Abstract
Background
Obesity is associated with progression of inflammatory bowel disease (IBD). Visceral adiposity may be a more meaningful measure of obesity compared with traditional measures such as body mass index (BMI). This study compared visceral adiposity vs BMI as predictors of time to IBD flare among patients with Crohn’s disease and ulcerative colitis.
Methods
This was a retrospective cohort study. IBD patients were included if they had a colonoscopy and computed tomography (CT) scan within a 30-day window of an IBD flare. They were followed for 6 months or until their next flare. The primary exposure was the ratio of visceral adipose tissue to subcutaneous adipose tissue (VAT:SAT) obtained from CT imaging. BMI was calculated at the time of index CT scan.
Results
A total of 100 Crohn’s disease and 100 ulcerative colitis patients were included. The median age was 43 (interquartile range, 31-58) years, 39% had disease duration of 10 years or more, and 14% had severe disease activity on endoscopic examination. Overall, 23% of the cohort flared with median time to flare 90 (interquartile range, 67-117) days. Higher VAT:SAT was associated with shorter time to IBD flare (hazard ratio of 4.8 for VAT:SAT ≥1.0 vs VAT:SAT ratio <1.0), whereas higher BMI was not associated with shorter time to flare (hazard ratio of 0.73 for BMI ≥25 kg/m2 vs BMI <25 kg/m2). The relationship between increased VAT:SAT and shorter time to flare appeared stronger for Crohn’s than for ulcerative colitis.
Conclusions
Visceral adiposity was associated with decreased time to IBD flare, but BMI was not. Future studies could test whether interventions that decrease visceral adiposity will improve IBD disease activity.
Keywords: visceral adiposity, Crohn’s disease, ulcerative colitis
Key Messages.
What is already known?
Obesity is associated with a more severe phenotype of inflammatory bowel disease (IBD).
Visceral adiposity may be a superior measure of obesity compared with body mass index for patient-centered outcomes among patients with IBD.
What is new here?
Increased visceral adiposity was associated with a shorter time to flare among patients with IBD, implying greater disease activity.
The relationship between visceral adiposity and time to flare appeared to be stronger for patients with Crohn’s disease compared with patients with ulcerative colitis.
Body mass index was not associated with time to flare in either Crohn’s disease or ulcerative colitis patients.
How can this study help patient care?
Measuring visceral adiposity may be a useful adjunctive tool when risk stratifying patients with IBD.
Interventions aimed at diminishing visceral adiposity may lead to an improvement in outcomes for IBD patients.
Introduction
Inflammatory bowel disease (IBD) is a chronic, progressive immune-mediated condition that results from a complex interplay of genetic and environmental risk factors. Despite an ever increasing armamentarium of immunomodulators, biologic and small molecule–targeted therapies, there is still no definite cure for disease, and an estimated 40% of Crohn’s disease (CD) and ulcerative colitis (UC) patients do not respond to treatment.1,2 Further investigation is necessary to risk stratify patients for disease progression and severity.
One risk factor that has been linked to IBD progression is obesity.3 Creeping fat around the small intestine was the first anatomic clue to CD and was described in the first definition of regional ileitis in the 1930s.4 Since then, obesity has been associated with an increased risk for surgery among patients started on anti-tumor necrosis factor alpha (TNF-α) therapy5 and with treatment failure of biologics.6 Visceral adiposity, which refers to the hormonally and metabolically active fat deposited around the viscera, may be the key player behind adverse outcomes in patients with obesity and IBD, because visceral fat can promote a proinflammatory state mediated by adipocyte-derived cytokines such as leptin, TNF-α and interleukin (IL)-6.7
Prior studies have independently associated visceral adiposity with stricturing in CD, elevated fecal calprotectin, and increased postoperative mortality following ileocolonic resection.8,9 On the other hand, prior studies testing the relationship between body mass index (BMI) and IBD outcomes have generally shown weaker relationships. A multicenter cohort study found that among IBD patients starting new biologic therapy, BMI was not associated with hospitalization, IBD-related surgery, or serious infection.10 Additionally, a pooled analysis of 4 clinical trials (ACCENT I [A randomized, double blind, placebo-controlled trial of anti-TNFalpha chimeric monoclonal antibody in the long term treatment of patients with moderately to severely active Crohn's disease], SONIC [The study of biologic and immunomodulator naive patients in Crohn's disease], ACT 1 and 2 [Active ulcerative colitis trials 1 and 2]) found that BMI was not associated with clinical remission, clinical response, or mucosal healing across both CD and UC.11 The same phenomenon, of BMI being outperformed by alternative measures for obesity, has been observed in other disease states.”12
Measuring the time between IBD flares is one way to assess disease activity, because a shorter time between flares implies more frequent flares over a lifetime and greater disease activity. To date, no studies have examined the effect of visceral adiposity on time to flare in IBD. This study evaluated visceral adiposity at the time of an index IBD flare and tested the association between visceral adiposity and the time to subsequent flare. We hypothesized that both CD and UC patients with higher visceral adiposity would have a decreased time to subsequent flare but that the same association would not be seen with BMI.
