Abstract
INTRODUCTION
This study aimed to assess whether social relationships in mid‐life reduce the risk of dementia related to amyloid burden.
METHODS
Participants in the Atherosclerosis Risk in Communities (ARIC) study were assessed for social support and isolation (visit 2; 1990–1992). A composite measure, “social relationships,” was generated. Brain amyloid was evaluated with florbetapir positron emission tomography (PET); (visit 5; 2012–2014). Incident dementia cases were identified following visit 5 through 2019 using ongoing surveillance. Relative contributions of mid‐life social relationships and elevated brain amyloid to incident dementia were evaluated with Cox regression models.
RESULTS
Among 310 participants without dementia, strong mid‐life social relationships were associated independently with lower dementia risk. Elevated late‐life brain amyloid was associated with greater dementia risk.
DISCUSSION
Although mid‐life social relationships did not moderate the relationship between amyloid burden and dementia, these findings affirm the importance of strong social relationships as a potentially protective factor against dementia.
Keywords: amyloid beta, Atherosclerosis Risk in Communities study, dementia, mid‐life, positron emission tomography, social relationships
1. BACKGROUND
Cortical amyloid beta (Aβ) deposition is highly associated with dementia incidence, yet not everyone with amyloid deposition will develop dementia in their lifetime. 1 Such discrepancies highlight the important role that risk factors may have in reducing the likelihood of dementia incidence.
Among these factors, psychosocial health has been identified as a modifiable risk factor that may be related to a reduction in dementia incidence. 2 , 3 , 4 , 5 The mechanism underlying this theory has been explored through the use of structural measures assessed via magnetic resonance imaging (MRI), cerebrospinal fluid (CSF) samples of proteins such as Aβ and tau, and neuropsychological tests which assess age‐related cognitive decline. 6 , 7 , 8 , 9 , 10 Likewise, other studies have shown significant relationships between psychosocial measures and Aβ burden measured using positron emission tomography (PET). 11 , 12 , 13 The premise in conducting many of these studies can be summarized as attempts to better discern the pathological mechanism in which older individuals are able to sustain cognitive function despite aging and the neuropathology that accompanies the aging process.
A recent study in the Atherosclerosis Risk in Communities (ARIC)–PET cohort showed that participants with stronger psychosocial health in mid‐life were less likely to have elevated amyloid burden in late life, compared to participants with weaker psychosocial health. 13 Findings of greater Aβ pathology in older adults who theoretically have a “protective” risk factor due to strong psychosocial health prompted the present study to further investigate whether lifetime dementia risk, canonically associated with amyloid burden, may be modified based upon this factor. We hypothesized that participants with strong social relationships in mid‐life would have a reduced risk of incident dementia associated with amyloid burden, relative to participants with poor social relationships in mid‐life. The measure of psychosocial health examined was “social relationships,” which was defined based upon measures of social support and social isolation assessed in the ARIC cohort.
2. METHODS
2.1. Study population
The ARIC study is an ongoing community‐based prospective cohort study that enrolled 15,792 adults (aged 45 to 64 years) from four U.S. communities at baseline (visit 1: 1987–1989). 14 Self‐reported questionnaires regarding psychosocial measures were collected at visit 2 (1990–1992). At visit 5 (2011–2013), the ARIC Neurocognitive Study (ARIC‐NCS) was initiated and a subset of ARIC participants without contraindications for imaging underwent brain MRI. 15 , 16 Among the subset who received brain MRI, 346 participants without dementia from three ARIC centers were recruited for florbetapir PET imaging (to occur within 1 year of cognitive testing) at visit 5 as part of the ARIC‐PET ancillary study. 17 Inclusion/exclusion criteria for the present study is shown (Figure 1). For participants with imaging at the Forsyth or Washington County sites, follow‐up for the development of incident dementia was administratively censored on December 31, 2019. For roughly 30% of the 119 participants who underwent imaging at the Jackson site, follow‐up data for this study were censored through December 31, 2017, due to administrative delays. The study was approved by Institutional Review Boards at each study center.
FIGURE 1.
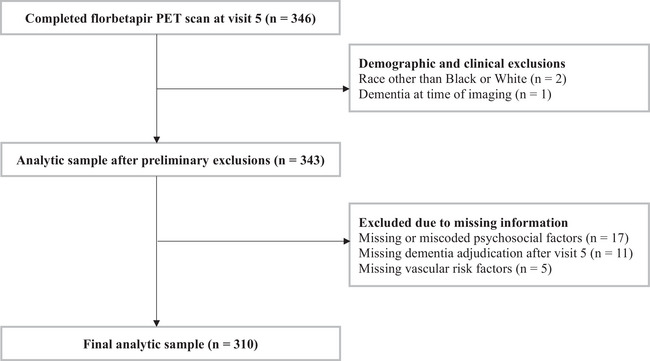
Study inclusion and exclusion criteria. PET, positron emission tomography.
