Abstract
Purpose
The hand motor cortex (HMC) is a reliable anatomical landmark for identifying the precentral gyrus. The current study aimed to investigate the morphology of HMC on axial MRI of glioma patients, propose a new morphological classification of HMC and analyze the effect of tumors on the morphology of HMC.
Methods
A retrospective study of 276 adult right-handed glioma patients was conducted. The morphology of HMC was assessed using T2 axial images. Subsequently, the distribution of morphological subtypes was compared between the bilateral hemispheres and the tumor-affected and healthy hemispheres. Finally, the influence of tumor pathology on the morphology of HMC was investigated.
Results
A new morphological classification of HMC with four subtypes (Ω, ε, Ω-ε and ε-Ω) was proposed. No significant difference was identified in the distribution of morphological subtypes between the bilateral hemispheres (p = 0.0901, Chi-square test), or between the tumor-affected and healthy hemispheres (p = 0.3507, Chi-square test), and the morphology of HMC between the bilateral hemispheres were consistent (p < 0.0001, Kappa test). In addition, a significant difference was identified in the distribution of morphological subtypes between astrocytic and oligodendroglial tumors (p = 0.0135, Chi-square test).
Conclusion
In the current study, we proposed a new morphological classification of HMC, and found that tumor could affect the morphology of HMC in glioma patients. The results can help our clinical practice, enabling us to further understand the spatial structure of the cerebral hemispheres.
Keywords: Glioma, Hand motor cortex, Magnetic resonance imaging, Morphological study, Hemisphere
1. Introduction
The cerebral cortex is anatomically complex and exhibits great variation among individuals. When there is a space-occupying lesion within the brain, such as a tumor or vascular malformation, the normal anatomical structure will be distorted, making it difficult to identify a specified cortical region. Magnetic resonance imaging (MRI) enables high-resolution, non-invasive investigation of cerebral cortical morphology, and functional MRI (fMRI) techniques can locate corresponding anatomical regions based on functional activation changes [1,2]. However, fMRI is difficult to be widely applied due to additional time for data acquisition and analysis and the requirement for high-field magnetic resonance equipment. Understanding the anatomical landmarks of the cerebral cortex plays quite an important role in clinical research and daily clinical work.
The hand motor cortex (HMC), which is also known as the “hand knob”, has been widely accepted as a reliable anatomical landmark for identifying the precentral gyrus on MRI [3,4]. To date, various modalities have been applied to locate HMC and investigate its morphological features [5,6,7,8]. In 1997, Yousry et al. first defined a knob-like structure for identifying the precentral gyrus directly, which is shaped like an omega (Ω) in the axial plane [9]. This characteristic inverted Ω shape is formed by two small fissures directly from the precentral sulcus. Occasionally, a third fissure occurs between the two fissures, which can lead to a horizontal epsilon (ε) shape appearance. According to previous studies, the region activated by hand movements is mainly located in the Ω region of the precentral gyrus [10,11,12]. Overall, on axial MRI images, HMC generally presents as a Ω shape and sometimes as a horizontal ε shape. While on sagittal MRI images, it could represent a typical shape of a hook, step, bayonet, or zigzag. In 2007, Caulo et al. performed a MRI study on the structure of the normal human brain and proposed that the morphology of HMC could be divided into five subtypes to better identify precentral gyrus: Ω, ε, laterally asymmetric ε, medially asymmetric ε and null [13].
Glioma is the most common type of primary intracranial malignant tumors, and gliomas involving eloquent areas, e.g., HMC, remain a great challenge for neurosurgeons [14,15]. Clinicians have already conducted some functional studies to investigate the impact of glioma on eloquent areas [16,17]. Nevertheless, morphological studies on the eloquent cortex affected by glioma are relatively rare. When gliomas involve the motor area, it compresses or infiltrates the cortex of motor area, causing deformation and even inability to recognize it. However, there is currently no research to prove whether glioma can still affect the morphology of HMC when they are far away from the motor area (more than 2 cm). Moreover, according to our previous research experience and clinical practice, the morphology of HMC is not fixed and unchanging. In some cases, the morphology of HMC varies at different slices of MRI scan, which is inconsistent with the common classification [13]. For the above two reasons, it is necessary to conduct a large sample study on the morphology of HMC in glioma patients.
