] [PubMed] [Google Scholar]
- 434.Huellen F, Patil R, Hamilton BA, Wright JF. A biosynthetic approach for CpG methylation of AAV expression cassettes (ASGCT abstract 211) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 435.Gursel I, et al. Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J. Immunol. 2003;171:1393–1400. doi: 10.4049/jimmunol.171.3.1393. [DOI] [PubMed] [Google Scholar]
- 436.Chan YK, et al. Engineering adeno-associated viral vectors to evade innate immune and inflammatory responses. Sci. Transl. Med. 2021;13:eabd3438. doi: 10.1126/scitranslmed.abd3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Kumar S, Li N, Herzog RW. Effectiveness of engineering AAV vector genomes to evade tlr9 signaling for prevention of CD8+ T cell responses in muscle gene transfer is vector dose dependent (ASGCT abstract 138) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 438.Majowicz A, et al. Efficacy of immunomodulatory regimens to prevent anti-AAV humoral response and to enable AAV re-administration in mouse gene transfer model (ASGCT abstract 712) Mol. Ther. 2022;30:S1–592. [Google Scholar]
- 439.Park M, Motwani M, Hennessy M, Choudhury SR. Specific pharmacological blockade of IRAK4 suppresses AAV induced immunogenicity (ASGCT abstract 725) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 440.Ertl HCJ. Immunogenicity and toxicity of AAV gene therapy. Front. Immunol. 2022;13:975803. doi: 10.3389/fimmu.2022.975803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Ertl HCJ. T cell-mediated immune responses to AAV and AAV vectors. Front. Immunol. 2021;12:666666. doi: 10.3389/fimmu.2021.666666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Fader KA, et al. Circulating neurofilament light chain as a promising biomarker of AAV-induced dorsal root ganglia toxicity in nonclinical toxicology species. Mol. Ther. Methods Clin. Dev. 2022;25:264–277. doi: 10.1016/j.omtm.2022.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Hordeaux J, et al. MicroRNA-mediated inhibition of transgene expression reduces dorsal root ganglion toxicity by AAV vectors in primates. Sci. Transl. Med. 2020;12:eaba9188. doi: 10.1126/scitranslmed.aba9188. [DOI] [PubMed] [Google Scholar]
- 444.Hordeaux J, et al. Toxicology study of intra-cisterna magna adeno-associated virus 9 expressing iduronate-2-sulfatase in Rhesus Macaques. Mol. Ther. Methods Clin. Dev. 2018;10:68–78. doi: 10.1016/j.omtm.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Mueller C, et al. SOD1 suppression with adeno-associated virus and MicroRNA in familial ALS. N. Engl. J. Med. 2020;383:151–158. doi: 10.1056/NEJMoa2005056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.O’Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Odom GL, et al. Deimmunized micro-dystrophin vectors blunt patient immunity in vitro & restore cardiac functional deficits in vivo in mdx4cv DMD mice (ASGCT abstract 39) Mol. Ther. 2022;30:S1–592. [Google Scholar]
- 448.Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annu. Rev. Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Ilyinskii PO, et al. ImmTOR nanoparticles enhance AAV transgene expression after initial and repeat dosing in a mouse model of methylmalonic acidemia. Mol. Ther. Methods Clin. Dev. 2021;22:279–292. doi: 10.1016/j.omtm.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Ilyinskii PO, et al. Enhancement of liver-directed transgene expression at initial and repeat doses of AAV vectors admixed with ImmTOR nanoparticles. Sci. Adv. 2021;7:eabd0321. doi: 10.1126/sciadv.abd0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Wang L, et al. Prednisolone reduces the interferon response to AAV in cynomolgus macaques and may increase liver gene expression. Mol. Ther. Methods Clin. Dev. 2022;24:292–305. doi: 10.1016/j.omtm.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Chai Z, et al. Dexamethasone transiently enhances transgene expression in the liver when administered at late-phase post long-term adeno-associated virus transduction. Hum. Gene Ther. 2022;33:119–130. doi: 10.1089/hum.2021.