Methods
Study Design and Population
This was a retrospective study using data from the electronic medical record at NewYork-Presbyterian/Columbia University Irving Medical Center. Consecutive adult patients ≥18 years of age with a diagnosis of CD or UC were selected for the study if they underwent a colonoscopy at the time of an IBD flare at our institution between January 2010 and March 2022. Patients were included if they had an abdominal CT scan within 30 days of their colonoscopy (either before or after). If more than 1 abdominal CT scan was obtained within the 30-day interval, the scan closer to the colonoscopy date was utilized. Starting 25 days after the index CT scan, patients were followed for up to 6 months from their index CT scan to determine if and when they developed their next IBD flare (Figure 1). The purpose of this 25-day window period was to allow time for recovery after the initial flare. Patients without a minimum of 6 months of longitudinal clinical follow-up were excluded. Records were reviewed until there were 100 adults with CD and 100 with UC who met study criteria. This study was approved by Columbia University’s Institutional Review Board.
Figure 1.
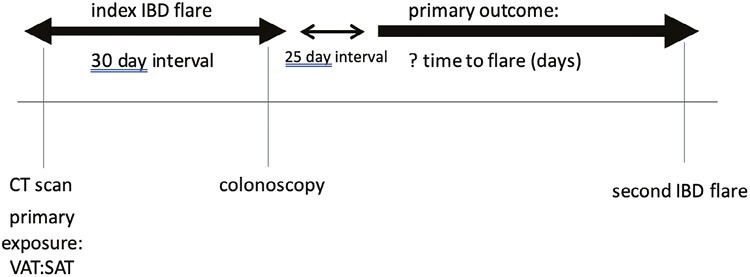
The initial computed tomography (CT) scan and colonoscopy had to be within a 30-day interval. The date of the CT scan was utilized for the index inflammatory bowel disease (IBD) flare. Disease activity was ascertained from the colonoscopy. The primary outcome was time to second flare. The second flare had to be at least 25 days after the index IBD flare and otherwise was not included. VAT:SAT, ratio of visceral adipose tissue to subcutaneous adipose tissue.
Primary Outcome
The primary outcome was IBD flare. The date and time of the index CT scan was considered time zero. An IBD flare was defined as a hospitalization or emergency room visit related to IBD, initiation of steroids, dose escalation in an IBD medication, change of IBD medication, or surgery for IBD that fell after the 25-day window period and before the end of the 6-month follow-up period. Identification of flare was based on manual chart review by one author (P.S.). This operationalization of IBD flare has been used in the past, specifically with relation to identifying risk factors for flare.13,14
Primary Exposure
The primary exposure was the ratio of visceral adipose tissue to subcutaneous adipose tissue (VAT:SAT), which was classified categorically into VAT:SAT <1.0 and ≥1.0 based on prior studies examining visceral adiposity.15-17 Elevated VAT:SAT has been previously linked to stricturing behavior in CD and is associated with worse outcomes across inflammatory diseases, including in rheumatoid arthritis.8,18 VAT:SAT was calculated based on computed tomography (CT) scan measurements as described subsequently. The indication for the CT scan was recorded based on the manual chart review and was adjusted for in the main analysis. BMI was extracted from the electronic medical record at the time of CT scan based on the recorded height and weight and was classified as normal (BMI <25 kg/m2) vs overweight (BMI ≥25 kg/m2). We chose to compare patients with a BMI above vs below 25 kg/m2 because this more evenly divided our data and improved power within this study (n = 100 for each BMI stratum). If we utilized a BMI of 30 kg/m2as the cutoff, we had 145 with a BMI <30 kg/m2 and only 55 with a BMI ≥30 kg/m2.
Body Composition Measurements
Body composition measurements, and in particular the exposure variable—VAT:SAT—were obtained via specialized software (TomoVision sliceOmatic Version 5.0). The axial slice of L3 vertebra was utilized to obtain the body composition measurements, and this location has been previously shown to represent adipose tissue.19 The radiographic slices for each patient was standardized to a thickness of 2.5 to 3.0 mm. Subsequently, measurements of adipose tissue were attained with thresholds of -150 to -50 Hounsfield units for VAT area and -190 to -30 Hounsfield units for SAT.20 All scans were analyzed by a single reader (S.S.). To ensure internal validity, a random selection of 40 scans (20% of the study population) was analyzed by a second reader (P.S.), and these scans demonstrated high interreader reliability, with intraclass correlations above 90% for all measures.
