Abstract
Graves' ophthalmopathy (GO) is an extrathyroidal manifestation of Graves' disease, Orbital fibroblasts (OFs) are recognized as key players in GO pathogenesis, involved in orbital inflammation, tissue remodeling, and fibrosis. This study offers a primary exploration of cell behavior and characteristics on OFs from GO (GO-OFs), and compared to OFs from healthy control (HC-OFs). Results reveal that GO-OFs exhibit delayed migration from tissue fragments, while no significant difference in cell proliferation is observed between GO-OFs and HC-OFs. Aberrant expression pattern of surface proteins Thy-1, TSHR, and IGF-1R suggests shared autoantigens and pathways between GO and GD, contributing to inflammation and fibrosis. Investigations into cytokine responses unveil elevated secretion of hyaluronic acid (HA) and prostaglandin E2 (PGE2) in GO-OFs, emphasizing their role in tissue remodeling. These findings deepen our understanding of OFs in GO pathogenesis, offering potential therapeutic avenues.
Keywords: Graves' ophthalmopathy, Orbital fibroblasts, TSHR, IGF-1R, Hyaluronic acid, Prostaglandin E2
1. Introduction
Graves' ophthalmopathy (GO) is extrathyroidal manifestation of Graves’ disease (GD), which is characterized by a complex interplay of orbital inflammation, tissue expansion, remodeling, and fibrosis [1,2]. As the most common autoimmune orbital disorder, GO exhibits an active phase marked by inflammation and tissue remodeling, followed by an inactive phase characterized by stabilization and remission. The pathophysiology of GO involves the infiltration of orbital tissues by various immune cells, including CD4+ and CD8+ T cells, mast cells, and B cells, leading to dysregulated immune responses and disordered accumulation of hyaluronan and glycosaminoglycan [3,4]. However, the mechanisms underlying GO remain incompletely known, factors underpinning the process of GO are still less well understood [5].
Central to the intricate cascade of events in GO is the role of orbital fibroblasts (OFs), which have recently gained prominence due to their pivotal functions in inflammation, tissue remodeling, and pathogenesis [3]. These resident OFs within the orbit are now recognized as key orchestrators of the disease process [6,7]. Through their surface receptors, activated OFs generate a cascade of inflammatory cytokines and chemokines that not only recruit and activate immune cells but also interact with them [[8], [9], [10], [11], [12]]. Furthermore, activated OFs contribute to orbital tissue expansion by intensifying the secretion of HA, promoting proliferation, and fostering adipogenesis [8,9,13]. Although OFs have been acknowledged as critical players in GO, their exact mechanisms and potential as therapeutic targets remain incompletely understood.
Understanding OFs' role in the pathogenesis of GO could potentially unveil novel therapeutic avenues for this debilitating disorder. To delve deeper into the intricate dynamics of GO, we conducted the present study to comparing OFs from GO (GO-OFs) and from healthy control (HC-OFs) from several aspects. By unraveling the unique attributes and behaviors of GO-OFs, we aim to shed light on the cellular mechanisms underpinning the pathophysiology of GO and pave the way for targeted therapeutic interventions.
2. Materials and methods
2.1. Patients and primary culture of orbital fibroblasts (OFs)
Orbital tissues were collected from six patients undergoing orbital decompression surgery due to severe GO (based on the European Group on Graves' Orbitopathy,EUGOGO). For comparison, age-and sex-matched HC orbital tissues (n = 6) were obtained from patient undergoing eyeball enucleation due to traumatic eyeball rupture, with no history of inflammatory diseases such as inflammatory pseudotumor, Sjogren's syndrome (SS), or rheumatoid arthritis (RA). All GO patients maintained stable euthyroidism for at least 6 months before surgery, with no history of receiving radiotherapy. Daily doses of methimazole (Tapazole)to maintain euthyroidism was 14.2 ± 7.4 mg per day. All GO patients were in active stage of GO, as determined by the clinical activity score (CAS), which was assessed based on the clinically validated scoring system proposed by Mourits et al. [14,15]. Preceding surgery, the GO patients had received systemic glucocorticoids treatment (Methylprednisone pulse therapy) at least three months earlier. In contrast, the control group subjects had received minimal glucocorticoids (Methylprednisone, 40 mg/day for 3 days) due to ocular trauma. Demographic information and clinical characteristics of the patients are presented in Table 1, Table 2.
Table 1.
Demographic information and clinical characteristics of patients.
| Group | Control | GO |
|---|---|---|
| Group size | 6 | 6 |
| Age (years) | 49.8 ± 8.2 | 52.3 ± 5.8 |
| Gender (Male) | 4(67.7%) | 3(50%) |
| Smoke history | 1(16.7%) | 2(33.3%) |
| CAS | NA | 4.8 ± 0.8 |
| Duration of GO (Months) | NA | 12.2 ± 6.5 |
| Glucocorticoid Therapy | 6(Methylprednisolone) | 6(Methylprednisolone) |
| Radiation Therapy | 0 | 0 |
NA = not applicable; CAS = clinical activity score.
Table 2.
Clinical characteristics of patients.
| #Patient | CAS | Glucocorticoid Therapy |
|---|---|---|
| #1 (Control) | NA | 120 mg |
| #2 (Control) | NA | 120 mg |
| #3(Control) | NA | 120 mg |
| #4(Control) | NA | 120 mg |
| #5 (Control) | NA | 120 mg |
| #6 (Control) | NA | 120 mg |
| #1 (GO) | 4 | 4.5 g |
| #2 (GO) | 5 | 3.0 g |
| #3 (GO) | 4 | 4.5 g |
| #4 (GO) | 5 | 4.5 g |
| #5 (GO) | 5 | 3.0 g |
| #6 (GO) | 6 | 4.5 g |
NA = not applicable; CAS = clinical activity score.