2.2. Psychosocial measures
Perceived social support was evaluated at visit 2 using the Interpersonal Support Evaluation List‐Short Form (ISEL‐SF; Figure S1). This 16‐item scale was constructed by ARIC investigators from the original 40‐item full scale and assesses perceived social support with four subscales including (1) appraisal support, (2) tangible assets support, (3) belonging support, and (4) self‐esteem support. The total score is an equally weighted sum, with scores ranging from 0 to 48. Higher scores indicate greater perceived social support. 18 , 19 Total ISEL‐SF score was categorized into distribution‐based tertiles of the total ARIC sample assessed at visit 2 (high ≥42, intermediate 36–41, and low ≤35). 13
Social isolation was also evaluated at visit 2 by use of the Lubben Social Network Scale (LSNS; Figure S2). This 10‐item scale assesses the size of the participant's active social network and the perceived social support received by family, friends, and neighbors. The total score is an equally weighted sum, with scores ranging from 0 to 50. Higher scores indicate lower risk of social isolation. 20 , 21 Although not evenly distributed, scores were divided into four categories based upon the Lubben criteria, which has been used in subsequent ARIC papers: ≤20 = isolated; 21–25 = high risk for isolation; 26–30 = moderate risk for isolation; ≥31 = low risk for isolation. 7 , 20 , 21 , 22 , 23
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using PubMed, specifically for studies examining psychosocial health and dementia risk. Studies examining psychosocial measures and long‐term health outcomes using a cross‐sectional design are widespread, but fewer examine how behaviors underlying social relationships, like social support and isolation, may modify cognitive outcomes and dementia incidence longitudinally.
Interpretation: Although mid‐life social relationships did not modify the association between late‐life amyloid burden and incident dementia, our results nonetheless emphasize the important independent contributions of mid‐life social relationships to dementia risk, even in the presence of amyloid pathology.
Future directions: Our findings support the protective role that strong social relationships in mid‐life may have on dementia risk. Future studies are needed to evaluate the most effective ways to measure social behaviors and how such factors may preserve cognition in the presence of brain pathology.
Following the categorization of both psychosocial measures, participants fell into 12 different groups. Performance on the ISEL‐SF and LSNS was paired to create a measure reflecting social relationships. Participants were classified as having strong, average, or poor social relationships in mid‐life (Figure 2). This conversion into a categorical composite measure that encompasses both psychosocial measures was conducted for several reasons: (1) because the relationship between continuous ISEL‐SF and LSNS scores in this sample was weak (Figure 3; R2 = 0.2009), which may indicate that feelings of social support and social isolation are not linear with one another; (2) to help offset the small number of participants who were categorized as “high risk of isolation” or “isolated”; and (3) to assess social relationships more globally. 24 , 25
FIGURE 2.
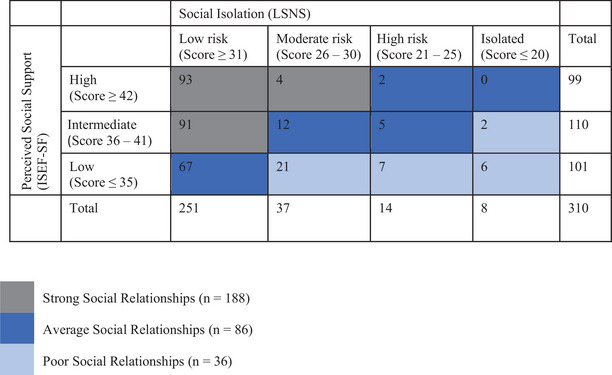
Categorization of social relationships. ISEL‐SF, Interpersonal Support Evaluation List‐Short Form; LSNS, Lubben Social Network Scale.
FIGURE 3.
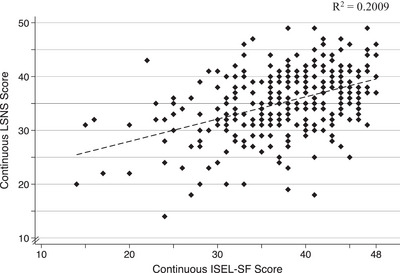
Two‐way scatterplot displaying continuous LSNS score and continuous ISEL‐SF score. Dashed line indicates the linear fit between continuous scores. ISEL‐SF, Interpersonal Support Evaluation List Short Form; LSNS, Lubben Social Network Scale.
2.3. Brain MRI and PET
Brain MRI scans, obtained at a 3T MRI facility near each field center, were read and preprocessed centrally at the Mayo Clinic. 16 , 26 The details of PET image processing and co‐registration with MRI, carried out at the Johns Hopkins University reading center, were described previously. 17 A global cortical measure of florbetapir uptake was used as a weighted average (based on region of interest size) of the orbitofrontal, prefrontal, and superior frontal cortices; the lateral temporal, parietal, and occipital lobes; the precuneus, anterior cingulate, and the posterior cingulate. An automated region for cerebellum gray was used as reference. Because continuous florbetapir standardized uptake values (SUVRs) are highly skewed, SUVRs were dichotomized at the sample median of >1.2. 17 Florbetapir PET scans were obtained within 1 year of MRI scans (ideally within 6 months).
2.4. Dementia
Participants with dementia at the time of the ARIC‐NCS visit 5 were previously excluded from the ARIC‐PET study and not part of the present analytic sample. As part of ARIC‐NCS visits 5–7, participants seen in‐person underwent detailed cognitive testing, and a subset had informant interviews. All participants were given a classification of normal cognition, mild cognitive impairment (MCI), or dementia following standard diagnostic criteria and a physician‐ and neuropsychologist‐led adjudication process. 15 , 27 Participants had to be seen in‐person at ARIC‐NCS visit 5 to be included in the primary ARIC‐PET sample but did not have to be seen in‐person at subsequent visits for inclusion in this sample. For post‐visit 5 incident dementia case identification, ongoing dementia ascertainment and surveillance in the parent ARIC cohort included in‐person visits and ongoing surveillance. 27 Dementia cases were identified through several sources, including, the administration of the Six Item Screener (SIS) annually by phone and the Ascertain Dementia 8‐Item Informant Questionnaire (AD8) when appropriate. 27 , 28 , 29 Hospitalization codes and death certificates identified additional cases of dementia during follow‐up. The date of dementia onset was defined as the earliest date of SIS/AD8 interviews, the date of hospitalization records with a dementia diagnosis, or the date of the in‐person visit when a participant was classified as having dementia. Of participants with diagnoses based on SIS/AD8, hospitalization records, or death certificates, dementia onset was defined as 180 days prior to the interview, hospitalization, or death.