Covariables
Prior to analysis, covariables age, sex, race, duration of CD or UC, IBD medication type, and disease activity were extracted from the electronic medical record for the final model. Disease activity was retrospectively scored by one reviewer (P.S.) via colonoscopy data using either the Simple Endoscopic Score for CD or the Mayo score for UC.21,22
Statistical Analyses
For continuous variables, mean ± SD was computed if data were normally distributed, with median (interquartile range [IQR]) if data were skewed. In the primary analysis, Cox proportional hazards modeling was used to test the relationship between VAT:SAT and time to disease flare, after adjusting for other factors. This model was created with all IBD patients, and subanalyses were also performed for both CD and UC patients. The main analysis was additionally repeated, substituting BMI for VAT:SAT as the exposure. All analyses were performed using STATA version 17.0 (StataCorp). Associations were considered statistically significant at a P value <.05.
Results
Population and Baseline Characteristics
A total of 200 patients were included in the final analysis, with 100 CD and 100 UC patients (Table 1). The median age of the population was 43 (IQR, 31-58) years. Of all participants, 41% were female, and most were White (55%) or Hispanic (26%). In CD patients, 37% had L1 disease (involving the terminal ileum), 20% had L2 disease (colonic), and 43% had L3 disease (ileocolonic). Furthermore, among those with CD, 43% had B1 disease (nonstricturing/nonpenetrating), and 56% had B2 disease (stricturing) or B3 disease (penetrating). Among those patients with UC, 5% had E1 disease (proctitis), 50% had E2 disease (left-sided colitis), and 45% had E3 disease (extensive colitis). Among all patients in the cohort, 45% were on a biologic agent. Disease activity was classified as severe for 14% of all patients (3% of CD and 25% of UC patients).
Table 1.
Characteristics at the time of the initial CT scan, stratified by Crohn’s disease vs ulcerative colitis.
| All (N = 200) | Crohn’s disease (n = 100) | Ulcerative colitis (n = 100) | |
|---|---|---|---|
| Age, y | 43 (31-58) | 41 (31-57.5) | 44 (29.5-59) |
| 18 to <30 y | 47 (23.5) | 22 (22) | 25 (25) |
| 30 to <65 y | 122 (61) | 65 (65) | 57 (57) |
| ≥65 y | 31 (15.5) | 13 (13) | 18 (18) |
| Sex | |||
| Male | 119 (59.5) | 63 (63) | 56 (56) |
| Female | 81 (40.50) | 37 (37) | 44 (44) |
| Race | |||
| White | 110 (55) | 56 (56) | 54 (54) |
| Hispanic | 51 (25.5) | 26 (26) | 25 (25) |
| Black | 25 (12.5) | 11 (11) | 14 (14) |
| Othera | 14 (7.0) | 7 (7) | 7 (7) |
| Disease subtype | |||
| L1 | — | 37 (37) | — |
| L2 | — | 20 (20) | — |
| L3 | — | 43 (43) | — |
| B1 | — | 43 (43) | — |
| B2 or B3 | — | 56 (56) | — |
| p | — | 13 (13) | — |
| E1 | — | — | 5 (5) |
| E2 | — | — | 50 (50) |
| E3 | — | — | 45 (45) |
| Duration of disease | |||
| 0 to <5 y | 98 (49) | 41 (41) | 57 (57) |
| 5 to <10 y | 24 (12) | 16 (16) | 8 (8) |
| ≥10 y | 78 (39) | 43 (43) | 35 (35) |
| Medication type | |||
| Steroid (budesonide) | 14 (7) | 7 (7) | 7 (7) |
| ASA only | 49 (24.5) | 11 (11) | 38 (38) |
| Immunomodulatorb | 10 (5) | 6 (6) | 4 (4) |
| Biologic | 86 (43) | 64 (64) | 22 (22) |
| Indication for CT scan | |||
| Concern for flare | 194 (97) | 98 (98) | 96 (96) |
| Concern for malignancy | 6 (3) | 2 (2) | 4 (4) |
| Patients with flare | 45 (23) | 21 (21) | 24 (24) |
| Time to flare, d | 90 (67-117) | 98 (69-137) | 85 (50-117) |
| Disease activity | |||
| Severe | 28 (14) | 3 (3)c | 25 (25)d |
| Moderate | 65 (32.5) | 35 (35)c | 30 (30)d |
| Mild | 69 (34.5) | 44 (44)c | 25 (25)d |
| Inactive | 38 (19) | 18 (18)c | 20 (20)d |
Values are median (interquartile range) or n (%). Disease subtype as per Montreal Classification: L1 = terminal ileum, L2 = colon, L3 = ileocolon; B1 = nonstricturing/nonpenetrating, B2 = structuring, B3 = penetrating; p = perianal disease.