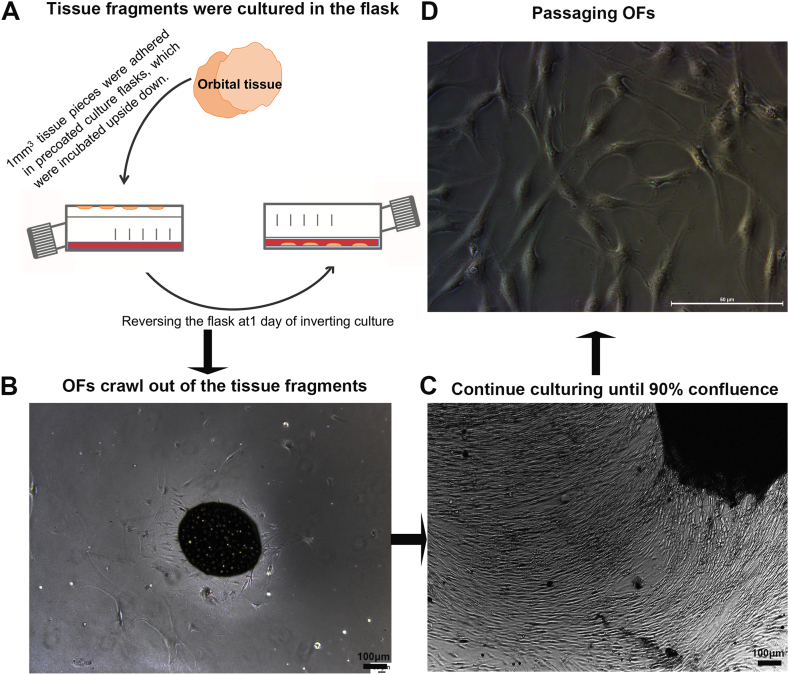
Immediately after removal, the orbital tissue samples were stored in Dulbecco's modified Eagle's medium (DMEM, Gibco, CA, USA) supplemented with 1% fetal bovine serum (FBS, Gibco, CA, USA), penicillin (100 U/mL), and gentamicin (20 μg/mL) (Gibco, CA, USA). Informed consent was obtained from all subjects, and the study was conducted in accordance with the principles of the Declaration of Helsinki. Ethical approval was granted by the ethical committee of the People's Hospital of Guangxi Region. OFs were cultured as previously described.[33] The primary human OFs were isolated and cultured following established explant techniques with minor modifications. The orbital tissues were aseptically collected and cut into 1 mm pieces. The tissue fragments were placed in culture flasks which had been precoated with FBS, which were initially incubated upside down with 5 mL complete DMEM with 10% FBS to prevent drying. After 12 h, the flasks were gently reversed, and the tissue fragments were immersed in complete DMEM medium (Fig. 1A). The OFs migrated out of the tissue fragments and attached to the plate (Fig. 1B). Upon reaching 90% confluence, the OFs were harvested and passaged (Fig. 1C and D).
Fig. 1.
The process of primary culture and passaging of Orbital Fibroblasts. (A) Schematic representation of primary culture using fragments of orbital tissue. (B) Representative image showed that some OFs start migrating from around the tissue fragments. (C) Representative image showed the proliferation of OFs, reaching 90–100% confluence. (D) Passaged OFs were cultured in another flask.
2.2. Cell proliferation assay and transwell migration assay
Passages 3–5 of OFs were used for the experiment. After 8 h of serum starvation in DMEM with 2% FBS, HC-OFs and GO-OFs were seeded in triplicate at a density of 5 × 10^3 cells per well in a 96-well plate and cultured in complete DMEM (10% FBS) for 48 h. Cell proliferation was assessed using the BrdU cell proliferation assay (Cell Signaling Technology, #6813) according to the manufacturer's instructions. Briefly, 10 μM BrdU was added to the plate, and the cells were incubated for 4 h. After fixation and incubation with BrdU antibody followed by horseradish peroxidase (HRP)-linked antibody, the absorbance at 450 nm was measured using the Synergy HTX Multi-Mode Microplate Reader (BioTek). The absorbance value (BrdU incorporation) served as an indicator of cell proliferation.
For the migration assay, OFs were suspended in DMEM with 2 % FBS, were seeded (5 × 10^3 cells) in the upper of chambers of transwell inserts in 24-well plates (8 μm pore, Falcon, BD Biosciences), the lower chambers contained 1 mL DMEM with 10% FBS. After incubation for 24 h, transwell inserts were removed, and fixed in 4% paraformaldehyde. Cells from the upper surface of the filters were removed by wiping with cotton swabs, followed by washing with PBS. Filter membranes were stained with DAPI and analyzed by fluorescence microscopy. The migrated cells on the lower surface were counted in 6 randomly-selected viewing fields per insert.
2.3. Immunofluorescence detection of fibronectin and α-SMA expression in OFs
OFs were seeded onto coverslips at a density of 1 × 10^4 cells/200 μL in complete medium. After 2 days in culture, the slides were fixed with 4% paraformaldehyde (PFA) and permeabilized with 0.2% Triton X-100. Following blocking, the cells were incubated with fibronectin antibody (1 μg/mL) and α-SMA antibody (5 μg/mL). Subsequently, the slides were stained with secondary antibodies (Alexa Fluor 488 at 1:300, Cell Signaling Technology, USA). Randomly selected images were captured using a Leica microscope with an FITC filter.
