Abstract
Glaucoma is a common and complex neurodegenerative disease characterized by progressive loss of retinal ganglion cells (RGCs) and axons. Currently, there is no effective method to address the cause of RGCs degeneration. However, studies on neuroprotective strategies for optic neuropathy have increased in recent years. Cell replacement and neuroprotection are major strategies for treating glaucoma and optic neuropathy. Regenerative medicine research into the repair of optic nerve damage using stem cells has received considerable attention. Stem cells possess the potential for multidirectional differentiation abilities and are capable of producing RGC-friendly microenvironments through paracrine effects. This article reviews a thorough researches of recent advances and approaches in stem cell repair of optic nerve injury, raising the controversies and unresolved issues surrounding the future of stem cells.
Keywords: stem cell, glaucoma, retinal ganglion cell, optic nerve, axon regeneration
INTRODUCTION
Glaucoma is a progressive neurodegenerative condition that can lead to irreversible blindness[1]–[2]. Retinal ganglion cells (RGCs) are neurons that project long axons to the central nervous system, transmitting visual signals from the retina to the brain[3]. It is characterized by changes in the structure of the optic nerve papilla and the progressive loss of RGCs[4]–[5]. Currently, 64.3 million people worldwide aged 40-80y suffer from glaucoma, making it the leading cause of irreversible blindness globally. By 2040, this number may increase to 111.8 million[4].
The mechanisms of optic nerve damage include neurotrophic factor (NTF) deprivation, glial activation, excitotoxicity, tumor necrosis factor (TNF) release, immune system dysregulation, and oxidative stress[6]–[7]. A sudden damage, such as an increase in the intraocular pressure (IOP), could disrupt the homeostasis and induce the resident neuronal glia to adopt a reactive, pro-inflammatory, degenerative state[8]. IOP is the greatest risk factor for oxidative stress and reactive neuroglial changes in axonal trans injury. Current treatments for glaucoma largely aim to lower IOP through pharmacological, surgical, or laser-based approaches[4]. The main focus of current treatments is to reduce IOP[9]. However, it seems that factors other than elevated IOP may contribute to the development and advancement of this degenerative condition[10]. Glia cells, as well as RGCs, respond to microenvironmental changes and can induce axonal damage during repair and remodeling. The activation of glial cells in response to injury is regulated by protein phosphorylation[11].
Stem cells are used in multiple pathways to achieve RGCs protection, such as paracrine function working in multiple pathways to protect the RGCs and preserve its function[7]. However, the sequence of events in axonal degeneration differs from that of somatic tissues, and therefore, strategies to protect axons may differ from those used to protect the cytosol. Neuroprotective approaches aimed at protecting the neuronal cell body may not be sufficient to improve function if the axon remains damaged and disconnected from its central nervous system targets[6]. Stem cell research has not yet resolved the complex afferent retinal connections and long axons of RGCs for precise projection to the brain[12]. Several neuroprotective strategies have been studied, such as peripheral nerve grafts, electrical stimulation, and the application of NTFs, including brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), glial cell-derived neurotrophic factor (GDNF), and nerve growth factor (NGF). Additionally, direct endogenous regenerative stimulation, RNA interference, and human adult stem cells have also been investigated[12]. Stem cell-based therapies can aid in retinal regeneration by addressing two primary issues: 1) preventing secondary degeneration of RGCs and preserving residual vision, 2) replacing degenerated RGCs and promoting regeneration of RGC axons in the damaged area. The initial approach, known as neuroprotective therapy, involves incorporating stem cells into the deteriorating retina to create a nourishing environment for damaged RGCs. This can result in both anatomical and functional improvements. The second approach, known as RGCs replacement therapy, aims to replace damaged RGCs with healthy RGCs or RGC precursors[13].
However, the sequence of events in axonal degeneration differs from that of somatic tissues, and therefore, strategies for protecting axons may differ from those used to protect the cytosol. Neuroprotective methods alone may not suffice to improve function if the axon remains damaged and disconnected from its central nervous system (CNS) targets, despite the protection of the neuronal cell body. It is important to note that different RGC subtypes exhibit varying axonal regenerative capacities[6]. Axonal regeneration is a complex process that involves many intrinsic factors and extrinsic environments, such as signaling pathways, epigenetic modifications, and inflammatory stimuli. It is suggested that no single factor is sufficient to promote regeneration. Identifying additional factors that regulate axonal regeneration will facilitate a better understanding of the underlying mechanisms. Therefore, many questions still need to be answered regarding the primary goal of axonal regeneration, which is to restore functional visual ability. Although regenerated RGC axons may be present at the target site in the brain, it does not necessarily imply the restoration of visual function[2].
CURRENT RESEARCH STATUS ON OPTIC NERVE INJURY
Damage to RGCs is linked to several optic nerve dysfunctions, including glaucoma, traumatic optic neuropathy (TON), and ischemic optic neuropathy. These diseases result in extracellular risk factors and intracellular death signals, leading to retrograde neuronal degeneration[14]. Recent studies on optic nerve injury have accumulated, and Liu et al[15] have highlighted the importance of phosphorylation-mediated cellular signaling in the growth of neurons and axon guidance in RGCs. According to Yuan et al[2], the regeneration of axons in RGC does not necessarily lead to the restoration of visual function. Currently, axonal regeneration experiments are conducted on rodents or cultured cells, and it is yet to be determined whether these manipulations are effective in primates and humans. Osborne et al[5] investigated the sustained enhancement of BDNF production by a new adeno-associated virus (AAV) gene therapy (AAV2 TrkB-2A-mBDNF), and the sustained survival signaling within RGCs by increased expression of the BDNF receptor (TrkB) in the inner layers of the retina, which was significantly elevated over a 6-month period. Transplantation of RGCs derived from male germline stem cells could be a potential treatment for glaucoma. Intravitreal injection of spermatogonial stem cell (SSC)-derived RGCs showed close proximity to host RGCs 10d after transplantation[16]. Inhibition of germinal cell kinase IV (GCK-IV) prevents CNS cell death and promotes axonal regeneration in CNS neurons[17]. The chemokine C-X3-C motif receptor 1 (CX3CR1) regulates microglia activation in hypertension. Thus, inhibition of microglia activation (paradoxically) appears to be a potential treatment to slow glaucoma progression and improve RGCs survival[10]. Park et al[18] proposed that both Wnt/β-catenin signaling and increased nuclear factor kappa-B (NF-κB) could rescue damaged RGCs through upregulation of neuroprotective factors, microglia engagement and anti-inflammatory regulation of human pluripotent stem cell-derived neural progenitor cells (NPCs). This suggests that human pluripotent stem cell-derived NPCs can be used in cell therapy for a variety of optic neuropathies, as well as cell therapy using mesenchymal stem cells (MSCs). The mechanism by which Wnt signaling promotes axon regeneration may include the induction of these axon growth-promoting genes. Zhang et al[1] found that intervening in RGCs using the transcription factors OCT4, SOX2 and KLF4 significantly promoted rejuvenation of senescent RGC phenotype and axon regeneration. Nascimento-dos-Santos et al[19] concluded that coupling of isolated mitochondrial organelles was critical for the mid-term effects on axons 28d after optic nerve extrusion. RGC-selective expression of Sirtuin 1 (SIRT1) provides a targeted therapy for animal models with significant ganglion cell loss. Overexpression of SIRT1 through AAV-mediated gene transduction suggests an RGC-selective component of neuroprotection using the optic nerve crush model[20].