Abbreviations: ASA, 5-aminosalicylic acid; CT, computed tomography.
aIncludes Asian.
bIncludes patients on immunomodulator + ASA (n = 2) and immunomodulator + biologic (n = 3) for ease of reporting.
cSimple Endoscopic Score for Crohn’s Disease.
dFull Mayo score.
Body Composition Measurements
The median VAT:SAT was 0.39 (IQR, 0.22-0.70) and the median BMI was 24 (IQR, 21-29) kg/m2 (Table 2). There was no difference in either VAT:SAT or BMI comparing patients with CD vs those with UC (rank sum P = .12 and .48, respectively).
Table 2:
Adiposity variables measured, stratified by Crohn’s disease vs ulcerative colitis.
| Adiposity variable | All (N = 200) | Crohn’s disease (n = 100) | Ulcerative colitis (n = 100) |
|---|---|---|---|
| VAT: SAT | 0.39 (0.22-0.70) | 0.49 (0.25-0.83) | 0.35 (0.12-0.59) |
| <1.0 | 171 (85.5) | 78 (78) | 93 (93) |
| ≥1.0 | 29 (14.5) | 22 (22) | 7 (7) |
| BMI, kg/m2 | 24 (21-29) | 234 (20-27) | 23.0 (21-29) |
| Underweight/normal (<25) | 100 (50) | 57 (57) | 43 (43) |
| Overweight (≥25) | 100 (50) | 43 (43) | 57 (57) |
| VAT, mm3 | 4727 (1845-14 238) | 6606 (2080-14 651) | 4438.5 (1407-11 440) |
| 0 to <2500 | 61 (30.5) | 30 (30) | 31 (31) |
| 2500-9000 | 66 (33) | 27 (27) | 39 (39) |
| ≥9000 | 73 (36.5) | 43 (43) | 30 (30) |
| SAT, mm3 | 13 241 (8615-24 611) | 12 955 (9292-23 986) | 13 621 (7232-25 294) |
| 0 to <9000 | 56 (28) | 22 (22) | 34 (34) |
| 9000-15 000 | 62 (31) | 38 (38) | 24 (24) |
| ≥15 000 | 82 (41) | 40 (40) | 42 (42) |
Values are median (interquartile range) or n (%).
Abbreviations: BMI, body mass index; VAT:SAT, ratio of visceral adipose tissue to subcutaneous adipose tissue.
VAT:SAT and Time to IBD Flare
Body composition measures were assessed for time to flare. Overall, 23% of the cohort had a second flare within 6 months. Among those who flared, the median time to flare was 90 (IQR, 67-117) days. In crude analysis, increased VAT:SAT was associated with shorter time to flare, with a median of 72 (IQR, 36-85) days among those with VAT:SAT ≥1.0. vs a median of 98 (IQR, 71-117) days among those with VAT:SAT <1.0 (log-rank P < .01) (Figure 2). A multivariable Cox proportional hazards model was constructed to examine the relationship between VAT:SAT and time to IBD flare among all 200 patients after adjusting for other factors. The final model included VAT:SAT and the covariables: age, sex, race, duration of disease, and IBD medication type. In the final model, a VAT:SAT ≥1.0 was strongly associated with time to flare (hazard ratio [HR], 4.8; 95% confidence interval [CI], 1.7-13; P < .01) (Table 3). Age ≥65 years, Black and Hispanic race, disease duration ≥5 years, and mesalamine and biologic use were also associated with time to flare. Because disease activity at the time of index flare was associated with body composition measures, we elected to exclude disease activity in the final model. Furthermore, when the final model was repeated excluding the patients who had the index CT scan performed for reasons other than IBD flare (n = 6), the results were not substantively changed.
Figure 2.
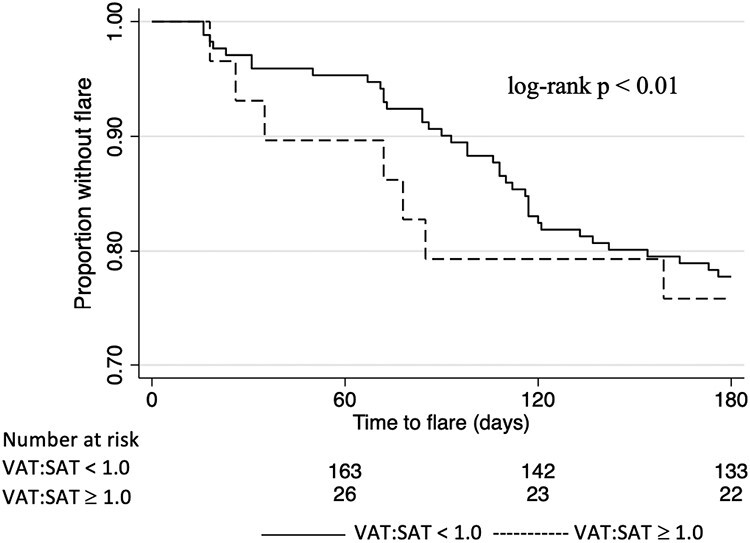
Time to second inflammatory bowel disease flare stratified by a ratio of visceral adipose tissue to subcutaneous adipose tissue (VAT:SAT) ≥1.0 and <1.0. A 25-day buffer window was maintained to allow for patient recovery from the initial inflammatory bowel disease flare.