2.4. Real-time quantitative RT-PCR for fibronectin and α-SMA expression
Total RNA was extracted from cultured OFs using TRIzol (Thermo Fisher, #15596026, USA) according to the manufacturer's protocol. cDNA was synthesized using the PrimeScript™ RT reagent Kit with gDNA Eraser (TAKARA, RR047A), followed by real-time PCR using SYBR Premix Ex Taq (TAKARA RR420A) on the Step One Plus Real-Time PCR System (Life Technologies). The PCR conditions involved an initial step at 95 °C for 30 s, followed by 40 cycles of denaturation at 95 °C for 5 s and annealing/extension at 60 °C for 30 s. The relative mRNA expression was normalized to GAPDH, and data were presented as relative expression units. PCR reactions were performed in triplicate, and the results were analyzed using the 2−ΔΔCt method with Ct < 35. Primer sequences for FN, α-SMA, and the housekeeping gene GAPDH were as follows: FN forward (5′- ACAACACCGAGGTGACTGAGAC -3′) and reverse (5′- GGACACAACGATGCTTCCTGAG -3′); α-SMA forward (5′- CTATGCCTCTGGACGCACAACT -3′) and reverse (5′- CAGATCCAGACGCATGATGGCA -3′); GAPDH forward (5′-GGTGAAGGTCGGAGTCAACGGA-3′) and reverse (5′-GAGGGATCTCGCTCCTGGAAGA-3′).
2.5. Quantification of surface protein expression using flow cytometry
OFs were trypsinized and suspended. For Thy-1 staining, 1 × 10^6 cells were incubated with the fluorescein-conjugated antibody (anti-human Thy-1-PE, #328109, Biolegend) in the dark for 1 h at room temperature. TSHR and IGF-1R were stained using primary and secondary antibodies. The cell suspension was incubated with anti-human TSHR (RD System, MAB65342) and anti-human IGF-1R (RD System, MAB391) for 1 h. Cells incubated with IgG were defined as isotype control. Then, cells were washed and incubated with fluorescein-conjugated antibodies in the dark for 20 min. The cells were immediately analyzed using standard flow cytometric techniques on a FACSC flow cytometer (BD Biosciences). The flow cytometry data were analyzed using FlowJo software.
2.6. Measurement of HA and PGE2 using ELISA
OFs from passages 3–6 were seeded into 6-well plates at a density of 1 × 10^6 cells per well in complete medium and allowed to reach 90% confluence. Various cytokines were added into the cell medium individually and incubated for 24 h. The final concentrations were as follow: IL-10 (100 ng/mL, R&D, Minneapolis, USA), TNF-α (10 ng/mL, R&D, Minneapolis, USA), IFN-γ (10 ng/mL, R&D, Minneapolis, USA), IL-1β (10 ng/mL R&D, Minneapolis, USA). The cell supernatants were collected and stored at −80 °C. The concentration of hyaluronic acid (HA) and PGE2 in the supernatants was measured using a human Hyaluronan ELISA kit (R&D, #DHYAL0, USA) and human PGE2 ELISA kit (R&D, #KGE004B, USA) according to the manufacturer's instructions. Samples were diluted 1:10 before analysis, and the mean value of triplicate samples was reported. The optical density (OD) was measured at 450 nm wavelength using a microplate reader (Molecular Devices, Sunnyvale, CA, USA). The HA and PGE2 concentration in each sample was determined by reference to a standard curve generated with known amounts of HA and PGE2.
2.7. Statistical analysis
All statistical analyses were conducted using SPSS Statistics version 20, and p-values less than 0.05 were considered statistically significant. The results were presented as means ± standard error of the mean (SEM). Differences among the groups were analyzed by a Mann-Whitney U test or t-test when comparing two groups. Mann-Whitney U test was used for analyze the data which did not follow normal distribution. The difference was considered significant if p value was less than 0.05.
3. Results
3.1. Primary culture of orbital fibroblasts and cell proliferation/migration
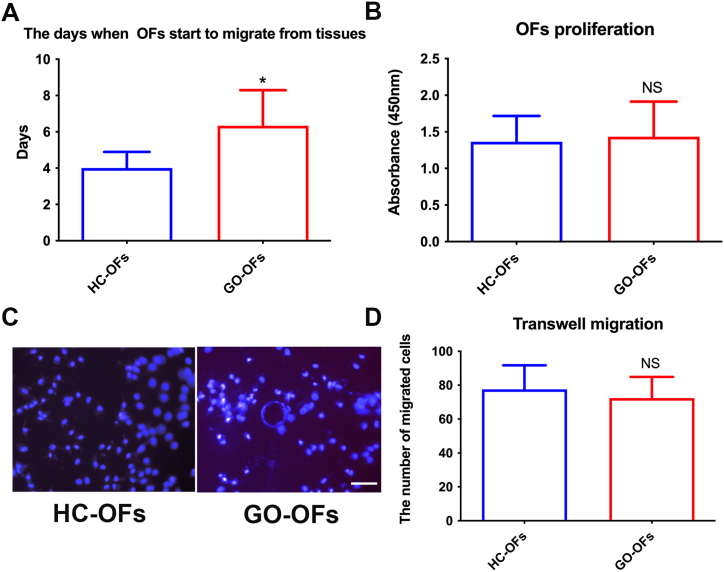
The spindle-shaped OFs began to emerge from tissue fragments within 3–9 days. The average duration for OFs to exit tissues was (4.00 ± 0.89) days in HC and (6.33 ± 1.97) days in GO patients. This difference was statistically significant (Fig. 2A), indicating that GO-OFs exhibited a slower migration from tissues compared to HC-OFs. Additionally, we compared the cell proliferation and migration between isolated HC-OFs and GO-OFs. However, as shown in Fig. 2B–D, there was no significant difference between the two groups.
Fig. 2.