The current study aimed to investigate the morphology of HMC on axial MRI of glioma patients, and propose a new morphological classification that is more suitable for clinical application. In addition, the morphology of HMC in the tumor-affected hemisphere was assessed to clarify the influence of tumor pathology on it.
2. Methods
2.1. Ethical standard
All studies on humans described in this article were carried out with the approval of the responsible ethics committee and in accordance with national law and the HELSINKI Declaration from 1964. Informed consent was obtained from all patients prior to participation in the study.
2.2. Data collection
Data of 276 diffuse glioma patients operated at our Hospital from August 2010 to May 2012 were systematically reviewed. Inclusion criteria: 1) age over 18 years; 2) right-handedness; 3) no pushing or infiltrating to the anterior central gyrus; 4) no previous history of craniotomy or biopsy; 5) no history of alcohol and drug abuse; 6) no history of other diseases which may affect brain morphology; 7) confirmed as diffuse gliomas by pathological diagnosis. Exclusion criteria: 1) blurred MRI; 2) long-term history of hypertension and/or diabetes; 3) pregnant women; 4) history of mental disease; 5) history of musical training or other activities involving hand dexterity. The enrolled patients included 176 males (63.8%) and 100 females (36.2%), and the average age of all patients was 44.59 ± 12.62 years old. The cohort had 126 cases of World Health Organization (WHO) grade II tumors, 67 cases of WHO grade III and 83 cases of WHO grade IV, with 154 tumors in the left hemisphere, and 122 tumors in the right hemisphere. The general clinical data of the patients are shown in Table 1.
Table 1.
Demographic and clinical data of the patients.
| Characteristics | Value |
|---|---|
| Age (yrs, mean ± SD) | 44.59 ± 12.62 |
| Sex | |
| Male | 176 (63.77%) |
| Female | 100 (36.23%) |
| Handness | |
| Left | 0 (0%) |
| Right | 276 (100%) |
| Hemisphere of Lesion | |
| Left | 154 (55.80%) |
| Right | 122 (44.20%) |
| Tumor histopathology | |
| WHO Grade II | 126 (45.65%) |
| WHO Grade III | 67 (24.28%) |
| WHO Grade IV | 83 (30.07%) |
| Origin of pathology | |
| A | 115 (41.67%) |
| O | 161 (58.33%) |
A: astrocytoma; O: oligodendroglioma.
2.3. Magnetic resonance imaging
All patients underwent a preoperative 3.0T MR scan. Conventional MRI sequences were performed in all patients and the parameters were as follows: repetition time: 5500 MS; echo time: 120 ms; field of view: 240 × 240 mm2; layer thickness: 5 mm; layer spacing: 1 mm; turning angle: 150°. All images were obtained by using the 512 × 512 voxel matrix. The voxel size was 0.47 × 0.47 × 6.0 mm3. T2 axial images were selected for study and the morphology of HMC was subject to mutual analysis by MRIcron software (http://www.nitrc.org/projects/mricron).
2.4. Morphological evaluation of HMC
Prior to the assessment of HMC, the clinical data of all patients were confidential. The researchers first identified the anterior central gyrus in two ways: 1) by identifying the relationship between the end of the anterior central gyrus and the posterior part of the inferior frontal gyrus; 2) by identifying the superior frontal gyrus and the anterior central sulcus. The cortex protruding from the middle and upper part of the anterior central gyrus to the central sulcus is considered the hand area. Meanwhile, it is necessary to adjust the number of axial slices to accurately evaluate the morphology of HMC. The evaluation was performed according to the actual observation, the changing trend of the shape (if any) should also be recorded. The assessment was conducted independently by two neurosurgeons, if their results were inconsistent, the conflicts would be resolved through group meetings with adding an experienced neuroradiologist.
2.5. Statistical analysis
Statistical analysis was performed using SPSS Statistics (Version 19.0, IBM Corp., Armonk, New York, USA) and GraphPad Prism (Version 8.0.1, GraphPad Software Inc., San Diego, California, USA). The chi-square test was used to detect the distribution of different morphological types in the cerebral hemisphere and its gender gap. The Kappa test was used to detect the morphological consistency of HMC between bilateral cerebral hemispheres. P < 0.05 was considered statistically significant.
