Abstract
AIM
To explore whether CD3ε is involved in the adaptive immunity of Aspergillus fumigatus (A. fumigatus) keratitis in mice and the role of innate and adaptive immunity in it.
METHODS
Mice models of A. fumigatus keratitis were established by intra-stromal injection and corneal epithelial scratching. Subconjunctival injections of natamycin, wedelolactone, LOX-1 inhibitor (poly I) or Dectin-1 inhibitor (laminarin) were used to treat mice with A. fumigatus keratitis. Mice were pretreated by intraperitoneal injection of anti-mouse CD3ε. We observed the corneal infection of mice under the slit lamp microscope and made a clinical score. The protein expression of CD3ε and interleukin-10 (IL-10) was determined by Western blotting.
RESULTS
With the disease progresses, the degree of corneal opacity and edema augmented. In the intra-stromal injection models, CD3ε protein expression began to increase significantly on the 2nd day. However, in the scraping epithelial method models, CD3ε only began to increase on the 3rd day. After natamycin treatment, the degree of corneal inflammation in mice was significantly attenuated on the 3rd day. After wedelolactone treatment, the severity of keratitis worsened. And the amount of CD3ε protein was also reduced, compared with the control group. By inhibiting LOX-1 and Dectin-1, there was no significant difference in CD3ε production compared with the control group. After inhibiting CD3ε, corneal ulcer area and clinical score increased, and IL-10 expression was downregulated.
CONCLUSION
As a pan T cell marker, CD3ε participate in the adaptive immunity of A. fumigatus keratitis in mice. In our mice models, the corneas will enter the adaptive immune stage faster. By regulating IL-10, CD3ε exerts anti-inflammatory and repairs effects in the adaptive immune stage.
Keywords: CD3ε, adaptive immunity, Aspergillus fumigatus keratitis, interleukin-10
INTRODUCTION
Fungal keratitis (FK) is currently one of the main blinding factors[1]. It usually occurs after corneal epithelial defects caused by vegetable trauma or other factors[2]. Pathogenic fungi invade the corneal stroma from the damaged site to grow and multiply, and finally cause necrosis and inflammation[3].
The occurrence of FK depends on the interaction of fungal pathogenicity and body immunity. The immune mechanism which body fights against pathogenic microorganism mainly includes innate immunity and adaptive immunity[4]. Studies have shown that when fungi invade cornea, they are recognized by pattern recognition receptors, initiating innate immunity, inducing local phagocytes (neutrophils, macrophages etc.) to release cytokines and chemokines, which can enhance fungal clearance[4]–[6]. Activated macrophages, as important antigen presenting cells, engulf apoptotic neutrophils and enhance T cell activation to initiate adaptive immunity. Once the adaptive immunity is activated, it will enhance the migration of activated macrophages and neutrophils, promote the outbreak of pro-inflammatory cytokines, trigger a strong hypersensitivity response, and even exacerbate corneal damage. Therefore, precise regulatory mechanisms are needed to regulate the innate and adaptive immunity of FK in order to develop new target therapies[7]–[9].
There is an important molecule in adaptive immunity— CD3 composed of γ, δ, ε, and ζ chains[10]. The TCR/CD3 complex formed by CD3 and T cell antigen receptor (TCR), which participates in antigen recognition and immune signal transduction, is a sign of initiating adaptive immunity[11]–[12]. CD3ε plays a core and multiple roles in the development and function of T cells[13]–[14]. However, it has not been reported whether CD3ε plays a role in the immune process of FK. Therefore, our experiment further analyzed the expression of CD3ε in FK to understand the T cell immune function in mice with FK.
MATERIALS AND METHODS
Ethical Approval
The treatment of mice complies with the Use of Animals in Ophthalmic and Vision Research published by the Association for Research in Vision and Ophthalmology (ARVO) and the regulations of the Chinese Ministry of Science and Technology Guidelines on the Humane Treatment of Laboratory Animals (vGKFCZ-2006–398).
Experimental Animals
Female 8-week-old C57BL/6 mice were purchased from the Changzhou Cavens Laboratory (Jiangsu Province, China).
Fungal Species
Aspergillus fumigatus (A. fumigatus) strain 3.0772 purchased from the China General Microbiological Culture Collection Center (Beijing, China) was cultured on sabouraud agar for 2-4d, and conidia were scraped with bacterial L-ring into phosphate buffered saline (PBS). It was then filtered with sterile cotton gauze to obtain a pure conidial suspension, and the concentration was adjusted to 5×107 cfu/mL to be reserved.
Murine Models of Fungal Corneal Inflammation
Mice were anesthetized by intraperitoneal injection of 8% chloral hydrate. Corneal intra-stromal injection method: a 33-gauge Hamilton syringe was used to inject 2.5 µL of conidia (5×107 cfu/mL) into the corneal intra-stromal to establish mouse FK models. Corneal epithelial scratch method: completely scrape the epithelial layer in the center of the cornea with a diameter of 4 mm. Spread the fungal colony evenly on the corneal surface where the epithelium has been scraped, then cover the homemade contact lens and suture the eyelids.
