Abstract
STUDY QUESTION
Which clinical and embryological factors should be considered to apply double embryo transfer (DET) instead of elective single embryo transfer (eSET)?
SUMMARY ANSWER
No clinical or embryological factor per se justifies a recommendation of DET instead of eSET in IVF/ICSI.
WHAT IS KNOWN ALREADY
DET is correlated with a higher rate of multiple pregnancy, leading to a subsequent increase in complications for both mother and babies. These complications include preterm birth, low birthweight, and other perinatal adverse outcomes. To mitigate the risks associated with multiple pregnancy, eSET is recommended by international and national professional organizations as the preferred approach in ART.
STUDY DESIGN, SIZE, DURATION
The guideline was developed according to the structured methodology for development and update of ESHRE guidelines. Literature searches were performed in PUBMED/MEDLINE and Cochrane databases, and relevant papers published up to May 2023, written in English, were included. Live birth rate, cumulative live birth rate, and multiple pregnancy rate were considered as critical outcomes.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Based on the collected evidence, recommendations were discussed until a consensus was reached within the Guideline Development Group (GDG). A stakeholder review was organized after the guideline draft was finalized. The final version was approved by the GDG and the ESHRE Executive Committee.
MAIN RESULTS AND THE ROLE OF CHANCE
The guideline provides 35 recommendations on the medical and non-medical risks associated with multiple pregnancies and on the clinical and embryological factors to be considered when deciding on the number of embryos to transfer. These recommendations include 25 evidence-based recommendations, of which 24 were formulated as strong recommendations and one as conditional, and 10 good practice points. Of the evidence-based recommendations, seven (28%) were supported by moderate-quality evidence. The remaining recommendations were supported by low (three recommendations; 12%), or very low-quality evidence (15 recommendations; 60%). Owing to the lack of evidence-based research, the guideline also clearly mentions recommendations for future studies.
LIMITATIONS, REASONS FOR CAUTION
The guideline assessed different factors one by one based on existing evidence. However, in real life, clinicians’ decisions are based on several prognostic factors related to each patient’s case. Furthermore, the evidence from randomized controlled trials is too scarce to formulate high-quality evidence-based recommendations.
WIDER IMPLICATIONS OF THE FINDINGS
The guideline provides health professionals with clear advice on best practice in the decision-making process during IVF/ICSI, based on the best evidence currently available, and recommendations on relevant information that should be communicated to patients. In addition, a list of research recommendations is provided to stimulate further studies in the field.
STUDY FUNDING/COMPETING INTEREST(S)
The guideline was developed and funded by ESHRE, covering expenses associated with the guideline meetings, the literature searches, and the dissemination of the guideline. The guideline group members did not receive payment. DPB declared receiving honoraria for lectures from Merck, Ferring, and Gedeon Richter. She is a member of ESHRE EXCO, and the Mediterranean Society for reproductive medicine and the president of the Croatian Society for Gynaecological Endocrinology and Reproductive Medicine. CDG is the past Chair of the ESHRE EIM Consortium and a paid deputy member of the Editorial board of Human Reproduction. IR declared receiving reimbursement from ESHRE and EDCD for attending meetings. She holds an unpaid leadership role in OBBCSSR, ECDC Sohonet, and AER. KAR-W declared receiving grants for clinical researchers and funding provision to the institution from the Swedish Cancer Society (200170F), the Senior Clinical Investigator Award, Radiumhemmets Forskningsfonder (Dnr: 201313), Stockholm County Council FoU (FoUI-953912) and Karolinska Institutet (Dnr 2020-01963), NovoNordisk, Merck and Ferring Pharmaceuticals. She received consulting fees from the Swedish Ministry of Health and Welfare. She received honoraria from Roche, Pfizer, and Organon for chairmanship and lectures. She received support from Organon for attending meetings. She participated in advisory boards for Merck, Nordic countries, and Ferring. She declared receiving time-lapse equipment and grants with payment to institution for pre-clinical research from Merck pharmaceuticals and from Ferring. SS-R received research funding from Roche Diagnostics, Organon/MSD, Theramex, and Gedeo-Richter. He received consulting fees from Organon/MSD, Ferring Pharmaceuticals, and Merck Serono. He declared receiving honoraria for lectures from Ferring Pharmaceuticals, Besins, Organon/MSD, Theramex, and Gedeon Richter. He received support for attending Gedeon Richter meetings and participated in the Data Safety Monitoring Board of the T-TRANSPORT trial. He is the Deputy of ESHRE SQART special interest group. He holds stock options in IVI Lisboa and received equipment and other services from Roche Diagnostics and Ferring Pharmaceuticals. KT declared receiving payment for honoraria for giving lectures from Merck Serono and Organon. She is member of the safety advisory board of EDQM. She holds a leadership role in the ICCBBA board of directors. ZV received reimbursement from ESHRE for attending meetings. She also received research grants from ESHRE and Juhani Aaltonen Foundation. She is the coordinator of EHSRE SQART special interest group. The other authors have no conflicts of interest to declare.
DISCLAIMER
This guideline represents the views of ESHRE, which were achieved after careful consideration of the scientific evidence available at the time of preparation. In the absence of scientific evidence on certain aspects, a consensus between the relevant ESHRE stakeholders has been obtained.
Adherence to these clinical practice guidelines does not guarantee a successful or specific outcome, nor does it establish a standard of care. Clinical practice guidelines do not replace the need for application of clinical judgement to each individual presentation, nor variations based on locality and facility type.
ESHRE makes no warranty, express or implied, regarding the clinical practice guidelines and specifically excludes any warranties of merchantability and fitness for a particular use or purpose (full disclaimer available at https://www.eshre.eu/Guidelines-and-Legal).
Keywords: IVF, ICSI, embryo transfer, single embryo transfer, double embryo transfer, live birth rate, multiple pregnancies, medical risks
Introduction
Elective single embryo transfer (eSET) is considered the preferable approach towards safe and effective ART. Currently, this is recommended by several international and national professional organizations (De los Santos et al., 2016; ASRM, 2021). In Europe, the recommendations led to a decrease in the proportion of double embryo transfer (DET) and an increase of the elective transfer of only one embryo at a time (Kupka et al., 2014; Wyns et al., 2021, 2022). However, the data show that there is still a considerable difference in the practice of eSET and the recommendations are not equally followed in all countries, as evident in annual reviews (Sunderam et al., 2022; Wyns et al., 2022).
With the aim of providing the healthcare professionals and patients with the best available evidence, ESHRE has developed a guideline on the number of embryos to transfer during IVF/ICSI. This guideline assesses the medical and non-medical factors that are to be taken into consideration when deciding on the number of embryos to transfer.
Materials and methods
The guideline was developed following a well-documented methodology that is universally used for ESHRE guidelines (Vermeulen et al., 2019). In summary, the Guideline Development Group (GDG) formulated 22 questions structured in PICO format (Patient, Intervention, Comparison, Outcome). Literature searches were conducted in databases (PUBMED/MEDLINE and the Cochrane library) from inception to May 2023, with a limitation to studies written in English. The critical outcomes considered in this guideline are the efficacy in terms of cumulative live birth rate (CLBR) per started cycle and LBR per started cycle, as well as multiple pregnancy rate. A total of 17 700 papers were screened, and relevant studies were selected based on the PICO questions, assessed for quality, and summarized in evidence tables and summary of findings tables. Three relevant papers published after May 2023 were selected by the GDG members and added where appropriate. During the GDG meetings, the evidence and draft recommendations were presented and discussed until consensus was reached within the group. Each recommendation was classified as strong or conditional (Fig. 1), and a grade was assigned (Andrews et al., 2013) based on the strength of the supporting evidence (High ⊕⊕⊕⊕—Moderate ⊕⊕⊕○—Low ⊕⊕○○—Very low ⊕○○○). In the absence of evidence, the GDG formulated no recommendation, or a good practice point (GPP) based on clinical expertise. The draft of the guideline and an invitation for stakeholder review were published on the ESHRE website. Personal invitations to review were sent to all relevant stakeholders, and a total of 71 comments were received from 19 reviewers representing 15 countries, including two national societies (Commission of the Spanish Society of Clinical Chemistry (SEQC) and Kazakhstan Association of the Reproductive Medicine (KARM)). All comments were processed by the GDG, either by adapting the content of the guideline or by providing responses to the reviewers. The review process is summarized in the review report, which is published on the ESHRE website (www.eshre.eu/Guidelines-and-Legal/Guidelines/Embryo-transfer). This guideline will be considered for an update 4 years after publication, with an intermediate assessment of the need for updating 2 years after publication.