The history of stem cell research has gone through the process of focusing on the differentiation of stem cells into neuronal cells to the role of paracrine effects, and nowadays the research on exosomes is very hot and has gained the recognition of others. In recent years, there has been a growing emphasis on the importance of functional analysis in cell transplantation therapy. The ultimate goal of this therapy is to restore function, rather than solely focusing on the morphological appearance of the tissue[21]. Recent studies indicate that stem cell-based therapy can restore visual function by replacing damaged RGCs through cell transplantation, providing trophic factors to damaged RGCs, and supplying healthy mitochondria and other cellular components to exert neuroprotection and mediate transdifferentiating of autologous retinal stem cells to achieve endogenous regeneration of RGCs. It is important to note that this is still an emerging field and further research is needed to fully understand the potential of this therapy[1]. Do et al[22] carried out a study targeting the survival and regeneration of RGCs. Human neural stem cells (NSCs) promote the modest survival and axonal regeneration of axotomized RGCs, which is partly mediated by diffusible NSC-derived factors mediated. In addition, NSCs integrate with damaged optic nerves and have the potential to form neuronal relays to restore off-retinal connections. MSC may improve mitochondrial function and attenuate oxidative damage by inhibiting reactive oxygen species (ROS) production and enhancing mitochondrial dynamics. Hypoxic preconditioning appears to enhance the therapeutic effects of MSC in neurological disorders and traumatic injuries[23]. Platelet-derived growth factor (PDGF) secretion may play an important role in MSC-mediated neuroprotection of RGCs, and may be an independent target for achieving neuroprotection of RGCs. Among the many factors secreted by MSC that appear to promote RGC survival, members of the PDGF family confer particularly strong neuroprotective effects both in vitro and in vivo, which appear to be dependent on phosphatidylinositide 3-kinases (PI3K) signaling. Future work will evaluate the translational potential of local MSC transplantation for glaucoma treatment, while targeting the PDGF signaling pathway through stem cell-independent approaches (e.g., through drug development)[24]. Cen et al[25] identified a novel mechanism in human adult stem cells whereby damaged retinas augmented BDNF secretion from human periodontal ligament-derived stem cells. The question of how this positive feedback stimulated by damage to the host retina enhances BDNF secretion from human periodontal ligament-derived stem cells and what factors and retinal cell types are involved in this stimulation needs to be further investigated. In addition, human periodontal ligament-derived stem cells transplantation induces mild inflammation in rats, and it is reported that inflammation promotes nerve survival and axonal regeneration. Whether xenograft-induced mild inflammation contributes to increased RGC survival and axonal regeneration remains to be elucidated. Cui et al[14] found that MSC-derived exosomes (MSC-exos) administration ameliorated apoptosis in RGCs by promoting Bcl-2/Bax ratio expression and inhibiting caspase-3 activation. Intravitreal injection of MSC-exos attenuates apoptosis and inflammatory response of RGCs in optic nerve crush rats through the PI3K/AKT pathway. No signs of immunogenicity, abnormal proliferation, or other significant complications were detected in eyes treated with exosomes. Reboussin et al[26] showed that in an ex vivo axotomy model leading to rapid RGC degeneration and neurogliosis induced by optic nerve transection and disruption of axonal transport allowed the study of neuroprotective or anti-inflammatory therapeutic compounds or stem cell transplantation therapies in human or rodent tissues. Current clinical trials of bone marrow-derived MSC (BMSC) have raised safety concerns that require further research. Additional experimental models that mimic glaucomatous retinal degeneration are needed.
Further approaches of combinations of different factors are going to be necessitated to develop effective future therapeutic strategies to promote eventual axonal and RGC regeneration and functional vision recovery after injury[2]. Bioengineering as a basis for the development of optimal cell culture biomaterials[27]. Nascimento-dos-Santos et al[6] highlighted the results of the study the synergistic effects of combined gene and cell therapy, relevant for future therapeutic interventions in optic nerve injury. Behtaj et al[28] found that stem cell RGC replacement therapy that can be supported by biomaterial scaffolds, poly(glycerol sebacate)/poly(ε-caprolactone) (PGS/PCL) scaffolds may promote the differentiation of human embryonic stem cells (ESCs) into RGC-like cells by mimicking the cellular active environment signals and promote the growth of RGCs neural synapses along their length. RGCs were generated from human induced pluripotent stem cells (iPSCs)-3D neural retinas. These RGCs were then seeded on biodegradable poly(lactic-co-glycolic acid) (PLGA) scaffolds to form engineered RGC scaffold biomaterials. The results indicate that the aligned PGS/PCL scaffolds promote the differentiation of hESCs into RGC-like cells by mimicking the cell survival environmental signals and promote the growth of RGC neurons along their length[1]. Kwon et al[29] proposed that hypoxia-preconditioned strain was more dependent on vascular endothelial growth factor (VEGF) than human placenta-derived stem cells (hPSCs) in hypoxia-injured R28 cells and rodent optic nerve compression model, emphasizing the role of vascular endothelial growth factor (VEGF) as a mediator. The study also demonstrated the potential for advanced cellular therapies using human placenta-derived stem cells (hPSCs) to rescue the optic nerve from injury. The emergence of 3D printing has enabled the creation of customized micron and nano-grooves in innovative scaffolds, which could aid in guiding the direction of axonal growth. These studies indicate that combining grafts with a nerve regeneration approach could offer therapeutic benefits in optic neuropathy[30]. Liu et al[31] propose that a significant challenge in stem cell transplantation is ascertaining whether transplanted neuronal cells can integrate into the host cell nuclear layer and establish appropriate synapses with targeted interneural cells, including bipolar, amacrine, and horizontal synapses. Additionally, the authors address the issue of how these cells can aid the survival and proper functioning of the transplanted cells. In recent years, a technique involving hypoxia or hypoxia preconditioning has been used to protect neurons. The advanced cell therapy approach involves the combination of stem cells and a hypoxia preconditioning bacterial strain to enhance stem cell functionality[29]. Intravitreal transplantation of human periodontal ligament-derived stem cells ameliorated RGC degeneration after optic nerve injury in rats and enhanced axonal regeneration through cell-cell interactions and NTFs secreted by human periodontal ligament-derived stem cells to promote nerve repair[25]. One strategy for slowing down degenerative processes involves virus-based gene therapy to express trophic factors that provide growth and survival signals. This approach has been shown to be effective for many trophic factors in animal models, including CNTF, pigment epithelium-derived factor (PEDF), BDNF, and optic rod cell-derived optic cone cell viability factor. Future alternatives could use cell mixtures for delivering or genetically modifying donor RGCs to provide sustained paracrine or autocrine delivery of NTFs[16]. Significant progress has been made since this goal was set a decade ago. It is likely that multiple technologies for restoring vision will be clinically applied within the next decade (Table 1)[6],[32]–[49].
Table 1. Recent findings on mesenchymal stem cells in optic nerve injury within the last 10y.
| Type of stem cell | Mechanism | Significance/findings |
| hWJ-MSCs[33] | Axon-target reconnection through glutamatergic synapses | For the first time have shown a long-term effect of hWJ-MSCs |
| hMSCs[6] | Overexpression of PEDF by intravitreal delivery of AAV2 vector | Combined PEDF gene therapy and hMSCs therapy |
| hUC-MSCs[34] | Increased the expression of BDNF and GDNF | Continuous secretion to protect optic nerve |
| BMSCs[35] | Increase the expression of FGF-2 and IL-1b | Labeled with a fluorescent dye or with superparamagnetic iron oxide nanoparticles |
| BMSCs[36] | Produce and secrete a battery of both neurotrophic factors and anti-inflammatory cytokines | A robust neuroprotective effect in a model, intravenous MSC delivery is unlikely to prove efficacious as a novel neuroprotective therapy in glaucoma |
| BMSCs[37] | Increase GAP-43 expression | The function of transplanted BMSCs may involve the regulation of immune system-related inflammation in the injured tissues |
| hUC-MSCs[38] | The potential anti-inflammatory effects of hUC-MSCs transplantation | Differentiation into glial tissues and mature astrocytes |
| UMSCs and UMSC-exos[39] | Promoted Brn3a+ RGCs survival and GFAP+ glia cells activation in retina | UMSC-exos may play a role in neuroprotection by promoting the RGCs survival and glia cells activation but not the axon regeneration |
| BMSCs[40] | The GAP-43 expressed vectors were transfected into the BMSCs and activated of MAPK pathway | GAP-43 could induce the differentiation of BMSCs into RGCs |
| DPSCs and BMSCs[41] | NGF, BDNF, and NT-3; neuritogenesis/axogenesis of bIII-tubulinþ retinal cells in vitro | Both DPSCs and BMSCs secreted NGF, BDNF, and NT-3, with DPSCs secreting significantly higher titers of NGF and BDNF than BMSCs |
| BMSCs[35] | FGF-2 and IL-1b participate in the trophic and immunomodulatory effect | Upregulation of trophic factors, such as FGF-2 |
| hUC-MSC[42] | Secrete anti-inflammatory molecules and trophic factors | Infiltration of Iba1+ cells from the choroid to the retina that alters the retinal structure |
| ADSCs[43] | Stem cell transplantation was able to resist the apoptosis of retinal cells | Demonstrated the positive effects of transplanted ADSCs on RGCs survival |
| Rat MSCs[44] | Secrete soluble factors or vesicles containing a variety of molecules, ranging from miRNA to trophic factors, that can be internalized by host cells | Rat MSCs-induced neuroprotection is sustained at longer time points, but the reconnection of RGCs with their targets was transitory |
| hWJ-MSCs[33] | hWJ-MSCs stimulated the neuroprotective and pro-regenerative of RGC axons | Human-derived MSCs promoted sustained neuroprotection and regeneration of RGCs after optic nerve injury |
| hUC-MSCs[45] | UTMD enhanced target-specific gene delivery, thus improving the effect of therapy | UTMD can remarkably enhance the therapeutic effect of hUC-MSCs |
| hPMSCs[46] | UBA2 played a key role in activating the Wnt/β-catenin signaling pathway | The first report on the therapeutic benefit that hPMSC-exos offer to protect retinal precursor cells after hypoxic injury |
| Rat BMSCs[47] | Decreased apoptotic rate and caspase-3 activity of RGCs | Exert a neuroprotective effect against hypoxia-induced apoptosis of RGCs, and cells from female rats have greater neuroprotective ability compared with males |
| hPMSCs[48] | Activated the pathway of NF-κB | Transactivation of NF-κB protein has a critical role in recovery mechanism by hPMSCs |
| hPDLSCs[49] | Direct cell-cell interaction and elevated brain-derived neurotrophic factor secretion | hPDLSCs strongly promoted RGCs survival and axonal regeneration both in vivo and in vitro |
MSCs: Mesenchymal stem cells; hWJ-MSCs: Human umbilical Wharton's jelly-derived mesenchymal stem cells; hMSCs: Human mesenchymal stem cells; hUC-MSCs: Human umbilical cord mesenchymal stem cells; BMSCs: Bone marrow-derived mesenchymal stem cells; UMSCs: Umbilical cord mesenchymal stem cells; UMSC-exos: UMSC-derived exosomes; hPMSCs: Human placenta derived mesenchymal stem cells; DPSCs: Dental pulp stem cells; ADSCs: Adipose-derived stem cells; hPDLSCs: Human periodontal ligament-derived stem cells; AAV: Adeno-associated virus; PEDF: Potential of pigment epithelium-derived factor; BDNF: Brain-derived neurotrophic factor; GDNF: Glial cell line-derived neurotrophic factor; FGF: Fibroblast growth factor; IL: Interleukin; GAP: Growth associated protein; RGCs: Retina ganglion cells; GFAP: Glial fibrillary acidic protein; NGF: Nerve growth factor; NT-3: Neurotrophin-3; UTMD: Ultrasound targeted microbubble destruction; UBA2: Ubiquitin like modifier activating enzyme 2; NF-κB: Nuclear factor kappa-B.
