Abstract
Background
Individuals with a history of depression/depressive symptoms are suspected to be at increased risk of incident inflammatory bowel diseases (IBDs).
Methods
We systematically searched MEDLINE/PubMed, Embase, and Scopus databases for longitudinal studies examining the association between depression/depressive symptoms and subsequent new-onset IBD (ie, Crohn’s disease and ulcerative colitis). We included studies in which the exposure was a confirmed diagnosis of depression/depressive symptoms measured through a validated scale. To limit concerns of diagnostic bias and reverse causality, and support temporality between exposure and outcomes, we synthesized estimates corresponding to the longest time lag reported. Two authors extracted study data independently and assessed each study’s risk of bias. Maximally adjusted relative risk (RR) estimates were synthesized using random- and fixed-effects models.
Results
Of 5307 records, 13 studies (8 cohort and 5 nested case-control studies; 9 million individuals) fulfilled the eligibility criteria. Depression was significantly associated with incident Crohn’s disease (RRrandom, 1.17; 95% confidence interval, 1.02-1.34; 7 studies, 17 676 cases) and ulcerative colitis (RRrandom, 1.21; 95% confidence interval, 1.10-1.33; 6 studies, 28 165 cases). The primary studies considered pertinent confounders. Several years, on average, separated exposure and outcomes. No evidence of important heterogeneity or publication bias was found. Summary estimates were at low risk of bias, and results were confirmed in multiple sensitivity analyses. No firm conclusions could be drawn regarding a dilution of the association over time.
Conclusions
Individuals with a history of depression may show small-to-moderate increased risk of IBD even when depression is diagnosed several years before new-onset IBD. Further epidemiological and mechanistic studies should clarify whether these associations are causal.
Keywords: depressive disorder, risk factors, inflammatory bowel diseases, bias, epidemiology
What is already known?
Conflicting evidence exists regarding the association between history of depression and subsequent development of inflammatory bowel disease (IBD).
What is new here?
Individuals with a history of depression may show a small-to-moderate increase in the risk of IBD, even when depression is diagnosed several years before new-onset IBD.
How can this study help patient care?
Our results can guide future research on mechanisms of the bidirectional brain-gut axis and inform clinicians and patients about possible prevention strategies.
INTRODUCTION
Inflammatory bowel diseases (IBDs) are chronic, disabling, immune-mediated disorders of the gastrointestinal tract comprising mainly Crohn’s disease (CD) and ulcerative colitis (UC).1,2 Patients with IBD are at high risk of developing depression and anxiety.3 Recent seminal studies have demonstrated that depression is associated with worse future prognosis of IBD.4,5 Although an increased susceptibility to depression in chronic diseases is often attributable to disability and decreased quality of life, an intricate communication system exists between the brain and the gut, and vice versa.6-8 This network comprises a complex signaling involving several metabolites, the autonomic nervous and neuroendocrine systems, the immune system, and the hypothalamic-pituitary-adrenal axis.9 Researchers have hypothesized that if the association between IBD and depression is bidirectional, depression may constitute a risk factor for developing new-onset IBD.10
Depression is prevalent in about 5% to 10% of the adult population.11 Accumulating evidence shows an association between depression or depressive symptoms and a number of incident diseases,12,13 including autoimmune14,15 and gastrointestinal conditions.16,17 Few studies have shown conflicting results regarding the influence of depression on development of new-onset IBD.10,18,19 Frolkis et al10 showed an approximately 2-fold higher risk of developing incident CD and UC among individuals with history of depression—a finding not confirmed, however, by Blackwell et al.19 Given the conflicting evidence in this area of research, we performed a systematic review and meta-analysis to clarify the association between history of depression and subsequent development of IBD. Significant diagnostic delay of IBD may render temporality between depressive symptoms and diagnosis of IBD challenging to assess20; hence, we synthesized stratified effect estimates corresponding to the longest time lag available between depression and incident IBD. Our findings may inform patients and clinicians, and guide future research on mechanisms and prevention strategies.
METHODS
The study protocol is registered with PROSPERO (CRD42022368356). We followed guidance published by the Cochrane collaboration21 and adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)22 and the MOOSE (Meta-analyses of Observational Studies in Epidemiology) guidelines.23
Search strategy
We systematically searched the MEDLINE/PubMed, Embase, and Scopus databases, from inception to October 5, 2022, to identify studies assessing whether individuals being diagnosed with depression (or showing symptoms of depression) are at increased risk of incident IBD, CD, or UC. Search terms included “inflammatory bowel disease,” “Crohn’s disease,” or “ulcerative colitis” combined with “depression,” “depressive,” “mood,” “depressive disorder,” “mood disorders,” or “self-harm.” We included relevant variants, acronyms, and truncated and MeSH (Medical Subject Headings) terms. The detailed search algorithms are presented in Supplementary Appendix 1. Reference lists of articles identified after screening titles and abstracts, including systematic and narrative reviews, were hand searched. The search strategy was complemented with a semi-structured search in Google Scholar. We excluded editorials and studies not conducted in humans. Language restrictions were not applied.
Study selection
We considered eligible any longitudinal study in which the temporal association between depressive symptoms (ie, first event, exposure) and IBD (ie, incident event, outcome) could be elucidated (ie, prospective or retrospective cohort studies and nested case-control studies).
To be eligible, studies were required to either measure depressive symptoms by using a validated scale (eg, Beck Depression Inventory, Mental Health Index, Hospital Anxiety and Depression Scale) or have registered a diagnosis of depression by using the International Classification of Diseases or an equivalent, validated record of diagnosis of depression at baseline. We included studies with radiologically, endoscopically, or histologically confirmed IBD or, alternatively, record-linkage studies using validated algorithms for the diagnosis of IBD. We did not consider studies in which the outcome was any measure of IBD activity (eg, relapses, hospitalizations, visits, fatigue). We excluded studies in which the measurement of depressive symptoms or diagnosis of depression co-occurred with the diagnosis of IBD and those not providing a minimum average of 1 year of follow-up after the exposure. In case of potentially eligible studies in which critical data were missing, we asked the authors to provide additional information.
Two investigators (D.P. and S.B.) independently screened titles and abstracts and excluded those that were clearly irrelevant. Potentially eligible articles were retrieved in full text and critically reviewed for inclusion. Any discrepancy was discussed and agreement was reached by consensus.
Data extraction
Two authors (D.P. and S.B.) extracted independently the following data from each study into a standardized data extraction form: citation data; study design; population characteristics; exposure(s) definition(s); outcome(s); number of exposed/unexposed individuals and person-time of follow-up; number of individuals who developed CD, UC, or IBD; follow-up length; lag-time/temporal distance between exposure and outcome; adjusted relative risk (RR) estimate(s) with 95% confidence intervals (CIs); method of control for confounding (ie, stratification, matching, restriction, or multivariable analysis); confounders considered; time period; geographic setting; and our evaluation of possible methodological limitations. Any pertinent effect estimate elucidating the association between depression and incident IBD at different temporal intervals was also extracted.