2.5. Covariates
Covariates included age (at visit 2), sex, education (less than high school, high school or equivalent, and greater than high school), race, and apolipoprotein E (APOE) ɛ4 genotype (0 or ≥1 allele). Of the 310 participants, five were missing APOE genotyping. Leisure‐Time Physical Activity (LTPA) was assessed at visit 1 using the Modified Baecke Physical Activity Questionnaire. 30 , 31 For this analysis, participants were defined as “inactive” if they reported 0 min/week of LTPA at visit 1. 30 , 31 , 32 The Maastricht Vital Exhaustion Questionnaire was administered at visit 2 to measure symptoms of depression and fatigue. 22 , 33 , 34 Questionnaire scores were dichotomized at ≥14 to indicate depressive and fatigue symptomology. 22 Marital status was self‐reported at visit 2 and characterized as married or not married (divorced, separated, widowed, or never married). Vascular risk factors, such as hypertension (systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, or use of antihypertensive medications), diabetes (fasting glucose ≥126 mg/dL, non‐fasting glucose ≥200 mg/dL, HbA1c ≥6.5, self‐report of physician‐diagnosed diabetes, or use of oral diabetes medications or insulin), smoking/drinking (self‐report, binarized into current vs non), obesity (BMI ≥30 kg/m2), and elevated total cholesterol (≥200 mg/dL) were all collected at visit 2. As described in Section 2.4 and because dementia was an exclusionary diagnosis for inclusion in the ARIC‐PET study, each participant's cognitive status at visit 5 was classified as normal cognition or MCI.
2.6. Statistical analysis
Participant characteristics were evaluated in the analytic sample (Table 1) and the total ARIC visit 2 sample (Table S1). Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between elevated global SUVR and incident dementia between ARIC‐PET visit 5 and December 31, 2019 (with visit 5 considered to be time 0). Within models, the contributions of mid‐life social relationships as a categorical measure (strong, average, or poor) in addition to dichotomous florbetapir SUVR were evaluated independently. To evaluate for effect modification, we tested for multiplicative interactions by including an interaction term between social relationships and elevated global SUVR. Model 1 was adjusted for demographics (age, sex, education, race, marital status) and APOE ε4 status. Model 2 further adjusted for depressive/fatigue symptoms, vascular risk factors, and physical inactivity. Model 3 additionally included cognitive status at visit 5. However, primary inferences should be drawn from model 2 because MCI is likely on the causal pathway between the exposures and incident dementia. Exploratory analyses examined the three‐way interaction between sex, amyloid burden, and dementia risk. Sensitivity analyses were conducted in models stratified by cognitive status at visit 5, excluding participants with prevalent stroke at visit 5, and using global SUVRs as a scaled continuous measure (scaled = SUVR × 10). Additional sensitivity models assessed the contributions of social support and social isolation as continuous and categorical measures. Because these factors were paired to create the “social relationships” variable, the purpose of these analyses was to see whether the observed effects of psychosocial measures on the association between amyloid burden with dementia were congruent across different behaviors related to psychosocial health. Stata SE, version 17 for Macintosh (Stata Corp) was used for all analyses; p < 0.05 was considered statistically significant, and testing was two‐sided.
TABLE 1.
Characteristics of the ARIC‐PET analytic sample at visit 2, stratified by levels of social relationships (n = 310).