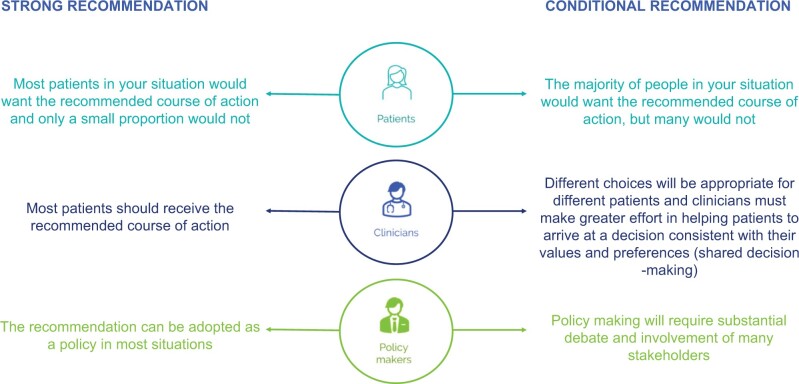
Figure 1.
Interpreting strong and conditional recommendations. Suggested interpretation of strong and conditional recommendations from the perspectives of patients (upper panel), clinicians (middle panel), and healthcare policymakers (lower panel).
Results
Key questions and recommendations
The ESHRE guideline on the numbers of embryos to transfer during IVF/ICSI provides 25 recommendations and 10 GPPs answering 22 key questions regarding clinical, embryological, and other factors to be considered in the decision-making process (The ESHRE Guideline Group on the Number of Embryos to Transfer During IVF/ICSI, 2023).
The current document summarizes all the key questions and the recommendations from the guideline. Further background information and the supporting evidence for each recommendation can be found in the full version of the guideline available at www.eshre.eu/Guidelines-and-Legal/Guidelines/Embryo-transfer.
Which pregnancy-related risks and issues should be considered before the transfer of more than one embryo?
Medical risks related to multiple pregnancy/birth
| Medical risks that should be considered before the transfer of more than one embryo are the higher rates of maternal, foetal, and neonatal complications (D’Souza et al., 1997; Makhseed et al., 1998; Pinborg et al., 2004; van Heesch et al., 2014; Li et al., 2015; Perkins et al., 2015; Bu et al., 2016; Santos-Ribeiro et al., 2016; Eapen et al., 2020; Gupta et al., 2020; Sites et al., 2020; Luke et al., 2021; Anzhel et al., 2022; Cirillo et al., 2022; Wang et al., 2022; Rodriguez-Wallberg et al., 2023). |
|
| The GDG recommends that whenever the transfer of >1 embryo is considered, the patient should be provided with clear information about the higher risk of pregnancy loss, ectopic pregnancy, pre-eclampsia, gestational diabetes, antepartum and postpartum haemorrhage, Caesarean section, stillbirth, preterm birth, low birthweight, neonatal intensive care admission, and neonatal death associated with multiple pregnancies. The GDG also recommends that the patients sign an additional consent form if >1 embryo is transferred. | GPP |
Financial issues of multiple pregnancy/birth
| It is recommended to consider the increased direct costs related to obstetric care of multiple pregnancies and paediatric care of twins and triplets (Gerris et al., 2004; Koivurova et al., 2004, 2007; Lukassen et al., 2004, 2005; Motohashi et al., 2004; Fiddelers et al., 2006; Kjellberg et al., 2006; Veleva et al., 2009; Chambers et al., 2014; Velez et al., 2014; Hernandez Torres et al., 2015; van Heesch et al., 2015; Carpinello et al., 2016). | Strong⊕⊕⊕○ |
| It is recommended to consider increased indirect costs with multiple pregnancies due to sick leave days, over-the-counter medication, and loss of productivity because of an ill child (Fiddelers et al., 2006; Kjellberg et al., 2006; Stillman et al., 2009). | Strong⊕⊕○○ |
| The GDG recommends that cost-related information should be provided and discussed with the patient(s) at the treatment planning stage. | GPP |
Psychological issues of multiple pregnancy/birth
| Clinicians should consider the possible complications of multiple pregnancies with regards to mental health postpartum, emotional distress, and possible marital problems, as well as the influence of personality characteristics, sociodemographic factors, and family functioning, on the mental health of parents and offspring regardless of the number of children born (Boivin et al., 2005; Golombok et al., 2007; Spinelli et al., 2013; Noy et al., 2014; Wenze et al., 2015; van den Akker et al., 2016; Anderson et al., 2017; De Roose et al., 2018; Porat-Zyman et al., 2018). | Strong⊕⊕○○ |
| The GDG recommends that information on possible psychosocial complications should be provided to patients at the treatment planning stage. | GPP |
Which personal, regulatory, and reimbursement factors are expected to affect the decision for the number of embryos to transfer?
Patient preferences, regulatory factors, and reimbursement policies have an impact on embryo transfer practices.
Social, legislative, and economic factors
| The GDG encourages legislative and health insurance policies that promote the practice of eSET. | GPP |
Which clinical criteria should be considered as factors when deciding to apply DET instead of (E)SET for couples/individuals undergoing ART?
Previous unsuccessful ART treatments
| The decision to perform DET instead of eSET should not be based on the number of previous unsuccessful ART treatments (Monteleone et al., 2016). | Strong⊕○○○ |
Duration of infertility
| The decision to perform DET instead of eSET should not be based on the duration of infertility (Yilmaz et al., 2013; Monteleone et al., 2016). | Strong⊕○○○ |
Previous pregnancy/live birth
| The decision to perform DET instead of eSET should not be based on previous pregnancies or live births from ART (Luke et al., 2015). | Strong⊕○○○ |
Female age
| The decision to perform DET instead of eSET should not be based on female age. | Strong⊕⊕○○ |
| Women aged less than 38 years should receive eSET (Veleva et al., 2006; Lawlor and Nelson, 2012; Niinimaki et al., 2013; Mancuso et al., 2016; Tannus et al., 2017; Arab et al., 2020; Ma et al., 2022). | Strong⊕⊕⊕○ |
| Women aged 38 years or more should receive eSET (Veleva et al., 2006; Niinimaki et al., 2013; Tannus et al., 2017; Mejia et al., 2021). | Strong⊕○○○ |
Ovarian response
| For normal responders, eSET is recommended (Moustafa et al., 2008). | Strong⊕○○○ |
|
| |
| The GDG recommends eSET in patients with low or high ovarian response. | GPP |
Criteria related to the endometrium
| The decision to perform DET instead of eSET in fresh embryo transfer cycles should not be based on endometrial characteristics (Huang et al., 2020). | Strong⊕○○○ |
|
| |
| The decision to perform DET instead of eSET in frozen embryo transfer cycles should not be based on endometrial characteristics (El-Toukhy et al., 2008). | Strong⊕○○○ |
Treatments with donor oocytes and donated embryos
| Only eSET should be practised for patients undergoing ART with donor oocytes (Clua et al., 2015; Acharya et al., 2016; Jeve et al., 2016; Fishel et al., 2017; Mersereau et al., 2017; Arab et al., 2020). | Strong⊕⊕⊕○ |
|
| |
| Only eSET should be practised for patients undergoing ART with donated embryos (Peigné et al., 2023). | Strong⊕○○○ |
Gestational carriers
| Only eSET should be practised for gestational carriers (Wang et al., 2016; Namath et al., 2021). | Strong⊕○○○ |
|
| |
| The GDG recommends that both gestational carriers and intended parents be counselled that DET is associated with greater risk of pregnancy and perinatal complications in surrogate pregnancies. | GPP |
Which embryo-related criteria should be considered as factors in deciding to apply DET instead of (E)SET for couples/individuals undergoing ART?