Study risk of bias
We used the Newcastle–Ottawa Scale24 to assess the risk of bias of observational studies on the basis of selection of study groups, comparability, ascertainment of exposure(s), and outcome(s). The risk of bias was rated as low (7-9 points), moderate (4-6 points), or high (<4 points).
Data synthesis and analysis
We extracted any pertinent estimate of RR available from each study but considered maximally adjusted estimates for the primary analysis. Besides age and sex, the most important confounders were deemed established risk factors for a diagnosis of IBD and conditions associated with depression and IBD (eg, smoking habits, socioeconomic status and income, presence of gastrointestinal symptoms, psychiatric and nonpsychiatric comorbidities).
We included studies reporting different measures of effect (ie, risk ratio, odds ratio, hazard ratio, and incidence rate ratio). As IBD is a rare outcome, these measures of RR yield very similar estimates; hence, a comprehensive quantitative evidence synthesis was deemed appropriate.
Significant diagnostic delay can occur in IBD, hence limiting a precise assessment of temporality between onset of depressive symptoms and confirmed diagnosis of IBD.19 In an attempt to resolve this issue, our primary analysis considered effect estimates corresponding to the longest lag time available between depression and incident IBD. Whenever possible, we conducted stratified analyses to elucidate any modifying effect of the reported timing between depression and subsequent IBD.
In case of substantial overlap in follow-up period and/or catching area across primary studies, we retained for meta-analysis the study including the largest number of incident events or, if similar, lower risk of bias. This was necessary to limit the risk of double counting individuals and artificially increase the precision of pooled estimates. We conducted sensitivity analyses using estimates extracted by the alternative study/studies (see Supplementary Appendix 2 for details).
We synthesized adjusted effect estimates by random-effects meta-analysis using the DerSimonian-Laird method yielding conservative 95% CIs in the presence of heterogeneity.25 We also calculated fixed-effects estimates, though our main conclusions were based on the random-effects model. Separate analyses were conducted for CD, UC, and IBD.
We investigated heterogeneity across studies using the Cochran’s Q test,26 with a conservative 0.10 threshold for significance, and quantified with the τ (standard deviation of the underlying effects across studies) and the I2 metric, reporting the proportion of the variability across studies that is due to between-study heterogeneity rather than to chance.27 If at least 10 studies were synthesized and in the presence of substantial heterogeneity (I2 > 60% or PCochran’s Q test < .10),27 we conducted subgroup analyses to investigate reasons for heterogeneity by geographical continent, study design, lag time between depression and incident IBD, and risk-of-bias assessment (Newcastle–Ottawa Scale). We tested whether estimates of associations between depression and subsequent IBD differed across strata using a test of interaction based on the Cochran’s Q test. Besides sensitivity analyses, we used the leave-one-out approach to further investigate the robustness of our results.28 We assessed small-study effect by visual inspection of the funnel plot and we applied the Egger’s test if at least 10 studies were available.29,30 Analyses were performed with Stata 17 software (StataCorp).
RESULTS
Literature Flow
We identified 5307 articles from the literature search. After detailed screening of 94 articles in full text, 13 eligible studies comprising over 9 million individuals were included.10,18,19,31-40 The search and selection process is shown in Supplementary Figure 1. The most commons reason for exclusion were that (1) the study was a systematic or narrative review and (2) depressive symptoms or depression were not assessed before diagnosis of IBD. Eight of 13 were prospective or retrospective cohort studies10,18,31,33,37,38,40 and 5 were nested case-control studies.19,32,35,36,39 Publication dates ranged from 2001 to 2022. Eight studies were conducted in Europe (United Kingdom [n = 5], Denmark [n = 2], Sweden),10,19,31,34-37,39 3 in North America (Canada [n = 2], United States),18,32,33 and 2 in Taiwan, Asia.38,40 All studies considered incident IBD occurring after a diagnosis of depression with an average follow-up time ranging from 2.235 to 13 years.18 Seven studies included 1 or more pertinent stratified analyses excluding patients diagnosed with IBD in proximity of previous depression (Table 1).10,18,19,32,34-36 One of the eligible studies was in the form of a letter34 including additional stratified analyses in respect to the original cohort study published by the same authors.10 Study characteristics are reported in Table 1. Varying degrees of population overlap were apparent between certain studies. Detailed reasons for selecting studies for data syntheses are reported in Supplementary Appendix 2.
Table 1.
Characteristics of eligible studies investigating the association between history of depression and incident CD, UC, or IBDs.