| Characteristics | Strong social relationships (n = 188) | Average social relationships (n = 86) | Poor social relationships (n = 36) | p value |
|---|---|---|---|---|
| Age, y, mean (SD) | 54.8 (5.2) | 55.7 (5.4) | 54.9 (4.3) | 0.40 |
| Race, N (%) | 0.94 | |||
| Black (n = 125) | 75 (39.9) | 36 (41.9) | 14 (38.9) | |
| White (n = 185) | 113 (60.1) | 50 (58.1) | 22 (61.1) | |
| Sex, N (%) | 0.002 | |||
| Female (n = 175) | 119 (63.3) | 44 (51.2) | 12 (33.3) | |
| Male (n = 135) | 69 (36.7) | 42 (48.8) | 24 (66.7) | |
| Field Center, N (%) | 0.68 | |||
| Forsyth | 47 (25.0) | 16 (18.6) | 6 (16.7) | |
| Jackson | 71 (37.8) | 34 (39.5) | 14 (38.9) | |
| Minneapolis | — | — | — | |
| Washington | 70 (37.2) | 36 (41.9) | 16 (44.4) | |
| APOE ε4 status, N (%) (n = 305) | 0.83 | |||
| Non‐carrier | 127 (67.6) | 58 (67.4) | 27 (75.0) | |
| Carrier | 58 (30.9) | 27 (31.4) | 8 (22.2) | |
| Education level, N (%) | 0.001 | |||
| Less than high school | 23 (12.2) | 21 (24.4) | 2 (5.6) | |
| High school or equivalent | 73 (38.8) | 44 (51.2) | 18 (50.0) | |
| More than high school | 92 (48.9) | 21 (24.4) | 16 (44.4) | |
| Not married, N (%) | 28 (14.9) | 16 (18.6) | 12 (33.3) | 0.03 |
|
Elevated florbetapir global SUVR (>1.2) at visit 5, N (%) |
101 (53.7) | 36 (41.9) | 13 (36.1) | 0.06 |
| Cognitive status of MCI at visit 5, N (%) | 42 (22.3) | 27 (31.4) | 13 (36.1) | 0.11 |
| Physically inactive at visit 1 (0 min/wk), N (%) | 71 (37.8) | 36 (41.9) | 13 (36.1) | 0.77 |
| Depressive/fatigue symptoms, N (%) | 42 (22.3) | 34 (39.5) | 15 (41.7) | 0.003 |
| Hypertension, N (%) | 50 (26.6) | 26 (30.2) | 15 (41.7) | 0.19 |
| Diabetes, N (%) | 14 (7.5) | 14 (16.3) | 4 (11.1) | 0.08 |
| Body mass index (≥30 kg/m2), N (%) | 50 (26.6) | 25 (29.1) | 14 (38.9) | 0.33 |
| Total cholesterol (≥200 mg/dL), N (%) | 93 (49.5) | 39 (45.4) | 14 (38.9) | 0.47 |
| Current smoker, N (%) | 28 (14.9) | 16 (18.6) | 5 (13.9) | 0.70 |
| Current drinker, N (%) | 103 (54.8) | 44 (51.2) | 17 (47.2) | 0.66 |
| Developed dementia by visit 7, N (%) | 25 (13.3) | 16 (18.6) | 7 (19.4) | 0.42 |
| Died prior to visit 7, N (%) | 13 (6.9) | 7 (8.1) | 3 (8.3) | 0.92 |
Note: Baseline differences assessed with analysis of variance (ANOVA) for continuous variables and χ2 tests for categorical values. No PET scans were conducted at Minneapolis ARIC Field Center.
Abbreviations: APOE, apolipoprotein E; MCI, mild cognitive impairment; SUVR, standardized uptake value ratio.
3. RESULTS
3.1. Characteristics of the analytic sample
A total of 310 participants comprised the analytic sample; 175 (56.5%) were women and 125 (40.3%) were Black (Table 1). The mean (SD) age of participants at visit 2 when psychosocial measures were evaluated was 55.1 (5.1) years and the mean age at visit 5 when PET imaging occurred was 77.8 (5.3) years. The median follow‐up after PET imaging was 4.7 years (interquartile cut‐points: 4.0 and 5.2 years), during which 48 participants (15.5%) developed dementia.
3.2. Contributions of social relationships and brain amyloid to dementia risk
Across models, participants with elevated global SUVR had greater risk of developing incident dementia (Table 2). Independent of this effect, model 2 further showed that participants with strong (HR 0.35, 95% 0.13–0.94) or average social relationships in mid‐life (HR 0.27, 95% CI 0.10–0.78), relative to those with poor social relationships in mid‐life, had a reduced risk of developing incident dementia (Table 2). The relative contributions of mid‐life social relationships and elevated global SUVR on dementia risk are shown (Figure 4). Although social relationships in mid‐life independently predicted dementia risk, this factor did not interact with elevated global SUVR on a multiplicative scale (p‐interaction values between 0.41 and 0.93). Consistent with published results, 27 older age, APOE ε4, and lower educational attainment were associated with elevated risk of incident dementia in late life (Table 2). Exploratory analyses that examined the three‐way interaction between sex, amyloid burden, and dementia risk were not statistically significant. Sex‐stratified model findings are shown (Table S2).
TABLE 2.
Adjusted hazard ratios for association of mid‐life social relationships and elevated florbetapir global SUVR with dementia risk (n = 305).
| Variables | Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 3 HR (95% CI) |
|---|---|---|---|
| Strong social relationships | 0.32 (0.12–0.86) * | 0.35 (0.13–0.94) * | 0.45 (0.16–1.23) |
| Average social relationships | 0.29 (0.10–0.80) * | 0.27 (0.10–0.78) * | 0.28 (0.10–0.80) * |
| Poor social relationships | Reference | Reference | Reference |
| Elevated florbetapir global SUVR >1.2 | 4.04 (1.93–8.46) ** | 4.14 (1.93–8.87) ** | 3.25 (1.47–7.17) ** |
| Age (per 1 year) | 1.15 (1.07–1.23) ** | 1.15 (1.07–1.23) ** | 1.16 (1.08–1.25) ** |
| Race (Black) | 1.71 (0.90–3.22) | 1.97 (1.01–3.82) * | 1.94 (0.99–3.80) |
| Sex (Female) | 0.55 (0.29–1.03) | 0.58 (0.28–1.18) | 0.56 (0.27–1.16) |
| APOE ε4 carrier | 2.27 (1.25–4.14) ** | 2.06 (1.11–3.81) * | 2.13 (1.15–3.94) * |
| Education | |||
| Less than high school | 2.80 (1.31–5.96) ** | 2.99 (1.27–7.01) * | 3.61 (1.51–8.65) ** |
| High school or equivalent | 0.79 (0.38–1.65) | 0.91 (0.40–2.04) | 1.06 (0.47–2.38) |
| More than high school | Reference | Reference | Reference |
| Not married | 1.17 (0.53–2.59) | 0.86 (0.35–2.10) | 1.02 (0.41–2.55) |
| Depressive/fatigue symptoms | — | 0.91 (0.43–1.94) | 0.98 (0.45–2.13) |
| Hypertension | — | 1.02 (0.54–1.95) | 1.20 (0.62–2.32) |
| Diabetes | — | 2.77 (1.20–6.37) * | 2.04 (0.84–4.96) |
| Body mass index (≥30 kg/m2) | — | 1.73 (0.88–3.40) | 1.88 (0.96–3.71) |
| Total cholesterol (≥200 mg/dL) | — | 0.52 (0.27–0.98) * | 0.54 (0.29–1.00) |
| Current smoker | — | 1.22 (0.48–3.10) | 1.50 (0.58–3.89) |
| Current drinker | — | 0.54 (0.28–1.05) | 0.68 (0.34–1.35) |
| Physical inactivity at visit | — | 0.75 (0.39–1.43) | 0.76 (0.40–1.45) |
| Having MCI at visit 5 | — | — | 2.47 (1.22–5.00) * |
Note: Model 1 adjusted for age, race, sex, APOE ε4, education, and marital status. Model 2 adjusted for model 1 covariates in addition to depressive/fatigue symptoms, vascular risk factors, and physical inactivity. Model 3 adjusted for model 2 covariates in addition to having MCI at visit 5.