Treatment
Natamycin eye drops (Natacyn, Alcon Laboratories) and wedelolactone (S9042, Selleck Chem) were used to treat keratitis[15]. Totally 5 µL of laminarin (SCH772984, Selleck Chem) or poly I (SM528906, Sigma) were injected under the conjunctiva. Before infection, mice were intraperitoneally injected with anti mouse CD3ε (1 mg/kg; A2104, Selleck Chem). The cornea of mice was observed daily under a slit lamp and photographed. According to the scoring criteria proposed by Wu et al[16], ocular disease was scored using clinical scores. The corneas of the mice collected were subjected to Western blot tests.
Western Blot
The corneas were collected and placed in RIPA buffer (Solarbio), PMSF (Solarbio), phosphatase inhibitor cocktail I (MedChemExpress) at a ratio of 98:1:1 and lysed by ultrasound. A BCA kit (Solarbio) was used to determine the protein concentration. Then the amount of the sample required was calculated and added to the MeilunGel protein precast gels (8%-16%, 10 wells, HEPES-Tris, 1.5 mmol/L; Meilunbio) to separate the protein sample. Proteins were transferred to a polyvinylidene difluoride membrane (PVDF; Solarbio). The PVDF membrane was blocked in a Western blocking buffer (Beyotime) at 37°C for 2h. The primary antibody was then added and incubated at 4°C overnight (<16h). After washing 3 times with PBST (PBS+0.05% Tween 20; Bio-Rad), the membrane and the corresponding secondary antibody were incubated for 1h at 37°C. Western ECL blotting substrate (Bio-Rad) was added to the PVDF membrane to develop blots. Digital images were analyzed using a Vilber Solo 4S chemiluminescence imaging system.
Primary antibodies against the following proteins were used: anti-CD3ε (Abcam), anti-IL-10 (Cell Signaling Technology) and anti-β-actin (Cell Signaling Technology). HRP-tagged secondary antibodies were purchased from Cell Signaling Technology.
Statistical Analysis
The statistical significance of experimental data were determined by an unpaired, two-tailed Student's t-test. P<0.05 indicated statistically significant differences. All experimental results are represented as mean±standard deviation (SD).
RESULTS
CD3ε Increased Earlier in Mice Corneas of A. fumigatus Keratitis Developed by Intra-stromal Injection than Corneal Epithelium Scratch
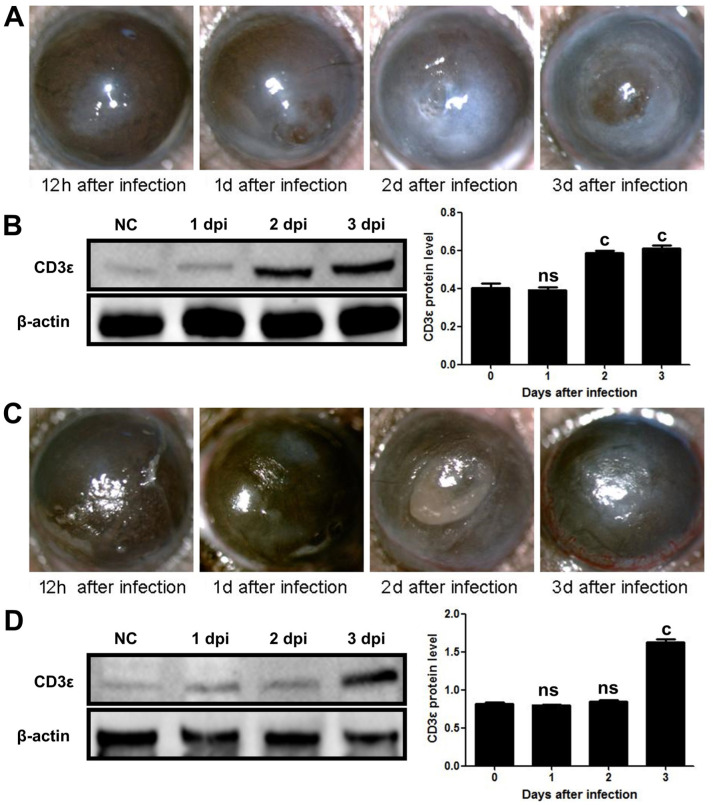
At present, there were two main methods for establishing models of keratitis, namely intra-stromal injection method and corneal epithelial scratch method. We established mouse models of A. fumigatus keratitis using two different methods to observe the expression of CD3ε at different stages of the disease. On 12h, 1, 2, and 3d after infection, the slit lamp examination revealed that the mouse cornea gradually became opaque and the ulcer area gradually increased (Figure 1A, 1C). CD3ε expression in mouse cornea with intra-stromal injection was obviously increased on the next day (Figure 1B). The difference was that in the corneal epithelial scratch group, CD3ε increased from the third day (Figure 1D). Thus, intra-stromal injection method were used for subsequent research.
Figure 1. CD3ε increased earlier in mice corneas of A. fumigatus keratitis developed by intra-stromal injection than corneal epithelium scratch.