Fresh embryo transfer cycles
Cleavage stage
| In fresh cleavage-stage embryo transfer, the decision to perform DET instead of eSET should not be based on embryo criteria (Martikainen et al., 2001; Thurin et al., 2004; Le Lannou et al., 2006; Fauque et al., 2010; Hatırnaz et al., 2016; Aldemir et al., 2020). | Strong⊕⊕⊕○ |
Blastocyst stage
| In fresh blastocyst transfer cycles, the decision to perform DET instead of eSET should not be based on blastocyst morphology/quality (Abuzeid et al., 2017; Aldemir et al., 2020; Hill et al., 2020; Theodorou et al., 2021). | Strong⊕⊕⊕○ |
Frozen embryo transfer cycles
| When reporting research on vitrified-warmed treatments, the GDG recommends including details on the minimal embryo criteria for vitrification and/or transfer as well as on the selection of devices or embryos for thawing and warming (e.g. randomly picked or according to quality criteria as choosing the first embryos with the best quality). | GPP |
|
| |
| The GDG recommends cryopreserving one embryo per device in order to facilitate the practice of SET and for traceability purposes. | GPP |
Cryopreserved-warmed cleavage stage
| In cryopreserved-warmed cleavage-stage embryo transfer cycles, the decision to perform DET instead of SET should not be based on embryo criteria (Thurin et al., 2004; Hydén-Granskog et al., 2005; Le Lannou et al., 2006; Salumets et al., 2006; Moustafa et al., 2008; López Regalado et al., 2014; Racca et al., 2020; Zhu et al., 2020). | Strong⊕⊕⊕○ |
Vitrified-warmed blastocyst stage
| In vitrified-warmed blastocyst transfer cycles, SET should be applied regardless of the quality of the vitrified blastocyst (Van Landuyt et al., 2011; Liu et al., 2014; Dobson et al., 2018; Park et al., 2019; Arab et al., 2020; Chen et al., 2020; Wang et al., 2020; Zhu et al., 2020). | Strong⊕○○○ |
Can time-lapse morphokinetics or preimplantation genetic testing outcomes be considered factors in decising to apply DET instead of (E)SET for couples/individuals undergoing ART?
Time-lapse morphokinetics
| Time-lapse imaging-derived parameters for embryo selection should not be considered a factor to perform DET instead of eSET (Fishel et al., 2017). | Strong⊕○○○ |
Preimplantation genetic testing
| Outcomes of preimplantation genetic testing for aneuploidies should not be considered when deciding to perform DET instead of eSET. | Strong⊕○○○ |
In any patient undergoing ART, should the transfer of more than two embryos be applied considering the risks of the higher order pregnancies?
Transfer of more than two embryos
| Transfer of more than two embryos is not recommended (Salha et al., 2000; Ng et al., 2001; Combelles et al., 2005; Elizur et al., 2005; Setti et al., 2005; Heijnen et al., 2006; Clayton et al., 2007; Berin et al., 2010; Sun et al., 2012; Li et al., 2015; Perkins et al., 2015; Bu et al., 2016; Richter et al., 2016; Ruhlmann et al., 2017; Pi et al., 2020; Anzhel et al., 2022; Cirillo et al., 2022). | Strong⊕○○○ |
In any patient undergoing ART, should the transfer of more than two embryos with embryo reduction after implantation be applied considering the risks of the procedure?
Foetal reduction
| In patients who conceived higher-order multiples (HOM) following multiple embryo transfer, foetal reduction can be considered to reduce the risk of maternal complications (Groutz et al., 1996; Anthoulakis et al., 2017; Zipori et al., 2017; Liu et al., 2019; Jin et al., 2020). | Conditional⊕○○○ |
|
| |
| The transfer of two or more embryos with the intention of performing foetal reduction in case of multiple embryo implantation instead of (e)SET is not recommended (van de Mheen et al., 2015; Kristensen et al., 2022; Wang et al., 2022; Yimin et al., 2022). | Strong⊕○○○ |
|
| |
| The GDG recommends against the transfer of more than two embryos with foetal reduction after multiple embryo implantation considering the high risks of the procedure. | GPP |
Which issues are crucial for decision-making regarding the number of embryos to transfer and how should they be discussed with the patients?
Patient counselling
|
GPP |
Discussion
The current paper summarizes the 35 recommendations (25 evidence-based recommendations and 10 GPPs) on the factors to consider when deciding on the number of embryos to transfer during IVF/ICSI, as developed in the ESHRE guideline on the number of embryos to transfer during IVF/ICSI. As a basis for the current guideline, a broad and formal literature review was conducted according to the ESHRE guidelines methodology (Vermeulen et al., 2019). The GDG identified a limited number of randomized controlled trials (12 RCTs across various key questions), with evidence for most interventions deriving from cohort studies.
The effects of different prognostic factors were revised one by one to facilitate a more tailored evidence-based decision-making process towards a successful pregnancy with minimal potential risks of complications. No clear indication in any single factor to favour DET over eSET was found.
DET should be avoided at all costs for treatments with donor oocytes, donated embryos or in gestational carriers because of clearly increased pregnancy complications risks. Instead, eSET is strongly recommended for these cases.
Blastocysts should also be transferred in a SET because of the higher monozygotic twin potential of blastocysts (Hviid et al., 2018) and because of the high risk of multiple pregnancy and complications after the transfer of two blastocysts, regardless of the developmental stage of the embryo at the time of cryopreservation or its quality (Van Landuyt et al., 2011; Liu et al., 2014; Abuzeid et al., 2017; Dobson et al., 2018; Park et al., 2019; Aldemir et al., 2020; Arab et al., 2020; Chen et al., 2020; Hill et al., 2020; Wang et al., 2020; Zhu et al., 2020; Theodorou et al., 2021).
The practical recommendation for all other treatments is a more nuanced one because, in real life, patient cases present as a combination of factors associated with either good or poor prognosis. However, the clinical reality is not well represented in scientific research and, currently, there are few studies comparing DET and eSET outcomes in the setting of a truly multivariate regression analysis. Moreover, complex settings frequently encountered in everyday clinical practice cannot be separated from clinic treatment policies, making multivariate studies susceptible to bias regarding ovarian stimulation, embryo selection for transfer, and preparation for frozen embryo transfer.
The number of previously failed treatments has long been recognized as a prognostic factor of poor outcomes (Templeton et al., 1996; Roberts et al., 2010). Each unsuccessful ART cycle has been shown to decrease the odds of ongoing implantation (Thurin et al., 2005). Two previous unsuccessful IVF treatments have been associated with lower chance for live birth when compared with no previous IVF (Strandell et al., 2000; McLernon et al., 2016).
The evidence indicated that DET is not associated with a higher cumulative live birth in poor prognosis patients when considering each factor separately. However, transferring two embryos to patients is sometimes opted for in those marginal cases where patients present several poor prognostic factors, including advanced age, poor-quality embryos, and a lack of live birth from previous ART cycles. Nevertheless, patients for whom DET is considered should be counselled not only regarding the chances their treatment will be successful, but also regarding short- and long-term medical risks, social and economic factors. This should preferably be done as part of shared decision-making.
Apart from the counselling regarding the risks of pregnancy complications with the transfer of more than one embryo, and resulting multiple gestations, patients for whom DET is considered should be aware that the risk of ectopic pregnancy increases along with the number of embryos transferred, up to about 20-fold (Li et al., 2015; Perkins et al., 2015; Bu et al., 2016; Santos-Ribeiro et al., 2016; Pi et al., 2020; Anzhel et al., 2022; Cirillo et al., 2022). The risk of extrauterine pregnancy is elevated after the transfer of two versus one embryo, regardless of development stage or freezing status (Santos-Ribeiro et al., 2016; Anzhel et al., 2022). The rate of ectopic pregnancy is higher after the transfer of non-top-quality embryos (Anzhel et al., 2022). Furthermore, even if a singleton pregnancy develops after DET, it is associated with an overall higher risk of neonatal death and a higher risk of low birthweight in frozen embryo transfer, compared with singleton pregnancies after SET (Rodriguez-Wallberg et al., 2023).
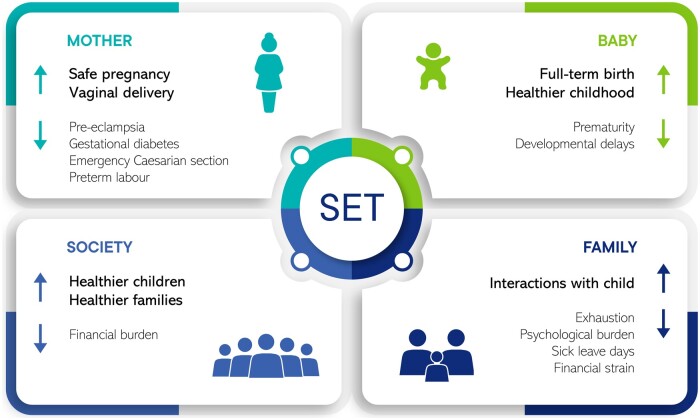
The guideline recommendations emphasize that eSET is the best practice for achieving a healthy pregnancy and minimizing the risk of multiple pregnancies (Fig. 2).