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Verdera HC, et al. Investigating the role of vector DNA sensing and transgene expression on innate immune signaling and cell toxicity upon AAV- mediated gene transfer in HIPSC-derived CNS models (ASGCT abstract 850) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 455.Durost PA, et al. Gene therapy with an adeno-associated viral vector expressing human interleukin-2 alters immune system homeostasis in humanized mice. Hum. Gene Ther. 2018;29:352–365. doi: 10.1089/hum.2017.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Im D-S, Muzyczka N. The AAV origin binding protein Rep68 is an ATP-dependent site-specific endonuclease with DNA helicase activity. Cell. 1990;61:447–457. doi: 10.1016/0092-8674(90)90526-K. [DOI] [PubMed] [Google Scholar]
- 457.Martins KM, et al. Prevalent and disseminated recombinant and wild-type adeno-associated virus integration in Macaques and Humans. Hum. Gene Ther. 2023;34:1081–1094. doi: 10.1089/hum.2023.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Tai PWL. Integration of gene therapy vectors: a risk factor for tumorigenesis or another commensal property of adeno-associated viruses that benefits long-term transgene expression? Hum. Gene Ther. 2023;34:1074–1076. doi: 10.1089/hum.2023.29255.pwl. [DOI] [PubMed] [Google Scholar]
- 459.Sabatino DE, et al. Evaluating the state of the science for adeno-associated virus integration: an integrated perspective. Mol. Ther. 2022;30:2646–2663. doi: 10.1016/j.ymthe.2022.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Nguyen GN, et al. A long-term study of AAV gene therapy in dogs with hemophilia A identifies clonal expansions of transduced liver cells. Nat. Biotechnol. 2021;39:47–55. doi: 10.1038/s41587-020-0741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Dalwadi DA, et al. Liver injury increases the incidence of HCC following AAV gene therapy in mice. Mol. Ther. 2021;29:680–690. doi: 10.1016/j.ymthe.2020.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Rosas LE, et al. Patterns of scAAV vector insertion associated with oncogenic events in a mouse model for genotoxicity. Mol. Ther. 2012;20:2098–2110. doi: 10.1038/mt.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Kang H-R, et al. Pathogenesis of hepatic tumors following gene therapy in murine and canine models of glycogen storage disease. Mol. Ther. Methods Clin. Dev. 2019;15:383–391. doi: 10.1016/j.omtm.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Ferla R, et al. Low incidence of hepatocellular carcinoma in mice and cats treated with systemic adeno-associated viral vectors. Mol. Ther. Methods Clin. Dev. 2021;20:247–257. doi: 10.1016/j.omtm.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Greig, J. A. et al. Integrated vector genomes may contribute to long-term expression in primate liver after AAV administration. Nat. Biotechnol. 1–11 10.1038/s41587-023-01974-7 (2023). [DOI] [PMC free article] [PubMed]
- 466.Nault J-C, et al. Recurrent AAV2-related insertional mutagenesis in human hepatocellular carcinomas. Nat. Genet. 2015;47:1187–1193. doi: 10.1038/ng.3389. [DOI] [PubMed] [Google Scholar]
- 467.Fujimoto A, et al. Whole-genome mutational landscape and characterization of noncoding and structural mutations in liver cancer. Nat. Genet. 2016;48:500–509. doi: 10.1038/ng.3547. [DOI] [PubMed] [Google Scholar]
- 468.Bayard Q, et al. Cyclin A2/E1 activation defines a hepatocellular carcinoma subclass with a rearrangement signature of replication stress. Nat. Commun. 2018;9:5235. doi: 10.1038/s41467-018-07552-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Kaeppel C, et al. A largely random AAV integration profile after LPLD gene therapy. Nat. Med. 2013;19:889–891. doi: 10.1038/nm.3230. [DOI] [PubMed] [Google Scholar]