A: Fungal keratitis models were established by intra-stromal injection of A. fumigatus spores, and mouse corneas were observed under a slit lamp at 12h, 1, 2, and 3d after infection. B: The mouse corneas (six per group) were taken at each time point for CD3ε Western blot. C: A. fumigatus keratitis models were established by scraping the epithelium, and mouse corneas were observed under a slit lamp at 1/2, 1, 2, and 3d after infection. D: The mouse corneas (six per group) were taken at each time point for CD3ε Western blot. dpi: Day post infection. (cP≤0.001, ns: P>0.05).
CD3ε was Down-regulated in Natamycin Treated A. fumigatus Keratitis Mice
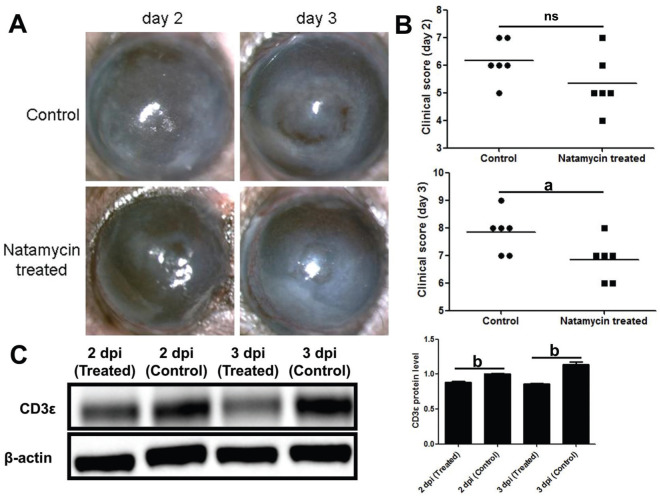
Compared with the control group, the natamycin treatment group had more transparent corneas (Figure 2A) and lower clinical scores (Figure 2B) on the third day. Western blotting showed the protein of CD3ε was down-regulated after natamycin treatment (Figure 2C).
Figure 2. CD3ε was down-regulated in natamycin treated A. fumigatus keratitis mice.
A, B: Subconjunctival injection of natamycin was used to treat fungal keratitis in mice for 2d and 3d. The mouse corneal slit lamp pictures (A) and clinical scores (B) were compared. C: After euthanasia, corneas (six per group) were taken for Western blotting to detect the protein of CD3ε. dpi: Day post infection. aP≤0.05, bP≤0.01, ns: P>0.05.
CD3ε was Down-regulated in Wedelolactone Treated A. fumigatus Keratitis Mice
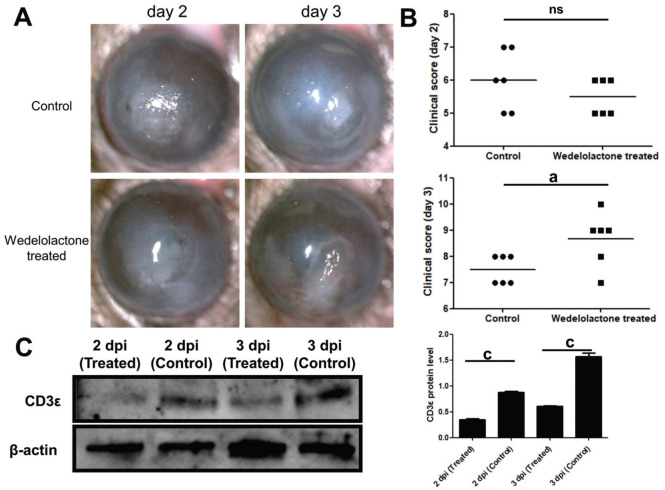
On the second day of wedelolactone treatment, there was no significant decrease in corneal condition and clinical score in the treatment group. On the contrary, on the third day after wedelolactone treatment, corneal ulcers (Figure 3A) worsened and clinical scores (Figure 3B) increased. Compared with the control group, the protein of CD3ε was significantly down-regulated after wedelolactone treatment on the second and third day (Figure 3C).
Figure 3. CD3ε was down-regulated in wedelolactone treated A. fumigatus keratitis mice.
A, B: Subconjunctival injection of wedelolactone was used to treat fungal keratitis in mice for 2d and 3d. The mouse corneal slit lamp pictures (A) and clinical scores (B) were compared. C: After euthanasia, corneas (six per group) were taken for Western blotting to detect the protein of CD3ε. Dpi: Day post infection. aP≤0.05, cP≤0.001, ns: P>0.05.