| Study, publication year | Country (database) | Study design (period) | Population | Exposure definition (depression/depressive symptoms) | Temporal relationship between exposure and outcome | Follow-up length (median) | Total study subjects | Total IBD/CD/UC subjects | Effect estimate (95% CI) | Control for confoundersa | Risk of biasb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ananthakrishnan et al, 201318 | United States (NHS I and II) | Prospective cohort (1992-2005) | NHS-I: Female nurses age 30-55 y at cohort start NHS-II: Female nurses age 25-42 y at cohort start |
Mental Health Index <53 evaluated at cohort entry | IBD diagnosis occurring after “remote depression” (median of 6 [IQR: 4 to 10] y between the 2 diagnoses) | 13 y (average) | 49 934 | CD (females): 60 | aHR, 1.62 (0.95-2.77) | 2-9 | 8 |
| UC (females): 60 | aHR, 1.07 (0.63-1.83) | ||||||||||
| Andersson et al, 201531 | Denmark | Retrospective cohort (1995-2012) | Any sex and age | ICD | IBD diagnosis occurring after depression diagnosis | 7 y (average) | 1 016 519 | CD: 1527 | aIRR, 1.36 (1.16-1.60) | 2,9,10,13 | 8 |
| UC: 2408 | aIRR, 1.17 (0.98-1.40)c | ||||||||||
| Bernstein et al, 201732 | Manitoba, Canada | Nested case-control (1989-2012) | Any sex and age | ICD | IBD diagnosis occurring ≥5 y after depression diagnosis | NR | 36 692 | IBD: 6119 | RR, 1.47 (1.32-1.63) | 2,9,10 | NA |
| Blackwell et al, 202119 | United Kingdom (CPRD) | Nested case-control (1998-2016) | Any sex and age | ICD | IBD diagnosis occurring at 4.5-5.5 y after depression diagnosis | 7.4 y | 9062 | CD: 4531 | aOR, 1.05 (0.78-1.42) | 2,3,9,11,12 | 9 |
| 21 658 | UC: 10 829 | aOR, 1.21 (0.99-1.47) | |||||||||
| Cawthorpe et al, 201533,d | Calgary, Canada | Retrospective cohort (1994-2009) | Any sex and age | ICD | UC diagnosis occurring after the diagnosis of any neurosis/depression diagnosis (4.7 y temporal distance on average) | NR | 413 644 | UC (females): 2334 | OR, 2.24 (2.04-2.47) | 9 | 5 |
| 348 638 | UC (males): 1612 | OR, 1.87 (1.70-2.07) | |||||||||
| Frolkis et al, 201910 and 202034 | United Kingdom (THIN) | Retrospective cohort (1986-2012) | Any sex, 10-90 y old | Diagnostic code for depressive disorder | IBD diagnosis occurring ≥2 y after the diagnosis of depression | 6.7 y | 5 727 651 | CD: 1792 | aHR, 1.36 (1.02-1.80) | 2,3,9,12,14,17,18 | 9 |
| UC: 5214 | aHR, 1.66 (1.40-1.97) | ||||||||||
| García Rodríguez et al, 200535 | United Kingdom (GPRD) | Nested case-control (1995-1997) | Any sex, 20-84 y old | Diagnostic code for depression | UC diagnosis occurring ≥2 y after the diagnosis of depression | 2.2 y (average) | ~10 000 | UC: 222 | aOR, 1.37 (0.90-2.08) | 2,3,9,17,19-27 | 6 |
| CD diagnosis occurring after the diagnosis of depression | CD: 171 | aOR, 0.94 (0.59-1.48) | |||||||||
| Kurina et al, 200136 | Oxford, United Kingdom (ORLS) | Nested case-control (1963-1999) | Any sex and age | ICD | IBD diagnosis occurring ≥5 y after the diagnosis of depression | NR | ~810 000 | UC: 7269 | RR, 1.49 (1.12-1.93) | 2,9,10,19 | 6 |
| CD: 5231 | RR, 1.15 (0.76-1.67) | ||||||||||
| Leone et al, 202137 | Sweden | Retrospective cohort (1987-2013) | Any sex and age | ICD | IBD diagnosis occurring after “youth depression” (depression onset between 5 and 19 y old) | NR | 1 487 964 | UC: 7377 | aHR, 1.13(0.94-1.37) | 2,9,13,19 | 7 |
| CD: 6052 | aHR, 1.00(0.81-1.23) | ||||||||||
| Lin et al, 201838 | Taiwan | Retrospective cohort (1996-2013) | Primiparous women ≥20 y old | ICD | CD diagnosis occurring after postpartum depression | 6.9 y (average) | 2707 | CD (females): 104 | aHR, 1.31 (0.82-2.09) | 2,9,14-16 | 8 |
| Torres et al, 202339 | Denmark | Nested case-control (1977-2018) | Any age and sexe | ICD | IBD diagnosis occurring after depression diagnosis | NR | 38 838 | IBD: 1254 | aOR, 1.29 (0.63-2.63) | 2,9,28,29 | 6 |
| UC: NR | aOR, 1.56 (0.63-3.90) | ||||||||||
| CD: NR | aOR, 0.79 (0.19-3.28) | ||||||||||
| Zhang et al, 202240 | Taiwan | Retrospective cohort (2001-2011) | Any sex and age | ICD | IBD diagnosis occurring after depression diagnosis | NR | 130 140 | IBD: 76 | aOR, 1.87 (1.07-3.26) | 2,9,14,30-33 | 6 |
This table reports maximally adjusted effect estimates corresponding to the longest time period available between depression and IBDs (ie, primary analysis). Variables controlled for were age,2 smoking,3 oral contraceptives,4 postmenopausal hormones,5 aspirin,6 nonsteoridal anti-inflammatory drugs,7 body mass index,8 sex,9 geographic area/municipality,10 gastrointestinal symptoms before depression,11 socioeconomic status,12 psychiatric comorbidities,13 nonpsychiatric comorbidities,14 alcohol use disorder,15 tobacco use disorders,16 anxiety diagnosis,17 antidepressant drug use,18 calendar year,19 osteoarthritis,20 rheumatoid arthritis,21 stress level,22 asthma,23 chronic obstructive pulmonary disorder,24 diabetes,25 irritable bowel disease,26 appendectomy,27 disease-free time at risk,28 birth order,29 income,30 urbanization,31 Charlson comorbidity index,32 and all-cause clinical visits.33.
Abbreviations: aHR, adjusted hazard ratio; aIRR, adjusted incidence rate ratio; aOR, adjusted odds ratio; CD, Crohn’s disease; CI, confidence interval; CPRD, Clinical Practice Research Datalink; GPRD, General Practice Research Database; IBD, inflammatory bowel disease; ICD, International Classification of Diseases; IQR, interquartile range; NA, not applicable; NHS, Nurses’ Health Study; NR, not reported; OR, odds ratio; ORLS, Oxford Record Linkage Study; RR, relative risk; THIN, The Health Improvement Network; UC, ulcerative colitis.
aThrough matching, stratification, restriction, or statistical analysis. This column refers to confounders considered in calculating the effect estimate of interest (ie, the one reported in the current table) and not necessarily to the primary analysis of the original study.
bRisk of bias assessment for observational studies: Newcastle–Ottawa Scale based on selection of study groups, comparability, ascertainment of exposure(s), and outcome(s). The risk of bias was rated as low (7-9 points), moderate (4-6 points), or high (<4 points).
cThe 95% CI corresponding to this effect estimate was originally asymmetric. We have corrected it (ie, made it symmetric) by deriving the standard error from the distance between the lower bound and the point estimate.
dIn this study, the defining criteria for exposure were broader than depression alone; hence, it was included only in sensitivity analyses. The study considered exposed subjects those with a diagnosis of major depressive disorder (ICD–Ninth Revision codes 296.2, 296.3), neurotic disorders (code 300), acute reaction to stress (code 308), adjustment reaction (code 309), or depression disorder not classified elsewhere (code 311).
eCases were individuals developing IBD in families in which at least 1 first-degree relative had IBD. Controls were individuals of other families matched 1:30 by age, sex, and disease-free time.
Risk-of-bias assessment
Seven studies scored 7 points or higher and were judged at low risk of bias.10,18,19,31,34,37,38 Other 5 studies were rated at moderate risk of bias (Table 1, Supplementary Table 1).33,35,36,39,40 The article retrieved as a conference abstract was not assessed,32 as critical appraisal of such studies is rather limited.