Table 3.
Cox proportional hazards model for predictors of time to flare among all 200 patients.
| Risk factor | Univariate analysis | Multiple regression model |
|---|---|---|
| VAT:SAT | ||
| <1.0 | Reference | Reference |
| ≥1.0 | 1.66 (0.73-3.79) | 4.8 (1.68-13.74) |
| Age | ||
| 18 to <30 y | Reference | Reference |
| 30 to <65 y | 1.10 (0.56-2.21) | 2.06 (0.78-5.43) |
| ≥65 y | 2.60 (1.0-6.72) | 10.03 (1.82-55.12) |
| Sex | ||
| Female | Reference | Reference |
| Male | 1.31 (0.70-2.46) | 1.32 (0.59-2.95) |
| Race | ||
| White | Reference | Reference |
| Hispanic | 0.61 (0.32-1.19) | 0.28 (0.11-0.71) |
| Black | 1.28 (0.43-3.78) | 0.06 (0.01-0.41) |
| Other | 0.75 (0.22-2.53) | 0.73 (0.20-2.76) |
| Duration | ||
| 0 to <5 y | Reference | Reference |
| 5 to <10 y | 1.62 (0.66-4.00) | 3.85 (1.20-12.37) |
| ≥10 y | 1.82 (0.94-3.52) | 2.83 (1.12-7.15) |
| Medication type | ||
| No medication | Reference | Reference |
| Steroid | 0.50 (0.14-1.72) | 0.19 (0.03-1.04) |
| ASA | 0.40 (0.14-1.18) | 0.19 (0.05-0.65) |
| Biologic | 0.35 (0.14-0.83) | 0.13 (0.04-0.50) |
Values are hazard ratio (HR) for univariate and multivariate analysis respectively, comparing elevated VAT:SAT greater than or equal to 1 to less than 1.
The colonoscopy and computed tomography scan for each patient were obtained within a 30-day interval of the index flare and patients were followed for up to 6 months or until the second flare.
Abbreviations: ASA, 5-aminosalicylic acid; VAT:SAT, ratio of visceral adipose tissue to subcutaneous adipose tissue.
Stratified Analyses
We hypothesized that the relationship between VAT:SAT and time to flare might differ among those with CD vs UC. To test this hypothesis, we stratified the results into CD and UC cohorts. Upon stratification, a strong association was seen between VAT:SAT ≥1.0 and time to flare for CD patients (adjusted HR [aHR], 7.7; 95% CI, 0.93-63.22; P = .058), whereas the relationship was less strong for those with UC (aHR, 0.26; 95% CI, 0.02-3.7; P = .32). Because there was the visual appearance of effect modification in VAT:SAT based on CD vs UC, we formally tested an interaction term representing IBD subtype in the final multivariable model. This interaction term was not statistically significant (P = .8), although this may be due to diminished sample size, as there were only 7 patients with UC who had elevated VAT:SAT.
BMI and Time to IBD Flare
Finally, we examined the relationship between BMI and time to IBD flare. When BMI was substituted for VAT:SAT as a predictor variable in the final model, there was no relationship between BMI and time to IBD flare (aHR, 0.73; 95% CI, 0.32-1.67 for BMI ≥25 kg/m2 vs BMI <25 kg/m2). Stratifying the cohort into CD vs UC, we found no relationship between elevated BMI and time to IBD flare either in those with CD (aHR, 1.06; 95% CI, 0.11-9.96) or in those with UC (aHR, 1.57; 95% CI, 0.27-9.16).
Discussion
This retrospective study assessed the relationship between visceral adiposity and IBD. We found that an elevated VAT:SAT was independently associated with a shorter time to flare across all IBD patients, although this relationship appeared to be stronger among those with CD compared with those with UC. Conversely, an elevated BMI was not associated with a shorter time to flare in IBD patients overall, or after stratifying the cohort into CD vs UC. These results suggest that visceral fat, perhaps because it represents more hormonally active tissue, may contribute more to the inflammatory process in IBD compared with overall adipose tissue. It also shows that BMI is not likely to be an important predictor for outcomes among patients with IBD.