CD3ε Up-regulated by A. fumigatus Infection was Independent of Dectin-1 and LOX-1
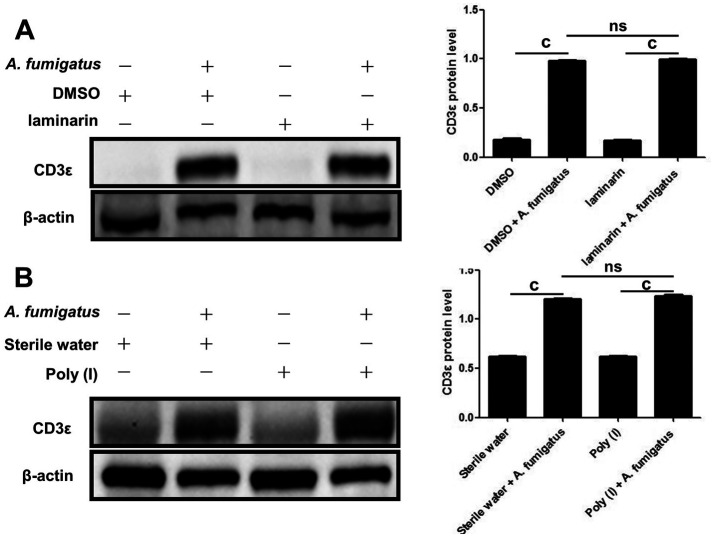
Our previous research demonstrated that Dectin-1 and LOX-1 were involved in the inflammatory response of A. fumigatus keratitis. We wanted to know whether Dectin-1 and LOX-1 regulated the expression of CD3ε. So subconjunctival injection of laminarin and poly I inhibited the production of LOX-1 (Figure 4A) and Dectin-1 (Figure 4B), but found that CD3ε did not decrease.
Figure 4. CD3ε up-regulation in response to A. fumigatus infection was independent of Dectin-1 and LOX-1.
The LOX-1 inhibitor (poly I; A) and Dectin-1 inhibitor (laminarin; B) were pretreated with mice. Two days later, mouse corneas (six per group) were taken for Western blotting to detect CD3ε (cP≤0.001, ns: P>0.05).
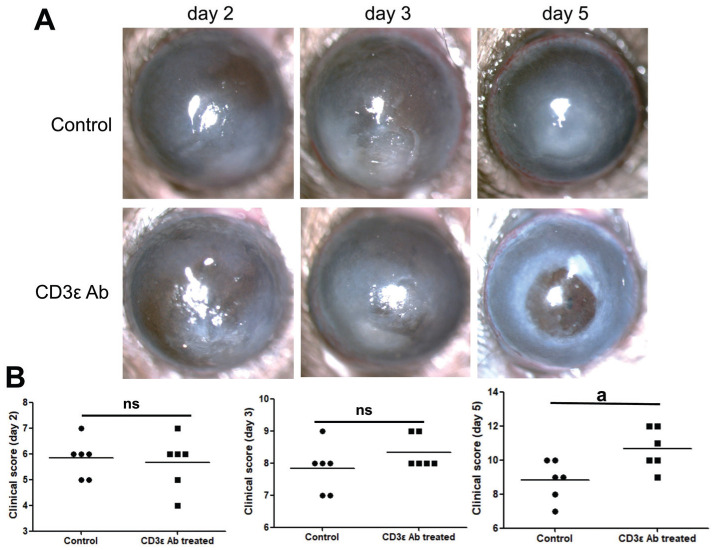
Disease Response after CD3ε Antibody
To further investigate the role of CD3ε in the progression of keratitis, we injected anti-mouse CD3ε (145-2C11) intraperitoneally. Compared with the control group, there were no significant changes in the cornea and clinical scores on the first three days of the disease course, but there was a significant deterioration on the fifth day (Figure 5A, 5B).
Figure 5. Disease response after CD3ε antibody treatment.
Intraperitoneal injection of anti-mouse CD3ε (145-2C11) was used to pretreat fungal keratitis in mice. The mouse corneal slit lamp pictures (A) and clinical scores (B) were compared on day 2, 3 and 5 (aP≤0.05, ns: P>0.05).
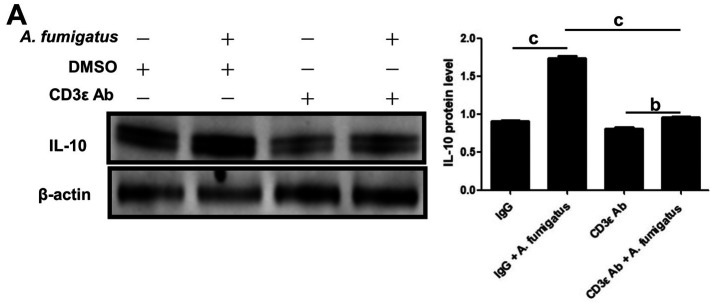
Blockade of CD3ε Decreased Expression of IL-10 in Mouse A. fumigatus Keratitis
In order to explore the mechanism of CD3ε chain in the adaptive immunity, Western blotting was performed on the corneas pretreated by intraperitoneal injection of CD3ε on the 5th day, and it was found that the expression of IL-10, an anti-inflammatory factor, was significantly downregulated (Figure 6A).
Figure 6. Blockade of CD3ε decreased expression of IL-10 in mouse A. fumigatus keratitis.
After euthanasia, corneas (six per group) were taken for Western blotting to detect the protein of IL-10 (bP≤0.01, cP≤0.001).