Crohn’s disease
An estimate of the association between depression and subsequent CD was reported by 10 studies (Table 1),10,18,19,31,34-39 Seven studies including over 3.3 million individuals and 17 676 cases of incident CD were suitable for data synthesis (see Supplementary Appendix 2 for details).18,19,31,35-38
In the primary analysis, we synthesized stratified effect estimates corresponding to the longest period of time available between depression and incident CD. History of depression was associated with an increased risk of subsequent CD (RR, 1.17; 95% CI, 1.02-1.34) (Figure 1, Table 2), though the magnitude of effect was small. We found no evidence of important heterogeneity (I2 = 29.1%; P = .21). Although a formal analysis of publication bias was not carried out due to a small number of studies, the funnel plot did not suggest any asymmetrical pattern (Supplementary Figure 2). Five studies were at low risk of bias and 2 were at moderate risk of bias. Meta-analysis of studies at low risk of bias was consistent with the primary analysis (RR, 1.20; 95% CI, 1.01-1.42) (Supplementary Figure 3).18,19,31,37,38 Sensitivity analyses conducted by selecting alternative studies in case of overlap in populations yielded random- and fixed-point effect estimates ranging from 1.20 to 1.26 and the same conclusions regarding statistical significance (Table 2, Supplementary Appendix 2, Supplementary Figures 4 and 5). In a secondary analysis pooling effect estimates considering patients with UC diagnosed at any time after depression (ie, not the longest time distance available), the association was compatible with the primary analysis (RR, 1.50; 95% CI, 1.11-2.03) (Table 2, Supplementary Figure 6), though large heterogeneity was noted (I2 = 83.8%; P < .01).
Figure 1.
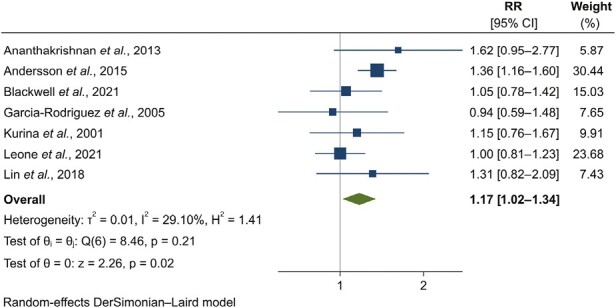
Forest plot for the association between history of depression and incident Crohn’s disease: results from individual studies and meta-analysis. The relative risk (RR) and 95% confidence interval (CI) for each study are displayed on a logarithmic scale.
Table 2.
Results of meta-analysis of studies investigating the association between history of depression and CD, UC, or IBDs.
| No. of studies | Pooled effect estimate: RR (95% CI) | Test of homogeneity | Risk of bias | Test of interaction (P value)a | |||||
|---|---|---|---|---|---|---|---|---|---|
| Random effects | Fixed effects | Q value | P value | I2 (%) | Studies at low risk of bias (n/N) | Overall judgment | |||
| CD | |||||||||
| Primary analysis | 7 | 1.17 (1.02-1.34) | 1.19 (1.07-1.32) | 8.5 | .21 | 29.1 | 5/7 | low | |
| Sensitivity 1b | 5 | 1.26 (1.07-1.48) | 1.25 (1.12-1.40) | 6.7 | .15 | 40.4 | 5/5 | low | |
| Sensitivity 2b | 8 | 1.20 (1.06-1.35) | 1.21 (1.10-1.33) | 9.2 | .24 | 24.0 | 6/8 | low | |
| Subgroups | |||||||||
| Risk-of-bias assessment c | .47 | ||||||||
| Studies rated at low risk of bias | 5 | 1.20 (1.01-1.42) | 1.21 (1.08-1.35) | 7.3 | .12 | 45.4 | 5/5 | low | |
| Studies rated at moderate risk of bias | 2 | 1.06 (0.78-1.42) | 1.06 (0.78-1.42) | 0.4 | .51 | 0.0 | 0/2 | moderate | |
| Study design | .27 | ||||||||
| Cohort studies | 4 | 1.24 (1.01-1.53) | 1.24 (1.10-1.39) | 6.4 | .10 | 52.8 | 4/4 | low | |
| Nested case-control studies | 3 | 1.05 (0.85-1.30) | 1.05 (0.85-1.30) | 0.4 | .81 | 0.0 | 1/3 | moderate | |
| Geographic area | .40 | ||||||||
| Europe | 5 | 1.13 (0.96-1.32) | 1.17 (1.05-1.30) | 6.9 | .14 | 42.1 | 3/5 | moderate | |
| North America | 1 | 1.62 (0.95-2.77) | 1.62 (0.95-2.77) | NA | NA | NA | 1/1 | low | |
| Asia | 1 | 1.31 (0.82-2.09) | 1.31 (0.82-2.09) | NA | NA | NA | 1/1 | low | |
| UC | |||||||||
| Primary analysis | 6 | 1.21 (1.10-1.33) | 1.21 (1.10-1.33) | 3.4 | .63 | 0.0 | 4/6 | low | |
| Sensitivity 1b | 4 | 1.27 (1.02-1.59) | 1.31 (1.18-1.45) | 11.8 | .01 | 74.7 | 4/4 | low | |
| Sensitivity 2b | 7 | 1.30 (1.14-1.49) | 1.31 (1.20-1.42) | 13.4 | .04 | 55.1 | 5/7 | low | |
| Sensitivity 3b | 7 | 1.43 (1.15-1.79) | 1.72 (1.62-1.82) | 86.5 | <.01 | 91.9 | 4/7 | moderate | |
| Subgroups | |||||||||
| Risk-of-bias assessment c | .08 | ||||||||
| Studies rated at low risk of bias | 4 | 1.16 (1.05-1.29) | 1.16 (1.05-1.29) | 0.3 | .95 | 0.0 | 4/4 | low | |
| Studies rated at moderate risk of bias | 2 | 1.45 (1.16-1.83) | 1.45 (1.16-1.83) | 0.1 | .74 | 0.0 | 0/2 | moderate | |
| Study design | .18 | ||||||||
| Cohort studies | 3 | 1.15 (1.01-1.30) | 1.15 (1.01-1.30) | 0.1 | .93 | 0.0 | 3/3 | low | |
| Nested case-control studies | 3 | 1.31 (1.13-1.52) | 1.31 (1.13-1.52) | 1.5 | .63 | 0.0 | 1/3 | Moderate | |
| Geographic area | .64 | ||||||||
| Europe | 5 | 1.22 (1.10-1.34) | 1.22 (1.10-1.34) | 3.2 | .52 | 0.0 | 3/5 | Moderate | |
| North America | 1 | 1.07 (0.63-1.82) | 1.07 (0.63-1.82) | NA | NA | NA | 1/1 | Low | |
| IBDs | |||||||||
| Primary analysis | 3 | 1.48 (1.33-1.64) | 1.48 (1.33-1.64) | 0.8 | .66 | 0.0 | 0/3 | Moderate | |
| Subgroups | |||||||||
| Risk-of-bias assessment c | .66 | ||||||||
| Studies rated at moderate risk of bias | 2 | 1.63 (1.05-2.52) | 1.63 (1.05-2.52) | 0.7 | .42 | 0.0 | 0/2 | Moderate | |
| NA | 1 | 1.47 (1.32-1.63) | 1.47 (1.32-1.63) | NA | NA | NA | 0/1 | NA | |
| Study design | .40 | ||||||||
| Cohort studies | 1 | 1.87 (1.07-3.26) | 1.87 (1.07-3.26) | NA | NA | NA | 0/1 | Moderate | |
| Nested case-control studies | 2 | 1.47 (1.32-1.63) | 1.47 (1.32-1.63) | 0.1 | .72 | 0.0 | 0/2 | NA | |
| Geographic area | .66 | ||||||||
| Europe | 1 | 1.29 (0.63-2.63) | 1.29 (0.63-2.63) | NA | NA | NA | 0/1 | Moderate | |
| North America | 1 | 1.47 (1.32-1.63) | 1.47 (1.32-1.63) | NA | NA | NA | NA | NA | |
| Asia | 1 | 1.87 (1.07-3.26) | 1.87 (1.07-3.26) | NA | NA | NA | 0/1 | Moderate | |
These analyses synthesized maximally adjusted effect estimates corresponding to the longest time period available between depression and IBDs. Primary, sensitivity, and subgroup analyses are presented.
