Abstract
Purpose
This report aims to highlight the wide spectrum of ophthalmic adverse events associated with erdafitinib, a fibroblast growth factor inhibitor that blocks activation of the mitogen-activated protein kinase kinase (MAPK/MEK) cascade. The purpose of this report is to describe a case of erdafitinib-associated bilateral outer retinal alterations in the MEK-associated retinopathy spectrum and rapid onset bilateral total cataracts following a 20-month course of erdafitinib therapy.
Observations
A 69 year old male with metastatic bladder cancer presented 47 days following treatment initiation with daily erdafitinib (8-mg) with mild new subretinal fluid and minimal associated subretinal debris in the left eye and accentuation/thickening of the interdigitation zone in the right eye. Over the course of treatment, improvements were noted, particularly with erdafitinib dose reduction. At 20 months, both eyes developed rapidly progressive mature cataracts with significant visual changes, necessitating bilateral cataract extraction.
Conclusions and importance
The potential stability of moderate outer retinal changes (i.e., ellipsoid zone/interdigitation zone, subretinal fluid) while continuing erdafitinib therapy is highlighted in this report. In addition, the importance of continued ophthalmic surveillance is emphasized given the possible association of anterior segment adverse events with long-term erdafitinib use.
Keywords: Erdafitinib, Fibroblast growth factor receptor inhibitor, Drug reactions, Cataracts
1. Introduction
Fibroblast growth factor receptors (FGFR) play an important role in cellular functioning and developmental processes, and pathway dysregulation has been linked to the progression and development of a variety of malignancies.1 Erdafitinib, a tyrosine kinase receptor inhibitor of FGFR 1–4, was the first targeted therapy approved for metastatic bladder cancer, and is involved in blocking activation of the mitogen-activated protein kinase kinase (MAPK/MEK) cascade.2 It was granted accelerated FDA approval in 2019 for patients with locally advanced or metastatic urothelial carcinoma with a susceptible FGFR2 or FGFR3 genetic alteration that has progressed during or following platinum-containing chemotherapy. In the phase II trial that led to erdafitinib's approval, 46% of the patients had reported treatment-related grade 3 or higher adverse events based on the Common Terminology Criteria for Adverse Events (CTCAE).3 Ocular related adverse events included dry eyes (28%) and “central serous retinopathy” (CSR, 25%), of which 6% symptomatic and 3% experienced marked decrease in visual acuity with best corrected visual acuity worse than 20/40 or more than 3 lines of decreased vision from known baseline.3,4 Although the label for the drug describes CSR-like changes, the literature supports the retinal findings to be more on the spectrum of MEK-associated retinopathy (MEKAR) than CSR. Previous reports have described the occurrence of anterior, and to a lesser extent posterior segment adverse events, however the literature regarding the ophthalmic impact of this drug remains sparse.3, 4, 5, 6, 7 Furthermore, to our knowledge, long-term sequelae beyond 12 months of erdafitinib treatment has not been documented outside of a clinical trial protocol. In this report, the longitudinal clinical course of a patient treated with erdafitinib over 20 months developed important adverse events: unilateral subretinal fluid, bilateral outer retinal alterations (i.e., enhanced visualization of the interdigitation zone), and rapid onset of bilateral total cataracts.
2. Case report
A 69 year old male diagnosed with metastatic bladder cancer previously treated with palliative pembrolizumab was started on erdafitinib (8mg) daily 47 days prior to presenting. The patient had been previously followed for retinal tear and mild epiretinal membrane in the left eye with associated minimal macular thickening (Fig. 1A). On presentation following erdafitinib initiation, visual acuity (VA) had decreased slightly from 20/25 to 20/30. Additional ocular history included bilateral radial keratotomy and mild bilateral nuclear sclerotic cataracts.