MECHANISMS OF STEM CELL THERAPY
The mechanism of stem cell repair of glaucomatous optic nerve damage is mainly due to their paracrine capacity, differentiation capacity, and immunomodulatory effects (Figure 1).
Figure 1. Mechanism of stem cells in repair endometrial damage.
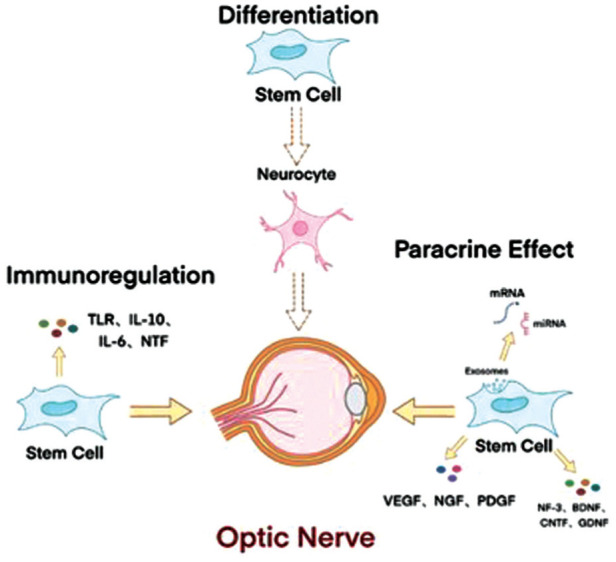
The potential ability to transdifferentiate into neurocytes is indicated by dashed arrows, as transdifferentiation is a controversial process in vivo.
Paracrine Effects of Mesenchymal Stem Cells
The beneficial effects of MSCs are primarily mediated by the secretion of paracrine signaling factors or cytokines[14]. MSCs produce a variety of cytokines and angiogenic factors that are released either directly in soluble form or in extracellular vesicles and exosomes[50]. They rescue neurons and oligodendrocytes from apoptosis by releasing trophic and anti-apoptotic molecules. Additionally, they have anti-inflammatory and proliferative effects on microglia and astrocytes, creating an induced neuroprotective microenvironment. Stem cells are capable of direct intercellular communication through gap junctions and synapses, as well as providing paracrine signaling and trophic support via secreted proteins and extracellular vesicles. They can be easily manipulated in vitro or in vivo to enhance the expression of NTFs[51] (Figure 1).
Immunomodulation
The effect of transplantation cells may be linked to the ability of MSC inflammatory mediators to modify the microenvironment of damaged tissues. After administration in vivo, MSCs induce peripheral tolerance, migrate to injured tissues, inhibit the release of pro-inflammatory cytokines, and promote the survival of damaged cells. MSCs can reduce the potential for an inflammatory response by inhibiting the production of TNF22[52]. MSCs possess the ability to migrate to the site of injury and promote wound healing and tissue regeneration. They also play a beneficial regulatory role by releasing immunomodulators and chemokines to recruit immune cells. Additionally, MSCs suppress immune responses by regulating the proliferation and function of innate and acquired immune cells, such as BMSCs[6],[53]. MSCs spontaneously produce transforming growth factor (TGF)-β, a potent negative regulator of the immune response, which indirectly contributes to MSCs-mediated immunosuppression. Additionally, MSCs have an anti-inflammatory effect through the secretion of cytokines, including interleukin (IL)-10, which promotes neurogenesis and prevents neurodegeneration. BMSCs also secrete IL-6 and monocyte chemotactic protein (MCP)-1, which contribute to promoting neurogenesis and preventing neurodegeneration[2],[50],[54]–[55]. MSCs have potent effects on immune cells. They provide trophic support to damaged retinal cells directly through secretion of NTF and potentially indirectly through the stimulation of endogenous neighborhood-resident retinal cells to enhance the survival of RGCs[56]. MSCs enhance the survival of RGCs through neuroprotective and neurogenic cytokines, promoting the improvement of damaged RGCs and reducing inflammation with the help of their anti-inflammatory and immunomodulatory properties[57]. Although inflammation can hinder axonal growth, it can also provide important signals that activate repair and regeneration pathways in RGCs[30]. Furthermore, MSC-exos have the ability to modulate the inflammatory state in retinal tissues by reducing the levels of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, IL-8, and MCP-1, while increasing the expression of the anti-inflammatory factor interleukin-10[10],[14],[54],[58].
Neurotrophic Factors
MSCs produce a range of growth factors, cytokines, chemokines, and proteases. One of the primary hypotheses for RGCs degeneration is the lack of NTFs that support neuronal survival, induce endogenous cell proliferation, and promote nerve fiber regeneration at the injury site. Modulating NTF delivery to promote donor RGCs survival may be an alternative or complementary approach to directly targeting cell-intrinsic death signaling cascades[16]. Transplanted stem cells have the potential to target multiple pro-survival pathways simultaneously through local delivery of secreted NTFs and regulation of the intraocular microenvironment. Additionally, neurotrophic and growth factors produced by stem cells may have synergistic and regulatory effects on stem cell behavior through paracrine activity. During tissue repair and regeneration, the regulation of growth factors is necessary. The main neuroprotective agents for RGCs are basic fibroblast growth factor (bFGF)[59], VEGF[60], and NGF[24]. For instance, BMSC can secrete cell growth factors, such as PDGF and TGF-β, which act on the recipient's cells and tissues. Neurotrophic factor (NT-3)[61] and CNTF[2] are potent survival factors for RGCs. BDNF is crucial for the neuroprotection of RGCs. Stem cells that are transplanted intravitreally express BDNF, which induces axonal growth. Additionally, they express GDNF, which promotes the survival of RGCs after optic nerve injury[4],[62]. Stem cells that have been transduced by NTFs secrete higher levels of these factors compared to normal stem cells[63]. Additionally, stem cells can be engineered to express specific genes or trophic factors that promote RGCs survival and axonal ontogeny. To improve transplantation efficiency, cell encapsulation and transplantation are promising alternative methods for NTF delivery[28]. MSCs treatment was found to increase FGF-2 expression in the RGC layer, indicating potential beneficial outcomes mediated by trophic factors. Growth associated protein-43 (GAP-43) is a protein expressed exclusively by neurons during axon growth. Changes in the phosphorylation state of GAP-43 are a crucial factor in promoting axon growth. Its expression can be used to assess neuronal growth and survival[16],[40]. Under normal physiological conditions, brain-derived BDNF supports RGCs survival and synaptogenesis through retrograde transport in the optic nerve. While systemically administered BDNF cannot cross the blood-brain barrier, intravitreal delivery of purified proteins or AAV-mediated overexpression can enhance RGCs survival in many optic nerve injury models. Transplantation of genetically engineered MSCs that oversecrete BDNF can protect RGCs in eyes with high IOP by taking advantage of this neurotrophic effect. Additionally, BDNF stimulates the release of GDNF from Müller cells and microglial cells[16].