Abbreviations: CD, Crohn’s disease; CI, confidence interval; IBD, inflammatory bowel disease; NA, not applicable; RR, relative risk; UC, ulcerative colitis.
aConsidering random-effect estimates in subgroups.
bSensitivity analyses are described in detail in Supplementary Appendix 2.
cNewcastle–Ottawa Scale based on selection of study groups, comparability, ascertainment of exposure(s), and outcome(s). The risk of bias was rated as low (7-9 points), moderate (4-6 points), or high (<4 points).
Ulcerative colitis
Of 10 studies investigating the association between depression and subsequent UC (Table 1),10,18,19,31,33-37,39 we synthesized 6 studies including about 3.4 million individuals and 28 165 cases of incident UC (see Supplementary Appendix 2 for details).18,19,31,35-37
When summarizing stratified effect estimates corresponding to the longest period of time between exposure and outcome, history of depression was associated with an increased risk of subsequent UC (RR, 1.21; 95% CI, 1.10-1.33) (Figure 2, Table 2). The magnitude of effect was modest. No evidence of heterogeneity was found (I2 = 0.0%; P = .63). Although a formal analysis of publication bias was not carried out due to the small number of studies, the funnel plot did not suggest any asymmetrical pattern (Supplementary Figure 7). Meta-analysis of the 4 studies at low risk of bias was consistent with the primary analysis (RR, 1.16; 95% CI, 1.05-1.29) (Supplementary Figure 8).18,19,31,37 Sensitivity analyses conducted by selecting alternative studies in case of overlap in populations yielded random- and fixed-summary-point estimates ranging from 1.27 to 1.72 and the same conclusions regarding statistical significance (Table 2, Supplementary Appendix 2, Supplementary Figures 9-11). In a secondary analysis pooling estimates considering patients with UC diagnosed at any time after depression (ie, not the longest time distance available), the association was compatible with the primary analysis (Table 2, Supplementary Figure 12). We noted very large heterogeneity in this secondary analysis (I2 = 93.1%; P < .01).
Figure 2.
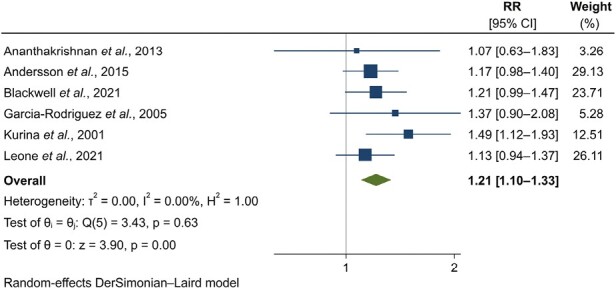
Forest plot for the association between history of depression and incident ulcerative colitis: results from individual studies and meta-analysis. The relative risk (RR) and 95% confidence interval (CI) for each study are displayed on a logarithmic scale.
Inflammatory bowel diseases
Three studies, including over 200 000 individuals and 7449 cases of incident IBD, investigated the association between depression and subsequent IBD.32,39,40 History of depression was a risk factor for incident IBD (RR, 1.48; 95% CI, 1.33-1.64) (Figure 3, Table 2). No heterogeneity was noted (I2 = 0.0%; P = .66). The risk of bias of 2 studies was moderate.39,40 One estimate derived from a published conference abstract.32 The random-effects estimate obtained by excluding the conference abstract was 1.63 (95% CI, 1.05-2.52) (Table 2).
Figure 3.
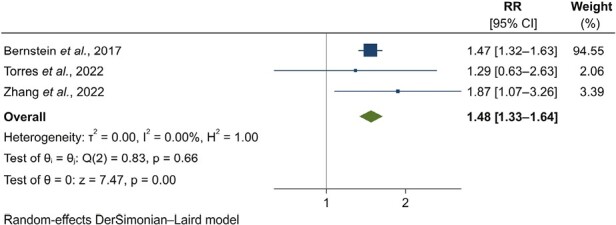
Forest plot for the association between history of depression and incident inflammatory bowel diseases: results from individual studies and meta-analysis. The relative risk (RR) and 95% confidence interval (CI) for each study are displayed on a logarithmic scale.
Robustness of results
Excluding the study by Andersson et al31 in leave-one-out meta-analysis pushed the summary effect estimate of the association between history of depression and incident CD to include the null (Supplementary Figure 13A), though the effect estimate remained relatively stable. The results of the primary analysis pertaining UC and IBD were robust (Supplementary Figure 13B, 13C). Primary results were not influenced significantly by study design, risk of bias, or geographical continent (P > .05) (Table 2).
Temporality of associations
We searched for any evidence of a differential association between history of depression and IBD over time. Six studies reported at least 2 effect estimates including cases of incident CD (5 studies) or UC (6 studies) diagnosed at different, definite time lags after depression.10,18,19,34-36 Effect estimates considering different temporal intervals and including largely overlapping populations precluded a formal data synthesis of stratified data (Table 3).
Table 3.