Comparison of Primary Culture and Cell Proliferation between GO-OFs and HC-OFs. (A) The time for spindle-shaped OFs to exit tissues was assessed. GO-OFs exhibited a delayed migration compared to HC-OFs. (B) Cell proliferation rates of isolated HC-OFs and GO-OFs were compared. (C) Representative images of migrated cells on the lower surface of filter membranes, stained with DAPI, Bar = 50 μm. (D) The number of migrated cells were compared between HC-OFs and GO-OFs. n = 6 (HC-OFs), n = 6(GO-OFs). Data was present as means ± standard error of the mean (SEM). t-test, *p < 0.05 was considered as significant.
3.2. Differential expression of cell surface markers
3.2.1. Expression of fibroblast surface markers on HC-OFs and GO-OFs
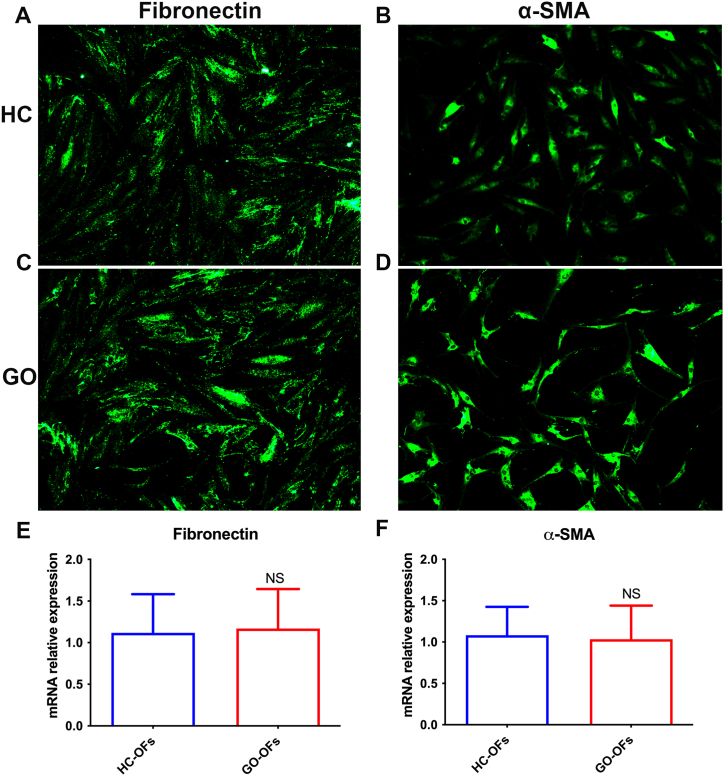
We initially examined the surface markers on freshly isolated orbital fibroblasts. Fibronectin (FN), a glycoprotein that binds to extracellular matrix (ECM) proteins and plays a crucial role in OFs migration and growth, was widely expressed in both HC-OFs (Fig. 3A) and GO-OFs (Fig. 3C). Quantitative PCR (qPCR) analysis also showed no difference in FN mRNA levels between the two groups (Fig. 3E). Additionally, we investigated the expression of α-smooth muscle actin (α-SMA), a definitive marker for mature fibroblasts' contractility. Similar to FN, there was no significant difference in α-SMA expression between HC-OFs and GO-OFs (Fig. 3B–D, and F).
Fig. 3.
Comparison of FN and α-SMA Expression on freshly isolated HC-OFs and GO-OFs. (A) FN was positive expressed in HC-OFs. (B) α-SMA was positive expressed in HC-OFs. (C) FN was positive expressed in GO-OFs. (D) α-SMA was positive expressed in GO-OFs. (E) qPCR showed no difference in FN mRNA levels between HC-OFs and GO-OFs. (F) qPCR showed no difference in α-SMA mRNA levels between HC-OFs and GO-OFs. n = 6 (HC-OFs), n = 6(GO-OFs). Data was present as means ± standard error of the mean (SEM). Mann-Whitney U test, NS p > 0.05 was considered as non-significant.
3.2.2. Abnormal expression of Thy-1, TSHR, and IGF-1R in GO-OFs
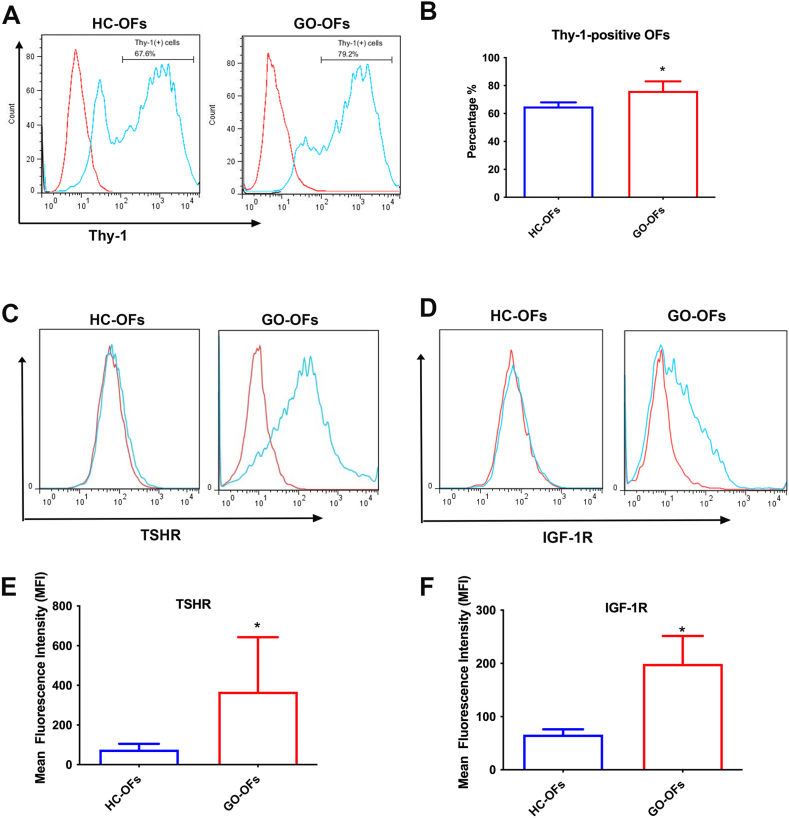
Orbital fibroblasts exhibit phenotypic heterogeneity based on surface receptor expression. Thy-1 (also known as CD90) is a surface protein that defines functionally distinct subpopulations of fibroblasts. Thy-1-positive fibroblasts are capable of myofibroblast differentiation, while Thy-1-negative fibroblasts can undergo adipogenesis. Flow cytometry analysis showed that GO-OFs had a significantly higher percentage of Thy-1-positive cells (average ± SD 76.17 ± 6.84%; range 65.5–85.6%) compared to HC-OFs (average ± SD 65.02 ± 2.95%; range 61.0–69.2%; p < 0.001, t-test, Fig. 4A and B). This suggests that GO-OFs have a higher potential to differentiate into mature myofibroblasts.