3. Results
3.1. Morphological classification of HMC
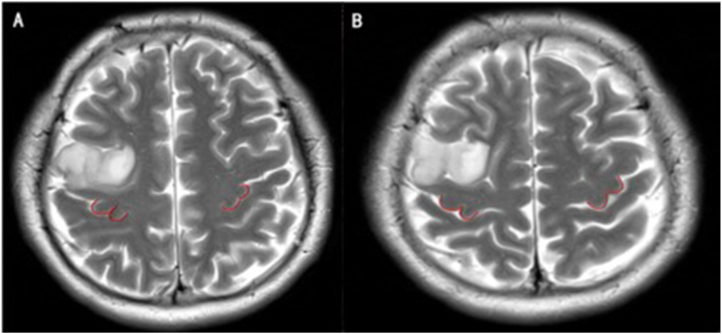
In all 276 glioma patients, 552 brain hemispheres were studied. Four morphological types were observed: Ω, ε, Ω-ε, ε-Ω. Among those four subtypes, Ω and ε were previously reported classical types, Ω-ε represented that the shape of HMC gradually changed from Ω to ε with the increase of MRI layers, and vice versa. The last two subtypes could only be seen in hemispheres affected by gliomas, and ε-Ω were only seen in the left affected cerebral hemisphere of male patients (Fig. 1, Fig. 2, Fig. 3, Fig. 4). The most common morphological subtypes from high to low were Ω, ε, Ω-ε, and ε-Ω.
Fig. 1.
The morphology of bilateral HMC. A) The 20th slice from the baseline of the magnetic resonance axial image, the morphology of the bilateral HMC is Ω. B) On the 21st slice, the morphology of the bilateral HMC is still Ω.
Fig. 2.
The morphology of bilateral HMC. A) The 20th slice from the baseline of the magnetic resonance axial image, the morphology of the bilateral HMC is ε. B) On the 21st slice, the morphology of the bilateral HMC is still ε.
Fig. 3.
The morphology of bilateral HMC. A) The 20th slice from the baseline of the magnetic resonance axial image, the morphology of the bilateral HMC is Ω. B) On the 21st slice, the morphology of the bilateral HMC is ε.
Fig. 4.
The morphology of bilateral HMC. A) The 20th slice from the baseline of the magnetic resonance axial image, the morphology of the left HMC is Ω. B) On the 21st slice, the morphology of the left HMC is ε.
3.2. Distribution of morphological subtypes
The distribution of morphological subtypes in the bilateral hemispheres was as follows: 1) in the left hemisphere, 153 cases of Ω (55.4%), 77 cases of ε (27.9%), 43 cases of Ω-ε (15.6%), 3 cases of ε-Ω (1.1%); 2) in the right hemisphere, 160 cases of Ω (58.0%), 87 cases of ε (31.5%), 29 cases of Ω-ε (10.5%). No significant difference was identified in the distribution of morphological subtypes between the bilateral hemispheres (p = 0.0901, Chi-square test, Table 2). In addition, in order to assess the effect of gliomas on the morphology of HMC, we compared the distribution of morphological subtypes between the tumor-affected and healthy hemispheres. The results were as follows: 1) in the tumor-affected hemisphere, 157 cases of Ω (56.9%), 79 cases of ε (28.6%), 37 cases of Ω-ε (13.4%), 3 cases of ε-Ω (1.1%); 2) in the healthy hemisphere, 156 cases of Ω (56.5%), 85 cases of ε (30.8%), 35 cases of Ω-ε (12.7%). No significant difference was identified in the distribution of morphological subtypes between the tumor-affected hemisphere and the healthy hemisphere (p = 0.3507, Chi-square test, Table 3).
Table 2.
Distribution of morphologic variants in HMC across both hemispheres in glioma patients.
| Right |
Total | |||||
|---|---|---|---|---|---|---|
| Ω | ε | Ω-ε | ε-Ω | |||
| Left | Ω | 128 (46.4) | 21 (7.6) | 4 (1.4) | 0 (0.0) | 153 (55.4) |
| ε | 17 (6.2) | 58 (21.0) | 2 (0.7) | 0 (0.0) | 77 (27.9) | |
| Ω-ε | 13 (4.7) | 7 (2.5) | 23 (8.4) | 0 (0.0) | 43 (15.6) | |
| ε-Ω | 2 (0.7) | 1 (0.4) | 0 (0.0) | 0 (0.0) | 3 (1.1) | |
| Total | 160 (58.0) | 87 (31.5) | 29 (10.5) | 0 (0.0) | 276 | |
No significant difference was observed in the incidence of morphological variants of HMC across bilateral cerebral hemispheres (p = 0.0901, Chi-square test).