Exosomes of Mesenchymal Stem Cell
Recently, there has been increasing attention on the role of exosomes secreted by MSCs in MSC-mediated neuroprotection. Exosomes are extracellular vesicles that have emerged as a valuable paracrine factor. They are composed of micro vesicles and apoptotic vesicles and are named exosomes or microvesicles depending on their size[61]. Endocytosed structures consist of proteins, lipids, mRNAs and miRNAs[7],[64], have a narrow diameter of about 100 nm (range 30-150 nm), are rich in cholesterol, sphingomyelin, ceramides and raft proteins, and regulate protein translation in target cells when delivered by cytosis or membrane fusion. Exosomes transport their contents to the inner layers of the retina, where the mRNA is then translated by recipient cells, while the miRNA regulates gene expression, a process that is critical for proper retinal function[1],[65]–[68]. Upon release into recipient cells, miRNAs regulate gene expression post-transcriptionally by base pairing with the 3′-untranslated regions (UTR)[69]. Exosomes contain a variety of cytokines and provide intercellular communication, affecting the microenvironment of stem cells and forming a unique ecological environment[60]. It has been shown that MSC-exos can elicit significant physiological and pathological cellular responses. These responses include reducing immune cell infiltration, attenuating retinal cell apoptosis, decreasing the expression of inflammatory mediators, promoting post-implantation RGCs survival, providing neuroprotection, aiding in tissue repair, and exhibiting immunomodulatory properties[4],[69]. The intravitreal injection of MSC-exos reduced RGC apoptosis and inflammatory responses through the PI3K/AKT pathway. Specifically, MSC-exos was able to ameliorate RGC apoptosis by promoting the expression of Bcl-2/Bax ratio and inhibiting caspase-3 activation[1],[14]. Because of their nano-size, MSC-exos can rapidly penetrate biological barriers and reach immune-privileged organs, allowing efficient delivery of therapeutic factors such as trophic agents and immunomodulators to ocular tissues that are often difficult to target with conventional therapies and MSC transplants. The use of exosomes as an alternative to MSC-based therapies may help to avoid potential risks such as allogeneic immune rejection, unwanted differentiation and small blood vessel obstruction from intravitreal MSC injections, which are critical to optimizing treatment outcomes. The use of extracellular vesicles minimizes the risks associated with MSC transplantation[69]. Exosomes derived from BMSC significantly promoted the survival and axonal regeneration of RGCs, while partially preventing axonal loss and dysfunction[65]. A growing body of evidence supports the emerging view that MSC-mediated therapeutic effects are created by their potent immunomodulatory/anti-inflammatory functions and pleiotropic properties[70].
Other Mechanisms
Stem cells can be induced in vitro and in vivo to differentiate in culture into non-mesenchymal derivatives, such as neural cells, a process known as stem cell plasticity. MSCs promote the proliferation and maturation of local neural precursor cells, allowing them to differentiate into mature neurons and oligodendrocytes[54]. However, it is partly argued that no evidence of neural transdifferentiating of MSCs has been shown. It has been proposed that bFGF-induced ESCs are able to differentiate and then generate RGC-like cells, which are able to integrate into the host retina[2]. Stem cells can also be used to support neuromodulation, remove toxins, alter the extracellular matrix, and promote vascular interactions[51]. The beneficial effects of MSCs can also be attributed to the membrane protein CD73[53].
EXPERIMENTAL METHODS ON GLAUCOMATOUS OPTIC NERVE INJURY WITH STEM CELLS
Glaucoma Models
Current glaucoma optic nerve models are categorized into acute and chronic, incorporating high IOP paradigms. Chronic IOP can be induced by laser-induced trabecular meshwork or extra scleral vein occlusion[24]. Injecting microbeads into the anterior chamber can lead to obstruction and increase IOP in the trabecular meshwork, resulting in RGC and its axonal degeneration[8]. The optic nerve was clamped with a forceps pressed on the optic nerve for 15s[33]. The temporal eyelid incision was made to expose the infraorbital optic nerve, which was clamped with a special clamp with a 40-g force and sutured with continuous pressure for 30s[34]. The optic nerve was then closed. However, the microenvironment suitable for survival, differentiation, and integration of transplanted stem cells varies with acute optic nerve injury and chronic neurodegenerative disease.
Stem Cell Types and Application Methods
Characteristics of stem cells
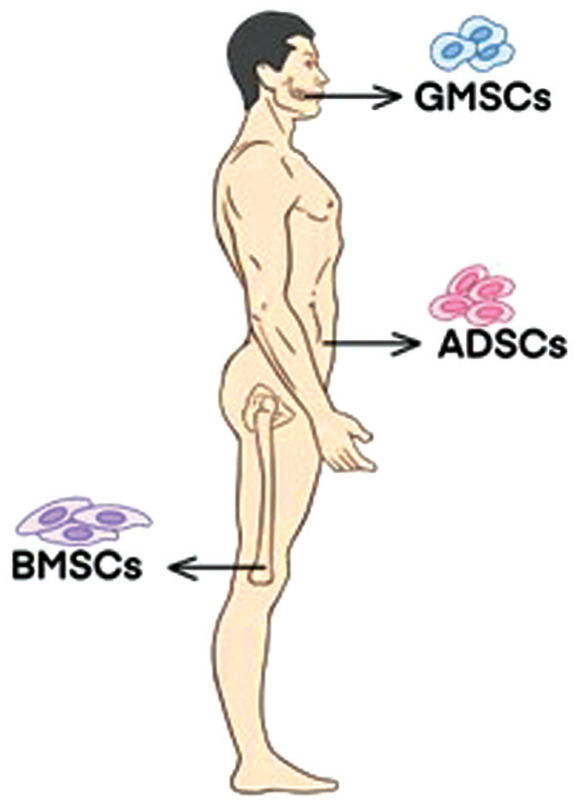
It is worth noting that stem cells can be sourced from various sources, including ESCs and iPSCs, as well as adult stem cells. In the field of ophthalmology, ESCs, limbal corneal stem cells (LSCs), and MSCs have been used as part of regenerative medicine for cell therapy. Stem cells are a class of cells with a high capacity for self-renewal and multidirectional proliferation and differentiation (Figure 2). Human umbilical cord blood stem cells (hUCBSC) are currently the preferred stem cell type for optic nerve injury due to their low inflammatory response, lack of graft rejection, and minimal post-transplantation reactions[38], periodontal ligament-derived stem cells[34], adipose-derived mesenchymal stem cells (ADSCs), and human placental mesenchymal stem cells (hPMSCs) have been shown to have potential for clinical treatment of optic nerve injuries and promotion of RGC survival[41],[71]. MSCs have been extensively studied in animal models of neurodegeneration due to their biological properties[13]. They have the potential for cell expansion, immunomodulation, and anti-inflammatory effects. MSCs are easy to isolate and grow rapidly after a short period of dormancy. They comply with ethical standards and have a low predisposition to tumor formation[52]. MSCs are a type of stromal stem cell that have been successfully isolated from various sources, including bone marrow, adipose tissue, umbilical cord blood, amniotic membrane, and dental pulp. They are known for their heterogeneity and have shown promising results in various studies[61],[72]. For instance, Gingival mesenchymal stem cells (GMSCs) are highly proliferative and tend to differentiate into neural cell lineages due to their neural crest origin[70]. MSCs are ideal for transplantation due to their strong immunosuppressive properties, which inhibit the release of pro-inflammatory cytokines. This allows for both autologous and allogeneic transplantation without the need for pharmacological immunosuppression. Moreover, MSCs can be transplanted directly without genetic modification or pretreatment and can migrate to sites of tissue injury without the risk of teratoma formation after transplantation. It is important to note that MSCs can be used for neuroprotection or can be induced into neuronal cells for alternative therapies[25]. These biological properties and expansion potential make MSCs a promising therapeutic option for the treatment of various human diseases, particularly degenerative diseases of the RGC[57]. Several MSCs derived from somatic cells have significant neuroprotective and axon-forming effects on RGCs. When using stem cells or organoids as a source of donor RGCs, it is important to maximize purity during the isolation process to limit abnormal tissue overgrowth or teratoma formation from undifferentiated cell populations[16].
Figure 2. The black arrows in the figure indicate the species that are more commonly used in MSC experiments: gingival mesenchymal stem cells (GMSCs); adipose-derived stem cells (ADSCs); bone marrow derived mesenchymal stem cells (BMSCs).
Application Methods of Stem Cells
Regenerative medicine based on stem cell repair
In addition to stem cell therapy used independently for the treatment of optic nerve damage in glaucoma, the synergistic effects of combined gene and cell therapy have been demonstrated to be beneficial for future interventions in the treatment of optic nerve injuries over the past two decades[73]. In addition to their potential for direct cellular therapy, MSCs are also utilized as vectors for gene therapy. The combination of gene and cell therapies has been shown to have synergistic effects, making it a promising intervention for optic nerve damage. In gene therapy, they are commonly employed in conjunction with techniques such as adenoviral gene editing and molecular transfection to enhance the efficiency of their binding with stem cells. Cell-free therapy involves the injection of stem cell-derived extracellular vesicles into the vitreous cavity for repairing optic nerve injuries. Gene therapy utilizes artificially modified AAV vectors to insert target genes into specific cells, replacing mutated genes and restoring normal cellular functions[74]. With breakthroughs and combined applications of single-cell genomics, gene editing, tissue engineering, nanotechnology, and cell-free therapy, the utilization of stem cells for protection, regeneration, and replacement of damaged RGCs will play a crucial role in restoring vision for glaucoma patients[1].