Effect estimates elucidating the temporal relationship between history of depression and incident CD or UC as reported by primary studies.
| Study, publication year | Population | Exposure definition (depression/depressive symptoms) | Stratified analysis (time between exposure and incident outcome) | Effect estimate (95% CI) | P for the differencea |
|---|---|---|---|---|---|
| Ananthakrishnan et al, 201313 | NHS-I: Female nurses age 30-55 y at cohort start NHS-II: Female nurses age 25-42 y at cohort start |
Mental Health Index < 53 evaluated at cohort entry | CD: any time CD: ≥2 y CD: remote depression (~6 y) UC: any time UC: ≥2 y UC: remote depression (~6 y) |
aHR, 2.36 (1.40-3.99) aHR, 2.13 (1.15-3.95) aHR, 1.62 (0.95-2.77) aHR, 1.14 (0.68-1.92) aHR, 1.31 (0.75-2.26) aHR, 1.07 (0.63-1.83) |
NAb |
| Blackwell et al, 202119 | Any sex and age | ICD | CD: 2-4 y CD: 4.5-5.5 y UC: 2-4 y UC: 4.5-5.5 y |
aOR, 1.08 (0.84-1.39) aOR, 1.05 (0.78-1.42) aOR, 1.23 (1.05-1.45) aOR, 1.21 (0.99-1.47) |
.89 .90 |
| Frolkis et al, 201910 and 202034 | Any sex, 10-90 y old | Diagnostic code for depressive disorder | CD: any time CD: ≥6 mo CD: ≥9 mo CD: ≥2 y UC: any time UC: ≥6 y UC: ≥9 y UC: ≥2 y |
aHR, 2.11 (1.65-2.70 aHR, 1.89 (1.47-2.44) aHR, 1.72 (1.33-2.24) aHR, 1.36 (1.02-1.80) aHR, 2.23 (1.92-2.60) aHR, 2.11 (1.81-2.46) aHR, 2.00 (1.71-2.34) aHR, 1.66 (1.40-1.97) |
NAb |
| García Rodríguez et al, 200535 | Any sex, 20-84 y old | Diagnostic code for depression | UC: 0-2 y UC: ≥2 y |
aOR, 1.39 (0.71-2.71) aOR, 1.37 (0.90-2.08) |
.97 |
| Kurina et al, 200136 | Any sex and age | ICD | CD: 0-1 y CD: 1-4 y CD: ≥5 y UC: 0-1 y UC: 1-4 y UC: ≥5 y |
RR, 1.66 (0.75-3.16) RR, 0.94 (0.51-1.58) RR, 1.15 (0.76-1.67) RR, 2.14 (1.22-3.49) RR, 1.01 (0.62-1.55) RR, 1.49 (1.12-1.93) |
.48 .10 |
Abbreviations: aHR, adjusted hazard ratio; aOR, adjusted odds ratio; CD, Crohn’s disease; CI, confidence interval; NA, not applicable; RR, relative risk; UC, ulcerative colitis.
aWe tested whether the RR estimates of associations between history of depression and incident CD or UC differed across strata using a test of interaction based on Cochran’s Q test.
bDue to multiple overlapping strata.
Ananthakrishnan et al18 reported estimates including all cases of CD diagnosed after depression (adjusted hazard ratio [aHR], 2.36; 95% CI, 1.40-3.99), restricting to patients diagnosed at least after 2 years (aHR, 2.13; 95% CI, 1.15-3.95), and using the longest period available (ie, the baseline questionnaire of the Nurses’ Health Studies, corresponding in most cases to a time distance of over 6 years; aHR, 1.62; 95% CI, 0.95-2.77). Although there was an apparent dilution of effect over time, no definite conclusion could be drawn given the vastly overlapping periods. This was not observed for UC.
The study by Frolkis et al34 was the one providing more evidence of a dilution of effect over time, though no definite conclusions could be drawn. Despite the apparent dilution, the estimates remained statistically significant when considering patients diagnosed with CD or UC at least 2 years after depression (aHRCD, 1.36; 95% CI, 1.02-1.80; aHRUC, 1.66; 95% CI, 1.40-1.97).34
In the study by Kurina et al,36 estimates considering patients diagnoses with CD or UC within 1 year from depression were apparently larger than those obtained excluding such patients. Nonetheless, no statistically significant difference was found across stratified effect estimates (Table 3).36 The studies by Blackwell et al19 and García Rodríguez et al35 did not show evidence of a differential association between depression and IBD over time.
Overall, no firm conclusions could be drawn regarding a dilution of the association over time.
DISCUSSION
In this systematic review and meta-analysis, individuals with a history of depression or depressive symptoms were at increased risk of developing subsequent new-onset IBD. In particular, depression was associated with a 17% increased risk of incident CD and a 21% increased risk of UC, even in case of large temporal distance between the 2 diagnoses. Our analyses included several millions of individuals at risk and dozens of thousands of incident diagnoses of IBD. Our conclusions are based on longitudinal studies designed to assess the temporal relationship between history of depression and IBD and employing homogeneous definitions of exposure and outcomes. Although the magnitude of association was overall modest, the primary analyses included mostly studies at low risk of bias and having adjusted by a considerable number of potential confounders. Results were consistent across sensitivity analyses, though the association observed with CD was slightly less robust than the one observed with UC. We carefully synthesized estimates corresponding to the longest time distance between exposure and outcomes. This was deemed necessary to limit concerns that a diagnostic delay of IBD may have masked a different temporal sequence of the 2 events.34 In fact, depression may co-occur after the first symptoms of IBD as a reaction to disability and impaired quality of life.3,20 Although assessing an association with certain lag times makes less likely such biases, a dilution of effect estimates at increasing time distances is plausible. Depression symptoms may remain undiagnosed for some time and become worse in severity nearing the time of diagnosis of IBD (ie, healthcare-seeking behavior),34 thus leading to the 2 conditions being diagnosed closer in time than otherwise apparent based on symptoms. Furthermore, depression may contribute to systemic and intestinal inflammation,6,8 which renders the coexistence of the 2 events more probable.
In the last decade, the prevalence of depression has increased, especially among young adults.41 Recent seminal studies have associated depression with several incident medical conditions, including autoimmune diseases.12-15,37,42 The associations observed are biologically plausible. There is an association between depression and inflammation mediated by an extensive cytokine signaling and the peripheral immune system.43 There are hints about the presence of a bidirectional pathway between intestinal inflammation and the brain.6,9 Animal models have shown that (1) inducing colitis leads to brain changes,44 (2) depression is associated with bowel inflammation,45 and (3) the gut microbiota may modulate the relationship between the brain and intestinal inflammation.6 Studies conducted in humans have partially confirmed the first46 and third47 previously mentioned findings. The current paradigm of a bidirectional gut-brain axis involves afferent and efferent vagal fibers, central inducible nitric oxide synthase, peripheral and central proinflammatory cytokines, and gut dysbiosis as a result of environmental stimuli in a plausible interplay with genetic factors.6,8,9,43
Landmark primary studies and meta-analyses have shown that depression is often found in patients with IBD and that patients with quiescent IBD and depression at baseline have a less favorable prognosis.3-5 This is the first systematic review and meta-analysis synthesizing longitudinal studies investigating history of depression as a possible risk factor for subsequent development of IBD. The study represents a further necessary step in clarifying the supposed bidirectional link between depression and IBD. Our work used a standardized methodology, a comprehensive search strategy in 3 databases, and followed a registered protocol. We used both random- and fixed-effects models but based our conclusions on random-effects estimates to provide a more conservative estimate of associations. Although plausible, we did not find evidence of heterogeneity, which renders the results more robust. Most studies included in the evidence syntheses were considered at low risk of bias.