The morphology of HMC in bilateral cerebral hemispheres demonstrated consistency (p < 0.0001, Kappa test).
Table 3.
Comparative analysis of HMC morphologic variants in glioma-affected and unaffected hemispheres.
| Unaffected |
Total | |||||
|---|---|---|---|---|---|---|
| Ω | ε | Ω-ε | ε-Ω | |||
| Affected | Ω | 128 (46.4) | 22 (8.0) | 7 (2.5) | 0 (0.0) | 157 (56.9) |
| ε | 16 (5.8) | 58 (21.0) | 5 (1.8) | 0 (0.0) | 79 (28.6) | |
| Ω-ε | 10 (3.6) | 4 (1.4) | 23 (8.4) | 0 (0.0) | 37 (13.4) | |
| ε-Ω | 2 (0.7) | 1 (0.4) | 0 (0.0) | 0 (0.0) | 3 (1.1) | |
| Total | 156 (56.5) | 85 (30.8) | 35 (12.7) | 0 (0.0) | 276 | |
No significant difference was observed in the morphological incidence of HMC between affected and unaffected cerebral hemispheres (p = 0.3507, Chi-square test).
3.3. Morphological consistency of HMC between the bilateral hemispheres
Among all 276 patients, 209 (75.8%) showed morphological consistency of HMC between the bilateral hemispheres: 128 cases of Ω (46.4%), 58 cases of ε (21.0%), 23 cases of Ω-ε (8.4%). In the 176 male patients, 133 (75.6%) showed morphological consistency of HMC, with 70 cases of Ω (39.8%), 46 cases of ε (26.1%), 17 cases of Ω-ε (9.7%). In comparison, in the 100 female patients, 76 (76.0%) showed morphological consistency of HMC, with 58 cases of Ω (58.0%), 12 cases of ε (12.0%), 6 cases of Ω-ε (6.0%). Overall, the morphological consistency of HMC between the bilateral hemispheres was significant, regardless of gender difference (p < 0.0001 for the whole cohort, males and females, Kappa test, Table 2, Table 4, Table 5).
Table 4.
Distribution of HMC morphology in bilateral cerebral hemispheres among male patients.
| Men | Right |
Total | ||||
|---|---|---|---|---|---|---|
| Ω | ε | Ω-ε | ε-Ω | |||
| Left | Ω | 70 (39.8) | 15 (8.5) | 4 (2.3) | 0 (0.0) | 89 (50.6) |
| ε | 9 (5.1) | 46 (26.1) | 2 (1.1) | 0 (0.0) | 57 (32.3) | |
| Ω-ε | 6 (3.4) | 4 (2.3) | 17 (9.7) | 0 (0.0) | 27 (15.4) | |
| ε-Ω | 2 (1.1) | 1 (0.6) | 0 (0.0) | 0 (0.0) | 3 (1.7) | |
| Total | 87 (49.4) | 66 (37.5) | 23 (13.1) | 0 (0.0) | 176 | |
No significant difference was observed in the incidence of morphological variants of HMC across bilateral cerebral hemispheres (p = 0.2613, Chi-square test).
Consistent morphology of HMC was demonstrated in bilateral cerebral hemispheres (p < 0.0001, Kappa test).
Table 5.
Distribution of HMC morphology in bilateral cerebral hemispheres among female patients.
| Women | Right |
Total | ||||
|---|---|---|---|---|---|---|
| Ω | ε | Ω-ε | ε-Ω | |||
| Left | Ω | 58 (58.0) | 6 (6.0) | 0 (0.0) | 0 (0.0) | 64 (64.0) |
| ε | 8 (8.0) | 12 (12.0) | 0 (0.0) | 0 (0.0) | 20 (20.0) | |
| Ω-ε | 7 (7.0) | 3 (3.0) | 6 (6.0) | 0 (0.0) | 16 (16.0) | |
| ε-Ω | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Total | 73 (73.0) | 21 (21.0) | 6 (6.0) | 0 (0.0) | 100 | |
No significant difference was found in the incidence of HMC morphological variants across bilateral cerebral hemispheres (p = 0.0757, Chi-square test).