- 470.Hanlon KS, et al. High levels of AAV vector integration into CRISPR-induced DNA breaks. Nat. Commun. 2019;10:4439. doi: 10.1038/s41467-019-12449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Nelson CE, et al. Long-term evaluation of AAV-CRISPR genome editing for duchenne muscular dystrophy. Nat. Med. 2019;25:427–432. doi: 10.1038/s41591-019-0344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Chand D, et al. Hepatotoxicity following administration of Onasemnogene Abeparvovec (AVXS-101) for the treatment of spinal muscular atrophy. J. Hepatol. 2021;74:560–566. doi: 10.1016/j.jhep.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 473.Wilson JM, Flotte TR. Moving forward after two deaths in a gene therapy trial of myotubular myopathy. Hum. Gene Ther. 2020;31:695–696. doi: 10.1089/hum.2020.182. [DOI] [PubMed] [Google Scholar]
- 474.Mingozzi F, et al. T cell responses to AAV vector capsid limit the duration of transgene expression in humans after liver-directed gene therapy. Blood. 2005;106:3055. doi: 10.1182/blood.V106.11.3055.3055. [DOI] [Google Scholar]
- 475.McFarlane PA, et al. Making the correct diagnosis in thrombotic microangiopathy: a narrative review. Can. J. Kidney Heal. Dis. 2021;8:20543581211008708. doi: 10.1177/20543581211008707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Young JA, Pallas CR, Knovich MA. Transplant-associated thrombotic microangiopathy: theoretical considerations and a practical approach to an unrefined diagnosis. Bone Marrow Transplant. 2021;56:1805–1817. doi: 10.1038/s41409-021-01283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Yazaki K, Sakuma S, Hikita N, Fujimaru R, Hamazaki T. Child neurology: pathologically confirmed thrombotic microangiopathy caused by Onasemnogene Abeparvovec treatment for SMA. Neurology. 2022;98:808–813. doi: 10.1212/WNL.0000000000200676. [DOI] [PubMed] [Google Scholar]
- 478.Hordeaux J, et al. Adeno-associated virus-induced dorsal root ganglion pathology. Hum. Gene Ther. 2020;31:808–818. doi: 10.1089/hum.2020.167. [DOI] [PubMed] [Google Scholar]
- 479.Buss N, et al. Characterization of AAV-mediated dorsal root ganglionopathy. Mol. Ther. - Methods Clin. Dev. 2022;24:342–354. doi: 10.1016/j.omtm.2022.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Hinderer C, et al. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum. Gene Ther. 2018;29:285–298. doi: 10.1089/hum.2018.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Tukov FF, et al. Single-dose intrathecal dorsal root ganglia toxicity of Onasemnogene Abeparvovec in cynomolgus monkeys. Hum. Gene Ther. 2022;33:740–756. doi: 10.1089/hum.2021.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Zerah M, et al. Intracerebral gene therapy using AAVrh.10-hARSA recombinant vector to treat patients with early-onset forms of metachromatic leukodystrophy: preclinical feasibility and safety assessments in nonhuman primates. Hum. Gene Ther. Clin. Dev. 2015;26:113–124. doi: 10.1089/humc.2014.139. [DOI] [PubMed] [Google Scholar]
- 483.Sondhi D, et al. Long-term expression and safety of administration of AAVrh.10hCLN2 to the Brain of rats and nonhuman primates for the treatment of late infantile neuronal ceroid lipofuscinosis. Hum. Gene Ther. B Methods. 2012;23:324–335. doi: 10.1089/hgtb.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Rosenberg JB, et al. Safety of direct intraparenchymal AAVrh.10-mediated central nervous system gene therapy for metachromatic leukodystrophy. Hum. Gene Ther. 2021;32:563–580. doi: 10.1089/hum.2020.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Sondhi D, et al. Slowing late infantile Batten disease by direct brain parenchymal administration of a rh.10 adeno-associated virus expressing CLN2. Sci. Transl. Med. 2020;12:eabb5413. doi: 10.1126/scitranslmed.abb5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Löser P, Jennings GS, Strauss M, Sandig V. Reactivation of the previously silenced cytomegalovirus major immediate-early promoter in the mouse liver: involvement of NFκB. J. Virol. 1998;72:180–190. doi: 10.1128/JVI.72.1.180-190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Chen X, Tai PW, Gao G, Xie J. Strong compact neuron-specific promoters for AAV gene transfer (ASGCT abstract 252) Mol. Ther. 2023;31:S1–794. [Google Scholar]
- 488.Heinke P, et al. Diploid hepatocytes drive physiological liver renewal in adult humans. Cell Syst. 2022;13:499–507.e12. doi: 10.1016/j.cels.2022.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Current clinical trials employing rAAV-based gene therapy for human diseases