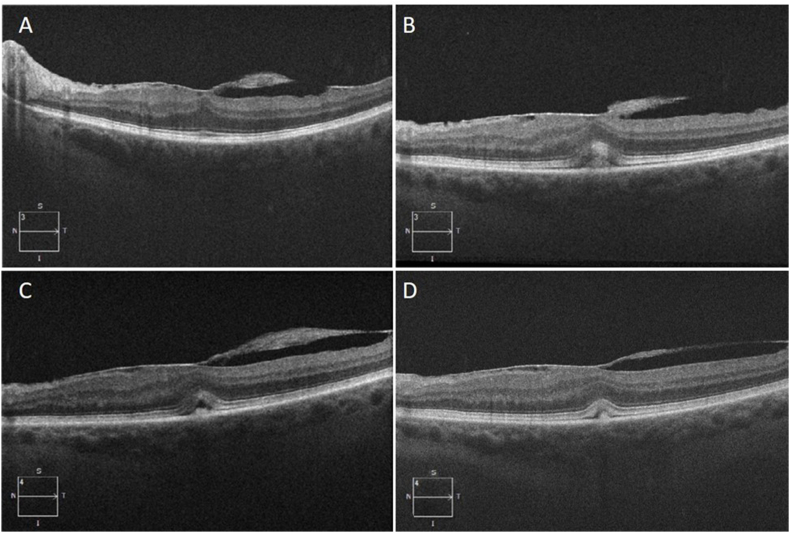
Fig. 1.
Optical coherence tomography imaging of the left eye at baseline and examinations following erdafitinib initiation. (A) Baseline OCT prior to erdafitinib initiation showing epiretinal membrane without significant outer retinal alterations. (B) 47 days after erdafitinib initiation, new subretinal material accumulation is present with EZ-RPE distance changes and EZ disruption. (C) Six month OCT with new SRF accumulation. (D) Twelve month OCT showing some improvement in outer retinal alterations following erdafitinib dose reduction.
Posterior exam was generally stable in both eyes. Optical coherence tomography (OCT) of the left eye demonstrated a stable epiretinal membrane with new mild subretinal fluid (SRF) and minimal associated subretinal debris. In addition, alterations in the outer retina were observed, with increased EZ-RPE height/distance and greater visualization/thickening of the interdigitation zone (IZ; Fig. 1) with possible subretinal material. Right eye visual acuity (20/20) and OCT remained initially unchanged from baseline. Given the unclear etiology (i.e., ERM traction-related vs erdafitinib side effect), minimal visual symptoms, and the potential systemic benefit of the medication, the decision was made to not withhold the drug at the time and follow closely at a monthly interval.
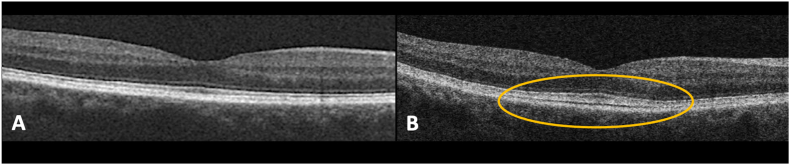
Four months after initiating erdafitinib, the dose was reduced to 5mg due to elevated phosphate levels. At the 6 month visit, the left eye OCT revealed reduction in SRF and EZ integrity improvements (Fig. 1C). Right eye OCT remained stable. Further erdafitinib dose reduction to 4 mg was performed at 11 months following initiation, due to additional systemic side effects. Overall, the left eye findings remained stable to improved with minimal residual SRF (Fig. 1D). However, prominence of the EZ with enhanced visualization/thickening of the interdigitation zone with possible material was noted in the right eye (Fig. 2B). BCVA in the right eye was 20/30 and 20/50–1 in the left. Periodic OCTs in the following months showed persistence of the findings from month 12 without major changes in the outer retinal alterations.
Fig. 2.
Optical coherence tomography imaging of the right eye at baseline and following erdafitinib initiation. (A) Baseline OCT. (B) Twelve month OCT showing expansion of the interdigitation zone with greater prominence of the EZ.
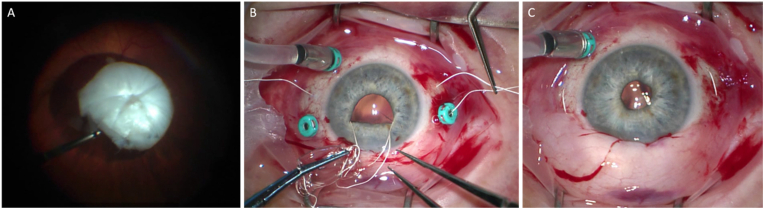
Twenty months after the initiation of erdafitinib therapy, the patient's best-corrected visual acuity (BCVA) rapidly declined from 20/25; 20/50 to hand motion (HM); 20/80–2 within approximately two months. Slit lamp exam revealed rapid progression of a white cataract with 4+ posterior subcapsular cataract in the right eye, and 2+ nuclear sclerosis, 2+ posterior subcapsular cataract in the left. Cataract surgery with phacoemulsification was performed in the right eye, but due to significant intracapsular tension, the posterior capsule ruptured and the nucleus dropped posteriorly. The patient subsequently underwent pars plana vitrectomy, phacofragmentation, and implantation of a scleral fixated Akreos lens using Gore-Tex suture (Fig. 3). Vision improved to 20/40 following surgery with stable posterior segment findings.