Consistent morphology of HMC was observed in bilateral cerebral hemispheres (p < 0.0001, Kappa test).
3.4. The influence of gender on the morphology of HMC
As previous studies suggested that gender differences could lead to different arrangements of sulcus and gyrus in the cerebral cortex and thus result in morphological differences, here we also tried to investigate the influence of gender on the morphology of HMC. In the 176 male patients, the distribution of morphological subtypes of HMC in the bilateral hemispheres was as follows: 1) in the left hemisphere, 89 cases of Ω (50.6%), 57 cases of ε (32.3%), 27 cases of Ω-ε (15.4%), 3 cases of ε-Ω (1.7%). 2) in the right hemisphere, 87 cases of Ω (49.4%), 66 cases of ε (37.5%), and 23 cases of Ω-ε (13.1%). In the 100 female patients, the distribution of morphological subtypes was as follows: 1) In the left hemisphere, 64 cases of Ω (64.0%), 20 cases of ε (20.0%), 16 cases of Ω-ε (16.0%). 2) In the right hemisphere, 73 cases of Ω (73.0%), 21 cases of ε (21.0%), and 6 cases of Ω-ε (6.0%). Overall, the difference is not statistically significant in the distribution of morphological subtypes between the bilateral hemispheres in either male or female patients (p = 0.2613 and 0.0758 for males and females, respectively, Chi-square test, Table 4). However, it seemed that the ε subtype was more common in male patients (p = 0.0002, Chi-square test, Table 6).
Table 6.
Overall incidence of HMC morphology in male and female patients.
| Ω | ε | Ω-ε | ε-Ω | Hemispheres | |
|---|---|---|---|---|---|
| Male | 176 (50.0) | 123 (34.9) | 50 (14.2) | 3 (0.9) | 352 |
| Female | 137 (68.5) | 41 (20.5) | 22 (11.0) | 0 (0.0) | 200 |
A significant difference was observed in the total incidence of four HMC types between male and female patients (p = 0.0002, Chi-square test)..
3.5. The influence of tumor pathology on the morphology of HMC in the tumor-affected hemisphere
The relationship between the morphology of HMC in the tumor-affected hemisphere and glioma grade was assessed. The results were as follows: 1) in patients with WHO grade II gliomas: 66 cases of Ω, 41 cases of ε, 17 cases of Ω-ε, 2 cases of ε-Ω; 2) in patients with WHO grade III gliomas: 41 cases of Ω, 21 cases of ε, 5 cases of Ω-ε, none of ε-Ω; 3) in patients with WHO grade IV gliomas: 50 cases of Ω, 17 cases of ε, 15 cases of Ω-ε, 1 case of ε-Ω. There was no significant difference in the distribution of morphological subtypes of the tumor-affected hemisphere (p = 0.2573, Chi-square test, Table 7).
Table 7.
Distribution of HMC morphology in affected cerebral hemispheres across different pathological grades.
| II | III | IV | Total | ||
|---|---|---|---|---|---|
| Ω | 66 | 41 | 50 | 157 | |
| ε | 41 | 21 | 17 | 79 | |
| Ω-ε | 17 | 5 | 15 | 37 | |
| ε-Ω | 2 | 0 | 1 | 3 | |
| Total | 126 | 67 | 83 | 276 |
No significant difference was observed in the morphological distribution of HMC (p = 0.2573, Chi-square test).
Glioma can originate from either astrocytes or oligodendrocytes. Here, we also investigated the correlation between the morphology of HMC in the tumor-affected hemisphere and tumor origin. Among the 276 tumors, 115 originated from astrocytes, while 161 originated from oligodendrocytes. In patients with astrocytic tumors, the distribution of morphological subtypes was as follows: 67 cases of Ω, 24 cases of ε, 23 cases of Ω-ε, 1 case of ε-Ω. In patients with oligodendroglial tumors, there are 90 cases of Ω, 55 cases of ε, 14 cases of Ω-ε, and 2 cases of ε-Ω. A significant difference was identified in the distribution of morphological subtypes of HMC in the tumor-affected hemisphere between patients with astrocytic tumors and those with oligodendroglial tumors (p = 0.0135, Chi-square test, Table 8).
Table 8.
