Take Home Message
For patients who underwent cytoreductive nephrectomy (CN), immunotherapy receipt was associated with better survival outcomes in comparison to tyrosine kinase inhibitor (TKI) therapy. Furthermore, systemic therapy initiation before versus after CN was associated with better outcomes. While further investigations are needed, our findings call into question the applicability of CN clinical trial data from the TKI era to current immunotherapy-based therapeutic protocols for metastatic renal cell carcinoma.
Keywords: Immunotherapy, Metastatic renal cell carcinoma, Nephrectomy, Tyrosine kinase inhibitor, Survival
Abstract
Background and objective
The role of cytoreductive nephrectomy (CN) in the treatment of metastatic renal cell carcinoma (mRCC) has been called into question on the basis of clinical trial data from the tyrosine kinase inhibitor (TKI) era. Comparative analyses of CN for patients treated with immuno-oncology (IO) versus TKI agents are sparse. Our objective was to compare CN timing and outcomes among patients who received TKI versus IO therapy.
Methods
This was a multicenter retrospective analysis of patients who underwent CN using data from the REMARCC (Registry of Metastatic RCC) database. The cohort was divided into TKI versus IO first-line therapy groups. The primary outcome was all-cause mortality (ACM). Secondary outcomes included cancer-specific mortality (CSM). Multivariable analysis was used to identify factors predictive for ACM and CSM. The Kaplan-Meier method was used to analyze 5-yr overall survival (OS) and cancer-specific survival (CSS) with stratification by primary systemic therapy and timing in relation to CN.
Key findings and limitations
We analyzed data for 189 patients (148 TKI + CN, 41 IO +CN; median follow-up 23.2 mo). Multivariable analysis revealed that a greater number of metastases (hazard ratio [HR] 1.06; p = 0.015), greater primary tumor size (HR 1.10; p = 0.043), TKI receipt (HR 2.36; p = 0.015), and initiation of systemic therapy after CN (HR 1.49; p = 0.039) were associated with worse ACM. A greater number of metastases at diagnosis (HR 1.07; p = 0.011), greater primary tumor size (HR 1.12; p = 0.018), TKI receipt (HR 5.43; p = 0.004), and initiation of systemic therapy after CN (HR 2.04; p < 0.001) were associated with worse CSM. Kaplan-Meier analyses revealed greater 5-yr rates for OS (51% vs 27%; p < 0.001) and CSS (83% vs 30%; p < 0.001) for IO +CN versus TKI + CN. This difference persisted in a subgroup analysis for patients with intermediate or poor risk, with 5-yr OS rates of 50% for IO + CN versus 30% for TKI + CN (p < 0.001). A subanalysis stratified by CN timing revealed better 5-yr rates for OS (50% vs 30%; p = 0.042) and CSS (90% vs 30%, p = 0.019) for delayed CN after IO therapy, but not after TKI therapy.
Conclusions and clinical implications
For patients who underwent CN, systemic therapy before CN was associated with better outcomes. In addition, IO therapy was associated with better survival outcomes in comparison to TKI therapy. Our findings question the applicability of clinical trial data from the TKI era to CN in the IO era for mRCC.
Patient summary
For patients with metastatic kidney cancer treated with surgery, better survival outcomes were observed for those who also received immunotherapy in comparison to therapy targeting specific proteins in the body (tyrosine kinase inhibitors, TKIs). Immunotherapy or TKI treatment resulted in better outcomes if it was received before rather than after surgery.
1. Introduction
Despite stage migration in renal cell carcinoma (RCC) because of greater incidental detection of lesions on cross-sectional imaging, up to 15% of patients diagnosed with RCC present with metastatic disease [1], [2]. Cytoreductive nephrectomy (CN) has been a cornerstone of therapy for metastatic RCC (mRCC) on the basis of clinical trial evidence from the cytokine era. This role was challenged by the recent publication of clinical trial results that cast doubt on the benefit of CN as an adjunct to TKI systemic therapy (CARMENA) or primary CN (SURTIME) [3], [4]. Nonetheless, there was a trend towards better outcomes for patients who underwent delayed nephrectomy (systemic therapy initiated before nephrectomy) in the SURTIME trial, which closed because of low accrual, with a lack of power leading to nonsignificant data. The emergence of immune checkpoint inhibitors (ICIs) as first-line options for mRCC heralded another paradigm shift in the field, with better outcomes observed in comparison to TKI-only regimens [5], [6]. These data, and the conceptual importance of cytoreduction in immunotherapeutic principles, call into question the applicability of data on CN + TKI in the immunotherapy era. Data on direct comparisons of outcomes for patients who underwent CN and were treated with TKIs or ICIs are sparse, particularly for systemic therapy before CN. Our aim was to compare CN outcomes in patient groups treated with TKIs or ICIs.
2. Patients and methods
2.1. Patient population
This is a retrospective international multi-institutional analysis using Registry of Metastatic RCC (REMARCC) data for patients presenting with metastatic RCC (mRCC) between January 2006 and October 2019 who underwent CN. Our methods have been described previously [7], [8]. Institutional review board approval was obtained at all participating centers. Patients with RCC underwent initial staging evaluation including computed tomography or magnetic resonance imaging of the chest and abdomen/pelvis, with additional studies as indicated [9], [10]. The decision to proceed with CN and systemic therapy involved interdisciplinary consultation between urologic oncologists and medical oncologists. The type of surgery, CN timing, and surgical approach were at the surgeon’s discretion. Patients received either TKI or ICI first-line therapy. The type of systemic therapy provided and the treatment protocol were chosen at an institutional level. Radiographic follow-up and determination of response were in accordance with Response Evaluation Criteria in Solid Tumors [11], [12].