Prior studies have also suggested that visceral adiposity is a superior marker compared with BMI for outcomes in IBD. For example, a single-center prospective study evaluating CD patients found that increasing VAT:SAT was associated with 70% increased risk for baseline stricturing behavior (B2).8 This was in contrast to the results for BMI, which was not associated with stricturing disease. This study also found that VAT:SAT was associated with increased fecal calprotectin at 0, 12, and 24 months on follow-up.8 Another retrospective cohort study examining the effect of visceral adiposity on response to anti-TNF-α induction found that those with increased visceral adiposity (≥3000 cm3) were 3.5 times more likely to have treatment response compared with those with decreased visceral adiposity (≥1500 cm3).5 However, this difference in response to anti-TNF-α agents was not seen with BMI or VAT:SAT. This study only included patients on anti-TNF-α agents. Because fat tissue sequesters biologic agents such as anti-TNF-α agents, some of the findings may be related to drug pharmacokinetics and underdosing, rather than to inflammation per se.23 In our study, only 29% of patients were on anti-TNF therapy, so our study extends these prior findings.
Our findings also indicate that visceral adiposity is more meaningful in the CD vs UC cohort with regard to time to flare. This is in keeping with prior literature and may be related to differences in pathophysiology between the 2 disease processes. It has been shown in CD that creeping fat (perhaps a locally restricted version of visceral adiposity) correlates with the extent of histologic inflammation and degree of lymphocyte or macrophage infiltration.24 In a 2013 study by Zulian et al,25 adipose tissue from CD and UC patients was compared and it was found that that the morphology and molecular profile of UC-associated adipose tissue was less inflamed than that of tissue resected from patients with CD. In this study, genes related to inflammation, bacterial response, chemotaxis, and angiogenesis were downregulated in UC as compared with CD. The investigators also compared an in vitro bacterial infection model across UC and CD adipose cells and found that the CD adipose cells were more densely colonized with bacterial cells, which in turn increased adipocyte proliferation. These prior studies offer insight into the mechanisms that may underly our differential findings for UC vs CD.
In contrast to our findings, a prospective study by Bryant et al8 reported no independent association between VAT and hospitalization or surgery over a 4-year extended follow-up period. This study, which was limited to CD, had fewer than 100 patients, and not all of them were followed for the complete 4-year study period. Their primary outcome—IBD hospitalization or surgery—may be a more specific but less sensitive way of operationalizing IBD disease activity. In a cross-sectional study, Yadav et al26 found no correlation between visceral adiposity and the extent of disease in UC or disease phenotype in patients with CD. This study took place at a single institution in India, with demographics that may not be applicable to the more heterogeneous IBD patient population in the United States. Another study with a contrasting result was by Thiberge et al,27 who found that IBD patients with decreased visceral adiposity were more likely to have adverse outcomes such as surgery or mortality at 6 months. Again, the difference in outcomes may have been responsible for the apparent contrast in findings. Overall, the existing literature for visceral adiposity and IBD is mixed, at least in part because of differences in study design and patient population. Our study findings, which include a relatively large effect size for VAT:SAT and IBD flare, provide evidence that visceral adiposity may meaningfully contribute toward IBD disease activity.
Our study has many strengths. This study longitudinally explores the relationship of VAT:SAT and time to flare in both CD and UC patients. We had excellent interreader reliability confirming internal validity of our primary exposure visceral adiposity measurements. And we compared VAT:SAT with BMI, a common measure of obesity.
The study also has certain limitations. Our cutoffs for VAT:SAT were derived from prior studies in the literature that utilized similar cutoffs; however, these are by no means standardized. Additionally, our study was unable to establish remission after the index flare, and patients who remained continually sick for >25 days from their initial flare may have been misclassified as having a second flare. Furthermore, because the study design required a CT scan to measure VAT:SAT, the study may have disproportionately selected patients with small bowel disease, which may limit the generalizability of our results. Furthermore, this study was retrospective, so we were not able to assess other measures of adiposity such as waist-to-hip ratio, which may be easier to obtain and may capture similar data. As CT scans can pose an increased radiation risk, future prospective studies can be conducted with the use of magnetic resonance imaging or waist-to-hip ratio, or seek to obtain and directly test VAT (eg, from patients who went to IBD surgery).28 Last, our study took place at a single academic tertiary institution and would be more generalizable if the results are replicated at other centers.
Conclusions
In summary, we found that visceral adiposity (measured by CT scan) is associated with time to flare among CD and UC patients. BMI was not associated with time to flare. Our study provides further insight into the notion that obesity is best measured via VAT volumetrics and not by BMI. These results come at a time when new pharmacological treatments that may decrease visceral adiposity are available. Future studies could prospectively test whether interventions that reduce visceral adiposity can influence the progression of IBD.
Contributor Information
Priya Sehgal, Division of Digestive and Liver Diseases, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY, USA.
Steven Su, Department of Medicine, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY, USA.
John Zech, Department of Radiology, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY, USA.
Yael Nobel, Division of Digestive and Liver Diseases, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY, USA.