Distribution of HMC morphology in affected cerebral hemispheres of various pathological origins.
| A | O | Total | ||
|---|---|---|---|---|
| Ω | 67 | 90 | 157 | |
| ε | 24 | 55 | 79 | |
| Ω-ε | 23 | 14 | 37 | |
| ε-Ω | 1 | 2 | 3 | |
| Total | 115 | 161 | 276 |
A significant difference was observed in the morphological distribution of HMC in the affected cerebral hemisphere (p = 0.0135, Chi-square test).
3.6. The influence of age on the morphology of HMC
To analyze whether age has an impact on the morphology of the HMC, we divided the subjects into two groups (20–40years, >40years). And we found that there was no significant difference in the distribution of morphology of HMC between the two groups (p = 0.4641, Chi-square test, Table 9).
Table 9.
The overall incidence of morphology of HMC in two groups.
| Ω | ε | Ω-ε | ε-Ω | Hemispheres | |
|---|---|---|---|---|---|
| 20–40y | 116 (53.2) | 73 (33.5) | 28 (12.8) | 1 (0.05) | 218 |
| >40y | 197 (58.9) | 91 (27.3) | 44 (13.2) | 2 (0.06) | 334 |
There is a no significant difference in the distribution of morphology of HMC between the two groups (20–40years, >40years) (p = 0.4641, Chi-square test)..
4. Discussion
HMC is a crucial anatomical structure that protrudes from the central anterior gyrus to the central sulcus. In the current study, we investigated the morphology of HMC on axial MRI in a large cohort of glioma patients, and proposed a new morphological classification of HMC with four subtypes: Ω, ε, Ω-ε (Ω convert to ε), ε-Ω (ε convert to Ω).
In this study, we only enrolled patients whose central anterior gyrus was not pushed or infiltrated by their tumors to exclude the direct effect of glioma on the morphology of HMC. We found no significant difference in the distribution of morphological subtypes in the bilateral hemispheres or between the affected and healthy hemispheres. It was worth noting that the ε-Ω subtype could be only found in the tumor-affected left hemisphere of male patients, but the incidence was extremely low. We speculated that this subtype might be accidental and could be caused by the potential indirect influence of tumor. As we know, the normal aging process can affects gyrification, and as age increases, the brain become more flattened, to avoid bias caused by age, we divided the subjects into two groups (20–40 years,>40 years). And we found that there was no significant difference in the distribution of morphology of HMC between the two groups.
Previous studies suggested that gender differences in the morphology of HMC, women were more likely to have the folding of the frontal and parietal cortex, and have higher complexity of the cerebral cortex [18,19]. Our study did not find significant differences in the distribution of morphological subtypes between male and female patients. However, ε subtype was more common in male patients, and ε-Ω could be seen only in male patients, either. If we consider that the ε subtype is a more complex morphological presentation of the hand motor cortex, such results were inconsistent with a study published by Louders et al. [19], but consistent with the results reported by Caulo et al. [13]. One of the reasons may be that Louders et al. used a three-dimensional analytic technique, while Caulo et al. and our study used a two-dimensional analytic technique.
Hemispheric asymmetry has been accepted as a typical feature of the human brain. In general, during the development of the human brain, the bilateral cerebral hemispheres remain relatively symmetrical, and due to some acquired reasons, such as gender, age, and environment, the bilateral cerebral hemispheres can appear asymmetric in certain areas. Some previous studies showed that asymmetry in the cerebral hemispheres was more common in males, and such asymmetry can be observed in the temporal surface, parietal lobe, and the posterior part of the end of the sylvian fissure [13,20]. However, in the current study, we found that the morphology of HMC between the bilateral hemispheres was generally consistent, regardless of gender difference. This may indicate that the morphology of HMC is not the main manifestation of hemispheric asymmetry.
There was no previous research on the relationship between the morphology of HMC and tumor pathology in glioma patients. In our study, we analyzed the influence of tumors on the morphology of HMC according to tumor grade and tumor origin. No difference was identified in the distribution of morphological subtypes of the tumor-affected hemisphere among different tumor grades. However, Ω-ε subtype had a sognificantly higher incidence in patients with astrocytic tumors. We speculated that there are two explanations for this result. The first possibility is that our sample size is not large enough, resulting in such statistical results. Secondly, it may be attributed to differences in the biological behaviors between astrocytic and oligodendroglial tumors. Astrocytic tumors are highly invasive and generally show a diffuse infiltrating growth pattern. On the contrary, oligodendroglial tumors often exert a squeezing effect on the surrounding brain tissue. Accordingly, in patients with astrocytic tumors, the morphology of HMC is more complicated. Although the tumor is far from HMC(>2 cm), the morphology of HMC will still be affected by tumors, it may be related to distant microinfiltration or growth factors secreted by the tumor, this requires further experimental verification.