Transplantation methods
In general, stem cell transplantation is mainly divided into systemic administration and local administration[33]. Most experiments have used local administration, mainly utilizing the paracrine effects of MSCs and the regulatory effects of host cells to repair optic nerve damage. Stem cells injected intravenously are mostly isolated in the lungs and rarely enter the ocular circulation via the systemic route[75]. The eye is an ideal target for stem cell transplantation due to its immune-privileged environment and non-invasive post-transplantation monitoring. The eye is an ideal target for stem cell transplantation due to its immune-privileged environment and non-invasive post-transplantation monitoring. Stem cells can be delivered to this restricted environment, which requires only a small number of stem cells. This makes the eye an excellent candidate for transplantation. The eye is an ideal target for stem cell transplantation due to its immune-privileged environment and non-invasive post-transplantation monitoring[31].
MSCs labeled with superparamagnetic iron oxide nanoparticles (SPION) were transplanted in vivo and imaged using magnetic resonance imaging (MRI)[4],[66]. In experimental studies, various injections have been used to target the extracellular matrix of the eye, including intravitreal injections, subretinal injections, subfascial injections, intravenous injections, peripapillary space injections[38], and intrathecal injections in the peripapillary space[18]. Among these, intravitreal injection, a minimally invasive surgical technique, has been widely used. Intravitreal injection of MSCs inhibits apoptosis of photoreceptor cells and attenuates degeneration of retinal morphology and function[69]. However, achieving adequate cellular localization and integration at the target site using this approach may pose challenges. Transplanting cells into the vitreous cavity provides direct access to the inner retina, but it also presents challenges such as cell dispersion in a large three-dimensional space, lack of isolation from neighboring retinal tissues, and the need to bypass physical barriers in the subretinal space[16]. Johnson et al[56] demonstrated that glial cell reactivity is the primary barrier to retinal integration of intravitreal transplanted stem cells, rather than the physical barrier contained within the inner limiting membrane (ILM). Both subconjunctival and retrobulbar injections are periocular routes that can be used to deliver the molecule to the posterior segment of the eye and avoid the adverse effects of intravitreal injections[33]. Recent studies have demonstrated the effectiveness of subconjunctival and periocular injections as alternative routes of administration for MSC-exos[69]. In the study by Park et al[18], PSCs or human pluripotent stem cell-derived NPCs was also injected via the subfascial route, which is considered less invasive and safer for repeated injections than other routes such as intravitreal or intravenous. Currently, some investigators suggest that the subfascial and suprachoroidal routes are appropriate for intraocular MSC implantation[76]. Therefore, it is important to explore and/or combine other delivery routes to optimize cell therapy for retinal and optic nerve injury[33]. Some researchers have advocated proximal injection of stem cells into the optic nerve as the best route, which has the potential to enhance stem cell accumulation around the optic nerve and minimize systemic spread. The advantage of stem cell transplantation is the long-term release of multiple therapeutic factors through a single injection, thereby reducing the risk of infection and bleeding associated with intraocular injections and easing the burden of patient compliance. The optimal transplantation method for optic nerve regeneration remains an active area of research, and the choice of method depends on factors such as the specific therapeutic goal, the type and severity of optic nerve injury, and the characteristics of the stem cells used. Each approach has its strengths and challenges, and its efficacy and safety need to be thoroughly evaluated through preclinical and clinical studies to determine the most appropriate approach for successful integration of stem cells. It is important to examine the survival of donor cells in all ocular tissues or limit the metric to a composite result of survival and retinal homing. The migration and survival of injected cells are understudied aspects of intraocular transplantation[16].
Migration location and survival time
The success of stem cell therapy depends on the donor cells' ability to survive, migrate, differentiate, and integrate into the desired locations. The role of the transplantation pathway is crucial in identifying the survival of donor cells[16]. Once positioned within the host's RGC layer, the donor RGCs must establish appropriate topographic spacing while targeting dendrites to specific sublayers of the inner plexiform layer (IPL) to form synaptic connections with non-long axon cells and bipolar cells[16]. Studies have shown that transplantation of MSCs not only significantly improves the survival rate of RGCs but also regenerates the original target area[77]. When periodontal ligament-derived stem cells were injected into the vitreous body, some of the transplanted cells migrated to the host retina and were mainly located in the nerve fiber layer and RGC layer, with few cells migrating to the inner plexiform layer and nuclear layer. Periodontal ligament-derived stem cells migrated along the nerve fibers towards the prelaminar and post-laminar regions of the optic nerve disc. A higher density of stem cells was observed in the peripheral area of the optic nerve injury compared to the central area, indicating that stem cells appeared to survive and migrate to the peripheral and central regions of the injured optic nerve[38]. Stem cells usually survive for 3-4wk after intravitreal transplantation[24]. Johanson et al[24] discovered that MSCs survived for more than 35d and migrated significantly to the retina after intravitreal transplantation in a rat model of glaucoma[78]. Although the transplanted MSCs could integrate into the RGC layer, they showed limited differentiation into RGCs. It is suggested that the therapeutic effects of MSC transplantation primarily result from the neuroprotective actions of NTFs secreted by MSCs and their regulatory effects on the microenvironment of neurons[1].
Assess optic nerve function after stem cell injection
The evaluation includes the ability to assess aspects such as intracellular migration, localization, synaptic formation, and axonogenesis. Chain reaction, enzyme-linked immunosorbent assay (ELISA), quantitative reverse transcription-polymerase chain reaction (RT-PCR), electroretinogram, immunohistochemistry, and Western blot have commonly been involved the use in the experiment to detect the expression of NTFs and growth trophic factors. Flash visual evoked potential (f-VEP) is applied to evaluate visual nerve function[79]. RGC survival rate is quantified through axonal quantification of the optic nerve. Optical coherence tomography (OCT) is used to measure retinal nerve fiber layer as a measurement of axonal atrophy at 7,14, and 21d after injury[41]. Fluorogold (FG) is used to retrogradely trace RGCs[48]. Impact of Embryonic stem cell-derived MSC extracellular vesicles on optic nerve function and potential long-term neuroprotection by assessing RGC survival, cognitive visual behavior, retinal nerve fiber layer (RNFL) thickness, and cis p-tau accumulation[57].
INFLAMMATORY RESPONSE AFTER TRANSPLANTATION
Several studies have raised concerns about the inflammatory response following stem cell transplantation. Allogeneic sources are increasingly used in MSC-based therapies due to their favorable safety and efficacy. Their main advantages include reduced immunogenicity, high accessibility, and increased capacity for high-quality donor selection. However, as with most allogeneic therapies, there is a possibility of immune rejection. In addition, selecting the best donor candidate for use in the disease of interest may present significant challenges due to donor-donor heterogeneity. Furthermore, the patient's system rapidly clears MSC after infusion. Autologous MSC sources are safer as they come directly from the patient and the MSC itself is immunocompromised. The text describes the drawbacks of long-term availability and potential disease candidate genes[69]. Zhang et al[16] suggest that allogeneic MSC transplantation can be harmful to recipients. Tassoni et al[80] report an intense inflammatory response accompanied by reactive gliosis after BMSC transplantation. Transplantation of human Wharton's jelly mesenchymal stem cell (hWJ-MSC) grafts in the vitreous cavity of normal Wistar rats resulted in allogeneic immune rejection, malignant transformation, and small vessel occlusion[14]. Wen et al[76] found that human leukocyte antigen DR (HLA-DR) positivity induced by the transplantation of hWJ-MSC grafts triggers severe inflammation and retinal damage.
Huang et al[81] demonstrated that intravitreal administration of rat and human MSCs induced retinal vascular degeneration, increased pericyte loss, noncellular capillary formation, cataracts, and retinal inflammatory responses in normal and diseased rat retinas. While the retinal neuronal function remained unaffected by the MSC injection, the injection did trigger immune and inflammatory responses. This was accompanied by the activation of glial cells and signaling, which were triggered by heat shock protein 90 (HSP90)-mediated pathways[81]. It has been reported that these responses may also occur with other cell types[16]. In a clinical study conducted by Pastor et al[82], intravitreal injections of allogeneic BMSCs were administered to treat patients with acute nonarthritic optic neuropathy. At the one-year follow-up, no significant inflammatory response was observed. MSC transplantation carries several risks, including vitreous clouding, vitreous hemorrhage, proliferative vitreoretinopathy leading to retinal detachment, alloimmune rejection, retinal arterial and venous obstruction, and malignant transformation. However, the use of MSC-exos can significantly reduce these potential complications. In this study, we used inbred Lewis rats for allogeneic transplantation without immunosuppression to limit graft rejection and avoid potential complications caused by immunosuppressive drugs. Homozygous transplantation resulted in graft survival for 12wk[12].