Lyndon Luk, Department of Radiology, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY, USA.
Ioannis Economou, Division of Colorectal Surgery, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY, USA.
Bo Shen, Division of Colorectal Surgery, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY, USA.
James D Lewis, Division of Gastroenterology and Hepatology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Daniel E Freedberg, Division of Digestive and Liver Diseases, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY, USA.
Author Contribution
P.S. and D.E.F.: Conceptualization: Equal; Data curation: Equal; Formal analysis: Equal; Investigation: Equal; Methodology: Equal; Project administration: Equal; Validation: Equal; Writing—original draft: Equal; Writing—review & editing: Equal. S.S. and J.Z.: Data curation: Equal. Y.N. and L.L.: Methodology: Equal. I.E., B.S., and J.L.: Conceptualization: Equal.
Funding
P.S. has received grant funding via the National Institutes of Health (5T32DK083256-13). D.E.F. has received funding from the Department of Defense (PR181960) and through a Columbia University Irving Scholar Award.
Conflicts of Interest
B.S. has received educational grants and personal fees from AbbVie and Janssen; personal fees from Takeda; and consulting fees from AbbVie, Janssen, and Takeda. J.D.L. has received personal fees from Johnson & Johnson Consumer Inc, Eli Lilly and Company, Samsung Bioepis, UCB, Bristol-Myers Squibb, Bridge Biotherapeutics, Celgene, Merck, Gilead, Arena Pharmaceuticals, and Protagonist Therapeutics; grants, personal fees, and other from Takeda Pharmaceuticals and Pfizer; personal fees and nonfinancial support from AbbVie; and grants and personal fees from Janssen Pharmaceuticals, Nestlé Health Science.
References
- 1. Katz S. “Mind the gap”: an unmet need for new therapy in IBD. J Clin Gastroenterol. 2007;41(9):799-809. doi: 10.1097/MCG.0b013e318033d71d [DOI] [PubMed] [Google Scholar]
- 2. Kayal M, Posner H, Spencer E, Colombel JF, Stalgis C, Ungaro RC.. Net remission rates with biologic and small molecule treatment in ulcerative colitis: a reappraisal of the clinical trial data. Clin Gastroenterol Hepatol. Published online January 12, 2023. doi: 10.1016/j.cgh.2023.01.005 [DOI] [PubMed] [Google Scholar]
- 3. Singh S, Dulai PS, Zarrinpar A, Ramamoorthy S, Sandborn WJ.. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017;14(2):110-121. doi: 10.1038/nrgastro.2016.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Campos FGMC, Kotze PG.. Burrill Bernard Crohn (1884-1983): the man behind the disease. Arq Bras Cir Dig. 2013;26(4):253-255. doi: 10.1590/s0102-67202013000400001 [DOI] [PubMed] [Google Scholar]
- 5. Gu P, Chhabra A, Chittajallu P, et al. Visceral adipose tissue volumetrics inform odds of treatment response and risk of subsequent surgery in IBD patients starting antitumor necrosis factor therapy. Inflamm Bowel Dis. 2022;28(5):657-666. doi: 10.1093/ibd/izab167 [DOI] [PubMed] [Google Scholar]
- 6. Bassi M, Singh S.. Impact of obesity on response to biologic therapies in patients with inflammatory bowel diseases. BioDrugs. 2022;36(2):197-203. doi: 10.1007/s40259-022-00522-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trayhurn P, Wood IS.. Adipokines: inflammation and the pleiotrophic role of white adipose tissue. Br J Nutr. 2004;92(3):347-355. [DOI] [PubMed] [Google Scholar]
- 8. Bryant RV, Schultz CG, Ooi S, et al. Visceral adipose tissue is associated with stricturing Crohn’s disease behavior, fecal calprotectin, and quality of life. Inflamm Bowel Dis. 2019;25(3):592-600. doi: 10.1093/ibd/izy278 [DOI] [PubMed] [Google Scholar]
- 9. Connelly TM, Juza RM, Sangster W, Sehgal R, Tappouni RF, Messaris E.. Volumetric fat ratio and not body mass index is predictive of ileocolectomy outcomes in Crohn’s disease patients . Dig Surg. 2014;31(3):219-224. doi: 10.1159/000365359 [DOI] [PubMed] [Google Scholar]
- 10. Gu P, Luo J, Kim J, et al. Effect of obesity on risk of hospitalization, surgery, and serious infection in biologic-treated patients with inflammatory bowel diseases: A CA-IBD Cohort Study. Am J Gastroenterol. 2022;117(10):1639-1647. doi: 10.14309/ajg.0000000000001855 [DOI] [PubMed] [Google Scholar]