Fig. 4.
(A) Representative image showed the expression of Thy-1 on the surface of OFs. (B) The percentage of Thy-1-positive cells in GO-OFs was significantly higher than that in HC-OFs. (C) Representative image showed the display of TSHR expression on the surface of OFs. The red histograms represent staining with isotype control Abs, and the green histogram represent fluorescein staining with anti-TSHR Abs.(D) Representative image showed the display of IGF-1R expression on the surface of OFs. The red histograms represent staining with isotype control Abs, and the green histogram represent fluorescein staining with anti-IGF-1R Abs. (E) The expression level of TSHR on the cell surface of GO-OFs were significantly higher than that in HC-OFs. (F) The expression level of IGF-1R on the cell surface of GO-OFs were significantly higher than that in HC-OFs. n = 6 (HC-OFs), n = 6(GO-OFs). t-test, *p < 0.05 was considered as significant. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Furthermore, since Grave's disease (GD) and GO share a pathogenic autoantigen, we investigated the expression of two main pathogenic targets, thyroid-stimulating hormone receptor (TSHR), and insulin-like growth factor-1 receptor (IGF-1R). Flow cytometry analysis revealed that GO-OFs displayed an increased percentage of TSHR-positive (Fig. 4C–E) and IGF-1R-positive cells (Fig. 4D–F), while all HC-OFs showed negative expression for these receptors. In summary, GO-OFs displayed an abnormal pattern of Thy-1, TSHR, and IGF-1R expression compared to HC-OFs.
3.3. Secretion of HA and prostaglandin E2 in response to inflammatory cytokines
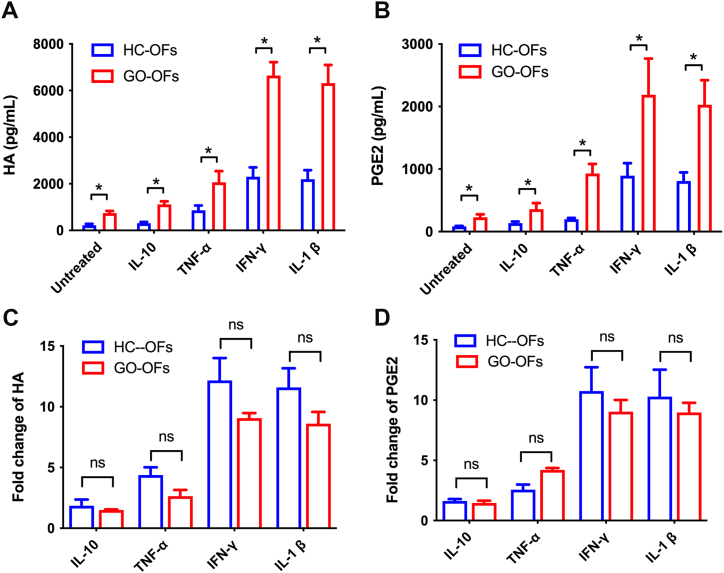
Disordered accumulation of hyaluronic acid (HA) and prostaglandin E2 (PGE2) produced by OFs contributes to the orbital tissue remodeling in GO. We investigated the HA and PGE2 expression induced by inflammatory cytokines between HC-OFs and GO-OFs.
At baseline, GO-OFs expressed significantly higher levels of HA and PGE2 compared to HC-OFs (Fig. 5A and B). In response to various cytokines, both HC-OFs and GO-OFs showed increased HA production. Interleukin-10 (IL-10) induced a slight stimulation of HA in both groups, but the increase was not significant (p > 0.05, paired t-test). Tumor necrosis factor-alpha (TNF-α) induced a significant increase in HA production in both HC-OFs and GO-OFs (p < 0.05, paired t-test). Interferon-gamma (IFN-γ) and interleukin-1beta (IL-1β) also significantly increased HA production in both groups (p < 0.05, paired t-test). The fold change of HA secretion in response to cytokines seemed slightly higher in HC-OFs, but the difference was not statistically significant (Fig. 5C, P > 0.05, Mann-Whitney U test).
Fig. 5.
Expression of soluble HA and PGE2 in the supernatants from cultured HC-OFs and GO-OFs following stimulation. (A) Comparison of the soluble HA concentration between the HC-OFs and GO-OFs following stimulation via cytokines. (B) Comparison of soluble PGE2 between the HC-OFs and GO-OFs after stimulation via cytokines. (C) Fold change of induced HA after stimulation (compared to untreated) was calculated and compared between HC-OFs and GO-OFs. (D) Fold change of induced PEG2 after stimulation (compared to untreated) was calculated and compared between HC-OFs and GO-OFs. n = 6 (HC-OFs), n = 6(GO-OFs). t-test was used for comparison of the concentration of HA and PGE2, Mann-Whitney U test was used for comparison of the fold change of induced HA and PGE2, *p < 0.05 was considered as significant.