Abbreviations: APOE, apolipoprotein E; CI, confidence interval; HR, hazard ratio; MCI, mild cognitive impairment; SUVR, standardized uptake value ratio.
p ≤ 0.05.
p ≤ 0.01.
FIGURE 4.
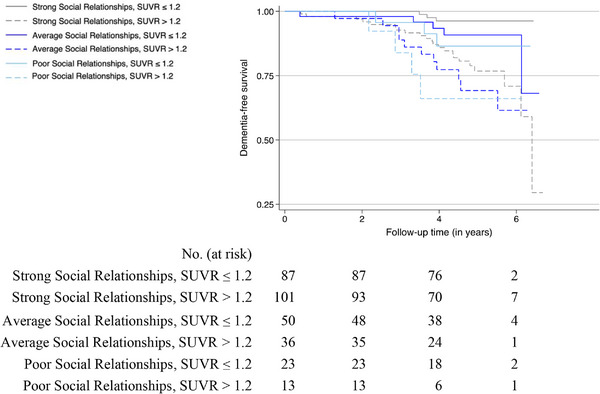
Kaplan‐Meier curves for dementia‐free survival by social relationships and elevated florbetapir global SUVR status. Follow‐up time begins at ARIC‐PET visit 5 (mean (SD) age 77.8 (5.3) years), whereas social relationships were measured in mid‐life at ARIC visit 2 (mean (SD) age 55.1 (5.1) years). Rates of dementia incidence differed significantly (log‐rank p < 0.001) in participants with varying levels of social relationships and elevated global SUVR. SUVR, standardized uptake value ratio.
3.3. Sensitivity analyses
Models stratified by cognitive status at visit 5 showed independent associations between elevated global SUVR with dementia risk in both cognitive groups, but otherwise the only significant association to persist was that between average social relationships in mid‐life and dementia risk in participants with MCI (Table S3). Notably in MCI participants, the interaction between mid‐life social relationships and elevated global SUVR approached statistical significance across models (p < 0.10), but given small numbers, this result should be interpreted with caution. In models that excluded participants with reported prevalent stroke at visit 5 (n = 13) or used scaled continuous global SUVRs (scaled = SUVR × 10), independent associations, like those reported in the full sample, were shown (Tables S4 and S5). However, the association between strong social relationships in mid‐life with dementia risk was no longer significant in models using scaled continuous SUVRs, and the effect size of the association between scaled continuous SUVR with dementia risk was smaller than that observed between binary elevated SUVR and dementia risk.
Models assessing the independent contributions of mid‐life measures of social support and social isolation on the association between elevated global SUVR with incident dementia risk are shown (Tables S6–S8). Across models, elevated global SUVR predicted dementia risk. Associations between continuous ISEL‐SF and LSNS scores with elevated global SUVR were non‐significant. In models which used the ISEL‐SF categorically, only participants with intermediate social support in mid‐life, relative to low social support, were less likely to develop dementia (models 1 and 2). In models which used the LSNS categorically, participants with low risk or high risk of social isolation in mid‐life were less likely to develop dementia, relative to participants who were categorized as isolated (models 1‐3). This association was not statistically significant in participants with a moderate risk of social isolation in mid‐life. No multiplicative interactions between mid‐life measures of social support (or social isolation) and amyloid burden with dementia risk were shown.
4. DISCUSSION
Contrary to our hypothesis we did not find evidence that strong social relationships in mid‐life significantly modify the relationship between amyloid burden and dementia risk. However, we found that mid‐life social relationships and elevated amyloid burden independently contribute to subsequent risk of dementia. Specifically, we observed that participants who were categorized as having strong or average social relationships in mid‐life had a lower risk of developing dementia, relative to participants who had poor social relationships in mid‐life. This finding is consistent with previous studies which have shown that strong social relationships help to preserve cognitive function, thus prolonging the development of dementia. 6 , 11 Furthermore, the finding that elevated global SUVR predicted increased risk of dementia is consistent with previous studies. 27 Model findings remained robust to adjustments for demographics, APOE ε4, depressive/fatigue symptoms, vascular risk factors, and physical inactivity. Associations between strong social relationships in mid‐life and dementia risk were no longer statistically significant when MCI was added to the model. This likely reflects that MCI is on the causal pathway to dementia and model 3 may be over‐adjusted as a result.