Figure 2.
The benefits of transferring only one embryo at a time.
Conclusion
As there is no evidence showing that CLBR in eSET is inferior to that in DET, and as published data clearly demonstrate that the multiple birth rate after DET significantly exceeds that after (e)SET, the GDG recommends eSET as the standard procedure whenever more than one embryo is available.
Acknowledgements
The Guideline Development Group acknowledges the help of 19 reviewers who provided feedback on the content of the guideline and submitted helpful comments to the draft version. A detailed list of all the reviewers can be found in the ESHRE guideline on the number of embryos to transfer on ESHRE website: www.eshre.eu/Guidelines-and-Legal/Guidelines/Embryo-transfer
Contributor Information
Alessandra Alteri, Department of Obstetrics and Gynaecology, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Gemma Arroyo, Reproductive Medicine Service, Dexeus Mujer, Dexeus University Hospital, Barcelona, Spain.
Giuliana Baccino, NewLifeBank, Madrid, Spain.
Laurentiu Craciunas, Department of Fertility Services and Gynaecology, Newcastle Fertility Centre, Newcastle upon Tyne, UK.
Christian De Geyter, Reproductive Medicine and Gynaecological Endocrinology (RME), University Hospital, University of Basel, Basel, Switzerland.
Thomas Ebner, Department of Gynaecology, Obstetrics and Gynaecological Endocrinology, Kepler University Hospital, Linz, Austria.
Martina Koleva, Patient Representative, Sofia, Bulgaria.
Klaudija Kordic, Patient Representative, Executive Committee, Fertility Europe, Brussels, Belgium.
Saria Mcheik, ESHRE, Central Office, Strombeek-Bever, Belgium.
Heidi Mertes, Department of Philosophy and Moral Sciences, Gent University, Gent, Belgium.
Dinka Pavicic Baldani, Division of Reproductive Medicine and Gynaecological Endocrinology, Department of Obstetrics and Gynaecology, Clinical Hospital Centre Zagreb, and School of Medicine, University of Zagreb, Zagreb, Croatia.
Kenny A Rodriguez-Wallberg, Laboratory of Translational Fertility Preservation, Department of Oncology-Pathology, Karolinska Institute, Stockholm, Sweden; Division of Gynaecology and Reproduction, Department of Reproductive Medicine, Karolinska University Hospital, Stockholm, Sweden.
Ioana Rugescu, Cells Department, National Transplant Agency, Bucharest, Romania.
Samuel Santos-Ribeiro, Department of Reproductive Medicine, Valencian Institute of Infertility in Lisbon (IVI-RMA Lisboa), Lisbon, Portugal.
Kelly Tilleman, Department of Reproductive Medicine, Gent University Hospital, Gent, Belgium.
Bryan Woodward, X&Y Fertility, Leicester, UK.
Nathalie Vermeulen, ESHRE, Central Office, Strombeek-Bever, Belgium.
Zdravka Veleva, Department of Obstetrics and Gynaecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, Finland.
Data availability
This article conducts a literature review of existing research records, and no new data were generated or analysed in support of this manuscript. A full literature search report can be found on the ESHRE website (https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Embryo-transfer).
Authors’ roles
Z.V. chaired the guideline development group and hence fulfilled a leading role in collecting the new evidence, rewriting the updated chapters, and dealing with reviewer comments. S.M. as methodological expert, performed all literature searches update for the guideline, provided methodological support and coordinated the guideline development. All other authors, listed in alphabetical order, as guideline group members, contributed equally to the article, by synthesizing the evidence, writing the guideline, and discussing the recommendations until consensus within the group was reached.
Funding
The guideline development was not supported by external funding; all costs for meetings and methodological support were covered by ESHRE.
Conflict of interest
D.P.B. declared receiving honoraria for lectures from Merck, Ferring, and Gedeon Richter. She is a member of ESHRE EXCO, and the Mediterranean Society for reproductive medicine and the president of the Croatian Society for Gynaecological Endocrinology and Reproductive Medicine. C.D.G. is the past Chair of the ESHRE EIM Consortium and a paid deputy member of the Editorial board of Human Reproduction. I.R. declared receiving reimbursement from ESHRE and EDCD for attending meetings. She holds an unpaid leadership role in OBBCSSR, ECDC Sohonet, and AER. K.A.R.-W. declared receiving grants for clinical researchers and funding provision to the institution from the Swedish Cancer Society (200170F), the Senior Clinical Investigator Award, Radiumhemmets Forskningsfonder (Dnr: 201313), Stockholm County Council FoU (FoUI-953912) and Karolinska Institutet (Dnr 2020-01963), NovoNordisk, Merck and Ferring Pharmaceuticals. She received consulting fees from the Swedish Ministry of Health and Welfare. She received honoraria from Roche, Pfizer, and Organon for chairmanship and lectures. She received support from Organon for attending meetings. She participated in advisory boards for Merck, Nordic countries, and Ferring. She declared receiving time-lapse equipment and grants with payment to institution for pre-clinical research from Merck pharmaceuticals and from Ferring. S.S.-R. received research funding from Roche Diagnostics, Organon/MSD, Theramex, and Gedeo-Richter. He received consulting fees from Organon/MSD, Ferring Pharmaceuticals, and Merck Serono. He declared receiving honoraria for lectures from Ferring Pharmaceuticals, Besins, Organon/MSD, Theramex, and Gedeon Richter. He received support for attending Gedeon Richter meetings and participated in the Data Safety Monitoring Board of the T-TRANSPORT trial. He is the Deputy of ESHRE SQART special interest group. He holds stock options in IVI Lisboa and received equipment and other services from Roche Diagnostics and Ferring Pharmaceuticals. K.T. declared receiving payment for honoraria for giving lectures from Merck Serono and Organon. She is member of the safety advisory board of EDQM. She holds a leadership role in the ICCBBA board of directors. Z.V. received reimbursement from ESHRE for attending meetings. She also received research grants from ESHRE and Juhani Aaltonen Foundation. She is the coordinator of ESHRE SQART special interest group. The other authors have no conflicts of interest to declare.