The study had its limitations. Firstly, it was performed only based on two-dimensional analysis. However, as axial images are commonly used in clinical practice and are convenient for neurosurgeons, such limitation was relatively acceptable. Secondly, only right-handed patients were included in the study. The relationship between the morphology of HMC and handedness should be further investigated. Thirdly, we did not conduct external verification to support our results. Finally, this study only found that gliomas may affect the morphology of HMC, and did not delve into its underlying mechanism. Of course, we are currently conducting research in an attempt to explain this phenomenon.
5. Conclusion
In the current study, we proposed a new morphological classification of HMC with four subtypes: Ω, ε, Ω-ε (Ω convert to ε), ε-Ω (ε convert to Ω). No significant difference was identified in the distribution of morphological subtypes between the bilateral hemispheres, or between the tumor-affected and healthy hemispheres, and the morphology of HMC between the bilateral hemispheres were generally consistent. Moreover, gender and tumor origin may affect the morphology of HMC. The results can help our clinical practice, enabling us to further understand the spatial structure of the cerebral hemispheres.
Availability of data and material
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Rongjie Wu: Writing – original draft, Methodology, Formal analysis, Data curation. Changtao Liu: Writing – original draft, Formal analysis. Congying Yang: Software, Methodology. Dezhi Xu: Validation, Software. Shiwei Yan: Visualization, Methodology. Xing Fan: Writing – review & editing, Validation, Supervision, Resources. Jingshan Liang: Writing – review & editing, Writing – original draft, Visualization, Software, Resources, Formal analysis.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Jingshan Liang reports financial support was provided by Project of Health and Science of Lianyungang City (202002). Jingshan Liang reports financial support was provided by Postdoctoral Research Funding of Jiangsu Province (SBSH01). If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Rongjie Wu, Email: chrio185@163.com.
Changtao Liu, Email: lctgz@163.com.
Congying Yang, Email: 1477673243@qq.com.
Dezhi Xu, Email: 470402350@qq.com.
Shiwei Yan, Email: yan18961328366@163.com.
Xing Fan, Email: xingkongyaoxiang@163.com.
Jingshan Liang, Email: neuroliang@163.com.
References
- 1.Vakamudi K., Posse S., Jung R., Cushnyr B., Chohan M.O. Real-time presurgical resting-state fMRI in patients with brain tumors: quality control and comparison with task-fMRI and intraoperative mapping. Hum. Brain Mapp. 2020;41:797–814. doi: 10.1002/hbm.24840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wengenroth M., Blatow M., Guenther J., Akbar M., Tronnier V.M., Stippich C. Diagnostic benefits of presurgical fMRI in patients with brain tumours in the primary sensorimotor cortex. Eur. Radiol. 2011;21:1517–1525. doi: 10.1007/s00330-011-2067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanakawa T., Parikh S., Bruno M.K., Hallett M. Finger and face representations in the ipsilateral precentral motor areas in humans. J. Neurophysiol. 2005;93:2950–2958. doi: 10.1152/jn.00784.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah K.B., Hayman L.A., Chavali L.S., Hamilton J.D., Prabhu S.S., Wangaryattawanich P., et al. Glial tumors in brodmann area 6: spread pattern and relationships to motor areas. Radiographics. 2015;35:793–803. doi: 10.1148/rg.2015140207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciavarro M., Grande E., Pavone L., Bevacqua G., De Angelis M., di Russo P., et al. Pre-surgical fMRI localization of the hand motor cortex in brain tumors: comparison between finger tapping task and a new visual-triggered finger movement task. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.658025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang S., Bai H.X., Fan X., Li S., Zhang Z., Jiang T., et al. A novel sequence: ZOOMit-blood oxygen level-dependent for motor-cortex localization. Neurosurgery. 2020;86:E124–E132. doi: 10.1093/neuros/nyz441. [DOI] [PubMed] [Google Scholar]