Similar to the null interaction effect in the present study, no effect modification was shown in a recent ARIC study that examined how mid‐life vascular risk factors, including hypertension, smoking, diabetes, obesity, and total cholesterol, may modify the association between amyloid burden and incident dementia. 27 This may reflect that mid‐life factors (including but not limited to psychosocial and/or vascular risk factors) are driving dementia risk through processes that are independent of amyloid and may include other mechanisms such as neuroinflammation, cerebrovascular burden, or tauopathy. Such ideas have been proposed by others studying protective risk factors for elevated amyloid burden and Alzheimer's disease (AD)–related pathology. 8 , 11 , 35 , 36 , 37 , 38
Across sensitivity analysis models using mid‐life social support and social isolation as exposure measures, elevated global SUVR predicted dementia risk. In models assessing mid‐life social support as a categorical measure, participants with intermediate social support, relative to low social support, were less likely to develop dementia. Models assessing levels of mid‐life social isolation with dementia risk showed that participants at “risk for social isolation,” compared to those categorized as “isolated,” were less likely to develop dementia. Altogether, these findings reiterate the protective effect psychosocial factors may have on dementia risk.
The rationale that led us to primarily examine social relationships as a composite measure was based upon the idea that social support and social isolation are independent constructs yet with underlying similarities. 24 , 25 The ISEL‐SF is thought to measure perceived social support, which has been shown to vary little over time, and focuses on the support that participants feel they are receiving with little emphasis on reciprocated social support. 25 The LSNS is largely objective and captures many aspects of social relationships including social support, relationship closeness with friends and family, and aspects of loneliness, all of which may vary situationally and temporally. 25 Both scales have a collective emphasis on relationships closeness with friends and family, with a lesser focus on how individuals see themselves and their role within their greater community. Continuing to view psychosocial health as a comprehensive measure will likely remain important as the field aims to design and tailor intervention methods that are able to successfully target many facets of psychosocial health. 4 , 37
Psychosocial measures were surveyed at visit 2 and amyloid burden was not measured until visit 5. We acknowledge that social relationships often change as individuals age from their mid‐50s to late 70s, and that psychosocial health in later life may have a stronger influence on amyloid burden. An optimal way to capture these changes would be to have psychosocial factors measured at multiple timepoints. Despite having psychosocial measures solely evaluated in mid‐life, the longitudinal design of this study can be considered a strength for several reasons. First, it means that there is a smaller chance that our results are reflecting reverse causation, which remains a major challenge in observational research. 37 Next, assessments of psychosocial factors cross‐sectionally are limited in their ability to make inferences about the life course or the mechanism by which such pathological changes occur over time. 3 , 4 , 5 Moreover, the assessment of psychosocial factors in late life is arguably too late for meaningful intervention. Relatedly, preliminary intervention efforts have favored a preventive approach, which suggests that engaging in meaningful relationships in mid‐life means individuals are more likely to retain those relationships and habits as they age. 4
There are limitations to the present study. Survival bias may have influenced our findings and the relationships observed in a few different ways. First, to be included in this analytic sample, participants had to be non‐demented and alive well into their 70s. Still, sensitivity analyses that stratified the sample by cognitive status at visit 5 indicated that associations between social relationships in mid‐life and dementia risk may have been driven by the portion of the sample that had MCI (n = 81). Although this can be viewed as a limitation, it may also indicate that in a sample with poorer overall health, mid‐life social relationships may have had larger implications on the association between amyloid burden and incident dementia risk. This was further supported by the marginally significant interaction effect between mid‐life social relationships and elevated global SUVR in MCI participants.
Next, we observed that the proportion of participants who developed incident dementia in this study sample (15.5%) is closely aligned with global estimates of AD in this age group, 39 , 40 whereas the prevalence of amyloid positivity in this sample is relatively high (48.4%). It remains possible that a clinical effect from amyloid burden would have emerged over a longer follow‐up period (median follow‐up from amyloid PET was 4.7 years). Taken together, these findings pose the question as to how many more participants in this group will eventually develop incident dementia. We recognize that there remain other factors that may impact amyloid burden, yet not dementia. Ultimately, such factors should be taken into consideration prior to generalizing the associations shown in this study to the entire population. Likewise, the sample represented in this study was limited to Black and White participants. We are hopeful that others will continue to build upon our findings and refine our measure of social relationships in additional ethnically diverse populations.
Finally, some of the associations shown between psychosocial measures with dementia risk lacked a clear dose‐response relationship; nor were the same associations consistently shown when examining categorical levels of social support, isolation, or relationships. This may reflect that the numeric cut‐offs used to distinguish levels of each measure are not sufficiently sensitive to pick up the degree of variation attributed to each. For this purpose, we also assessed findings with the ISEL‐SF and LSNS scored continuously but did not find significant associations with amyloid burden or effect modification on dementia risk.
In conclusion, stronger social relationships in mid‐life were associated with a lower risk of developing dementia, independent of elevated global SUVR. Despite no statistical evidence of an interaction, our results nonetheless emphasize the important independent contributions of mid‐life social relationships to dementia risk, even in the presence of amyloid pathology. Future studies are needed to evaluate the most effective ways to measure social behaviors and how such factors may preserve cognition in the presence of brain pathology.