We must acknowledge some limitations. The small overall number of studies did not allow a formal assessment of publication bias, though the visual inspection of funnel plots was reassuring. There were overlaps in populations across certain estimates, thus we had to exclude valid studies from the data synthesis to avoid providing unrealistically precise summary estimates. Nonetheless, we described transparently the entire selection process. Sensitivity analyses summarizing studies previously excluded confirmed the primary analyses. One potential limitation is that the primary studies synthesized showed some differences in populations. The study by Lin et al38 considered postpartum depression and the study by Cawthorpe et al33 considered depression and neuroses. Therefore, these studies may have contributed to heterogeneity in effect estimates. Given the broader outcome definition of the study by Cawthorpe et al, this study was not included in the primary analysis. Few authors reported data sufficient to assess whether the association between depression and IBD changed over time, and these findings were partially conflicting. Hence, more studies are needed to this regard.
The associations found were of modest magnitude. This was partially expected. Our findings are compatible with results of stratified analyses reported by prominent primary studies in the area of IBD18,19,34 and with the effect sizes reported by landmark studies investigating the association between mood disorders or depression and other chronic medical conditions.12,13 We synthesized estimates corresponding, on average, to a distance of several years between the 2 conditions, hence strongly reducing biases that would have falsely inflated the association.
Given the high prevalence of depression, our findings might have relevance for prevention. Frolkis et al10 suggested that antidepressants may mitigate the risk of developing IBD among individuals diagnosed with underlying depression. This observation may have a possible biological basis. Patients with IBD show low colon mucosal norepinephrine and serotonin levels,48,49 which may be rescued by certain classes of antidepressants.10 Further epidemiological and mechanistic studies are needed to clarify this important aspect.
CONCLUSIONS
The results of the current systematic review and meta-analysis are compatible with a small to moderate increased risk of IBD among individuals with depression, even in case of large temporal distance between the 2 diagnoses. The findings are in agreement with an increasing body of epidemiological evidence linking depression with several incident medical conditions,12-15,37,42 and with mechanistic studies investigating the systemic and gut-specific consequences of depressive symptoms.6,9,44-47 Further rigorous cohort studies are needed to confirm these results and help clarifying further the temporal association between exposure and outcomes and whether prevention measures may be useful. Additional studies should elucidate mechanisms linking mood disorders and depression with development of IBD. More and innovative research is needed to clarify the role of mood disorders on gut inflammation and vice versa and to determine whether peculiar genetic susceptibilities can affect this association. Our results may prompt and orient future research on mechanisms and prevention strategies.
Supplementary Material
Contributor Information
Daniele Piovani, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Italy; IRCCS Humanitas Research Hospital, Rozzano, Italy.
Alessandro Armuzzi, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Italy; IBD Center, IRCCS Humanitas Research Hospital, Rozzano, Italy.
Stefanos Bonovas, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Italy; IRCCS Humanitas Research Hospital, Rozzano, Italy.
Author Contribution
D.P. was involved with the study conceptualization and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and statistical analysis. A.A. was involved with the study conceptualization and design, interpretation of data, and critical revision of the manuscript for important intellectual content. S.B. was involved with the study conceptualization and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and study supervision.
Funding
This work was funded by BIBLIOSAN.
Conflicts of Interest
A.A. has received consulting fees from AbbVie, Allergan, Amgen, Arena, Biogen, Bristol-Myers Squibb, Celgene, Celltrion, Eli Lilly, Ferring, Galapagos, Gilead, Janssen, MSD, Mylan, Pfizer, Protagonist Therapeutics, Roche, Samsung Bioepis, Sandoz, and Takeda; speaker fees from AbbVie, Amgen, Arena, Biogen, Bristol-Myers Squibb, Eli Lilly, Ferring, Galapagos, Gilead, Janssen, MSD, Novartis, Pfizer, Roche, Samsung Bioepis, Sandoz, Takeda, and Tigenix; and research support from MSD, Takeda, Pfizer, and Biogen. The other authors declare no financial disclosures or conflicts of interest.
Data Availability
The data underlying this article are available in the article and in its online supplementary material.
References
- 1. Torres J, Mehandru S, Colombel J-F, Peyrin-Biroulet L.. Crohn’s disease. Lancet. 2017;389(10080):1741-1755. [DOI] [PubMed] [Google Scholar]
- 2. Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet. 2017;389(10080):1756-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barberio B, Zamani M, Black CJ, Savarino EV, Ford AC.. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6(5):359-370. [DOI] [PubMed] [Google Scholar]
- 4. Fairbrass KM, Lovatt J, Barberio B, Yuan Y, Gracie DJ, Ford AC.. Bidirectional brain-gut axis effects influence mood and prognosis in IBD: a systematic review and meta-analysis. Gut. 2022;71(9):1773-1780. [DOI] [PubMed] [Google Scholar]
- 5. Gracie DJ, Guthrie EA, Hamlin PJ, Ford AC.. Bi-directionality of brain-gut interactions in patients with inflammatory bowel disease. Gastroenterology. 2018;154(6):1635-1646.e3. [DOI] [PubMed] [Google Scholar]
- 6. Bisgaard TH, Allin KH, Keefer L, Ananthakrishnan AN, Jess T.. Depression and anxiety in inflammatory bowel disease: epidemiology, mechanisms and treatment. Nat Rev Gastroenterol Hepatol. 2022;19(11):717-726. [DOI] [PubMed] [Google Scholar]
- 7. Peppas S, Pansieri C, Piovani D, et al. The brain-gut axis: psychological functioning and inflammatory bowel diseases. J Clin Med. 2021;10(3):377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carloni S, Bertocchi A, Mancinelli S, et al. Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science. 2021;374(6566):439-448. [DOI] [PubMed] [Google Scholar]
- 9. Morais LH, Schreiber HL, Mazmanian SK.. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19(4):241-255. [DOI] [PubMed] [Google Scholar]
- 10. Frolkis AD, Vallerand IA, Shaheen A-A, et al. Depression increases the risk of inflammatory bowel disease, which may be mitigated by the use of antidepressants in the treatment of depression. Gut. 2019;68(9):1606-1612. [DOI] [PubMed] [Google Scholar]
- 11. Brody DJ, Pratt LA, Hughes JP.. Prevalence of depression among adults aged 20 and over: United States, 2013-2016. NCHS Data Brief. 2018(303):1-8. [PubMed] [Google Scholar]