2.2. Data collection
Data were entered into institutional data sets by database managers. Variables collected included demographic data at the time of diagnosis (age, sex, body mass index [BMI]), baseline laboratory values (hemoglobin, creatinine), TNM stage, Motzer risk category, and clinical disease characteristics (performance status, number and location of metastases) [13], [14]. Treatment data (primary surgery, systemic therapy) and operative variables (estimated blood loss, complications) were also collected [15]. Survival outcomes, including progression-free survival (PFS), disease-free survival, and overall survival (OS) at last follow-up, were recorded.
2.3. Data analysis
The primary outcome was all-cause mortality (ACM)/OS measured from the date of diagnosis to the date of last follow-up. Secondary outcomes included cancer-specific mortality (CSM)/cancer-specific survival (CSS) and disease progression/PFS. The cohort was divided into patients who received a TKI versus an immuno-oncology (IO) agent as first-line systemic treatment. Descriptive analyses were conducted using Student’s t test or analysis of variance for continuous variables, and Fisher’s exact test for categorical variables. Multivariable proportional-hazards regression analysis was conducted for ACM and CSM. The Kaplan-Meier method was used to analyze median OS and CSS, with stratification by type of systemic therapy and the timing of CN. SPSS v.26 (IBM, Armonk, NY, USA) was used for statistical analyses, with p < 0.05 considered statistically significant.
3. Results
We analyzed data for 189 patients (148 TKI vs 41 IO). Median follow-up for the cohort was 23.2 mo (interquartile range 12.3–40.2). Table 1 lists demographic data and clinical disease characteristics for the study population. There were no differences between the TKI and IO groups with respect to age at diagnosis (p = 0.710), male sex (p = 0.063), Motzer risk category (p = 0.719), BMI (p = 0.479), clinical T stage of the primary tumor (p = 0.727), primary tumor size (9.0 vs 9.3 cm; p = 0.603), or median number of metastases at diagnosis (4 vs 3; p = 0.354). A greater proportion of patients had a lower Eastern Cooperative Oncology Group performance status (ECOG PS) score in the TKI cohort (p = 0.037). A greater proportion of patients in the TKI cohort had lung metastases (62.2% vs 56.1%; p = 0.030), but there was no significant difference between the groups in the proportion of patients with liver (p = 0.980), bone (p = 0.426), or brain (0.622) metastases.
Table 1.
Patient demographics and clinical disease characteristics
| Variable | Tyrosine kinase inhibitor (n = 148) |
Immunotherapy (n = 41) |
p value |
|---|---|---|---|
| Mean age, yr (standard deviation) | 62.29 (11.08) | 61.56 (11.14) | 0.710 |
| Sex, n (%) | 0.063 | ||
| Female | 39 (26.4) | 5 (12.2) | |
| Male | 109 (73.6) | 36 (87.8) | |
| Median body mass index, kg/m2 (interquartile range) | 25.5 (23.0–29.2) | 26.1 (24.4–30.4) | 0.479 |
| ECOG PS score, n (%) | 0.037 | ||
| 0 | 83 (56.1) | 16 (39.0) | |
| 1 | 49 (33.1) | 17 (41.5) | |
| 2 | 11 (7.4) | 8 (19.5) | |
| 3 | 5 (3.4) | 0 (0) | |
| Motzer risk category, n (%) | 0.719 | ||
| Low risk | 18 (12.2) | 4 (9.8) | |
| Intermediate risk | 101 (68.7) | 27 (65.9) | |
| High risk | 28 (19.1) | 10 (24.4) | |
| Data missing | 1 | – | |
| Clinical T stage of the primary tumor, n (%) | 0.727 | ||
| cT1 | 20 (13.5) | 7 (17.5) | |
| cT2 | 38 (25.7) | 12 (30.0) | |
| cT3 | 74 (50.0) | 16 (40.0) | |
| cT4 | 16 (10.8) | 5 (12.5) | |
| Data missing | – | 1 | |
| Median primary tumor size, cm (interquartile range) | 9.0 (7.0–12.0) | 9.3 (7.0–11.75) | 0.603 |
| Clinical N stage, n (%) | 0.011 | ||
| N0 | 84 (56.8) | 23 (56.1) | |
| N1 | 64 (43.2) | 15 (36.6) | |
| NX | 0 (0) | 3 (7.3) | |
| Median no. of metastases at diagnosis (interquartile range) | 4 (2–8) | 3 (1–7) | 0.354 |
| Location of metastases at diagnosis, n (%) | |||
| Lungs | 92 (62.2) | 23 (56.1) | 0.030 |
| Liver | 11 (7.4) | 3 (7.3) | 0.980 |
| Bone | 37 (25.0) | 13 (31.7) | 0.426 |
| Brain | 1 (0.6) | 2 (4.9) | 0.323 |
| Cytoreductive approach, n (%) | 0.457 | ||
| Open surgery | 98 (66.2) | 26 (63.4) | |
| Minimally invasive surgery | 50 (33.3) | 15 (36.6) | |
| Median estimated blood loss, ml (interquartile range) | 400 (150–800) | 475 (150–1000) | 0.672 |
| Perioperative complications, n (%) | 41 (27.7) | 6 (14.6) | 0.637 |
| Timing of systemic therapy initiation, n (%) | 0.008 | ||
| Before cytoreductive nephrectomy | 44 (29.7) | 4 (9.8) | |
| After cytoreductive nephrectomy | 104 (70.3) | 37 (90.2) | |
| Median systemic therapy duration, mo (interquartile range) | 6.0 (2.0–12.3) | 8.0 (3.0–18.6) | 0.612 |
| Median follow-up, mo (interquartile range) | 22.3 (12.4–40.0) | 27.0 (12.3–53.6) | 0.696 |
| Disease progression, n (%) | 126 (85.1) | 31 (75.6) | 0.162 |
| Cancer-specific deaths, n (%) | 86 (58.1) | 6 (14.6) | <0.001 |
| All-cause deaths, n (%) | 104 (70.3) | 15 (36.7) | <0.001 |
ECOG PS = Eastern Cooperative Oncology Group performance status.