DISCUSSION
In agriculture-based developing countries, plant-induced corneal damage is the main risk factor for FK[17]. Fungal infections of the eye can cause corneal edema, opacity, ulcers and even perforations, which can lead to decreased vision and even blindness[18]–[19].
The CD3 present on the surface of T cells is a characteristic sign of mature T cells, transducing the activation signal generated by TCR recognition antigen[20]. The combination of CD3 and TCR leads to the differentiation and activation of T cells and changes in the expression of cytokines and effectors[21]–[22]. The ε, γ, δ, and ζ chains of CD3 each contained one (ε, γ, and δ) or three (ζ) tyrosine-based immunoreceptor activation motifs (ITAM) are phosphorylated by the Src kinase Lck, which initiates the downstream T cell signaling cascade[14],[23]. The studies we had performed found that the expression of CD3ε chain, an important signal molecule in TCR signal, was significantly increased in FK. Elevated CD3ε chain expression late in the disease course seemed to indicate the differentiation and activation of T cells, which indicated the adaptive immune stage. Compared with the scraping epithelial method to establish models, by the intra-stromal injection, the CD3ε expression in the corneal increased relatively earlier and entered the adaptive immune stage faster. This may be due to the fact that inherent phagocytes residing in the corneal stroma layer can quickly recognize fungi injected into the stroma, thereby presenting antigens to activate T cells and trigger applicable immunity more quickly.
Natamycin is currently the first-line drug for the treatment of FK[24]–[25]. As reported by others, natamycin binded to the ergosterol on the fungal cell membrane to change the permeability, which inhibited the growth of the fungus[26]–[27]. Consistent with our observation, natamycin enhanced the cornea transparency with FK. Our experiment also found that the CD3ε expression was decreased after natamycin treatment. The direct bactericidal effect of natamycin only activated an appropriate amount of phagocytes, these phagocytes cleared the residue, and avoided excessive adaptive immune activation, which significantly improved FK. This explained CD3ε as a activation marker for T cell was decreased after treatment with natamycin.
Our laboratory previous studies found that wedelolactone exerted a protective effect on the first day after the remedy of FK, while cornea began to worsen significantly on the third day after consecutive treatment. It was related to the reduction of inflammatory cell infiltration during the early immune response stage, inhibiting caspase-1 and thus inhibiting IL-1β release[15]. Wedelolactone had a certain anti-inflammatory effect in the innate immune stage, avoiding immune storms and leading to good corneal transparency in the early stage of treatment. This explained the decrease of CD3ε in the early stages of treatment. However, the corneal transparency was significantly reduced from the third day of the wedelolactone treatment, while CD3ε was still expressed at a low level. We suspected that this result was related to the corresponding lag of adaptive immunity after medication. That was to say, whether it was an inhibitor of caspase-11 on the non-classical pyroptosis pathway that inhibited pyroptosis[28] or exerted anti-inflammatory effects by inhibiting the activation of inflammasomes[15], wedelolactone would delay T cell activation mediated by CD3ε, thereby affecting subsequent adaptive immunity. A small amount of T cell activation was not sufficient to limit fungal proliferation and tissue repair, thereby exacerbating the symptoms of keratitis and gradually leading to perforation. Previous studies have also shown that the combination of wedelolactone and natamycin treatment significantly reduced the severity of the entire course of A. fumigatus keratitis[15]. Therefore, maintaining the balance between innate and adaptive immunity is the key to curing FK.
In recent years, it has been the focus of our laboratory to determine the signals and effectors of the innate immune response in the mouse FK model. However, the impact of innate and adaptive immunity on FK is still of great concern. Much information about the role of their links in the pathogenesis remains to be elucidated.
Dectin-1 and LOX-1, both belonging to pattern recognition receptors and expressed in multiple types of cells in keratitis, including corneal epithelial cells, macrophages, neutrophils, etc., mediate the activation of innate immunity to eliminate fungi[29]–[30]. Previous studies in our laboratory have found that the corneal epithelium expressed and up-regulated LOX-1 after A. fumigatus infection, and inhibiting LOX-1 reduced the infiltration of polymorphonuclear neutrophils (PMN) and monocytes, as well as IL-1β activation[31]–[33]. Dectin-1 which could recognize the β-glucan in fungal cell walls, was necessary for immunity against fungi, triggering phagocyte activation and activating cytokines including IL-1β, IL-6, CXCL 1, CCL 2 and others[34]–[36]. So far, it was known that the connection of fungal components with Dectin-1 triggered Th1 and Th17-mediated immune responses against fungi[6],[36]–[37]. Dectin-1 deficiency leaded to impaired T cell-mediated immune function and loss of control of fungal infections[38].
However, our experiments found that LOX-1 and Dectin-1 did not participate in the regulation of CD3ε expression in A. fumigatus keratitis. This may be due to the following reasons: In FK, the transmission of immune signals mediated by LOX-1 and Dectin-1 may be conducted by CD3γ, δ and ζ instead of CD3ε, which further activates the adaptive immunity of downstream T cells. These also need our follow-up experiments to verify.