- 12. Momen NC, Plana-Ripoll O, Agerbo E, et al. Association between mental disorders and subsequent medical conditions. N Engl J Med. 2020;382(18):1721-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bobo WV, Grossardt BR, Virani S, St Sauver JL, Boyd CM, Rocca WA.. Association of depression and anxiety with the accumulation of chronic conditions. JAMA Netw Open. 2022;5(5):e229817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roberts AL, Kubzansky LD, Malspeis S, Feldman CH, Costenbader KH.. Association of depression with risk of incident systemic lupus erythematosus in women assessed across 2 decades. JAMA Psychiatry 2018;75(12):1225-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Siegmann EM, Müller HHO, Luecke C, Philipsen A, Kornhuber J, Grömer TW.. Association of depression and anxiety disorders with autoimmune thyroiditis: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75(6):577-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levenstein S, Ackerman S, Kiecolt-Glaser JK, Dubois A.. Stress and peptic ulcer disease. JAMA. 1999;281(1):10-11. [DOI] [PubMed] [Google Scholar]
- 17. Levy RL, Olden KW, Naliboff BD, et al. Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterology. 2006;130(5):1447-1458. [DOI] [PubMed] [Google Scholar]
- 18. Ananthakrishnan AN, Khalili H, Pan A, et al. Association between depressive symptoms and incidence of Crohn’s disease and ulcerative colitis: results from the Nurses’ Health Study. Clin Gastroenterol Hepatol. 2013;11(1):57-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blackwell J, Saxena S, Petersen I, et al. ; POP-IBD study group. Depression in individuals who subsequently develop inflammatory bowel disease: a population-based nested case-control study. Gut. 2021;70(9):1642-1648. [DOI] [PubMed] [Google Scholar]
- 20. Vavricka SR, Spigaglia SM, Rogler G, et al. ; Swiss IBD Cohort Study Group. Systematic evaluation of risk factors for diagnostic delay in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18(3):496-505. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3. Cochrane Collaboration; 2022. [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012. [DOI] [PubMed] [Google Scholar]
- 23. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. [DOI] [PubMed] [Google Scholar]
- 24. Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale for assessing the quality of non-randomised studies in meta-analyses. Accessed June 01, 2023. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 25. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. [DOI] [PubMed] [Google Scholar]
- 26. Cochran WG. The comparison of percentages in matched samples. Biometrika 1950;37(3-4):256-266. [PubMed] [Google Scholar]
- 27. Higgins JP, Thompson SG, Deeks JJ, Altman DG.. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Greenhouse JB, Iyengar S.. Sensitivity analysis and diagnostics. In: Cooper HM, Hedges LV, Valentine JC, eds. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. Russell Sage Foundation; 2009:423-424. [Google Scholar]
- 29. Sterne JA, Gavaghan D, Egger M.. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53(11):1119-1129. [DOI] [PubMed] [Google Scholar]
- 30. Egger M, Davey Smith G, Schneider M, Minder C.. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andersson NW, Gustafsson LN, Okkels N, et al. Depression and the risk of autoimmune disease: a nationally representative, prospective longitudinal study. Psychol Med. 2015;45(16):3559-3569. [DOI] [PubMed] [Google Scholar]
- 32. Bernstein CN, Walker J, Bolton J, et al. Prevalence of psychiatric disorders is increased five years before the diagnosis in IBD. Gastroenterology. 2017;152(5):S973. [Google Scholar]
- 33. Cawthorpe D, Davidson M.. Temporal comorbidity of mental disorder and ulcerative colitis. Perm J. 2015;19(1):52-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Frolkis AD, Vallerand IA, Patten SB, Kaplan GG.. Depression in inflammatory bowel disease: risk factor, prodrome or extraintestinal manifestation? Reply from authors. Gut. 2020;69(3):611-612. [DOI] [PubMed] [Google Scholar]
- 35. García Rodríguez LA, González-Pérez A, Johansson S, Wallander MA.. Risk factors for inflammatory bowel disease in the general population. Aliment Pharmacol Ther. 2005;22(4):309-315. [DOI] [PubMed] [Google Scholar]
- 36. Kurina LM, Goldacre MJ, Yeates D, Gill LE.. Depression and anxiety in people with inflammatory bowel disease. J Epidemiol Community Health. 2001;55(10):716-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leone M, Kuja-Halkola R, Leval A, et al. Association of youth depression with subsequent somatic diseases and premature death. JAMA Psychiatry. 2021;78(3):302-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lin CY, Li CK, Liu JM, Hsu RJ, Chuang HC, Chang FW.. Postpartum depression and subsequent autoimmune diseases in Taiwan. Int J Environ Res Public Health. 2018;15(8):1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Torres J, Gomes C, Jensen C, et al. Risk factors for developing inflammatory bowel disease within and across families with family history of IBD. J Crohns Colitis. 2023;17(1):30-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang B, Wang HE, Bai YM, et al. Bidirectional association between inflammatory bowel disease and depression among patients and their unaffected siblings. J Gastroenterol Hepatol. 2022;37(7):1307-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goodwin RD, Dierker LC, Wu M, Galea S, Hoven CW, Weinberger AH.. Trends in U.S. Depression prevalence from 2015 to 2020: the widening treatment gap. Am J Prev Med. 2022;63(5):726-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Harshfield EL, Pennells L, Schwartz JE, et al. ; Emerging Risk Factors Collaboration. Association between depressive symptoms and incident cardiovascular diseases. JAMA. 2020;324(23):2396-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW.. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ghia JE, Blennerhassett P, Deng Y, Verdu EF, Khan WI, Collins SM.. Reactivation of inflammatory bowel disease in a mouse model of depression. Gastroenterology. 2009;136(7):2280-2288.e1. [DOI] [PubMed] [Google Scholar]
- 45. Gao X, Cao Q, Cheng Y, et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc Natl Acad Sci U S A. 2018;115:E2960-E2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Agostini A, Filippini N, Cevolani D, et al. Brain functional changes in patients with ulcerative colitis: a functional magnetic resonance imaging study on emotional processing. Inflamm Bowel Dis. 2011;17(8):1769-1777. [DOI] [PubMed] [Google Scholar]
- 47. Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186-194. [DOI] [PubMed] [Google Scholar]
- 48. Magro F, Vieira-Coelho MA, Fraga S, et al. Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease. Dig Dis Sci. 2002;47(1):216-224. [DOI] [PubMed] [Google Scholar]
- 49. Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126(7):1657-1664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.