There were no significant differences between the groups with respect to surgical approach (p = 0.457), estimated blood loss (p = 0.672), or perioperative complications (p = 0.637). A greater percentage of patients in the TKI cohort had systemic therapy before CN (29.7% vs 9.8%; p = 0.008). However, there were no significant differences in median systemic therapy duration (TKI 6.0 mo vs IO 8.0 mo; p = 0.612) or the percentage of patients who underwent metastasectomy (TKI 20.9% vs IO 17.0%).
Table 2 lists multivariable analysis results for factors associated with survival outcomes. A greater number of metastases at diagnosis (hazard ratio [HR] 1.06; p = 0.015), greater primary tumor size (HR 1.10; p = 0.043), TKI versus IO therapy (HR 2.36; p = 0.015), and initiation of systemic therapy after versus before CN (HR 1.49; p = 0.039) were associated with worse ACM, while higher BMI was associated with better ACM (HR 0.93; p = 0.006). A greater number of metastases at diagnosis (HR 1.07; p = 0.011), greater primary tumor size (HR 1.12; p = 0.018), TKI versus IO therapy (HR 5.43; p = 0.004), and initiation of systemic therapy after versus before CN (HR 2.04; p < 0.001) were also independently associated with worse CSM, while higher BMI was associated with better CSM (HR 0.93; p = 0.014). Male sex (HR 2.90; p < 0.001), worse ECOG PS score (HR 3.66; p = 0.032), a greater number of metastases at diagnosis (HR 1.10; p < 0.001), and initiation of systemic therapy after versus before CN (HR 1.47; p = 0.041) were independently associated with worse progression.
Table 2.
Multivariable analysis results for all-cause mortality, cancer-specific mortality, and disease progression
| Variable | All-cause mortality |
Cancer-specific mortality |
Disease progression |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Age | 1.03 (0.99–1.05) | 0.061 | 1.01 (0.99–1.04) | 0.280 | 0.99 (0.97–1.01) | 0.273 |
| Sex (male vs female) | 1.04 (0.670–1.91) | 0.860 | 0.93 (0.88–0.99) | 0.014 | 2.90 (1.64–5.13) | <0.001 |
| Body mass index | 0.93 (0.88–0.98) | 0.006 | 2.26 (0.41–7.60) | 0.443 | 0.98 (0.92–1.05) | 0.557 |
| ECOG PS (0/1 vs 2/3) | 1.54 (0.40–5.88) | 0.528 | 3.66 (1.12–11.97) | 0.032 | ||
| Location of metastases | ||||||
| Lung | Reference | Reference | Reference | |||
| Liver | 1.23 (0.67–1.23) | 0.948 | 1.04 (0.87–1.11) | 0.961 | 1.45 (0.65–3.95) | 0.255 |
| Bone | 0.95 (0.54–1.70) | 0.868 | 0.99 (0.54–1.82) | 0.969 | 1.84 (0.79–4.26) | 0.158 |
| Brain | 1.11 (0.46–2.65) | 0.817 | 1.13 (0.45–2.86) | 0.793 | 2.32 (0.85–6.35) | 0.102 |
| Other | 0.98 (0.91–1.34) | 0.768 | 1.67 (0.34–4.10) | 0.267 | 1.36 (0.79–4.50) | 0.345 |
| Motzer risk category | ||||||
| Low risk | Reference | Reference | Reference | |||
| Intermediate risk | 1.44 (0.36–5.52) | 0.617 | 1.45 (0.33–6.38) | 0.627 | 1.57 (0.40–6.21) | 0.518 |
| High risk | 1.11 (0.38–3.26) | 0.852 | 1.27 (0.38–4.22) | 0.701 | 1.99 (0.71–5.61) | 0.193 |
| PO complications | 1.00 (0.582–1.72) | 0.998 | – | 1.06 (0.60–1.88) | 0.846 | |
| Estimated blood loss | 1.00 (0.99–1.00) | 0.546 | 1.01 (0.99–1.02) | 0.112 | 1.00 (0.99–1.01) | 0.228 |
| MIS (vs open surgery) | – | – | 1.40 (0.61–3.20) | 0.426 | ||
| Primary tumor size | 1.10 (0.58–1.71) | 0.043 | 1.12 (1.02–1.24) | 0.018 | 1.06 (0.97–1.16) | 0.187 |
| No. of metastases at Dx | 1.06 (1.01–1.11) | 0.015 | 1.07 (1.02–1.12) | 0.011 | 1.10 (1.05–1.16) | <0.001 |
| TKI treatment (vs IO) | 2.36 (1.14–4.89) | 0.021 | 10.0 (0.77–77.0) | 0.027 | 0.63(0.21–1.89) | 0.411 |
| STx before CN (vs after) | 0.67 (0.46–0.98) | 0.039 | 0.49 (0.32–0.74) | <0.001 | 0.68 (0.48–0.99) | 0.041 |
CI = confidence interval; CN = cytoreductive nephrectomy; Dx = diagnosis; ECOG PS = Eastern Cooperative Oncology Group performance status; HR = hazard ratio; IO = immuno-oncology agent; MIS = minimally invasive surgery; PO = perioperative; STx = systemic therapy; TKI = tyrosine kinase inhibitor.