IL-10 is a major anti-inflammatory cytokine produced by different types of immune cells, including neutrophils, macrophages, dendritic cell, B cells, and a variety of T cell subset[39]. Due to the importance of IL-10 in the immune response and its deficiency association with certain autoimmune, inflammatory diseases, and cancer[40]–[44], understanding the molecular and cellular mechanisms regulating this cytokine in FK is crucial. In this study, CD3ε was associated with the regulation of IL-10 in FK. Preprocessing suppressed CD3ε expression, thus inhibiting the activation of some T cells in the early stages. Due to the fact that the body primarily activated innate immunity in the early stage, the keratitis did not seem to have significantly worsened. However, as the course of keratitis entered its later stages, adaptive immunity began to play a greater role. T cells that mainly secrete IL-10 were repressed due to the suppression of CD3ε. The expression of IL-10 was reduced, unable to exert anti-inflammatory and tissue repair functions, leading to worsening of corneal ulcers in the later stage, swelling of the posterior elastic layer, and even corneal perforation. However, which types of immune T cells, activated by CD3ε, regulating IL-10 secretion still require our subsequent experiments to verify.
In summary, our study revealed that CD3ε, as an important marker of T cell activation, playing an anti-inflammatory and repair role by regulating IL-10 secretion, was involved in the adaptive immune stage of A. fumigatus keratitis in mice. However, LOX-1 and Dectin-1 did not participate in regulation of CD3ε expression in A. fumigatus keratitis. Maintaining the balance between innate immunity and adaptive immunity was the key to treating FK.
Footnotes
Foundations: Supported by the National Natural Science Foundation of China (No.82171019); the Natural Science Foundation of Shandong Province (No.ZR2021MH368); Traditional Chinese Medicine Research Project of Qingdao (No.2020-zyy055); Shandong Qingdao Outstanding Health Professional Development Fund.
Conflicts of Interest: Shi WH, None; Wang LM, None; Yan HJ, None; Liu SL, None; Yang X, None; Yang XJ, None; Che CY, None.
REFERENCES
- 1.Hoffman JJ, Arunga S, Mohamed Ahmed AHA, Hu VH, Burton MJ. Management of filamentous fungal keratitis: a pragmatic approach. J Fungi. 2022;8(10):1067. doi: 10.3390/jof8101067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown L, Kamwiziku G, Oladele RO, Burton MJ, Prajna NV, Leitman TM, Denning DW. The case for fungal keratitis to be accepted as a neglected tropical disease. J Fungi. 2022;8(10):1047. doi: 10.3390/jof8101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills B, Radhakrishnan N, Karthikeyan Rajapandian SG, Rameshkumar G, Lalitha P, Prajna NV. The role of fungi in fungal keratitis. Exp Eye Res. 2021;202:108372. doi: 10.1016/j.exer.2020.108372. [DOI] [PubMed] [Google Scholar]
- 4.Karthikeyan RS, Leal SM, Jr, Prajna NV, Dharmalingam K, Geiser DM, Pearlman E, Lalitha P. Expression of innate and adaptive immune mediators in human corneal tissue infected with Aspergillus or fusarium. J Infect Dis. 2011;204(6):942–950. doi: 10.1093/infdis/jir426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbondante S, Leal SM, Clark HL, Ratitong B, Sun Y, Ma LJ, Pearlman E. Immunity to pathogenic fungi in the eye. Semin Immunol. 2023;67:101753. doi: 10.1016/j.smim.2023.101753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z, Lu G, Meng GX. Pathogenic fungal infection in the lung. Front Immunol. 2019;10:1524. doi: 10.3389/fimmu.2019.01524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang RB, Wu LP, Lu XX, Zhang C, Liu H, Huang Y, Jia Z, Gao YC, Zhao SZ. Immunologic mechanism of fungal keratitis. Int J Ophthalmol. 2021;14(7):1100–1106. doi: 10.18240/ijo.2021.07.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng LL, Zhong J, Xiao YC, Wang BW, Li SQ, Deng YQ, He DL, Yuan J. Therapeutic effects of an anti-IL-6 antibody in fungal keratitis: Macrophage inhibition and T cell subset regulation. Int Immunopharmacol. 2020;85:106649. doi: 10.1016/j.intimp.2020.106649. [DOI] [PubMed] [Google Scholar]