Similarly, the trend for PGE2 secretion in response to cytokines was similar to HA. Both HC-OFs and GO-OFs showed increased PGE2 production when treated with IL-10, TNF-α, IFN-γ, and IL-1β (Fig. 5B and D, p < 0.05, paired t-test). The fold change of PGE2 secretion in response to cytokines between GO-OFs and HC-OFs was not statistically significant (P > 0.05, Mann-Whitney U test). Overall, these findings suggest that both GO-OFs and HC-OFs could be induced to overexpress HA and PGE2 when exposed to proinflammatory cytokines, particularly to IFN-γ and IL-1β. And the similar fold change of increased HA and PGE2 in GO-OFs and HC-OFs showed that they respond equally.
4. Discussion
The findings of this study provide a primary exploration of orbital fibroblast behavior and characteristics in GO. In the realm of cell behavior and proliferation, our results reveal that GO-OFs exhibit a delayed migration from tissue fragments. And no significant difference was observed in cell proliferation between GO-OFs and HC-OFs. The abnormal expression pattern of surface proteins Thy-1, TSHR, and IGF-1R were observed in GO-OFs, implies these shared autoantigens and pathways between GO and GD contributing to fibrosis and inflammation in GO. Additionally, our investigations into cytokine responses demonstrate elevated secretion of HA and PGE2 in GO-OFs under cytokine stimulation, reflecting their pivotal role in tissue remodeling. These findings collectively contribute to our understanding of the OFs in GO pathogenesis.
Intriguingly, our study revealed a discrepancy in the proliferative activity of GO-OFs compared to previous research, challenging established findings. A study by Armin E has previously indicated that OFs exhibit heightened proliferative activity in GO [12,16]. While studies by Roberta Botta [17] and S. Lisi [18] did not directly compare the proliferative capacity of OFs from GO and normal controls, the basal proliferative capacity of GO-OFs appeared to be slightly higher than that of HC-OFs based on the figures they presented, however, the authors did not compare the significance of the difference. Contrary to a prior investigation, our current observations found comparable rates of cell proliferation and migration between GO-OFs and HC-OFs. This divergence might stem from variations in the severity or disease states of the GO subjects in these studies. Specifically, our study enrolled individuals with severe active GO in the active phase, differing from subjects in the earlier study, which lacked clinical characterization details in studies by Armin E and Roberta Botta. Subjects were in inactive stage of GO in study by S. Lisi. Additionally, the history of glucocorticoid treatment might potentially influence OFs' proliferation and migration, but the impact remains unclear. Although glucocorticoids were also administered to patients in the HC group, the dosage was substantially lower than that in the GO group, who were administered Methylprednisone pulse therapy at least three months before orbital compression surgery. However, whether this prior steroid treatment continues to influence the proliferation of OFs in orbital tissues after three months remains an intriguing question warranting further investigation.
Specifically, we have discovered that patients with GO-OFs demonstrate a postponed migration from tissue fragments compared to HC-OFs. This delay in migration may potentially be attributed to elevated fibrosis and inflammatory edema within orbital tissues of severe active GO, which could impede the movement of OFs. Additionally, it's noteworthy that the administration of glucocorticoids might contribute to this finding.
Considering fibroblast marker expression, our study results compared the expression of FN and α-SMA in the two OFs. FN, a glycoprotein present in the extracellular matrix, is essential for cell adhesion, migration, and tissue repair. On the other hand, α-SMA, a definitive marker of mature fibroblasts, is implicated in fibroblast contractility and tissue contraction. In our findings, these markers displayed consistent expression across both GO-OFs and HC-OFs, emphasizing that they share fundamental fibroblast behavior. It is worth noting that the existing literature on this subject presents varying findings. For instance, a study conducted by Xin Qi et al. [19] reported elevated levels of FN and α-SMA in GO-OFs compared to healthy controls, which was inconsistent with our findings. Importantly, this discrepancy may be attributed to variations in the stage and grade of GO among the subjects. Conversely, another study [20] supported our observation, indicating no significant difference in FN and α-SMA expression in orbital fibroblasts between control and severe GO subjects, which was align with our study.
The observations presented in our study regarding the abnormal expression patterns of Thy-1, TSHR, and IGF-1R in GO-OFs offer a significant contribution to the expanding body of knowledge on GO. The heightened Thy-1 expression in GO-OFs in comparison to HC-OFs suggests that the inclination of GO-OFs to differentiate into mature myofibroblasts [21,22]. This finding correlates well with a previous study, which proposed enhanced Thy-1 expression in fibroblast populations from GO [21]. Furthermore, previous studies have reported that inflammatory cytokines, such as IL-1β and TNF-α, can upregulate Thy-1 expression in cultured microvascular endothelial cells [23].However, we did not observe the significant upregulate Thy-1 expression in both GO-OFs and HC-OFs induced by cytokines.
The activation of TSHR and IGF-1R by their respective autoantibodies (TSH and IGF-1) in OFs of GO initiates a cascade of events, including OF proliferation, inflammatory cytokine production, and HA secretion. These processes contribute significantly to the development of orbital tissue edema and inflammation [24,25]. Our identification of elevated TSHR and IGF-1R expression in GO-OFs aligns with prior research, emphasizing the central roles of these receptors in the pathogenesis of GO [24,26]. Notably, a study [27] reported a subpopulation of cells exhibiting the CD34+Col1+ phenotype within the orbit of GO during flow cytometric analysis, which suggests that the TSHR + subpopulation of GO-OFs might represent CD34+ fibrocytes originating from peripheral blood mononuclear cells (PBMCs). Cumulatively,CD34+ fibrocytes with high expression of TSHR among the GO-OFs might be the core participants in the pathogenesis of GO [28].