Fig. 3.
Pars plana vitrectomy combined with implantation of a scleral fixated Akreos lens. (A) Dropped lens on the retinal surface. (B) Akreos lens with Gore-Tex suture implantation technique. (C) Successful implantation of lens in the posterior chamber.
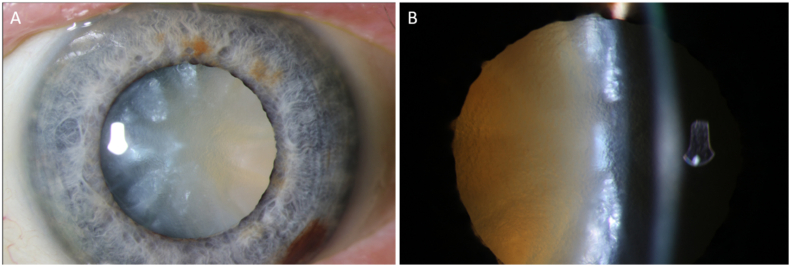
During the postoperative period, the left eye visual acuity rapidly decreased from 20/80 to counting fingers due to rapid cataract progression within 2 months. Slit lamp exam revealed an intumescent cataract with significant anterior chamber shallowing (Fig. 4). At this point erdafitinib was stopped due to cumulative toxicity. Phacoemulsification with depressurization of the intracapsular contents using the phaco probe was performed without complications. The vision recovered to 20/60 with stable posterior segment findings.
Fig. 4.
Left eye intumescent cataract. (A) Anterior chamber photo showing white cataract. (B) Slit-lamp examination showing anterior chamber shallowing, lens swelling, and white cataract.
3. Discussion
In this report, we describe a patient with erdafitinib-related retinopathy and bilateral cataract formation. The presence of the retinopathy starting 47 days after erdafitinib initiation is consistent with previously reported presentation of retinal-related adverse events.3, 4, 5, 6 In the erdafitinib phase II clinical trial, central serous retinopathy and retinal pigment epithelial detachment were reported in 25% of patients with a median time to first onset of 50 days.3 While the patient's history of left sided epiretinal membrane may have been a contributing factor in the OCT changes observed, the lack of obvious traction on OCT, the stable epiretinal membrane over time, and the acute change after starting erdafitinib point to erdafitinib as the likely underlying cause. Additionally, similar erdafitinib-related development of subretinal material with localized SRF was reported by Perensky et al.5 and Ramtohul et al.,6 who described their findings as pseudovitelliform lesions. The underlying cause for this lesion may be related to the FGFR-MAPK pathway. FGFR is an activator of MAPK which plays a prominent role in the maintenance, survival and repair of the RPE.8 While on OCT, the pseudovitelliform lesions do not present the same as central serous retinopathy or classic MEK inhibitor-associated retinopathy, the subretinal material observed may be an indicator of RPE dysfunction.5
Furthermore, in this report the patient developed rapidly progressive bilateral mature/white cataracts 20 months after erdafitinib initiation. While erdafitinib-induced dry eyes are the most common ocular side effect, anterior segment complications appear to be less common. In long-term follow-up of the erdafitinib phase II clinical trial recently published, 2 out of 101 patients with a median treatment exposure of 5.4 months developed cataracts.4 This is in contrast to the 25% of patients who developed central serous retinopathy previously mentioned. While the cataract's severity and grading were not described, Bauters et al.7 presented a case series of three patients, all with severe dry eyes, two of whom developed bilateral white cataract. One patient developed cataracts 11 months after erdafitinib initiation, and one patient who was on erdafitinib for 6 months presented with bilateral cataracts two months following cessation of the drug. Similar to its role in RPE homeostasis, FGFR signaling has been linked to multiple aspects of lens development, including lens cell proliferation and overall survival.9,10
The findings of this case study provide additional support of the importance of ongoing ophthalmic surveillance of patients while on erdafitinib and the wide spectrum of ophthalmic adverse events. The rapid progression of bilateral cataracts was particularly unique. In addition, this report highlights the potential stability of mild retinal findings while maintaining treatment with erdafitinib. The functional/anatomic consequence of the enhanced visualization/thickening interdigitation zone and expansion of the ellipsoid zone remain unknown. While the improvement in outer retinal alterations after erdafitinib dose reductions may suggest a dose-dependent etiology, it is possible that anterior segment complications are more associated with long-term erdafitinib use and have an underlying time-dependent adverse effect. To gain a better understanding of these adverse events, more studies and long-term follow-up are needed, particularly of patients with a history of long-term erdafitinib use.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Patient consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
CRediT authorship contribution statement
Antoine G. Sassine: Data curation, Formal analysis, Investigation, Writing – original draft. Yavuz Cakir: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. Lyndsey Della Vecchia: Data curation, Investigation, Writing – review & editing. Justis P. Ehlers: Conceptualization, Data curation, Software, Validation, Writing – review & editing.