References
- Abuzeid OM, Deanna J, Abdelaziz A, Joseph SK, Abuzeid YM, Salem WH, Ashraf M, Abuzeid MI.. The impact of single versus double blastocyst transfer on pregnancy outcomes: a prospective, randomized control trial. Facts Views Vis Obgyn 2017;9:195–206. [PMC free article] [PubMed] [Google Scholar]
- Acharya KS, Keyhan S, Acharya CR, Yeh JS, Provost MP, Goldfarb JM, Muasher SJ.. Do donor oocyte cycles comply with ASRM/SART embryo transfer guidelines? An analysis of 13,393 donor cycles from the SART registry. Fertil Steril 2016;106:603–607. [DOI] [PubMed] [Google Scholar]
- Aldemir O, Ozelci R, Baser E, Kaplanoglu I, Dilbaz S, Dilbaz B, Tekin OM.. Impact of transferring a poor quality embryo along with a good quality embryo on pregnancy outcomes in IVF/ICSI cycles: a retrospective study. Geburtshilfe Frauenheilkd 2020;80:844–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KN, Rueter MA, Connor JJ, Koh BD.. Observed mother- and father-child interaction differences in families with medically assisted reproduction-conceived twins and singletons. Fam Process 2017;56:997–1011. [DOI] [PubMed] [Google Scholar]
- Andrews JC, , SchünemannHJ, , OxmanAD, , PottieK, , MeerpohlJJ, , CoelloPA, , RindD, , MontoriVM, , BritoJP, , Norris S. et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation's direction and strength. J Clin Epidemiol 2013;66:726–735. [DOI] [PubMed] [Google Scholar]
- Anthoulakis C, Dagklis T, Mamopoulos A, Athanasiadis A.. Risks of miscarriage or preterm delivery in trichorionic and dichorionic triplet pregnancies with embryo reduction versus expectant management: a systematic review and meta-analysis. Hum Reprod 2017;32:1351–1359. [DOI] [PubMed] [Google Scholar]
- Anzhel S, Mäkinen S, Tinkanen H, Mikkilä T, Haltia A, Perheentupa A, Tomás C, Martikainen H, Tiitinen A, Tapanainen JS. et al. Top-quality embryo transfer is associated with lower odds of ectopic pregnancy. Acta Obstet Gynecol Scand 2022;101:779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arab S, Badegiesh A, Aldhaheri S, Son WY, Dahan MH.. What are the live birth and multiple pregnancy rates when 1 versus 2 low-quality blastocysts are transferred in a cryopreserved cycle? A retrospective cohort study, stratified for age, embryo quality, and oocyte donor cycles. Reprod Sci 2020;28:1403–1411. [DOI] [PubMed] [Google Scholar]
- ASRM. Guidance on the limits to the number of embryos to transfer: a committee opinion. Fertil Steril 2021;116:651–654. [DOI] [PubMed] [Google Scholar]
- Berin I, Engmann LL, Benadiva CA, Schmidt DW, Nulsen JC, Maier DB.. Transfer of two versus three embryos in women less than 40 years old undergoing frozen transfer cycles. Fertil Steril 2010;93:355–359. [DOI] [PubMed] [Google Scholar]
- Boivin M, Pérusse D, Dionne G, Saysset V, Zoccolillo M, Tarabulsy GM, Tremblay N, Tremblay RE.. The genetic-environmental etiology of parents’ perceptions and self-assessed behaviours toward their 5-month-old infants in a large twin and singleton sample. J Child Psychol Psychiatry 2005;46:612–630. [DOI] [PubMed] [Google Scholar]
- Bu Z, Xiong Y, Wang K, Sun Y.. Risk factors for ectopic pregnancy in assisted reproductive technology: a 6-year, single-center study. Fertil Steril 2016;106:90–94. [DOI] [PubMed] [Google Scholar]
- Carpinello OJ, Casson PR, Kuo CL, Raj RS, Sills ES, Jones CA.. Cost implications for subsequent perinatal outcomes after IVF stratified by number of embryos transferred: a five year analysis of vermont data. Appl Health Econ Health Policy 2016;14:387–395. [DOI] [PubMed] [Google Scholar]
- Chambers GM, Hoang VP, Lee E, Hansen M, Sullivan EA, Bower C, Chapman M.. Hospital costs of multiple-birth and singleton-birth children during the first 5 years of life and the role of assisted reproductive technology. JAMA Pediatr 2014;168:1045–1053. [DOI] [PubMed] [Google Scholar]
- Chen S, Du H, Liu J, Liu H, Li L, He Y.. Live birth rate and neonatal outcomes of different quantities and qualities of frozen transferred blastocyst in patients requiring whole embryo freezing stratified by age. BMC Pregnancy Childbirth 2020;20:655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo F, Paladino I, Ronchetti C, Busnelli A, Morenghi E, Grilli L, Patrizio P, Zannoni E, Levi-Setti PE.. Ectopic pregnancy risk factors in infertile patients: a 10-year single center experience. Sci Rep 2022;12:20473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton HB, Schieve LA, Peterson HB, Jamieson DJ, Reynolds MA, Wright VC.. A comparison of heterotopic and intrauterine-only pregnancy outcomes after assisted reproductive technologies in the United States from 1999 to 2002. Fertil Steril 2007;87:303–309. [DOI] [PubMed] [Google Scholar]
- Clua E, Tur R, Coroleu B, Rodríguez I, Boada M, Gómez MJ, Barri PN, Veiga A.. Is it justified to transfer two embryos in oocyte donation? A pilot randomized clinical trial. Reprod Biomed Online 2015;31:154–161. [DOI] [PubMed] [Google Scholar]
- Combelles CM, Orasanu B, Ginsburg ES, Racowsky C.. Optimum number of embryos to transfer in women more than 40 years of age undergoing treatment with assisted reproductive technologies. Fertil Steril 2005;84:1637–1642. [DOI] [PubMed] [Google Scholar]
- D’Souza SW, Rivlin E, Cadman J, Richards B, Buck P, Lieberman BA.. Children conceived by in vitro fertilisation after fresh embryo transfer. Arch Dis Child Fetal Neonatal Ed 1997;76:F70–F74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De los Santos MJ, Apter S, Coticchio G, Debrock S, Lundin K, Plancha CE, Prados F, Rienzi L, Verheyen G, Woodward B. et al. Revised guidelines for good practice in IVF laboratories (2015). Hum Reprod 2016;31:685–686. [DOI] [PubMed] [Google Scholar]
- De Roose M, Beeckman D, Eggermont K, Vanhouche E, Van Hecke A, Verhaeghe S.. Level of parenting stress in mothers of singletons and mothers of twins until one year postpartum: a cross-sectional study. Women Birth 2018;31:e197–e203. [DOI] [PubMed] [Google Scholar]
- Dobson SJA, Lao MT, Michael E, Varghese AC, Jayaprakasan K.. Effect of transfer of a poor quality embryo along with a top quality embryo on the outcome during fresh and frozen in vitro fertilization cycles. Fertil Steril 2018;110:655–660. [DOI] [PubMed] [Google Scholar]
- Eapen A, Ryan GL, Ten Eyck P, Van Voorhis BJ.. Current evidence supporting a goal of singletons: a review of maternal and perinatal outcomes associated with twin versus singleton pregnancies after in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril 2020;114:690–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizur SE, Lerner-Geva L, Levron J, Shulman A, Bider D, Dor J.. Factors predicting IVF treatment outcome: a multivariate analysis of 5310 cycles. Reprod Biomed Online 2005;10:645–649. [DOI] [PubMed] [Google Scholar]
- El-Toukhy T, Coomarasamy A, Khairy M, Sunkara K, Seed P, Khalaf Y, Braude P.. The relationship between endometrial thickness and outcome of medicated frozen embryo replacement cycles. Fertil Steril 2008;89:832–839. [DOI] [PubMed] [Google Scholar]
- Fauque P, Jouannet P, Davy C, Guibert J, Viallon V, Epelboin S, Kunstmann J-M, Patrat C.. Cumulative results including obstetrical and neonatal outcome of fresh and frozen-thawed cycles in elective single versus double fresh embryo transfers. Fertil Steril 2010;94:927–935. [DOI] [PubMed] [Google Scholar]
- Fiddelers AA, van Montfoort AP, Dirksen CD, Dumoulin JC, Land JA, Dunselman GA, Janssen JM, Severens JL, Evers JL.. Single versus double embryo transfer: cost-effectiveness analysis alongside a randomized clinical trial. Hum Reprod 2006;21:2090–2097. [DOI] [PubMed] [Google Scholar]
- Fishel S, Campbell A, Montgomery S, Smith R, Nice L, Duffy S, Jenner L, Berrisford K, Kellam L, Smith R. et al. Live births after embryo selection using morphokinetics versus conventional morphology: a retrospective analysis. Reprod Biomed Online 2017;35:407–416. [DOI] [PubMed] [Google Scholar]