HA and PGE2 are key molecules involved in the tissue remodeling process within GO [29,30]. Previous studies have shown that IL-1β can trigger the synthesis of both HA and PGE2 in orbital fibroblasts from GO. In our study, we comprehensively compared the induction capabilities of multiple cytokines on the secretion of HA and PGE2 in both GO-OFs and HC-OFs. Notably, we found that IFN-γ and IL-1β exhibited equally induction capabilities, and were much higher than TNF-α and IL10. Additionally, we assessed the sensitivity of GO-OFs and HC-OFs to inflammatory stimuli by calculating the fold change of increase in HA and PGE2 after stimulation. Contrary to our initial hypothesis, the fold change in GO-OFs was slightly lower than that in HC-OFs, while statistical analysis did not reveal a significant difference. This result may be attributed to the substantially higher baseline levels of GO-OFs compared to HC-OFs. In summary, these results indicate that GO-OFs and HC-OFs might exhibit similar sensitivity to inflammatory stimuli, highlighting comparable responsiveness to inflammation despite variations in baseline levels.
4.1. Limitations
While this study deepens our understanding of GO, its limitations must be acknowledged. The sample size, though carefully selected, remains modest. Larger sample sizes would offer more robust statistical power and a comprehensive representation of the population. Furthermore, the behavior of isolated OFs from orbital tissue may not fully encapsulate the intricacies of true cellular behavior, and the utilization of exposed cytokines might fall short of replicating the complete inflammatory microenvironment characteristic of GO.
5. Conclusion
To conclude, our findings contribute significantly to the understanding of GO's pathophysiology by highlighting the significance of fibroblast behavior, surface protein expression, and cytokine responses. These findings hold promise for the development of precise therapeutic interventions targeting on orbital fibroblasts within the context of GO.
Ethics declarations
This study was reviewed and approved by ethical committee of the People's Hospital of Guangxi Region, with the approval number: [KY-GZR-2023-106]. All participants provided informed consent to participate in the study.
Data availability statement
Data will be made available on request.
Funding
The work was supported by the National Natural Science Foundation of China (No.82000925 and 82101232),Natural Science Foundation of Guangxi Province (2021GXNSFBA075051), and Guangxi clinical ophthalmic research center (No. Guike AD19245193).
CRediT authorship contribution statement
Yu Wu: Visualization, Validation, Investigation. Jiuming Zhang: Visualization, Validation, Investigation. Wen Deng: Writing – review & editing, Visualization, Methodology. Chaoting Mo: Writing – review & editing. Yumei Liang: Writing – review & editing. Kongqian Huang: Writing – review & editing. Fan Xu: Funding acquisition, Conceptualization. Fen Tang: Writing – original draft, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Fan Xu, Email: oph_fan@163.com.
Fen Tang, Email: tangfen8002@163.com.
References
- 1.Smith T.J., Bahn R.S., Gorman C.A. Connective tissue, glycosaminoglycans, and diseases of the thyroid. Endocr. Rev. 1989;10:366–391. doi: 10.1210/edrv-10-3-366. 1989/08/01. [DOI] [PubMed] [Google Scholar]
- 2.Hufnagel T.J., Hickey W.F., Cobbs W.H., et al. Immunohistochemical and ultrastructural studies on the exenterated orbital tissues of a patient with Graves' disease. Ophthalmology. 1984;91:1411–1419. doi: 10.1016/s0161-6420(84)34152-5. 1984/11/01. [DOI] [PubMed] [Google Scholar]
- 3.Smith T.J., Hegedus L. Graves' disease. N. Engl. J. Med. 2016;375:1552–1565. doi: 10.1056/NEJMra1510030. 2016/11/01. [DOI] [PubMed] [Google Scholar]
- 4.Smith T.J., Janssen J.A. Building the Case for insulin-like growth factor receptor-I involvement in thyroid-associated ophthalmopathy. Front. Endocrinol. 2016;7:167. doi: 10.3389/fendo.2016.00167. 2017/01/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viard J.P., Gilquin J. Graves' disease. N. Engl. J. Med. 2017;376:184–185. doi: 10.1056/NEJMc1614624. 2017/01/13. [DOI] [PubMed] [Google Scholar]
- 6.Kuriyan A.E., Woeller C.F., O'Loughlin C.W., et al. Orbital fibroblasts from thyroid eye disease patients differ in proliferative and adipogenic responses depending on disease subtype. Invest. Ophthalmol. Vis. Sci. 2013;54:7370–7377. doi: 10.1167/iovs.13-12741. 2013/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahn R.S. Clinical review 157: pathophysiology of Graves' ophthalmopathy: the cycle of disease. J. Clin. Endocrinol. Metab. 2003;88:1939–1946. doi: 10.1210/jc.2002-030010. 2003/05/03. [DOI] [PubMed] [Google Scholar]
- 8.van Steensel L., Dik W.A. The orbital fibroblast: a key player and target for therapy in graves' ophthalmopathy. Orbit. 2010;29:202–206. doi: 10.3109/01676831003668443. 2010/09/04. [DOI] [PubMed] [Google Scholar]