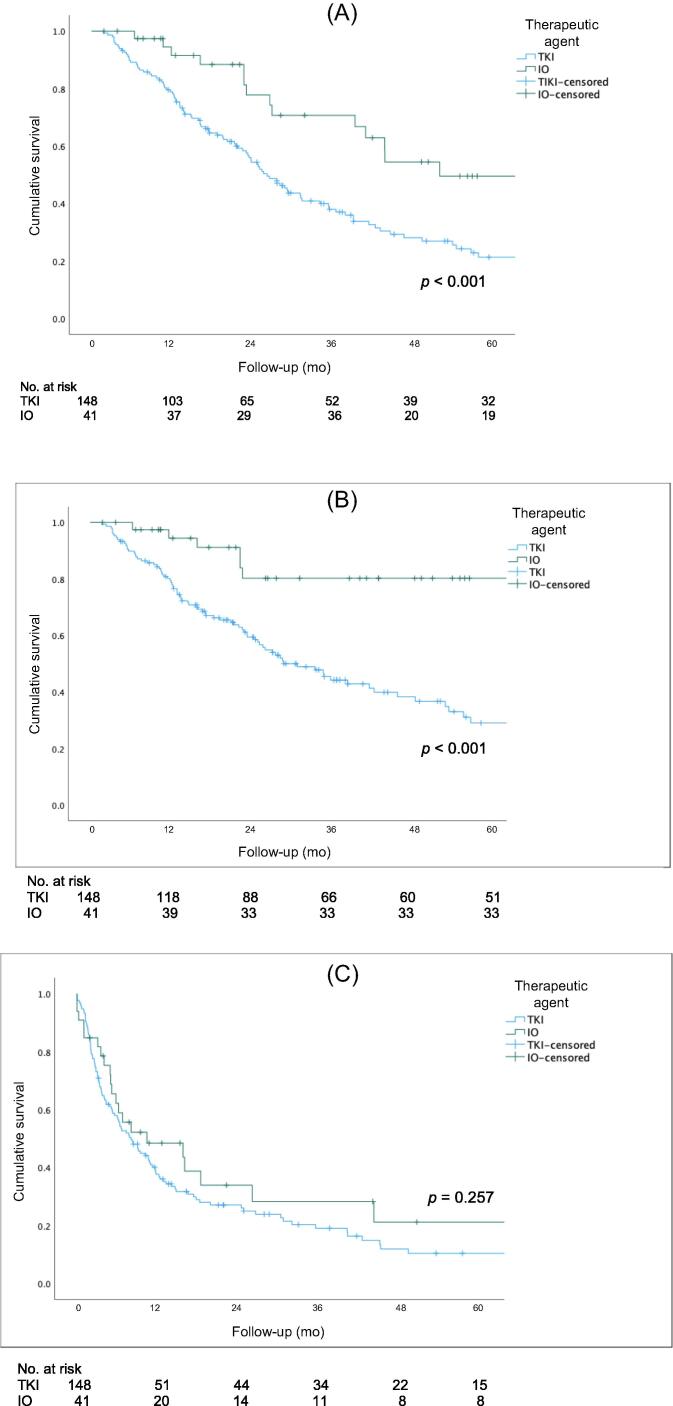
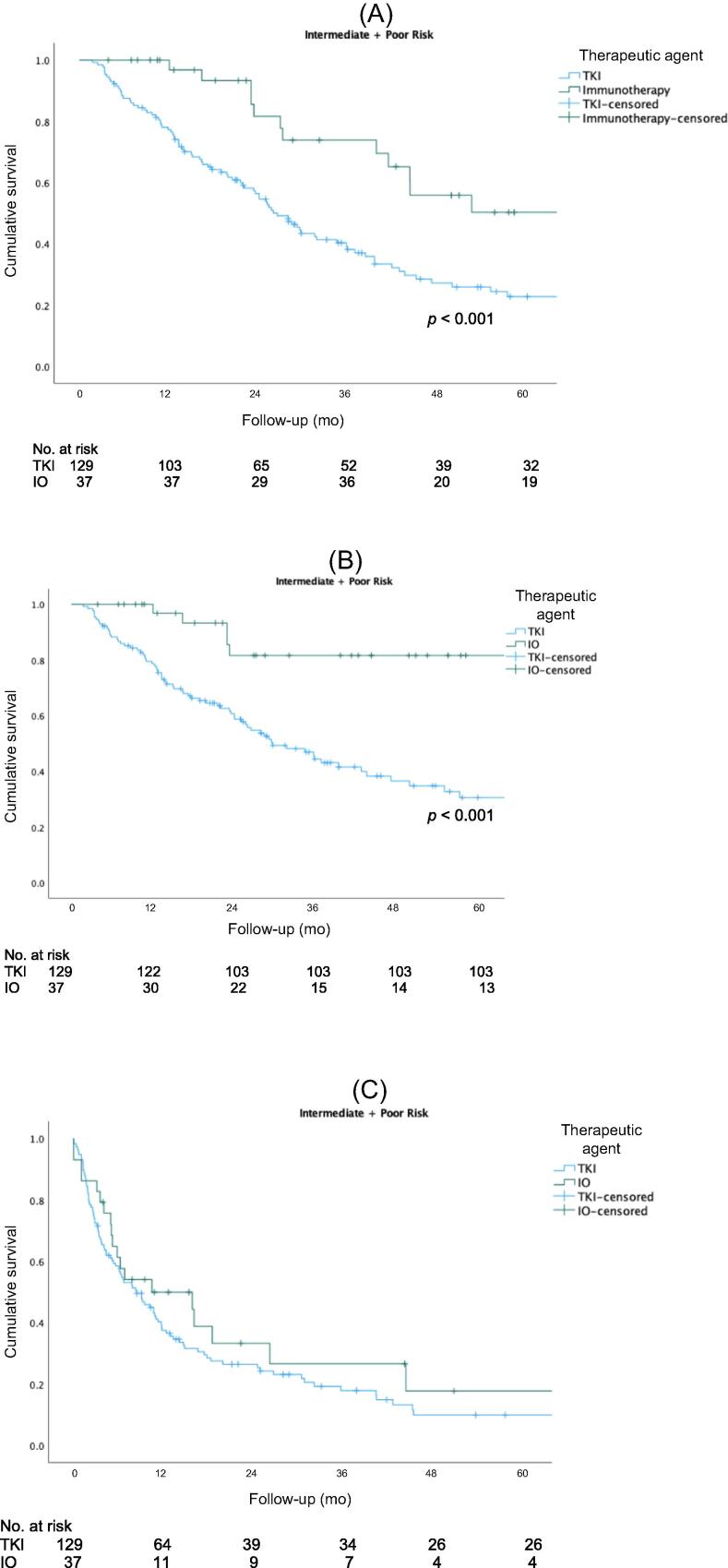
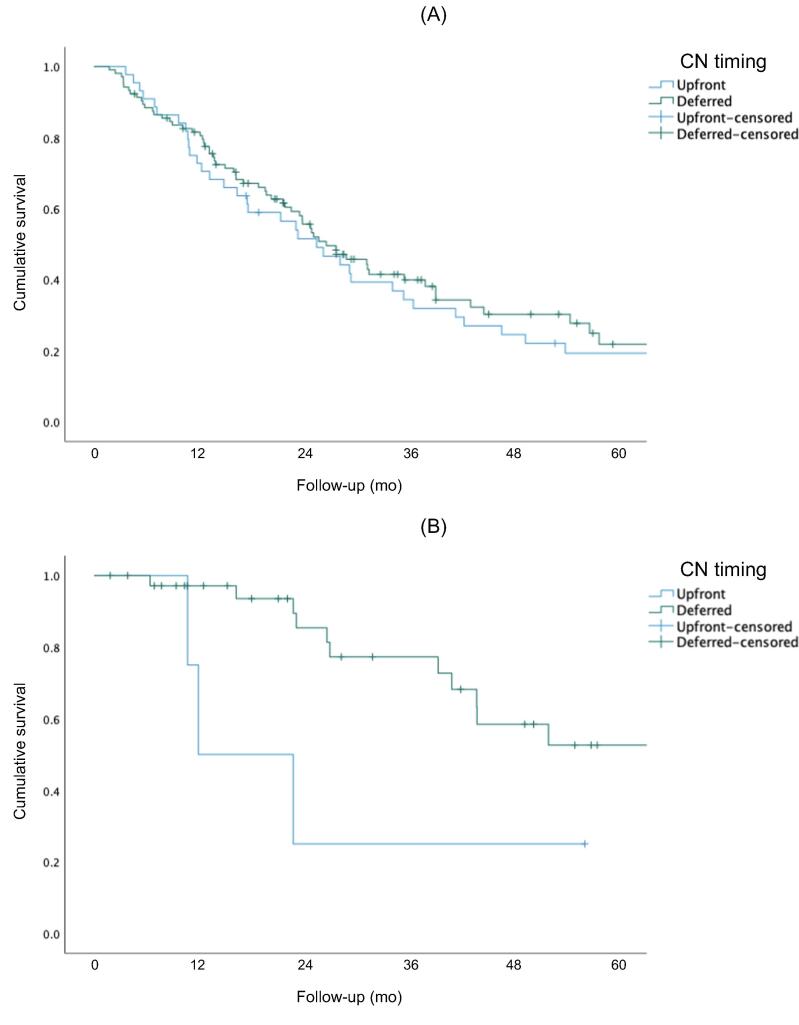
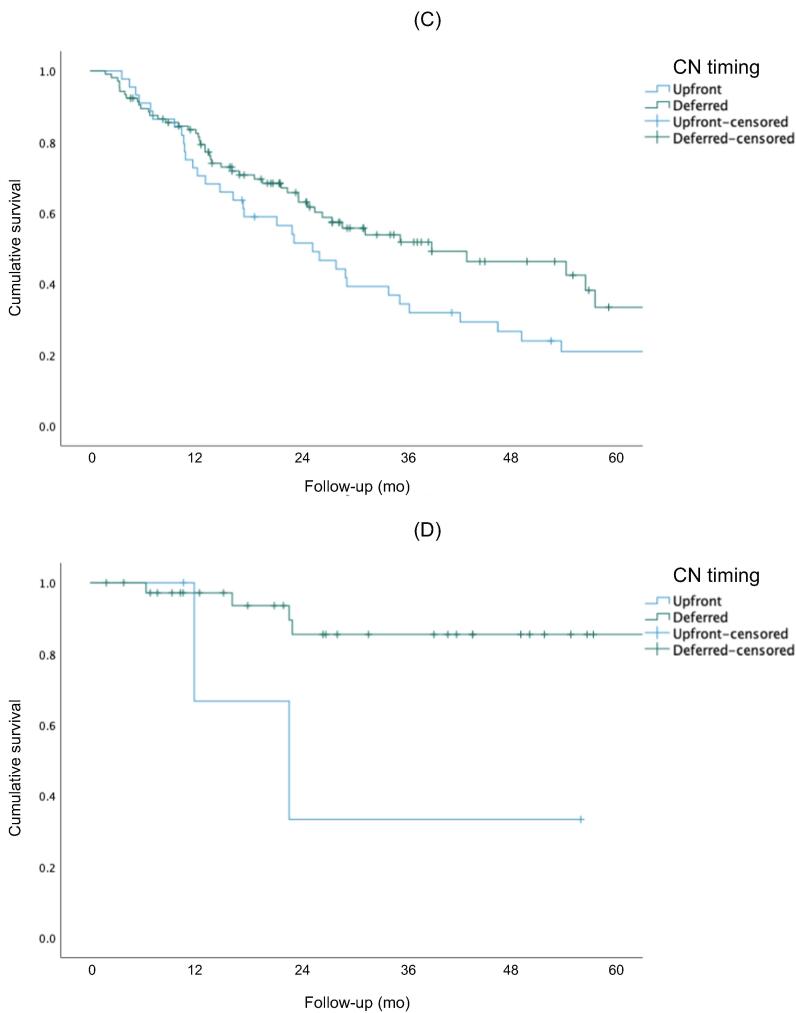
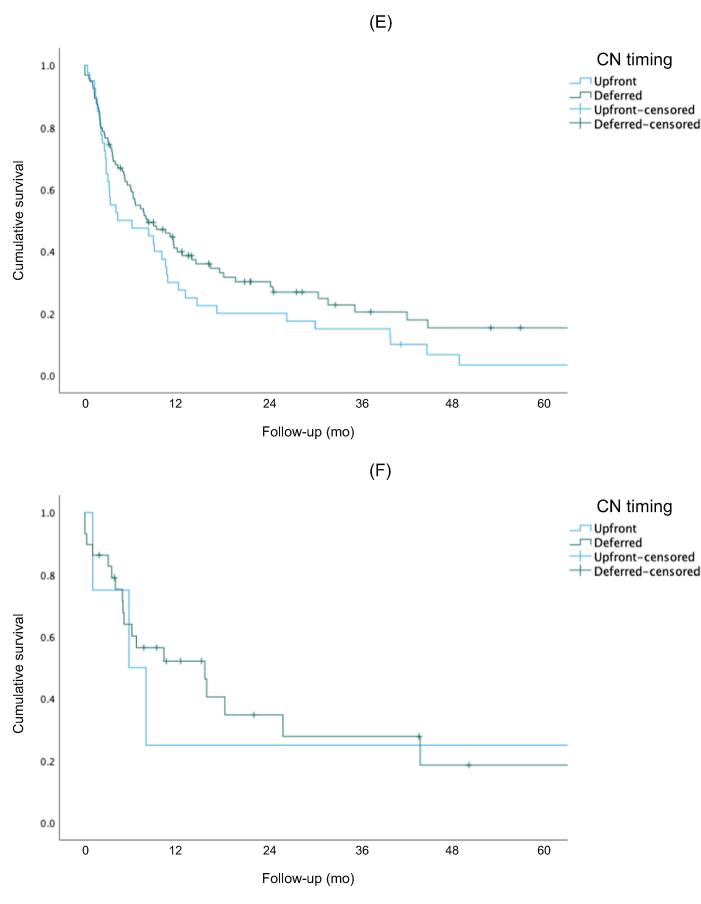
Figure 1 shows Kaplan-Meier curves for OS, CSS, and PFS for the overall cohort. The 5-yr rates for OS (51% vs 27%, p<0.001) and CSS (83% vs 30%, p < 0.001) were higher for the IO group than for the TKI group. There was no significant difference in PFS between the two groups (p = 0.257). Figure 2 shows Kaplan-Meier OS curves for the subcohort of patients with intermediate- or poor-risk disease. The 5-yr rates for OS (50% vs 23%; p < 0.001) and CSS (80% vs 30%; p < 0.001) were higher for the IO group than