CONFLICT OF INTEREST STATEMENT
Renée C. Groechel, Albert C. Liu, Chelsea Liu, Silvia Koton, Anna M. Kucharska‐Newton, Pamela L. Lutsey, Thomas H. Mosley Jr., Priya Palta, A. Richey Sharrett, Keenan A. Walker, Dean F. Wong, Rebecca F. Gottesman: no conflicts to report. David S. Knopman: serves on a data safety monitoring board for the Dominantly Inherited Alzheimer Network Treatment Unit study. He served on a data safety monitoring board for a tau therapeutic for Biogen (until 2021) but received no personal compensation. He is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the University of Southern California. He has served as a consultant for Roche, Samus Therapeutics, Magellan Health, Biovie, and Alzeca Biosciences but receives no personal compensation. He attended an Eisai advisory board meeting for lecanemab in December 2, 2022, but received no compensation directly or indirectly. He receives funding from the NIH. Author disclosures are available in the Supporting Information.
CONSENT STATEMENT
The study was approved by each institutional review board. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Diversity, equity, and inclusion (DEI) was addressed in the study design, execution, and interpretation. Participants came from multiple study centers across the United States characterized by varying economic and racial profiles. When analyzing and interpreting the results, the study accounted for differences in age, sex, and race.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The authors thank the staff and participants of the Atherosclerosis Risk in Communities (ARIC) study for their important contributions. The ARIC study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (NHLBI) (75N92022D00001, 75N92022D00002, 75N92022D00003, 75N92022D00004, 75N92022D00005). The ARIC Neurocognitive Study is supported by U01HL096812, U01HL096814, U01HL096899, U01HL096902, and U01HL096917 from the National Institutes of Health (NHLBI, NINDS, National Institute on Aging [NIA], and National Institute on Deafness and Communications Disorders [NIDCD]). The authors thank the staff and participants of the ARIC study for their important contributions. The ARIC‐Positron Emission Tomography (PET) study is funded by the NIA (R01AG040282). Avid Radiopharmaceuticals provided the florbetapir isotope for the study. Renée C. Groechel and Rebecca F. Gottesman were supported by the NINDS Intramural Research Program.
Groechel RC, Liu AC, Liu C, et al. Social relationships, amyloid burden, and dementia: The ARIC‐PET study. Alzheimer's Dement. 2024;16:e12560. 10.1002/dad2.12560
REFERENCES
- 1. Boyle PA, Yu L, Leurgans SE, et al. Attributable risk of Alzheimer's dementia attributed to age‐related neuropathologies. Ann Neurol. 2019;85(1):114‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Duffner LA, DeJong N, Jansen JF, et al. Associations between social health factors, cognitive activity and neurostructural markers for brain health–a systematic literature review and meta‐analysis. Ageing Res Rev. 2023;89:101986. [DOI] [PubMed] [Google Scholar]
- 3. Fratiglioni L, Marseglia A, Dekhtyar S. Ageing without dementia: can stimulating psychosocial and lifestyle experiences make a difference? Lancet Neurol. 2020;19(6):533‐543. [DOI] [PubMed] [Google Scholar]
- 4. Holt‐Lunstad J. Why social relationships are important for physical health: a systems approach to understanding and modifying risk and protection. Annu Rev Psychol. 2018;69:437‐458. [DOI] [PubMed] [Google Scholar]
- 5. Sachdev SP. Social health, social reserve and dementia. Curr Opin Psychiatry. 2022;35(2):111‐117. doi: 10.1097/YCO.0000000000000779 [DOI] [PubMed] [Google Scholar]
- 6. Chan D, Shafto M, Kievit R, et al. Lifestyle activities in mid‐life contribute to cognitive reserve in late‐life, independent of education, occupation, and late‐life activities. Neurobiol Aging. 2018;70:180‐183. doi: 10.1016/j.neurobiolaging.2018.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kats D, Patel MD, Palta P, et al. Social support and cognition in a community‐based cohort: the Atherosclerosis Risk in Communities (ARIC) study. Age Ageing. 2016;45(4):475‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ma YH, Wang YY, Tan L, et al. Social networks and cerebrospinal fluid biomarkers of Alzheimer's disease pathology in cognitively intact older adults: the CABLE study. J Alzheimers Dis. 2021;81(1):263‐272. doi: 10.3233/JAD-201426 [DOI] [PubMed] [Google Scholar]
- 9. Pettigrew C, Soldan A, Zhu Y. Cognitive reserve and cortical thickness in preclinical Alzheimer's disease. Brain Imaging Behav. 2017;11:357‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Serra L, Cercignani M, Petrosini L, et al. Neuroanatomical correlates of cognitive reserve in Alzheimer disease. Rejuvenation Res. 2011;14(2):143‐151. [DOI] [PubMed] [Google Scholar]
- 11. Biddle KD, Uquillas FDO, Jacobs HI, et al. Social relationships and amyloid‐β‐related cognitive decline in cognitively normal older adults. Am J Geriatr Psychiatry. 2019;27(11):1247‐1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donovan NJ, Okereke OI, Vannini P, et al. Association of higher cortical amyloid burden with loneliness in cognitively normal older adults. JAMA Psychiatry. 2016;73(12):1230‐1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Groechel RC, Liu AC, Koton S, et al. Associations between mid‐life psychosocial measures and estimated late life amyloid burden: the Atherosclerosis Risk in Communities (ARIC)‐PET Study. J Alzheimers Dis. 2024;97(4):1901‐1911. doi: 10.3233/JAD-231218 [DOI] [PubMed] [Google Scholar]