- 9.Hu JZ, Hu YF, Chen SK, Dong CH, Zhang JJ, Li YL, Yang J, Han XL, Zhu XJ, Xu GX. Role of activated macrophages in experimental Fusarium solani keratitis. Exp Eye Res. 2014;129:57–65. doi: 10.1016/j.exer.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Gaud G, Achar S, Bourassa FXP, Davies J, Hatzihristidis T, Choi S, Kondo T, Gossa S, Lee J, Juneau P, Taylor N, Hinrichs CS, McGavern DB, François P, Altan-Bonnet G, Love PE. CD3ζ ITAMs enable ligand discrimination and antagonism by inhibiting TCR signaling in response to low-affinity peptides. Nat Immunol. 2023;24(12):2121–2134. doi: 10.1038/s41590-023-01663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang YB, Becker D, Vass T, White J, Marrack P, Kappler JW. A conserved CXXC motif in CD3ε is critical for T cell development and TCR signaling. PLoS Biol. 2009;7(12):e1000253. doi: 10.1371/journal.pbio.1000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zorn JA, Wheeler ML, Barnes RM, et al. Humanization of a strategic CD3 epitope enables evaluation of clinical T-cell engagers in a fully immunocompetent in vivo model. Sci Rep. 2022;12(1):3530. doi: 10.1038/s41598-022-06953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gil D, Schamel WW, Montoya M, Sánchez-Madrid F, Alarcón B. Recruitment of Nck by CD3 epsilon reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell. 2002;109(7):901–912. doi: 10.1016/s0092-8674(02)00799-7. [DOI] [PubMed] [Google Scholar]
- 14.Bettini ML, Guy C, Dash P, Vignali KM, Hamm DE, Dobbins J, Gagnon E, Thomas PG, Wucherpfennig KW, Vignali DA. Membrane association of the CD3ε signaling domain is required for optimal T cell development and function. J Immunol. 2014;193(1):258–267. doi: 10.4049/jimmunol.1400322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng M, Lin J, Li C, Zhao WY, Yang H, Lv LY, Che CY. Wedelolactone suppresses IL-1β maturation and neutrophil infiltration in Aspergillus fumigatus keratitis. Int Immunopharmacol. 2019;73:17–22. doi: 10.1016/j.intimp.2019.04.050. [DOI] [PubMed] [Google Scholar]
- 16.Wu TG, Wilhelmus KR, Mitchell BM. Experimental keratomycosis in a mouse model. Invest Ophthalmol Vis Sci. 2003;44(1):210–216. doi: 10.1167/iovs.02-0446. [DOI] [PubMed] [Google Scholar]
- 17.Wang YN, Chen HB, Wang FY, Dong YL. Six-year analysis of the pathogenic spectrum, risk factors, and prognosis of non-traumatic fungal keratitis in Northern China. Clin Exp Ophthalmol. 2024;52(1):111–113. doi: 10.1111/ceo.14314. [DOI] [PubMed] [Google Scholar]
- 18.Weiss K, Ardjomand N, El-Shabrawi Y. Mycotic infections of the eye. Wien Med Wochenschr. 2007;157(19-20):517–521. doi: 10.1007/s10354-007-0468-9. [DOI] [PubMed] [Google Scholar]
- 19.Niu LZ, Liu X, Ma ZM, Yin Y, Sun LX, Yang LF, Zheng YJ. Fungal keratitis: Pathogenesis, diagnosis and prevention. Microb Pathog. 2020;138:103802. doi: 10.1016/j.micpath.2019.103802. [DOI] [PubMed] [Google Scholar]
- 20.van der Donk LEH, Ates LS, van der Spek J, Tukker LM, Geijtenbeek TBH, van Heijst JWJ. Separate signaling events control TCR downregulation and T cell activation in primary human T cells. Immun Inflamm Dis. 2021;9(1):223–238. doi: 10.1002/iid3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alcover A, Alarcón B, Di Bartolo V. Cell biology of T cell receptor expression and regulation. Annu Rev Immunol. 2018;36:103–125. doi: 10.1146/annurev-immunol-042617-053429. [DOI] [PubMed] [Google Scholar]
- 22.Wucherpfennig KW, Gagnon E, Call MJ, Huseby ES, Call ME. Structural biology of the T-cell receptor: insights into receptor assembly, ligand recognition, and initiation of signaling. Cold Spring Harb Perspect Biol. 2010;2(4):a005140. doi: 10.1101/cshperspect.a005140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin YT, Sun ZS, Wang W, Xu JW, Wang BJ, Jia Z, Li X, Wang JY, Gao Q, Chen XH, Zou J. Characterization of CD3γ/δ+ cells in grass carp (Ctenopharyngodon idella) Dev Comp Immunol. 2021;114:103791. doi: 10.1016/j.dci.2020.103791. [DOI] [PubMed] [Google Scholar]
- 24.Guo Y, Karimi F, Fu Q, G Qiao G, Zhang H. Reduced administration frequency for the treatment of fungal keratitis: a sustained natamycin release from a micellar solution. Expert Opin Drug Deliv. 2020;17(3):407–421. doi: 10.1080/17425247.2020.1719995. [DOI] [PubMed] [Google Scholar]
- 25.Retamal J, Ordenes-Cavieres G, Grau-Diez A. Natamycin versus voriconazole for fungal keratitis. Medwave. 2018;18(8):e7388. doi: 10.5867/medwave.2018.08.7387. [DOI] [PubMed] [Google Scholar]
- 26.te Welscher YM, Jones L, van Leeuwen MR, Dijksterhuis J, de Kruijff B, Eitzen G, Breukink E. Natamycin inhibits vacuole fusion at the priming phase via a specific interaction with ergosterol. Antimicrob Agents Chemother. 2010;54(6):2618–2625. doi: 10.1128/AAC.01794-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai JJ, Yang CW, Wei QH, Lian HF, An L, Zhang R. Natamycin versus natamycin combined with voriconazole in the treatment of fungal keratitis. Pak J Med Sci. 2023;39(3):775–779. doi: 10.12669/pjms.39.3.6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu S, Liu XT, Liu XT, Shi Y, Jin X, Zhang N, Li XY, Zhang H. Wedelolactone ameliorates Pseudomonas aeruginosa-induced inflammation and corneal injury by suppressing caspase-4/5/11/GSDMD-mediated non-canonical pyroptosis. Exp Eye Res. 2021;211:108750. doi: 10.1016/j.exer.2021.108750. [DOI] [PubMed] [Google Scholar]