- 9.Smith T.J., Tsai C.C., Shih M.J., et al. Unique attributes of orbital fibroblasts and global alterations in IGF-1 receptor signaling could explain thyroid-associated ophthalmopathy. Thyroid. 2008;18:983–988. doi: 10.1089/thy.2007.0404. 2008/09/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang F., Chen X., Mao Y., et al. Orbital fibroblasts of Graves' orbitopathy stimulated with proinflammatory cytokines promote B cell survival by secreting BAFF. Mol. Cell. Endocrinol. 2017;446:1–11. doi: 10.1016/j.mce.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y., Fang S., Li D., et al. The involvement of T cell pathogenesis in thyroid-associated ophthalmopathy. Eye (Lond) 2019;33:176–182. doi: 10.1038/s41433-018-0279-9. 2018/12/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dik W.A., Virakul S., van Steensel L. Current perspectives on the role of orbital fibroblasts in the pathogenesis of Graves' ophthalmopathy. Exp. Eye Res. 2016;142:83–91. doi: 10.1016/j.exer.2015.02.007. 2015/12/18. [DOI] [PubMed] [Google Scholar]
- 13.Bahn R.S. Graves' ophthalmopathy. N. Engl. J. Med. 2010;362:726–738. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartalena L., Baldeschi L., Dickinson A., et al. Consensus statement of the European Group on Graves' orbitopathy (EUGOGO) on management of GO. Eur. J. Endocrinol. 2008;158:273–285. doi: 10.1530/EJE-07-0666. [DOI] [PubMed] [Google Scholar]
- 15.Mourits M.P., Prummel M.F., Wiersinga W.M., et al. Clinical activity score as a guide in the management of patients with Graves' ophthalmopathy. Clin. Endocrinol. 1997;47:9–14. doi: 10.1046/j.1365-2265.1997.2331047.x. [DOI] [PubMed] [Google Scholar]
- 16.Heufelder A.E., Bahn R.S. Modulation of Graves' orbital fibroblast proliferation by cytokines and glucocorticoid receptor agonists. Invest. Ophthalmol. Vis. Sci. 1994;35:120–127. [PubMed] [Google Scholar]
- 17.Botta R., Lisi S., Marcocci C., et al. Enalapril reduces proliferation and hyaluronic acid release in orbital fibroblasts. Thyroid. 2013;23:92–96. doi: 10.1089/thy.2012.0373. [DOI] [PubMed] [Google Scholar]
- 18.Lisi S., Botta R., Lemmi M., et al. Quercetin decreases proliferation of orbital fibroblasts and their release of hyaluronic acid. J. Endocrinol. Invest. 2011;34:521–527. doi: 10.3275/7321. 20101027. [DOI] [PubMed] [Google Scholar]
- 19.Qi X., Luo B., Deng M., et al. Botox-A improve the thyroid-associated ophthalmopathy (TAO) orbital fibroblast activation through inhibiting the TGF-beta/Smad signaling. Exp. Eye Res. 2022;217 doi: 10.1016/j.exer.2022.108971. 20220131. [DOI] [PubMed] [Google Scholar]
- 20.Kim B.Y., Choi S.H., Kim J.Y., et al. Potential therapeutic role of bone Morphogenic protein 7 (BMP7) in the pathogenesis of graves' orbitopathy. Invest. Ophthalmol. Vis. Sci. 2022;63:7. doi: 10.1167/iovs.63.6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khoo T.K., Coenen M.J., Schiefer A.R., et al. Evidence for enhanced Thy-1 (CD90) expression in orbital fibroblasts of patients with Graves' ophthalmopathy. Thyroid. 2008;18:1291–1296. doi: 10.1089/thy.2008.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woeller C.F., O'Loughlin C.W., Pollock S.J., et al. Thy1 (CD90) controls adipogenesis by regulating activity of the Src family kinase, Fyn. FASEB J. 2015;29:920–931. doi: 10.1096/fj.14-257121. 20141121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee W.S., Jain M.K., Arkonac B.M., et al. Thy-1, a novel marker for angiogenesis upregulated by inflammatory cytokines. Circ. Res. 1998;82:845–851. doi: 10.1161/01.res.82.8.845. [DOI] [PubMed] [Google Scholar]
- 24.Cui X., Wang F., Liu C. A review of TSHR- and IGF-1R-related pathogenesis and treatment of Graves' orbitopathy. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1062045. 20230119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girnita L., Smith T.J., Janssen J. It Takes two to Tango: IGF-I and TSH receptors in thyroid eye disease. J. Clin. Endocrinol. Metab. 2022;107:S1–S12. doi: 10.1210/clinem/dgac045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krieger C.C., Place R.F., Bevilacqua C., et al. TSH/IGF-1 receptor cross talk in graves' ophthalmopathy pathogenesis. J. Clin. Endocrinol. Metab. 2016;101:2340–2347. doi: 10.1210/jc.2016-1315. 20160404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Douglas R.S., Afifiyan N.F., Hwang C.J., et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J. Clin. Endocrinol. Metab. 2010;95:430–438. doi: 10.1210/jc.2009-1614. 20091106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith T.J. Potential role for bone marrow-derived fibrocytes in the orbital fibroblast heterogeneity associated with thyroid-associated ophthalmopathy. Clin. Exp. Immunol. 2010;162:24–31. doi: 10.1111/j.1365-2249.2010.04219.x. 20100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galgoczi E., Jeney F., Gazdag A., et al. Cell density-dependent stimulation of PAI-1 and hyaluronan synthesis by TGF-beta in orbital fibroblasts. J. Endocrinol. 2016;229:187–196. doi: 10.1530/JOE-15-0524. 20160315. [DOI] [PubMed] [Google Scholar]
- 30.Raychaudhuri N., Douglas R.S., Smith T.J. PGE2 induces IL-6 in orbital fibroblasts through EP2 receptors and increased gene promoter activity: implications to thyroid-associated ophthalmopathy. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015296. 20101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.