- Gerris J, De Sutter P, De Neubourg D, Van Royen E, Vander Elst J, Mangelschots K, Vercruyssen M, Kok P, Elseviers M, Annemans L. et al. A real-life prospective health economic study of elective single embryo transfer versus two-embryo transfer in first IVF/ICSI cycles. Hum Reprod 2004;19:917–923. [DOI] [PubMed] [Google Scholar]
- Golombok S, Olivennes F, Ramogida C, Rust J, Freeman T; Follow-Up Team. Parenting and the psychological development of a representative sample of triplets conceived by assisted reproduction. Hum Reprod 2007;22:2896–2902. [DOI] [PubMed] [Google Scholar]
- Groutz A, Yovel I, Amit A, Yaron Y, Azem F, Lessing JB.. Pregnancy outcome after multifetal pregnancy reduction to twins compared with spontaneously conceived twins. Hum Reprod 1996;11:1334–1336. [DOI] [PubMed] [Google Scholar]
- Gupta R, Sardana P, Arora P, Banker J, Shah S, Banker M.. Maternal and neonatal complications in twin deliveries as compared to singleton deliveries following in vitro fertilization. J Hum Reprod Sci 2020;13:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatırnaz S, Hatırnaz E, Dahan MH, Tan SL, Ozer A, Kanat-Pektas M, Ata B.. Is elective single-embryo transfer a viable treatment policy in in vitro maturation cycles? Fertil Steril 2016;106:1691–1695. [DOI] [PubMed] [Google Scholar]
- Heijnen EM, Klinkert ER, Schmoutziguer AP, Eijkemans MJ, Te Velde ER, Broekmans FJ.. Prevention of multiple pregnancies after IVF in women 38 and older: a randomized study. Reprod Biomed Online 2006;13:386–393. [DOI] [PubMed] [Google Scholar]
- Hernandez Torres E, Navarro-Espigares JL, Clavero A, Lopez-Regalado M, Camacho-Ballesta JA, Onieva-Garcia M, Martinez L, Castilla JA.. Economic evaluation of elective single-embryo transfer with subsequent single frozen embryo transfer in an in vitro fertilization/intracytoplasmic sperm injection program. Fertil Steril 2015;103:699–706. [DOI] [PubMed] [Google Scholar]
- Hill MJ, Eubanks AE, Csokmay JM, Christy AY, Jahandideh S, DeCherney AH, Devine K, Levens ED, Connell MT.. Is transferring a lower-quality embryo with a good-quality blastocyst detrimental to the likelihood of live birth? Fertil Steril 2020;114:338–345. [DOI] [PubMed] [Google Scholar]
- Huang X, Liu R, Shen W, Cai Y, Ding M, Sun H, Zhou J.. An elective single cleavage embryo transfer strategy to minimize twin live birth rate based on a prediction model from double cleavage embryos transfer patients. J Matern Fetal Neonatal Med 2020;35:1775–1782. [DOI] [PubMed] [Google Scholar]
- Hviid KVR, Malchau SS, Pinborg A, Nielsen HS.. Determinants of monozygotic twinning in ART: a systematic review and a meta-analysis. Hum Reprod Update 2018;24:468–483. [DOI] [PubMed] [Google Scholar]
- Hydén-Granskog C, Unkila-Kallio L, Halttunen M, Tiitinen A.. Single embryo transfer is an option in frozen embryo transfer. Hum Reprod 2005;20:2935–2938. [DOI] [PubMed] [Google Scholar]
- Jeve YB, Potdar N, Opoku A, Khare M.. Donor oocyte conception and pregnancy complications: a systematic review and meta-analysis. BJOG 2016;123:1471–1480. [DOI] [PubMed] [Google Scholar]
- Jin B, Huang Q, Ji M, Yu Z, Shu J.. Perinatal outcomes in dichorionic diamniotic twins with multifetal pregnancy reduction versus expectant management: a systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e20730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellberg AT, Carlsson P, Bergh C.. Randomized single versus double embryo transfer: obstetric and paediatric outcome and a cost-effectiveness analysis. Hum Reprod 2006;21:210–216. [DOI] [PubMed] [Google Scholar]
- Koivurova S, Hartikainen AL, Gissler M, Hemminki E, Järvelin MR.. Post-neonatal hospitalization and health care costs among IVF children: a 7-year follow-up study. Hum Reprod 2007;22:2136–2141. [DOI] [PubMed] [Google Scholar]
- Koivurova S, Hartikainen AL, Gissler M, Hemminki E, Klemetti R, Järvelin MR.. Health care costs resulting from IVF: prenatal and neonatal periods. Hum Reprod 2004;19:2798–2805. [DOI] [PubMed] [Google Scholar]
- Kristensen SE, Ekelund CK, Sandager P, Jørgensen FS, Hoseth E, Sperling L, Balaganeshan SB, Hjortshøj TD, Gadsbøll K, Wright A. et al. Risks and pregnancy outcome after fetal reduction in dichorionic twin pregnancies: a Danish national retrospective cohort study. Am J Obstet Gynecol 2022;228:590.e1. [DOI] [PubMed] [Google Scholar]
- Kupka MS, Ferraretti AP, de Mouzon J, Erb K, D’Hooghe T, Castilla JA, Calhaz-Jorge C, De Geyter C, Goossens V; European IVF-Monitoring Consortium, for the European Society of Human Reproduction and Embryology. Assisted reproductive technology in Europe, 2010: results generated from European registers by ESHRE†. Hum Reprod 2014;29:2099–2113.25069504 [Google Scholar]
- Lawlor DA, Nelson SM.. Effect of age on decisions about the numbers of embryos to transfer in assisted conception: a prospective study. Lancet 2012;379:521–527. [DOI] [PubMed] [Google Scholar]
- Le Lannou D, Griveau J-F, Laurent M-C, Gueho A, Veron E, Morcel K.. Contribution of embryo cryopreservation to elective single embryo transfer in IVF-ICSI. Reprod Biomed Online 2006;13:368–375. [DOI] [PubMed] [Google Scholar]
- Li Z, Sullivan EA, Chapman M, Farquhar C, Wang YA.. Risk of ectopic pregnancy lowest with transfer of single frozen blastocyst. Hum Reprod 2015;30:2048–2054. [DOI] [PubMed] [Google Scholar]
- Liu L, Li Y-H, Ding X-F, Geng Y-H, Chen C-Y, Gao Y.. Influence of blastocysts morphological score on pregnancy outcomes in frozen-thawed blastocyst transfers: a retrospective study of 741 cycles. J Huazhong Univ Sci Technolog Med Sci 2014;34:750–754. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shen Y, Zhang H, Tang Y, Lu G, Lin G, Gong F.. Clinical outcomes of multifetal pregnancy reduction in trichorionic and dichorionic triplet pregnancies: a retrospective observational study. Taiwan J Obstet Gynecol 2019;58:133–138. [DOI] [PubMed] [Google Scholar]
- López Regalado ML, Clavero A, Gonzalvo MC, Serrano M, Martínez L, Mozas J, Rodríguez-Serrano F, Fontes J, Romero B, Castilla JA.. Cumulative live birth rate after two single frozen embryo transfers (eSFET) versus a double frozen embryo transfer (DFET) with cleavage stage embryos: a retrospective cohort study. J Assist Reprod Genet 2014;31:1621–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukassen HG, Braat DD, Wetzels AM, Zielhuis GA, Adang EM, Scheenjes E, Kremer JA.. Two cycles with single embryo transfer versus one cycle with double embryo transfer: a randomized controlled trial. Hum Reprod 2005;20:702–708. [DOI] [PubMed] [Google Scholar]
- Lukassen HG, Schönbeck Y, Adang EM, Braat DD, Zielhuis GA, Kremer JA.. Cost analysis of singleton versus twin pregnancies after in vitro fertilization. Fertil Steril 2004;81:1240–1246. [DOI] [PubMed] [Google Scholar]
- Luke B, Brown MB, Wantman E, Forestieri NE, Browne ML, Fisher SC, Yazdy MM, Ethen MK, Canfield MA, Nichols HB. et al. Risks of nonchromosomal birth defects, small-for-gestational age birthweight, and prematurity with in vitro fertilization: effect of number of embryos transferred and plurality at conception versus at birth. J Assist Reprod Genet 2021;38:835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke B, Brown MB, Wantman E, Stern JE, Baker VL, Widra E, Coddington CC III, Gibbons WE, Van Voorhis BJ, Ball GD.. Application of a validated prediction model for in vitro fertilization: comparison of live birth rates and multiple birth rates with 1 embryo transferred over 2 cycles vs 2 embryos in 1 cycle. Am J Obstet Gynecol 2015;212:676.e1–676.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Peng Y, Hu L, Wang X, Xiong Y, Tang Y, Tan J, Gong F.. Comparisons of benefits and risks of single embryo transfer versus double embryo transfer: a systematic review and meta-analysis. Reprod Biol Endocrinol 2022;20:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhseed M, Al-Sharhan M, Egbase P, Al-Essa M, Grudzinskas JG.. Maternal and perinatal outcomes of multiple pregnancy following IVF-ET. Int J Gynaecol Obstet 1998;61:155–163. [DOI] [PubMed] [Google Scholar]