- 29.Dambuza IM, Brown GD. C-type lectins in immunity: recent developments. Curr Opin Immunol. 2015;32:21–27. doi: 10.1016/j.coi.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Che C, Li C, Lin J, Zhang J, Jiang N, Yuan K, Zhao G. Wnt5a contributes to dectin-1 and LOX-1 induced host inflammatory response signature in Aspergillus fumigatus keratitis. Cell Signal. 2018;52:103–111. doi: 10.1016/j.cellsig.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 31.Zhu GQ. Regulation of LOX-1 on adhesion molecules and neutrophil infiltration in mouse Aspergillus fumigatus keratitis. Int J Ophthalmol. 2020;13(6):870–878. doi: 10.18240/ijo.2020.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang JQ, Li C, Cui CX, Ma YN, Zhao GQ, Peng XD, Xu Q, Wang Q, Zhu GQ, Li CY. Inhibition of LOX-1 alleviates the proinflammatory effects of high-mobility group box 1 in Aspergillus fumigatus keratitis. Int J Ophthalmol. 2019;12(6):898–903. doi: 10.18240/ijo.2019.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W, Zou J. Recent progress in N6-methyladenosine modification in ocular surface diseases. Int J Ophthalmol. 2023;16(4):645–651. doi: 10.18240/ijo.2023.04.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang M, Jin X, Yang YF. β-Glucan from Saccharomyces cerevisiae induces SBD-1 production in ovine ruminal epithelial cells via the Dectin-1-Syk-NF-κB signaling pathway. Cell Signal. 2019;53:304–315. doi: 10.1016/j.cellsig.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 35.Jin Y, Li P, Wang F. β-glucans as potential immunoadjuvants: a review on the adjuvanticity, structure-activity relationship and receptor recognition properties. Vaccine. 2018;36(35):5235–5244. doi: 10.1016/j.vaccine.2018.07.038. [DOI] [PubMed] [Google Scholar]
- 36.Chen SM, Zou Z, Qiu XR, Hou WT, Zhang Y, Fang W, Chen YL, Wang YD, Jiang YY, Shen H, An MM. The critical role of Dectin-1 in host controlling systemic Candida krusei infection. Am J Transl Res. 2019;11(2):721–732. [PMC free article] [PubMed] [Google Scholar]
- 37.Martínez A, Bono C, Gozalbo D, Goodridge HS, Gil ML, Yáñez A. TLR2 and dectin-1 signaling in mouse hematopoietic stem and progenitor cells impacts the ability of the antigen presenting cells they produce to activate CD4 T cells. Cells. 2020;9(5):1317. doi: 10.3390/cells9051317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ren A, Li Z, Zhang X, Deng R, Ma Y. Inhibition of dectin-1 on dendritic cells prevents maturation and prolongs murine islet allograft survival. J Inflamm Res. 2021;14:63–73. doi: 10.2147/JIR.S287453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10(3):170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 40.Kubo M, Motomura Y. Transcriptional regulation of the anti-inflammatory cytokine IL-10 in acquired immune cells. Front Immunol. 2012;3:275. doi: 10.3389/fimmu.2012.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dibra D, Li S. The cell-to-cell coordination between activated T cells and CpG-stimulated macrophages synergistically induce elevated levels of IL-10 via NF-κB1, STAT3, and CD40/CD154. Cell Commun Signal. 2013;11:95. doi: 10.1186/1478-811X-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuang PP, Liu XQ, Li CG, He BX, Xie YC, Wu ZC, Li CL, Deng XH, Fu QL. Mesenchymal stem cells overexpressing interleukin-10 prevent allergic airway inflammation. Stem Cell Res Ther. 2023;14(1):369. doi: 10.1186/s13287-023-03602-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang M, Du GC, Fang JN, Wang LS, Guo QQ, Zhang TT, Li RG. UGT440A1 is associated with motility, reproduction, and pathogenicity of the plant-parasitic nematode Bursaphelenchus xylophilus. Front Plant Sci. 2022;13:862594. doi: 10.3389/fpls.2022.862594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu Q, Wang Q, Hu LT, Lin J, Jiang N, Peng XD, Li C, Zhao GQ. NADPH oxidase 2 plays a protective role in experimental Aspergillus fumigatus keratitis in mice through killing fungi and limiting the degree of inflammation. Int J Ophthalmol. 2022;15(7):1044–1052. doi: 10.18240/ijo.2022.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]