- 11. Singh S, Proudfoot J, Xu R, Sandborn WJ.. Obesity and response to infliximab in patients with inflammatory bowel diseases: pooled analysis of individual participant data from clinical trials. Am J Gastroenterol. 2018;113(6):883-889. doi: 10.1038/s41395-018-0104-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wei J, Liu X, Xue H, Wang Y, Shi Z.. Comparisons of visceral adiposity index, body shape index, body mass index and waist circumference and their associations with diabetes mellitus in adults. Nutrients. 2019;11(7):1580. doi: 10.3390/nu11071580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Varma S, Faye AS, Kannan A, et al. Patients with more severe IBD get Clostridioides difficile rather than Clostridioides difficile increasing the severity of IBD. Dig Dis Sci. 2021;66(9):3113-3123. doi: 10.1007/s10620-020-06504-y [DOI] [PubMed] [Google Scholar]
- 14. Kulmala KA, Björk J, Andersson S, et al. Older age is a risk factor for inadequate energy intake during acute, severe IBD and is associated with shorter time to relapse. Scand J Gastroenterol. 2020;55(10):1185-1192. doi: 10.1080/00365521.2020.1818119 [DOI] [PubMed] [Google Scholar]
- 15. Wang FH, Meng LY, Yu TY, et al. Associations of abdominal visceral fat content and plasma adiponectin level with intracranial atherosclerotic stenosis: a cross-sectional study. Front Neurol. 2022;13(1):893401. doi: 10.3389/fneur.2022.893401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim JH, Kim J, Lee WJ, et al. A high visceral-to-subcutaneous fat ratio is an independent predictor of surgical site infection after gastrectomy. J Clin Med. 2019;8(4):494494. doi: 10.3390/jcm8040494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El-Serag HB, Hashmi A, Garcia J, et al. Visceral abdominal obesity measured by CT scan is associated with an increased risk of Barrett’s oesophagus: a case-control study. Gut. 2014;63(2):220-229. doi: 10.1136/gutjnl-2012-304189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giles JT, Allison M, Blumenthal RS, et al. Abdominal adiposity in rheumatoid arthritis: Association with cardiometabolic risk factors and disease characteristics. Arthritis Rheum. 2010;62(11):3173-3182. doi: 10.1002/art.27629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Irlbeck T, Massaro JM, Bamberg F, O’Donnell CJ, Hoffmann U, Fox CS.. Association between single-slice measurements of visceral and abdominal subcutaneous adipose tissue with volumetric measurements: the Framingham Heart Study. Int J Obes (Lond). 2010;34(4):781-787. doi: 10.1038/ijo.2009.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nobel YR, Su SH, Anderson MR, et al. Relationship between body composition and death in patients with COVID-19 differs based on the presence of gastrointestinal symptoms. Dig Dis Sci. 2022;67(9):4484-4491. doi: 10.1007/s10620-021-07324-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koutroumpakis E, Katsanos KH.. Implementation of the simple endoscopic activity score in Crohn’s disease. Saudi J Gastroenterol. 2016;22(3):183-191. doi: 10.4103/1319-3767.182455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mohammed Vashist N, Samaan M, Mosli MH, et al. Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst Rev. 2018;1(1):CD011450. doi: 10.1002/14651858.CD011450.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lim Z, Welman CJ, Raymond W, Thin L.. The effect of adiposity on anti-tumor necrosis factor-alpha levels and loss of response in Crohn’s disease patients. Clin Transl Gastroenterol. 2020;11(9):e00233. doi: 10.14309/ctg.0000000000000233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kredel LI, Siegmund B.. Adipose-tissue and intestinal inflammation - visceral obesity and creeping fat. Front Immunol. 2014;5:462. doi: 10.3389/fimmu.2014.00462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zulian A, Cancello R, Ruocco C, et al. Differences in visceral fat and fat bacterial colonization between ulcerative colitis and Crohn’s disease. An in vivo and in vitro study. PLoS One. 2013;8(10):e78495. doi: 10.1371/journal.pone.0078495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yadav DP, Kedia S, Madhusudhan KS, et al. Body composition in Crohn’s disease and ulcerative colitis: correlation with disease severity and duration. Can J Gastroenterol Hepatol. 2017;2017:1215035. doi: 10.1155/2017/1215035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thiberge C, Charpentier C, Gillibert A, et al. Lower subcutaneous or visceral adiposity assessed by abdominal computed tomography could predict adverse outcome in patients with Crohn’s disease. J Crohns Colitis. 2018;12(12):1429-1437. doi: 10.1093/ecco-jcc/jjy124 [DOI] [PubMed] [Google Scholar]
- 28. Rowan CR, McManus J, Boland K, O’Toole A.. Visceral adiposity and inflammatory bowel disease. Int J Colorectal Dis. 2021;36(11):2305-2319. doi: 10.1007/s00384-021-03968-w [DOI] [PubMed] [Google Scholar]