for the TKI group. There was no significant difference in PFS between the groups (p = 0.304). Subgroup analysis stratified by CN timing revealed better 5-yr rates for OS (50% vs 30%; p = 0.042) and CSS (90% vs 30%; p = 0.019) for delayed versus upfront CN in the IO cohort, but not in the TKI cohort (Fig. 3).
Fig. 1.
(A) Overall survival, (B) cancer-specific survival, and (C) progression-free survival for the entire cohort. IO = immuno-oncology; TKI = tyrosine kinase inhibitor.
Fig 2.
(A) Overall survival, (B) cancer-specific survival, and (C) progression-free survival for patients with intermediate- or poor-risk disease. IO = immuno-oncology; TKI = tyrosine kinase inhibitor.
Fig. 3.
Overall survival for the (A) TKI and (B) IO therapy groups. Cancer-specific survival for the (C) TKI and (D) IO therapy groups. Progression-free survival for the (E) TKI and (F) IO therapy groups. IO = immuno-oncology; TKI = tyrosine kinase inhibitor.
4. Discussion
We compared CN outcomes for groups of patients who received TKI versus IO systemic treatment. Our results suggest that for patients who underwent CN, initiation of systemic therapy before versus after CN was associated with better outcomes. Furthermore, IO systemic therapy was associated with better survival outcomes in comparison to TKI therapy. Our findings call into question the applicability of CN clinical trial data from the TKI era to CN in the IO era.