DISCUSSION
Stem cells have multiple functions that make them promising for treating optic nerve damage caused by glaucoma. Future research and development should focus on the following. 1) Evaluate whether the generation of an inflammatory response after transplanting stem cells into the eye is related to the cell type, the number and dose of grafts, the method of transplantation, and the mechanism by which the inflammatory response is triggered. Additionally, determine which MSCs source and type are most suitable for neuroprotection. Currently, there are limited cross-sectional comparisons available on this issue. 2) The aim of this study is to investigate whether there is an optimal time window for intravitreal transplantation of stem cells in cases of glaucomatous optic nerve injury, and to determine the most effective timing for intervention. 3) Additionally, gradient experiments will be conducted to determine the optimal dosage of stem cells. 4) The aim of this study is to analyze exosomes secreted by stem cells at the transplantation site both qualitatively and quantitatively. Additionally, the safety and long-term efficacy of stem cell transplantation as a therapeutic approach will be determined, along with the development of reliable cell delivery and integration techniques[42]. The study will also investigate whether there is dose-dependent neuroprotection from stem cells and which transplantation route is most appropriate to use in models of glaucoma-induced optic nerve damage.
CONCLUSIONS
Based on existing research, stem cells and stem cell exosomes show promise for potential future therapies. Some progress has been made in the field of stem cell repair of optic nerve damage in glaucoma. However, there are still a number of issues and limitations that need to be addressed. In addition to demonstrating effectiveness after transplantation, regenerating and effectively projecting RGCs axons remains a major challenge. As imaging techniques improve, we will make significant progress in assessing migration, localization, synaptogenesis, and axonogenesis in vivo. It is unclear to what extent transplanted human stem cell-derived RGCs resemble developing or mature neurons. Selection of appropriate stem cell types and improved therapeutic efficacy through appropriate preparation methods and therapeutic dosages may be necessary for different types of neurological injuries. Furthermore, the current research on stem cell therapy for optic nerve injury faces the challenge of a lack of comprehensive understanding of the mechanism of action of stem cells. Although some progress has been made, further basic research and clinical trials are required for the development of stem cell therapy for optic nerve injury. The field of optic nerve regeneration has become possible due to advances in stem cell biology and regenerative neuroscience[16].
Footnotes
Foundation: Supported by Science & Technology Department of Sichuan Province (No.2021YFS0214).
Conflicts of Interest: Hu BY, None; Xin M, None; Chen M, None; Yu P, None; Zeng LZ, None.
REFERENCES
- 1.Zhang J, Wu S, Jin ZB, Wang N. Stem cell-based regeneration and restoration for retinal ganglion cell: recent advancements and current challenges. Biomolecules. 2021;11(7):987. doi: 10.3390/biom11070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan F, Wang M, Jin K, Xiang M. Advances in regeneration of retinal ganglion cells and optic nerves. Int J Mol Sci. 2021;22(9):4616. doi: 10.3390/ijms22094616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donahue RJ, Maes ME, Grosser JA, Nickells RW. BAX-depleted retinal ganglion cells survive and become quiescent following optic nerve damage. Mol Neurobiol. 2020;57(2):1070–1084. doi: 10.1007/s12035-019-01783-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coulon SJ, Schuman JS, Du YQ, Bahrani Fard MR, Ethier CR, Stamer WD. A novel glaucoma approach: stem cell regeneration of the trabecular meshwork. Prog Retin Eye Res. 2022;90:101063. doi: 10.1016/j.preteyeres.2022.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osborne A, Khatib TZ, Songra L, Barber AC, Hall K, Kong GYX, Widdowson PS, Martin KR. Neuroprotection of retinal ganglion cells by a novel gene therapy construct that achieves sustained enhancement of brain-derived neurotrophic factor/tropomyosin-related kinase receptor-B signaling. Cell Death Dis. 2018;9(10):1007. doi: 10.1038/s41419-018-1041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nascimento-Dos-Santos G, Teixeira-Pinheiro LC, da Silva-Júnior AJ, Carvalho LRP, Mesentier-Louro LA, Hauswirth WW, Mendez-Otero R, Santiago MF, Petrs-Silva H. Effects of a combinatorial treatment with gene and cell therapy on retinal ganglion cell survival and axonal outgrowth after optic nerve injury. Gene Ther. 2020;27(1-2):27–39. doi: 10.1038/s41434-019-0089-0. [DOI] [PubMed] [Google Scholar]
- 7.Mead B, Tomarev S. Extracellular vesicle therapy for retinal diseases. Prog Retin Eye Res. 2020;79:100849. doi: 10.1016/j.preteyeres.2020.100849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wareham LK, Liddelow SA, Temple S, Benowitz LI, Di Polo A, Wellington C, Goldberg JL, He ZG, Duan X, Bu GJ, Davis AA, Shekhar K, Torre A, Chan DC, Canto-Soler MV, Flanagan JG, Subramanian P, Rossi S, Brunner T, Bovenkamp DE, Calkins DJ. Solving neurodegeneration: common mechanisms and strategies for new treatments. Mol Neurodegener. 2022;17(1):23. doi: 10.1186/s13024-022-00524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu F, Wang Y, Huang CQ, Lin SJ, Gao RX, Wu RY. Neuroprotective effect of mesenchymal stem cell-derived extracellular vesicles on optic nerve injury in chronic ocular hypertension. Neural Regen Res. 2023;18(10):2301–2306. doi: 10.4103/1673-5374.369121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mélik Parsadaniantz S, Réaux-le Goazigo A, Sapienza A, Habas C, Baudouin C. Glaucoma: a degenerative optic neuropathy related to neuroinflammation? Cells. 2020;9(3):535. doi: 10.3390/cells9030535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mallick S, Sharma M, Kumar A, Du Y. Cell-based therapies for trabecular meshwork regeneration to treat glaucoma. Biomolecules. 2021;11(9):1258. doi: 10.3390/biom11091258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson TV, Bull ND, Martin KR. Transplantation prospects for the inner retina. Eye (Lond) 2009;23(10):1980–1984. doi: 10.1038/eye.2008.376. [DOI] [PubMed] [Google Scholar]
- 13.Behtaj S, Rybachuk M. Strategies on the application of stem cells based therapies for the treatment of optic neuropathies. Neural Regen Res. 2021;16(6):1190–1191. doi: 10.4103/1673-5374.300343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui Y, Liu C, Huang L, Chen J, Xu N. Protective effects of intravitreal administration of mesenchymal stem cell-derived exosomes in an experimental model of optic nerve injury. Exp Cell Res. 2021;407(1):112792. doi: 10.1016/j.yexcr.2021.112792. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Zhong H, Bussan EL, Pang IH. Early phosphoproteomic changes in the retina following optic nerve crush. Exp Neurol. 2020;334:113481. doi: 10.1016/j.expneurol.2020.113481. [DOI] [PubMed] [Google Scholar]
- 16.Zhang KY, Aguzzi EA, Johnson TV. Retinal ganglion cell transplantation: approaches for overcoming challenges to functional integration. Cells. 2021;10(6):1426. doi: 10.3390/cells10061426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel AK, Broyer RM, Lee CD, Lu TL, Louie MJ, La Torre A, Al-Ali H, Vu MT, Mitchell KL, Wahlin KJ, Berlinicke CA, Jaskula-Ranga V, Hu Y, Duan X, Vilar S, Bixby JL, Weinreb RN, Lemmon VP, Zack DJ, Welsbie DS. Inhibition of GCK-IV kinases dissociates cell death and axon regeneration in CNS neurons. Proc Natl Acad Sci U S A. 2020;117(52):33597–33607. doi: 10.1073/pnas.2004683117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park M, Kim HM, Shin HA, Lee SH, Hwang DY, Lew H. Human pluripotent stem cell-derived neural progenitor cells promote retinal ganglion cell survival and axon recovery in an optic nerve compression animal model. Int J Mol Sci. 2021;22(22):12529. doi: 10.3390/ijms222212529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nascimento-Dos-Santos G, de-Souza-Ferreira E, Lani R, Faria CC, Araújo VG, Teixeira-Pinheiro LC, Vasconcelos T, Gonçalo T, Santiago MF, Linden R, Galina A, Petrs-Silva H. Neuroprotection from optic nerve injury and modulation of oxidative metabolism by transplantation of active mitochondria to the retina. Biochim Biophys Acta Mol Basis Dis. 2020;1866(5):165686. doi: 10.1016/j.bbadis.2020.165686. [DOI] [PubMed] [Google Scholar]