- 14. Wright JD, Folsom AR, Coresh J, et al. The ARIC (Atherosclerosis Risk in Communities) Study: JACC focus seminar 3/8. J Am Coll Cardiol. 2021;77(23):2939‐2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities neurocognitive study. Alzheimer's Dement Diagnosis Assess Dis Monit. 2016;2:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Knopman DS, Griswold ME, Lirette ST, et al. Vascular imaging abnormalities and cognition: mediation by cortical volume in nondemented individuals: Atherosclerosis Risk in Communities‐neurocognitive Study. Stroke. 2015;46(2):433‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gottesman RF, Schneider ALC, Zhou Y, et al. The ARIC‐PET amyloid imaging study: brain amyloid differences by age, race, sex, and APOE. Neurology. 2016;87(5):473‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cohen S, Hoberman HM. Positive events and social supports as buffers of life change stress. J Appl Soc Psychol. 1983;13(2):99‐125. [Google Scholar]
- 19. Payne TJ, Andrew M, Butler KR, Wyatt SB, Dubbert PM, Mosley TH Jr. Psychometric evaluation of the interpersonal support evaluation list–short form in the ARIC Study Cohort. SAGE Open. 2012;2(3):2158244012461923. [Google Scholar]
- 20. Lubben JE. Assessing social networks among elderly populations. Fam Community Health. 1988;11(3):42‐52. [Google Scholar]
- 21. Lubben J, Blozik E, Gillmann G, et al. Performance of an abbreviated version of the Lubben Social Network Scale among three European community‐dwelling older adult populations. Gerontologist. 2006;46(4):503‐513. [DOI] [PubMed] [Google Scholar]
- 22. Honda Y, Mok Y, Mathew L, et al. Psychosocial factors and subsequent risk of hospitalizations with peripheral artery disease: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2021;329:36‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nagayoshi M, Everson‐Rose SA, Iso H, Mosley TH, Rose KM, Lutsey PL. Social network, social support, and risk of incident stroke. Stroke. 2014;45(10):2868‐2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu J, Fitzgerald SM, Owen AJ, et al. Social isolation, social support, loneliness and cardiovascular disease risk factors: a cross‐sectional study among older adults. Int J Geriatr Psychiatry. 2021;36(11):1795‐1809. doi: 10.1002/gps.5601 [DOI] [PubMed] [Google Scholar]
- 25. Sakr‐Ashour F. Concept clarification and assessment of social isolation and social support in older adults. Administration for Community Living Office of Performance and Evaluation. New Editions Consulting, Inc; 2021. [Google Scholar]
- 26. Jack CR Jr, Bernstein MA, Borowski BJ, et al. Update on the magnetic resonance imaging core of the Alzheimer's disease neuroimaging initiative. Alzheimer Dement. 2010;6(3):212‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gottesman RF, Wu A, Coresh J, et al. Associations of vascular risk and amyloid burden with subsequent dementia. Ann Neurol. 2022;92:607‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carpenter CR, DesPain B, Keeling TN, Shah M, Rothenberger M. The Six‐Item Screener and AD8 for the detection of cognitive impairment in geriatric emergency department patients. Ann Emerg Med. 2011;57(6):653‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559‐564. [DOI] [PubMed] [Google Scholar]
- 30. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936‐942. [DOI] [PubMed] [Google Scholar]
- 31. Richardson MT, Ainsworth BE, Wu HC, Jacobs DR Jr, Leon AS. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke questionnaire to assess leisure–time physical activity. Int J Epidemiol. 1995;24:685‐693. [DOI] [PubMed] [Google Scholar]
- 32. Palta P, Sharrett AR, Gabriel KP, et al. Prospective analysis of leisure‐time physical activity in midlife and beyond and brain damage on MRI in older adults. Neurology. 2021;96(7):e964‐e974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Appels A, Hoppener P, Mulder PA. A questionnaire to assess premonitory symptoms of myocardial infarction. Int J Cardiol. 1987;17(1):15‐24. [DOI] [PubMed] [Google Scholar]
- 34. Williams JE, Mosley TH Jr, Kop WJ, Couper DJ, Welch VL, Rosamond WD. Vital exhaustion as a risk factor for adverse cardiac events (from the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol. 2010;105(12):1661‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arenaza‐Urquijo EM, Bejanin A, Gonneaud J, et al. Association between educational attainment and amyloid deposition across the spectrum from normal cognition to dementia: neuroimaging evidence for protection and compensation. Neurol Aging. 2017;59:72‐79. [DOI] [PubMed] [Google Scholar]
- 36. Jagust WJ, Mormino EC. Lifespan brain activity, β‐amyloid, and Alzheimer's disease. Trends Cogn Sci. 2011;15(11):520‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sommerlad A, Kivimäki M, Larson EB, et al. Social participation and risk of developing dementia. Nature Aging. 2023;3(5):532‐545. [DOI] [PubMed] [Google Scholar]
- 38. Vemuri P, Knopman DS, Lesnick TG, et al. Evaluation of amyloid protective factors and Alzheimer disease neurodegeneration protective factors in elderly individuals. JAMA Neurol. 2017;74(6):718. doi: 10.1001/jamaneurol.2017.0244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alzheimer's Association . 2023 Alzheimer's disease facts and figures. Alzheimers Dement. 2023;19(4):1598‐1695. doi: 10.1002/alz.13016 [DOI] [PubMed] [Google Scholar]
- 40. Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. Population estimate of people with clinical Alzheimer's disease and mild cognitive impairment in the United States (2020‐2060). Alzheimers Dement. 2021;17(12):1966‐1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