Historically, CN has been a cornerstone of mRCC management, with initial reports describing spontaneous regression of metastases following primary CN, which led to elaboration of the concept of immunological debulking [16]. The role of CN was seemingly cemented by findings reported by Flanigan et al [17], who demonstrated that CN followed by interferon α was associated with a 2-mo improvement in OS in comparison to interferon α alone (11.1 mo vs 9.1 mo; p = 0.05). With the introduction of TKI therapy and improvements in objective response rates, as well as data suggesting that upfront systemic therapy is a more effective strategy, the role of CN and its timing became more uncertain [18].
CARMENA demonstrated the noninferiority of sunitinib in comparison to upfront CN followed by sunitinib. In this clinical trial involving 450 patients with intermediate- or poor-risk metastatic clear-cell RCC randomized to upfront CN followed by sunitinib or to sunitinib alone, Méjean et al [3] found that while OS did not significantly differ between the groups (HR 0.89, 95% confidence interval [CI] 0.71–1.10), median PFS was longer with sunitinib alone (8.3 mo, 95% CI 6.2–9.9) than with CN followed by sunitinib (7.2 mo, 95% CI 6.7–8.5). Our analysis revealed significantly higher 3-yr OS rates for CN + TKI (45%) and CN + IO (70%) than the 25.9% for CN followed by sunitinib in CARMENA. While this may be attributable to the inclusion of mainly patients with favorable risk in our study, similar findings were noted for our subanalysis of patients with intermediate or poor risk, with better 3-yr OS rates for these subgroups (TKI + CN 40%, IO + CN 73%) in comparison to CARMENA. Taken together, our findings suggest that addition of IO-based protocols to CN results in a significant improvement in outcomes in comparison to CN + TKI, even in intermediate- or poor-risk mRCC.
SURTIME evaluated the timing of CN (before or after sunitinib) and found that immediate surgery did not impart a survival benefit [4]. This randomized clinical trial of 99 patients followed over 5.7 yr demonstrated that deferred CN did not improve the PFS rate at 28 wk (42% for immediate CN vs 43% for deferred CN; p = 0.61). However, the OS rate was better with deferred CN (albeit not statistically significant), which the authors posit may be attributable to greater eligibility for sunitinib therapy in this group (87% in the upfront CN group vs 98% in the delayed CN group). Our findings suggest better outcomes for patients who underwent CN after systemic therapy, which confirms the trend observed in SURTIME. In our study, deferred CN was independently associated with better OS (HR 0.67; p = 0.039), CSS (HR 0.49; p < 0.001), and PFS (HR 0.68; p = 0.041). Considering the failure to enroll the required number of patients in SURTIME, with lack of power leading to nonsignificant data, and the lack of similar data for patients treated with IO agents, our study adds important information about the optimal timing of CN and confirms results from SURTIME and other studies suggesting that CN after primary systemic therapy may be associated with better outcomes.
With the paradigm shift of the IO era, a re-examination of the impact of CN in IO treated is a topic of emerging investigation. In a study involving 433 patients, Ghatalia et al [19] found that upfront CN + IO had an OS benefit over IO alone (26.6 vs 14.6 mo; p < 0.001). There was no difference in median OS between upfront and delayed CN in this IO setting (HR 1.00; p = 0.99). Similarly, in a study involving 391 patients from the National Cancer Data Base, Singla et al [20] found that patients treated with CN and immunotherapy had better OS survival than patients treated with immunotherapy alone (HR 0.23; p < 0.001). In this cohort the OS rate at 30 mo was ∼50% for CN + IO compared to 23% for IO alone. In a recently published study by the International Metastatic RCC Database Consortium, Bakouny et al [21] analyzed 4639 patients who received TKI or ICI therapy with or without upfront CN. A survival benefit with upfront CN was observed for both the TKI group (HR 0.72; p < 0.001) and the ICI group (HR 0.61; p = 0.013). There was no significant difference in the improvement in OS between ICI and TKI therapy (interaction p = 0.6). These findings emphasize the impact of primary CN. By contrast, a significant proportion of patients in our cohort received upfront systemic therapy (IO or TKI) before CN and the improvement in OS for this group (HR 0.67; p = 0.039) suggests a beneficial impact of primary systemic therapy followed by CN. Ultimately, questions remain regarding the role of CN in the IO era, particularly with respect to surgical timing and patient selection; it is hoped that these questions will be answered by actively recruiting clinical trials, including PROBE and NORDIC SUN [22], [23].
Our results are subject to the inherent limitations of a retrospective study. The multicenter and multinational setting involves potential confounding because of nonstandardized therapeutic approaches and differences in follow-up protocols. The TKI and IO agents used and the duration of therapy varied by center. In addition, we have no information regarding whether CN was performed for palliative or debulking/consolidative indications. We also acknowledge that patient selection for CN may differ between the TKI and IO eras.
Our results suggesting better outcomes for CN + IO therapy in comparison to TKI-only regimens warrant further trials on the utility and timing of CN in protocols involving IO or combined IO-TKI treatment for mRCC.
5. Conclusions
For patients who underwent CN, initiation of systemic therapy before CN was associated with better outcomes. Furthermore, IO therapy was associated with better survival outcomes in comparison to TKI therapy. While further investigations are needed, our findings call into question the applicability of CN clinical trial data from the TKI era to current IO-based therapeutic protocols for mRCC.
Author contributions: Ithaar H. Derweesh had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Derweesh, Meagher.
Acquisition of data: Minervini, Mir, Cerrato, Rebez, Hampton, Campi, Kriegmair, Linares, Hevia, Musquera, D’Anna, Roussel, Albersen, Pavan, Claps, Antonelli, Marchioni, Paksoy, Erdem.
Analysis and interpretation of data: Meagher, Derweesh.