- 7.Sahin N., Mohan S., Maralani P.J., Duddukuri S., O'Rourke D.M., Melhem E.R., et al. Assignment confidence in localization of the hand motor cortex: comparison of structural imaging with functional MRI. AJR Am. J. Roentgenol. 2016;207:1263–1270. doi: 10.2214/AJR.15.15119. [DOI] [PubMed] [Google Scholar]
- 8.Tirakotai W., Hellwig D., Bertalanffy H., Riegel T. Localization of precentral gyrus in image-guided surgery for motor cortex stimulation. Acta Neurochir. Suppl. 2007;97:75–79. doi: 10.1007/978-3-211-33081-4_9. [DOI] [PubMed] [Google Scholar]
- 9.Yousry T.A., Schmid U.D., Alkadhi H., Schmidt D., Peraud A., Buettner A., et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120(Pt 1):141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- 10.Dubbioso R., Madsen K.H., Thielscher A., Siebner H.R. The myelin content of the human precentral hand knob reflects interindividual differences in manual motor control at the physiological and behavioral level. J. Neurosci. 2021;41:3163–3179. doi: 10.1523/JNEUROSCI.0390-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H., Kim J., Lee H.J., Lee J., Na Y., Chang W.H., et al. Optimal stimulation site for rTMS to improve motor function: anatomical hand knob vs. hand motor hotspot. Neurosci. Lett. 2021;740 doi: 10.1016/j.neulet.2020.135424. [DOI] [PubMed] [Google Scholar]
- 12.Willoughby W.R., Thoenes K., Bolding M. Somatotopic arrangement of the human primary somatosensory cortex derived from functional magnetic resonance imaging. Front. Neurosci. 2020;14 doi: 10.3389/fnins.2020.598482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caulo M., Briganti C., Mattei P.A., Perfetti B., Ferretti A., Romani G.L., et al. New morphologic variants of the hand motor cortex as seen with MR imaging in a large study population. AJNR Am. J. Neuroradiol. 2007;28:1480–1485. doi: 10.3174/ajnr.A0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang S., Li Y., Wang Y., Zhang Z., Jiang T. Awake craniotomy for gliomas involving motor-related areas: classification and function recovery. J. Neuro Oncol. 2020;148:317–325. doi: 10.1007/s11060-020-03520-w. [DOI] [PubMed] [Google Scholar]
- 15.Ostrom Q.T., Bauchet L., Davis F.G., Deltour I., Fisher J.L., Langer C.E., et al. The epidemiology of glioma in adults: a "state of the science" review. Neuro Oncol. 2014;16:896–913. doi: 10.1093/neuonc/nou087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavrador J.P., Gioti I., Hoppe S., Jung J., Patel S., Gullan R., et al. Altered motor excitability in patients with diffuse gliomas involving motor eloquent areas: the impact of tumor grading. Neurosurgery. 2020;88:183–192. doi: 10.1093/neuros/nyaa354. [DOI] [PubMed] [Google Scholar]
- 17.Niu C., Wang Y., Cohen A.D., Liu X., Li H., Lin P., et al. Machine learning may predict individual hand motor activation from resting-state fMRI in patients with brain tumors in perirolandic cortex. Eur. Radiol. 2021 doi: 10.1007/s00330-021-07825-w. [DOI] [PubMed] [Google Scholar]
- 18.Awate S.P., Yushkevich P., Licht D., Gee J.C. Gender differences in cerebral cortical folding: multivariate complexity-shape analysis with insights into handling brain-volume differences. Med. Image Comput. Comput. Assist. Interv. 2009;12:200–207. doi: 10.1007/978-3-642-04271-3_25. [DOI] [PubMed] [Google Scholar]
- 19.Luders E., Narr K.L., Thompson P.M., Rex D.E., Jancke L., Steinmetz H., et al. Gender differences in cortical complexity. Nat. Neurosci. 2004;7:799–800. doi: 10.1038/nn1277. [DOI] [PubMed] [Google Scholar]
- 20.Kong X.Z., Mathias S.R., Guadalupe T., Group E.L.W., Glahn D.C., Franke B., et al. Mapping cortical brain asymmetry in 17,141 healthy individuals worldwide via the ENIGMA Consortium. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E5154–E5163. doi: 10.1073/pnas.1718418115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.