Declaration of competing interest
There are no direct competing interests related to this article. Just the appropriate financial disclosures, listed below:
JPE: Consultant: Zeiss, Leica/Bioptigen, Alcon, Beyeonics. Allergan, Allegro, Adverum, Regeneron, Roche, Genentech, RegenxBIO, Iveric Bio, Boehringer Ingelheim, Apellis, Novartis, Boehringer Ingelheim, Stealth Biotherapeutics, Perceive Biotherapeutics, Exegenesis, Ophthalytics, Eyepoint, AbbVie, Bayer, BVI, Alexion; Grant support: Regeneron, Genentech, Oxurion/Thrombogenics, Alcon, Aerpio, Allergan, Roche, Iveric Bio, Boehringer Ingelheim, Adverum, Novartis, Zeiss, Stealth Biotherapeutics, Perceive Biotherapeutics, Alexion, Beyeonics; Patents/Intellectual Property/Licensing: Bioptigen/Leica.
The following authors have no financial disclosures: AGS, YC, LD.
References
- 1.Mahipal A., Tella S.H., Kommalapati A., Yu J., Kim R. Prevention and treatment of FGFR inhibitor-associated toxicities. Crit Rev Oncol Hematol. 2020;155 doi: 10.1016/j.critrevonc.2020.103091. [DOI] [PubMed] [Google Scholar]
- 2.FDA grants accelerated approval to erdafitinib for metastatic urothelial carcinoma. U.S. Food and Drug Administration. Published April 12, 2019. Accessed March 25, 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-erdafitinib-metastatic-urothelial-carcinoma.
- 3.Loriot Y., Necchi A., Park S.H., et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med. 2019;381(4):338–348. doi: 10.1056/nejmoa1817323. [DOI] [PubMed] [Google Scholar]
- 4.Siefker-Radtke A.O., Necchi A., Park S.H., et al. Efficacy and safety of erdafitinib in patients with locally advanced or metastatic urothelial carcinoma: long-term follow-up of a phase 2 study. Lancet Oncol. 2022;23(2):248–258. doi: 10.1016/S1470-2045(21)00660-4. [DOI] [PubMed] [Google Scholar]
- 5.Prensky C., Marlow E., Gupta M., Sales C., Kiss S., D'Amico D.J. Reversible macular lesions in the setting of oral pan-fibroblast growth factor inhibitor for the treatment of bladder cancer. Journal of VitreoRetinal Diseases. 2018;2(2):111–114. doi: 10.1177/2474126417751724. [DOI] [Google Scholar]
- 6.Ramtohul P., Denis D., Comet A. Pseudovitelliform maculopathy associated with FGFR inhibitor therapy. Ophthalmology Retina. 2021;5(2) doi: 10.1016/j.oret.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Bauters G., Paques M., Borderie V., Bouheraoua N. Reversible corneal stromal thinning, acute-onset white cataract and angle-closure glaucoma due to erdafitinib, a fibroblast growth factor receptor inhibitor: report of three cases. J Fr Ophtalmol. 2021;44(1):67–75. doi: 10.1016/j.jfo.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 8.van der Noll R., Leijen S., Neuteboom G.H.G., Beijnen J.H., Schellens J.H.M. Effect of inhibition of the FGFR-MAPK signaling pathway on the development of ocular toxicities. Cancer Treat Rev. 2013;39(6):664–672. doi: 10.1016/j.ctrv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Robinson M.L. An essential role for FGF receptor signaling in lens development. Semin Cell Dev Biol. 2006;17(6):726–740. doi: 10.1016/j.semcdb.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovicu F.J., McAvoy J.W., de Iongh R.U. Understanding the role of growth factors in embryonic development: insights from the lens. Philos Trans R Soc Lond B Biol Sci. 2011;366(1568):1204–1218. doi: 10.1098/rstb.2010.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]