- Mancuso AC, Boulet SL, Duran E, Munch E, Kissin DM, Van Voorhis BJ.. Elective single embryo transfer in women less than age 38 years reduces multiple birth rates, but not live birth rates, in United States fertility clinics. Fertil Steril 2016;106:1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martikainen H, Tiitinen A, Tomás C, Tapanainen J, Orava M, Tuomivaara L, Vilska S, Hydén-Granskog C, Hovatta O, Finnish ETSG; Finnish ET Study Group. One versus two embryo transfer after IVF and ICSI: a randomized study. Hum Reprod 2001;16:1900–1903. [DOI] [PubMed] [Google Scholar]
- McLernon DJ, Steyerberg EW, Te Velde ER, Lee AJ, Bhattacharya S.. Predicting the chances of a live birth after one or more complete cycles of in vitro fertilisation: population based study of linked cycle data from 113 873 women. BMJ 2016;362:k3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia RB, Capper EA, Summers KM, Ten Eyck P, Van Voorhis BJ.. Elective transfer of one embryo is associated with a higher cumulative live birth rate and improved perinatal outcomes compared to the transfer of two embryos with in vitro fertilization. F S Rep 2021;2:50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersereau J, Stanhiser J, Coddington C, Jones T, Luke B, Brown MB.. Patient and cycle characteristics predicting high pregnancy rates with single-embryo transfer: an analysis of the Society for Assisted Reproductive Technology outcomes between 2004 and 2013. Fertil Steril 2017;108:750–756. [DOI] [PubMed] [Google Scholar]
- Monteleone PA, Mirisola RJ, Goncalves SP, Baracat EC, Serafini PC.. Outcomes of elective cryopreserved single or double embryo transfers following failure to conceive after fresh single embryo transfer. Reprod Biomed Online 2016;33:161–167. [DOI] [PubMed] [Google Scholar]
- Motohashi T, Honda T, Hasegawa M, Uchida T, Kanamoto N, Koizumi K, Beppu M, Nakahori T, Takahashi A.. Costs of maternal and neonatal medical care for triplet and quadruplet pregnancies in Japan. Reprod Med Biol 2004;3:159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa MK, Sheded SA, El Aziz Mousta MA.. Elective single embryo transfer versus double embryo transfer in assisted reproduction. Reprod Biomed Online 2008;17:82–87. [DOI] [PubMed] [Google Scholar]
- Namath A, Jahandideh S, Devine K, O’Brien JE, Stillman RJ.. Gestational carrier pregnancy outcomes from frozen embryo transfer depending on the number of embryos transferred and preimplantation genetic testing: a retrospective analysis. Fertil Steril 2021;115:1471–1477. [DOI] [PubMed] [Google Scholar]
- Ng EH, Lau EY, Yeung WS, Ho PC.. Transfer of two embryos instead of three will not compromise pregnancy rate but will reduce multiple pregnancy rate in an assisted reproduction unit. J Obstet Gynaecol Res 2001;27:329–335. [DOI] [PubMed] [Google Scholar]
- Niinimaki M, Suikkari AM, Makinen S, Soderstrom-Anttila V, Martikainen H.. Elective single-embryo transfer in women aged 40-44 years. Hum Reprod 2013;28:331–335. [DOI] [PubMed] [Google Scholar]
- Noy A, Taubman-Ben-Ari O, Kuint J.. Well-being and distress in mothers of two-year-old singletons and twins. Women Health 2014;54:317–335. [DOI] [PubMed] [Google Scholar]
- Park DS, Kim JW, Chang EM, Lee WS, Yoon TK, Lyu SW.. Strategies in the transfer of varying grades of vitrified-warmed blastocysts in women aged over 35 years: a propensity-matched analysis. J Obstet Gynaecol Res 2019;45:849–857. [DOI] [PubMed] [Google Scholar]
- Peigné M, de Mouzon J, Khiel A, Fraissinet A, Maget V, Saïas-Magnan J, Mathieu-D’Argent E, Gervereau O, Letur H.. Donated-embryo pregnancies are associated with increased risk of hypertensive disorders even for young recipients: a retrospective matched-cohort study. Fertil Steril 2023;119:69–77. [DOI] [PubMed] [Google Scholar]
- Perkins KM, Boulet SL, Kissin DM, Jamieson DJ; National ART Surveillance (NASS) Group. Risk of ectopic pregnancy associated with assisted reproductive technology in the United States, 2001-2011. Obstet Gynecol 2015;125:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi R, Liu Y, Zhao X, Liu P, Qi X.. Tubal infertility and pelvic adhesion increase risk of heterotopic pregnancy after in vitro fertilization: a retrospective study. Medicine (Baltimore) 2020;99:e23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinborg A, Loft A, Schmidt L, Langhoff-Roos J, Andersen AN.. Maternal risks and perinatal outcome in a Danish national cohort of 1005 twin pregnancies: the role of in vitro fertilization. Acta Obstet Gynecol Scand 2004;83:75–84. [DOI] [PubMed] [Google Scholar]
- Porat-Zyman G, Taubman-Ben-Ari O, Morag I, Kuint J.. Maternal mental health over the course of 4 years following childbirth: the contribution of birth circumstances and psycho-social factors. Women Health 2018;58:72–91. [DOI] [PubMed] [Google Scholar]
- Racca A, Drakopoulos P, Van Landuyt L, Willem C, Santos-Ribeiro S, Tournaye H, Blockeel C, Polyzos NP.. Single and double embryo transfer provide similar live birth rates in frozen cycles. Gynecol Endocrinol 2020;36:824–828. [DOI] [PubMed] [Google Scholar]
- Richter KS, Ginsburg DK, Shipley SK, Lim J, Tucker MJ, Graham JR, Levy MJ.. Factors associated with birth outcomes from cryopreserved blastocysts: experience from 4,597 autologous transfers of 7,597 cryopreserved blastocysts. Fertil Steril 2016;106:354–362.e2. [DOI] [PubMed] [Google Scholar]
- Roberts SA, Hirst WM, Brison DR, Vail A; towardSET Collaboration. Embryo and uterine influences on IVF outcomes: an analysis of a UK multi-centre cohort. Hum Reprod 2010;25:2792–2802. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Wallberg KA, Palomares AR, Nilsson HP, Oberg AS, Lundberg F.. Obstetric and perinatal outcomes of singleton births following single- vs double-embryo transfer in Sweden. JAMA Pediatr 2023;177:149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhlmann C, Molina L, Tessari G, Ruhlmann F, Tessari L, Gnocchi D, Cattaneo A, Irigoyen M, Martínez AG.. Optimizing the number of embryos to transfer on day 5: two should be the limit. JBRA Assist Reprod 2017;21:7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salha O, Dada T, Levett S, Allgar V, Sharma V.. The influence of supernumerary embryos on the clinical outcome of IVF cycles. J Assist Reprod Genet 2000;17:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salumets A, Suikkari A-M, Mäkinen S, Karro H, Roos A, Tuuri T.. Frozen embryo transfers: implications of clinical and embryological factors on the pregnancy outcome. Hum Reprod 2006;21:2368–2374. [DOI] [PubMed] [Google Scholar]
- Santos-Ribeiro S, Tournaye H, Polyzos NP.. Trends in ectopic pregnancy rates following assisted reproductive technologies in the UK: a 12-year nationwide analysis including 160 000 pregnancies. Hum Reprod 2016;31:393–402. [DOI] [PubMed] [Google Scholar]
- Setti PE, Cavagna M, Albani E, Morreale G, Novara PV, Cesana A, Parini V.. Outcome of assisted reproductive technologies after different embryo transfer strategies. Reprod Biomed Online 2005;11:64–70. [DOI] [PubMed] [Google Scholar]
- Sites CK, Wilson D, Bernson D, Boulet S, Zhang Y.. Number of embryos transferred and diagnosis of preeclampsia. Reprod Biol Endocrinol 2020;18:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli M, Poehlmann J, Bolt D.. Predictors of parenting stress trajectories in premature infant-mother dyads. J Fam Psychol 2013;27:873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman RJ, Richter KS, Banks NK, Graham JR.. Elective single embryo transfer: a 6-year progressive implementation of 784 single blastocyst transfers and the influence of payment method on patient choice. Fertil Steril 2009;92:1895–1906. [DOI] [PubMed] [Google Scholar]