- 20.Ross AG, McDougald DS, Khan RS, Duong TT, Dine KE, Aravand P, Bennett J, Chavali VRM, Shindler KS. Rescue of retinal ganglion cells in optic nerve injury using cell-selective AAV mediated delivery of SIRT1. Gene Ther. 2021;28(5):256–264. doi: 10.1038/s41434-021-00219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grozdanic SD, Ast AM, Lazic T, Kwon YH, Kardon RH, Sonea IM, Sakaguchi DS. Morphological integration and functional assessment of transplanted neural progenitor cells in healthy and acute ischemic rat eyes. Exp Eye Res. 2006;82(4):597–607. doi: 10.1016/j.exer.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Do JL, Allahwerdy S, David RCC, Weinreb RN, Tuszynski MH, Welsbie DS. Optic nerve engraftment of neural stem cells. Invest Ophthalmol Vis Sci. 2021;62(9):30. doi: 10.1167/iovs.62.9.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park M, Shin HA, Duong VA, Lee H, Lew H. The role of extracellular vesicles in optic nerve injury: neuroprotection and mitochondrial homeostasis. Cells. 2022;11(23):3720. doi: 10.3390/cells11233720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson TV, DeKorver NW, Levasseur VA, Osborne A, Tassoni A, Lorber B, Heller JP, Villasmil R, Bull ND, Martin KR, Tomarev SI. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain. 2014;137(Pt 2):503–519. doi: 10.1093/brain/awt292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cen LP, Ng TK. Stem cell therapy for retinal ganglion cell degeneration. Neural Regen Res. 2018;13(8):1352–1353. doi: 10.4103/1673-5374.235237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reboussin É, Buffault J, Brignole-Baudouin F, Goazigo ARL, Riancho L, Olmiere C, Sahel JA, Parsadaniantz SM, Baudouin C. Evaluation of neuroprotective and immunomodulatory properties of mesenchymal stem cells in an ex vivo retinal explant model. J Neuroinflammation. 2022;19(1):63. doi: 10.1186/s12974-022-02418-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miotti G, Parodi PC, Zeppieri M. Stem cell therapy in ocular pathologies in the past 20 years. World J Stem Cells. 2021;13(5):366–385. doi: 10.4252/wjsc.v13.i5.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behtaj S, Karamali F, Najafian S, Masaeli E, Esfahani MN, Rybachuk M. The role of PGS/PCL scaffolds in promoting differentiation of human embryonic stem cells into retinal ganglion cells. Acta Biomater. 2021;126:238–248. doi: 10.1016/j.actbio.2021.03.036. [DOI] [PubMed] [Google Scholar]
- 29.Kwon H, Park M, Nepali S, Lew H. Hypoxia-preconditioned placenta-derived mesenchymal stem cells rescue optic nerve axons via differential roles of vascular endothelial growth factor in an optic nerve compression animal model. Mol Neurobiol. 2020;57(8):3362–3375. doi: 10.1007/s12035-020-01965-8. [DOI] [PubMed] [Google Scholar]
- 30.Gokoffski KK, Peng M, Alas B, Lam P. Neuro-protection and neuro-regeneration of the optic nerve: recent advances and future directions. Curr Opin Neurol. 2020;33(1):93–105. doi: 10.1097/WCO.0000000000000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Lee RK. Cell transplantation to replace retinal ganglion cells faces challenges - the Switchboard Dilemma. Neural Regen Res. 2021;16(6):1138–1143. doi: 10.4103/1673-5374.300329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Gelder RN, Chiang MF, Dyer MA, Greenwell TN, Levin LA, Wong RO, Svendsen CN. Regenerative and restorative medicine for eye disease. Nat Med. 2022;28(6):1149–1156. doi: 10.1038/s41591-022-01862-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.da Silva-Junior AJ, Mesentier-Louro LA, Nascimento-Dos-Santos G, Teixeira-Pinheiro LC, Vasques JF, Chimeli-Ormonde L, Bodart-Santos V, de Carvalho LRP, Santiago MF, Mendez-Otero R. Human mesenchymal stem cell therapy promotes retinal ganglion cell survival and target reconnection after optic nerve crush in adult rats. Stem Cell Res Ther. 2021;12(1):69. doi: 10.1186/s13287-020-02130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao T, Li Y, Tang L, Li Y, Fan F, Jiang B. Protective effects of human umbilical cord blood stem cell intravitreal transplantation against optic nerve injury in rats. Graefes Arch Clin Exp Ophthalmol. 2011;249(7):1021–1028. doi: 10.1007/s00417-011-1635-7. [DOI] [PubMed] [Google Scholar]
- 35.Mesentier-Louro LA, Zaverucha-do-Valle C, da Silva-Junior AJ, Nascimento-Dos-Santos G, Gubert F, de Figueirêdo AB, Torres AL, Paredes BD, Teixeira C, Tovar-Moll F, Mendez-Otero R, Santiago MF. Distribution of mesenchymal stem cells and effects on neuronal survival and axon regeneration after optic nerve crush and cell therapy. PLoS One. 2014;9(10):e110722. doi: 10.1371/journal.pone.0110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51(4):2051–2059. doi: 10.1167/iovs.09-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan H, Kang X, Lu S, Liu L. The therapeutic effects of bone marrow mesenchymal stem cells after optic nerve damage in the adult rat. Clin Interv Aging. 2015;10:487–490. doi: 10.2147/CIA.S75319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Looi SY, Bastion MC, Leow SN, Luu CD, Hairul NMH, Ruhaslizan R, Wong HS, Wan Haslina AH, Ng MH, Idrus Ruszymah BH, Then KY. Therapeutic potential of human umbilical cord-derived mesenchymal stem cells transplantation in rats with optic nerve injury. Indian J Ophthalmol. 2022;70(1):201–209. doi: 10.4103/ijo.IJO_473_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan D, Chang X, Xu M, Zhang M, Zhang S, Wang Y, Luo X, Xu J, Yang X, Sun X. UMSC-derived exosomes promote retinal ganglion cells survival in a rat model of optic nerve crush. J Chem Neuroanat. 2019;96:134–139. doi: 10.1016/j.jchemneu.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Ma C, Nie L. GAP-43 induces the differentiation of bone marrow-derived mesenchymal stem cells into retinal ganglial-like cells. Comput Math Methods Med. 2022;2022:4949206. doi: 10.1155/2022/4949206. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest Ophthalmol Vis Sci. 2013;54(12):7544–7556. doi: 10.1167/iovs.13-13045. [DOI] [PubMed] [Google Scholar]
- 42.Millán-Rivero JE, Nadal-Nicolás FM, García-Bernal D, Sobrado-Calvo P, Blanquer M, Moraleda JM, Vidal-Sanz M, Agudo-Barriuso M. Human Wharton's jelly mesenchymal stem cells protect axotomized rat retinal ganglion cells via secretion of anti-inflammatory and neurotrophic factors. Sci Rep. 2018;8(1):16299. doi: 10.1038/s41598-018-34527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X, Zhao S, Wang L. Therapeutic effect of adipose-derived stem cell transplantation on optic nerve injury in rats. Mol Med Rep. 2018;17(2):2529–2534. doi: 10.3892/mmr.2017.8103. [DOI] [PubMed] [Google Scholar]
- 44.Mesentier-Louro LA, Teixeira-Pinheiro LC, Gubert F, Vasques JF, Silva-Junior AJ, Chimeli-Ormonde L, Nascimento-Dos-Santos G, Mendez-Otero R, Santiago MF. Long-term neuronal survival, regeneration, and transient target reconnection after optic nerve crush and mesenchymal stem cell transplantation. Stem Cell Res Ther. 2019;10(1):121. doi: 10.1186/s13287-019-1226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu T, Huang X, Peng S, Ye Y, Zhao J. Ultrasound targeted microbubble destruction promotes the therapeutic effect of HUMSC transplantation on glaucoma-caused optic nerve injury in rabbits. Transl Vis Sci Technol. 2022;11(5):12. doi: 10.1167/tvst.11.5.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koh K, Park M, Bae ES, Duong VA, Park JM, Lee H, Lew H. UBA2 activates Wnt/β-catenin signaling pathway during protection of R28 retinal precursor cells from hypoxia by extracellular vesicles derived from placental mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):428. doi: 10.1186/s13287-020-01943-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan J, Yu JX. Gender difference in the neuroprotective effect of rat bone marrow mesenchymal cells against hypoxia-induced apoptosis of retinal ganglion cells. Neural Regen Res. 2016;11(5):846–853. doi: 10.4103/1673-5374.182764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park M, Kim HC, Kim O, Lew H. Human placenta mesenchymal stem cells promote axon survival following optic nerve compression through activation of NF-κB pathway. J Tissue Eng Regen Med. 2018;12(3):e1441–e1449. doi: 10.1002/term.2561. [DOI] [PubMed] [Google Scholar]