Drafting of the manuscript: Meagher.
Critical revision of the manuscript for important intellectual content: Mir, Autorino.
Statistical analysis: Meagher.
Obtaining funding: Derweesh.
Administrative, technical, or material support: None.
Supervision: Derweesh.
Other: None.
Financial disclosures: Ithaar H. Derweesh certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This work was supported by the Stephen Weissman Kidney Cancer Research Fund. The funding body played a role in management and analysis of the data.
Associate Editor: Jochen Walz
References
- 1.Cooperberg M.R., Mallin K., Kane C.J., Carroll P.R. Treatment trends for stage I renal cell carcinoma. J Urol. 2011;186:394–399. doi: 10.1016/j.juro.2011.03.130. [DOI] [PubMed] [Google Scholar]
- 2.Kane C.J., Mallin K., Ritchey J., Cooperberg M.R., Carroll P.R. Renal cell cancer stage migration. Cancer. 2008;113:78–83. doi: 10.1002/cncr.23518. [DOI] [PubMed] [Google Scholar]
- 3.Méjean A., Ravaud A., Thezenas S., et al. Sunitinib alone or after nephrectomy in metastatic renal cell carcinoma. N Engl J Med. 2018;379:417–427. doi: 10.1056/NEJMoa1803675. [DOI] [PubMed] [Google Scholar]
- 4.Bex A., Mulders P., Jewett M., et al. Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib: the SURTIME randomized clinical trial. JAMA Oncol. 2019;5:164–170. doi: 10.1001/jamaoncol.2018.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mennitto A., Grassi P., Ratta R., Verzoni E., Prisciandaro M., Procopio G. Nivolumab in the treatment of advanced renal cell carcinoma: clinical trial evidence and experience. Ther Adv Urol. 2016;8:319–326. doi: 10.1177/1756287216656811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Popovic M., Matovina-Brko G., Jovic M., Popovic L.S. Immunotherapy: a new standard in the treatment of metastatic clear cell renal cell carcinoma. World J Clin Oncol. 2022;13:28–38. doi: 10.5306/wjco.v13.i1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roussel E., Campi R., Larcher A., et al. Rates and predictors of perioperative complications in cytoreductive nephrectomy: analysis of the Registry for Metastatic Renal Cell Carcinoma. Eur Urol Oncol. 2020;3:523–529. doi: 10.1016/j.euo.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Meagher M.F., Mir M.C., Autorino R., et al. Impact of metastasectomy on cancer specific and overall survival in metastatic renal cell carcinoma: analysis of the REMARCC registry. Clin Genitourin Cancer. 2022;20:326–333. doi: 10.1016/j.clgc.2022.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Motzer R.J., Jonasch E., Michaelson M.D., et al. NCCN guidelines insights: kidney cancer, version 2.2020. J Natl Compr Cancer Netw. 2019;17:1278–1285. doi: 10.6004/jnccn.2019.0054. [DOI] [PubMed] [Google Scholar]
- 10.Ljungberg B., Albiges L., Abu-Ghanem Y., et al. European Association of Urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol. 2019;75:799–810. doi: 10.1016/j.eururo.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz L.H., Mazumdar M., Wang L., et al. Response assessment classification in patients with advanced renal cell carcinoma treated on clinical trials. Cancer. 2003;98:1611–1619. doi: 10.1002/cncr.11712. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P., Arbuck S.G., Eisenhauer E.A., et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Motzer R.J., Jonasch E., Agarwal N., et al. Kidney cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2022;20:71–90. doi: 10.6004/jnccn.2022.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motzer R.J., Bacik J., Murphy B.A., Russo P., Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–296. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 15.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abou Youssif T., Tanguay S. Nephrectomy is necessary in the treatment of metastatic renal cell carcinoma. Can Urol Assoc J. 2010;4:65–67. doi: 10.5489/cuaj.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flanigan R.C., Salmon S.E., Blumenstein B.A., et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–1659. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 18.Bindayi A., Hamilton Z.A., McDonald M.L., et al. Neoadjuvant therapy for localized and locally advanced renal cell carcinoma. Urol Oncol. 2018;36:31–37. doi: 10.1016/j.urolonc.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Ghatalia P., Handorf E.A., Geynisman D.M., et al. The role of cytoreductive nephrectomy in metastatic renal cell carcinoma: a real-world multi-institutional analysis. J Urol. 2022;208:71–79. doi: 10.1097/JU.0000000000002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singla N., Hutchinson R.C., Ghandour R.A., et al. Improved survival after cytoreductive nephrectomy for metastatic renal cell carcinoma in the contemporary immunotherapy era: an analysis of the National Cancer Database. Urol Oncol. 2020;38:604.e9–604.e17. doi: 10.1016/j.urolonc.2020.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bakouny Z., El Zarif T., Dudani S., et al. Upfront cytoreductive nephrectomy for metastatic renal cell carcinoma treated with immune checkpoint inhibitors or targeted therapy: an observational study from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol. 2023;83:145–151. doi: 10.1016/j.eururo.2022.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Fristrup N. Deferred cytoreductive nephrectomy in synchronous metastatic renal cell carcinoma: the NORDIC-SUN trial. https://clinicaltrials.gov/study/NCT03977571. [DOI] [PMC free article] [PubMed]
- 23.Bell H., Cotta B.H., Salami S.S., Kim H., Vaishampayan U. “PROBE”ing the role of cytoreductive nephrectomy in advanced renal cancer. Kidney Cancer J. 2022;6:3–9. doi: 10.3233/kca-210010. [DOI] [PMC free article] [PubMed] [Google Scholar]