- Strandell A, Bergh C, Lundin K.. Selection of patients suitable for one-embryo transfer may reduce the rate of multiple births by half without impairment of overall birth rates. Hum Reprod 2000;15:2520–2525. [DOI] [PubMed] [Google Scholar]
- Sun Y, Feng Y, Zhang A, Lu X, Niu Z, Gu R.. Frozen-thawed embryo transfer cycles in China: clinical outcomes of two and three multicellular embryos transfers. J Assist Reprod Genet 2012;29:417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderam S, Kissin DM, Zhang Y, Jewett A, Boulet SL, Warner L, Kroelinger CD, Barfield WD.. Assisted reproductive technology surveillance—United States, 2018. MMW Surveill Summ 2022;71:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannus S, Cohen Y, Son WY, Shavit T, Dahan MH.. Cumulative live birth rate following elective single blastocyst transfer compared with double blastocyst transfer in women aged 40 years and over. Reprod Biomed Online 2017;35:733–738. [DOI] [PubMed] [Google Scholar]
- Templeton A, Morris JK, Parslow W.. Factors that affect outcome of in-vitro fertilisation treatment. Lancet 1996;348:1402–1406. [DOI] [PubMed] [Google Scholar]
- The ESHRE Guideline Group on the Number of Embryos to Transfer During IVF/ICSI; Alteri A, Arroyo G, Baccino G, Craciunas L, De Geyter C, Ebner T, Koleva M, Kordic K, Mcheik S, Mertes H, Pavicic Baldani D, Rodriguez-Wallberg K, Rugescu I. et al. Evidence-based guideline: number of embryos to transfer during IVF/ICSI. 2023. https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Embryo-transfer. [DOI] [PMC free article] [PubMed]
- Theodorou E, Jones BP, Cawood S, Heath C, Serhal P, Ben-Nagi J.. Adding a low-quality blastocyst to a high-quality blastocyst for a double embryo transfer does not decrease pregnancy and live birth rate. Acta Obstet Gynecol Scand 2021;100:1124–1131. [DOI] [PubMed] [Google Scholar]
- Thurin A, Hardarson T, Hausken J, Jablonowska B, Lundin K, Pinborg A, Bergh C.. Predictors of ongoing implantation in IVF in a good prognosis group of patients. Hum Reprod 2005;20:1876–1880. [DOI] [PubMed] [Google Scholar]
- Thurin A, Hausken J, Hillensjö T, Jablonowska B, Pinborg A, Strandell A, Bergh C.. Elective single-embryo transfer versus double-embryo transfer in in vitro fertilization. N Engl J Med 2004;351:2392–‐2402. [DOI] [PubMed] [Google Scholar]
- van de Mheen L, Everwijn SM, Knapen MF, Haak MC, Engels MA, Manten GT, Zondervan HA, Wirjosoekarto SA, van Vugt JM, Erwich JJ. et al. Pregnancy outcome after fetal reduction in women with a dichorionic twin pregnancy. Hum Reprod 2015;30:1807–1812. [DOI] [PubMed] [Google Scholar]
- van den Akker O, Postavaru GI, Purewal S.. Maternal psychosocial consequences of twins and multiple births following assisted and natural conception: a meta-analysis. Reprod Biomed Online 2016;33:1–14. [DOI] [PubMed] [Google Scholar]
- van Heesch MM, Evers JL, Dumoulin JC, van der Hoeven MA, van Beijsterveldt CE, Bonsel GJ, Dykgraaf RH, van Goudoever JB, Koopman-Esseboom C, Nelen WL. et al. A comparison of perinatal outcomes in singletons and multiples born after in vitro fertilization or intracytoplasmic sperm injection stratified for neonatal risk criteria. Acta Obstet Gynecol Scand 2014;93:277–286. [DOI] [PubMed] [Google Scholar]
- van Heesch MM, Evers JL, van der Hoeven MA, Dumoulin JC, van Beijsterveldt CE, Bonsel GJ, Dykgraaf RH, van Goudoever JB, Koopman-Esseboom C, Nelen WL. et al. Hospital costs during the first 5 years of life for multiples compared with singletons born after IVF or ICSI. Hum Reprod 2015;30:1481–1490. [DOI] [PubMed] [Google Scholar]
- Van Landuyt L, Stoop D, Verheyen G, Verpoest W, Camus M, Van de Velde H, Devroey P, Van den Abbeel E.. Outcome of closed blastocyst vitrification in relation to blastocyst quality: evaluation of 759 warming cycles in a single-embryo transfer policy. Hum Reprod 2011;26:527–534. [DOI] [PubMed] [Google Scholar]
- Veleva Z, Karinen P, Tomas C, Tapanainen JS, Martikainen H.. Elective single embryo transfer with cryopreservation improves the outcome and diminishes the costs of IVF/ICSI. Hum Reprod 2009;24:1632–1639. [DOI] [PubMed] [Google Scholar]
- Veleva Z, Vilska S, Hyden-Granskog C, Tiitinen A, Tapanainen JS, Martikainen H.. Elective single embryo transfer in women aged 36-39 years. Hum Reprod 2006;21:2098–2102. [DOI] [PubMed] [Google Scholar]
- Velez MP, Connolly MP, Kadoch IJ, Phillips S, Bissonnette F.. Universal coverage of IVF pays off. Hum Reprod 2014;29:1313–1319. [DOI] [PubMed] [Google Scholar]
- Vermeulen N, Le Clef N, Veleva Z, D’Angelo A, Tilleman K.. European recommendations for good practice in addition to an evidence-based guidelines programme: rationale and method of development. BMJ Evid Based Med 2019;24:30–34. [DOI] [PubMed] [Google Scholar]
- Wang AY, Dill SK, Bowman M, Sullivan EA.. Gestational surrogacy in Australia 2004-2011: treatment, pregnancy and birth outcomes. Aust N Z J Obstet Gynaecol 2016;56:255–259. [DOI] [PubMed] [Google Scholar]
- Wang W, Cai J, Liu L, Xu Y, Liu Z, Chen J, Jiang X, Sun X, Ren J.. Does the transfer of a poor quality embryo with a good quality embryo benefit poor prognosis patients? Reprod Biol Endocrinol 2020;18:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhu H, Tong X, Jiang L, Wei Q, Zhang S.. Clinical outcomes after elective double-embryo transfer in frozen cycles for women of advanced maternal age: a retrospective cohort study. Medicine (Baltimore) 2022;101:e28992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenze SJ, Battle CL, Tezanos KM.. Raising multiples: mental health of mothers and fathers in early parenthood. Arch Womens Ment Health 2015;18:163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyns C, De Geyter C, Calhaz-Jorge C, Kupka MS, Motrenko T, Smeenk J, Bergh C, Tandler-Schneider A, Rugescu IA, Goossens V; European IVF Monitoring Consortium (EIM), for the European Society of Human Reproduction and Embryology (ESHRE). ART in Europe, 2018: results generated from European registries by ESHRE. Hum Reprod Open 2022;2022:hoac022.35795850 [Google Scholar]
- Wyns C, De Geyter C, Calhaz-Jorge C, Kupka MS, Motrenko T, Smeenk J, Bergh C, Tandler-Schneider A, Rugescu IA, Vidakovic S. et al. ART in Europe, 2017: results generated from European registries by ESHRE. Hum Reprod Open 2021;2021:hoab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz N, Engin-Üstün Y, Inal H, Gorkem U, Bardakci Y, Gulerman C.. The impact of single embryo transfer policy on pregnancy outcomes after legislative change. Gynecol Endocrinol 2013;29:600–602. [DOI] [PubMed] [Google Scholar]
- Yimin Z, Minyue T, Yanling F, Huanmiao Y, Saijun S, Qingfang L, Xiaoling H, Lanfeng X.. Fetal reduction could improve but not completely reverse the pregnancy outcomes of multiple pregnancies: experience from a single center. Front Endocrinol (Lausanne) 2022;13:851167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Lin J, Gao H, Wang N, Wang B, Wang Y.. The association between embryo quality, number of transferred embryos and live birth rate after vitrified cleavage-stage embryos and blastocyst transfer. Front Physiol 2020;11:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipori Y, Haas J, Berger H, Barzilay E.. Multifetal pregnancy reduction of triplets to twins compared with non-reduced triplets: a meta-analysis. Reprod Biomed Online 2017;35:296–304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article conducts a literature review of existing research records, and no new data were generated or analysed in support of this manuscript. A full literature search report can be found on the ESHRE website (https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Embryo-transfer).