- 49.Cen LP, Ng TK, Liang JJ, Zhuang X, Yao X, Yam GH, Chen H, Cheung HS, Zhang M, Pang CP. Human periodontal ligament-derived stem cells promote retinal ganglion cell survival and axon regeneration after optic nerve injury. Stem Cells. 2018;36(6):844–855. doi: 10.1002/stem.2812. [DOI] [PubMed] [Google Scholar]
- 50.Zhu Y, Ge J, Huang C, Liu H, Jiang H. Application of mesenchymal stem cell therapy for aging frailty: from mechanisms to therapeutics. Theranostics. 2021;11(12):5675–5685. doi: 10.7150/thno.46436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakowski SA, Chen KS. Stem cell therapy for central nervous system disorders: metabolic interactions between transplanted cells and local microenvironments. Neurobiol Dis. 2022;173:105842. doi: 10.1016/j.nbd.2022.105842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 53.Yu B, Li XR, Zhang XM. Mesenchymal stem cell-derived extracellular vesicles as a new therapeutic strategy for ocular diseases. World J Stem Cells. 2020;12(3):178–187. doi: 10.4252/wjsc.v12.i3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holan V, Hermankova B, Krulova M, Zajicova A. Cytokine interplay among the diseased retina, inflammatory cells and mesenchymal stem cells - a clue to stem cell-based therapy. World J Stem Cells. 2019;11(11):957–967. doi: 10.4252/wjsc.v11.i11.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao C, Tarlé S, Kaigler D. Characterization of the immunomodulatory properties of alveolar bone-derived mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):102. doi: 10.1186/s13287-020-01605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson TV, Bull ND, Martin KR. Identification of barriers to retinal engraftment of transplanted stem cells. Invest Ophthalmol Vis Sci. 2010;51(2):960–970. doi: 10.1167/iovs.09-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seyedrazizadeh SZ, Poosti S, Nazari A, Alikhani M, Shekari F, Pakdel F, Shahpasand K, Satarian L, Baharvand H. Extracellular vesicles derived from human ES-MSCs protect retinal ganglion cells and preserve retinal function in a rodent model of optic nerve injury. Stem Cell Res Ther. 2020;11(1):203. doi: 10.1186/s13287-020-01702-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mead B, Chamling X, Zack DJ, Ahmed Z, Tomarev S. TNFα-mediated priming of mesenchymal stem cells enhances their neuroprotective effect on retinal ganglion cells. Invest Ophthalmol Vis Sci. 2020;61(2):6. doi: 10.1167/iovs.61.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109(12):1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 60.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11):1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mead B, Amaral J, Tomarev S. Mesenchymal stem cell-derived small extracellular vesicles promote neuroprotection in rodent models of glaucoma. Invest Ophthalmol Vis Sci. 2018;59(2):702–714. doi: 10.1167/iovs.17-22855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo Y, Saloupis P, Shaw SJ, Rickman DW. Engraftment of adult neural progenitor cells transplanted to rat retina injured by transient ischemia. Invest Ophthalmol Vis Sci. 2003;44(7):3194–3201. doi: 10.1167/iovs.02-0875. [DOI] [PubMed] [Google Scholar]
- 63.Levkovitch-Verbin H, Sadan O, Vander S, Rosner M, Barhum Y, Melamed E, Offen D, Melamed S. Intravitreal injections of neurotrophic factors secreting mesenchymal stem cells are neuroprotective in rat eyes following optic nerve transection. Invest Ophthalmol Vis Sci. 2010;51(12):6394–6400. doi: 10.1167/iovs.09-4310. [DOI] [PubMed] [Google Scholar]
- 64.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mead B, Tomarev S. Bone marrow-derived mesenchymal stem cells-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms. Stem Cells Transl Med. 2017;6(4):1273–1285. doi: 10.1002/sctm.16-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mesentier-Louro LA, Zaverucha-do-Valle C, Rosado-de-Castro PH, Silva-Junior AJ, Pimentel-Coelho PM, Mendez-Otero R, Santiago MF. Bone marrow-derived cells as a therapeutic approach to optic nerve diseases. Stem Cells Int. 2016;2016:5078619. doi: 10.1155/2016/5078619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mathew B, Ravindran S, Liu XR, Torres L, Chennakesavalu M, Huang CC, Feng L, Zelka R, Lopez J, Sharma M, Roth S. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials. 2019;197:146–160. doi: 10.1016/j.biomaterials.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thomaidou AC, Goulielmaki M, Tsintarakis A, Zoumpourlis P, Toya M, Christodoulou I, Zoumpourlis V. miRNA-guided regulation of mesenchymal stem cells derived from the umbilical cord: paving the way for stem-cell based regeneration and therapy. Int J Mol Sci. 2023;24(11):9189. doi: 10.3390/ijms24119189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu KY, Ahmad H, Lin G, Carbonneau M, Tran SD. Mesenchymal stem cell-derived exosomes in ophthalmology: a comprehensive review. Pharmaceutics. 2023;15(4):1167. doi: 10.3390/pharmaceutics15041167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim D, Lee AE, Xu Q, Zhang Q, Le AD. Gingiva-derived mesenchymal stem cells: potential application in tissue engineering and regenerative medicine - A comprehensive review. Front Immunol. 2021;12:667221. doi: 10.3389/fimmu.2021.667221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang LJ, Liu LP, Gu XL, Wang M, Liu LM. Implantation of adipose-derived stem cells cures the optic nerve injury on rats through inhibiting the expression of inflammation factors in the TLR4 signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22(5):1196–1202. doi: 10.26355/eurrev_201803_14458. [DOI] [PubMed] [Google Scholar]
- 72.Cen J, Zhang Y, Bai Y, Ma S, Zhang C, Jin L, Duan S, Du Y, Guo Y. Research progress of stem cell therapy for endometrial injury. Mater Today Bio. 2022;16:100389. doi: 10.1016/j.mtbio.2022.100389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, Zhang K, Yeung C, Feng G, Yankner BA, He Z. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature. 2011;480(7377):372–375. doi: 10.1038/nature10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chamling X, Sluch VM, Zack DJ. The potential of human stem cells for the study and treatment of glaucoma. Invest Ophthalmol Vis Sci. 2016;57(5):ORSFi1–ORSFi6. doi: 10.1167/iovs.15-18590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li N, Li XR, Yuan JQ. Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefes Arch Clin Exp Ophthalmol. 2009;247(4):503–514. doi: 10.1007/s00417-008-1009-y. [DOI] [PubMed] [Google Scholar]
- 76.Wen YT, Ho YC, Lee YC, Ding DC, Liu PK, Tsai RK. The benefits and hazards of intravitreal mesenchymal stem cell (MSC) based-therapies in the experimental ischemic optic neuropathy. Int J Mol Sci. 2021;22(4):2117. doi: 10.3390/ijms22042117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zwart I, Hill AJ, Al-Allaf F, Shah M, Girdlestone J, Sanusi ABR, Mehmet H, Navarrete R, Navarrete C, Jen LS. Umbilical cord blood mesenchymal stromal cells are neuroprotective and promote regeneration in a rat optic tract model. Exp Neurol. 2009;216(2):439–448. doi: 10.1016/j.expneurol.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 78.Daliri K, Ljubimov AV, Hekmatimoghaddam S. Glaucoma, stem cells, and gene therapy: where are we now? Int J Stem Cells. 2017;10(2):119–128. doi: 10.15283/ijsc17029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chung S, Rho S, Kim G, Kim SR, Baek KH, Kang M, Lew H. Human umbilical cord blood mononuclear cells and chorionic plate-derived mesenchymal stem cells promote axon survival in a rat model of optic nerve crush injury. Int J Mol Med. 2016;37(5):1170–1180. doi: 10.3892/ijmm.2016.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tassoni A, Gutteridge A, Barber AC, Osborne A, Martin KR. Molecular mechanisms mediating retinal reactive gliosis following bone marrow mesenchymal stem cell transplantation. Stem Cells. 2015;33(10):3006–3016. doi: 10.1002/stem.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang H, Kolibabka M, Eshwaran R, Chatterjee A, Schlotterer A, Willer H, Bieback K, Hammes HP, Feng Y. Intravitreal injection of mesenchymal stem cells evokes retinal vascular damage in rats. FASEB J. 2019;33(12):14668–14679. doi: 10.1096/fj.201901500R. [DOI] [PubMed] [Google Scholar]
- 82.Pastor JC, Pastor-Idoate S, López-Paniagua M, Para M, Blazquez F, Murgui E, García V, Coco-Martín RM. Intravitreal allogeneic mesenchymal stem cells: a non-randomized phase II clinical trial for acute non-arteritic optic neuropathy. Stem Cell Res Ther. 2023;14(1):261. doi: 10.1186/s13287-023-03500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


